- 1CSIR-Centre for Cellular and Molecular Biology, Hyderabad, India
- 2CSIR-Indian Institute of Chemical Technology, Hyderabad, India
The role of Y chromosome in sex determination and male fertility is well established. It is also known that infertile men are prone to psychological disturbances. Earlier studies in the laboratory identified genes expressed in testes that are putatively regulated by Y chromosome in man and mouse. With the availability of a Y-deleted mouse model, that is subfertile, we studied the effect of a partial deletion of Y-chromosomal heterochromatin on mouse behavior when compared to its wild type. The partial Y-deleted mice exhibited anxiety like phenotype under stress when different anxiety (open field test and elevated plus maze, EPM test) and depression related tests (tail suspension and force swim) were performed. The mutant mice also showed reduction in hippocampal neurogenesis and altered expression of neurogenesis markers such as Nestin, Sox2, Gfap, NeuroD1 and Dcx using quantitative real time PCR (qPCR) analysis. The genes with altered expression contained short stretches of homology to Y-derived transcripts only in their Untranslated Regions (UTRs). Our study suggests putative regulation of these genes by the Y chromosome in mouse brain altering stress related behavior.
Introduction
The differences between male and female of any species is well established. They differ with respect to morphology, physiology, behavior and the male/female determining chromosomes (Lovell-Badge, 2005). The male sex hormone androgen can modulate neuronal activity, stem cell self-renewal, differentiation in brain and acts synergistically with genes on Y chromosome to induce aggressive behavior in males. Some Y-chromosomal genes like Sry and a few noncoding RNAs are expressed both in testes and in brain (Mayer et al., 2000; Reisert et al., 2002). Such Y-chromosomal genes could affect male behavior. Y-linked genes have been shown to affect rodent brain development and behavior (De Vries et al., 2002; Arnold and Burgoyne, 2004). There are conflicting reports on the effect of Y chromosome on aggressive behavior in mice (Roubertoux et al., 1994; Guillot and Chapouthier, 1998; Sluyter et al., 1999). Roubertoux et al. (1994) observed that pseudoautosomal region of the Y and autosomes had additive effects on the initiation of attack behavior. A number of reproductive behaviors are affected by the male specific region in the Y chromosome (Guillot and Chapouthier, 1998). There are contradictory reports of aggressive behavior in XYY men (Lovell-Badge, 2005). Several studies show that overexpression of Y chromosome leads to the development of aggressive phenotype (Miczek et al., 2001). Individuals with 47, XYY are reported to be at elevated risk for developing anti-social aggressive behavior (Stochholm et al., 2012). A case study on a boy with Attention deficit/hyperactivity disorder (ADHD) showed that a rare deletion of Yq with duplication of Yp made him susceptible to develop ADHD (Liu et al., 2017). De Vries et al. (2002) are of the opinion that much more research is required for strong conclusions and care must be taken to reduce the effects of genetic background. This motivated us to explore whether partial deletion of Y chromosome impacts mouse behavior and susceptibility to neuropsychiatric disorders, using a mutant strain mouse with partial deletion of Y-long arm (XYRIIIqdel) and its wild type counterpart RIII (XYRIII). In general, we noticed that the XYRIIIqdel mice are hyperactive compared to the wild type, XYRIII animals. Therefore, in the present study Y-deleted (XYRIIIqdel) male mice were evaluated for their basic mood status, degree of stress responsiveness and also to uncover if it is linked with the hippocampal neurogenesis, which gets affected in rodent models of chronic stress-induced mood disorders (Gould and Tanapat, 1999; Gould and Gross, 2002; Yun et al., 2016).
XYRIIIqdel mice exhibit severe morphological and motility-related abnormalities of spermatozoa, reduced sperm count and subfertility compared to its wild type, XYRIII (Conway et al., 1994; Touré et al., 2004; Burgoyne and Mitchell, 2007). Yet another strain of Y-deleted mutant strain B10.BR-Y-del shows impaired spermatogenesis and subfertility compared to its wild type, B10.BR/SgSn (Styrna et al., 1991, 2002). Men with microdeletions in the euchromatic long arm of human Y chromosome suffer from varying degrees of infertility (Najmabadi et al., 1996; Vogt et al., 1996). Partial deletion of the Y chromosome also affects reproductive efficiency of females sired by the Y-deleted male (Kotarska and Styrna, 2012; Kotarska et al., 2013, 2015). Over all, most of the reports dealt with and were rather restricted to reproduction related anomalies only, which eventually concluded that deletion of Y chromosome has strong connections with sub-fertility.
Psychological disturbances like anxiety and depression have been reported in infertile men (Sahin et al., 2017; Yang et al., 2017). Sustained grief could lead to major depression as per definition consistent with Diagnostic and Statistical Manual of Mental Disorders, 4th edn, DSM-IV (Ln, 2006). Additionally, if we look through some interesting similarities between the brain and the testes, for instance, both have barriers (the blood-brain barrier and the blood-testes barrier) that restrict the entry of large molecules from the bloodstream, and both organs are very high in cholesterol. In fact, an in-silico analysis indicates that human testis and brain share the highest similarity of gene expression patterns (Guo et al., 2003). However, there is not much information on the regulation by Y-derived transcripts of neural genes involved in behavior, similar to the recently reported putative regulation of testicular autosomal genes by Y chromosomes in mouse, human and Drosophila (Vigneault and Zouros, 1986; Jehan et al., 2007; Bhattacharya et al., 2013). In the light of all this information it was legitimate to hypothesize that XYRIIIqdel mice may be more prone to show stress vulnerability. Using XYRIIIqdel mice, our study tried to address this aspect too, and we report an interesting finding here. The genes that have altered expression in hippocampal neurogenesis in XYRIIIqdel mice contain short stretches of homology to Y-derived transcripts in their Untranslated Regions (UTRs). This might have implication for anxiety and related neuropsychiatric disorders.
Materials and Methods
Animals
Adult male mice, wild type XYRIII and XYRIIIqdel mice around 10 weeks old, were used as required in various behavioral and molecular experiments. Animals were obtained originally from Prof. Paul S. Burgoyne, MRC Laboratory, UK and later bred and maintained in Animal House facility of Centre for Cellular and Molecular Biology, Hyderabad, India. The animal room was maintained at 25°C with a 12 h:12 h light-dark cycle (lights off between 18.0 h to 6.0 h). Food and water were available to the animals, ad libitum. All animal procedures were carried out in accordance with the approved guidelines of the Institutional Animal Ethics Committee of the Centre for Cellular and Molecular Biology, Hyderabad, India.
Behavioral Procedure
The mice were subjected to behavior tests that measure anxiety in animals through open field and elevated plus maze (EPM) tests. For all the tests, in-between trials with each mouse, the area was cleaned with 70% alcohol, dried for the next one. A flowchart showing the scheme of experiments has been provided as Supplementary Figure S1.
Open Field Test
This test was performed according to the previous report in rats (Reddy et al., 2016) with appropriate modifications for mouse. Briefly, each mouse was introduced to the corner of an open field arena (40 × 40 × 40 cm) for this test and was allowed to explore the arena for 5 min. The open-field box floor was virtually divided into 16 equal squares where the central four squares were considered the center zone whereas the twelve peripheral squares were designated as peripheral zone. The amount of time spent in peripheral zone vs. the amount of time spent in the central zone was measured by using video tracking software (Ethovision 3.1 software, Noldus, Netherlands).
Elevated Plus-Maze Test
This test, to evaluate anxiety status of the animal, was performed with the help of an EPM as reported earlier (LaPlant et al., 2009; Pathak et al., 2017). In detail, EPM consisted of a plus-shaped wooden apparatus elevated at 100 cm above the ground, with two open (33 cm long × 5 cm wide) and two closed arms (with 25 cm tall walls on the sides) and a central region at their intersection. Experimental mice were individually placed in the central region of the EPM and allowed to explore for 5 min, where time spent by mice in each arm was measured using Ethovision 3.1 (Noldus, Netherlands).
Forced Swim Test
The forced swim test was performed according to previously published protocols (LaPlant et al., 2009; Pathak et al., 2017). Briefly, mice were tested in a 10-L Pyrex glass beaker, filled up to approximately 23 cm with normal water having a temperature of 25 ± 2°C, for 5 min. The entire swimming test session was recorded with a video camera and then was scored manually for the time spent immobile. Total immobility was measured as the time spent without noticeable movement, except for single limb paddling to maintain flotation.
Tail Suspension Test
The test of tail suspension is also a classic paradigm for assessing depression-like phenotype in mice. It is considered as a “dry” version of usual forced swim test and often used as alternative (Porsolt et al., 2001). Here, mice were individually suspended by means of their tails from a height of around 40 cm from ground for a period of 5 min. Depression was assessed as the measure of total time of immobilization by not trying climbing/escaping from the hanging situation.
Experimental Stress Paradigm
After conducting all the tests, selected for anxiety and depression in the behavioral battery at basic level, to know how these mice would perform under stress the mice of both groups were subjected to forced swim stress for 10 min for two consecutive days. Twenty-four hours after the last stress episode, we performed again open field test, EPM (for anxiety) and tail suspension test (for depression) to evaluate the degree of response of XYRIIIqdel mice as compared to wild type (XYRIII) mice, under stress.
Before starting first stress episode we have waited for 2–3 weeks to completely diminish the effect of forced swim test as stressor because to check the basal response level of despair behavior we have used the same procedure as one of the behavioral tests. With the same reason keeping in mind that stressor cannot be included as behavioral despair test also, while performing the tests after stress episodes we kept only “tail suspension test” as despair test which is known to be a dry version of behavior despair model (Porsolt et al., 2001; Supplementary Figure S1).
