
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Behav. Neurosci., 15 January 2019
Sec. Learning and Memory
Volume 13 - 2019 | https://doi.org/10.3389/fnbeh.2019.00001
This article is part of the Research Topic10 Years of Impactful, Open NeuroscienceView all 47 articles
 Daisuke Ishii1,2*
Daisuke Ishii1,2* Kotaro Takeda3*
Kotaro Takeda3* Satoshi Yamamoto4
Satoshi Yamamoto4 Akira Noguchi5
Akira Noguchi5 Kiyoshige Ishibashi6
Kiyoshige Ishibashi6 Kenya Tanamachi6
Kenya Tanamachi6 Arito Yozu1
Arito Yozu1 Yutaka Kohno1
Yutaka Kohno1The integration of multiple sensory modalities allows us to adapt to the environment of the outside world. It is widely known that visual stimuli interfere with the processing of auditory information, which is involved in the ability to pay attention. Additionally, visuospatial attention has the characteristic of laterality. It is unclear whether this laterality of visuospatial attention affects the processing of auditory stimuli. The sensorimotor gating system is a neurological process, which filters out unnecessary stimuli from environmental stimuli in the brain. Prepulse inhibition (PPI) is an operational measure of the sensorimotor gating system, which a weaker prestimulus (prepulse), such as a visual stimulus, inhibits the startle reflex elicited by a subsequent robust startling stimulus (pulse) such as a tone. Therefore, we investigated whether the visual stimulus from the left or right visual space affects the sensorimotor gating system in a “rest” task (low attentional condition) and a “selective attention” task (high attentional condition). In the selective attention task, we found that the target prepulse presented in the left and bilateral visual fields suppressed the startle reflex more than that presented in the right visual field. By contrast, there was no laterality of PPI in the no-target prepulse condition, and there was no laterality of PPI in the rest task. These results suggest that the laterality of visuospatial attention affects the sensorimotor gating system depending on the attentional condition. Moreover, the process of visual information processing may differ between the left and right brain.
We recognize the environment of the outside world through multiple sensory modalities, such as the visual and auditory modalities. Adaptation to the environment is achieved by integrating these multiple sensory modalities. It has been shown that the processing of visual and auditory information interfere with each other, which is involved in the ability to pay attention (Richard et al., 1988; Pomper and Chait, 2017).
The right hemisphere plays an important role in visuospatial and auditory attention (Kinsbourne, 1977; Corbetta et al., 1993; Thiebaut de Schotten et al., 2011; Duecker and Sack, 2015). In the visuospatial attention, the hemispatial neglect is most common in damage to the right cerebral hemisphere, which causes visual neglect of the left visual space (Stone et al., 1992). These studies have indicated that the visuospatial attention exhibits laterality.
The sensorimotor gating system is a neurological process that filters out unnecessary stimuli from environmental stimuli in the brain. Prepulse inhibition (PPI) of the startle reflex is an operational measure of the sensorimotor gating system. PPI reduces the amplitude of the startle reflex that occurs when a prepulse (visual, auditory, or tactile stimulus) is presented prior to the startling stimulus (Graham, 1975; Blumenthal and Gescheider, 1987; Luthy et al., 2003). The attention to a prepulse or negative emotional experience has been shown to enhance PPI (Luthy et al., 2003; Ishii et al., 2010), suggesting that the sensorimotor gating system changes with a variety of internal and external conditions. It is unclear whether this laterality of the visuospatial attention affects the sensorimotor gating system.
Given this background, we investigated whether visual stimuli from the left or right visual space affect the sensorimotor gating system using two tasks, a “rest” task (low attentional condition) and a “selective attention” task (high attentional condition). In the future, it will be important to investigate the effects of visuospatial-attention laterality on the processing of other sensory modalities, in order to elucidate the pathological mechanisms of attention-related illnesses such as hemispatial neglect or attention disorders, which make it difficult to properly process information from the environment.
