
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 23 June 2020
Sec. Neurocognitive Aging and Behavior
Volume 12 - 2020 | https://doi.org/10.3389/fnagi.2020.00165
This article is part of the Research TopicPotential Neurotoxic Effects of Perioperative FactorsView all 25 articles
 Rui Gao1†
Rui Gao1† Chan Chen1*†
Chan Chen1*† Qi Zhao1
Qi Zhao1 Ming Li1
Ming Li1 Qiao Wang1
Qiao Wang1 Lu Zhou1
Lu Zhou1 Erya Chen1
Erya Chen1 Hai Chen1
Hai Chen1 Yue Zhang1
Yue Zhang1 Xingwei Cai1
Xingwei Cai1 Changliang Liu1
Changliang Liu1 Xu Cheng1
Xu Cheng1 Shu Zhang2
Shu Zhang2 Xiaobo Mao3
Xiaobo Mao3 Yanhua Qiu1
Yanhua Qiu1 Lu Gan1
Lu Gan1 Hai Yu1
Hai Yu1 Jin Liu1
Jin Liu1 Tao Zhu1*
Tao Zhu1*Background: Postoperative cognitive dysfunction (POCD) is one of the severe complications after surgery, inducing low life quality and high mortality, especially in elderly patients. However, the underlying molecular mechanism of POCD remains largely unknown, and the ideal biomarker for clinical diagnosis and prognosis is lacking. Circular RNAs (circRNAs), as a unique class of non-coding RNAs, were characterized by its stability and conservativeness, serving as novel biomarkers in various diseases. Nevertheless, the role of circRNAs in the occurrence of POCD remains elusive.
Methods: To investigate the differentially expressed circRNAs in the serum of POCD patients and its potential role in the development of POCD, we performed a circRNA microarray to screen the differentially expressed circRNAs in the serum samples from three patients of the POCD group and three paired patients of the non-POCD group. Subsequently, quantitative real-time polymerase chain reaction analysis (qRT-PCR) was utilized to verify the microarray data with the serum samples from 10 paired patients. Cytoscape software was used to construct the circRNA–miRNA–mRNA network for circRNAs with different expression levels as well as the target genes. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses showed the biological functions of the differentially expressed circRNAs target genes.
Results: In total, we have analyzed 10,198 circRNAs through the microarray. Compared with the non-POCD patient group, there were 210 differentially expressed circRNAs with 133 upregulated and 77 downregulated in the POCD group (≥2-fold differential expression, P ≤ 0.05). The qRT-PCR confirmed 10 circRNAs with different expressed levels, and the results were consistent with the microarray findings. Among them, hsa_circRNA_001145, hsa_circRNA_101138, and hsa_circRNA_061570 had the highest magnitude of change. The GO analysis showed that the differentially expressed circRNAs were associated with the regulation of the developmental process, cell-to-cell adhesion, and nervous system development. The KEGG analysis showed that the target genes of circRNAs were enriched in the MAPK signaling pathway and RAS signaling pathway. According to the targetscan7.1 and mirdbV5 databases, the circRNA–miRNA–mRNA network was constructed, and these results provided a vital landscape of circRNA expression profile in POCD.
Conclusions: Our study provides an essential perspective for the differential expression of circRNAs in POCD patients. Further studies need to be performed to explore their potential therapeutic roles in the development of POCD.
Postoperative cognitive dysfunction (POCD) is a severe and well-known complication following cardiac surgery with poor outcomes, especially in elderly patients (Steinmetz et al., 2009). What is worse, the neurocognitive function impairment after surgery can be persistent and result to a declined quality of life for the patients (Ballard et al., 2012). As one type of mild cognitive impairment, POCD could potentially increase the risk of Alzheimer’s disease (AD) (Grundman et al., 2004; Ward et al., 2012; Kapila et al., 2014). Therefore, many studies have been performed to explore its possible molecular mechanism. Recently, neuroinflammation, neuronal apoptosis, amyloid-Aβ (Aβ) deposition, and hyperphosphorylation of tau proteins have been reported as the main mediators in POCD (Xie et al., 2013; Chen C. et al., 2019; Li et al., 2019). Nevertheless, the pathogenesis of POCD remains elusive.
As a unique class of non-coding RNAs (ncRNAs), circular RNAs (circRNAs) are characterized by a covalently closed continuous loop, playing an important role in inhibiting the function of microRNAs (miRNAs), regulating the activity of RNA polymerase, binding the promoter sequence of corresponding genes, and influencing the expression of corresponding genes (Memczak et al., 2013; Bahn et al., 2015). Besides that, it has the characteristics of universality, conservativeness, and tissue specificity. Compared with linear RNAs, the stability of circRNAs is much higher so that it can exist stably in body fluid, serving as a valuable novel biomarker (Li P. et al., 2015; Li Y. et al., 2015; Xuan et al., 2016). Previous studies showed that circRNAs were involved in regulating various pathological processes of the central nerve cells and mediating the occurrence and the development of neurodegenerative diseases such as Parkinson’s disease and AD (Kumar et al., 2017; Piwecka et al., 2017). Meanwhile, the circRNA ciRS-7 has been found to promote the degradation of APP and BACE1 proteins and reduce the aberrant accumulation of β-amyloid peptide acting as a sponge adsorbent of microRNA-7 in AD (Shi et al., 2017). Furthermore, accumulative studies have reported similar pathomechanisms of persistent POCD as that of AD, such as the cell apoptosis, inflammation, and accumulation of misfolded proteins in the aging brain (Bilotta et al., 2010). However, the role of circRNAs in POCD is largely unknown.
Therefore, in our study, we performed circRNAs microarray to investigate the differential expression profiles of circRNAs from serum samples between the POCD and the paired non-POCD elderly patients undergoing cardiac valve replacement surgery under cardiopulmonary bypass. Subsequently, the miRNA binding sites and the related mRNAs were predicted by bioinformatics analysis. Besides that, we constructed the predicted circRNA–miRNA–mRNA co-expression network and investigated the principal functions and the pathways of target genes. These results may help to provide insights for clinical circRNA biomarkers and therapeutic targets for POCD.
The study was approved by the Ethics Committee of West China Hospital, Sichuan University, China (ChiCTR-OOC-16008289). Written informed consent was obtained from all the participants before the surgery. The patients scheduled to undergo an elective cardiac surgery under general anesthesia and cardiopulmonary bypass were enrolled in this study. The inclusion criteria were (1) more than 50 years of age, (2) American Society of Anesthesiologists physical status I–III, and (3) scheduled for cardiac surgery under general anesthesia. The exclusion criteria were (1) patients with second surgery, (2) ejection fraction less than 40%, (3) preoperative Mini-Mental State Examination (MMSE) scores less than 24, (4) patients with psychiatric disease or central nervous system disease, and (5) patients who were unable or rejected to complete preoperative neuropsychological testing.
To assess patient cognitive function, a series of neuropsychological tests was performed on the day before surgery and 7 days after surgery by an experienced investigator. Various cognitive domains were evaluated by several neuropsychological tests including (1) MMSE, (2) Word Memory Test, a test used to assess the short-term and the long-term memory, (3) Digit Span Test, a test of the Wechsler memory scale used to measure concentration and attention, (4) Brief Visuospatial Memory Test—Revised (BVMT—R), a test to measure the immediate visuospatial and learning memory, (5) Symbol–Digit Modalities Test, a measure for psychomotor speed such that high scores indicated the better function, (6 and 7) BVMT—R Delayed Recall Test and BVMT—R Discrimination Index, the test used to estimate delayed recall abilities, (8) Trail Making Test, used to measure hand–eye coordination, concentration, and attention, and (9) Verbal Fluency Test, used to assess fluency and executive function. MMSE was performed to exclude patients with severe cognitive impairment. Then, all the subjects’ postoperative defects were defined as negative change scores, and the absolute value of each change score was greater than the standard deviation (SD) of the same cognitive test baseline score. A patient with two or more neuropsychological test defects was considered to be in the POCD group (Zhang et al., 2012; Saleh et al., 2015); otherwise, the patient was assigned to the non-POCD (NPOCD) group.
All the patients underwent the same preoperative preparation, including corrective electrolysis disorder, maintenance of cardiac function, preoperative intramuscular injection of 3 mg scopolamine and 5 mg morphine, radial artery puncture catheterization, venous access, electrocardiogram, and oxygen saturation monitoring. We used midazolam (0.2–0.3 mg/kg), sufentanil (0.5–0.8 μg/kg), and cis-atracurium (2–3 mg/kg) to induction. The anesthesia was maintained by a continuous intravenous infusion of propofol and remifentanil, with an intermittent addition of midazolam, sufentanil, and cis-atracurium. Anesthesia machine was used for mechanical ventilation, such that the respiratory rate was 12 times per minute, and the tidal volume was 6–8 mlkg. After the anesthesia, central venous catheterization and manometry were performed. Mean artery pressure was controlled at 50–80 mmHg during a cardiopulmonary bypass. The perfusion dosage was 2.0–2.6 L/min/m2, and the hematocrit was 25–30%, with moderate hemodilution. After a cardiopulmonary bypass, protamine was used to neutralize heparin, such that the ratio was 1:1. The patients were transferred to the intensive care unit for further treatment after surgery.
