- 1Vascular Biology Center, Division of Hematology, Oncology and Transplantation, Department of Medicine, University of Minnesota, Minneapolis, MN, United States
- 2Department of Neurosurgery, University of California, San Diego, San Diego, CA, United States
Mast cells (MCs) are located in the periphery as well as the central nervous system (CNS). Known for sterile inflammation, MCs play a critical role in neuroinflammation, which is facilitated by their close proximity to nerve fibers in the periphery and meninges of the spinal cord and the brain. Multifaceted activation of MCs releasing neuropeptides, cytokines and other mediators has direct effects on the neural system as well as neurovascular interactions. Emerging studies have identified the release of extracellular traps, a phenomenon traditionally meant to ensnare invading pathogens, as a cause of MC-induced neural injury. In this review article, we will discuss mechanisms of MC interaction with the nervous system through degranulation, de novo synthesis, extracellular vesicles (EVs), tunneling nanotubes, and extracellular traps with implications across a variety of pathological conditions.
Introduction
Mast cells (MCs) are proinflammatory cells that are the first responders of the immune system (Galli et al., 2005; Gupta and Harvima, 2018). MCs localize in proximity to afferent fibers innervating the periphery, visceral organs and meninges. MC proximity to the external environment makes them prime candidates for a rapid response against external stimuli and internal microenvironment. In addition to their function against the external environment, MCs are also sensitive to the endogenous environment and may thereby contribute to multiple pathobiologies, including pain, itch, and disorders of the nervous system (Mattila et al., 2011; Xanthos et al., 2011; Arac et al., 2014; Kempuraj et al., 2017; Gupta and Harvima, 2018). It is suggested that MCs contribute to pathology through interaction with the vasculature and the central nervous system (CNS). Upon activation, MCs quickly release substances from preformed granules including proteoglycans, proteases, leukotrienes, biogenic amines, and cytokines (Vukman et al., 2017). In addition to degranulation, MCs have a delayed response leading to the release of cytokines, neuropeptides, and chemokines by de novo synthesis. Noxious (toxic or injurious) substances released include but are not limited to histamine (Sjoerdsma et al., 1957), tryptase (Glenner and Cohen, 1960), chymase (Benditt and Arase, 1959), tumor necrosis factor α (TNFα; Gordon and Galli, 1990), and interleukin (IL-6), IL1β (Bradding et al., 1993; Nakamura et al., 2012), monocyte chemoattractant protein 1 (Vincent et al., 2013), nerve growth factor (Leon et al., 1994), brain-derived neurotrophic factor (Yuan et al., 2010), gonadotropin-releasing hormone (Khalil et al., 2003) and substance P (SP; Vincent et al., 2013; Taracanova et al., 2018). In this review article, we will examine the different ways in which MC activation interacts with the nervous system and their pathological implications.
Localization of Mast Cells
MCs are specifically located in the dura mater/meninges of the spinal cord and brain (Khalil et al., 2007). Within the CNS, MCs are located on the abluminal side of the blood brain barrier (BBB) in apposition to astrocytes and neurons (Manning et al., 1994; Florenzano and Bentivoglio, 2000; Silverman et al., 2000). Increased BBB permeability can lead to MCs crossing into the CNS (Silverman et al., 2000). In addition, MCs can cross through the blood-spinal cord barrier (Dong et al., 2014). MCs are found in close proximity to sensory nerve endings, and their degranulation can modulate the excitability of nociceptors. In the spinal cord, white matter separates the dura from the lumbar dorsal horn allowing MC mediators to reach the superficial laminae, which is a key relay station that modulates synaptic transmission and nociception (Xanthos et al., 2011). It has been found that MCs can induce persistent nociception and long-term potentiation at spinal C-fiber synapses (Xanthos et al., 2011). This is mediated through SP release, which mediates pain sensation through unmyelinated C fibers (Tore and Tuncel, 2009). In most tissues, MCs and nerves have a gap of 20 nm allowing MCs to immediately act on peripheral nerves following degranulation. A correlation has been shown between MC proximity to nerve fibers and complex regional pain syndrome (Morellini et al., 2018). MCs also co-localize with astrocytes and may modulate the behavior of astrocytes to release more mediators through the release of histamine from MCs (Skaper and Facci, 2012).
