- 1Division of Developmental Pediatrics, Department of Pediatrics, and Child Health, Red Cross War Memorial Children's Hospital, University of Cape Town, Cape Town, South Africa
- 2Department of Psychiatry and Mental Health, University of Cape Town, Cape Town, South Africa
- 3Unit on Risk and Resilience in Mental Disorders, South African Medical Research Council (SAMRC), Cape Town, South Africa
Aim: This paper systematically reviews the literature on the effects of prenatal alcohol exposure on early child development from birth to 5 years with the aim to synthesize the developmental outcomes associated with prenatal alcohol exposure, and inform further research to improve our knowledge of the manifestations of prenatal alcohol exposure.
Methods: Electronic databases (MEDLINE, Psych INFO, and Psych ARTICLES) were searched to find papers on the developmental outcomes of prenatal alcohol exposure in neonates, infants and toddlers and pre-school aged children. Studies were selected based on participants self-reporting alcohol consumption during pregnancy (either prospectively or retrospectively) and/or children being diagnosed with FASD based on a standardized assessment that includes a dysmorphology examination. The search was limited to peer-reviewed, English language studies involving human subjects, up to 5.5 years old.
Results: Out of the 1,684 titles screened, a total of 71 papers were identified as relevant and included in this review. The majority of studies were prospective longitudinal studies. A range of assessment modalities (or tools) was used to determine neurodevelopmental outcomes of prenatal exposure to alcohol in the age group under review, the most frequently described being the Bayley Scales of Infant and Toddler Development (BSID) (n = 19). Studies varied in terms of the dose, frequency, and timing of alcohol consumption during pregnancy and methodology used to assess alcohol consumption. Findings demonstrate extensive evidence for poor global developmental outcomes in children prenatally exposed to alcohol, particularly with moderate to severe levels of prenatal alcohol exposure.
Conclusion: The outcomes related to lower levels of prenatal alcohol exposure as well as outcomes in specific developmental domains, are poorly understood. Further research should aim to clarify the more subtle or less easily measurable manifestations of prenatal alcohol exposure on early development when the potential for greatest impact of interventions is highest.
Drinking of alcohol during pregnancy is known to have widespread negative effects on fetal growth and development (1–4). The term “Fetal alcohol spectrum disorder” (FASD) encompasses the broad spectrum of effects associated with prenatal alcohol exposure. Deficits include severe growth delay, facial dysmorphology, and cognitive and behavioral impairment (i.e., fetal alcohol syndrome; FAS) as well as the presence of cognitive or behavioral deficits without the presence of facial dysmorphology (i.e., alcohol-related neurodevelopmental disorder; ARND) (4, 5). Global estimates of prevalence range from 2 to 5% of births for FASD (6–9), with rates as high as 18 to 26% in certain regions in South Africa (6, 10–13). FASDs thus present a serious public health and economic burden (14, 15) and are recognized as a major cause of developmental disability and loss of developmental potential in children (16).
A growing body of literature documents poor global developmental outcomes (4, 17, 18), as well as domain specific deficits in motor, learning, attention, processing speed, language, and executive functioning (19–21) amongst children with prenatal alcohol exposure. Previous research has also highlighted that the severity and type of impact associated with prenatal alcohol exposure depends on several factors, including but not only related to metrics of alcohol exposure. These factors include the quantity, frequency, and timing of alcohol consumption during pregnancy (21–24), maternal risk factors (25–27), and genetic predispositions (28). In addition, postnatal socioeconomic and environmental influences may compound the outcomes related to prenatal alcohol exposure (29, 30). Due to the above mentioned influencing factors as well as variations in study design and methodologies, there are currently many knowledge gaps on the outcomes associated with prenatal alcohol exposure and research has yet to establish whether FASDs exhibit a unique neurocognitive profile (31).
FASDs are difficult to diagnose in early childhood and as a result the manifestations of prenatal alcohol exposure during early childhood are particularly poorly understood (32). There remain inconsistencies in what is known regarding the early developmental outcomes related to maternal alcohol consumption during pregnancy. Namely, findings on the dose specific effects of alcohol exposure are not clear (33, 34) and there is a lack of clarity on the manifestations of outcomes associated with prenatal alcohol exposure at specific stages during early development. The early developmental years are a unique period where trajectories are being established and children are most likely to benefit from interventions (35), there is thus a crucial need for research that broadens current knowledge on the early developmental outcomes related to prenatal alcohol exposure.
Recent reviews on prenatal alcohol exposure primarily focus on the effects of varying quantities of prenatal alcohol exposure (21, 36) or the effects of prenatal alcohol exposure on specific outcomes (33) throughout childhood (37). There has however been little focus on synthesizing the developmental manifestations of prenatal alcohol exposure across the duration of the early childhood period. This paper aims to systematically review the literature on the effects of prenatal alcohol exposure on developmental outcomes during early childhood. Consolidating the existing literature on the effects of prenatal alcohol exposure during infancy and early childhood helps provide clarity on our current understanding on prenatal alcohol exposure and provides directions for future research.
Methods
We conducted an electronic search through the following databases: MEDLINE, Psych INFO, and Psych ARTICLES. An inclusive search using the following keywords were used to identify relevant studies: “prenatal alcohol exposure,” “fetal alcohol exposure,” “fetal alcohol spectrum disorders,” “fetal alcohol syndrome,” “FAS,” “FASD,” “maternal alcohol consumption,” and “maternal drinking.” The search was not restricted to starting limits and was extended to 31 October 2017. The search was restricted to include case-control or follow-up studies including human subjects, up to 5.5 years old, published in peer-reviewed, English language journals. Case studies, case reports, reviews, and studies that fell out of our age range were excluded. Additional studies were identified through the reference lists of studies identified in the initial database search. Developmental outcomes were broadly defined to include global, cognitive, motor, behavior, socio-emotional, language, executive functions, information processing, and attentional domains. Studies were selected based on participants self-reporting alcohol consumption during pregnancy (either prospectively or retrospectively) or children being diagnosed with FASD.
Given that heterogeneities in methodology and outcome measures used amongst studies makes quantitative comparisons between studies challenging, we chose a systematic qualitative approach for this review. Furthermore, since this review aims to address and synthesize the broad range developmental outcomes associated with prenatal alcohol exposure, we found a narrative approach more appropriate than a meta-analytic approach that requires more stringent study selection criteria.
Results
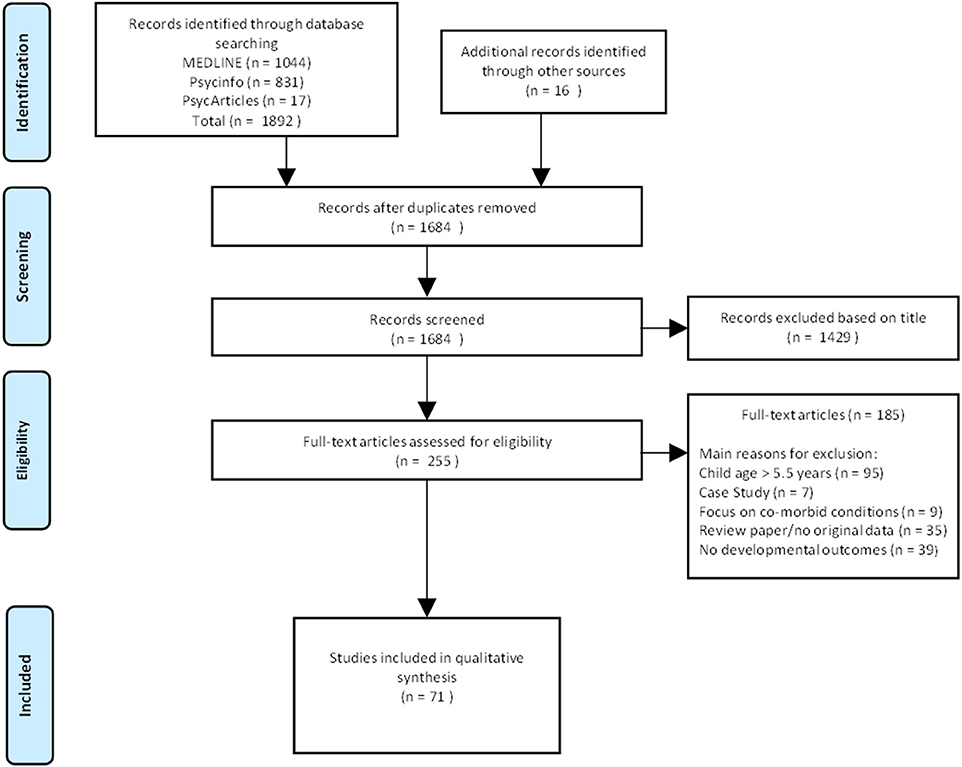
Seventy one papers met our criteria and were included in this review (see Figure 1). Selected studies are listed in Table 1. The majority of studies (n = 57) drew samples from prospective longitudinal cohorts. Most studies were conducted in the United States of America (USA, n = 34). Other publications included various countries within Europe (n = 20), Canada (n = 10), South Africa, (n = 5), and Australia (n = 2). Generally, studies compared children with prenatal alcohol exposure with a control group (either abstainers or light drinkers) with less focus on children with an established FASD diagnosis. Only 7 studies focused on children diagnosed with FASDs (17, 29, 53, 66, 81, 102, 103), negative developmental outcomes associated with prenatal alcohol exposure were evident in all 7 studies. With the exception of nationally-representative population-based studies (24, 49, 71, 75, 78, 82, 95, 97, 101, 104–107) and a few studies that recruited exclusively middle-income participants (43–45), most samples were recruited from clinics that served low socioeconomic status (SES) communities. Negative effects of prenatal alcohol exposure were more apparent in studies conducted in low income countries (17, 29, 67) than studies conducted in high income countries (24, 75, 78, 101, 108). Studies ranged in the pattern of alcohol exposure including low to moderate levels of exposure (75), isolated binge drinking (82, 95), frequent heavy drinking (46) as well as the duration of exposure including early (49) and prolonged (72) exposure.
