- 1Department of Neurology, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Neurology, Zhongshan Hospital Fudan University, Shanghai, China
- 3Biostatistics Office of Clinical Research Center, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Accumulating evidence suggests that freezing of gait (FOG) is a unique gait disturbance in Parkinson’s disease (PD), and its pathophysiology is not fully elucidated. The present study aims to investigate the clinical and oculomotor associations with FOG in Chinese PD patients. From Jan 2017 to Dec 2019, a total of 210 PD patients were consecutively registered for FOG evaluation based on item-3 of the Freezing of Gait Questionnaire (FOGQ). We explored the demographic, motor, and non-motor symptom differences in FOG positive (PD+FOG, n = 45) vs. negative (PD-FOG, n = 165) group. In addition, 40 PD patients and 37 healthy controls (HC) also underwent oculomotor test via videonystagmography (VNG). Visually guided saccade (VGS) latency, saccade accuracy and gain in smooth pursuit eye movement (SPEM) at three frequencies of horizontal axis were compared among PD+FOG (n = 20), PD-FOG (n = 20), and HC (n = 37). Compared with PD-FOG, PD+FOG had longer disease duration, more severe motor symptoms, lower cognitive scores, more severe depressive and autonomic impairments, as well as higher daily levodopa equivalent dosage. FOG occurred more frequently in patients with wearing-off. VNG subgroup analysis demonstrated that PD+FOG had prolonged saccade latency and decreased saccade accuracy relative to PD-FOG or HC. SPEM gain at 0.1 and 0.2 Hz was also decreased in PD+FOG compared with HC. Furthermore, prolonged saccade latency was correlated with higher FOGQ scores in PD patients. Our results verify that PD with FOG patients suffer from more severe motor and non-motor symptoms, indicating more extensive neurodegeneration. Prolonged saccade latency could be a practical oculomotor parameter both for identification and progression of FOG in PD.
Introduction
Freezing of gait (FOG) is defined as a brief, episodic absence or marked reduction of forward progression of the feet despite having the intention to walk. It usually occurs relatively in late stage of Parkinson’s Disease (PD) and increases the risk of falls, contributing to impaired quality of life (Nieuwboer and Giladi, 2013). FOG in PD has been reported to be associated with several clinical features such as more severe motor symptoms, lower limb onset, cognitive impairment, and higher daily dose of levodopa (Giladi et al., 2001; Perez-Lloret et al., 2014; Amboni et al., 2015). However, the detailed motor and non-motor clinical correlates are not well investigated in China, and the potential pathophysiology is not fully understood yet. We currently lack biomarkers to predict this subset.
Accumulating evidence since 1983 showed that PD patients also had eye movement abnormalities including saccadic and smooth pursuit eye movement (SPEM) system (White et al., 1983) and the increased inhibition to superior colliculus (SC) activity from basal ganglia output may be the cause (Anderson and MacAskill, 2013). However, the clinical implication of these oculomotor impairments is less investigated in PD. Oculomotor impairment may influence balance and posture/gait control of a subject. It is reported that PD with postural instability had increased anti-saccade latency (Ewenczyk et al., 2017); and PD with FOG had reduced postural control (Schlenstedt et al., 2016). We hypothesized that PD with FOG may have more obvious oculomotor deficits.
Therefore, the present study firstly investigated the motor and non-motor symptom correlations with FOG by detailed scales in Shanghai, China; In addition, we tried to explore the oculomotor associations with FOG by videonystagmography (VNG), aiming to find biomarkers for this unique subset of PD.
Materials and Methods
Subjects
From Jan 2017 to Dec 2019, a total of 210 PD patients, who met the Movement Disorder Society (MDS) clinical diagnostic criteria (Postuma et al., 2015) were consecutively recruited from the movement disorders clinic (Department of Neurology, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China). None of the patients had undergone functional neurosurgery for PD. We excluded individuals with other chronic or acute brain diseases. 37 healthy controls were recruited from the community nearby. The age of all the participants ranged from 50 to 80 years old. All patients and controls underwent the Chinese equivalent of the Mini Mental State Examination (Katzman et al., 1988). We excluded those whose MMSE score fell below 20 for individuals that received 6 years of schooling or less (primary school education) and below 24 for individuals that received more than 6 years of schooling (junior high and above). Written informed consents were obtained from all participants. The study was approved by the Ethics Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine.
