- 1IMT School for Advanced Studies Lucca, Lucca, Italy
- 2Laboratory of Neuropsychiatry, IRCCS Santa Lucia Foundation, Rome, Italy
- 3Department of Neurosciences, Psychology, Drug Research and Child Health, University of Florence, Florence, Italy
- 4Division of Neuropsychiatry, Menninger Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, TX, United States
The dynamics of the environment where we live in and the interaction with it, predicting events, provided strong evolutionary pressures for the brain functioning to process temporal information and generate timed responses. As a result, the human brain is able to process temporal information and generate temporal patterns. Despite the clear importance of temporal processing to cognition, learning, communication and sensory, motor and emotional processing, the basal mechanisms of how animals differentiate simple intervals or provide timed responses are still under debate. The lesson we learned from the last decade of research in neuroscience is that functional and structural brain connectivity matter. Specifically, it has been accepted that the organization of the brain in interacting segregated networks enables its function. In this paper we delineate the route to a promising approach for investigating timing mechanisms. We illustrate how novel insight into timing mechanisms can come by investigating brain functioning as a multi-layer dynamical network whose clustered dynamics is bound to report the presence of metastable states. We anticipate that metastable dynamics underlie the real-time coordination necessary for the brain's dynamic functioning associated to time perception. This new point of view will help further clarifying mechanisms of neuropsychiatric disorders.
The View
Timing is an umbrella term that encompasses a variety of processes based on the prediction and estimation of temporal intervals across a wide range of scales, from hundreds of milliseconds to seconds. Theoretical models, mainly based on the existence of an internal clock (Gibbon, 1977), have been challenged by compelling behavioral findings that enhance suspects about its biological plausibility (Karmarkar and Buonomano, 2007). Alternate models have been proposed, describing timing as an ensemble of neural processes emerging from the activity of neural circuits inherently capable of temporal processing as a result of the complexity of cortical networks coupled with the presence of time-dependent neuronal properties (Buonomano and Maass, 2009). In this view, neural systems can benefit from the temporal evolution of their states, caused by the variation in neural and synaptic properties. The overall effect results in an adaptation of cerebral networks that could be tuned to discriminate temporal intervals (Bueno et al., 2017). State-dependent models can be extended to be consistent with the majority of timing models (Hass and Durstewitz, 2016), with the different models indicating specific constraints on what would collapse the state space. Although a route is traced toward a comprehensive description of timing, it is still unclear whether brain networks states are part of a coding scheme used to track time or a by-product of other processes that could generate a time-decodable signal. A possible theoretical framework could be the multi-scale description of brain networks both in space and time. On one hand it would be able to capture the local-to-global properties of neural processes that give rise to timing, on the other hand it would allow to grasp the integration processes among brain regions responsible for timing by means of metastability of network states (Friston, 1997; Fingelkurts and Fingelkurts, 2004, 2017; Deco and Kringelbach, 2016). Accordingly, our perspective view about the best strategy able to provide a coherent and complete description of timing can be divided in three steps: (1) the choice of tasks involving different aspects of timing (Coull and Nobre, 1998, 2008; Coull, 2004; Coull et al., 2013; Ciullo et al., 2018a) to be administered on a steady-state fashion (Gonzalez-Castillo and Bandettini, 2018; Tommasin et al., 2018) in order to saturate the activity of the areas interacting during the specific task; (2) the brain activity should be monitored by means of different techniques able to highlight different temporal and spatial scales (e.g., fMRI, hd-EEG, MEG). Specifically the different scales can be cast in a common framework according to the multilayer representation (De Domenico et al., 2013) (different spatial scales for the same time scale or different temporal scales for the same spatial one); (3) the temporal dynamics from each task will be finally analyzed and fitted to theoretical models of neuronal synchronization (Deco et al., 2017; Cavanna et al., 2018) in order to cluster the dynamics of brain's activity during time processing.
In the following paragraphs the core of each step is clarified and a review of the state-of-the-art is proposed.
Timing in Human and Non—Human Animals
The perception of what happens around us and the capacity to respond to it are crucially based on our ability of keeping track of time. Since both perception and action change over time, timing is necessary to estimate environmental dynamics, evaluate interplay between events and predict the consequences of our actions. Throughout normal development we acquire a sense of duration and rhythm that is basic to many behavioral aspects (Allman et al., 2012). Even if there is no specific system that senses time, human and non-human animals can estimate temporal intervals across a wide range of scales (Mauk and Buonomano, 2004; Buhusi and Meck, 2005). Intervals ranging from hundreds of milliseconds to seconds are typically associated with sensory and motor processing, learning, cognition and emotional processing (Figure 1), while larger intervals include processes that range from decision making to sleep-wake cycles (Buhusi and Meck, 2005). There is experimental evidence that timing is an intrinsic computational ability of every circuit in the cortex and that it can be performed locally. This notion implies that during perception tasks cortical networks can tell time as a result of time-dependent changes in synaptic properties, which influence any population response to sensory events in a history dependent fashion (Karmarkar and Buonomano, 2007). Furthermore, with the above mentioned sensory timing, motor timing is supposed to depend on the activity of highly connected cortical recurrent networks able to self-sustain activity (Mauk and Buonomano, 2004).
