
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Aging Neurosci. , 25 May 2020
Sec. Neurocognitive Aging and Behavior
Volume 12 - 2020 | https://doi.org/10.3389/fnagi.2020.00138
As a component of traditional Chinese medicine (TCM), acupuncture has the potential to lower blood pressure (BP) in patients with hypertension. Emerging evidence indicates that the acupuncture-induced inhibition of high BP occurs through the activation of the pathway in the afferent, central, and efferent pathways. An increasing number of studies have demonstrated that acupuncture not only activates distinct brain regions under conditions of hypertension caused by an imbalance between the sympathetic and parasympathetic systems but also modulates neurotransmitters in related brain regions to alleviate the autonomic response. The activity of these pathways can be assessed by injecting agonists or inhibitors or by performing neurotomy. This review focuses on the clinical and mechanistic studies of acupuncture in modulating BP, which might provide a neurobiological foundation for the effects of acupuncture. Although many mechanisms underlying the effects of acupuncture on cardiovascular function have been identified, further investigation is warranted.
Hypertension has an overall prevalence of 46% in the general adult population in the United States and is thus the dominant preventable risk factor for premature death and disability worldwide (Table 1, Forouzanfar et al., 2015; Muntner et al., 2018). Hypertension causes nearly 9.4 million deaths per year from cardiovascular-related mortality (Yang et al., 2017) people aged ≥60 years constituted >16.2% of the Chinese population in 2017, but the percentage predicted by the UN will increase to 35.1% by the year 2050 (United Nations, 2017). Currently, there are numerous recommendations for controlling hypertension, including improving lifestyle, such as doing more exercise, keeping a balanced diet, and having pharmacological treatment. However, drugs that nonspecifically block receptors may have multiple side effects, and most individuals find it challenging to change their long-formed lifestyle; therefore, there is a growing need to explore alternative medical procedures for treating hypertension worldwide (Li and Longhurst, 2010; Li et al., 2010a).
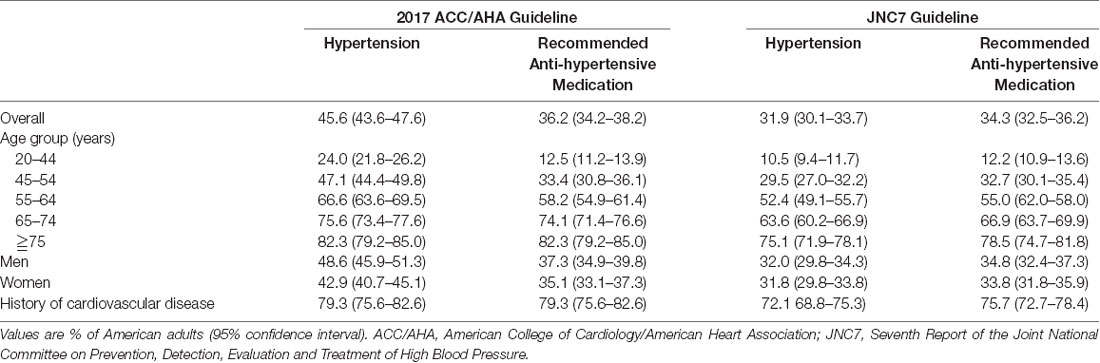
Table 1. Percentage of American adults meeting the definition for hypertension and recommended antihypertensive medication.
Acupuncture has been reported to have roles in treating hypertension (Abdi et al., 2017). This ancient treatment technique in traditional chinese medicine (TCM) has a much lower (0.13%) incidence of side effects (MacPherson et al., 2001) than traditional Western medicine (WM). In recent years, several randomized controlled trials have shown that acupuncture alleviates blood pressure (BP) in patients (Wang et al., 2016; Zheng et al., 2016). Animal experiments (Yang et al., 2017) have also shown that acupuncture has a significant effect on the BP of spontaneously hypertensive rats (SHRs). Since Besedovsky and Sorkin (1977) proposed the neuroendocrine network theory, scholars have examined this research topic, which provides new clues for understanding hypertension; the complex network of neurotransmitters and the nervous system are often disordered in the context of hypertension. Experts have shown that acupuncture plays a role in lowering BP through effects on the nervous system [including the afferent pathway, central nervous system (CNS), and efferent pathways] and neurotransmitters. Hence, this review summarizes the results of clinical and mechanistic studies to explain the antihypertensive effect of acupuncture.
Most studies have shown that acupuncture can decrease BP in hypertensive patients. Zheng et al. (2019) reported that compared with participants who received sham acupuncture and those on a waiting-list group, individuals who received active acupuncture (three sessions per week, 6-week treatment) showed better improvements in systolic blood pressure (SBP) and diastolic blood pressure (DBP) at weeks 6, 9 and 12. These data suggest that although the beneficial effects do not appear immediately, they persist for a longer period. Also, a meta-analysis (Chen et al., 2018) demonstrated that acupuncture used together with antihypertensive drugs achieve better results than antihypertensive drugs alone in reducing SBP and DBP. Similar results were observed in experiments that compared a combination of acupuncture and medication to that of sham acupuncture and medication in the reduction of SBP and DBP. These findings are supported by systematic reviews (SRs). Two SRs (Zhang et al., 2012; Zhao et al., 2015) found that acupuncture combined with WM had better outcomes than WM alone in reducing SBP. One SR (Zhao et al., 2015) found that a combination of acupuncture and WM was superior to WM alone in treating SBP and DBP, concerning efficacy rate. These data indicate that acupuncture is effective at reducing BP in patients with hypertension.
However, trials estimating the BP-lowering effects of acupuncture have shown diverse results. Although the Stop Hypertension With the Acupuncture Research Program (SHARP) was conducted by the United States, this acupunctural trial’s real effect of antihypertension is still debatable (Macklin et al., 2006). There was no significant difference in BP reduction, which between the acupuncture group and the sham acupuncture group from its baseline to 10 weeks. The reason why no such difference may be the frequency (the number of acupuncture treatments per week). Although these experimental results are controversial, however, most of the other experiments indicate that acupuncture plays a role in reducing BP.
As acupuncture has garnered worldwide acceptance, it is important to define and study acupoints, which are located on a conceptual model of meridians and collaterals. This concept indicates that meridians make the human body an organic whole, and connect the viscera, body surface, and body parts (Bianco, 2019). Acupoints are the site through which the Qi of the internal organs and channels is transported to the body surface. These acupoints are used to transmit feedback, induce stimulation, and regulate physiologic functions. Chinese medicine (CM) identifies 12 bilateral meridians and eight extra meridians that connect internal splanchnic organs to external regions (Cheng et al., 2015). Hypertensive patients without medication received electroacupuncture (EA) at P5-6+ST36-37 (Neiguan-Jianshi+Zusanli-Shangjuxu, Figure 1) and then were assessed with 24 h by ambulatory BP monitoring. After treatment for 8 weeks, these patients showed significant reductions in SBP/24 h and DBP/24 h. Patients treated at the control acupoints G37-39+Li6-7 (Guangming-Xuanzhong+ Pianli-Wenliu, Figure 1) showed no reductions in SBP/24 h or DBP/24 h (Li et al., 2015). Similarly, while exercising, healthy individuals had inhibited stress-induced increases in SBP, mean blood pressure (MBP) and the double product (SBP×heart rate, reflecting myocardial oxygen consumption) following 30 min of EA at the P5–6 acupoints (Figure 1; Longhurst et al., 1980; Li et al., 2004). However, EA at the G37–39 (Figure 1) control acupoints did not alter the exercise-related increase in BP or the double product. The strong effect at P5–6 on BP and the absence of an influence of acupuncture at G37–39 provide further evidence for the acupoint-specific effects of acupuncture on hypertension, suggesting a close relation between acupoint specificity and meridians.
