- 1Institute of Neuroscience and Department of Neurology of the Second Affiliated Hospital of Guangzhou Medical University, Key Laboratory of Neurogenetics and Channelopathies of Guangdong Province and the Ministry of Education of China, Guangzhou, China
- 2Department of Neurology, Guangzhou First People's Hospital, School of Medicine, South China University of Technology, Guangzhou Medical University, Guangzhou, China
- 3Shanghai Tissue Bank Biotechnology Co., Ltd, Shanghai, China
- 4The First Affiliated Hospital of Xinxiang Medical University, Xinxiang, China
- 5Guangdong 999 Brain Hospital, Guangzhou, China
- 6Epilepsy Center and Department of Neurosurgery, The First Affiliated Hospital, Jinan University, Guangzhou, China
Antiepileptic drugs frequently cause cutaneous adverse reactions (cADRs). Numerous studies have reported associations between human leukocyte antigen (HLA) alleles and cADRs caused by single antiepileptic drug in Southern Han Chinese people. However, the relationship between the HLA allele and cADRs sequentially induced by two or more antiepileptic drugs (AEDs-induced cross-reactivity) is unclear. To explore the associations between HLA alleles and AEDs-induced cross-reactivity, we prospectively recruited patients with AEDs-induced cross-reactivity from 2009 to 2017 and performed high-resolution genotyping to detect the HLA-A, B, C, and DRB1 alleles in patients for comparison with normal controls. To verify the important genotype, we compared its presence in patients with cross-reactivity to enlarged normal controls, and its presence in patients with carbamazepine (CBZ)-induced maculopapular exanthema (MPE) to CBZ-tolerant controls. Further, the important allele was replicated by meta-analysis. Twenty-three patients with AED-induced cross-reactivity and 500 healthy individuals were enrolled from Southern China. All patients had a mild rash without mucosal or systemic involvement. The HLA-B*13:01 allele was present in 34.78% (8/23) of patients, 14.60% (73/500) of healthy individuals, and 14.5% (763/5,270) healthy individuals, revealing a significant association (8/23 vs. 73/500; P = 0.02; OR: 3.12; 95% CI: 1.28–7.62; 8/23 vs. 763/5,270; P = 0.014; OR: 3.15; 95% CI: 1.33–7.46). HLA-B*13:01 was presented numerically higher in CBZ-induced MPE than that in CBZ-tolerant individuals without statistical significance (33/145, 22.76%, vs. 28/179, 15.64%; P = 0.103). Meta-analysis revealed an association between HLA-B*13:01 and cADRs induced by single AEDs or/and non-AEDs in Chinese and Thai populations (P = 0.000). This study suggests that HLA-B*13:01 is potentially associated with AED-cADRs in general, possibly with stronger effect in cross-reactivity. Screening for HLA-B*13:01 prior to starting AEDs therapy may help to avoid cADRs. However, this association requires further analysis in a multi-center study with a larger sample size.
Introduction
Carbamazepine (CBZ), lamotrigine (LTG), oxcarbazepine (OXC), phenytoin (PHT), and phenobarbital (PB) share a similar aromatic structure and are widely used antiepileptic drugs (AEDs) to control seizures, trigeminal neuralgia, etc. However, they frequently lead to cutaneous adverse reactions (cADRs) (1, 2). cADRs comprise 10–30% of all reported adverse drug reaction (3–5), which vary from mild maculopapular eruption (MPE), hypersensitivity syndrome (HSS), to Stevens-Johnson syndrome (SJS) and/or toxic epidermal necrolysis (TEN) (6). The most severe phenotypes SJS and TEN show high morbidity and mortality (7). It has been reported that 3.61% of patients treated with AEDs experience a skin rash induced by single AED, while 0.48% of patients show cross-reactivity sequentially induced by two or more AEDs (AEDs-induced cross-reactivity) in Han Chinese people, including aromatic and non-aromatic AEDs such as valproic acid, levetiracetam, and topiramate (8). cADRs frequently result in drug withdrawal, which increases the risk of seizure worse and medical costs (9, 10).
Although the mechanism of AEDs-induced cADRs is unclear, numerous studies have suggested that the major histocompatibility complex (also known as human leukocyte antigen, HLA) is involved in cADRs (9). Several HLA alleles were found to be associated with cADRs caused by single drug. The HLA-B*15:02 allele is a strong risk factor for CBZ-induced SJS in Han Chinese people (11, 12), HLA-A*24:02 is a common risk factor for aromatic AEDs induced SJS in the Southern Han Chinese people (13), and HLA-A*31:01 is associated with CBZ-induced cADRs in Japanese and Caucasian people (14, 15). However, the HLA-related genetic risk factors of AEDs-induced cross-reactivity are unclear.
Here, we performed a case-control study to investigate the relationship between AEDs-induced cross-reactivity and HLA alleles in a Southern Han Chinese population.
Materials and Methods
Participants
Individuals were enrolled in southern China from 2009 to 2017, and treated at the Second Affiliated Hospital of Guangzhou Medical University, Guangzhou First People's Hospital, the First Affiliated Hospital of Jinan University, and Guangdong 999 Brain Hospital in Guangzhou. The study was approved by the hospital ethics committee. All participants or their parents provided written informed consent in accordance with the Declaration of Helsinki.
Cases with cross-reactivity were defined as sequential rashes in response to two or more AEDs in the same individual. For example, when a patient experienced a skin rash induced by CBZ, CBZ was discontinued, and 2 weeks later the patient was treated with LTG, then rashes appeared again. The diagnosis of AEDs-induced cross-reactivity was confirmed by a dermatologist based on the clinical and morphological characteristics of the patients' skin. MPE was defined as skin eruptions without mucosal or systemic involvement. SJS and TEN were diagnosed according to Roujeau's diagnostic criteria. Hypersensitivity syndrome was defined as a fever, rash, eosinophilia, and systemic manifestation (e.g., hepatitis and nephritis).
