- 1Department of Neurology, Geisinger Medical Center, Danville, PA, United States
- 2Department of Bioinformatics, Geisinger Medical Center, Danville, PA, United States
- 3Freeman College of Management, Bucknell University, Lewisburg, PA, United States
Background and Objective: Although the risk of recurrent cerebral ischemia is higher after a transient ischemic attack (TIA), there is limited data on the outcome of TIA mimics. The goal of this study is to compare the 6-month outcome of patients with negative and positive diffusion-weighted imaging (DWI) TIAs (DWI-neg TIA vs. DWI-pos TIA) and also TIA mimics.
Methods: We prospectively studied consecutive patients with an initial diagnosis of TIA in our tertiary stroke centers in a 2-year period. Every included patient had an initial magnetic resonance (MR) with DWI and one-, three-, and six-month follow-up visits. The primary outcome was defined as the composition of intracerebral hemorrhage, ischemic stroke, TIA, coronary artery disease, and death.
Results: Out of 269 patients with the initial diagnosis of TIA, 259 patients (mean age 70.5 ± 15.0 [30–100] years old, 56.8% men) were included in the final analysis. Twenty-one (8.1%, 95% confidence interval [CI] 5.1-12.1%) patients had a composite outcome event within the six-month follow-up. Five (23.8%) and 13 (61.9%) composite outcome events occurred in the first 30 and 90 days, respectively. Among patients with DWI-neg TIA, the one- and six-month ischemic stroke rate was 1.5 and 4.6%, respectively. The incidence proportion of composite outcome event was significantly higher among patients who had the diagnosis of DWI-neg TIA compared with those who had the diagnosis of TIA mimics (12.2 vs. 2.1%—relative risk 5.9; 95% CI, 1.4–25.2). In our univariable analysis among patients with DWI-neg TIA and DWI-pos TIA, age (P = 0.017) was the only factor that was significantly associated with the occurrence of the composite outcome.
Conclusion: Our study indicated that the overall six-month rate of the composite outcome among patients DWI-neg TIA, DWI-pos TIA, and TIA mimics were 12.2, 9.7, and 2.1%, respectively. Age was the only factor that was significantly associated with the occurrence of the composite outcome.
Introduction
After a transient ischemic attack (TIA), the risk of ischemic stroke is high and ranging from 9 to 20% within the first 3 months (1–3). Patients suffering from TIA also have higher risks of myocardial infarction (4), disability, and death (5, 6). A long-term outcome study of patients with TIA has shown that the 10-year risk of stroke can be up to 19% while combined outcome risks including stroke, myocardial infarction, and death can be as high as 43% (7).
Although there is not a widely accepted definition for TIA mimics and there is only limited number of studies on patients with TIA mimics (3, 8), reports are indicating that more than 50% of patients who are referred to TIA clinics are TIA mimics (9, 10). Patients who are classified as TIA mimics have the heterogeneous etiologies ranging from cardiac ischemic events, dementia, strokes, to benign conditions (11, 12).
There is limited evidence on the outcome of TIA mimics. At the same time, some of the previous TIA outcome studies used the TIA clinical definition that alone cannot differentiate between a DWI-neg (DWI: diffusion-weighted imaging, neg: negative) TIA and DWI-pos (pos: positive) TIA. In this prospective study, we aimed to assess and compare the 6-month outcome of patients with DWI-neg TIA and TIA mimics, as well as patients with DWI-pos TIA.
Materials and Methods
We prospectively studied consecutive patients with an admission diagnosis of TIA in one of Geisinger three tertiary stroke centers or referral TIA diagnosis in our single TIA clinic in northeast Pennsylvania in a 2-year period (2016–2018). Geisinger is a comprehensive healthcare system with several hospitals and clinics, and over one million active patients of around 90% white ethnicity in central and northeast Pennsylvania, as well as southeast New Jersey.
Patient Inclusion Criteria
Included patients in our study had the admission diagnosis of TIA in one of our three tertiary stroke centers or had been referred to our dedicated TIA clinic with the referral diagnosis of TIA. Every included patient had an interpretable initial brain magnetic resonance imaging (MRI). Each patient had presented with a transient focal neurological deficit that lasted <24 h. All hospitalized patients were evaluated by an attending neurologist within 24 h. Every patient had either follow-up visits at our TIA/stroke clinic or phone encounter in the following intervals: 1 month, three, and 6 months ± 7 days following the index TIA event. We excluded patients who missed to complete the 6-month follow-up course.
Diagnostic Process
The final diagnoses of DWI-neg TIA, TIA mimics, or DWI-pos TIA were made independent of the hospital discharge and clinic diagnoses at the end of a 6-month follow-up period. Although there is no clear definition for TIA mimics, we concluded patients with “TIA mimics” when the diagnosis of TIA was ruled out, and a different diagnosis (e.g., seizure, migraine headache) was made. Patients with “DWI-neg TIA” diagnosis included patients who had no alternative diagnosis or patients with probable TIA diagnosis with negative findings in DWI. Patients with the diagnosis of “DWI-pos TIA” had their symptoms resolved within 24 h; however, they had a positive DWI for acute ischemic stroke. We independently reviewed each case, and final diagnoses were made based on all the clinical information after the completion of all follow-ups and consensus between our stroke research fellow (AS), and one of our vascular neurologists (NE). In the absence of a diagnostic consensus, our second vascular neurologist (RZ) reviewed the patient medical record and acted as a tiebreaker.
