- Department of Neurology, Harvard Medical School, Neurological Clinical Research Institute, Massachusetts General Hospital, Boston, MA, United States
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disorder with limited treatment options. Despite decades of therapeutic development, only two modestly efficacious disease-modifying drugs—riluzole and edaravone—are available to ALS patients. Biomarkers that can facilitate ALS diagnosis, aid in prognosis, and measure drug pharmacodynamics are needed to accelerate therapeutic development for patients with ALS. Positron emission tomography (PET) imaging has promise as a biomarker for ALS because it permits visualization of central nervous system (CNS) pathology in individuals living with ALS. The availability of PET radioligands that target a variety of potential pathophysiological mechanisms—including cerebral metabolism, neuroinflammation, neuronal dysfunction, and oxidative stress—has enabled dynamic interrogation of molecular changes in ALS, in both natural history studies and human clinical trials. PET imaging has potential as a diagnostic biomarker that can establish upper motor neuron (UMN) pathology in ALS patients without overt UMN symptoms, as a prognostic biomarker that might help stratify patients for clinical trials, and as a pharmacodynamic biomarker that measures the biological effect of investigational drugs in the brain and spinal cord. In this Review, we discuss progress made with 30 years of PET imaging studies in ALS and consider future research needed to establish PET imaging biomarkers for ALS therapeutic development.
Introduction
Amyotrophic lateral sclerosis (ALS) is a devastating disorder characterized by degeneration of motor neurons in the brain and spinal cord. It is clinically heterogeneous and shares clinical and pathological features with frontotemporal dementia (FTD). ALS invariably leads to weakness and death; ~70–80% of ALS patients die within 5 years of symptom onset (1).
Riluzole and edaravone are currently the only disease-modifying treatments for ALS. More efficacious therapy is urgently needed. Fortunately, the recent expansion of knowledge about genetics and pathophysiology of ALS (2) has generated a large pipeline of potential therapeutic agents to be tested in ALS. Biomarkers for ALS are now urgently needed to stratify patients for trial enrollment, to demonstrate biological drug effect, and to guide dose-selection and go-no-go decisions in early phase clinical trials.
Multiple types of biomarkers are being developed for use in ALS (3–5). Electrophysiological biomarkers of the upper motor neurons (UMNs) [transcranial magnetic stimulation (6)] and lower motor neurons (LMN) [motor unit number index (7) and electrical impedance myography (8)] directly quantify physiology of diseased tissues. Biological fluid-based biomarkers such as phosphorylated neurofilament heavy chain in cerebrospinal fluid (CSF) (9, 10), neurofilament light chain from CSF or serum (10–14), and urine p75 neurotrophin receptor extracellular domain (15) are being evaluated as markers of neuronal degeneration. Neuroimaging biomarkers using magnetic resonance imaging (MRI) or positron emission tomography (PET) techniques can objectively visualize changes associated with the disease processes and help to understand the mechanisms of neurodegeneration in vivo (16).
This Review will focus on development of PET molecular imaging biomarkers for ALS. References for this Review were identified by searching PubMed for the terms “amyotrophic lateral sclerosis” or “ALS” or “motor neuron disease” or “MND” AND “PET” or “positron emission tomography.” As of October 11, 2018, 222 articles were identified. We excluded articles that were not focused on motor neuron diseases (17), were animal or post-mortem studies (18), were not focused on PET imaging (19), were not dedicated to brain or spinal cord (2), were not written in English (12), were inaccessible (7), studied fewer than 5 ALS or MND cases (20), or were literature reviews or guidelines (21), resulting in 48 papers.
The Development of PET Imaging in ALS
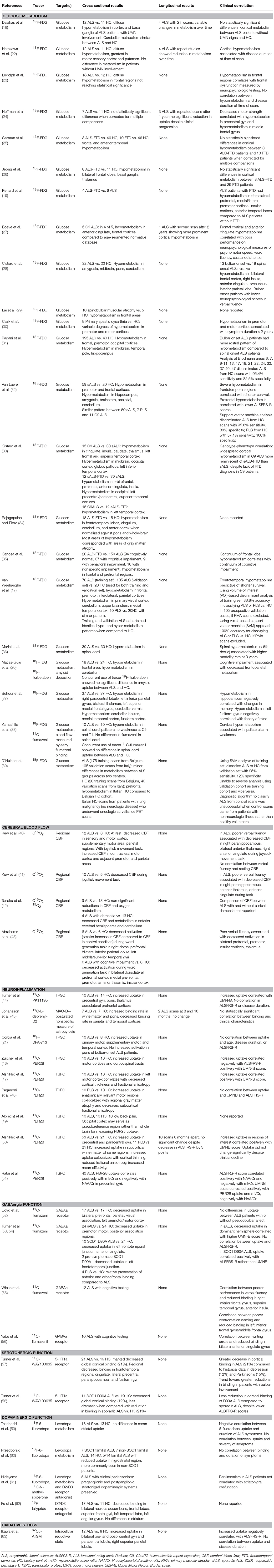
PET imaging uses positron-emitting radioisotopes that are incorporated into molecules of interest (“tracers”), which are injected intravenously and enter the central nervous system (CNS). When positrons encounter electrons, they annihilate and emit pairs of gamma rays that travel away from one another at a 180° angle. The detection of gamma ray pairs by the PET camera enables localization of the annihilation event and subsequent three-dimensional reconstruction of radiotracer distribution in the tissue of interest (16). The development of PET tracers that permit visualization of glucose metabolism, cerebral blood flow, neurotransmitter metabolism, neuroreceptor binding, inflammation, and oxidative stress have permitted a deep investigation into the molecular pathophysiology of ALS in vivo (Table 1).
