
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurol. , 24 October 2018
Sec. Neurotrauma
Volume 9 - 2018 | https://doi.org/10.3389/fneur.2018.00885
This article is part of the Research Topic Developing Successful Neuroprotective Treatments for TBI: Translational Approaches, Novel Directions, Opportunities and Challenges View all 24 articles
 Marco Carbonara1
Marco Carbonara1 Francesca Fossi2,3
Francesca Fossi2,3 Tommaso Zoerle1
Tommaso Zoerle1 Fabrizio Ortolano1
Fabrizio Ortolano1 Federico Moro2
Federico Moro2 Francesca Pischiutta2
Francesca Pischiutta2 Elisa R. Zanier2*
Elisa R. Zanier2* Nino Stocchetti1,4
Nino Stocchetti1,4Traumatic brain injury (TBI) is a leading cause of death and disability worldwide. In the last 30 years several neuroprotective agents, attenuating the downstream molecular and cellular damaging events triggered by TBI, have been extensively studied. Even though many drugs have shown promising results in the pre-clinical stage, all have failed in large clinical trials. Mesenchymal stromal cells (MSCs) may offer a promising new therapeutic intervention, with preclinical data showing protection of the injured brain. We selected three of the critical aspects identified as possible causes of clinical failure: the window of opportunity for drug administration, the double-edged contribution of mechanisms to damage and recovery, and the oft-neglected role of reparative mechanisms. For each aspect, we briefly summarized the limitations of previous trials and the potential advantages of a newer approach using MSCs.
Traumatic brain injury (TBI) is the leading cause of mortality and morbidity across all ages in all countries. In Europe, it is estimated that 2.5 million people suffer a TBI each year, 1 million are admitted to hospital, and 57,000 die (1). TBI survivors have to deal with chronic post-injury motor, cognitive, and neuropsychological symptoms/dysfunctions. Even in the milder cases, TBI substantially increases the risk of epilepsy, stroke, and late-life neurodegenerative diseases (1). TBI thus implies a huge burden for patients, their families, and society.
Trauma causes primary damage to the brain by multiple mechanisms, including tearing, shearing, and stretching forces. Consequently, a cascade of metabolic, biochemical, and inflammatory changes is initiated, leading to secondary damage. Then second insults, both intracranial and systemic such as hypoxia, hypotension and intracranial hypertension, may worsen the progression of the injury.
Treatment of TBI patients has not changed much in the last 20 years, consisting only in supportive therapy directed at prevention, early detection and treatment of second insults, since all pharmacological trials testing neuroprotective agents have failed (2–5). This translational defeat may have several explanations, analyzed in numerous papers (6–8). In these critical reappraisals, many factors were identified at preclinical and clinical levels as area of improvement. They included, but were not limited to, pharmacokinetics and pharmacodynamics, inadequate sample sizes, heterogeneity of TBI populations, the lack of relevant mechanistic early endpoints and insensitivity of global outcome measures (9).
Mesenchymal stromal cells (MSCs) may offer a promising strategy, with preclinical data showing that MSCs of human origin protect the injured brain by acting on multiple mechanisms of protection and repair (10–13), with potential advantages in terms of therapeutic window.
After initial expectations about the possibility of MSC trans-differentiation through neuronal lineage for brain reconstruction, decades of experimental data mainly show that MSCs do not protect the TBI brain through cell replacement, but by stimulating neuroprotective and endogenous neuroreparative mechanisms that this narrative review will discuss. We shall focus on three flaws of past trials that MSC-based therapy has the potential to overcome: the “window of opportunity” for drug administration, the double-edged contribution of mechanisms to damage and recovery, and the important, but often neglected, role of reparative mechanisms.
The biochemical mechanisms of progressive brain damage are set in motion immediately after TBI as a consequence of the external force applied to the head. Using microdialysis in a rodent model of concussion, Katayama demonstrated a surge of extracellular potassium in the first minutes after injury, parallel with massive release of glutamate—up to 10–100 times the normal concentration (14). The time-resolution of the method, however, was limited (1 min of dead space for dialysate collection); when electrodes were used, almost immediate K+ release was demonstrated after trauma (15).
Early mechanisms of cellular injury act in minutes-to-hours after injury. The massive release of excitatory neurotransmitters, spreads energy failure and overload of free radicals from the contused tissue to surrounding brain regions. Energy crisis alters cell permeability, causing calcium inflow, which triggers mitochondrial dysfunction, with consequent energy failure, and apoptotic/necrotic death. Primary axotomy is uncommon, even in the case of traumatic axonal injury; the alteration of membrane permeability induces edema and impairs axonal transport, making axons more vulnerable to secondary axotomy and demyelination. These cascades clearly indicate how mechanical forces applied to the brain may evolve and propagate to healthy, potentially salvable tissue (16, 17).
The progress of secondary injury, in its sequence of deleterious events over time, is the theoretical basis for neuroprotective strategies. When neuroprotectant drugs were tested under experimental conditions, it became evident that their maximum potential was exploited by early administration or—when possible—by pre-treating the brain before insults (18). In general, however, later exposure to a protective compound gave less or no benefit (19–21).
These findings shaped the design of clinical trials, where drugs had to be administered in the first hours after injury, when there was felt to be a “window of opportunity.” A recent review (5) of 16 robust trials testing neuroprotective agents in TBI indicated a window of opportunity of 4 h in three, 6 h in two, 8 h in seven, and 12 h in one trial, while only three trials tested treatment up to 24 h after injury (Table 1). This narrow window of opportunity makes clinical trials more challenging, reducing enrolment rates and increasing complexity. Patients need to be rescued, stabilized, centralized to the study center, evaluated clinically and by imaging; relatives must be contacted for consent procedures so, finally, randomization and drug preparation and administration can start within the few hours permitted by the protocol. It is no surprise that a number of cases failed to meet the time limit: in several trials ~20% of potential candidates could not be enrolled because of the narrow time window (36). In the same trials the duration of the pharmacological intervention was limited to the first days after ICU admission (Table 1).