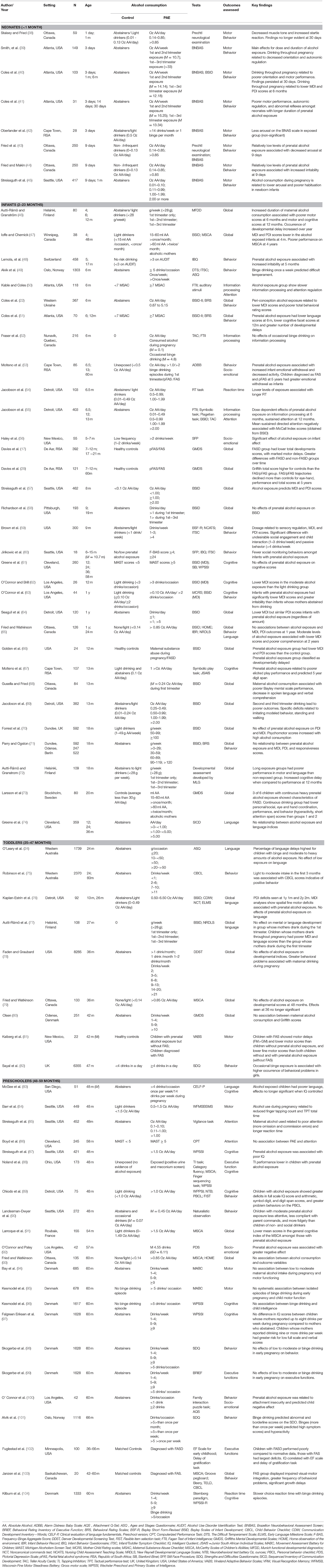
Regarding the age groups investigated in studies, the neonatal age-group was the area with least investigation (n = 7), followed by toddlers (i.e., 24–47 months; n = 10). The majority of studies (n = 32) investigated outcomes in infants (i.e., 4–23 months) and pre-school aged children (i.e., 48–66 months; n = 27). Most studies assessed global developmental outcomes using assessment tools that combine outcomes across several developmental domains to provide a general indication of development. The most frequently used assessment tool was the Bayley Scales of Infant and Toddler Development infants (22, 41, 47, 51, 57–59, 62, 64–66, 68–71, 77, 79). A number of other tools were also used in studies in this review and are listed in Table 1.
Compared to studies focusing on global developmental outcomes, less research focused on the effects of prenatal alcohol exposure on specific domains such as motor (81, 84, 95), language (24, 74, 76, 77, 83), executive functions (88, 99, 102), and aspects of emotional and behavioral functioning (48, 49, 53, 56, 59, 60, 63, 75, 82, 89, 90, 92, 98, 100, 101, 103). A small body of research focused on specific functional outcomes of interest in executive function such as attention span (67), sustained attention (86), processing speed (52, 55, 109), and reaction time (54, 85, 104).
Discussion
This review investigated the effects of prenatal alcohol exposure on developmental outcomes during early childhood. The following section will discuss summary findings from the literature on the general developmental outcomes and domain-specific effects of prenatal alcohol exposure on neonates, infants, toddlers, and preschool age children with the aim to highlight the gaps in our understanding and directions for future research.
Effects of Prenatal Alcohol Exposure on Neonatal Outcomes
The fewest number of studies focused on the effects of prenatal alcohol exposure on neonates. Effects associated with abnormal motor and behavioral functioning amongst neonates with prenatal alcohol exposure include decreased arousal (42–45), decreased orientation (39, 40), decreased habituation (45), abnormal reflexes (38, 40), increased irritability (44), and decreased muscle tone (38). Although the majority of studies within this age group demonstrate that poorer outcomes are related to longer duration and greater dose of exposure (38–40, 42), it appears that negative outcomes such as poorer arousal and increased irritability can be detected with exposure as low as 1 drink per day (44).
Findings from apical assessments such as the BNBAS typically provide a general indication of atypical functioning, rather than a behavioral profile specific to the effects of prenatal substance exposure (110) and therefore have limitations in aiding clinicians' diagnosis of FASDs. Findings from this review do however indicate that the central nervous system deficits detectable in neonates may be predictive of delayed or atypical development later in infancy. For example, Brazelton Neurobehavioral Assessment Screen (BNBAS) scores measured by Coles, Smith (41) at 3 days predicted infants' mental and psychomotor development index scores on the BSID at 6 months. These findings suggest that the poor developmental outcomes associated with alcohol exposure may be identifiable in the first days and weeks of life before higher order cognitive functions emerge and postnatal environmental factors confound developmental functioning (45). The value of these neonatal studies in predicting cognitive, motor, and behavioral developmental outcomes later in childhood, support the crucial need for the use of a standardized screening protocol to help clinicians identify neonates who may have been exposed to alcohol prenatally and will thus benefit from closer monitoring of their development (8).
Relative to older infants and children, relatively little research has investigated the early neurobehavioral effects of prenatal alcohol exposure, and several research gaps remain. The majority of studies investigating outcomes in neonates drew samples from North American populations mostly consisting of middle-income participants. Further research drawing from a wider population base including a greater range of socio-economic and cultural environments is required to clarify the generalisability of the existing findings. Similarly, evidence on how outcomes identifiable during the neonatal period may predict functioning later in infancy in relation to alcohol exposure patterns remains limited (40). Furthermore, the actual mechanisms by which the effects translate into real life developmental and neurobehavioral outcomes are poorly understood and are compounded and confounded by the complexity of the CNS, rapid body change with time, and other factors.
Effects of Prenatal Alcohol Exposure on Infant Development
Relative to other developmental stages, there is extensive literature on the effects of prenatal alcohol exposure in the infant age-group. The majority of findings from developmental assessments are suggestive of global developmental impairment (i.e., impairment to both cognitive and motor domains) and developmental delay amongst infants with prenatal alcohol exposure (17, 29, 41, 46, 47, 51, 57, 59, 66, 72, 73, 76). However, developmental assessments of children in this age group did not always find impairments to both cognitive and motor domains. Three studies utilizing the BSID for example only found lower MDI (cognitive) scores amongst infants, between 1 and 2 years of age, with prenatal alcohol exposure (64, 65, 68) while PDI (performance/motor) outcomes were non-significant when compared to unexposed controls. Four studies found marked motor delays, such as delayed sitting, standing, and walking in infants between 6 and 18 months (17, 46, 69, 72) with prenatal alcohol exposure compared to controls. These delays appear apparent amongst infants with longer duration (i.e., exposure throughout pregnancy) and heavier doses of prenatal alcohol exposure (69, 72), confirming known trends on the dose-response relationship between the amount and duration of prenatal alcohol exposure and the likelihood of motor delays.
Looking at specific domains, some investigators found evidence that poor language outcomes could be identified in infants as young as 6 months of age (51), while others described older infants with prenatal alcohol exposure having poorer comprehension and spoken language when compared to healthy controls (17, 68, 72). These investigations demonstrate that the poor language abilities in infants with prenatal alcohol exposure are present across social and linguistic contexts in the USA, Western Europe, and rural communities within South Africa and appear in studies with low (68) and heavier (17) doses of alcohol exposure. Studies tended to use different methods to assess language however, making comparisons on language development within this age band difficult.