Glucose Metabolism and Cerebral Blood Flow
The first PET study in ALS, conducted in 1987, used the tracer 18F-fluorodeoxyglucose ([18F]-FDG) to demonstrate that ALS patients with UMN involvement had diffuse cortical hypometabolism compared to healthy controls (18). Subsequent [18F]-FDG PET studies found variable cortical hypometabolism in ALS (22–24). PET studies using radiolabeled carbon dioxide (C[15O]2), which detects alterations in regional cerebral blood flow (40–42), revealed decreased cerebral blood flow to the prefrontal cortex (41–43) and thalamus (41, 43) that correlated with cognitive impairment in ALS. These early PET findings suggested that ALS pathology expanded outside the motor cortex, years before ALS was widely accepted as a disorder on the same spectrum as frontotemporal dementia (FTD).
The 2011 discovery that C9orf72 hexanucleotide repeat expansions cause both ALS and FTD (64–66) motivated new [18F]-FDG PET studies that explored genotype-phenotype correlations and cognition in ALS. One study suggested that ALS patients with C9orf72 expansions had more widespread cortical hypometabolism than sporadic ALS patients (33), though this finding was not replicated (32). Other studies demonstrated frontal and prefrontal hypometabolism in patients with sporadic ALS-FTD compared to ALS patients without FTD (33–35).
In recent years, large cross-sectional [18F]-FDG PET studies have established that sporadic ALS is associated with hypometabolism in the premotor and frontal cortices and hypermetabolism in the brainstem (28, 31, 32). There is now interest in spinal cord imaging: two [18F]-FDG PET studies demonstrated hypermetabolism in the cervical cords of ALS patients (36, 38). These findings suggest potential differences between cortical vs. brainstem and spinal cord metabolism that warrant further exploration.
Neuroinflammation
Neuroinflammation, specifically microglial activation, is a pathological hallmark of ALS (67, 68) and is associated with rate of disease progression (69). The 18 kD translocator protein (TSPO) is highly expressed on activated microglia and astrocytes (70, 71). Radiotracers that bind to TSPO thus can visualize neuroinflammation and gliosis in vivo. Indeed, early PET studies of neuroinflammation in ALS used the first-generation TSPO ligands [11C]-PK11195 (44) and [18F]-DPA-714 (21) to demonstrate the presence of widespread glial activation in brains of ALS patients compared to healthy controls.
The second-generation TSPO tracer [11C]-PBR28, which binds TSPO with an 80-fold higher specificity than [11C]-PK11195 (72), has enabled more precise PET evaluation of glial activation. Several [11C]-PBR28 PET studies demonstrated increased tracer uptake isolated to the motor cortices of ALS patients compared with controls (46, 47, 50). Areas of increased uptake correlated positively with Upper Motor Neuron Burden Scale and negatively with ALS Functional Rating Scale-Revised (ALSFRS-R) scores (46, 47, 50). Integrated [11C]-PBR28 PET and MRI scans established that areas of increased uptake co-localize with areas of cortical thinning and reduced fractional anisotropy (47, 50).
[11C]-PBR28 PET studies in patients with primary lateral sclerosis (PLS) found a pattern of glial activation similar to that seen in ALS patients, though tracer uptake was greatest in subcortical white matter in PLS patients and in cortical gray matter in ALS patients (48). The differences between ALS and PLS scans highlight the increased specificity of [11C]-PBR28 tracer and merit further investigation into why such differences in glial activation might exist in these two conditions.
In the largest longitudinal ALS PET study to date, 10 patients underwent [11C]-PBR28 PET scans twice over 6 months. Tracer uptake remained stable despite disease progression, as measured by a 3-point decrease in ALSFRS-R (50). This stability may mirror the pattern of beta-amyloid brain deposition in Alzheimer's disease, as measured by Pittsburg compound B (PiB) PET imaging: PiB uptake rises in patients developing mild cognitive impairment, then plateaus upon development of Alzheimer's dementia (73). Alternatively, it may reflect a bias toward recruitment of slowly-progressive patients into longitudinal neuroimaging studies. Longitudinal studies with larger sample sizes, rapidly progressing patients, and patients early in the disease course are needed to determine the natural history of glial activation in ALS.
GABAergic Function
Cortical excitability is altered in ALS (6). To evaluate whether loss of GABAergic inhibition contributes to cortical hyperexcitability in ALS, PET studies were conducted using the GABAA receptor ligand [11C]-Flumazenil. These studies showed widespread reductions in binding in ALS patients compared to controls (52), and found that reduced binding in the frontal lobes (55) and anterior cingulate gyri (56) in ALS patients correlated with poorer performance on language tasks. Additionally, patients with slowly progressive ALS caused by SOD1 D90A mutations had smaller reductions in binding compared to sporadic ALS patients (53). Taken together, these findings could suggest that loss of GABAergic cortical inhibition is part of ALS pathogenesis, though it is also possible that it reflects generalized cortical neuronal loss rather than specific loss of GABAergic inhibition.
Serotonergic Function
The serotonin 5-hydroxytryptamine (5-HT1a) receptor is expressed widely in the cortex, including on layer III and V pyramidal neurons in the cortex (74). A PET imaging study using the 5-HT1a ligand [11C]-WAY100635 demonstrated decreased tracer binding in the frontotemporal regions, precentral, cingulate, parahippocampal, and fusiform gyri in non-depressed ALS patients compared to healthy controls (57). A follow up study reported smaller reductions in [11C]-WAY100635 uptake in patients with slowly progressive SOD1 D90A genetic ALS compared to sporadic ALS (58). Like the studies using GABAA ligands, these studies suggest widespread neuronal loss or dysfunction in ALS patients that is less apparent in slowly progressive disease.
Dopaminergic Function
Evidence of extramotor involvement in ALS has raised questions about its overlap with neurodegenerative disorders such as Parkinson's disease. Rare patients with ALS have parkinsonism, and post-mortem evaluation has revealed degeneration of the substantia nigra in ALS (75). To evaluate whether dopaminergic dysfunction plays a role in ALS pathogenesis, several PET studies were conducted using ligands that interrogate levodopa metabolism [[18F]-fluorodopa (59, 61)], bind to dopamine receptors in the striatum [[11C]-N-methylspiperone (61)], and bind to dopamine receptors in the cortex [[18F]-fallypride (62)]. The [18F]-fluorodopa and [11C]-N-methylspiperone studies showed no significant difference in levodopa metabolism or dopamine receptor binding in the striatum of ALS vs. control subjects, even in patients with overt parkinsonism (59, 61). Conversely, the [18F]-fallypride PET study showed decreased dopamine binding in the cortex of ALS patients (62), even though the patients were not noted to have clinical parkinsonism. One interpretation of these studies is that ALS is associated with cortical rather than striatal dopaminergic dysfunction. However, PET studies demonstrating decreased cortical binding of GABAergic, serotonergic, and now dopaminergic ligands in ALS patients argues against dopamine-specific pathogenesis of ALS and supports a generalized cortical neuronal loss or dysfunction in disease.