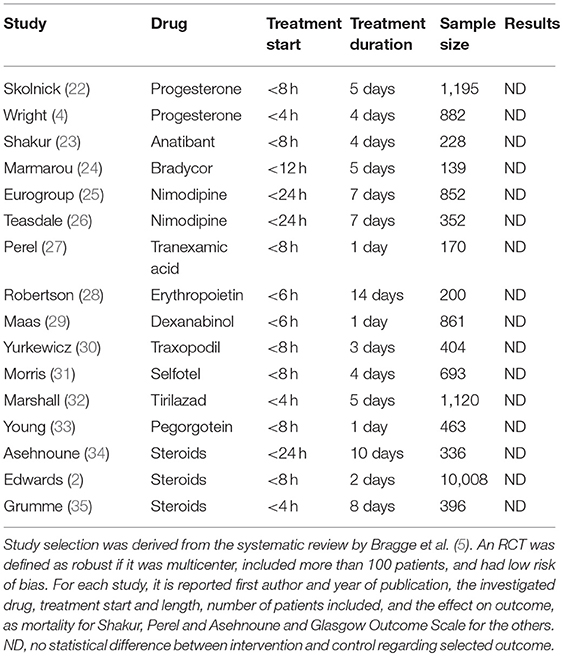
Table 1. Major randomized clinical trials (RCT) evaluating pharmacological treatments for acute moderate/severe TBI.
However, mounting evidences indicate that pathophysiologic processes caused by the initial injury do not exhaust themselves in the first days but persist for months or years, and that TBI survivors are at risk of late neurodegenerative diseases (including Alzheimer's and Parkinson's disease, chronic traumatic encephalopathy) (37). For example, Johnson et al. reported immunohistochemical evidence of microglia activation and white matter degradation in subjects who died many years after TBI (38); and in patients there is a relation between chronic inflammation detected by positron emission tomography, up to 17 years post-TBI, and worse cognitive outcomes (39).
Inflammation is an important beneficial mechanism for clearing pathological debris and effecting repair (40–44); however, if dysregulated, it may also contribute to neuronal damage. The relative positive or negative effects of inflammation in relation to time from injury are still far from certain, and a threat of neurodegeneration associated with late microglia inhibition has recently been reported in TBI subjects chronically treated with minocycline, an antibiotic that can inhibit microglia activation (45). With this in mind, immunomodulatory rather than inhibitory strategies contributing to the resolution of inflammatory changes may prove effective, with a wide therapeutic window.
MSCs have high immunomodulatory potential both in vitro and in vivo. It has been suggested that in response to injury these cells can sense the injured environment, leading to the promotion of injury resolution and regenerative processes through the secretion of immunomodulatory bioactive factors and trophic molecules including growth factors, cytokines, and antioxidants (46–48), that may vary in relation to the needs of the tissue and the time from injury.
Preclinical studies in rodents address the effects of MSCs in a wide range of TBI-to-therapy intervals, with consistent data from several laboratories showing their efficacy when infused 24 h post-TBI. Both central (49–60) and systemic (60–75) administration of MSCs 24 h post-TBI have resulted in early and persistent improvements of functional and structural outcomes. It was recently shown that a double systemic infusion of MSC (at 4 and 24 h) post-TBI was more effective than a single dose at 24 h (76). The authors showed 4 and 24 h post-TBI peaks of IL1β, TNFα, and IFNγ, suggesting that a shorter lag time between TBI and treatment may be important to counteract early pro-inflammatory changes. Whether this gain in protection was due to multiple doses or the earlier treatment still needs to be fully investigated.
MSC infusion has also given protective effects when delivered in the sub-acute phase (between 2 and 7 days after injury) either systemically (77, 78) or centrally (79–81). Kota et al. administered bone-marrow MSCs (BM-MSCs) 3 days after TBI, showing IFN-γ and TNF-α reductions of ~50% (82), with significant inhibition of brain permeability, edema, microglial activation and systemic levels of norepinephrine, while promoting neurogenesis (83).
Effect size relative to the time from experimental TBI to MSC administration has been evaluated in a recent meta-analysis (10). The analysis confirms MSC efficacy when infused from 2 h up to 7 days, with no significant differences in effect sizes relative to the time from TBI to intervention.
So far there are only few reports of MSC given in the chronic phase of TBI. At 2 months post-TBI, MSC transplant into the lesion core improved sensorimotor deficits and promoted neuro-restorative processes (84, 85). However, at this stage iv injection was not effective (86), suggesting that MSC may act through complementary mechanisms when infused locally into the brain or systemically, the latter no longer being sufficient at later stages.
Compelling data on MSC rationale, efficacy, immune tolerance, and feasibility are fostering the design of clinical studies. Pragmatically, to optimize the reduction of toxic cascades and the promotion of endogenous reparative mechanisms, an administration of MSC within 48 h from TBI would seem to be desirable. However, only data from clinical trials will provide a definitive answer in term of the best timing for intervention.
Counteracting specific damage pathways should reduce the extent of tissue injury, and ultimately contribute to a better outcome. This is the logic behind several lines of investigation in TBI, from studies on calcium blockers to N-methyl-D-aspartate (NMDA) antagonists.
Under physiological conditions, glutamate plays an important role as a neurotransmitter; it is also involved in coupling glucose utilization and neuronal activity (87). However, high concentrations of glutamate (100–500 micromoles) are lethal for neurons in vitro, and have been measured in the extracellular space of experimental TBI in rodents. In humans too there is evidence of high extracellular concentrations of glutamate after TBI, particularly in the elderly (88). This supported the hypothesis that blocking glutamate receptors (like the NMDA subtype) might attenuate the deleterious effects of the glutamate surge induced by TBI. Several compounds inhibiting the NMDA receptors, with competitive or non-competitive mechanisms, have therefore been tested in experimental and clinical settings. While in the laboratory evidence of neuroprotection was found (89, 90), clinical trials all failed to show benefit (91). Among the possible explanations for these repeated failures, there is the hypothesis that NMDA receptor activity is essential for neuronal function and integrity, so that NMDA blockage at critical time points (92), especially in vulnerable phases after TBI, could be deleterious rather than protective.
Another failed neuroprotective treatment is corticosteroids, which were tested in the first mega-trial in TBI at the end of the last century, “Corticosteroid randomization after significant head injury” (CRASH) (2). The hypothesis leading to this trial was that inflammation is a key component of the brain response to TBI and that blocking the inflammatory cascade could therefore be protective. Soluble mediators and cellular components of inflammation were investigated and related to the extent of brain damage. Unfortunately, however, the CRASH results showed no improvement in favorable outcomes and there was in fact a higher risk of mortality in the treatment group, not fully explained by systemic complications (such as infection and gastric bleeding); this may call into play the complex double-edged function of inflammation involved not only in toxic but also in regenerative processes, as discussed above. In this context MSCs affect the biology of the injured cells and tissue through the secretion of cytokines, morphogens, small molecules, and cargo-bearing exosomes (93, 94), which skew the activation of immune cells from a toxic to a more permissive phenotype, thus contributing to injury resolution and tissue repair (55, 58).