Brain Tissue Collections
Animals were rapidly decapitated; brains were removed and placed on a sterile platform on ice. Hippocampus (bilateral) was micro-dissected with the help of fine-tipped dissection tools and quickly frozen in liquid nitrogen and stored at −80°C until RNA was extracted.
BrdU Treatment, Immunostaining and Counting
A separate group of animals (XYRIII and XYRIIIqdel, 4–5 in each group), were injected with BrdU (50 mg/Kg, intraperitoneally), a thymine analog that gets incorporated in DNA of proliferating cells, twice daily starting 2 days prior to the sacrifice. The mice were intra-cardially perfused with paraformaldehyde and brains were collected and processed for immunostaining as reported earlier (Suri et al., 2013; Chakravarty et al., 2015; Joshi et al., 2017) with minor modifications. In brief, Vectastain ABC kit (Vector Labs) was used and sections were developed with diaminobenzidine (DAB) before mounting in DPX to count BrdU positive cells observed in sub granular zone of hippocampus. Total six sections were used per mouse. Regarding region, approximately following coordinates: anterioposterior = −2 mm from Bregma; lateral = ± 1.6 mm; ventral = 2.5 mm (Ikrar et al., 2013), were used.
Quantitative mRNA Expression of Neurogenesis Markers Using Real-Time PCR
For molecular study, hippocampal region from XYRIII and XYRIIIqdel brain tissues were excised out and stored in RNA later (Ambion), until used. The total RNA from each tissue was extracted using Trizol method (Ambion). The purity and quantity of the extracted RNA was checked using Nanodrop (NANODROP 2000, Thermo Scientific) and Qubit (Thermo Scientific). One microgram of isolated RNA was reverse transcribed to cDNA using Verso cDNA synthesis kit (Thermo Scientific). Quantitative Real time PCR (qPCR) was performed using SYBR green master mix (Roche) and analyzed in Roche Light Cycler LC480. The primer sequences corresponding to the neurogenesis markers used are listed in Table 1. The thermal cycling conditions included an initial denaturation for 5 min at 95°C followed by 45 cycles of denaturation at 95°C for 10 s annealing at 58°C for 20 s and elongation at 72°C for 30 s. The amplification of specific product was confirmed by melting curve profile (cooling the sample to 65°C for 1 min and heating slowly with an increase in temperature of 5°C at each step till 95°C, with continuous measurement of fluorescence). The relative fold change in expression was estimated based on Livak method (2−ΔΔCt).
Statistical Analysis
Mean differences between groups were determined by either a two-tailed unpaired Student’s t-test with confidence intervals of 95% or two-way analysis of variance (ANOVA) followed by Bonferroni post hoc analysis with confidence intervals of 95%. Data were tested for outliers by means of the Grubbs test before performing ANOVA. This test is based on the difference between the average in a group and the most extreme data point in the group which differs significantly, considering the standard deviation (Grubbs, 1969). For individual variation in each group scatter bar plots were also made and displayed in appropriate places in the figures. Statistical analysis was performed using the software GraphPad Prism 6.0 (San Diego, CA, USA). Results are depicted as means with standard errors (mean ± SEM). Statistical significance was set at P < 0.05 “*”, P < 0.01 “**” and P < 0.001 “***” represents significant difference from the respective controls. “#” represents showing a trend but not significant P < 0.09.
Results
XYRIIIqdel Mice Apparently Did Not Show Any Difference in Mood Status Except Slight Hyperactivity
To check if there is any basic difference in general mood status of XYRIIIqdel mice from the XYRIII, we performed few specific anxiety (open field test and EPM test) and depression (Tail suspension and forced swim) tests. We did not find any significant change in anxiety level as animals showed no difference in spending time at center in open field test and in open arm of (Figures 1A,C) EPM (Figures 2A,C) test duration. In case of depression specific tests, interestingly we observed in both the despair tests (tail suspension and forced swim tests) XYRIIIqdel mice showed significantly less immobility percentage as compared to wild type XYRIII (tail suspension test: Figures 3A,C; forced swim test: Supplementary Figures S2A,C) before stress. This might be an indication of hyperactivity of these mice. To confirm this hyperactivity, we precisely checked their latency to get first time immobility and to our surprise we have noticed XYRIIIqdel mice had significantly higher latency than wild type XYRIII in the despair tests (tail suspension: Figure 4A; however, the forced swim test did not show a clear difference: Supplementary Figures S2B,D).
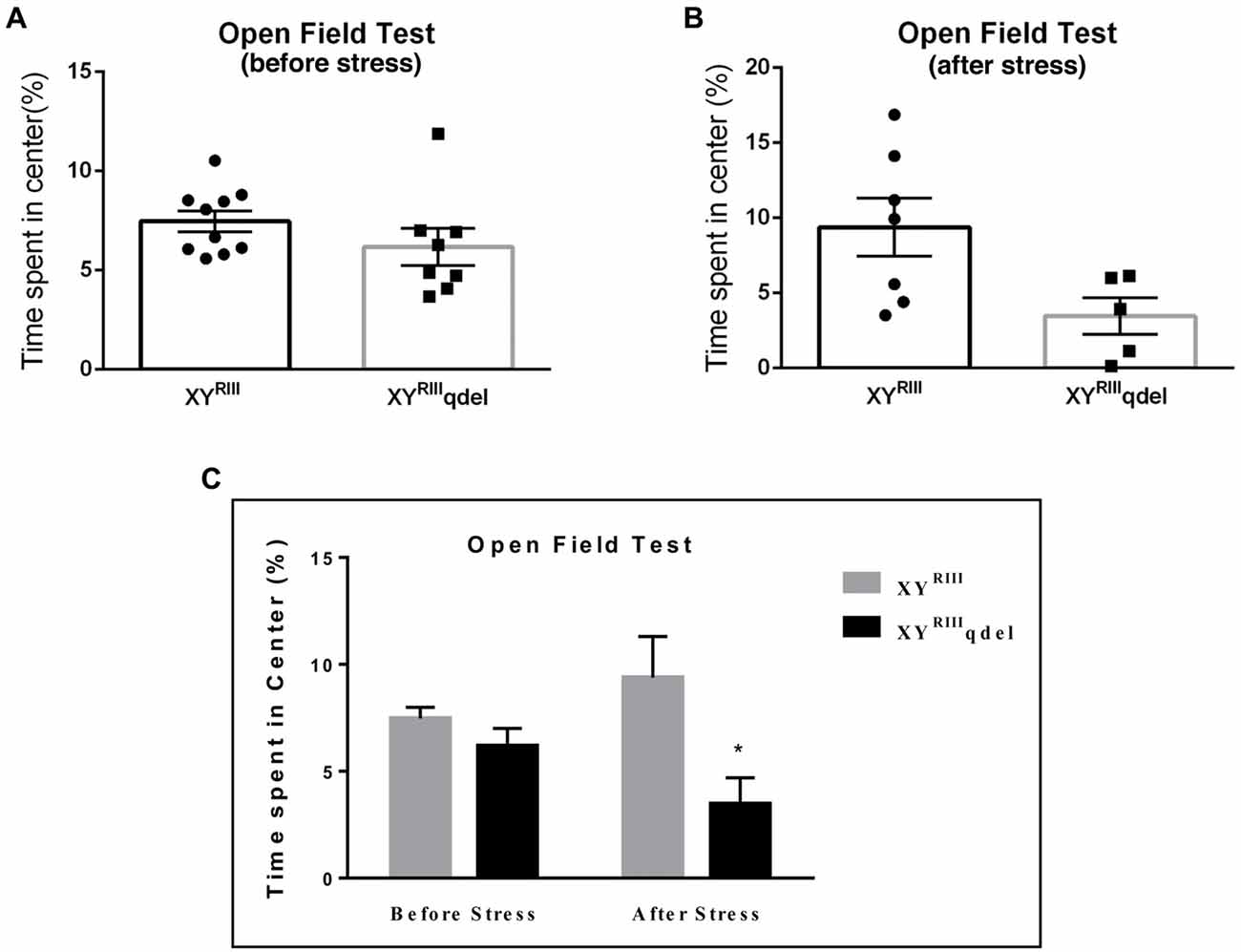
Figure 1. The status of anxiety in XYRIIIqdel and XYRIII mice was evaluated by open field test before and after the exposure of the stressors. Before the mice have undergone stress paradigm, the status of anxiety did not differ from the wild type as shown by scatter bar plot (A), whereas in the same test they showed significantly more anxiety phenotype by spending remarkably less time in center on stress response (B). Combined bar graph is shown to compare the effect of before and after stress (C). Data are expressed as the mean ± SEM, *P < 0.05, n = 8–10 per group in before stress, n = 6–8 per group in after stress experiments.
