- 1Institute of Sports Science, Leibniz University Hannover, Hanover, Germany
- 2Consultation Division, Program Management Discovery Sciences, RSGBIOGEN, New Delhi, India
Patients undergoing chemotherapy, radiotherapy, and immunotherapy experience neurotoxic changes in the central and peripheral nervous system. These neurotoxic changes adversely affect functioning in the sensory, motor, and cognitive domains. Thereby, considerably affecting autonomic activities like gait and posture. Recent evidence from a range of systematic reviews and meta-analyses have suggested the beneficial influence of music-based external auditory stimulations i.e., rhythmic auditory cueing and real-time auditory feedback (sonification) on gait and postural stability in population groups will balance disorders. This perspective explores the conjunct implications of auditory stimulations during cancer treatment to simultaneously reduce gait and posture related deficits. Underlying neurophysiological mechanisms by which auditory stimulations might influence motor performance have been discussed. Prompt recognition of this sensorimotor training strategy in future studies can have a widespread impact on patient care in all areas of oncology.
Introduction
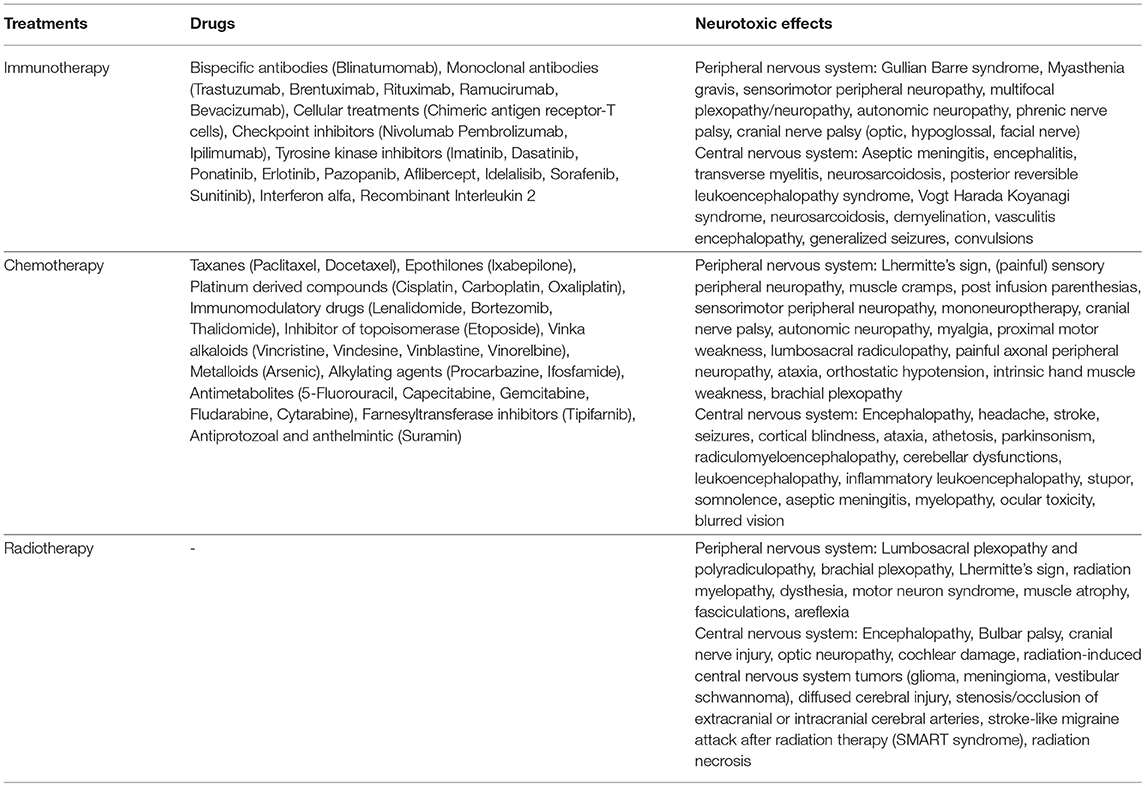
Pharmacological treatment of cancer is varying dramatically with benefits for better patient outcomes and ease, but also with new toxicity profiles (1–3). Neurotoxicity is an unavoidable complication of life-saving cancer treatments, such as chemotherapy, radiotherapy, and immunotherapy (4, 5). Typically, treatment with immunotherapeutic agents involves activation of the body's own immune system for targeting malignant cells (6) (Table 1). During the treatment cross-adverse reactions with existing neural cells result in heightened neurotoxicity (7–9). Topp et al. (10) for instance, reported that approximately >50% of patients receiving Blinatumomab for acute B-lymphoblastic leukemia exhibited movement disorders, encephalopathic changes, cerebellar dysfunctions, and seizures. Similarly, chemotherapy acts by instigating damage to the structural composition of the DNA, and by also disrupting DNA repair and microtubule functioning. During its functioning the chemotherapeutic agents impart non-specific damage on the cells of the nervous system, thereby resulting in neurotoxicity (9) (Table 1). The most commonly used class of chemotherapy drugs include Vinca alkaloids. This class of drugs has been reported to disrupt microtubule functioning, promote degeneration and axonal atrophy in dosages more than 2 mg/m3 (11). Furthermore, radiotherapy inhibits cell division and promotes neurotoxicity by inducing vascular damage, hormonal disruption, alteration in cytokine expression, neural stem cell deletion, neural fibrosis (12, 13) (Table 1) [for a detailed review see (14)]. Several factors can influence the extent of neurotoxicity induced by radiation therapy i.e., volume of brain irradiated, fraction (>200cGy), cumulative radiation dosage (<5,000cGy), simultaneous administration of chemotherapy, administration of therapy in age groups <7 years old or more than 60 years old and pre-existence of stroke (15). Despite precarious planning to irradiate specific parts and minimize neuropathy, radiation-induced neurotoxicity is still prevalent in several parts of the neural axis (12).
There are several pathophysiological mechanisms by which neurotoxicity can be induced. For instance, therapeutic interventions can impart direct damage to the neuron, glia, and modify the cerebral microvasculature (8, 16–18). Moreover, pathological analysis has also suggested that onset of neural necrosis, axonal degeneration due to microtubular and secondary myelin disruptions (19), can result in central and peripheral nervous system neurotoxicity. Although, several sensory, motor, and cognitive deficits have been discussed in the published literature that can result due to neurotoxicity. In this present perspective our objectives are:
a) Outline the impact of cancer treatment-induced neurotoxicity on gait and posture.
b) Discuss the applicability of music-based external auditory stimulations for facilitating gait and postural recovery in cancer patients.
