
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Neurosci. , 11 June 2020
Sec. Non-Neuronal Cells
Volume 14 - 2020 | https://doi.org/10.3389/fncel.2020.00156
This article is part of the Research Topic Recent Advances in Myelin Plasticity View all 9 articles
Plasticity in the central nervous system (CNS) allows for responses to changing environmental signals. While the majority of studies on brain plasticity focus on neuronal synapses, myelin plasticity has now begun to emerge as a potential modulator of neuronal networks. Oligodendrocytes (OLs) produce myelin, which provides fast signal transmission, allows for synchronization of neuronal inputs, and helps to maintain neuronal function. Thus, myelination is also thought to be involved in learning. OLs differentiate from oligodendrocyte precursor cells (OPCs), which are distributed throughout the adult brain, and myelination continues into late adulthood. This process is orchestrated by numerous cellular and molecular signals, such as axonal diameter, growth factors, extracellular signaling molecules, and neuronal activity. However, the relative importance of, and cooperation between, these signaling pathways is currently unknown. In this review, we focus on the current knowledge about myelin plasticity in the CNS. We discuss new insights into the link between this type of plasticity, learning and behavior, as well as mechanistic aspects of myelin formation that may underlie myelin plasticity, highlighting OPC diversity in the CNS.
In the central nervous system (CNS), myelin is produced by oligodendrocytes (OLs) that differentiate from oligodendrocyte precursor cells (OPCs) (Fields, 2014). OPCs are distributed throughout the adult brain and represent the main self-renewing population of cells in the CNS (Dawson et al., 2003). Myelination has generally been studied in a developmental context and is often described as a process that terminates after juvenile development. However, recent work shows that myelination continues into late adulthood, with adult OPCs providing a continuous supply of new myelinating OLs (Rivers et al., 2008; Hughes et al., 2013, 2018; Young et al., 2013; Hill et al., 2018). This suggests that protracted myelination may allow for fine-tuning of neural circuits throughout life. While studies of brain plasticity mostly focus on neuronal synapses, myelin plasticity, defined as the myelination of previously unmyelinated axons or changes in the structure of already-myelinated axons (e.g., ion channel surface expression, changes in internode number and length, myelin thickness or geometry of the nodal area), is now also thought to modulate neural networks (Sampaio-Baptista et al., 2013; Gibson et al., 2014; McKenzie et al., 2014; Pajevic et al., 2014). Indeed, changes in myelin sheath stability, length and thickness can alter conduction velocity, and therefore modulate input synchronization (Waxman, 1997; Fields, 2015). While myelin plasticity is a novel field of study, and the mechanisms underlying it are poorly understood, it is likely that several processes governing developmental myelination are applicable in the context of plasticity (Figure 1). In particular, developmental myelination is thought to occur either in a neuronal activity-independent or -dependent mode (Lundgaard et al., 2013). Here, we will briefly review both modes of myelination, along with the role of motor, cognitive and sensory learning, and OPC diversity, in the context of myelin plasticity.
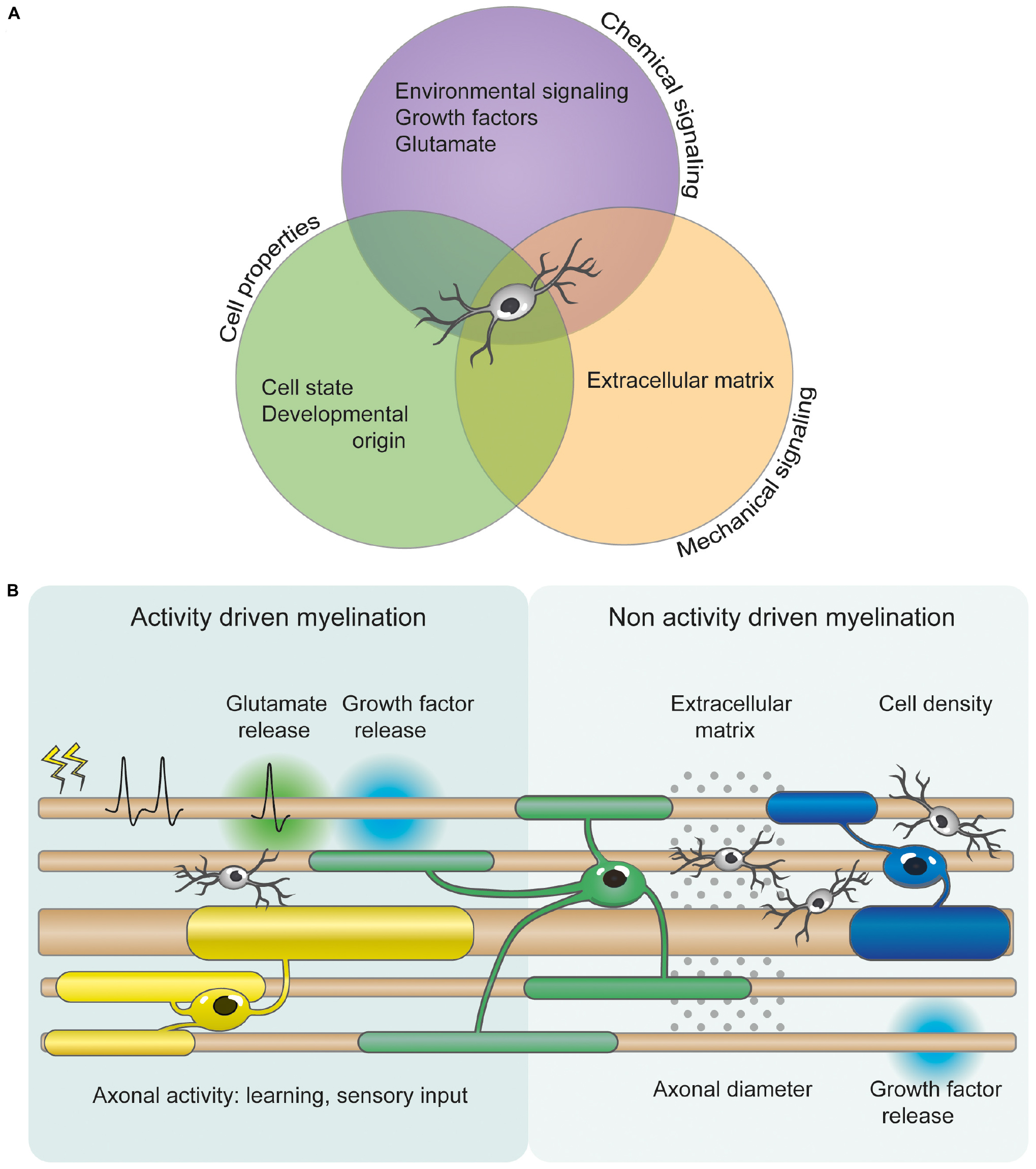
Figure 1. OPC heterogeneity and axonal factors allow for differential myelination and myelin plasticity. (A) OPC proliferation, differentiation and myelination are orchestrated by numerous mechanical, cellular, and chemical signals. These include axonal diameter, growth factors, extracellular signaling molecules, extracellular matrix composition, cellular intrinsic deposition, neurotransmitters (such as glutamate), and neuronal activity. However, the relative importance of and cooperation between these signaling pathways is currently unclear. (B) Several studies indicate that myelination can be modified by activity- and experience-driven mechanisms. Glutamate and growth factor release from electrically active neurons can regulate OPC proliferation, differentiation and myelination. Additionally, motor and possibly cognitive learning, and sensory experience also influence myelination changes. However, myelination can also occur independently of neuronal activity. Non activity driven myelination could be regulated by the physical and mechanical properties of the extracellular environment, such as cellular density and extracellular matrix. OPCs are depicted in light gray. OLs are represented in different colors to illustrate the differential myelination.