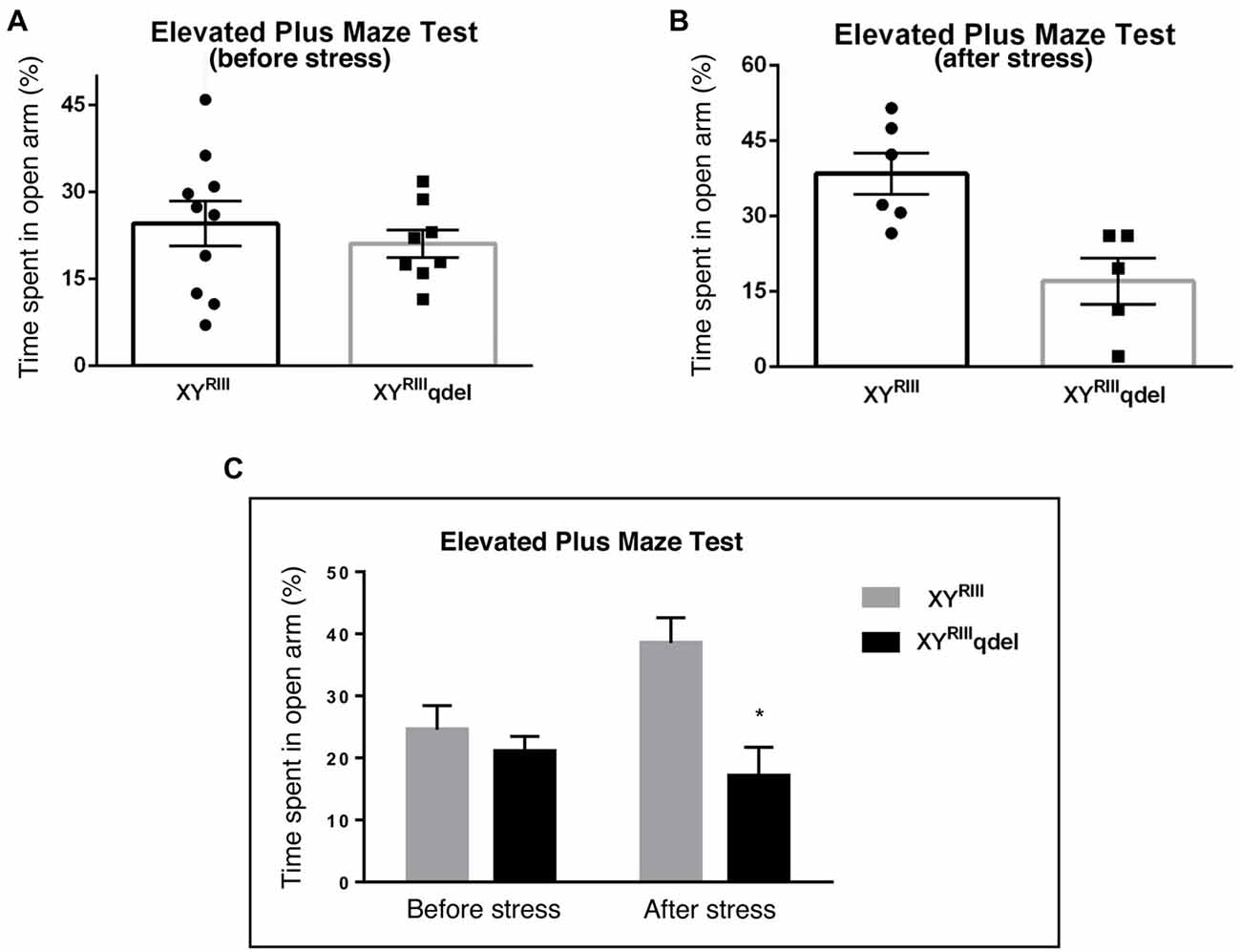
Figure 2. The status of anxiety in XYRIIIqdel and XYRIII mice was evaluated by elevated plus maze test (EPM) before and after the exposure of the stressors. Before the mice have undergone stress paradigm, the status of anxiety in XYRIIIqdel did not differ from the wild type as shown by scatter bar plot (A) whereas in the same test they showed significantly more anxiety phenotype by spending remarkably less time in open arm on stress response (B). Combined bar graph is shown to compare the effect of before and after stress (C). Data are expressed as the mean ± SEM, *P < 0.05, n = 8–10 per group in before stress, n = 6–8 per group in after stress experiments.
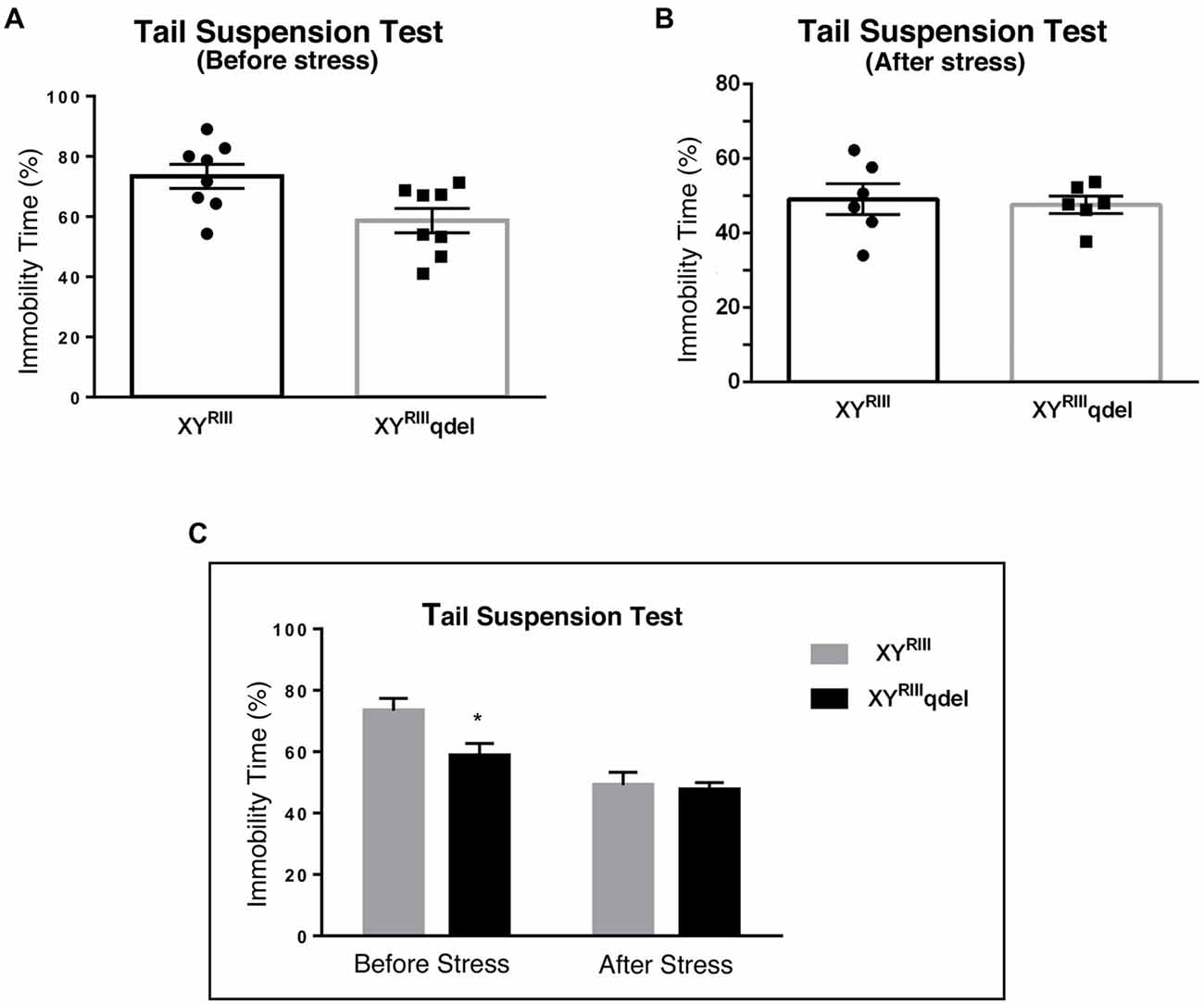
Figure 3. The status of depression in XYRIIIqdel and XYRIII mice was evaluated by tail suspension test before and after the exposure of the stressors. Before the mice have undergone stress paradigm, XYRIIIqdel exhibited significant hyperactivity by showing remarkable less immobility time in percentage out of total test period (A,C). Please notice after stress this hyperactivity was leveled as shown by scatter bar plot (B) of the same test. Combined bar graph is shown to compare the effect of before and after stress for the same parameter (C). Data are expressed as the mean ± SEM, *P < 0.05, n = 8–10 per group in before stress, n = 6–8 per group in after stress experiments.