Outcome Measures
The primary outcome was defined as the composition of intracerebral hemorrhage (ICH), ischemic stroke, TIA, coronary artery disease (CAD), and all-cause death. Patients had an ischemic stroke outcome when they presented with a focal neurological deficit and a confirmatory cerebral MRI or CT Scan. All cases of recurrent stroke and DWI-neg TIA were concluded. Patients with ICH had confirmatory cerebral MRI or CT Scan, as well. Patients with CAD had signs of typical chest pain confirmed by ST-T wave changes in ECG or high blood troponin levels. We also reviewed cardiology notes for further confirmation. We calculated total composite outcome events for the studied cohort in the following intervals: 30, 90, and 180 days ± 7 days following the index event. We recorded all the patients' medical record information including demographics, presenting symptoms, past medical history, medication list, clinical work-up, and imaging study results. We calculated ABCD2 risk score for every patient (13). We also reviewed DWI, FLAIR, T2*-weighted gradient recalled echo (GRE) sequences, and CT scan. We applied Fazekas scale for white matter lesions scoring (14, 15). This study was part of the ongoing Geisinger stroke registry and approved by institutional review board of Geisinger.
Statistical Analysis
We summarized all continuous variables as mean ± standard deviation (normal distribution) and as median with Inter Quantile Range (IQR, for skewed distribution). We summarized all categorical variables as percentages with their corresponding 95% Confidence Intervals. We applied Kruskal–Wallis test for continuous variables. We performed statistical comparisons between three groups using the χ2 test or, in the case of small expected frequencies, Fisher's exact test. We performed multivariable analysis of logistic binary regression model. We performed Kaplan-Meier survival analysis to evaluate “time-to-composite outcome event” information and also the Log-Rank statistical test was applied for evaluation of differences among studied groups (P < 0.05). We used SPSS 24.0 (Chicago, Ill., USA) for all our statistical analysis. Incidence rates were calculated using MedCalc software (16).
Results
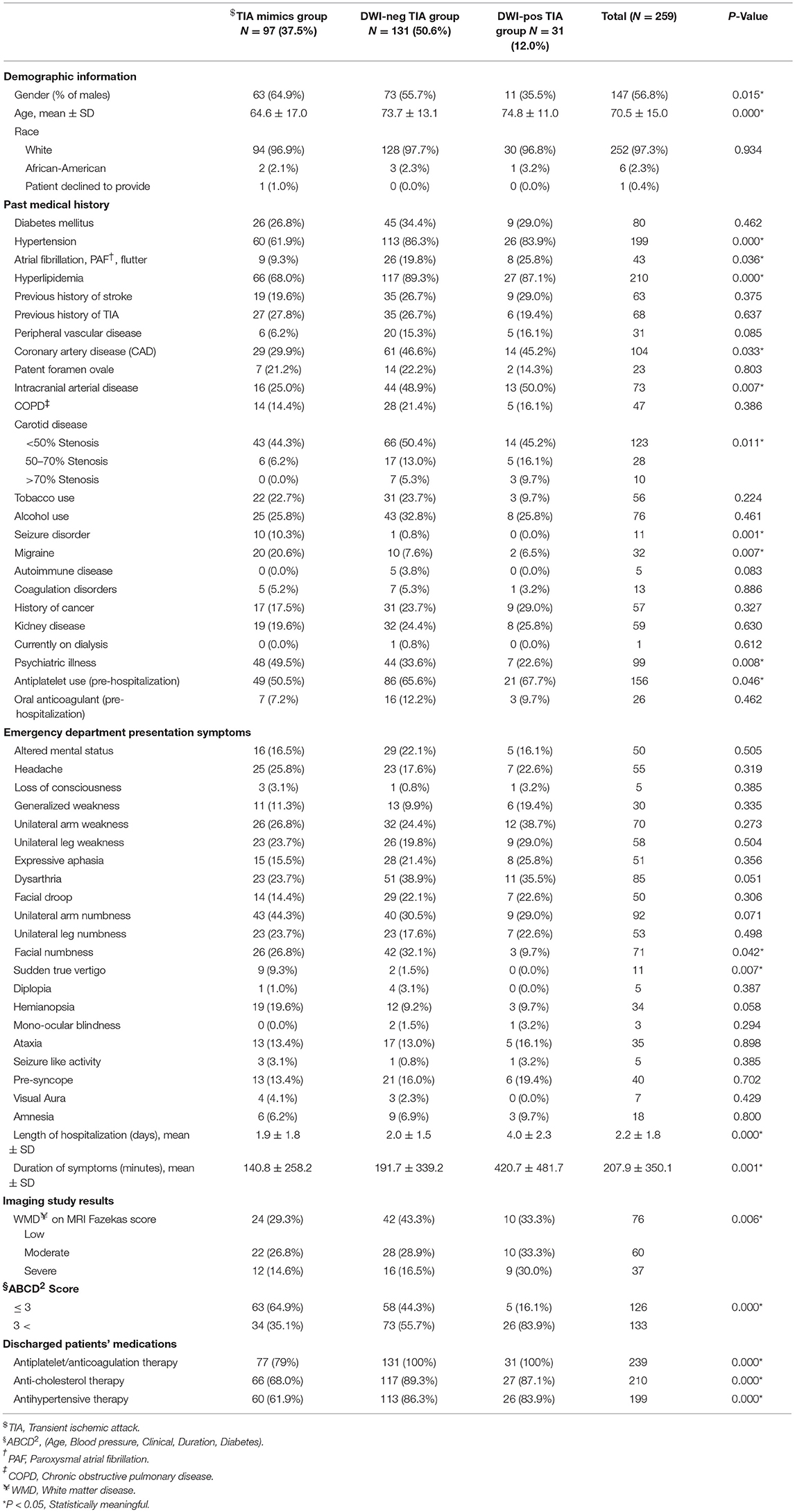
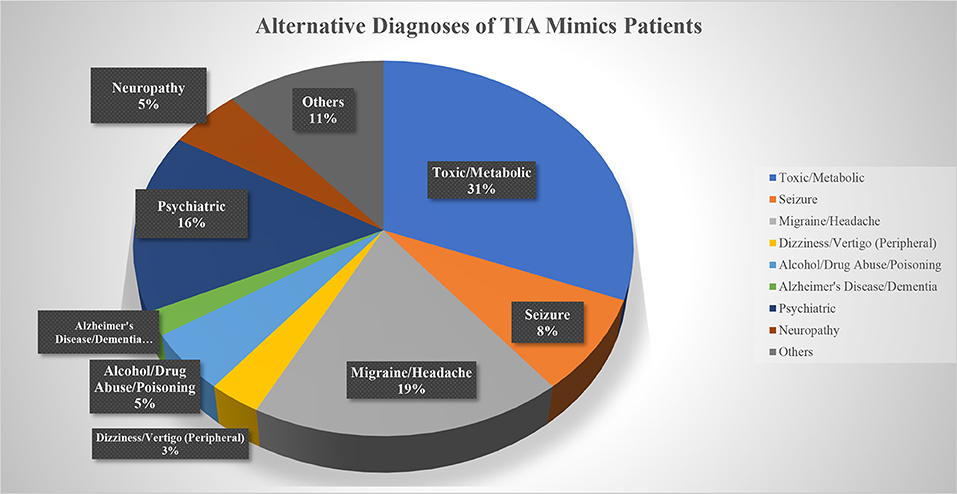
Out of 269 patients with the initial diagnosis of TIA, 259 patients (mean age 70.5 ± 15.0 [30–100] years old, 56.8% men) were included in the final analysis (Table 1). Ten patients (2 patients with a diagnosis of TIA mimics and 8 patients with a diagnosis of DWI-neg TIA) who missed to complete 180 days follow-up course were excluded. Out of 259 patients, 97 (37.4%) patients had the diagnosis of TIA mimics, 131 (50.6%) patients had a DWI-neg TIA diagnosis, and 31 (12.0%) patients had the diagnosis of DWI-pos TIA. Carotid endarterectomy was performed only for three (1.2%) patients in the group of DWI-neg TIA. The most common alternative diagnosis in the TIA mimics group was toxic/metabolic diseases followed by migraine (Figure 1).