Studies investigating outcomes in this age group also suggest that infants with prenatal alcohol exposure may display a wide range of emotional and behavioral deficits identified using observational self-report rating scales (22) and experimentally induced paradigms such as the still face procedure (56, 60). These deficits include poor emotional regulation (51), emotional withdrawal (53), few social monitoring behaviors (60) increased irritability (48, 63), difficult temperament (49), passive behavior and lack of social engagement (22, 59).
Prenatal alcohol exposed children at-risk for developmental delays, or who meet the diagnostic classification for FAS (17, 66), are those whose mothers consumed alcohol frequently, for prolonged periods, at moderate-to-heavy doses (46, 72), and binges (51). However, there is evidence that some functions related to information processing such as reaction time may be sensitive to much lower doses (i.e., doses equivalent to 1 drink per day) of exposure (54) and are observable at this early age. Other aspects of information processing such as fixation duration measured on a visual recognition memory paradigm (111) appear more apparent when infants are exposed to heavy frequent exposure (50, 55) as opposed to heavy infrequent exposure such as during occasional binge drinking (52). Thus, while the overall pattern of results is indicative of a dose-response relationship between prenatal alcohol exposure and developmental outcomes, research gaps remain in identifying whether there is a threshold level of effects associated maternal drinking during pregnancy (112).
Effects of Prenatal Alcohol Exposure on Toddler Development
A number of studies investigated outcomes associated with prenatal alcohol exposure within this age group. Although the majority of studies demonstrate poorer cognitive scores in toddlers with prenatal alcohol exposure (65, 77, 113), developmental assessment findings on toddlers with prenatal alcohol exposure compared to their unexposed counterparts are not entirely consistent in the literature (see 48, 114). Findings demonstrating no effects of prenatal alcohol exposure on developmental outcomes were more common in high income countries where children are exposed to fewer risk factors (114) than in settings where FASDs are prevalent (12). Alcohol consumption patterns in these contexts are also less detrimental to fetal health than in contexts where heavy, binge drinking on weekends is common (115, 116).
Conducting studies on 2–3 year olds when key and more complex domains start developing broadens the range of deficits that can be assessed in children with prenatal alcohol exposure. Identifying developmental delays related to prenatal alcohol exposure amongst children during this period may allow clinicians to identify children who are “at risk” and may benefit from interventions targeted the specific developmental outcomes that they may struggle with at school age. A greater proportion of literature on 2–3 years olds report domain specific findings such as gross motor (81) fine motor (76, 81), and behavioral problems such as hyperactivity, difficulty managing behavior, and tantrums (78, 82) in 3 year olds with prenatal alcohol exposure. Faden and Graubard (78) found that behavioral problems in their sample were present despite there being no other effects of prenatal alcohol exposure on children's developmental indices. It is possible that carer-observed behavioral problems associated with prenatal alcohol exposure may be easier to identify than overall developmental problems which are in comparison less apparent and may require clinical expertise to formally assess.
Regarding the impact of prenatal alcohol syndrome on language abilities, evidence is mixed and only two studies demonstrate that 2–3 year old children with prenatal alcohol exposure perform poorly on language tasks measuring expressive and comprehensive language abilities (77, 113). It is possible that these effects are dose specific where lower levels of alcohol exposure are more subtle and difficult to detect on language tests amongst this age group as results from studies on toddlers suggest that language delays are not apparent in children with lower levels of exposure (< 1 drink on a weekly basis or less) (24) but are more evident in children with binge pattern exposure (24) and longer durations of exposure (77).
Some research also demonstrated poorer language outcomes in both controls and alcohol exposed infants from low socioeconomic environments, suggesting that poorer language outcomes may result from post-natal environmental factors rather than the teratogenic effects of alcohol on language functioning (76). There is evidence on the strong negative effect of low maternal education and SES in South African cohorts in older children (25). Other research suggests that language deficits in children with prenatal alcohol exposure is in keeping with children's general intellectual functioning, and the effects of prenatal alcohol exposure on language are no longer evident when IQ is controlled (83). Since higher functions have not yet emerged during these early years, research conducted on young children may not be able to establish whether the impairments amongst children with prenatal alcohol exposure are a product of generalized cognitive difficulties (measured by Intelligent Quotient testing) or occur independent of children's overall IQ (31). Findings from child data does, however does provide guidance to researchers interested in the trajectories of prenatal alcohol exposure regarding development domains that should receive focus at later time-points.
Regardless of the underlying nature of the language difficulties amongst children with FASDs, language impairments amongst children exposed to alcohol prenatally are likely to impact other aspects of their functioning such as their working memory (117) abilities and their social and interpersonal abilities (118). Impaired language abilities may be especially significant in inhibiting the adaptive functioning of children with prenatal alcohol exposure during the pre-school period where children are prepared for the transition to school. Thus, language functioning amongst young children with prenatal alcohol exposure remains an important area of investigation.
Effects of Prenatal Alcohol Exposure on Preschool Age Development
A substantial body of literature has investigated a wide range of outcomes associated with prenatal alcohol exposure amongst preschool age children. Findings on general development in 4–5 year old children with prenatal alcohol exposure are variable. Whilst some studies indicate lower IQ scores on the WPSSI in children with prenatal alcohol exposure (87, 89), an equal number of studies did not find any differences in IQ scores between those with and those without prenatal alcohol exposure (61, 97). Falgreen Eriksen, Mortensen (97), however, found that risk for poor performance was dose-dependent and children with higher levels of exposure (i.e., mothers who consumed 9 or more drinks per week) was associated with lower IQ scores. Investigations that used the McCarthy Scales of Children's Abilities appear more consistent and demonstrate lower scores in the cognitive index (91) and overall scores (47) in children prenatally exposed to alcohol and children diagnosed with FAS (103).
Regarding the effects of prenatal alcohol exposure on motor functioning, some studies suggest that social drinking (i.e., consuming 1 drink per day) (84), but not isolated episodes of binge drinking (i.e., consuming 5 or more drinks per occasion), (95) is associated with poorer gross and fine motor abilities. Deficits in attention, social and behavioral and executive functioning that are well-established in older children with prenatal alcohol exposure also appear prominent amongst preschool aged children with prenatal alcohol exposure (119, 120). Studies that investigated social and behavior functioning demonstrate greater negative affect (92, 100), emotional and conduct problems (101), and greater frequency of insecure attachment (100) amongst children with prenatal alcohol exposure and a greater frequency of behavioral problems in children with FAS (103).
Emerging executive functioning difficulties in children with prenatal alcohol exposure include lack of inhibition [measured in a tapping task (88), cognitive flexibility, and delay of gratification (102)]. These difficulties along with poorer sustained attention (85) and choice reaction time (104) amongst children with prenatal alcohol exposure may play a key role in the adaptive functioning difficulties evident in children and adolescents with prenatal alcohol exposure (121, 122). Cognitive and behavioral difficulties during 4–5 years of age are a key limitation in children's ability to cope with the demands of the pre-school curriculum, which in turn negatively impacts their ability to cope with the cognitive and social demands of the schooling environment. The presence of young children with FASDs and cognitive and behavioral difficulties are especially challenging in resource-limited regions with high prevalence rates of FASDs that are heavily burdened by the effects of impoverished conditions and have (17). It is imperative that early interventions to ameliorate the effects of prenatal alcohol exposure are suitable and feasible for low-income contexts where the majority of cases of prenatal alcohol exposure are present.
Longitudinal Investigations on the Effects of Prenatal Alcohol Exposure on Early Development
A number of papers within this review comprised of cross-sectional analyses from longitudinal studies (17, 44, 51, 123, 124). Longitudinal studies have been conducted in various population groups including both middle class (38) and low income groups (86, 125) These studies highlight the enduring effects of prenatal alcohol exposure through the duration of childhood and provide key information on how socio economic factors may interact with the effects of alcohol exposure in impacting children's developmental trajectories (29). They also highlight the complexities in identifying the effects of prenatal alcohol exposure over the developmental trajectory and the need for early intervention that mitigates the negative enduring effects of prenatal alcohol exposure. Given that the bulk of longitudinal investigation have been conducted in North American contexts, relatively less is known on the longitudinal outcomes associated with prenatal alcohol exposure in lower-middle income contexts.
Limitations and Directions for Future Research
Studies in this review demonstrate clear global impairment related to FAS (17, 29, 66, 102, 103) but several inconsistencies in research evidence regarding the global and specific effects of low to moderate alcohol exposure on early development (34, 58, 61, 71, 74, 80, 86, 95). In order to gain clarity on the effects of light drinking during pregnancy on child developmental outcomes, further research should aim to address the potential methodological challenges that may contribute to these inconsistent findings on the effects of low doses of prenatal alcohol exposure on child development.