Motor Deficits (Gait and Posture)
Research has conclusively demonstrated that joint dysfunctions in sensory, motor and cognitive domains due to neurotoxicity can affect activities of daily living, such as gait (5, 20, 21), posture (22), and promote falls. Epidemiological evidence suggests that the majority of the diagnosed patients are geriatrics i.e., 60–70 years old (23, 24). Spoelstra et al. (25), for instance, reported that geriatric patients with a history of cancer were more likely to fall (33%) as compared to patients with no history of cancer (29%). This higher risk of fall can be due to joint additional neurological deficits imposed by drug-induced neurotoxicity and an age-associated neurological decline (2, 25). Studies analyzing the spatiotemporal gait parameters have also reported larger decrements in gait performance for cancer patients (2, 20, 26). Marshall et al. (2), reported a significantly reduced gait velocity, step length, and an increased duration in timed up and go test in patients with cancer as compared to their healthy counterparts (5, 27). Similarly, kinematic discrepancies during gait performance are also documented. Wright et al. (28) analyzed gait performance (3-D motion analysis, EMG) following treatment for acute lymphoblastic leukemia. The authors reported a significant reduction in peak hip extension, knee flexion during the loading phase, plantarflexion during pre-swing, dorsiflexion during initial heel contact, lower ankle moments, and power outputs. The authors also reported that the patients exhibited excessive co-activations and an atypical “out of phase” motor unit firing of gastrocnemius during the late swing and premature firing of tibialis anterior during terminal stance.
Monfort et al. (22) too in a longitudinal analysis reported a significant decrease in balance (center of pressure perturbations in medioateral direction) in breast cancer patients receiving taxane-based chemotherapy. The authors further correlated this decrease in balance with patient-reported outcomes i.e., EORTC QLQ-CIPN20 subscales (European Organization for Research and treatment of Cancer Quality of Life Questionnaire Chemotherapy Induced Peripheral Neuropathy) i.e., increased pain, fatigue, and disruption in physical functioning reported with the treatment progression.
Cognitive Deficits
In addition to the motor deficits, patients receiving cancer treatment also exhibit heightened cognitive deficits [see chemobrain or chemofog (29)]. These deficits can persist years after the treatment and can considerably affect a patient's quality of life (30). A wide range of cognitive disorders are manifested by patients i.e., disruptions in executive functions, multitasking, concentration, attentional allocation, even memory recall, visuospatial function, and more (29–31). The pathophysiological changes which account for such deficits include white matter abnormality, regional brain volume differences in superior and middle frontal gyri, parahippocampal gyrus, cingulate gyrus, and precuneus (32, 33). Silverman et al. (34), in a PET study, reported that breast cancer patients who received chemotherapy 5–10 years prior had differences in inferior frontal gyrus, contralateral posterior cerebellum, and left inferior frontal gyrus. The authors also implied the onset of cognitive overload by reporting a larger activation pattern of frontal cortical structures i.e., pre-frontal cortex during a memory task (34). This decline in cognitive performance due to adverse neurotoxic effects of oncologic therapy in our opinion might be amplified when coupled with an age-associated decline in cognition. This, then, might promote a major decline in cognitive performance, further affecting autonomic functions such as posture, gait (35). For instance, this reduced cognitive functioning might limit a patient's ability to effectively allocate attentional resources for instance in high-stress environments and instigate falls (36, 37).
Sensory Deficits
A wide range of sensory deficits are accounted in patients due to neurotoxic effects on the nervous system (8). Evidence of optic neuropathy have been extensively documented due to radiotherapy, intra-arterial administration of drugs such as Carmustine, Oxaliplatin, Tamoxifen, and more (38, 39). Likewise, deficits in vestibular (40), and proprioceptive signaling (41), are also well reported. Vincent et al. (41) for instance, reported that administration of Oxaliplatin drug promoted the onset of movement disorders. The authors suggested that possibly neurotoxic changes impaired specific ionic current channels (NaPIC) on the sensory terminals of muscle proprioceptors further leading to a modified sensory encoding which could have affected motor functioning (41). Additionally, axonal degeneration of sensory neurons, which promotes receptor denervation, have also been associated with sensorimotor aberrations that affect motor execution (41–43). Bibi (44), for instance, reported that cancer therapy-induced neurotoxic changes can also promote pervasive deterioration in the autonomic mechanisms for sensory gating and sensory memory mechanisms. This contextual decline in the available state of sensory information might affect the state of a system to integrate sensorimotor information and develop internal models (45–47). Here, a mismatch incongruency of sensorimotor information or a decrease in the quality of perceptual information could promote sensorimotor deficits, further affecting motor planning, execution during gait, and postural performance (48, 49).
Conventional Rehabilitation Interventions
A few rehabilitation strategies have been discussed in the published literature that can enhance gait and balance dysfunctions in patients with cancer. These strategies include physiotherapy, physical exercises, virtual reality and more (21, 50, 51) (see Table 2). Moreover, to the best of our knowledge, only one recent systematic review has analyzed the influence of exercise rehabilitation interventions for managing deficits in gait and postural stability in cancer patients undergoing chemotherapy (21). Despite having a high prevalence for inducing fall-related morbidity and mortality (63), such a limited amount of research is a matter of concern for medical practitioners dealing with cancer patients. Therefore, the development of additional rehabilitation interventions that can be applied as an adjunct to conventional pharmacological interventions is strongly warranted.
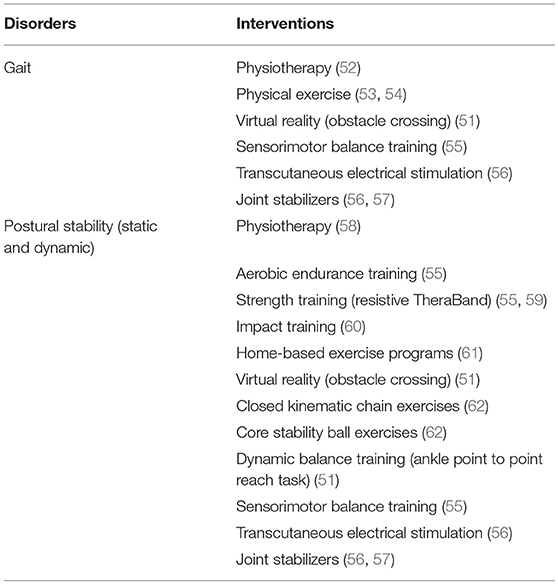
Table 2. Conventional rehabilitation approaches for managing gait and postural deficits associated with neurotoxicity.
Prospective Role of Music-Based Therapies: External Auditory Stimulations
Music therapy has been extensively studied in cancer management [for detailed reviews see (64–66)]. This therapy has been reported to decrease pain, stress, anxiety associated with cancer treatment and has also been documented to improve mood, relaxation, and quality of life (66). The studies predominantly deal with either active or passive types of music therapies (64–66). Here, the active therapy signifies playing musical instruments, improvisation, singing, and passive therapy signify listening to music, imagination (2, 3). Although the outcomes of these cumulative studies comprehend the beneficial psychological aspects of music therapy, the aim of this present study is to explore as to how motor rehabilitation might be facilitated by the application of music-based auditory stimulations?