Clinical Profiles
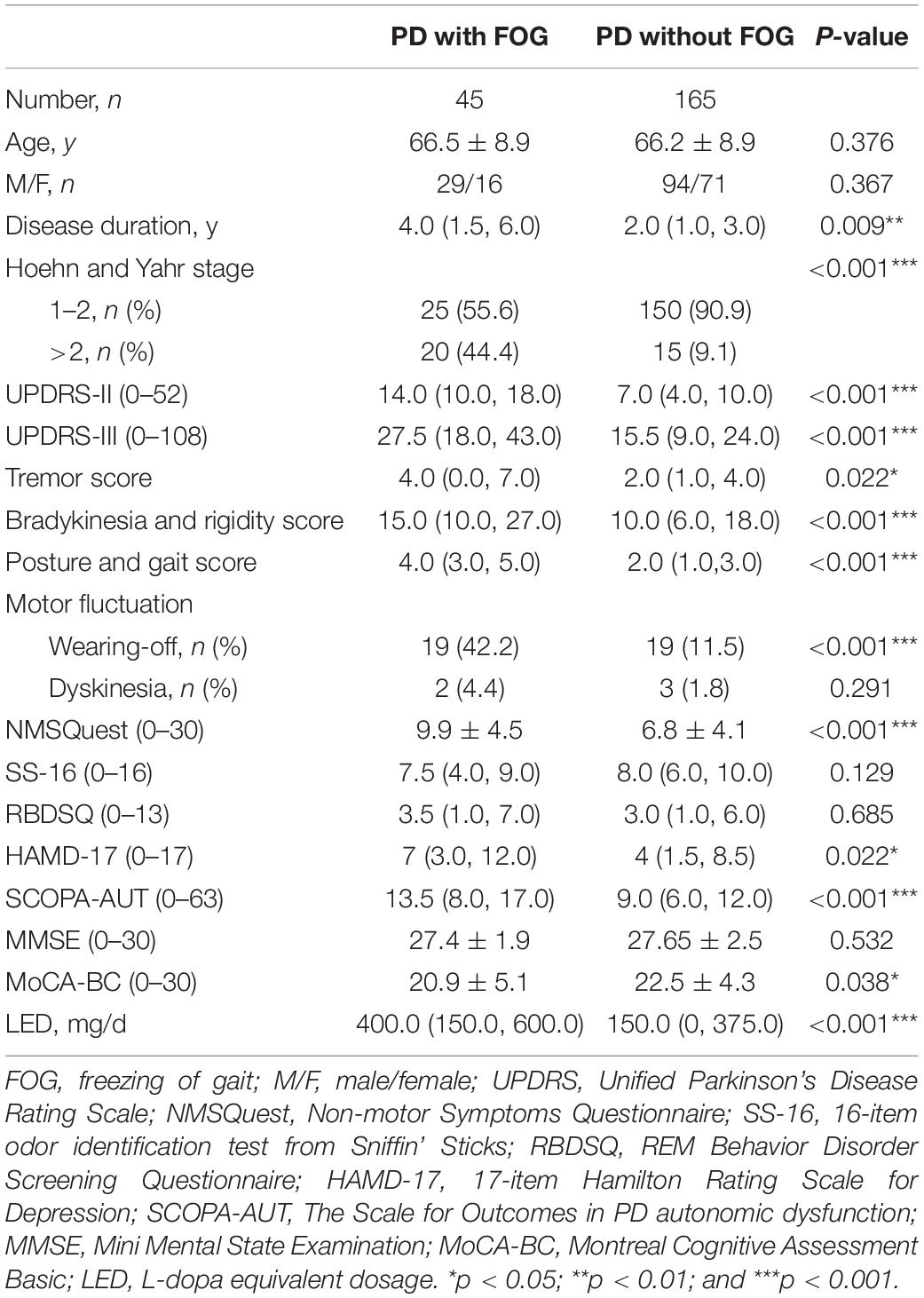
Demographic and clinical characteristics of PD were listed in Table 1. For these patients, motor severity was measured with modified Hoehn and Yahr (H&Y) stage (Hoehn and Yahr, 1967), and Unified Parkinson’s Disease Rating Scale part III (UPDRS-III; Richards et al., 1994) performed in the “on” state. Tremor, akinetic/rigid, postural, and gait instability scores were further calculated according to the report from Kang et al. (2005). UPDRS-II was used to evaluate activities of daily living. FOG was considered present, when subjects had a score of >0 on item 3 of Freezing of Gait questionnaire (FOG-Q; Giladi et al., 2000). The total burden of non-motor symptoms was measured with Non-Motor Symptoms Questionnaire (NMSQuest; Richards et al., 1994). Olfactory function was assessed by SS-16 (Burghart Messtechnik, Wedel, Germany) as our previous report (Chen et al., 2012). REM Behavior Disorder Screening Questionnaire (RBDSQ) was used to screen clinical possible RBD (cpRBD; Nomura et al., 2011). The severity of depressive symptom was assessed by 17-item Hamilton Rating Scale for Depression (HAMD-17; Hamilton, 1960). The Scales for Outcomes in PD Autonomic Dysfunction (SCOPA-AUT) was used as a measurement of autonomic disfunction (Verbaan et al., 2007). Total cognitive function was assessed by MMSE (Katzman et al., 1988) and Montreal Cognitive Assessment Basic (MoCA-BC; Chen et al., 2016). Daily L-dopa equivalent dosage (LED) was calculated as reported (Tomlinson et al., 2010).
Videonystagmography Evaluation
To minimize the influence of involuntary movements, subjects with head tremor or dyskinesia were excluded for oculomotor evaluation. Finally, 40 out of 210 PD patients and 37 healthy controls underwent oculomotor test by a VisualEyes 4 channel VNG (Micromedical Technologies), which acquired binocular movement samples at 120 Hz. Subjects remained seated in darkness for 2 min before testing. Horizontal VGS and SPEM at three frequencies (0.1, 0.2, and 0.4 Hz) were performed in all participants. We recorded these oculomotor parameters: saccade latency, saccade accuracy, and gain of SPEM at three different frequencies separately.