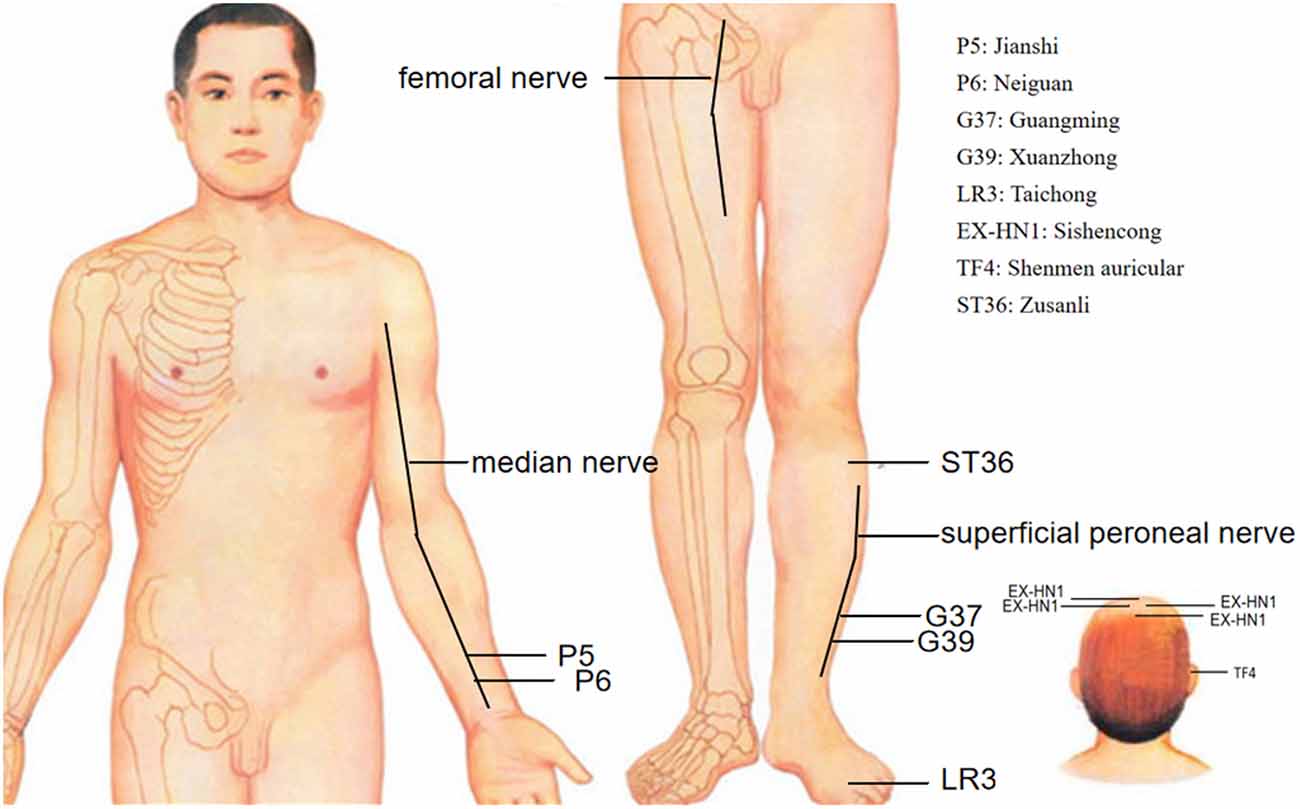
Figure 1. Effective and ineffective acupoints in acupuncture inhibition of hypertension. • Effective acupoints; ° Ineffective acupoints.
In recent years, mechanistic studies have greatly improved our understanding of hypertension. Our previous study (Wang et al., 2018) showed that acupuncture exerts an antihypertensive effect by ameliorating oxidative stress and the redox-sensitive pathway in the rostral ventral lateral medulla (RVLM) of SHRs. At the same time, there is increasing mechanistic research on acupuncture therapy for hypertension. The effects of acupuncture on hypertension encompass a holistic regulatory process. Anatomically, the afferent pathway, efferent pathway, CNS, and neurotransmitters can be distinguished. Hence, we need to explain why acupuncture lowers BP from the perspective of each of these components.
Acupuncture stimulates specific but poorly defined sites called acupoints (Kim et al., 2017), and this is the starting point for research on the mechanism of acupuncture. TCM describes the special communication between each acupoint and a specific visceral organ; an acupoint can reflect the status of specific visceral organs, and visceral disorders can be treated by manipulating these acupoints (Stux and Pomeranz, 1998; Rong et al., 2011). Acupoints are a part of the afferent pathway. Although there has been a great deal of effort in identifying acupoints, their anatomical structures remain unknown. Acupoint specificity was regarded as a major scientific issue in acupuncture practice at the Society for Acupuncture Research international symposium in 2007 and the American Association of Acupuncture conference in 2010.
Acupoints are always located in areas abundant in nerves, blood vessels, and lymph vessels, and under physiological conditions, these sites contain densely distributed nerve endings and receptors, mast cells and blood vessels and highly concentrated neural and neuroactive components (Figure 2; Zhang et al., 2012). It is generally accepted based on circumstantial evidence that acupoints become hypersensitive when pathological conditions develop in visceral organs (Ben et al., 2012) and have higher electrical conductance than the surrounding tissue (Ahn and Martinsen, 2007; Ben et al., 2012). Similarly, a previous study in a rat model of hypertension reported that the high conductance at acupoints is attributed to the release of the neuropeptide substance P (SP) and calcitonin gene-related peptide (CGRP; Fan et al., 2018). This study would help solve some of the controversial issues concerning the electrical properties of acupoints. Kim et al. (2017) showed that consistent with the physiological characteristics of acupoints, neurogenic spots caused by activation of somatic afferents during visceral disorders: (1) are most frequently located in the same anatomical location as traditional acupoints; (2) have high electrical conductance; and (3) show mechanical hypersensitivity.
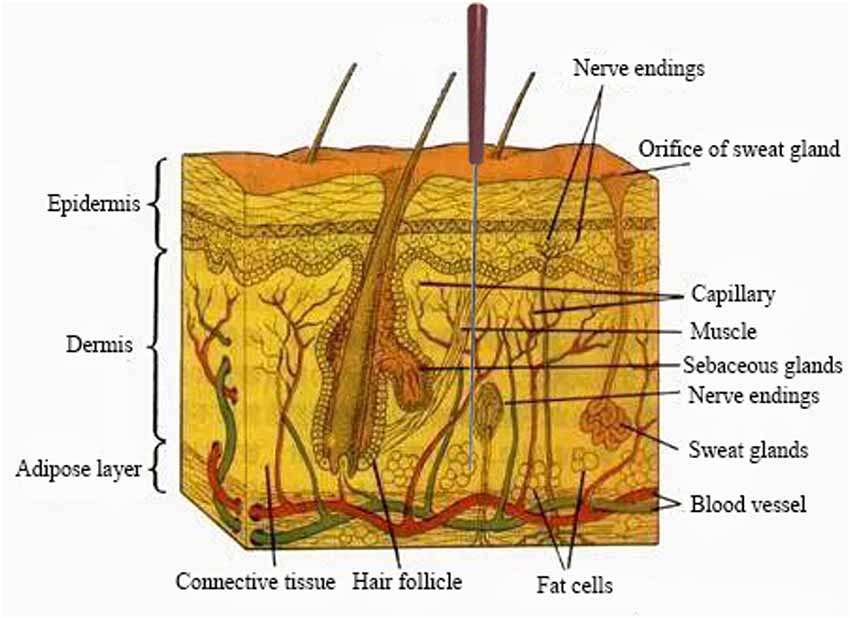
Figure 2. A representative region that is abundant in nerve endings, blood vessels etc. In response to acupuncture stimulation.