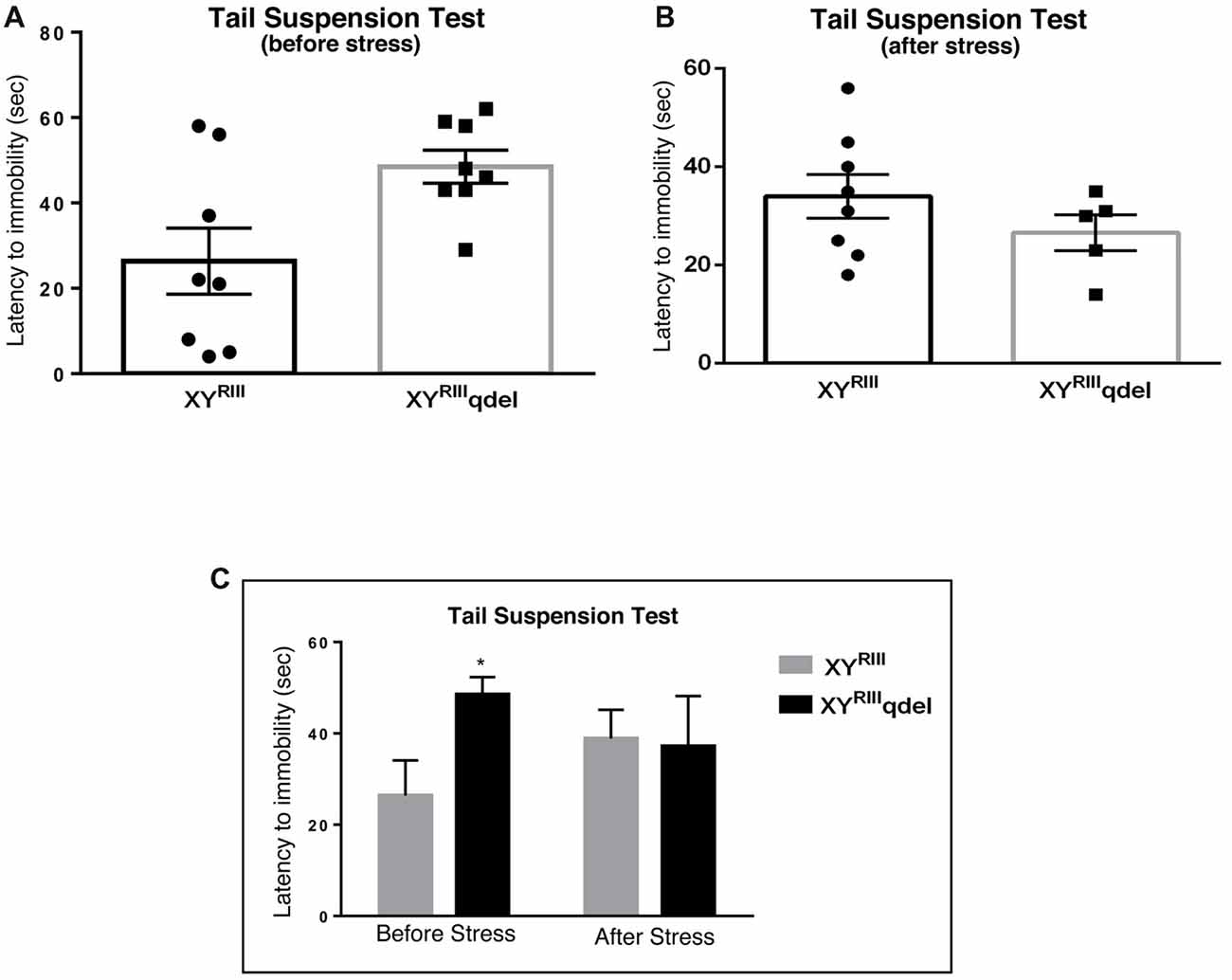
Figure 4. The latency to immobility in XYRIIIqdel and XYRIII mice was evaluated by tail suspension test before and after the exposure of the stressors. Before the mice have undergone stress paradigm, XYRIIIqdel exhibited significant hyperactivity by showing remarkably more time in attaining first time immobility (A,C). Please notice after stress this hyperactivity was leveled as shown by scatter bar plot (B) of the latency. Combined bar graph is shown to compare the effect of before and after stress in latency (C). Data are expressed as the mean ± SEM, *P < 0.05, n = 8–10 per group in before stress, n = 6–8 per group in after stress experiments.
XYRIIIqdel Mice Showed More Vulnerability to Stress by Showing Severe Anxiety (Affective) Phenotype but Not Despair
In order to find out if the XYRIIIqdel mice differ in their degree of response to stress from their wild type counterpart, the same behavioral tests were repeated after a stress paradigm. Interestingly, we observed XYRIIIqdel mice developed anxiety phenotype in both the anxiety-specific tests (open field and EPM); they spent significantly less time in open area than the control animals after the stress episodes (open field test: Figures 1B,C and elevated plus test: Figures 2B,C). Beside this, we also found their hyperactivity showing more mobility during despair tests, (Figures 3A,C and Supplementary Figure S2A) also was remarkably reduced under the stress as compared to their wild type control counterparts (tail suspension test: Figures 3B,C). However, forced swim test was not done after stress because we have used the same test protocol as stressor. To take a detail look in their stress-induced amelioration in hyperactivity we carefully observed the latency to immobility for each individual from the videos. We clearly found that there is no visible difference anymore in latency of XYRIIIqdel mice to attain first time immobility after stress in TST as compared to wild type ones (Figures 4B,C).
XYRIIIqdel Mice Failed to Show Any Visible Difference in Locomotion as Such but Exhibited Difference After Stress
Once we found an indication that XYRIIIqdel mice might be having some hyperactivity in the despair tests (Figure 4A and Supplementary Figure S2A), we evaluated their normal locomotor status using open field test area. Unlike TST and FST, we could not notice any significant change in locomotion (neither in velocity: Figures 5A–C nor in distance traveled: Figures 6A–C) of XYRIIIqdel as compared to wild type ones, however after stress they showed a noticeable reduction in overall distance traveled compared to their counterpart XYRIII mice (Figure 6C). In fact, there was a uniform difference observed in velocity of both the groups of individuals after stress; the XYRIIIqdel animals were not found to be specifically affected as far as the velocity is concerned (Figures 5B,C). Whereas, after the mice have undergone stress paradigm, XYRIIIqdel showed a strong tendency of difference from the wild type in distance traveled (P < 0.09, #; Figure 6C). The representative tracks of the animal groups before and after stress can be referred for clarity (Figure 6D).
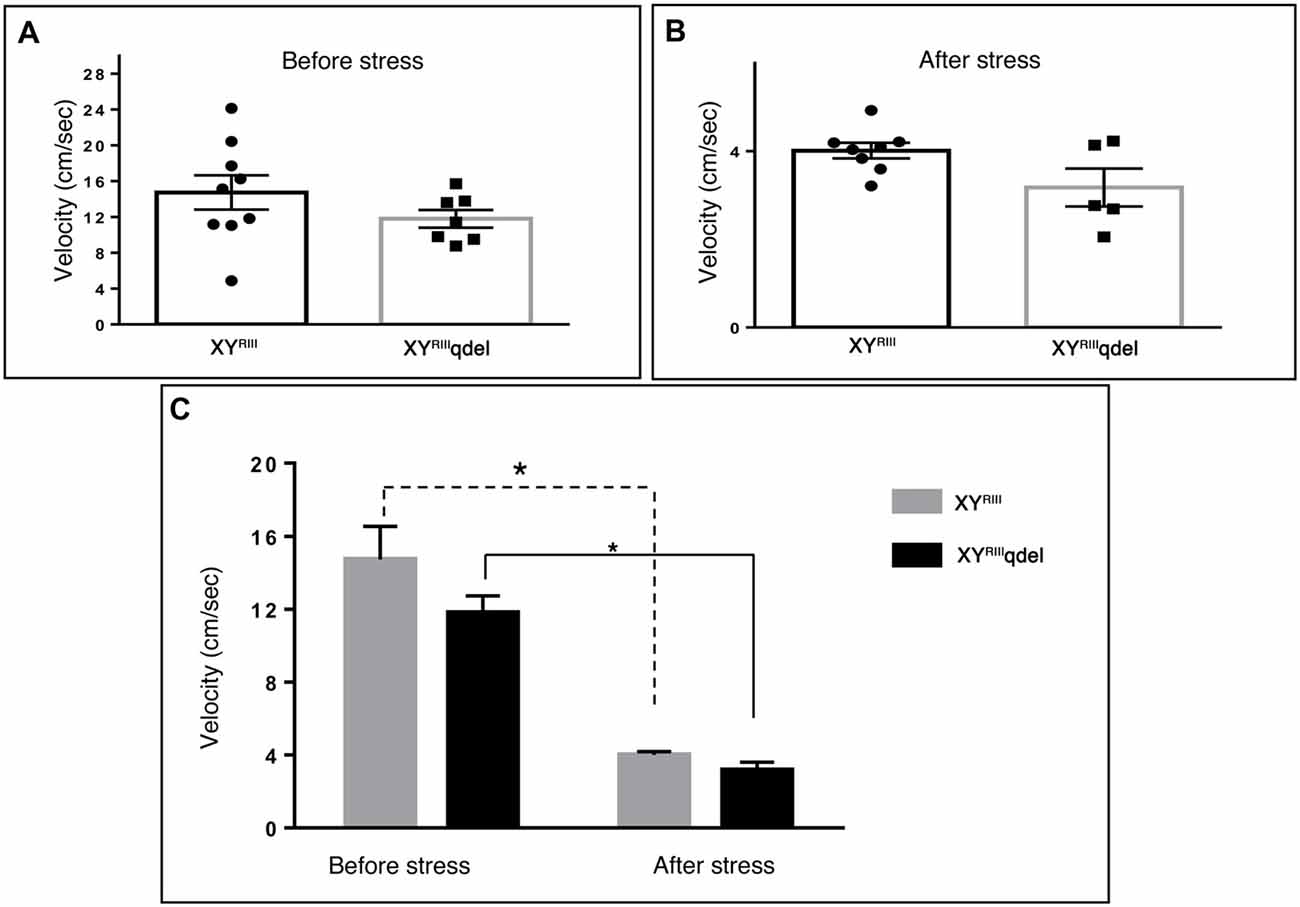
Figure 5. The status of normal locomotion in XYRIIIqdel and XYRIII mice before and after stress: velocity. Before and after stress the mice did not show any difference in the velocity of their movement as such in between genotypes (A,B) whereas while comparing movements before and after stress episodes both the groups have shown significant reduction (C). Data are expressed as the mean ± SEM. Solid line represents the difference between XYRIIIqdel groups and dotted line represents the wild type before and after stress. *P < 0.05, n = 8–10 per group in before stress, n = 6–8 per group in after stress experiments.