Statistical Analysis
Statistical analyses were performed utilizing SPSS (version 23.0 for Windows) and MedCalc (version 18.0 for Windows). GraphPad Prism (version 5.0 for Windows) was used for plotting the graphics. Shapiro–Wilk test was used to determine whether sample data is normally distributed. We used mean and standard deviation (SD) for numerical variables (age, etc.) with normal distribution; median and interquartile range (IQR, 25th–75th percentile) for those with skewed distributions. To compare categorical data among groups, we applied the chi-square test or Fisher’s exact test. We analyzed the continuous variables by in-dependent t test, one-way analysis of variance (ANOVA) or non-parametric Kruskal–Wallis test, depending on whether the data were normally distributed or not. The correlation between oculomotor dynamics and clinical parameters was analyzed by Spearman rank correlation. A 95% confidence interval was calculated for the correlation coefficient. Binary logistic regression analysis by forward stepwise was used to determine the independent associated factors of FOG in PD patients. The input variables were those with significant difference in univariate analyses. Odds ratios (ORs) and 95% confidence intervals (CIs) were reported accordingly. In all analyses, a two-tailed p-value < 0.05 was considered statistically significant.
Results
Demographic and Clinical Features of PD Patients With FOG
The age at examination of the patients was 65.49 ± 8.88 years, and 123 (58.57%) of whom were males. The disease duration was 3.06 ± 3.34 years. Thirty-seven (17.62%) of them reported family history. One hundred and seventy-five (83.33%) patients had H&Y stage 1–2; whereas 35 (16.67%) patients had H&Y stage >2. Thirty-eight (18.10%) patients had wearing-off and 5 (2.38%) had dyskinesia.
There were 45 patients with FOG positive (PD+FOG), occupying 21.4% of all enrolled PD. Compared with PD-FOG, PD+FOG had longer disease duration (p = 0.009), and more severe motor symptoms as revealed by UPDRS-III score (p < 0.001), and H&Y stage (p < 0.001); In addition, PD with FOG had more non-motor symptom burden as shown with higher score of NMSQuest (p < 0.001), HAMD-17 (p = 0.022), SCOPA-AUT (p < 0.001), and lower score of MoCA-BC (p = 0.038). Regarding medications, PD with FOG had more daily LED (p < 0.001; Table 1).
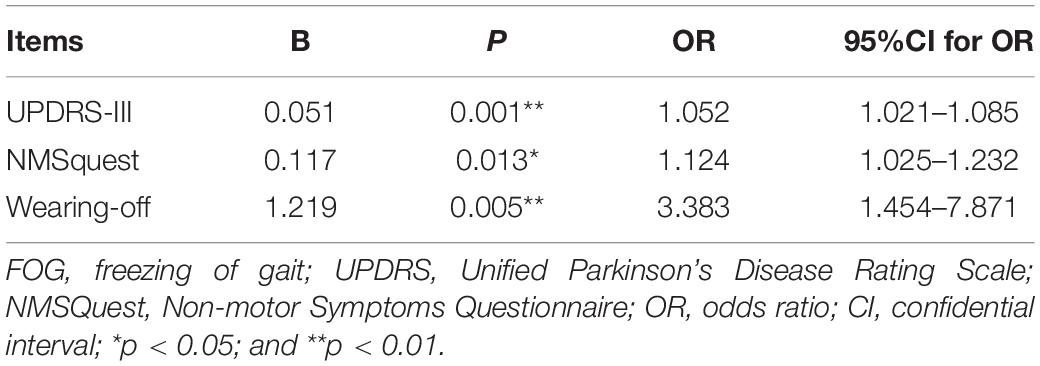
Binary logistic regression analysis showed that UPDRS-III (OR = 1.052; 95%CI: 1.021–1.085; p = 0.001), NMSquest (OR = 1.124; 95%CI:1.025–1.232; p = 0.013) and wearing- off (OR = 3.383; 95%CI:1.454–7.871; p = 0.005) were independent associated factors for FOG (Table 2).