Besides toxic cascades TBI also induces neuro-restorative processes including neurogenesis, gliogenesis, angiogenesis, synaptic plasticity, and axonal sprouting (95–97). These events are induced by biochemical factors such as growth factors, steroids, and neurotransmitters, released in response to injury, with the potential for counteracting progression of the injury and contributing to functional recovery. However, all these spontaneous processes are short-lived and the efficacy of the self-repair responses is limited. Providing the injured tissue with a facilitatory milieu that increases endogenous reparative mechanisms may open up new therapeutic opportunities.
In the adult brain the subventricular zone (SVZ) and the subgranular zone (SGZ) of the dentate gyrus (DG) are populated by neural stem cells, which can differentiate into functional neurons (95, 98). Proliferation in the DG is age-dependent, with higher potential in the juvenile brains. The new cells can differentiate into functional mature neurons, involved in higher functions.
The neurogenic response after TBI comprises three phases: proliferation of precursors/progenitors cells, migration to injured tissue, and differentiation into proper cell types (99). An increased proliferative response in the hippocampus 2 days after TBI, with a peak in the first week after injury, has been described in different TBI models (100). These proliferating cells may differentiate into astrocytes, oligodendrocytes, and neurons, and extend projections alongside the hippocampal mossy fibers participating in recovery of function.
TBI induces a proliferative response in the neurogenic niche in the SVZ and hippocampus (101), under stimulation by growth factors. Preclinical studies have shown that intracerebral administration of single growth factors including fibroblast growth factor 2 (FGF-2) and epidermal growth factor (EGF) can promote endogenous neurogenesis after TBI (102, 103) and improve cognitive outcome. Similarly, infusion of VEGF into the lateral brain ventricle in TBI mice promotes cell proliferation in the SVZ and the peri-lesional cortex after TBI (104); VEGF in fact mediates the survival of newly generated neurons rather than proliferation of neuroblasts (105).
On account of their neurogenic and neuroprotective effects, growth factors are an interesting tool to stimulate reparative processes after TBI. However, their administration after injury is linked to temporal issues related to their rapid kinetics and limited effects. Studies in TBI rodents have shown increased amounts of growth factors after MSC treatment (52, 55, 64, 106–108), leading to the promotion of endogenous restorative processes and suggesting that MSCs may act as a local bioreactor able to produce and release a multitude of growth factors, depending on the specific requirements of the injured tissue.
It has been shown that MSCs stimulate endogenous neurogenesis with an higher proliferation rate in the SVZ and SGZ (64) and an increased number of developing neurons in the SVZ (detected as doublecortin marker) (57, 60); they also stimulate axonal regeneration, as documented by increased GAP-43 expression (58, 107) in MSC-treated TBI animals. Likewise, their ability to promote plasticity in TBI has been documented by infusing a fluorescent dye into the contralateral cortex 5 weeks after injury and measuring its transport from the injection site to the injured hemisphere through the corpus callosum 1 week later (79). Functional outcome and axonal fiber length were increased in MSC-treated animals, suggesting an MSC mediated effect on neuronal connectivity by directing axonal projections, neurite outgrowth and elongation in the injured cortex.
Another aspect linked to neuroplastic processes is represented by glial activation and extracellular matrix composition, both aspects possibly being modulated by MSCs. Acute glial activation is needed to clear excessive glutamate release and remove cellular debris (109, 110). However, at chronic stages, excessive gliotic scar may hamper remodeling processes (111). MSCs reduce the gliotic scar surrounding the contusion 1 month after TBI and this effect is associated with a smaller lesion and better functional recovery (55, 57). MSCs can also alter the extracellular matrix composition, allowing restorative plasticity by circuit reorganization (112).
MSCs act also on vascular cerebral compartment, increasing vessel density in the pericontusional tissue after acute (24 h post-TBI) (57, 60, 113, 114), sub-acute (7 days) (115), and chronic (84) administrations. This suggests that rescue effects on injured vessels as well as regenerative action on brain vasculature involve mechanisms stimulated by cell therapy. In fact, gene expression microarray analysis showed MSC expression of genes involved in angiogenic processes possibly sustaining both neurovascular repair in the acute phase after injury and neovascularization later on (81).
Despite its high prevalence and heavy social burden, TBI remains a neglected syndrome. Acute care for TBI patients relies on maintenance of cerebral and body homeostasis, blunting or avoiding further insults. After more than 30 years looking for treatments, broadly defined as neuroprotective strategies, to reverse or mitigate injury progression in TBI, we still lack any effective therapy. The reasons for this failure have been extensively analyzed, and new therapeutic approaches for dealing with them could have higher translational potential. Experimental studies support the hypothesis that MSCs may overcome three of the major limitations. First, MSCs in animal models show efficacy when administered within the acute, sub-acute and delayed phases post-TBI, not being limited by a narrow window of opportunity. Second, preclinical data support the notion that MSCs influence a complex pathway such as inflammation, favoring restorative over deleterious aspects. Finally, the recognition that MSCs act on the injured environment fostering reparative processes, relies on a new paradigm, exploitation of the endogenous ability of self-repair.
In conclusion, MSCs have the potential to be the next candidate for neuroprotective trials in TBI patients.
MC, TZ, ERZ, and NS designed the review, assembled a preliminary draft, and incorporated further contributions from each author into subsequent versions. All the authors revised it critically for important intellectual content and approved the final version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We acknowledge the contribution of D. Fattori for drawing Figure 1.