Developing OPCs are proliferative, self-renewing cells that possess the capability to differentiate into myelinating OLs (Rosenberg et al., 2008). Notwithstanding, several external cues regulate this differentiation capacity. Neuronal activity, through neurotransmitter signaling, is a regulatory signal for OPC proliferation and differentiation. However, OPCs can differentiate into OLs that wrap inert fibers with compact myelin, and have internodes of expected lengths, clearly indicating that the initiation of OL differentiation and some forms of myelination does not require neuronal activity (Rosenberg et al., 2008; Lee et al., 2012) (Table 1). Nevertheless, the presence of the axon, or an axon-like structure, remains a strong inductive signal for differentiation, suggesting that the biophysical characteristics of the axon, such as the shape and the caliber, regulate OPC differentiation. Axon caliber has been shown to influence myelin thickness (Voyvodic, 1989) and the internodal distribution (Trapp and Kidd, 2000). Similarly, increasing axonal diameter by knocking out Pten in axons induces myelination of normally unmyelinated parallel fibers (Goebbels et al., 2017), and retinal ganglion axons following enucleation (i.e., the surgical removal of one eye) (Mayoral et al., 2018). Eye enucleation in a non-degenerative mouse model reduces axonal diameter and myelination, supporting the notion that axon caliber is a main regulatory factor of myelination. Although there is a correlation between axon diameter and myelination, it is important to note that knocking out Pten alters growth factor signaling (Goebbels et al., 2017), and that enucleation alters spontaneous firing in the control eye (Failor et al., 2018), raising the possibility that diameter alone is not the only mechanism regulating myelination (Friede, 1972; Lee et al., 2012), or that different axons are myelinated by different mechanisms (Koudelka et al., 2016). Additionally, diameter alone does not explain how the same axon can be differentially myelinated along its length, nor how axons of the same diameter can be either myelinated or remain unmyelinated (Tomassy et al., 2014). Using a neuron-free in vitro system in which OPCs from either the spinal cord or cortex differentiate into OLs that ensheath inert fibers, it has been found that spinal cord OLs produced longer sheaths along the microfibers than cortical OLs, consistent with their length in the CNS (Bechler et al., 2015). These data suggest that sheath length may be intrinsic, region-specific, and programmed before differentiation. Intriguingly, Schwann cells, the myelinating glia of the peripheral nervous system (PNS), were not able to myelinate the microfibers, implying that the capacity to myelinate without axonal cues is exclusive to the CNS. However, Schwann cells usually myelinate larger diameter axons than OLs, thus another interpretation could be that the size of the fibers used in this study was insufficient to initiate Schwann cell myelination. This region-specific property points to local cues which regulate OL lineage progression and OL properties.

Table 1. Summary of current literature on activity-independent and activity-dependent myelination in the CNS.
The physical properties of the microenvironment, such as proximity to an axon or cellular density, may induce differentiation by altering OPC size or shape, thus generating structural rearrangement within the cells (Ingber, 1997; McBeath et al., 2004). This rearrangement could allow for interactions between different effectors in a signaling pathway, and thus, promote differentiation (Boudreau and Jones, 1999). Another potential explanation is that changes in cell shape might directly modify the nuclear size or structure, inducing the transcriptional activity necessary for OPC differentiation (Maniotis et al., 1997). This possibility is supported by work showing that mechanically deforming OPCs or plating them in the presence of neurons, beads, or at high density, promotes differentiation by altering the chromatin structure (Hernandez et al., 2016).
A recent study by the Chalut group further supports the idea that the mechanical environment modulates OPC function. By mimicking the stiffness of young brains using scaffolds in culture, they demonstrated that OPCs isolated from aged rats and cultured in these softer conditions became molecularly and functionally similar to neonatal OPCs. Disrupting mechanical signaling in these aged OPCs increased their proliferation and differentiation rate, indicating that increasing brain stiffening with age downregulates the proliferation and differentiation potential of OPCs (Segel et al., 2019). During development, the maturation of the extracellular matrix (ECM) stabilizes neural networks by limiting changes in synaptic connectivity (Bikbaev et al., 2015). Conversely, removing the ECM promotes synaptic plasticity (Lazarevich et al., 2020). It is possible that ECM maturation also limits differentiation and myelination rates with aging to prevent hypermyelination and stabilize neural networks. However, local changes in the ECM may allow for local differentiation and could therefore be a mechanism underlying myelin plasticity.
Changes in the physical environment can also affect the chemical signaling by altering the extra cellular volume, for instance altering growth factor concentration. This could influence OPC development as platelet-derived growth factor (PDGF) activates the α receptor (PDGFRα) on OPCs and regulates both their proliferation and survival (Raff et al., 1988; Richardson et al., 1988; Barres and Raff, 1993). However, not all OPCs respond equally to PDGF. Although PDGFRα protein expression is similar in both gray and white matter OPCs, cells in the white matter of early postnatal organotypic slice cultures proliferate more in response to PDGF than those in the gray matter (Hill et al., 2013). Consistent with this finding, it has been shown that while all adult OPCs continue to divide, white matter cells divide at a higher rate than gray matter cells (Young et al., 2013). It could be argued that the differential response of gray and white matter OPCs to PDGF stems from the microenvironment (physical properties) rather than a cell intrinsic process. Addressing this question, the Nishiyama group showed, by using small tissue section transplant experiments, that regional identity, and not environment, determined the proliferative response to PDGF (Hill et al., 2013). On the other hand, studies looking into differences due to the developmental origin of OPCs, with a transgenic approach (Psachoulia et al., 2009), or regional identity using cell transplantation (Vigano et al., 2013), have failed to find differences in OPC proliferation. A possible explanation of the difference in results is that using small tissue sections, instead of isolated cells, may have provided sufficient environmental signals of the original region to influence OPCs’ response to PDGF in the transplanted area, and, given a longer period of time for the section to integrate into the host slice, these experiments may have yielded a different result. These studies show that physical properties and environment influence OPC proliferation, differentiation and myelination. Although it is unclear whether these properties can mediate myelin plasticity in response to learning and sensory inputs, their contribution cannot be ruled out. Another mechanism such as neuronal activity, known to influence physical properties (Lazarevich et al., 2020), release of growth factors (Barres and Raff, 1993; Balkowiec and Katz, 2000) and regulate myelination, is perhaps more amenable to plasticity changes, as we shall discuss in the next paragraph.