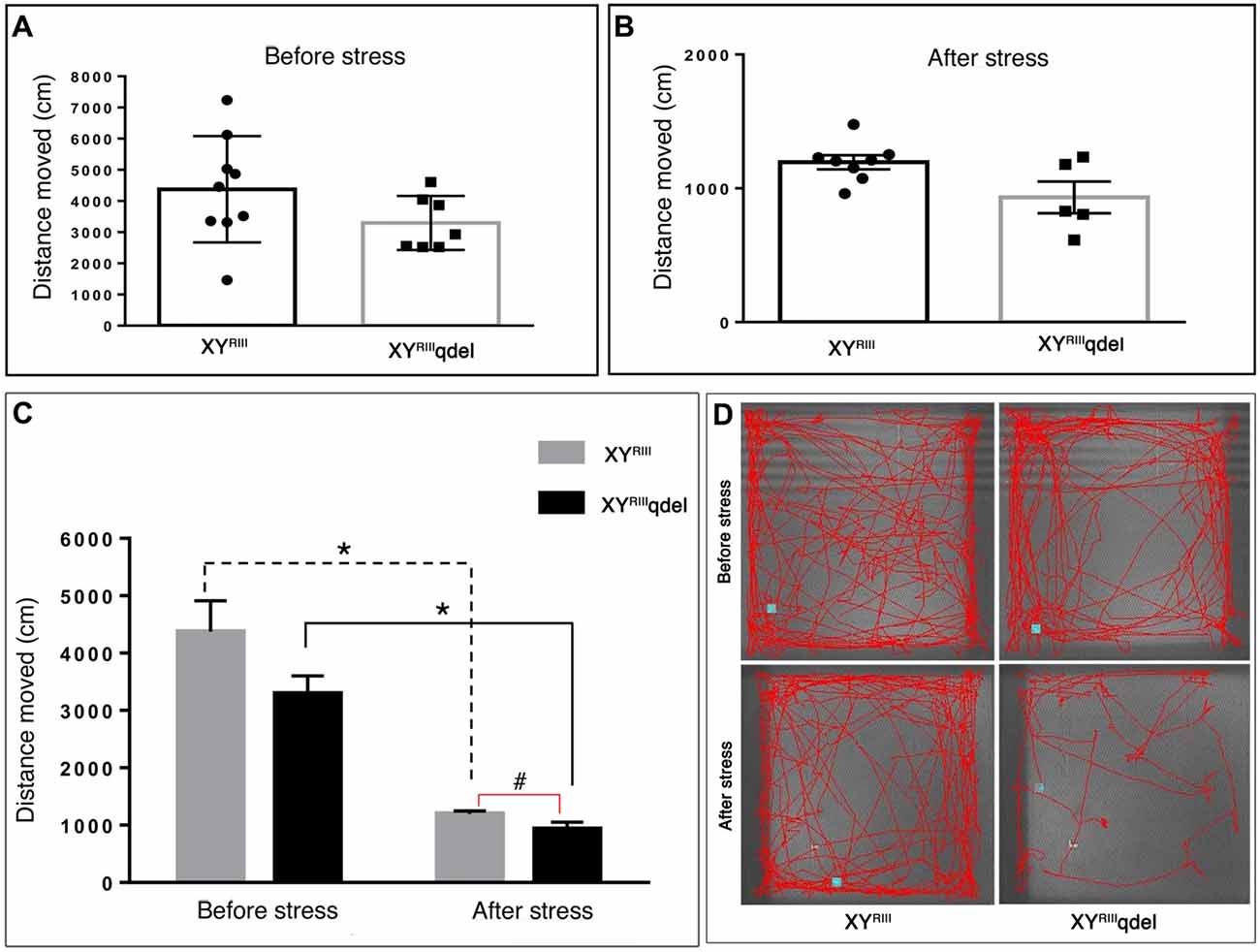
Figure 6. The status of normal locomotion in XYRIIIqdel and XYRIII mice before and after stress: distance moved. Before and after stress the mice did not show any difference in total distance moved during the test period as such in between genotypes (A,B) whereas, after the mice have undergone stress paradigm, XYRIIIqdel showed a strong tendency of difference from the wild type in distance traveled (P < 0.09) (C). Similar to velocity data (Figure 5) on comparison of movements before and after stress episodes both the groups have shown significant reduction in the same parameter. Data are expressed as the mean ± SEM. Solid line represents the difference between XYRIIIqdel groups and dotted line represents the wild type before and after stress. *P < 0.05, #P < 0.09, n = 8–10 per group in before stress, n = 6–8 per group in after stress experiments. Representative image of animal’s track from each group shown (D).
XYRIIIqdel Mice Exhibited Poor Hippocampal Neurogenesis
In order to evaluate the status of basal level of hippocampal neurogenesis, we quantified the number of BrdU positive cells in subgranular layer of dentate gyrus from each mouse. The data suggest that XYRIIIqdel mice indeed have remarkably reduced turnover of neural stem cells/neural progenitor cells as compared to the XYRIII mice (*P < 0.05; Figure 7). It is pertinent to mention here that before performing the final set of experiments described here, a pilot experiment was performed after FST stress. Unfortunately, after stress (for 2 days) hippocampal neurogenesis of deletion group was reduced to such an extent where it was hard to detect any proliferation (data not shown).
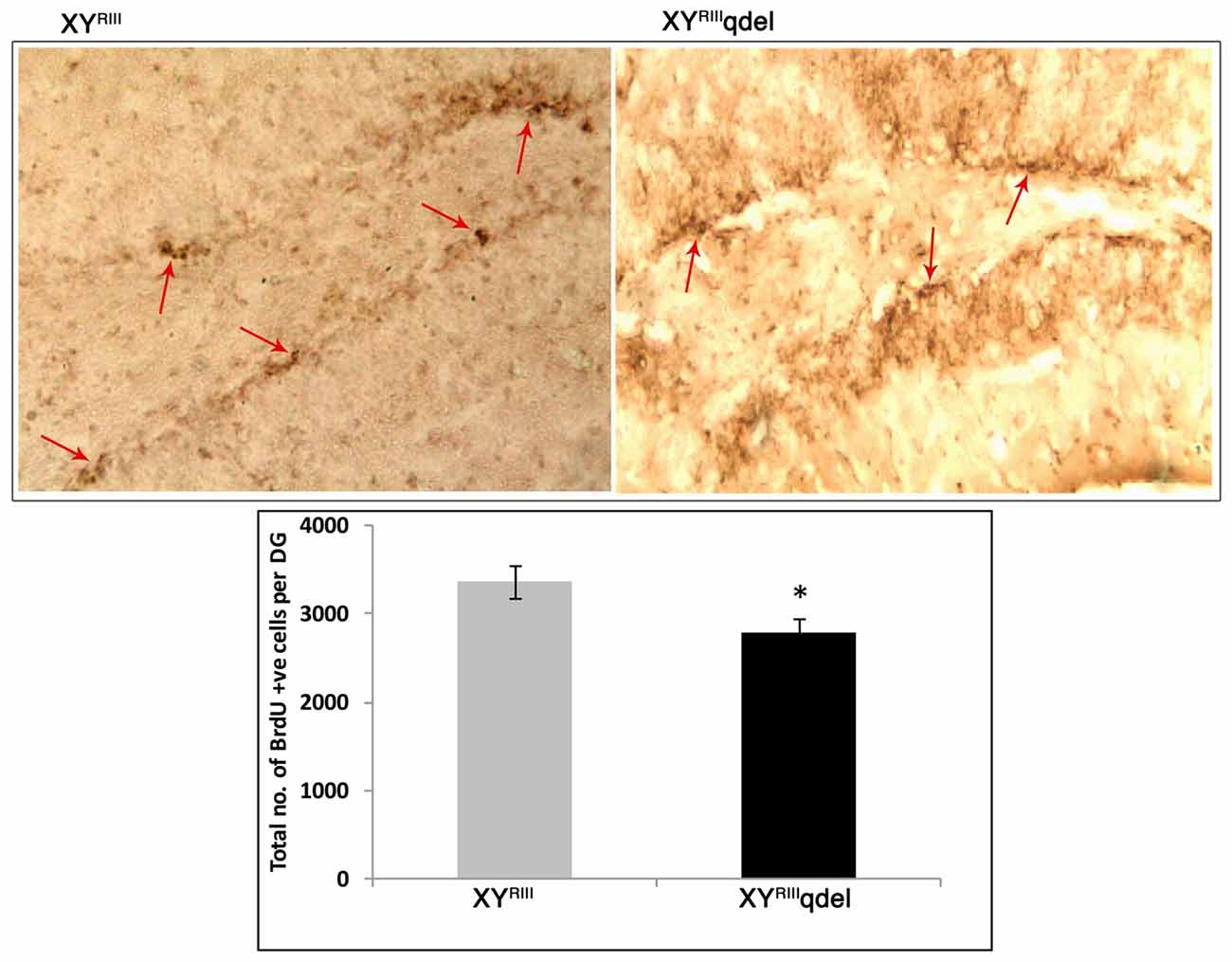
Figure 7. Hippocampal neurogenesis in XYRIIIqdel and wild type mice. Number of BrdU-positive cells in the sub-granular zone of the dentate gyrus. Upper panel shows representative photomicrographs and lower panel shows the actual counting (n = 4–5/group) *P < 0.05.