Oxidative Stress
Oxidative stress is considered one of the pathogenic mechanisms underlying neurodegeneration in ALS (76) and is the proposed target of edaravone, a free radical scavenger recently approved for treatment of ALS (77). The PET ligand [62Cu]-ATSM is a copper-linked small molecule structurally similar to superoxide dismutase (78). It distributes to areas of hypoxia and oxidative stress in PET studies of patients with Parkinson's disease (79) and mitochondrial diseases (80). One [62Cu]-ATSM PET study in ALS showed increased tracer accumulation in the motor cortices, paracentral lobules, and right superior parietal lobule in ALS patients compared to controls (63). Areas of increased uptake negatively correlated with ALSFRS-R score.
Notably, Cu-ATSM was selected as an investigational drug for ALS because human [62Cu]-ATSM PET studies demonstrated effective penetration into the brain. Cu-ATSM's proposed mechanism of action is free radical scavenging and delivery of copper into the CNS (81). Cu-ATSM slowed disease progression in SODG93A mouse models of ALS (81, 82) and is now entering phase I human clinical trials for ALS (Clinicaltrials.gov NCT02870634).
Challenges and Opportunities in the Development of Molecular Imaging Biomarkers for ALS
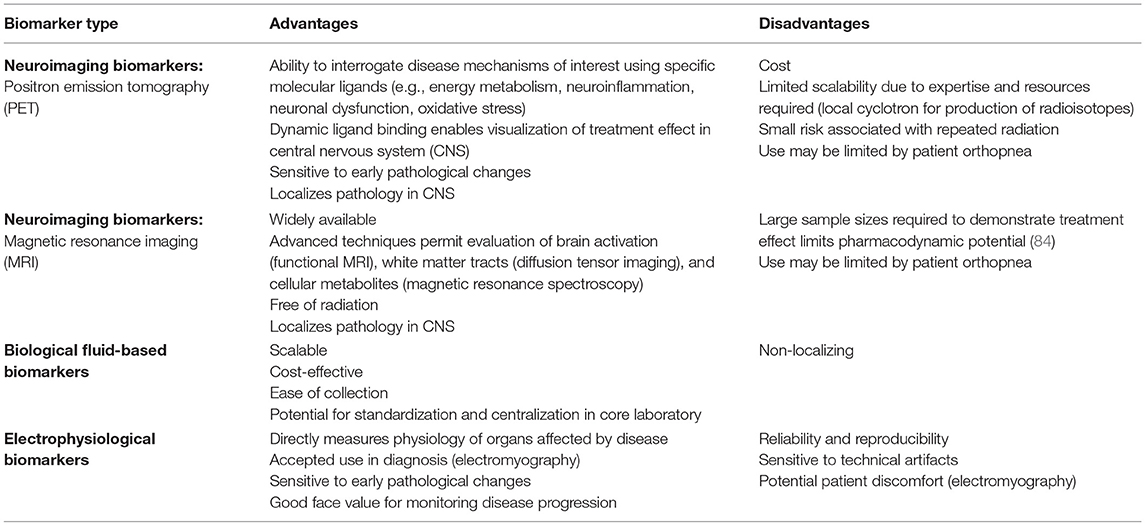
The FDA-NIH Biomarker Working Group defines a biomarker as a “characteristic that is measured as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions” (83). Because PET imaging can localize molecular changes in the brain, it has unique promise for use as a diagnostic, prognostic, and pharmacodynamic biomarker. Its advantages and disadvantages complement other biomarkers being developed for ALS (Table 2).
PET Imaging as a Diagnostic Biomarker
Mounting evidence of quantifiable PET imaging differences between ALS and control brains has generated interest in using PET as a diagnostic biomarker for ALS. Indeed, the sensitivity of PET makes it uniquely positioned to detect or confirm UMN dysfunction in suspected ALS patients, which has traditionally been difficult to measure.
Three successive studies recently assessed the diagnostic potential of [18F]-FDG PET in ALS (17, 32, 39). In these studies, the authors used group differences in scans from ALS and control subjects to generate algorithms (“diagnostic algorithms”) for classifying individual scans as ALS vs. control. Group-level differences in FDG uptake between ALS and control scans were consistent across time and between two imaging centers. Within one center, the diagnostic algorithm generated from a training cohort achieved high accuracy when classifying scans from a validation cohort (as ALS or control), though accuracy decreased when scans from PLS patients were included in the analysis (17). However, in a multicenter study, the diagnostic algorithm derived from one center's scans (training cohort) achieved 94.8% sensitivity but only 12.5% specificity in classifying scans from a second center (validation cohort) as ALS or control (39). The low specificity was attributed to relative frontal hypometabolism in the validation control scans, compared to training control scans. The validation control scans came from patients with non-neurologic malignancies undergoing surveillance brain PET, whereas the training control scans came from healthy volunteers.
These studies highlight the challenges in translating population-level PET data into diagnostic criteria for individual patients. While progress is being made, PET is not yet a valid diagnostic biomarker for ALS. Validation will require longitudinal studies to determine whether prospectively collected scans of patients undergoing evaluation for ALS can distinguish UMN dysfunction before clinical signs emerge. The studies will also need to distinguish motor neuron disease not just from healthy volunteers, but also from disease mimics. If validated as a diagnostic biomarker, PET imaging could shorten the time from ALS symptom onset to diagnosis and facilitate earlier intervention in the neurodegenerative process.