Findings indicative of null effects related to prenatal alcohol exposure appear prevalent in studies where participants report low levels of alcohol exposure (61, 79). It is possible that the developmental tools commonly used to identify global developmental delays common in children with heavier exposure/diagnoses of FAS are less sensitive in detecting the specific effects of prenatal alcohol exposure commonly associated with lower levels of exposure (69, 126). Given that some domains are sensitive to low doses of exposure (69), further research should investigate the suitability of tools that provide a more detailed assessment on aspects of functioning (such as behavior and language) that are less easily detected on general functioning tests (78).
In population-based studies where negative outcomes associated with prenatal alcohol exposure have been reported, studies have frequently reported on a small proportion of participants with moderate to severe prenatal alcohol exposure and are thus often unable to reliably measure the outcomes associated with various doses of alcohol exposure. Given that drinking during pregnancy is heavily stigmatized, participants within these studies may be likely to underreport their alcohol consumption. In addition, studies often lack detailed information on the potential confounding effects of sociodemographic and psychosocial factors that play a key role when investigating the outcomes related to prenatal alcohol exposure. For example, further investigation suggests that the better neurodevelopmental outcomes in children with prenatal alcohol exposure compared to controls in the sample assessed by Bay and colleagues (94) may likely be due to increased socioeconomic protective factors (e.g., higher maternal education levels) amongst the prenatal alcohol exposure group (127). Better understanding the contribution of the role of confounding factors on the outcomes related to prenatal alcohol exposure may help understand some of the inconsistencies in research studies published to date.
The pattern of some inconsistencies in the findings described in this review suggest that outcomes related to prenatal alcohol exposure may be more apparent at particular stages of early development. For example, Fried and colleagues research demonstrate that findings evident in their sample at 36 months were no longer evident at 48 or 60 months (79, 93). In addition the inconsistent findings on the effects of prenatal alcohol exposure on IQ findings amongst preschool age children may suggest that the negative effects of prenatal alcohol exposure on IQ functioning are more identifiable when children reach school age when cognitive demands are higher (128). Furthermore, longitudinal findings also suggest that developments tools such as the BSID may be less able to detect less apparent effects of prenatal alcohol exposure at certain developmental periods (126). Similarly, given that decreased IQ scores amongst school-aged children with prenatal alcohol exposure in comparison to healthy controls are common, current IQ tools may be limited in establishing children's IQ abilities during the pre-school years (21). These studies suggests the importance of investigating the timing during development at which specific developmental abnormalities can be identified and highlight the importance of understanding the trajectory of the developmental outcomes related to prenatal alcohol exposure.
Research that investigates the longitudinal trajectory of prenatal alcohol exposure demonstrate that the negative effects of prenatal alcohol exposure tend to endure as cognitive demands increase over childhood (123, 124). Recent experimental findings in rodents demonstrating the persistent effects of binge-type alcohol exposure on pyramidal neurons in the somatosensory cortex support these findings (129). Deficits in developmental domains identified earlier in infancy may also compound the development of other developmental domains. For example, the poor attention span present during infancy (55, 73) impacts the development of early executive functioning abilities that often require sustained attention as a prerequisite (102). The developmental effects of prenatal alcohol exposure may be exacerbated by risk factors related to low socio economic status (e.g., poor nutrition, lack of stimulation, exposure to violence) (17, 29). These risk factors related to the postnatal environment however, may mask the effects of the teratogenic influences of alcohol exposures and thus add to the methodological challenges in investigating the effects of prenatal alcohol exposure on developmental outcomes. The increased occurrence of null findings in older ages groups in this review supports the possibility of socioeconomic influences masking the differences between children prenatally exposed to alcohol and matched controls.
Future longitudinal research is warranted to address the paucity of research conducted in low income settings to improve our understanding of the interaction between prenatal alcohol exposure and potential socioeconomic factors on developmental outcomes (130). Understanding how these outcomes manifest during early childhood will be crucial in designing interventions that are feasible in low-income settings and able to minimize the enduring effects of prenatal alcohol exposure on adaptive functioning throughout childhood (121, 122). Further longitudinal investigations that investigates the early developmental trajectories outcomes related to prenatal alcohol may also provide an important direction for research that aims to delineate a cognitive profile of FASDs (31, 131).
In comparison to knowledge on the cognitive and behavioral developmental outcomes, there is less knowledge on other aspects of development that are influenced by maternal alcohol consumption during pregnancy. Exposure to alcohol in-utero affects multiple organs and children with prenatal alcohol exposure are at increased risk for developing comorbid conditions including hearing impairments (132), visual impairments (133), and sensory processing disorders (134). These disorders further hinder children's developmental potential and results in difficulties coping with the school environment (135), thus further research on these comorbid conditions will be highly beneficial to improving long-term outcomes amongst children with FASDs. In addition while some studies within this review reported growth outcomes amongst their samples (17, 29, 52), the majority of studies did not include child growth outcomes in their analyses of developmental outcomes. Since growth restrictions may not always be present in children with prenatal alcohol exposure (136) and growth restrictions in young children may be a result of many factors other than prenatal alcohol exposure (e.g., nutrition status and genetics), poorer growth outcomes alone may be insufficient to identify children who may have been exposed to alcohol in-utero. Regardless, understanding the growth trajectory of children exposed to alcohol in-utero may be of clinical value as findings demonstrate that growth trajectories may serve as an important biomarker to identify children at greatest risk for the cognitive deficits associated with prenatal alcohol exposure (51, 137).
Another key avenue for further research is to explore where alcohol consumption during pregnancy affects males and females differently. Studies within this review suggests that girls exposed to alcohol in-utero are more vulnerable to the effects of prenatal alcohol exposure (82), and that patterns of stress reactivity differ between girls and boys with prenatal alcohol exposure (56). Although several animal studies have explored the effects of prenatal alcohol exposure on males and females, this area of research has been less investigated in humans (138, 139). Given that these findings have implications in the diagnosis and intervention of FASD's, further research should aim to clarify and further understand these findings.
Although this review followed a systematic approach, it has several methodological limitations. First, several studies within this review (e.g., birth cohort studies) drew their data from the same pool of participants and findings from those samples may be repeated and overrepresented within this review. Although evidence from longitudinal studies is valuable in tracking the developmental manifestations of prenatal alcohol exposure throughout development, a significant proportion of evidence on the effects of prenatal alcohol exposure within this review comes from investigations with potential overlap between samples. Second, heterogeneity in the design and methodologies of studies included in this review limits our ability to interpret results across cohorts and studies and in being able to fully interpret inconsistencies this review. For example, due to cultural variations in early language acquisitions and challenges in interpreting outcomes on translation tests, findings on the effects of prenatal alcohol exposure on language outcomes, are difficult to compare and interpret (140). In comparison to neonatal studies, studies on infants and older age groups have greater heterogeneity due to a wider range of methodologies used to assess developmental outcomes. Finally, current findings are also difficult to interpret as studies use a wide range of systems to classify alcohol exposure. Greater standardization in the classification of alcohol exposure may aid the understanding of the developmental outcomes associated with prenatal alcohol exposure in infants, particularly in ascertaining the effects associated with low and moderate doses of alcohol use during pregnancy. The use of biomarkers may provide an objective means to identify infants exposed to lower doses alcohol in-utero (141).
In sum, studies within this review highlight the complex relationship between dose of prenatal alcohol exposure and its various associated developmental outcomes. This review shows that the cognitive and behavioral effects of prenatal alcohol exposure seen in older age groups are apparent during the early childhood period. The specific outcomes of lower to moderate doses prenatal alcohol exposure on specific outcomes such as early language development remains poorly understood. Further research should aim to clarify the more subtle manifestations of prenatal alcohol exposure on early development as well as core early deficits which predict later functional outcomes.
Author Contributions
SS selected the literature, extracted data, and compiled the first draft of the manuscript. EE assisted with the literature search, data extraction, and provided critical review of the manuscript. KD conceptualized the review and provided critical review of the manuscript. CA and DS provided critical review of manuscript drafts. All authors approved the final draft.
