
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Aging Neurosci., 17 January 2020
Sec. Alzheimer's Disease and Related Dementias
Volume 11 - 2019 | https://doi.org/10.3389/fnagi.2019.00366
 Elisabetta Maffioletti1,2
Elisabetta Maffioletti1,2 Elena Milanesi3
Elena Milanesi3 Abulaish Ansari2
Abulaish Ansari2 Orazio Zanetti4
Orazio Zanetti4 Samantha Galluzzi5
Samantha Galluzzi5 Cristina Geroldi4
Cristina Geroldi4 Massimo Gennarelli1,2*
Massimo Gennarelli1,2* Luisella Bocchio-Chiavetto1,6
Luisella Bocchio-Chiavetto1,6miR-146a is a microRNA (miRNA) involved in neuroinflammation and aging; alterations in its expression were described in Alzheimer’s disease (AD). However, most of the studies conducted so far on this miRNA included a limited number of participants and produced contradictory results. We compared miR-146a levels in plasma from 33 AD patients vs. 28 age-matched non-affected controls (CTRL) through quantitative real-time polymerase chain reaction (qRT-PCR). No difference between the case and the control group was evidenced, but a correlation was detected between miR-146a levels and subjects’ age (p < 0.001) as well as between miR-146a levels and patients’ Mini-Mental State Examination (MMSE) scores (p = 0.011), in an enlarged group of 51 AD patients and 45 CTRL supporting a role for this miRNA in aging processes and disease progression.
Dementia has become an important public health, social and economic issue and represents an increasing focus for policymakers, health institutions, and researchers. The most common form of dementia is late-onset Alzheimer’s disease (AD), a heterogeneous pathology caused by the combination of genetic, environmental, and lifestyle risk factors. microRNAs (miRNAs) are small non-coding RNAs which regulate gene expression by binding to the 3′-UTR of their target messenger RNAs (mRNAs) and altering their stability and/or inhibiting translation (O’Carroll and Schaefer, 2013). Almost 50% of miRNAs are expressed in the central nervous system where they play important roles in basic brain functions, in normal aging, and in different psychiatric and neurological disorders, including AD (Maffioletti et al., 2014). Studies conducted in animal models of AD and in post-mortem brains from patients indicated a dysregulation in the levels of several miRNAs, many of which are implicated in neuroprotective/neurodegenerative processes and target key disease genes (Reddy et al., 2017). Besides their production in cells, miRNAs are also released in several body fluids, including cerebrospinal fluid (CSF), blood and its derived products (plasma and serum). AD-related alterations in circulating miRNAs have been described by several studies, and they have been suggested as non-invasive and sensitive biomarkers which could help both in the diagnosis and prognosis of AD (Batistela et al., 2017). miR-146a (miR-146a-5p) is an immune system regulator involved in neuroinflammatory processes associated with several central nervous system diseases, including AD, and has been implicated in aging processes in animal models (Jiang et al., 2012; Deng et al., 2017). Disease-related alterations in the expression of miR-146a were described in AD, both in peripheral tissues as CSF, serum, and plasma and in post-mortem brains, although with contradictory results (Kiko et al., 2014; Müller et al., 2014, 2016; Denk et al., 2015; Dong et al., 2015).
Moreover, increased peripheral miR-146a levels have been shown to predict the conversion to AD in subjects with mild cognitive impairment (MCI; Ansari et al., 2019).
The aim of this study was to extend previous findings by comparing miR-146a levels in plasma samples from AD patients vs. non-affected controls, considering a much larger cohort than previous research conducted on this biological matrix (Kiko et al., 2014).
Fifty-one AD patients were selected from a prospective study on the natural history of cognitive impairment, the Translational Outpatient Memory Clinic (TOMC) study, carried out in the outpatient facility of the National Institute for the Research and Care of AD (IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia, Italy; Frisoni et al., 2009). The sample consisted of individuals with a diagnosis of probable AD according to the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) and the Alzheimer’s Disease and Related Disorders Association (ADRDA) criteria (McKhann et al., 1984). All patients were on stable pharmacological treatment since at least 3 months. Forty-five non-affected controls (CTRL) were also enrolled in the study. The Mini-Mental State Examination (MMSE) was performed on all study participants; for controls, a cut-off of ≥27 was applied. Age- and education-adjusted MMSE scores were calculated and used for subsequent statistical analysis. The study was approved by the local ethics committee, and all the participants and/or caregivers provided written informed consent. Blood was collected in the morning, after an overnight fast, in EDTA-containing tubes for plasma preparation. miRNAs were isolated from plasma with the kit NucleoSpin miRNA plasma (Macherey-Nagel, Düren, Germany) after having added the spike-in control cel-miR-39 (Qiagen, Hilden, Germany). The extracted miRNAs were reverse-transcribed with the TaqMan MicroRNA Reverse Transcription Kit and quantitative real-time polymerase chain reaction (qRT-PCR) was conducted on the StepOnePlus instrument using TaqMan MicroRNA Assays (Applied Biosystems, Foster City, CA, USA, assay IDs: 000468 and 000200 for miR-146a and cel-miR-39, respectively). The Ct values were normalized according to the deltaCt (dCt) method on the spike-in control cel-miR-39. The statistical analyses were performed with the software IBM SPSS Statistics; data normality was assessed through the Kolmogorov–Smirnov test. Differences in continuous and discrete variables between groups were evaluated through t-test (in case of significantly unequal variances, Welch’s t-test was applied) and chi-square test, respectively. Correlations were assessed through Pearson’s bivariate correlation analysis or, when controlling for covariates, through partial correlation.
