- 1Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China
- 2Advanced Center of Stroke, Beijing Institute for Brain Disorders, Beijing, China
- 3Department of China-America Institute of Neuroscience, Xuanwu Hospital, Capital Medical University, Beijing, China
- 4Department of Neurosurgery, Wayne State University School of Medicine, Detroit, MI, United States
- 5Department of Neurosurgery, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, Buffalo, NY, United States
- 6Department of Neurosurgery, Gates Vascular Institute at Kaleida Health, Buffalo, NY, United States
- 7Department of Cardiology, Anzhen Hospital, Capital Medical University, Beijing, China
- 8Department of Ultrasonography, Tiantan Hospital, Capital Medical University, Beijing, China
- 9School of Basic Medical Sciences, Capital Medical University, Beijing, China
Background and purpose: Stroke is a leading cause of death and acquired disability in adults today. Inflammation plays an important role in the pathophysiology of stroke. The peripheral neutrophil-to-lymphocyte ratio (NLR) is an important global inflammatory indicator becoming more mainstream in stroke care. This meta-analysis aims to evaluate the relationship between the baseline NLR and acute ischemic and hemorrhagic stroke, as well as define the clinical significance of NLR in subtypes of ischemic stroke.
Methods: This meta-analysis was registered in PROSPERO with the number CRD42018105305. We went through relevant articles from PubMed Central (PMC) and EMBASE. Prospective and retrospective studies were included if related to baseline NLR levels prior to treatment in patients with ischemic or hemorrhagic stroke. Studies were identified up until April 2019. The cutoff value for NLR and the sources of odds ratios (ORs)/risk ratios (RRs) were measured. Modified Rankin Scale (mRS) was used to investigate the outcomes during clinical follow-up. Predefined criteria were used to evaluate the risk of bias in eligible studies. P-values < 0.05 were considered statistically significant. STATA version 14.0 (STATA, College Station, TX) was used in all statistical analyses.
Results: Thirty-seven studies with 43,979 individuals were included in the final analysis. Higher NLR levels were correlated with increased risk of ischemic stroke (ORs/RRs = 1.609; 95% CI = 1.283–2.019), unfavorable functional outcome at 3 months (ORs/RRs = 1.851; 95% CI = 1.325–2.584), and increased mortality in patients with ischemic stroke (ORs/RRs = 1.068; 95% CI = 1.027–1.111). While in terms of hemorrhagic stroke (including SAH and ICH), elevated NLR levels only had deleterious effects on mortality (ORs/RRs = 1.080; 95% CI = 1.018–1.146).
Conclusions: Baseline NLR level is a promising predictor of the clinical outcomes in both ischemic and hemorrhagic stroke. In addition, elevated NLR is also associated with a high risk of ischemic stroke occurrence. However, future studies are needed to demonstrate the underlying mechanisms and further explain this association.
Background
Stroke is a leading cause of death and acquired disability in adults (1). The major subtypes of stroke are ischemic stroke and hemorrhagic stroke, representing approximately 80% and 20% of types, respectively (2). In recent years, inflammation has been shown to have a strong relationship with the occurrence of stroke, and negative effects in both experimental and clinical data (3, 4). The inflammatory process is mediated by numerous inflammatory mediators including adhesion molecule (e.g., P-selectin), cytokines (e.g., IL-1, IL-6), chemokine (e.g., CCL2), and protease (e.g., matrix metalloproteinase-9). Furthermore, all brain cells (such as glial cells, endothelial cells, and neurons) and peripheral immune cells (such as neutrophils and lymphocytes) are contributors to the post-stroke inflammation (5, 6).
Neutrophil to lymphocyte ratio (NLR) as a reflection of innate (neutrophilic) and adaptive (lymphocytic) immune responses have been widely studied due to their convenience to obtain from peripheral blood. The increased NLR level with neutrophilic elevation and lymphocytic depletion indicates the imbalanced interaction between stroke-induced central inflammation and peripheral inflammation. Numerous studies have demonstrated that baseline NLR levels are higher in cohorts of ischemic stroke (7, 8) than hemorrhagic stroke (9, 10). Furthermore, it is suggested that higher NLR levels are correlated with poor outcomes and stroke occurrence (11–13). Several meta-analyses have indicated that increased NLR is a negative prognostic indicator in acute ischemic stroke (AIS) and spontaneous intra-cerebral hemorrhage (ICH) (14–16). Isolated analysis of ischemic and hemorrhagic stroke has created limitations in result interpretation. However, despite the different symptomology between these two subtypes of stroke, a similar pathological inflammatory pathway remains. Whether there is difference between ischemic stroke and hemorrhagic stroke with regard to prognostic value of NLR is still unclear. Elucidation of the clinical significance of NLR is needed to further explore the prognostic potential of this biomarker and its conveyed relative risk, such that it can be followed for treatment response. Our aim was to conduct a comprehensive evaluation of the relationship between baseline NLR and stroke, followed by a comparison of the prognostic value of NLR in the two main subtypes of stroke.
Methods
Search Strategy
This meta-analysis was registered in PROSPERO with the number CRD42018105305. Databases PubMed Central (PMC) and EMBASE were searched to identify studies for inclusion through April 2019. We used Medical subject headings and Emtree headings combined with the following keywords: “neutrophil to lymphocyte ratio OR NLR OR neutrophil OR lymphocyte” and “prognosis OR prognostic OR survival OR outcome” and “stroke OR Brain Ischemia OR Brain Infarction OR cerebral infarction OR intra-cerebral hemorrhage OR intracranial hemorrhage.” The full search strategy is presented in Supplementary Table 1.
Study Selection
We included both prospective and retrospective studies that evaluated baseline NLR levels prior to any treatment in patients with definitive diagnosis of ischemic or hemorrhagic stroke. Eligible studies were selected if they provided an odds ratio (OR) or risk ratio (RR) with 95% confidence interval (CI) for clinical outcomes or risk of stroke incidence, or enough data to calculate these quantities. Exclusion was made if the population of study was complicated with autoimmune disorders (e.g., inflammatory bowel, primary or secondary vasculitis, rheumatoid arthritis, or anti-phospholipid syndrome) and systematic inflammatory disorders (e.g., malignancy, end stage liver disease or renal disease, or recent infection). Conference abstracts, review articles, case reports, letters, animal studies, or in vitro studies were not eligible for our analysis. Studies with duplicate or overlapping data were also excluded. Two reviewers (SY-S and XX-Z) independently performed the study selection and resolved any disagreements via discussion.
Data Extraction
Data from all included studies were extracted by one author (SY-S) and was cross-checked by another author (XX-Z). The data were extracted using the name of the first author, year of publication, country, study characteristics (sample size, age, and gender), clinical characteristics (the type and subtype, severity, time of onset, comorbid status, and initial therapy of the stroke), sample time, and statistical methods used. Moreover, female-to-male gender ratio (F/M gender ratio) was calculated to precisely assess the various gender distributions among the included cohorts, which ranged from 0 to 1.8. The F/M ratio of a female-dominant composition was more than 1.2, whereas that of male-dominant cohorts was <0.8. The definition of limit interval was based on average population size in the following subgroup analysis. ORs/RRs and 95% CIs were extracted for mortality (short term or long term), functional outcome, risk of stroke incidence, and risk of post-ischemic stroke complication incidence (symptomatic intracranial hemorrhage or parenchymal hematoma). We used SPSS 19.0 to calculate RRs and 95% CIs based on the available data in studies if we received no response from the investigators after two requests. All disagreements were resolved by consensus.
Outcomes
Outcomes were measured by the modified Rankin Scale (mRS) during clinical follow-up. Death was defined as an mRS of 6 points while unfavorable functional outcome was identified as an mRS of 3–6 points.
Statistical Analyses
STATA version 14.0 (STATA, College Station, TX) was utilized in all analyses. Multivariate-adjusted ORs/RRs were used when possible, and univariate ORs/RRs were included in the meta-analysis if multivariate-adjusted ORs/RRs were missing. Pooled estimates with 95% CIs were derived using the Mantel-Haenszel method. We assumed that an OR is a good approximation to RR in our study due to large sample size; therefore, we pooled ORs and RRs together and simplified the description as ORs/RRs. Furthermore, we explored heterogeneity comprehensively through subgroup analysis and sensitivity analysis. Heterogeneity was assessed using the χ2 test and expressed as the I2 index (25% = low, 50% = medium, 75% = high) (17). When heterogeneity was more than 50%, random effects model was conducted. Assessment of publication bias was done by visual inspection of funnel plots, combined with Begg's test and Egger's test (18, 19). In addition, we applied Duval and Tweede's trim and fill method to estimate corrected effect size after adjustment for publication bias (20). Predefined criteria were used to evaluate the quality of eligible studies (21, 22). P-values < 0.05 were considered statistically significant.