Among studied cohort (N = 259), the univariate analysis revealed a significant difference (P < 0.05) among the three groups (TIA mimics, DWI-neg TIA, and DWI-pos TIA) in terms of age, history of hypertension, atrial fibrillation, hyperlipidemia, coronary artery disease, intracranial arterial disease, psychiatric illness, carotid disease, and antiplatelet use (Table 1). Patients with DWI-pos TIA had longer symptoms' duration and higher ABCD2 score (Table 1).
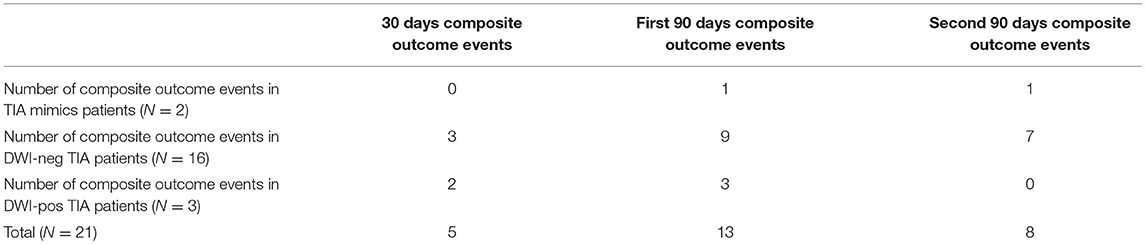
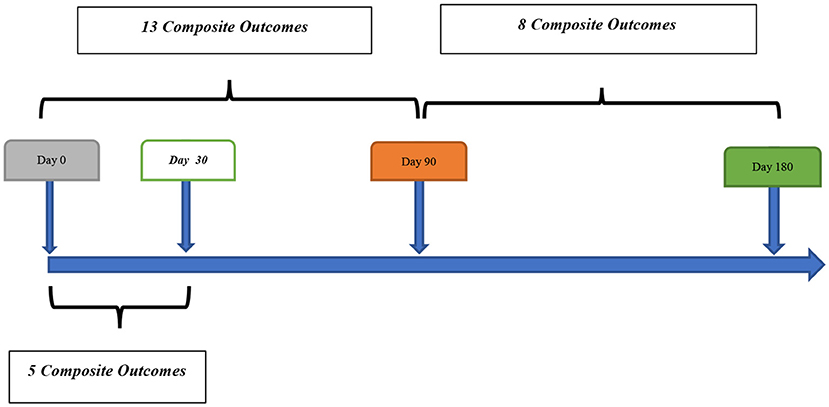
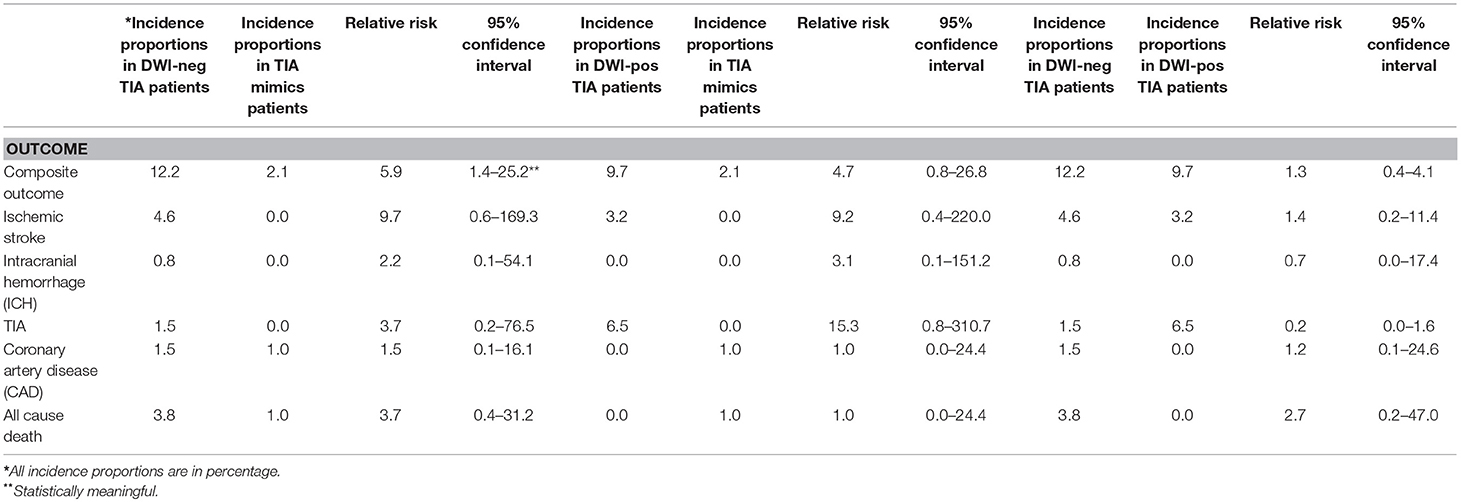
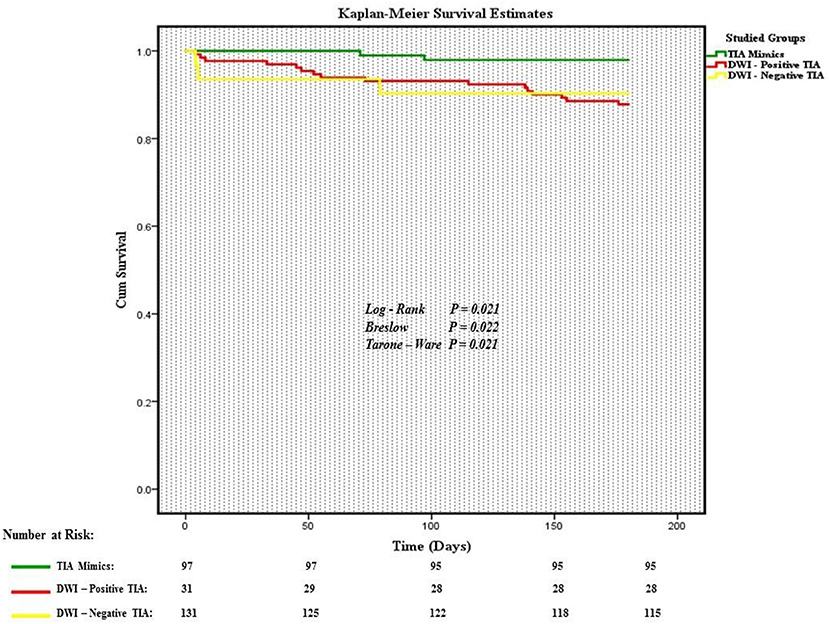
Out of 259 patients, 21 (8.1%, 95% confidence interval [CI] 5.1–12.1%) patients had a composite outcome event within the 6-month follow-up. Although several patients had readmission for other reasons, we did not have any patient who had more than one composite outcome event during the 6 months follow-up. Five (23.8%) and 13 (61.9%) composite outcome events occurred in the first 30 and 90 days, respectively (Table 2). We also observed 8 (38.1%) composite outcome events in the second 90 days (Figure 2). Overall, patients with a diagnosis of DWI-neg TIA had a higher rate (12.2%) of composite outcome events followed by DWI-pos TIA (9.7%) and TIA mimics cohort (2.1%) (Table 3).