All the patients signed an informed consent before the neuropsychological tests, and the blood samples were collected on the day before surgery and 7 days after surgery. Meanwhile, all the blood samples were examined, and it was made sure that they were not in the hemolysis state. After having been collected by one professional registered nurse, the whole blood was left undisturbed at room temperature for about 30 min. Following centrifugation at 3,000 × g for 10 min at 4°C, the supernatant (serum) was collected. We also confirmed that the color of the serum was clear and yellowish. Then, the supernatants (serum) were transferred to a refrigerator at −80°C for further analysis. The total RNA was extracted using the miRNeasy Serum/Plasma Kit (50) (Qiagen, Germany). The concentrations of the RNA samples were determined spectrophotometrically at 260, 280, and 230 nm by using a NanoDrop ND-1000 instrument.
The case–control matching of POCD and NPOCD groups was performed based on age (±2) and sex. Three pairs of serum samples were randomly selected for the microarray detection. Sample preparation and microarray hybridization were carried out in accordance with the standard protocol of Arraystar (Arraystar Inc., United States). In short, total RNAs were digested by RNase R (Epicentre, Inc., United States) to remove linear RNA and enrich circular RNAs. A random priming method was used to amplify and transcribe the enriched circular RNAs into fluorescent cRNAs (Arraystar Super RNA Labeling Kit; Arraystar). Then, the labeled cRNAs were purified by RNeasy Mini Kit (Qiagen, Germany). Five microliters of 10 × blocking agent and 1 μl of 25× fragmentation buffer were added in the labeled cRNAs, and then the mixture was heated at 60°C for 30 min. Finally, the labeled cRNAs were diluted by 25 μl of 2 × hybridizing buffer. Then, 50 μl of hybridizing solution was injected into the spacer slide and assembled on the circRNA expression microarray slide. The slides were incubated in an Agilent incubator at 65°C for 17 h. Agilent Scanner G2505C was utilized to wash, fix, and scan the hybridized arrays (Agilent Technologies, Santa Clara, CA, United States).
The quantile standardization of the original data and the subsequent data processing were carried out by using the R software package through the log2 ratio. After quantile normalization of the original data, low-intensity filtering was performed, and the circRNAs with “P” or “M” marks (“All Target Values”) were retained for further analysis in three of at least six samples. When comparing the contour differences between the two groups, the “fold change” (i.e., the ratio of the group averages) between the groups for each circRNA was calculated. The statistical significance of the difference was conveniently estimated by t-test. The circRNAs with fold changes greater or equal to two and P-value ≤0.05 were chosen as the significant differential expression.
Total RNA was extracted from serum using the miRNeasy Serum/Plasma Kit (50) (Qiagen, Germany). Quantitative real-time PCR (qRT-PCR) was conducted with an Eppendorf RT-PCR system (Hauppauge, NY, United States). We used Caenorhabditis elegans-miRNA 39 mimic (Ce-miR-39) as miRNeasy Serum Spike-In control. The specific primer pairs used in the study are listed in Table 1.
The circRNA–miRNA interaction was predicted with Arraystar’s (Rockville, MD, United States) miRNA target prediction software, according to TargetScan (Enright et al., 2003) & miRanda (Pasquinelli, 2012). The target miRNAs were predicted by miRNA support vector regression (mirSVR) algorithm to determine their score and rank the efficiency. Thus, five miRNAs were identified for each differentially expressed circRNA according to the mirSVR score. The network of the top five highest miRNA connecting to one circRNA was constructed.
We used targetscan7.11 and mirdbV52 databases to predict miRNAs’ target genes. We generally accept the overlapping results of two databases. To construct the interaction network, the cumulative weight context++ score <−0.3 and target score >70 were set as the cutoff. Then, Cytoscape software was used to construct the circRNA–miRNA–mRNA network for the top 10 differentially expressed circRNAs that we had validated as well as their predicted target genes. In addition, we performed gene ontology (GO) analysis3 to explore the potential functional roles of the target genes, including molecular functions (MF), cellular components (CC), and biological processes (BP). The potential biological pathway analysis related to circRNAs target genes was performed using the KEGG database. The p-value (EASE score, Fisher P-value, or hypergeometric P-value) indicated the significant correlation between the pathway and the conditions. The lower the P-value, the more significant the pathway is. P ≤ 0.05 showed that the GO terms and the KEGG pathways of the differentially expressed genes were significantly enriched. The microarray analysis was performed by Kang Cheng Bio-tech (Shanghai, China).
The categorical variables were presented as frequencies and analyzed using the chi-square test. The data are expressed as mean ± SD. All statistical data analyses were performed using GraphPad Prism 6.0. An analysis of the qRT-PCR validation between the POCD and the NPOCD groups was performed using Student’s t-test. The case–control matching was performed using SPSS 22.0 software. A rate value of less than 0.05 (P < 0.05) was considered as statistically significant.
In total, there were 98 patients finally included who completed all perioperative cognitive tests and blood collections (Figure 1). Among them, 39 patients (39.8%) developed POCD, and 59 patients were assigned to the NPOCD group. No difference was observed between the two groups in terms of age, gender, body mass index, education level, and the baseline of each neuropsychological test (Table 2).
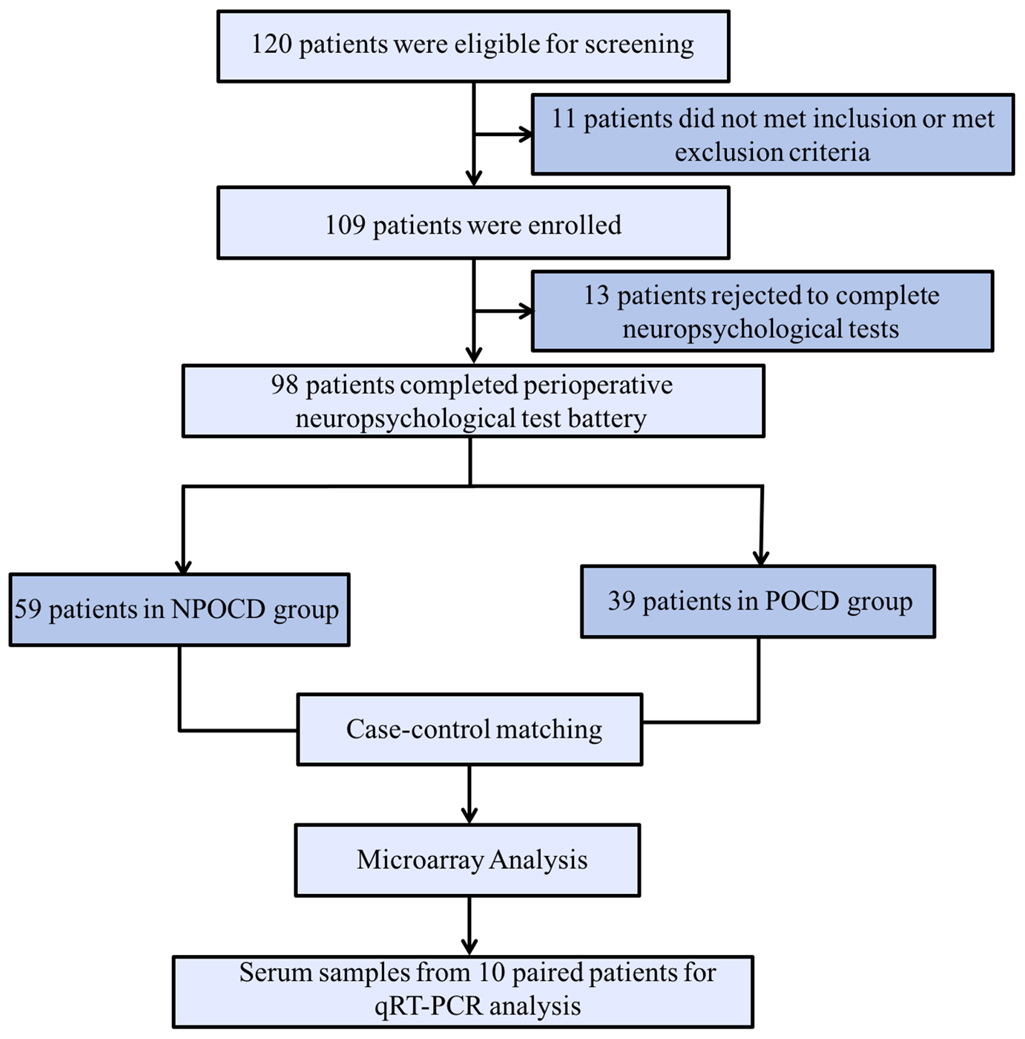
Figure 1. The process flow diagram of the experiment. POCD, postoperative cognitive dysfunction; NPOCD, the patients without postoperative cognitive decline; qRT-PCR, quantitative real-time PCR.