Five-hundred healthy volunteers who have not taken AEDs were recruited as normal controls. To verify the significant allele, we further increased the sample size of normal controls from 500 to 5,270. All healthy volunteers were recruited from the Healthy Physical Examination Center in the Second Affiliated Hospital of Guangzhou Medical University, and had not taken AEDs. Because most of the patients had taken CBZ, we recruited another cohort comprising of individuals with CBZ-induced MPE and CBZ-tolerant to clarify whether AEDs-induced cross-reactivity and CBZ-induced MPE have the same risk HLA allele. The cases with CBZ-induced MPE and CBZ tolerant controls were recruited from the Second Affiliated Hospital of Guangzhou Medical University between 2007 and 2018. CBZ tolerant controls were participants with epilepsy who took CBZ for at least 3 months without evidence of cutaneous adverse reactions.
All cases and controls were Southern Han Chinese.
Sequence based high-resolution HLA-A/B/C/DRB1 genotyping was performed by Shanghai Tissue Bank Biotechnology Co., Ltd. (Shanghai, China).
Statistical Analysis
We performed statistical analyses using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). Fisher's exact tests were used to assess the difference in the presence of HLA alleles between the case and control groups. Because CBZ and LTG were the most common culprit drugs, we conducted an independent t-test to compare the mean age, latency to cADRs, and initial and maximum dosages between one group of CBZ/LTG as the first culprit drug and the other group of CBZ/LTG as the second culprit drug. P < 0.05 (two-sided) were considered significantly different. The corrected P (Pc) values were estimated by Bonferroni's correction for multiple comparisons (n = 12, 16, and 14 for HLA-A, HLA -B, and HLA-C alleles, respectively).
Meta-Analysis
We performed meta-analyses on data obtained from other studies to investigate the relationship between HLA-B*13:01 allele and cADRs induced by AEDs or/and non-AEDs. A complete search of online databases, including MEDLINE, EMBASE, Google Scholar, was conducted.
The following terms were used in our searches: “HLA-B*13:01” or “human leukocyte antigen B*13:01,” “Stevens–Johnson syndrome” or “SJS,” “toxic epidermal necrolysis” or “TEN,” “cutaneous adverse drug reactions” or “cADRs,” “maculopapular eruption” or “MPE,” “Antiepileptic drugs” or “AEDs.” The latest search was conducted on April1, 2018.
Criteria for the selection of studies were: (1) the report was of a case-control study on association between HLA-B*13:01 and cADRs induced by AEDs or non-AEDs; (2) the genotyping method and ethnicity were provided; (3) the presence of HLA-B*13:01 in the cases, either the population controls or tolerant controls was reported or could be obtained from the authors or other sources; (4) the most recent publication with the largest number of samples was selected when duplicate publications were identified. Exclusion criteria were: (1) reports were not of case-control studies; (2) repeated studies; (3) studies did not indicate the presence of HLA-B*13:01 in the case group and control group; (4) abstracts and reviews; (5) non-human studies.
The following information were extracted: the first author of the study, publication year, ethnicity of the study population, presence of HLA-B*13:01 allele among cADRs cases and controls, total number of cADRs cases and controls, and main results. The methodological quality was assessed according to the recommendations of the Cochrane Collaboration Handbook (https://www.cochrane.de).
Data managements and analyses were conducted using STATA (Version 10.1 Stata Corp LP, College Station, TX, USA). Odds ratios (ORs) with corresponding 95% confidence intervals (CIs) were calculated to verify the association between the HLA-B*13:01 allele and drugs-induced cADRs. Begg's test was used to evaluate publication bias (16). Statistical heterogeneity among studies was assessed via the Q statistic and I2 tests (17). A P < 0.1 and an I2-value >50% were defined as evidence of statistical heterogeneity, under which the association was assessed using the random model. Analyses were also performed separately on studies using different types of control patients (e.g., drug-tolerant or normal controls) and different drugs induced cADRs.
Seven articles met the criteria. Data from 8 studies, including the present study, were used for meta-analysis. The data included the populations from Thailand and China.
Results
Characteristics of Patients
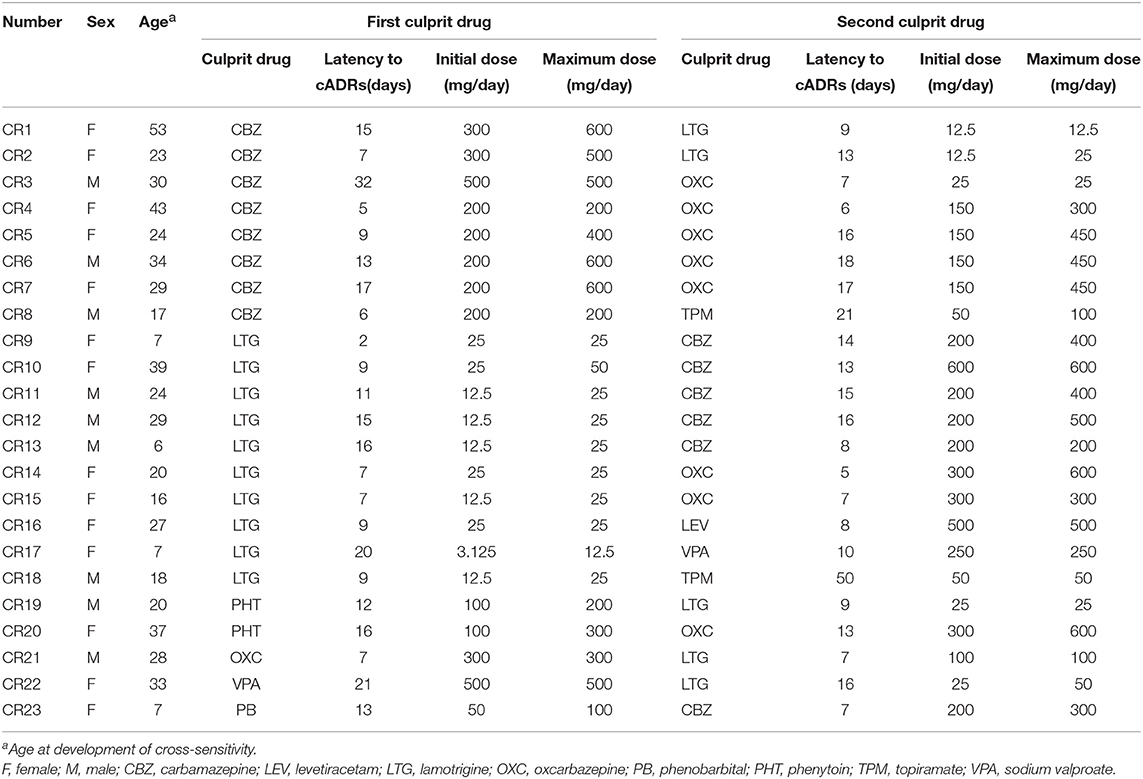
A total of 23 patients with AEDs-induced cross-reactivity and 500 healthy volunteers were recruited in this study. The demographic variables and clinical manifestations of cases are summarized in Table 1. There were 14 females and 9 males. The average age was 24 years (range 6–53 years). All cases occurred as sequential rashes induced by two of the following drugs: CBZ, LTG, OXC, levetiracetam, topiramate, valproic acid, PB, and PHT. The phenotype of AED-induced cross-reactivity in this cohort was MPE without mucosal or systemic involvement.
