
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Neurol. , 10 December 2018
Sec. Movement Disorders
Volume 9 - 2018 | https://doi.org/10.3389/fneur.2018.01084
This article is part of the Research Topic Pathophysiologic Insights from Biomarker Studies in Neurological Disorders View all 33 articles
 Christian Schlenstedt*
Christian Schlenstedt* Steffen Paschen
Steffen Paschen Jana Seuthe
Jana Seuthe Jan Raethjen
Jan Raethjen Daniela Berg
Daniela Berg Walter Maetzler
Walter Maetzler Günther Deuschl
Günther DeuschlBackground and Aim: Individuals with Parkinson's disease (PD) and Freezing of Gait (FOG) have impaired postural control, which relate to the severity of FOG. The aim of this study was to analyze whether a moderate frequency resistance (RT) and balance training (BT), respectively, are effective to diminish FOG.
Methods: This post-hoc sub-analysis of a randomized controlled training intervention study of PD patients with and without FOG reports about results from FOG patients. Twelve FOG patients performed RT and 8 BT (training 2x/week, 7 weeks). Testing was performed prior and post intervention. FOG was assessed with the FOG Questionnaire (FOGQ) and with the FOG score of a FOG provoking walking course. Balance performance was evaluated with the Fullerton Advanced Balance (FAB) scale. Tests were conducted by raters blinded to group allocation and assessment time point (only FOG score and FAB scale).
Results: For the FOGQ and FOG score, no significant differences were found within and between the two training groups (p > 0.05) and effect sizes for the improvements were small (r < 0.1). Groups did not significantly improve in the FAB scale. FOG score changes and FAB scale changes within the RT group showed a trend toward significant negative correlation (Rho = −0.553, p = 0.098).
Conclusions: Moderate frequency RT and BT was not effective in reducing FOG in this pilot study. The trend toward negative correlation between changes in FOG score and FAB scale suggests an interaction between balance (improvement) and FOG (improvement). Future studies should include larger samples and high frequency interventions to investigate the role of training balance performance to reduce the severity of FOG.
Freezing of gait (FOG) in Parkinson's disease (PD) is a disabling symptom which is defined as the “brief, episodic absence or marked reduction of forward progression of the feet despite the intention to walk” (1). It has been shown that FOG-specific training interventions, such as cueing, can reduce FOG (2–4). It is however unclear whether non-FOG-specific exercises which target FOG-related deficits also alleviate FOG.
Individuals with PD with FOG (PD + FOG) have postural control deficits (5, 6) and the severity of FOG relates to the degree of postural instability (5). Recently it has been shown that PD+FOG have smaller anticipatory postural adjustments (APAs) when preparing for step initiation compared to patients without FOG (PD-FOG) and that the size of medio-lateral APAs was positively correlated with FOG severity (7). It has been suggested that reducing the size of APA might be a compensatory strategy addressing postural control deficits (7). Further, Plotnik et al. (8) proposed that FOG might be a result of multiple with FOG associated motor impairments such as dynamic postural control, gait asymmetry, and gait variability. According to this framework, FOG might occur if enough of these features deteriorate. It is unclear, whether an improvement of postural control as one of these FOG related features might diminish FOG.
In a recent study we compared resistance training (RT) with balance training (BT) to improve postural control in PD and we showed that RT was beneficial to improve balance performance. This sub-analysis has two aims: first, to test whether RT or BT is effective to reduce the severity of FOG and second, whether an improvement in FOG is related to improved postural control.
This study is a sub-analysis (only PD + FOG, N = 20) of a randomized controlled trial that investigated the efficacy of RT vs. BT to improve postural control in PD (N = 40) (9).
Inclusion criteria were the diagnosis of idiopathic PD as defined by the UK Brain Bank criteria, FOG based on the FOG Questionnaire (10) (FOGQ) (item 3 > 0) and postural instability [Fullerton Advanced Balance (FAB) scale <26 points (11)]. Details about the exclusion criteria are reported in Schlenstedt et al. (9). Individuals had to be on stable medication during the training and assessment periods.