Proliferative Markers in Hippocampus Also Showed Significant Alteration
After observing a marked reduction in hippocampal neurogenesis in XYRIIIqdel mice, we wanted to see if the expression of genes involved in proliferation in the hippocampus also reflect the same without stress. We selected few known proliferation/differentiation gene markers and compared them with a house-keeping gene, Gapdh. From the qPCR results, it was observed that the mRNA expression level of proliferation markers nestin and Sox-2 decreased significantly (P < 0.001) in XYRIIIqdel compared to XYRIII, with reference to Gapdh. Yet, XYRIIIqdel mice showed significant increase in the expression of Gfap (P < 0.01), Dcx (P < 0.01) and NeuroD1 (P < 0.001), in comparison to XYRIII. It was interesting to observe that the NeuroD1 expression was nine-fold higher in XYRIIIqdel compared to XYRIII. However, no significant change in expression was observed between XYRIII and XYRIIIqdel for NeuN, a marker for mature neurons (Figure 8).
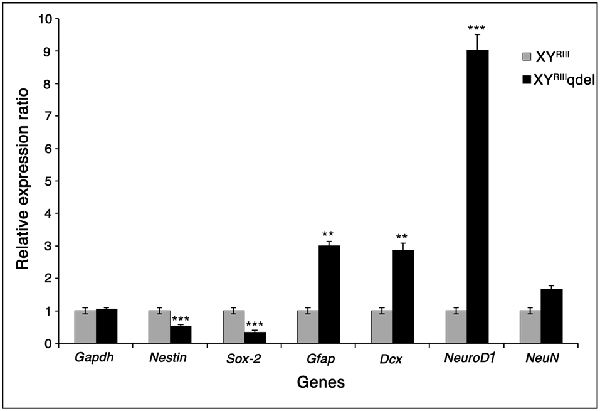
Figure 8. The mRNA expression level of neurogenesis markers. This figure shows the m-RNA expression level of neurogenesis markers in XYRIII and XYRIIIqdel mice, with reference to Gapdh. The proliferation markers Nestin and Sox-2 (P < 0.001, ***) showed significant reduction in expression while the differentiation markers were upregulated in XYRIIIqdel compared to XYRIII mice. Gfap and Dcx show upregulation at a significance level of P < 0.01, ** and NeuroD1, P < 0.001, ***). Students t-test was used to calculate the statistical significance.
Y Chromosomal Homology
The six genes studied here in mouse hippocampal neurogenesis, Nestin, Sox2, Gfap, Dcx, NeuroD1 and NeuN, were checked for homology to Y-derived transcripts. The coding and UTR (both 5’ and 3’) sequences of the corresponding genes were obtained from Ensembl. BLASTN of these sequences were performed against the Y-derived transcripts using the parameters of word size 10, E-value 10 with the DUST filter off. All the genes had homology to the Y transcripts specifically in their UTRs (either 5’ or 3’), with no homology in the corresponding coding regions (Figure 9). Nestin and Dcx had unique hits in both the UTRs whereas Gfap had one unique hit only in its 5’ UTR; Sox2, NeuroD1 and NeuN had unique hits only in their 3’ UTRs.

Figure 9. Y-chromosomal homology in untranslated regions (UTRs) of deregulated genes in hippocampus. The short stretches of homology observed between the Y-chromosomal transcripts (red color) and the UTRs of the deregulated genes are represented in the figure. Nestin and Dcx had hits in both 5’ and 3’UTRs; Gfap had homology only in the 5’ UTR whereas Sox2, NeuroD1 and NeuN had unique hits only in their 3’ UTRs.
We demonstrate here that mouse Y heterochromatin has defined effects on behavior. A strain of mouse partially deleted in repeats present on the Y chromosome exhibits anxiety phenotype under stress. These mice also have reduced neurogenesis in the hippocampal region. Real time PCR analysis reveals reduced expression of neurogenesis markers and increased expression of differentiation markers. The genes that are deregulated in XYRIIIqdel hippocampus have small stretches of homology from Y heterochromatin transcripts specifically in their UTRs. Thus, this study elucidates a novel role in behavior for mouse Y-heterochromatin.
Discussion
We observed behavioral anomalies pertaining to stress in a strain of mouse with partial deletion of Y-heterochromatin. Decrease in hippocampal neurogenesis in the XYRIIIqdel mice could partially be responsible for the behavioral anomalies observed. This is in line with the established fact in the field that anxiety and related mood disorders are associated with altered hippocampal neurogenesis (Sahay and Hen, 2008; Lee et al., 2013). This decrease in hippocampal neurogenesis is also reflected by significant decrease in the expression of neural stem or progenitor proliferative markers Nestin and Sox-2 in our study, as shown by others (Lagace et al., 2007b). Decrease in neural stem or progenitor cell proliferation appears to be compensated by increased differentiation of stem or progenitor cells, which is reflected by significant increase in the differentiation markers Dcx (early neuronal marker), Gfap (glial marker) and NeuroD1 (transcription factor for differentiation). This could indicate a shift in homeostasis between proliferation and differentiation in hippocampus on deletion of Y-heterochromatin.
The presence of Y-homologous sequences in the UTRs of deregulated genes analyzed in this study could indicate regulation of these genes by Y chromosome. Further this elicits the concept of regulation of hippocampal neurogenesis by Y-heterochromatin. This points to the importance of Y chromosomal transcripts and repeat elements in regulating genes expressed during adult hippocampal neurogenesis. This also substantiates our previous finding that Y chromosome plays a prominent role in regulating autosomal genes (Jehan et al., 2007; Bhattacharya et al., 2013).Our recent molecular data from a study using XYRIII and XYRIIIqdel mice indicate putative regulation of autosomal genes expressed in testis by Y-derived noncoding RNAs (Reddy et al., 2018).
The behavioral assessment of XYRIIIqdel mice using a battery of assays showed behavioral changes pertaining to anxiety and hyperactivity. Moreover, they became vulnerable to stress. Since depression and other neuropsychiatric disorders are associated with decreased hippocampal neurogenesis (Boulle et al., 2014; Levone et al., 2015), we anticipate that this might possibly play a major role in making the XYRIIIqdel mice more susceptible to stress. Our findings are supported by the recent report that the terminal Azoospermia factor (AZFb+c) deletions are associated with abnormal neuropsychiatric condition (Castro et al., 2017). In patients with Yq microdeletions, there is a copy number variation of genes in the Pseudo-autosomal region (PAR; Jorgez et al., 2011). Though the functional role of genes in PAR is less explored, some genes are known to be involved in neuropsychiatric disorders. The Acetylserotonin O-Methyltransferase (ASMT) gene located in PAR, which is involved in circadian rhythm abnormalities, is considered as a candidate gene for bipolar depression (Flaquer et al., 2010; Etain et al., 2012). Deletion of AZFb+c is found to influence the PAR gene expression, thus altering neuropsychiatric condition (Castro et al., 2017).
There is no sex-specific difference in hippocampal neurogenesis between male and female (Lagace et al., 2007a). But male and female differ in their susceptibility to neuropsychiatric disorders, which suggests direct/indirect involvement of the sex-linked genes in the onset of these brain and behavior disorders (Trent and Davies, 2012). Gene expression in adult human brain has been shown to be different between males and females i.e., the XY and XX genotypes for some genes (Trabzuni et al., 2013). This could be because of the regulation of those genes by Y chromosome in males. Further, we observed a difference in neural gene expression when part of the Y chromosome is deleted. The fact that the genes that are up/downregulated in hippocampus of the XYRIIIqdel mice exhibit homology to Y-chromosomal transcripts in their UTRs shows a possibility for these genes involved in regulation of hippocampal neurogenesis to be regulated by the Y chromosome. Y-linked genes have been explored for the influence on neural masculinization and their contribution for the sexual differentiation of the brain development and function (Kopsida et al., 2009). The Y-linked genes including the six NRY genes and Sry are shown to contribute to neural sexual differentiation (Xu et al., 2002). Our findings would extend the knowledge that apart from the well-established Y-linked genes, there are other possible Y-derived transcripts and repeat elements which could regulate the genes involved in hippocampal neurogenesis and thus affect brain and behavioral function.
The behavioral data in our study shows there are some differences between the wild type and XYRIIIqdel mice to begin with, i.e., when the animals are not exposed to stress. In unstressed situation, XYRIIIqdel mice do not exhibit the phenotype of mood related disorders. However, in TST and FST these mutant animals show less immobility, i.e., hyperactivity, compared to their wild type counterparts. Upon stress exposure, the pre-stress level hyperactivity in XYRIIIqdel mice goes down significantly thus suggesting vulnerability to stress, in addition to increase in anxiety, which the mutants show following stress-exposure.
Regarding the observation that locomotion was significantly reduced in both groups of animals after stress, it is well known that the stress responses differ in acute and chronic stress specially with respect to locomotory behavior in the mouse (Cabib et al., 1988), where they have shown a reduced locomotion on acute stress and increased locomotion after chronic stress (Cabib et al., 1988). Hyper-locomotion also was reported in chronic stress mouse models (Ito et al., 2010). In the present study, we have given 2 days of Forced Swim stressor, which can be considered as “Acute” stress. This could be the reason behind the observation of reduced locomotion in both the groups, where both the groups have undergone the same stress paradigm.
It is well established that chronic long-term stress leads to anxiety and depression. The clinical symptoms of stress, anxiety and depression and its presentation are different in males compared to females (Martin et al., 2013). There is no hormonal ebbs and flows in men, which are the major reasons attributed to behavioral changes in female (Soares and Zitek, 2008). The only hormonal link that is predicted as a possible contributor in neuropsychiatric disorder in males is the association of decreased testosterone level with depression (Kenny et al., 2004). Therefore, there is an urgent need for the identification of male specific regulatory elements, in particular noncoding RNAs in neurodegenerative and neuropsychiatric diseases, for which Y chromosome is a rich source. A deeper understanding of this Y chromosome induced regulation of autosomal genes in brain might also answer why certain neuropsychiatric disorders are gender biased. Our findings might open up new doors for better understanding of neuropsychiatric and neurodegenerative etiology. However, in the light of our behavioral studies, pre-stress hippocampal neuronal gene expression and neurogenesis changes, post-stress gene expression and neurogenesis profiling would have strengthened the behavioral data. Further studies in these lines are warranted for more definite conclusions.