Mast Cell Activation and the Nervous System
Mast Cell Degranulation
Degranulation occurs within minutes of activation and results in the rapid release of substances from pre-formed granules. Degranulation begins with the activation of receptors with high affinity for IgE (FcεR1) and rearrangement of F-actin and microtubule formation. FcεR1 activates Fyn/Gab2/RhoA tyrosine kinases and leads to microtubule polymerization and the shuttling of secretory granules to the plasma membrane (Figure 1; Nishida et al., 2005). Tyrosine kinases Lyn and Syk of the activated FcεR1 cause calcium mobilization, vesicle fusion, and exocytosis of the granule through the protein kinase B (PBK)/Akt pathway and upregulation of peptidylarginine deiminase-4 (PAD-4) activation (Figure 1; Doyle et al., 2013; Aich et al., 2015). A variant of degranulation called transgranulation is associated with neuropathic pain (Keith et al., 1995; Wilhelm et al., 2005). Transgranulation occurs when MCs are in direct contact with other cells and thereby transfer the granules into nearby cells through exocytosis of the MCs and intake by the recipient cell. The effect is furthered through MC cytoplasmic extensions similar to pseudopodia that increase the range a MC can act through transgranulation (Barbara et al., 2004; Wilhelm et al., 2005). Because MCs are located closely to vasculature and nerve fibers, transgranulation can have a disruptive effect on those cells contributing to vascular dysfunction and neuropathic pain, respectively.
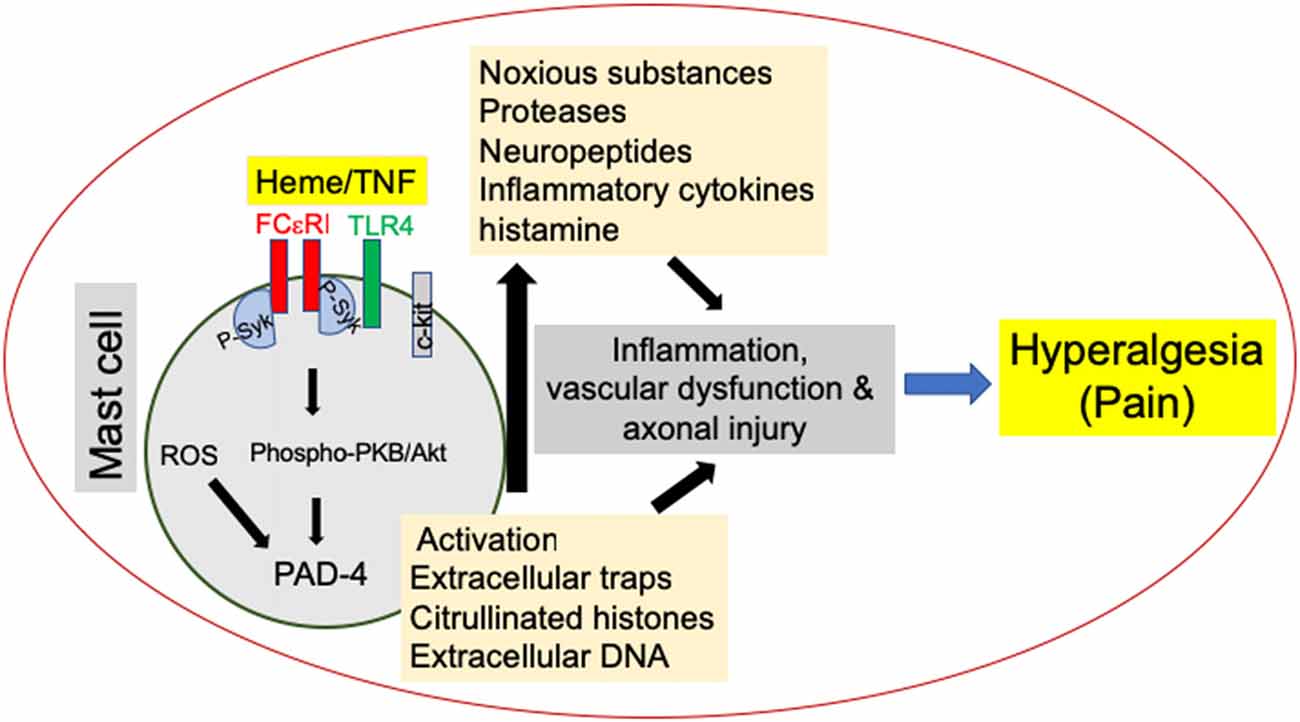
Figure 1. Mast cell (MC) activation promotes hyperalgesia in sickle cell disease (SCD). MC activation leads to the release of noxious substances such as proteases, neuropeptides and cytokines as well as the release of MC extracellular traps (MCETs) with citrullinated H3 histones and DNA, which cause noxious as well as physical injury to the vasculature and nerve bundles, leading to hyperalgesia in SCD. Emerging data suggest that this process is mediated by endoplasmic reticulum (ER) stress/reactive oxygen species (ROS) production and may engage FcεR1 and toll-like receptor 4 (TLR4) leading to Syk associated downstream protein kinase B (PKB/Akt) signaling. Consequent peptidylarginine deiminase-4 (PAD-4) activation may stimulate the release of extracellular traps, while inflammasome signaling may further augment inflammation.