Numerous studies have shown that neuronal activity can regulate myelination (Table 1). In addition to growth factors, glutamate release from active neurons is a likely mechanism underlying activity-dependent myelination, as OPCs receive synaptic inputs from neurons and express glutamate receptors (Bergles et al., 2000; Karadottir et al., 2005, 2008; Micu et al., 2006; Kukley et al., 2007; Ziskin et al., 2007; Lundgaard et al., 2013; Gautier et al., 2015; Spitzer et al., 2019), allowing them to monitor and respond to neuronal activity. However, it is important to note that OPCs display a range of electrophysiological profiles, with ion channel and glutamate receptor densities varying with age and brain region. OPCs acquire voltage-gated potassium channels (KV) and sodium channels (NaV), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), kainate receptors (KARs), and N-methyl-D-aspartate receptors (NMDARs) during development, but at different rates, which appear to be regulated by the environment (Spitzer et al., 2019).
Neuronal activity regulates OPC proliferation in vivo (Barres and Raff, 1993; Mangin et al., 2012; Gibson et al., 2014; Mitew et al., 2018) (Table 1). Numerous studies show that blocking activity blocks proliferation (Barres and Raff, 1993), while increasing activity promotes proliferation (Gibson et al., 2014; Mitew et al., 2018). Axonal activity also regulates differentiation (Table 1): decreasing activity by whisker removal or raising mice in social isolation impedes differentiation (Liu et al., 2012, 2016; Makinodan et al., 2012; Hill et al., 2014), although ocular deprivation leads to enhanced differentiation, albeit with reduced internode length (Etxeberria et al., 2016). However, it is important to note that ocular deprivation did not block neuronal firing, but rather altered it (Etxeberria et al., 2016), suggesting that changes in activity modulate differentiation. Similarly, enhancing activity with optogenetics, chemogenetics, receptor agonists/antagonists, or physiological manipulations, promotes differentiation (Gibson et al., 2014; Mitew et al., 2018), or enhances myelination (Tauber et al., 1980; Demerens et al., 1996; Mensch et al., 2015; Mitew et al., 2018). However, other studies using similar approaches have failed to show an effect of neuronal activity on developmental myelination (Fukui et al., 1991; Colello et al., 1995; Shrager and Novakovic, 1995). Nonetheless, blocking neuronal activity decreases myelination and prevents myelin repair after demyelination (Gyllensten and Malmfors, 1963; Demerens et al., 1996; Stevens et al., 1998; Gautier et al., 2015; Mensch et al., 2015; Mitew et al., 2018) while enhancing activity improves remyelination (Ortiz et al., 2019).
Neuronal activity not only promotes myelination through glutamate signaling, but also induces a switch to the activity-dependent mode of myelination. Indeed, activity-dependent release of neuregulin (NRG) or brain-derived neurotrophic factor (BDNF) enhances NMDAR functional expression in OPCs and switches myelination to an activity-dependent mode in neuron-OPC co-cultures (Lundgaard et al., 2013). Activity-dependent myelination occurs faster than activity-independent myelination, and crucially, blocking neuronal activity, NMDARs, or AMPARs once the activity-dependent mode is activated by NRG or BDNF significantly reduces myelination (Lundgaard et al., 2013). However, blocking activity, NMDARs or AMPARs does not affect myelination in the absence of NRG or BDNF (Lundgaard et al., 2013). In mice, blocking activity-dependent BDNF release or deleting the BDNF receptor TrkB in OPCs blocks activity-dependent myelination (Geraghty et al., 2019). Nevertheless, NRG/ErbB (the NRG receptor) signaling plays a complicated role in the CNS (Lyons et al., 2005; Brinkmann et al., 2008). Despite evidence indicating a function in OL differentiation, myelination, and survival in vitro (Flores et al., 2000; Kim et al., 2003; Taveggia et al., 2008), knocking out ErbB3 and ErbB4 in OL lineage cells does not prevent myelination (Brinkmann et al., 2008), although it is unclear whether there was a delay in myelination, it does seem to prevent experience-dependent myelination (Makinodan et al., 2012). The controversial actions of NRG/ErbB signaling can be explained by the ability of NRG to switch OPCs from activity-independent myelination to the activity-dependent mode through the increase of NMDAR-mediated currents in OL lineage cells (Lundgaard et al., 2013). It is likely that in the absence of ErbB3 and ErbB4, NRG could not enhance NMDAR expression in OPCs, and therefore, could not induce the switch to activity-dependent myelination. Therefore, a delay in myelination, but no myelination defect, would be expected, although this was not tested. Moreover, the release of NRG itself is activity-dependent (Ozaki et al., 2004). The resulting interaction of NRG with glutamate signaling increases myelination on active neurons, providing a mechanism by which activity plays a role in myelination and myelin plasticity (Spitzer et al., 2016).
The importance of glutamate signaling in activity-dependent myelination is revealed by studies of myelin repair following demyelinating lesions. Blocking neuronal activity, vesicular release, AMPARs or NMDARs at the lesion site impedes myelin regeneration after toxin-induced demyelination (Lundgaard et al., 2013; Gautier et al., 2015). However, the exact role of glutamate signaling in vivo remains elusive. In vitro studies report that AMPA/KAR activation reduces both proliferation and differentiation (Gallo et al., 1996; Yuan et al., 1998; Fannon et al., 2015), while activating NMDARs promotes myelination (Wake et al., 2011; Cavaliere et al., 2012; Li et al., 2013; Lundgaard et al., 2013). Nevertheless, the importance of glutamate signaling through AMPARs and NMDARs for myelination in vivo is disputed due to the mild deficits observed in the respective knockouts (De Biase et al., 2011; Guo et al., 2012; Saab et al., 2016; Kougioumtzidou et al., 2017). However, both receptors may have been knocked out in OPCs prior to the activation of AMPA/KARs or NMDARs (Spitzer et al., 2019). Even if neurons released NRG or BDNF onto OPCs, myelination would not have switched to the activity-dependent mode, as OPCs did not have NMDARs or AMPARs, and therefore, NRG could not enhance NMDAR expression. If this were the case, a delay in myelination would be expected, due to compensation by the slower activity-independent mode of myelination. While this was not tested in all of the studies above, two groups found that both the AMPAR and NMDAR knockouts lead to a delay in myelination (Saab et al., 2016; Kougioumtzidou et al., 2017). As activity-independent myelination occurs slower than activity-dependent myelination, these studies indicate that it is possible that compensation may have occurred by defaulting to the slower activity-independent myelination mode. Whereas, altering AMPAR receptors, postnatally, during the peak of the myelination period increased OPC proliferation while reducing their differentiation (Chen et al., 2018), suggesting that modifying receptor properties at specific timepoints can alter OPC dynamics. These studies indicate that glutamate signaling through AMPA/KARs and NMDARs depends on a complex interplay of factors, such as the receptor subtype and density, the frequency, the amount, and probability of glutamate release from active neurons. Nonetheless, glutamate signaling remains an integral mechanism of activity-dependent myelination in the context of both normal developmental myelination and myelin plasticity.