Ten healthy right-handed male subjects (mean age: 28.9 years, range: 20–40 years) participated in this study after giving informed consent. None of the participants had a history of neurological or psychiatric disease or any condition associated with auditory and visual system abnormalities, as determined by a non-structured interview. All subjects were able to understand the instructions and gave written informed consent. Subjects were excluded if they did not satisfy the following criterion: the average integrated electromyogram (iEMG; startle reflex) within 40–140 ms from the onset of startle stimuli in each prepulse-pulse condition was less than the mean + 3 standard deviations of the baseline (iEMG for 100 ms just before the onset). The study was performed in accordance with the Declaration of Helsinki. Approval of the protocol was obtained from the ethics committee of the Ibaraki Prefectural University of Health Sciences (approval number: 800).
All participants underwent two experimental tasks (rest task and selective attention task) in random order; these were counterbalanced across subjects on two separate days with an interval of at least three days between the tasks. Subjects were asked about their smoking on the experimental day as well as their sleeping hours the night before the experiment. Moreover, before the session, alertness was assessed using the Stanford Sleepiness Scale (Hoddes et al., 1973; Rumpf et al., 2017).
Acoustic stimuli were administered binaurally through headphones (ATH-PRO5MK3; Audio-Technica, Tokyo, Japan). We used two acoustic stimuli: a tone burst stimulus (white noise, 110 dB SPL; duration = 40 ms; rise/fall time = 5 ms) as a pulse to elicit a startle reflex and continuous background white noise (68 dB). Visual stimuli were presented on an LCD monitor with a 60 Hz refresh rate (XU2390HS; MouseComputer, Tokyo, Japan) at a viewing distance of 100 cm. All stimuli appeared white against a uniformly black background. We used six visual stimuli as prepulses (Figure 1A). All prepulses appeared at symmetrical locations in the left and right visual fields at the same height as the fixation cross. All stimuli were randomly presented across the experiments using PsychToolBox for Matlab R2017b (MathWorks, Natick, MA, United States).
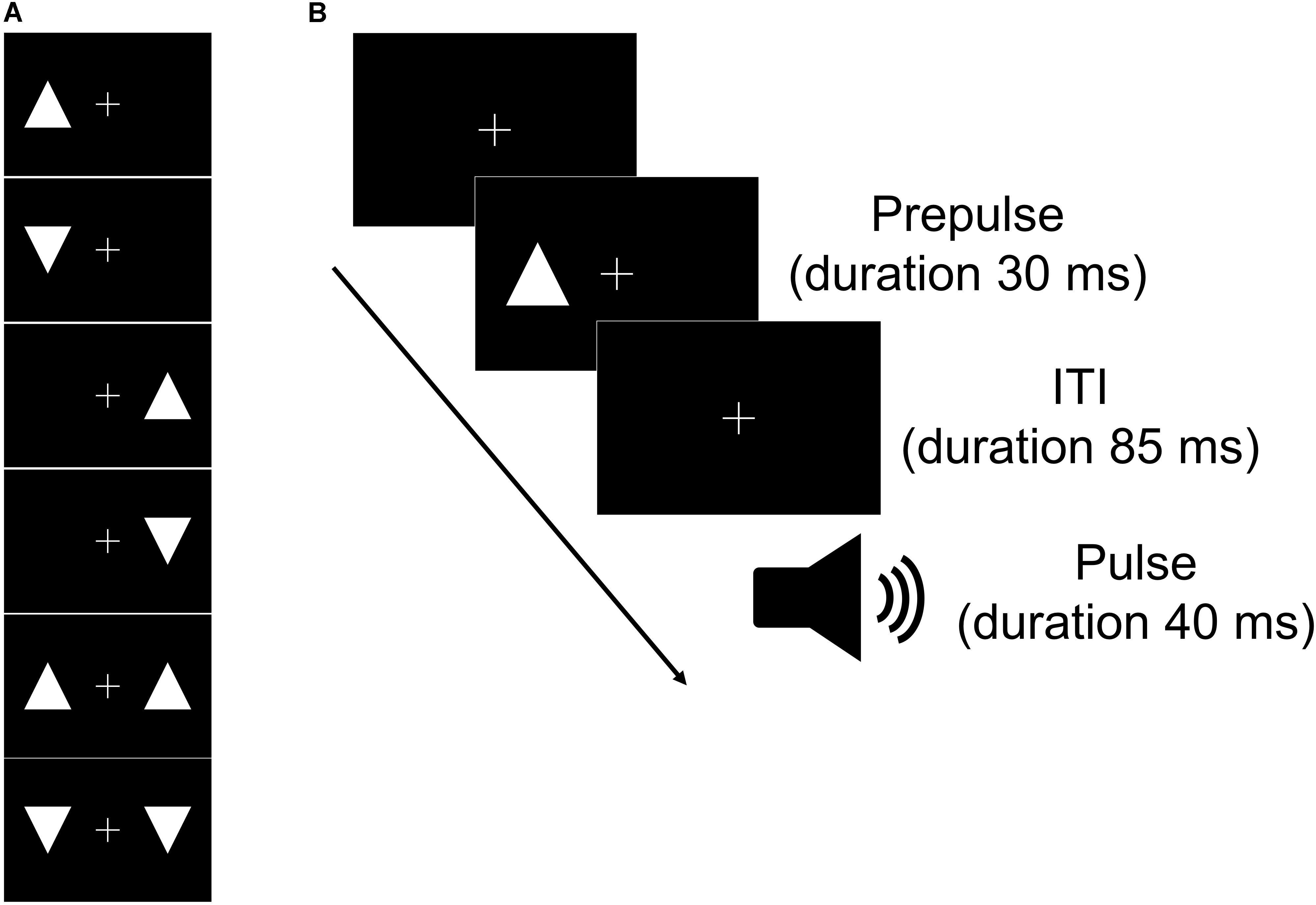