It is generally acknowledged that stimulation of the skin and muscles through acupuncture either electrically or with noxious or non-noxious stimuli can induce various somatomotor and autonomic responses (Kagitani et al., 2010). This implies that acupuncture acts by exciting cutaneous and/or muscular afferent nerve fibers. Studies show that manual acupuncture-like stimulation of a hindleg in rats modifies BP, heart rate, and the secretion rate of adrenal medullary catecholamine hormones (adrenaline and noradrenaline; Uchida et al., 2008). Neuronal activity underlying the acupoints is eliminated by surgical denervation of the median and ulnar nerves or inactivating TRPV receptors on small diameter afferent fibers of the median and ulnar nerves with resiniferatoxin (Fan et al., 2018). The effects of acupuncture were lost after the somatic afferent nerves [femoral nerve (Figure 1) and sciatic nerve] innervating the needle insertion site were severed surgically. Our research data showed (Wang et al., 2018) that surgical or chemical ablation (capsaicin) of sciatic nerve signals abolishes the antihypertensive effect of acupuncture at LR3 (Taichong, Figure 1). These findings reveal that afferent fibers are involved in the ability of acupuncture to lower BP. Afferent fibers are damaged, such as trauma, which may influence the hypotensive effect of acupuncture.
Previous studies have shown that somatosensory afferents are stimulated during acupuncture treatment. There are different types of afferent fibers, including thick myelinated Aα and Aβ (groups I and II), thin myelinated Aδ (group III), and thinner unmyelinated C (group IV) fibers. According to the study by Tjen-A-Looi (Tjen-A-Looi et al., 2005), unmyelinated fibers in the median nerve activated by P5–6 acupoint stimulation also participate in the inhibitory effects of EA on cardiovascular responses. Investigators (Uchida et al., 2003) have concluded that group III fibers are involved in the actions of EA. The inhibitory effect of acupuncture at P5–6 (Figure 1) on cardiovascular excitatory reflexes involves both thinly myelinated and unmyelinated fibers (Tjen-A-Looi et al., 2004).
The efferent pathway mainly depends on the sympathetic and vagus nerves, which originate in the spinal cord or a certain nucleus of the brain and form the peripheral nervous system of vertebrates. The sympathetic and vagus nerves, as the operating system of humans, permeate all organ systems, and are thus involved in virtually all diseases (Ziemssen and Siepmann, 2019). Acupuncture influences BP mainly through these two types of efferent nerves.
The sympathetic mechanism of action modulated by acupuncture was shown through in vivo experiments (Li et al., 2002; Tjen-A-Looi et al., 2003, 2004): (1) EA at P5–6 along the pericardial meridian reduces sympathoexcitatory-related increases in BP; and (2) EA induces the inhibition of evoked premotor sympathetic RVLM neuronal discharge. These data suggest that acupuncture lowers BP through sympathetic nerves.
Clinical trials and animal experiments have been conducted to investigate cardiovascular vagal regulation by acupuncture (Hsu et al., 2007; La Marca et al., 2010; Gao et al., 2011, 2012). Auricular EA stimulation was found to have a positive effect on respiratory sinus arrhythmia adjusted for tidal volume, indicating an increase in vagal activity, and therefore, this modality has potential as a treatment or preventive method for diseases such as hypertension and arrhythmia (La Marca et al., 2010). Hsu et al.’s (2007) study, acupuncture at TF4 (shenmen acupoint, Figure 1) and EX-HN1 (Sishencong acupoint, Figure 1) reduced BP, slowed the heart rate and activated the vagal nerves. Acupuncture at the auricular point “Heart” in anesthetized Sprague–Dawley rats had a significant inhibitory effect on arterial pressure and heart rate by activating the vagus nerve (Gao et al., 2011); therefore, acupuncture led to more vagal and less sympathetic activity, which contributed to the reduction of hypertension. Activated sympathetic and decreased vagal activities are an attractive target for future therapies for hypertension.
The importance of the CNS in cardiovascular regulation is well established (Figure 3; Guyenet, 2006; Malpas, 2010). The CNS is mainly composed of the spinal cord and the brain, which are recognized as the most important and complicated parts of the nervous system (Wang et al., 2018). Acupuncture is effective at improving BP because it affects the CNS (Hua and Jie, 2019).
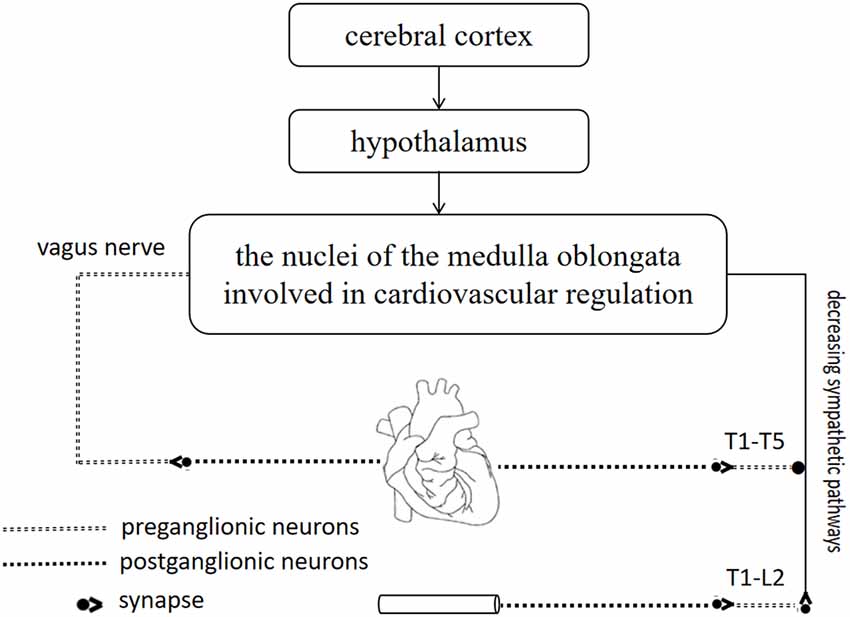
Figure 3. The importance of central nervous system (CNS) in cardiovascular regulation. The cerebral cortex and hypothalamus are closely related to the nuclei of the medulla oblongata, where is coordinated and integrated with input from baroreceptors and chemoreceptors. Sympathetic neurons and vagus nerve are involved in cardiovascular regulation.
The spinal cord has various functions, including conveying afferent sensory information received from the peripheral nervous system to the brain (England, 2008; Joshua, 2019), transmitting signals from the motor cortex of the brain to the periphery (Kinaan, 2019) and serving as the center of reflex responses (Chu and Ng, 2018). Reflex responses that lead to activation of somatic and visceral afferents are integrated into cardiovascular supraspinal regions and the spinal cord. Outputs are relayed by the spinal cord from the CNS to effector organs involved in cardiovascular reflex regulation (Longhurst, 2003). One main component of the spinal cord associated with hypertension is the sympathetic nerve pathway.
