
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cell. Neurosci., 20 September 2019
Sec. Non-Neuronal Cells
Volume 13 - 2019 | https://doi.org/10.3389/fncel.2019.00405
This article is part of the Research TopicFundamentals of 21st Century NeuroscienceView all 32 articles
The blood–brain barrier (BBB) helps maintain a tightly regulated microenvironment for optimal central nervous system (CNS) homeostasis and facilitates communications with the peripheral circulation. The brain endothelial cells, lining the brain’s vasculature, maintain close interactions with surrounding brain cells, e.g., astrocytes, pericytes and perivascular macrophages. This function facilitates critical intercellular crosstalk, giving rise to the concept of the neurovascular unit (NVU). The steady and appropriate communication between all components of the NVU is essential for normal CNS homeostasis and function, and dysregulation of one of its constituents can result in disease. Among the different brain regions, and along the vascular tree, the cellular composition of the NVU varies. Therefore, differential cues from the immediate vascular environment can affect BBB phenotype. To support the fluctuating metabolic and functional needs of the underlying neuropil, a specialized vascular heterogeneity is required. This is achieved by variances in barrier function, expression of transporters, receptors, and adhesion molecules. This mini-review will take you on a journey through evolving concepts surrounding the BBB, the NVU and beyond. Exploring classical experiments leading to new approaches will allow us to understand that the BBB is not merely a static separation between the brain and periphery but a closely regulated and interactive entity. We will discuss shifting paradigms, and ultimately aim to address the importance of BBB endothelial heterogeneity with regard to the function of the BBB within the NVU, and touch on its implications for different neuropathologies.
The central nervous system (CNS) needs a highly controlled microenvironment for optimal functioning. Several barriers of the CNS, including the cerebral endothelial cells (CECs) of the blood brain barrier (BBB) tightly regulate transport into and out of the CNS. An early indication of a barrier at the cerebral blood vessels was recorded by Ridley (1653–1708) after injecting wax and mercury, resulting in three-dimensional vessel casts. While trying to improve histological tissue staining, the classical experiments by Ehrlich and Goldman, during the late 1800s and early 1900s, also suggested a separation between the CNS and peripheral circulation. During that same era, Lewandowsky tested neuro-pharmacologically active substances in animals, and observed neurological effects of only a subset. He hypothesized that, in order to shuttle them into the CNS, the brain vessel wall displayed a specific affinity for these select substances. Studies on the movement of substances between peripheral blood, cerebrospinal fluid and brain, led Lina Stern and collaborators (1918–1934) to the conclusion that CECs played a dual role in both protecting and metabolically supporting the CNS, thereby effectively proposing the BBB concept. The introduction of the transmission electron microscope allowed Reese and Karnovsky (1967) to show that electron dense tracers were not able to penetrate in-between adjacent CECs, hence pointing to the actual site of the barrier. Subsequent studies with tracers and micro-electrodes, confirmed the low BBB permeability, and demonstrated its high trans-endothelial electrical resistance (Crone and Olesen, 1982). In the late 1980s it was discovered that transmembrane multi-protein tight junctional complexes at CEC-CEC borders conferred to the BBB its barrier function. Freeze facture studies, initially carried out by Farquhar and Palade (1963), revealed the complex belt-like networks of these cell–cell junctions. Analysis, first on epithelial cells and later confirmed for brain endothelium, identified individual junctional components, including claudins, occludins, junctional adhesion molecules, AJ (e.g., VE-cadherin, N-cadherin, and β-catenin) and cytoplasmic adaptors, such as zona occludens proteins. Due to its stringent barrier function and low vesicle transport activity, the passage of nutrients and waste products across the BBB was found to be regulated by polarized transporters on CECs; with efflux transporters, such as the ATP-binding cassette transporter family, usually at the luminal membrane, and solute carriers delivering essential nutrients into the CNS, such as GLUT-1, predominantly localized on the abluminal side (Bendayan et al., 2006; Roberts et al., 2008). Together, these classical experiments established the concept of a tight BBB at endothelial junctions. In-depth reviews on the history of the BBB (Liddelow, 2011; Saunders et al., 2014) and BBB-endothelial junctions (Haseloff et al., 2015; Bauer and Traweger, 2016; Stamatovic et al., 2016) are suggested.