Figure 1. Schematic of the types of prepulse (A) and the time course of one trial of the task (B). (A) Six visual stimuli were used in the experiment as prepulses. Upward and/or downward triangles were presented on the left and/or right across a fixation cross for the visual stimuli. (B) The prepulse (visual stimulus) could appear following the fixation cross. The pulse (tone) was presented at 115 ms after the prepulse presentation. ITI, interstimulus interval.
The rest task began with a 2-min acclimation period consisting of 68 dB continuous white noise via headphones. After the acclimation period, the subjects received one tone burst stimulus. The initial tone burst stimulus was followed by 7 trials in six blocks (a total of 42 trials). Each block had a pulse alone trial and six visual prepulse-pulse trials in which a prepulse preceded the pulse at 85-ms intervals ordered pseudo-randomly. The sequence of events for prepulse-pulse trials is shown in Figure 1B. After the 43 trials, the subjects received one tone burst stimulus. During the task, the subjects were instructed to relax and maintain their gaze on the central cross. The session lasted approximately 12 min (interstimulus interval = 9–22 s).
In the selective attention task, the apparatus and stimuli were identical to those of the rest task. The subjects were instructed to silently count the number of occurrences of a target prepulse (white upward triangle or white downward triangle) while maintaining their gaze on the central cross. The target prepulse was randomly selected for each participant.
Disposable gelled EMG electrodes (Mets, Tokyo, Japan) were placed on the left orbicularis oculi muscle. The ground electrode was attached to the forehead. EMG data were collected at a sampling frequency of 10 kHz with custom-made LabView software (National Instruments Japan, Tokyo, Japan) from –100 to 200 ms of the onset of the pulse stimulus. A bandpass filter was set at 28 Hz to 500 kHz. A notch filter was also applied to eliminate the 50 Hz line noise. The EMG signals were rectified, integrated with a time constant of 10 ms (iEMG), and then averaged for each condition. The first eight and the last trials were excluded from the average. When the excessive activity due to eye-blinking overlapped with baseline period or startle reflex phase, these trials were also excluded from the average. The peak in the averaged iEMG within 40–140 ms from the onset of the startle stimuli was determined as the maximal amplitude for the startle reflex. All the signal processing was performed using MATLAB R2017b.
