- 1Center for Neurophysics, Physiology, Pathology, CNRS UMR 8119, Université Paris Descartes, Sorbonne Paris Cité, Paris, France
- 2Centre National D'Etudes Spatiales, Paris, France
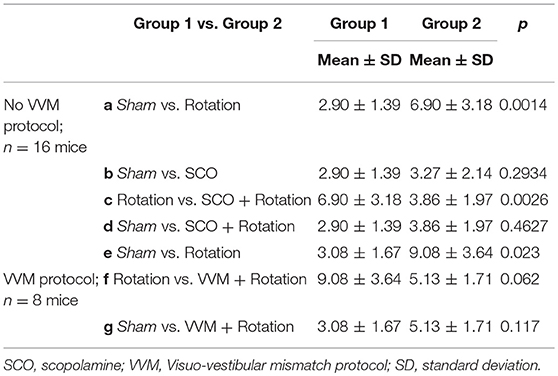
Motion sickness occurs when the vestibular system is subjected to conflicting sensory information or overstimulation. Despite the lack of knowledge about the actual underlying mechanisms, several drugs, among which scopolamine, are known to prevent or alleviate the symptoms. Here, we aim at better understanding how motion sickness affects the vestibular system, as well as how scopolamine prevents motion sickness at the behavioral and cellular levels. We induced motion sickness in adult mice and tested the vestibulo-ocular responses to specific stimulations of the semi-circular canals and of the otoliths, with or without scopolamine, as well as the effects of scopolamine and muscarine on central vestibular neurons recorded on brainstem slices. We found that both motion sickness and scopolamine decrease the efficacy of the vestibulo-ocular reflexes and propose that this decrease in efficacy might be a protective mechanism to prevent later occurrences of motion sickness. To test this hypothesis, we used a behavioral paradigm based on visuo-vestibular interactions which reduces the efficacy of the vestibulo-ocular reflexes. This paradigm also offers protection against motion sickness, without requiring any drug. At the cellular level, we find that depending on the neuron, scopolamine can have opposite effects on the polarization level and firing frequency, indicating the presence of at least two types of muscarinic receptors in the medial vestibular nucleus. The present results set the basis for future studies of motion sickness counter-measures in the mouse model and offers translational perspectives for improving the treatment of affected patients.
Introduction
Motion sickness (MS) is a disease that occurs when the brain cannot track the movement of the self in a given environment. Motion sickness is experienced by up to 15% of the humans subjects traveling by air, sea or on ground (1–3). What are the physiological causes for MS? While many theories are still debated (4, 5), it is mostly accepted that MS results from a mismatch between motion-derived neural signals, as for instance a conflict between visual and vestibular inputs experienced while reading in a moving car or on a sailing boat (6). Notably, the conflict between motion-sensitive signals can also be limited to a single sensory modality: vestibular-only motion sickness results from a conflict between semicircular canals signals and otolith signals. Vestibular-only motion sickness incapacitates the brain to integrate angular and linear acceleration in order to efficiently reconstruct the orientation of the head in space (7, 8).
To prevent the onset of motion sickness, medications have been empirically developed and documented for at least a century and probably used for much longer (9). To date, one of the most efficient drugs to prevent in particular space motion sickness (10, 11) is scopolamine (12–14), a muscarinic antagonist commonly administered through transdermal patches. While its molecular effects are well characterized, its putative action on the peripheral and/or central vestibular system, at the neuronal (15) and behavioral levels (14, 16) have still to be specified. Several studies have also tried non-pharmacological approaches to help prevent motion sickness by habituating the system to vestibular stimulation (17–20). Habituation to visual stimulation was also promising because its effects were demonstrated to be long-lasting (1).
While the interactions between the vestibular system, motion sickness and pharmacological treatments have been widely studied in humans, similar studies are conducted on animal models to understand their correlate at the cellular and molecular levels. Here we use the mouse model to investigate the interplay between vestibular reflexes, motion sickness and different counter-measures by addressing several related questions.
1. what are the consequences of motion sickness on the efficacy of the vestibular system?
2. does scopolamine protect mice against MS, as it does in humans?
3. can a non-pharmacological, preemptive adaptation reduce the occurrence of mice MS?
4. what are the direct pharmacological effects of scopolamine on the electrophysiological properties of central vestibular neurons recorded in vitro?
We find that motion sickness leads to a general decrease in the efficacy of vestibulo-ocular reflexes (VOR). When administrated before the occurrence of MS, scopolamine decreases the efficacy of the vestibulo-ocular reflexes and prevents the occurrence of symptoms normally associated with MS. Then, we tested the effect of a long-lasting VOR gain-down reduction protocol and validated that this reduction offers a protection against MS. At the cellular level, we demonstrate that muscarinic antagonists have heterogeneous effects on the neuron's electrophysiological parameters suggesting that the action of scopolamine on central vestibular neurons is differentially affecting subpopulations of neurons.
Materials and Methods
Ethics
Animals were used in accordance with the European Communities Council Directive 2010/63/EU. All efforts were made to minimize suffering and reduce the number of animals included in the study. All procedures were approved by the ethical committee for animal research of the University Paris Descartes (CEEA.34).
Surgical Procedures
Surgical preparation and postoperative care for head implant surgery have been described previously (21, 22). Gas anesthesia was induced using isoflurane. The head was shaved using an electric razor. Lidocaine hydrochloride (2%; 2 mg/Kg) was injected locally before a longitudinal incision of about 2 cm was performed into the skin to expose the skull. A small custom-built head holder (3 × 3 × 5 mm) was fixed using dental cement (C&B Metabond; Parkellinc, Edgewood, NY, United States) to the skull just anterior to the lambda landmark. Following the surgery, animals were isolated and closely surveyed for 48 h. Buprenorphine (0.05 mg/kg) was provided for postoperative analgesia and care was taken to avoid hypothermia and dehydration.
Behavioral Measures
The vestibulo-ocular pathway works as an open-loop: the vestibular signals trigger compensatory eye movements to stabilize gaze in the absence of sensory feedback. As a consequence, any imbalance or modification in the vestibular inputs leads to alteration of the eye movements triggered by head movements. This makes video-oculography the main tool used in hospitals to measure vestibular function. Eye movements were therefore used as a proxy to evaluate the efficacy of the vestibular system by quantification of the vestibulo-ocular reflexes of the mice.
Video-Oculography Procedure
Eye movements were recorded using non-invasive video-oculography (23). The experimental set-up, apparatus and methods of data acquisition are similar to those previously described (22, 24). Briefly, mice were head-fixed at a ~30° nose-down position to align the horizontal canals in the yaw plane (25, 26). Animals were placed in a custom-built Plexiglas tube secured on the superstructure of a vestibular stimulator. The VOR performance was tested in a temperature-controlled room (21°C) with all sources of light turned off except for computer screens. The turntable was further surrounded with a closed box to isolate the animal from remaining light, with a final luminance inside the box <0.02 lux.
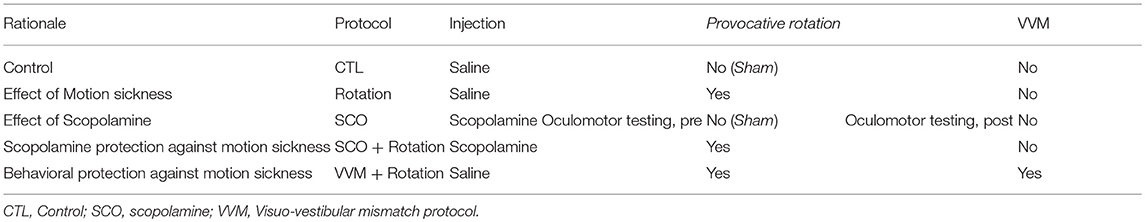
To prevent excessive pupil dilatation in dark, a topical application of a combination of pilocarpine (inducing a miosis via local muscarinic stimulation) and Combigan (brimonidine 0.2% + timolol 0.5%, preventing the mydriasis by locally blocking the adrenergic pathways) was used. The addition of Combigan on top of the usually used pilocarpine is necessary to counteract locally the miotic effect of the systemic scopolamine injected in some protocols (cf. Table 1). To avoid introducing a bias between experiments with and without scopolamine, the combination of Combigan and pilocarpine was used in all experiments.
Vestibulo-Ocular Reflex Tests and Analysis
To evaluate the canalar and otolithic contributions to the VOR, different vestibular stimulations were used.
1. The eye movements evoked by an angular stimulation of the horizontal canals (aVOR) were tested. The animal was rotated around a vertical axis with sinusoidal movements at frequency of 0.2, 0.5, 1 Hz with a peak velocity of 25°/s. The angular amplitude of the movement was adjusted accordingly. At least 60 cycles were produced for each frequency. Two parameters were extracted from the recordings: the gain (aVOR_G) and the phase (aVOR_φ). The gain is the ratio between the amplitude of the eye (response) and head (stimulus) rotations. Since the animal is head-fixed to the rotating table, head movements and table movements are identical. The phase is the temporal shift between the eye and table rotations, expressed as ratio of the sinusoidal cycle (2 pi). Details for gain and phase calculation are reported in Carcaud et al. (24).