Myelin plasticity includes both de novo myelination and structural changes to existing myelin. De novo myelination is thought to occur through the differentiation of adult OPCs, which receive cues – presumably axon-derived – following motor, sensory or social experience. Thus, to study myelin plasticity, we must investigate how OPCs integrate these cues. This is made more complex by several groups reporting that OPCs are a heterogeneous population, with differences in their proliferation and differentiation potentials with age or between brain regions (Rivers et al., 2008; Vigano et al., 2013; Young et al., 2013; Moshrefi-Ravasdjani et al., 2017; Spitzer et al., 2019). In addition, bulk-RNA sequencing shows that OPCs exhibit age- related changes in transcriptome (Marques et al., 2018; Spitzer et al., 2019), and single-cell experiments suggest that proliferation and differentiation gene expression is altered with age (Marques et al., 2016, 2018). OPCs also display differential responses to growth factors and cytokines (Mason and Goldman, 2002; Lin et al., 2009; Hill et al., 2013; Lentferink et al., 2018). Furthermore, in zebrafish, two populations of OPCs were identified in the spinal cord: a population that mostly proliferates in response to activity, but does not differentiate, and a second population arising from the first one, which differentiates into myelinating OLs (Marisca et al., 2020). These differences must be considered, especially when attempting to study myelin plasticity through the lens of developmental myelination (Table 2).
Given the role of neuronal activity, via glutamate signaling, in regulating OPC proliferation, differentiation, and myelination, and its potential role in myelin plasticity regulation, it is important to understand if all OPCs display the same physiological properties. While some reports indicate that OPCs from the hippocampus and corpus callosum are homogeneous (De Biase et al., 2010; Clarke et al., 2012), differences in ion channels between gray and white matter OPCs have been described (Chittajallu et al., 2004; Spitzer et al., 2019). Furthermore, age-dependent changes in ion channels have also been described (Karram et al., 2008; Moshrefi-Ravasdjani et al., 2017; Spitzer et al., 2019) (Table 2). An in-depth study of mouse OPC membrane properties in different brain regions between embryonic day 13 and postnatal day 330 indicates that the density of NaV, KV, AMPA/KARs and NMDARs differs. Specifically, at embryonic day 13, when they first appear in the brain, OPCs have no ion channels or glutamate receptors, and acquire them with age at different rates, and differentially between brain regions (Spitzer et al., 2019). Functional expression of ion channels and glutamate receptors can be linked to the proliferation and differentiation potential of OPCs (Spitzer et al., 2019).
These data led to the identification of several OPC states. First, embryonic-like “naïve” OPCs, lacking ion channels and glutamate receptors, which cannot sense neuronal activity. Second, “highly proliferative” OPCs, with KV, AMPA/KARs, and a high density of NaV. Third, OPCs that are “primed” for differentiation, with KV, AMPA/KARs, a high NaV density, and a high density of NMDARs, indicative of a high sensitivity to neuronal activity. Lastly, “quiescent” OPCs, who have lost NMDARs, and have acquired a high density of AMPA/KARs (Spitzer et al., 2019). Importantly, at every postnatal time point and brain region tested, a range of electrophysiological profiles of OPCs can be detected, although in differing proportions, suggesting that this functional diversity may represent cell states rather than heterogeneity.
Understanding OPC states is crucial for our understanding of both activity-dependent and activity-independent myelination. For instance, most embryonic OPCs are naïve, yet proliferate, and, in the spinal cord, have begun to differentiate, perhaps indicating that early developmental myelination may proceed in an activity-independent mode, presumably to ensure that critical processes like breathing are functional by birth (Foran and Peterson, 1992; Spitzer et al., 2019). In addition, the majority of OPCs are in the primed state during the first three postnatal months, at the time where differentiation and myelination are proceeding at the highest rate, and NMDAR expression is highest, suggesting that at this time, myelination is activity-dependent (Spitzer et al., 2019). Most studies on motor or sensory myelination and myelin plasticity have been performed at this time. It is therefore not surprising that activity-dependent myelination is thought to underlie myelin plasticity.
This poses the problem of what happens in mature brains, once most OPCs have become quiescent. Does myelination stop, and does myelin plasticity remain possible? Is plasticity limited to a critical window, defined by OPC ion channel expression? The majority of OPCs were described as quiescent by nine months, yet OPC differentiation and myelination have been reported to continue in the mouse cortex until 2 years of age (Young et al., 2013; Hill et al., 2018; Hughes et al., 2018; Spitzer et al., 2019). In addition, a study examining plasticity in adult mice showed that sensory enrichment increased the formation of new myelin in the somatosensory cortex of 10–14 month old mice (Hughes et al., 2018). The signaling mechanism driving this plasticity was not investigated, but sensory enrichment alters neuronal activity, which may in turn lead to the release of NRG or BDNF from neurons, promoting NMDAR functional expression in OPCs, and a shift to the primed state (Lundgaard et al., 2013; Spitzer et al., 2019). Indeed, glutamate receptors in OPCs can be regulated by growth factors (Gallo et al., 1994; Lundgaard et al., 2013). Thus, growth factors may regulate state transitions, and allow for activity-dependent myelination following major sensory events.
OPC states may also influence the different myelination strategies employed by different brain regions. For instance, in the rodent optic nerve, myelin tends to be remodeled (with changes on already myelinated axons), while in the corpus callosum, the tendency is more toward de novo myelination of unmyelinated axons (Young et al., 2013). It is therefore critical that we understand both OPC states and their regulators to better understand myelination, and myelin plasticity.
Recent evidence suggests that myelination may be dynamically regulated by learning and experience, and may therefore play a role in learning (Table 3). Structural changes in human white matter occur with learning new tasks, such as playing the piano (Bengtsson et al., 2005) or learning how to juggle (Scholz et al., 2009), though whether these changes indicate myelin remodeling remains unclear (Zatorre et al., 2012; Walhovd et al., 2014). Nevertheless, experiments combining diffusion MRI fractional anisotropy (as in human studies) and immunohistochemistry have shown that motor learning in adult mice leads to white matter structural changes which correlate with an increased myelin density (Sampaio-Baptista et al., 2013). Furthermore, the Richardson group showed that motor learning increases OPC differentiation into myelinating OLs in the motor cortex and corpus callosum, and that motor learning is in fact dependent on this (McKenzie et al., 2014; Xiao et al., 2016).
A current outstanding question in the field is whether myelination is initiated only during sensory-motor learning or in all types of learning. In human studies, changes in white matter have been observed following reading (Carreiras et al., 2009) or learning a second language (Schlegel et al., 2012), suggesting that modifications in myelin may also occur following cognitive learning. In addition to various reports demonstrating that sensory experience or neuronal activity modulate myelination in the somatosensory system (Hughes et al., 2018; Mitew et al., 2018), a recent publication suggests that myelin plasticity is important for normal cognitive function. Activity-regulated myelination fails in a model of chemo-therapy-related cognitive impairment (CRCI), a syndrome characterized by deficits in attention and memory (Koppelmans et al., 2012), and this is linked to a reduced BDNF-TrkB signaling in OPCs, as demonstrated by the deficits in cognitive behavioral performance following the OPC-specific TrkB receptor loss (Geraghty et al., 2019).
It seems that changes in myelination as a response to the environment have important long-term behavioral and cognitive consequences. Indeed, rearing juvenile mice in social isolation alters myelin in the medial prefrontal cortex (mPFC) (Liu et al., 2012; Makinodan et al., 2012). In one of these studies, NRG was shown to be decreased following social isolation, and the modifications in myelin were phenocopied by an OL ErbB3 receptor knockout (Makinodan et al., 2012). Together, these data indicate that social experience, presumably via neuronal activity, regulates myelination and that this is important for normal cognitive function.