This study was carried out in accordance with the recommendations of Ethik-Kommission, Universitätsklinikum Schleswig-Holstein, Campus Kiel, Arnold-Heller-Straße 3, 24105 Kiel, Germany, with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Ethik-Kommission, Universitätsklinikum Schleswig-Holstein, Campus Kiel, Arnold-Heller-Straße 3, 24105 Kiel, Germany.
Participants were randomized into either RT or BT (7 weeks, 2x/week, 60 min per session) within the original study. There was no stratification for FOG in the original randomization. Training was conducted in groups of 4–5 people. Each session started with a warm-up (10 min) followed by either RT or BT. In brief, RT consisted of lower limb muscle strength exercises and participants' own weight, cuff weights, and elasticated bands were used as resistance. Squats, knee extensions, toe/calf raises, hip abductions, and other exercises were performed [for details see (9)].
BT consisted of static and dynamic postural control tasks. Participants were asked to train their limits of stability by leaning forward/backward/sideward. Reactive postural control was trained by shoulder pulls. One option to reach training progression was the inclusion of unstable surfaces on which the participants had to stand or walk [see Schlenstedt et al. (9) for further details about training progression].
Participants were tested 1 week prior (PRE) and 1 week post (POST) intervention. Testing was conducted in the ON state of medication at the same time of a day for each participant. Severity of FOG was assessed with the FOGQ (10) and with the FOG score by Ziegler et al. (12). The FOGQ was conducted by an assessor blinded to group allocation. Trials of the FOG score were video-recorded and videos were rated by an independent rater, also blinded to assessment time point and group allocation.
Furthermore, the following tests were included in the analysis: FAB scale (to assess postural control) (11, 13), Unified Parkinson's Disease Rating Scale (UPDRS), and Mini Mental State Examination.
Demographic and baseline differences between groups were analyzed with a Mann-Whitney-U-Test (except for gender: Chi-Square Test). As data were not normally distributed, non-parametric tests were used. A Wilcoxon-Signed-Rank-Test was conducted to analyze the changes from PRE to POST within one group. To compare the different training types, the differences from PRE to POST were calculated and the magnitude of change were compared between the two groups were analyzed with using the Mann-Whitney-U-Test. Effect sizes were calculated (r = z-score/(n)∧1/2). We considered effect sizes to be small with 0.1 < r < 0.3, medium with 0.3 < r < 0.5 and large with r > 0.5 (14). The magnitude of change in FOG severity was correlated [Spearman's rank correlation coefficient [Rho] with the change in balance performance (FAB scale). Level of significance was set at p < 0.05. Statistical analysis was performed with R (version 1.1.442) (15).
Table 1 shows the participant characteristics. RT and BT groups neither significantly differed in any demographic variable, nor with regard to severity of FOG. Both training types had no significant effect on FOGQ and FOG score (Table 2 and Figure 1) (p < 0.05). The effect sizes for the slight improvements within the RT group were small (r < 0.1) (14). Within this sample, the groups did not improve significantly in postural control as measured with the FAB scale (p < 0.05). Although statistically not significant within this sample, a large effect was found within the RT group when relating the change in balance performance (FAB scale) with the change in FOG score (Rho = −0.553, p = 0.098). A similar trend was found when calculating this correlation taken both groups together (p = 0.11, Rho = −0.4). A medium effect was found within the BT group (p = 0.426, Rho = 0.361). Changes in FOGQ was not related to the change in FAB scale (RT: p = 0.948; BT: p = 0.612). The exclusion of outliers did not relevantly affect our results.
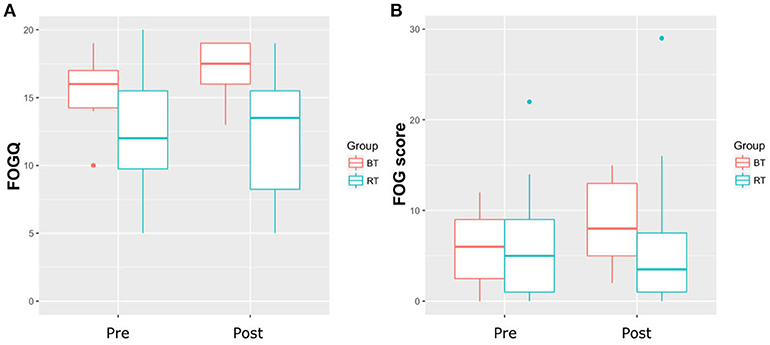
Figure 1. (A) Results of the Freezing of Gait Questionnaire (FOGQ). (B) Results of the Freezing of Gait score; BT, Balance Training; RT, Resistance Training.
