- 1The National Clinical Research Center for Mental Disorders and Beijing Key Laboratory of Mental Disorders, Beijing Anding Hospital, Capital Medical University, Beijing, China
- 2Advanced Innovation Center for Human Brain Protection, Capital Medical University, Beijing, China
- 3Qingdao Municipal Hospital, Qingdao, China
- 4School of Life Sciences, University of Science and Technology of China, Hefei, China
- 5Center for Hormone Advanced Science and Education, Roskamp Institute, Sarasota, FL, United States
- 6Beijing Institute for Brain Disorders, Capital Medical University, Beijing, China
The main purpose of this study is to explore sex differences in the antidepressant effect of sertraline in genetic knockout or overexpression estrogen-synthesizing enzyme aromatase (Ar) gene mouse models in the forced swim test (FST). Our results demonstrated a significant reduction of depression-like behavior in the mice with overexpression of brain aromatase (Thy1-Ar) compared to sex- and age-matched Ar+/− mice or wild type control mice. Using HPLC analysis, we also found an association between the brain estrogen-related antidepressive behavior and the regulation of serotonin (5-HT) system. Interestingly, a single dose administration of sertraline (10 mg/kg, i.p.) induced reduction of immobility time was found in all genotypes, except male Ar+/− mice. While the underlying mechanisms of sex-specific response on antidepressive effect of sertraline remain to be investigated, our data showed that female mice appear to be more sensitive to sertraline-induced changes of 5-HT system than male mice in the prefrontal cortex (PFC) and the hippocampus (HPC). Further investigation of sex-specific effect of brain estrogen on antidepressant is needed.
Introduction
The prevalence of major depressive disorder (MDD) is more common in women than men (Pearson et al., 2013). Sex hormones may play an important role in this gender difference as increased likelihood of depression in menopausal women as well as postpartum depression occurs soon after giving birth (Soares, 2014). While lower estrogen levels have been specifically implicated in this increased risk in post-menopausal women (Albert et al., 2015), the hypothesis of sex hormone-related depression is also supported by animal studies, such as more depression-like behaviors were seen in the ovariectomized (OVX) rodents vs. intact rodents (Ye et al., 2016). Furthermore, similar to the human clinical practice of treating depression with estrogen (Toffol et al., 2015), many animal studies suggest that estrogen supplementation can prolong the swimming time in the forced swim test (FST) as a support of estrogen antidepressive effect (Walf and Frye, 2010a; Saravi et al., 2016).
In addition to sex difference in depression, interestingly, clinical studies showed that women with depression may respond better to serotonin reuptake inhibitors (SRIs) than depressive men (Baca et al., 2004; Berlanga and Flores-Ramos, 2006). Similar sex differences in SRIs response are also seen in animal tests (Fernandez-Guasti et al., 2017). While very little is known about the mechanisms of this sex-dependent differential response, we hypothesized that estrogen level might be responsible for the sex-dependent SRIs action.
Aromatase is the rate-limiting enzyme that catalyzes the conversion of androstenedione or testosterone to estrogens (Di Nardo and Gilardi, 2013). The level of estrogen in the brain depends largely on the expression of aromatase (Prange-Kiel and Rune, 2006). Brain-derived estrogen has a greater impact on brain function and neuronal diseases than the estrogen in circulation (Yue et al., 2005). This suggests that investigation of brain aromatase may clarify the mechanism of differences in gender response.
In the current study, we investigated gender differences to SRI response with a genetic mouse model of estrogen deficiency and a transgenic mouse mode of brain estrogen overexpression. We first examined the antidepressive effect of sertraline, one of classic SRIs, in behavioral test such as immobility time in the FST. Then we investigated brain monoamine system such as serotonin (5-HT), dopamine (DA), and their metabolites in the prefrontal cortex (PFC) and the hippocampus (HPC) of the experimental mice. Lastly, we analyzed the association between sex hormone levels and the antidepressive effect of sertraline in both male and female transgenic mice.
Materials and Methods
Animals
Aromatase-knockout (Ar−/−) mice (background: C57Bl/6Jmice) were generated by deleting exons1 and 2 encoded by the CYP19 gene as described (Honda et al., 1998). Heterozygous males and females were generated when breeding a homozygous-null male mouse to a wild type (WT) female mouse. Neuron-specific aromatase expression mice (Thy1-Ar) were generated by modifying marine thy1.2 genomic expression cassette for driving human aromatase expression in the nervous system. All mice (8–12 weeks old) were maintained in groups-housed four per cage, a 12 h light-dark cycle (lights on at 0800), and kept at a constant 24 ± 1°C temperature. Food and drinking water were unlimited. Mice were randomly divided into sertraline treatment group and vehicle treatment group. Each experimental group consisted of 6–7 mice. All animal studies and experimental procedures were in conformity to National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Ethical Committee for Animal Use, University of Science and Technology of China.
Genotyping
Genomic DNA extraction from toe tissue was performed by incubating samples in NaOH (50 mM) at 99°C for 30 min. Samples were centrifuged and Tris-HCL (1M; PH 7.4) was added. One μl of the supernatant was used for PCR amplification. Primers specific for the Ar+/− mice (sense: 5′-CTTGT CTAAG TGTCC AATCAC-3′; antisense: 5′-TTACC ATGTC CTAAT CTTCAC-3′), and primers specific for Thy1-Ar mice (sense: 5′-AGCCC TCAAG GTAAA TGGGGA-3; antisense: 5′-GAGGA TGTGC CCTCA TAATTCC-3′) were used. After initial denaturation at 94°C for 5 min, reactions were subjected to 35 cycles of 94, 60, and 72°C for 30 s each. PCR products were assessed by electrophoresis on agarose gel with concentration of 2% and detected by gel imaging analysis system (CLINX, Shanghai).
Drug Treatment
Mice received a single injection intraperitoneally (i.p.) of distilled water (10 ml/kg) or sertraline hydrochloride (Sigma, United States, dissolved in distilled water) (1 mg/ml), in a volume of 10 ml/kg of body weight. The dose was chosen on the basis of previous studies (Mouri et al., 2012).
Forced Swim Test (FST)
The FSTs were conducted in keeping with previous method with minor modifications (Autry et al., 2011). The experimental mice were placed in the behavioral test room for 2 h before the FST. Individual mice were placed in a cylindrical tank (25 cm tall × 14 cm diameter) containing15 cm of water with temperature at 24 ± 2°C. Thirty minutes after injection, mice were videotaped in the FST for 6 min. During the last 4 min, floating and remaining motionless were defined as the immobile and the duration was recorded. In order to minimize circadian influence, FSTs were consistently performed between 2100 and 2400 h. Mice were sacrificed by decapitation immediately after FST. The HPC and the PFC of mice were dissected on an ice-cold plate and stored at −80°Cfor later tissue analysis.
Spontaneous Locomotor Activity
Prior to FST, each animal was put into the center of the open-field square (50 cm × 50 cm × 50 cm) 30 min after administration with sertraline or vehicle. Between each mouse exposure the apparatus was cleaned with 10% ethanol and rinsed with hot water twice. The distance traveled (cm) was recorded for 5 min using a digital video-camera. A video tracking software EthoVision XT (Noldus, Netherlands) was used to analyze the horizontal locomotor activity of each mouse.
High-Performance Liquid Chromatography (HPLC)
Brain homogenates underwent a deproteinization step with 0.2 mL ice-cold perchloric acid (0.4 M) and then centrifugation for 20 min (12,000 × g, 4°C). Twenty μl clear supernatant were filtered by 0.22 mm Cellulose filters (Millipore, United States). The resulting sample was injected into the HPLC system with electrochemical detection (Model 5600A; Coularray Detector System, ESA, Chelmsford, MA, United States). The analytic results consisted of DA, 3,4-Dihydroxyphenylacetic acid (DOPAC), 5-HT, 5-Hydroxyindoleacetic acid (5-HIAA) and homovanillic acid (HVA), were expressed as μg/g protein to present. The ratios of DOPAC plus HVA to DA and 5-HIAA to 5-HT are an index of DA and 5-HT turnover rate, respectively (Santos et al., 2010).
Statistical Analysis
Data were evaluated using SPSSv.20 statistical software (IBM; United States). Two-way analysis of variance (ANOVA) considering genotype as one factor and treatment (vehicle, sertraline) as the other factor followed by Bonferroni post hoc test was performed for depression-like behaviors and neurotransmitters. All data were presented as means ± SEM and P < 0.05 were deemed statistical significance.