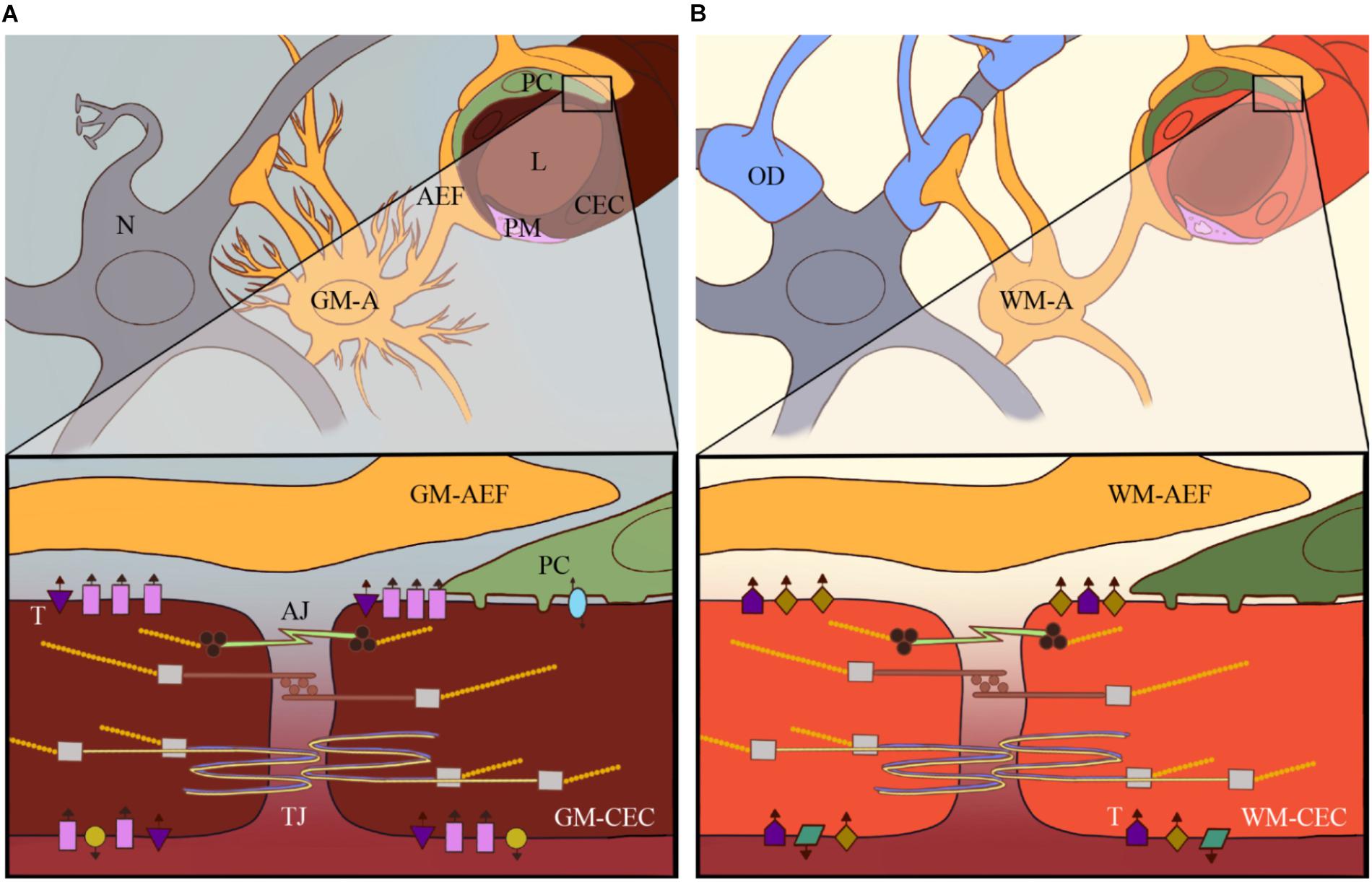
Figure 1. Heterogeneity of the neurovascular unit (NVU): current research has revealed high heterogeneity of e.g., astrocytes and pericytes within the CNS and among different brain areas. The heterogeneity of these different cellular components of the NVU in, for example, the GM (A) versus the WM (B) contributes to brain vascular heterogeneity to support local physiological and metabolic needs for that particular part of the brain. This includes differential expression of specific receptors and transporters on CECs, such as GLUT-1, Pgp and Na+/K+-ATPase, which are represented by the different shapes and colors in GM (A) versus WM-CECs (B).
The modulating influence of the neural environment on the BBB was first suggested by Stewart and Wiley (1981) in a series of reverse grafting experiments of neural and non-neural tissues. Ultrastructural studies revealed close apposition of AEF to CECs, and cell transplantation experiments and trypan blue exclusion confirmed that astrocytes contributed to the BBB phenotype (Janzer and Raff, 1987). Subsequent studies showed that astrocyte-derived signaling to CECs leading to increased expression of tight-junctions was mediated by, e.g., transforming growth factor beta (TGFβ), Sonic hedgehog and Wnt signaling (Siddharthan et al., 2007; Alvarez et al., 2011; Wang et al., 2018; Benz et al., 2019). Moreover, astrocytes regulate expression of alkaline phosphatase and Na-ATPase on CECs via cAMP and IL6, suggesting astrocytes regulate ionic homeostasis (Beuckmann et al., 1995; Sun et al., 1997).
Besides astrocytes, pericytes also influence the BBB phenotype. Pericytes are mural cells that wrap around the abluminal surface of cerebral microvessels. Due to the presence of smooth muscle-like fibers, contractile characteristics and expression of vaso-active mediators, pericytes were initially thought to regulate microvascular hemodynamics (Balabanov and Dore-Duffy, 1998). Subsequent ultrastructural studies revealed that pericytes intercalate with AEF, covering up to 60–70% of CECs, and maintain close physical contacts with CECs via gap junctions and “peg-socket” structures, indicative of communicative functions. In the CNS, pericyte loss and low pericyte coverage correlate with increased BBB permeability, implicating their involvement in regulating BBB-barrier functions (Winkler et al., 2011). Combined in vivo murine models and in vitro studies confirmed the ability of pericytes to directly modulate BBB phenotype by regulating, e.g., Wnt and Notch signaling, caveolar transport across the BBB through the expression of the lysolipid transporter mfsd2a (Ben-Zvi et al., 2014; Sweeney et al., 2016), and the expression of GLUT-1 and transferrin receptor CD71 (Liebner et al., 2011). Pericyte CD146, together with PDGF act via TGFβ, contributing to CECs barrier function (Armulik et al., 2010; Sa-Pereira et al., 2012; Chen et al., 2017). Indirectly, pericytes target CECs by inducing polarization of AQP4, Kir4.1, and laminin-α2 on AEF and thus indirectly affect permeability by restricting vesicular transport across CECs (Hori et al., 2004; Armulik et al., 2010; Daneman et al., 2010). In vitro data suggests pericyte involvement in immune function, through modulation of phagocytosis, expression of αSMA and ICAM-1 and supporting ICAM-1-mediated neutrophil transmigration in response to pro-inflammatory stimuli and the generation of mediators such as iNOS, ROS, COX2, MHCII, (Pieper et al., 2014). Reviews on pericyte-neurovascular unit (NVU) interactions (De Bock et al., 2017; Zhao et al., 2018) and signaling (Sweeney et al., 2016) are suggested for more in-depth information.
Neuronal effects on the BBB-CEC phenotype include release of growth factors, such as neuregulin and brain-derived neurotrophic factor, (Gauthier et al., 2013). Neuronal activity and neurotransmitter release can regulate BBB permeability through, e.g., glutamate-activating CEC-NMDA receptors and modulate transport of insulin-like growth factor, across the BBB (Nishijima et al., 2010; Vazana et al., 2016).
Taken together, the recognition of the contribution of inter-cellular communication between astrocytes, pericytes and neurons to the specific phenotype of CECs led to the formulation of the concept of the NVU. This model emphasizes the maintenance of CNS homeostasis through multidimensional, continuous and reciprocal communication among all NVU members by means of either physical contacts and/or the release of signaling mediators. Hence, dysregulation of one of the NVU components could lead to neuro-disease (Iadecola, 2017; Sweeney et al., 2019).