Results
Study Characteristics
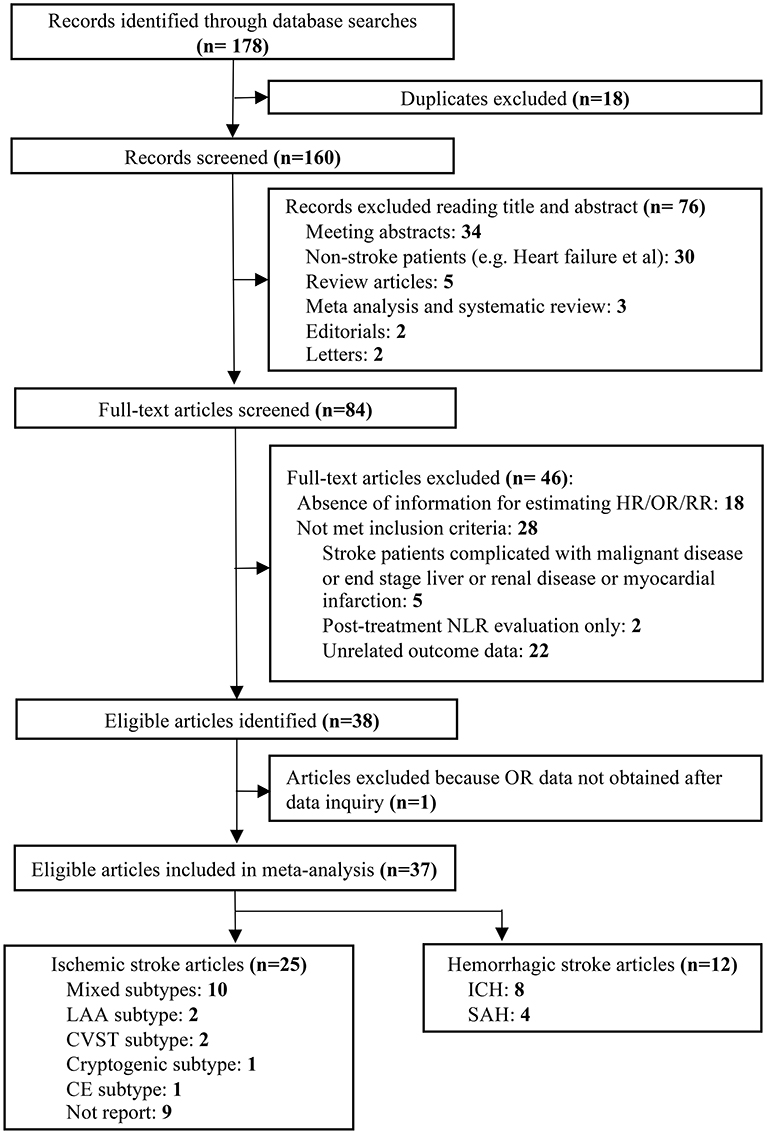
Our literature search identified 178 potentially relevant records. Eighteen duplicates were removed and then a total of 160 articles were screened by titles and abstracts. Seventy-six studies with irrelevant content were excluded. Furthermore, we reviewed the remaining 84 articles with full texts. In sum, 37 studies with 43,979 patients were finally included in our analysis according to the inclusion and exclusion criteria (Figure 1).
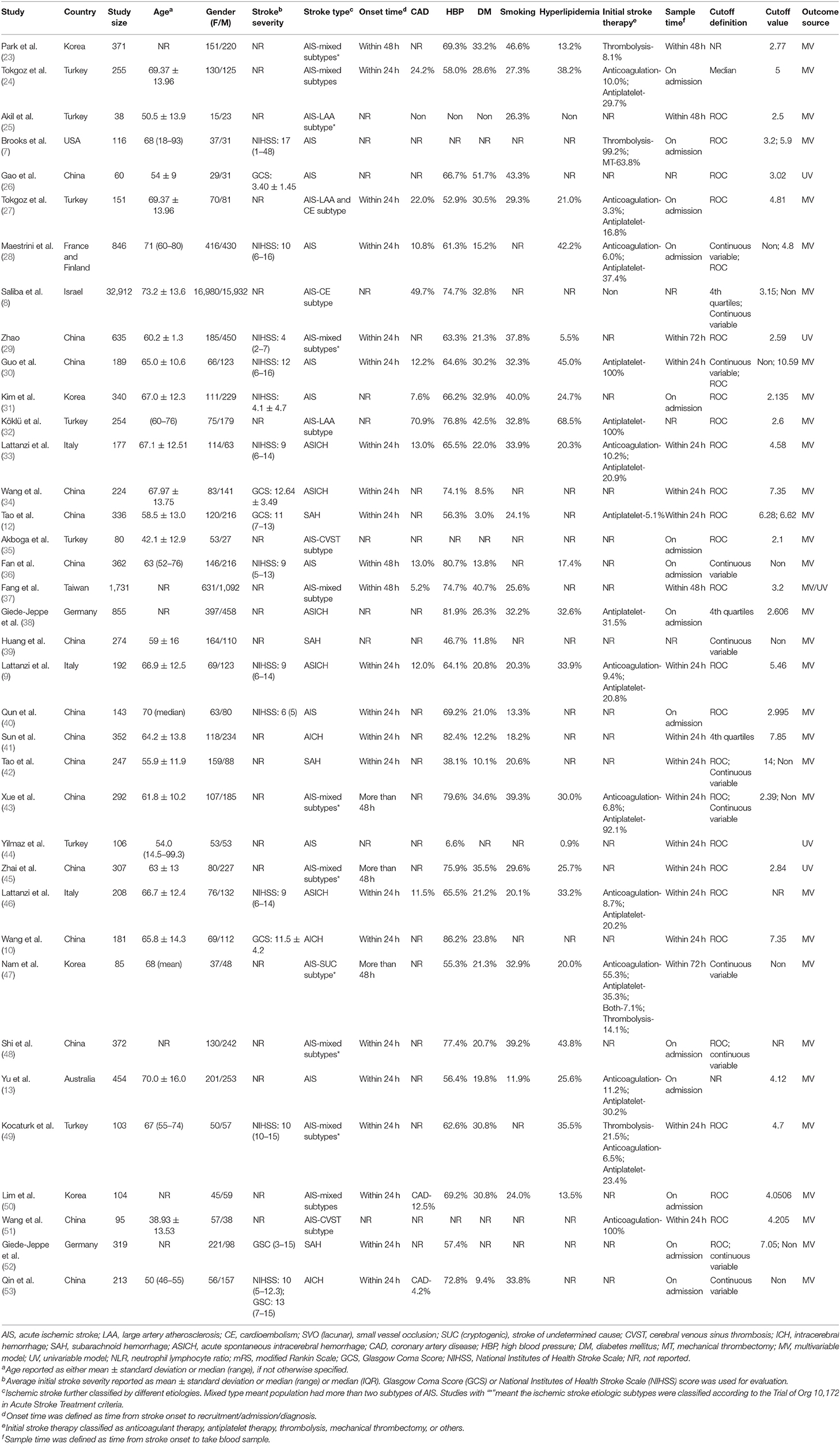
The characteristics of the included studies are shown in Table 1 (7–10, 12, 13, 23–53). Mortality, functional outcome, risks of ischemic stroke, and post-stroke complication were reported in 20, 17, 7, and 2 articles, respectively. For ischemic stroke, 25 studies included populations with AIS. The majority of studies enrolled patients with mixed stroke subtypes (n = 10), including large artery atherosclerosis (LAA) type, cardioembolism (CE) type, small vessel occlusion (SVC) type, cryptogenic type, and cerebral venous sinus thrombosis (CVST) type. However, several studies only evaluated specific subtypes of AIS, which were LAA subtype (n = 2), CVST subtype (n = 2), CE subtype (n = 1), and cryptogenic subtype (n = 1). For hemorrhagic stroke, a total of 12 studies reported clinical outcomes. The most frequently evaluated subtype of hemorrhagic stroke was ICH (n = 8) and subarachnoid hemorrhage (n = 4). In terms of comorbid status, a large number of studies evaluated the presence of hypertension (n = 33), diabetes mellitus (DM) (n = 31), and hyperlipidemia (n = 21) in their populations. Fifteen articles reported the presence of vascular disease. Current smoking status was described in 25 studies. Initial stroke therapy included antiplatelet (n = 14), anticoagulation (n = 11), thrombolysis (n = 4), and mechanical thrombectomy (n = 1). Blood samples were mostly drawn on admission (n = 14) or in the first 24 h after admission (n = 15). Four different methods for defining cutoff values were observed in the included studies. Region under the curve (ROC) analysis was used most frequently (n = 28), followed by the continuous (n = 11) and 4th quartiles (n = 3). Cutoff values of NLR varied between studies, ranging from 2.1 to 14, with respect to demographic characteristics among the cohorts, such as age, gender, and country of origin. Sixteen studies enrolled elderly population, the median or mean age of whom was >65 years. More than 50% of the included cohorts were with male dominant composition (n = 22). The number of cohorts originally from Eastern countries (n = 21) was nearly equal to that of cohorts from Western countries (n = 16). Twenty-one studies had quality scores more than 7, while the remaining 16 studies had scores ≤7 (Supplementary Table 3).
Overall Prognostic Analysis
Seventeen studies with 5,858 patients provided ORs/RRs and 95% CIs for functional outcome. Unfavorable functional outcome was related to increased NLR in patients with stroke (ORs/RRs = 1.423; 95% CI = 1.218–1.662; I2 = 89.5%; P < 0.001; Figure 2). The negative effect of increased NLR levels was more pronounced in ischemic stroke (ORs/RRs = 1.609; 95% CI = 1.283–2.019) than in hemorrhagic stroke (ORs/RRs = 1.523; 95% CI = 0.590–3.931; Figure 2).