CBZ and LTG were the most common culprit drugs. With respect to the sex ratio, mean age, latency to cADRs, and initial and maximum dosages, no significant difference was found between one group of CBZ/LTG as the first culprit drug and the other group of CBZ/LTG as the second culprit drug.
HLA-B*13:01 Is Associated With Antiepileptic Drugs-Induced Cross-Reactivity
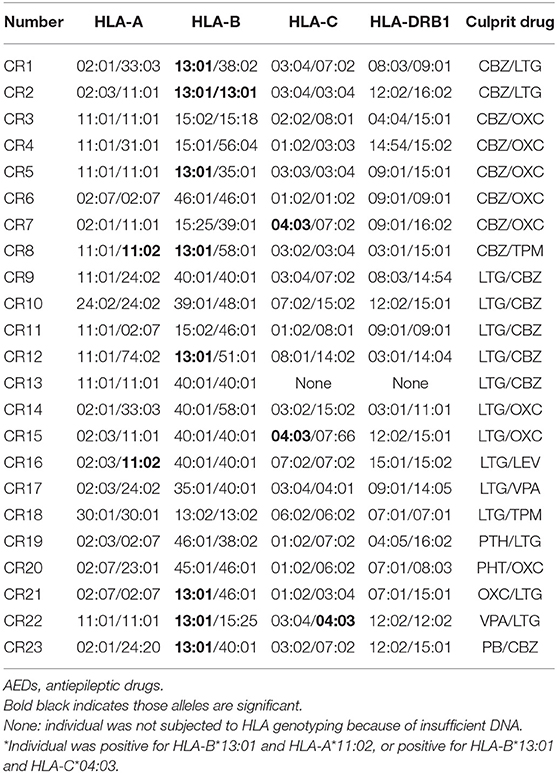
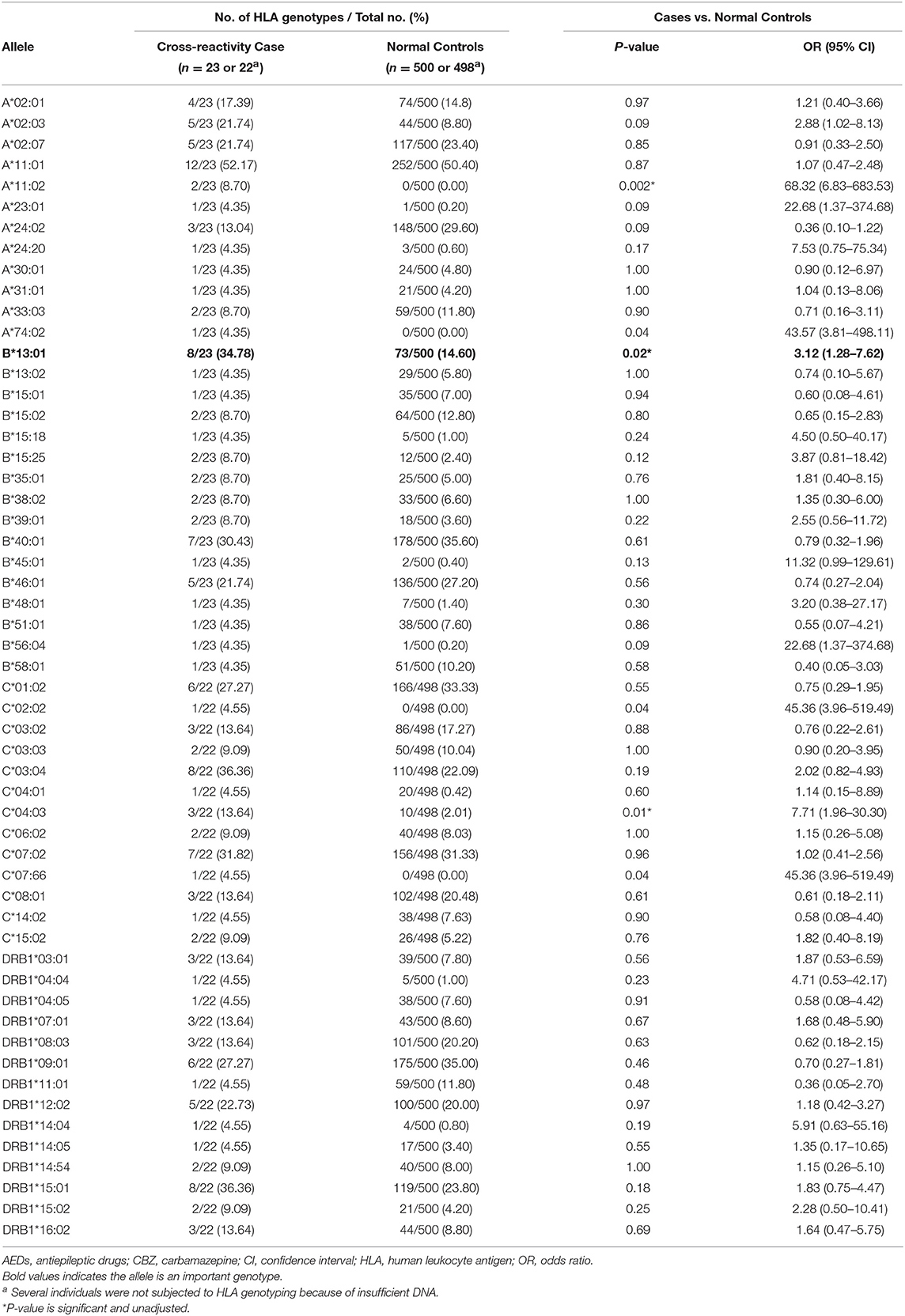
The HLA genotypes of patients with AEDs-induced cross-reactivity are shown in Table 2. The distribution of HLA alleles in the 23 cases and 500 normal controls is shown in Table 3. Three alleles were significantly associated with AEDs-induced cross-reactivity. The HLA-B*13:01 allele was found in 8 (34.78%) of the 23 AEDs-induced cross-reactivity patients and 73 (14.60%) of the 500 normal controls (P = 0.02; OR: 3.12; 95% CI: 1.28–7.62; Pc = 0.32, n = 16 for HLA-B*13:01 correction). The HLA-A*11:02 allele was found in two (8.70%) of the 23 AEDs-induced cross-reactivity patients, and in none (0%) of the 500 normal controls (P = 0.002; OR: 68.32; 95% CI: 6.83–683.53; Pc = 0.02, n = 12 for HLA-A*11:02 correction). The HLA-C*04:03 allele was found in three (13.64%) of the 22 AED-induced cross-reactivity patients and 10 (2.01%) of the 498 normal controls (P = 0.01; OR: 7.71; 95% CI: 1.96-30.3; Pc = 0.14, n = 14 for HLA-C*04:03 correction). Furthermore, one case with HLA-A*11:02 and one case with HLA-C*04:03 were positive for HLA-B*13:01. If excluding these cases, the presence of HLA-A*11:02 and C*04:03 in the patients was 4.35% (1/23) and 9.09% (2/22), respectively. The presence of the two alleles HLA-A*11:02 and C*04:03 is very low in the normal Chinese population (http://www.allelefrequencies.net, such as 0 and 2.01% in this cohort). These results suggested HLA-B*13:01 should be considered further. To exclude the possibility of lost significance, we compared the presence of HLA-B*13:01 between the 23 patients of AEDs-induced cross-reactivity and a larger control cohort containing 5,270 normal individuals, which revealed a significant association between HLA-B*13:01 and AEDs-induced cross-reactivity (8/23, 34.78%, vs. 763/5,270, 14.48%; P = 0.014, OR:3.15, 95%CI: 1.33–7.46).
Since 18 subjects (18/23, 78.26%) showed cross-reactivity induced by two aromatic AEDs, we compared the presence of HLA-B*13:01 between the 18 patients of aromatic AEDs-induced cross-reactivity (6/18, 33.33%) and the two normal control cohorts, which showed no significant difference (6/18 vs. 73/500, P = 0.066; 6/18 vs. 763/5,270, P = 0.054, respectively).