Differences in morphology, cellular content, and microvascular density have been observed among different brain regions and are especially apparent in white matter (WM) versus grey matter (GM). Historically, astroglial classifications were based on their morphology and anatomical position; as is the case with “fibrous astrocytes” with long processes in WM and more star-shaped “protoplasmic astrocytes” in GM. However, GM and WM astrocytes have extensive functional differences, as indicated by differential expression of transporters, including glutamate-transporters and GLUT-1 subtypes. In addition, they respond differently to in vitro stimuli (de Graaf et al., 2001; Goursaud et al., 2009). Hippocampal astroglia display differential ion channel expression and GABA responses (Cavaglia et al., 2001), confirming the regional heterogeneity of astroglial populations. The overall transcriptomes of GM and WM were shown to be unique and corroborate functional heterogeneities (Mills et al., 2013). Further development of technical abilities and “big data” processing revealed a large heterogeneity of individual NVU members. RNA-seq studies of populations of brain cells exposed high heterogeneity in both morphology of astrocytes, glia, endothelial cells and pericytes and in physiological properties, metabolic processes and functions (Zhang and Barres, 2010; Zhang et al., 2014; Li et al., 2019).
Single cell transcriptomics also indicated high diversity of glial sub-populations throughout the CNS, including in immunologic profiles (Batiuk et al., 2018). Clear region-specific differences, with a predominance of type-I interferon genes in GM, versus NFκB-signaling in WM was observed. Similarly, RNAseq showed that, with respect to immunological responses, isolated microglia clustered into at least nine transcriptionally different states (Hammond et al., 2019). Likewise, transcriptional profiling highlighted large differences in microglial populations derived from WM versus GM, with amoeboid-type microglia in WM regions (Verdonk et al., 2016; van der Poel et al., 2019). Transcriptional profiling also provided additional insights into the heterogeneity of the spatiotemporal responses of microglia to disease (Masuda et al., 2019). For example, metabolically active amoeboid microglia with phagocytic capability share gene signatures with those associated with degenerative disease (Li et al., 2019; Staszewski and Hagemeyer, 2019). These diverse populations also have different roles during neuronal plasticity as shown by their variances in response to injury and disease (Tay et al., 2018; Masuda et al., 2019). Such diversity may differentially affect phenotype and function of CECs, thus contributing to vascular heterogeneity.
Perivascular macrophages are differentially distributed along the vascular tree. They aid in preserving BBB integrity and contribute to regulating vascular tone (Goldmann et al., 2016; Hoeffel and Ginhoux, 2018). Under inflammatory conditions, PMs respond quickly but differentially (He et al., 2016; Verdonk et al., 2016; van der Poel et al., 2019). However, their interactions within the NVU and their implications for BBB-phenotype are under-investigated. The pericyte population is heterogeneous, not just between macro- versus micro-vessels but also among capillaries. For example, pericyte arms are fewer and shorter in post-capillary venules, leading to differences in pre- versus post-capillary contraction capacity (Itoh and Suzuki, 2012). Pericyte coverage differs among brain regions affecting BBB permeability in a regional-dependent manner, although other mechanisms are involved (Winkler et al., 2018). Coverage is also higher in the cerebral cortex compared to the spinal cord, suggesting region-dependent differential regulation of the CECs phenotype and function (Wilhelm et al., 2016). As alluded above, pericyte-derived factors directly influence BBB function and affect AEF polarization, thus indirectly restricting vesicular transport across CECs. Although RNA-seq studies indicated heterogeneity among pericytes (Zhang et al., 2014), it is essential to exploit novel methods for isolation, characterization and analysis of pericytes from different brain regions. This will shed more light on the functional heterogeneity of pericytes, as well as on their contributions to vascular heterogeneity (Crouch and Doetsch, 2018; Dore-Duffy and Esen, 2018).
Oligodendrocytes are more prevalent in WM compared to GM brain regions, therefore their function was traditionally viewed as to myelinate neuronal processes and facilitate neural transmission. In the 1920s Rio Hortega described four types of oligodendroglia, based on the number of axons they myelinated and their location; perineural or perivascular. Recently, single-cell RNA sequencing with Fluidigm-C1 technology revealed 12 clusters of heterogeneous oligodendrocyte populations or states. Their functional heterogeneity among brain regions may be related to different progenitor lineages (Dimou and Simons, 2017; Trotter and Mittmann, 2019). Although little is known about the communication between oligodendrocytes and CECs, the survival and proliferation of oligodendrocyte precursor cells is influenced by factors released from CECs, such as brain-derived neurotropic growth factor (Arai and Lo, 2009; Hamanaka et al., 2018). Oligodendrocyte progenitors can modulate BBB integrity via secretion of TGFβ-1, resulting in the upregulation of junctional proteins in CECs (Seo et al., 2014). Pericytes also influence progenitor development and neuronal myelination via Lama2 and VEGF signaling and by regulating the bioavailability of PDGF and TGFβ (De La Fuente et al., 2017; Girolamo et al., 2019). Due to the high oligodendrocyte prevalence in WM, CECs in WM are likely to have differential cellular interactions than CECs residing in GM. More research on the interactions and communications between oligodendrocytes and CECs and the consequences for specific phenotypes of CECs in WM is needed.
Besides cellular interactions within the NVU, physiological differences, such as blood flow, affect CEC-vascular phenotype. For the brain’s blood supply, large vessel branches penetrate the brain parenchyma, morphing into a dense network of small arteries, arterioles, capillaries, and venules. Compared to larger vessels, the microvasculature does not contain smooth muscles but pericytes, indicating differences in regulation of vaso-reactivity, blood flow, and shear stress (Cipolla, 2016; Mikhail Kellawan et al., 2017). Shear stress has been shown to affect expression of transporters, ion channels, and of tight- and adherens junction proteins on CECs (Cucullo et al., 2011).
Dependent on brain area, the vasculature displays differential densities and spatial orientation. In mouse frontal cortex, GM vessels are perpendicular to the pyramidal cell layer, whereas WM vessels are orientated parallel to axonal fibers (Itoh and Suzuki, 2012; Murugesan et al., 2012). The capillary density in the GM is greater than in the WM, reflecting different regional metabolic and energy demands, such as in synaptically active regions (e.g., cerebral cortex) versus fiber tract heavy regions (e.g., corpus callosum) (Cavaglia et al., 2001; Itoh and Suzuki, 2012; Wilhelm et al., 2016). To support metabolic needs in areas with low vascular density, an increased presence of transcellular pathways, gap-junctions and specific expression of receptors and transporters on CECs is needed (Cavaglia et al., 2001; Keaney and Campbell, 2015). Along the vascular tree, from large to small vessels, heterogeneity in the expression of various genes and expression of claudin-5 is evident (Macdonald et al., 2010; Paul et al., 2013; Sabbagh et al., 2018). Transcripts for junctional proteins occludin, claudin-5, and α-catenin were increased in WM-CECs compared to GM-CECs (Nyul-Toth et al., 2016).