Sensory experience also influences myelin plasticity. Whisker deprivation, by unilateral removal, leads to a decrease in the number of mature OLs, but an increase in OPC density and proliferation (Mangin et al., 2012; Hill et al., 2014). However, whisker deprivation also increases apoptosis of proliferating OPCs (Hill et al., 2014). Thus, these data suggest that whisker deprivation leads to a decrease in mature OL numbers, which may in turn lead to over proliferation of OPCs, although the increase in apoptosis may be a mechanism to maintain homeostasis (Hill et al., 2014).
The surprisingly rapid dynamics of OL production in response to motor learning (within 2 h) (Xiao et al., 2016) and myelin basic protein (MBP) translation in response to neuronal activity (within minutes to hours) (Wake et al., 2011) occur on a timescale that is similar to that of dendritic spine changes underlying synaptic plasticity (Xu et al., 2009). Like synaptic plasticity, myelin plasticity following motor or cognitive learning and sensory experience is thought to be regulated by activity-dependent myelination, as motor, cognitive and sensory events lead to changes in neuronal activity (although the contribution of activity-independent myelination cannot be excluded) (Figure 1). These data suggest that myelin plasticity and synaptic plasticity may be complementary mechanisms underlying learning and memory.
Until recently, myelination was considered a static process, and studies examining circuit function and plasticity mostly focused on synaptic plasticity. However, a number of studies described above demonstrate that myelination is far from static, and does not only change in response to injury, but also as a result of motor, sensory and cognitive events. Although some progress has been recently made, our knowledge of myelin plasticity and the mechanisms underlying it remains restricted by regional differences, OPC diversity, a lack of understanding of the neuronal dynamics required to regulate OL lineage progression, and a limited comprehension of how OL lineage cells integrate the various activity-independent and activity-dependent signals (Figure 1).
The study of myelin plasticity requires morphological analyses, both at the sub microscopic scale and the macroscopic scale, but also a combination of behavioral, electrophysiological and molecular analyses. This can only be achieved by combining both in vitro and in vivo experimental models.
One area that deserves major investigation is examining whether activity-dependent myelination proceeds similarly in different brain regions. From the studies reviewed in this paper, it appears that, akin to synaptic plasticity, neuronal activity-dependent myelin plasticity may be an important mechanism underlying learning and cognition. Activity-dependent release of growth factors and glutamate may be particularly important for this process, and thus, it is crucial to understand these mechanisms of myelination. Moreover, dynamic myelin changes in the hippocampus and mPFC, two regions that are comprised of both gray and white matter, are likely to have long-lasting effects on brain function. In humans, myelination of these regions continues for decades, suggesting that lifelong myelination and myelin plasticity tune neuronal networks and regulate normal brain function.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
This work was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Programme (Grant Agreement No. 771411; RK, GB, and KE); a Wellcome Studentship (102160/Z/13/Z; YK), the Fonds de recherche du Québec-Santé, a scholarship (YK); the Cambridge Commonwealth European and International Trust, a scholarship (YK); and the Lister Institute, a Research Prize (RK).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Balkowiec, A., and Katz, D. M. (2000). Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. J. Neurosci. 20, 7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000
Barres, B. A., and Raff, M. C. (1993). Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 361, 258–260. doi: 10.1038/361258a0
Bechler, M. E., Byrne, L., and Ffrench-Constant, C. (2015). CNS myelin sheath lengths are an intrinsic property of oligodendrocytes. Curr. Biol. 25, 2411–2416. doi: 10.1016/j.cub.2015.07.056
Bengtsson, S. L., Nagy, Z., Skare, S., Forsman, L., Forssberg, H., and Ullen, F. (2005). Extensive piano practicing has regionally specific effects on white matter development. Nat. Neurosci. 8, 1148–1150. doi: 10.1038/nn1516
Bergles, D. E., Roberts, J. D., Somogyi, P., and Jahr, C. E. (2000). Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 405, 187–191. doi: 10.1038/35012083
Bikbaev, A., Frischknecht, R., and Heine, M. (2015). Brain extracellular matrix retains connectivity in neuronal networks. Sci. Rep. 5:14527. doi: 10.1038/srep14527
Boudreau, N. J., and Jones, P. L. (1999). Extracellular matrix and integrin signalling: the shape of things to come. Biochem. J. 339, 481–488. doi: 10.1042/bj3390481
Brinkmann, B. G., Agarwal, A., Sereda, M. W., Garratt, A. N., Müller, T., Wende, H., et al. (2008). Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron 59, 581–595. doi: 10.1016/j.neuron.2008.06.028
Carreiras, M., Seghier, M. L., Baquero, S., Estévez, A., Lozano, A., Devlin, J. T., et al. (2009). An anatomical signature for literacy. Nature 461, 983–986. doi: 10.1038/nature08461
Cavaliere, F., Urra, O., Alberdi, E., and Matute, C. (2012). Oligodendrocyte differentiation from adult multipotent stem cells is modulated by glutamate. Cell Death Dis. 3:e268. doi: 10.1038/cddis.2011.144
Chen, T. J., Kula, B., Nagy, B., Barzan, R., Gall, A., Ehrlich, I., et al. (2018). In vivo regulation of oligodendrocyte precursor cell proliferation and differentiation by the AMPA-receptor subunit GluA2. Cell Rep. 25, 852–861. doi: 10.1016/j.celrep.2018.09.066
Chittajallu, R., Aguirre, A., and Gallo, V. (2004). NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J. Physiol. 561, 109–122. doi: 10.1113/jphysiol.2004.074252
Clarke, L. E., Young, K. M., Hamilton, N. B., Li, H., Richardson, W. D., and Attwell, D. (2012). Properties and fate of oligodendrocyte progenitor cells in the corpus callosum, motor cortex, and piriform cortex of the mouse. J. Neurosci. 32, 8173–8185. doi: 10.1523/JNEUROSCI.0928-12.2012