Results
Enriched Brain Estrogen Synthesis Expressed Antidepressive-Like Behavior in Male and Female Thy1-Ar Mice During the FST
Mice with three genotypes as WT, Ar+/− and Thy1-Ar were used to study the effect of endogenous estrogen on depression-like behaviors. As shown in Figure 1C, vehicle-treated Thy1-Ar mice showed a significant less immobility time (∼30% in females, ∼40% in males) compared to sex-matched WT mice. There is no difference in immobility time between male and female mice in all three genotypes. Unexpectedly, we found no effect of endogenous estrogen deficiency on immobile time in the FST in both male and female Ar+/− mice compared to sex-matched WT animals. These results imply that enriched brain estrogen promotes antidepressive-like behavior, whether reduction of endogenous estrogen failed to alter immobility time during the FST.
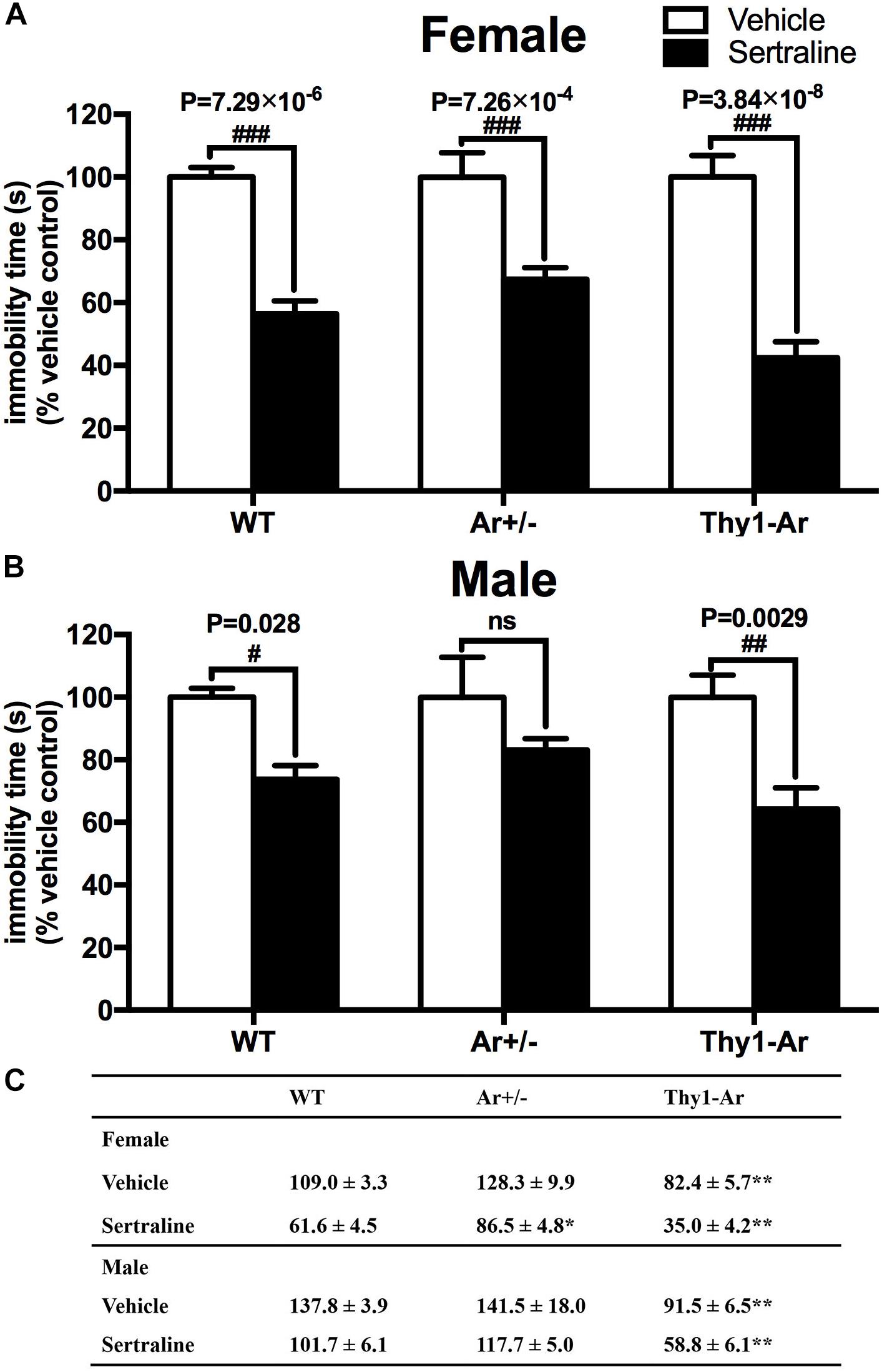
Figure 1. Enriched brain estrogen synthesis expressed antidepressive-like behavior and promoted antidepressive effect of sertraline in both male and female mice. Effect of sertraline on percentage change of immobility time during the FST from vehicle treated mice in both (A) females and (B) males. Mean immobility duration (C) in sec. of female and male mice after sertraline administration or vehicle during the FST. Data represent means ± SEM (N = 6–7 mice/group), as evidenced by two-way ANOVA. #P < 0.05, ##P < 0.01, ###P < 0.001 versus vehicle-treated group; ∗P < 0.05, ∗∗P < 0.01 versus WT mice.
Enriched Brain Estrogen Promotes Antidepressive Effect of Sertraline in Both Male and Female Mice
Sertraline significantly reduced the immobility time during FST in all female animals when compared with their vehicle treated counterparts (Figure 1A). Sertraline administration induced much greater reduction of immobility time in female Thy1-Ar mice (P = 3.8 × 10−8) while female Ar+/− mice showed less reduction of immobility time (P = 7.3 × 10−4) compared to that in sex-matched WT mice (P = 7.3 × 10−6). Interestingly, male animals responded to the sertraline administration differently. First, while both male and female Thy1-Ar mice responded to sertraline-induced antidepressive effect in immobility time, male Thy1-Ar mice showed less sensitive to sertraline treatment than female Thy1-Ar mice (P = 0.0029 vs. P = 0.000000038) compared to sex-matched WT mice. In addition, we found sertraline administration induced no significant reduction of depressive-like behavior in male Ar+/− mice (Figure 1B), while female Ar+/− mice had significant but less response to sertraline than sex-matched WT and Thy1-Ar mice (Figure 1A).
Sertraline Did Not Alter Spontaneous Locomotor Activity in All Three Genotypes Regardless of Sex Difference
To examine whether the endogenous estrogen-related behaviors is depressive specific, we also included open field test for locomotor activity in all three genotypes mice. As shown in Figure 2, there were no differences in distance moved in the open field behavioral test among the WT, Ar+/−, Thy1-Ar mice regardless sexes. In addition, sertraline treatment did not alter the spontaneous locomotor activity in all of the experimental mice. Our data suggested that endogenous estrogen induced no significant drug-effect or sex-effect on spontaneous locomotion.