Several studies have reported that a large component of motor (re)learning is dependent upon the extent of sensorimotor integration (67, 68). Here, amplification of sensorimotor representations by enhancing the salience of sensory afferent information while minimally engaging the deficit cognitive resources should be a major objective (69–71). This enhanced sensorimotor representations of body schematics and executed movements could facilitate the development of efficient internal models (46, 72). Thereby, enhancing the system's ability to acquire, process, and execute a skill in an efficient manner (73–75). In the published literature, movement sonification and rhythmic auditory cueing are two well-studied auditory stimulations that have been demonstrated to incur beneficial effects in motor performance by jointly targeting sensorimotor and cognitive deficits (76–83).
Rhythmic auditory cueing can be defined as a repetitive isosynchronous auditory stimulation applied with an aim to simultaneously synchronize motor execution (74, 84). Real-time kinematic auditory feedback (movement sonification) on the other hand is a comparatively new approach (85). Such type of an intervention involves mapping of movement parameters on the sound components, such as pitch, amplitude with a very minimal or no latency (72) [for differential effects of auditory cueing and sonification please see (82)]. Recent systematic reviews and meta-analyses have conclusively demonstrated the benefits of these auditory stimulations on gait and postural stability with aging (77), in neurological disorders such as stroke (82, 83, 86), parkinsonism (78), cerebral palsy (80), and multiple sclerosis (81). Findings from these reviews can have widespread implications for counteracting neurotoxicity related motor deficits in cancer patients.
For instance, rhythmic auditory cueing has been reported to enhance gait, and postural stability performance across all age groups (77). We have previously stated that the majority of the affected cancer population groups are geriatrics and that this factor according to several studies accounts for the majority of fall-related morbidity and mortality (25). Likewise, stroke, a common neurotoxic manifestation also account for widespread movement, cognitive disorders (2). Ghai (82) has demonstrated that both rhythmic and real-time auditory stimulations can benefit stroke patients in recovering their motor and cognitive performances. Additionally, we also presume that damage induced by white matter deficits, which are a prominent manifestation of neurotoxicity can also be supplemented by the application of auditory stimulations (32, 87). Ghai and Ghai (81) recently demonstrated the beneficial effects of auditory cueing on patients with multiple sclerosis (a multifocal white matter disease). The authors stated evidence which supports the possibility of white matter re-organization with auditory-motor training [see (88)].
Likewise, Ghai et al. (78), demonstrated the beneficial effects of auditory cueing on movement disorders exhibited during parkinsonism. Chemotherapy, for instance with Metoclopramide (dopamine receptor antagonist) has been associated with inhibition of D2 receptors in putamen (89). This disruption has been reported to result in movement disorders which are identical to that exhibited by a patient in Parkinson's disease (90). Here, dysfunctions between the striatopallidal projections could affect the internal timing mechanism of a patient in a similar manner as of a patient with Parkinson's disease. In this instance, the application of external auditory stimulations could assist in movement execution by providing an external cue to time movements. The external cueing can effectively bypass the deficit internal cueing pathway (Cerebellum-putamen-thalamus-pre supplementary motor area-supplementary motor area-primary motor area) through an alternative preserved pathway between (cerebellum-thalamus-parietal cortex-premotor area-primary motor area) and facilitate motor activity (91) (Figure 1).
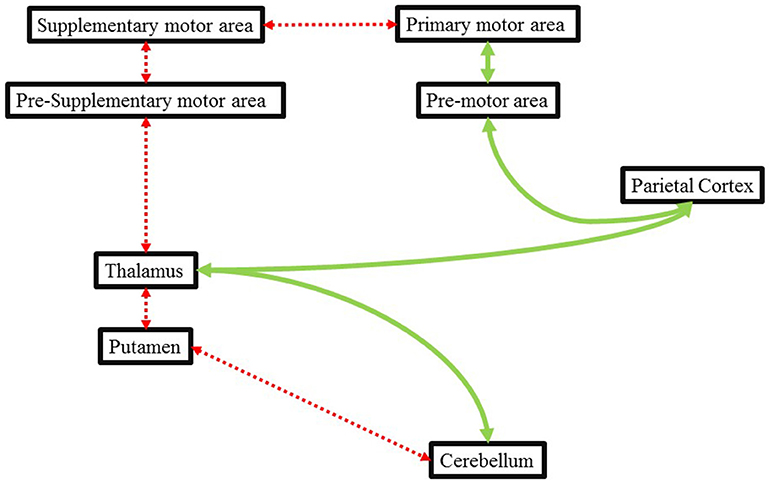
Figure 1. Illustrating the deficit internal cueing pathways (dotted red line) and how external cueing might bypass this deficit pathway via an alternative route (green line). Adapted from Nombela et al. (91) and Ghai et al. (78).
Furthermore, we presume that the auditory stimulations could counteract sensory-perceptual deficits i.e., hearing, visual loss by enhancing the salience of sensory afferent information and aiding in the development of sensorimotor representations. For instance, Schmitz et al. (92) in a neuroimaging study reported that observation of a convergent sensory feedback can enhance activations in frontoparietal networks, action observation system i.e., superior temporal sulcus, Broadman area 44, 6, insula, precentral gyrus, cerebellum, thalamus, and basal ganglia (92). The activations in these areas are associated with biological motion perception, thereby suggesting an enhancement in sensorimotor representation that might strengthen the perceptual analysis of a movement, ultimately resulting in efficient motor planning and execution (92).
Recent evidence has also demonstrated that auditory stimulations can even facilitate proprioceptive perceptions (93). Ghai et al. (94) demonstrated that concurrent auditory feedback can facilitate enhancements in knee-proprioception. Hasegawa et al. (95) too demonstrated that auditory biofeedback training resulted in enhanced spatiotemporal components of postural stability. Therefore, practical implications can be derived for cancer survivors, where deficits in proprioceptive perceptions are quite prominent (94, 96). According to Hasegawa et al. (95), auditory-motor training promoted a challenging environment that could have facilitated proprioceptive integration [for further insights on neuroimaging data see (97)]. Additional mechanisms by which auditory stimulations can facilitate motor performance are that they can provide explicit guidance to time/execute movements (94), reduce variability in musculoskeletal co-activation (98, 99), provide error feedback (100), enhance auditory-motor imagery (101, 102), allow cortical re-organization (103, 104), facilitate neural plasticity (105, 106), and even facilitate neural regeneration (107–109).