Because most of the AEDs-induced cross-reactivity patients (14/23, 60.9%) had taken CBZ, we recruited another cohort comprising of individuals with CBZ-induced MPE (145) and CBZ-tolerant controls (179) to clarify whether AEDs-induced cross-reactivity and CBZ-induced MPE have the same risk HLA allele. The HLA-B*13:01 presence was significantly higher in AEDs-induced cross-reactivity individuals than in CBZ-tolerant individuals (8/23, 34.78%, vs. 28/179, 15.64%; P = 0.049, OR: 2.88, 95%CI: 1.11–7.42), while its presence in CBZ-induced MPE was numerically higher than that in CBZ-tolerant individuals without statistical significance (33/145, 22.76%, vs. 28/179, 15.64%; P = 0.103) (Supplementary Table 1).
In addition, in this cohort, two of 23 AEDs-induced cross-reactivity patients (2/23) were positive for HLA-B*15:02, three of 23 patients (3/23) were positive for HLA-A*24:02, and one patient (1/23) was positive for HLA-A*31:01. Compared to the normal controls, there was no significant difference in the presence of HLA-B*15:02, HLA-A*24:02, or HLA-A*31:01 (8.7% vs. 12.80%, P = 0.80; 13.04% vs. 29.60%, P = 0.09; 4.35% vs. 4.20%, P = 1.0, respectively) (Table 3).
Meta-Analysis: HLA-B*13:01 and cADRs
To clarify the relationship between HLA-B*13:01 and cADRs, a meta-analysis was performed using data from different populations. Seven case-control studies including Thailand and China population met the criteria (Supplementary Table 2). The meta-analysis analyzed 8 studies, including the present study. The data covered two aspects, i.e., HLA-B*13:01 is associated with cADRs induced by single AED, and HLA-B*13:01 is associated with cADRs induced by single non-AED. There was a total of 254 cases with cADRs induced by single AED, 446 AEDs tolerant controls, and 6,678 normal controls, and there was a significant association between HLA-B*13:01 and cADRs induced by single AED (P = 0.000), compared to tolerant controls and normal controls (Figure 1A). There was a total of 117 cases with cADRs induced by single non-AED, 1,195 non-AEDs tolerant controls, and 3,309 normal controls, and there was a significant association between HLA-B*13:01 and cADRs induced by single non-AED (P = 0.000), compared to tolerant controls and normal controls (Figure 1B). We further combined the cases of cADRs induced by AEDs with the case of cADRs induced by non-AEDs, and there was a significant association between HLA-B*13:01 and cADRs in Thai and Chinese populations (P = 0.000), compared to tolerant controls and normal controls (Figure 1C).