In the bladder, bidirectional communication between nerves and MCs using lamellipodia that enclosed fibers within a cell have been observed (Keith et al., 1995). Retrograde transport of MC mediators has also been shown in dorsal root ganglia (DRG; Murphy et al., 1999). In this study, a nerve injected with a MC degranulation product increased the expression of IL-6 mRNA in sensory neurons, and blockade of retrograde axonal transport attenuated the induction of IL-6 mRNA in primary sensory neurons.
De novo Synthesis
As a late response, MCs release cytokines and chemokines that are synthesized de novo. The mechanism for activation is thus complex and involves multiple receptors. Immunoglobulin E (IgE) receptors are involved in mediating response in cooperation with the toll-like receptor (TLR). TLRs also recruit CD14 or CD48 for the effect of TLR ligands (Marshall, 2004).
IgE independent receptors also regulate MC de novo synthesis. This involves the receptor tyrosine kinase kit (c-kit), which is a receptor tyrosine kinase for stem cell factor (SCF; Mitsui et al., 1993). SCF promotes the development of more MCs. The large number of cytokines, chemokines, and neuropeptides released from MCs along with their proximity to axonal processes may be a large contributor to neuropathy and inflammation.
Extracellular Vesicles
Extracellular vesicle (EV) release is common to many different cells. EVs may be formed in endosomes and released through exocytosis. MC exosomes are known to interact with sensory nerves through the release of SP (Azimi et al., 2017). In turn, SP interacts with Mas-related G protein-coupled receptors (Mrgprs) to activate DRG neurons in mice (Azimi et al., 2017). Neurokinin 1 (NK1) receptors and Mrgprs were activated in mice and then pain behaviors were measured in vivo and DRG activation was measured in culture leading to the conclusion that SP activates DRG in culture through Mrgprs not NK-1 receptors (Azimi et al., 2017).
Because of their small size, EVs can travel long distances and influence synaptic transmission in the CNS. EVs may also participate in reuptake by local neurons as EVs can be localized as MC granules (Groot Kormelink et al., 2016). Groot Kormelink et al. (2016) found that upon activation of MCs a CD63-positive subset of EVs is released. Upon phospholipid and proteome analysis of these EVs, it was found that the EVs contain MC-specific mediators (Groot Kormelink et al., 2016).
MC-released EVs can influence dendritic cell maturation via immunomodulatory exogenously encountered antigens (Ags; Skokos et al., 2003). Ags induce phenotypic changes in dendritic cell maturation by up-regulating MHC class II, CD80, CD86, and CD40 molecules. These molecules stimulate T lymphocytes and induce Ag-specific immune responses which could impact the function of dendritic cells.
MicroRNAs contained in EVs may also participate in neuropathy. MiRNA-let-7b causes rapid excitation in DRG neurons via TLR7 and transient receptor potential ankyrin 1 (TRPA1; Park et al., 2014). MiRNA-let-7b causes rapid inward currents and exciting the DRG neurons and inducing pain via TLR7 and TRPA1 (Park et al., 2014).