2. The eye movements evoked by a specific stimulation of the otoliths (maculo-ocular reflexes, MOR) were tested (27) using off-vertical axis rotation (OVAR) as previously described (22). Briefly, the axis of rotation was tilted by 17° with respect to the vertical. Rotations were performed at constant speed (50°/s) for at least 10 rotations both in the clockwise (cw) and the counterclockwise (ccw) directions. Due to the inertial nature of the angular movement detection, a rotation at constant speed elicits a combined canalar and otolithic response at the beginning of the trace, however after a few seconds only the otolithic component remains (22, 28). Since gravitational acceleration acts vertically, this stimulation is equivalent to a continuous rotation (at 0.14 Hz) around the mouse head of a 17° tilted constant linear acceleration stimulus [see Figure 2B in Beraneck et al. (22)]. For horizontal OVAR responses, quick-phases were identified and removed. During rotations, the velocity of horizontal slow phases is modulated (modulation, μ) around a constant bias (β). Both parameters (μ and β) were calculated from the sinusoidal fit of eye horizontal slow-phase velocity using the least-squares optimization of the equation:
where SP(t) is slow-phase velocity, β is the steady-state bias slow phase velocity, μ is the modulation of eye velocity, f0 is the frequency of table rotation, td is the dynamic lag time (in ms) of the eye movement with respect to the head movement. The bias (Maculo-ocular reflex Bias; MORβ) is reported here as the main index of otolithic response (22, 27).
Motion Sickness Generation
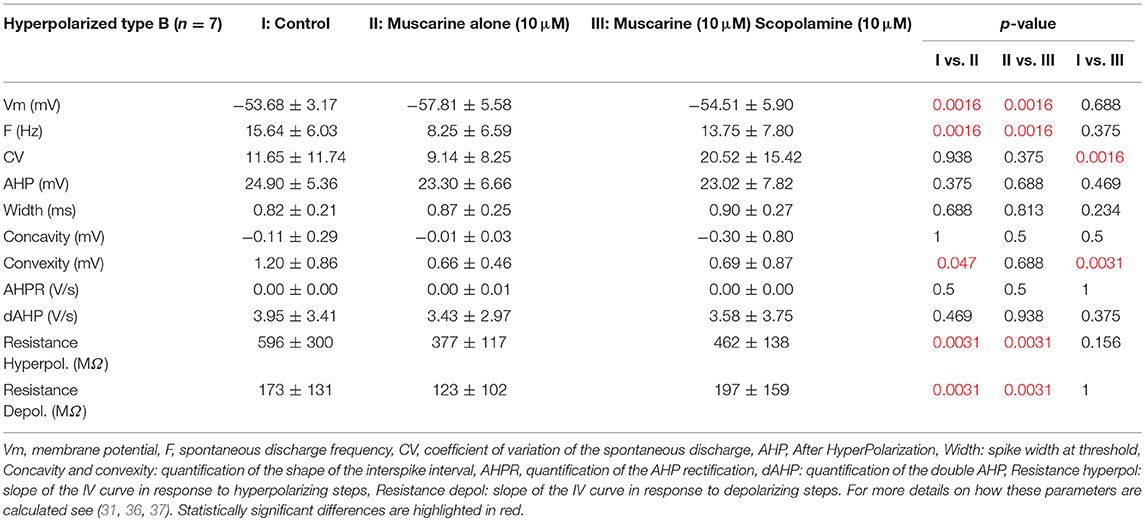
Motion sickness was induced in mice using a double provocative rotation comparable to the one used in rats by Morita et al. (29). Animals were tested one at a time. Each animal was rotated for 30 min in the home-made motion sickness generating device, under room lighting (300 lux). This device is composed of one central axis rotating clockwise a 30 cm-long arm at 60°/s constant velocity. At the distal extremity of the arm is a second axis, which rotates the box containing the animal counter-clockwise with a sinusoidally-modulated speed (range 5–55°/s; Figure 1A). The box containing the non-restrained mouse had a padded floor. The padding was changed before each test to prevent any olfactory signaling within the box. The top part of the box was transparent.
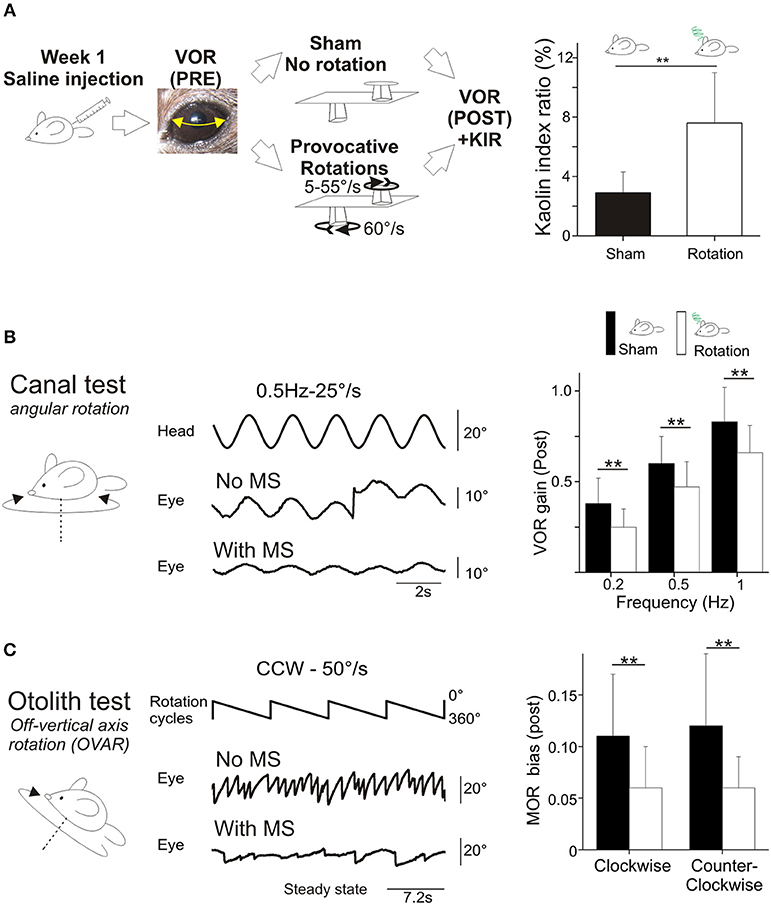
Figure 1. (A) Rotation induces motion sickness. Left, scheme of the protocol designed for intra-individual comparison. Animals received saline injection and VOR was tested before and after a sham experiment and a provocative double-rotation. Right, Pica behavior quantified as a Kaolin Index Ratio was quantified before and after rotation. (B) Motion sickness reduces angular horizontal vestibulo-ocular reflex. Left panel, raw traces of the eye movement observed during sinusoidal rotation of the turntable after the Sham or provocative rotation session. Right panel, intra-individual comparison of the VOR gain measured with or without MS. (C) Motion sickness reduces the maculo-ocular reflex. Off-vertical axis rotation was performed at velocities of 50°/s. A sample of 4 over 10 cycles of 360° rotations at constant velocity are presented. Left, raw traces of the eye movements evoked with or without MS. Right, intra-individual comparison of the MOR gain measured with or without MS. In this and all figures, plots represent mean ± standard deviation. Asterisks indicate statistically significant differences with Holm-Bonferroni correction, *p < 0.05; **p < 0.01; ***p < 0.001 respectively. For table position up is left; for ease of reading, eye position is inverted (up is right).
Motion Sickness Evaluation
Kaolin is a mineral clay commonly used in animal feed. Preparation of a mix of kaolin (Sigma Aldrich #18672) and 1% w/w arabic gum (Sigma Aldrich #G9752-500G), hereafter referred to as “kaolin”, was similar to that reported by Yu et al. (30). To quantify the occurrence of MS, we measured the changes in alimentary preferences observed following an aversive stimulus. Affected mice eat less of the regular food and instead turn to kaolin, which has no nutritional value.
Each mouse was housed individually for the entire time of the experiment, with ad libitum access to water, regular food, and kaolin. Individual consumption of food (F) and kaolin (K) was measured daily. The kaolin intake ratio (KIR) is calculated as K/(K+F) and expressed in percent.
Protective Protocols
Scopolamine dynamics
To test whether the effects of scopolamine were lasting during the entire experiment, we measured the pupil dilation under constant artificial lighting (300 lux) in a separate group of animals (n = 12). Animals were injected with scopolamine (Sigma Aldrich # S1875-1G; 0.3 μg/g of corporal mass, in saline solution) and the individual duration of the pupil dilation was measured. During this preliminary experiment, the scopolamine effects were found to peak within minutes and then to slowly fade: significant pupil dilatation was seen after 5–7 min and this dilation lasted for at least 90 min, i.e., longer than the duration of the experimental protocols (see Table 1 below). No change in the pupil size was observed when animals were injected with saline in the same configuration.
Visuo-vestibular mismatch protocol
To test if decreasing the efficacy of the vestibular system is causally linked to the protective effect of scopolamine, we took advantage of a behavioral protocol recently developed [see Figure 2 in Carcaud et al. (24)] that leads to a decrease of VOR gain. A custom-built device was secured on top of the head holder for 14 days. The device consisted of a “helmet” (size: 2.2 cm width × 1.5 cm depth × 1.5 cm length; weight 2 g) that completely covered the mouse's head. The front of the device was adapted to the mouse anatomy so that the nose was not covered, and its width allowed for grooming and barbering behaviors. To preserve light-dependent physiology and nychthemeral rythm, the device was made of non-opaque plastic with a thickness of 0.3 mm. In addition, 3 mm large vertical black stripes were drawn on the external surface. When the mouse moves its head, the highly contrasted head-fixed stripes generate a visuo-vestibular mismatch (VVM). After 2 weeks, we reported a long-lasting gain-down reduction of the angular VOR of about 50% [range tested 0.2–2 Hz for velocities of 10–50°/s; see results in Carcaud et al. (24)]. Here, we take advantage of this protocol to test the interactions between the VOR and motion sickness.
Design of the Study
Different procedures were designed to test, on one hand, the functional consequences of motion sickness or of scopolamine on the vestibular system and, on the other hand, the influence of scopolamine or of the visuo-vestibular mismatch on motion sickness.