The physiological/metabolic needs of the highly active neural milieu are in constant flux. Demands for exchange of nutrients, solutes, water and oxygen are conveyed through cues to the brain microvasculature. As discussed above, there is high diversity in the cellular composition of the NVU, which includes a significant heterogeneity of astrocytes, pericytes and oligodendrocytes in different brain areas. Taking into account the reciprocal interactions of these brain cells within the NVU and differing metabolic needs and differences in blood flow/shear stress, the microvascular phenotype must differ between different brain regions, especially between WM and GM regions. Characterizing and understanding the implications of regional heterogeneity of the brain microvasculature in health and disease is a new frontier for brain vascular research.
The critical role of brain vascular pathology recently emerged in studies of various neurological diseases, e.g., multiple sclerosis, infections including cerebral malaria and HIV, neurodegenerative disorders, including Alzheimer’s disease, traumatic brain injuries and in some psychiatric diseases. Small vessel disease exhibits brain vascular pathologies associated with either focal or generalized changes in different brain regions (Varghese et al., 2017; Kealy et al., 2018), comprised of WM lesions, cerebral micro-bleeds (Pantoni, 2010) with BBB opening and vasogenic edema resulting from oxygen loss and vascular inflammatory responses, including MMP release (Yang and Rosenberg, 2011). In multiple sclerosis, microvascular centered inflammatory lesions involve BBB breakdown and are associated with foci of demyelination in both GM and WM regions (Prins et al., 2015). In WM, early demyelination, the loss of WM volume and leukocyte infiltrations are associated with local BBB damage (Lucchinetti et al., 2011; Popescu et al., 2011; Prins et al., 2015; Granberg et al., 2017). Cerebral malaria, a severe neurological complication of malaria, and post-CM sequelae present a clear example of differential WM and GM pathology associated with BBB permeability and hemorrhagic punctae which predominate in WM but not in GM (Taylor and Molyneux, 2015). Alzheimer’s is regarded mainly as a GM disease with heterogeneous findings that include small vessel damage, cerebral amyloid angiopathy, inflammation, and hypercoagulability (Zamolodchikov and Strickland, 2016). In addition, differences in vascular pathologies between different lobes and WM versus GM are reported, with the occipital lobe more severely affected by cerebral amyloid angiopathies, followed by frontal-, temporal-, and parietal lobes (Vinters et al., 1996; Attems and Jellinger, 2014). Recent studies show clear vascular pathologies in traumatic brain injury, involving neurovascular inflammation, ROS, MMP’s resulting in a loss of junctional integrity (Abdul-Muneer et al., 2015). Major depressive disorder and attention deficit-hyperactivity disorder also exhibit signs of vascular pathology. In schizophrenia, evidence is mounting for NVU involvement (Najjar et al., 2017). Here, selected regions of GM are predominately affected (Cannon et al., 2002; Vita et al., 2012), whereas WM involvement is limited to select tracts (Davis et al., 2003; Hercher et al., 2014). Postmortem brain samples of schizophrenic patients also revealed ultrastructural differences in brain capillaries (Uranova et al., 2010).
Several brain diseases have underlying vasculopathic mechanisms and the vascular heterogeneity may lead to differential neuropathologies, especially in GM versus WM. To understand the contribution of the brain’s vascular heterogeneity and dysregulation of the NVU to neuro-pathogenesis, besides animal models, appropriate in vitro models are needed. Traditionally, isolated single CECs cultures or combinations with astrocytes and/or pericytes have been used for in vitro BBB modeling. More recently, inducible progenitor cells have been used for BBB-models, which show a low permeability and express drug transporters. An additional advantage is that patient material can be differentiated into homologous multi-cell BBB-models (DeStefano et al., 2018). However, considering NVU-cell heterogeneity, it is not clear which part of the BBB vasculature is represented; e.g., WM or GM. Depending on the scientific questions involved, “simple” in vitro BBB models may suffice or to better recapitulate the complexity of the NVU interactions, multi-cellular, multi-compartment micro-fluidic models, or organ-on-a-chip approaches may be more suitable (Noumbissi et al., 2018). However, the incorporation of NVU heterogeneity, including diversity of astrocytes and pericytes derived from GM versus WM areas and the potential role of oligodendrocytes, has thus far, been neglected in BBB-model designs. When studying neuropathologies that differ in their presentation in various brain regions, it is particularly important to benchmark in vitro BBB models to the in vivo vasculature of the region of interest. Therefore, considering brain cell heterogeneity in experimental design may lead to BBB models better reflecting WM versus GM vasculature.
This mini-review aims to highlight the region-specific heterogeneity of the brain’s vasculature and is not meant to be an exhaustive list. Additional BBB topics, including other barrier sites, immune interactions or BBB-development have been recently reviewed (Forrester et al., 2018; Mastorakos and McGavern, 2019; Saunders et al., 2019). Understanding the contributions of cellular diversity of the NVU micro-environment to phenotypic and functional heterogeneity of the brain’s vasculature will aid in elucidating differing region-dependent neuropathologies. This can be achieved by combining basic research and clinical approaches with large scale genetic/RNA-seq and proteomic analysis of regional microvasculature and other NVU components. Ultimately, this may inform us of novel targets for designing region-specific neuro-therapeutics.
Cerebral endothelial cells forming a low permeability barrier between the peripheral blood circulation and the CNS. Presence of TJ and polarized transporters tightly regulate passage of molecules into and out of the CNS.
The concept that CECs, astroglial cells, pericytes, PMs, and neurons communicate together to maintain brain homeostasis for optimal functioning of the organism. Dysfunction of any one component affects another and can lead to neuro-disease.
Heterogeneity in morphological-, molecular phenotype, and function of brain cells (e.g., glia, neurons). As these cells interact with the brain vascular CECs, they can influence the CECs phenotype and function, reflecting the needs of the underlying brain tissue.
Variances in the anatomical, cellular and molecular composition of the vasculature. Differential interactions with adjacent brain tissue can lead to a heterogeneity of the BBB phenotype and function not only along the vascular tree, e.g., large to small vessels, but also in different brain regions.
AV-R, CE, and MS wrote the manuscript. MS and CP conceptualized and edited the manuscript. All authors contributed equally to this manuscript.
This work was supported by the NIH RO1 HL 130649, the Johns Hopkins Malaria Research Institute, and Bloomberg Philanthropies to MS. CP was supported by the Johns Hopkins Project Restore and the Bart Mclean Fund for Neuroimmunology Research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Hector Fabian Plata Santos for his input and help as a graphical designer in producing the artwork for the Graphical Abstract and Figure 1.