Oculomotor Performances in PD Patients With FOG
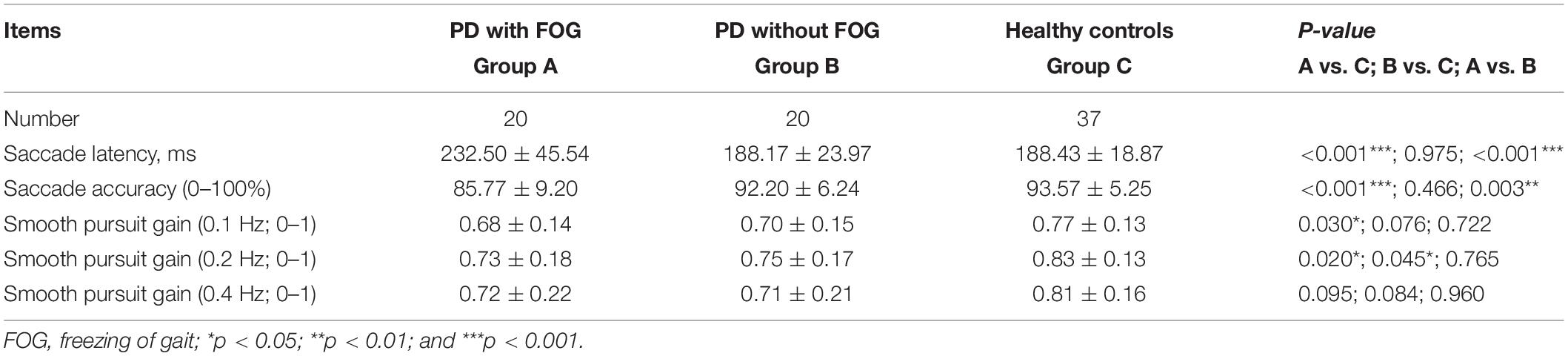
Forty PD patients underwent oculomotor evaluation, the current age of whom at examination was 66.35 ± 5.07 years, and 57.50% (n = 23) were males. The disease duration was 4.75 ± 3.16 years. Twenty-six (65.00%) patients had H&Y stage 1–2; whereas 14 (35.00%) patients had H&Y stage >2. Seventeen (42.50%) patients had wearing-off (Supplementary Table 1). There were no significant differences in age, gender distribution or MMSE score among the three groups: PD+FOG, PD-FOG, and HC (Supplementary Table 1). Also, the disease duration, H&Y stage and UPDRS-III was comparable between PD with and without FOG (Supplementary Table 1).
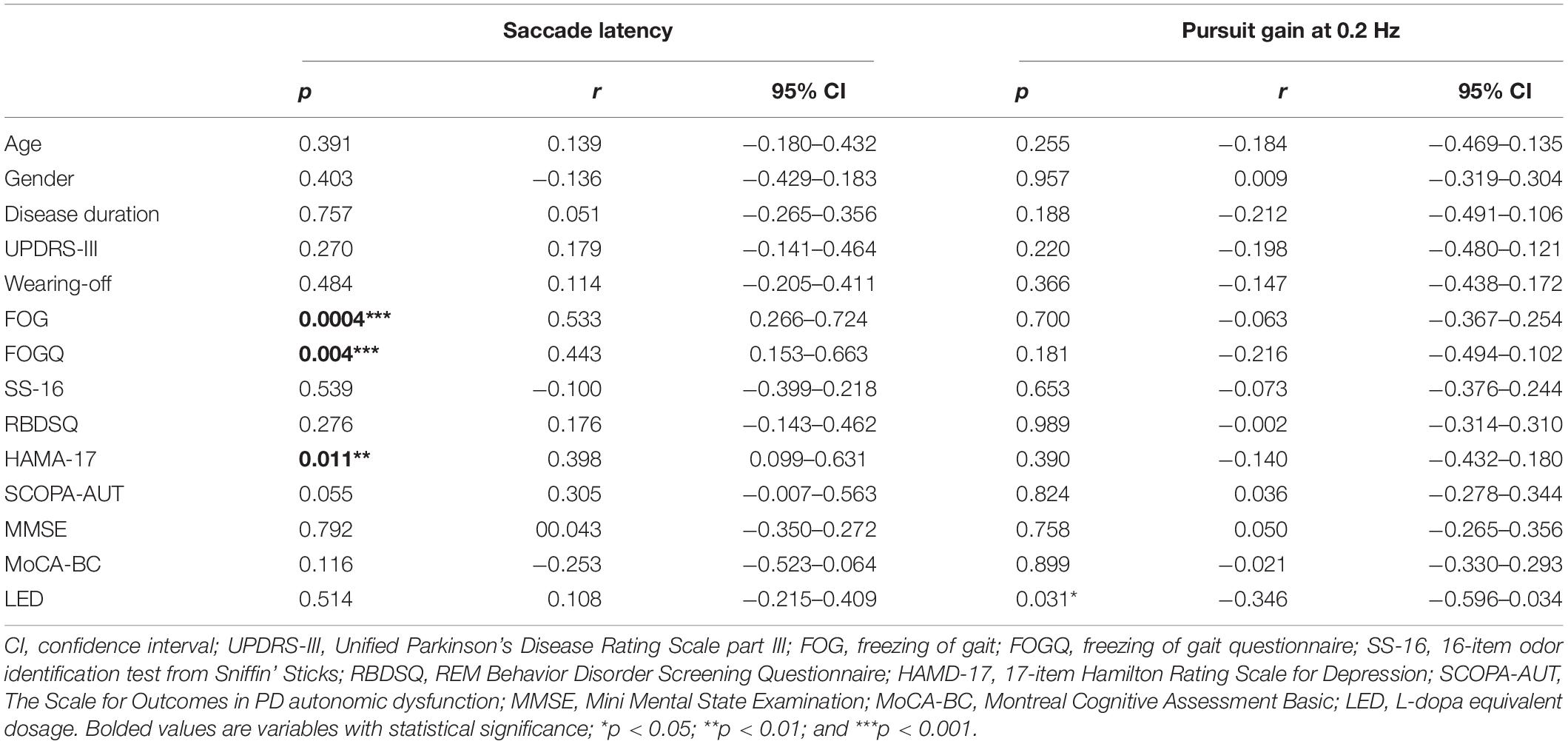
Oculomotor analysis revealed that saccade latency was not associated with other oculomotor dynamics such as saccade accuracy or SPEM gain. PD+FOG group had prolonged saccade latency (both p < 0.001) and decreased saccade accuracy (p = 0.003, p < 0.001, respectively) relative to PD-FOG or HC group (Table 3 and Figures 1A,B). Meanwhile, SPEM gain at 0.1 Hz (p = 0.03), and 0.2 Hz (p = 0.02) was decreased in PD+FOG compared with HC group (Table 3 and Figures 1D,E). PD-FOG also had decreased SPEM gain at 0.2 Hz (p = 0.045; Table 3 and Figure 1E) in comparison with HC. A marginal trend was noted for SPEM gain at 0.4 Hz (Table 3 and Figure 1F). There was a moderate positive correlation (r = 0.44, p = 0.004) between saccade latency and FOGQ score in PD patients (Table 4 and Figure 1C).