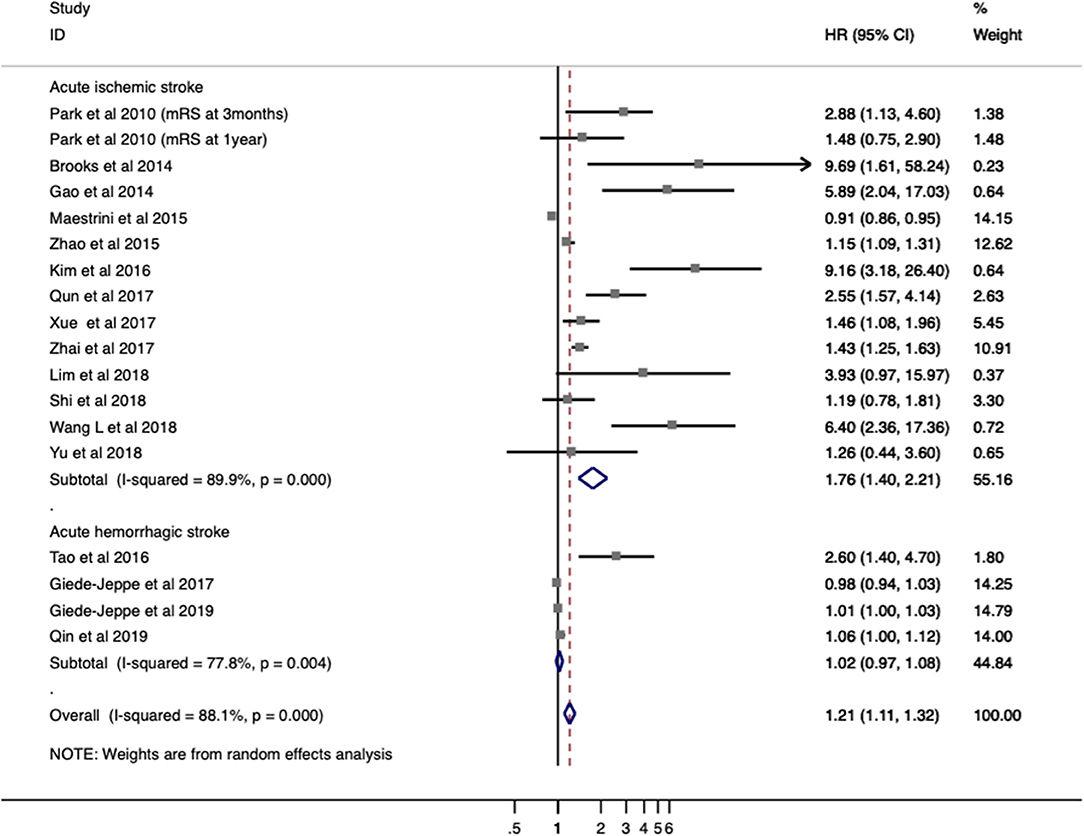
Figure 2. Meta-analysis of the association between NLR and modified Rankin Scale (mRS) functional outcome in patients. Results are presented as individual and pooled risk ratios (RRs) with 95% confidence intervals (CIs).
Twenty studies with 7,517 patients were analyzed for overall mortality. The pooled ORs/RRs of higher baseline NLR level was 1.067 (95% CI = 1.030–1.105; I2 = 83.9%; P < 0.001; Figure 3). Elevated NLR levels were associated with increased mortality in both ischemic stroke (ORs/RRs = 1.068; 95% CI = 1.027–1.111) and hemorrhagic stroke (ORs/RRs = 1.080; 95% CI = 1.018–1.146; Figure 3).
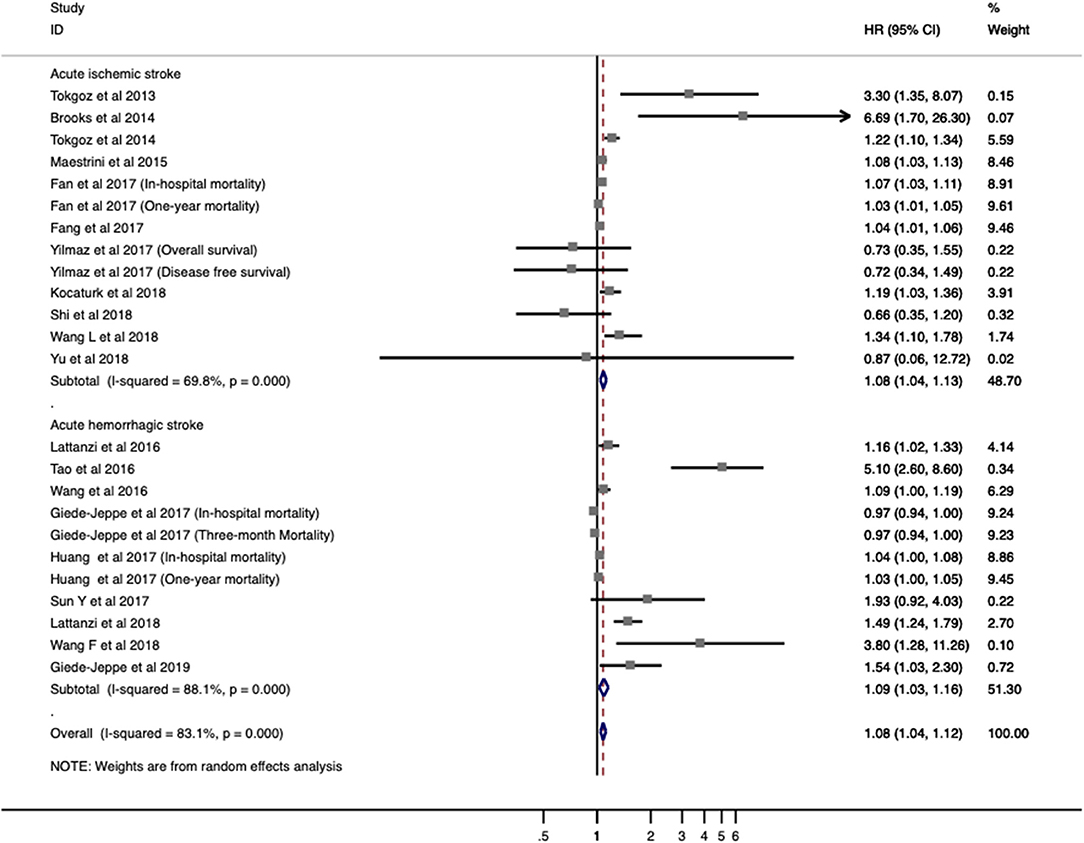
Figure 3. Meta-analysis of the association between NLR and mortality in patients. Results are presented as individual and pooled risk ratios (RRs) with 95% confidence intervals (CIs).