Spinal sympathetic preganglionic neurons (SPNs) are mainly located within the lateral horns of spinal gray matter in spinal segments T1-L2. SPN axons exit the spinal cord through ventral roots and synapses on the sympathetic ganglionic neurons within the paravertebral chain ganglia or prevertebral ganglia (Krassioukov and Weaver, 1995). Furthermore, opioid and nociceptin-like immunoreactivities exist in the spinal sympathetic region (Dun et al., 1997; Pomeranz and Cheng, 1979). EA reduces visceral sympathoexcitation via opioid and nonopioid (nociceptin) mechanisms in the spinal cord dorsal horn and intermediolateral column (IML). Thus, this EA action in the IML shows that acupuncture also modulates sympathetic outflow from the spinal cord (Tjen-A-Looi et al., 2004; Zhou et al., 2009), suggesting that sympathetic nerves in the spinal cord play a role in BP regulation.
There are few reports on sympathetic nerve blockage of the spinal cord in hypertension, and such investigations have mainly focused on spinal cord injury (SCI) and drug inhibition. In a rat model of upper thoracic SCI has been established, rats with severe hypotension and bradycardia exhibited classic characteristics of a sympathetic nerve block (Krassioukov and Claydon, 2006; Krassioukov et al., 2007). In terms of drug inhibition of the sympathetic ganglion, NaCl was administered via intracerebroventricular infusions of 0.6 mol/l or 1 mol/l after sympathetic ganglionic blockade with chlorisondamine to assess the contribution of sympathetic nerve activity (SNA) to NaCl-induced pressor responses (Blaustein et al., 2012; Stocker et al., 2013). Stocker et al. (2015) showed that the intravenous injection of chlorisondamine decreased the mean arterial pressure (MAP), SNA, tachycardia, and pressor response induced by 0.6 mol/l and 1.0 mol/l NaCl. In another study (Ma et al., 2004), renal SNA and arterial pressure were recorded in anesthetized R+A+ mice (transgenic mice overexpressing renin and angiotensinogen) and littermate control mice before and after sympathetic ganglionic blockade with chlorisondamine. Ganglionic blockade reduced the SNA and BP in R+A+ mice. A literature review (Houghtling and Bayer, 2002; Li et al., 2013; Na et al., 2014; Lowrance et al., 2016) revealed that, in addition to surgical removal of superficial cervical sympathetic ganglia, the other sympathetic ganglia can be treated with intravenous or intraperitoneal injections of antagonists. The above experiments showed that blocking the sympathetic nerves in the spinal cord leads to a drop in BP. However, this method of blocking sympathetic ganglia in the spinal cord has the disadvantage of systemic rather than specific inhibition. The spinal cord has been shown to play a role in sympathoexcitatory responses including reflex responses and sustained elevated BPs that are reduced with acupuncture (Zhou et al., 2009). The above studies provide a theoretical basis for the mechanism of acupuncture.
The brain plays a very important role in the maintenance of BP homeostasis in the CNS (Drummond, 2019). The brain can adjust various components of the cardiovascular system to maintain homeostasis by modulating the release of critical hormonal factors and neurotransmitters, and the connections between brain nuclei (Rahmouni, 2016). Numerous types of cardiovascular nucleus dysfunction can affect BP. Here, we provide a review of the existing literature supporting a potentially important role for different brain nuclei in BP dysfunction, including the arcuate nucleus (ARC), midbrain ventrolateral periaqueductal gray (vlPAG), and RVLM.
The ARC, located at the bottom of the hypothalamus, surrounds the ventral part of the third ventricle extending from the retrochiasmatic to the premammillary regions (Chronwall, 1985). The first direct evidence implicating the ARC in BP control derives from the work of Brody et al. (1984), which showed that direct electrical stimulation of the ARC evokes a frequency-dependent increase in arterial pressure and renal, mesenteric, and vascular resistance in anesthetized and conscious rats. Studies by Li et al. (2009) have shown that EA at P5–6 (Figure 1) activates ARC neurons, which then excite the vlPAG and subsequently inhibit cardiovascular sympathoexcitatory neurons in the RVLM. Micropipettes were inserted into the ARC, vlPAG, and RVLM for neural recording to determine whether or not the ARC inhibits RVLM activity directly or indirectly. The caudal vlPAG was shown to be required for the ARC-mediated inhibition of RVLM neuronal activation that is subsequently inhibited by EA. Although there are direct projections from the vlPAG to the RVLM, it remains unclear whether an indirect pathway through the nucleus raphe pallidus (NRP) plays an important role in vlPAG-RVLM cardiovascular modulation. EA at P5–6 for 30 min increased in the NRP response to splanchnic nerve (SN) stimulation, an effect that could be blocked by the microinjection of kynurenic acid (KA, a glutamatergic antagonist) into the caudal vlPAG. Also, the reflex increase in BP induced by bradykinin application to the gallbladder and the RVLM cardiovascular parasympathetic neuronal response to SN stimulation was inhibited by the injection of DL-homocysteic acid (DLH, a glutamate agonist) into the vlPAG, and these responses were reversed by injecting KA into the NRP. The results show that EA activates the vlPAG, which in turn excites the NRP to then inhibit RVLM parasympathetic neurons and reflex cardiovascular sympathoexcitatory responses (Figure 4; Li et al., 2010b).