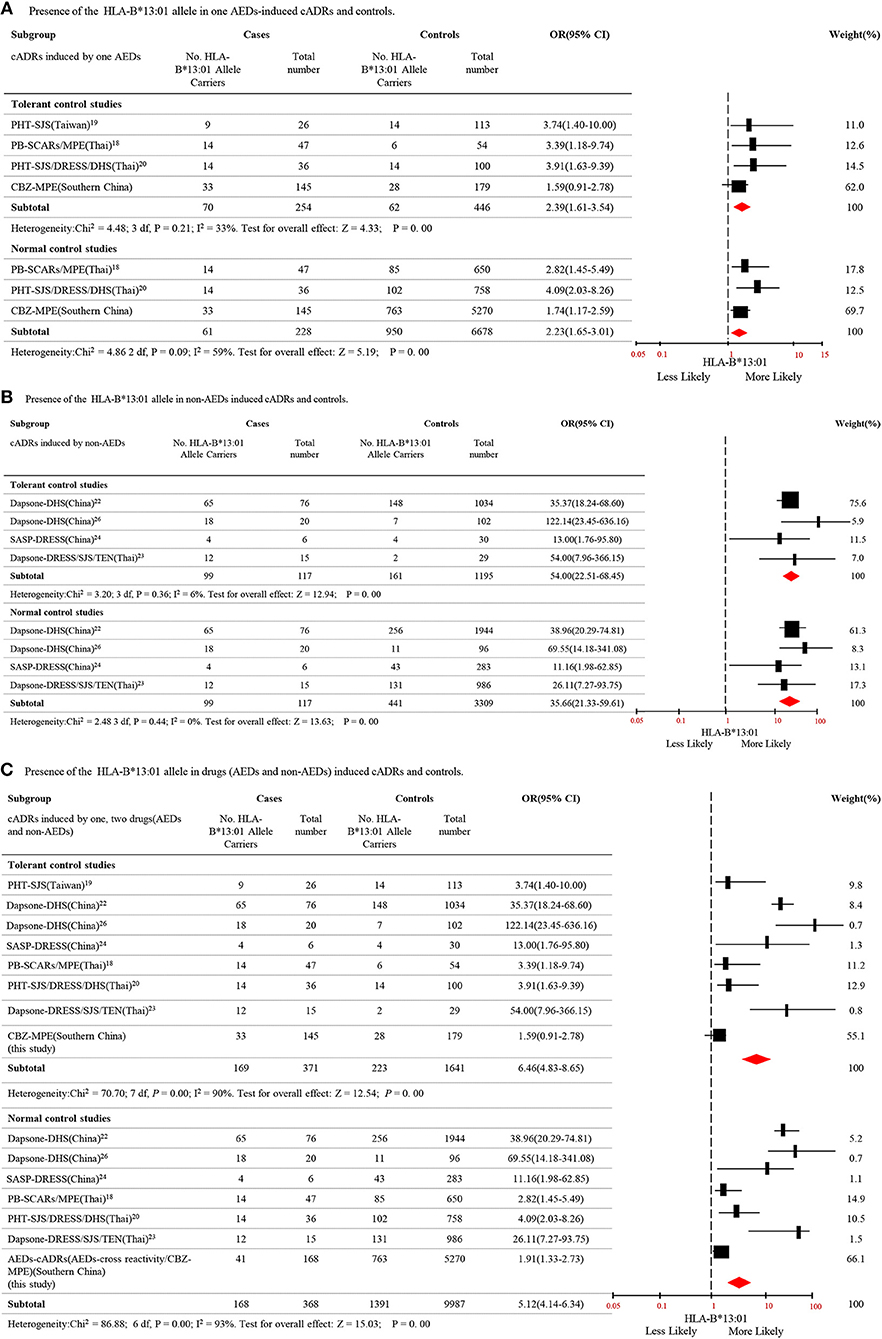
Figure 1. Distribution of HLA-B*13:01 allele in cutaneous adverse reactions induced by AEDs and non-AEDs. (A) Presence of the HLA-B* 13:01 allele in one AEDs-induced cADRs and controls. (B) Presence of the HLA-B* 13:01 allele in non-AEDs induced cADRs and controls. (C) Presence of the HLA-B* 13:01 allele in drugs (AEDs and non-AEDs) induced cADRs and controls.
Discussion
In the present study, we found that the HLA-B*13:01 allele was significantly associated with AEDs-induced cross-reactivity with a sensitivity of 34.78% and specificity of 85.40%. If Bonferroni's correction was performed for multiple comparisons, the significance of HLA-B*13:01 would be lost [Pc = 0.32 (16 × 0.02)]. Considering the HLA-B was an independent variable, while the frequency of each allele was not. To exclude the possibility of lost significance, we compared the presence of HLA-B*13:01 between the 23 patients with AEDs-induced cross-reactivity and a larger control cohort containing 5,270 normal individuals. A significant difference was found between the two groups, suggesting a significant association between HLA-B*13:01 and AEDs-induced cross-reactivity. Considering most of the patients (14/23, 60.9%) had taken CBZ, we recruited another cohort comprised of individuals with CBZ-induced MPE (145) and CBZ-tolerant (179) to clarify whether AEDs-induced cross-reactivity and CBZ-induced MPE have the same risk HLA allele. We found that HLA-B*13:01 presence was significantly higher in AEDs-induced cross-reactivity individuals than in CBZ-tolerant individuals, while its presence in CBZ-induced MPE was numerically higher than that in CBZ-tolerant control without reaching statistical significance. These results suggest that HLA-B*13:01 is a relatively higher risk factor for AEDs-induced cross-reactivity.