qRT-PCR data (dCts) were normally distributed; we therefore conducted all the subsequent analyses applying parametric tests. Two samples were identified as outliers and excluded; the final analyses were conducted on 49 AD patients and 45 CTRL. In the whole group (AD + CTRL, n = 94) and in the CTRL group only (n = 45), a positive correlation between miR-146a levels and age was evidenced (r = −0.432, p = 1.4*10−5 and r = −0.512, p = 0.0003, respectively; the values of r are negative since the correlations were calculated on miR-146a levels expressed as dCts, which inversely represent miRNA levels). The same was not observed in the AD group only (n = 49, r = 0.002, p = 0.991; Figure 1A). We also highlighted sex-related differences in miR-146a levels, which were lower in females (F) compared to males (M) in the whole group (F: 0.14 ± 2.13, M: 1.60 ± 2.12; p = 0.002) and in the CTRL group (F: 0.31 ± 2.84, M: 2.36 ± 2.04; p = 0.009), but not in the AD group (p = 0.282). Even when controlling for sex, the correlation with age was confirmed (AD + CTRL: r = −0.403, p = 6.3*10−5; CTRL: r = −0.476, p = 0.001).
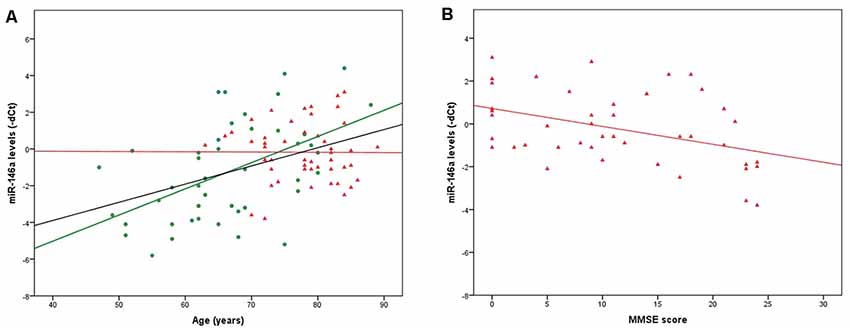
Figure 1. Figure 1. (A) Significant correlations between age and miR-146a levels in the whole group of CTRL + Alzheimer’s disease (AD) patients (n = 94, black line) and in the CTRL group (n = 45, green dots, green line). Although the correlation is not significant, the regression line for the AD group (n = 49, red triangles, red line) is also shown. (B) Significant correlation between Mini-Mental State Examination (MMSE) scores and miR-146a levels in the AD group (n = 43, red triangles, red line). For clarity, microRNA (miRNA) levels are indicated as negative deltaCts (dCts) since dCts inversely represent miRNA quantity.
Since AD patients and CTRL were not age-matched (AD: 77.67 ± 5.91 years; CTRL: 66.33 ± 9.61 years; p < 2.4*10−9; conversely, the two groups were sex-matched: AD: 34 F/15 M; CTRL: 24 F/21 M), to test possible differences in miR-146a levels between cases and controls we selected a subgroup including 33 AD patients and 28 CTRL matched for age (AD: 74.6 ± 4.6 years, CTRL: 72.0 ± 6.7 years); the two groups were also sex-matched (AD: 25 F/8 M, CTRL: 17 F/11 M). The comparison of miR-146a levels between AD patients and CTRL revealed no significant difference (p = 0.884, Supplementary Figure S1). Interestingly, by taking into account the whole group, with no matching for age between cases and controls, significantly higher miR-146a levels were observed in AD patients vs. CTRL (p = 0.020); however, the significance was lost when age was introduced as a covariate (p = 0.924), indicating a confounding effect of this variable.