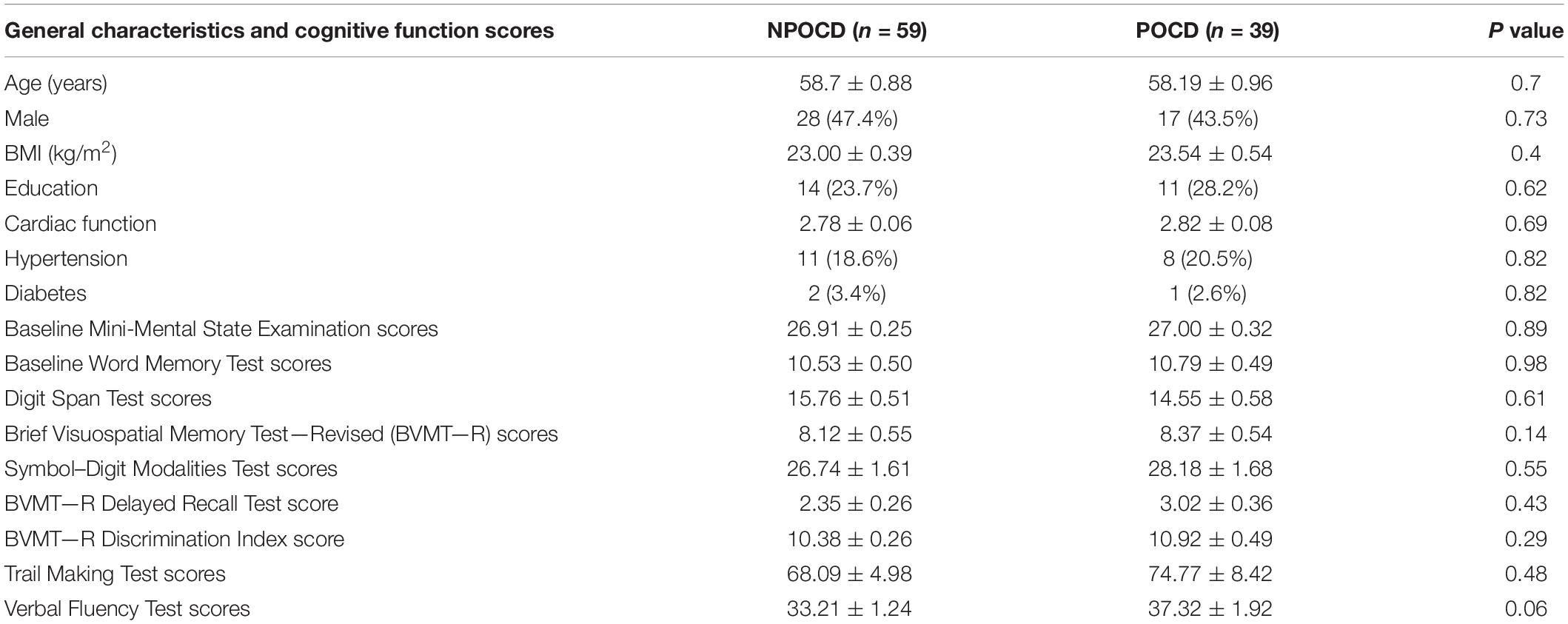
Table 2. General characteristics and cognitive functions of the participants from the postoperative cognitive dysfunction (POCD) and non-POCD groups at baseline.
Total RNAs were extracted from the serum samples of the two groups. The OD260/OD280 ratios of each sample were between 1.8 and 2.1, and the OD260/OD230 ratios were greater than 1.8, which demonstrated the RNA quality (Supplementary Figure 1). Using a human circRNA microarray, we drew the box plot to show the distribution of the intensities. We observed that the distribution of log2 ratios was similar after normalization in the tested samples (Figure 2A). The results of hierarchical clustering showed distinguishable circRNA profiling from six samples based on their expression level, indicating that circRNAs have different expression patterns in POCD patients compared with those in NPOCD patients (Figures 2B,C). The Volcano plot was performed to visualize the significant differences between POCD and NPOCD groups (fold change ≥2.0, P-value ≤0.05) (Figure 2D). Besides that, the distribution of the circRNAs differentially expressed in chromosomes showed that most of the circRNAs were transcribed from chr1, chr2, chr5, chr10, chr11, and chr16 and seldom from chr8, chr18, chr21, and chrY (Figure 2E). These data indicated that circRNAs have a different expression pattern in POCD serum compared with that in NPOCD serum. The microarray data also showed 210 circRNAs that were differentially expressed, among which 133 circRNAs were upregulated and 77 were downregulated in POCD patients’ serum. Among them, hsa_circ_001145 was most unregulated, and hsa_circ_005537 was most downregulated. Here we presented the top five upregulated and downregulated circRNAs (Table 3).
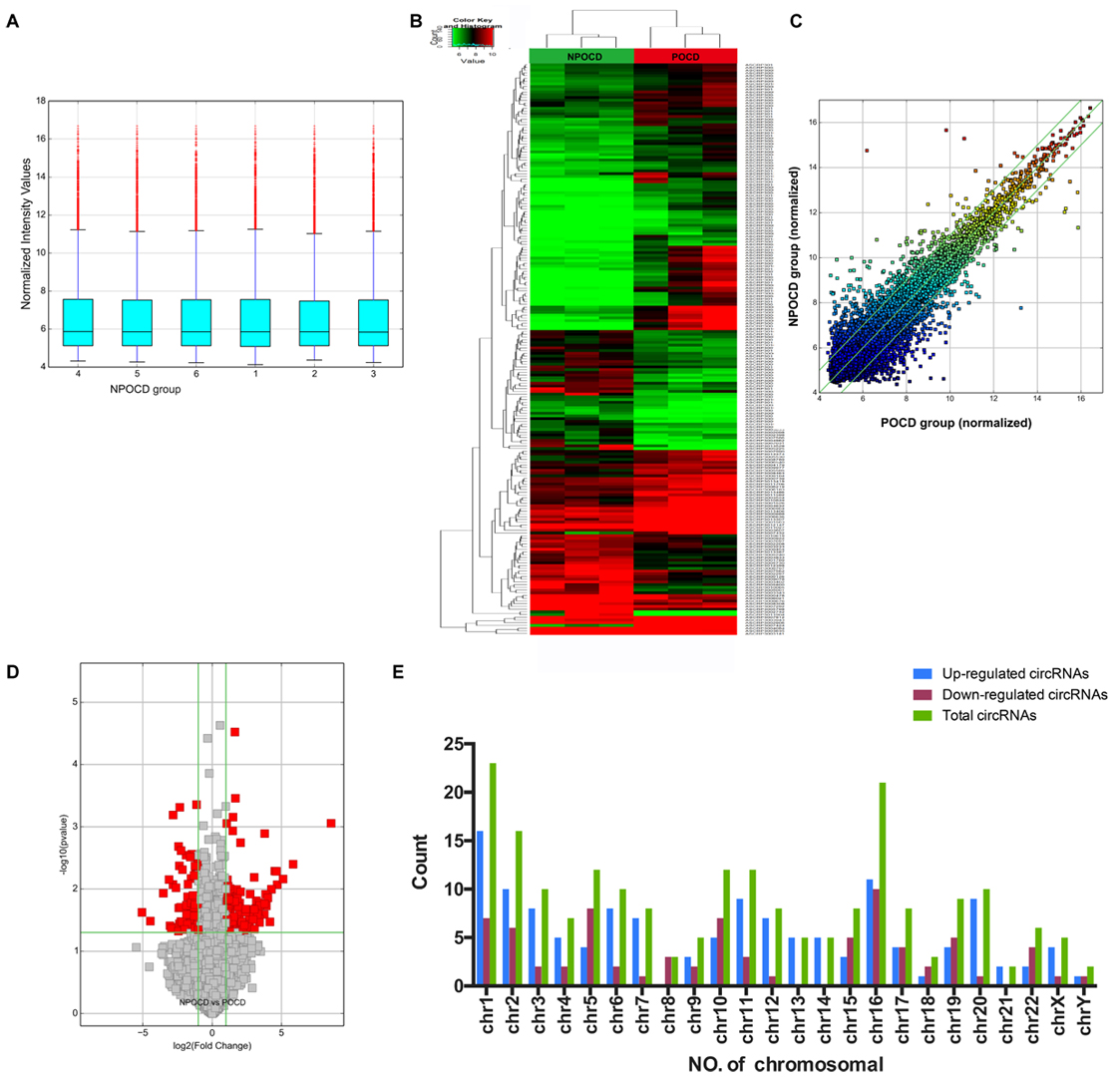
Figure 2. Differentially expressed profile of circRNAs and characterization between the serum of postoperative cognitive dysfunction (POCD) and non-POCD patients. (A) Box plots showing the distribution of circRNAs between the serum samples. (B,C) Hierarchical clustering plot showing the differentially expressed circRNA profiles in the six samples. “Red” represents the higher expression, while “green” represents the lower expression level. (D) Volcano plots visualizing the distinguishable circRNA expression. (E) Chromosomal distributions of circRNAs in the two groups.