PET Imaging as a Prognostic Biomarker
PET imaging has potential for prognostic use in ALS. Two studies in ALS patients found an association between mortality rate and presence of extensive frontotemporal hypometabolism on [18F]-FDG PET scans (17, 32). Conversely, patients with spinal cord hypermetabolism in the top 20% of one study cohort had a significantly higher mortality rate compared to the rest of the cohort (36). Further longitudinal studies that evaluate whether PET imaging findings can predict the likelihood of future events (such as survival, development of cognitive impairment, or spread of disease from one anatomical region to another) are needed to establish valid prognostic PET biomarkers in ALS.
One intriguing potential use for prognostic PET imaging is in identifying when asymptomatic ALS gene carriers enter a high-risk period for developing clinical disease (“phenoconversion”). Rising levels of serum neurofilament light chain can detect neurodegeneration ~1 year before phenoconversion in asymptomatic ALS gene mutation carriers (13). To evaluate whether PET imaging can detect also changes that predict phenoconversion, longitudinal [11C]-PBR28 PET studies are being conducted in asymptomatic gene mutation carriers to look for neuroinflammation before disease onset. Prognostic biomarkers of phenoconversion may facilitate development of gene therapy trials designed to prevent ALS, which may be the best opportunity for treating or even curing certain genetic forms of ALS.
PET Imaging as a Pharmacodynamic Biomarker
PET imaging has value as a pharmacodynamic marker in ALS because it can rapidly measure and localize biological activity of investigational agents in the target tissue—the brain. The variety of available PET ligands may enable direct visualization of multiple pharmacologic targets. PET imaging's sensitivity to molecular changes can increase statistical power to detect a drug effect.
[11C]-PBR28 PET is an appealing pharmacodynamic biomarker for ALS clinical trials because binding is dynamic and rapidly responsive to treatment: in Parkinson's disease (85) and traumatic brain injury (86) patients, anti-inflammatory treatment reduced cortical [11C]-PBR28 binding in as little as 4 weeks (85). Additionally, the stability of [11C]-PBR28 uptake in ALS over 6 months of disease progression permits a marked reduction in sample size needed to determine drug effect. A simulated sample size and power calculation using longitudinal [11C]-PBR28 PET data found that 30 participants are needed in a single-arm ALS clinical trial to show a 2% change in [11C]-PBR28 uptake after drug treatment, whereas hundreds of participants are needed to show a 30% reduction in ALSFRS-R slope (50). Currently, four ongoing clinical trials are using [11C]-PBR28 PET as a pharmacodynamic biomarker to assess the biological activity of investigational treatments in ALS (Clinicaltrials.gov NCT02714036, NCT02469896, NCT03127514, NCT03456882) (87).
In the future, PET imaging using an array of ligands will enable efficient evaluation of multiple pharmacologic targets. Pharmacodynamic data from PET studies may help confirm the biological activity of ALS drugs in the target tissue and inform dose selection based on biological activity. Data derived from these trials will enable deeper understanding of the role of different molecular mechanisms in disease pathogenesis.
Conclusions and Future Directions
Thirty years of PET imaging has shed light on the pathophysiology of ALS and the expanding boundaries of cortical dysfunction in disease. Because PET imaging can localize molecular changes in the CNS in vivo, it has the potential to fill a critical gap in our armamentarium of diagnostic, prognostic, and pharmacodynamic biomarkers for ALS. To realize this potential, major limitations of the research to date will need to be addressed. First, most PET studies in ALS were small. Only 7 published studies enrolled more than 50 ALS patients (17, 31–33, 35, 39, 50), which raises concern for false positive and/or negative findings generated by studies with small sample sizes. Second, minimal longitudinal PET data exists in the ALS literature. A total of 24 ALS patients have had longitudinal PET scans in published studies (18, 22, 24, 27, 45, 50). Third, clinical-radiological correlations reported in the literature are insufficiently characterized and often contradictory. To address these limitations, we must conduct collaborative, multicenter longitudinal studies to collect PET imaging and clinical data in large patient cohorts. Moreover, to ascertain accurate clinical-radiologic correlations, clinical data should be captured by validated instruments that separate motor and cognitive deficits and reliably measure UMN dysfunction.
From a practical standpoint, the widespread use of PET imaging is presently limited by cost, need for expertise and local production of radioactive isotopes. Therefore, PET imaging currently is most useful as a pharmacodynamic biomarker for early clinical trials in ALS. Future multicenter longitudinal studies will allow us to establish the relationship between PET imaging findings and meaningful clinical outcomes, and thus develop and validate the PET imaging biomarkers that can accelerate drug development and advance care for people with ALS.
Disclosure
NA receives consulting fees from Biogen Idec and MT Pharma.
Author Contributions
SC and NA drafted the manuscript. Both authors made a direct and intellectual contribution to the work and approved it for publication.
Funding
SC is supported by the Anne B. Young Neuroscience Translational Medicine Fellowship Award (Biogen MA, Inc.). NA is supported by The ALS Association (16-TACL-238), ALS Finding A Cure, ALS ONE, Muscular Dystrophy Association (511619), and the National Institute of Neurological Disorders and Stroke (K23NS083715). The content is of this work is solely the responsibility of the authors and does not necessarily represent the official views of these sponsors.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We acknowledge the generosity of our patients and volunteers for participating in research studies.