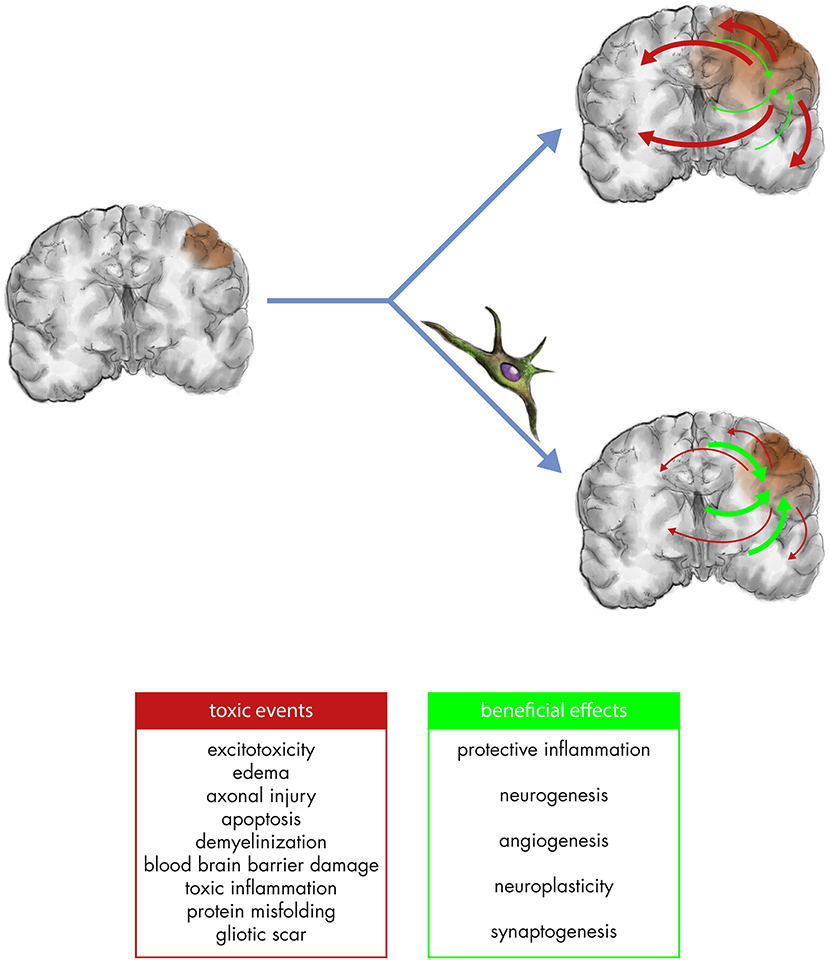
Figure 1. After the biomechanical impact (left panel), toxic secondary cascades including excitotoxicity, axonal injury, apoptosis, demyelinization, blood/brain barrier damage, toxic inflammation, protein misfolding, and gliotic scar (red arrows) contribute to the amplification of brain damage. Endogenous responses in TBI also comprise potentially beneficial mechanisms of protective inflammation, neurogenesis, angiogenesis, neuroplasticity, synaptogenesis (green arrows) but are too weak and short-lived to counteract the toxic cascades (right upper panel). MSC can mitigate toxic cascades and foster the regenerative ones, contributing to both neuroprotection and neurorestoration (right lower panel).
1. Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. (2017) 16:987–1048. doi: 10.1016/S1474-4422(17)30371-X
2. Edwards P, Arango M, Balica L, Cottingham R, El-Sayed H, Farrell B, et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet Lond Engl. (2005) 365:1957–9. doi: 10.1016/S0140-6736(05)66552-X
3. Temkin NR, Anderson GD, Winn HR, Ellenbogen RG, Britz GW, Schuster J, et al. Magnesium sulfate for neuroprotection after traumatic brain injury: a randomised controlled trial. Lancet Neurol. (2007) 6:29–38. doi: 10.1016/S1474-4422(06)70630-5
4. Wright DW, Yeatts SD, Silbergleit R, Palesch YY, Hertzberg VS, Frankel M, et al. Very early administration of progesterone for acute traumatic brain injury. N Engl J Med. (2014) 371:2457–66. doi: 10.1056/NEJMoa1404304
5. Bragge P, Synnot A, Maas AI, Menon DK, Cooper DJ, Rosenfeld JV, et al. A state-of-the-science overview of randomized controlled trials evaluating acute management of moderate-to-severe traumatic brain injury. J Neurotrauma (2016) 33:1461–78. doi: 10.1089/neu.2015.4233
6. Hawryluk GWJ, Bullock MR. Past, present, and future of traumatic brain injury research. Neurosurg Clin N Am. (2016) 27:375–96. doi: 10.1016/j.nec.2016.05.002
7. Maas AIR, Roozenbeek B, Manley GT. Clinical trials in traumatic brain injury: past experience and current developments. Neurother J Am Soc Exp Neurother. (2010) 7:115–26. doi: 10.1016/j.nurt.2009.10.022
8. Zoerle T, Carbonara M, Zanier ER, Ortolano F, Bertani G, Magnoni S, et al. Rethinking neuroprotection in severe traumatic brain injury: toward bedside neuroprotection. Front Neurol. (2017) 8:354. doi: 10.3389/fneur.2017.00354
9. Stocchetti N, Taccone FS, Citerio G, Pepe PE, Le Roux PD, Oddo M, et al. Neuroprotection in acute brain injury: an up-to-date review. Crit Care Lond Engl. (2015) 19:186. doi: 10.1186/s13054-015-0887-8
10. Peng W, Sun J, Sheng C, Wang Z, Wang Y, Zhang C, et al. Systematic review and meta-analysis of efficacy of mesenchymal stem cells on locomotor recovery in animal models of traumatic brain injury. Stem Cell Res Ther. (2015) 6:47. doi: 10.1186/s13287-015-0034-0
11. Gennai S, Monsel A, Hao Q, Liu J, Gudapati V, Barbier EL, et al. Cell-based therapy for traumatic brain injury. Br J Anaesth. (2015) 115:203–12. doi: 10.1093/bja/aev229
12. Hasan A, Deeb G, Rahal R, Atwi K, Mondello S, Marei HE, et al. Mesenchymal stem cells in the treatment of traumatic brain injury. Front Neurol. (2017) 8:28. doi: 10.3389/fneur.2017.00028
13. Dekmak A, Mantash S, Shaito A, Toutonji A, Ramadan N, Ghazale H, et al. Stem cells and combination therapy for the treatment of traumatic brain injury. Behav Brain Res. (2018) 340:49–62. doi: 10.1016/j.bbr.2016.12.039
14. Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg. (1990) 73:889–900. doi: 10.3171/jns.1990.73.6.0889
15. DeSalles AAF, Jenkins LW, Anderson RL, Opoku-Edusei J, Marmarou A, Hayes RL. Extracellular potassium activity following concussion. A microelectrode study in the cat. Soc Neurosci. (1986) 12:967.
16. Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nat Rev Neurosci. (2013) 14:128–42. doi: 10.1038/nrn3407
17. Pischiutta F, Micotti E, Hay JR, Marongiu I, Sammali E, Tolomeo D, et al. Single severe traumatic brain injury produces progressive pathology with ongoing contralateral white matter damage one year after injury. Exp Neurol. (2018) 300:167–78. doi: 10.1016/j.expneurol.2017.11.003
18. McCulloch J, Ozyurt E, Park CK, Nehls DG, Teasdale GM, Graham DI. Glutamate receptor antagonists in experimental focal cerebral ischaemia. Acta Neurochir Suppl. (1993) 57:73–9.