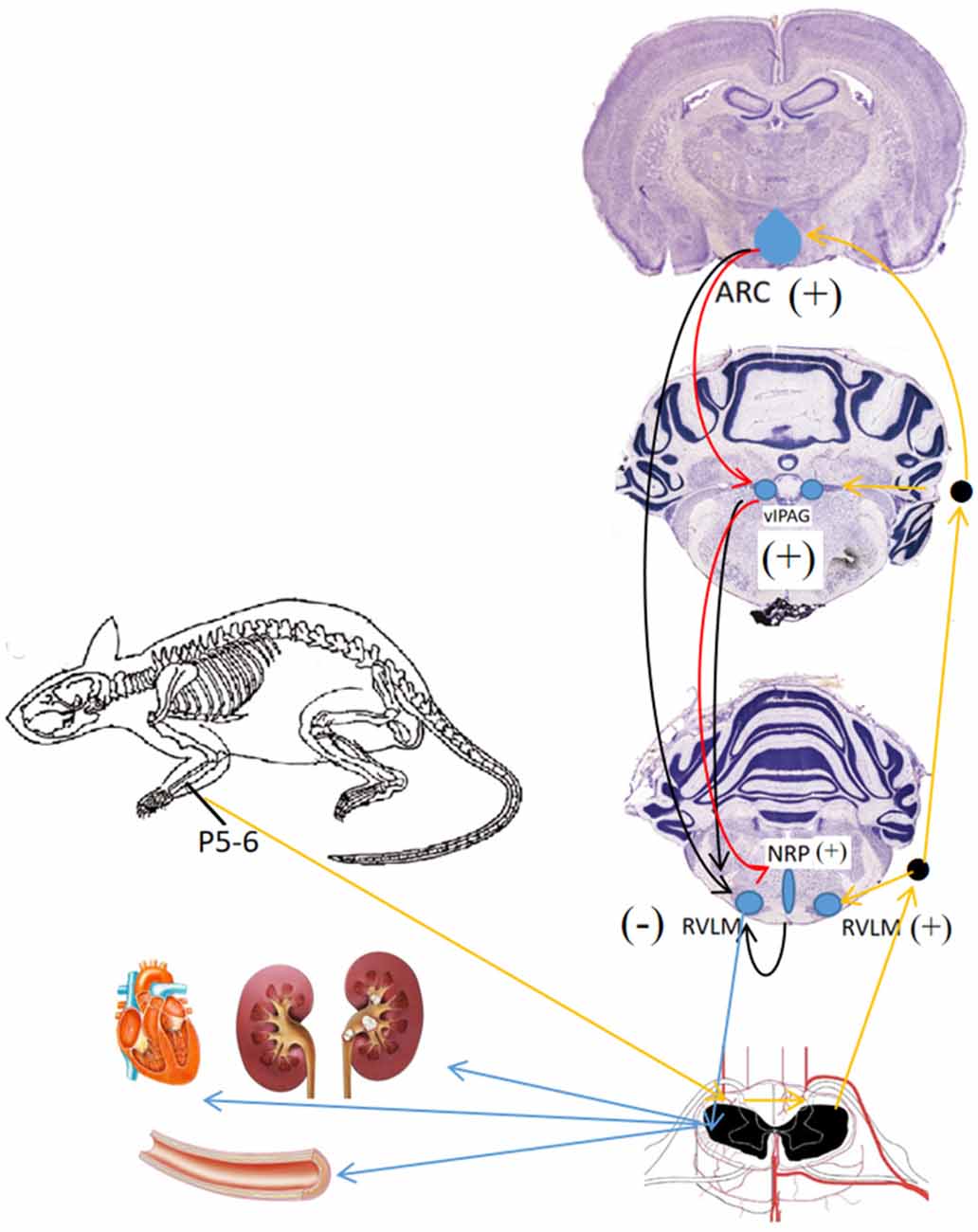
Figure 4. Neuronal pathways and mechanisms underlying the effect of acupuncture for different brain nuclei in blood pressure (BP) regulation. Black arrows, →, indicate inhibition effect from ARC, vIPAG, NRP; red arrows,  , indicate activation effect from ARC, blue arrows,
, indicate activation effect from ARC, blue arrows,  indicate efferent projections from different brain nuclei to heart, blood vessels and kidney; orange arrows,
indicate efferent projections from different brain nuclei to heart, blood vessels and kidney; orange arrows,  , indicate afferent input from P 5–6 acupoints.
, indicate afferent input from P 5–6 acupoints.
Neurotransmitters are involved in the action of acupuncture on modulating BP (Flachskampf et al., 2007). Similarly, a series of studies have shown that several neurotransmitters participate in the inhibitory effects of acupuncture (Tjen-A-Looi et al., 2007, 2009; Guo et al., 2008; Fu and Longhurst, 2009).
Recent research and available evidence show that acupuncture activates distinct brain regions in cases of diseases caused by imbalances between sympathetic and parasympathetic activity and modulates neurotransmitters in related brain regions to alleviate autonomic responses (Flachskampf et al., 2007; Li Q. Q. et al., 2013). EA inhibition of reflex autonomic responses in cats is related to the activation of μ- and δ-opioid receptors in the RVLM, suggesting that endorphins, enkephalins and perhaps endomorphins, except for dynorphin, are responsible for the EA-mediated modulation of cardiovascular responses (Li et al., 2001). Improving the effects of EA at ST36 (Zusanli) on gastric motility might activate central opioids that, in turn, inhibit sympathetic outflow (Yin et al., 2010). Although acupuncture plays a significant role in decreasing BP and heart rate in pentobarbital-anesthetized rats, these responses are related to the activation of GABAergic neurons instead of opioid neurons (Uchida et al., 2008). Uchida et al. (2010) noted that opioid receptor-mediated transmission can be unrelated to the antihypertensive effect of acupuncture-like stimulation. The findings suggest that acupuncture may be related to different neurotransmitters, which is following the holistic view of acupuncture in TCM theory.
EA activates enkephalinergic neurons and endorphinergic neurons in several brain areas that regulate BP, including the ARC, vlPAG, and RVLM (Guo and Longhurst, 2007; Li et al., 2010). By this fact, Longhurst (Guo et al., 2004; Guo and Longhurst, 2007; Guo et al., 2008) found enkephalinergic neurons in the RVLM and endorphinergic neurons in the ARC that project directly to the RVLM; both neurotransmitter systems are activated by EA. Moreover, a previous study (Guo and Longhurst, 2010) showed the presence of reciprocal glutamatergic projections (excitatory neurotransmitters) between the ARC and vlPAG that participate in inhibiting elevations in BP. Additionally, data suggest that these reciprocal projections may include a cholinergic component in the ARC but not necessarily in the vlPAG. These findings also illustrate that connections in different brain regions are mediated by different neurotransmitters involved in regulating BP, including both excitatory and inhibitory neurotransmitters.
The sympathetic nervous system, via norepinephrine, regulates adrenergic receptor (AR) expression, and sympathetic activation causes sustained increases in BP by enhanced norepinephrine release. The long-lasting inhibition of sympathetic activity by EA was confirmed in EA-treated hypertensive patients with decreased levels of norepinephrine and renin (Cheng et al., 2015). Our previous study (Yang et al., 2017) indicated that renal sympathetic activation-induced upregulation of epinephrine, norepinephrine, and renin content, which were attenuated by acupuncture in SHRs. The decreased norepinephrine we observed might be involved in the beneficial effect of acupuncture on hypertension.
An increasing amount of evidence shows that the effect of acupuncture on reducing BP occurs through various pathways, including acupoints, afferent nerves, the CNS, efferent nerves, and neurotransmitters. However, it remains unclear whether different pathways are activated by specific acupoints from the same meridian, including local points and distant points, or by acupoints from different meridians. Rigorous clinical studies and mechanistic studies are required to design future protocols for using acupuncture to lower BP. Such research would allow us to understand the relationship between acupoints and BP. Thus, acupuncture, as a general alternative therapy, can be used appropriately to treat hypertension.
HF, JH, and C-ZL were responsible for the study concept and design. L-QW and YW participated in collecting literature. HF and L-LL wrote the draft manuscript. J-WY and NZ were responsible for revising the manuscript.
This work was supported by the Beijing University of Chinese Medicine Science Fund for Distinguished Young Scholars (Grant No. BUCM-2019-JCRC011) and the Fundamental Research Funds for the Central Universities (Grant No. 2019-JYB-XJSJJ-31).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ARC, arcuate nucleus; BP, blood pressure; CGRP, calcitonin gene-related peptide; CM, Chinese medicine; CNS, central nervous system; CVD, cardiovascular disease; DBP, diastolic blood pressure; DLH, DL-homocysteic acid; EA, electroacupuncture; EX-HN1, sishencong acupoint; IML, intermediolateral column; KYN, kynurenic acid; LR3, Taichong acupoint; MAP, mean arterial pressure; MBP, mean blood pressure; NRP, nucleus raphe pallidus; P5, Jianshi acupoint; P6, Neiguan acupoint; RVLM, rostral ventral lateral medulla; SBP, systolic blood pressure; SCI, spinal cord injury; SHR, spontaneously hypertensive rat; SN, splanchnic nerve; SNA, sympathetic nerve activity; SP, substance P; SPN, sympathetic preganglionic neuron; SR, systematic review; TCM, Traditional Chinese Medicine; TF4, shenmen acupoint; vlPAG, ventrolateral periaqueductal gray; WM, Western medicine.