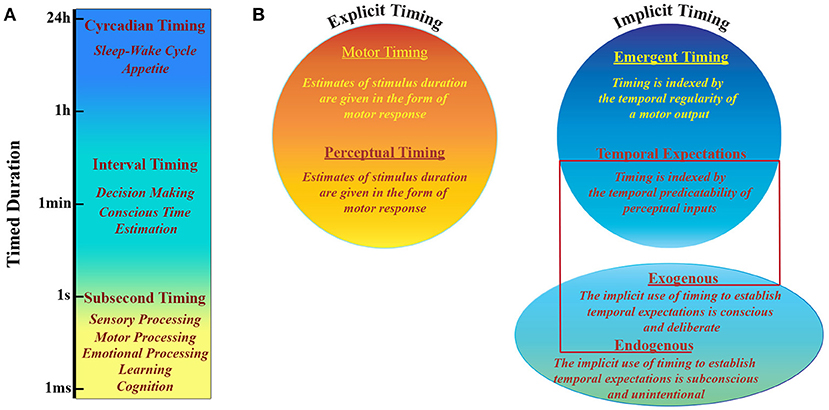
Figure 1. Timing taxonomy. (A) Human and non-human animals have developed multiple systems able to perform different tasks that are based on timing processing at different scales, that range over more than 10 orders of magnitude. (B) Explicit vs. Implicit timing. Explicit timing is engaged by tasks requiring either motor production (motor timing) or perceptual discrimination (perceptual timing) of a timed duration. Implicit timing is engaged as a product of the temporal regularity of either a motor output (emergent timing) or a perceptual input (temporal expectation). The latter can be established either incidentally via a temporally regular stimulus structure (exogenous temporal expectation) or deliberately via informative pre-cues (endogenous temporal expectation). Adapted from (Coull and Nobre, 2008).
Psychophysical experiments suggest that sensory timing can be local (Johnston et al., 2006; Burr et al., 2007; van Wassenhove and Nagarajan, 2007), even if other results suggest that temporal performance variability in different contexts may be better described by a hybrid model (Merchant et al., 2008). Neuroimaging research suggests that a partially distributed timing mechanism sustains contextual flexibility. It is supposed to be integrated by core structures such as the cortico-thalamic-basal ganglia (CTBG) circuit and regions that are selectively engaged by different behavioral contexts (Buhusi and Meck, 2005; Coull et al., 2011). Cell activity changes, associated with temporal processing in behaving monkeys, have been described in areas composing different circuits responsible for sensorimotor processing via the skeletomotor or oculomotor effector systems (Perrett, 1998; Lebedev et al., 2007; Tanaka, 2007; Genovesio et al., 2009; Mita et al., 2009). Most of these studies reported climbing activity during different timing conditions: discrimination of time, time estimation, single interval reproduction and delay related response. Specifically, Merchant et al. (2013) showed a variable discharge rate of cells of Medial Premotor Cortex (MPC) as a function of interval durations with a synchronization-continuation tapping task. This suggested the MPC might contain a representation of interval duration, in the hundred of milliseconds, where diverse populations of interval-tuned cells are typically activated according to the duration of the produced interval. Ramping activity of MPC cells encodes either the elapsed or the remaining time for a temporalized movement such that the dynamic organization of motor intentions and action is sustained by ramping cells. Accordingly, interval tuning on the overall discharge rate affects more cognitive facets of temporal processing.
By moving to larger temporal and spatial scales, functional magnetic resonance imaging (fMRI) studies in humans showed that interval timing is regulated by distributed brain networks whose involvement is flexibly adapted according to task demands: timing emerges from the interaction among diverse brain regions rather than from processing in a specific one (Livesey et al., 2007; Coull et al., 2008; Harrington et al., 2010; Fingelkurts, 2014). For example pattern of timing-related activation in bilateral caudate and putamen was found to be distinguished from that found for most other brain regions in time-perception tasks. Only the anterior insula was found to exhibit the same activation pattern. This region crucially integrates processing from disparate domains (e.g., interoception, emotion, and cognition), including time (Kosillo and Smith, 2010; Wittmann et al., 2010), via its dense pattern of connections with most association areas in the basal ganglia and the occipital, temporal and prefrontal cortex. The connectedness of anterior insula with frontal cognitive control areas suggests that it supports the perceptual integration of sensory information (Eckert et al., 2009). By stimulating the supramarginal gyrus of the right hemisphere with transcranial magnetic stimulation (TMS) a dilation of perceived duration was induced because of its effect on interval encoding (Wiener et al., 2012). This result indicates that the neural circuitry that encodes time crucially includes the right supramarginal gyrus, confirming the detrimental effect of right parietal damage on time perception (Harrington et al., 1998). These findings support also the hypothesis of a network of multiple central clocks and distributed processes of timing mechanisms (Merchant et al., 2008). The ability to organize behaviors within periods in the range of seconds to minutes, depends on a cognitive system that requires multiple neuropsychological functions (Buhusi and Meck, 2005; Coull and Nobre, 2008), consequently pathophysiological distortions in time might reflect neuropsychological deficits typical of definite neuropsychiatric disorders as schizophrenia (Ciullo et al., 2016, 2018a), acquired brain injury (Piras et al., 2014), Parkinson's disease (Wearden et al., 2008), Huntington's disease (Beste et al., 2007) and attention-deficit hyperactivity disorder (Zelaznik et al., 2012). Thus, the understanding of timing mechanisms and of the related cognitive processes may also allow the realization of a model system aiming to characterize cognitive dysfunctions in order to define novel tools for early diagnosis and to develop novel targeted cognitive therapies. However, despite intensive investigations and substantial progress, the absence of a definitive framework encompassing the multifaceted nature of timing processes indicate that our understanding of the principles and mechanisms underlying brain functioning during time perception remains still incomplete. Nonetheless, all the results described above emphasize the role of interactions among distributed neuronal populations at different spatiotemporal scales in enabling flexible cognitive operations that give rise to sense of time (Fingelkurts and Fingelkurts, 2006). Given the functional specialization and integration that sustain the sense of time, a promising framework able to provide a modeling of time perception in the brain from an explicitly integrative perspective is represented by complex network theory. Recent developments in the quantitative analysis of complex networks, based largely on graph theory, have been rapidly translated to studies of brain network organization. Accordingly, the brain is described as a network of nodes and edges, while analytic advancements in network science and statistics allow us to represent and quantify functional interactions among brain regions of interest in order to make inferences about its organizational properties both at rest and as a function of cognitive demands. To our knowledge, a network based description of brain regions integration in timing is still largely incomplete and actually available only in Ciullo et al. (2018b) and Ghaderi et al. (2018).
This kind of cerebral systems modeling (Bassett and Sporns, 2017), will be crucially beneficial in the close future to an organic description of brain functioning during the estimation of temporal intervals and eventually to a better description of disorders characterized by impaired time perception.