Subgroup Prognostic Analysis in Ischemic Stroke
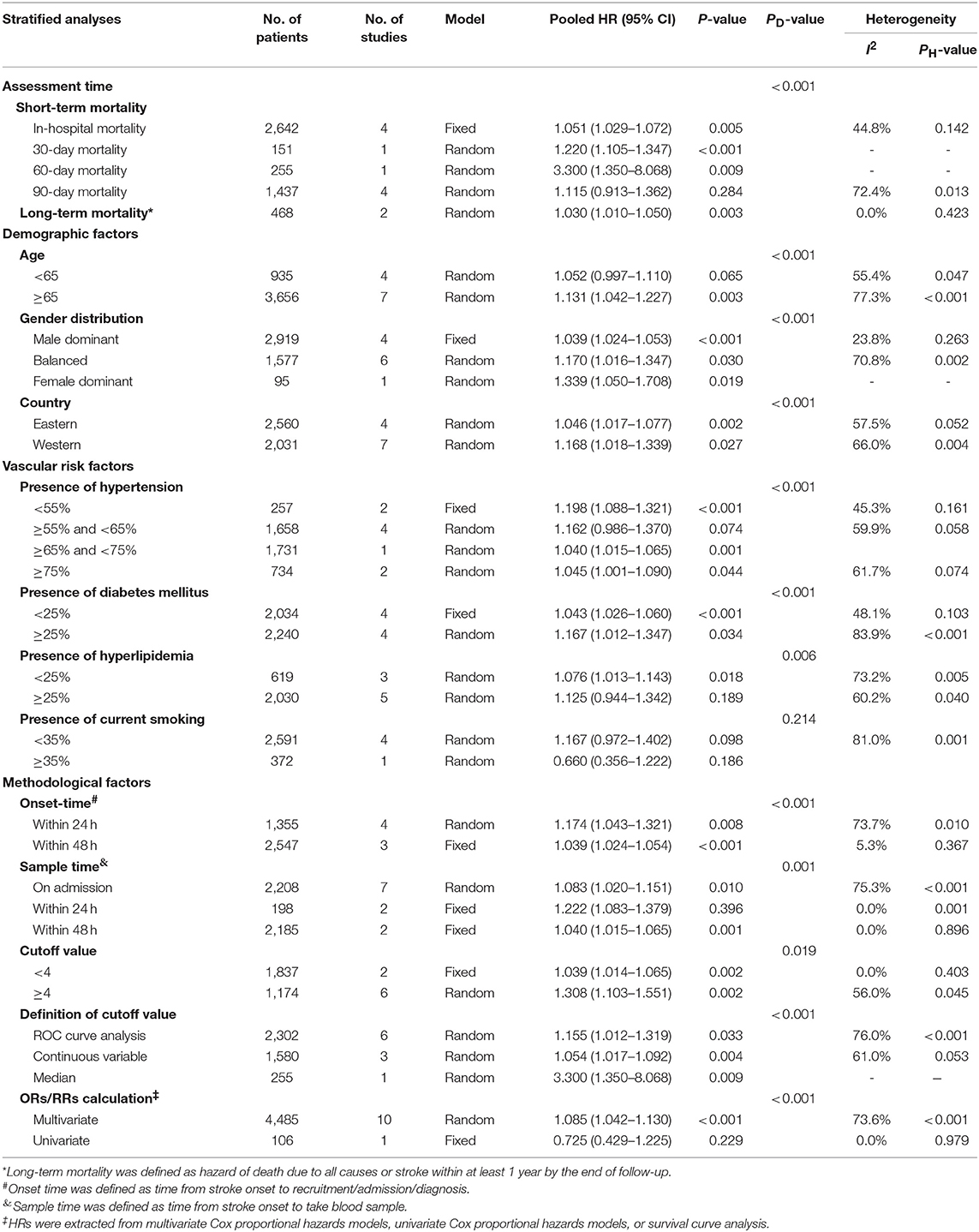
In subgroup analysis, functional outcome (Table 2) in ischemic stroke was according to four major factors, including assessment time, demographic factors (baseline NIHSS score, age, gender distribution, country), vascular risk factors (presence of hypertension, DM, hyperlipidemia, and current smoking) and methodological factors (onset time, sample time, cutoff value, definition of cutoff value, and ORs/RRs calculation). The poor prognostic effect of high NLR levels was only seen at 3 months (ORs/RRs = 1.851; 95% CI = 1.325–2.584; I2 = 91.7%; P < 0.001). Combined ORs/RRs remained significant in subgroups of male dominant populations and eastern countries. Poor functional outcomes were observed in non-elderly or elderly individuals with high NLR. Stroke severity with elevated NLR was not related to worse outcome. Furthermore, cohorts with higher presence of hypertension, DM, and current smoking were more likely to have unfavorable outcomes. With regard to methodological factors, we explored the relationship between the temporal profile of plasma NLR and functional outcomes. A poor prognosis was found in populations with continuously high NLR level at 48 h or long after stroke onset (ORs/RRs = 1.432; 95% CI = 1.266–1.619). The subgroup with higher plasma NLR on admission had the worst functional outcome (ORs/RRs = 3.291; 95% CI = 1.514–7.157). Cutoff values of plasma NLR varied among studies, and those with a cutoff value more than 4 were associated with worse ORs/RRs (ORs/RRs = 3.469; 95% CI = 1.904–6.320). ROC analysis was the most widely used method of assessment and had a relatively close relationship with worse outcomes (ORs/RRs = 2.306; 95% CI = 1.685–3.155). Finally, the estimated ORs/RRs from multivariate and univariate models were 2.076 (1.384–3.112) and 1.706 (1.200–2.426), respectively. In sensitivity analysis under “one study removed” model, the pooled ORs/RRs were significantly affected by exclusion of Maestrini et al. (28) (Supplementary Table 4). After removal of this study, heterogeneity decreased by 10% and the pooled ORs/RRs remained significant (ORs/RRs = 1.963; 95% CI = 1.526–2.524).
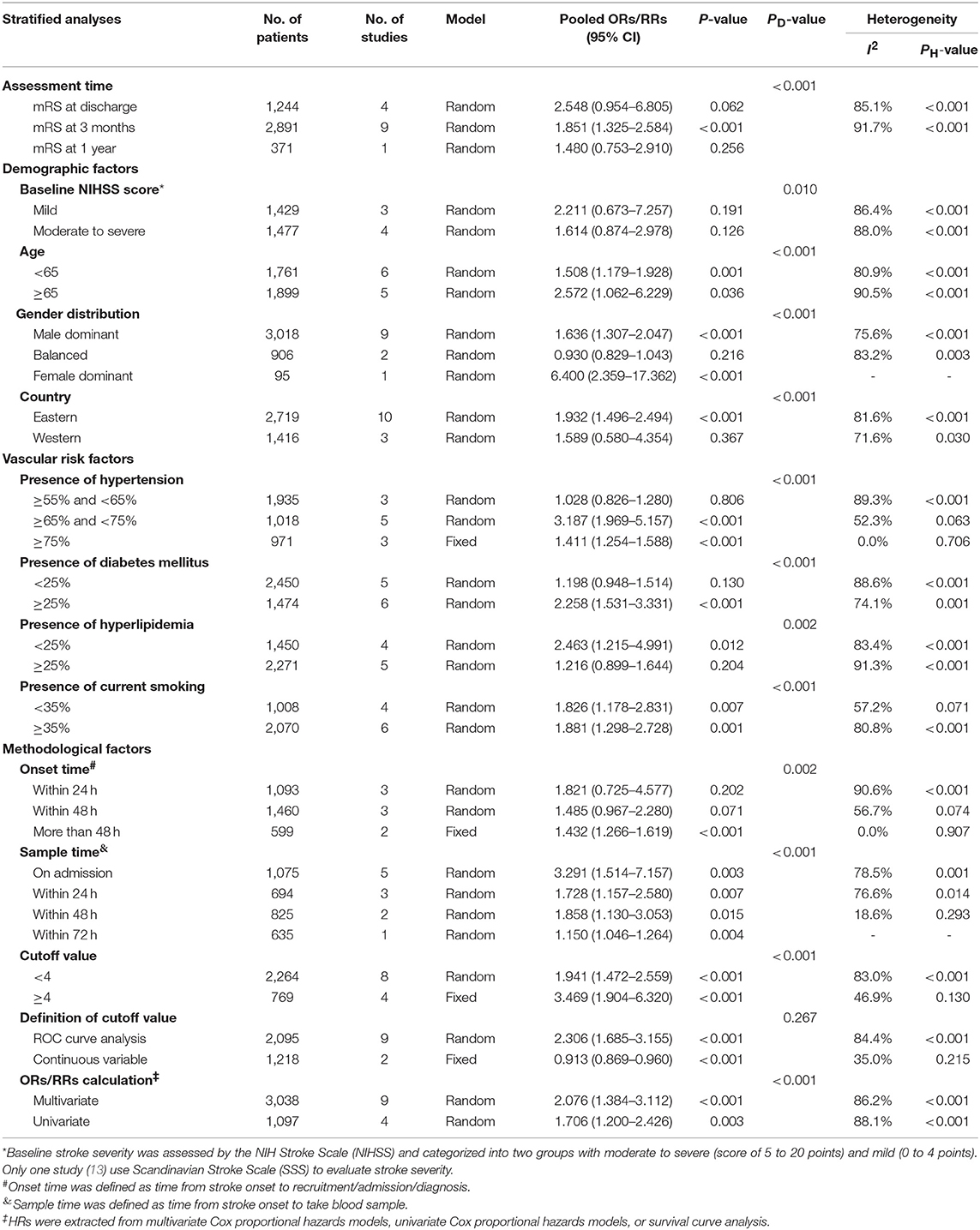
Table 2. Subgroup analyses of the associations between NLR and modified Rankin Scale (mRS) assessed functional outcome in ischemic stroke.
Table 3 demonstrates the relationship between NLR and mortality in ischemic stroke. Subgroups analysis was stratified by the four aforementioned major factors. Higher NLR levels were associated with both in-hospital mortality and long-term mortality. The elderly subgroup showed comparatively worse ORs/RRs (ORs/RRs = 1.131; 95% CI = 1.042–1.227). Pooled ORs/RRs from eastern countries was 1.046 (95% CI = 1.017–1.077), and that from western countries was 1.168 (95% CI = 1.018–1.339). ORs/RRs remained significant in subgroups stratified by all methodological factors.
Patients had increased risk of hemorrhagic transformation after thrombolysis in ischemic stroke. Herein, we further evaluated the relationship between NLR levels and post-stroke complications. Higher NLR levels posed a higher risk of spontaneous ICH with thrombolysis (RRs = 1.290; 95% CI = 1.063–1.565; I2 = 87.1%; P < 0.001; Supplementary Figure 2).