19. Sullivan PG, Rabchevsky AG, Hicks RR, Gibson TR, Fletcher-Turner A, Scheff SW. Dose-response curve and optimal dosing regimen of cyclosporin A after traumatic brain injury in rats. Neuroscience (2000) 101:289–95. doi: 10.1016/S0306-4522(00)00380-8
20. Sullivan PG, Sebastian AH, Hall ED. Therapeutic window analysis of the neuroprotective effects of cyclosporine A after traumatic brain injury. J Neurotrauma (2011) 28:311–8. doi: 10.1089/neu.2010.1646
21. Wang B, Kang M, Marchese M, Rodriguez E, Lu W, Li X, et al. Beneficial effect of erythropoietin short peptide on acute traumatic brain injury. Neurother J Am Soc Exp Neurother. (2016) 13:418–27. doi: 10.1007/s13311-015-0418-y
22. Skolnick BE, Maas AI, Narayan RK, van der Hoop RG, MacAllister T, Ward JD, et al. Synapse trial investigators. A clinical trial of progesterone for severe traumatic brain injury. N Engl J Med. (2014) 371:2467-76. doi: 10.1056/NEJMoa1411090
23. Shakur H, Andrews P, Asser T, Balica L, Boeriu C, Quintero JD, et al. The brain trial: a randomised, placebo controlled trial of a Bradykinin B2 receptor antagonist (Anatibant) in patients with traumatic brain injury. Trials (2009) 10:109. doi: 10.1186/1745-6215-10-109
24. Marmarou A, Nichols J, Burgess J, Newell D, Troha J, Burnham D, et al. Effects of the bradykinin antagonist BradycorTM (deltibant, CP-1027) in severe traumatic brain injury: results of a multi-center, randomized, placebo-controlled trial. American Brain Injury Consortium Study Group. J Neurotrauma (1999) 16:431–44. doi: 10.1089/neu.1999.16.431
25. The European Study Group on Nimodipine in Severe Head Injury. A multicenter trial of the efficacy of nimodipine on outcome after severe head injury. J Neurosurg. (1994) 80:797–804. doi: 10.3171/jns.1994.80.5.0797
26. Teasdale G, Bailey I, Bell A, Gray J, Gullan R, Heiskanan O, et al. A randomized trial of nimodipine in severe head injury: HIT I. British/Finnish Co-operative Head Injury Trial Group. J Neurotrauma (1992) 9(Suppl 2):S545–50.
27. Perel P, Al-Shahi Salman R, Kawahara T, Morris Z, Prieto-Merino D, Roberts I, et al. CRASH-2 (Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage) intracranial bleeding study: the effect of tranexamic acid in traumatic brain injury–a nested randomised, placebo-controlled trial. Health Technol Assess. (2012) 16:1–54. doi: 10.3310/hta16130
28. Robertson CS, Hannay HJ, Yamal JM, Gopinath S, Goodman JC, Tilley BC, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. JAMA (2014) 312:36–47. doi: 10.1001/jama.2014.6490
29. Maas AI, Murray G, Henney H III, Kassem N, Legrand V, Mangelus M, et al. Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol. (2006) 5:38–45. doi: 10.1016/S1474-4422(05)70253-2
30. Yurkewicz L, Weaver J, Bullock MR, Marshall LF. The effect of the selective NMDA receptor antagonist traxoprodil in the treatment of traumatic brain injury. J Neurotrauma (2005) 22:1428–43. doi: 10.1089/neu.2005.22.1428
31. Morris GF, Bullock R, Marshall SB, Marmarou A, Maas A, Marshall LF. Failure of the competitive N-methyl-D-aspartate antagonist Selfotel (CGS 19755) in the treatment of severe head injury: results of two phase III clinical trials. The Selfotel Investigators. J Neurosurg. (1999) 91:737–43. doi: 10.3171/jns.1999.91.5.0737
32. Marshall LF, Maas AI, Marshall SB, Bricolo A, Fearnside M, Iannotti F, et al. A multicenter trial on the efficacy of using tirilazad mesylate in cases of head injury. J Neurosurg. (1998) 89:519–25. doi: 10.3171/jns.1998.89.4.0519
33. Young B, Runge JW, Waxman KS, Harrington T, Wilberger J, Muizelaar JP, et al. Effects of pegorgotein on neurologic outcome of patients with severe head injury. A multicenter, randomized controlled trial. JAMA (1996) 276:538–43. doi: 10.1001/jama.1996.03540070034027
34. Asehnoune K, Seguin P, Allary J, Feuillet F, Lasocki S, Cook F, et al. Hydrocortisone and fludrocortisone for prevention of hospital-acquired pneumonia in patients with severe traumatic brain injury (Corti-TC): a double-blind, multicentre phase 3, randomised placebo-controlled trial. Lancet Respir Med. (2014) 2:706–16. doi: 10.1016/S2213-2600(14)70144-4
35. Grumme T, Baethmann A, Kolodziejczyk D, Krimmer J, Fischer M, von Eisenhart Rothe B, et al. Treatment of patients with severe head injury by triamcinolone: a prospective, controlled multicenter clinical trial of 396 cases. Res Exp Med (Berl). (1995) 195:217–29. doi: 10.1007/BF02576791
36. Roozenbeek B, Maas AIR, Marmarou A, Butcher I, Lingsma HF, Lu J, et al. The influence of enrollment criteria on recruitment and outcome distribution in traumatic brain injury studies: results from the impact study. J Neurotrauma (2009) 26:1069–75. doi: 10.1089/neu.2008.0569
37. Simon DW, McGeachy MJ, Bayir H, Clark RSB, Loane DJ, Kochanek PM. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat Rev Neurol. (2017) 13:171–91. doi: 10.1038/nrneurol.2017.13
38. Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol. (2013) 246:35–43. doi: 10.1016/j.expneurol.2012.01.013
39. Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, et al. Inflammation after trauma: microglial activation and traumatic brain injury. Ann Neurol. (2011) 70:374–83. doi: 10.1002/ana.22455
40. Neumann H, Kotter MR, Franklin RJM. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain J Neurol. (2009) 132:288–95. doi: 10.1093/brain/awn109
41. Nielsen HH, Ladeby R, Fenger C, Toft-Hansen H, Babcock AA, Owens T, et al. Enhanced microglial clearance of myelin debris in T cell-infiltrated central nervous system. J Neuropathol Exp Neurol. (2009) 68:845–56. doi: 10.1097/NEN.0b013e3181ae0236
42. Lozano D, Gonzales-Portillo GS, Acosta S, de la Pena I, Tajiri N, Kaneko Y, et al. Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr Dis Treat. (2015) 11:97–106. doi: 10.2147/NDT.S65815