Among all the patients, we randomly selected 10 pairs of serum samples from the POCD group and the NPOCD group. By using qRT-PCR, we verified 10 typically differentially expressed circRNAs. Meanwhile, the PCR results were identified by melt curve analysis such that the primers could specifically amplify the back-splice sites of circRNAs (Figure 3A). We found that the expression of hsa_circ_001145, hsa_circ_101138, hsa_circ_030050, hsa_circ_061570, and hsa_circ_050545 in the POCD group was significantly higher than those in the NPOCD group, while the expression of hsa_circ_005537, hsa_circ_092522, and hsa_circ_005458 was considerably downregulated in the POCD group. Therefore, the expression level of the abovementioned circRNAs was consistent with the analysis results from the microarray, validating the reliability of our microarray data (Figure 3B, ****P < 0.0001).
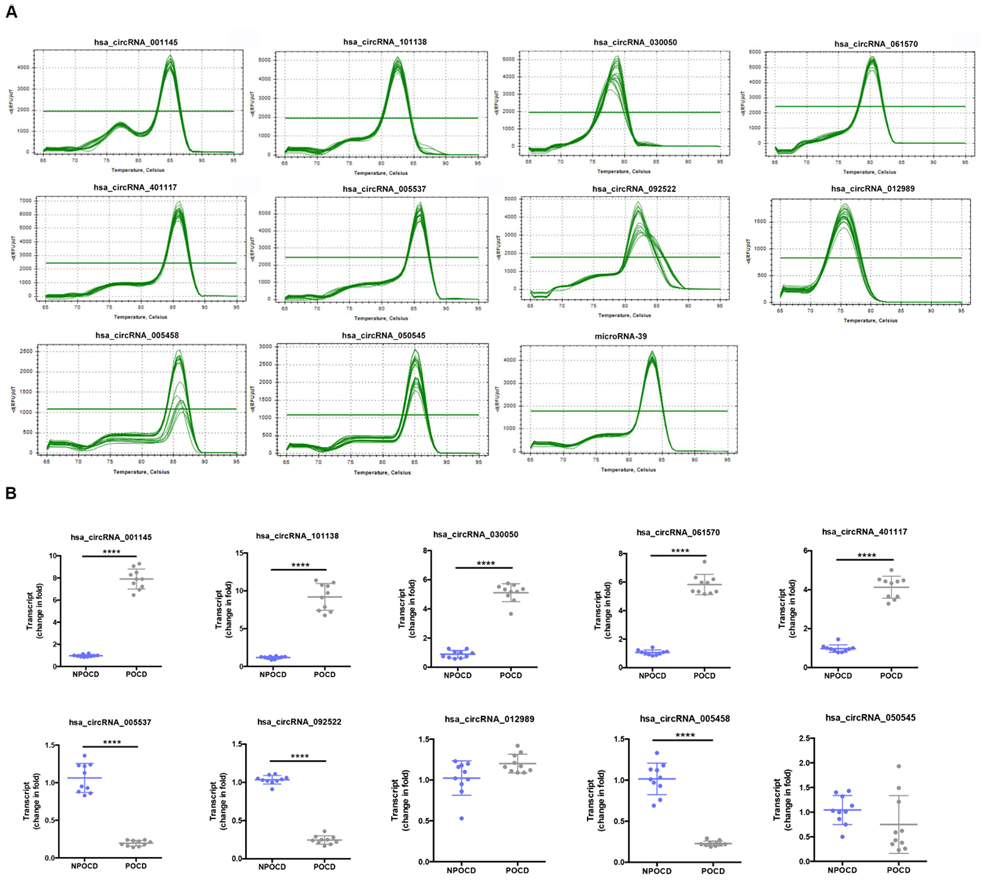
Figure 3. Amplification and identification of differentially expressed circRNAs. (A) The melt curves of the identified distinguishable expressed circRNAs. (B) qRT-PCR showing the expression levels of circRNAs between the two groups. Data are presented as means ± SD. ****P < 0.0001, N = 10.
The GO analysis for the differentially expressed circRNAs showed the top 10 significantly enriched target genes in terms of BP (Figures 4A,B), CC, and MF (Supplementary Figure 2). These results showed that the upregulated circRNAs had a strong relationship with the regulations of the developmental process (GO:0032502), cell communication (GO:0010646), nervous system development (GO:0007399), and so on (Figure 4A). Meanwhile, all downregulated expressed circRNAs were associated with cell-to-cell adhesion (GO:0098609), nervous system development (GO:0007399), and so on (Figure 4B). The KEGG analysis for differentially expressed circRNAs was performed to explore the top 10 significantly enriched pathways (Figure 4C). Especially, the target genes of upregulated circRNAs were mostly enriched in the MAPK signaling pathway, while those of downregulated circRNAs were enriched in the RAS signaling pathway (Supplementary Figures 3, 4). Of note is that these processes and pathways were reported to be associated with inflammation and apoptosis in POCD (Li et al., 2014; Wang et al., 2018).
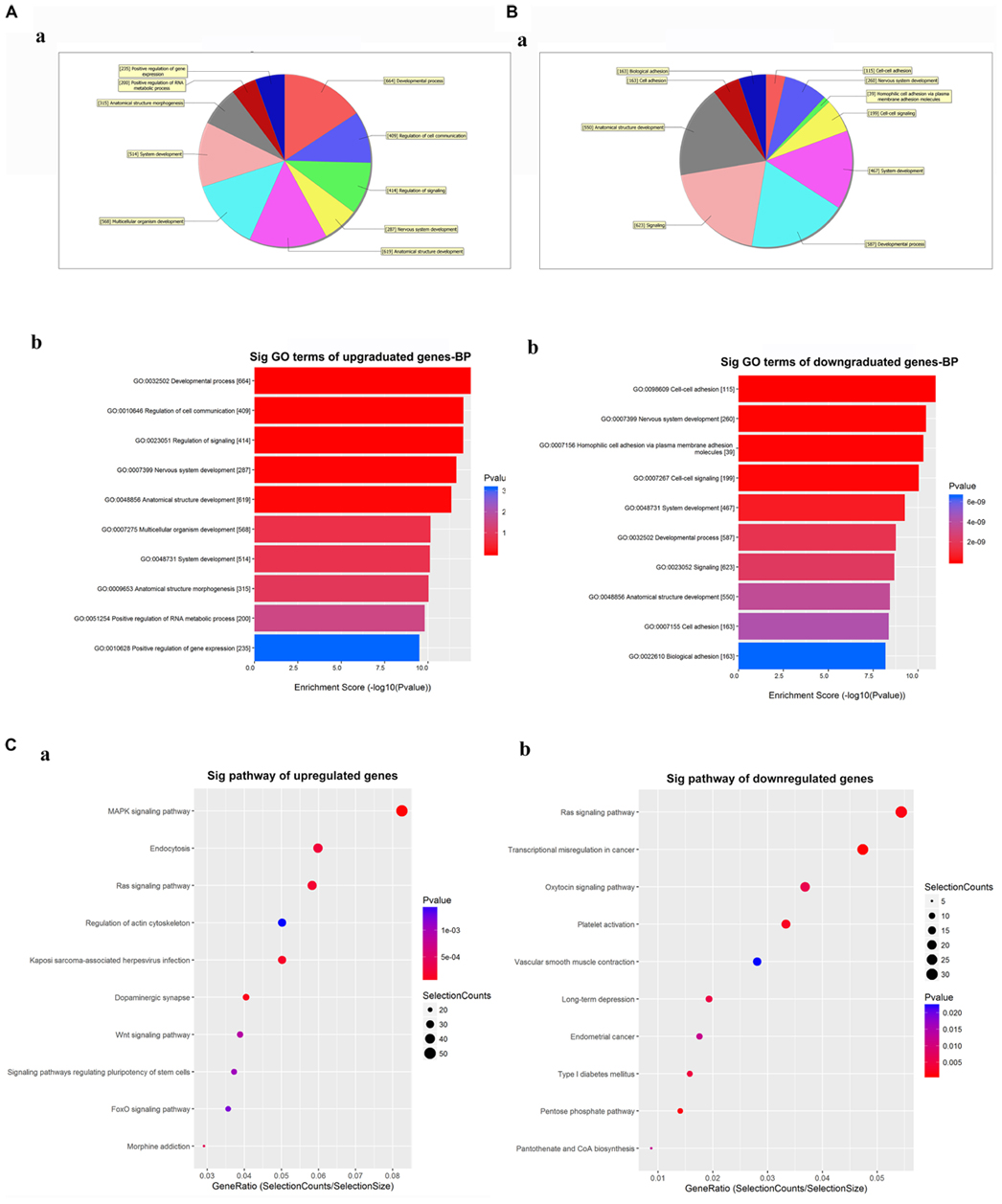
Figure 4. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses for the top five upregulated and downregulated circRNAs. (A,B) GO analysis showing the biological processes enriched by the target genes of the top five upregulated and downregulated circRNAs: a—classification of the predicted biological processes; b—the top 10 significantly enriched genes based on their enrichment scores. (C) KEGG pathway analysis showing the top 10 significantly enriched pathways and their scores. GO, gene ontology; Sig, significantly; BP, biological processes. Selection Counts, Count of the genes’ entities directly associated with the listed pathway ID; Selection Size, the total number of the genes’ entities.