%PPI was calculated as {[(Peak of the averaged iEMG in pulse-alone trial) – (Peak of the averaged iEMG in prepulse-pulse trial)]/Peak of the averaged iEMG in pulse-alone trial}× 100. We performed a one-way repeated-measures analysis of variance (ANOVA) on the %PPI to compare among the prepulse conditions for each task. Bonferroni’s correction was used for post hoc comparisons when ANOVA revealed statistically significant differences. The level of statistical significance was set at p < 0.05. All the analyses were performed using SPSS Statistics 23.0 for Mac (IBM, Armonk, NY, United States).
In the rest task, the average total sleeping time on the day before the experiment was 6.55 h (SD 0.96). The median Stanford sleepiness scale was 2 (interquartile range, 1.8 to 3.0). In the selective attention task, the sleeping time was 6.25 h (1.32). The median Stanford sleepiness scale was 2 (1.0 to 3.0). There was no significant difference of the sleeping time between the experimental tasks (paired t-test). Three subjects smoked on each experimental day.
One-way repeated measures ANOVA revealed a significant main effect of the prepulse conditions (F(2, 18) = 4.377, p < 0.05, η2p= 0.33) (Figure 2A). By contrast, a post hoc comparison indicated that there was no difference in %PPI in the rest task among the different prepulse conditions (Bonferroni corrected p > 0.05; left vs. right, d = 0.37; left vs. bilateral, d = 0.66; right vs. bilateral, d = 0.36).
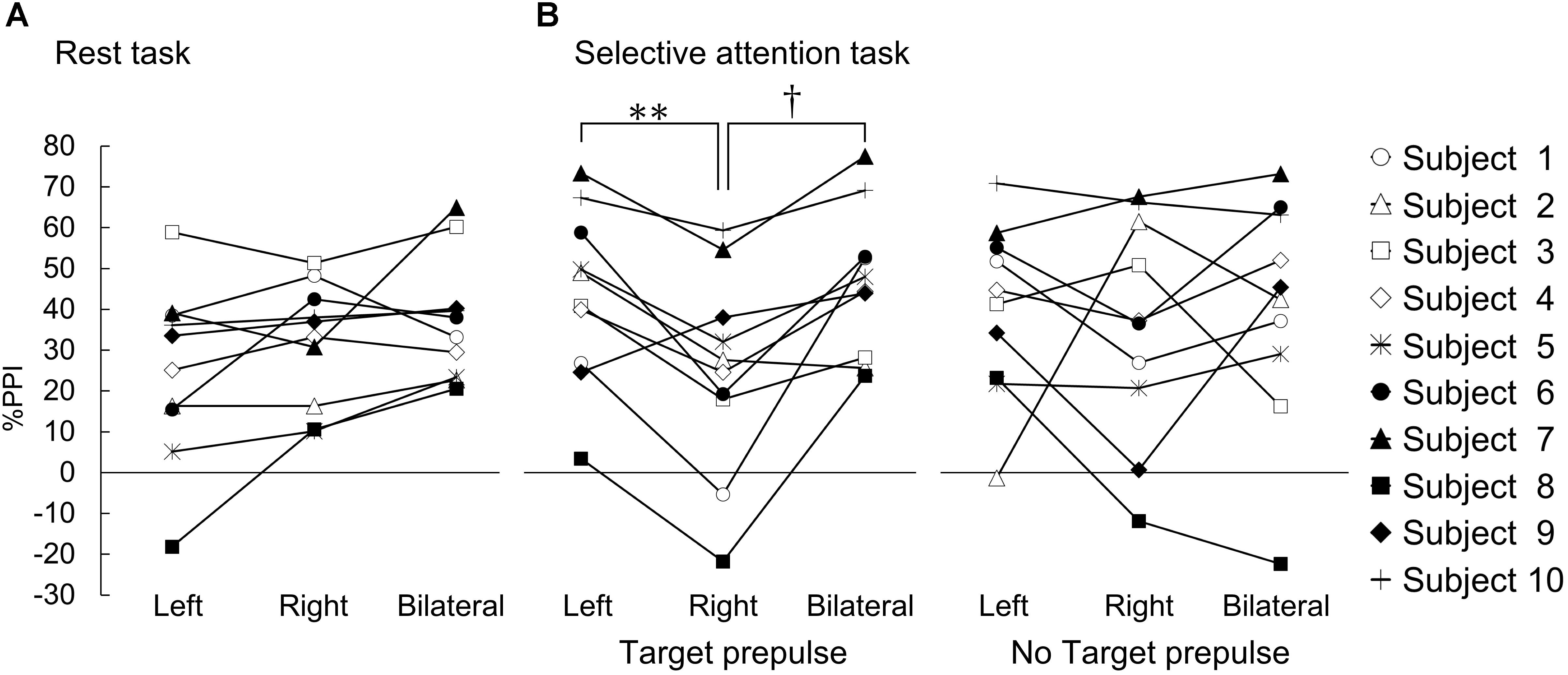
Figure 2. %PPI in the rest task and the selective attention task. The results for each individual subject are shown. %PPI in the rest task (A) and the selective attention task (B). (A) %PPI in each prepulse (left, prepulse presented on the left visual field; right, prepulse presented on the right visual field; bilateral, prepulse presented on both visual fields). (B) %PPI under the target prepulse (left panel) and no-target prepulse (right panel) conditions. ∗∗, †Bonferroni-corrected p < 0.01 and p < 0.05, respectively.
In the responses to the target prepulse, one-way ANOVA revealed a significant main effect of the prepulse conditions (F(2, 18) = 10.645, p < 0.01, η2p = 0.54) (Figure 2B). A post hoc comparison indicated that the %PPI at the target prepulse presented in the right visual space was significantly lower than the %PPIs for the target prepulses presented in the left and bilateral visual spaces (right vs. left Bonferroni corrected p < 0.01, d = 0.82; right vs. bilateral, Bonferroni corrected p < 0.05, d = 1.02). On the other hand, there was no difference between the left and bilateral visual spaces (left vs. bilateral, d = 0.16).