AEF, astrocyte endfeet; AJ, adherens junctions; GM-A, gray matter astrocyte; GM-CEC, cerebral endothelial cell in gray matter; L, lumen of brain microvessel; N, neurons; OD, oligodendrocyte; PC, pericyte; PM, perivascular macrophage; T, transporters; TJ, tight junctions; WM-A, white matter astrocyte; WM-CEC, cerebral endothelial cell in white matter
Abdul-Muneer, P. M., Chandra, N., and Haorah, J. (2015). Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol. Neurobiol. 51, 966–979. doi: 10.1007/s12035-014-8752-3
Alvarez, J. I., Dodelet-Devillers, A., Kebir, H., Ifergan, I., Fabre, P. J., Terouz, S., et al. (2011). The hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 334, 1727–1731. doi: 10.1126/science.1206936
Arai, K., and Lo, E. H. (2009). An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. J. Neurosci. 29, 4351–4355. doi: 10.1523/JNEUROSCI.0035-09.2009
Armulik, A., Genove, G., Mae, M., Nisancioglu, M. H., Wallgard, E., Niaudet, C., et al. (2010). Pericytes regulate the blood-brain barrier. Nature 468, 557–561.
Attems, J., and Jellinger, K. A. (2014). The overlap between vascular disease and Alzheimer’s disease–lessons from pathology. BMC Med. 12:206. doi: 10.1186/s12916-014-0206-2
Balabanov, R., and Dore-Duffy, P. (1998). Role of the CNS microvascular pericyte in the blood-brain barrier. J. Neurosci. Res. 53, 637–644. doi: 10.1002/(sici)1097-4547(19980915)53:6<637::aid-jnr1>3.0.co;2-6
Batiuk, M., Martirosyan, A., Voet, T., Ponting, C., Belgard, T. G., and Holt, M. G. (2018). Molecularly distinct astrocyte subpopulations spatially pattern the adult mouse brain. bioRxiv [Preprint]. doi: 10.1101/317503
Bauer, H., and Traweger, A. (2016). Tight junctions of the blood-brain barrier - a molecular gatekeeper. CNS Neurol. Disord. Drug Targets 15, 1016–1029. doi: 10.2174/1871527315666160915142244
Bendayan, R., Ronaldson, P. T., Gingras, D., and Bendayan, M. (2006). In situ localization of P-glycoprotein (ABCB1) in human and rat brain. J. Histochem. Cytochem. 54, 1159–1167. doi: 10.1369/jhc.5a6870.2006
Benz, F., Wichitnaowarat, V., Lehmann, M., Germano, R. F., Mihova, D., Macas, J., et al. (2019). Low wnt/beta-catenin signaling determines leaky vessels in the subfornical organ and affects water homeostasis in mice. eLife 8:e43818. doi: 10.7554/eLife.43818
Ben-Zvi, A., Lacoste, B., Kur, E., Andreone, B. J., Mayshar, Y., Yan, H., et al. (2014). Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 509, 507–511. doi: 10.1038/nature13324
Beuckmann, C., Hellwig, S., and Galla, H. J. (1995). Induction of the blood/brain-barrier-associated enzyme alkaline phosphatase in endothelial cells from cerebral capillaries is mediated via CAMP. Eur. J. Biochem. 229, 641–644. doi: 10.1111/j.1432-1033.1995.0641j.x
Cannon, T. D., Thompson, P. M., Van Erp, T. G., Toga, A. W., Poutanen, V. P., Huttunen, M., et al. (2002). Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 99, 3228–3233. doi: 10.1073/pnas.052023499
Cavaglia, M., Dombrowski, S. M., Drazba, J., Vasanji, A., Bokesch, P. M., and Janigro, D. (2001). Regional variation in brain capillary density and vascular response to ischemia. Brain Res. 910, 81–93. doi: 10.1016/s0006-8993(01)02637-3
Chen, J., Luo, Y., Hui, H., Cai, T., Huang, H., Yang, F., et al. (2017). CD146 coordinates brain endothelial cell-pericyte communication for blood-brain barrier development. Proc. Natl. Acad. Sci. U.S.A. 114, E7622–E7631. doi: 10.1073/pnas.1710848114
Cipolla, M. J. (2016). The Cerebral Circulation, Colloquium Series on Integrated Systems Physiology: From Molecule to Function, 2nd Edn. Vol. 8. San Rafael, CA: Morgan & Claypool Life Sciences, 1–80.
Crone, C., and Olesen, S. P. (1982). Electrical resistance of brain microvascular endothelium. Brain Res. 241, 49–55. doi: 10.1016/0006-8993(82)91227-6
Crouch, E. E., and Doetsch, F. (2018). Facs isolation of endothelial cells and pericytes from mouse brain microregions. Nat. Protoc. 13, 738–751. doi: 10.1038/nprot.2017.158
Cucullo, L., Hossain, M., Puvenna, V., Marchi, N., and Janigro, D. (2011). The role of shear stress in blood-brain barrier endothelial physiology. BMC Neurosci. 12:40. doi: 10.1186/1471-2202-12-40
Daneman, R., Zhou, L., Kebede, A. A., and Barres, B. A. (2010). Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562–566. doi: 10.1038/nature09513
Davis, K. L., Stewart, D. G., Friedman, J. I., Buchsbaum, M., Harvey, P. D., Hof, P. R., et al. (2003). White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch. Gen. Psychiatry 60, 443–456.