Colello, R. J., Devey, L. R., Imperato, E., and Pott, U. (1995). The chronology of oligodendrocyte differentiation in the rat optic nerve: evidence for a signaling step initiating myelination in the CNS. J. Neurosci. 15, 7665–7672. doi: 10.1523/jneurosci.15-11-07665.1995
Dawson, M. R., Polito, A., Levine, J. M., and Reynolds, R. (2003). NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell. Neurosci. 24, 476–488. doi: 10.1016/s1044-7431(03)00210-0
De Biase, L. M., Kang, S. H., Baxi, E. G., Fukaya, M., Pucak, M. L., Mishina, M., et al. (2011). NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. J. Neurosci. 31, 12650–12662. doi: 10.1523/JNEUROSCI.2455-11.2011
De Biase, L. M., Nishiyama, A., and Bergles, D. E. (2010). Excitability and synaptic communication within the oligodendrocyte lineage. J. Neurosci. 30, 3600–3611. doi: 10.1523/JNEUROSCI.6000-09.2010
Demerens, C., Stankoff, B., Logak, M., Anglade, P., Allinquant, B., Couraud, F., et al. (1996). Induction of myelination in the central nervous system by electrical activity. Proc. Natl. Acad. Sci. U.S.A. 93, 9887–9892. doi: 10.1073/pnas.93.18.9887
Etxeberria, A., Hokanson, K. C., Dao, D. Q., Mayoral, S. R., Mei, F., Redmond, S. A., et al. (2016). Dynamic modulation of myelination in response to visual stimuli alters optic nerve conduction velocity. J. Neurosci. 36, 6937–6948. doi: 10.1523/JNEUROSCI.0908-16.2016
Failor, S. W., Ng, A., and Cheng, H. J. (2018). Monocular enucleation alters retinal waves in the surviving eye. Neural Dev. 13:4. doi: 10.1186/s13064-018-0101-1
Fannon, J., Tarmier, W., and Fulton, D. (2015). Neuronal activity and AMPA-type glutamate receptor activation regulates the morphological development of oligodendrocyte precursor cells. Glia 63, 1021–1035. doi: 10.1002/glia.22799
Fields, R. D. (2014). Myelin - More than insulation. Science 344, 264–266. doi: 10.1126/science.1253851
Fields, R. D. (2015). A new mechanism of nervous system plasticity: activity-dependent myelination. Nat. Rev. Neurosci. 16, 756–767. doi: 10.1038/nrn4023
Flores, A. I., Mallon, B. S., Matsui, T., Ogawa, W., Rosenzweig, A., Okamoto, T., et al. (2000). Akt-mediated survival of oligodendrocytes induced by neuregulins. J. Neurosci. 20, 7622–7630. doi: 10.1523/JNEUROSCI.20-20-07622.2000
Foran, D. R., and Peterson, A. C. (1992). Myelin acquisition in the central nervous system of the mouse revealed by an MBP-Lac Z transgene. J. Neurosci. 12, 4890–4897. doi: 10.1523/jneurosci.12-12-04890.1992
Friede, R. L. (1972). Control of myelin formation by axon caliber. (With a model of the control mechanism). J. Comp. Neurol. 144, 233–252. doi: 10.1002/cne.901440207
Fukui, Y., Hayasaka, S., Bedi, K. S., Ozaki, H. S., and Takeuchi, Y. (1991). Quantitative study of the development of the optic nerve in rats reared in the dark during early postnatal life. J. Anat. 174, 37–47.
Gallo, V., Wright, P., and McKinnon, R. D. (1994). Expression and regulation of a glutamate receptor subunit by bFGF in oligodendrocyte progenitors. Glia 10, 149–153. doi: 10.1002/glia.440100209
Gallo, V., Zhou, J. M., McBain, C. J., Wright, P., Knutson, P. L., and Armstrong, R. C. (1996). Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J. Neurosci. 16, 2659–2670. doi: 10.1523/JNEUROSCI.16-08-02659.1996
Gautier, H. O., Evans, K. A., Volbracht, K., James, R., Sitnikov, S., Lundgaard, I., et al. (2015). Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors. Nat. Commun. 6:8518. doi: 10.1038/ncomms9518
Geraghty, A. C., Gibson, E. M., Ghanem, R. A., Greene, J. J., Ocampo, A., Goldstein, A. K., et al. (2019). Loss of adaptive myelination contributes to methotrexate chemotherapy-related cognitive impairment. Neuron 103, 250–265.e8. doi: 10.1016/j.neuron.2019.04.032
Gibson, E. M., Purger, D., Mount, C. W., Goldstein, A. K., Lin, G. L., Wood, L. S., et al. (2014). Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344:1252304. doi: 10.1126/science.1252304
Goebbels, S., Wieser, G. L., Pieper, A., Spitzer, S., Weege, B., Yan, K., et al. (2017). A neuronal PI(3,4,5)P 3 -dependent program of oligodendrocyte precursor recruitment and myelination. Nat. Neurosci. 20, 10–15. doi: 10.1038/nn.4425
Guo, F., Maeda, Y., Ko, E. M., Delgado, M., Horiuchi, M., Soulika, A., et al. (2012). Disruption of NMDA receptors in oligodendroglial lineage cells does not alter their susceptibility to experimental autoimmune encephalomyelitis or their normal development. J. Neurosci. 32, 639–645. doi: 10.1523/JNEUROSCI.4073-11.2012
Gyllensten, L., and Malmfors, T. (1963). Myelinization of the optic nerve and its dependence on visual function–a quantitative investigation in mice. J. Embryol. Exp. Morphol. 11, 255–266.
Hernandez, M., Patzig, J., Mayoral, S. R., Costa, K. D., Chan, J. R., and Casaccia, P. (2016). Mechanostimulation promotes nuclear and epigenetic changes in oligodendrocytes. J. Neurosci. 36, 806–813. doi: 10.1523/JNEUROSCI.2873-15.2016
Hill, R. A., Li, A. M., and Grutzendler, J. (2018). Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat. Neurosci. 21, 683–695. doi: 10.1038/s41593-018-0120-6
Hill, R. A., Patel, K. D., Goncalves, C. M., Grutzendler, J., and Nishiyama, A. (2014). Modulation of oligodendrocyte generation during a critical temporal window after NG2 cell division. Nat. Neurosci. 17, 1518–1527. doi: 10.1038/nn.3815
Hill, R. A., Patel, K. D., Medved, J., Reiss, A. M., and Nishiyama, A. (2013). NG2 cells in white matter but not gray matter proliferate in response to PDGF. J. Neurosci. 33, 14558–14566. doi: 10.1523/JNEUROSCI.2001-12.2013
Hughes, E. G., Kang, S. H., Fukaya, M., and Bergles, D. E. (2013). Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci. 16, 668–676. doi: 10.1038/nn.3390
Hughes, E. G., Orthmann-Murphy, J. L., Langseth, A. J., and Bergles, D. E. (2018). Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci. 21, 696–706. doi: 10.1038/s41593-018-0121-5
Ingber, D. E. (1997). Tensegrity: the architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 59, 575–599. doi: 10.1146/annurev.physiol.59.1.575
Karadottir, R., Cavelier, P., Bergersen, L. H., and Attwell, D. (2005). NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 438, 1162–1166. doi: 10.1038/nature04302
Karadottir, R., Hamilton, N. B., Bakiri, Y., and Attwell, D. (2008). Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat. Neurosci. 11, 450–456. doi: 10.1038/nn2060
Karram, K., Goebbels, S., Schwab, M., Jennissen, K., Seifert, G., Steinhauser, C., et al. (2008). NG2-expressing cells in the nervous system revealed by the NG2-EYFP-knockin mouse. Genesis 46, 743–757. doi: 10.1002/dvg.20440