Multiscale Brain Networks
A tentative modeling of time perception processes necessarily points to a description of brain functioning based on the interplay of multi-scale brain networks (Fingelkurts et al., 2010; Bassett and Siebenhühner, 2013). The meaning of “scale” can vary according to the context: (i) a network's spatial scale, which refers to the resolution at which its connected regions of interest (nodes) and connections (edges) are defined, and can range from individual cells and synapses size (Jarrell et al., 2012; Shimono and Beggs, 2015; Lee et al., 2016), to brain regions and fiber tracts (Bullmore and Bassett, 2011) and (ii) temporal scales with precision ranging from sub-millisecond (Burns et al., 2014), to lifetime (Betzel et al., 2014; Gu et al., 2015). Although it is important to understand the functioning of individual elements, at each scale it is crucial to understand the sets of pair-wise relations that arrange the elements into the larger description of a totally interconnected system, namely the local and global topology of the network (Fingelkurts et al., 2010; Barabasi, 2016). Together these scales define a three-dimensional space in which the evolution of the brain network complexity is reported, being each point identified by three coordinates: space, time, and topology (Betzel and Bassett, 2017). Most descriptions of time perception mechanisms exist as single points in this space being based on analyses focused on networks defined singularly at one spatial, temporal, and topological scale. We anticipate that, while such studies have proven illuminating, in order to better understand the brain's true multi-scale, multi-level nature, it is essential that analyses begin to form bridges that link different scales to one another in order to offer a comprehensive description of the mechanisms that govern timing.
One promising approach to study a network that changes over multiple timescales is to make use of multi-layer network models of temporal networks (De Domenico et al., 2013; Kivelä et al., 2014). The multi-layer network model can treat estimates of the network's topology at different points of the time-scale as “layers.” This implies the necessity to integrate different modalities of investigation spanning different time-scales. It could be done by creating a multi-layer from different non-invasive neuroimaging techniques: from high-density electroencephalography (hd-EEG) (Liu et al., 2017), to magnetoencephalography (MEG) (de Pasquale et al., 2010), fast fMRI (Lewis et al., 2016), classical fMRI (Telesford et al., 2016) and combined EEG-fMRI (Mullinger and Bowtell, 2010; Yu et al., 2016). On the other hand, invasive approaches are able to detect multiple single neuron signals in non-human animals (Logothetis, 2012) and in human patients that need deep brain stimulation (Okun, 2014). Traditional analysis would characterize each layer independently of one another, while multi-layer network analysis treats the ensemble of layers as a single unit, characterizing its structure as a whole to explicitly bridge multiple temporal scales. Since the multi-layer network model doesn't depend on the timescales represented by each layer, it can include any timescale made accessible using neuroimaging technologies.
As well as for time, the space dimension can be also investigated at multiple scales (Figure 2A). MEG and fMRI analyses of human brain networks are limited by the accuracy of the inverse source localization of signal generators (MEG), and the spatial granularity of the individual voxel (fMRI). Nonetheless, it is possible to probe multiple spatial scales by appropriately aggregating the minimal units of interest into parcels or regions of interest. Several parcellation approaches have been proposed, distinguishing to one another according to different criteria as spatial variation in functional connectivity, myelination, cytoarchitectonics, etc. (Tzourio-Mazoyer et al., 2002; Craddock et al., 2012; Wang et al., 2015; Glasser et al., 2016; Gordon et al., 2016). Since the choice of parcellation conditions the network's topology (Wang et al., 2009; Zalesky et al., 2010), it must be checked if any result is not driven by the specific choice of parcellation, and is reproducible (at least qualitatively) using a different set of parcels at the same resolution (Bassett et al., 2011). A route for future research is to apply multi-scale topological analysis to voxel-level networks during the execution of tasks. It will allow identifying different parcels differentially involved in different brain states in order to sub-divide specific brain areas responsible for sustaining different cognitive engagements.
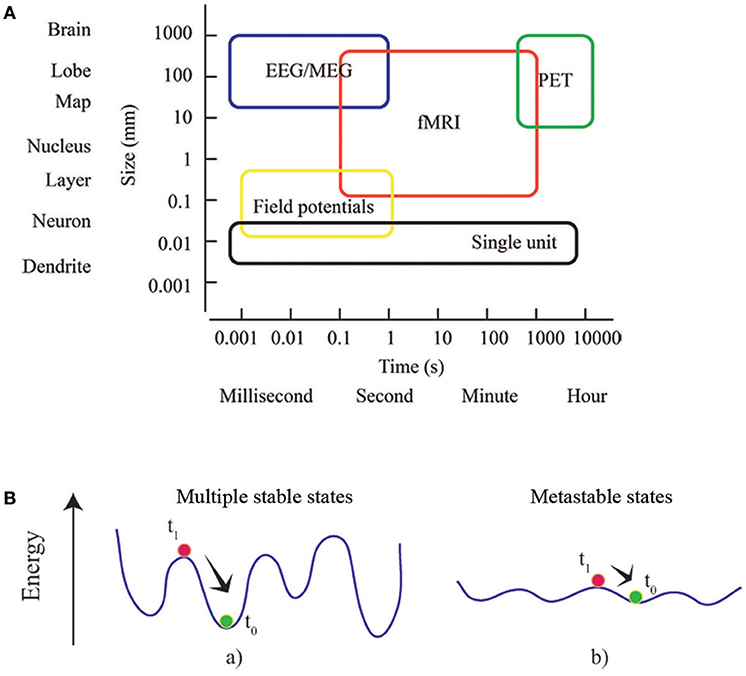
Figure 2. Multiscale and multistable nature of the brain. (A) Rather than considering the brain as a list of parts defined at a particular scale, brain network theory take advantage of the complexity of the interactions between the parts, and identifies the dependence of phenomena across scales. Box dimensions give outer bounds of the spatial and temporal scales at which relational data are measured and interactions unfold. Adapted from an image of neuroscience recording methods (Sejnowski et al., 2014). (B) The concepts of energy landscape and metastability. Points of these landscapes correspond to particular states of the system. The system at equilibrium (green) is perturbed (at t0) toward a state (red) that subsequently (at t1) relaxes. In (a) the system is stable and local minima (equilibrium points) are deep: dynamics are rapidly restored and the effects of perturbation are short-lasting. In (b) the energy landscape is almost flat and the stability of local minima decreases: the system can easily explore different (metastable) states without an external driving or endogenous fluctuation.