Abdi, H., Tayefi, M., Moallem, S. R., Zhao, B., Fayaz, M., Ardabili, H. M., et al. (2017). Abdominal and auricular acupuncture reduces blood pressure in hypertensive patients. Complement. Ther. Med. 31, 20–26. doi: 10.1016/j.ctim.2017.01.003
Ahn, A. C., and Martinsen, O. G. (2007). Electrical characterization of acupuncture points: technical issues and challenges. J. Altern. Complement. Med. 13, 817–824. doi: 10.1089/acm.2007.7193
Ben, H., Li, L., Rong, P. J., Jin, Z. G., Zhang, J. L., Li, Y. H., et al. (2012). Observation of pain-sensitive points along the meridians in patients with gastric ulcer or gastritis. Med. Princ. Pract. 2012:130802. doi: 10.1155/2012/130802
Besedovsky, H., and Sorkin, E. (1977). Network of immune-neuroendocrine interactions. Clin. Exp. Immunol. 27, 1–12.
Bianco, G. (2019). Fascial neuromodulation: an emerging concept linking acupuncture, fasciology, osteopathy and neuroscience. Eur. J. Transl. Myol. 29:8331. doi: 10.4081/ejtm.2019.8331
Blaustein, M. P., Leenen, F. H., Chen, L., Golovina, V. A., Hamlyn, J. M., Pallone, T. L., et al. (2012). How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am. J. Physiol. Heart Circ. Physiol. 302, H1031–H1049. doi: 10.1152/ajpheart.00899.2011
Brody, M. J., O’Neill, T. P., and Porter, J. P. (1984). Role of central catecholaminergic systems in pathogenesis and treatment of hypertension. J. Cardiovasc. Pharmacol. 6, S727–S741. doi: 10.1097/00005344-198400065-00002
Chen, H., Shen, F. E., Tan, X. D., Jiang, W. B., and Gu, Y. H. (2018). Efficacy and safety of acupuncture for essential hypertension: a meta-analysis. Med. Sci. Monit. 24, 2946–2969. doi: 10.12659/msm.909995
Cheng, L., Li, P., Tjen-A-Looi, S. C., and Longhurst, J. C. (2015). What do we understand from clinical and mechanistic studies on acupuncture treatment for hypertension? Chin. Med. 10:36. doi: 10.1186/s13020-015-0070-9
Chronwall, B. M. (1985). Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides 6, 1–11. doi: 10.1016/0196-9781(85)90128-7
Chu, E. C. P., and Ng, M. (2018). Long-term relief from tension-type headache and major depression following chiropractic treatment. J. Family Med. Prim. Care 7, 629–631. doi: 10.4103/jfmpc.jfmpc_68_18
Drummond, J. C. (2019). Blood pressure and the brain: how low can you go? Anesth. Analg. 128, 759–771. doi: 10.1213/ane.0000000000004034
Dun, N., Dun, S. L., and Hwang, L. L. (1997). Nociceptin-like immunoreactivity in autonomic nuclei of the rat spinal cord. Neurosci. Lett. 234, 95–98. doi: 10.1016/s0304-3940(97)00666-6
England, S. (2008). Voltage-gated sodium channels: the search for subtype-selective analgesics. Expert Opin. Investig. Drugs 17, 1849–1864. doi: 10.1517/13543780802514559
Fan, Y., Kim, D. H., Ryu, Y., Chang, S., Lee, B. H., Yang, C. H., et al. (2018). Neuropeptides SP and CGRP underlie the electrical properties of acupoints. Front. Neurosci. 12:907. doi: 10.3389/fnins.2018.00907
Flachskampf, F. A., Gallasch, J., Gefeller, O., Gan, J., Mao, J., Pfahlberg, A. B., et al. (2007). Randomized trial of acupuncture to lower blood pressure. Circulation 115, 3121–3129. doi: 10.1161/CIRCULATIONAHA.106.661140
Fu, L. W., and Longhurst, J. C. (2009). Electroacupuncture modulates vlPAG release of GABA through presynaptic cannabinoid CB1 receptors. J. Appl. Physiol. 106, 1800–1809. doi: 10.1152/japplphysiol.91648.2008
Gao, X. Y., Li, Y. H., Liu, K., Rong, P. J., Ben, H., Li, L., et al. (2011). Acupuncture-like stimulation at auricular point heart evokes cardiovascular inhibition via activating the cardiac-related neurons in the nucleus tractus solitarius. Brain Res. 1397, 19–27. doi: 10.1016/j.brainres.2011.04.034
Gao, X. Y., Liu, K., Zhu, B., and Litscher, G. (2012). Sino-European transcontinental basic and clinical high-tech acupuncture studies-part 1, auricular acupuncture increases heart rate variability in anesthetized rats. Evid. Based Complement. Alternat. Med. 2012:817378. doi: 10.1155/2012/817378
GBD 2013 Risk Factors Collaborators, Forouzanfar, M. H., Alexander, L., Anderson, H. R., Bachman, V. F., Biryukov, S., et al. (2015). Global, regional and national comparative risk assessment of 79 behavioural, environmental and occupational and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 386, 2287–2323. doi: 10.1016/S0140-6736(15)00128-2
Guo, Z. L., and Longhurst, J. C. (2007). Expression of c-Fos in arcuate nucleus induced by electroacupuncture: relations to neurons containing opioids and glutamate. Brain Res. 1166, 65–76. doi: 10.1016/j.brainres.2007.06.042
Guo, Z. L., and Longhurst, J. C. (2010). Activation of reciprocal pathways between arcuate nucleus and ventrolateral periaqueductal gray during electroacupuncture: involvement of VGLUT3. Brain Res. 1360, 77–88. doi: 10.1016/j.brainres.2010.08.102
Guo, Z. L., Moazzami, A. R., and Longhurst, J. C. (2004). Electroacupuncture induces c-Fos expression in the rostral ventrolateral medulla and periaqueductal gray in cats: relation to opioid containing neurons. Brain Res. 1030, 103–115. doi: 10.1016/j.brainres.2004.09.059
Guo, Z. L., Moazzami, A. R., Tjen-A-Looi, S., and Longhurst, J. C. (2008). Responses of opioid and serotonin containing medullary raphe neurons to electroacupuncture. Brain Res. 1229, 125–136. doi: 10.1016/j.brainres.2008.07.020
Guyenet, P. G. (2006). The sympathetic control of blood pressure. Nat. Rev. Neurosci. 7, 335–346. doi: 10.1038/nrn1902
Houghtling, R. A., and Bayer, B. M. (2002). Rapid elevation of plasma interleukin-6 by morphine is dependent on autonomic stimulation of adrenal gland. J. Pharmacol. Exp. Ther. 300, 213–219. doi: 10.1124/jpet.300.1.213
Hsu, C. C., Weng, C. S., Sun, M. F., Shyu, L. Y., Hu, W. C., and Chang, Y. H. (2007). Evaluation of scalp and auricular acupuncture on EEG, HRV, and PRV. Am. J. Chin. Med. 35, 219–230. doi: 10.1142/s0192415x0700476x
Hua, Z., and Jie, J. (2019). Progress of researches on acupuncture treatment of diseases by regulating endoplasmic reticulum stress. Zhen Ci Yan Jiu 44, 75–79. doi: 10.13702/j.1000-0607.170860
Kagitani, F., Uchida, S., and Hotta, H. (2010). Afferent nerve fibers and acupuncture. Auton. Neurosci. 157, 2–8. doi: 10.1016/j.autneu.2010.03.004
Kim, D. H., Ryu, Y., Hahm, D. H., Sohn, B. Y., Shim, I., Kwon, O. S., et al. (2017). Acupuncture points can be identified as cutaneous neurogenic inflammatory spots. Sci. Rep. 7:15214. doi: 10.1038/s41598-017-14359-z
Krassioukov, A. V., and Claydon, V. E. (2006). The clinical problems in cardiovascular control following spinal cord injury: an overview. Prog. Brain Res. 152, 223–229. doi: 10.1016/s0079-6123(05)52014-4