Kim, J. Y., Sun, Q., Oglesbee, M., and Yoon, S. O. (2003). The role of ErbB2 signaling in the onset of terminal differentiation of oligodendrocytes in vivo. J. Neurosci. 23, 5561–5571. doi: 10.1523/jneurosci.23-13-05561.2003
Koppelmans, V., Breteler, M. M. B., Boogerd, W., Seynaeve, C., Gundy, C., and Schagen, S. B. (2012). Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J. Clin. Oncol. 30, 1080–1086. doi: 10.1200/JCO.2011.37.0189
Koudelka, S., Voas, M. G., Almeida, R. G., Baraban, M., Soetaert, J., Meyer, M. P., et al. (2016). Individual neuronal subtypes exhibit diversity in CNS myelination mediated by synaptic vesicle release. Curr. Biol. 26, 1447–1455. doi: 10.1016/j.cub.2016.03.070
Kougioumtzidou, E., Shimizu, T., Hamilton, N. B., Tohyama, K., Sprengel, R., Monyer, H., et al. (2017). Signalling through AMPA receptors on oligodendrocyte precursors promotes myelination by enhancing oligodendrocyte survival. eLife 6:e28080. doi: 10.7554/eLife.28080
Kukley, M., Capetillo-Zarate, E., and Dietrich, D. (2007). Vesicular glutamate release from axons in white matter. Nat. Neurosci. 10, 311–320. doi: 10.1038/nn1850
Lazarevich, I., Stasenko, S., Rozhnova, M., Pankratova, E., Dityatev, A., and Kazantsev, V. (2020). Activity-dependent switches between dynamic regimes of extracellular matrix expression. PLoS One 15:e0227917. doi: 10.1371/journal.pone.0227917
Lee, S., Leach, M. K., Redmond, S. A., Chong, S. Y. C., Mellon, S. H., Tuck, S. J., et al. (2012). A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat. Methods 9, 917–922. doi: 10.1038/nmeth.2105
Lentferink, D. H., Jongsma, J. M., Werkman, I., and Baron, W. (2018). Grey matter OPCs are less mature and less sensitive to IFNgamma than white matter OPCs: consequences for remyelination. Sci. Rep. 8:2113. doi: 10.1038/s41598-018-19934-6
Li, C., Xiao, L., Liu, X., Yang, W., Shen, W., Hu, C., et al. (2013). A functional role of NMDA receptor in regulating the differentiation of oligodendrocyte precursor cells and remyelination. Glia 61, 732–749. doi: 10.1002/glia.22469
Lin, G., Mela, A., Guilfoyle, E. M., and Goldman, J. E. (2009). Neonatal and adult O4(+) oligodendrocyte lineage cells display different growth factor responses and different gene expression patterns. J. Neurosci. Res. 87, 3390–3402. doi: 10.1002/jnr.22065
Liu, J., Dietz, K., DeLoyht, J. M., Pedre, X., Kelkar, D., Kaur, J., et al. (2012). Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 15, 1621–1623. doi: 10.1038/nn.3263
Liu, J., Dupree, J. L., Gacias, M., Frawley, R., Sikder, T., Naik, P., et al. (2016). Clemastine enhances myelination in the prefrontal cortex and rescues behavioral changes in socially isolated mice. J. Neurosci. 36, 957–962. doi: 10.1523/JNEUROSCI.3608-15.2016
Lundgaard, I., Luzhynskaya, A., Stockley, J. H., Wang, Z., Evans, K. A., Swire, M., et al. (2013). Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol. 11:e1001743. doi: 10.1371/journal.pbio.1001743
Lyons, D. A., Pogoda, H. M., Voas, M. G., Woods, I. G., Diamond, B., Nix, R., et al. (2005). erbb3 and erbb2 are essential for Schwann cell migration and myelination in zebrafish. Curr. Biol. 15, 513–524. doi: 10.1016/j.cub.2005.02.030
Makinodan, M., Rosen, K. M., Ito, S., and Corfas, G. (2012). A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 337, 1357–1360. doi: 10.1126/science.1220845
Mangin, J.-M., Li, P., Scafidi, J., and Gallo, V. (2012). Experience-dependent regulation of NG2 progenitors in the developing barrel cortex. Nat. Neurosci. 15, 1192–1194. doi: 10.1038/nn.3190
Maniotis, A. J., Chen, C. S., and Ingber, D. E. (1997). Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl. Acad. Sci. U.S.A. 94, 849–854. doi: 10.1073/pnas.94.3.849
Marisca, R., Hoche, T., Agirre, E., Hoodless, L. J., Barkey, W., Auer, F., et al. (2020). Functionally distinct subgroups of oligodendrocyte precursor cells integrate neural activity and execute myelin formation. Nat. Neurosci. 23, 363–374. doi: 10.1038/s41593-019-0581-2
Marques, S., van Bruggen, D., Vanichkina, D. P., Floriddia, E. M., Munguba, H., Varemo, L., et al. (2018). Transcriptional convergence of oligodendrocyte lineage progenitors during development. Dev. Cell 46, 504–517.e7. doi: 10.1016/j.devcel.2018.07.005
Marques, S., Zeisel, A., Codeluppi, S., van Bruggen, D., Falcão, A. M., Xiao, L., et al. (2016). Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352, 1326–1329. doi: 10.1126/science.aaf6463
Mason, J. L., and Goldman, J. E. (2002). A2B5+ and O4+ Cycling Progenitors in the Adult Forebrain White Matter Respond Differentially to PDGF-AA, FGF-2, and IGF-1. Mol. Cell. Neurosci. 20, 30–42. doi: 10.1006/mcne.2002.1114
Mayoral, S. R., Etxeberria, A., Shen, Y. A. A., and Chan, J. R. (2018). Initiation of CNS myelination in the optic nerve is dependent on axon caliber. Cell Rep. 25, 544–550. doi: 10.1016/j.celrep.2018.09.052
McBeath, R., Pirone, D. M., Nelson, C. M., Bhadriraju, K., and Chen, C. S. (2004). Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495. doi: 10.1016/S1534-5807(04)00075-9
McKenzie, I. A., Ohayon, D., Li, H., de Faria, J. P., Emery, B., Tohyama, K., et al. (2014). Motor skill learning requires active central myelination. Science 346, 318–322. doi: 10.1126/science.1254960
Mensch, S., Baraban, M., Almeida, R., Czopka, T., Ausborn, J., El Manira, A., et al. (2015). Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat. Neurosci. 18, 628–630. doi: 10.1038/nn.3991
Micu, I., Jiang, Q., Coderre, E., Ridsdale, A., Zhang, L., Woulfe, J., et al. (2006). NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature 439, 988–992. doi: 10.1038/nature04474
Mitew, S., Gobius, I., Fenlon, L. R., McDougall, S. J., Hawkes, D., Xing, Y. L., et al. (2018). Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat. Commun. 9:306. doi: 10.1038/s41467-017-02719-2
Moshrefi-Ravasdjani, B., Dublin, P., Seifert, G., Jennissen, K., Steinhäuser, C., Kafitz, K. W., et al. (2017). Changes in the proliferative capacity of NG2 cell subpopulations during postnatal development of the mouse hippocampus. Brain Struct. Funct. 222, 831–847. doi: 10.1007/s00429-016-1249-2
Ortiz, F. C., Habermacher, C., Graciarena, M., Houry, P. Y., Nishiyama, A., Nait Oumesmar, B., et al. (2019). Neuronal activity in vivo enhances functional myelin repair. JCI Insight 5:e123434. doi: 10.1172/jci.insight.123434