Subgroup Prognostic Analysis in Hemorrhagic Stroke
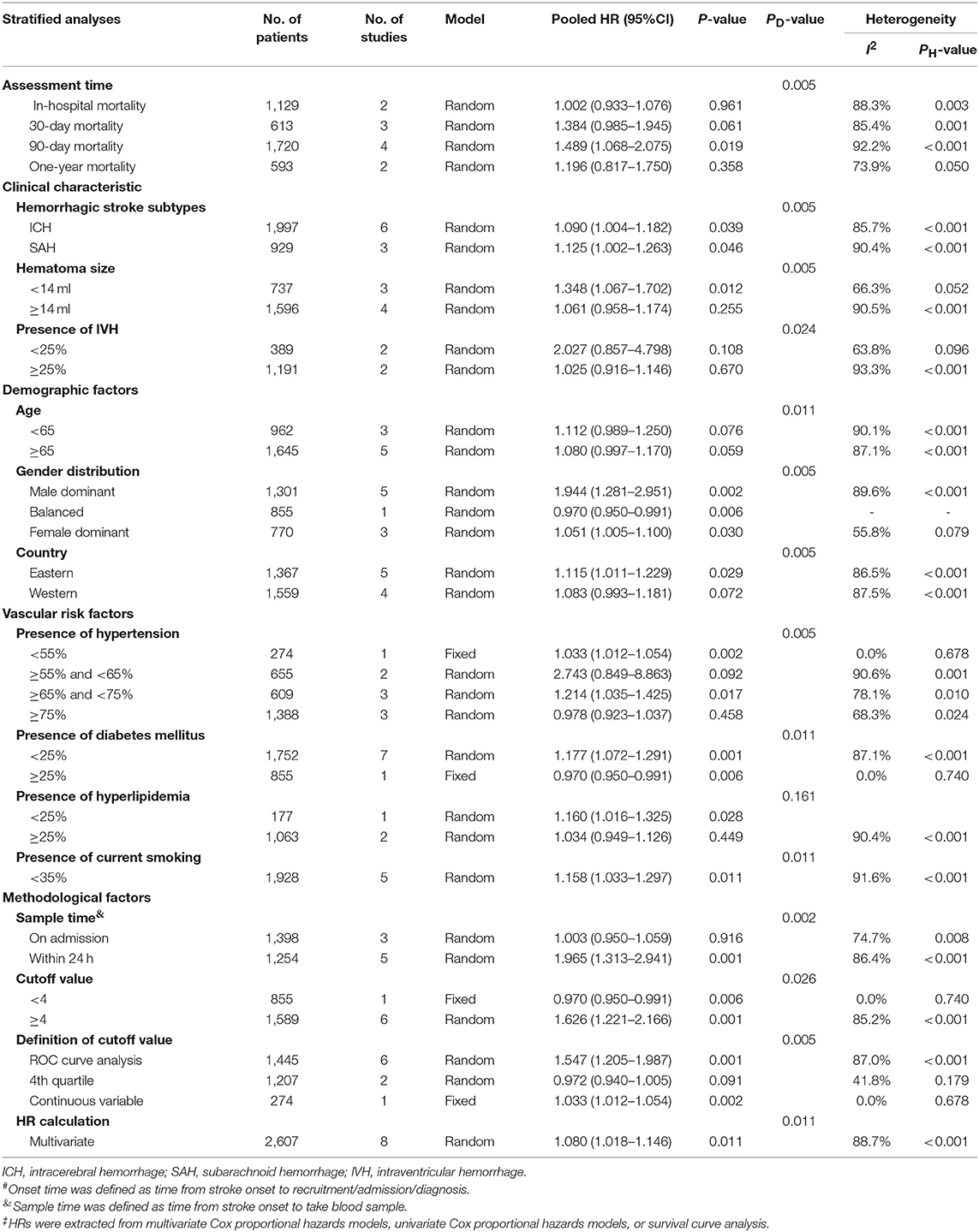
We only conducted subgroup analysis of mortality in hemorrhagic stroke (Table 4) as higher NLR level was not associated with poor functional outcomes in overall analysis (ORs/RRs = 1.523; 95% CI = 0.590–3.931). Elevated NLR was a negative prognostic factor in 90-day mortality. Increased mortality was observed in two types of hemorrhagic stroke, which are ICH (ORs/RRs = 1.090; 95% CI = 1.004–1.182) and SAH (ORs/RRs = 1.125; 95% CI = 1.002–1.263). Male dominant cohorts with high NLR had higher mortality rates (ORs/RRs = 1.944; 95% CI = 1.281–2.951). In addition, studies using ROC analysis to define optimal cutoff values were associated with negative pooled ORs/RRs. The estimated ORs/RRs of subgroups with cutoff values more than 4 was 1.547 (95% CI = 1.205–1.987).
Association of NLR and Risk of Ischemic Stroke
Seven articles reporting data from 35,367 subjects were estimated to evaluate the relationship between NLR and risk of ischemic stroke. We found a high statistically significant risk of ischemic stroke among individuals with elevated NLR levels (RRs = 2.074; 95% CI = 1.485–2.896; I2 = 93.6%; P < 0.001; Supplementary Figure 1). In addition, we explored the high heterogeneity by subgroup analysis stratified by ischemic stroke subtypes, demographic factors, vascular risk factors, and methodological factors (Supplementary Table 2). There was a negative relationship between risk of all subtypes of ischemic stroke and increased NLR levels. Risk of ischemic stroke was elevated when the population had high baseline NLR levels comorbid with higher presence of hypertension (RRs = 2.312; 95% CI = 1.238–4.321), DM (RRs = 1.942; 95% CI = 1.371–2.752), hyperlipidemia (RRs = 2.156; 95% CI = 1.204–3.861), and current smoking (RRs = 1.047; 95% CI = 1.011–1.084). Cutoff values of these articles were all <4. Majority of cutoff values were defined by ROC analysis. The combined RRs was 2.795 (95% CI = 1.685–4.636) in subgroup of ROC analysis.
Publication Bias
We observed evidence of publication bias in studies providing functional outcomes in ischemic stroke (Supplementary Table 5) as well as mortality in hemorrhagic stroke (Supplementary Table 6) by Egger's test. Then, we applied the trim and fill method to address these problems. After the adjustment, the combined ORs/RRs of higher baseline NLR level were 1.088 (0.869–1.361) and 1.027 (0.957–1.102), respectively (Supplementary Tables 5, 6).
Discussion
Literatures on NLR, as an inflammatory biomarker in cancer and cardiovascular disease, have grown exponentially over the past 5 years. Our meta-analysis evaluates the clinical significance of the NLR in stroke and adds a comprehensive systematic review to the cerebrovascular field. NLR is an easily acquired, non-invasive, and inexpensive marker, which can be used routinely to indicate systematic inflammatory status in clinical work. This is the first meta-analysis to comprehensively assess the clinical significance of NLR in both ischemic and hemorrhagic stroke under consistent methodology. In the setting of ischemic stroke, higher NLR levels were correlated with increased risk of stroke, unfavorable functional outcome at 3 months, and increased mortality, while in terms of hemorrhagic stroke (including SAH and ICH), elevated NLR levels only had deleterious effects on mortality.
The mechanism underlying the clinical significance of NLR on stroke is due to a central role of inflammation in all types of stroke from its initiation, progression of injury, and recovery (54–56). The inflammation cascade is initiated immediately by stagnant blood flow resulting from either ischemic or hemorrhagic lesion (5, 11). Release of proinflammatory mediators, such as TNF-α, IL-1, IL-6, and matrix metalproteinase-9 (MMP-9) from endothelium and brain parenchyma further potentiates tissue injury. Moreover, danger-/damage-associated molecular patterns (DAMP) are produced from injured and dying neurons. The main target of inflammation is the disruption of the brain–blood barrier (BBB) or neurovascular unit. Older animal studies have reported a biphasic behavior of BBB damage. However, recent human and animal studies indicate that BBB permeability remains elevated especially in the acute phase (6–48 h after stroke onset) due to the inflammatory cascade (57). Therefore, DAMP and proinflammatory mediators could gain access to the systemic circulation through the disrupted BBB or the cerebrospinal fluid (CSF) drainage system. Once in circulation, the systematic inflammatory response is potentiated. Among various types of peripheral inflammatory cells, neutrophils are the first to infiltrate the lesion (30 min to a few hours), peak earlier (24–72 h) and decrease rapidly with time (58). Locally, neutrophils participate in brain injury by exacerbating oxidative stress and BBB damage (59–61). The consequence of BBB breakdown is related to the many complications of stroke. Most commonly, pathologic cerebral edema results from increased BBB permeability and tends to develop within the first 24 to 48 h in AIS (62) or within the first 24 h in ICH (63). Breakdown of BBB is also associated with elevated risk of hemorrhagic transformation in AIS. Furthermore, inflammation is involved in the restoration of BBB function. After the production of proinflammatory factor peak and neutrophils in the acute/subacute phase (from onset to more than 48 h), neutrophil levels fall. This decrease during stroke recovery may help BBB integrity and be associated with good prognosis (64, 65). Therefore, the post-stroke inflammatory response has become a therapeutic target, as an adjacent treatment to reperfusion therapy using thrombolysis or intravascular clot removal (54, 66). Several drugs have been tested in randomized trials such as Fingolimod (67, 68), Natalizumab (69), Interleukin-1 receptor antagonist (IL-1ra) (70), and Minocycline (ACTRN12611001053910). The findings are anticipated to improve treatment options and clinical outcomes in of patients with acute stroke (59). Moreover, suppression of inflammation is also beneficial in models of cerebral hemorrhage (71). However, systemic immunosuppression follows after acute phase due to disturbed brain-immune interaction (4). Increased released glucocorticoids by the hypothalamic–pituitary axis and circulating epinephrine produced by the adrenal medulla or via the dense innervation by postganglionic sympathetic fibers of lymphoid organs are the major pathways to decrease lymphocyte counts, especially T cells and natural killer cells (3). Accordingly, infection is the most prevalent complication after stroke and contributes to the main cause of in-hospital death (66, 72, 73). This is consistent with our results that higher NLR levels were especially related to in-hospital mortality in ischemic stroke. Completing the cycle, NLR levels are elevated because of increased neutrophil counts and downsized lymphocyte counts in the post-stroke stage. Furthermore, elevated NLR levels had detrimental effects on prognosis due to secondary brain injury by neutrophil activation and increased risk of infection by lymphocyte suppression. Given the success of mechanical thrombectomy for large vessel occlusions, it would stand to reason that the NLR would fall in successful recanalization, given a lack of stagnating clot and reperfusion with less loss of BBB integrity. Abdalla et al. (74) reported their results with successful TICI 2b/3 recanalization and reported NLR fall 72 h post successful recanalization. The lower NLR level correlated directly with 90-day functional outcomes. Furthermore, an elevated neutrophil count was noted to be an independent predictor of poor outcome (>mRS3) at 90 days despite TICI 2b/3 recanalization by Bouisseau et al. (75) with higher infarct volumes. Thus, post-stroke NLR may serve as a marker of patients who may require hemicraniectomy for large infarcts despite recanalization. Recanalization of low-ASPECTS score, large-core strokes has been shown to decrease the rate of malignant transformation requiring hemicraniectomy, and reperfusion with decreasing NLR counts may be one explanation/marker (76). However, our meta-analysis was unable to evaluate the prognostic value of NLR in patients with a certain type of stroke treatment or with different infarct sizes due to insufficient data. We highly suggest that future studies could pay more attention on these issues.