References
1. Traynor BJ, Codd MB, Corr B, Forde C, Frost E, Hardiman OM. Clinical features of amyotrophic lateral sclerosis according to the El Escorial and Airlie House diagnostic criteria: a population-based study. Arch Neurol. (2000) 57:1171–6. doi: 10.1001/archneur.57.8.1171
2. Taylor JP, Brown RH Jr, Cleveland DW. Decoding ALS: from genes to mechanism. Nature. (2016) 539:197–206. doi: 10.1038/nature20413
3. Turner MR, Kiernan MC, Leigh PN, Talbot K. Biomarkers in amyotrophic lateral sclerosis. Lancet Neurol. (2009) 8:94–109. doi: 10.1016/s1474-4422(08)70293-x
4. Chiò A, Pagani M, Agosta F, Calvo A, Cistaro A, Filippi M. Neuroimaging in amyotrophic lateral sclerosis: insights into structural and functional changes. Lancet Neurol. (2014) 13:1228–40. doi: 10.1016/s1474-4422(14)70167-x
5. Benatar M, Boylan K, Jeromin A, Rutkove SB, Berry J, Atassi N, et al. ALS biomarkers for therapy development: state of the field and future directions. Muscle Nerve. (2016) 53:169–82. doi: 10.1002/mus.24979
6. Vucic S, Ziemann U, Eisen A, Hallett M, Kiernan MC. Transcranial magnetic stimulation and amyotrophic lateral sclerosis: pathophysiological insights. J Neurol Neurosurg Psychiatry. (2013) 84:1161–70. doi: 10.1136/jnnp-2012-304019
7. Nandedkar SD, Nandedkar DS, Barkhaus PE, Stalberg EV. Motor unit number index (MUNIX). IEEE Trans Biomed Eng. (2004) 51:2209–11. doi: 10.1109/tbme.2004.834281
8. Rutkove SB, Caress JB, Cartwright MS, Burns TM, Warder J, David WS, et al. Electrical impedance myography correlates with standard measures of ALS severity. Muscle Nerve. (2014) 49:441–3. doi: 10.1002/mus.24128
9. Boylan KB, Glass JD, Crook JE, Yang C, Thomas CS, Desaro P, et al. Phosphorylated neurofilament heavy subunit (pNF-H) in peripheral blood and CSF as a potential prognostic biomarker in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. (2013) 84:467–72. doi: 10.1136/jnnp-2012-303768
10. Feneberg E, Oeckl P, Steinacker P, Verde F, Barro C, Van Damme P, et al. Multicenter evaluation of neurofilaments in early symptom onset amyotrophic lateral sclerosis. Neurology. (2018) 90:e22–30. doi: 10.1212/WNL.0000000000004761
11. Gaiottino J, Norgren N, Dobson R, Topping J, Nissim A, Malaspina A, et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS ONE. (2013) 8:e75091. doi: 10.1371/journal.pone.0075091
12. Gaiani A, Martinelli I, Bello L, Querin G, Puthenparampil M, Ruggero S, et al. Diagnostic and prognostic biomarkers in amyotrophic lateral sclerosis: neurofilament light chain levels in definite subtypes of disease. JAMA Neurol. (2017) 74:525–32. doi: 10.1001/jamaneurol.2016.5398
13. Benatar M, Wuu J, Andersen PM, Lombardi V, Malaspina A. Neurofilament light: a candidate biomarker of presymptomatic amyotrophic lateral sclerosis and phenoconversion. Ann Neurol. (2018) 84:130–9. doi: 10.1002/ana.25276
14. Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. (2018) 14:577–89. doi: 10.1038/s41582-018-0058-z
15. Shepheard SR, Wuu J, Cardoso M, Wiklendt L, Dinning PG, Chataway T, et al. Urinary p75(ECD): a prognostic, disease progression, and pharmacodynamic biomarker in ALS. Neurology. (2017) 88:1137–43. doi: 10.1212/wnl.0000000000003741
16. Menke RA, Agosta F, Grosskreutz J, Filippi M, Turner MR. Neuroimaging endpoints in amyotrophic lateral sclerosis. Neurotherapeutics. (2017) 14:11–23. doi: 10.1007/s13311-016-0484-9
17. Van Weehaeghe D, Ceccarini J, Delva A, Robberecht W, Van Damme P, Van Laere K. Prospective validation of 18F-FDG brain PET discriminant analysis methods in the diagnosis of amyotrophic lateral sclerosis. J Nucl Med. (2016) 57:1238–43. doi: 10.2967/jnumed.115.166272
18. Dalakas MC, Hatazawa J, Brooks RA, Di Chiro G. Lowered cerebral glucose utilization in amyotrophic lateral sclerosis. Ann Neurol. (1987) 22:580–6. doi: 10.1002/ana.410220504
19. Renard D, Collombier L, Castelnovo G, Fourcade G, Kotzki PO, LaBauge P. Brain FDG-PET changes in ALS and ALS-FTD. Acta Neurol Belg. (2011) 111, 306–9.
20. Matias-Guiu JA, Pytel V, Cabrera-Martin MN, Galan L, Valles-Salgado M, Guerrero A, et al. Amyloid- and FDG-PET imaging in amyotrophic lateral sclerosis. Eur J Nucl Med Mol Imaging. (2016) 43:2050–60. doi: 10.1007/s00259-016-3434-1
21. Corcia P, Tauber C, Vercoullie J, Arlicot N, Prunier C, Praline J, et al. Molecular imaging of microglial activation in amyotrophic lateral sclerosis. PLoS ONE. (2012) 7:e52941. doi: 10.1371/journal.pone.0052941
22. Hatazawa J, Brooks RA, Dalakas MC, Mansi L, Di Chiro G. Cortical motor-sensory hypometabolism in amyotrophic lateral sclerosis: a PET study. J Comput Assist Tomogr. (1988) 12:630–6.
23. Ludolph AC, Langen KJ, Regard M, Herzog H, Kemper B, Kuwert T, et al. Frontal lobe function in amyotrophic lateral sclerosis: a neuropsychologic and positron emission tomography study. Acta Neurol Scand. (1992) 85, 81–9.
24. Hoffman JM, Mazziotta JC, Hawk TC, Sumida R. Cerebral glucose utilization in motor neuron disease. Arch Neurol. (1992) 49:849–54.
25. Garraux G, Salmon E, Degueldre C, Lemaire C, Franck G. Medial temporal lobe metabolic impairment in dementia associated with motor neuron disease. J Neurol Sci. (1999) 168:145–50.