Previous studies demonstrated that HLA-B*13:01 is associated with PHT-SJS/TEN in Han Chinese people, and with severe cADRs induced by PHT or PB in Thai people (18–21). Meta-analysis of data from independent studies including studies conducted in Thailand and China revealed that HLA-B*13:01 may be a potential risk factor for cADRs induced by AEDs (Figure 1A). Additionally, the association with cADRs was not limited to AEDs, and studies reported that HLA-B*13:01 was associated with cADRs induced by dapsone and salazosulfapyridine (22–26). Thus, we conducted a meta-analysis to confirm that HLA-B*13:01 was associated with cADRs induced by non-AEDs in Chinese and Thai populations (Figure 1B). We further demonstrated that HLA-B*13:01 was associated with cADRs induced by drugs including AEDs and non-AEDs (Figure 1C). The HLA-B*13:01 allele is present as a medium common allele in Asian populations (https://www.allelefrequencies.net). Based on these data, HLA-B*13:01 may be a common genetic marker for predicting cADRs in Asian populations.
cADRs were considered as idiosyncratic immune responses involving the T cell-mediated drug hypersensitivities. At present, the mechanism underlying the HLA involved in AEDs-induced cADRs was unclear, and even for AEDs-induced cross-sensitivity. Previous studies demonstrated that abacavir can bind within the F pocket of the peptide-binding groove of HLA-B*57:01 and then alter the repertoire of self-peptides presented to T cells, resulted in abacavir-induced cADRs (27, 28). Specific T-cell receptor is crucial for CBZ-induced SJS in individuals with HLA-B*15:02 (29). Symptoms are relieved upon cessation of culprit drug administration, which is consistent with removal of the antigen; however, reintroduction of the drug or a drug with a similar structure can induce an allergic reaction, presumably by rapidly activating memory T cells (30–32). Several studies demonstrated that certain HLAgenotypes can induce T-cell activation in response to a specific drug, resulting in an immune response. The function of the classical HLA molecules is to present the drug for immune surveillance, followed by activation and clonal expansion of CD8+T or CD4+T cells to trigger immunological responses (33). Activated CD8+T or CD4+T cells have been detected in the epidermis and dermis of patients with cADRs (34). Further studies are required to explore the mechanism of HLA-B*13:01 involvement in AEDs-induced cross-sensitivity.
Additionally, HLA-A*11:02 and HLA-C*04:03 showed significant association with AEDs-induced cross reactivity. However, one of two cases carrying HLA-A*11:02 was positive for HLA-B*13:01 and one of three cases carrying HLA-C*04:03 was positive for HLA-B*13:01. After excluding cases that were also positive for the HLA-B*13:01, there was only one patient positive for HLA-A*11:02 and two patients positive for HLA-C*04:03. HLA-A*11:02 and HLA-C*04:03 are rare alleles and their frequency were low to 0% in Han Chinese populations (https://www.allelefrequencies.net). Hence, the relationship between HLA-A*11:02 or HLA-C*04:03 and AEDs-induced cross-reactivity requires further analysis.
Previous studies demonstrated that HLA-B*15:02 is specifically associated with CBZ-induced SJS/TEN, HLA-A*24:02 is commonly associated with AEDs-induced SJS/TEN in the Han Chinese (13, 35), HLA-A*24:02 may be associated with the cross-reactivity of DRESS/MPE induced by PHT and LTG, and HLA-B*38:01 may be a major responsible allele for the cross-reactivity of SJS/TEN induced by PHT and LTG in Spanish people (36). However, we failed to find that these alleles were associated with AEDs-induced cross reactivity, the phenotype of the cross-reactivity in this cohort was MPE, which may explain this result. Additionally, a previous study reported an association between the specific haplotype HLA-A*02:01/-B*35:01/-C*04:01 and LTG-induced MPE in Mexican Mestizo people (37), indicating that further studies should explore the relationship between the specific HLA haplotype and cross-reactivity induced by AEDs.
The rate of cross-reactivity caused by two aromatic AEDs was higher than that caused by one aromatic drug and one non-aromatic drug. It was reported that cross-sensitivity induced by aromatic AEDs (CBZ, LTG, OXC, PHT, and PB) occurred in 40–58% of patients because of their similar chemical structures and intermediary metabolism molecules (38). If detoxification of this toxic metabolite is insufficient, it may bind to cellular macromolecules, causing cell necrosis or a secondary immunological response. Among the aromatic drugs, CBZ and OXC, as well as LTG and CBZ, were administered more frequently than the other drugs. Thus, clinicians should use caution when determining replacement drug-therapy among aromatic AEDs; particularly, replace CBZ with OXC, and LTG with CBZ.
We found no significant difference in terms of the sex ratio, mean age, latency to cADRs, and initial and maximum dosages between CBZ/LTG as the first culprit drug and CBZ/LTG as the second culprit drug. Because of the different chemical and pharmacological properties of the causative drug, and heterogeneity of the clinical presentations, it is not surprising that idiosyncratic reactions involve a broad range of mechanism, and that more than one risk alleles may be involved in any single event.
Further Improvement
This study had several limitations. First, we did not divide the cases into subgroups according to cross-reactivity induced by the same order of drug prescribed due to the limit sample. Second, we did not exactly match the drug tolerant subgroup. To decrease deviation, we investigated the significance of the association by comparison with a large number of normal individuals. A further study with a larger samples size and cases from multiple centers along with exactly matched tolerant controls should be conducted to confirm that the HLA-B*13:01 allele is a risk factor for AEDs-induced cross-reactivity, and a common risk factor for cADRs.
Conclusion
The HLA-B*13:01 allele is potentially associated with AED-cADRs in general, possibly with stronger effect in cross-reactivity. Our finding suggests that screening for HLA-B*13:01 prior to starting AEDs therapy may help to avoid AEDs-induced cross-reactivity. HLA-A*31:01 testing can alert clinicians patients to patients who are at an increased risk of AEDs-induced cross-reactivity, and patients who have already experienced cADRs can be advised to avoid structurally related drugs.
Ethics Statement
The study was approved by the Second Affiliated Hospital of Guangzhou Medical University ethics committee. All participants or their parents gave written informed consent in accordance with the Declaration of Helsinki.