All behavioral measures reported were performed on n = 24 mice. 12 additional animals were used in preliminary experiments to determine the exact parameters used but were not included in the study.
During the first week, the susceptibility of the 24 mice to provocative rotations was tested following an injection of a saline solution (Figure 1A). To account for the inter-animal variability and non-specific effects, each animal was tested in 2 sessions: vestibulo-ocular reflexes were tested a first time in the dark (aVOR and MOR testing, pre). Then, the animal was put into the motion sickness generating device either activated (i.e., provocative rotation condition) or not (Sham condition). Finally, the same vestibulo-ocular reflexes were recorded a second time in the dark (aVOR and MOR testing, post; Figure 1A).
The effects of scopolamine were tested in a subset of mice (n = 16). The mice received a scopolamine injection (0.3 μg/g of corporal mass, in saline solution). The mice were then tested again in the Sham and provocative rotations conditions (Figure 2A).
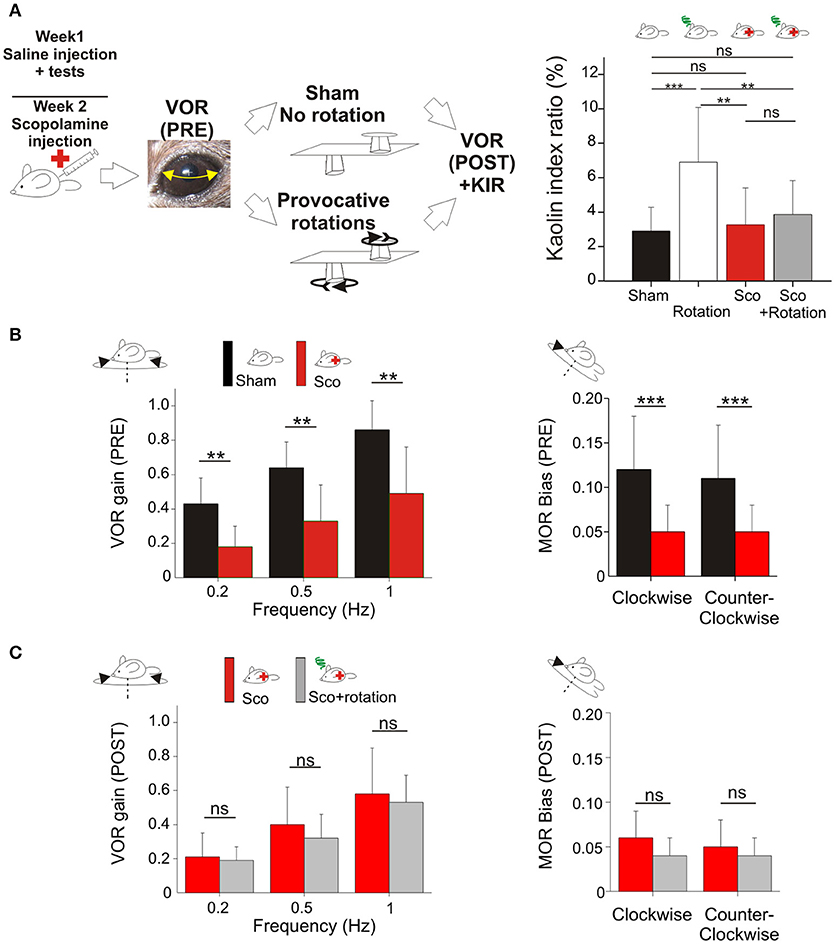
Figure 2. (A) Scopolamine protects against motion sickness. Left panels, animals already tested with saline received scopolamine injection; VOR was tested before and after a Sham experiment and a provocative double-rotation experiment. Right, Pica behavior demonstrated the protective effects of scopolamine against motion sickness. (B) Scopolamine reduces vestibular sensitivity. Left, plots of angular VOR gain show significant reduction at all tested frequencies before provocative stimulation (Pre measurements). Right, plots of MOR bias shows significant reduction under scopolamine treatment. (C) No additional reduction of vestibular sensitivity was observed following provocative rotation protocol (Post measurements). Left, plots of angular VOR. Right, plots of MOR bias. Asterisks indicate statistically significant differences with Holm-Bonferroni correction, *p < 0.05; **p < 0.01; ***p < 0.001 respectively.
To test whether motion sickness could be prevented without scopolamine, the remaining 8 mice were included in the VVM gain-decrease experiment (Figure 3A). After the initial motion sickness tests, the helmet was put on the mouse's head for 2 weeks (see section Visuo-vestibular mismatch protocol). Following this perturbation period, the VOR was recorded immediately after removing the helmet, and again after the provocative rotation stimulation. No sham condition was recorded to prevent de-adaptation of the VOR.
Figure 3. (A) Visuo-vestibular mismatch reduces vestibular sensitivity. Left, picture of a mouse during the visuo-vestibular conflict protocol. The helmet is kept for 2 weeks. Right panel, Pica behavior demonstrated the protective effect of VVM protocol against MS induced by the double-rotation (B). Left panels, plots of angular VOR of the n = 8 mice before the provocative rotations (black and deep blue bars) and after the provocative rotations (white and light blue bars). Right panels, MOR bias ratios in the same conditions. No additional reduction of vestibular sensitivity was induced by the rotation, suggesting protective effects of the VVM protocol. Asterisks indicate statistically significant differences with Holm-Bonferroni correction, *p < 0.05; **p < 0.01; ***p < 0.001 respectively.
The different protocols are summarized in Table 1.
Electrophysiological Experiments
To measure the neuronal effects of scopolamine, 220 μm-thick coronal brainstem slices were obtained from 5-week-old male C57BL/6J mice (n = 18) (24, 31). A total of 51 medial vestibular nuclei neurons (MVNn) were recorded with patch-clamp electrodes. The artificial cerebrospinal fluid (aCSF) used during the dissection and slicing is composed of (in mM): NaCl (120), NaHCO3 (25), NaH2PO4 (1), KCl (2.5), MgCl2 (3), CaCl2 (0), glucose (10), sucrose (240). The recording solution differs only for NaCl (120), MgCl2 (2), CaCl2 (1) and sucrose (0). Analysis of resting discharge parameters, spike shape and classification of type A vs. type B neurons are similar to those previously reported (31). The intrinsic properties, as well as the responses to hyperpolarizing and depolarizing steps were compared between control conditions, or during pharmacological testing by the addition of muscarine (10 μM), or addition of muscarine (10 μM) + scopolamine (10 μM) to the bath. All chemicals were purchased from Sigma-Aldrich.
Statistics
All mice were first tested during the Control protocol (Figure 1), then during one of the two counter-measure protocols (scopolamine, Figure 2, or visuo-vestibular mismatch, Figure 3). This approach allowed performing statistical analyses based on within-subjects models to account for non-specific and inter-individual variations. Since not all parameters were normally distributed (as tested with a Lilliefors test), we used the same non-parametric paired-test (Wilcoxon signed-rank test) to evaluate statistical significance in all conditions. When appropriate, a one-tail version was used to account for prior knowledge about the alternative hypothesis [e.g., the effect of a treatment on the parameters as the expected reduction of the VOR gain by the VVM protocol, Carcaud et al. (24)]. The thresholds for the statistical tests were adjusted using the Holm-Bonferroni method to account for the numerosity of the planed multiple comparisons. Although adjusted p-value thresholds were used to define the level of significance of the statistical tests, for ease of reading we report in the results section the corresponding uncorrected value noted with the ¢ symbol. All results in both the text and the figures are reported as mean ± standard deviation.
Results
Effect of Rotation on the Behavior of Control Mice
Induction and Quantification of MS
In response to motion sickness (MS), mice do not vomit (30, 32); however behavioral proxies can be used in rodents to assess the debilitating effects associated with MS. Following the provocative double-rotation protocol, qualitative symptoms such as urination, piloerection or tremor were observed, suggesting that MS had been induced. To quantify the occurrence of MS, we measured the “Pica” behavior: changes in alimentary preferences observed following an aversive stimulus (33, 34). Affected mice eat less of the regular food and instead turn to a substance referred to as “Kaolin” which has no nutritional value.
Mice food consumption was measured before and after their exposure to the Sham condition or provocative rotation condition (Figure 1A). The quantity of food and of Kaolin was then compared and used to calculate the Kaolin Index Ratio (KIR). As expected, the Pica behavior was observed in all mice (n = 24) following MS induction and the KIR was significantly increased (Figure 1A; p < 0.01¢; Table 2a,e for the different protocols).
Sustained Rotation Decreases the Efficacy of the Vestibular Reflexes
To assess the interplay between vestibular responses and MS syndrome, various components of vestibulo-ocular reflexes were tested during passive head-fixed movements performed in the dark.
First, to determine possible non-specific effects of the protocol, mice were tested in a Sham condition (put in the device after saline injection, but not rotated; Figure 1A, left panel). There was a diminution of aVOR gain by ~10% (15% at 0.2 Hz, p < 0.05¢; 9% at 0.5 Hz, p < 0.05¢; 5% at 1 Hz, p > 0.1¢) during the second measure. To account for this effect, we compare below and in Figure 1 the different protocols from similar conditions (e.g., Sham Pre vs. rotated Pre; Sham Post vs. rotated Post).