De Bock, M., Leybaert, L., and Giaume, C. (2017). Connexin channels at the glio-vascular interface: gatekeepers of the brain. Neurochem. Res. 42, 2519–2536. doi: 10.1007/s11064-017-2313-x
de Graaf, R. A., Pan, J. W., Telang, F., Lee, J. H., Brown, P., Novotny, E. J., et al. (2001). Differentiation of glucose transport in human brain gray and white matter. J. Cereb. Blood Flow Metab. 21, 483–492. doi: 10.1097/00004647-200105000-00002
De La Fuente, A. G., Lange, S., Silva, M. E., Gonzalez, G. A., Tempfer, H., Van Wijngaarden, P., et al. (2017). Pericytes stimulate oligodendrocyte progenitor cell differentiation during CNS remyelination. Cell Rep. 20, 1755–1764. doi: 10.1016/j.celrep.2017.08.007
DeStefano, J. G., Jamieson, J. J., Linville, R. M., and Searson, P. C. (2018). Benchmarking in vitro tissue-engineered blood-brain barrier models. Fluids Barriers CNS 15:32. doi: 10.1186/s12987-018-0117-2
Dimou, L., and Simons, M. (2017). Diversity of oligodendrocytes and their progenitors. Curr. Opin. Neurobiol. 47, 73–79. doi: 10.1016/j.conb.2017.09.015
Dore-Duffy, P., and Esen, N. (2018). The microvascular pericyte: approaches to isolation, characterization, and cultivation. Adv. Exp. Med. Biol. 1109, 53–65. doi: 10.1007/978-3-030-02601-1_5
Farquhar, M. G., and Palade, G. E. (1963). Junctional complexes in various epithelia. J. Cell Biol. 17, 375–412. doi: 10.1083/jcb.17.2.375
Forrester, J. V., Mcmenamin, P. G., and Dando, S. J. (2018). CNS infection and immune privilege. Nat. Rev. Neurosci. 19, 655–671. doi: 10.1038/s41583-018-0070-8
Gauthier, M. K., Kosciuczyk, K., Tapley, L., and Karimi-Abdolrezaee, S. (2013). Dysregulation of the neuregulin-1-ErbB network modulates endogenous oligodendrocyte differentiation and preservation after spinal cord injury. Eur. J. Neurosci. 38, 2693–2715. doi: 10.1111/ejn.12268
Girolamo, F., Errede, M., Longo, G., Annese, T., Alias, C., Ferrara, G., et al. (2019). Defining the role of NG2-expressing cells in experimental models of multiple sclerosis. A biofunctional analysis of the neurovascular unit in wild type and NG2 null mice. PLoS One 14:e0213508. doi: 10.1371/journal.pone.0213508
Goldmann, T., Wieghofer, P., Jordao, M. J., Prutek, F., Hagemeyer, N., Frenzel, K., et al. (2016). Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat. Immunol. 17, 797–805. doi: 10.1038/ni.3423
Goursaud, S., Kozlova, E. N., Maloteaux, J.-M., and Hermans, E. (2009). Cultured astrocytes derived from corpus callosum or cortical grey matter show distinct glutamate handling properties. J. Neurochem. 108, 1442–1452. doi: 10.1111/j.1471-4159.2009.05889.x
Granberg, T., Fan, Q., Treaba, C. A., Ouellette, R., Herranz, E., Mangeat, G., et al. (2017). In vivo characterization of cortical and white matter neuroaxonal pathology in early multiple sclerosis. Brain 140, 2912–2926. doi: 10.1093/brain/awx247
Hamanaka, G., Ohtomo, R., Takase, H., Lok, J., and Arai, K. (2018). White-matter repair: interaction between oligodendrocytes and the neurovascular unit. Brain Circ. 4, 118–123. doi: 10.4103/bc.bc_15_18
Hammond, T. R., Dufort, C., Dissing-Olesen, L., Giera, S., Young, A., Wysoker, A., et al. (2019). Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50, 253.e6–271.e6. doi: 10.1016/j.immuni.2018.11.004
Haseloff, R. F., Dithmer, S., Winkler, L., Wolburg, H., and Blasig, I. E. (2015). Transmembrane proteins of the tight junctions at the blood-brain barrier: structural and functional aspects. Semin. Cell Dev. Biol. 38, 16–25. doi: 10.1016/j.semcdb.2014.11.004
He, H., Mack, J. J., Guc, E., Warren, C. M., Squadrito, M. L., Kilarski, W. W., et al. (2016). Perivascular macrophages limit permeability. Arterioscler. Thromb. Vasc. Biol. 36, 2203–2212. doi: 10.1161/atvbaha.116.307592
Hercher, C., Chopra, V., and Beasley, C. L. (2014). Evidence for morphological alterations in prefrontal white matter glia in schizophrenia and bipolar disorder. J. Psychiatry Neurosci. 39, 376–385. doi: 10.1503/jpn.130277
Hoeffel, G., and Ginhoux, F. (2018). Fetal monocytes and the origins of tissue-resident macrophages. Cell Immunol. 330, 5–15. doi: 10.1016/j.cellimm.2018.01.001
Hori, S., Ohtsuki, S., Hosoya, K., Nakashima, E., and Terasaki, T. (2004). A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J. Neurochem. 89, 503–513. doi: 10.1111/j.1471-4159.2004.02343.x
Iadecola, C. (2017). The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96, 17–42. doi: 10.1016/j.neuron.2017.07.030
Itoh, Y., and Suzuki, N. (2012). Control of brain capillary blood flow. J. Cereb. Blood Flow Metab. 32, 1167–1176. doi: 10.1038/jcbfm.2012.5
Janzer, R. C., and Raff, M. C. (1987). Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 325, 253–257. doi: 10.1038/325253a0
Kealy, J., Greene, C., and Campbell, M. (2018). Blood-brain barrier regulation in psychiatric disorders. Neurosci. Lett. doi: 10.1016/j.neulet.2018.06.033 [Epub ahead of print].