As there are specific binding sites of miRNAs in the circRNA sequence, circRNAs can interact with miRNAs by miRNA response elements. Five miRNAs with the highest mirSVR scores regarding the differentially expressed circRNAs, including the top five upregulated and downregulated circRNAs, are shown in Table 4. We assumed that the differentially expressed circRNAs act as a miRNA sponge to regulate its circRNA–miRNA–mRNA network. We then predicted the target genes of the top 5 miRNAs utilizing TargetScan & miRanda. Based on the overlapping results of targetscan7.1 and mirdbV5, there were 1,987 target genes for the top five upregulated circRNAs and 1,941 target genes for the top five downregulated circRNAs (Supplementary Figure 5). Based on our validation results of qRT-PCR, three circRNAs with the most significant change folds, hsa_circRNA_001145, hsa_circRNA_101138, and hsa_circRNA_061570, were used to construct the circRNA–miRNA–mRNA interaction network (Figure 5A). In contrast, an additional network diagram, considering all the rest of the validated circRNAs, was provided (Supplementary Figure 6). Utilizing the public databases (circBase4), the targeted miRNAs were screened, and the results were displayed according to their specific base pairing and seed sequence (Figure 5B).
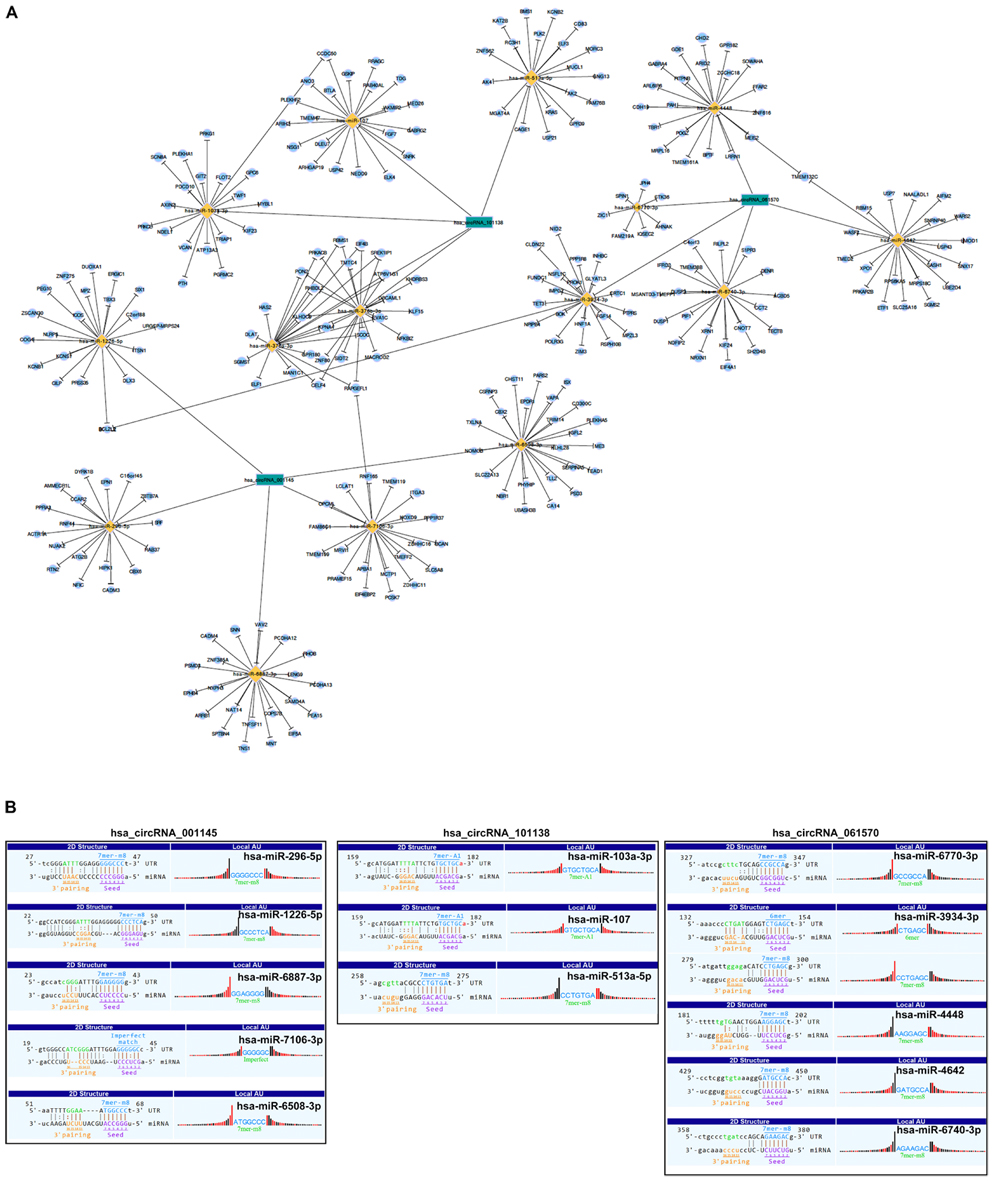
Figure 5. Representative circRNA–miRNA–mRNA network and sequence pairing predictions for circRNAs and miRNAs. (A) Based on the circRNA microarray profile results, the co-expression network drawn by Cytoscape software showing the dysregulated circRNAs, hsa_circ_001145, hsa_circ_101138, and hsa_circ_061570 (green nodes), having the highest magnitude of change and which were predicted to be functionally connected with their targeted miRNAs in the network. (B) Seed sequence matching predicted the direct interaction of the abovementioned circRNAs with their related miRNAs.
In our study, we performed a circRNA microarray of the serum from clinical patients and found that the expression of circRNAs in POCD patients was significantly different from that in NPOCD patients. Among the 210 differentially expressed circRNAs, there were 133 circRNAs upregulated and 77 were downregulated. Next, we validated the typical circRNA expression levels in the two groups, and the results were consistent with the microarray analysis, showing the accuracy and the reliability of our microarray findings. Subsequently, our results showed the top five upregulated and downregulated circRNAs and predicted five miRNAs with the highest mirSVR scores for each circRNA, indicating the possible miRNAs involved in POCD. Then, we utilized GO and KEGG analyses to enrich the target genes of differentially expressed circRNAs in their biological functions and pathways. Finally, we constructed the circRNA–miRNA–mRNA interaction network for the three circRNAs with the most robust differential expression. Therefore, in our present study, through the human circRNA microarray and bioinformatics analysis, we have found the circRNAs with differential expression levels in POCD patients as well as the possible functions and pathways that their target genes might be involved in, thus trying to provide a preliminary perspective for the potential roles of circRNAs in POCD.