Krassioukov, A. V., Karlsson, A. K., Wecht, J. M., Wuermser, L. A., Mathias, C. J., Marino, R. J., et al. (2007). Assessment of autonomic dysfunction following spinal cord injury: rationale for additions to International Standards for Neurological Assessment. J. Rehabil. Res. Dev. 44, 103–112. doi: 10.1682/jrrd.2005.10.0159
Krassioukov, A. V., and Weaver, L. C. (1995). Reflex and morphological changes in spinal preganglionic neurons after cord injury in rats. Clin. Exp. Hypertens. 17, 361–373. doi: 10.3109/10641969509087077
La Marca, R., Nedeljkovic, M., Yuan, L., Maercker, A., and Elhert, U. (2010). Effects of auricular electrical stimulation on vagal activity in healthy men: evidence from a three-armed randomized trial. Clin. Sci. 118, 537–546. doi: 10.1042/cs20090264
Li, P., Ayannusi, O., Reid, C., and Longhurst, J. C. (2004). Inhibitory effect of electroacupuncture (EA) on the pressor response induced by exercise stress. Clin. Auton. Res. 14, 182–188. doi: 10.1007/s10286-004-0175-1
Li, P., and Longhurst, J. C. (2010). Neural mechanism of electroacupuncture’s hypotensive effects. Auton. Neurosci. 157, 24–30. doi: 10.1016/j.autneu.2010.03.015
Li, P., Rowshan, K., and Crisostomo, M. (2002). Effect of electroacupuncture on pressor reflex during gastric distention. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R1335–R1345. doi: 10.1152/ajpregu.00192.2002
Li, P., Tjen-A-Looi, S. C., Cheng, L., Liu, D., Painovich, J., Vinjamury, S., et al. (2015). Long-lasting reduction of blood pressure by electroacupuncture in patients with hypertension: randomized controlled trial. Med. Acupunct. 27, 253–266. doi: 10.1089/acu.2015.1106
Li, P., Tjen-A-Looi, S. C., Guo, Z. L., Fu, L. W., and Longhurst, J. C. (2009). Long-loop pathways in cardiovascular electroacupuncture responses. J. Appl. Physiol. 106, 620–630. doi: 10.1152/japplphysiol.91277.2008
Li, P., Tjen-A-Looi, S. C., Guo, Z. L., and Longhurst, J. C. (2010a). An arcuate-ventrolateral periaqueductal gray reciprocal circuit participates in electroacupuncture cardiovascular inhibition. Auton. Neurosci. 158, 13–23. doi: 10.1016/j.autneu.2010.05.006
Li, P., Tjen-A-Looi, S. C., and Longhurst, J. C. (2010b). Nucleus raphe pallidus participates in midbrain-medullary cardiovascular sympathoinhibition during electroacupuncture. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R1369–R1376. doi: 10.1152/ajpregu.00361.2010
Li, P., Tjen-A-Looi, S. C., and Longhurst, J. C. (2001). Rostral ventrolateral medullary opioid receptor subtypes in the inhibitory effect of electroacupuncture on reflex autonomic response in cats. Auton. Neurosci. 89, 38–47. doi: 10.1016/s1566-0702(01)00247-8
Li, B., Shi, Z., Cassaglia, P. A., and Brooks, V. L. (2013). Leptin acts in the forebrain to differentially influence baroreflex control of lumbar, renal, and splanchnic sympathetic nerve activity and heart rate. Hypertension 61, 812–819. doi: 10.1161/hypertensionaha.111.00518
Li, M., Tjen-A-Looi, S. C., and Longhurst, J. C. (2010). Electroacupuncture enhances preproenkephalin mRNA expression in rostral ventrolateral medulla of rats. Neurosci. Lett. 477, 61–65. doi: 10.1016/j.neulet.2010.04.025
Li, Q. Q., Shi, G. X., Xu, Q., Wang, J., Liu, C. Z., and Wang, L. P. (2013). Acupuncture effect and central autonomic regulation. Evid. Based Complement. Alternat. Med. 2013:267959. doi: 10.1155/2013/267959
Longhurst, J. C., Kelly, A. R., Gonyea, W. J., and Mitchell, J. H. (1980). Cardiovascular responses to static exercise in distance runners and weight lifters. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 49, 676–683. doi: 10.1152/jappl.1980.49.4.676
Lowrance, S. A., Ionadi, A., McKay, E., Douglas, X., and Johnson, J. D. (2016). Sympathetic nervous system contributes to enhanced corticosterone levels following chronic stress. Psychoneuroendocrinology 68, 163–170. doi: 10.1016/j.psyneuen.2016.02.027
Ma, X., Sigmund, C. D., Hingtgen, S. D., Tian, X., Davisson, R. L., Abboud, F. M., et al. (2004). Ganglionic action of angiotensin contributes to sympathetic activity in renin-angiotensinogen transgenic mice. Hypertension 43, 312–316. doi: 10.1161/01.hyp.0000111835.16662.43
Macklin, E. A., Wayne, P. M., Kalish, L. A., Valaskatgis, P., Thompson, J., Pian-Smith, M. C., et al. (2006). Stop Hypertension with the Acupuncture Research Program (SHARP): results of a randomized, controlled clinical trial. Hypertension 48, 838–845. doi: 10.1161/01.HYP.0000241090.28070.4c
MacPherson, H., Thomas, K., Walters, S., and Fitter, M. (2001). A prospective survey of adverse events and treatment reactions following 34,000 consultations with professional acupuncturists. Acupunct. Med. 19, 93–102. doi: 10.1136/aim.19.2.93
Malpas, S. C. (2010). Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol. Rev. 90, 513–557. doi: 10.1152/physrev.00007.2009
Muntner, P., Carey, R. M., Gidding, S., Jones, D. W., Taler, S. J., Wright, J. T., et al. (2018). Potential U.S. population impact of the 2017 ACC/AHA high blood pressure guideline. J. Am. Coll. Cardiol. 71, 109–118. doi: 10.1016/j.jacc.2017.10.073
Na, S., Kim, O. S., Ryoo, S., Kweon, T. D., Choi, Y. S., Shim, H. S., et al. (2014). Cervical ganglion block attenuates the progression of pulmonary hypertension via nitric oxide and arginase pathways. Hypertension 63, 309–315. doi: 10.1161/hypertensionaha.113.01979
Pomeranz, B., and Cheng, R. (1979). Suppression of noxious responses in single neurons of cat spinal cord by electroacupuncture and its reversal by the opiate antagonist naloxone. Exp. Neurol. 64, 327–341. doi: 10.1016/0014-4886(79)90273-5
Rahmouni, K. (2016). Cardiovascular regulation by the arcuate nucleus of the hypothalamus: neurocircuitry and signaling systems. Hypertension 67, 1064–1071. doi: 10.1161/hypertensionaha.115.06425
Rong, P., Zhu, B., Li, Y., Gao, X., Ben, H., Li, Y., et al. (2011). Mechanism of acupuncture regulating visceral sensation and mobility. Front. Med. 5, 151–156. doi: 10.1007/s11684-011-0129-7
Stocker, S. D., Lang, S. M., Simmonds, S. S., Wenner, M. M., and Farquhar, W. B. (2015). Cerebrospinal fluid hypernatremia elevates sympathetic nerve activity and blood pressure via the rostral ventrolateral medulla. Hypertension 66, 1184–1190. doi: 10.1161/hypertensionaha.115.05936
Stocker, S. D., Monahan, K. D., and Browning, K. N. (2013). Neurogenic and sympathoexcitatory actions of NaCl in hypertension. Curr. Hypertens. Rep. 15, 538–546. doi: 10.1007/s11906-013-0385-9
Stux, G., and Pomeranz, B. (1998). In Acupuncture: Textbook and Atlas Ch. 5, 55–76. New York, NY: Springer-Verlag.