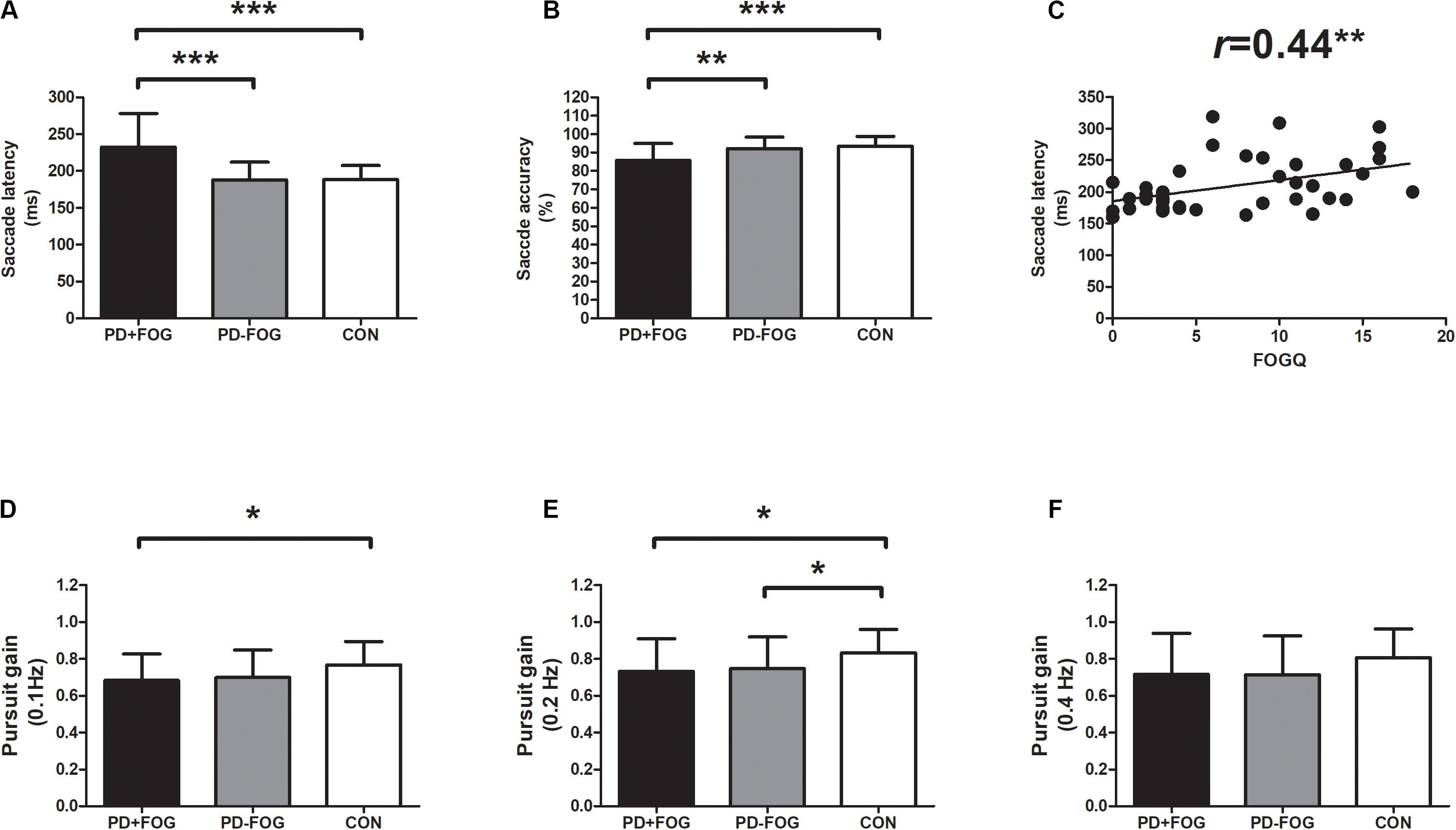
Figure 1. Oculomotor parameters in PD with FOG. (A) Saccade latency was increased in PD+FOG group, compared with PD-FOG or healthy control group; (B) Saccade accuracy was decreased both in PD+FOG or PD-FOG group, compared with healthy control group; (C) saccade latency was positively associated with FOGQ score in PD; (D,E) PD had decreased gain of smooth pursuit eye movement at 0.1 Hz (D) and 0.2 Hz (E) relative to healthy controls; (F) PD+FOG group had marginally decreased gain of smooth pursuit eye movement at 0.4 Hz, relative to healthy control group (p = 0.095). Notes: PD+FOG, Parkinson’s disease with freezing of gait; PD-FOG, Parkinson’s disease without freezing of gait; CON, healthy controls. Error bar represents standard deviation. *p < 0.05; **p < 0.01; and ***p < 0.001.