Funding
The authors receive support from the South African NRF and MRC, by the NIAAA via (R21AA023887) and by the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD) developmental grant (U24 AA014811).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Jones K, Smith D. Recognition of the fetal alcohol syndrome in early infancy. Lancet (1973) 302:999–1001. doi: 10.1016/S0140-6736(73)91092-1
2. Riley EP, Infante MA, Warren KR. Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev. (2011) 21:73–80. doi: 10.1007/s11065-011-9166-x
3. Jones K, Smith D, Ulleland C, Streissguth A. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet (1973) 301:1267–71. doi: 10.1016/S0140-6736(73)91291-9
4. Mattson SN, Ph D, Roesch SC, Ph D, Fagerlund Å, Autti I, et al. Towards a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res. (2011) 34:1640–50. doi: 10.1111/j.1530-0277.2010.01250.x
5. Hoyme HE, Kalberg WO, Elliott AJ, Blankenship J, Buckley D, Marais A-S, et al. Updated clinical guidelines for diagnosing fetal alcohol spectrum disorders. Pediatrics (2016) 138:e20154256. doi: 10.1542/peds.2015-4256
6. May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, et al. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. (2009) 15:176–92. doi: 10.1002/ddrr.68
7. May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, et al. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics (2014) 134:855–66. doi: 10.1542/peds.2013-3319
8. Popova S, Lange S, Shield K, Mihic A, Chudley AE, Mukherjee RAS, et al. Comorbidity of fetal alcohol spectrum disorder: a systematic review and meta-analysis. Lancet (2016) 387:978–87. doi: 10.1016/S0140-6736(15)01345-8
9. Roozen S, Peters GJY, Kok G, Townend D, Nijhuis J, Curfs L. Worldwide prevalence of fetal alcohol spectrum disorders: A systematic literature review including meta-analysis. Alcohol Clin Exp Res. (2016) 40:18–32. doi: 10.1111/acer.12939
10. Olivier L, Viljoen D, Curfs L. Fetal alcohol spectrum disorders: prevalence rates in South Africa: the new millennium. South African Med J. (2016) 106(Suppl. 1):103–6. doi: 10.7196/SAMJ.2016.v106i6.11009
11. May PA, de Vries MM, Marais A-S, Kalberg WO, Adnams CM, Hasken JM, et al. The continuum of fetal alcohol spectrum disorders in four rural communities in South Africa: Prevalence and characteristics. Drug Alcohol Depend. (2016) 159:207–18. doi: 10.1016/j.drugalcdep.2015.12.023
12. May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Barnard R, et al. Approaching the prevalence of the Full Spectrum of Fetal Alcohol Spectrum Disorders in a South African population-based study. Alcohol Clin Exp Res. (2013) 37:818–30. doi: 10.1111/acer.12033
13. Urban MF, Olivier L, Viljoen D, Lombard C, Louw JG, Drotsky LM, et al. Prevalence of fetal alcohol syndrome in a South African city with a predominantly Black African population. Alcohol Clin Exp Res. (2015) 39:1016–26. doi: 10.1111/acer.12726
14. Lupton C, Burd L, Harwood R editors. Cost of fetal alcohol spectrum disorders. Am J Med Genet C Semin Med Genet. (2004) 127C:42–50. doi: 10.1002/ajmg.c.30015
15. Popova S, Lange S, Burd L, Rehm J. Burden and social cost of fetal alcohol spectrum disorders. In: Oxford Handbooks Online. New York, NY (2016). doi: 10.1093/oxfordhb/9780199935291.013.78
16. Abuse CoS, Disabilities CoCw. Fetal alcohol syndrome and alcohol-related neurodevelopmental disorders. Pediatrics (2000) 106:358–61. doi: 10.1542/peds.106.2.358
17. Davies L, Dunn M, Chersich M, Urban M, Chetty C, Olivier L, et al. Developmental delay of infants and young children with and without fetal alcohol spectrum disorder in the Northern Cape Province, South Africa. African J Psychiatry (2011) 14:298–305. doi: 10.4314/ajpsy.v14i4.7
18. Streissguth AP, Sampson PD, Barr HM. Neurobehavioral dose-response effects of prenatal alcohol exposure in humans from infancy to adulthood. Annal NY Acad Sci. (1989) 562:145–58. doi: 10.1111/j.1749-6632.1989.tb21013.x
19. Gray R, Mukherjee RA, Rutter M. Alcohol consumption during pregnancy and its effects on neurodevelopment: what is known and what remains uncertain. Addiction (2009) 104:1270–3. doi: 10.1111/j.1360-0443.2008.02441.x
20. Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: Neuropsychological and behavioral features. Neuropsychol Rev. (2011) 21:81–101. doi: 10.1007/s11065-011-9167-9
21. Flak AL, Su S, Bertrand J, Denny CH, Kesmodel US, Cogswell ME. The association of mild, moderate, and binge prenatal alcohol exposure and child neuropsychological outcomes: a meta-analysis. Alcohol Clin Exp Res. (2014) 38:214–26. doi: 10.1111/acer.12214
22. Coles CD, Kable JA, Keen CL, Jones KL, Wertelecki W, Granovska IV, et al. Dose and timing of prenatal alcohol exposure and maternal nutritional supplements: developmental effects on 6-month-old infants. Mater Child Health J. (2015) 19:2605–14. doi: 10.1007/s10995-015-1779-x
23. Jacobson JL, Jacobson SW. Prenatal alcohol exposure and neurobehavioral development: where is the threshold? Alcohol Res Health (1994) 18:30.
24. O'Leary C, Zubrick SR, Taylor CL, Dixon G, Bower C. Prenatal alcohol exposure and language delay in 2-year-old children: the importance of dose and timing on risk. Pediatrics (2009) 123:547–54. doi: 10.1542/peds.2008-0459
25. May P, Tabachnick B, Gossage J. Maternal factors predicting cognitive and behavioral characteristics of children with fetal alcohol spectrum disorders. J Dev Behav Pediatr. (2013) 34:314–25. doi: 10.1097/DBP.0b013e3182905587
26. Chiodo LM, da Costa DE, Hannigan JH, Covington CY, Sokol RJ, Janisse J, et al. The impact of maternal age on the effects of prenatal alcohol exposure on attention. Alcohol Clin Exp Res. (2010) 34:1813–21. doi: 10.1111/j.1530-0277.2010.01269.x
27. May PA, Gossage JP, Marais A-S, Hendricks LS, Snell CL, Tabachnick BG, et al. Maternal risk factors for fetal alcohol syndrome and partial fetal alcohol syndrome in South Africa: a third study. Alcohol Clin Exp Res. (2008) 32:738–53. doi: 10.1111/j.1530-0277.2008.00634.x
28. Warren KR, Li TK. Genetic polymorphisms: impact on the risk of fetal alcohol spectrum disorders. Birth Defects Res A Clin Mol Teratol. (2005) 73:195–203. doi: 10.1002/bdra.20125
29. Davies L-A, Cockcroft K, Olinger L, Chersich M, Urban M, Chetty Makkan CM, et al. Alcohol exposure during pregnancy altered childhood developmental trajectories in a rural South African community. Acta Paediatr. (2017) 106:1802–10. doi: 10.1111/apa.13978
30. Abel EL, Hannigan JH. Maternal risk factors in fetal alcohol syndrome: provocative and permissive influences. Neurotoxicol Teratol.. (1995) 17:445–62. doi: 10.1016/0892-0362(95)98055-6
31. Kodituwakku PW. Neurocognitive profile in children with fetal alcohol spectrum disorders. Dev Disabil Res Rev. (2009) 15:218–24. doi: 10.1002/ddrr.73
32. Burd L, Cotsonas-Hassler TM, Martsolf JT, Kerbeshian J. Recognition and management of fetal alcohol syndrome. Neurotoxicol Teratol.. (2003) 25:681–8. doi: 10.1016/j.ntt.2003.07.020
33. Testa M, Quigley BM, Eiden RD. The effects of prenatal alcohol exposure on infant mental development: a meta-analytical review. Alcohol Alcohol. (2003) 38:295–304. doi: 10.1093/alcalc/agg087
34. Valenzuela CF, Morton RA, Diaz MR, Topper L. Does moderate drinking harm the fetal brain? Insights from animal models. Trends Neurosci. (2012) 35:284–92. doi: 10.1016/j.tins.2012.01.006
35. Britto PR, Lye SJ, Proulx K, Yousafzai AK, Matthews SG, Vaivada T, et al. Nurturing care: promoting early childhood development. Lancet (2017) 389:91–102. doi: 10.1016/S0140-6736(16)31390-3
36. Henderson J, Kesmodel U, Gray R. Systematic review of the fetal effects of prenatal binge-drinking. J Epidemiol Commun Health. (2007) 61:1069–73. doi: 10.1136/jech.2006.054213
37. Bay B, Kesmodel US. Prenatal alcohol exposure - A systematic review of the effects on child motor function. Acta Obstetr Gynecol Scand. (2011) 90:210–26. doi: 10.1111/j.1600-0412.2010.01039.x
38. Staisey NL, Fried PA. Relationships between Moderate Maternal Alcohol Consumption during pregnancy and infant neurological development. J Stud Alcohol. (1983) 44:262–70.
39. Smith IE, Coles CD, Lancaster J, Fernhoff PM. The effect of volume and duration of prenatal ethanol exposure on neonatal physical and behavioral development. Neurobehav Toxicol Teratol. (1986) 8:375–81.