Tjen-A-Looi, S. C., Fu, L. W., Zhou, W., Syuu, Z., and Longhurst, J. C. (2005). Role of unmyelinated fibers in electroacupuncture cardiovascular responses. Auton. Neurosci. 118, 43–50. doi: 10.1016/j.autneu.2004.12.006
Tjen-A-Looi, S. C., Li, P., and Longhurst, J. C. (2003). Prolonged inhibition of rostral ventral lateral medullary premotor sympathetic neuron by electroacupuncture in cats. Auton. Neurosci. 106, 119–131. doi: 10.1016/s1566-0702(03)00076-6
Tjen-A-Looi, S. C., Li, P., and Longhurst, J. C. (2004). Medullary substrate and differential cardiovascular response during stimulation of specific acupoints. Am. J. Physiol. 287, R852–R862. doi: 10.1152/ajpregu.00262.2004
Tjen-A-Looi, S. C., Li, P., and Longhurst, J. C. (2007). Role of medullary GABA, opioids and nociceptin in prolonged inhibition of cardiovascular sympathoexcitatory reflexes during electroacupuncture in cats. Am. J. Physiol. Heart Circ. Physiol. 293, H3627–H3635. doi: 10.1152/ajpheart.00842.2007
Tjen-A-Looi, S. C., Li, P., and Longhurst, J. C. (2009). Processing cardiovascular information in the vlPAG during electroacupuncture in rats: roles of endocannabinoids and GABA. J. Appl. Physiol. 106, 1793–1799. doi: 10.1152/japplphysiol.00142.2009
Uchida, Y., Nishigori, A., Takeda, D., Ohshiro, M., Ueda, Y., Ohshima, M., et al. (2003). Electroacupuncture induces the expression of Fos in rat dorsal horn via capsaicin-insensitive afferents. Brain Res. 978, 136–140. doi: 10.1016/s0006-8993(03)02800-2
Uchida, S., Kagitani, F., and Hotta, H. (2008). Mechanism of the reflex inhibition of heart rate elicited by acupuncture-like stimulation in anesthetized rats. Auton. Neurosci. 143, 12–19. doi: 10.1016/j.autneu.2008.06.005
Uchida, S., Kagitani, F., and Hotta, H. (2010). Neural mechanisms of reflex inhibition of heart rate elicited by acupuncture-like stimulation in anesthetized rats. Auton. Neurosci. 157, 18–23. doi: 10.1016/j.autneu.2010.03.021
Wang, Y., Tan, H., and Hui, X. (2018). Biomaterial scaffolds in regenerative therapy of the central nervous system. Biomed Res. Int. 2018:7848901. doi: 10.1155/2018/7848901
Wang, X. R., Yang, J. W., Ji, C. S., Zeng, X. H., Shi, G. X., Fisher, M., et al. (2018). Inhibition of NADPH oxidase-dependent oxidative stress in the rostral ventrolateral medulla mediates the antihypertensive effects of acupuncture in spontaneously hypertensive rats. Hypertension 71, 356–365. doi: 10.1161/hypertensionaha.117.09759
Wang, Y., Zheng, Y., Qu, S., Zhang, J., Zhong, Z., Zhang, J., et al. (2016). Cerebral targeting of acupuncture at combined acupoints in treating essential hypertension: an Rs-fMRI study and curative effect evidence. Evid. Based Complement. Alternat. Med. 2016:5392954. doi: 10.1155/2016/5392954
Yang, J. W., Ye, Y., Wang, X. R., Li, F., Xiao, L. Y., Shi, G. X., et al. (2017). Acupuncture attenuates renal sympathetic activity and blood pressure via β-adrenergic receptors in spontaneously hypertensive rats. Neural Plast. 2017:8696402. doi: 10.1155/2017/8696402
Yang, Z. Q., Zhao, Q., Jiang, P., Zheng, S. B., and Xu, B. (2017). Prevalence and control of hypertension among a community of elderly population in changning district of shanghai: a cross-sectional study. BMC Geriatr. 17:296. doi: 10.1186/s12877-017-0686-y
Yin, J., Chen, J., and Chen, J. D. (2010). Ameliorating effects and mechanisms of electroacupuncture on gastric dysrhythmia, delayed emptying and impaired accommodation in diabetic rats. Am. J. Physiol. 298, G563–G570. doi: 10.1152/ajpgi.00252.2009
Zhang, Z. J., Wang, X. M., and McAlonan, G. M. (2012). Neural acupuncture unit: a new concept for interpreting effects and mechanisms of acupuncture. Evid. Based Complement. Alternat. Med. 2012:429412. doi: 10.1155/2012/429412
Zhao, X. F., Hu, H. T., Li, J. S., Shang, H. C., Zheng, H. Z., Niu, J. F., et al. (2015). Is acupuncture effective for hypertension? A systematic review and meta-analysis. PLoS One 10:e0127019. doi: 10.1371/journal.pone.0127019
Zheng, H., Li, J., Li, Y., Zhao, L., Wu, X., Chen, J., et al. (2019). Acupuncture for patients with mild hypertension: a randomized controlled trial. J. Clin. Hypertens. 21, 412–420. doi: 10.1111/jch.13490
Zheng, Y., Zhang, J., Wang, Y., Wang, Y., Lan, Y., Qu, S., et al. (2016). Acupuncture decreases blood pressure related to hypothalamus functional connectivity with frontal lobe, cerebellum and insula: a study of instantaneous and short-term acupuncture treatment in essential hypertension. Evid. Based Complement. Alternat. Med. 2016:6908710. doi: 10.1155/2016/6908710
Zhou, W., Mahajan, A., and Longhurst, J. C. (2009). Spinal nociceptin mediates electroacupuncture-related modulation of visceral sympathoexcitatory reflex responses in rats. Am. J. Physiol. 297, H859–H865. doi: 10.1152/ajpheart.00149.2009
Keywords: acupuncture, blood pressure, clinical study, hypertension, mechanism study
Citation: Fan H, Yang J-W, Wang L-Q, Huang J, Lin L-L, Wang Y, Zhang N and Liu C-Z (2020) The Hypotensive Role of Acupuncture in Hypertension: Clinical Study and Mechanistic Study. Front. Aging Neurosci. 12:138. doi: 10.3389/fnagi.2020.00138
Received: 21 December 2019; Accepted: 23 April 2020;
Published: 25 May 2020.
Edited by:
Daniel Ortuño-Sahagún, Universidad de Guadalajara, MexicoReviewed by:
Stephanie Tjen-A-Looi, University of California, Irvine, United StatesCopyright © 2020 Fan, Yang, Wang, Huang, Lin, Wang, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cun-Zhi Liu, bGN6NjIzNzgwQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.