Outcome Events in Patients With the Diagnosis of TIA Mimics
The overall composite outcome rate was 2.1% (2 out of 97 patients) in this group. There was not any composite outcome event in the first 30 days after initial admission. There was only one composite outcome event (CAD) in the first 90 days followed by one composite outcome event of death due to cancer in the second 90 days (97th day) (Figure 3).
Outcome Events in Patients With the Diagnosis of DWI-neg TIA
The overall composite outcome rate was 12.2% (16 out of 131 patients) in this group. More than half of the composite outcome events (9 events; 56.3%) occurred in the first 90 days. The single ICH outcome event occurred in this cohort. Our earliest outcome event (ischemic stroke) occurred on day 4. The one-month and six-month ischemic stroke rate was 1.5 and 4.6%, respectively. The one-month and six-month mortality rate were 0.0 and 3.8%, respectively (Table 3).
Outcome Events in Patients With the Diagnosis of DWI-pos TIA
The composite outcome rate was 9.7% (3 out of 31 patients) among patients with DWI-pos TIA. There was no death in this group. We had one patient with TIA outcome event on day 4 after initial admission. We had only one (3.2%) patient with recurrent ischemic stroke outcome event (Table 3).
The incidence proportion of composite outcome event was higher among patients who had the diagnosis of DWI-neg TIA compared with those who had the diagnosis of TIA mimics (12.2 vs. 2.1%), the difference was significant (relative risk 5.9; 95% CI, 1.4–25.2). Although there was not a significant difference in the incidence proportion of composite outcome events between patients with the diagnosis of DWI-pos TIA and patients with TIA mimics (relative risk 4.7; 95% CI, 0.82–26.8), there was a trend for a higher rate of composite outcome events in patients with DWI-pos TIA (9.7 vs. 2.1%) (Table 3).
We performed a univariable analysis among patients with DWI-neg TIA and DWI-pos TIA (Table 4). Although we observed a higher rate of hypertension (P = 0.078), the severity of white matter disease (P = 0.076), and peripheral vascular disease (P = 0.082) among patients with the positive composite outcome, the difference was not significant. In our analysis, age (P = 0.017) was the only factor that was significantly associated with the occurrence of the composite outcome. We performed a multivariable binary logistic regression analysis for our composite outcomes based on univariable analysis results. All variables with P < 0.15 (Table 4) in the univariate model including: age, hypertension, peripheral vascular disease, intracranial arterial disease, seizure disorder, and white matter disease were included in the model. None of the variables including age was found to be significantly associated with the outcome.
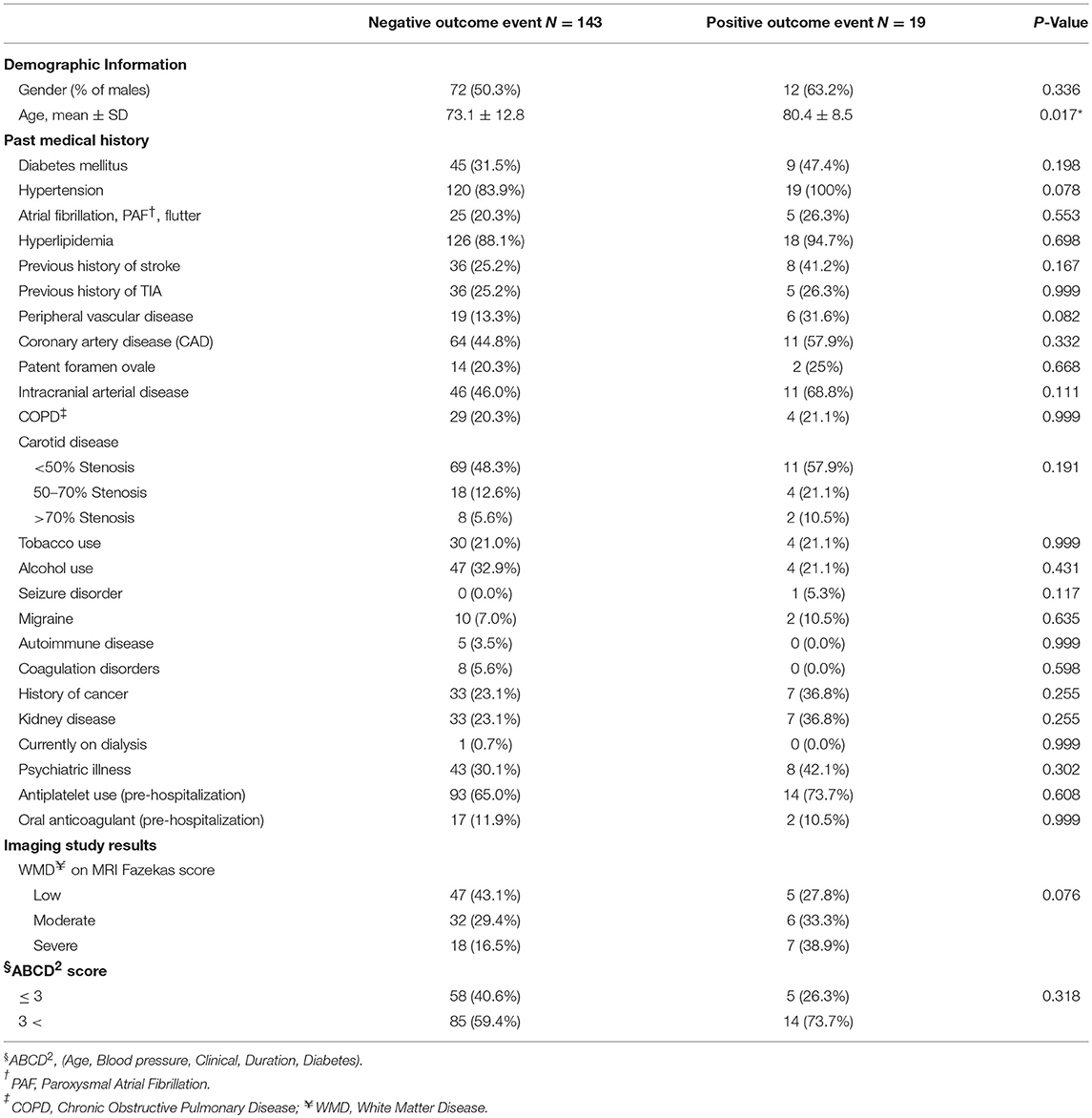
Table 4. Univariate analysis: composite outcome and associated factors among patients with DWI-neg TIA and DWI-pos TIA.