To further investigate the correlation between oculomotor dynamics and clinical parameters in PD, Spearman rank correlation tests were employed, We found that saccade latency was closely related to FOG status and FOGQ, In addition, it was also significantly correlated with HAMA-17 score (r = 0.398, p = 0.011; Table 4); SPEM gain at 0.2 Hz was negatively associated with LED (r = −0.346, p = 0.031; Table 4). However, saccade latency, as well as SPEM gain, was not correlated with other clinical parameter such as age, disease duration, motor, or cognitive impairments in PD.
Discussion
This is a cross-sectional study with both clinical and ocular movement evaluation from a cohort of PD patients in Shanghai, China. Based on 210 PD patients with detailed phenotype analysis, we verified that PD+FOG had more severe motor and non-motor symptom burden in comparison with PD-FOG; VNG subgroup analysis found that PD+FOG had prolonged saccade latency relative to PD-FOG and HC, and delayed saccade latency was correlated with higher FOGQ scores in PD patients.
Given that PD with freezers had longer disease duration, more severe motor symptom and more frequency of wearing-off in our results, confirming that striatal dopaminergic denervation is critical in the pathophysiology of FOG in PD. Besides motor symptoms, non-motor symptoms, such as cognitive, affective, and autonomic functions were also more severely implicated in freezers compared to non-freezers in our PD cohort. These findings are consistent with other longitudinal studies in other Countries (Ehgoetz Martens et al., 2018; Kim et al., 2018). Base on a PPMI cohort, presynaptic striatal dopaminergic depletion could predict the later development of FOG in de novo PD, patients with severe DAT uptake in striatum had a high incidence of FOG during follow-up (Kim et al., 2018). Besides, extra-striatal pathological conditions may also contribute to FOG. As shown in an in-vivo PET study, PD with freezers had more severe loss of striatal dopaminergic and cortical cholinergic binding, and the presence of cortical amyloidopathy, compared with non-freezers (Bohnen et al., 2014). These clinical and neuroimaging studies indicated that FOG is not merely a gait problem, but may represent a unique entity in PD with extensive neurodegeneration both in dopaminergic and non-dopaminergic system.
This is the first study in China to show that PD with FOG had increased latency of VGS. Previously, Nemanich et al. in 2016 from United States found that PD with FOG had prolonged saccade latency (Nemanich and Earhart, 2016) relative to PD without FOG and healthy controls. Ours is the second one worldwide to indicate such association. In addition, we also found that increased saccade latency was associated with a higher FOGQ score in PD, indicating saccade latency was fit for identification and progression of FOG in PD. It is well-known that frontal and parietal eye fields are the key regions for saccade generation. Saccade latency in VGS is negatively associated with gray matter volume in frontal and parietal eye fields and cerebellar vermis, as demonstrated in one saccade-neuroimaging study (Perneczky et al., 2011). Accumulating neuroimaging evidence indicated that PD with FOG had widespread structural and functional impairment in cortical and subcortical brain structures, especially the specific nodes of frontoparietal cortex, basal ganglia, mesencephalic, and cerebellar locomotor region (Bharti et al., 2019). Whereas, functional connectivity abnormality of cerebellar locomotor region was also critical to lesion-induced FOG patients, as disclosed in a lesion network mapping study (Fasano et al., 2017). Therefore, structural and functional impairment in frontoparietal cortex or cerebellar locomotor region could impact both oculomotor and FOG networks, which may explain the close association between prolonged saccade latency and FOG phenotype in PD. Multimodal MRI studies are warranted in future to verify this hypothesis.
Our result has important clinical implications. As VGS is relatively easy to perform and saccade latency is an objective oculomotor parameter, VNG may provide important information for detection and progression of FOG. There are some methodological limitations in the present study. As a cross-sectional study, only associated factor with FOG in PD could be summarized and we didn’t further stratify the FOG subtypes. Also, the sample size of FOG positive group in VNG study is limited. Studies with a larger sample size is warranted in future to verify the diagnostic value of saccade latency in FOG identification. Besides, wearable devices in combination with FOGQ could be more objective to investigate the close relationship between gait parameters and oculomotor dynamics (Iijima et al., 2017).