40. Coles CD, Smith IE, Falek A. Prenatal alcohol exposure and infant behavior: immediate effects and implications for later development. Adv Alcohol Subs Abuse (1987) 6:87–104. doi: 10.1300/J251v06n04_07
41. Coles CD, Smith IE, Lancaster JS, Falek A. Persistence over the first month of neurobehavioral differences in infants exposed to alcohol prenatally. Infant Behav Dev. (1987) 10:23–37. doi: 10.1016/0163-6383(87)90004-X
42. Oberlander TF, Jacobson SW, Weinberg J, Grunau RE, Molteno CD, Jacobson JL. Prenatal alcohol exposure alters biobehavioral reactivity to pain in newborns. Alcohol Clin Exp Res. (2010) 34:681–92. doi: 10.1111/j.1530-0277.2009.01137.x
43. Fried PA, Watkinson B, Dillon RF, Dulberg CS. Neonatal neurological status in a low-risk population after prenatal exposure to cigarettes, marijuana, and alcohol. J Dev Behav Pediatr. (1987) 8:318–26. doi: 10.1097/00004703-198712000-00003
44. Fried PA, Makin J. Neonatal behavioural correlates of prenatal exposure to marihuana, cigarettes and alcohol in a low risk population. Neurotoxicol Teratol. (1987) 9:1–7. doi: 10.1016/0892-0362(87)90062-6
45. Streissguth AP, Barr HM, Martin DC. Maternal alcohol use and neonatal habituation assessed with the Brazelton scale. Child Dev. 1983:1109–18. doi: 10.2307/1129667
46. Autti-Rämö I, Granström ML. The psychomotor development during the first year of life of infants exposed to intrauterine alcohol of various duration*-fetal alcohol exposure and development. Neuropediatrics (1991) 22:59–64. doi: 10.1055/s-2008-1071418
47. Ioffe S, Chernick V. Prediction of subsequent motor and mental retardation in newborn infants exposed to alcohol in utero by computerized EEG analysis. Neuropediatrics (1990) 21:11–7. doi: 10.1055/s-2008-1071450
48. Lemola S, Stadlmayr W, Grob A. Infant irritability: the impact of fetal alcohol exposure, maternal depressive symptoms, and low emotional support from the husband. Infant Ment Health J. (2009) 30:57–81. doi: 10.1002/imhj.20203
49. Alvik A, Torgersen AM, Aalen OO, Lindemann R. Binge alcohol exposure once a week in early pregnancy predicts temperament and sleeping problems in the infant. Early Hum Dev. (2011) 87:827–33. doi: 10.1016/j.earlhumdev.2011.06.009
50. Kable Ja, Coles CD. The impact of prenatal alcohol exposure on neurophysiological encoding of environmental events at six months. Alcohol Clin Exp Res. (2004) 28:489–96. doi: 10.1097/01.ALC.0000117837.66107.64
51. Coles CD, Kable JA, Drews-Botsch C, Falek A. Early identification of risk for effects of prenatal alcohol exposure. J Stud Alcohol (2000) 61:607–16. doi: 10.15288/jsa.2000.61.607
52. Fraser SL, Muckle G, Abdous BB, Jacobson JL, Jacobson SW. Effects of binge drinking on infant growth and development in an Inuit sample. Alcohol (2012) 46:277–83. doi: 10.1016/j.alcohol.2011.09.028
53. Molteno CD, Jacobson JL, Carter RC, Dodge NC, Jacobson SW. Infant emotional withdrawal: A precursor of affective and cognitive disturbance in fetal alcohol spectrum disorders. Alcohol Clin Exp Res. (2014) 38:479–88. doi: 10.1111/acer.12240
54. Jacobson SW, Jacobson JL, Sokol RJ. Effects of fetal alcohol exposure on infant reaction time. Alcohol Clin Exp Res. (1994) 18:1125–32. doi: 10.1111/j.1530-0277.1994.tb00092.x
55. Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Ager JW. Prenatal alcohol exposure and infant information processing ability. Child Dev. (1993) 64:1706–21. doi: 10.2307/1131464
56. Haley DW, Handmaker NS, Lowe J. Infant stress reactivity and prenatal alcohol exposure. Alcohol Clin Exp Res. (2006) 30:2055–64. doi: 10.1111/j.1530-0277.2006.00251.x
57. Streissguth AP, Barr HM, Martin DC, Herman CS. Effects of maternal alcohol, nicotine, and caffeine use during pregnancy on infant mental and motor development at eight months. Alcohol Clin Exp Res. (1980) 4:152–64. doi: 10.1111/j.1530-0277.1980.tb05630.x
58. Richardson GA, Day NL, Goldschmidt L. Prenatal alcohol, marijuana, and tobacco use: Infant mental and motor development. Neurotoxicol Teratol. (1995) 17:479–87. doi: 10.1016/0892-0362(95)00006-D
59. Brown CW, Olson HC, Croninger RG. Maternal alcohol consumption during pregnancy and infant social, mental, and motor development. J Early Intervent. (2010):110–26. doi: 10.1177/1053815110366654
60. Jirikowic T, Chen M, Nash J, Gendler B, Olson HC. Regulatory behaviors and stress reactivity among infants at high risk for fetal alcohol spectrum disorders: an exploratory study. J Mental Health Res. Intellect Disabil. (2016) 9:171–88. doi: 10.1080/19315864.2016.1183246
61. Greene T, Ernhart CB, Ager J, Sokol R, Martier S, Boyd T. Prenatal alcohol exposure and cognitive development in the preschool years. Neurotoxicol Teratol. (1991) 13:57–68. doi: 10.1016/0892-0362(91)90028-U
62. O'Connor MJ, Sigman M, Brill N. Disorganization of attachment in relation to maternal alcohol consumption. J Consul Clin Psychol. (1987) 55:831–6. doi: 10.1037/0022-006X.55.6.831
63. O'Connor MJ, Sigman M, Kasari C. Interactional model for the association among maternal alcohol use, mother-infant interaction, and infant cognitive development. Infant Behav Dev. (1993) 16:177–92. doi: 10.1016/0163-6383(93)80016-2
64. Seagull FN, Mowery JL, Simpson PM, Robinson TR, Martier SS, Sokol RJ, et al. Maternal assessment of infant development: associations with alcohol and drug use in pregnancy. Clin Pediatric. (1996) 35:621–8. doi: 10.1177/000992289603501203
65. Fried PA, Watkinson B. 12-and 24-month neurobehavioural follow-up of children prenatally exposed to marihuana, cigarettes and alcohol. Neurotoxicol Teratol. (1988) 10:305–13. doi: 10.1016/0892-0362(88)90032-3
66. Golden NL, Sokol RJ, Kuhnert BR, Bottoms S. Maternal alcohol use and infant development. Pediatrics (1982) 70:931–4.
67. Molteno CD, Jacobson SW, Carter RC, Jacobson JL. Infant symbolic play as an early indicator of fetal alcohol-related deficit. Infancy (2010) 15:586–607. doi: 10.1111/j.1532-7078.2010.00031.x
68. Gusella JL, Fried PA. Effects of maternal social drinking and smoking on offspring at 13 months. Neurobehav Toxicol Teratol. (1983) 6:13–7.
69. Jacobson JL, Jacobson SW, Sokol RJ, Martier SS, Ager JW, Kaplan-Estrin MG. Teratogenic effects of alcohol on infant development. Alcohol Clin Exp Res. (1993) 17:174–83. doi: 10.1111/j.1530-0277.1993.tb00744.x
70. Forrest F, Florey CD, Taylor D, McPherson F, Young JA. Reported social alcohol consumption during pregnancy and infants' development at 18 months. Bmj. (1991) 303:22–6. doi: 10.1136/bmj.303.6793.22
71. Parry GJ, Ogston SA. Child Development at Age 18 months. International Journal of Epidemiology. (1992) 21:S72–8. doi: 10.1093/ije/21.Supplement_1.S72
72. Autti-Rämö I, Granström ML. The effect of intrauterine alcohol exposition in various durations on early cognitive development. Neuropediatrics (1991) 22:203–10. doi: 10.1055/s-2008-1071442
73. Larsson G, Bohlin AB, Tunell R. Prospective study of children exposed to variable amounts of alcohol in utero. Arch Dis Child. (1985) 60:316–21. doi: 10.1136/adc.60.4.316
74. Greene T, Ernhart CB, Martier S, Sokol R, Ager J. Prenatal alcohol exposure and language development. Alcohol Clin Exp Res. (1990) 14:937–45. doi: 10.1111/j.1530-0277.1990.tb01842.x
75. Robinson M, Oddy WH, McLean NJ, Jacoby P, Pennell CE, Klerk NHD, et al. Low – moderate prenatal alcohol exposure and risk to child behavioural development: a prospective cohort study. BJOG (2010):1139–52. doi: 10.1111/j.1471-0528.2010.02596.x
76. Kaplan-Estrin M, Jacobson SW, Jacobson JL. Neurobehavioral effects of prenatal alcohol exposure at 26 Months. Neurotoxicol Teratol. (1999) 21:503–11. doi: 10.1016/S0892-0362(99)00031-8
77. Autti-Rämö I, Korkman M, Hilakivi-Clarke L, Lehtonen M, Halmensmäki E, Granström ML. Mental development of 2-year-old children exposed to alcohol in utero. The Journal of pediatrics. (1992) 120:740–6. doi: 10.1016/S0022-3476(05)80237-9
78. Faden VB, Graubard BI. Maternal substance use during pregnancy and developmental outcome at age three. J Subs Abuse (2000) 12:329–40. doi: 10.1016/S0899-3289(01)00052-9
79. Fried PA, Watkinson B. 36- and 48-month neurobehavioral follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol. J Dev Behav Pediatr. (1990) 11:49–58.