26. Jeong Y, Park KC, Cho SS, Kim EJ, Kang SJ, Kim SE, et al. Pattern of glucose hypometabolism in frontotemporal dementia with motor neuron disease. Neurology. (2005) 64:734–6. doi: 10.1212/01.wnl.0000152047.58767.9d
27. Boeve BF, Boylan KB, Graff-Radford NR, DeJesus-Hernandez M, Knopman DS, Pedraza O, et al. Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain. (2012) 135(Pt 3):765–83. doi: 10.1093/brain/aws004
28. Cistaro A, Valentini MC, Chio A, Nobili F, Calvo A, Moglia C, et al. Brain hypermetabolism in amyotrophic lateral sclerosis: a FDG PET study in ALS of spinal and bulbar onset. Eur J Nucl Med Mol Imaging. (2012) 39:251–9. doi: 10.1007/s00259-011-1979-6
29. Lai TH, Liu RS, Yang BH, Wang PS, Lin KP, Lee YC, et al. Cerebral involvement in spinal and bulbar muscular atrophy (Kennedy's disease): a pilot study of PET. J Neurol Sci. (2013) 335:139–44. doi: 10.1016/j.jns.2013.09.016
30. Clark HM, Duffy JR, Whitwell JL, Ahlskog JE, Sorenson EJ, Josephs KA. Clinical and imaging characterization of progressive spastic dysarthria. Eur J Neurol. (2014) 21:368–76. doi: 10.1111/ene.12271
31. Pagani M, Chio A, Valentini MC, Oberg J, Nobili F, Calvo A, et al. Functional pattern of brain FDG-PET in amyotrophic lateral sclerosis. Neurology. (2014) 83:1067–74. doi: 10.1212/wnl.0000000000000792
32. Van Laere K, Vanhee A, Verschueren J, De Coster L, Driesen A, Dupont P, et al. Value of 18fluorodeoxyglucose-positron-emission tomography in amyotrophic lateral sclerosis: a prospective study. JAMA Neurol. (2014) 71:553–61. doi: 10.1001/jamaneurol.2014.62
33. Cistaro A, Pagani M, Montuschi A, Calvo A, Moglia C, Canosa A, et al. The metabolic signature of C9ORF72-related ALS: FDG PET comparison with nonmutated patients. Eur J Nucl Med Mol Imaging. (2014) 41:844–52. doi: 10.1007/s00259-013-2667-5
34. Rajagopalan V, Pioro EP. Comparing brain structural MRI and metabolic FDG-PET changes in patients with ALS-FTD: ‘the chicken or the egg?' question. J Neurol Neurosurg Psychiatry. (2015) 86:952–8. doi: 10.1136/jnnp-2014-308239
35. Canosa A, Pagani M, Cistaro A, Montuschi A, Iazzolino B, Fania P, et al. 18F-FDG-PET correlates of cognitive impairment in ALS. Neurology. (2016) 86:44–9. doi: 10.1212/wnl.0000000000002242
36. Marini C, Cistaro A, Campi C, Calvo A, Caponnetto C, Nobili FM, et al. A PET/CT approach to spinal cord metabolism in amyotrophic lateral sclerosis. Eur J Nucl Med Mol Imaging. (2016) 43:2061–71. doi: 10.1007/s00259-016-3440-3
37. Buhour MS, Doidy F, Mondou A, Pelerin A, Carluer L, Eustache F, et al. Voxel-based mapping of grey matter volume and glucose metabolism profiles in amyotrophic lateral sclerosis. EJNMMI Res. (2017) 7:21. doi: 10.1186/s13550-017-0267-2
38. Yamashita T, Hatakeyama T, Sato K, Fukui Y, Hishikawa N, Ohta Y, et al. Flow-metabolism uncoupling in the cervical spinal cord of ALS patients. Neurol Sci. (2017) 38:659–65. doi: 10.1007/s10072-017-2823-y
39. D'Hulst L, Van Weehaeghe D, Chio A, Calvo A, Moglia C, Canosa A, et al. Multicenter validation of [18F]-FDG PET and support-vector machine discriminant analysis in automatically classifying patients with amyotrophic lateral sclerosis versus controls. Amyotroph Lateral Scler Frontotemporal Degener. (2018) 19:570–7. doi: 10.1080/21678421.2018.1476548
40. Kew JJ, Leigh PN, Playford ED, Passingham RE, Goldstein LH, Frackowiak RS, et al. Cortical function in amyotrophic lateral sclerosis. A positron emission tomography study. Brain. (1993) 116 (Pt 3):655–80.
41. Kew JJ, Goldstein LH, Leigh PN, Abrahams S, Cosgrave N, Passingham RE, et al. The relationship between abnormalities of cognitive function and cerebral activation in amyotrophic lateral sclerosis. A neuropsychological and positron emission tomography study. Brain. (1993) 116 (Pt 6):1399–423.
42. Tanaka M, Kondo S, Hirai S, Sun X, Yamagishi T, Okamoto K. Cerebral blood flow and oxygen metabolism in progressive dementia associated with amyotrophic lateral sclerosis. J Neurol Sci. (1993) 120:22–8.
43. Abrahams S, Goldstein LH, Kew JJ, Brooks DJ, Lloyd CM, Frith CD, et al. Frontal lobe dysfunction in amyotrophic lateral sclerosis. A PET study. Brain. (1996) 119 (Pt 6):2105–20.