Tunneling Nanotubules
MC form tunneling nanotubules (TNTs), which are F-actin structures that form in response to reticulation. TNTs are similar to pseudopodia described earlier, except they are not adherent, and can span distances longer than pseudopodia. MC-microglia interactions have been found to be involved in brain inflammation (Skaper and Facci, 2012). MCs cultured in medium containing MC activators were found to rapidly form TNTs to transport mitochondrial and secretory granule particles to other MCs and glioblastoma, implicating TNTs in MC-microglia interactions (Weng et al., 2016).
Mast Cell Extracellular Traps
Extracellular trap formation is a process to ensnare external organisms for self-defense. This process involves citrullination of histone proteins, resulting in disassembly of DNA and ejection of web-like contents (Jorch and Kubes, 2017).
MC extracellular trap (MCET) formation is dependent on reactive oxygen species (ROS) formation and engagement of TLR4 (Figure 1; Stoiber et al., 2015). In addition to DNA, MCETs also contain fibers with tryptase. MCETs have been suggested to contain chemokines and cytokines necessary for an inflammatory response, which could lead to tissue damage, inflammation, and neuronal activation (Schauer et al., 2014; Möllerherm et al., 2016). IL-17 and IL-8 have been shown to be released by MCETs. MCs are the majority of IL-17-containing cells in control and psoriatic skin (Lin et al., 2011). Interestingly, MCETs showed bright costaining for IL-17. MCs and neutrophils have been reported to release IL-17, which contributes to the pathology of psoriasis.
Neurovascular Interactions and Pathological Outcomes
It is well documented that MC mediators contribute to endothelial dysfunction in the vasculature (Kunder et al., 2011). Excess of MC mediators can cause an increase in BBB permeability. It is known that activation of MCs locally increases BBB permeability (Zhuang et al., 1996). Zhuang et al. (1996) found that upon MC degranulation by C48/80 treatment, BBB permeability was increased.
MCs operate in a feed-forward mechanism. Inflammation caused by MCs can further activate the MCs in an autocrine manner. Mediators released by MCs such as IL33 and tumor necrosis factor alpha (TNFα) have been known to activate MCs (Taracanova et al., 2017). SP along with IL33 causes MCs to increase secretion and gene expression of IL-1β (Taracanova et al., 2018). These responses were mediated by SP and IL-33 receptors, NK1 and small conductance Ca2+-activated K+ (SK2), respectively on MCs. Receptors were inhibited by methoxyluteolin inhibiting IL33 decreased IL-1β release (Taracanova et al., 2017).
MCs have been found to increase vascular permeability in the skin of mice with sickle cell disease (SCD; Vincent et al., 2013). Treatment of mice with a MC inhibitor cromolyn or imatinib or cannabinoids reduced vascular permeability in these mice (Vincent et al., 2016). These observations led to the suggestion that MC activation leads to the release of SP which activates protease-activated receptor 2 on the peripheral nerve endings, which in turn release more neuropeptides including SP leading to vascular dilatation and increased permeability. Thus MC activation leads to neurogenic inflammation involving neurovascular interactions.
Increased vascular permeability has been found in the CNS where MC degranulation compromises the BBB and allows further entry of inflammatory substances into the brain (Zhuang et al., 1996). Acute stress has pro-inflammatory effects that are mediated through the activation of MCs via corticotropin-releasing hormone (Esposito et al., 2001). Additionally, external and internal ROS formation contributes to changes in endothelial cell-cell interactions (van Wetering et al., 2002), BBB integrity (Lehner et al., 2011), and the disruption of tight junctions (Schreibelt et al., 2007). Increase in BBB permeability is associated with higher levels of neuroinflammation and brain dysfunction. Additionally, BBB disruption may further changes by systemic inflammation (Dénes et al., 2011; Knowland et al., 2014). Dénes et al. (2011) found that systemic inflammation compromises survivability after stroke which also augments BBB damage. Activation of meningeal MCs has been shown to worsen stroke pathology in mice (Arac et al., 2014). Therefore, activation of MCs in the periphery, as well as CNS, has implications in altering the neural activity and function directly and/or via neurovascular interactions.
Conclusion
MCs contribute to neural and vascular injury directly as well as induce neurovascular interactions. The complex milieu of multiple mediators released from MCs via diverse mechanisms alters the microenvironment leading to a hypersensitized system. Recent advances in MC-CNS interactions demonstrate that MC-induced hypersensitivity also contributes to the CNS disorders and pain. Thus, targeting of MCs provides a potentially treatable target for the disorders of the CNS and pain.