80. Olsen J. Effects of moderate alcohol consumption during pregnancy on child development at 18 and 42 months. Alcohol Clin Exp Res. (1994) 18:1109–13. doi: 10.1111/j.1530-0277.1994.tb00089.x
81. Kalberg WO, Provost B, Tollison SJ, Tabachnick BG, Robinson LK, Eugene Hoyme H, et al. Comparison of motor delays in young children with fetal alcohol syndrome to those with prenatal alcohol exposure and with no prenatal alcohol exposure. Alcohol Clin Exp Res. (2006) 30:2037–45. doi: 10.1111/j.1530-0277.2006.00250.x
82. Sayal K, Heron J, Golding J, Alati R, Smith GD, Gray R, et al. Binge pattern of alcohol consumption during pregnancy and childhood mental health outcomes: longitudinal population-based study. Pediatrics (2009) 123:e289–e96. doi: 10.1542/peds.2008-1861
83. McGee CL, Bjorkquist OA, Riley EP, Mattson SN. Impaired language performance in young children with heavy prenatal alcohol expsosure. Neurotoxicol Teratol. (2009) 70:646–56. doi: 10.1016/j.ntt.2008.09.004
84. Barr HM, Darby BL, Sampson PD. Prenatal Exposure to Alcohol, Caffeine, Tobacco, and Aspirin: Effects on Fine and Gross Motor Performance in 4-Year-Old Children. (1990) 26:339–48.
85. Streissguth AP, Martin DC, Barr HM, Sandman BM, Kirchner GL, Darby BL. Intrauterine alcohol and nicotine exposure: attention and reaction time in 4-year-old children. Dev Psychol. (1984) 20:533–41. doi: 10.1037/0012-1649.20.4.533
86. Boyd TA, Ernhart CB, Greene TH, Sokol RJ, Martier S. Prenatal alcohol exposure and sustained attention in the preschool years. Neurotoxicol Teratol. (1991) 13:49–55. doi: 10.1016/0892-0362(91)90027-T
87. Streissguth AP, Ban HM, Sampson PD, Darby BL, Martin DC, Robinson NM, et al. IQ at Age 4 in relation to maternal alcohol use and smoking during pregnancy. Dev Psychol. (1989) 25:3–11.
88. Noland JS, Singer LT, Arendt RE, Minnes S, Short EJ, Bearer CF. Executive functioning in preschool-age children prenatally exposed to alcohol, cocaine, and marijuana. Alcohol Clin Exp Res. (2008) 27:647–56. doi: 10.1097/01.ALC.0000060525.10536.F6
89. Chiodo LM, Janisse J, Delaney-Black V, Sokol RJ, Hannigan JH. A metric of maternal prenatal risk drinking predicts neurobehavioral outcomes in preschool children. Alcohol Clin Exp Res. (2009) 33:634–44. doi: 10.1111/j.1530-0277.2008.00878.x
90. Landesman-Dwyer S. Maternal drinking and pregnancy outcome. Appl Res Mental Retard. (1982) 3:241–63. doi: 10.1016/0270-3092(82)90018-2
91. Larroque B, Kaminski M, Dehaene P, Subtil D, Delfosse M-J, Querleu D. Moderate prenatal alcohol exposure and psychomotor development at preschool age. Am J Public Health (1995) 85:1654–61. doi: 10.2105/AJPH.85.12.1654
92. O'Connor MJ, Paley B. The relationship of prenatal alcohol exposure and the postnatal environment to child depressive symptoms. J Pediatr Psychol. (2006) 31:50–64. doi: 10.1093/jpepsy/jsj021
93. Fried PA, O'Connell CM, Watkinson B. 60- and 72-month follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol: cognitive and language assessment. J Dev Behav Pediatr. (1992) 13:383–91. doi: 10.1097/00004703-199212000-00001
94. Bay B, Støvring H, Wimberley T, Denny CH, Mortensen EL, Eriksen HLF, et al. Low to moderate alcohol intake during pregnancy and risk of psychomotor deficits. Alcohol Clin Exp Res. (2012) 36:807–14. doi: 10.1111/j.1530-0277.2011.01657.x
95. Kesmodel US, Bay B, Wimberley T, Eriksen HLF, Mortensen EL. Does binge drinking during early pregnancy increase the risk of psychomotor deficits? Alcohol Clin Exp Res. (2013) 37:1204–12. doi: 10.1111/acer.12072
96. Kesmodel US, Eriksen HLF, Underbjerg M, Kilburn TR, Støvring H, Wimberley T, et al. The effect of alcohol binge drinking in early pregnancy on general intelligence in children. BJOG (2012) 119:1222–31. doi: 10.1111/j.1471-0528.2012.03395.x
97. Falgreen Eriksen HL, Mortensen EL, Kilburn T, Underbjerg M, Bertrand J, Støvring H, et al. The effects of low to moderate prenatal alcohol exposure in early pregnancy on IQ in 5-year-old children. BJOG (2012) 119:1191–200. doi: 10.1111/j.1471-0528.2012.03394.x
98. Skogerbø Å, Kesmodel US, Denny CH, Kjaersgaard MIS, Wimberley T, Landrø NI, et al. The effects of low to moderate alcohol consumption and binge drinking in early pregnancy on behaviour in 5-year-old children: a prospective cohort study on 1628 children. BJOG (2013) 120:1042–50. doi: 10.1111/1471-0528.12208
99. Skogerbø Å, Kesmodel US, Wimberley T, Støvring H, Bertrand J, Landrø NI, et al. The effects of low to moderate alcohol consumption and binge drinking in early pregnancy on executive function in 5-year-old children. BJOG (2012) 119:1201–10. doi: 10.1111/j.1471-0528.2012.03397.x
100. O'Connor MJ, Kogan N, Findlay R. Prenatal alcohol exposure and attachment behavior in children. Alcohol Clin Exp Res. (2002) 26:1592–602. doi: 10.1111/j.1530-0277.2002.tb02460.x
101. Alvik A, Aalen OO, Lindemann R. Early fetal binge alcohol exposure predicts high behavioral symptom scores in 5.5-year-old children. Alcohol Clin Exp Res. (2013) 37:1954–62. doi: 10.1111/acer.12182
102. Fuglestad AJ, Whitley ML, Carlson SM, Boys CJ, Eckerle JK, Fink BA, et al. Executive functioning deficits in preschool children with Fetal Alcohol Spectrum Disorders. Child Neuropsychol. (2015) 21:716–31. doi: 10.1080/09297049.2014.933792
103. Janzen LA, Nanson JL, Block GW. Neuropsychological evaluation of preschoolers with fetal alcohol syndrome. Neurotoxicol Teratol. (1995) 17:273–9. doi: 10.1016/0892-0362(94)00063-J
104. Kilburn TR, Eriksen H-LF, Underbjerg M, Thorsen P, Mortensen EL, Landrø NI, et al. Low to moderate average alcohol consumption and binge drinking in early pregnancy: effects on choice reaction time and information processing time in five-year-old children. PLoS ONE (2015) 10:e0138611. doi: 10.1371/journal.pone.0138611
105. Underbjerg M, Kesmodel US, Landrø NI, Bakketeig L, Grove J, Wimberley T, et al. The effects of low to moderate alcohol consumption and binge drinking in early pregnancy on selective and sustained attention in 5-year-old children. BJOG (2012) 119:1211–21. doi: 10.1111/j.1471-0528.2012.03396.x
106. Kelly Y, Sacker A, Gray R, Kelly J, Wolke D, Quigley MA. Light drinking in pregnancy, a risk for behavioural problems and cognitive deficits at 3 years of age? Int J Epidemiol. (2008) 38:129–40. doi: 10.1093/ije/dyn230
107. Kelly YJ, Sacker A, Gray R, Kelly J, Wolke D, Head J, et al. Light drinking during pregnancy: still no increased risk for socioemotional difficulties or cognitive deficits at 5 years of age? J Epidemiol Commun Health. (2010) 66:41–8. doi: 10.1136/jech.2009.103002
108. Ogston SA, Parry GJ. EUROMAC. A European concerted action: maternal alcohol consumption and its relation to the outcome of pregnancy and child development at 18 months. Results–strategy of analysis and analysis of pregnancy outcome. Int J Epidemiol. (1992) (21 Suppl. 1):S45–71. doi: 10.1093/ije/21.Supplement_1.S45
109. Coles C, Kable JA, Coles CD. The impact of prenatal alcohol exposure on neurophysiological encoding of environmental events at six months. Alcohol Clin Exp Res. (2004) 28:489–96. doi: 10.1097/01.ALC.0000117837.66107.64·
110. Tronick EZ. The neonatal behavioral assessment scale as a biomarker of the effects of environmental agents on the newborn. Environ Health Perspect. (1987) 74:185. doi: 10.1289/ehp.8774185