Discussion
In our study, the overall six-month rate of the composite outcome (ICH, ischemic stroke, TIA, CAD, and all-caused death) among patients with DWI-neg TIA, DWI-pos TIA, and TIA mimic were 12.2, 9.7, and 2.1%, respectively. The six-month rate of the composite outcome of combined DWI-neg TIA and DWI-pos TIA was 11.7%. More than half of composite outcome events occurred within 3 months following the index event. Patients with TIA mimics had no stroke or ICH event at 6 months follow-up course. Our results highlight the value of a multi-resolution view, where high-fidelity data at multiple time points are used, for better identification of more efficient care plans for this at-risk patient population.
Over the past few decades, there have been multiple studies measuring the rate of stroke following a TIA event. Older studies indicated a range of 9–15% (17–20) within 3 months after a TIA event; however, newer studies reported a lower range of 0.9–4.3% (8, 21–23). In our cohort, patients with DWI-neg TIA had 4.6% risk stroke occurrence at 6 months. Although there has been considerable heterogeneity among studies due to different TIA phenotype and outcome definition, the calculated risk of stroke (4.6%) in our study is similar to recent studies. There are limited literature on TIA mimics; however, we found two studies (3, 8), that evaluated the outcome of patients with a TIA mimics. Our TIA mimics patients had no stroke outcome event at 6 months follow-up course which was similar to the 3 months TIA mimics outcome results in the other two studies (3, 8). Our TIA mimics patients had a composite outcome rate of 2.1% at 6 months including 1.0% all-cause death rate and 1.0% CAD that were closely similar to the retrospective study by Dutta et al. (8) with the all-cause death rate of 0.6% and CAD rate of 0.6% at 3 months follow-up course. It is noteworthy that the lack of a widely approved definition for TIA mimics and heterogeneous nature of TIA mimics should be taken into account for interpretation of outcome in different studies.
The decremental trend of the cerebral ischemia recurrence could be attributed to the changes in the definition of TIA, better secondary prevention, and faster evaluation of TIA patients (24). Recent studies have shown that over the past decade patients with primary TIA or stroke in developed countries are older probably due to improved primary prevention. The concept of rapid access TIA clinic or urgent in-hospital care might have also played an important role in reducing recurrent ischemic events.
In our study, patients with DWI-pos TIA had a 9.7% rate of the composite outcome within 6 months follow-up course. Comparison of our results with 1-year outcome assessment of a large international cohort (22) shows a barely similar composite outcome rate (6.2%). One important difference between these two studies is that we used MRI to differentiate between DWI-pos TIA and DWI-neg TIA while in the other study a mixed pool of DWI-neg TIA or DWI-pos TIA patients were considered for outcome assessment. We did not have any death during the 6-month follow-up course while in the other study all-cause death rate was 1.8%. This difference could be easily attributed to the studied population size and longer follow-up course. The most common single outcome event in our DWI-pos TIA patients was TIA (6.5%) which was similar to the other study result (7.4%).
As it has shown in our study and other studies, patients with DWI-pos TIA or DWI-neg TIA have the highest risk of recurrent ischemic stroke event in the first 30 and 90 days following index event (25), this highlights the importance of rapid and proper evaluation and treatment of the initial ischemic event. The role of hypertension as an important modifiable risk factor for stroke has been known, however, in recent metanalysis hypertension is found to be the most important modifiable risk factor for stroke recurrence, as well (26). In our cohort, we had a meaningful difference among patients with diagnoses of DWI-neg TIA and DWI-neg TIA and patients with a TIA mimic in terms of hypertension. We also observed a higher rate of hypertension (P = 0.078) among patients with an outcome event; however, the difference did not reach a significant level. Taken altogether, strict hypertension control along with other modifiable risks management in the early phase after index event could take a unique role in the secondary stroke prevention. Although in our cohort more than 60% of our composite outcome events occurred in the first 90 days, which is consistent with previous studies, we still need to answer an important question about the value of risk factor modification beyond the early follow-up course (27). Finally, the multi-resolution design in this study was important to draw some of these important conclusions.