Author Contributions
F-LM and B-JM contributed equally to this work. ZL and Y-WS conceived and designed the experiments. F-LM, B-JM, JW, and Z-ZZ performed the experiments. F-LM, B-JM, BQ, and Y-WS analyzed the data. F-LM, B-JM, BQ, Y-MO, JW, Y-HY, W-PL, C-XF, NH, and R-YC collected samples and clinical data. F-LM, B-JM, and Y-WS wrote the paper. All authors have been involved in the study and have approved the final paper.
Funding
This work was funded by the National Natural Science Foundation of China (grant no. 81571274 and 81601136), the Science and Technology Project of Guangdong Province (grant nos. 2017B09090436 and 2017B030314159), the Science and Technology Project of Guangzhou (grant no. 201904010275), and the Yangcheng Scholar Research Project of Guangzhou Municipal College (grant no. 12A017G). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest Statement
Z-ZZ was employed by Shanghai Tissue Bank Biotechnology Co. Ltd, China.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer PK declared a past co-authorship with several of the authors, BQ, JW, and NH, to the handling editor.
Acknowledgments
We are grateful to the He Shanheng Charity Foundation for contributing to the development of this institute.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2019.00614/full#supplementary-material
Abbreviations
CBZ, carbamazepine; LTG, lamotrigine; OXC, oxcarbazepine; PHT, phenytoin; PB, phenobarbital; AED, antiepileptic drug; cADRs, cutaneous adverse reactions; MPE, maculopapular exanthema; HSS, hypersensitivity syndrome; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis; VPA, valproic acid; LEV, levetiracetam; TPM, topiramate; HLA, human leukocyte antigen.
References
1. Leeder JS. Mechanisms of idiosyncratic hypersensitivity reactions to antiepileptic drugs. Epilepsia. (1998) 39(Suppl. 7):S8–16. doi: 10.1111/j.1528-1157.1998.tb01679.x
2. Toledano R, Gil-Nagel A. Adverse effects of antiepileptic drugs. Semin Neurol. (2008) 28:317–27. doi: 10.1055/s-2008-1079336
3. Stewart RB, May FE, Cullen SI. Dermatologic adverse drug reactions in hospitalized patients. Am J Hosp Pharm. (1979) 36:609–12. doi: 10.1093/ajhp/36.5.609
4. Faich GA, Knapp D, Dreis M, Turner W. National adverse drug reaction surveillance: 1985. JAMA. (1987) 257:2068–70. doi: 10.1001/jama.1987.03390150084040
5. Naldi L, Conforti A, Venegoni M, Troncon MG, Caputi A, Ghiotto E, et al. Cutaneous reactions to drugs. An analysis of spontaneous reports in four Italian regions. Br J Clin Pharmacol. (1999) 48:839–46. doi: 10.1046/j.1365-2125.1999.00096.x
6. Roujeau JC. The spectrum of Stevens-Johnson syndrome and toxic epidermal necrolysis: a clinical classification. J Invest Dermatol. (1994) 102:28S−30S. doi: 10.1111/1523-1747.ep12388434
7. Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med. (1994) 331:1272–85. doi: 10.1056/NEJM199411103311906
8. Wang XQ, Lang SY, Shi XB, Tian HJ, Wang RF, Yang F. Cross-reactivity of skin rashes with current antiepileptic drugs in Chinese population. Seizure. (2010) 19:562–6. doi: 10.1016/j.seizure.2010.09.003
9. Roujeau JC, Bracq C, Huyn NT, Chaussalet E, Raffin C, Duedari N. HLA phenotypes and bullous cutaneous reactions to drugs. Tissue Antigens. (1986) 28:251–4. doi: 10.1111/j.1399-0039.1986.tb00491.x
10. Severino G, Del Zompo M. Adverse drug reactions: role of pharmacogenomics. Pharmacol Res. (2004) 49:363–73. doi: 10.1016/j.phrs.2003.05.003
11. Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, Ho HC, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. (2004) 428:486. doi: 10.1038/428486a
12. Chen P, Lin JJ, Lu CS, Ong CT, Hsieh PF, Yang CC, et al. Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med. (2011) 364:1126–33. doi: 10.1056/NEJMoa1009717
13. Shi YW, Min FL, Zhou D, Qin B, Wang J, Hu FY, et al. HLA-A*24:02 as a common risk factor for antiepileptic drug-induced cutaneous adverse reactions. Neurology. (2017) 88:2183–91. doi: 10.1212/WNL.0000000000004008
14. McCormack M, Alfirevic A, Bourgeois S, Farrell JJ, Kasperaviciute D, Carrington M, et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. (2011) 364:1134–43. doi: 10.1056/NEJMoa1013297
15. Ozeki T, Mushiroda T, Yowang A, Takahashi A, Kubo M, Shirakata Y, et al. Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum Mol Genet. (2011) 20:1034–41. doi: 10.1093/hmg/ddq537
16. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
17. Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics. (2005) 21:3672–3. doi: 10.1093/bioinformatics/bti536
18. Hung SI, Chung WH, Liu ZS, Chen CH, Hsih MS, Hui RC, et al. Common risk allele in aromatic antiepileptic-drug induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Han Chinese. Pharmacogenomics. (2010) 11:349–56. doi: 10.2217/pgs.09.162
19. Chung WH, Chang WC, Lee YS, Wu YY, Yang CH, Ho HC, et al. Genetic variants associated with phenytoin-related severe cutaneous adverse reactions. JAMA. (2014) 312:525–34. doi: 10.1001/jama.2014.7859