Conclusion
In summary, our results verify that FOG is a unique entity in PD with more severe motor and non-motor symptoms. Prolonged saccade latency could be a practical oculomotor parameter both for identification and progression of FOG in PD.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LW contributed to conception, organization, and execution of research project, design, execution, and review and critique of statistical analysis, and writing of the first draft of the manuscript. QW contributed to conception of research project, design, execution, and review and critique of statistical analysis, and writing of the first draft of the manuscript. LZ contributed to organization and execution of research project, design, execution, and review and critique of statistical analysis, and writing of the first draft of the manuscript. C-YJ and QX contributed to organization and execution of research project. S-CW contributed to execution and review and critique of statistical analysis. Y-RD contributed to organization and execution of research project and review and critique of the manuscript. QH contributed to organization of research project, design and execution of statistical analysis, and writing of the first draft and review and critique of the manuscript. WC contributed to conception and organization of research project, execution and review and critique of statistical analysis, and writing of the first draft and review and critique of the manuscript. J-RL contributed to conception of research project, review and critique of statistical analysis, and review and critique of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
WC received grants from National Natural Science Foundation of China (81401039), Shanghai Pujiang Program (18PJD023), and Shanghai medical guidance program (17411964000). QH received grants from National Natural Science Foundation of China (81501085). LW received grants from Shanghai Sailing Program (18YF1412600) and the Interdisciplinary Program of Shanghai Jiao Tong University (YG2017QN13).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank all the subjects participating in the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2020.00237/full#supplementary-material
References
Amboni, M., Stocchi, F., Abbruzzese, G., Morgante, L., Onofrj, M., Ruggieri, S., et al. (2015). Prevalence and associated features of self-reported freezing of gait in Parkinson disease: the DEEP FOG study. Parkinsonism Relat. Disord. 21, 644–649. doi: 10.1016/j.parkreldis.2015.03.028
Anderson, T. J., and MacAskill, M. R. (2013). Eye movements in patients with neurodegenerative disorders. Nat. Rev. Neurol. 9, 74–85. doi: 10.1038/nrneurol.2012.273
Bharti, K., Suppa, A., Tommasin, S., Zampogna, A., Pietracupa, S., Berardelli, A., et al. (2019). Neuroimaging advances in Parkinson’s disease with freezing of gait: a systematic review. NeuroImage Clin. 24:102059. doi: 10.1016/j.nicl.2019.102059
Bohnen, N. I., Frey, K. A., Studenski, S., Kotagal, V., Koeppe, R. A., Constantine, G. M., et al. (2014). Extra-nigral pathological conditions are common in Parkinson’s disease with freezing of gait: an in vivo positron emission tomography study. Mov. Disord. 29, 1118–1124. doi: 10.1002/mds.25929
Chen, K.-L., Xu, Y., Chu, A.-Q., Ding, D., Liang, X.-N., Nasreddine, Z. S., et al. (2016). Validation of the chinese version of montreal cognitive assessment basic for screening mild cognitive impairment. J. Am. Geriatr. Soc. 64, e285–e290. doi: 10.1111/jgs.14530
Chen, W., Chen, S., Kang, W.-Y., Li, B., Xu, Z.-M., Xiao, Q., et al. (2012). Application of odor identification test in Parkinson’s disease in China: a matched case-control study. J. Neurol. Sci. 316, 47–50. doi: 10.1016/j.jns.2012.01.033
Ehgoetz Martens, K. A., Lukasik, E. L., Georgiades, M. J., Gilat, M., Hall, J. M., Walton, C. C., et al. (2018). Predicting the onset of freezing of gait: a longitudinal study. Mov. Disord. 33, 128–135. doi: 10.1002/mds.27208
Ewenczyk, C., Mesmoudi, S., Gallea, C., Welter, M.-L., Gaymard, B., Demain, A., et al. (2017). Antisaccades in Parkinson disease: a new marker of postural control? Neurology 88, 853–861. doi: 10.1212/WNL.0000000000003658
Fasano, A., Laganiere, S. E., Lam, S., and Fox, M. D. (2017). Lesions causing freezing of gait localize to a cerebellar functional network. Ann. Neurol. 81, 129–141. doi: 10.1002/ana.24845
Giladi, N., McDermott, M. P., Fahn, S., Przedborski, S., Jankovic, J., Stern, M., et al. (2001). Freezing of gait in PD: prospective assessment in the DATATOP cohort. Neurology 56, 1712–1721. doi: 10.1212/wnl.56.12.1712