Author Contributions
SD, AKamle, RD, SMT and SC performed experiments. SRT did bioinformatics analysis. AKumar, SC and RJ conceived the article and wrote the manuscript.
Funding
This project was initiated with funding from the Department of Biotechnology, Ministry of Science and Technology (BT/PR6123) to RJ and later supported by one of the Council of Scientific and Industrial Research (CSIR) network projects (BSC0115-miND) to RJ, SC and AKumar.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to acknowledge the gift of the XYRIII and XYRIIIqdel mice used in this study from Prof. Paul S. Burgoyne. We would like to thank Dr. Sunayana MR for help with preparation of figures and editing the manuscript. We also acknowledge Mr. Dwaraknath and Mr. Sai Ram for animal care and maintenance throughout the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnbeh.2018.00215/full#supplementary-material
References
Arnold, A. P., and Burgoyne, P. S. (2004). Are XX and XY brain cells intrinsically different? Trends Endocrinol. Metab. 15, 6–11. doi: 10.1016/j.tem.2003.11.001
Bhattacharya, R., Devi, M. S., Dhople, V. M., and Jesudasan, R. A. (2013). A mouse protein that localizes to acrosome and sperm tail is regulated by Y-chromosome. BMC Cell Biol. 14:50. doi: 10.1186/1471-2121-14-50
Boulle, F., Massart, R., Stragier, E., Païzanis, E., Zaidan, L., Marday, S., et al. (2014). Hippocampal and behavioral dysfunctions in a mouse model of environmental stress: normalization by agomelatine. Transl. Psychiatry 4:e485. doi: 10.1038/tp.2014.125
Burgoyne, P. S., and Mitchell, M. J. (2007). “The roles of mouse Y chromosome genes in spermatogenesis,” in Y Chromosome and Male Germ Cell Biology, ed. W.-Y. C. Yun-Fai (Hackensack , NJ: World Scientific Publishers), 27–45.
Cabib, S., Kempf, E., Schleef, C., Mele, A., and Puglisi-Allegra, S. (1988). Different effects of acute and chronic stress on two dopamine-mediated behaviors in the mouse. Physiol. Behav. 43, 223–227. doi: 10.1016/0031-9384(88)90242-9
Castro, A., Rodríguez, F., Flórez, M., López, P., Curotto, B., Martínez, D., et al. (2017). Pseudoautosomal abnormalities in terminal AZFb+c deletions are associated with isochromosomes Yp and may lead to abnormal growth and neuropsychiatric function. Hum. Reprod. 32, 465–475. doi: 10.1093/humrep/dew333
Chakravarty, S., Maitra, S., Reddy, R. G., Das, T., Jhelum, P., Kootar, S., et al. (2015). A novel natural product inspired scaffold with robust neurotrophic, neurogenic and neuroprotective action. Sci. Rep. 5:14134. doi: 10.1038/srep14134
Conway, S. J., Mahadevaiah, S. K., Darling, S. M., Capel, B., Rattigan, A. M., and Burgoyne, P. S. (1994). Y353/B: a candidate multiple-copy spermiogenesis gene on the mouse Y chromosome. Mamm. Genome 5, 203–210. doi: 10.1007/BF00360546
De Vries, G. J., Rissman, E. F., Simerly, R. B., Yang, L. Y., Scordalakes, E. M., Auger, C. J., et al. (2002). A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J. Neurosci. 22, 9005–9014. doi: 10.1523/jneurosci.22-20-09005.2002
Etain, B., Dumaine, A., Bellivier, F., Pagan, C., Francelle, L., Goubran-Botros, H., et al. (2012). Genetic and functional abnormalities of the melatonin biosynthesis pathway in patients with bipolar disorder. Hum. Mol. Genet. 21, 4030–4037. doi: 10.1093/hmg/dds227
Flaquer, A., Jamra, R. A., Etterer, K., Díaz, G. O., Rivas, F., Rietschel, M., et al. (2010). A new susceptibility locus for bipolar affective disorder in PAR1 on Xp22.3/Yp11.3. Am. J. Med. Genet. B Neuropsychiatr. Genet. 153B, 1110–1114. doi: 10.1002/ajmg.b.31075
Gould, E., and Gross, C. G. (2002). Neurogenesis in adult mammals: some progress and problems. J. Neurosci. 22, 619–623. doi: 10.1523/jneurosci.22-03-00619.2002
Gould, E., and Tanapat, P. (1999). Stress and hippocampal neurogenesis. Biol. Psychiatry 46, 1472–1479. doi: 10.1016/S0006-3223(99)00247-4
Grubbs, F. E. (1969). Procedures for ditecting outlying observations in samples. Technometrics 11, 1–21.
Guillot, P. V., and Chapouthier, G. (1998). Intermale aggression, GAD activity in the olfactory bulbs and Y chromosome effect in seven inbred mouse strains. Behav. Brain Res. 90, 203–206. doi: 10.1016/s0166-4328(97)00110-1
Guo, J., Zhu, P., Wu, C., Yu, L., Zhao, S., and Gu, X. (2003). In silico analysis indicates a similar gene expression pattern between human brain and testis. Cytogenet. Genome Res. 103, 58–62. doi: 10.1159/000076290
Ikrar, T., Guo, N., He, K., Besnard, A., Levinson, S., Hill, A., et al. (2013). Adult neurogenesis modifies excitability of the dentate gyrus. Front. Neural Circuits 7:204. doi: 10.3389/fncir.2013.00204
Ito, H., Nagano, M., Suzuki, H., and Murakoshi, T. (2010). Chronic stress enhances synaptic plasticity due to disinhibition in the anterior cingulate cortex and induces hyper-locomotion in mice. Neuropharmacology 58, 746–757. doi: 10.1016/j.neuropharm.2009.12.011
Jehan, Z., Vallinayagam, S., Tiwari, S., Pradhan, S., Singh, L., Suresh, A., et al. (2007). Novel noncoding RNA from human Y distal heterochromatic block (Yq12) generates testis-specific chimeric CDC2L2. Genome Res. 17, 433–440. doi: 10.1101/gr.5155706
Jorgez, C. J., Weedin, J. W., Sahin, A., Tannour-Louet, M., Han, S., Bournat, J. C., et al. (2011). Aberrations in pseudoautosomal regions (PARs) found in infertile men with Y-chromosome microdeletions. J. Clin. Endocrinol. Metab. 96, E674–E679. doi: 10.1210/jc.2010-2018
Joshi, P. C., Samineni, R., Bhattacharya, D., Reddy, B. R., Veeraval, L., Das, T., et al. (2017). A 2-oxa-spiro[5.4]decane scaffold displays neurotrophic, neurogenic and anti-neuroinflammatory activities with high potential for development as a versatile CNS therapeutic. Sci. Rep. 7:1492. doi: 10.1038/s41598-017-01297-z
Kenny, A. M., Fabregas, G., Song, C., Biskup, B., and Bellantonio, S. (2004). Effects of testosterone on behavior, depression and cognitive function in older men with mild cognitive loss. J. Gerontol. A Biol. Sci. Med. Sci. 59, 75–78. doi: 10.1093/gerona/59.1.m75
Kopsida, E., Stergiakouli, E., Lynn, P. M., Wilkinson, L. S., and Davies, W. (2009). The role of the Y chromosome in brain function. Open Neuroendocrinol. J. 2, 20–30. doi: 10.2174/1876528900902010020
Kotarska, K., Galas, J., Przybyło, M., Bilińska, B., and Styrna, J. (2015). Increased progesterone production in cumulus-oocyte complexes of female mice sired by males with the Y-chromosome long arm deletion and its potential influence on fertilization efficiency. Reprod. Sci. 22, 242–249. doi: 10.1177/1933719114537717
Kotarska, K., Lenartowicz, M., Przybyło, M., Gołas, A., and Styrna, J. (2013). Increased prostaglandin E(2)-EP2 signalling in cumulus cells of female mice sired by males with the Y-chromosome long-arm deletion. Reprod. Fertil. Dev. 25, 900–906. doi: 10.1071/RD12086
Kotarska, K., and Styrna, J. (2012). Can the partial deletion in the Y chromosome of male mice affect the reproductive efficiency of their daughters? Syst. Biol. Reprod. Med. 58, 81–87. doi: 10.3109/19396368.2011.638969
Lagace, D. C., Fischer, S. J., and Eisch, A. J. (2007a). Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus 17, 175–180. doi: 10.1002/hipo.20265
Lagace, D. C., Whitman, M. C., Noonan, M. A., Ables, J. L., DeCarolis, N. A., Arguello, A. A., et al. (2007b). Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J. Neurosci. 27, 12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007
LaPlant, Q., Chakravarty, S., Vialou, V., Mukherjee, S., Koo, J. W., Kalahasti, G., et al. (2009). Role of nuclear factor kappaB in ovarian hormone-mediated stress hypersensitivity in female mice. Biol. Psychiatry 65, 874–880. doi: 10.1016/j.biopsych.2009.01.024
Lee, M. M., Reif, A., and Schmitt, A. G. (2013). Major depression: a role for hippocampal neurogenesis? Curr. Top. Behav. Neurosci. 14, 153–179. doi: 10.1007/7854_2012_226
Levone, B. R., Cryan, J. F., and O’leary, O. F. (2015). Role of adult hippocampal neurogenesis in stress resilience. Neurobiol. Stress 1, 147–155. doi: 10.1016/j.ynstr.2014.11.003
Liu, L., Cheng, J., Li, H., Su, Y., Sun, L., Yang, L., et al. (2017). Association of Y-linked variants with impulsivity and aggression in boys with attention-deficit/hyperactivity disorder of Chinese Han descent. Psychiatry Res. 252, 185–187. doi: 10.1016/j.psychres.2017.02.055
Ln, W. K. Z. (2006). Psychopathology and Psychopharmacology in the Infertile Patient. Cambridge: Cambridge University Press.