Metastability: A Resource of Brain Networks for Sustaining Time Perception Mechanisms
Large-scale brain networks have been showed to be organized according to multiple segregated sub-networks of interacting areas. It has been suggested that a dynamic, adaptable brain network arrangement in response to environmental stimulations underlies successful cognition (Bressler and Kelso, 2001; Fries, 2005). Dynamic combination of responses to sensory inputs, and spontaneous processing is at the core of brain activity, where task evoked responses should not be interpreted only in terms of localized processing, but should also take into account distributed processing occurring as activity flow across intrinsic networks (Smith et al., 2009; Zalesky et al., 2014; Sadaghiani et al., 2015; Cole et al., 2016; Shine et al., 2016). This allows a description of brain functioning in terms of a continuous recruitment of neuronal populations in a temporally coordinated fashion both during tasks execution, and at rest (Fingelkurts and Fingelkurts, 2005). Recently, it has been found that the neuronal engagement follows a precise hierarchy, according to two distinct sets of networks, or metastates, that the brain tends to cycle within (Vidaurre et al., 2017).
Metastates or metastable cerebral states are the core of a prominent conceptual framework known as Metastability (Scott Kelso, 1995; Fingelkurts and Fingelkurts, 2004, 2017; Freeman and Holmes, 2005; Werner, 2007). It offers a description of the reciprocal influence among interconnected parts and processes when pure synchronization does not occur. In coordination dynamics, such synchronization corresponds to stable fixed points of collective states (Friston, 1997). Metastability can be better understood by defining an energy landscape for the ensemble of possible states experienced by the brain: the phase space of the brain system (Fingelkurts and Fingelkurts, 2004, 2017). Generally, a system dynamically evolves attracted toward states of minimum energy, which can be either local or global. After being temporarily attracted toward a local state of minimum energy, an externally driven system can flee the basin of attraction and experience other equilibrium states. The dynamics of a metastable system is characterized by states that only transiently attract the dynamics. Since during its dynamic evolution the system tends to linger around these metastable states, the idea of a repertoire of conditions or configurations can be introduced (Figure 2B). Consequently, components are able to influence each other's destiny without being caught in a sustained state of synchronization, unable to create collectively new information Scott Kelso, 1995; Tognoli and Kelso, 2014). The emergence of metastable dynamics has been theoretically showed to be contingent upon the coupling between modules of a dynamical system (Friston, 1997; Strogatz, 2001; Shanahan, 2010; Cabral et al., 2011). Specifically, dynamic patterns of functional brain networks, consistent with metastable dynamics, come out when coupling is topologically characterized by short average path lengths and high clustering (Wildie and Shanahan, 2012) of modules. The efficiency of task-related brain activity has been showed to depend on metastability of spontaneous brain activity, which allows for optimal experience of the dynamical repertoire (Cabral et al., 2014). Recently metastability in brain networks has been investigated in aging, consciousness and neuronal communication in healthy subjects (Deco and Kringelbach, 2016; Deco et al., 2017; Naik et al., 2017; Cavanna et al., 2018) and in Schizophrenia and Alzheimer's disease patients (Córdova-Palomera et al., 2017; Koutsoukos and Angelopoulos, 2018). A variety of methods are described in order to capture synchronization and metastability in brain functioning.
Since metastability is a fundamental concept to grasp the behavior of complex systems theoretically and empirically, we anticipate that a form of metastability exists in time processing systems that parallels the metastability observed in many other aspects of brain functioning. The need for metastability in time perception modeling follows right from the definition. Metastability is the simultaneous occurrence of two competing tendencies: the inclination of individual components to exist as interacting entities and the propensity for the components to be characterized just by their independent behavior (Kelso, 2012). As a consequence metastability may be thought as a dynamical condition that allows the coordination of heterogeneous elements as it happens during time perception (brain areas having disparate intrinsic dynamics or brain areas whose activity is associated with different sensory, motor and cognitive processes) (Fingelkurts and Fingelkurts, 2006; Fingelkurts, 2014). Metastable brain theory may ameliorate timing modeling as it does not favor extremes, e.g., integrated vs. segregated processes, but it tends to reconcile them. Since metastability is a characteristic of the full complexity of the brain, it reaches a maximum when the balance between segregative and integrative forces is found. Furthermore, metastability doesn't need active induction since no disengagement mechanisms are required, as it happens in timing processing (Kononowicz et al., 2016). Finally, the crucial importance of time to perception and action necessitates metastability, in order to explain the ease with which timing can be performed by a range of different neural architectures. Clustering the dynamics of brain's activity during time processing may unearth the presence of metastable states associated with this specific aspect of cognition.
Conclusion
Here we propose that the route along which future research will find novel insight into timing mechanisms is drawn in the direction of brain investigation as a multi-layer dynamical network whose clustered dynamics unavoidably reports the presence of metastable states. This perspective paves the way for future investigations into both the role of timing in other cognitive domains, from learning to agency, and the role that temporal dependency of brain network states has in cognition, elucidating the general characteristics of human cognitive activity that exists at a wide range of spatiotemporal scales. At the same time, our better understanding of dysfunctional timing processes will crucially allow us to develop novel diagnostics of neuropsychiatric diseases, and to design personalized therapeutics for rehabilitation and treatment of brain disorders characterized by distorted time perception.
Author Contributions
TG and GS conceived the paper. TG organized the paper. TG and VC wrote the first draft of the paper and collected and filtered the references. GS supervised the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was conducted within the project Multidimensional study of timing abilities and sense of agency in schizophrenia and bipolar patients funded through 5Xmille 2016 from the Italian Ministry of Health.