Although ischemic and hemorrhagic stroke shared similar inflammatory reaction (6), we found that prognosis of hemorrhagic stroke was weakly predicted by NLR level in contrast with that of ischemic stroke. Higher NLR levels were associated with increased risk of ischemic stroke. These results may be due to prothrombotic state induced by inflammation responsible for ischemic stroke prodrome. During inflammation, leukocytes interact with platelets, endothelium, and coagulation factors and have been widely recognized as important contributors to facilitating hemostasis in physiological and pathological conditions. This mechanism can also explain similar results in other clinical articles. For example, leukocytosis does not independently predict poor ICH prognosis when controlling for other outcome determinants including age, baseline hematoma volume, and admission Glasgow Coma Scale (77). Similarly, as hematoma expansion is related to poor outcome in hemorrhagic stroke (78), the inverse relationship between neutrophil counts and risk of hematoma expansion might relate to better prognosis (79). However, interestingly, elevated baseline NLR levels were also correlated with higher risk of hemorrhagic transformation after thrombolysis in ischemic stroke. This may be associated with antithrombotic effect of thrombolysis vs. a leaky BBB integrity. Thus, further experimental and clinical studies are needed to evaluate the predicting role of NLR in patients after thrombolysis.
In subgroup analysis, we found that prognostic value of NLR in stroke remained significant in subgroups of more than 65 years, male dominant composition, and patients from eastern countries, which are consistent with prior studies (14–16). Furthermore, as thromboembolism is the most common cause of ischemic stroke, we evaluated the vascular risk factors among the included studies. Cohorts with higher presence of hypertension (>65%), DM (>25%), and current smoking (>35%) tended to have more unfavorable functional outcomes in ischemic stroke. Cutoff values varied between studies due to different definitive methods, blood sampling time, and capacity of immune system (16). A higher cutoff value (>4) indicated poorer prognosis in stroke. In addition, we observed that cutoff values defined by ROC curves were more likely to predict poor clinical outcomes. Thus, future studies are suggested to determine their specific cutoff values by ROC curves. Temporal dynamics of neutrophil and lymphocyte counts have been described in previous studies (3, 80). Therefore, we conducted subgroup analysis stratified by onset time and sample time. Shorter time from stroke onset to admission (within 24 h), and quicker procurement of the blood sample (within 72 h) were beneficial to record the NLR level at early stages of stroke-induced inflammation and helped predict negative prognosis.
In this meta-analysis, baseline NLR was identified as a robust predictor of ischemic stroke occurrence and prognosis. However, there are several limitations. Firstly, considerable heterogeneity was found when combined ORs/RRs for functional outcomes and mortality were assessed. In the setting of ischemic stroke, heterogeneity was tremendously decreased to <50% after subgroup analysis of mortality assessment time, age, gender, country, and vascular risk factor. We further conducted sensitivity analysis of studies reporting functional outcomes in ischemic stroke and the outcomes had no significant change after excluding a single study. Secondly, publication bias existed in studies providing functional outcomes in ischemic stroke as well as mortality in hemorrhagic stroke. The negative effect of higher NLR was slightly reduced after adjustment of publication bias by the trim and fill method. Therefore, future studies are encouraged to publish null results to avoid overestimation of clinical significance of NLR. We excluded studies if their populations were complicated with autoimmune disorders or systematic inflammatory disorders to avoid the influence of chronic inflammatory status on NLR value (3, 81). However, it is also worth evaluating the clinical significance of NLR in patients with inflammatory conditions prior to enrollment as stroke can also manifest as a complication of inflammation. Finally, we observed that our included studies only reported the negative effect of high baseline NLR on all-cause mortality. As NLR is a reflection of inflammatory status, we highly suggest that future studies could specify the cause of death related to inflammation in post-stroke patients, such as infection-related death.
Conclusions
Baseline NLR level is a promising predictor of ischemic or hemorrhagic stroke prognosis. Elevated NLR is also associated with high risk of ischemic stroke occurrence. Shorter time from stroke onset to admission (within 24 h) and timely procurement of blood samples may help to reflect the early inflammatory response of neutrophils and lymphocytes, which may predict clinical outcomes. Cutoff values of more than 4 may be related to worse prognosis. Future studies are needed to improve the aforementioned limitations and demonstrate the underlying mechanisms of our work here.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The corresponding local ethics committee approved this study and all participants provided informed consent.
Author Contributions
RM: manuscript drafting and revision, and study concept and design. S-YS: manuscript drafting and revision, study concept and design, collection, assembly, and interpretation of the data. X-XZ: collection, assembly, and interpretation of the data. RM, S-YS, X-XZ, CH, RK, and YH: manuscript writing and final approval of manuscript. GR and YD deeply edited the revised version and contributed critical revision.
Funding
This study was sponsored by the National Key R&D Program of China (2017YFC1308400), the National Natural Science Foundation (81371289), and the Project of Beijing Municipal Top Talent for Healthy Work of China (2014-2-015).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank all authors who reported their relevant data on PubMed Central (PMC) and EMBASE.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2019.01032/full#supplementary-material
Abbreviations
NLR, neutrophil-to-lymphocyte ratio; AIS, acute ischemic stroke; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; GCS, Glasgow Coma Score; OR, odds ratio; RR, risk ratio; SAH, subarachnoid hemorrhage; ICH, intra-cerebral hemorrhage; IL, interleukin; CCL, chemokine (C-C motif) ligand 2; MMP-9, matrix metalloproteinase-9; PMC, PubMed Central; CI, confidence interval; LAA, large artery atherosclerosis; CE, cardioembolism; SVC, small vessel occlusion; CVST, cerebral venous sinus thrombosis; DM, diabetes mellitus; ROC, region under the curve; DAMP, danger-/damage-associated molecular patterns; BBB, brain–blood barrier; CSF, cerebrospinal fluid; ROS, reactive oxygen species; RNS, reactive nitrogen species; TICI, Thrombolysis in cerebral infarction; ASPECTS, Alberta Stroke Program Early CT Score.