Giladi, N., Shabtai, H., Simon, E., Biran, S., Tal, J., and Korczyn, A. (2000). Construction of freezing of gait questionnaire for patients with Parkinsonism. Parkinsonism Relat. Disord. 6, 165–170. doi: 10.1016/s1353-8020(99)00062-0
Hamilton, M. (1960). A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62. doi: 10.1136/jnnp.23.1.56
Hoehn, M. M., and Yahr, M. D. (1967). Parkinsonism: onset, progression and mortality. Neurology 17, 427–442. doi: 10.1212/wnl.17.5.427
Iijima, M., Mitoma, H., Uchiyama, S., and Kitagawa, K. (2017). Long-term monitoring gait analysis using a wearable device in daily lives of patients with parkinson’s disease: the efficacy of selegiline hydrochloride for gait disturbance. Front. Neurol. 8:542. doi: 10.3389/fneur.2017.00542
Kang, G. A., Bronstein, J. M., Masterman, D. L., Redelings, M., Crum, J. A., and Ritz, B. (2005). Clinical characteristics in early Parkinson’s disease in a central California population-based study. Mov. Disord. 20, 1133–1142. doi: 10.1002/mds.20513
Katzman, R., Zhang, M. Y., Ouang, Y.-Q., Wang, Z. Y., Liu, W. T., and Yu, E. (1988). A Chinese version of the mini-mental state examination; impact of illiteracy in a Shanghai dementia survey. J. Clin. Epidemiol. 41, 971–978. doi: 10.1016/0895-4356(88)90034-0
Kim, R., Lee, J., Kim, Y., Kim, A., Jang, M., Kim, H.-J., et al. (2018). Presynaptic striatal dopaminergic depletion predicts the later development of freezing of gait in de novo Parkinson’s disease: an analysis of the PPMI cohort. Parkinsonism Relat. Disord. 51, 49–54. doi: 10.1016/j.parkreldis.2018.02.047
Nemanich, S. T., and Earhart, G. M. (2016). Freezing of gait is associated with increased saccade latency and variability in Parkinson’s disease. Clin. Neurophysiol. 127, 2394–2401. doi: 10.1016/j.clinph.2016.03.017
Nieuwboer, A., and Giladi, N. (2013). Characterizing freezing of gait in Parkinson’s disease: models of an episodic phenomenon. Mov. Disord. 28, 1509–1519. doi: 10.1002/mds.25683
Nomura, T., Inoue, Y., Kagimura, T., Uemura, Y., and Nakashima, K. (2011). Utility of the REM sleep behavior disorder screening questionnaire (RBDSQ) in Parkinson’s disease patients. Sleep Med. 12, 711–713. doi: 10.1016/j.sleep.2011.01.015
Perez-Lloret, S., Negre-Pages, L., Damier, P., Delval, A., Derkinderen, P., Destée, A., et al. (2014). Prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson disease. JAMA Neurol. 71, 884–890. doi: 10.1001/jamaneurol.2014.753
Perneczky, R., Ghosh, B. C. P., Hughes, L., Carpenter, R. H. S., Barker, R. A., and Rowe, J. B. (2011). Saccadic latency in Parkinson’s disease correlates with executive function and brain atrophy, but not motor severity. Neurobiol. Dis. 43, 79–85. doi: 10.1016/j.nbd.2011.01.032
Postuma, R. B., Berg, D., Stern, M., Poewe, W., Olanow, C. W., Oertel, W., et al. (2015). MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601. doi: 10.1002/mds.26424
Richards, M., Marder, K., Cote, L., and Mayeux, R. (1994). Interrater reliability of the Unified Parkinson’s Disease Rating Scale motor examination. Mov. Disord. 9, 89–91. doi: 10.1002/mds.870090114
Schlenstedt, C., Muthuraman, M., Witt, K., Weisser, B., Fasano, A., and Deuschl, G. (2016). Postural control and freezing of gait in Parkinson’s disease. Parkinsonism Relat. Disord. 24, 107–112. doi: 10.1016/j.parkreldis.2015.12.011
Tomlinson, C. L., Stowe, R., Patel, S., Rick, C., Gray, R., and Clarke, C. E. (2010). Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 25, 2649–2653. doi: 10.1002/mds.23429
Verbaan, D., Marinus, J., Visser, M., van Rooden, S. M., Stiggelbout, A. M., and van Hilten, J. J. (2007). Patient-reported autonomic symptoms in Parkinson disease. Neurology 69, 333–341. doi: 10.1212/01.wnl.0000266593.50534.e8
Keywords: Parkinson’s disease, freezing of gait, videonystagmography, saccade latency, non-motor symptom
Citation: Wu L, Wang Q, Zhao L, Jiang C-Y, Xu Q, Wu S-C, Dong Y-R, He Q, Chen W and Liu J-R (2020) Clinical and Oculomotor Correlates With Freezing of Gait in a Chinese Cohort of Parkinson’s Disease Patients. Front. Aging Neurosci. 12:237. doi: 10.3389/fnagi.2020.00237
Received: 03 May 2020; Accepted: 08 July 2020;
Published: 31 July 2020.
Edited by:
Xiao-Xin Yan, Central South University, ChinaReviewed by:
Rosario Vasta, University of Turin, ItalyJuan Rafael Orozco-Arroyave, University of Antioquia, Colombia
Copyright © 2020 Wu, Wang, Zhao, Jiang, Xu, Wu, Dong, He, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing He, aGVxaW5nMTk4MzA1MzFAMTYzLmNvbQ==; Wei Chen, ZGF2aWRfY2hlbjgxMDZAaG90bWFpbC5jb20=
†These authors have contributed equally to this work
 Li Wu
Li Wu Qin Wang2†
Qin Wang2† Wei Chen
Wei Chen