Keaney, J., and Campbell, M. (2015). The dynamic blood-brain barrier. FEBS J. 282, 4067–4079. doi: 10.1111/febs.13412
Li, Q., Cheng, Z., Zhou, L., Darmanis, S., Neff, N. F., Okamoto, J., et al. (2019). Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron 101, 207.e10–223.e10. doi: 10.1016/j.neuron.2018.12.006
Liddelow, S. A. (2011). Fluids and barriers of the CNS: a historical viewpoint. Fluids Barriers CNS 8:2. doi: 10.1186/2045-8118-8-2
Liebner, S., Czupalla, C. J., and Wolburg, H. (2011). Current concepts of blood-brain barrier development. Int. J. Dev. Biol. 55, 467–476. doi: 10.1387/ijdb.103224sl
Lucchinetti, C. F., Popescu, B. F., Bunyan, R. F., Moll, N. M., Roemer, S. F., Lassmann, H., et al. (2011). Inflammatory cortical demyelination in early multiple sclerosis. N. Engl. J. Med. 365, 2188–2197. doi: 10.1056/NEJMoa1100648
Macdonald, J. A., Murugesan, N., and Pachter, J. S. (2010). Endothelial cell heterogeneity of blood-brain barrier gene expression along the cerebral microvasculature. J. Neurosci. Res. 88, 1457–1474. doi: 10.1002/jnr.22316
Mastorakos, P., and McGavern, D. (2019). The anatomy and immunology of vasculature in the central nervous system. Sci. Immunol. 4:eaav0492. doi: 10.1126/sciimmunol.aav0492
Masuda, T., Sankowski, R., Staszewski, O., Bottcher, C., Amann, L., and Sagar, et al. (2019). Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 566, 388–392. doi: 10.1038/s41586-019-0924-x
Mikhail Kellawan, J., Harrell, J. W., Roldan-Alzate, A., Wieben, O., and Schrage, W. G. (2017). Regional hypoxic cerebral vasodilation facilitated by diameter changes primarily in anterior versus posterior circulation. J. Cereb. Blood Flow Metab. 37, 2025–2034. doi: 10.1177/0271678X16659497
Mills, J. D., Kavanagh, T., Kim, W. S., Chen, B. J., Kawahara, Y., Halliday, G. M., et al. (2013). Unique transcriptome patterns of the white and grey matter corroborate structural and functional heterogeneity in the human frontal lobe. PLoS One 8:e78480. doi: 10.1371/journal.pone.0078480
Murugesan, N., Demarest, T. G., Madri, J. A., and Pachter, J. S. (2012). Brain regional angiogenic potential at the neurovascular unit during normal aging. Neurobiol. Aging 33, 1004.e1–1004.e16. doi: 10.1016/j.neurobiolaging.2011.09.022
Najjar, S., Pahlajani, S., De Sanctis, V., Stern, J. N. H., Najjar, A., and Chong, D. (2017). Neurovascular unit dysfunction and blood-brain barrier hyperpermeability contribute to schizophrenia neurobiology: a theoretical integration of clinical and experimental evidence. Front. Psychiatry 8:83. doi: 10.3389/fpsyt.2017.00083
Nishijima, T., Piriz, J., Duflot, S., Fernandez, A. M., Gaitan, G., Gomez-Pinedo, U., et al. (2010). Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron 67, 834–846. doi: 10.1016/j.neuron.2010.08.007
Noumbissi, M. E., Galasso, B., and Stins, M. F. (2018). Brain vascular heterogeneity: implications for disease pathogenesis and design of in vitro blood-brain barrier models. Fluids Barriers CNS 15:12. doi: 10.1186/s12987-018-0097-2
Nyul-Toth, A., Suciu, M., Molnar, J., Fazakas, C., Hasko, J., Herman, H., et al. (2016). Differences in the molecular structure of the blood-brain barrier in the cerebral cortex and white matter: an in silico, in vitro, and ex vivo study. Am. J. Physiol. Heart Circ. Physiol. 310, H1702–H1714. doi: 10.1152/ajpheart.00774.2015
Pantoni, L. (2010). Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 9, 689–701. doi: 10.1016/S1474-4422(10)70104-6
Paul, D., Cowan, A. E., Ge, S. J., and Pachter, J. S. (2013). Novel 3D analysis of Claudin-5 reveals significant endothelial heterogeneity among CNS microvessels. Microvasc. Res. 86, 1–10. doi: 10.1016/j.mvr.2012.12.001
Pieper, C., Marek, J. J., Unterberg, M., Schwerdtle, T., and Galla, H. J. (2014). Brain capillary pericytes contribute to the immune defense in response to cytokines or LPS in vitro. Brain Res. 1550, 1–8. doi: 10.1016/j.brainres.2014.01.004
Popescu, B. F., Bunyan, R. F., Parisi, J. E., Ransohoff, R. M., and Lucchinetti, C. F. (2011). A case of multiple sclerosis presenting with inflammatory cortical demyelination. Neurology 76, 1705–1710. doi: 10.1212/WNL.0b013e31821a44f1
Prins, M., Schul, E., Geurts, J., Van Der Valk, P., Drukarch, B., and Van Dam, A. M. (2015). Pathological differences between white and grey matter multiple sclerosis lesions. Ann. N. Y. Acad. Sci. 1351, 99–113. doi: 10.1111/nyas.12841
Reese, T. S., and Karnovsky, M. J. (1967). Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 34, 207–217. doi: 10.1083/jcb.34.1.207
Roberts, L. M., Black, D. S., Raman, C., Woodford, K., Zhou, M., Haggerty, J. E., et al. (2008). Subcellular localization of transporters along the rat blood-brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience 155, 423–438. doi: 10.1016/j.neuroscience.2008.06.015
Sabbagh, M. F., Heng, J. S., Luo, C. Y., Castanon, R. G., Nery, J. R., Rattner, A., et al. (2018). Transcriptional and epigenomic landscapes of CNS and non-CNS vascular endothelial cells. eLife 7:e36187. doi: 10.7554/eLife.36187
Sa-Pereira, I., Brites, D., and Brito, M. A. (2012). Neurovascular unit: a focus on pericytes. Mol. Neurobiol. 45, 327–347. doi: 10.1007/s12035-012-8244-2
Saunders, N. R., Dreifuss, J. J., Dziegielewska, K. M., Johansson, P. A., Habgood, M. D., Mollgard, K., et al. (2014). The rights and wrongs of blood-brain barrier permeability studies: a walk through 100 years of history. Front. Neurosci. 8:404. doi: 10.3389/fnins.2014.00404
Saunders, N. R., Dziegielewska, K. M., Mollgard, K., and Habgood, M. D. (2019). Recent developments in undertsanding barrier mechanisms in the developing brain:drugs and drug transporters in pregnancy, susceptibility or protection in the fetal brain? Annu. Rev. Pharmacol. Toxicol. 59, 487–505. doi: 10.1146/annurev-pharmtox-010818-021430
Seo, J. H., Maki, T., Maeda, M., Miyamoto, N., Liang, A. C., Hayakawa, K., et al. (2014). Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-beta signaling. PLoS One 9:e103174. doi: 10.1371/journal.pone.0103174
Siddharthan, V., Kim, Y. V., Liu, S., and Kim, K. S. (2007). Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res. 1147, 39–50. doi: 10.1016/j.brainres.2007.02.029
Stamatovic, S. M., Johnson, A. M., Keep, R. F., and Andjelkovic, A. V. (2016). Junctional proteins of the blood-brain barrier: new insights into function and dysfunction. Tissue Barriers 4:e1154641. doi: 10.1080/21688370.2016.1154641
Staszewski, O., and Hagemeyer, N. (2019). Unique microglia expression profile in developing white matter. BMC Res. Notes 12:367. doi: 10.1186/s13104-019-4410-1
Stewart, P. A., and Wiley, M. J. (1981). Developing nervous-tissue induces formation of blood-brain-barrier characteristics in invading endothelial-cells - a study using quail-chick transplantation chimeras. Dev. Biol. 84, 183–192. doi: 10.1016/0012-1606(81)90382-1
Sun, D., Lytle, C., and O’donnell, M. E. (1997). Il-6 secreted by astroglial cells regulates Na-K-Cl cotransport in brain microvessel endothelial cells. Am. J. Physiol. 272, C1829–C1835.