Although evaluation of the outcome among patients with diagnosis of TIA mimics, considering the heterogenic nature of the condition, is difficult, the very low rate of adverse outcome among patients in this group can be clinically important. It can further highlight the importance of triaging, and proper diagnosis of these patients as many of these patients might not require urgent clinical evaluation and hospitalization. Some of our patients in this group had the alternative diagnoses of toxic or metabolic encephalopathy that encompasses a wide variety of diseases that require a different treatment plan. The main etiology of single death outcome event in this group was cancer, not cerebrovascular disease; however, the most frequently defined alternative diagnosis was migraine similar to a previous report (28). Altogether, it suggests that outcome study in TIA mimics is challenging however it confirms that majority of these patients might not benefit from a hospital admission.
The ABCD2 score has been widely used, since its introduction, in the primary care setting for identification of the patients who have a higher risk of stroke recurrence. Although we found a significantly lower ABCD2 score among patients with TIA mimics, the score failed to predict the risk of the composite outcome occurrence at 6 months. Our finding is similar to the results of recent studies (29, 30), which showed that the ABCD2 score might not be a reliable tool to define a higher risk of the recurrent stroke.
Our study has some limitations. Although the patients were recruited from different hospitals in rural and urban areas in central and northeast Pennsylvania, the study was a single healthcare system study. We had a relatively small sample size with lack of ethnic diversity in the study population. Nevertheless, the lack of diversity could also be considered as a strength of this study due to reduced heterogeneity and higher degrees of generalizability to a similar population.
In conclusion, our study indicated that the overall six-month rate of the composite outcome (ICH, ischemic stroke, TIA, CAD, and all-cause death) among patients with DWI-neg TIA, DWI-pos TIA, and TIA mimics were 12.2, 9.7, and 2.1%, respectively. More than half of the composite outcome events occurred within 90 days following the index event. Age was the only factor that was significantly associated with the occurrence of the composite outcome.
Data Availability
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
This study was part of the ongoing Geisinger stroke registry and approved by institutional review board of Geisinger.
Author Contributions
ASa: chart review, data gathering, clinical diagnosis, statistical analysis, manuscript writing. VA: data gathering, data archive designer, statistical analysis, manuscript review. ASt, MB, and NH: manuscript review. RZ: study designer, clinical diagnosis, statistical analysis, manuscript writing. NE: chart review, clinical diagnosis.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research work was part of a larger research study which was funded through Bucknell Geisinger Research Initiative (BGRI).
References
1. Coutts SB, Eliasziw M, Hill MD, Scott JN, Subramaniam S, Buchan AM, et al. An improved scoring system for identifying patients at high early risk of stroke and functional impairment after an acute transient ischemic attack or minor stroke. Int J Stroke. (2008) 3:3–10. doi: 10.1111/j.1747-4949.2008.00182.x
2. Ois A, Gomis M, Rodríguez-Campello A, Cuadrado-Godia E, Jiménez-Conde J, Pont-Sunyer C, et al. Factors associated with a high risk of recurrence in patients with transient ischemic attack or minor stroke. Stroke. (2008) 39:1717–21. doi: 10.1161/STROKEAHA.107.505438
3. Amort M, Fluri F, Schäfer J, Weisskopf F, Katan M, Burow A, et al. Transient ischemic attack versus transient ischemic attack mimics: frequency, clinical characteristics and outcome. Cerebrovasc Dis. (2011) 32:57–64. doi: 10.1159/000327034
4. Elkins JS, Sidney S, Gress DR, Go AS, Bernstein AL, Johnston SC. Electrocardiographic findings predict short-term cardiac morbidity after transient ischemic attack. Arch Neurol. (2002) 59:1437–41. doi: 10.1001/archneur.59.9.1437
5. Pettersen R, Dahl T, Wyller TB. Prediction of long-term functional outcome after stroke rehabilitation. Clin Rehabil. (2002) 16:149–59. doi: 10.1191/0269215502cr482oa
6. Dhamoon MS, Sciacca RR, Rundek T, Sacco RL, Elkind MSV. Recurrent stroke and cardiac risks after first ischemic stroke: the Northern Manhattan Study. Neurology. (2006) 66:641–6. doi: 10.1212/01.wnl.0000201253.93811.f6
7. Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. (2016) 133:e38–360. doi: 10.1161/CIR.0000000000000350
8. Dutta D, Bowen E, Foy C. Four-year follow-up of transient ischemic attacks, strokes, and mimics: a retrospective transient ischemic attack clinic cohort study. Stroke. (2015) 46:1227–32. doi: 10.1161/STROKEAHA.114.008632
9. Ferro JM, Falcão I, Rodrigues G, Canhão P, Melo TP, Oliveira V, et al. Diagnosis of transient ischemic attack by the nonneurologist. A validation study. Stroke. (1996) 27:2225–9.