In the responses to the “no-target” prepulse, the one-way ANOVA showed no significant main effect of the prepulse conditions (F(2, 18) = 0.204, η2p = 0.02) (Figure 2B).
The average correct answer rate of the selective attention task was 91.7% (SD 10.2; range 72.2–100%).
This study revealed that the lower %PPI could be shown only in the prepulse presented in the right visual field in the selective attention task. This result suggests that visual stimuli from the left and right visual fields have different effects on the sensorimotor gating system under the high attentional condition. Additionally, there was no laterality of the interference effect of the prepulse on the sensorimotor gating system when attention was not directed to the prepulse or the subjects were asked to ignore the prepulse.
It is known that paying attention to a prepulse enhances PPI (Luthy et al., 2003). Additionally, healthy humans show a leftward bias in visuospatial tasks (Jewell and McCourt, 2000). For the visuospatial attention, the right hemisphere plays an important role, and the attention exhibits right hemisphere dominance (Kinsbourne, 1977; Corbetta et al., 1993; Thiebaut de Schotten et al., 2011). The current study showed that there was no laterality of PPI under the lower attentional conditions. Our study and previous studies suggest that the laterality of the visuospatial attention is not always present.
In the pointing reaction to the visual target, the reaction time for the left hand has been reported to be shorter than that for the right hand, suggesting that the processing of visual information related to the spatial parameterization of the movement is faster in the right hemisphere than in the left hemisphere (Carson et al., 1990). In our study, the higher %PPI could be seen in the prepulse presented in the left visual field in the selective attention task. Our results and this previous study suggest that the processing of visual information in the right brain may be faster than that in the left brain under the high attentional condition and have a great effect on subsequent reaction.