Ozaki, M., Itoh, K., Miyakawa, Y., Kishida, H., and Hashikawa, T. (2004). Protein processing and releases of neuregulin-1 are regulated in an activity-dependent manner. J. Neurochem. 91, 976–988. doi: 10.1111/j.1471-4159.2004.02719.x
Pajevic, S., Basser, P. J., and Fields, R. D. (2014). Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience 276, 135–147. doi: 10.1016/j.neuroscience.2013.11.007
Psachoulia, K., Jamen, F., Young, K. M., and Richardson, W. D. (2009). Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biol. 5, 57–67. doi: 10.1017/S1740925X09990354
Raff, M. C., Lillien, L. E., Richardson, W. D., Burne, J. F., and Noble, M. D. (1988). Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature 333, 562–565. doi: 10.1038/333562a0
Richardson, W. D., Pringle, N., Mosley, M. J., Westermark, B., and Dubois-Dalcg, M. (1988). A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell 53, 309–319. doi: 10.1016/0092-8674(88)90392-3
Rivers, L. E., Young, K. M., Rizzi, M., Jamen, F., Psachoulia, K., Wade, A., et al. (2008). PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat. Neurosci. 11, 1392–1401. doi: 10.1038/nn.2220
Rosenberg, S. S., Kelland, E. E., Tokar, E., De La Torre, A. R., and Chan, J. R. (2008). The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc. Natl. Acad. Sci. U.S.A. 105, 14662–14667. doi: 10.1073/pnas.0805640105
Saab, A. S., Tzvetavona, I. D., Trevisiol, A., Baltan, S., Dibaj, P., Kusch, K., et al. (2016). Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron 91, 119–132. doi: 10.1016/j.neuron.2016.05.016
Sampaio-Baptista, C., Khrapitchev, A. A., Foxley, S., Schlagheck, T., Scholz, J., Jbabdi, S., et al. (2013). Motor skill learning induces changes in white matter microstructure and myelination. J. Neurosci. 33, 19499–19503. doi: 10.1523/JNEUROSCI.3048-13.2013
Schlegel, A. A., Rudelson, J. J., and Tse, P. U. (2012). White matter structure changes as adults learn a second language. J. Cogn. Neurosci. 24, 1664–1670. doi: 10.1162/jocn_a_00240
Scholz, J., Klein, M. C., Behrens, T. E. J., and Johansen-Berg, H. (2009). Training induces changes in white-matter architecture. Nat. Neurosci. 12, 1370–1371. doi: 10.1038/nn.2412
Segel, M., Neumann, B., Hill, M. F. E., Weber, I. P., Viscomi, C., Zhao, C., et al. (2019). Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature 573, 130–134. doi: 10.1038/s41586-019-1484-9
Shrager, P., and Novakovic, S. D. (1995). Control of myelination, axonal growth, and synapse formation in spinal cord explants by ion channels and electrical activity. Dev. Brain Res. 88, 68–78. doi: 10.1016/0165-3806(95)00081-N
Spitzer, S., Volbracht, K., Lundgaard, I., and Káradóttir, R. T. (2016). Glutamate signalling: a multifaceted modulator of oligodendrocyte lineage cells in health and disease. Neuropharmacology 110, 574–585. doi: 10.1016/j.neuropharm.2016.06.014
Spitzer, S. O., Sitnikov, S., Kamen, Y., Evans, K. A., Kronenberg-Versteeg, D., Dietmann, S., et al. (2019). Oligodendrocyte progenitor cells become regionally diverse and heterogeneous with age. Neuron 101, 459–471. doi: 10.1016/j.neuron.2018.12.020
Stevens, B., Tanner, S., and Fields, R. D. (1998). Control of myelination by specific patterns of neural impulses. J. Neurosci. 18, 9303–9311. doi: 10.1523/JNEUROSCI.18-22-09303.1998
Tauber, H., Waehneldt, T. V., and Neuhoff, V. (1980). Myelination in rabbit optic nerves is accelerated by artificial eye opening. Neurosci. Lett. 16, 235–238. doi: 10.1016/0304-3940(80)90003-8
Taveggia, C., Thaker, P., Petrylak, A., Caporaso, G. L., Toews, A., Falls, D. L., et al. (2008). Type III neuregulin-1 promotes oligodendrocyte myelination. Glia 56, 284–293. doi: 10.1002/glia.20612
Tomassy, G. S., Berger, D. R., Chen, H. H., Kasthuri, N., Hayworth, K. J., Vercelli, A., et al. (2014). Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 344, 319–324. doi: 10.1126/science.1249766
Trapp, B. D., and Kidd, G. J. (2000). Axo-glial septate junctions: the maestro of nodal formation and myelination? J. Cell Biol. 150, F97–F100. doi: 10.1083/jcb.150.3.F97
Vigano, F., Mobius, W., Gotz, M., and Dimou, L. (2013). Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat. Neurosci. 16, 1370–1372. doi: 10.1038/nn.3503
Voyvodic, J. T. (1989). Target size regulates calibre and myelination of sympathetic axons. Nature 342, 430–433. doi: 10.1038/342430a0
Wake, H., Lee, P. R., and Fields, R. D. (2011). Control of local protein synthesis and initial events in myelination by action potentials. Science 333, 1647–1651. doi: 10.1126/science.1206998
Walhovd, K. B., Johansen-Berg, H., and Káradóttir, R. T. (2014). Unraveling the secrets of white matter - Bridging the gap between cellular, animal and human imaging studies. Neuroscience 276, 2–13. doi: 10.1016/j.neuroscience.2014.06.058
Waxman, S. G. (1997). Axon-glia interactions: building a smart nerve fiber. Curr. Biol. 7, 406–410. doi: 10.1016/s0960-9822(06)00203-x
Xiao, L., Ohayon, D., McKenzie, I. A., Sinclair-Wilson, A., Wright, J. L., Fudge, A. D., et al. (2016). Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat. Neurosci. 19, 1210–1217. doi: 10.1038/nn.4351
Xu, T., Yu, X., Perlik, A. J., Tobin, W. F., Zweig, J. A., Tennant, K., et al. (2009). Rapid formation and selective stabilization of synapses for enduring motor memories. Nature 462, 915–919. doi: 10.1038/nature08389
Young, K. M., Psachoulia, K., Tripathi, R. B., Dunn, S. J., Cossell, L., Attwell, D., et al. (2013). Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–885. doi: 10.1016/j.neuron.2013.01.006
Yuan, X., Eisen, A. M., McBain, C. J., and Gallo, V. (1998). A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development 125, 2901–2914.
Zatorre, R. J., Fields, R. D., and Johansen-Berg, H. (2012). Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat. Neurosci. 15, 528–536. doi: 10.1038/nn.3045
Keywords: myelin plasticity, oligodendrocyte, oligodendrocyte precursor cell, myelin, glutamate
Citation: Bonetto G, Kamen Y, Evans KA and Káradóttir RT (2020) Unraveling Myelin Plasticity. Front. Cell. Neurosci. 14:156. doi: 10.3389/fncel.2020.00156
Received: 15 March 2020; Accepted: 11 May 2020;
Published: 11 June 2020.
Edited by:
Pascale Durbec, UMR 7288 Institut de Biologie du Développement de Marseille (IBDM), FranceReviewed by:
Wendy Xin, University of California, San Francisco, United StatesCopyright © 2020 Bonetto, Kamen, Evans and Káradóttir. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ragnhildur Thóra Káradóttir, cmszODVAY2FtLmFjLnVr
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.