POCD is a serious perioperative complication with the main clinical manifestations of impaired memory, attention, language comprehension, and social skills after surgery in elderly patients, especially in heart, orthopedic, and abdominal surgery (Evered et al., 2011; Quan et al., 2019). It is of great clinical significance to find and screen out a molecule that can predict the occurrence of POCD at an early stage. Xie and his colleagues found that the ratio of Aβ to Tau protein in the cerebrospinal fluid (CSF) was closely related to neurocognitive dysfunction after surgery, which helped to identify POCD patients at an early stage (Xie et al., 2013). Besides that, the previous study also suggested that the CSF biomarkers in POCD were similar to that in AD. However, in clinical practice, the acquisition of CSF is invasive and difficult to implement. As a body fluid, blood can also carry a large number of valuable information molecules between the peripheral organs and the central brain. Therefore, searching for disease-related molecules based on blood detection and exploring the relevant regulation pathways are essential to find effective preventions for the development of POCD.
In recent years, accumulative studies have suggested that, in addition to the traditional roles of protein synthesis or enzyme-like catalytic activity, RNAs could also act as communicators between cells in physiopathological processes. Extracellular RNAs could be released from damaged cells under stress conditions, such as trauma, ischemia, and hypoxia (Kosaka et al., 2013). Additionally, the coding and the non-coding RNAs could transfer to the distant cells carried by extracellular microvesicles (Wei Z. et al., 2017). Furthermore, the protective role of ribonuclease (RNase), the counterpart of RNAs, was reported in several POCD models (Chen et al., 2015; Ma G. et al., 2017; Ma Y. et al., 2017). The application of RNase could reduce the amount of extracellular RNAs released from the damaged cells and then attenuate inflammation and apoptosis in the central nervous system. In our previous study, postoperatively increased double-stranded RNAs could activate toll-like receptor 3 and induce neuroinflammation in the hippocampus in an aged mouse model of POCD (Chen C. et al., 2019). Besides that, Chen and colleagues found that miRNA-146a could protect the cognitive decline induced by surgery trauma by suppressing neuroinflammation in mice (Chen L. et al., 2019). Also, emerging studies showed the differentially expressed lncRNAs and mRNAs and their potential roles in POCD disease as analyzed by microarray (Wei C. et al., 2017; Zhang et al., 2018; Li et al., 2019). Thus, it is necessary to investigate whether the circRNAs, a unique class of ncRNAs, participate in the pathogenesis of POCD.
CircRNAs exist ubiquitously in eukaryotic cells with high stability and evolutionary conservatism (Werfel et al., 2016). Additionally, it was reported that the expression level of circRNAs was much higher than that of corresponding linear mRNA in body fluid (Jeck et al., 2013). Because of their stability, conservativeness, and ubiquity, circRNAs were expected to become a new ideal marker for the diagnosis of diseases. Up to now, circRNAs were demonstrated to play vital roles in various conditions, including cancers, cardiovascular diseases, neurodegenerative diseases, and so on (Lukiw, 2013; Hancock, 2014; You et al., 2015; Wang et al., 2016; Zhou and Yu, 2017). However, the clinical value and the role of circRNAs in POCD have remained mostly unknown. Wang and colleagues performed a circRNA microarray based on the patients’ serum exosomes and predicted that circRNA-089763 might be the key circRNA in POCD development (Wang et al., 2019). Consistently, in our study, circRNA-089763 was also significantly upregulated, with 2.62 fold change in the microarray results, which was not shown in the manuscript. However, during the validation process, we verified a total of 10 circRNAs, which are all consistent with the microarray results. In comparison, they measured 15 circRNAs in which only the expression of circRNA-089763 was consistent with their microarray findings. Of note is that the pathomechanisms of diseases were complicated, while one gene would be regulated by a series of RNAs. The results of our microarray might help to expand the scope of other possible critical circRNAs involved in the pathogenesis of POCD and their potential communicable mechanism. Additionally, their study has focused on exosomal exRNAs, which might partially represent the differentially expressed circRNAs in the serum of POCD patients.
In general, circRNAs play the roles through negatively regulating the miRNAs upon target mRNAs as sponges or function with RNA binding protein (RBP) to regulate their parent genes (Chen, 2016). In this study, the highest mirSVRs of the top five upregulated and downregulated expressed circRNAs were identified by TargetScan and miRanda. Several miRNAs and target genes had been reported for their roles in neurodegenerative diseases. As shown in the circRNA–miRNA–mRNA network (Figure 5A), the predicted miRNA for hsa_circRNA_101138 included hsa-miR-107 in our study, and it was suggested to be the potential biomarker that would be downregulated early in AD (Swarbrick et al., 2019). Meanwhile, the numbers of potential target genes of hsa_circRNA_101138-hsa-miR-107 were also shown in the network, such as NEDD9 which was associated with AD genetically in Chinese (Xing et al., 2011) and GSKIP which functioned as anchoring proteins to strengthen the cAMP/PKA/Tau axis signaling during AD pathogenesis in CSF. Notably, Xie and his colleagues had found the import roles of Tau protein in CSF to identify POCD patients (Xie et al., 2013).
Besides that, hsa_circRNA_101138 could also regulate hsa-miR-376a-3p and hsa-miR-376b-3p targeting on the HAS2 gene shown in the network, its potential role of which was recently suggested in tau protein pathogenesis in AD related to cognitive dysfunction (Li et al., 2017). Additionally, hsa_circRNA_001145 was shown to potentially function with miR-1226-5p as sponge upon the ITSN1 gene in the circRNA–miRNA–mRNA network. At the same time, ITSN1 was also the parent target gene of hsa_circRNA_061570, as shown in Table 3, and might be regulated by hsa_circRNA_061570 through functioning with RBP. ITSN1 was reported to activate RAS-JNK signaling, thus damaging synaptic plasticity and reducing learning and memory functions in AD (Yarza et al., 2015). As previously reported, ITSN1 could predict neurodegenerative diseases early, such as AD and Down syndrome (Murphy et al., 1990).
Similarly, UBE3B was not only the parent target gene of hsa_circRNA_101138 but also the targeted gene of hsa-miR-1226-5p, which might be regulated by hsa_circRNA_001145. UBE3B was associated with speech ability and intelligence development (Cheon et al., 2019). Thus, one target gene involved in POCD might be regulated by several circRNAs through different mechanisms. From the network of circRNA–miRNA–mRNA, we also found that several target genes were co-targeted by different circRNAs. For example, BCL2L2 was the potential target gene of both hsa_circRNA_001145 and hsa_circRNA_061570, the differential expression of which was reported to induce systemic inflammation and cognitive decline (Pathak et al., 2019). Therefore, the circRNAs might function together through the communicable mechanism and the complicated networks, such as circRNA–miRNA–mRNA or RBP mechanism. In the present study, we just provide an expanded prospective for possible circRNAs and mechanisms promoting the development of POCD, and it is worthwhile to further investigate their potential roles.
Then, GO analysis showed that the target genes of these circRNAs were involved in nervous system development (GO:0007399) and synapse (GO:0045202) in terms of BP and CC, indicating their potential roles in influencing nervous development in the progress of POCD. A recent study has revealed that circRNAs could affect neuronal development and plasticity (van Rossum et al., 2016), which are also crucial in learning and memory (Amtul and Atta Ur, 2015). For example, EphB4, one potential target gene of hsa_circRNA_001145, was among several Eph gene families which were reported to be involved in the development of AD by influencing neuronal synapses formation (Dalva et al., 2000; Nolt et al., 2011; Zhang et al., 2016). Meanwhile, KEGG analysis enriched these genes in MAPK and RAS signaling pathways that were both reported to have vital roles in inflammation and apoptosis, inducing the cognitive impairment in POCD (Li et al., 2014; Wang et al., 2018). The parent gene of hsa_circRNA_061570, ITSN1, also was reported to activate RAS-JNK signaling, damaging synaptic plasticity and reducing learning and memory functions in AD (Yarza et al., 2015). The difference in our GO and KEGG analyses from Wang’s research was the gene quantities. More target genes regulated by several circRNAs were involved in POCD and would be enriched in different signaling pathways.
Our study also has some potential limitations. Firstly, we just provide an expanded prospective for possible circRNAs and potential mechanisms promoting the development of POCD. Thus, the blood samples were only collected at one time point after surgery when learning and memory dysfunction was observed. Further verifying of these screened-out circRNAs on several time points after surgery might provide more clinical significance for diagnosis or prediction. Secondly, the possible roles of circRNA in the development of POCD were based on bioinformatics prediction. Thus, further experiments in vivo would be performed to identify how these differentially expressed circRNAs initiate the POCD. Third, currently we could not distinguish the source of these differentially expressed circRNAs; more experiments might be performed to explore the relationship between POCD and the circRNAs originating from different organs or tissues.