43. Ertürk A, Mentz S, Stout EE, Hedehus M, Dominguez SL, Neumaier L, et al. Interfering with the chronic immune response rescues chronic degeneration after traumatic brain injury. J Neurosci Off J Soc Neurosci. (2016) 36:9962–75. doi: 10.1523/JNEUROSCI.1898-15.2016
44. Stocchetti N, Zanier ER. Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review. Crit Care Lond Engl. (2016) 20:148. doi: 10.1186/s13054-016-1318-1
45. Scott G, Zetterberg H, Jolly A, Cole JH, De Simoni S, Jenkins PO, et al. Minocycline reduces chronic microglial activation after brain trauma but increases neurodegeneration. Brain J Neurol. (2018) 141:459–71. doi: 10.1093/brain/awx339
46. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. (2008) 8:726–36. doi: 10.1038/nri2395
47. Carty F, Mahon BP, English K. The influence of macrophages on mesenchymal stromal cell therapy: passive or aggressive agents? Clin Exp Immunol. (2017) 188:1–11. doi: 10.1111/cei.12929
48. Xu C, Fu F, Li X, Zhang S. Mesenchymal stem cells maintain the microenvironment of central nervous system by regulating the polarization of macrophages/microglia after traumatic brain injury. Int J Neurosci. (2017) 127:1124–35. doi: 10.1080/00207454.2017.1325884
49. Mahmood A, Lu D, Yi L, Chen JL, Chopp M. Intracranial bone marrow transplantation after traumatic brain injury improving functional outcome in adult rats. J Neurosurg. (2001) 94:589–95. doi: 10.3171/jns.2001.94.4.0589
50. Mahmood A, Lu D, Wang L, Chopp M. Intracerebral transplantation of marrow stromal cells cultured with neurotrophic factors promotes functional recovery in adult rats subjected to traumatic brain injury. J Neurotrauma (2002) 19:1609–17. doi: 10.1089/089771502762300265
51. Kojima R, Kami M, Kanda Y, Kusumi E, Kishi Y, Tanaka Y, et al. Comparison between reduced intensity and conventional myeloablative allogeneic stem-cell transplantation in patients with hematologic malignancies aged between 50 and 59 years. Bone Marrow Transplant. (2005) 36:667–74. doi: 10.1038/sj.bmt.1705122
52. Chen Q, Long Y, Yuan X, Zou L, Sun J, Chen S, et al. Protective effects of bone marrow stromal cell transplantation in injured rodent brain: synthesis of neurotrophic factors. J Neurosci Res. (2005) 80:611–9. doi: 10.1002/jnr.20494
53. Mori K, Iwata J, Miyazaki M, Nakao Y, Maeda M. Functional recovery of neuronal activity in rat whisker-barrel cortex sensory pathway from freezing injury after transplantation of adult bone marrow stromal cells. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. (2005) 25:887–98. doi: 10.1038/sj.jcbfm.9600083
54. Walker PA, Harting MT, Jimenez F, Shah SK, Pati S, Dash PK, et al. Direct intrathecal implantation of mesenchymal stromal cells leads to enhanced neuroprotection via an NFkappaB-mediated increase in interleukin-6 production. Stem Cells Dev. (2010) 19:867–76. doi: 10.1089/scd.2009.0188
55. Zanier ER, Montinaro M, Vigano M, Villa P, Fumagalli S, Pischiutta F, et al. Human umbilical cord blood mesenchymal stem cells protect mice brain after trauma. Crit Care Med. (2011) 39:2501–10. doi: 10.1097/CCM.0b013e31822629ba
56. Wang Z, Yao W, Deng Q, Zhang X, Zhang J. Protective effects of BDNF overexpression bone marrow stromal cell transplantation in rat models of traumatic brain injury. J Mol Neurosci MN (2013) 49:409–16. doi: 10.1007/s12031-012-9908-0
57. Pischiutta F, D'Amico G, Dander E, Biondi A, Biagi E, Citerio G, et al. Immunosuppression does not affect human bone marrow mesenchymal stromal cell efficacy after transplantation in traumatized mice brain. Neuropharmacology (2014) 79:119–26. doi: 10.1016/j.neuropharm.2013.11.001
58. Zanier ER, Pischiutta F, Riganti L, Marchesi F, Turola E, Fumagalli S, et al. Bone marrow mesenchymal stromal cells drive protective M2 microglia polarization after brain trauma. Neurotherapeutics (2014) 11:679–95. doi: 10.1007/s13311-014-0277-y
59. Mastro-Martínez I, Pérez-Suárez E, Melen G, González-Murillo, Á, Casco F, Lozano-Carbonero N, et al. Effects of local administration of allogenic adipose tissue-derived mesenchymal stem cells on functional recovery in experimental traumatic brain injury. Brain Inj. (2015) 29:1497–510. doi: 10.3109/02699052.2015.1053525
60. Pischiutta F, Brunelli L, Romele P, Silini A, Sammali E, Paracchini L, et al. Protection of brain injury by amniotic mesenchymal stromal cell-secreted metabolites. Crit Care Med. (2016) 44:e1118–e1131. doi: 10.1097/CCM.0000000000001864
61. Lu D, Li Y, Wang L, Chen J, Mahmood A, Chopp M. Intraarterial administration of marrow stromal cells in a rat model of traumatic brain injury. J Neurotrauma (2001) 18:813–9. doi: 10.1089/089771501316919175
62. Mahmood A, Lu D, Wang L, Li Y, Lu M, Chopp M. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery (2001) 49:1196–204. doi: 10.1097/00006123-200111000-00031
63. Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery (2003) 53:697–703. doi: 10.1227/01.NEU.0000079333.61863.AA
64. Mahmood A, Lu D, Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J Neurotrauma (2004) 21:33–9. doi: 10.1089/089771504772695922
65. Harting MT, Jimenez F, Xue H, Fischer UM, Baumgartner J, Dash PK, et al. Intravenous mesenchymal stem cell therapy for traumatic brain injury. J Neurosurg. (2009) 110:1189–97. doi: 10.3171/2008.9.JNS08158
66. Kim H-J, Lee J-H, Kim S-H. Therapeutic effects of human mesenchymal stem cells on traumatic brain injury in rats: secretion of neurotrophic factors and inhibition of apoptosis. J Neurotrauma (2010) 27:131–8. doi: 10.1089/neu.2008.0818
67. Menge T, Zhao Y, Zhao J, Wataha K, Gerber M, Zhang J, et al. Mesenchymal stem cells regulate blood-brain barrier integrity through TIMP3 release after traumatic brain injury. Sci Transl Med. (2012) 4:161ra150. doi: 10.1126/scitranslmed.3004660