The transcallosal fibers that connect the two hemispheres of the cerebral cortex mediate interhemispheric inhibition (Asanuma and Okuda, 1962; Ferbert et al., 1992). The right human posterior parietal cortex associated with the visuospatial attention exerts strong inhibitory activity over the contralateral homologous area (Heilman and Valenstein, 1972; Hilgetag et al., 2001; Cazzoli et al., 2009; Koch et al., 2011). We speculate that the right hemisphere, with increased excitability, under the high attentional condition may inhibit the activity of the left hemisphere through the transcallosal fibers, which may induce laterality of the visuospatial attention.
A previous study revealed that PPI can be enhanced by smoking (Kumari et al., 1996). This effect is canceled by short-term abstinence from smoking (Della Casa et al., 1998). In our study, smokers had abstained from smoking for 3 h before the experiments. Additionally, the data of smokers were not obviously different from that of non-smokers in the experiments. There was also no significant difference in the sleeping status between the experiments. These results suggested that the laterality of the PPI was not due to a change in smoking status or vigilance.
We showed that the interference effects of visual stimuli on the auditory gating system changed dynamically depending on the attentional conditions. Our study might shed light on the pathology of attention-related illnesses such as hemispatial neglect or attention disorders.
DI, KoT, SY, AN, and YK conceived of the presented idea and designed the experiments. DI, SY, and AN carried out the experiments. DI, KoT, and KeT analyzed the data. DI, KI, AY, and YK interpreted the results. DI and KoT drafted the manuscript.
This research was partly supported by JSPS KAKENHI grant numbers JP 15K16360, 17H05901, and 18K17725.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Asanuma, H., and Okuda, O. (1962). Effects of transcallosal volleys on pyramidal tract cell activity of cat. J. Neurophysiol. 25, 198–208. doi: 10.1152/jn.1962.25.2.198
Blumenthal, T. D., and Gescheider, G. A. (1987). Modification of the acoustic startle reflex by a tactile prepulse: the effects of stimulus onset asynchrony and prepulse intensity. Psychophysiology 24, 320–327. doi: 10.1111/j.1469-8986.1987.tb00302.x
Carson, R. G., Chua, R., Elliott, D., and Goodman, D. (1990). The contribution of vision to asymmetries in manual aiming. Neuropsychologia 28, 1215–1220. doi: 10.1016/0028-3932(90)90056-T
Cazzoli, D., Wurtz, P., Muri, R. M., Hess, C. W., and Nyffeler, T. (2009). Interhemispheric balance of overt attention: a theta burst stimulation study. Eur. J. Neurosci. 29, 1271–1276. doi: 10.1111/j.1460-9568.2009.06665.x
Corbetta, M., Miezin, F. M., Shulman, G. L., and Petersen, S. E. (1993). A PET study of visuospatial attention. J. Neurosci. 13, 1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993
Della Casa, V., Hofer, I., Weiner, I., and Feldon, J. (1998). The effects of smoking on acoustic prepulse inhibition in healthy men and women. Psychopharmacology 137, 362–368. doi: 10.1007/s002130050631
Duecker, F., and Sack, A. T. (2015). The hybrid model of attentional control: new insights into hemispheric asymmetries inferred from TMS research. Neuropsychologia 74, 21–29. doi: 10.1016/j.neuropsychologia.2014.11.023
Ferbert, A., Priori, A., Rothwell, J. C., Day, B. L., Colebatch, J. G., and Marsden, C. D. (1992). Interhemispheric inhibition of the human motor cortex. J. Physiol. 453, 525–546. doi: 10.1113/jphysiol.1992.sp019243