For all tested frequencies, mice had similar angular VOR gain (aVOR_G; range 0.45 ± 0.13–0.87 ± 0.19; p > 0.1¢) and phase (range 20.6 ± 7.2 to −2.5 ± 5.8; p > 0.1¢) responses before the protocols (Pre values). Following the MS protocol however, there was a significant decrease in the aVOR gain (Figure 1B; p < 0.01¢ for all frequencies). When the responses before and after the rotation were compared, the mean decrease in aVOR gain reached about ~30% at 0.2 Hz and ~20% at 0.5 and 1 Hz (Figure 1B, right panel). There was also significant changes in the timing of the aVOR (aVOR_φ) toward greater phase leads, particularly for the low and middle frequencies (0.2 Hz: Δphase = +5°, p < 0.05¢; 0.5 Hz: Δphase = +3°, p < 0.05¢ at 0.5 Hz; 1 Hz: Δphase = +2.5°, p > 0.1¢ at 1 Hz).
As for the angular VOR, Sham condition was first tested for the maculo-ocular reflex (MOR; Figure 1C) and no significant differences were found. Before the MS protocol, the efficacy of the MOR tested in clockwise and counterclockwise direction was similar in all mice (MORβ: 0.11 ± 0.06 vs. 0.12 ± 0.07; n = 24).
Following the MS protocol however, a significant decrease of ~50% in the efficacy of the MOR was evidenced (p < 0.001¢; Figure 1C), in both CW (MORβ POST: 0.06 ± 0.04) and CCW directions (0.06 ± 0.03).
Overall, these results demonstrate that the provocative rotation induces motion sickness-associated behavior and affects the vestibular system by decreasing its response to motion. This decrease is observed when canal-dependent (aVOR) or otolith-dependent (MOR) reflexes are recorded; suggesting that sensitivity to angular and linear motion is affected.
Scopolamine Prevents Motion Sickness-Related Changes in the Vestibular System
Scopolamine is known to help preventing motion sickness in humans. To determine if scopolamine has a comparable effect on mice, the effect of an injection of scopolamine on VOR and motion sickness were investigated on a subset of the animals (n = 16).
First, the effect of scopolamine on the Pica behavior was measured. Injection of scopolamine did not significantly change the baseline of the KIR in Sham condition (Table 2b, Figure 2A black vs. red bar; p > 0.1¢). Then, the protective effects were tested by rotating the scopolamine-injected mice. While the rotation was efficient in provoking motion sickness in the absence of scopolamine (higher KIR with the MS protocol; Table 2a, Figure 2A, white bar; p < 0.05¢), the KIR remained low when scopolamine was preemptively administrated (Table 2c, Figure 2A, gray bar). The KIR of scopolamine-injected mice after rotation was not different from that of the Control condition (no scopolamine; no rotation; Table 2d, Figure 2A black vs. gray bars; p > 0.1¢). Thus, while rotation provoked the Pica behavior in these mice, preemptive injection of scopolamine protected them against MS.
To determine the interplay between motion sickness and vestibular sensitivity, the VOR and MOR of mice injected with scopolamine was recorded. Following the injection of scopolamine, but in the absence of rotation (no MS induction), the angular VOR gain of mice was decreased (p < 0.001¢ at all tested frequencies; Figure 2B left panel). The decrease was in range ~60% at 0.2 Hz and ~40% at 0.5 and 1 Hz. Modifications of aVOR also affected the timing of the response with a tendency toward greater phase lead at 0.2 Hz (Δphase = +9°, p < 0.10¢) and 0.5 Hz (Δphase = +15°, p < 0.01¢). Notably, this effect of scopolamine was consistently observed in all injected mice tested before provocative rotation. Similarly, the MOR of scopolamine-injected mice was significantly reduced in both CW and CCW direction (Figure 2B, right panel; p < 0.01¢ for both directions).
Since motion sickness and scopolamine injection both induce reduction of the vestibular gain and increase in phase leads, we asked whether their combination would lead to a greater attenuation of vestibular reflexes. When scopolamine-injected animals were provocatively rotated, the gain of the aVOR was found to stay significantly lower compared to control conditions (p < 0.01¢ at all frequencies; Figure 2B, left panel). However, there was no additional decrease between the scopolamine and scopolamine+rotation groups (Figure 2C, left panel; all frequencies >0.05¢). A similar result was found for the MORβ which was significantly decreased by the scopolamine injection (Figure 2B, right panel, p < 0.001¢) but was not different in scopolamine-injected mice tested with or without rotation (Figure 2C, right panel). This result demonstrates that the preemptive modification of the vestibular reflexes by scopolamine injection has occluded the effects on the VOR normally observed following rotation (MS induction).
Since the scopolamine injected groups did not show any obvious sign of MS (see behavioral proxies, Figure 2A), we interpret that in saline-injected mice the reduction of the vestibular reflexes could be causally related to the occurrence of motion sickness following rotation. Since scopolamine-injected mice do not suffer from motion sickness, we hypothesize that the diminution of the vestibular sensitivity (by scopolamine injection in this case) could act as a protective mechanism against motion sickness.
Drugless Protection Against Motion Sickness
To test this hypothesis, we took advantage of a new methodology based on a long-lasting (14 days) visuo-vestibular mismatch (VVM, see Methods) which leads to a significant decrease in the gain of the VOR (24). Another subset of the mice (n = 8) was initially tested in control conditions and exposed to the provocative protocol. Before VVM, these mice had normal KIR, which again significantly increased following MS induction (Table 2e; p < 0.05¢). Following these initial tests, the animals were left unperturbed for 48 h, before to start the VVM protocol. This methodology consists in putting on the head of the mouse a device which creates a visuo-vestibular mismatch. For 2 weeks, the animals were left in their home-cage with the apparatus on the head [see Figure 3A and protocol in Carcaud et al. (24)].
How does the VVM protocol affect vestibulo-ocular reflexes? As expected, the VVM protocol significantly reduced the gain of the VOR compared to pre-VVM values at all tested frequencies by >50% (Figure 3B compare black and dark blue bars; p < 0.05¢). We then compared the maculo-ocular responses of mice before and after the VVM protocol. The MOR responses of the mice post-VVM were also significantly reduced compared to pre-VVM condition by about 50% in both clockwise and counterclockwise directions (p < 0.05¢ for both directions; Figure 3B right panel). This result demonstrates that the VOR reduction following the long-lasting visuo-vestibular mismatch already reported for the canal-dependent pathway similarly reduces the otolith-dependent pathways, possibly through central mechanisms [see discussion in Carcaud et al. (24)].
Could the reduction of vestibular sensitivity following the VVM protocol prevent motion sickness? As expected the KIR of these mice was increased by the rotations before VVM (Table 2e, Figure 3A, right panel). After the VVM, the KIR was not significantly different from control conditions (p > 0.1¢; compare black and deep blue bars on Figure 3A). When VVM mice were rotated (light blue bar), their KIR remained low, tended to be smaller compared to that of Shams (p > 0.1¢) and similar to the non-rotated condition (p > 0.1¢). The KIR in the VVM conditions tended to remain smaller from that of rotated mice (p < 0.10¢ for both non-rotated and rotated VVM conditions), suggesting a protective effect of the VVM against MS.
To prevent de-adaptation of the reflexes, no Sham condition were attempted after removal of the device. VOR and MOR of the VVM mice were thus recorded immediately after removing the device (Pre values), and again immediately after MS rotation (Post values; Figure 3A). The effects of the provocative rotation were evaluated by comparing the PRE and POST effects (Figure 3B dark vs. white bars). As previously described (Figures 1B,C), the provocative rotation induced a reduction of angular VOR and MOR in control condition seen as a significant reduction in most tested conditions (compare black and white bars). Following the VVM protocol, rotation did no longer affect the efficacy of the vestibular reflexes, so that VOR gains and MOR bias all remained low and non significantly different between the VVM and VVM + rotated conditions (Figure 3B, compare blue and light blue bars).
Overall, these results show that rotations trigger in control mice MS symptoms (KIR increase) and lead to a reduction of the aVOR gain with increased phase lead, and to a decrease of MOR bias. Scopolamine or visuo-vestibular mismatch protocols both reduce the efficacy of the reflexes and offer some protection against motion sickness symptoms.
To understand the cellular mechanisms of scopolamine, pharmacological experiments were then conducted on vestibular neurons recorded on brainstem slices.
Electrophysiological Results
Scopolamine Is Specifically Acting on MVNn Muscarinic Receptors
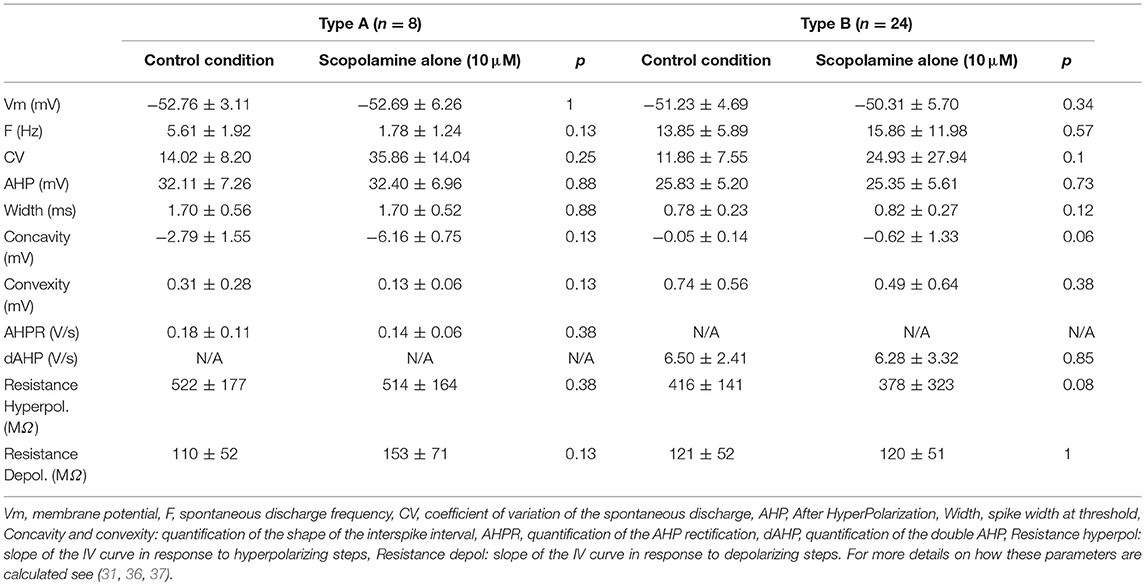
Thirty-two medial vestibular nuclei neurons (MVNn) were recorded in standard ACSF solution. MVNn can be segregated into subpopulations based on the characteristics of the after hyperplorization and inter-spike interval (35, 36). Table 3 summarizes the membrane properties computed from spontaneous discharge (pacemaker activity) of the neurons, i.e., in the absence of any electrical stimulation. Here, from the 32 neurons recorded, 8 were type A neurons characterized by a single, deep afterhyperpolarization (AHP) and 24 were type B neurons characterized by a biphasic AHP. Apart from the a priori differences (Concavity, convexity and AHP parameters), only the firing frequency differed significantly (p < 0.05) between the 2 subpopulations recorded in control conditions.