111. Fagan JF, Singer LT. Infant recognition memory as a measure of intelligence. Adv Infan Res. (1983) 2:31–78.
112. Comasco E, Rangmar J, Eriksson U, Oreland L. Neurological and neuropsychological effects of low and moderate prenatal alcohol exposure. Acta Physiol. (2018) 222:e12892. doi: 10.1111/apha.12892
113. Fried PA, Watkinson B. 36- and 48-month neurobehavioral follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol. J Dev Behav Pediatr. (1990) 11:49–58. doi: 10.1097/00004703-199004000-00003
114. Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, et al. Child development: risk factors for adverse outcomes in developing countries. Lancet (2007) 369:145–57. doi: 10.1016/S0140-6736(07)60076-2
115. Abel EL. Fetal alcohol syndrome: The ‘American paradox.’ Alcohol Alcohol. (1998) 33:195–201. doi: 10.1093/oxfordjournals.alcalc.a008382
116. May P, Gossage J. Maternal risk factors for fetal alcohol spectrum disorders: not as simple as it might seem. Alcohol Res Health (2011) 34:15–26.
117. Montgomery JW. Working memory and comprehension in children with specific language impairment: what we know so far. J Commun Disorder. (2003) 36:221–31. doi: 10.1016/S0021-9924(03)00021-2
118. McCabe PC, Meller PJ. The relationship between language and social competence: how language impairment affects social growth. Psychol Schools (2004) 41:313–21. doi: 10.1002/pits.10161
119. Kingdon D, Cardoso C, McGrath JJ. Research Review: Executive function deficits in fetal alcohol spectrum disorders and attention-deficit/hyperactivity disorder–a meta-analysis. J Child Psychol Psychiatry Allied Discipl. (2016) 57:116–31. doi: 10.1111/jcpp.12451
120. Mattson SN, Goodman AM, Caine C, Delis DC, Riley EP. Executive functioning in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. (1999) 23:1808–15. doi: 10.1111/j.1530-0277.1999.tb04077.x
121. Whaley SE, O'Connor MJ, Gunderson B. Comparison of the adaptive functioning of children prenatally exposed to alcohol to a nonexposed clinical sample. Alcohol Clin Exp Res. (2001) 25:1018–24. doi: 10.1111/j.1530-0277.2001.tb02311.x
122. Schonfeld AM, Paley B, Frankel F, O'Connor MJ. Executive functioning predicts social skills following prenatal alcohol exposure. Child Neuropsychol. (2006) 12:439–52. doi: 10.1080/09297040600611338
123. Autti-Rämö I. Twelve-year follow-up of children exposed to alcohol in utero. Dev Med Child Neurol. (2000) 42:406–11. doi: 10.1017/S0012162200000748
124. Streissguth AP. Offspring effects of prenatal alcohol exposure from birth to 25 years: the seattle prospective longitudinal study. J Clin Psychol Med Settings (2007) 14:81–101. doi: 10.1007/s10880-007-9067-6
125. Kaplan-Estrin M, Jacobson S, Jacobson J. Neurobehavioral effects of prenatal alcohol exposure at 26 months. Neurotoxicol Teratol. (1999) 21:503–11.
126. Streissguth AP, Barr HM, Sampson PD, Bookstein FL. Prenatal alcohol and offspring development: the first fourteen years. Drug Alcohol Depend. (1994) 36:89–99. doi: 10.1016/0376-8716(94)90090-6
127. Niclasen J. Drinking or not drinking in pregnancy: the multiplicity of confounding influences. Alcohol Alcohol. (2014) 49:349–55. doi: 10.1093/alcalc/agt141
128. Streissguth AP, Barr HM, Sampson PD. Moderate prenatal alcohol exposure: effects on child IQ and learning problems at age 7½ years. Alcohol Clin Exp Res. (1990) 14:662–9.
129. Delatour LC, Yeh PW, Yeh HH. Ethanol exposure in utero disrupts radial migration and pyramidal cell development in the somatosensory cortex. Cerebr Cortex (2018). doi: 10.1093/cercor/bhy094. [Epub ahead of print].
130. Adnams CM. Fetal alcohol spectrum disorder in Africa. Curr Opin Psychiatry (2017) 30:108–12. doi: 10.1097/YCO.0000000000000315
131. Thomas MS, Annaz D, Ansari D, Scerif G, Jarrold C, Karmiloff-Smith A. Using developmental trajectories to understand developmental disorders. J Speech Lang Hear Res. (2009) 52:336–58. doi: 10.1044/1092-4388(2009/07-0144)
132. Yoshida S, Wilunda C, Kimura T, Takeuchi M, Kawakami K. Prenatal alcohol exposure and suspected hearing impairment among children: a population-based retrospective cohort study. Alcohol Alcohol. (2017) 53:221–7. doi: 10.1093/alcalc/agx092
133. Strömland K. Visual impairment and ocular abnormalities in children with fetal alcohol syndrome. Addict Biol. (2004) 9:153–7. doi: 10.1080/13556210410001717024
134. Carr JL, Agnihotri S, Keightley M. Sensory processing and adaptive behavior deficits of children across the fetal alcohol spectrum disorder continuum. Alcohol Clin Exp Res. (2010) 34:1022–32. doi: 10.1111/j.1530-0277.2010.01177.x
135. Green JH. Fetal alcohol spectrum disorders: Understanding the effects of prenatal alcohol exposure and supporting students. J School Health (2007) 77:103–8. doi: 10.1111/j.1746-1561.2007.00178.x
136. Kuehn D, Aros S, Cassorla F, Avaria M, Unanue N, Henriquez C, et al. A prospective cohort study of the prevalence of growth, facial, and central nervous system abnormalities in children with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. (2012) 36:1811–9. doi: 10.1111/j.1530-0277.2012.01794.x
137. Carter RC, Jacobson JL, Molteno CD, Dodge NC, Meintjes EM, Jacobson SW. Fetal alcohol growth restriction and cognitive impairment. Pediatrics (2016) 138:1–9. doi: 10.1542/peds.2016-0775
138. Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicol Teratol. (2000) 22:143–9. doi: 10.1016/S0892-0362(99)00073-2
139. Weinberg J. Prenatal ethanol effects: sex differences in offspring stress responsiveness. Alcohol (1992) 9:219–23. doi: 10.1016/0741-8329(92)90057-H
140. Hoff E. How social contexts support and shape language development. Dev Rev. (2006) 26:55–88. doi: 10.1016/j.dr.2005.11.002
Keywords: early childhood, developmental outcomes, fetal alcohol spectrum disorders, prenatal alcohol exposure, neurodevelopment
Citation: Subramoney S, Eastman E, Adnams C, Stein DJ and Donald KA (2018) The Early Developmental Outcomes of Prenatal Alcohol Exposure: A Review. Front. Neurol. 9:1108. doi: 10.3389/fneur.2018.01108
Received: 31 August 2018; Accepted: 04 December 2018;
Published: 18 December 2018.
Edited by:
Carl E. Stafstrom, Johns Hopkins Medicine, United StatesReviewed by:
Eileen M. Moore, San Diego State University, United StatesAlberto Granato, Catholic University of Sacred Heart, Italy
Copyright © 2018 Subramoney, Eastman, Adnams, Stein and Donald. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kirsten A. Donald, a2lyc3R5LmRvbmFsZEB1Y3QuYWMuemE=
 Sivenesi Subramoney
Sivenesi Subramoney Emma Eastman1
Emma Eastman1 Colleen Adnams
Colleen Adnams Dan J. Stein
Dan J. Stein Kirsten A. Donald
Kirsten A. Donald