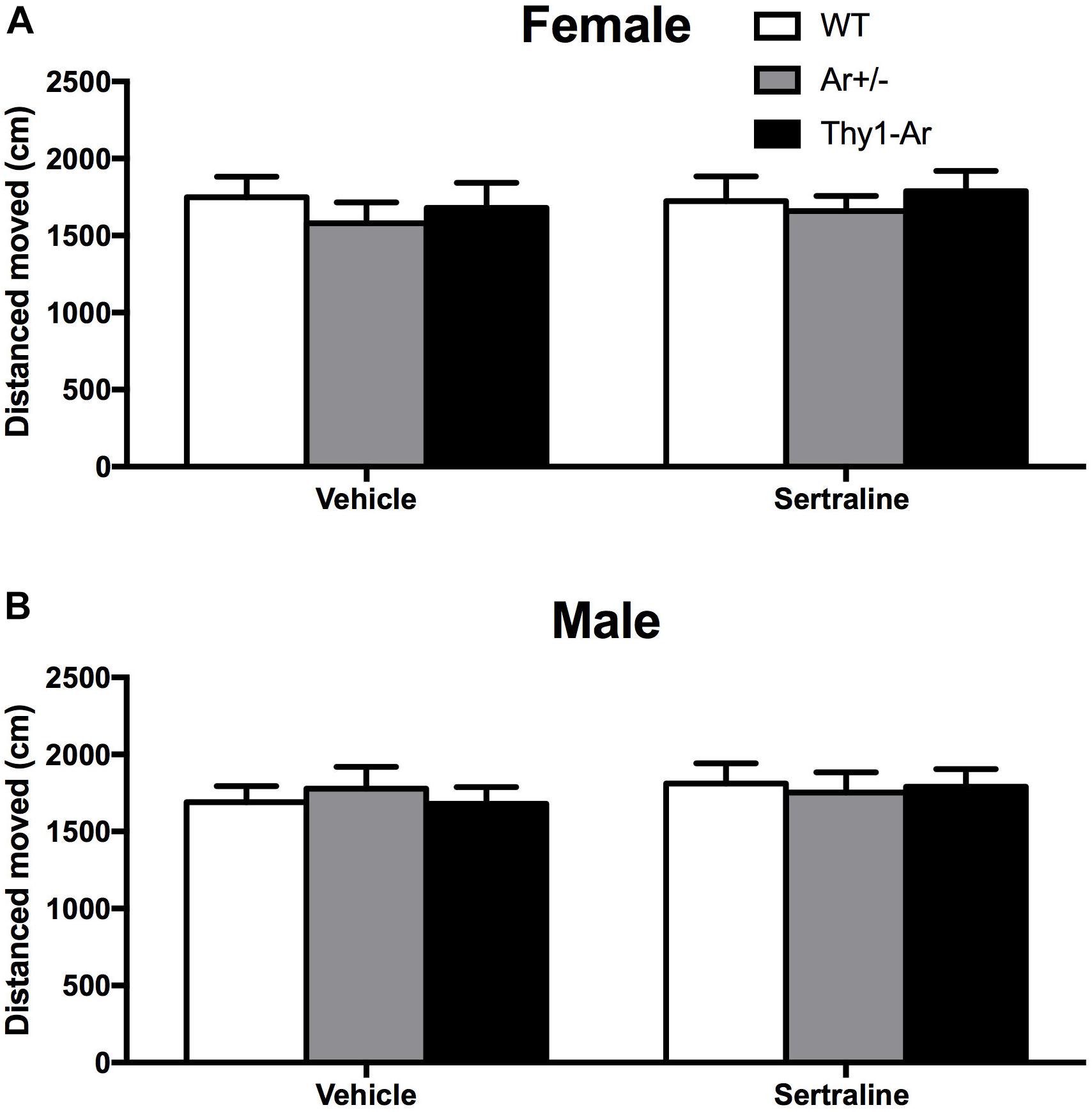
Figure 2. Sertraline did not alter spontaneous locomotor activity in all three genotypes regardless of sex difference. No differences were found in total distance moved in spontaneous locomotor activity in both (A) female and (B) male mice treated by vehicle or sertraline. Data represent means ± SEM (N = 6–7 mice/group), as evidenced by the two-way ANOVA.
Endogenous Estrogen Induced Sex- and Brain Region-Specific Alterations in 5-TH Systems
To further understand the role of endogenous estrogen in depressive behaviors, we also measured neurotransmitters DA and 5-HT and their metabolites in the HPC and the PFC of mice. As shown in Figure 3, we found no significant effect of endogenous estrogen on DA, DOPAC, HVA, and DA index in regardless sexes and brain regions. For the 5-HT system, our data showed an elevated level of 5-HIAA and 5-HT turnover rate without the change of 5-HT in the PFC of female Ar+/− mice compared to that of WT females (Figure 3A), while a higher 5-HT level and lower 5-HT turnover rate were observed in the HPC of female Thy1-Ar mice (Figure 3B). On the other hand, no differences were detected in 5-HT or 5HIAA levels in all male genotypes regardless brain regions (Figures 3C,D), except a significantly decreased 5-HT index was observed in the PFC of male Thy1-Ar mice compared to male WT mice.
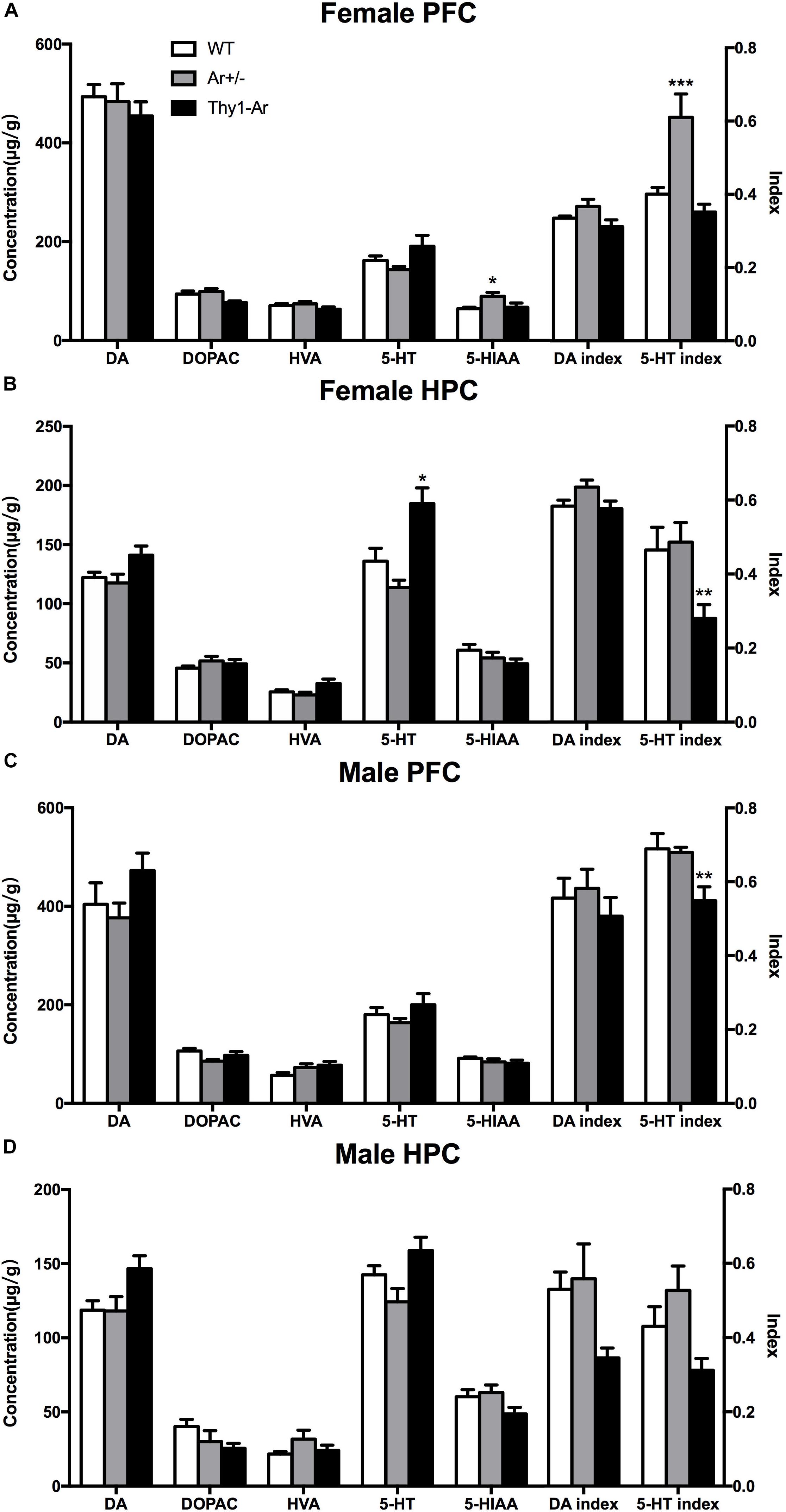
Figure 3. Endogenous estrogen induced sex- and brain region-specific alterations in 5-TH systems. The concentration (μg/g) of DA and 5-TH related neurotransmitters affected by endogenous estrogen in (A) female PFC, (B) female HPC, (C) male PFC, and (D) male HPC. Data represent means ± SEM (N = 6–7 mice/group), as evidenced by the two-way ANOVA followed by Bonferroni post hoc test. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 versus WT mice.