Then, scopolamine (10 μM) was applied to the bath. Notably, the addition of scopolamine did not have any effect on either type A or type B neurons (Table 3). Since scopolamine acts as an antagonist of muscarinic receptors, and because muscarinic receptors have been reported in MVNn (38), we hypothesized that this absence of modulatory effect could be due to the in vitro slices recording conditions and in particular to the non-activation of the muscarinic receptors. This result suggests that the putative action of scopolamine on MVNn is specific and restricted to its action on muscarinic receptors.
Muscarine Application Can Either Depolarize or Hyperpolarize the Cells
A second set of 19 neurons (18 type B and 1 type A) was recorded in presence of cholinergic agonists (Figure 4A). Since only one type A was recorded, no interpretation can be made about the effects of muscarine on this subpopulation.
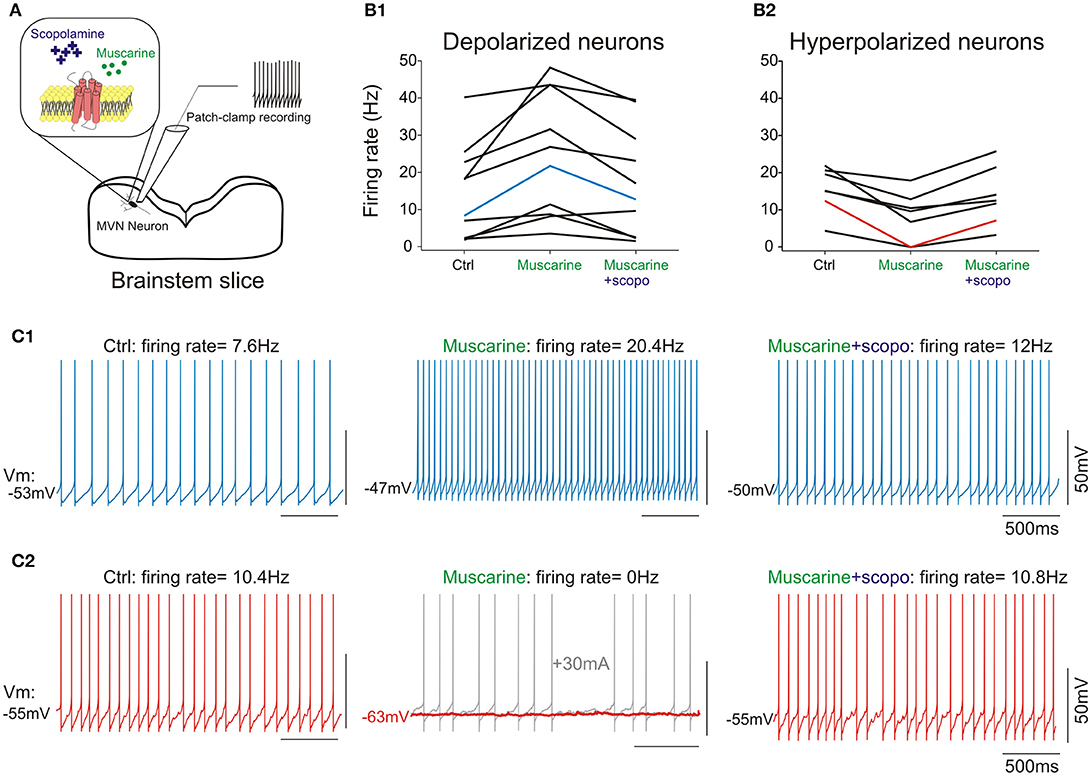
Figure 4. (A) scheme of the pharmacology patch-clamp experiment. (B1), cells depolarized by muscarine; (B2), cells hyperpolarized by muscarine. Scopolamine counteracts muscarine effect at cellular level, irrespective of initial response to muscarine. (C1) Raw traces from a type B neuron in control condition (left panel), depolarized following addition of muscarine (middle panel), and re-polarized by the addition of scopolamine (right panel). (C2) Raw traces from a type B neuron in control condition (left panel), hyperpolarized by muscarine (middle panel) and re-polarized by the addition of scopolamine (right panel). Note that the hyperpolarization induced by muscarine silenced the neuron (middle panel, red trace). A holding current (gray trace) was injected to ensure the neuron was still correctly recorded. Neurons presented in (C1,C2) are highlighted in (B1,B2). All numbers and statistics for the electrophysiology experiments are further reported in Tables 3–5.
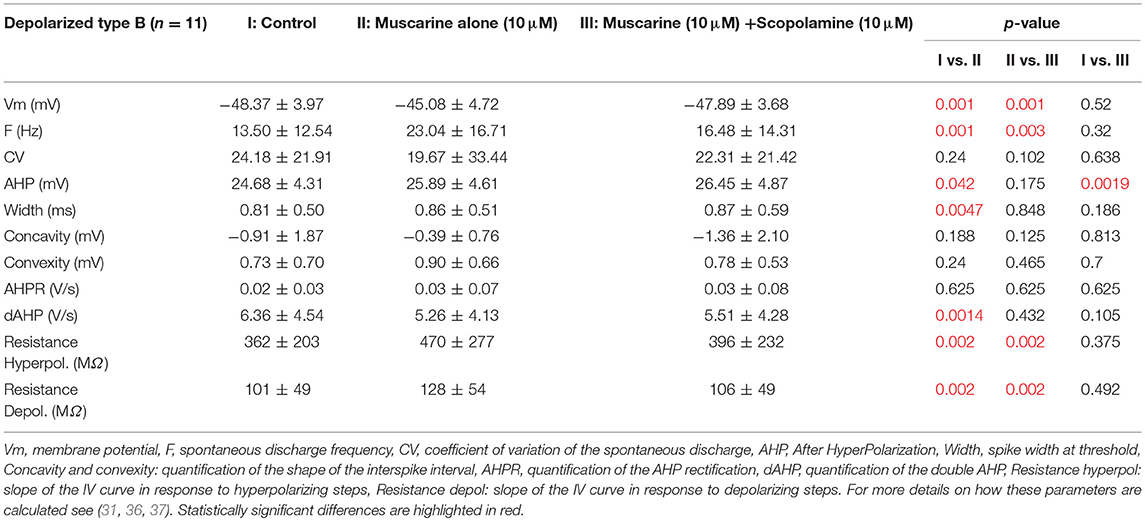
Muscarine depolarized 11 type B neurons by ~3 mV. Application of muscarine strikingly modified the frequency of the spontaneous discharge which nearly doubled (Figures 4B1,C1). In addition, it slightly but significantly increased the amplitude of the AHP and the width of the action potential. Finally, the cellular resistance measured both in presence and in absence of action potentials significantly increased by ~30% (Table 4).
Conversely, application of muscarine hyperpolarized the 7 remaining type B neurons by ~4 mV (Figures 4B2,C2), while the frequency of the spontaneous discharge was almost halved and the cellular resistance measured in presence and in absence of action potentials decreased significantly by ~40 and 30%, respectively (Table 5).
Scopolamine Counteracts an Activated Cholinergic System
What are the effects of scopolamine on both subpopulations? When applied on the depolarized neurons, scopolamine reversed all the effects of the muscarine application such that neurons membrane potential, frequency of discharge, spike parameters and resistance were all back to normal range and no longer statistically significantly modified compared to control condition (Table 4).
When scopolamine was applied on the hyperpolarized neurons, it also significantly reversed the effects of muscarine on the membrane potential, frequency of discharge, and resistance. Compared to control conditions, only the regularity of the discharge (CV) and interspike interval (Convexity) were still significantly different compared to control condition (Table 5).
Overall, these electrophysiological data show that (i) cholinergic stimulation has opposite effects on specific subpopulations of type B neurons, suggesting that each might express specific type of muscarinic receptors, (ii) scopolamine effects on vestibular neurons depends on cholinergic activation, is direct and specific, (iii) scopolamine acts as an antagonist which completely abolished the various cholinergic responses on all type B neurons tested.