We would also like to draw the reader's attention toward literature suggesting how auditory stimulations might act by counteracting deficits in cognitive processing. Firstly, auditory stimulations have been suggested to strengthen attentional allocation (97). This might allow a patient to effectively switch between different tasks at hand without experiencing cognitive overload and/or movement failure. Secondly, enhanced cross-modal processing between auditory and proprioceptive signals can also circumvent cognitive overload and alleviate motor performance (94, 110). Thirdly, adjoining auditory stimulations with music can be an additional way to overcome cognitive deficits. For instance, coupling the auditory stimulations with musical mnemonics might facilitate synchronization of the oscillatory network in the prefrontal regions (111). Here, Thaut et al. (111) has reported that mnemonics might facilitate “deep encoding” during the acquisition phase of learning and might also amplify the internal timings of neural dynamics in the brain which are normally degraded by demyelination process in multiple sclerosis [also see (81)]. As demyelination is also a prominent neurotoxic manifestation of radiotherapy (8), transferrable beneficial effects on cognitive performance could be expected. Moreover, recent research also suggests that in addition to reducing cognitive overload in patients with stroke, the external auditory cueing via music might facilitate, reorganize deficit cortical structures (107–109). For instance, merging the external auditory stimuli with music can allow facilitation of neural network including prefrontal, and limbic cortex this, in turn, has been associated with cognitive and emotional recovery (109). Likewise, incorporating the component of music with external auditory stimulations might yield additional benefits in terms of reducing anxiety and stress (112). Studies have demonstrated that music therapy can allow a reduction in pain, fear-related stress [reduced salivary cortisol (113)], and anxiety outcomes (112). This can allow increased patient adherence toward medical procedures involved during cancer therapies and screening, for instance, screening mammography (114), sigmoidoscopy (115), colonoscopy (113), and even prostate biopsy (112, 116). Facilitation in the functioning of these mechanisms can have widespread influence on the regulation of cancer patient-related outcome and even the disease progression.
An additional outcome that can have important implications in management with auditory stimulations is the length of auditory-motor training duration. Here, interpretations can be drawn from neuroimaging research by Bangert and Altenmüller (117), and Ross et al. (106). Both the studies report that an auditory-motor training facilitates learning by acting on the rich neuroanatomical interconnectivity between the respective regions. The authors report a brief training duration lasting between 20 and 30 min to facilitate plasticity. Likewise, several of the published reviews and meta-analyses have also suggested a similar temporal course i.e., training session lasting for 25–40 min for auditory-motor training regimens (77, 78, 118). This training duration is relatively smaller as compared to conventional physiotherapy and physical exercise strategies discussed in the review by Duregon et al. (21). Therefore, beneficial implications in terms of cost-effectiveness and an enhanced prognosis in cancer survivors can be expected. Furthermore, we would also like to emphasize on the viability of the auditory stimulations, as a home-based intervention. Developing home-based interventions, are efficient for population groups in developing countries where lack of proper medical exposure accounts for widespread cancer-related morbidity and mortality (23). Wonders et al. (61) have also reported that home-based interventions can indeed impart beneficial effects in cancer survivors by reducing the peripheral neuropathic symptoms and enhancing the quality of life. We propose that in a home-based scenario patients can be taught by medical experts to utilize established smartphone rhythmic auditory cueing applications, such as Walkmate (119) to train gait effectively.
Finally, this perspective is a preliminary attempt to instigate scientific discussions for developing efficient rehabilitation protocols while using auditory neuroprosthetics based rehabilitation approach for enhancing motor recovery in patients with cancer. Incorporating these rehabilitation protocols with other sensory augmentation strategies such as virtual reality (120), joint prostheses (121–123), electrical stimulations (124) might have additional implications for enhancing the prognosis during cancer therapy. We have mentioned several mechanisms and findings from our previous review work, which could serve as the groundwork for future studies that could help design sensorimotor training regimens for the benefit of cancer population groups. Future studies are strongly recommended to analyze the effects of gait training with music-based auditory neuroprosthetics as a possible mechanism to counteract neurotoxic deficits because of cancer treatment.
Author Contributions
SG conceptualized the perspective article. IG contributed in the formulation of the manuscript. Both authors approved the final draft.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The publication of this article was funded by the Open Access fund of Leibniz Universität Hannover.
References
1. Kroschinsky F, Stölzel F, von Bonin S, Beutel G, Kochanek M, Kiehl M, et al. New drugs, new toxicities: severe side effects of modern targeted and immunotherapy of cancer and their management. Critical Care (2017) 21:89. doi: 10.1186/s13054-017-1678-1
2. Marshall TF, Zipp GP, Battaglia F, Moss R, Bryan S. Chemotherapy-induced-peripheral neuropathy, gait and fall risk in older adults following cancer treatment. J Cancer Res Pract. (2017) 4:134–8. doi: 10.1016/j.jcrpr.2017.03.005
3. Park SB, Goldstein D, Krishnan AV, Lin CSY, Friedlander ML, Cassidy J, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J. (2013) 63:419–37. doi: 10.3322/caac.21204
4. Kolb NA, Smith A, Singleton JR, Beck SL, Stoddard GJ, Brown S, et al. The association of chemotherapy-induced peripheral neuropathy symptoms and the risk of falling. JAMA Neurol. (2016) 73:860–6. doi: 10.1001/jamaneurol.2016.0383
6. Rosenberg SA. Immunotherapy of cancer using interleukin 2: current status and future prospects. Immunol Today (1988) 9:58–62.
7. Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, et al. Endothelial activation and blood–brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. (2017) 7:1–16. doi: 10.1158/2159-8290.CD-17-0698
8. Stone JB, DeAngelis LM. Cancer-treatment-induced neurotoxicity—focus on newer treatments. Nat Rev Clin Oncol. (2016) 13:92. doi: 10.1038/nrclinonc.2015.152
9. Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell (2008) 132:645–60. doi: 10.1016/j.cell.2008.01.033
10. Topp MS, Gökbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. (2015) 16:57–66. doi: 10.1016/S1470-2045(14)71170-2