Sweeney, M. D., Ayyadurai, S., and Zlokovic, B. V. (2016). Pericytes of the neurovascular unit: key functions and signaling pathways. Nat. Neurosci. 19, 771–783. doi: 10.1038/nn.4288
Sweeney, M. D., Zhao, Z., Montagne, A., Nelson, A. R., and Zlokovic, B. V. (2019). Blood-brain barrier: from physiology to disease and back. Physiol. Rev. 99, 21–78. doi: 10.1152/physrev.00050.2017
Tay, T. L., Sagar, Dautzenberg, J., Grun, D., and Prinz, M. (2018). Unique microglia recovery population revealed by single-cell RNASEQ following neurodegeneration. Acta Neuropathol. Commun. 6:87. doi: 10.1186/s40478-018-0584-3
Taylor, T. E., and Molyneux, M. E. (2015). The pathogenesis of pediatric cerebral malaria: eye exams, autopsies, and neuroimaging. Ann. N. Y. Acad. Sci. 1342, 44–52. doi: 10.1111/nyas.12690
Trotter, J., and Mittmann, T. (2019). Diversity in the oligodendrocyte lineage: current evidence. Neuron 101, 356–357. doi: 10.1016/j.neuron.2019.01.032
Uranova, N. A., Zimina, I. S., Vikhreva, O. V., Krukov, N. O., Rachmanova, V. I., and Orlovskaya, D. D. (2010). Ultrastructural damage of capillaries in the neocortex in schizophrenia. World J. Biol. Psychiatry 11, 567–578. doi: 10.3109/15622970903414188
van der Poel, M., Ulas, T., Mizee, M. R., Hsiao, C. C., Miedema, S. S. M., and Adelia, et al. (2019). Transcriptional profiling of human microglia reveals grey-white matter heterogeneity and multiple sclerosis-associated changes. Nat. Commun. 10:1139. doi: 10.1038/s41467-019-08976-7
Varghese, M., Keshav, N., Jacot-Descombes, S., Warda, T., Wicinski, B., Dickstein, D. L., et al. (2017). Autism spectrum disorder: neuropathology and animal models. Acta Neuropathol. 134, 537–566. doi: 10.1007/s00401-017-1736-4
Vazana, U., Veksler, R., Pell, G. S., Prager, O., Fassler, M., Chassidim, Y., et al. (2016). Glutamate-mediated blood-brain barrier opening: implications for neuroprotection and drug delivery. J. Neurosci. 36, 7727–7739. doi: 10.1523/JNEUROSCI.0587-16.2016
Verdonk, F., Roux, P., Flamant, P., Fiette, L., Bozza, F. A., Simard, S., et al. (2016). Phenotypic clustering: a novel method for microglial morphology analysis. J. Neuroinflamm. 13:153. doi: 10.1186/s12974-016-0614-7
Vinters, H. V., Wang, Z. Z., and Secor, D. L. (1996). Brain parenchymal and microvascular amyloid in Alzheimer’s disease. Brain Pathol. 6, 179–195. doi: 10.1111/j.1750-3639.1996.tb00799.x
Vita, A., De Peri, L., Deste, G., and Sacchetti, E. (2012). Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Transl. Psychiatry 2:e190. doi: 10.1038/tp.2012.116
Wang, Y., Cho, C., Williams, J., Smallwood, P. M., Zhang, C., Junge, H. J., et al. (2018). Interplay of the Norrin and Wnt7a/Wnt7b signaling systems in blood-brain barrier and blood-retina barrier development and maintenance. Proc. Natl. Acad. Sci. U.S.A. 115, E11827–E11836.
Wilhelm, I., Nyul-Toth, A., Suciu, M., Hermenean, A., and Krizbai, I. A. (2016). Heterogeneity of the blood-brain barrier. Tissue Barriers 4:e1143544. doi: 10.1080/21688370.2016.1143544
Winkler, E. A., Bell, R. D., and Zlokovic, B. V. (2011). Central nervous system pericytes in health and disease. Nat. Neurosci. 14, 1398–1405. doi: 10.1038/nn.2946
Winkler, E. A., Birk, H., Burkhardt, J. K., Chen, X., Yue, J. K., Guo, D., et al. (2018). Reductions in brain pericytes are associated with arteriovenous malformation vascular instability. J. Neurosurg. 129, 1464–1474. doi: 10.3171/2017.6.JNS17860
Yang, Y., and Rosenberg, G. A. (2011). Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 42, 3323–3328. doi: 10.1161/STROKEAHA.110.608257
Zamolodchikov, D., and Strickland, S. (2016). A possible new role for Abeta in vascular and inflammatory dysfunction in Alzheimer’s disease. Thromb. Res. 141(Suppl. 2), S59–S61. doi: 10.1016/S0049-3848(16)30367-X
Zhang, Y., and Barres, B. A. (2010). Astrocyte heterogeneity: an underappreciated topic in neurobiology. Curr. Opin. Neurobiol. 20, 588–594. doi: 10.1016/j.conb.2010.06.005
Zhang, Y., Chen, K., Sloan, S. A., Bennett, M. L., Scholze, A. R., O’keeffe, S., et al. (2014). An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 34, 11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014
Keywords: cerebral endothelial cells, blood–brain barrier, neurovascular unit, brain cellular heterogeneity, vascular heterogeneity
Citation: Villabona-Rueda A, Erice C, Pardo CA and Stins MF (2019) The Evolving Concept of the Blood Brain Barrier (BBB): From a Single Static Barrier to a Heterogeneous and Dynamic Relay Center. Front. Cell. Neurosci. 13:405. doi: 10.3389/fncel.2019.00405
Received: 02 June 2019; Accepted: 23 August 2019;
Published: 20 September 2019.
Edited by:
Francisco F. De-Miguel, National Autonomous University of Mexico, MexicoReviewed by:
Britta Engelhardt, University of Bern, SwitzerlandCopyright © 2019 Villabona-Rueda, Erice, Pardo and Stins. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Monique F. Stins, bXN0aW5zQGpobWkuZWR1
†Present address: Andres Villabona-Rueda, Department of Pulmonary and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, United States; Clara Erice, Sandra Rotman Centre for Global Health, Toronto General Hospital, University Health Network, Toronto, ON, Canada
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.