Author Contributions
AM wrote the manuscript and prepared for submission. VS edited the manuscript. MG wrote and edited the manuscript. KG conceived, designed, and supervised the manuscript writing and editing and prepared the figure.
Funding
This work was supported by National Institutes of Health (NIH) UO1 HL117664 to KG.
Conflict of Interest Statement
KG is a Consultant for Tau Tona Group and Novartis but it does not have conflict with the present work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are thankful to Huy Tran and Julia Nguyen for fruitful discussion and constructive criticism of the Review. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
Ags, exogenously encountered antigens; BBB, blood brain barrier; c-kit, receptor tyrosine kinase kit; CNS, central nervous system; DRG, dorsal root ganglion; EV, extracellular vesicle; FcεR1, receptors with high affinity for IgE; IgE, immunoglobulin E; IL, interleukin; MC, mast cell; MCET, mast cell extracellular trap; MS, multiple sclerosis; NK1, neurokinin 1; PAD, peptidylarginine deiminase; PBK/Akt, protein kinase B; ROS, reactive oxygen species; SCF, stem cell factor; SCD, sickle cell disease; SK2, small conductance Ca2+-activated K+; TLR, toll-like receptor; TNFα, tumor necrosis factor α; TNT, tunneling nanotubules; TRPA1, transient receptor potential ankyrin 1.
References
Aich, A., Afrin, L., Gupta, K., Aich, A., Afrin, L. B., and Gupta, K. (2015). Mast cell-mediated mechanisms of nociception. Int. J. Mol. Sci. 16, 29069–29092. doi: 10.3390/ijms161226151
Arac, A., Grimbaldeston, M. A., Nepomuceno, A. R. B., Olayiwola, O., Pereira, M. P., Nishiyama, Y., et al. (2014). Evidence that meningeal mast cells can worsen stroke pathology in mice. Am. J. Pathol. 184, 2493–2504. doi: 10.1016/j.ajpath.2014.06.003
Azimi, E., Reddy, V. B., Pereira, P. J. S., Talbot, S., Woolf, C. J., and Lerner, E. A. (2017). Substance P activates Mas-related G protein-coupled receptors to induce itch. J. Allergy Clin. Immunol. 140, 447–453.e3. doi: 10.1016/j.jaci.2016.12.980
Barbara, G., Stanghellini, V., De Giorgio, R., Cremon, C., Cottrell, G. S., Santini, D., et al. (2004). Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 126, 693–702. doi: 10.1053/j.gastro.2003.11.055
Benditt, E. P., and Arase, M. (1959). An enzyme in mast cells with properties like chymotrypsin. J. Exp. Med. 110, 451–460. doi: 10.1084/jem.110.3.451
Bradding, P., Feather, I. H., Wilson, S., Bardin, P. G., Heusser, C. H., Holgate, S. T., et al. (1993). Immunolocalization of cytokines in the nasal mucosa of normal and perennial rhinitic subjects. The mast cell as a source of IL-4, IL-5 and IL-6 in human allergic mucosal inflammation. J. Immunol. 151, 3853–3865.