11. Yarbro CH, Wujcik D, Gobel BH. Cancer Nursing: Principles and Practice. Jones & Bartlett Learning (2011).
12. Dropcho EJ. Neurotoxicity of radiation therapy. Neurol Clin. (2010) 28:217–34. doi: 10.1016/j.ncl.2009.09.008
13. Smart D. Radiation Toxicity in the central nervous system: mechanisms and strategies for injury reduction. Semin Radiat Oncol. (2017) 27:332–9. doi: 10.1016/j.semradonc.2017.04.006
14. Soussain C, Ricard D, Fike JR, Mazeron JJ, Psimaras D, Delattre JY. CNS complications of radiotherapy and chemotherapy. Lancet (2009) 374:1639–51. doi: 10.1016/S0140-6736(09)61299-X
16. Plotkin SR, Wen PY. Neurologic complications of cancer therapy. Neurol Clin. (2003) 21:279–318. doi: 10.1016/S0733-8619(02)00034-8
17. Giglio P, Gilbert MR. Neurologic complications of cancer and its treatment. Curr Oncol Rep. (2010) 12:50–9. doi: 10.1007/s11912-009-0071-x
18. Belka C, Budach W, Kortmann R, Bamberg M. Radiation induced CNS toxicity–molecular and cellular mechanisms. Br J Cancer (2001) 85:1233. doi: 10.1054/bjoc.2001.2100
19. Toopchizadeh V, Barzegar M, Rezamand A, Feiz AH. Electrophysiological consequences of vincristine contained chemotherapy in children: a cohort study. J Pediatr Neurol. (2009) 7:351–6. doi: 10.3233/JPN-2009-0333
20. Pamoukdjian F, Paillaud E, Zelek L, Laurent M, Lévy V, Landre T, et al. Measurement of gait speed in older adults to identify complications associated with frailty: a systematic review. J Geriatr Oncol. (2015) 6:484–96. doi: 10.1016/j.jgo.2015.08.006
21. Duregon F, Vendramin B, Bullo V, Gobbo S, Cugusi L, Di Blasio A, et al. Effects of exercise on cancer patients suffering chemotherapy-induced peripheral neuropathy undergoing treatment: a systematic review. Crit Rev Oncol Hematol. (2018) 121:90–100. doi: 10.1016/j.critrevonc.2017.11.002
22. Monfort SM, Pan X, Patrick R, Ramaswamy B, Wesolowski R, Naughton MJ, et al. Gait, balance, and patient-reported outcomes during taxane-based chemotherapy in early-stage breast cancer patients. Breast Cancer Res Treat. (2017) 164:69–77. doi: 10.1007/s10549-017-4230-8
23. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. (2016) 66:7–30. doi: 10.3322/caac.21332
24. Howlader N, Noone A, Krapcho M, Garshell J, Miller D, Altekruse S, et al. SEER Cancer Statistics Review, 1975–2012. Bethesda, MD: National Cancer Institute (2015).
25. Spoelstra SL, Given BA, Schutte DL, Sikorskii A, You M, Given CW. Do older adults with cancer fall more often? A comparative analysis of falls in those with and without cancer. Oncol Nurs Forum (2013) 40:E69–78. doi: 10.1188/13.ONF.E69-E78
26. Niederer D, Schmidt K, Vogt L, Egen J, Klingler J, Hübscher M, et al. Functional capacity and fear of falling in cancer patients undergoing chemotherapy. Gait Post. (2014) 39:865–9. doi: 10.1016/j.gaitpost.2013.11.014
27. Gilchrist L, Tanner L. Gait patterns in children with cancer and vincristine neuropathy. Pediatr Phys Ther. (2016) 28:16–22. doi: 10.1097/PEP.0000000000000208
28. Wright MJ, Twose DM, Gorter JW. Gait characteristics of children and youth with chemotherapy induced peripheral neuropathy following treatment for acute lymphoblastic leukemia. Gait Post. (2017) 58:139–45. doi: 10.1016/j.gaitpost.2017.05.004
29. Raffa R. Is a picture worth a thousand (forgotten) words?: neuroimaging evidence for the cognitive deficits in ‘chemo-fog’/'chemo-brain'. J Clin Pharmacy Therapeut. (2010) 35:1–9. doi: 10.1111/j.1365-2710.2009.01044.x
30. Scherling CS, Smith A. Opening up the window into “chemobrain”: a neuroimaging review. Sensors (2013) 13:3169–203. doi: 10.3390/s130303169
31. Raffa RB, Duong PV, Finney J, Garber DA, Lam LM, et al. Is ‘chemo-fog’/‘chemo-brain’ caused by cancer chemotherapy? J Clin Phar Therapeut. (2006) 31:129–38. doi: 10.1111/j.1365-2710.2006.00726.x
32. Stemmer SM, Stears JC, Burton BS, Jones RB, Simon JH. White matter changes in patients with breast cancer treated with high-dose chemotherapy and autologous bone marrow support. Am J Neuroradiol. (1994) 15:1267–73.
33. Brown MS, Stemmer SM, Simon JH, Stears JC, Jones RB, Cagnoni PJ, et al. White matter disease induced by high-dose chemotherapy: longitudinal study with MR imaging and proton spectroscopy. Am J Neuroradiol. (1998) 19:217–21.
34. Silverman DH, Dy CJ, Castellon SA, Lai J, Pio BS, Abraham L, et al. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res Treat. (2007) 103:303–11. doi: 10.1007/s10549-006-9380-z
35. Rosso AL, Verghese J, Metti AL, Boudreau RM, Aizenstein HJ, Kritchevsky S, et al. Slowing gait and risk for cognitive impairment. The hippocampus as a shared neural substrate. Neurology. (2017) 89:336–42. doi: 10.1212/WNL.0000000000004153
36. Ghai S, Ghai I, Effenberg AO. Effects of dual tasks and dual-task training on postural stability: a systematic review and meta-analysis. Clin Intervent Aging (2017) 12:557–77. doi: 10.2147/CIA.S125201
37. Holtzer R, Wang C, Verghese J. The relationship between attention and gait in aging: facts and fallacies. Motor Control (2012) 16:64–80. doi: 10.1123/mcj.16.1.64
38. Reddy MA, Naeem Z, Duncan C, Robertson F, Herod J, Rennie A, et al. Reduction of severe visual loss and complications following intra-arterial chemotherapy (IAC) for refractory retinoblastoma. Br J Ophthalmol. (2017) 101:1704–8. doi: 10.1136/bjophthalmol-2017-310294
39. Omoti AE, Omoti CE. Ocular toxicity of systemic anticancer chemotherapy. Pharm Pract. (2006) 4:55–9.
41. Vincent JA, Wieczerzak KB, Gabriel HM, Nardelli P, Rich MM, Cope TC. A novel path to chronic proprioceptive disability with oxaliplatin: distortion of sensory encoding. Neurobiol Dis. (2016) 95:54–65. doi: 10.1016/j.nbd.2016.07.004
42. Argyriou AA, Polychronopoulos P, Iconomou G, Chroni E, Kalofonos HP. A review on oxaliplatin-induced peripheral nerve damage. Cancer Treat Rev. (2008) 34:368–77. doi: 10.1016/j.ctrv.2008.01.003
43. Burakgazi AZ, Messersmith W, Vaidya D, Hauer P, Hoke A, Polydefkis M. Longitudinal assessment of oxaliplatin-induced neuropathy. Neurology (2011) 77:980–6. doi: 10.1212/WNL.0b013e31822cfc59
44. Bibi R. Effects of Chemotherapy on Sensory Inhibition and Sensory Memory in Breast Cancer Survivor. CUNY Academic Works (2013). Available online at: http://academicworks.cuny.edu/cc_etds_theses
45. Thaler DS. Design for an aging brain. Neurobiol Aging (2002) 23:13–15. doi: 10.1016/S0197-4580(01)00262-7
46. Wolpert DM, Diedrichsen J, Flanagan JR. Principles of sensorimotor learning. Nat Rev Neurosci. (2011) 12:739–51. doi: 10.1038/nrn3112
47. Haggard P, Wolpert DM. Disorders of body scheme. In: Freund, HJ, Jeannerod M, Hallett M, and Leiguarda R, editors. Higher-Order Motor Disorders. Oxford: Citeseer (2005).