References
Allman, M. J., Pelphrey, K. A., and Meck, W. H. (2012). Developmental neuroscience of time and number: implications for autism and other neurodevelopmental disabilities. Front. Integr. Neurosci. 6:7. doi: 10.3389/fnint.2012.00007
Bassett, D. S., Brown, J. A., Deshpande, V., Carlson, J. M., and Grafton, S. T. (2011). Conserved and variable architecture of human white matter connectivity. Neuroimage 54, 1262–1279. doi: 10.1016/j.neuroimage.2010.09.006
Bassett, D. S., and Siebenhühner, F. (2013). “Multiscale network organization in the human brain,” in Multiscale Analysis and Nonlinear Dynamics: From Genes to the Brain, ed M. M. Pesenson (Weinheim: Wiley-VCH), 179–204.
Bassett, D. S., and Sporns, O. (2017). Network neuroscience. Nat. Neurosci. 20, 353–364. doi: 10.1038/nn.4502
Beste, C., Saft, C., Andrich, J., Müller, T., Gold, R., and Falkenstein, M. (2007). Time processing in Huntington's disease: A group-control study. PLoS ONE 2:e1263. doi: 10.1371/journal.pone.0001263
Betzel, R. F., and Bassett, D. S. (2017). Multi-scale brain networks. Neuroimage 160, 73–83. doi: 10.1016/j.neuroimage.2016.11.006
Betzel, R. F., Byrge, L., He, Y., Goñi, J., Zuo, X. N., and Sporns, O. (2014). Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage 102, 345–357. doi: 10.1016/j.neuroimage.2014.07.067
Bressler, S. L., and Kelso, J. A. (2001). Cortical coordination dynamics and cognition. Trends Cogn. Sci. 5, 26–36. doi: 10.1016/S1364-6613(00)01564-3
Bueno, F. D., Morita, V. C., De Camargo, R. Y., Reyes, M. B., Caetano, M. S., and Cravo, A. M. (2017). Dynamic representation of time in brain states. Sci. Rep. 7:46053. doi: 10.1038/srep46053
Buhusi, C. V., and Meck, W. H. (2005). What makes us tick? Functional and neural mechanisms of interval timing. Nat. Rev. Neurosci. 6, 755–765. doi: 10.1038/nrn1764
Bullmore, E. T., and Bassett, D. S. (2011). Brain graphs: graphical models of the human brain connectome. Annu. Rev. Clin. Psychol. 7, 113–140. doi: 10.1146/annurev-clinpsy-040510-143934
Buonomano, D. V., and Maass, W. (2009). State-dependent computations: spatiotemporal processing in cortical networks. Nat. Rev. Neurosci. 10, 113–125. doi: 10.1038/nrn2558
Burns, S. P., Santaniello, S., Yaffe, R. B., Jouny, C. C., and Crone, N. E. (2014). Network dynamics of the brain and influence of the epileptic seizure onset zone. Proc. Natl. Acad. Sci. U.S.A. 111, E5321–5330. doi: 10.1073/pnas.1401752111
Burr, D., Tozzi, A., and Morrone, M. C. (2007). Neural mechanisms for timing visual events are spatially selective in real-world coordinates. Nat. Neurosci. 10, 423–425. doi: 10.1038/nn1874
Cabral, J., Hugues, E., Sporns, O., and Deco, G. (2011). Role of local network oscillations in resting-state functional connectivity. Neuroimage 57, 130–139. doi: 10.1016/j.neuroimage.2011.04.010
Cabral, J., Kringelbach, M. L., and Deco, G. (2014). Exploring the network dynamics underlying brain activity during rest. Prog Neurobiol. 114, 102–131. doi: 10.1016/j.pneurobio.2013.12.005
Cavanna, F., Vilas, M. G., Palmucci, M., and Tagliazucchi, E. (2018). Dynamic functional connectivity and brain metastability during altered states of consciousness. Neuroimage. 180, 383–395. doi: 10.1016/j.neuroimage.2017.09.065
Ciullo, V., Piras, F., Vecchio, D., Banaj, N., Coull, J. T., and Spalletta, G. (2018a). Predictive timing disturbance is a precise marker of schizophrenia. Schizophr. Res. Cogn. 12, 42–49. doi: 10.1016/j.scog.2018.04.001
Ciullo, V., Spalletta, G., Caltagirone, C., Jorge, R. E., and Piras, F. (2016). Explicit time deficit in schizophrenia: systematic review and meta-analysis indicate it is primary and not domain specific. Schizophr. Bull. 42, 505–518. doi: 10.1093/schbul/sbv104
Ciullo, V., Vecchio, D., Gili, T., Spalletta, G., and Piras, F. (2018b). Segregation of Brain Structural Networks Supports Spatio-Temporal Predictive Processing. Front. Hum. Neurosci. 12:212. doi: 10.3389/fnhum.2018.00212
Cole, M. W., Ito, T., Bassett, D. S., and Schultz, D. H. (2016). Activity flow over resting-state networks shapes cognitive task activations. Nat. Neurosci. 19, 1718–1726. doi: 10.1038/nn.4406
Córdova-Palomera, A., Kaufmann, T., Persson, K., Alnæs, D., Doan, N. T., Moberget, T., et al. (2017). Disrupted global metastability and static and dynamic brain connectivity across individuals in the Alzheimer's disease continuum. Sci. Rep. 7:40268. doi: 10.1038/srep40268
Coull, J., and Nobre, A. (2008). Dissociating explicit timing from temporal expectation with fMRI. Curr. Opin. Neurobiol. 18, 137–144. doi: 10.1016/j.conb.2008.07.011
Coull, J. T. (2004). Functional anatomy of the attentional modulation of time estimation. Science 303, 1506–1508. doi: 10.1126/science.1091573
Coull, J. T., Cheng, R.-K., and Meck, W. H. (2011). Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology 36, 3–25. doi: 10.1038/npp.2010.113
Coull, J. T., Davranche, K., Nazarian, B., and Vidal, F. (2013). Functional anatomy of timing differs for production versus prediction of time intervals. Neuropsychologia 51, 309–319. doi: 10.1016/j.neuropsychologia.2012.08.017
Coull, J. T., Nazarian, B., and Vidal, F. (2008). Timing, storage, and comparison of stimulus duration engage discrete anatomical components of a perceptual timing network. J. Cogn. Neurosci. 20, 2185–2197. doi: 10.1162/jocn.2008.20153
Coull, J. T., and Nobre, A. C. (1998). Where and when to pay attention: the neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J. Neurosci. 18, 7426–7435.