Discussion
Rodent Models for Studying Motion Sickness Using Combined Genetic, Molecular and Physiological Approaches
Motion sickness is a disease associated with discomfort, and often mistaken with the emetic reflex. While the association of MS and emesis is common in humans, it was demonstrated that rodents actually lack the brainstem neurological components responsible for emesis (32). However, the illness-response behavior known as Pica was identified as an analogous to vomiting, observed both in response to intoxication (33) and to provocative vestibular stimuli (29). The Pica behavior has since the 90's extensively been used as an index of rat motion sickness [e.g., (34, 39–41)] and was later validated in mice (42, 43). In both species, the causal relation between an intact vestibular system and the Pica behavior following challenging rotational stimuli was demonstrated (29, 43). Here, we have shown that in mice, Pica behavior can serve as a reliable index of motion sickness induced by a double-rotation paradigm similar to the one originally used in rats (29). We note that other behavioral symptoms such as piloerection, tremble, and abnormal urination were also frequently observed, although not quantified here. The stimulation protocol used is particularly efficient in generating combined canalar and otolithic overstimulation. Because of the possibility to use genetically-engineered mice and to conduct molecular studies, rodent models have recently attracted the attention of many research groups. Wang et al. (44–46) have studied in rats the inter-individual differences and the implication of the vestibulo-thalamic pathway in the habituation to provocative rotation. They also demonstrated that otoconia-deficient mice (het) are less susceptible to vestibular MS, indicating the pivotal role of otolithic overstimulation in MS generation. Wang and colleagues (47) took advantage of the mouse model to study the genetic susceptibility to MS by generating MS-susceptible or MS-resistant mouse strains. This recent work suggests the implication of a new protein, the swiprosin-1, in the vestibular-dependent response to MS. Collectively these studies demonstrate that Pica behavior constitute a reliable index of MS and reveal the high potential for combined genetic, molecular and physiological approaches in rodent models of MS.
Relation Between Vestibulo-Ocular Reflexes and Motion Sickness
Many studies have investigated the correlation between VOR characteristics and the susceptibility of the subject to MS, in order to use the VOR as a predictive measurement of MS. Overall, contradictory results were reported regarding angular VOR and occurrence of MS (48, 49). Ventre-dominey and colleagues (50) reported that MS susceptibility co-occurs with decreasing time constant of the VOR and with the increasing eye velocity during otolith-specific stimulation (OVAR); however other studies contradicted this result (51) and rather suggested an implication of the velocity storage in the genesis of MS during OVAR. Recently, Clement and Reschke (52) reported a correlation between MS susceptibility and the phase lead of the VOR at low frequency, with no correlation with VOR gain. Overall, studies in humans suggest a closer relationship between MS and VOR dynamic properties (phase) rather than VOR sensitivity (gain). Notably, human studies were conducted in order to evoke some degree of discomfort, but experiments were stopped just before or as soon as the subject reached sickness (52), limiting the exposure to motion to typically few minutes, which differs from our protocol. We note also that the rotation protocol used here (combination of 2 opposite directions of rotation with sinusoidal variation in angular speed) is more challenging than the protocols used in humans. Our results suggest that a lasting provocative vestibular stimulation leading to the occurrence of MS drives a significant decrease in the gain of vestibulo-ocular reflexes associated with an increase in the phase lead. This decrease in gain similarly concerned the semi-circular canals (angular VOR) and the otoliths (Maculo-ocular reflex), compatible with the hypothesis of a central common mechanism. Reduction of motion sickness following habituation was associated with a decrease in the time constant of the velocity storage (51, 53–56) and there is evidence for angular VOR gain reduction correlated with MS reduction in expert subjects, as for instance in skaters (57) or in sailors (58). A link between a higher aVOR gain and an increase in phase lead was also suggested as an indication of higher seasickness susceptibility (48). Within this framework, the general decrease in gain and increase in phase lead in the vestibulo-ocular responses we report, putatively associated with a decrease in the general sensitivity of the vestibular system, might reduce the sensitivity to the conflicting sensory inputs, and thus putatively help preventing later occurrence of MS.
Visual and Vestibular Interactions and Motion Sickness Prevention
If interactions between the VOR main parameters and MS exist, then it might be possible to act on the reflexes to manipulate the susceptibility of the patients. Dai et al. (1) demonstrated in a group of MS-susceptible patients that a visuo-vestibular iterated training protocol could reduce MS sensitivity for several weeks following the habituation sessions. We took advantage of a long-lasting visuo-vestibular mismatch to induce a reduction in the vestibulo-ocular reflexes that again affected equally both canals- and otolith-based reflexes. We demonstrated (24) that this protocol leads to a reduction of the neural responses in the direct VOR pathway. The cellular mechanisms associated to this decrease were a reduction in the synaptic efficiency between the vestibular afferent and the central vestibular neurons and a decrease in the excitability of subpopulations of central vestibular neurons (24). In other term, the long-lasting visual perturbation reduced the brainstem sensitivity to vestibular inputs. Here, we show in mice that the visually-induced reduction in the vestibular reflexes offers a protection against MS. Our results suggest that this effect lasts for at least 3 days, although longer term effects are possible and would deserve dedicated experiments. Overall, our neural and behavioral evidence support the possibility of using visuo-vestibular protocols to habituate susceptible patients to MS induced by vestibular overstimulation or by visuo-vestibular sensory conflicts. For example, since myopic people who wear glasses (but not lenses) have lower angular VOR gains (59), it would be interesting to test whether they are less susceptible to MS than myopic people wearing lenses, and even less than hyperopic people corrected with glasses, whose angular VOR gain is enhanced because of their high positive lenses. Because changes in the efficacy of gaze stabilizing systems are often associated with oscillopsia (60, 61), it would be interesting to study if patients under anti-motion sickness treatments report greater oscillopsia during active head motions.
Scopolamine Effects on the Vestibular Reflexes and on Motion Sickness
Scopolamine is well-known as being among the most efficient anti-MS drugs in humans. It is commonly used in particular during space flight as a counter-measure against space motion sickness. In a series of experiments performed on humans in the 80's, Pyykkö et al. (62–64) demonstrated that patches of scopolamine prevented motion sickness by reducing the vestibular and optokinetic gains and suggested that the drug acted on the integrative function of the central vestibular nuclei. More recently, Werts et al. (14) reported a reduction of the angular VOR and caloric response following intranasal administration of scopolamine. Scopolamine had a depressant action on the response of the semicircular canals, postulated to be a combination of peripheral and central effects while it had little effect on the saccular reflex tested with cervico Vestibular Evoked Myogenic Potentials (cVEMP). On the other hand, Tal et al. (65) reported a significant decrease in cVEMP p13 latency following scopolamine administration. Bestaven et al. (16) demonstrated a significant reduction of ~30% of the vestibulo-spinal reflexes following galvanic vestibular stimulation associated with a decrease in balance test and vertical perception. In cat, no direct effect of scopolamine on the VOR was found at low doses, while at high doses the effects were confounded by sedation (66). To our knowledge, our experiments for the first time demonstrate in rodents that the prophylactic effect of scopolamine is associated with a reduction of vestibular sensitivity that concerns not only the semi-circular canal but also the otolith signals. We further show that the preemptive reduction of the vestibular reflexes by scopolamine injection can occlude the reduction of the VOR normally observed following MS. This occlusion suggests that both phenomena rely on a single mechanism or that, if the two processes are distinct, they converge on the same neuronal elements that cannot be adapted below a certain threshold.
Neuronal Mechanisms and Motion Sickness
What are the neuronal mechanisms associated with MS? Experimental evidence suggests that the processing of divergent sensory inputs in various brain areas (e.g., cerebellum; thalamus) contributes to patients' MS and also impacts the functioning of many cortical areas (67, 68). A key observation which emphasizes the instrumental role of the vestibular system may be, however, that patients with a total loss of labyrinthine function do not get motion sick [review in Lackner (5)]. In addition, in most instances it is the exposure to passive, rather than active, motion that leads to MS (69). In the vestibular nuclei and in the fastigial nuclei of the cerebellum, neurons categorized as “vestibular-only” were demonstrated to differentially encode passive and active movements (70–74). The proposed mechanism is termed “reafference cancelation.” It suggests that the vestibulo-cerebellum is using an internal model to predict the consequences of active, voluntary movements and substract this reafference signal from the signal sensed by the vestibular organs, termed exafference signal. As a result of the substraction of reafference and exafference, the discharge of vestibular-only neurons would represent the difference between the expected movement and the actual movement. Their discharge thus codes the “unexpected,” passive part of head movements. Vestibular-only neurons are implicated in vestibulo-spinal and vestibulo-sympathic pathway and are nowadays the best candidate for motion sickness generation within the vestibulo-cerebellum (73).
The identification of vestibular-only neurons in vitro still remains to be done. However, recent data have suggested that type B neurons constitute the vestibular-projection neurons while type A neurons would constitute the interneurons implicated in local regulation of activity (24, 35). It was also demonstrated that VN neurons that project to the cerebellum and are implicated in vestibulo-cerebellar regulatory loops are glutamatergic, so there is a high probability that vestibular-only neurons and neurons that project on the cerebellum are dominantly type B neurons. Here, all tested type B neurons were found to be modulated by cholinergic stimulation. The presence of nicotinic and muscarinic acetylcholine receptors (mAChR) in the vestibular nucleus with high density in the medial vestibular nucleus is well documented (75–79). Two distinct populations of type B neurons were found based on their modulation by the cholinergic system. Acetylcholine had opposite effects on these subpopulations, suggesting the existence of different receptors. Zhu et al. (38) reported that among the five mAChR subtypes, M2 and M3 may be the most highly expressed in the rat MVN. Interestingly, M2 is linked to the excitatory Gq/11 proteins, while M3 is coupled to the inhibitory Gi/o proteins (80, 81), and both receptors could play distinct roles in regulating vestibular afferent activity onto MVN neurons and activity of cerebellum-projecting neurons (38). The potential of mACHR subtype-specific agonists and antagonists as counter-measures again MS should be the focus of future studies.
Author Contributions
EI and MB: Designed research. EI: Performed research. EI, MT, and MB: Analyzed data and wrote the paper.
Funding
This work was funded by the Centre National des Etudes Spatiales, the University Paris Descartes, and the CNRS. EI received a post-doctoral CNES fellowship. MT and MB research is supported through CNES DAR grants.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Patrice Jegouzo for his help in technical developments during this study, and generally over the several years of collaboration.