Finally, a negative correlation was highlighted between miR-146a levels and MMSE scores in the whole AD group (n = 43, since for six subjects the MMSE score was not available; r = 0.409, p = 0.007; the value of r is positive since the correlation was calculated on miR-146a levels expressed as dCts, which inversely represent miRNA levels; Figure 1B). The same was confirmed when controlling for sex (r = 0.422, p = 0.005).
In summary, no significant difference in plasma levels of miR-146a comparing AD patients vs. non-affected controls was detected in the present study. A positive correlation was evidenced between miR-146a levels and age, in line with animal studies which described an implication of this miRNA in aging processes, such as age-related macrophagic dysfunction, senescence and apoptosis (Jiang et al., 2012; Deng et al., 2017). An effect of sex was also observed, with females showing lower miR-146a levels. This was in line with a previous study (Zheng et al., 2018) and with other evidence indicating an influence of sex adjustment on the analysis results (Bae and Lee, 2018; Meerson et al., 2019).
A previous study reported a decrease of miR-146a levels in the plasma of AD patients compared to non-affected controls (Kiko et al., 2014). However, this investigation was conducted on a quite small sample size (10 AD patients and 10 controls) and, importantly, did not consider age as a confounding factor, although it was different between cases and controls. Since age could affect the statistical analysis when comparing groups in which this variable is different, we suggest that the influence of aging should be considered when evaluating peripheral levels of miR-146a. We also observed a negative correlation between miR-146a plasma levels and MMSE scores, indicating that, even though they do not differ between cases and controls, the levels of this miRNA could vary in relation to disease severity. It can be hypothesized that alterations in miR-146a levels during progressive illness stages could exert an influence on the expression of genes which represent important players in AD. Indeed, a validated target gene of miR-146a is the toll-like receptor (TLR) 2, which encodes a primary receptor for beta-amyloid (Jurkin et al., 2010). Other AD-associated targets of miR-146a are the chemokine receptor 4 (CXCR4), the Fas-Associated Death Domain (FADD; validated targets), and the microtubule-associated protein tau (MAPT; predicted target according to TargetScan1). However, a limitation of the present study is represented by the fact that AD diagnosis was made clinically and lacked neuropathological or biomarker confirmation. Because of the low specificity of the NINCDS-ADRDA criteria for AD, we cannot exclude the co-occurrence of neurodegenerative conditions other than AD in our patients’ cohort.
In conclusion, the results here presented indicated no expression alterations of miR-146a in AD patients’ plasma, but revealed a correlation with age and illness severity as well as an effect of sex. Further investigations in larger cohorts are needed to replicate these findings and to clarify the role of miR-146a in aging processes and AD progression.
The datasets generated for this study are available on request to the corresponding author.
The studies involving human participants were reviewed and approved by CEIOC IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli, Brescia. The patients/participants provided their written informed consent to participate in this study.
EMa and LB-C conceived the study. OZ, SG, and CG recruited and clinically characterized the patients. EMi and AA conducted the experiments. EMa, EMi and LB-C analyzed the data. EMa, LB-C and MG prepared the manuscript.
This research was funded by the Italian Ministry of Health (Ricerca Corrente) and Ricerca Finalizzata 2013 (Grant: RF-2013-02356444). EMi is recipient of a contract of the European Regional Development Fund, Competitiveness Operational Program 2014-2020, through the grant P_37_732/2016 REDBRAIN.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2019.00366/full#supplementary-material.