Craddock, R. C., James, G. A., Holtzheimer, P. E., Hu, X. P., and Mayberg, H. S. (2012). A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum. Brain Mapp. 33, 1914–1928. doi: 10.1002/hbm.21333
De Domenico, M., Solé-Ribalta, A., Cozzo, E., Kivelä, M., Moreno, Y., Porter, M. A., et al. (2013). Mathematical formulation of multilayer networks. Phys. Rev. X 3:041022. doi: 10.1103/PhysRevX.3.041022
de Pasquale, F., Della Penna, S., Snyder, A. Z., Lewis, C., Mantini, D., Marzetti, L., et al. (2010). Temporal dynamics of spontaneous MEG activity in brain networks. Proc. Natl. Acad. Sci.U.S.A. 107, 6040–6045. doi: 10.1073/pnas.0913863107
Deco, G., and Kringelbach, M. L. (2016). Metastability and coherence: extending the communication through coherence hypothesis using a whole-brain computational perspective. Trends Neurosci. 39, 125–135. doi: 10.1016/j.tins.2016.01.001
Deco, G., Kringelbach, M. L., Jirsa, V. K., and Ritter, P. (2017). The dynamics of resting fluctuations in the brain: metastability and its dynamical cortical core. Sci. Rep. 7:3095. doi: 10.1038/s41598-017-03073-5
Eckert, M. A., Menon, V., Walczak, A., Ahlstrom, J., Denslow, S., Horwitz, A., et al. (2009). At the heart of the ventral attention system: the right anterior insula. Hum. Brain Mapp. 30, 2530–2541. doi: 10.1002/hbm.20688
Fingelkurts, A., and Fingelkurts, A. (2017). Information flow in the brain: ordered sequences of metastable states. Information 8:22. doi: 10.3390/info8010022
Fingelkurts, A. A. (2014). Present moment, past, and future: mental kaleidoscope. Front. Psychol. 5:395. doi: 10.3389/fpsyg.2014.00395
Fingelkurts, A. A., and Fingelkurts, A. A. (2004). Making complexity simpler: multivariability and metastability in the brain. Int. J. Neurosci. 114, 843–862. doi: 10.1080/00207450490450046
Fingelkurts, A. A., and Fingelkurts, A. A. (2005). “Mapping of brain operational architectonics,” in Focus on Brain Mapping Research, ed F. J. Chen (New York, NY Nova Science Publishers, Inc).
Fingelkurts, A. A., and Fingelkurts, A. A. (2006). Timing in cognition and EEG brain dynamics: discreteness versus continuity. Cogn. Process. 7, 135–162. doi: 10.1007/s10339-006-0035-0
Fingelkurts, A. A., Fingelkurts, A. A., and Neves, C. F. H. (2010). Natural world physical, brain operational, and mind phenomenal space-time. Phys. Life Rev. 7, 195–249. doi: 10.1016/j.plrev.2010.04.001
Freeman, W. J., and Holmes, M. D. (2005). Metastability, instability, and state transition in neocortex. Neural Networks 18, 497–504. doi: 10.1016/j.neunet.2005.06.014
Fries, P. (2005). A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci. 9, 474–480. doi: 10.1016/j.tics.2005.08.011
Friston, K. J. (1997). Transients, metastability, and neuronal dynamics. Neuroimage 5, 164–171. doi: 10.1006/nimg.1997.0259
Genovesio, A., Tsujimoto, S., and Wise, S. P. (2009). Feature- and order-based timing representations in the frontal cortex. Neuron 63, 254–266. doi: 10.1016/j.neuron.2009.06.018
Ghaderi, A. H., Moradkhani, S., Haghighatfard, A., Akrami, F., Khayyer, Z., and Balc,i, F. (2018). Time estimation and beta segregation: an EEG study and graph theoretical approach. PLoS ONE 13:e0195380. doi: 10.1371/journal.pone.0195380
Gibbon, J. (1977). Scalar expectancy theory and Weber's law in animal timing. Psychol. Rev. 84, 279–325. doi: 10.1037/0033-295X.84.3.279
Glasser, M. F., Coalson, T. S., Robinson, E. C., Hacker, C. D., Harwell, J., Yacoub, E., et al. (2016). A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178. doi: 10.1038/nature18933
Gonzalez-Castillo, J., and Bandettini, P. A. (2018). Task-based dynamic functional connectivity: recent findings and open questions. Neuroimage 180, 526–533. doi: 10.1016/j.neuroimage.2017.08.006
Gordon, E. M., Laumann, T. O., Adeyemo, B., Huckins, J. F., Kelley, W. M., and Petersen, S. E. (2016). Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb. Cortex 26, 288–303. doi: 10.1093/cercor/bhu239
Gu, S., Satterthwaite, T. D., Medaglia, J. D., Yang, M., Gur, R. E., Gur, R. C., et al. (2015). Emergence of system roles in normative neurodevelopment. Proc. Natl. Acad. Sci.U.S.A. 112, 13681–13686. doi: 10.1073/pnas.1502829112
Harrington, D. L., Haaland, K. Y., and Knight, R. T. (1998). Cortical networks underlying mechanisms of time perception. J. Neurosci. 18, 1085–1095
Harrington, D. L., Zimbelman, J. L., Hinton, S. C., and Rao, S. M. (2010). Neural modulation of temporal encoding, maintenance, and decision processes. Cereb. Cortex 20, 1274–1285. doi: 10.1093/cercor/bhp194
Hass, J., and Durstewitz, D. (2016). Time at the center, or time at the side? Assessing current models of time perception. Curr. Opin. Behav. Sci. 8, 238–244. doi: 10.1016/j.cobeha.2016.02.030
Jarrell, T. A., Wang, Y., Bloniarz, A. E., Brittin, C. A., Xu, M., Thomson, J. N., et al. (2012). The connectome of a decision-making neural network. Science 337, 437–444. doi: 10.1126/science.1221762
Johnston, A., Arnold, D. H., and Nishida, S. (2006). Spatially localized distortions of event time. Curr. Biol. 16, 472–479. doi: 10.1016/j.cub.2006.01.032
Karmarkar, U. R., and Buonomano, D. V. (2007). Timing in the absence of clocks: encoding time in neural network states. Neuron 53, 427–438. doi: 10.1016/j.neuron.2007.01.006
Kelso, J. A. (2012). Multistability and metastability: understanding dynamic coordination in the brain. Philos. Trans. R. Soc. B Biol. Sci. 367, 906–918. doi: 10.1098/rstb.2011.0351
Kivelä, M., Arenas, A., Barthelemy, M., Gleeson, J. P., Moreno, Y., and Porter, M. A. (2014). Multilayer networks. J. Complex Networks 2, 203–271. doi: 10.1093/comnet/cnu016
Kononowicz, T. W., van Rijn, H., and Meck, W. H. (2016). Timing and time perception: a critical review of neural timing signatures before, during, and after the To-Be-Timed Interval, in Stevens' Handbook of Experimental Psychology and Cognitive Neuroscience, 4th Edn. ed S. Ghetti (New York, NY: John Wiley & Sons), 1–48.