Sex- and Brain Region-Specific Differences in DA and 5-TH Systems Following Sertraline Administration
To investigate changes of neurotransmitters induced by SRI in males and females, relevant values of DA and 5-HT system were measured by HPLC analyses after sertraline administration. For the DA system, sertraline showed no effect on DA level and its metabolites in the PFC of all three genotype female mice (Figure 4A), while sertraline showed a mild elevation of DA level and reduction of DA index in the HPC of female Thy1-Ar mice, and a marginally significant reduction of DOPAC and DA index levels in the HPC of female Ar+/− mice as compared to the vehicle-treated genotype-matched female mice (Figure 4B). In males, sertraline was associated with elevated DA levels only in both brain regions of Thy1-Ar mice, while the enhanced HVA levels were found only in the PFC of WT and Thy1-Ar males (Figures 4C,D). Meanwhile, male Thy1-Ar mice responded better to sertraline treatment implemented by increasing DA level in both areas and decreasing DA index only in the PFC as compared with male WT mice. It is worth to note that male mice with estrogen deficiency failed to respond to sertraline treatment in DA relevant values (Figures 4C,D). For the 5-HT system, our data showed that sertraline administration also significantly promoted the levels of 5-HT and 5-HIAA from female animals regardless of genotypes. Decreased ratio of 5-HIAA/5-HT induced by sertraline was observed in both PFC and HPC of female Thy1-Ar mice, while such result was observed only in the PFC of WT and Ar+/− females (Figures 4A,B). Interestingly, the sertraline-induced elevation of 5-HT and 5-HIAA levels were only obtained in the HPC of male mice regardless of genotypes, while no changes were found in the PFC except in male Thy1-Ar mice (Figures 4C,D).
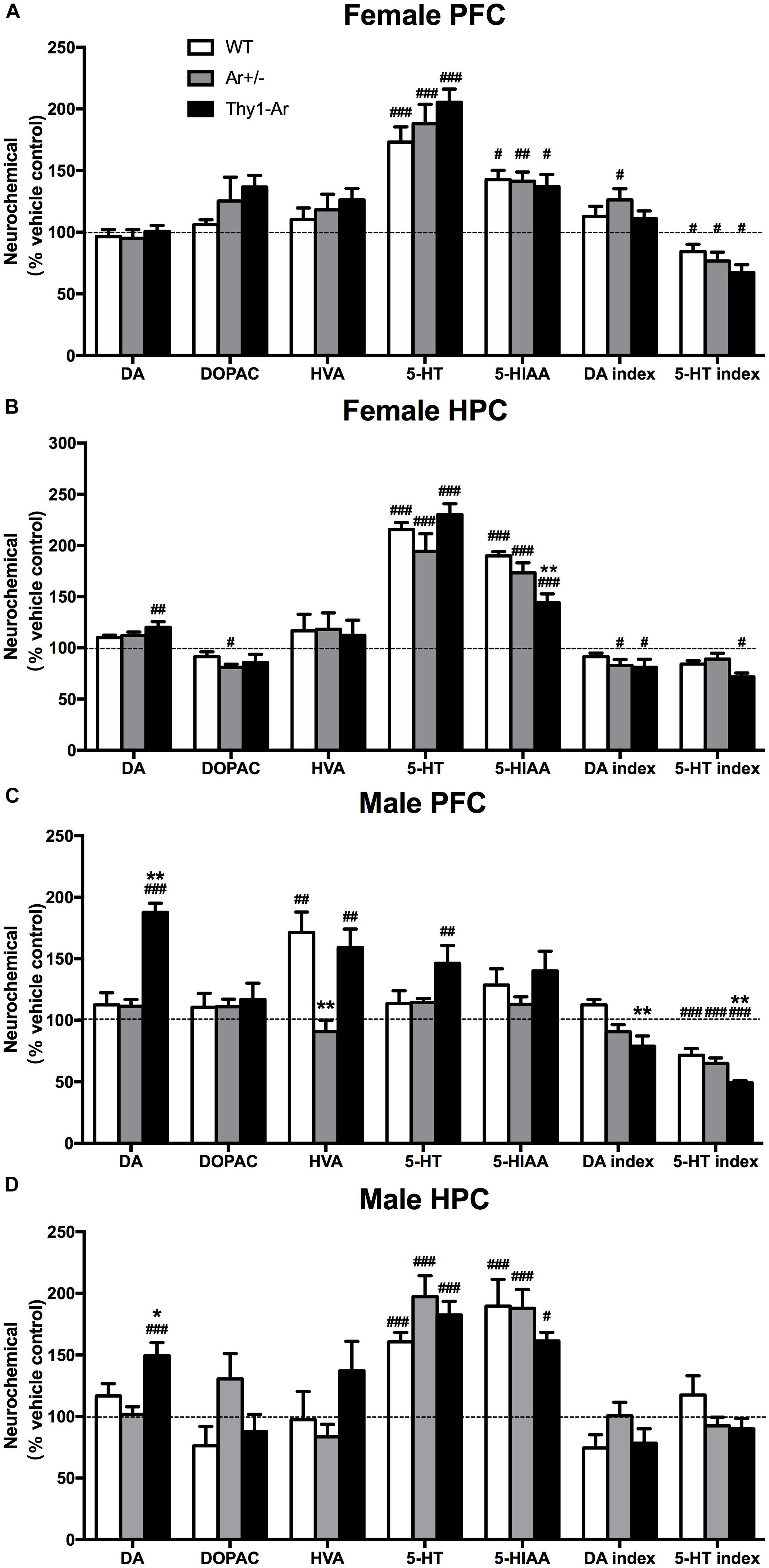
Figure 4. Sex- and brain region- specific differences in DA and 5-TH systems following sertraline administration. The percentage of DA and 5-TH related neurotransmitters for sertraline-treated and vehicle-treated in (A) female PFC, (B) female HPC, (C) male PFC, and (D) male HPC. Data represent means ± SEM (N = 6–7 mice/group), as evidenced by the two-way ANOVA followed by Bonferroni post hoc test. #P < 0.05, ##P < 0.01, ###P < 0.001 versus vehicle group; ∗P < 0.05, ∗∗P < 0.01 versus WT mice.
Discussion
In this study, we used transgenic mouse models to examine the effect of estrogen on sertraline-induced behavioral and biochemical changes. First, we demonstrated that an elevation of brain estrogen level in Thy1-Ar mice (Supplementary Figure S1) exhibited less depression-like behaviors in the FST than sex-matched WT mice (Figure 1). This estrogen enriched antidepressive-like behaviors were consistent with reports from other group which demonstrated that increased estrogen levels by estrogen supplementation could reverse depression-like behavior in ovariectomized rodents (Walf and Frye, 2010b). Instead of giving estrogen treatment, our study used the newly developed brain-specific aromatase transgenic mice, Thy1-Ar, reported that elevation of brain estrogen level can reduce depression-like behavior in both sexes. However, the Ar+/− mice, a model of estrogen deficiency as we previous published (Yue et al., 2005; McAllister et al., 2010), showed no changes in immobility time compared to sex-matched WT mice (Figure 1). There are several possible explanations for the weak effect of Ar+/− mice on depressive behaviors. For example, we think the level of estrogen reduction is critical for depressive-like behavior. While our data showed that increase brain estrogen (Thy1-Ar female mice) had less depression-like behavior, other report demonstrated a significant increase in depression-like behavior in homozygous aromatase knockout (Ar−/−) female mice compared to WT (Dalla et al., 2004). However, how essential does the level of endogenous estrogen plays in depression is unclear since studies demonstrated estrogen level-related mood and behaviors changes during the menstrual cycle in reproductive women (Jang and Elfenbein, 2018), as well as in female animals (Estrada-Camarena et al., 2011). As the changes of endogenous estrogen level in Ar+/− and Thy1-Ar mice are much greater than that occurred in regular estrous cycles for WT mice, we hypothesize that the behaviors changes in FST from these aromatase animal models might be influenced not only by the level of estrogen, but also the imbalanced sex hormones in our study. Another explanation for the weak effect of Ar+/− mice on depressive behavior is the duration of estrogen deficiency. As the reduction of endogenous estrogen in aged Ar+/− female mice (12 months) was sufficient enough to promote early development of Alzheimer’s disease pathology in APP mice as we previously published (Yue et al., 2005), the less response to the estrogen deficiency-related depressive-like behavior in FST in Ar+/− mice might be related to the mice at younger age (3 months) and shorter duration of estrogen deficiency (Estrada-Camarena et al., 2011). Together, although females showed higher risk of depression in human clinical studies, our data suggested that brain estrogen is important in suppressing depressive-like behaviors in both males and females in animal models.