10. Prabhakaran S, Silver AJ, Warrior L, McClenathan B, Lee VH. Misdiagnosis of transient ischemic attacks in the emergency room. Cerebrovasc Dis. (2008) 26:630–5. doi: 10.1159/000166839
11. Paul NLM, Simoni M, Rothwell PM, Oxford Vascular Study. Transient isolated brainstem symptoms preceding posterior circulation stroke: a population-based study. Lancet Neurol. (2013) 12:65–71. doi: 10.1016/S1474-4422(12)70299-5
12. Bos MJ, van Rijn MJE, Witteman JCM, Hofman A, Koudstaal PJ, Breteler MMB. Incidence and prognosis of transient neurological attacks. JAMA. (2007) 298:2877–85. doi: 10.1001/jama.298.24.2877
13. Johnston SC, Rothwell PM, Nguyen-Huynh MN, Giles MF, Elkins JS, Bernstein AL, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet. (2007) 369:283–92. doi: 10.1016/S0140-6736(07)60150-0
14. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. Am J Roentgenol. (1987) 149:351–6.
15. Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjögren M, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. (2001) 32:1318–22. doi: 10.1161/01.STR.32.6.1318
17. Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. (2000) 284:2901–6. doi: 10.1001/jama.284.22.2901
18. Hill MD, Yiannakoulias N, Jeerakathil T, Tu JV, Svenson LW, Schopflocher DP. The high risk of stroke immediately after transient ischemic attack: a population-based study. Neurology. (2004) 62:2015–20. doi: 10.1212/01.WNL.0000129482.70315.2F
19. Kleindorfer D, Panagos P, Pancioli A, Khoury J, Kissela B, Woo D, et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. (2005) 36:720–3. doi: 10.1161/01.STR.0000158917.59233.b7
20. Lin H-J, Yeh P-S, Tsai T-C, Cheng T-J, Ke D, Lin K-C, et al. Differential risks of subsequent vascular events for transient ischaemic attack and minor ischaemic stroke. J Clin Neurosci. (2007) 14:17–21. doi: 10.1016/j.jocn.2005.07.026
21. Sundararajan V, Thrift AG, Phan TG, Choi PM, Clissold B, Srikanth VK. Trends over time in the risk of stroke after an incident transient ischemic attack. Stroke. (2014) 45:3214–8. doi: 10.1161/STROKEAHA.114.006575
22. Amarenco P, Lavallée PC, Labreuche J, Albers GW, Bornstein NM, Canhão P, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med. (2016) 374:1533–42. doi: 10.1056/NEJMoa1412981
23. Park H-K, Kim BJ, Han M-K, Park J-M, Kang K, Lee SJ, et al. One-year outcomes after minor stroke or high-risk transient ischemic attack: Korean multicenter stroke registry analysis. Stroke. (2017) 48:2991–8. doi: 10.1161/STROKEAHA.117.018045
24. Rothwell PM, Giles MF, Chandratheva A, Marquardt L, Geraghty O, Redgrave JN, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. (2007) 370:1432–42. doi: 10.1016/S0140-6736(07)61448-2
25. Khanevski AN, Bjerkreim AT, Novotny V, Naess H, Thomassen L, Logallo N, et al. Thirty-day recurrence after ischemic stroke or TIA. Brain Behav. (2018) 8:e01108. doi: 10.1002/brb3.1108
26. Zheng S, Yao B. Impact of risk factors for recurrence after the first ischemic stroke in adults: a systematic review and meta-analysis. J Clin Neurosci. (2019) 60:24–30. doi: 10.1016/j.jocn.2018.10.026
27. Amarenco P, Lavallée PC, Monteiro Tavares L, Labreuche J, Albers GW, Abboud H, et al. Five-year risk of stroke after TIA or minor ischemic stroke. N Engl J Med. (2018) 378:2182–90. doi: 10.1056/NEJMc1808913
28. Nadarajan V, Perry RJ, Johnson J, Werring DJ. Transient ischaemic attacks: mimics and chameleons. Pract Neurol. (2014) 14:23–31. doi: 10.1136/practneurol-2013-000782
29. Chiu LHS, Yau WH, Leung LP, Pang P, Tsui CT, Wan KA, et al. Short-term prognosis of transient ischemic attack and predictive value of the ABCD2 Score in Hong Kong Chinese. Cerebrovasc Dis Extra. (2014) 4:40–51. doi: 10.1159/000360074
Keywords: DWI-negative transient ischemic attack, transient ischemic attack mimics, DWI-positive transient ischemic attack, composite outcome, follow-up study
Citation: Sadighi A, Abedi V, Stanciu A, El Andary N, Banciu M, Holland N and Zand R (2019) Six-Month Outcome of Transient Ischemic Attack and Its Mimics. Front. Neurol. 10:294. doi: 10.3389/fneur.2019.00294
Received: 08 January 2019; Accepted: 06 March 2019;
Published: 27 March 2019.
Edited by:
Yannick Béjot, Centre Hospitalier Regional Universitaire De Dijon, FranceReviewed by:
Linxin Li, University of Oxford, United KingdomAristeidis H. Katsanos, University of Ioannina, Greece
Copyright © 2019 Sadighi, Abedi, Stanciu, El Andary, Banciu, Holland and Zand. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ramin Zand, cmFtaW4uemFuZEBnbWFpbC5jb20=
 Alireza Sadighi1
Alireza Sadighi1 Vida Abedi
Vida Abedi Alia Stanciu
Alia Stanciu Mihai Banciu
Mihai Banciu Ramin Zand
Ramin Zand