In conclusion, our study has validated the significant differentially expressed circRNAs and predicted miRNAs and genes in the POCD patients. Our study could provide a preliminary perspective for circRNAs with different expressions and predict their potential participation mechanisms in POCD. In the future, further studies are still needed to investigate how these circRNAs function on genes regulating POCD development and become novel potential peripheral clinical biomarkers and therapeutic targets for POCD.
The raw datasets used and/or analyzed in the current study are have been uploaded in to Gene Expression Omnibus (GEO), and the GEO accession number is GSE147277.
The studies involving human participants were reviewed and approved by Ethics committee of West China Hospital, Sichuan University, China (ChiCTR-OOC-16008289). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
RG, CC, TZ, and JL designed the experiments. RG, QZ, LZ, QW, YQ, YZ, XCa, XCh, CL, and HC performed the experiments. RG, CC, XM, SZ, TZ, and EC analyzed the data. RG, CC, TZ, LG, XM, and HY wrote the main manuscript text. All the authors reviewed the manuscript.
This work was supported by the National Natural Science Foundation of China (Nos. 81870858 and 81500937 to CC), the National Key R&D Program of China (No. 2018YFC2001800 to TZ), and the National Natural Science Foundation of China (No. 81671062 to TZ), China Postdoctoral Science Foundation (Grant No. 2017M610603 to CC), and the Postdoctoral Science Foundation of Sichuan University (Grant No. 2017SCU12030 to CC).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors wish to thank Qianyao Deng and Zheng Zhang for their assistance with the clinical samples.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2020.00165/full#supplementary-material
Amtul, Z., and Atta Ur, R. (2015). Neural plasticity and memory: molecular mechanism. Rev. Neurosci. 26, 253–268. doi: 10.1515/revneuro-2014-0075
Bahn, J. H., Zhang, Q., Li, F., Chan, T. M., Lin, X., Kim, Y., et al. (2015). The landscape of microRNA, Piwi-interacting RNA, and circular RNA in human saliva. Clin. Chem. 61, 221–230. doi: 10.1373/clinchem.2014.230433
Ballard, C., Jones, E., Gauge, N., Aarsland, D., Nilsen, O. B., Saxby, B. K., et al. (2012). Optimised anaesthesia to reduce post operative cognitive decline (POCD) in older patients undergoing elective surgery, a randomised controlled trial. PLoS One 7:e37410. doi: 10.1371/journal.pone.0037410
Bilotta, F., Doronzio, A., Stazi, E., Titi, L., Fodale, V., Di Nino, G., et al. (2010). Postoperative cognitive dysfunction: toward the Alzheimer’s disease pathomechanism hypothesis. J. Alzheimers Dis. 22, (Suppl. 3), 81–89. doi: 10.3233/JAD-2010-100825
Chen, C., Cai, J., Zhang, S., Gan, L., Dong, Y., Zhu, T., et al. (2015). Protective effect of rnase on unilateral nephrectomy-induced postoperative cognitive dysfunction in aged mice. PLoS One 10:e0134307. doi: 10.1371/journal.pone.0134307
Chen, C., Gao, R., Li, M., Wang, Q., Chen, H., Zhang, S., et al. (2019). Extracellular RNAs-TLR3 signaling contributes to cognitive decline in a mouse model of postoperative cognitive dysfunction. Brain Behav. Immun. 80, 439–451. doi: 10.1016/j.bbi.2019.04.024
Chen, L., Dong, R., Lu, Y., Zhou, Y., Li, K., Zhang, Z., et al. (2019). MicroRNA-146a protects against cognitive decline induced by surgical trauma by suppressing hippocampal neuroinflammation in mice. Brain Behav. Immun. 78, 188–201. doi: 10.1016/j.bbi.2019.01.020
Chen, L. L. (2016). The biogenesis and emerging roles of circular RNAs. Nat. Rev. Mol. Cell Biol. 17, 205–211. doi: 10.1038/nrm.2015.32
Cheon, S., Kaur, K., Nijem, N., Tuncay, I. O., Kumar, P., Dean, M., et al. (2019). The ubiquitin ligase UBE3B, disrupted in intellectual disability and absent speech, regulates metabolic pathways by targeting BCKDK. Proc. Natl. Acad. Sci. U.S.A. 116, 3662–3667. doi: 10.1073/pnas.1818751116
Dalva, M. B., Takasu, M. A., Lin, M. Z., Shamah, S. M., Hu, L., Gale, N. W., et al. (2000). EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell 103, 945–956. doi: 10.1016/s0092-8674(00)00197-5
Enright, A. J., John, B., Gaul, U., Tuschl, T., Sander, C., and Marks, D. S. (2003). MicroRNA targets in Drosophila. Genome Biol. 5:R1. doi: 10.1186/gb-2003-5-1-r1
Evered, L., Scott, D. A., Silbert, B., and Maruff, P. (2011). Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth. Analg. 112, 1179–1185. doi: 10.1213/ANE.0b013e318215217e
Grundman, M., Petersen, R. C., Ferris, S. H., Thomas, R. G., Aisen, P. S., Bennett, D. A., et al. (2004). Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch. Neurol. 61, 59–66. doi: 10.1001/archneur.61.1.59
Hancock, J. M. (2014). Circles within circles: commentary on Ghosal et al. (2013) “Circ2Traits: a comprehensive database for circular RNA potentially associated with disease and traits”. Front. Genet. 5:459. doi: 10.3389/fgene.2014.00459
Jeck, W. R., Sorrentino, J. A., Wang, K., Slevin, M. K., Burd, C. E., Liu, J., et al. (2013). Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 19, 141–157. doi: 10.1261/rna.035667.112
Kapila, A. K., Watts, H. R., Wang, T., and Ma, D. (2014). The impact of surgery and anesthesia on post-operative cognitive decline and Alzheimer’s disease development: biomarkers and preventive strategies. J. Alzheimers Dis. 41, 1–13. doi: 10.3233/JAD-132258
Kosaka, N., Yoshioka, Y., Hagiwara, K., Tominaga, N., Katsuda, T., and Ochiya, T. (2013). Trash or Treasure: extracellular microRNAs and cell-to-cell communication. Front. Genet. 4:173. doi: 10.3389/fgene.2013.00173
Kumar, L., Shamsuzzama, L., Haque, R., Baghel, T., and Nazir, A. (2017). Circular RNAs: the emerging class of non-coding rnas and their potential role in human neurodegenerative diseases. Mol. Neurobiol. 54, 7224–7234. doi: 10.1007/s12035-016-0213-8
Li, M., Chen, C., Zhang, W., Gao, R., Wang, Q., Chen, H., et al. (2019). Identification of the potential key long non-coding RNAs in aged mice with postoperative cognitive dysfunction. Front. Aging Neurosci. 11:181. doi: 10.3389/fnagi.2019.00181
Li, P., Chen, S., Chen, H., Mo, X., Li, T., Shao, Y., et al. (2015). Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Clin. Chim. Acta 444, 132–136. doi: 10.1016/j.cca.2015.02.018
Li, Y., Zheng, Q., Bao, C., Li, S., Guo, W., Zhao, J., et al. (2015). Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 25, 981–984. doi: 10.1038/cr.2015.82
Li, Y., Li, Z. X., Jin, T., Wang, Z. Y., and Zhao, P. (2017). Tau pathology promotes the reorganization of the extracellular matrix and inhibits the formation of perineuronal nets by regulating the expression and the distribution of hyaluronic acid synthases. J. Alzheimers Dis. 57, 395–409. doi: 10.3233/JAD-160804
Li, Z., Cao, Y., Li, L., Liang, Y., Tian, X., Mo, N., et al. (2014). Prophylactic angiotensin type 1 receptor antagonism confers neuroprotection in an aged rat model of postoperative cognitive dysfunction. Biochem. Biophys. Res. Commun. 449, 74–80. doi: 10.1016/j.bbrc.2014.04.153
Lukiw, W. J. (2013). Circular RNA (circRNA) in Alzheimer’s disease (AD). Front. Genet. 4:307. doi: 10.3389/fgene.2013.00307
Ma, G., Chen, C., Jiang, H., Qiu, Y., Li, Y., Li, X., et al. (2017). Ribonuclease attenuates hepatic ischemia reperfusion induced cognitive impairment through the inhibition of inflammatory cytokines in aged mice. Biomed. Pharmacother. 90, 62–68. doi: 10.1016/j.biopha.2017.02.094