48. Skoura X, Papaxanthis C, Vinter A, Pozzo T. Mentally represented motor actions in normal aging: I. Age effects on the temporal features of overt and covert execution of actions. Behav Brain Res. (2005) 165:229–39. doi: 10.1016/j.bbr.2005.07.023
49. Boisgontier MP, Nougier V. Ageing of internal models: from a continuous to an intermittent proprioceptive control of movement. Age (2013) 35:1339–55. doi: 10.1007/s11357-012-9436-4
50. Ospina PA, McComb A, Wiart LE, Eisenstat DD, McNeely ML. Physical therapy interventions, other than general physical exercise interventions, in children and adolescents before, during and following treatment for cancer. Cochrane Database Syst Rev. (2018) CD012924. doi: 10.1002/14651858.CD012924
51. Schwenk M, Grewal GS, Holloway D, Muchna A, Garland L, Najafi B. Interactive Sensor-based balance training in older cancer patients with chemotherapy-induced peripheral neuropathy: a randomized controlled trial. Gerontology (2016) 62:553–63. doi: 10.1159/000442253
52. Rizzo A. The role of exercise and rehabilitation in the cancer care plan. J Advanc Practit Oncol. (2016) 7:339. doi: 10.6004/jadpro.2016.7.3.20
53. Andersen C, Rørth M, Ejlertsen B, Stage M, Møller T, Midtgaard J, et al. The effects of a six-week supervised multimodal exercise intervention during chemotherapy on cancer-related fatigue. Eur J Oncol Nurs. (2013) 17:331–9. doi: 10.1016/j.ejon.2012.09.003
54. Dalzell M, Smirnow N, Sateren W, Sintharaphone A, Ibrahim M, Mastroianni L, et al. Rehabilitation and exercise oncology program: translating research into a model of care. Curr Oncol. (2017) 24: e191–8. doi: 10.3747/co.24.3498
55. Streckmann F, Kneis S, Leifert J, Baumann F, Kleber M, Ihorst G, et al. Exercise program improves therapy-related side-effects and quality of life in lymphoma patients undergoing therapy. Ann Oncol. (2014) 25:493–99. doi: 10.1093/annonc/mdt568
56. Kumar SP. Cancer pain: a critical review of mechanism-based classification and physical therapy management in palliative care. Indian J Palliat Care (2011) 17:116–26. doi: 10.4103/0973-1075.84532
57. Paice JA. Mechanisms and management of neuropathic pain in cancer. J Support Oncol. (2003) 1:107–20.
58. Angin S, Karadibak D, Yavuzşen T, Demirbüken I. Unilateral upper extremity lymphedema deteriorates the postural stability in breast cancer survivors. Contem Oncol. (2014) 18:279–84. doi: 10.5114/wo.2014.44120
59. Mizrahi D, Broderick C, Friedlander M, Ryan M, Harrison M, Pumpa K, et al. An exercise intervention during chemotherapy for women with recurrent ovarian cancer: a feasibility study. Int J Gynecol Cancer (2015) 25:985–92. doi: 10.1097/IGC.0000000000000460
60. Winters-Stone K, Dobek J, Nail L, Bennett J, Leo M, Torgrimson-Ojerio B, et al. Impact+ resistance training improves bone health and body composition in prematurely menopausal breast cancer survivors: a randomized controlled trial. Osteop Int. (2013) 24:1637–46. doi: 10.1007/s00198-012-2143-2
61. Wonders KY, Whisler G, Loy H, Holt B, Bohachek K, Wise R. Ten weeks of home-based exercise attenuates symptoms of chemotherapy-induced peripheral neuropathy in breast cancer patients. Health Psychol Res. (2013) 1:e28. doi: 10.4081/hpr.2013.e28
62. Fernandes J, Kumar S. Effect of lower limb closed kinematic chain exercises on balance in patients with chemotherapy-induced peripheral neuropathy: a pilot study. Int J Rehabil Res. (2016) 39:368–71. doi: 10.1097/MRR.0000000000000196
63. Huang MH, Shilling T, Miller KA, Smith K, LaVictoire K. History of falls, gait, balance, fall risks in older cancer survivors living in the community. Clin Intervent Aging (2015) 10:1497. doi: 10.2147/CIA.S89067
64. Boyde C, Linden U, Boehm K, Ostermann T. The use of music therapy during the treatment of cancer patients: a collection of evidence. Glob Adv Health Med. (2012) 1:24–9. doi: 10.7453/gahmj.2012.1.5.009
65. Archie P, Bruera E, Cohen L. Music-based interventions in palliative cancer care: a review of quantitative studies and neurobiological literature. Support Care Cancer (2013) 21:2609–24. doi: 10.1007/s00520-013-1841-4
66. Stanczyk MM. Music therapy in supportive cancer care. Rep Pract Oncol Radiother. (2011) 16:170–2. doi: 10.1016/j.rpor.2011.04.005
67. Bolognini N, Russo C, Edwards DJ. The sensory side of post-stroke motor rehabilitation. Restor Neurol Neurosci. (2016) 34:571–86. doi: 10.3233/RNN-150606
68. Ghai S, Schmitz G, Hwang T-H, Effenberg AO. Training proprioception with sound: effects of real-time auditory feedback on intermodal learning. Ann NY Acad Sci. (2018). doi: 10.1111/nyas.13967 [Epub ahead of print].
71. Effenberg AO, Schmitz G. Acceleration and deceleration at constant speed: systematic modulation of motion perception by kinematic sonification. Ann NY Acad Sci. (2018) 1425:56–9. doi: 10.1111/nyas.13693
72. Effenberg AO, Fehse U, Schmitz G, Krueger B, Mechling H. Movement sonification: effects on motor learning beyond rhythmic adjustments. Front Neurosci. (2016) 10:67. doi: 10.3389/fnins.2016.00219
73. Wolpert DM, Ghahramani Z, Jordan MI. An internal model for sensorimotor integration. Science (1995) 269:1880. doi: 10.1126/science.7569931
74. Schaefer RS. Auditory rhythmic cueing in movement rehabilitation: findings and possible mechanisms. Philos Trans R Soc B Biol Sci. (2014) 369:20130402. doi: 10.1098/rstb.2013.0402
75. Thaut MH, Abiru M. Rhythmic auditory stimulation in rehabilitation of movement disorders: a review of current research. Music Percept. (2010) 27:263–9. doi: 10.1525/mp.2010.27.4.263
76. Thaut MH. Rhythm, Music, and the Brain: Scientific Foundations and Clinical Applications. New York, NY: Routledge (2005).