68. Watanabe J, Shetty AK, Hattiangady B, Kim D-K, Foraker JE, Nishida H, et al. Administration of TSG-6 improves memory after traumatic brain injury in mice. Neurobiol Dis. (2013) 59:86–99. doi: 10.1016/j.nbd.2013.06.017
69. Zhang R, Liu Y, Yan K, Chen L, Chen X-R, Li P, et al. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflamm. (2013) 10:106. doi: 10.1186/1742-2094-10-106
70. Tajiri N, Acosta SA, Shahaduzzaman M, Ishikawa H, Shinozuka K, Pabon M, et al. Intravenous transplants of human adipose-derived stem cell protect the brain from traumatic brain injury-induced neurodegeneration and motor and cognitive impairments: cell graft biodistribution and soluble factors in young and aged rats. J Neurosci Off J Soc Neurosci. (2014) 34:313–26. doi: 10.1523/JNEUROSCI.2425-13.2014
71. Darkazalli A, Ismail AAO, Abad N, Grant SC, Levenson CW. Use of human mesenchymal stem cell treatment to prevent anhedonia in a rat model of traumatic brain injury. Restor Neurol Neurosci. (2016) 34:433–41. doi: 10.3233/RNN-150628
72. Guo S, Zhen Y, Wang A. Transplantation of bone mesenchymal stem cells promotes angiogenesis and improves neurological function after traumatic brain injury in mouse. Neuropsychiatr Dis Treat. (2017) 13:2757–65. doi: 10.2147/NDT.S141534
73. Mishra SK, Rana P, Khushu S, Gangenahalli G. Therapeutic prospective of infused allogenic cultured mesenchymal stem cells in traumatic brain injury mice: a longitudinal proton magnetic resonance spectroscopy assessment. Stem Cells Transl Med. (2017) 6:316–29. doi: 10.5966/sctm.2016-0087
74. Darkazalli A, Vied C, Badger C-D, Levenson CW. Human mesenchymal stem cell treatment normalizes cortical gene expression after traumatic brain injury. J Neurotrauma (2017) 34:204–12. doi: 10.1089/neu.2015.4322
75. Shi X, Bai Y, Zhang G, Liu Y, Xiao H, Liu X, et al. Effects of over-expression of SOD2 in bone marrow-derived mesenchymal stem cells on traumatic brain injury. Cell Tissue Res. (2017) 372:67–75. doi: 10.1007/s00441-017-2716-7
76. Kim C, Park J-M, Kong T, Lee S, Seo K-W, Choi Y, et al. Double-injected human stem cells enhance rehabilitation in TBI mice via modulation of survival and inflammation. Mol Neurobiol. (2018) 55:4870–84. doi: 10.1007/s12035-017-0683-3
77. Li L, Jiang Q, Qu CS, Ding GL, Li QJ, Wang SY, et al. Transplantation of marrow stromal cells restores cerebral blood flow and reduces cerebral atrophy in rats with traumatic brain injury: in vivo MRI study. J Neurotrauma (2011) 28:535–45. doi: 10.1089/neu.2010.1619
78. Matsumoto M, Imura T, Fukazawa T, Sun Y, Takeda M, Kajiume T, et al. Electrical stimulation enhances neurogenin2 expression through β-catenin signaling pathway of mouse bone marrow stromal cells and intensifies the effect of cell transplantation on brain injury. Neurosci Lett. (2013) 533:71–6. doi: 10.1016/j.neulet.2012.10.023
79. Xiong Y, Qu C, Mahmood A, Liu Z, Ning R, Li Y, et al. Delayed transplantation of human marrow stromal cell-seeded scaffolds increases transcallosal neural fiber length, angiogenesis, and hippocampal neuronal survival and improves functional outcome after traumatic brain injury in rats. Brain Res. (2009) 1263:183–91. doi: 10.1016/j.brainres.2009.01.032
80. Mahmood A, Qu C, Ning R, Wu H, Goussev A, Xiong Y, et al. Treatment of TBI with collagen scaffolds and human marrow stromal cells increases the expression of tissue plasminogen activator. J Neurotrauma (2011) 28:1199–207. doi: 10.1089/neu.2010.1694
81. Qu C, Mahmood A, Liu XS, Xiong Y, Wang L, Wu H, et al. The treatment of TBI with human marrow stromal cells impregnated into collagen scaffold: functional outcome and gene expression profile. Brain Res. (2011) 1371:129–39. doi: 10.1016/j.brainres.2010.10.088
82. Kota DJ, Prabhakara KS, Toledano-Furman N, Bhattarai D, Chen Q, DiCarlo B, et al. Prostaglandin E2 indicates therapeutic efficacy of mesenchymal stem cells in experimental traumatic brain injury. Stem Cells Dayt Ohio. (2017) 35:1416–30. doi: 10.1002/stem.2603
83. Kota DJ, Prabhakara KS, van Brummen AJ, Bedi S, Xue H, DiCarlo B, et al. Propranolol and mesenchymal stromal cells combine to treat traumatic brain injury. Stem Cells Transl Med. (2016) 5:33–44. doi: 10.5966/sctm.2015-0065
84. Bonilla C, Zurita M, Otero L, Aguayo C, Vaquero J. Delayed intralesional transplantation of bone marrow stromal cells increases endogenous neurogenesis and promotes functional recovery after severe traumatic brain injury. Brain Inj. (2009) 23:760–9. doi: 10.1080/02699050903133970
85. Bonilla Horcajo C, Zurita, Castillo M, Vaquero Crespo J. Platelet-rich plasma-derived scaffolds increase the benefit of delayed mesenchymal stromal cell therapy after severe traumatic brain injury. Cytotherapy (2018) 20:314–21. doi: 10.1016/j.jcyt.2017.11.012
86. Bonilla C, Zurita M, Otero L, Aguayo C, Rico MA, Rodríguez A, et al. Failure of delayed intravenous administration of bone marrow stromal cells after traumatic brain injury. J Neurotrauma (2012) 29:394–400. doi: 10.1089/neu.2011.2101
87. Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. (1994) 91:10625–9.
88. Mellergård P, Sjögren F, Hillman J. The cerebral extracellular release of glycerol, glutamate, and FGF2 is increased in older patients following severe traumatic brain injury. J Neurotrauma (2012) 29:112–8. doi: 10.1089/neu.2010.1732
89. Eshhar N, Striem S, Kohen R, Tirosh O, Biegon A. Neuroprotective and antioxidant activities of HU-211, a novel NMDA receptor antagonist. Eur J Pharmacol. (1995) 283:19–29.
90. Shohami E, Novikov M, Bass R. Long-term effect of HU-211, a novel non-competitive NMDA antagonist, on motor and memory functions after closed head injury in the rat. Brain Res. (1995) 674:55–62.
91. Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. (2002) 1:383–6. doi: 10.1016/S1474-4422(02)00164-3
92. Ikonomidou C, Stefovska V, Turski L. Neuronal death enhanced by N-methyl-D-aspartate antagonists. Proc Natl Acad Sci USA. (2000) 97:12885–90. doi: 10.1073/pnas.220412197
93. Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. (2014) 15:1009–16. doi: 10.1038/ni.3002
94. Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell (2018) 22:824–33. doi: 10.1016/j.stem.2018.05.004
95. Richardson RM, Sun D, Bullock MR. Neurogenesis after traumatic brain injury. Neurosurg Clin N Am. (2007) 18:169–81. doi: 10.1016/j.nec.2006.10.007
96. Quintard H, Heurteaux C, Ichai C. Adult neurogenesis and brain remodelling after brain injury: From bench to bedside? Anaesth Crit Care Pain Med. (2015) 34:239–45. doi: 10.1016/j.accpm.2015.02.008
97. Sun D. Endogenous neurogenic cell response in the mature mammalian brain following traumatic injury. Exp Neurol. (2016) 275:405–10. doi: 10.1016/j.expneurol.2015.04.017
98. Kokaia Z, Lindvall O. Neurogenesis after ischaemic brain insults. Curr Opin Neurobiol. (2003) 13:127–32. doi: 10.1016/S0959-4388(03)00017-5
99. Hallbergson AF, Gnatenco C, Peterson DA. Neurogenesis and brain injury: managing a renewable resource for repair. J Clin Invest. (2003) 112:1128–33. doi: 10.1172/JCI20098
100. Patel K, Sun D. Strategies targeting endogenous neurogenic cell response to improve recovery following traumatic brain injury. Brain Res. (2016) 1640:104–13. doi: 10.1016/j.brainres.2016.01.055
101. Chang EH, Adorjan I, Mundim MV, Sun B, Dizon MLV, Szele FG. Traumatic brain injury activation of the adult subventricular zone neurogenic niche. Front Neurosci. (2016) 10:332. doi: 10.3389/fnins.2016.00332
102. Sun D, Bullock MR, Altememi N, Zhou Z, Hagood S, Rolfe A, et al. The effect of epidermal growth factor in the injured brain after trauma in rats. J Neurotrauma (2010) 27:923–38. doi: 10.1089/neu.2009.1209
103. Sun D, Bullock MR, McGinn MJ, Zhou Z, Altememi N, Hagood S, et al. Basic fibroblast growth factor-enhanced neurogenesis contributes to cognitive recovery in rats following traumatic brain injury. Exp Neurol. (2009) 216:56–65. doi: 10.1016/j.expneurol.2008.11.011
104. Thau-Zuchman O, Shohami E, Alexandrovich AG, Leker RR. Vascular endothelial growth factor increases neurogenesis after traumatic brain injury. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. (2010) 30:1008–16. doi: 10.1038/jcbfm.2009.271
105. Lee C, Agoston DV. Vascular endothelial growth factor is involved in mediating increased de novo hippocampal neurogenesis in response to traumatic brain injury. J Neurotrauma (2010) 27:541–53. doi: 10.1089/neu.2009.0905
106. Wang Z, Wang Y, Wang Z, Gutkind JS, Wang Z, Wang F, et al. Engineered mesenchymal stem cells with enhanced tropism and paracrine secretion of cytokines and growth factors to treat traumatic brain injury. Stem Cells Dayt Ohio. (2015) 33:456–67. doi: 10.1002/stem.1878
107. Shen Q, Yin Y, Xia Q-J, Lin N, Wang Y-C, Liu J, et al. Bone marrow stromal cells promote neuronal restoration in rats with traumatic brain injury: involvement of GDNF regulating BAD and BAX signaling. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. (2016) 38:748–62. doi: 10.1159/000443031
108. Feng Y, Ju Y, Cui J, Wang L. Bone marrow stromal cells promote neuromotor functional recovery, via upregulation of neurotrophic factors and synapse proteins following traumatic brain injury in rats. Mol Med Rep. (2017) 16:654–60. doi: 10.3892/mmr.2017.6619
109. Laird MD, Vender JR, Dhandapani KM. Opposing roles for reactive astrocytes following traumatic brain injury. Neurosignals (2008) 16:154–64. doi: 10.1159/000111560
110. Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurother J Am Soc Exp Neurother. (2010) 7:366–77. doi: 10.1016/j.nurt.2010.07.002
111. Iseki K, Hagino S, Nikaido T, Zhang Y, Mori T, Yokoya S, et al. Gliosis-specific transcription factor OASIS coincides with proteoglycan core protein genes in the glial scar and inhibits neurite outgrowth. Biomed Res Tokyo Jpn. (2012) 33:345–53. doi: 10.2220/biomedres.33.345
112. Sammali E, Alia C, Vegliante G, Colombo V, Giordano N, Pischiutta F, et al. Intravenous infusion of human bone marrow mesenchymal stromal cells promotes functional recovery and neuroplasticity after ischemic stroke in mice. Sci Rep. (2017) 7:6962. doi: 10.1038/s41598-017-07274-w
113. Mahmood A, Lu D, Qu C, Goussev A, Zhang ZG, Lu C, et al. Treatment of traumatic brain injury in rats with erythropoietin and carbamylated erythropoietin. J Neurosurg. (2007) 107:392–7. doi: 10.3171/JNS-07/08/0392
114. Qu C, Mahmood A, Lu D, Goussev A, Xiong Y, Chopp M. Treatment of traumatic brain injury in mice with marrow stromal cells. Brain Res. (2008) 1208:234–9. doi: 10.1016/j.brainres.2008.02.042
Keywords: traumatic brain injury, mesenchymal stromal cells, brain protection, brain repair, vulnerability
Citation: Carbonara M, Fossi F, Zoerle T, Ortolano F, Moro F, Pischiutta F, Zanier ER and Stocchetti N (2018) Neuroprotection in Traumatic Brain Injury: Mesenchymal Stromal Cells can Potentially Overcome Some Limitations of Previous Clinical Trials. Front. Neurol. 9:885. doi: 10.3389/fneur.2018.00885
Received: 25 June 2018; Accepted: 01 October 2018;
Published: 24 October 2018.
Edited by:
Stefania Mondello, Università degli Studi di Messina, ItalyReviewed by:
Eric Peter Thelin, University of Cambridge, United KingdomCopyright © 2018 Carbonara, Fossi, Zoerle, Ortolano, Moro, Pischiutta, Zanier and Stocchetti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elisa R. Zanier, ZWxpc2EuemFuaWVyQG1hcmlvbmVncmkuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.