Then we examined the estrogen-dependency in response to the dose of 10 mg/kg sertraline treatment between male and female mice. We selected the lowest effective dosage of sertraline (10 mg/kg) as our sertraline administration based on the dose-dependent response curve as shown in the Supplementary Figure S2. As shown in Figure 1, all mice responded to sertraline treatments by significant reduction of immobility time in the FST, except male Ar+/− mice. The effect of sertraline administration induced a genotype-specific effect on immobility time. There was a significant less immobility time in both male and female Thy1-Ar mice, and a significant more immobility time were found in Ar+/− female mice compared to sertraline-treated WT mice. Consistent with human studies in women, postmenopausal women with low level of estrogen showed worse response to SRIs treatment than premenopausal women (Pinto-Meza et al., 2006). In addition, clinical studies also showed that the combination of hormone replacement therapy (HRT) with fluoxetine (an SRI), was more effective in depression treatment than HRT (Liu et al., 2004) or fluoxetine alone (Yu et al., 2004) in post-menopausal women. The age- and estrogen-related responses to antidepressants are also reported in different clinical studies. For example, MDD patients at reproductive age were associated with a better response to SRIs while older MDD patients were associated with a superior TCAs response (Parker et al., 2003). Moreover, female MDD patients at younger age (<44 years) had significant higher rates of remission after treatment of SRIs than older (>50 years) MDD patients (Grigoriadis et al., 2003). These findings partially supported estrogen-dependent effect on antidepressants. In the human studies, antidepressants need to evoke adaptive changes after 2–4 weeks of application. However, in animal studies, acute and chronic treatment with SRIs may lead to different behavioral and neurochemical changes (Burghardt and Bauer, 2013), which offer us a clear mechanistic insight into the antidepressive actions of drugs. In our present animal study, we try to focus on the impact of estrogen levels on the acute anti-depressive effect of SRIs. We will extend our study in the future to investigate the clinical effect of sertraline in our animal models. In our current study, we showed that sertraline induced the greatest reduction of depressive-like behaviors in the Thy1-Ar mice (∼57.5% in females, ∼35.7% in males) compared to the vehicle treatment, while Ar+/− mice showed the least response to the sertraline treatment (∼32.6% in females, 16.8% in males) as shown in Figure 1. Our results not only supported the estrogen-dependent effect on depression as reported by others, but also suggested that enriched brain estrogen benefits the antidepressive effect of sertraline and estrogen deficiency reduces the antidepressive effect of sertraline in our animal model. While the results from scientific experiments answer some questions, it often left new puzzles for further investigations. For example, it is not clear why male Ar+/− mice did not respond significantly to sertraline in our study (Figure 1). It is possible that knocking out aromatase may also increase basal testosterone levels in males not in females, which may have affected the outcome (Amano et al., 2017). Such a sex-specific effect of the Ar+/− animal model in cognitive behaviors has been reported in our previous publications as only male Ar+/− mice express higher level of testosterone than age- and sex-matched WT controls and showed neuroprotective effect on Alzheimer’s related cognitive impairment as well as Alzheimer’s pathology at age of 12 months (McAllister et al., 2010), while female Ar+/− mice developed early Alzheimer’s brain pathology at same age (Yue et al., 2005). Instead, females Ar+/− mice are more vulnerable to the reduction of estrogen level which overrides the potential effect (if there is one) of testosterone on behavior and brain pathology. Interestingly, the estrogen-dependent influence in depressive-like behavior is not caused by potential changes of spontaneous locomotive activities in the mice. As shown in Figure 2, all three genotypes of mice showed the similar travel distance in the open field test regardless sexes.
To further understand the neuronal mechanisms of the sex difference in depression, we examined the 5-HT and DA metabolism in two brain regions in our animals. We included the PFC and HPC as the targeted brain regions to study sertraline antidepressant effect (Arnsten, 2009; Brezun and Daszuta, 2015). As showed in Figure 3, a significant elevation of 5-HIAA level and 5-HT turnover rate was found in female Ar+/− mice compared to the sex-matched WT mice (Figure 3A), while higher 5-HT level and lower 5-HT turnover rate were observed in the HPC of Thy1-Ar females (Figure 3B). No changes of 5-HT or its metabolite were identified in male mice regardless genotypes and brain areas, except a reduction of 5-HT index was observed in the PFC of Thy1-Ar male mice (Figures 3C,D). Our data on the estrogen-dependent regulation of 5-HT metabolisms were consistent with previous reports from other groups, such as higher level of endogenous estrogen was associated with elevation in the level of 5-HT, and lower 5-HT turnover rate in the PFC of female mice (Kiss et al., 2012). However, estrogen injection was associated with a decrease of 5-HT levels and an increased 5-HT turnover rate (Pandaranandaka et al., 2006), or showed no differences in OVX female rat (Lu et al., 1998). It is worth to note that most of the estrogen-dependent changes of 5-HT-related depressive behaviors in animals were associated with estrogen administration acutely or chronically (Shah and Frazer, 2014) which might be different from endogenous estrogen in related to the serotonergic system (Pandaranandaka et al., 2009). Instead of treating animals with exogenous estrogen, studies of 5-HT metabolism in female animals at young, middle and old ages demonstrated that young female animals exhibited higher hippocampal 5-HT concentration than middle-age animals (Kiss et al., 2012), and women over 60 years of age had less platelet 5-HT content than younger age (Guicheney, 1988). Together, our data were in line with other reports and suggested that higher endogenous estrogen levels (younger age) might be associated with higher level of 5-HT and vice versa. Our animal models presented a unique endogenous estrogen deficiency or overexpression system for investigating the relationship between endogenous estrogen and depression. These results, combined with our behavioral data which showed reduced depression-like symptoms related to overexpression of brain estrogen and vice versa, suggested that the 5-HT system may be modulated by endogenous brain estrogen, independent of gender. Therefore, our data provided first line neurochemistry evidence of the linkage between estrogen and depressive behaviors.
As sertraline is a SRI, we extended our investigation of sex difference in the response to sertraline to brain regional changes of 5-HT and its metabolites in our animals. As shown in Figure 4, sertraline caused an increase in 5-HT and 5-HIAA levels in both brain regions of all the female mice regardless genotypes (Figures 4A,B). However, sertraline in males showed brain regional effects, such as sertraline induced greater increase of 5-HT and 5-HIAA levels in HPC than that in the PFC, while much significant reduction of 5-HIAA/5-HT ratio was found in the PFC than in the HPC compared to vehicle treated male group (Figures 4C,D). Male Thy1-Ar mice responded better to sertraline than male WT mice in terms of 5-HT and DA level changes, particularly a significant reduction of 5-HT index in the PFC from Thy1-Ar males compared to sex-matched WT males (Figure 4C). These changes suggested that sertraline induced sex-specific effect on DA and 5-HT metabolisms in the PFC and HPC and enriched brain estrogen only associated with the reduction of 5-HT index in males. However, in our experiments, the absence of the levels of 5-HT after treatment with sertraline in the PFC of male mice seems contradictory with some previous works. For example, studies of male rats demonstrated a time-dependent curve of SRI (fluoxetine) treatment on PFC monoamine levels and showed an elevation of 5-HT was started 1 h and reached to the peak at 2.5 h after the single injection of fluoxetine (Zocchi et al., 2003; Beyera, 2008). In our experiment, mice were terminated less than 1 h (∼40 min) after the sertraline injection which might be too early to observe the significant change of 5-HT levels in the PFC. Other possibilities include a variation of regional response to SRIs treatment in the PFC (the medial PFC is more sensitive than lateral PFC to SRIs-induced 5-HT) and regional 5-HT1A autoreceptors feedback mechanisms (Beyera, 2008). While we examined the PFC as a whole, the regional-specific response could be bleached out which might be partially responsible for the absence change of 5-HT induced by sertraline.