Dénes, Á., Ferenczi, S., and Kovács, K. J. (2011). Systemic inflammatory challenges compromise survival after experimental stroke via augmenting brain inflammation, blood- brain barrier damage and brain oedema independently of infarct size. J. Neuroinflammation 8:164. doi: 10.1186/1742-2094-8-164
Dong, H., Zhang, X., and Qian, Y. (2014). Mast cells and neuroinflammation. Med. Sci. Monit. Basic Res. 20, 200–206. doi: 10.12659/MSMBR.893093
Doyle, H., Yang, M.-L., Raycroft, M. T., Gee, R. J., and Mamula, M. (2013). Autoantigens: novel forms and presentation to the immune system. Autoimmunity 47, 220–233. doi: 10.3109/08916934.2013.850495
Esposito, P., Gheorghe, D., Kandere, K., Pang, X., Connolly, R., Jacobson, S., et al. (2001). Acute stress increases permeability of the blood-brain-barrier through activation of brain mast cells. Brain Res. 888, 117–127. doi: 10.1016/s0006-8993(00)03026-2
Florenzano, F., and Bentivoglio, M. (2000). Degranulation, density and distribution of mast cells in the rat thalamus: a light and electron microscopic study in basal conditions and after intracerebroventricular administration of nerve growth factor. J. Comp. Neurol. 424, 651–669. doi: 10.1002/1096-9861(20000904)424:4%3C651::AID-CNE7%3E3.0.CO;2-G
Galli, S. J., Nakae, S., and Tsai, M. (2005). Mast cells in the development of adaptive immune responses. Nat. Immunol. 6, 135–142. doi: 10.1038/ni1158
Glenner, G. G., and Cohen, L. A. (1960). Histochemical demonstration of a species-specific trypsin-like enzyme in mast cells. Nature 185, 846–847. doi: 10.1038/185846a0
Gordon, J. R., and Galli, S. J. (1990). Mast cells as a source of both preformed and immunologically inducible TNF-α/cachectin. Nature 346, 274–276. doi: 10.1038/346274a0
Groot Kormelink, T., Arkesteijn, G. J. A., van de Lest, C. H., Geerts, W. J., Goerdayal, S. S., Altelaar, M. A. F., et al. (2016). Mast cell degranulation is accompanied by the release of a selective subset of extracellular vesicles that contain mast cell-specific proteases. J. Immunol. 197, 3382–3392. doi: 10.4049/jimmunol.1600614
Gupta, K., and Harvima, I. T. (2018). Mast cell-neural interactions contribute to pain and itch. Immunol. Rev. 282, 168–187. doi: 10.1111/imr.12622
Jorch, S. K., and Kubes, P. (2017). An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 23, 279–287. doi: 10.1038/nm.4294
Keith, I. M., Jin, J., and Saban, R. (1995). Nerve-mast cell interaction in normal guinea pig urinary bladder. J. Comp. Neurol. 363, 28–36. doi: 10.1002/cne.903630104
Kempuraj, D., Selvakumar, G. P., Thangavel, R., Ahmed, M. E., Zaheer, S., Raikwar, S. P., et al. (2017). Mast cell activation in brain injury, stress and post-traumatic stress disorder and Alzheimer’s disease pathogenesis. Front. Neurosci. 11:703. doi: 10.3389/fnins.2017.00703
Khalil, M., Ronda, J., Weintraub, M., Jain, K., Silver, R., and Silverman, A.-J. (2007). Brain mast cell relationship to neurovasculature during development. Brain Res. 1171, 18–29. doi: 10.1016/j.brainres.2007.07.034
Khalil, M. H., Silverman, A.-J., and Silver, R. (2003). Mast cells in the rat brain synthesize gonadotropin-releasing hormone. J. Neurobiol. 56, 113–124. doi: 10.1002/neu.10220
Knowland, D., Arac, A., Sekiguchi, K. J., Hsu, M., Lutz, S. E., Perrino, J., et al. (2014). Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron 82, 603–617. doi: 10.1016/j.neuron.2014.03.003
Kunder, C. A., St John, A. L., and Abraham, S. N. (2011). Mast cell modulation of the vascular and lymphatic endothelium. Blood 118, 5383–5393. doi: 10.1182/blood-2011-07-358432
Lehner, C., Gehwolf, R., Tempfer, H., Krizbai, I., Hennig, B., Bauer, H.-C., et al. (2011). Oxidative stress and blood-brain barrier dysfunction under particular consideration of matrix metalloproteinases. Antioxid. Redox Signal. 15, 1305–1323. doi: 10.1089/ars.2011.3923