We could not show that a moderate frequency RT and BT is effective to diminish FOG in people with PD in the present pilot study. As FOG-specific training interventions such as cueing did indeed show statistically significant reduction in FOG severity (2–4), our study might indirectly supports the hypothesis that exercises specifically designed to target FOG might be more beneficial than non-FOG-specific interventions. We acknowledge that our sample was small and results have to be interpreted cautiously; however, due to the low effect sizes we do not expect reaching significant results with this training protocol even with a larger sample. We rather believe that increasing the intensity and frequency of training is required to see a relevant effect and this has been suggested by other larger trials (16).
As FOG is related to postural control deficits (5, 6) the idea of this project was that improved postural control might lead to a reduction in FOG episodes. In the original study, participants of the RT group significantly improved postural control whereas the group of BT did not. We found a large effect when correlating the change in balance performance with the change in FOG severity within the RT group, indicating that those participants who improved postural control may also benefit with respect to FOG, supporting our hypothesis with respect to study aim II. However, this failed to reach statistical significance within this sample and the subgroup of participants with FOG did not significantly improve their postural control in this sub-analysis. This might be explained by the low training frequency (2x/week) and by the small subsample size, as in the original study on all participants of the RT balance performance improved significantly (9). Thus, the impact of training balance performance on FOG cannot clearly be answered with this study.
The following limitations have to be mentioned: Sample size is small and results therefore have to be interpreted cautiously. This study did not include a non-exercise control group which would give additional information with respect to the training effects.
A moderate frequency RT and BT was not effective to diminish FOG within this small sample. This pilot study might help designing future studies which should include larger samples and higher training frequency to investigate the role of training balance performance to reduce FOG occurrence in PD.
CS: Study design, study conduction, data collection, statistical analysis, and writing of first draft; SP and JS: Data analysis and manuscript critical revision; JR and GD: Study design and manuscript critical revision; DB and WM: Manuscript critical revision.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank our participant for donating their time to volunteer. We thank Annika Kruse and Anna Krebs for helping in conducting the training sessions and in data collection. This publication was made possible with support from the Coppenrath-Foundation, Geeste/Groß-Hesepe, Niedersachsen, Germany and Krumme-Stiftung, Eckernförde, Schleswig-Holstein, Germany (Schlenstedt). We acknowledge financial support by Land Schleswig-Holstein within the funding programme Open Access Publikationsfonds.