References
1. Dai M, Raphan T, Cohen B. Prolonged reduction of motion sickness sensitivity by visual-vestibular interaction. Exp Brain Res. (2011) 210:503–13. doi: 10.1007/s00221-011-2548-8
2. Lawther A, Griffin MJ. A survey of the occurrence of motion sickness amongst passengers at sea. Aviation Space Environ Med. (1988) 59:399–406.
3. Förstberg J, Andersson E, Ledin T. Influence of different conditions for tilt compensation on symptoms of motion sickness in tilting trains. Brain Res Bull. (1998) 47:525–35.
4. Oman CM. Are evolutionary hypotheses for motion sickness “Just-so” stories? J Vesti Res. (2012) 22:117–27. doi: 10.3233/VES-2011-0432
5. Lackner JR. Motion sickness: more than nausea and vomiting. Exp Brain Res. (2014) 232:2493–510. doi: 10.1007/s00221-014-4008-8
6. Bertolini G, Straumann D. Moving in a moving world: a review on vestibular motion sickness. Front Neurol. (2016) 7:14. doi: 10.3389/fneur.2016.00014
7. Merfeld DM. Rotation otolith tilt-translation reinterpretation (ROTTR) hypothesis: a new hypothesis to explain neurovestibular spaceflight adaptation. J Vesti Res. (2003) 13:309–20.
8. Merfeld DM, Park S, Gianna-Poulin C, Black FO, Wood S. Vestibular perception and action employ qualitatively different mechanisms. II VOR and Perceptual Responses during Combined Tilt&Translation. J Neurophysiol. (2005) 94:199–205. doi: 10.1152/jn.00905.2004
9. Soto E, Vega R. Neuropharmacology of vestibular system disorders. Curr Neuropharmacol. (2010) 8:26–40. doi: 10.2174/157015910790909511
10. Davis JR, Vanderploeg JM, Santy PA, Jennings RT, Stewart DF. Space motion sickness during 24 flights of the space shuttle. Aviation Space Environ Med. (1988) 59:1185–9.
11. Heer M, Paloski WH. Space motion sickness. Exp Brain Res. (2006) 175:377–99. doi: 10.1007/s00221-006-0697-y
13. Putcha L, Berens KL, Marshburn TH, Ortega HJ, Billica RD. Pharmaceutical use by U.S. astronauts on space shuttle missions. Aviation Space Environ Med. (1999) 70:705–8.
14. Weerts AP, Putcha L, Hoag SW, Hallgren E, Van Ombergen A, et al. Intranasal scopolamine affects the semicircular canals centrally and peripherally. J Appl Physiol. 119:213–18. doi: 10.1152/japplphysiol.00149.2015
15. Matsuoka I, Domino EF. Cholinergic mechanisms in the cat vestibular system. Neuropharmacology (1975) 14:201–10.
16. Bestaven E, Kambrun C, Guehl D, Cazalets JR, Guillaud E. The influence of scopolamine on motor control and attentional processes. Peer J. (2016) 4:e2008. doi: 10.7717/peerj.2008
17. Blair S, Gavin M. Modification of the Macaque's Vestibulo-Ocular Reflex after ablation of the cerebellar vermis. Acta Oto-Laryngologica (1979) 88:235–43.
18. Jäger J, Henn V. Vestibular habituation in man and monkey during sinusoidal rotation. Ann N Y Acad Sci. (1981) 374:330–9.
19. Cohen H, Cohen B, Raphan T, Waespe W. Habituation and adaptation of the vestibuloocular reflex: a model of differential control by the vestibulocerebellum'. Exp Brain Res. (1992) 90:526–38.
20. Clément G, Tilikete C, Courjon JH. Retention of habituation of vestibulo-ocular reflex and sensation of rotation in humans. Exp Brain Res. (2008) 190:307–15. doi: 10.1007/s00221-008-1471-0
21. Beraneck M, Cullen KE. Activity of vestibular nuclei neurons during vestibular and optokinetic stimulation in the alert mouse. J Neurophysiol. (2007) 98:1549–65. doi: 10.1152/jn.00590.2007
22. Beraneck M, Bojados M, Le Séac'h A, Jamon M, Vidal PP. Ontogeny of mouse vestibulo-ocular reflex following genetic or environmental alteration of gravity sensing. PLoS ONE (2012) 7:e40414. doi: 10.1371/journal.pone.0040414
23. Stahl JS, van Alphen AM, De Zeeuw CI. A comparison of video and magnetic search coil recordings of mouse eye movements. J Neurosci Methods (2000) 99:101–10. doi: 10.1016/S0165-0270(00)00218-1
24. Carcaud J, França de Barros F, Idoux E, Eugène D, Reveret L, Moore LE, et al. Long-lasting visuo-vestibular mismatch in freely-behaving mice reduces the vestibulo-ocular reflex and leads to neural changes in the direct vestibular pathway. eNeuro (2017) 4:ENEURO.0290-16.2017. doi: 10.1523/ENEURO.0290-16.2017
25. Calabrese DR, Hullar TE. Planar relationships of the semicircular canals in two strains of mice. J Assoc Res Otolaryngol. (2006) 7:151–9. doi: 10.1007/s10162-006-0031-1
26. Oommen BS, Stahl JS. Eye orientation during static tilts and its relationship to spontaneous head pitch in the laboratory mouse. Brain Res. (2008) 1193:57–66. doi: 10.1016/j.brainres.2007.11.053
27. Hess BJM, Dieringer N. Spatial organization of the maculo-ocular reflex of the rat: responses during off-vertical axis rotation. Eur J Neurosci. (1990) 2:909–19.
28. Romand R, Krezel W, Beraneck M, Cammas L, Fraulob V, Messaddeq N, et al. Retinoic acid deficiency impairs the vestibular function. J Neurosci. (2013) 33:5856–66. doi: 10.1523/JNEUROSCI.4618-12.2013
29. Morita M, Takeda N, Kubo T, Matsunaga T. Pica as an index of motion sickness in rats. ORL (1988) 50:188–92. doi: 10.1159/000275989
30. Yu XH, Cai GJ, Liu AJ, Chu ZX, Su DF. A novel animal model for motion sickness and its first application in rodents. Physiol Behav. (2007) 92:702–7. doi: 10.1016/j.physbeh.2007.05.067
31. Idoux E, Eugène D, Chambaz A, Magnani C, White JA, Moore LE. Control of neuronal persistent activity by voltage-dependent dendritic properties. J Neurophysiol. (2008) 100:1278–86. doi: 10.1152/jn.90559.2008
32. Horn CC, Kimball BA, Wang H, Kaus J, Dienel S, Nagy A, et al. Why can't rodents vomit? A comparative, behavioral anatomical, and physiological study. PloS ONE (2013) 8:e60537. doi: 10.1371/journal.pone.0060537
33. Mitchell D, Wells C, Hoch N, Lind K, Woods SC, Mitchell LK. Poison induced pica in rats. Physiol Behav. (1976) 17:691–7. doi: 10.1016/0031-9384(76)90171-2
34. Uno A, Takeda N, Horii A, Sakata Y, Yamatodani A, Kubo T. Effects of amygdala or hippocampus lesion on hypergravity-induced motion sickness in rats. Acta Oto-Laryngologica (2000) 120:860–5.
35. Beraneck M, Idoux E. Reconsidering the role of neuronal intrinsic properties and neuromodulation in vestibular homeostasis. Front Neurol. (2012) 3:25. doi: 10.3389/fneur.2012.00025
36. Beraneck M, Hachemaoui M, Idoux E, Ris L, Uno A, Godaux E, et al. Long-term plasticity of ipsilesional medial vestibular nucleus neurons after unilateral labyrinthectomy. J Neurophysiol. (2003) 90:184–203. doi: 10.1152/jn.01140.2002
37. Beraneck M, Idoux E, Uno A, Vidal PP, Moore LE, Vibert N. Unilateral labyrinthectomy modifies the membrane properties of contralesional vestibular neurons. J Neurophysiol. (2004) 92:1668–84. doi: 10.1152/jn.00158.2004
38. Zhu Y, Chen SR, Pan HL. Muscarinic receptor subtypes differentially control synaptic input and excitability of cerebellum-projecting medial vestibular nucleus neurons. J Neurochem. (2016) 137:226–39. doi: 10.1111/jnc.13554
39. Takeda N, Horii A, Uno A, Morita M, Mochizuki T, Yamatodani A, et al. A ground-based animal model of space adaptation syndrome. J Vesti Res. (1996) 6:403–9.
40. Takeda N, Hasegawa S, Morita M, Matsunagac T. Pica in Rats Is Analogous to Emesis: an animal model in emesis research. Pharmacol Biochem Behav. (1993) 45:817–21.
41. Takeda N, Morita M, Hasegawa S, Horii A, Kubo T, Matsunaga T. Neuropharmacology of motion sickness and emesis. A Review. Acta Oto-Laryngol Suppl. (1993) 501:10–5.