Kosillo, P., and Smith, A. T. (2010). The role of the human anterior insular cortex in time processing. Brain Struct. Funct. 214, 623–628. doi: 10.1007/s00429-010-0267-8
Koutsoukos, E., and Angelopoulos, E. (2018). Investigating the role of metastability in the disturbed brain functional connectivity observed under the experience of thought blocks in schizophrenia. Schizophr. Res. 193, 98–99. doi: 10.1016/j.schres.2017.07.055
Lebedev, M. A., O'Doherty, J. E., and Nicolelis, M. A. (2007). Decoding of temporal intervals from cortical ensemble activity. J. Neurophysiol. 99, 166–186. doi: 10.1152/jn.00734.2007
Lee, W. C., Bonin, V., Reed, M., Graham, B. J., Hood, G., Glattfelder, K., et al. (2016). Anatomy and function of an excitatory network in the visual cortex. Nature 532, 370–374. doi: 10.1038/nature17192
Lewis, L. D., Setsompop, K., Rosen, B. R., and Polimeni, J. R. (2016). Fast fMRI can detect oscillatory neural activity in humans. Proc. Natl. Acad. Sci.U.S.A. 113, E6679–E6685. doi: 10.1073/pnas.1608117113
Liu, Q., Farahibozorg, S., Porcaro, C., Wenderoth, N., and Mantini, D. (2017). Detecting large-scale networks in the human brain using high-density electroencephalography. Hum. Brain Mapp. 38, 4631–4643. doi: 10.1002/hbm.23688
Livesey, A. C., Wall, M. B., and Smith, A. T. (2007). Time perception: manipulation of task difficulty dissociates clock functions from other cognitive demands. Neuropsychologia 45, 321–331. doi: 10.1016/j.neuropsychologia.2006.06.033
Logothetis, N. K. (2012). Intracortical recordings and fMRI: an attempt to study operational modules and networks simultaneously. Neuroimage 62, 962–969. doi: 10.1016/j.neuroimage.2012.01.033
Mauk, M. D., and Buonomano, D. V. (2004). The neural basis of temporal processing. Annu. Rev. Neurosci. 27, 307–340. doi: 10.1146/annurev.neuro.27.070203.144247
Merchant, H., Pérez, O., Zarco, W., and Gámez, J. (2013). Interval tuning in the primate medial premotor cortex as a general timing mechanism. J. Neurosci. 33, 9082–9096. doi: 10.1523/JNEUROSCI.5513-12.2013
Merchant, H., Zarco, W., Bartolo, R., and Prado, L. (2008). The context of temporal processing is represented in the multidimensional relationships between timing tasks. PLoS ONE 3:e3169. doi: 10.1371/journal.pone.0003169
Mita, A., Mushiake, H., Shima, K., Matsuzaka, Y., and Tanji, J. (2009). Interval time coding by neurons in the presupplementary and supplementary motor areas. Nat. Neurosci. 12, 502–507. doi: 10.1038/nn.2272
Mullinger, K., and Bowtell, R. (2010). Combining EEG and fMRI. Methods Mol. Biol. 711, 303–326. doi: 10.1007/978-1-61737-992-5_15
Naik, S., Banerjee, A., Bapi, R. S., Deco, G., and Roy, D. (2017). Metastability in senescence. Trends Cogn. Sci. 21, 509–521. doi: 10.1016/j.tics.2017.04.007
Okun, M. S. (2014). Deep-brain stimulation — entering the era of human neural-network modulation. N. Engl. J. Med. 371, 1369–1373. doi: 10.1056/NEJMp1408779
Perrett, S. P. (1998). Temporal discrimination in the cerebellar cortex during conditioned eyelid responses. Exp. Brain Res. 121, 115–124. doi: 10.1007/s002210050443
Piras, F., Piras, F., Ciullo, V., Danese, E., Caltagirone, C., and Spalletta, G. (2014). Time dysperception perspective for acquired brain injury. Front. Neurol. 4:217. doi: 10.3389/fneur.2013.00217
Sadaghiani, S., Poline, J.-B., Kleinschmidt, A., and D'Esposito, M. (2015). Ongoing dynamics in large-scale functional connectivity predict perception. Proc. Natl. Acad. Sci. U.S.A. 112, 8463–8468. doi: 10.1073/pnas.1420687112
Scott Kelso, J. A. (1995). “The self-organization of brain and behavior,” in Dynamic Patterns (Cambridge, MA: MIT Press), 1–28.