A variety of potential mechanisms have been proposed to explain the effect of estrogen levels on depression-like behaviors. First, estrogen may ameliorate the dysfunctional serotonergic activity contributing to depression-like behavior. For example, estrogen increased the mRNA expression of 5-HT synthesis key enzyme – tryptophan hydroxylase 2 (TPH2) in rat midbrain raphe nucleus (Hiroi et al., 2006). And estrogen could also upregulate the expression of 5-HT2A receptor (Sumner et al., 1999) and downregulate the expression of 5-HT1A and 5-HT1B autoreceptor in female rats (Osterlund and Hurd, 1998; Hiroi and Neumaier, 2009) to exert antidepressant effect. Besides, estrogen exhibits antidepressive action in part through neurotrophic factors and anti-inflammatory actions. While it is known that MDD often expresses a reduction of brain-derived neurotrophic factor (BDNF), study reported that estrogen treatment can increase BDNF level through binding to estrogen response element which is located on the BDNF gene (Franklin and Perrot-Sinal, 2006). Our unpublished data showed that both female and male Thy1-Ar mice showed significant increase of BDNF protein expression in HPC compared to WT. In addition, estrogens could downregulate pro-inflammatory cytokines, such as interferon gamma (IFN-γ) and interleukin-6 (IL-6) in MDD through the transcription factor NF-κB (Pozzi et al., 2006; Xu et al., 2015).
Changes of 5-HT metabolisms in specific brain regions have been also proposed in depression and response to antidepressants. Decreased ratio of 5-HIAA/5-HT in the PFC was essential for the improvement of depressive symptoms of rats (McNamara et al., 2010). Studies showed that the 5-HT index in the PFC was elevated by negative emotions (Park et al., 2012) and the 5-HT level in the HPC is known to be negatively correlation with depression-like behavior and positively correlation with antidepressant treatment (Mahar et al., 2014). In line with many published findings, estrogen appeared to facilitate the 5-HT action of SRIs and reinforced the antidepressant of SRIs via increasing 5-HT in synaptic. While short-term administration of sertraline played an essential role in 5-HT conversion in various brain regions (Muneoka et al., 2009), estrogen acted on estrogen receptor and regulated the 5-HT metabolisms indirectly through the 5-HT1A receptor (Li et al., 2013). Furthermore, estrogen can also alter the effects of SRIs on serotonin clearance through the MAPK/ERK1/2 and PI3K/Akt pathways (Benmansour et al., 2014). The metabolism of sertraline was controlled extensively by CYP 2D6 and 2C19, especially for CYP 2C19 (Probst-Schendzielorz et al., 2015). In other words, poor activity in CYP 2D6 and CYP 2C19 might elevate concentrations of sertraline, while estrogen reduced the biological function of CYP 2D6 and CYP 2C19 were suggested (Mwinyi et al., 2010; Pan and Jeong, 2015).
Several potential factors might contribute to the relationship between sex hormone and antidepressant effect in male Ar+/− mice. As we mentioned before, in male Ar+/− mice, in addition to reduction of endogenous estrogen, there is a great increase in endogenous testosterone level. High level of testosterone combined with fluoxetine was ineffective in alleviating depressive symptoms in male rats (Martinez-Mota and Fernandez-Guasti, 2004). Testosterone injection may cause the dysfunction of serotonin system in animals (Hernandez-Rauda and Aldegunde, 2002) and chronic testosterone administration abolished the antidepressant effect of exercise which accompanied with the improvement of oxidative damage in male rat hippocampus (Joksimović et al., 2017). Clinical research also implies that neither testosterone nor testosterone supplementation to SRIs had a positive effect on adult men with MDD (Seidman et al., 2005; Pope et al., 2010). On the other hand, testosterone administration augments the antidepressive effect in hypogonadal men (Seidman and Rabkin, 1998). This collaborative effect may due to the basal low testosterone levels in hypogonadal men. In summary, endogenous estrogen alone, but not testosterone, enhances the antidepressant of sertraline. However, the elevation of testosterone level in male Ar+/− mice may influence 5-HT metabolism and eliminates the effectiveness of sertraline treatment. Sertraline may have effects on dopamine reuptake in addition to serotonin reuptake inhibition (Kitaichi et al., 2010). The dopaminergic action caused by sertraline is deemed to conduce to its positive effect on depression (Dunlop and Nemeroff, 2007), particularly in animals who are receiving close to four times the highest human dose, but unlikely in humans due to the relatively low affinity to the dopamine transporter. Our data showed that male Thy1-Ar mice are much sensitive to sertraline-induced elevation of DA levels in both PFC and HPC. Whether the sex difference in response to sertraline treatment in mice were related to the sex hormones and/or different neurotransmitters remain further investigation.
Conclusion
Our present study indicates that endogenous estrogen has both an antidepressant-like effect as well as a beneficial effect on the efficacy of SRIs in both males and females. The neurotransmitter alterations showed that these effects were mainly associated with changes of 5-HT metabolism in both PFC and HPC. Moreover, the response of 5-HT system in female mice to sertraline treatment was more pronounced than males. However, endogenous estrogen deficiency eliminated the antidepressant effect of sertraline in FST only in male mice, suggesting that there was an interaction between estrogen, testosterone and SRIs treatment.
Author Contributions
LM designed, conducted, and analyzed the experiments. YX, WJ, YL, and XZ contributed mice and designed the experiments. GW and RL designed and conceived the experiments. All authors discussed the results and contributed to writing the manuscript.
Funding
The research was supported by National Key R&D Program of China (2016YFC1307200), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201607), Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20151801), and National Natural Science Foundation of China (81671248).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to thank Jie Zhang, Danlei Bi, and Feng Gao from the University of Science and Technology of China for insightful comments provided during the preparation of this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fncel.2019.00024/full#supplementary-material
References
Albert, K., Pruessner, J., and Newhouse, P. (2015). Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology 59, 14–24. doi: 10.1016/j.psyneuen.2015.04.022
Amano, A., Kondo, Y., Noda, Y., Ohta, M., Kawanishi, N., Machida, S., et al. (2017). Abnormal lipid/lipoprotein metabolism and high plasma testosterone levels in male but not female aromatase-knockout mice. Arch. Biochem. Biophys. 622, 47–58. doi: 10.1016/j.abb.2017.03.007
Arnsten, A. F. T. (2009). Stress signaling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 10, 410–422. doi: 10.1038/nrn2648
Autry, A. E., Adachi, M., Nosyreva, E., Na, E. S., Los, M. F., Cheng, P. F., et al. (2011). NMDA receptor blockade at rest triggers rapid behavioral antidepressant responses. Nature 475, 91–95. doi: 10.1038/nature10130
Baca, E., Garcia-Garcia, M., and Porras-Chavarino, A. (2004). Gender differences in treatment response to sertraline versus imipramine in patients with nonmelancholic depressive disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 28, 57–65. doi: 10.1016/S0278-5846(03)00177-5