42. Yamamoto K, Matsunaga S, Matsui M, Takeda N, Yamatodani A. Pica in mice as a new model for the study of emesis. Methods Find Exp Clin Pharmacol. (2002) 24:135–8. doi: 10.1358/mf.2002.24.3.802297
43. Li Z, Zhang X, Zheng J, Huang M. Pica behavior induced by body rotation in mice. ORL (2008) 70:162–7. doi: 10.1159/000124289
44. Wang JQ, Li HX, Chen XM, Mo FF, Qi RR, Guo JS, Cai YL. (2012) Temporal change in NMDA receptor signaling and GABAA receptor expression in rat caudal vestibular nucleus during motion sickness habituation. Brain Res. (2012) 1461:30–40. doi: 10.1016/j.brainres.2012.04.041
45. Wang JQ, Qi RR, Zhou W, Tang YF, Pan LL, Cai YL. Differential gene expression profile in the rat caudal vestibular nucleus is associated with individual differences in motion sickness susceptibility. PLoS ONE (2015) 10:e0124203. doi: 10.1371/journal.pone.0124203
46. Wang J, Liu J, Pan L, Qi R, Liu P, Zhou W. Storage of passive motion pattern in hippocampal CA1 region depends on CaMKII/CREB signaling pathway in a motion sickness rodent model. Sci Rep. (2017) 7:43385. doi: 10.1038/srep43385
47. Wang ZB, Han P, Tong LC, Luo Y, Su WH, Wei X, et al. Low level of swiprosin-1/EFhd2 in vestibular nuclei of spontaneously hypersensitive motion sickness mice. Sci Rep. (2017) 7:40986. doi: 10.1038/srep40986
48. Gordon CR, Spitzer O, Doweck I, Shupak A, Gadoth N. The vestibulo-ocular reflex and seasickness susceptibility. J Vesti Res. (1996) 6:229–33.
49. Quarck G, Etard O, Oreel M, Denise P. Motion sickness occurrence does not correlate with nystagmus characteristics. Neurosci Lett. (2000) 287:49–52. doi: 10.1016/S0304-3940(00)01140-X
50. Ventre-Dominey J, Luyat M, Denise P, Darlot C. Motion sickness induced by otolith stimulation is correlated with otolith-induced eye movements. Neuroscience (2008) 155:771–9. doi: 10.1016/j.neuroscience.2008.05.057
51. Dai M, Sofroniou S, Kunin M, Raphan T, Cohen B. Motion sickness induced by off-vertical axis rotation (OVAR). Exp Brain Res. (2010) 204:207–22. doi: 10.1007/s00221-010-2305-4
52. Clément G, Reschke MF. Relationship between motion sickness susceptibility and vestibulo-ocular reflex gain and phase. J Vestib Res. (2018). doi: 10.3233/VES-180632
53. Clément G, Deguine O, Parant M, Costes-Salon MC, Vasseur-Clausen P, Pavy-LeTraon A. Effects of cosmonaut vestibular training on vestibular function prior to spaceflight. Eur J Appl Physiol. (2001) 85:539–45. doi: 10.1007/s004210100494
54. Dai M, Kunin M, Raphan T, Cohen B. The relation of motion sickness to the spatial-temporal properties of velocity storage. Exp Brain Res. (2003) 151:173–89. doi: 10.1007/s00221-003-1479-4
55. Cohen B, Dai M, Raphan T. The critical role of velocity storage in production of motion sickness. Ann N Y Acad Sci. (2003) 1004:359–76. doi: 10.1196/annals.1303.034
56. Cohen B, Dai M, Yakushin SB, Raphan T. Baclofen, Motion Sickness Susceptibility and the Neural Basis for Velocity Storage. Progr Brain Res. (2008) 171:543–53. doi: 10.1016/S0079-6123(08)00677-8
57. Tanguy S, Quarck G, Etard O, Gauthier A, Denise P. (2008). Vestibulo-ocular reflex and motion sickness in figure skaters. Eur J Appl Physiol. 104:1031–7. doi: 10.1007/s00421-008-0859-7
58. Shupak A, Kerem D, Gordon C, Spitzer O, Mendelowitz N, Melamed Y. Vestibulo-ocular reflex as a parameter of seasickness susceptibility. Ann Otol Rhinol Laryngol. (1990) 99:131–1336.
59. Cannon SC, Leigh RJ, Zee DS, Abel LA. The effect of the rotational magnification of corrective spectacles on the quantitative evaluation of the VOR. Acta Oto-Laryngologica (1985) 100:81–8.
60. Straube A, Bronstein A, Straumann D. European Federation of Neurologic Societies. Nystagmus and oscillopsia Eur J Neurol. (2012) 19:6–14. doi: 10.1111/j.1468-1331.2011.03503.x
61. Sanderson J, Oman CM, Harris LR. Measurement of oscillopsia induced by vestibular Coriolis stimulation. J Vestib Res. (2007) 17:289–99.
62. Pyykkö I, Schalén L, Jäntti V, Magnusson M. A reduction of vestibulo-visual integration during transdermally administered scopolamine and dimenhydrinate. A presentation of gain control theory in motion sickness. Acta Oto-Laryngologica. Supplementum (1984) 406:167–73.
63. Pyykkö I, Padoan S, Schalén L, Lyttkens L, Magnusson M, Henriksson NG. The effects of TTS-scopolamine, dimenhydrinate, lidocaine, and tocainide on motion sickness, vertigo, and nystagmus. Aviation Space Environ Med. (1985) 56:777–82.
64. Pyykkö I, Schalén L, Jäntti V. Transdermally administered scopolamine vs. dimenhydrinate. I. effect on nausea and vertigo in experimentally induced motion sickness. Acta Oto-Laryngologica (1985) 99: 588–96.
65. Tal D, Shemy S, Kaminski-Graif G, Wiener G, Hershkovitz D. Vestibular evoked myogenic potentials and motion sickness medications. Clin Neurophysiol. (2016) 127:2350–4. doi: 10.1016/j.clinph.2016.03.010
66. Schultheis LW, Robinson DA. The effect of scopolamine on the vestibuloocular reflex, gain adaptation, and the optokinetic response. Ann N Y Acad Sci. (1992) 656:880–3.
67. Miyazaki J, Yamamoto H, Ichimura Y, Yamashiro H, Murase T, Yamamoto T. Inter-hemispheric desynchronization of the human MT + during visually induced motion sickness. Exp. Brain Res. (2015) 233:2421–31 doi: 10.1007/s00221-015-4312-y
68. Toschi N, Kim J, Sclocco R, Duggento A, Barbieri R, Kuo B, et al. Motion sickness increases functional connectivity between visual motion and nausea-associated brain regions. Auton Neurosci. (2017) 202:108–13. doi: 10.1016/j.autneu.2016.10.003
69. Oman CM. Motion sickness: a synthesis and evaluation of the sensory conflict theory. Canad J Physiol Pharmacol. (1990) 68:294–303.
70. Roy JE, Cullen KE. Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei. J Neurosci. (2004) 24:2102–11. doi: 10.1523/JNEUROSCI.3988-03.2004
71. Cullen KE. The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci. (2012) 35:185–96. doi: 10.1016/j.tins.2011.12.001
72. Jerome C, Brooks JX, Cullen KE. Multimodal integration of self-motion cues in the vestibular system: active versus passive translations. J Neurosci. (2013) 33:19555–66. doi: 10.1523/JNEUROSCI.3051-13.2013
73. Oman CM, Cullen KE. Brainstem processing of vestibular sensory exafference: implications for motion sickness Etiology. Exp Brain Res. (2014) 232:2483–92. doi: 10.1007/s00221-014-3973-2
74. Brooks JX, Cullen KE. The primate cerebellum selectively encodes unexpected self-motion. Curr Biol. (2013) 23:947–55. doi: 10.1016/j.cub.2013.04.029
75. Burke RE, Fahn S. Choline acetyltransferase activity of the principal vestibular nuclei of rat, Studied by Micropunch Technique. Brain Res. (1985) 328:196–9.
76. Rotter A, Birdsall NJ, Burgen AS, Field PM, Hulme EC, Raisman G. Muscarinic receptors in the central nervous system of the rat. I. technique for autoradiographic localization of the binding of [3H]propylbenzilylcholine mustard and its distribution in the forebrain. Brain Res. (1979) 180:141–65.
77. Schwartz RD. Autoradiographic distribution of high affinity muscarinic and nicotinic cholinergic receptors labeled with [3H]acetylcholine in rat brain. Life Sci. (1986) 38:2111–9.
78. Wamsley JK, Lewis MS, Young WS, Kuhar MJ. Autoradiographic localization of muscarinic cholinergic receptors in rat brainstem. J Neurosci. (1981) 1:176–91.
79. Zanni M, Giardino L, Toschi L, Galetti G, Calzà L. Distribution of neurotransmitters, neuropeptides, and receptors in the vestibular nuclei complex of the rat: an immunocytochemical, in situ hybridization and quantitative receptor autoradiographic study. Brain Res Bull. (1995) 36:443–52.
80. Felder CC. Muscarinic acetylcholine receptors: signal transduction through multiple effectors. FASEB J. (1995) 9:619–25.
Keywords: vestibular, motion sickness, scopolamine, VOR, neurons, mouse, spatial orientation, visuo-vestibular
Citation: Idoux E, Tagliabue M and Beraneck M (2018) No Gain No Pain: Relations Between Vestibulo-Ocular Reflexes and Motion Sickness in Mice. Front. Neurol. 9:918. doi: 10.3389/fneur.2018.00918
Received: 21 August 2018; Accepted: 10 October 2018;
Published: 12 November 2018.
Edited by:
Christophe Lopez, Centre National de la Recherche Scientifique (CNRS), FranceReviewed by:
Andreas Zwergal, Ludwig-Maximilians-Universität München, GermanyHans Straka, Ludwig-Maximilians-Universität München, Germany
Copyright © 2018 Idoux, Tagliabue and Beraneck. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erwin Idoux, ZWlkb3V4QGJ1LmVkdQ==
Mathieu Beraneck, bWF0aGlldS5iZXJhbmVja0BwYXJpc2Rlc2NhcnRlcy5mcg==
 Erwin Idoux
Erwin Idoux Michele Tagliabue
Michele Tagliabue Mathieu Beraneck
Mathieu Beraneck