Ansari, A., Maffioletti, E., Milanesi, E., Marizzoni, M., Frisoni, G. B., Blin, O., et al. (2019). miR-146a and miR-181a are involved in the progression of mild cognitive impairment to Alzheimer’s disease. Neurobiol. Aging 82, 102–109. doi: 10.1016/j.neurobiolaging.2019.06.005
Bae, S. C., and Lee, Y. H. (2018). MiR-146a levels in rheumatoid arthritis and their correlation with disease activity: a meta-analysis. Int. J. Rheum. Dis. 21, 1335–1342. doi: 10.1111/1756-185x.13338
Batistela, M. S., Josviak, N. D., Sulzbach, C. D., and de Souza, R. L. (2017). An overview of circulating cell-free microRNAs as putative biomarkers in Alzheimer’s and Parkinson’s diseases. Int. J. Neurosci. 127, 547–558. doi: 10.1080/00207454.2016.1209754
Deng, S., Wang, H., Jia, C., Zhu, S., Chu, X., Ma, Q., et al. (2017). MicroRNA-146a induces lineage-negative bone marrow cell apoptosis and senescence by targeting polo-like kinase 2 expression. Arterioscler. Thromb. Vasc. Biol. 37, 280–290. doi: 10.1161/atvbaha.116.308378
Denk, J., Boelmans, K., Siegismund, C., Lassner, D., Arlt, S., and Jahn, H. (2015). MicroRNA profiling of CSF reveals potential biomarkers to detect Alzheimer’s disease. PLoS One 10:e0126423. doi: 10.1371/journal.pone.0126423
Dong, H., Li, J., Huang, L., Chen, X., Li, D., Wang, T., et al. (2015). Serum microRNA profiles serve as novel biomarkers for the diagnosis of Alzheimer’s disease. Dis. Markers 2015:625659. doi: 10.1155/2015/625659
Frisoni, G. B., Prestia, A., Zanetti, O., Galluzzi, S., Romano, M., Cotelli, M., et al. (2009). Markers of Alzheimer’s disease in a population attending a memory clinic. Alzheimers Dement. 5, 307–317. doi: 10.1016/j.jalz.2009.04.1235
Jiang, M., Xiang, Y., Wang, D., Gao, J., Liu, D., Liu, Y., et al. (2012). Dysregulated expression of miR-146a contributes to age-related dysfunction of macrophages. Aging Cell 11, 29–40. doi: 10.1111/j.1474-9726.2011.00757.x
Jurkin, J., Schichl, Y. M., Koeffel, R., Bauer, T., Richter, S., Konradi, S., et al. (2010). miR-146a is differentially expressed by myeloid dendritic cell subsets and desensitizes cells to TLR2-dependent activation. J. Immunol. 184, 4955–4965. doi: 10.4049/jimmunol.0903021
Kiko, T., Nakagawa, K., Tsuduki, T., Furukawa, K., Arai, H., and Miyazawa, T. (2014). MicroRNAs in plasma and cerebrospinal fluid as potential markers for Alzheimer’s disease. J. Alzheimers Dis. 39, 253–259. doi: 10.3233/jad-130932
Maffioletti, E., Tardito, D., Gennarelli, M., and Bocchio Chiavetto, L. (2014). Micro spies from the brain to the periphery: new clues from studies on microRNAs in neuropsychiatric disorders. Front. Cell. Neurosci. 8:75. doi: 10.3389/fncel.2014.00075
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., and Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology 34, 939–944. doi: 10.1212/wnl.34.7.939
Meerson, A., Najjar, A., Saad, E., Sbeit, W., Barhoum, M., and Assy, N. (2019). Sex differences in plasma microRNA biomarkers of early and complicated diabetes mellitus in Israeli Arab and Jewish patients. Noncoding RNA 5:E32. doi: 10.3390/ncrna5020032
Müller, M., Kuiperij, H. B., Claassen, J. A., Küsters, B., and Verbeek, M. M. (2014). MicroRNAs in Alzheimer’s disease: differential expression in hippocampus and cell-free cerebrospinal fluid. Neurobiol. Aging 35, 152–158. doi: 10.1016/j.neurobiolaging.2013.07.005
Müller, M., Kuiperij, H. B., Versleijen, A. A., Chiasserini, D., Farotti, L., Baschieri, F., et al. (2016). Validation of microRNAs in cerebrospinal fluid as biomarkers for different forms of dementia in a multicenter study. J. Alzheimers Dis. 52, 1321–1333. doi: 10.3233/jad-160038
O’Carroll, D., and Schaefer, A. (2013). General principals of miRNA biogenesis and regulation in the brain. Neuropsychopharmacology 38, 39–54. doi: 10.1038/npp.2012.87
Reddy, P. H., Tonk, S., Kumar, S., Vijayan, M., Kandimalla, R., Kuruva, C. S., et al. (2017). A critical evaluation of neuroprotective and neurodegenerative microRNAs in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 483, 1156–1165. doi: 10.1016/j.bbrc.2016.08.067
Keywords: microRNA, miR-146a, Alzheimer’s disease, aging, plasma, blood
Citation: Maffioletti E, Milanesi E, Ansari A, Zanetti O, Galluzzi S, Geroldi C, Gennarelli M and Bocchio-Chiavetto L (2020) miR-146a Plasma Levels Are Not Altered in Alzheimer’s Disease but Correlate With Age and Illness Severity. Front. Aging Neurosci. 11:366. doi: 10.3389/fnagi.2019.00366
Received: 24 October 2019; Accepted: 16 December 2019;
Published: 17 January 2020.
Edited by:
Robert Petersen, Central Michigan University, United StatesReviewed by:
Roberta Parolisi, University of Turin, ItalyCopyright © 2020 Maffioletti, Milanesi, Ansari, Zanetti, Galluzzi, Geroldi, Gennarelli and Bocchio-Chiavetto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimo Gennarelli, bWFzc2ltby5nZW5uYXJlbGxpQHVuaWJzLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.