44. Turner MR, Cagnin A, Turkheimer FE, Miller CC, Shaw CE, Brooks DJ, et al. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol Dis. (2004) 15:601–9. doi: 10.1016/j.nbd.2003.12.012
45. Johansson A, Engler H, Blomquist G, Scott B, Wall A, Aquilonius SM, et al. Evidence for astrocytosis in ALS demonstrated by [11C](L)-deprenyl-D2 PET. J Neurol Sci. (2007) 255:17–22. doi: 10.1016/j.jns.2007.01.057
46. Zurcher NR, Loggia ML, Lawson R, Chonde DB, Izquierdo-Garcia D, Yasek JE, et al. Increased in vivo glial activation in patients with amyotrophic lateral sclerosis: assessed with [(11)C]-PBR28. Neuroimage Clin. (2015) 7:409–14. doi: 10.1016/j.nicl.2015.01.009
47. Alshikho MJ, Zurcher NR, Loggia ML, Cernasov P, Chonde DB, Izquierdo Garcia D, et al. Glial activation colocalizes with structural abnormalities in amyotrophic lateral sclerosis. Neurology. (2016) 87:2554–61. doi: 10.1212/wnl.0000000000003427
48. Paganoni S, Alshikho MJ, Zurcher NR, Cernasov P, Babu S, Loggia ML, et al. Imaging of glia activation in people with primary lateral sclerosis. Neuroimage Clin. (2018) 17:347–53. doi: 10.1016/j.nicl.2017.10.024
49. Albrecht DS, Normandin MD, Shcherbinin S, Wooten DW, Schwarz AJ, Zurcher NR, et al. Pseudoreference regions for glial imaging with (11)C-PBR28: investigation in 2 clinical cohorts. J Nucl Med. (2018) 59:107–14. doi: 10.2967/jnumed.116.178335
50. Alshikho MJ, Zurcher NR, Loggia ML, Cernasov P, Reynolds B, Pijanowski O, et al. Integrated magnetic resonance imaging and [(11) C]-PBR28 positron emission tomographic imaging in amyotrophic lateral sclerosis. Ann Neurol. (2018) 83:1186–97. doi: 10.1002/ana.25251
51. Ratai EM, Alshikho MJ, Zurcher NR, Loggia ML, Cebulla CL, Cernasov P, et al. Integrated imaging of [(11)C]-PBR28 PET, MR diffusion and magnetic resonance spectroscopy (1)H-MRS in amyotrophic lateral sclerosis. Neuroimage Clin. (2018) 20:357–64. doi: 10.1016/j.nicl.2018.08.007
52. Lloyd CM, Richardson MP, Brooks DJ, Al-Chalabi A, Leigh PN. Extramotor involvement in ALS: PET studies with the GABA(A) ligand [(11)C]flumazenil. Brain. (2000) 123 (Pt 11):2289–96. doi: 10.1093/brain/123.11.2289
53. Turner MR, Hammers A, Al-Chalabi A, Shaw CE, Andersen PM, Brooks DJ, et al. Distinct cerebral lesions in sporadic and ‘D90A' SOD1 ALS: studies with [11C]flumazenil PET. Brain. (2005) 128(Pt 6):1323–9. doi: 10.1093/brain/awh509
54. Turner MR, Hammers A, Al-Chalabi A, Shaw CE, Andersen PM, Brooks DJ, et al. Cortical involvement in four cases of primary lateral sclerosis using [(11)C]-flumazenil PET. J Neurol. (2007) 254:1033–6. doi: 10.1007/s00415-006-0482-7
55. Wicks P, Turner MR, Abrahams S, Hammers A, Brooks DJ, Leigh PN, et al. Neuronal loss associated with cognitive performance in amyotrophic lateral sclerosis: an (11C)-flumazenil PET study. Amyotroph Lateral Scler. (2008) 9:43–9. doi: 10.1080/17482960701737716
56. Yabe I, Tsuji-Akimoto S, Shiga T, Hamada S, Hirata K, Otsuki M, et al. Writing errors in ALS related to loss of neuronal integrity in the anterior cingulate gyrus. J Neurol Sci. (2012) 315:55–9. doi: 10.1016/j.jns.2011.11.039
57. Turner MR, Rabiner EA, Hammers A, Al-Chalabi A, Grasby PM, Shaw CE, et al. [11C]-WAY100635 PET demonstrates marked 5-HT1A receptor changes in sporadic ALS. Brain. (2005) 128(Pt 4):896–905. doi: 10.1093/brain/awh428
58. Turner MR, Rabiner EA, Al-Chalabi A, Shaw CE, Brooks DJ, Leigh PN, et al. Cortical 5-HT1A receptor binding in patients with homozygous D90A SOD1 vs. sporadic. ALS Neurol. (2007) 68:1233–5. doi: 10.1212/01.wnl.0000259083.31837.64
59. Takahashi H, Snow BJ, Bhatt MH, Peppard R, Eisen A, Calne DB. Evidence for a dopaminergic deficit in sporadic amyotrophic lateral sclerosis on positron emission scanning. Lancet. (1993) 342:1016–8.
60. Przedborski S, Dhawan V, Donaldson DM, Murphy PL, McKenna-Yasek D, Mandel FS, et al. Nigrostriatal dopaminergic function in familial amyotrophic lateral sclerosis patients with and without copper/zinc superoxide dismutase mutations. Neurology. (1996) 47:1546–51.