Sejnowski, T. J., Churchland, P. S., and Movshon, J. A. (2014). Putting big data to good use in neuroscience. Nat. Neurosci. 17, 1440–1441. doi: 10.1038/nn.3839
Shanahan, M. (2010). Metastable chimera states in community-structured oscillator networks. Chaos 20:013108. doi: 10.1063/1.3305451
Shimono, M., and Beggs, J. M. (2015). Functional clusters, hubs, and communities in the cortical microconnectome. Cereb. Cortex 25, 3743–3757. doi: 10.1093/cercor/bhu252
Shine, J. M., Bissett, P. G., Bell, P. T., Koyejo, O., Balsters, J. H., Gorgolewski, K. J., et al. (2016). The dynamics of functional brain networks: integrated network states during cognitive task performance. Neuron 92, 544–554. doi: 10.1016/j.neuron.2016.09.018
Smith, S. M., Fox, P. T., Miller, K. L., Glahn, D. C., Fox, P. M., Mackay, C. E., et al. (2009). Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci.U.S.A. 106, 13040–13045. doi: 10.1073/pnas.0905267106
Tanaka, M. (2007). Cognitive signals in the primate motor thalamus predict saccade timing. J. Neurosci. 27, 12109–12118. doi: 10.1523/JNEUROSCI.1873-07.2007
Telesford, Q. K., Lynall, M.-E., Vettel, J., Miller, M. B., Grafton, S. T., and Bassett, D. S. (2016). Detection of functional brain network reconfiguration during task-driven cognitive states. Neuroimage 142, 198–210. doi: 10.1016/j.neuroimage.2016.05.078
Tognoli, E., and Kelso, J. A. (2014). The metastable brain. Neuron 81, 35–48. doi: 10.1016/j.neuron.2013.12.022
Tommasin, S., Mascali, D., Moraschi, M., Gili, T., Hassan, I. E., Fratini, M., et al. (2018). Scale-invariant rearrangement of resting state networks in the human brain under sustained stimulation. Neuroimage 179, 570–581. doi: 10.1016/j.neuroimage.2018.06.006
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289. doi: 10.1006/nimg.2001.0978
van Wassenhove, V., and Nagarajan, S. S. (2007). Auditory cortical plasticity in learning to discriminate modulation rate. J. Neurosci. 27, 2663–2672. doi: 10.1523/JNEUROSCI.4844-06.2007
Vidaurre, D., Smith, S. M., and Woolrich, M. W. (2017). Brain network dynamics are hierarchically organized in time. Proc. Natl. Acad. Sci.U.S.A. 114, 12827–12832. doi: 10.1073/pnas.1705120114
Wang, D., Buckner, R. L., Fox, M. D., Holt, D. J., Holmes, A. J., Stoecklein, S., et al. (2015). Parcellating cortical functional networks in individuals. Nat. Neurosci. 18, 1853–1860. doi: 10.1038/nn.4164
Wang, J., Wang, L., Zang, Y., Yang, H., Tang, H., Gong, Q., et al. (2009). Parcellation-dependent small-world brain functional networks: a resting-state fmri study. Hum. Brain Mapp. 30, 1511–1523. doi: 10.1002/hbm.20623
Wearden, J. H., Smith-Spark, J. H., Cousins, R., Edelstyn, N. M., Cody, F. W., and O'Boyle, D. J. (2008). Stimulus timing by people with Parkinson's disease. Brain Cogn. 67, 264–279. doi: 10.1016/j.bandc.2008.01.010
Werner, G. (2007). Metastability, criticality and phase transitions in brain and its models. BioSystems. 90, 496–508. doi: 10.1016/j.biosystems.2006.12.001
Wiener, M., Kliot, D., Turkeltaub, P. E., Hamilton, R. H., Wolk, D. A., and Coslett, H. B. (2012). Parietal influence on temporal encoding indexed by simultaneous transcranial magnetic stimulation and electroencephalography. J. Neurosci. 32, 12258–12267. doi: 10.1523/JNEUROSCI.2511-12.2012
Wildie, M., and Shanahan, M. (2012). Metastability and chimera states in modular delay and pulse-coupled oscillator networks. Chaos 22:043131. doi: 10.1063/1.4766592
Wittmann, M., Simmons, A. N., Aron, J. L., and Paulus, M. P. (2010). Accumulation of neural activity in the posterior insula encodes the passage of time. Neuropsychologia 48, 3110–3120. doi: 10.1016/j.neuropsychologia.2010.06.023
Yu, Q., Wu, L., Bridwell, D. A., Erhardt, E. B., Du, Y., He, H., et al. (2016). Building an EEG-fMRI multi-modal brain graph: a concurrent EEG-fMRI study. Front. Hum. Neurosci. 10:476. doi: 10.3389/fnhum.2016.00476
Zalesky, A., Fornito, A., Cocchi, L., Gollo, L. L., and Breakspear, M. (2014). Time-resolved resting-state brain networks. Proc. Natl. Acad. Sci. U.S.A. 111, 10341–10346. doi: 10.1073/pnas.1400181111
Zalesky, A., Fornito, A., Harding, I. H., Cocchi, L., Yücel, M., Pantelis, C., et al. (2010). Whole-brain anatomical networks: does the choice of nodes matter? Neuroimage 50, 970–983. doi: 10.1016/j.neuroimage.2009.12.027
Keywords: brain networks, multiscale modeling, metastable state brain dynamics, timing and time perception, functional MRI, electrophysiology
Citation: Gili T, Ciullo V and Spalletta G (2018) Metastable States of Multiscale Brain Networks Are Keys to Crack the Timing Problem. Front. Comput. Neurosci. 12:75. doi: 10.3389/fncom.2018.00075
Received: 27 April 2018; Accepted: 17 August 2018;
Published: 11 September 2018.
Edited by:
Daya Shankar Gupta, Camden County College, United StatesCopyright © 2018 Gili, Ciullo and Spalletta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tommaso Gili, dG9tbWFzby5naWxpQGltdGx1Y2NhLml0
Gianfranco Spalletta, Zy5zcGFsbGV0dGFAaHNhbnRhbHVjaWEuaXQ=
 Tommaso Gili
Tommaso Gili Valentina Ciullo
Valentina Ciullo Gianfranco Spalletta
Gianfranco Spalletta