77. Ghai S, Ghai I, Effenberg AO. Effect of rhythmic auditory cueing on aging gait: a systematic review and meta-analysis. Aging Dis. (2017) 9:901–23. doi: 10.14336/AD.2017.1031
78. Ghai S, Ghai I, Schmitz G, Effenberg AO. Effect of rhythmic auditory cueing on parkinsonian gait: a systematic review and meta-analysis. Sci Rep. (2018) 8:506. doi: 10.1038/s41598-017-16232-5
79. Ghai S, Ghai I, Effenberg AO. “Low road” to rehabilitation: a perspective on subliminal sensory neuroprosthetics. Neuropsychiat Dis Treat. (2018) 14:301–7. doi: 10.2147/NDT.S153392
80. Ghai S, Ghai I, Effenberg AO. Effect of rhythmic auditory cueing on gait in cerebral palsy: a systematic review and meta-analysis. Neuropsyc Dis Treat. (2018) 14:43–59. doi: 10.2147/NDT.S148053
81. Ghai S, Ghai I. Effects of rhythmic auditory cueing in gait rehabilitation for multiple sclerosis: a mini systematic review and meta-analysis. Front Neurol. (2018) 9:386. doi: 10.3389/fneur.2018.00386
82. Ghai S. Effects of real-time (sonification) and rhythmic auditory stimuli on recovering arm function post stroke: a systematic review & meta-analysis. Front Neurol. (2018) 9:488. doi: 10.3389/fneur.2018.00488
83. Yoo GE, Kim SJ. Rhythmic auditory cueing in motor rehabilitation for stroke patients: systematic review and meta-analysis. J Music Therapy (2016) 53:149–77. doi: 10.1093/jmt/thw003
84. Thaut MH, Hoemberg V. Handbook of Neurologic Music Therapy. Oxford: Oxford University Press (2014).
85. Höner O, Hunt A, Pauletto S, Röber N, Hermann T, Effenberg AO. Aiding movement with sonification in “exercise, play and sport”. In: Hermann T, Hunt A, Neuhoff JG, editors. The Sonification Handbook. Berlin: Logos (2011). p. 525–553.
86. Ghai S, Ghai I. Effects of (music-based) rhythmic auditory cueing training on gait & posture post-stroke: a systematic review & dose response meta-analysis. Sci Rep. (in press). doi: 10.1038/s41598-019-38723-3
87. Correa D, Wang Y, West J, Peck K, Root J, Baser R, et al. Prospective assessment of white matter integrity in adult stem cell transplant recipients. Brain Imaging Behav. (2016) 10:486–96. doi: 10.1007/s11682-015-9423-3
88. Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullén F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. (2005) 8:1148–50. doi: 10.1038/nn1516
89. Fernández-Seara MA, Aznárez-Sanado M, Mengual E, Irigoyen J, Heukamp F, et al. Effects on resting cerebral blood flow and functional connectivity induced by metoclopramide: a perfusion MRI study in healthy volunteers. Br J Pharmacol. (2011) 163:1639–52. doi: 10.1111/j.1476-5381.2010.01161.x
90. Feng DD, Cai W, Chen X. The associations between Parkinson's disease and cancer: the plot thickens. Trans Neurodegen. (2015) 4:20. doi: 10.1186/s40035-015-0043-z
91. Nombela C, Hughes LE, Owen AM, Grahn JA. Into the groove: can rhythm influence Parkinson's disease? Neurosci Biobehav Rev. (2013) 37:2564–70. doi: 10.1016/j.neubiorev.2013.08.003
92. Schmitz G, Mohammadi B, Hammer A, Heldmann M, Samii A, Münte TF, et al. Observation of sonified movements engages a basal ganglia frontocortical network. BMC Neurosci. (2013) 14:32. doi: 10.1186/1471-2202-14-32
93. Effenberg AO, Hwang T-H, Ghai S, Schmitz G. Auditory modulation of multisensory representations. In: Aramaki M, Davis M, Kronland-Martinet R, Ystad S. editors. Music Technology WITH SWING. CMMR. 2017. Lecture Notes in Computer Science, Vol. 11265. Cham: Springer (2018). doi: 10.1007/978-3-030-01692-0_20
94. Ghai S, Schmitz G. T., Hwang H, Effenberg AO. Auditory proprioceptive integration: Effects of real-time kinematic auditory feedback on knee proprioception. Front Neurosci. (2018) 12:142. doi: 10.3389/fnins.2018.00142
95. Hasegawa N, Takeda K, Sakuma M, Mani H, Maejima H, Asaka T. Learning effects of dynamic postural control by auditory biofeedback versus visual biofeedback training. Gait Post. (2017) 58:188–93. doi: 10.1016/j.gaitpost.2017.08.001
96. Danna J, Velay JL. On the auditory-proprioception substitution hypothesis: movement sonification in two deafferented subjects learning to write new characters. Front Neurosci. (2017) 11:137. doi: 10.3389/fnins.2017.00137
97. Ronsse R, Puttemans V, Coxon JP, Goble DJ, Wagemans J, Wenderoth N, Swinnen SP. Motor learning with augmented feedback: modality-dependent behavioral and neural consequences. Cereb Cortex (2011) 21:1283–94. doi: 10.1093/cercor/bhq209
98. Thaut MH, McIntosh GC, Hoemberg V. Neurobiological foundations of neurologic music therapy: rhythmic entrainment and the motor system. Front Psychol. (2014) 5:1185. doi: 10.3389/fpsyg.2014.01185
99. Thaut M, Schleiffers S, Davis W. Analysis of EMG activity in biceps and triceps muscle in an upper extremity gross motor task under the influence of auditory rhythm. J Music Therapy (1991) 28:64–88. doi: 10.1093/jmt/28.2.64
100. van Vugt FT, Tillmann B. Auditory feedback in error-based learning of motor regularity. Brain Res. (2015) 1606:54–67. doi: 10.1016/j.brainres.2015.02.026
101. Heremans E, Nieuwboer A, Spildooren J, De Bondt S, D'hooge AM, Helsen W, et al. Cued motor imagery in patients with multiple sclerosis. Neuroscience (2012) 206:115–21. doi: 10.1016/j.neuroscience.2011.12.060
102. Heremans E, Helsen WF, De Poel HJ, Alaerts K, Meyns P, Feys P. Facilitation of motor imagery through movement-related cueing. Brain Res. (2009) 1278:50–8. doi: 10.1016/j.brainres.2009.04.041