61. Hideyama T, Momose T, Shimizu J, Tsuji S, Kwak S. A positron emission tomography study on the role of nigral lesions in parkinsonism in patients with amyotrophic lateral sclerosis. Arch Neurol. (2006) 63:1719–22. doi: 10.1001/archneur.63.12.1719
62. Fu X, Zhu W, Guo Z, Shu G, Cui F, Yang F, et al. (18)F-fallypride PET-CT of dopamine D2/D3 receptors in patients with sporadic amyotrophic lateral sclerosis. J Neurol Sci. (2017) 377:79–84. doi: 10.1016/j.jns.2017.03.013
63. Ikawa M, Okazawa H, Tsujikawa T, Matsunaga A, Yamamura O, Mori T, et al. Increased oxidative stress is related to disease severity in the ALS motor cortex: a PET study. Neurology. (2015) 84:2033–9. doi: 10.1212/wnl.0000000000001588
64. DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. (2011) 72:245–56. doi: 10.1016/j.neuron.2011.09.011
65. Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. (2011) 72:257–68. doi: 10.1016/j.neuron.2011.09.010
66. Gijselinck I, Van Langenhove T, van der Zee J, Sleegers K, Philtjens S, Kleinberger G, et al. A C9orf72 promoter repeat expansion in a Flanders-Belgian cohort with disorders of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum: a gene identification study. Lancet Neurol. (2012) 11:54–65. doi: 10.1016/s1474-4422(11)70261-7
67. Sargsyan SA, Monk PN, Shaw PJ. Microglia as potential contributors to motor neuron injury in amyotrophic lateral sclerosis. Glia. (2005) 51:241–53. doi: 10.1002/glia.20210
68. Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. (2006) 312:1389–92. doi: 10.1126/science.1123511
69. Brettschneider J, Toledo JB, Van Deerlin VM, Elman L, McCluskey L, Lee VM, et al. Microglial activation correlates with disease progression and upper motor neuron clinical symptoms in amyotrophic lateral sclerosis. PLoS ONE. (2012) 7:e39216. doi: 10.1371/journal.pone.0039216
70. Brown AK, Fujita M, Fujimura Y, Liow JS, Stabin M, Ryu YH, et al. Radiation dosimetry and biodistribution in monkey and man of 11C-PBR28: a PET radioligand to image inflammation. J Nucl Med. (2007) 48:2072–9. doi: 10.2967/jnumed.107.044842
71. Lavisse S, Guillermier M, Herard AS, Petit F, Delahaye M, Van Camp N, et al. Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. J Neurosci. (2012) 32:10809–18. doi: 10.1523/jneurosci.1487-12.2012
72. Kreisl WC, Fujita M, Fujimura Y, Kimura N, Jenko KJ, Kannan P, et al. Comparison of [(11)C]-(R)-PK 11195 and [(11)C]PBR28, two radioligands for translocator protein (18 kDa) in human and monkey: implications for positron emission tomographic imaging of this inflammation biomarker. Neuroimage. (2010) 49:2924–32. doi: 10.1016/j.neuroimage.2009.11.056
73. Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer's disease. Nature. (2009) 461:916–22. doi: 10.1038/nature08538
74. Azmitia EC, Gannon PJ, Kheck NM, Whitaker-Azmitia PM. Cellular localization of the 5-HT1A receptor in primate brain neurons and glial cells. Neuropsychopharmacology. (1996) 14:35–46. doi: 10.1016/s0893-133x(96)80057-1
75. Williams TL, Shaw PJ, Lowe J, Bates D, Ince PG. Parkinsonism in motor neuron disease: case report and literature review. Acta Neuropathol. (1995) 89:275–83.
76. D'Amico E, Factor-Litvak P, Santella RM, Mitsumoto H. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic Biol Med. (2013) 65:509–27. doi: 10.1016/j.freeradbiomed.2013.06.029
77. Takei K, Watanabe K, Yuki S, Akimoto M, Sakata T, Palumbo J. Edaravone and its clinical development for amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. (2017) 18:5–10. doi: 10.1080/21678421.2017.1353101
78. Wada K, Fujibayashi Y, Tajima N, Yokoyama A. Cu-ATSM, an intracellular-accessible superoxide dismutase (SOD)-like copper complex: evaluation in an ischemia-reperfusion injury model. Biol Pharm Bull. (1994) 17:701–4.
79. Ikawa M, Okazawa H, Kudo T, Kuriyama M, Fujibayashi Y, Yoneda M. Evaluation of striatal oxidative stress in patients with Parkinson's disease using [62Cu]ATSM PET. Nucl Med Biol. (2011) 38:945–51. doi: 10.1016/j.nucmedbio.2011.02.016
80. Ikawa M, Okazawa H, Arakawa K, Kudo T, Kimura H, Fujibayashi Y, et al. PET imaging of redox and energy states in stroke-like episodes of MELAS. Mitochondrion. (2009) 9:144–8. doi: 10.1016/j.mito.2009.01.011
81. Williams JR, Trias E, Beilby PR, Lopez NI, Labut EM, Bradford CS, et al. Copper delivery to the CNS by CuATSM effectively treats motor neuron disease in SOD(G93A) mice co-expressing the Copper-Chaperone-for-SOD. Neurobiol Dis. (2016) 89:1–9. doi: 10.1016/j.nbd.2016.01.020
82. Vieira FG, Hatzipetros T, Thompson K, Moreno AJ, Kidd JD, Tassinari VR, et al. CuATSM efficacy is independently replicated in a SOD1 mouse model of ALS while unmetallated ATSM therapy fails to reveal benefits. IBRO Rep. (2017) 2:47–53. doi: 10.1016/j.ibror.2017.03.001
83. FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource [Internet]. Silver Spring, MD: Food and Drug Administration (US) (2016).
84. Zhang Y, Schuff N, Woolley SC, Chiang GC, Boreta L, Laxamana J, et al. Progression of white matter degeneration in amyotrophic lateral sclerosis: a diffusion tensor imaging study. Amyotroph Lateral Scler. (2011) 12:421–9. doi: 10.3109/17482968.2011.593036
85. Jucaite A, Svenningsson P, Rinne JO, Cselenyi Z, Varnas K, Johnstrom P, et al. Effect of the myeloperoxidase inhibitor AZD3241 on microglia: a PET study in Parkinson's disease. Brain. (2015) 138(Pt 9):2687–700. doi: 10.1093/brain/awv184
86. Scott G, Zetterberg H, Jolly A, Cole JH, De Simoni S, Jenkins PO, et al. Minocycline reduces chronic microglial activation after brain trauma but increases neurodegeneration. Brain. (2018) 141:459–71. doi: 10.1093/brain/awx339
Keywords: amyotrophic lateral sclerosis, neuroimaging, positron emission tomography, biomarker, diagnostic biomarker, pharmacodynamic biomarker, therapeutic development
Citation: Chew S and Atassi N (2019) Positron Emission Tomography Molecular Imaging Biomarkers for Amyotrophic Lateral Sclerosis. Front. Neurol. 10:135. doi: 10.3389/fneur.2019.00135
Received: 20 December 2018; Accepted: 01 February 2019;
Published: 01 March 2019.
Edited by:
Peter Bede, Trinity College Dublin, IrelandReviewed by:
Cristina Moglia, University of Turin, ItalyAndrew W. Barritt, University of Sussex, United Kingdom
Copyright © 2019 Chew and Atassi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheena Chew, c2NoZXcxQHBhcnRuZXJzLm9yZw==
 Sheena Chew
Sheena Chew Nazem Atassi
Nazem Atassi