20. Manuyakorn W, Mahasirimongkol S, Likkasittipan P, Kamchaisatian W, Wattanapokayakit S, Inunchot W, et al. Association of HLA genotypes with phenobarbital hypersensitivity in children. Epilepsia. (2016) 57:1610–6. doi: 10.1111/epi.13509
21. Yampayon K, Sukasem C, Limwongse C, Chinvarun Y, Tempark T, Rerkpattanapipat T, et al. Influence of genetic and non-genetic factors on phenytoin-induced severe cutaneous adverse drug reactions. Eur J Clin Pharmacol. (2017) 73:855–65. doi: 10.1007/s00228-017-2250-2
22. Wang H, Yan L, Zhang G, Chen X, Yang J, Li M, et al. Association between HLA-B*1301 and dapsone-induced hypersensitivity reactions among leprosy patients in China. J Invest Dermatol. (2013) 133:2642–4. doi: 10.1038/jid.2013.192
23. Zhang FR, Liu H, Irwanto A, Fu XA, Li Y, Yu GQ, et al. HLA-B*13:01 and the dapsone hypersensitivity syndrome. N Engl J Med. (2013) 369:1620–8. doi: 10.1056/NEJMoa1213096
24. Yang F, Gu B, Zhang L, Xuan J, Luo H, Zhou P, et al. HLA-B*13:01 is associated with salazosulfapyridine-induced drug rash with eosinophilia and systemic symptoms in Chinese Han population. Pharmacogenomics. (2014) 15:1461–9. doi: 10.2217/pgs.14.69
25. Tempark T, Satapornpong P, Rerknimitr P, Nakkam N, Saksit N, Wattanakrai P, et al. Dapsone-induced severe cutaneous adverse drug reactions are strongly linked with HLA-B*13: 01 allele in the Thai population. Pharmacogenet Genomics. (2017) 27:429–37. doi: 10.1097/FPC.0000000000000306
26. Wu X, Yang F, Chen S, Xiong H, Zhu Q, Gao X, et al. Clinical, viral and genetic characteristics of drug reaction with eosinophilia and systemic symptoms (DRESS) in Shanghai, China. Acta Derm Venereol. (2017) 98:401–05. doi: 10.2340/00015555-2867
27. Illing PT, Vivian JP, Dudek NL, Kostenko L, Chen Z, Bharadwaj M, et al. Immune self-reactivity triggered by drug-modified HLA-peptide repertoire. Nature. (2012) 486:554–8. doi: 10.1038/nature11147
28. Ostrov DA, Grant BJ, Pompeu YA, Sidney J, Harndahl M, Southwood S, et al. Drug hypersensitivity caused by alteration of the MHC-presented self-peptide repertoire. Proc Natl Acad Sci USA. (2012) 109:9959–64. doi: 10.1073/pnas.1207934109
29. Ko TM, Chen YT. T-cell receptor and carbamazepine-induced Stevens-Johnson syndrome and toxic epidermal necrolysis: understanding a hypersensitivity reaction. Expert Rev Clin Immunol. (2012) 8:467–77. doi: 10.1586/eci.12.31
30. Arroyo S, de la Morena A. Life-threatening adverse events of antiepileptic drugs. Epilepsy Res. (2001) 47:155–74. doi: 10.1016/S0920-1211(01)00306-0
31. Hetherington S, McGuirk S, Powell G, Cutrell A, Naderer O, Spreen B, et al. Hypersensitivity reactions during therapy with the nucleoside reverse transcriptase inhibitor abacavir. Clin Ther. (2001) 23:1603–14. doi: 10.1016/S0149-2918(01)80132-6
32. Pichler WJ. Pharmacological interaction of drugs with antigen-specific immune receptors: the p-i concept. Curr Opin Allergy Clin Immunol. (2002) 2:301–5. doi: 10.1097/00130832-200208000-00003
33. Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. (2010) 5:39. doi: 10.1186/1750-1172-5-39
34. Wu Y, Farrell J, Pirmohamed M, Park BK, Naisbitt DJ. Generation and characterization of antigen-specific CD4+, CD8+, and CD4+CD8+ T-cell clones from patients with carbamazepine hypersensitivity. J Allergy Clin Immunol. (2007) 119:973–81. doi: 10.1016/j.jaci.2006.12.617
35. Hung SI, Chung WH, Jee SH, Chen WC, Chang YT, Lee WR, et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics. (2006) 16:297–306. doi: 10.1097/01.fpc.0000199500.46842.4a
36. Ramirez E, Bellon T, Tong HY, Borobia AM, de Abajo FJ, Lerma V, et al. Significant HLA class I type associations with aromatic antiepileptic drug (AED)-induced SJS/TEN are different from those found for the same AED-induced DRESS in the Spanish population. Pharmacol Res. (2017) 115:168–78. doi: 10.1016/j.phrs.2016.11.027
37. Fricke-Galindo I, Martinez-Juarez IE, Monroy-Jaramillo N, Jung-Cook H, Falfan-Valencia R, Ortega-Vazquez A, et al. HLA-A*02:01:01/-B*35:01:01/-C*04:01:01 haplotype associated with lamotrigine-induced maculopapular exanthema in Mexican Mestizo patients. Pharmacogenomics. (2014) 15:1881–91. doi: 10.2217/pgs.14.135
Keywords: antiepileptic drugs, cross-reactivity, HLA allele, HLA-B*13:01, genetic marker
Citation: Min F-L, Mao B-J, Zheng Z-Z, He N, Fan C-X, Cai R-Y, Wang J, Ou Y-M, Qin B, Liao W-P, Yi Y-H, Li Z and Shi Y-W (2019) HLA-B*13:01 as a Risk Allele for Antiepileptic Drugs-Induced Cutaneous Adverse Reactions: Higher Risk for Cross-Reactivity? Front. Neurol. 10:614. doi: 10.3389/fneur.2019.00614
Received: 17 July 2018; Accepted: 24 May 2019;
Published: 11 June 2019.
Edited by:
Udaya Seneviratne, Monash Medical Centre, AustraliaReviewed by:
Maheedhar Kodali, Texas A&M Health Science Center, United StatesPatrick Kwan, Monash University, Australia
Copyright © 2019 Min, Mao, Zheng, He, Fan, Cai, Wang, Ou, Qin, Liao, Yi, Li and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ze Li, bGl6ZTIwMTgwMzEyQDE2My5jb20=; Yi-Wu Shi, c3RvbmV5aXd1QDE2My5jb20=; c2hpeWl3dUBnemhtdS5lZHUuY24=
†These authors have contributed equally to this work
 Fu-Li Min1,2†
Fu-Li Min1,2† Yi-Wu Shi
Yi-Wu Shi