103. Whitall J, McCombe Waller S, Sorkin JD, Forrester LW, Macko RF, Hanley DF, et al. Bilateral and unilateral arm training improve motor function through differing neuroplastic mechanisms: a single-blinded randomized controlled trial. Neurorehabil Neural Repair (2011) 25:118–29. doi: 10.1177/1545968310380685
104. Luft AR, McCombe-Waller S, Whitall J, Forrester LW, Macko R, Sorkin JD, et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. J Am Med Associat. (2004) 292:1853–61. doi: 10.1001/jama.292.15.1853
105. Fujioka T, Ween JE, Jamali S, Stuss DT, Ross B. Changes in neuromagnetic beta-band oscillation after music-supported stroke rehabilitation. Ann NY Acad Sci. (2012) 1252:294–304. doi: 10.1111/j.1749-6632.2011.06436.x
106. Ross B, Barat M, Fujioka T. Sound-making actions lead to immediate plastic changes of neuromagnetic evoked responses and induced beta-band oscillations during perception. J Neurosci. (2017) 37:5948–59. doi: 10.1523/JNEUROSCI.3613-16.2017
107. Särkämö T, Ripollés P, Vepsäläinen H, Autti T, Silvennoinen HM, Salli E, et al. Structural changes induced by daily music listening in the recovering brain after middle cerebral artery stroke: a voxel-based morphometry study. Front Hum Neurosci. (2014) 8:245. doi: 10.3389/fnhum.2014.00245
108. Särkämö T, Altenmüller E, Rodríguez-Fornells A, Peretz I. Editorial: music, brain, and rehabilitation: emerging therapeutic applications and potential neural mechanisms. Front Hum Neurosci. (2016) 10:103. doi: 10.3389/fnhum.2016.00103
109. Sihvonen AJ, Särkämö T, Leo V, Tervaniemi M, Altenmüller E, Soinila S. Music-based interventions in neurological rehabilitation. Lancet Neurol. (2017) 16:648–60. doi: 10.1016/S1474-4422(17)30168-0
110. Hopkins K, Kass SJ, Blalock LD, Brill JC. Effectiveness of auditory and tactile crossmodal cues in a dual-task visual and auditory scenario. Ergonomics (2017) 60:692–700. doi: 10.1080/00140139.2016.1198495
111. Thaut MH, Peterson DA, McIntosh GC, Hoemberg V. Music mnemonics aid verbal memory and induce learning – related brain plasticity in multiple sclerosis. Front Hum Neurosci. (2014) 8:395. doi: 10.3389/fnhum.2014.00395
112. Pichler A, Pichler M. Music therapy in cancer patients: fact or fiction? Fut Oncol. (2014) 10:2409–11. doi: 10.2217/fon.14.181
113. Uedo N, Ishikawa H, Morimoto K, Ishihara R, Narahara H, Akedo I, et al. Reduction in salivary cortisol level by music therapy during colonoscopic examination. Hepatogastroenterology (2004) 51:451–3.
114. Zavotsky KE, Adrienne Banavage M, Patricia James R, Kathy Easter M, Pontieri-Lewis V, et al. The effects of music on pain and anxiety during screening mammography. Clin J Oncol Nurs. (2014) 18:E45. doi: 10.1188/14.CJON.E45-E49
115. Chlan L, Evans D, Greenleaf M, Walker J. Effects of a single music therapy intervention on anxiety, discomfort, satisfaction, and compliance with screening guidelines in outpatients undergoing flexible sigmoidoscopy. Gastroenterol Nurs. (2000) 23:148–56. doi: 10.1097/00001610-200007000-00003
116. Tsivian M, Qi P, Kimura M, Chen VH, Chen SH, Gan TJ, et al. The effect of noise-cancelling headphones or music on pain perception and anxiety in men undergoing transrectal prostate biopsy. Urology (2012) 79:32–6. doi: 10.1016/j.urology.2011.09.037
117. Bangert M, Altenmüller EO. Mapping perception to action in piano practice: a longitudinal DC-EEG study. BMC Neurosci. (2003) 4:26. doi: 10.1186/1471-2202-4-26
118. Nascimento LR, de Oliveira CQ, Ada L, Michaelsen SM, Teixeira-Salmela LF. Walking training with cueing of cadence improves walking speed and stride length after stroke more than walking training alone: a systematic review. J Physiother. (2015) 61:10–5. doi: 10.1016/j.jphys.2014.11.015
119. Hove MJ, Suzuki K, Uchitomi H, Orimo S, Miyake Y. Interactive rhythmic auditory stimulation reinstates natural 1/f timing in gait of Parkinson's patients. PLoS ONE (2012) 7:e32600. doi: 10.1371/journal.pone.0032600
120. Ghai S, Ghai I. Virtual reality enhances gait recovery in cerebral palsy: a training dose-response meta-analysis. Front Neurol. (2019).
121. Ghai S, Driller MW, Masters RSW. The influence of below-knee compression garments on knee-joint proprioception. Gait Posture (2018) 60:258–61. doi: 10.1016/j.gaitpost.2016.08.008
122. Ghai S, Driller M, Ghai I. Effects of joint stabilizers on proprioception and stability: a systematic review and meta-analysis. Phys Ther Sport (2017) 25:65–75. doi: 10.1016/j.ptsp.2016.05.006
123. Ghai S. Proprioception and Performance: The Role of Below-Knee Compression Garments and Secondary Tasks. Hamilton: University of Waikato (2016). Available online at: https://hdl.handle.net/10289/10575
Keywords: cueing, chemotherapy, stability, rehabilitation, performance, balance, perception
Citation: Ghai S and Ghai I (2019) Role of Sonification and Rhythmic Auditory Cueing for Enhancing Gait Associated Deficits Induced by Neurotoxic Cancer Therapies: A Perspective on Auditory Neuroprosthetics. Front. Neurol. 10:21. doi: 10.3389/fneur.2019.00021
Received: 02 July 2018; Accepted: 08 January 2019;
Published: 29 January 2019.
Edited by:
Michael H. Thaut, University of Toronto, CanadaReviewed by:
Graziella Madeo, National Institutes of Health (NIH), United StatesPedro Ribeiro, Universidade Federal do Rio de Janeiro, Brazil
Copyright © 2019 Ghai and Ghai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shashank Ghai, c2hhc2hhbmsuZ2hhaUBzcG9ydHdpc3MudW5pLWhhbm5vdmVyLmRl
 Shashank Ghai
Shashank Ghai Ishan Ghai2
Ishan Ghai2