Leon, A., Buriani, A., Dal Toso, R., Fabris, M., Romanello, S., Aloe, L., et al. (1994). Mast cells synthesize, store and release nerve growth factor. Proc. Natl. Acad. Sci. U S A 91, 3739–3743. doi: 10.1073/pnas.91.9.3739
Lin, A. M., Rubin, C. J., Khandpur, R., Wang, J. Y., Riblett, M., Yalavarthi, S., et al. (2011). Mast cells and neutrophils release il-17 through extracellular trap formation in psoriasis. J. Immunol. 187, 490–500. doi: 10.4049/jimmunol.1100123
Manning, K. A., Pienkowski, T. P., and Uhlrich, D. J. (1994). Histaminergic and non-histamine-immunoreactive mast cells within the cat lateral geniculate complex examined with light and electron microscopy. Neuroscience 63, 191–206. doi: 10.1016/0306-4522(94)90016-7
Marshall, J. S. (2004). Mast-cell responses to pathogens. Nat. Rev. Immunol. 4, 787–799. doi: 10.1038/nri1460
Mattila, O. S., Strbian, D., Saksi, J., Pikkarainen, T. O., Rantanen, V., Tatlisumak, T., et al. (2011). Cerebral mast cells mediate blood-brain barrier disruption in acute experimental ischemic stroke through perivascular gelatinase activation. Stroke 42, 3600–3605. doi: 10.1161/STROKEAHA.111.632224
Mitsui, H., Furitsu, T., Dvorak, A. M., Irani, A. M., Schwartz, L. B., Inagaki, N., et al. (1993). Development of human mast cells from umbilical cord blood cells by recombinant human and murine c-kit ligand. Proc. Natl. Acad. Sci. U S A 90, 735–739. doi: 10.1073/pnas.90.2.735
Möllerherm, H., von Köckritz-Blickwede, M., and Branitzki-Heinemann, K. (2016). Antimicrobial activity of mast cells: role and relevance of extracellular DNA traps. Front. Immunol. 7:265. doi: 10.3389/fimmu.2016.00265
Morellini, N., Finch, P. M., Goebel, A., and Drummond, P. D. (2018). Dermal nerve fibre and mast cell density and proximity of mast cells to nerve fibres in the skin of patients with complex regional pain syndrome. Pain 159, 2021–2029. doi: 10.1097/j.pain.0000000000001304
Murphy, P. G., Borthwick, L. S., Johnston, R. S., Kuchel, G., and Richardson, P. M. (1999). Nature of the retrograde signal from injured nerves that induces interleukin-6 mRNA in neurons. J. Neurosci. 19, 3791–3800. doi: 10.1523/jneurosci.19-10-03791.1999
Nakamura, Y., Franchi, L., Kambe, N., Meng, G., Strober, W., and Núñez, G. (2012). Critical role for mast cells in interleukin-1β-driven skin inflammation associated with an activating mutation in the nlrp3 protein. Immunity 37, 85–95. doi: 10.1016/j.immuni.2012.04.013
Nishida, K., Yamasaki, S., Ito, Y., Kabu, K., Hattori, K., Tezuka, T., et al. (2005). FcεRI-mediated mast cell degranulation requires calcium-independent microtubule-dependent translocation of granules to the plasma membrane. J. Cell Biol. 170, 115–126. doi: 10.1083/jcb.200501111
Park, C.-K., Xu, Z.-Z., Berta, T., Han, Q., Chen, G., Liu, X.-J., et al. (2014). Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron 82, 47–54. doi: 10.1016/j.neuron.2014.02.011
Schauer, C., Janko, C., Munoz, L. E., Zhao, Y., Kienhöfer, D., Frey, B., et al. (2014). Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 20, 511–517. doi: 10.1038/nm.3547
Schreibelt, G., Kooij, G., Reijerkerk, A., Van Doorn, R., Gringhuis, S. I., van Der Pol, S., et al. (2007). Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase and PKB signaling. FASEB J. 21, 3666–3676. doi: 10.1096/fj.07-8329com
Silverman, A. J., Sutherland, A. K., Wilhelm, M., and Silver, R. (2000). Mast cells migrate from blood to brain. J. Neurosci. 20, 401–408. doi: 10.1523/jneurosci.20-01-00401.2000
Sjoerdsma, A., Waalkes, T. P., and Weissbach, H. (1957). Serotonin and histamine in mast cells. Science 125, 1202–1203. doi: 10.1126/science.125.3259.1202
Skaper, S. D., and Facci, L. (2012). Mast cell-glia axis in neuroinflammation and therapeutic potential of the anandamide congener palmitoylethanolamide. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 3312–3325. doi: 10.1098/rstb.2011.0391