Lovell-Badge, R. (2005). Aggressive behaviour: contributions from genes on the Y chromosome. Novartis Found. Symp. 268, 20–33; discussion 33–41, 96–99.
Martin, L. A., Neighbors, H. W., and Griffith, D. M. (2013). The experience of symptoms of depression in men vs. women: analysis of the National Comorbidity Survey Replication. JAMA Psychiatry 70, 1100–1116. doi: 10.1001/jamapsychiatry.2013.1985
Mayer, A., Mosler, G., Just, W., Pilgrim, C., and Reisert, I. (2000). Developmental profile of Sry transcripts in mouse brain. Neurogenetics 3, 25–30. doi: 10.1007/s100480000093
Miczek, K. A., Maxson, S. C., Fish, E. W., and Faccidomo, S. (2001). Aggressive behavioral phenotypes in mice. Behav. Brain Res. 125, 167–181. doi: 10.1016/s0166-4328(01)00298-4
Najmabadi, H., Huang, V., Yen, P., Subbarao, M. N., Bhasin, D., Banaag, L., et al. (1996). Substantial prevalence of microdeletions of the Y-chromosome in infertile men with idiopathic azoospermia and oligozoospermia detected using a sequence-tagged site-based mapping strategy. J. Clin. Endocrinol. Metab. 81, 1347–1352. doi: 10.1210/jcem.81.4.8636331
Pathak, S. S., Maitra, S., Chakravarty, S., and Kumar, A. (2017). Histone Lysine demethylases of JMJD2 or KDM4 family are important epigenetic regulators in reward circuitry in the etiopathology of depression. Neuropsychopharmacology 42, 854–863. doi: 10.1038/npp.2016.231
Porsolt, R. D., Brossard, G., Hautbois, C., and Roux, S. (2001). Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Neurosci. Chapter 8:Unit 8. 10A. doi: 10.1002/0471142301.ns0810as14
Reddy, H. M., Bhattacharya, R., Jehan, Z., Mishra, K., Annapurna, P., and Tiwari, S. (2018). Y chromosomal noncoding RNA regulates autosomal gene expression via piRNAs in mouse testis. bioRXiv:285429 [Preprint]. doi: 10.1101/285429
Reddy, B. R., Maitra, S., Jhelum, P., Kumar, K. P., Bagul, P. K., Kaur, G., et al. (2016). Sirtuin 1 and 7 mediate resveratrol-induced recovery from hyper-anxiety in high-fructose-fed prediabetic rats. J. Biosci. 41, 407–417. doi: 10.1007/s12038-016-9627-8
Reisert, I., Karolczak, M., Beyer, C., Just, W., Maxson, S. C., and Ehret, G. (2002). Sry does not fully sex-reverse female into male behavior towards pups. Behav. Genet. 32, 103–111. doi: 10.1023/A:1015297622509
Roubertoux, P. L., Carlier, M., Degrelle, H., Haas-Dupertuis, M. C., Phillips, J., and Moutier, R. (1994). Co-segregation of intermale aggression with the pseudoautosomal region of the Y chromosome in mice. Genetics 136, 225–230. doi: 10.1002/1098-2337(1994)20:5<379::aid-ab2480200505>3.0.co;2-b
Sahay, A., and Hen, R. (2008). Hippocampal neurogenesis and depression. Novartis Found. Symp. 289, 152–160; discussion 160–164, 193–195. doi: 10.1002/9780470751251.ch12
Sahin, A., Urkmez, A., Verit, A., Yuksel, O. H., and Verit, F. F. (2017). Psychologic and sexual dysfunction in primary and secondary infertile male patients. Arch. Ital. Urol. Androl. 89, 120–124. doi: 10.4081/aiua.2017.2.120
Sluyter, F., Korte, S. M., Van Baal, G. C., De Ruiter, A. J., and Van Oortmerssen, G. A. (1999). Y chromosomal and sex effects on the behavioral stress response in the defensive burying test in wild house mice. Physiol. Behav. 67, 579–585. doi: 10.1016/s0031-9384(99)00101-8
Soares, C. N., and Zitek, B. (2008). Reproductive hormone sensitivity and risk for depression across the female life cycle: a continuum of vulnerability? J. Psychiatry Neurosci. 33, 331–343.
Stochholm, K., Bojesen, A., Jensen, A. S., Juul, S., and Gravholt, C. H. (2012). Criminality in men with Klinefelter’s syndrome and XYY syndrome: a cohort study. BMJ Open 2:e000650. doi: 10.1136/bmjopen-2011-000650
Styrna, J., Bilińska, B., and Krzanowskaa, H. (2002). The effect of a partial Y chromosome deletion in B10.BR-Ydel mice on testis morphology, sperm quality and efficiency of fertilization. Reprod. Fertil. Dev. 14, 101–108. doi: 10.1071/rd01089
Styrna, J., Klag, J., and Moriwaki, K. (1991). Influence of partial deletion of the Y chromosome on mouse sperm phenotype. J. Reprod. Fertil. 92, 187–195. doi: 10.1530/jrf.0.0920187
Suri, D., Veenit, V., Sarkar, A., Thiagarajan, D., Kumar, A., Nestler, E. J., et al. (2013). Early stress evokes age-dependent biphasic changes in hippocampal neurogenesis, BDNF expression and cognition. Biol. Psychiatry 73, 658–666. doi: 10.1016/j.biopsych.2012.10.023
Touré, A., Szot, M., Mahadevaiah, S. K., Rattigan, A., Ojarikre, O. A., and Burgoyne, P. S. (2004). A new deletion of the mouse Y chromosome long arm associated with the loss of Ssty expression, abnormal sperm development and sterility. Genetics 166, 901–912. doi: 10.1534/genetics.166.2.901
Trabzuni, D., Ramasamy, A., Imran, S., Walker, R., Smith, C., Weale, M. E., et al. (2013). Widespread sex differences in gene expression and splicing in the adult human brain. Nat. Commun. 4:2771. doi: 10.1038/ncomms3771
Trent, S., and Davies, W. (2012). The influence of sex-linked genetic mechanisms on attention and impulsivity. Biol. Psychol. 89, 1–13. doi: 10.1016/j.biopsycho.2011.09.011
Vigneault, G., and Zouros, E. (1986). The genetics of asymmetrical male sterility in Drosophila mojavensis and Drosophila arizonensis hybrids: interactions between the Y-chromosome and autosomes. Evolution 40, 1160–1170. doi: 10.1111/j.1558-5646.1986.tb05741.x
Vogt, P. H., Edelmann, A., Kirsch, S., Henegariu, O., Hirschmann, P., Kiesewetter, F., et al. (1996). Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Hum. Mol. Genet. 5, 933–943. doi: 10.1093/hmg/5.7.933
Xu, J., Burgoyne, P. S., and Arnold, A. P. (2002). Sex differences in sex chromosome gene expression in mouse brain. Hum. Mol. Genet. 11, 1409–1419. doi: 10.1093/hmg/11.12.1409
Yang, B., Zhang, J., Qi, Y., Wang, P., Jiang, R., and Li, H. (2017). Assessment on occurrences of depression and anxiety and associated risk factors in the infertile Chinese men. Am. J. Mens Health 11, 767–774. doi: 10.1177/1557988317695901
Keywords: Y-deleted mouse, behavior, anxiety, neurogenesis, Y chromosomal homology
Citation: Dey SK, Kamle A, Dereddi RR, Thomas SM, Thummala SR, Kumar A, Chakravarty S and Jesudasan RA (2018) Mice With Partial Deletion of Y-Heterochromatin Exhibits Stress Vulnerability. Front. Behav. Neurosci. 12:215. doi: 10.3389/fnbeh.2018.00215
Received: 29 March 2018; Accepted: 27 August 2018;
Published: 21 September 2018.
Edited by:
Xiao-Dong Wang, Zhejiang University, ChinaReviewed by:
Tao Tan, Sichuan Provincial Hospital for Women and Children, ChinaZhimin Song, Emory University, United States
Copyright © 2018 Dey, Kamle, Dereddi, Thomas, Thummala, Kumar, Chakravarty and Jesudasan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sumana Chakravarty, c3VtYW5hOThAZ21haWwuY29t; c3VtYW5hY2hha0BpaWN0LnJlcy5pbg==
Rachel A. Jesudasan, cmFjaGVsbGlrZUBnbWFpbC5jb20=; cmFjaGVsQGNzaXJjY21iLm9yZw==
 Sandeep Kumar Dey
Sandeep Kumar Dey Avijeet Kamle1
Avijeet Kamle1 Ram Reddy Dereddi
Ram Reddy Dereddi Shiju M. Thomas
Shiju M. Thomas Shashi Rekha Thummala
Shashi Rekha Thummala Sumana Chakravarty
Sumana Chakravarty Rachel A. Jesudasan
Rachel A. Jesudasan