Benmansour, S., Privratsky, A. A., Adeniji, O. S., and Frazer, A. (2014). Signaling mechanisms involved in the acute effects of estradiol on 5-HT clearance. Int. J. Neuropsychopharmacol. 17, 765–777. doi: 10.1017/S146114571300165X
Berlanga, C., and Flores-Ramos, M. (2006). Different gender response to serotonergic and noradrenergic antidepressants. A comparative study of the efficacy of citalopram and reboxetine. J. Affect. Disord. 95, 119–123. doi: 10.1016/j.jad.2006.04.029
Beyera, C. E. (2008). Do selective serotonin reuptake inhibitors acutely increase frontal cortex levels of serotonin? Eur. J. Pharmacol. 580, 350–354. doi: 10.1016/j.ejphar.2007.11.028
Brezun, J. M., and Daszuta, A. (2015). Serotonergic reinnervation reverses lesion-induced decreases in PSA-NCAM labeling and proliferation of hippocampal cells in adult rats. Hippocampus 10, 37–46. doi: 10.1002/(SICI)1098-1063(2000)10:1<37::AID-HIPO4>3.0.CO;2-C
Burghardt, N. S., and Bauer, E. P. (2013). Acute and chronic effects of selective serotonin reuptake inhibitor treatment on fear conditioning: implications for underlying fear circuits. Neuroscience 247, 253–272. doi: 10.1016/j.neuroscience.2013.05.050
Dalla, C., Antoniou, K., Papadopouloudaifoti, Z., Balthazart, J., and Bakker, J. (2004). Oestrogen-deficient female aromatase knockout (ArKO) mice exhibit depressive-like symptomatology. Eur. J. Neurosci. 20, 217–228. doi: 10.1111/j.1460-9568.2004.03443.x
Di Nardo, G., and Gilardi, G. (2013). Human aromatase: perspectives in biochemistry and biotechnology. Biotechnol. Appl. Biochem. 60, 92–101. doi: 10.1002/bab.1088
Dunlop, B. W., and Nemeroff, C. B. (2007). The role of dopamine in the pathophysiology of depression. Arch. Gen. Psychiatry 64, 327–337. doi: 10.1001/archpsyc.64.3.327
Estrada-Camarena, E., López-Rubalcava, C., Hernández-Aragón, A., Mejía-Mauries, S., and Picazo, O. (2011). Long-term ovariectomy modulates the antidepressant-like action of estrogens, but not of antidepressants. J. Psychopharmacol. 25, 1365–1377. doi: 10.1177/0269881111408456
Fernandez-Guasti, A., Olivares-Nazario, M., Reyes, R., and Martinez-Mota, L. (2017). Sex and age differences in the antidepressant-like effect of fluoxetine in the forced swim test. Pharmacol. Biochem. Behav. 152, 81–89. doi: 10.1016/j.pbb.2016.01.011
Franklin, T. B., and Perrot-Sinal, T. S. (2006). Sex and ovarian steroids modulate brain-derived neurotrophic factor (BDNF) protein levels in rat hippocampus under stressful and non-stressful conditions. Psychoneuroendocrinology 31, 38–48. doi: 10.1016/j.psyneuen.2005.05.008
Grigoriadis, S., Kennedy, S. H., and Bagby, R. M. (2003). A comparison of antidepressant response in younger and older women. J. Clin. Psychopharmacol. 23, 405–407. doi: 10.1097/01.jcp.0000085415.08426.c6
Guicheney, P. (1988). Human platelet serotonin content: methodological aspects and physiological variations. Methods Find. Exp Clin. Pharmacol. 10, 253–258. doi: 10.1002/med.2610080206
Hernandez-Rauda, R., and Aldegunde, M. (2002). Effects of acute 17α-methyltestosterone, acute 17β-estradiol, and chronic 17α-methyltestosterone on dopamine, norepinephrine and serotonin levels in the pituitary, hypothalamus and telencephalon of rainbow trout (Oncorhynchus mykiss). J. Comp. Physiol. B 172, 659–667. doi: 10.1007/s00360-002-0282-z
Hiroi, R., Mcdevitt, R. A., and Neumaier, J. F. (2006). Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: association between gene expression and anxiety behavior in the open field. Biol. Psychiatry 60, 288–295. doi: 10.1016/j.biopsych.2005.10.019
Hiroi, R., and Neumaier, J. F. (2009). Estrogen decreases 5-HT 1B autoreceptor mRNA in selective subregion of rat dorsal raphe nucleus: inverse association between gene expression and anxiety behavior in the open field. Neuroscience 158, 456–464. doi: 10.1016/j.neuroscience.2008.10.016
Honda, S., Harada, N., Ito, S., Takagi, Y., and Maeda, S. (1998). Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the CYP19 gene. Biochem. Biophys. Res. Commun. 252, 445–449. doi: 10.1006/bbrc.1998.9672
Jang, D., and Elfenbein, H. A. (2018). Menstrual cycle effects on mental health outcomes: a meta-analysis. Arch. Suicide Res. 20, 1–21. doi: 10.1080/13811118.2018.1430638
Joksimović, J., Selaković, D., Jakovljević, V., Mihailović, V., Katanić, J., Boroja, T., et al. (2017). Alterations of the oxidative status in rat hippocampus and prodepressant effect of chronic testosterone enanthate administration. Mol. Cell. Biochem. 433, 41–50. doi: 10.1007/s11010-017-3014-0
Kiss, Á., Delattre, A. M., Pereira, S. I. R., Carolino, R. G., Szawka, R. E., Anselmo-Franci, J. A., et al. (2012). 17β-Estradiol replacement in young, adult and middle-aged female ovariectomized rats promotes improvement of spatial reference memory and an antidepressant effect and alters monoamines and BDNF levels in memory- and depression-related brain areas. Behav. Brain Res. 227, 100–108. doi: 10.1016/j.bbr.2011.10.047
Kitaichi, Y., Inoue, T., Nakagawa, S., Boku, S., Kakuta, A., Izumi, T., et al. (2010). Sertraline increases extracellular levels not only of serotonin, but also of dopamine in the nucleus accumbens and striatum of rats. Eur. J. Pharmacol. 647, 90–96. doi: 10.1016/j.ejphar.2010.08.026
Li, Q., Sullivan, N. R., McAllister, C. E., Van de Kar, L. D., and Muma, N. A. (2013). Estradiol accelerates the effects of fluoxetine on serotonin 1A receptor signaling. Psychoneuroendocrinology 38, 1145–1157. doi: 10.1016/j.psyneuen.2012.11.005
Liu, P., He, F. F., Bai, W. P., Yu, Q., Shi, W., Wu, Y. Y., et al. (2004). Menopausal depression: comparison of hormone replacement therapy and hormone replacement therapy plus fluoxetine. Chin. Med. J. 117, 189–194.
Lu, H., Yuri, K., Ito, T., Yoshimoto, K., and Kawata, M. (1998). The effects of oestrogen and progesterone on serotonin and its metabolite in the lateral septum, medial preoptic area and ventromedial hypothalamic nucleus of female rats. J. Neuroendocrinol. 10, 919–926. doi: 10.1046/j.1365-2826.1998.00280.x
Mahar, I., Bambico, F. R., Mechawar, N., and Nobrega, J. N. (2014). Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci. Biobehav. Rev. 38, 173–192. doi: 10.1016/j.neubiorev.2013.11.009
Martinez-Mota, L., and Fernandez-Guasti, A. (2004). Testosterone-dependent antidepressant-like effect of noradrenergic but not of serotonergic drugs. Pharmacol. Biochem. Behav. 78, 711–718. doi: 10.1016/j.pbb.2004.05.016
McAllister, C., Long, J., Bowers, A., Walker, A., Cao, P., Honda, S., et al. (2010). Genetic targeting aromatase in male amyloid precursor protein transgenic mice down-regulates beta-secretase (BACE1) and prevents alzheimer-like pathology and cognitive impairment. J. Neurosci. 30, 7326–7334. doi: 10.1523/JNEUROSCI.1180-10.2010
McNamara, R. K., Able, J. A., Rider, T., Tso, P., and Jandacek, R. (2010). Effect of chronic fluoxetine treatment on male and female rat erythrocyte and prefrontal cortex fatty acid composition. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 1317–1321. doi: 10.1016/j.pnpbp.2010.07.016
Mouri, A., Sasaki, A., Watanabe, K., Sogawa, C., Kitayama, S., Mamiya, T., et al. (2012). MAGE-D1 regulates expression of depression-like behavior through serotonin transporter ubiquitylation. J. Neurosci. 32, 4562–4580. doi: 10.1523/Jneurosci.6458-11.2012
Muneoka, K., Shirayama, Y., Takigawa, M., and Shioda, S. (2009). Brain region-specific effects of short-term treatment with duloxetine, venlafaxine, milnacipran and sertraline on monoamine metabolism in rats. Neurochem. Res. 34, 542–555. doi: 10.1007/s11064-008-9818-2
Mwinyi, J., Cavaco, I., Pedersen, R. S., Persson, A., Burkhardt, S., Mkrtchian, S., et al. (2010). Regulation of CYP2C19 expression by estrogen receptor α: implications for estrogen-dependent inhibition of drug metabolism. Mol. Pharmacol. 78, 886–894. doi: 10.1124/mol.110.065540