1. Giladi N, Nieuwboer A. Understanding and treating freezing of gait in parkinsonism, proposed working definition, and setting the stage. Mov Disord. (2008) 23(Suppl. 2):S423–5. doi: 10.1002/mds.21927
2. Fietzek UM, Schroeteler FE, Ziegler K, Zwosta J, Ceballos-Baumann AO. Randomized cross-over trial to investigate the efficacy of a two-week physiotherapy programme with repetitive exercises of cueing to reduce the severity of freezing of gait in patients with Parkinson's disease. Clin Rehabil. (2014) 28:902–11. doi: 10.1177/0269215514527299
3. Plotnik M, Shema S, Dorfman M, Gazit E, Brozgol M, Giladi N, et al. A motor learning-based intervention to ameliorate freezing of gait in subjects with Parkinson's disease. J Neurol. (2014) 261:1329–39. doi: 10.1007/s00415-014-7347-2
4. Barthel C, Nonnekes J, van Helvert M, Haan R, Janssen A, Delval A, et al. The laser shoes: a new ambulatory device to alleviate freezing of gait in Parkinson disease. Neurology (2018) 90:e164–71. doi: 10.1212/WNL.0000000000004795
5. Schlenstedt C, Muthuraman M, Witt K, Weisser B, Fasano A, Deuschl G. Postural control and freezing of gait in Parkinson's disease. Parkinsonism Relat Disord. (2016) 24:107–12. doi: 10.1016/j.parkreldis.2015.12.011
6. Bekkers EMJ, Dijkstra BW, Dockx K, Heremans E, Verschueren SMP, Nieuwboer A. Clinical balance scales indicate worse postural control in people with Parkinson's disease who exhibit freezing of gait compared to those who do not: a meta-analysis. Gait Posture (2017) 56:134–40. doi: 10.1016/j.gaitpost.2017.05.009
7. Schlenstedt C, Mancini M, Nutt J, Hiller AP, Maetzler W, Deuschl G, et al. Are hypometric anticipatory postural adjustments contributing to freezing of gait in Parkinson's disease? Front Aging Neurosci. (2018) 10:36. doi: 10.3389/fnagi.2018.00036
8. Plotnik M, Giladi N, Hausdorff JM. Is freezing of gait in Parkinson's disease a result of multiple gait impairments? Implications for treatment. Parkinsons Dis. (2012) 2012:459321. doi: 10.1155/2012/459321
9. Schlenstedt C, Paschen S, Kruse A, Raethjen J, Weisser B, Deuschl G. Resistance versus balance training to improve postural control in Parkinson's disease: a randomized rater blinded controlled study. PLoS ONE (2015) 10:e0140584. doi: 10.1371/journal.pone.0140584
10. Giladi N, Tal J, Azulay T, Rascol O, Brooks DJ, Melamed E, et al. Validation of the freezing of gait questionnaire in patients with Parkinson's disease. Mov Disord. (2009) 24:655–61. doi: 10.1002/mds.21745
11. Schlenstedt C, Brombacher S, Hartwigsen G, Weisser B, Moller B, Deuschl G. Comparing the fullerton advanced balance scale with the mini-BESTest and Berg Balance Scale to assess postural control in patients with Parkinson disease. Arch Phys Med Rehabil. (2015) 96:218–25. doi: 10.1016/j.apmr.2014.09.002
12. Ziegler K, Schroeteler F, Ceballos-Baumann AO, Fietzek UM. A new rating instrument to assess festination and freezing gait in Parkinsonian patients. Mov Disord. (2010) 25:1012–8. doi: 10.1002/mds.22993
13. Rose DJ, Lucchese N, Wiersma LD. Development of a multidimensional balance scale for use with functionally independent older adults. Arch Phys Med Rehabil. (2006) 87:1478–85. doi: 10.1016/j.apmr.2006.07.263
15. Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2005).
16. Clarke CE, Patel S, Ives N, Rick CE, Woolley R, Wheatley K, et al. Clinical effectiveness and cost-effectiveness of physiotherapy and occupational therapy versus no therapy in mild to moderate Parkinson's disease: a large pragmatic randomised controlled trial (PD REHAB). Health Technol Assess. (2016) 20:1–96. doi: 10.3310/hta20630
Keywords: freezing, postural control, balance, Parkinson's disease, exercise, training
Citation: Schlenstedt C, Paschen S, Seuthe J, Raethjen J, Berg D, Maetzler W and Deuschl G (2018) Moderate Frequency Resistance and Balance Training Do Not Improve Freezing of Gait in Parkinson's Disease: A Pilot Study. Front. Neurol. 9:1084. doi: 10.3389/fneur.2018.01084
Received: 29 August 2018; Accepted: 27 November 2018;
Published: 10 December 2018.
Edited by:
Tobias Warnecke, Universitätsklinikum Münster, GermanyReviewed by:
Simon Steib, Friedrich-Alexander-Universität Erlangen-Nürnberg, GermanyCopyright © 2018 Schlenstedt, Paschen, Seuthe, Raethjen, Berg, Maetzler and Deuschl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian Schlenstedt, Yy5zY2hsZW5zdGVkdEBuZXVyb2xvZ2llLnVuaS1raWVsLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.