Graham, F. K. (1975). Presidential address, 1974. The more or less startling effects of weak prestimulation. Psychophysiology 12, 238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x
Heilman, K. M., and Valenstein, E. (1972). Frontal lobe neglect in man. Neurology 22, 660–664. doi: 10.1212/WNL.22.6.660
Hilgetag, C. C., Theoret, H., and Pascual-Leone, A. (2001). Enhanced visual spatial attention ipsilateral to rTMS-induced ‘virtual lesions’ of human parietal cortex. Nat. Neurosci. 4, 953–957. doi: 10.1038/nn0901-953
Hoddes, E., Zarcone, V., Smythe, H., Phillips, R., and Dement, W. C. (1973). Quantification of sleepiness: a new approach. Psychophysiology 10, 431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x
Ishii, D., Matsuzawa, D., Fujita, Y., Sutoh, C., Ohtsuka, H., Matsuda, S., et al. (2010). Enhancement of acoustic prepulse inhibition by contextual fear conditioning in mice is maintained even after contextual fear extinction. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 183–188. doi: 10.1016/j.pnpbp.2009.10.023
Jewell, G., and McCourt, M. E. (2000). Pseudoneglect: a review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia 38, 93–110. doi: 10.1016/S0028-3932(99)00045-7
Koch, G., Cercignani, M., Bonni, S., Giacobbe, V., Bucchi, G., Versace, V., et al. (2011). Asymmetry of parietal interhemispheric connections in humans. J. Neurosci. 31, 8967–8975. doi: 10.1523/JNEUROSCI.6567-10.2011
Kumari, V., Checkley, S. A., and Gray, J. A. (1996). Effect of cigarette smoking on prepulse inhibition of the acoustic startle reflex in healthy male smokers. Psychopharmacology 128, 54–60. doi: 10.1007/s002130050109
Luthy, M., Blumenthal, T. D., Langewitz, W., Kiss, A., Keller, U., and Schachinger, H. (2003). Prepulse inhibition of the human startle eye blink response by visual food cues. Appetite 41, 191–195. doi: 10.1016/S0195-6663(03)00080-1
Pomper, U., and Chait, M. (2017). The impact of visual gaze direction on auditory object tracking. Sci. Rep. 7:4640. doi: 10.1038/s41598-017-04475-1
Richard, R., Christian, C., and Michel, F. (1988). Reciprocal effects of visual and auditory stimuli in a spatial compatibility situation. Bull. Psychon. Soc. 26, 350–352. doi: 10.3758/BF03337679
Rumpf, J. J., Wegscheider, M., Hinselmann, K., Fricke, C., King, B. R., Weise, D., et al. (2017). Enhancement of motor consolidation by post-training transcranial direct current stimulation in older people. Neurobiol. Aging 49, 1–8. doi: 10.1016/j.neurobiolaging.2016.09.003
Stone, S. P., Patel, P., Greenwood, R. J., and Halligan, P. W. (1992). Measuring visual neglect in acute stroke and predicting its recovery: the visual neglect recovery index. J. Neurol. Neurosurg. Psychiatry 55, 431–436. doi: 10.1136/jnnp.55.6.431
Keywords: prepulse inhibition, sensorimotor gating, visuospatial attention, laterality, startle reflex, visual prepulse
Citation: Ishii D, Takeda K, Yamamoto S, Noguchi A, Ishibashi K, Tanamachi K, Yozu A and Kohno Y (2019) Effect of Visuospatial Attention on the Sensorimotor Gating System. Front. Behav. Neurosci. 13:1. doi: 10.3389/fnbeh.2019.00001
Received: 03 October 2018; Accepted: 03 January 2019;
Published: 15 January 2019.
Edited by:
Nuno Sousa, University of Minho, PortugalReviewed by:
Roberto Frau, Università di Cagliari, ItalyCopyright © 2019 Ishii, Takeda, Yamamoto, Noguchi, Ishibashi, Tanamachi, Yozu and Kohno. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daisuke Ishii, ZC1pc2hpaUB1bWluLmFjLmpw Kotaro Takeda, a3Rha2VkYUBmdWppdGEtaHUuYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.