References
1. Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2011) 42:517–84. doi: 10.1161/STR.0b013e3181fcb238
2. Ikram MA, Wieberdink RG, Koudstaal PJ. International epidemiology of intracerebral hemorrhage. Curr Atheroscler Rep. (2012) 14:300–6. doi: 10.1007/s11883-012-0252-1
3. Macrez R, Ali C, Toutirais O, Le Mauff B, Defer G, Dirnagl U, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. (2011) 10:471–80. doi: 10.1016/S1474-4422(11)70066-7
4. Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. (2005) 6:775–86. doi: 10.1038/nrn1765
5. Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics. (2016) 13:661–70. doi: 10.1007/s13311-016-0483-x
6. Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage secondary brain injury. Stroke. (2011) 42:1781–6. doi: 10.1161/STROKEAHA.110.596718
7. Brooks SD, Spears C, Cummings C, VanGilder RL, Stinehart KR, Gutmann L, et al. Admission neutrophil-lymphocyte ratio predicts 90 day outcome after endovascular stroke therapy. J NeuroIntervent Surg. (2014) 6:578–83. doi: 10.1136/neurintsurg-2013-010780
8. Saliba W, Barnett-Griness O, Elias M, Rennert G. Neutrophil to lymphocyte ratio and risk of a first episode of stroke in patients with atrial fibrillation: a cohort study. J Thromb Haemost. (2015) 13:1971–9. doi: 10.1111/jth.13006
9. Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Neutrophil-to-lymphocyte ratio and neurological deterioration following acute cerebral hemorrhage. Oncotarget. (2017) 8:57489–94. doi: 10.18632/oncotarget.15423
10. Wang F, Wang L, Jiang TT, Xia JJ, Xu F, Shen LJ, et al. Neutrophil-to-lymphocyte ratio is an independent predictor of 30-day mortality of intracerebral hemorrhage patients: a validation cohort study. Neurotoxicity Res. (2018) 34:347–52. doi: 10.1007/s12640-018-9890-6
11. Lattanzi S, Brigo F, Trinka E, Cagnetti C, Di Napoli M, Silvestrini M. Neutrophil-to-lymphocyte ratio in acute cerebral hemorrhage: a system review. Transl Stroke Res. (2019) 10:137–45. doi: 10.1007/s12975-018-0649-4
12. Tao C, Hu X, Wang J, Ma J, Li H, You C. Admission neutrophil count and neutrophil to lymphocyte ratio predict 90-day outcome in intracerebral hemorrhage. Biomarkers Med. (2016) 11:33–42. doi: 10.2217/bmm-2016-0187
13. Yu S, Arima H, Bertmar C, Clarke S, Herkes G, Krause M. Neutrophil to lymphocyte ratio and early clinical outcomes in patients with acute ischemic stroke. J Neurolo Sci. (2018) 387:115–8. doi: 10.1016/j.jns.2018.02.002
14. Zhang J, Ren Q, Song Y, He M, Zeng Y, Liu Z, et al. Prognostic role of neutrophil–lymphocyte ratio in patients with acute ischemic stroke. Medicine. (2017) 96:e8624. doi: 10.1097/MD.0000000000008624
15. Zhang J, Cai L, Song Y, Shan B, He M, Ren Q, et al. Prognostic role of neutrophil lymphocyte ratio in patients with spontaneous intracerebral hemorrhage. Oncotarget. (2017) 8:77752–60. doi: 10.18632/oncotarget.20776
16. Ye Z, Ai X, Fang F, Hu X, Faramand A, You C. The use of neutrophil to lymphocyte ratio as a predictor for clinical outcomes in spontaneous intracerebral hemorrhage. Oncotarget. (2017) 8:90380–9. doi: 10.18632/oncotarget.20120
17. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
18. Stuck AE, Rubenstein LZ, Wieland D. Bias in meta-analysis detected by a simple, graphical test. Asymmetry detected in funnel plot was probably due to true heterogeneity. BMJ. (1998) 316:469; author reply 470–1. doi: 10.1136/bmj.316.7129.469
19. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
20. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
21. Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. (2006) 144:427–37. doi: 10.7326/0003-4819-144-6-200603210-00010
22. Altman DG. Systematic reviews of evaluations of prognostic variables. BMJ. (2001) 323:224–8. doi: 10.1136/bmj.323.7306.224
23. Park J-K, O H-G, Park T-H. Neutrophil to lymphocyte ratio at admission: prognostic factor in patients with acute ischemic stroke. J Korean Neurol Assoc. (2010) 28:172–8.
24. Tokgoz S, Kayrak M, Akpinar Z, Seyithanoglu A, Güney F, Yürüten B. Neutrophil lymphocyte ratio as a predictor of stroke. J Stroke Cerebrovasc Dis. (2013) 22:1169–74. doi: 10.1016/j.jstrokecerebrovasdis.2013.01.011
25. Akil E, Akil MA, Varol S, Özdemir HH, Yücel Y, Arslan D, et al. Echocardiographic epicardial fat thickness and neutrophil to lymphocyte ratio are novel inflammatory predictors of cerebral ischemic stroke. J Stroke Cerebrovasc Dis. (2014) 23:2328–34. doi: 10.1016/j.jstrokecerebrovasdis.2014.04.028
26. Gao Wei HZ, Yongsheng D, Xubin W, Yong Z. Association between neutrophil lymphocyte ratio and prognosis of acute ischemic stroke. J Clin Pathol Res. (2014) 34:509–13.
27. Tokgoz S, Keskin S, Kayrak M, Seyithanoglu A, Ogmegul A. Is neutrophil/lymphocyte ratio predict to short-term mortality in acute cerebral infarct independently from infarct volume? J Stroke Cerebrovasc Dis. (2014) 23:2163–8. doi: 10.1016/j.jstrokecerebrovasdis.2014.04.007
28. Maestrini I, Strbian D, Gautier S, Haapaniemi E, Moulin S, Sairanen T, et al. Higher neutrophil counts before thrombolysis for cerebral ischemia predict worse outcomes. Neurology. (2015) 85:1408–16. doi: 10.1212/WNL.0000000000002029
29. Zhao LL, Chen XL, Xu XM. Predictive value of leukocyte differential count in patients with acute cerebral infarction. J Med Postgrad. (2015) 28:1148–51.
30. Guo Z, Yu S, Xiao L, Chen X, Ye R, Zheng P, et al. Dynamic change of neutrophil to lymphocyte ratio and hemorrhagic transformation after thrombolysis in stroke. J Neuroinflamm. (2016) 13:199. doi: 10.1186/s12974-016-0680-x
31. Kim MK, Ha YS, Yoo BG. Neutrophil-to-lymphocyte ratio predicts short-term functional outcome in acute ischemic stroke with diabetes mellitus. ARC J Neurosci. (2017) 2:30. doi: 10.20431/2456-057X.0203005
32. Köklü E, Yüksel IÖ, Arslan S, Bayar N, Çagirci G, Gencer ES, et al. Is elevated neutrophil-to-lymphocyte ratio a predictor of stroke in patients with intermediate carotid artery stenosis? J Stroke Cerebrovasc Dis. (2016) 25:578–84. doi: 10.1016/j.jstrokecerebrovasdis.2015.10.031
33. Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Neutrophil-to-lymphocyte ratio predicts the outcome of acute intracerebral hemorrhage. Stroke. (2016) 47:1654–7. doi: 10.1161/STROKEAHA.116.013627
34. Wang F, Hu S, Ding Y, Ju X, Wang L, Lu Q, et al. Neutrophil-to-lymphocyte ratio and 30-day mortality in patients with acute intracerebral hemorrhage. J Stroke Cerebrovasc Dis. (2016) 25:182–7. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.013
35. Akboga YE, Bektas H, Anlar O. Usefulness of platelet to lymphocyte and neutrophil to lymphocyte ratios in predicting the presence of cerebral venous sinus thrombosis and in-hospital major adverse cerebral events. J Neurol Sci. (2017) 380:226–9. doi: 10.1016/j.jns.2017.07.036
36. Fan L, Gui L, Chai EQ, Wei CJ. Routine hematological parameters are associated with short- and long-term prognosis of patients with ischemic stroke. J Clin Lab Analysis. (2017) 32:22244. doi: 10.1002/jcla.22244
37. Fang YN, Tong MS, Sung PH, Chen YL, Chen CH, Tsai NW, et al. Higher neutrophil counts and neutrophil-to-lymphocyte ratio predict prognostic outcomes in patients after non-atrial fibrillation-caused ischemic stroke. Biomed J. (2017) 40:154–62. doi: 10.1016/j.bj.2017.03.002
38. Giede-Jeppe A, Bobinger T, Gerner ST, Sembill JA, Sprügel MI, Beuscher VD, et al. Associations of neutrophil-to-lymphocyte ratio with mortality in spontaneous intracerebral hemorrhage. Cerebrovasc Dis. (2017) 43:79.
39. Huang YL, Han ZJ, Hu ZD. Red blood cell distribution width and neutrophil to lymphocyte ratio are associated with outcomes of adult subarachnoid haemorrhage patients admitted to intensive care unit. Ann Clin Biochem. (2017) 54:696–701. doi: 10.1177/0004563216686623
40. Qun S, Tang Y, Sun J, Liu Z, Wu J, Zhang J, et al. Neutrophil-to-lymphocyte ratio predicts 3-month outcome of acute ischemic stroke. Neurotoxicity Res. (2017) 31:444–52. doi: 10.1007/s12640-017-9707-z
41. Sun Y, You S, Zhong C, Huang Z, Hu L, Zhang X, et al. Neutrophil to lymphocyte ratio and the hematoma volume and stroke severity in acute intracerebral hemorrhage patients. Am J Emerg Med. (2017) 35:429–33. doi: 10.1016/j.ajem.2016.11.037
42. Tao C, Wang J, Hu X, Ma J, Li H, You C. Clinical value of neutrophil to lymphocyte and platelet to lymphocyte ratio after aneurysmal subarachnoid hemorrhage. Neurocrit Care. (2017) 26:393–401. doi: 10.1007/s12028-016-0332-0
43. Xue J, Huang W, Chen X, Li Q, Cai Z, Yu T, et al. Neutrophil-to-lymphocyte ratio is a prognostic marker in acute ischemic stroke. J Stroke Cerebrovasc Dis. (2017) 26:650–7. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.010
44. Yilmaz E, Bayram Kacar A, Bozpolat A, Zararsiz G, Gorkem BS, Karakukcu M, et al. The relationship between hematological parameters and prognosis of children with acute ischemic stroke. Child Nerv Syst. (2018) 34:655–61. doi: 10.1007/s00381-017-3673-x
45. Zhai M, Wang J, Yu L, Fu X, Li L. Neutrophil and lymphocyte ratios for the predictive analysis of the prognosis in patients with acute cerebral infarction. Chin J Cerebrovasc Dis. (2017) 14:82–6.