Ma, Y., Chen, C., Zhang, S., Wang, Q., Chen, H., Dong, Y., et al. (2017). RNase alleviates neurological dysfunction in mice undergoing cardiac arrest and cardiopulmonary resuscitation. Oncotarget 8, 53084–53099. doi: 10.18632/oncotarget.18088
Memczak, S., Jens, M., Elefsinioti, A., Torti, F., Krueger, J., Rybak, A., et al. (2013). Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495, 333–338. doi: 10.1038/nature11928
Murphy, G. M. Jr., Eng, L. F., Ellis, W. G., Perry, G., and Meissner, L. C. (1990). Antigenic profile of plaques and neurofibrillary tangles in the amygdala in Down’s syndrome: a comparison with Alzheimer’s disease. Brain Res. 537, 102–108. doi: 10.1016/0006-8993(90)90345-c
Nolt, M. J., Lin, Y., Hruska, M., Murphy, J., Sheffler-Colins, S. I., Kayser, M. S., et al. (2011). EphB controls NMDA receptor function and synaptic targeting in a subunit-specific manner. J. Neurosci. 31, 5353–5364. doi: 10.1523/JNEUROSCI.0282-11.2011
Pasquinelli, A. E. (2012). MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat. Rev. Genet. 13, 271–282. doi: 10.1038/nrg3162
Pathak, G. A., Silzer, T. K., Sun, J., Zhou, Z., Daniel, A. A., Johnson, L., et al. (2019). Genome-wide methylation of mild cognitive impairment in mexican americans highlights genes involved in synaptic transport, alzheimer’s disease-precursor phenotypes, and metabolic morbidities. J. Alzheimers Dis. 72, 733–749. doi: 10.3233/JAD-190634
Piwecka, M., Glazar, P., Hernandez-Miranda, L. R., Memczak, S., Wolf, S. A., Rybak-Wolf, A., et al. (2017). Loss of a mammalian circular RNA locus causes miRNA deregulation and affects brain function. Science 357:8526. doi: 10.1126/science.aam8526
Quan, C., Chen, J., Luo, Y., Zhou, L., He, X., Liao, Y., et al. (2019). BIS-guided deep anesthesia decreases short-term postoperative cognitive dysfunction and peripheral inflammation in elderly patients undergoing abdominal surgery. Brain Behav. 9:e01238. doi: 10.1002/brb3.1238
Saleh, A. J., Tang, G. X., Hadi, S. M., Yan, L., Chen, M. H., Duan, K. M., et al. (2015). Preoperative cognitive intervention reduces cognitive dysfunction in elderly patients after gastrointestinal surgery: a randomized controlled trial. Med. Sci. Monit. 21, 798–805. doi: 10.12659/MSM.893359
Shi, Z., Chen, T., Yao, Q., Zheng, L., Zhang, Z., Wang, J., et al. (2017). The circular RNA ciRS-7 promotes APP and BACE1 degradation in an NF-kappaB-dependent manner. FEBS J. 284, 1096–1109. doi: 10.1111/febs.14045
Steinmetz, J., Christensen, K. B., Lund, T., Lohse, N., Rasmussen, L. S., and Group, I. (2009). Long-term consequences of postoperative cognitive dysfunction. Anesthesiology 110, 548–555. doi: 10.1097/ALN.0b013e318195b569
Swarbrick, S., Wragg, N., Ghosh, S., and Stolzing, A. (2019). Systematic review of miRNA as biomarkers in Alzheimer’s Disease. Mol. Neurobiol. 56, 6156–6167. doi: 10.1007/s12035-019-1500-y
van Rossum, D., Verheijen, B. M., and Pasterkamp, R. J. (2016). Circular RNAs: novel regulators of neuronal development. Front. Mol. Neurosci. 9:74. doi: 10.3389/fnmol.2016.00074
Wang, K., Long, B., Liu, F., Wang, J. X., Liu, C. Y., Zhao, B., et al. (2016). A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur. Heart J. 37, 2602–2611. doi: 10.1093/eurheartj/ehv713
Wang, M., Su, P., Liu, Y., Zhang, X., Yan, J., An, X., et al. (2019). Abnormal expression of circRNA_089763 in the plasma exosomes of patients with postoperative cognitive dysfunction after coronary artery bypass grafting. Mol. Med. Rep. 20, 2549–2562. doi: 10.3892/mmr.2019.10521
Wang, W. X., Wu, Q., Liang, S. S., Zhang, X. K., Hu, Q., Chen, Q. H., et al. (2018). Dexmedetomidine promotes the recovery of neurogenesis in aged mouse with postoperative cognitive dysfunction. Neurosci. Lett. 677, 110–116. doi: 10.1016/j.neulet.2018.03.043
Ward, A., Arrighi, H. M., Michels, S., and Cedarbaum, J. M. (2012). Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 8, 14–21. doi: 10.1016/j.jalz.2011.01.002
Wei, C., Luo, T., Zou, S., Zhou, X., Shen, W., Ji, X., et al. (2017). Differentially expressed lncRNAs and miRNAs with associated ceRNA networks in aged mice with postoperative cognitive dysfunction. Oncotarget 8, 55901–55914. doi: 10.18632/oncotarget.18362
Wei, Z., Batagov, A. O., Schinelli, S., Wang, J., Wang, Y., El Fatimy, R., et al. (2017). Coding and noncoding landscape of extracellular RNA released by human glioma stem cells. Nat. Commun. 8:1145. doi: 10.1038/s41467-017-01196-x
Werfel, S., Nothjunge, S., Schwarzmayr, T., Strom, T. M., Meitinger, T., and Engelhardt, S. (2016). Characterization of circular RNAs in human, mouse and rat hearts. J. Mol. Cell Cardiol. 98, 103–107. doi: 10.1016/j.yjmcc.2016.07.007
Xie, Z., McAuliffe, S., Swain, C. A., Ward, S. A., Crosby, C. A., Zheng, H., et al. (2013). Cerebrospinal fluid abeta to tau ratio and postoperative cognitive change. Ann. Surg. 258, 364–369. doi: 10.1097/SLA.0b013e318298b077
Xing, Y. Y., Yu, J. T., Yan, W. J., Chen, W., Zhong, X. L., Jiang, H., et al. (2011). NEDD9 is genetically associated with Alzheimer’s disease in a Han Chinese population. Brain Res. 1369, 230–234. doi: 10.1016/j.brainres.2010.10.113
Xuan, L., Qu, L., Zhou, H., Wang, P., Yu, H., Wu, T., et al. (2016). Circular RNA: a novel biomarker for progressive laryngeal cancer. Am. J. Transl. Res. 8, 932–939.
Yarza, R., Vela, S., Solas, M., and Ramirez, M. J. (2015). c-Jun N-terminal Kinase (JNK) signaling as a therapeutic target for Alzheimer’s disease. Front. Pharmacol. 6:321. doi: 10.3389/fphar.2015.00321
You, X., Vlatkovic, I., Babic, A., Will, T., Epstein, I., Tushev, G., et al. (2015). Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat. Neurosci. 18, 603–610. doi: 10.1038/nn.3975
Zhang, B., Tian, M., Zhen, Y., Yue, Y., Sherman, J., Zheng, H., et al. (2012). The effects of isoflurane and desflurane on cognitive function in humans. Anesth. Analg. 114, 410–415. doi: 10.1213/ANE.0b013e31823b2602
Zhang, Y., Li, P., Feng, J., and Wu, M. (2016). Dysfunction of NMDA receptors in Alzheimer’s disease. Neurol. Sci. 37, 1039–1047. doi: 10.1007/s10072-016-2546-5
Zhang, Y., Liu, Y. X., Xiao, Q. X., Liu, Q., Deng, R., Bian, J., et al. (2018). Microarray expression profiles of lncRNAs and mRNAs in postoperative cognitive dysfunction. Front. Neurosci. 12:694. doi: 10.3389/fnins.2018.00694
Keywords: circular RNAs, microarray, postoperative cognitive dysfunction, aging, microRNAs
Citation: Gao R, Chen C, Zhao Q, Li M, Wang Q, Zhou L, Chen E, Chen H, Zhang Y, Cai X, Liu C, Cheng X, Zhang S, Mao X, Qiu Y, Gan L, Yu H, Liu J and Zhu T (2020) Identification of the Potential Key Circular RNAs in Elderly Patients With Postoperative Cognitive Dysfunction. Front. Aging Neurosci. 12:165. doi: 10.3389/fnagi.2020.00165
Received: 18 November 2019; Accepted: 13 May 2020;
Published: 23 June 2020.
Edited by:
Zhongcong Xie, Harvard Medical School, United StatesReviewed by:
Bhaskar Roy, The University of Alabama at Birmingham, United StatesCopyright © 2020 Gao, Chen, Zhao, Li, Wang, Zhou, Chen, Chen, Zhang, Cai, Liu, Cheng, Zhang, Mao, Qiu, Gan, Yu, Liu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chan Chen, eHljaGVuY2hhbkBnbWFpbC5jb20=; Tao Zhu, c2N1d2NodGFvemh1QGZveG1haWwuY29t; eHd0YW9femh1QHlhaG9vLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.