Skokos, D., Botros, H. G., Demeure, C., Morin, J., Peronet, R., Birkenmeier, G., et al. (2003). Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J. Immunol. 170, 3037–3045. doi: 10.4049/jimmunol.170.6.3037
Stoiber, W., Obermayer, A., Steinbacher, P., and Krautgartner, W. D. (2015). The role of reactive oxygen species (ROS) in the formation of extracellular traps (ETs) in humans. Biomolecules 5, 702–723. doi: 10.3390/biom5020702
Taracanova, A., Alevizos, M., Karagkouni, A., Weng, Z., Norwitz, E., Conti, P., et al. (2017). SP and IL-33 together markedly enhance TNF synthesis and secretion from human mast cells mediated by the interaction of their receptors. Proc. Natl. Acad. Sci. U S A 114, E4002–E4009. doi: 10.1073/pnas.1524845114
Taracanova, A., Tsilioni, I., Conti, P., Norwitz, E. R., Leeman, S. E., and Theoharides, T. C. (2018). Substance P and IL-33 administered together stimulate a marked secretion of IL-1β from human mast cells, inhibited by methoxyluteolin. Proc. Natl. Acad. Sci. U S A 115, E9381–E9390. doi: 10.1073/pnas.1810133115
Tore, F., and Tuncel, N. (2009). Mast cells: target and source of neuropeptides. Curr. Pharm. Des. 15, 3433–3445. doi: 10.2174/138161209789105036
van Wetering, S., van Buul, J. D., Quik, S., Mul, F. P. J., Anthony, E. C., Ten Klooster, J.-P., et al. (2002). Reactive oxygen species mediate Rac-induced loss of cell-cell adhesion in primary human endothelial cells. J. Cell Sci. 115, 1837–1846.
Vincent, L., Vang, D., Nguyen, J., Benson, B., Lei, J., and Gupta, K. (2016). Cannabinoid receptor-specific mechanisms to ameliorate pain in sickle cell anemia via inhibition of mast cell activation and neurogenic inflammation. Haematologica 101, 566–577. doi: 10.3324/haematol.2015.136523
Vincent, L., Vang, D., Nguyen, J., Gupta, M., Luk, K., Ericson, M. E., et al. (2013). Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood 122, 1853–1862. doi: 10.1182/blood-2013-04-498105
Vukman, K. V., Försönits, A., Oszvald, Á., Tóth, E. Á., and Buzás, E. I. (2017). Mast cell secretome: soluble and vesicular components. Semin. Cell Dev. Biol. 67, 65–73. doi: 10.1016/j.semcdb.2017.02.002
Weng, Z., Zhang, B., Tsilioni, I., and Theoharides, T. C. (2016). Nanotube formation: a rapid form of “alarm signaling”? Clin. Ther. 38, 1066–1072. doi: 10.1016/j.clinthera.2016.02.030
Wilhelm, M., Silver, R., and Silverman, A. J. (2005). Central nervous system neurons acquire mast cell products via transgranulation. Eur. J. Neurosci. 22, 2238–2248. doi: 10.1111/j.1460-9568.2005.04429.x
Xanthos, D. N., Gaderer, S., Drdla, R., Nuro, E., Abramova, A., Ellmeier, W., et al. (2011). Central nervous system mast cells in peripheral inflammatory nociception. Mol. Pain 7:42. doi: 10.1186/1744-8069-7-42
Yuan, H., Zhu, X., Zhou, S., Chen, Q., Zhu, X., Ma, X., et al. (2010). Role of mast cell activation in inducing microglial cells to release neurotrophin. J. Neurosci. Res. 88, 1348–1354. doi: 10.1002/jnr.22304
Keywords: blood brain barrier, mast cell, endothelial cell, pain, inflammation, nervous system
Citation: Mittal A, Sagi V, Gupta M and Gupta K (2019) Mast Cell Neural Interactions in Health and Disease. Front. Cell. Neurosci. 13:110. doi: 10.3389/fncel.2019.00110
Received: 18 January 2019; Accepted: 06 March 2019;
Published: 20 March 2019.
Edited by:
Francesco Moccia, University of Pavia, ItalyReviewed by:
Elsa Fabbretti, University of Trieste, ItalyKempuraj Duraisamy, University of Missouri, United States
Copyright © 2019 Mittal, Sagi, Gupta and Gupta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kalpna Gupta, Z3VwdGEwMTRAdW1uLmVkdQ==
 Aditya Mittal
Aditya Mittal Varun Sagi1
Varun Sagi1 Mihir Gupta
Mihir Gupta Kalpna Gupta
Kalpna Gupta