46. Lattanzi S, Cagnetti C, Rinaldi C, Angelocola S, Provinciali L, Silvestrini M. Neutrophil-to-lymphocyte ratio improves outcome prediction of acute intracerebral hemorrhage. J Neurol Sci. (2018) 387:98–102. doi: 10.1016/j.jns.2018.01.038
47. Nam KW, Kim TJ, Kim CK, Mo H, Jeong HY, Kang MK, et al. Temporal changes in the neutrophil to lymphocyte ratio and the neurological progression in cryptogenic stroke with active cancer. PLoS ONE. (2018) 13:e0194286. doi: 10.1371/journal.pone.0194286
48. Shi J, Peng H, You S, Liu Y, Xu J, Xu Y, et al. Increase in neutrophils after recombinant tissue plasminogen activator thrombolysis predicts poor functional outcome of ischaemic stroke: a longitudinal study. Eu J Neurol. (2018) 25:687-e45. doi: 10.1111/ene.13575
49. Kocaturk O, Besli F, Gungoren F, Kocaturk M, Tanriverdi Z. The relationship among neutrophil to lymphocyte ratio, stroke territory, and 3-month mortality in patients with acute ischemic stroke. Neurol Sci. (2019) 40:139–46. doi: 10.1007/s10072-018-3604-y
50. Lim HH, Jeong IH, An GD, Woo KS, Kim KH, Kim JM, et al. Early prediction of severity in acute ischemic stroke and transient ischemic attack using platelet parameters and neutrophil-to-lymphocyte ratio. J Clin Lab Anal. (2019) 33:e22714. doi: 10.1002/jcla.22714
51. Wang L, Duan J, Bian T, Meng R, Wu L, Zhang Z, et al. Inflammation is correlated with severity and outcome of cerebral venous thrombosis. J Neuroinflamm. (2018) 15:329. doi: 10.1186/s12974-018-1369-0
52. Giede-Jeppe A, Reichl J, Sprügel MI, Lücking H, Hoelter P, Eyüpoglu IY, et al. Neutrophil-to-lymphocyte ratio as an independent predictor for unfavorable functional outcome in aneurysmal subarachnoid hemorrhage. J Neurosurg. (2019) 2019:1–8. doi: 10.3171/2018.9.JNS181975
53. Qin Q, Luo X, Wei J, Zhu Y, Wen X, Song S, et al. Acetonitrile activation: an effective two-carbon unit for cyclization. Angew Chem Int Ed Engl. (2019) 58:4376–80. doi: 10.1002/anie.201900947
54. Shekhar S, Cunningham MW, Pabbidi MR, Wang S, Booz GW, Fan F. Targeting vascular inflammation in ischemic stroke: recent developments on novel immunomodulatory approaches. Eur J Pharmacol. (2018) 833:531–44. doi: 10.1016/j.ejphar.2018.06.028
55. Wilkinson DA, Pandey AS, Thompson BG, Keep RF, Hua Y, Xi G. Injury mechanisms in acute intracerebral hemorrhage. Neuropharmacology. (2018) 134(Pt B):240–8. doi: 10.1016/j.neuropharm.2017.09.033
56. Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol. (2010) 92:463–77. doi: 10.1016/j.pneurobio.2010.08.001
57. Merali Z, Huang K, Mikulis D, Silver F, Kassner A. Evolution of blood-brain-barrier permeability after acute ischemic stroke. PLoS ONE. (2017) 12:e0171558. doi: 10.1371/journal.pone.0171558
58. Jin R, Liu L, Zhang S, Nanda A, Li G. Role of inflammation and its mediators in acute ischemic stroke. J Cardiovasc Transl Res. (2013) 6:834–51. doi: 10.1007/s12265-013-9508-6
59. Chamorro Á, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. (2016) 15:869–81. doi: 10.1016/S1474-4422(16)00114-9
60. Justicia C, Panés J, Solé S, Cervera A, Deulofeu R, Chamorro A, et al. Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J Cereb Blood Flow Metab. (2003) 23:1430–40. doi: 10.1097/01.WCB.0000090680.07515.C8
61. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. (2013) 13:159–75. doi: 10.1038/nri3399
62. Singh V, Edwards NJ. Advances in the critical care management of ischemic stroke. Stroke Res Treat. (2013) 2013:510481. doi: 10.1155/2013/510481
63. Gebel JM, Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S, et al. Natural history of perihematomal edema in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. (2002) 33:2631–5. doi: 10.1161/01.STR.0000035284.12699.84
64. Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke. (2004) 35:2220–5. doi: 10.1161/01.STR.0000138023.60272.9e
65. Jiang X, Andjelkovic AV, Zhu L, Yang T, Bennett MVL, Chen J, et al. Blood–brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol. (2018) 163–164:144–71. doi: 10.1016/j.pneurobio.2017.10.001
66. Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. (2011) 17:796–808. doi: 10.1038/nm.2399
67. Zhu Z, Fu Y, Tian D, Sun N, Han W, Chang G, et al. Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: a pilot trial. Circulation. (2015) 132:1104–12. doi: 10.1161/CIRCULATIONAHA.115.016371
68. Fu Y, Zhang N, Ren L, Yan Y, Sun N, Li YJ, et al. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc Natl Acad Sci USA. (2014) 111:18315–20. doi: 10.1073/pnas.1416166111
69. Llovera G, Hofmann K, Roth S, Salas-Pérdomo A, Ferrer-Ferrer M, Perego C, et al. Results of a preclinical randomized controlled multicenter trial (pRCT): anti-CD49d treatment for acute brain ischemia. Sci Transl Med. (2015) 7:299ra121. doi: 10.1126/scitranslmed.aaa9853
70. Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. (2005) 5:629–40. doi: 10.1038/nri1664
71. Lee ST, Chu K, Jung KH, Kim SJ, Kim DH, Kang KM, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. (2008) 131(Pt 3):616–29. doi: 10.1093/brain/awm306
72. Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. (2011) 11:110. doi: 10.1186/1471-2377-11-110
73. Shim R, Wong CH. Ischemia, immunosuppression and infection—Tackling the predicaments of post-stroke complications. Int J Mol Sci. (2016) 17:64. doi: 10.3390/ijms17010064
74. Abdalla R, Darwish M, Aly M, Potts M, Jahromi B, Shaibani A. E-098 Effect of endovascular revascularization on neutrophil–lymphocyte ratio and relationship to 90 day outcome. J Neurointervent Surg. (2019) 11 (Suppl. 1):A102. doi: 10.1136/neurintsurg-2019-SNIS.173
75. Boisseau W, Desilles JP, Fahed R, Kyheng M, Zuber K, Sabben C, et al. Neutrophil count predicts poor outcome despite recanalization after endovascular therapy. Neurology. (2019) 93:e467–75. doi: 10.1212/WNL.0000000000007859
76. Broocks G, Hanning U, Flottmann F, Schönfeld M, Faizy TD, Sporns P, et al. Clinical benefit of thrombectomy in stroke patients with low ASPECTS is mediated by oedema reduction. Brain. (2019) 142:1399–407. doi: 10.1093/brain/awz057
77. Di Napoli M, Godoy DA, Campi V, del Valle M, Piñero G, Mirofsky M, et al. C-reactive protein level measurement improves mortality prediction when added to the spontaneous intracerebral hemorrhage score. Stroke. (2011) 42:1230–6. doi: 10.1161/STROKEAHA.110.604983
78. Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE, et al. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. (2011) 76:1238–44. doi: 10.1212/WNL.0b013e3182143317
79. Morotti A, Phuah CL, Anderson CD, Jessel MJ, Schwab K, Ayres AM, et al. Leukocyte count and intracerebral hemorrhage expansion. Stroke. (2016) 47:1473–8. doi: 10.1161/STROKEAHA.116.013176
80. Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. (2009) 40:1849–57. doi: 10.1161/STROKEAHA.108.534503
Keywords: neutrophil-to-lymphocyte ratio, stroke, mortality, functional outcome, meta-analysis
Citation: Song S-Y, Zhao X-X, Rajah G, Hua C, Kang R, Han Y, Ding Y and Meng R (2019) Clinical Significance of Baseline Neutrophil-to-Lymphocyte Ratio in Patients With Ischemic Stroke or Hemorrhagic Stroke: An Updated Meta-Analysis. Front. Neurol. 10:1032. doi: 10.3389/fneur.2019.01032
Received: 23 June 2019; Accepted: 11 September 2019;
Published: 04 October 2019.
Edited by:
Alejandro Bustamante, Vall d'Hebron University Hospital, SpainReviewed by:
Elena Zapata-Arriaza, Virgen del Rocío University Hospital, SpainSheila Gillard Crewther, La Trobe University, Australia
Copyright © 2019 Song, Zhao, Rajah, Hua, Kang, Han, Ding and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ran Meng, cmFubWVuZzIwMTFAcGt1Lm9yZy5jbg==
 Si-Ying Song
Si-Ying Song Xiao-Xi Zhao1
Xiao-Xi Zhao1 Ran Meng
Ran Meng