Osterlund, M. K., and Hurd, Y. L. (1998). Acute 17 beta-estradiol treatment down-regulates serotonin 5HT1A receptor mRNA expression in the limbic system of female rats. Brain Res. Mol. Brain Res. 55, 169–172. doi: 10.1016/s0169-328x(98)00018-7
Pan, X., and Jeong, H. (2015). Estrogen-induced cholestasis leads to repressed CYP2D6 expression in CYP2D6-humanized mice. Mol. Pharmacol. 88, 106–112. doi: 10.1124/mol.115.098822
Pandaranandaka, J., Poonyachoti, S., and Kalandakanond-Thongsong, S. (2006). Anxiolytic property of estrogen related to the changes of the monoamine levels in various brain regions of ovariectomized rats. Physiol. Behav. 87, 828–835. doi: 10.1016/j.physbeh.2006.02.002
Pandaranandaka, J., Poonyachoti, S., and Kalandakanond-Thongsong, S. (2009). Differential effects of exogenous and endogenous estrogen on anxiety as measured by elevated T-maze in relation to the serotonergic system. Behav. Brain Res. 198, 142–148. doi: 10.1016/j.bbr.2008.10.043
Park, H., Yoo, D., Kwon, S., Yoo, T. W., Park, H. J., Hahm, D. H., et al. (2012). Acupuncture stimulation at HT7 alleviates depression-induced behavioral changes via regulation of the serotonin system in the prefrontal cortex of maternally-separated rat pups. J. Physiol. Sci. 62, 351–357. doi: 10.1007/s12576-012-0211-1
Parker, G., Parker, K., Austin, M. P., Mitchell, P., and Brotchie, H. (2003). Gender differences in response to differing antidepressant drug classes: two negative studies. Psychol. Med. 33, 1473–1477. doi: 10.1017/S0033291703007918
Pearson, C., Janz, T., and Ali, J. (2013). Mental and Substance Use Disorders in Canada. Available at: http://www150.statcan.gc.ca/n1/pub/82-624-x/2013001/article/11855-eng.htm
Pinto-Meza, A., Usall, J., Serrano-Blanco, A., Suarez, D., and Haro, J. M. (2006). Gender differences in response to antidepressant treatment prescribed in primary care. Does menopause make a difference? J. Affect. Disord. 93, 53–60. doi: 10.1016/j.jad.2006.02.010
Pope, HG Jr, Amiaz, R., Brennan, B. P., Orr, G., Weiser, M., Kelly, J. F., et al. (2010). Parallel-group placebo-controlled trial of testosterone gel in men with major depressive disorder displaying an incomplete response to standard antidepressant treatment. J. Clin. Psychopharmacol. 30, 126–134. doi: 10.1097/JCP.0b013e3181d207ca
Pozzi, S., Benedusi, V., Maggi, A., and Vegeto, E. (2006). Estrogen action in neuroprotection and brain inflammation. Ann. N. Y. Acad. Sci. 1089, 302–323. doi: 10.1196/annals.1386.035
Prange-Kiel, J., and Rune, G. M. (2006). Direct and indirect effects of estrogen on rat hippocampus. Neuroscience 138, 765–772. doi: 10.1016/j.neuroscience.2005.05.061
Probst-Schendzielorz, K., Viviani, R., and Stingl, J. C. (2015). Effect of Cytochrome P450 polymorphism on the action and metabolism of selective serotonin reuptake inhibitors. Expert Opin. Drug Metab.Toxicol. 11, 1219–1232. doi: 10.1517/17425255.2015.1052791
Santos, I. M., Freitas, R. L., Saldanha, G. B., Tomé, A. R., Jordán, J., and Freitas, R. M. (2010). Alterations on monoamines concentration in rat hippocampus produced by lipoic acid. Arq. Neuropsiquiatr. 68, 362–366. doi: 10.1590/s0004-282x2010000300006
Saravi, S. S. S., Arefidoust, A., Yaftian, R., Saravi, S. S. S., and Dehpour, A. R. (2016). 17 alpha-ethinyl estradiol attenuates depressive-like behavior through GABA(A) receptor activation/nitrergic pathway blockade in ovariectomized mice. Psychopharmacology 233, 1467–1485. doi: 10.1007/s00213-016-4242-9
Seidman, S. N., Miyazaki, M., and Roose, S. P. (2005). Intramuscular testosterone supplementation to selective serotonin reuptake inhibitor in treatment-resistant depressed men: randomized placebo-controlled clinical trial. J. Clin. Psychopharmacol. 25, 584–588. doi: 10.1097/01.jcp.0000185424.23515.e5
Seidman, S. N., and Rabkin, J. G. (1998). Testosterone replacement therapy for hypogonadal men with SSRI-refractory depression. J. Affect. Disord. 48, 157–161. doi: 10.1016/s0165-0327(97)00168-7
Shah, A., and Frazer, A. (2014). Influence of acute or chronic administration of ovarian hormones on the effects of desipramine in the forced swim test in female rats. Psychopharmacology 231, 3685–3694. doi: 10.1007/s00213-014-3510-9
Soares, C. N. (2014). Mood disorders in midlife women: understanding the critical window and its clinical implications. Menopause 21, 198–206. doi: 10.1097/GME.0000000000000193
Sumner, B. E. H., Grant, K. E., Rosie, R., Hegele-Hartung, C., Fritzemeier, K. H., and Fink, G. (1999). Effects of tamoxifen on serotonin transporter and 5-hydroxytryptamine 2A receptor binding sites and mRNA levels in the brain of ovariectomized rats with or without acute estradiol replacement. Brain Res. Mol. Brain Res. 73, 119–128. doi: 10.1016/s0169-328x(99)00243-0
Toffol, E., Heikinheimo, O., and Partonen, T. (2015). Hormone therapy and mood in perimenopausal and postmenopausal women: a narrative review. Menopause 22, 564–578. doi: 10.1097/gme.0000000000000323
Walf, A. A., and Frye, C. A. (2010a). Estradiol reduces anxiety- and depression-like behavior of aged female mice. Physiol. Behav. 99, 169–174. doi: 10.1016/j.physbeh.2009.09.017
Walf, A. A., and Frye, C. A. (2010b). Raloxifene and/or estradiol decrease anxiety-like and depressive-like behavior, whereas only estradiol increases carcinogen-induced tumorigenesis and uterine proliferation among ovariectomized rats. Behav. Pharmacol. 21, 231–240. doi: 10.1097/FBP.0b013e32833a5cb0
Xu, Y., Sheng, H., Tang, Z., Lu, J., and Ni, X. (2015). Inflammation and increased IDO in hippocampus contribute to depression-like behavior induced by estrogen deficiency. Behav. Brain Res. 288, 71–78. doi: 10.1016/j.bbr.2015.04.017
Ye, Y., Liu, C., Liu, X., and Huang, S. (2016). Ovariectomy changes the response to antidepressant drugs in tail suspension test in mice. Gynecol. Endocrinol. 32, 986–990. doi: 10.1080/09513590.2016.1197197
Yu, Q., Yin, C. X., Hui, Y., Yu, J., He, F. F., Wei, J., et al. (2004). Comparison of the effect of fluoxetine combined with hormone replacement therapy (HRT) and single HRT in treating menopausal depression. Zhonghua Fu Chan Ke Za Zhi 39, 461–464.
Yue, X., Lu, M., Lancaster, T., Cao, P., Honda, S., Staufenbiel, M., et al. (2005). Brain estrogen deficiency accelerates abeta plaque formation in an alzheimer’s disease animal model. Proc. Natl. Acad. Sci. U.S.A. 102, 19198–19203. doi: 10.1073/pnas.0505203102
Zocchi, A., Varnier, G., Arban, R., Griffante, C., Zanetti, L., Bettelini, L., et al. (2003). Effects of antidepressant drugs and GR 205171, an neurokinin-1 (NK1) receptor antagonist, on the response in the forced swim test and on monoamine extracellular levels in the frontal cortex of the mouse. Neurosci. Lett. 345, 73–76. doi: 10.1016/S0304-3940(03)00305-7
Keywords: sex difference, sertraline, 5-HT, DA, estrogen
Citation: Ma L, Xu Y, Jiang W, Li Y, Zhang X, Wang G and Li R (2019) Sex Differences in Antidepressant Effect of Sertraline in Transgenic Mouse Models. Front. Cell. Neurosci. 13:24. doi: 10.3389/fncel.2019.00024
Received: 23 September 2018; Accepted: 18 January 2019;
Published: 01 February 2019.
Edited by:
Tommaso Pizzorusso, Italian National Research Council (CNR), ItalyReviewed by:
Elisabetta Ciani, University of Bologna, ItalyGrzegorz Kreiner, Polish Academy of Sciences, Poland
Copyright © 2019 Ma, Xu, Jiang, Li, Zhang, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Wang, Z2FuZ3dhbmdkb2NAZ21haWwuY29t Rena Li, cmxpQHJmZG4ub3Jn
 Lei Ma
Lei Ma Yong Xu
Yong Xu Wei Jiang4
Wei Jiang4 Rena Li
Rena Li