
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 27 August 2019
Sec. Neurocognitive Aging and Behavior
Volume 11 - 2019 | https://doi.org/10.3389/fnagi.2019.00228
This article is part of the Research TopicCutting-Edge Approaches for CNS Protection and Repair: Focus on Vascular and Degenerative DisordersView all 83 articles
Background: Non-invasive brain stimulation (NIBS) has been widely used to treat mild cognitive impairment (MCI). However, there exists no consensus on the best stimulation sites.
Objective: To explore potential stimulation locations for NIBS treatment in patients with MCI, combining meta- and resting state functional connectivity (rsFC) analyses.
Methods: The meta-analysis was conducted to identify brain regions associated with MCI. Regions of interest (ROIs) were extracted based on this meta-analysis. The rsFC analysis was applied to 45 MCI patients to determine brain surface regions that are functionally connected with the above ROIs.
Results: We found that the dorsolateral prefrontal cortex (DLPFC) and inferior frontal gyrus (IFG) were the overlapping brain regions between our results and those of previous studies. In addition, we recommend that the temporoparietal junction (including the angular gyrus), which was found in both the meta- and rsFC analysis, should be considered in NIBS treatment of MCI. Furthermore, the bilateral orbital prefrontal gyrus, inferior temporal gyrus, medial superior frontal gyrus, and right inferior occipital gyrus may be potential brain stimulation sites for NIBS treatment of MCI.
Conclusion: Our results provide several potential sites for NIBS, such as the DLFPC and IFG, and may shed light on the locations of NIBS sites in the treatment of patients with MCI.
Mild cognitive impairment (MCI) is defined as subjective memory impairment without dementia or loss of function (Bruscoli and Lovestone, 2004). Literature suggests that MCI can be the early expression of Alzheimer’s disease (AD) and that up to 14.9% of MCI patients older than 65 will develop dementia. However, pharmacologic treatment for MCI is far from satisfactory (Petersen et al., 2018).
Recently, non-invasive brain stimulation (NIBS), such as repetitive transcranial magnetic stimulation (rTMS) and transcranial direct/alternating current stimulation (tDCS/tACS), has been widely used in the treatment of MCI (Brunoni et al., 2014; Nardone et al., 2014; Birba et al., 2017; Padala et al., 2018; Rajji, 2019). While most NIBS studies have targeted brain areas such as the dorsolateral prefrontal cortex (DLPFC), inferior frontal gyrus (IFG), inferior parietal lobule (IPL), and superior temporal gyrus (STG) (Elder and Taylor, 2014; Cappon et al., 2016; Birba et al., 2017), no stimulation location has been universally agreed upon (Pievani et al., 2017).
With the aid of cutting edge brain-imaging tools, investigators have found that many brain regions and networks are involved in the pathology of MCI (Cai et al., 2017; Melrose et al., 2018). These findings provide a basis for exploring new stimulation locations for NIBS. However, some of these regions pose a challenge for NIBS application, as they are located beneath the brain surface and are therefore difficult to access (Pievani et al., 2017).
In past decades, resting state functional connectivity (rsFC) has been widely used in brain research and in identifying NIBS locations. Functional connectivity is defined as the temporal correlation or coherence of a neurophysiological index measured in different brain areas that may show similar functional properties among these brain regions (Friston et al., 1993; Biswal et al., 1997). In a previous study, Fox used a connectivity-based approach to explore the underlying mechanisms of different TMS targets, which demonstrated the potential of using a connectivity-based targeting strategy to optimize TMS locations and increase clinical response (Fox et al., 2012).
In this study, we combined brain imaging meta- and rsFC analyses to explore brain locations for NIBS treatment of MCI. Specifically, we first performed a meta-analysis to identify surface brain areas associated with MCI and then selected these regions as potential NIBS targets. Further, we selected the key MCI-associated brain areas (including both superficial and deep structures) as regions of interest (ROIs) based on the meta-analysis. These ROIs were then used as seeds in the following rsFC analysis to further identify the functionally connected brain regions that are proximal to brain surface cortex in a cohort of MCI patients. We hypothesized that areas on the brain surface that are functionally connected with the deep brain structures involved in MCI pathology (meta-analysis map) may also be used as NIBS target locations in MCI treatment.
In the present study, we first used Neurosynth1 to conduct a forward inference meta-analysis at the threshold of p < 0.01 FDR-corrected and identify key brain regions involved in the pathophysiology of MCI. Neurosynth is a platform for automatically synthesizing the results of many different neuroimaging studies. Using the Neurosynth framework, a researcher can conduct large-scale automated neuroimaging meta-analyses of broad psychological concepts. The ability of Neurosynth to quantitatively distinguish forward inference from reverse inference allows researchers to assess the specificity of mappings between neural and cognitive functions (Yarkoni et al., 2011).
We then used three different pipelines (methods) to explore potential locations for NIBS in MCI patients. In Pipeline 1, we directly selected clusters on the brain surface of the original forward inference map from the meta-analysis as potential NIBS target regions. In Pipelines 2 and 3, we applied seed-based rsFC analysis to further explore the potential brain surface locations for NIBS in MCI patients. To do this, we first chose 20 ROIs from the meta-analysis map. In Pipeline 2, the group-level correlation maps of each ROI were saved to a binary mask (with positive and negative functional connectivity separated). The 20 positive and 20 negative correlation maps were added to obtain third level maps, and 4–6 surface clusters were selected from the third level maps as potential stimulation locations. In Pipeline 3, after 20 ROIs were picked, we combined them into a single ROI. The group-level seed-based functional connectivity map was created, and 4–6 surface clusters were chosen as potential stimulation locations.
In the Neurosynth platform, we used “MCI” as the search term to conduct forward inference analysis on all neuroimaging studies in the database that were available until February 2018. The forward brain maps were downloaded from the platform. Potential NIBS locations were chosen based on the report, and they were visually confirmed using BrainNet view2 (Xia et al., 2013), as well as MRcon_GL and MRcron_ice toolboxes. These potential locations were mapped onto a standard brain map and standard head map with the international 10–20 system in MNI space (Cutini et al., 2011).
The structural and resting state functional MRI data of MCI patients used in the present study were obtained from large multicenter Alzheimer’s Disease Neuroimaging Initiative (ADNI) studies3. The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessments can be combined to measure the progression of MCI and early AD. A total of 45 MCI patients were included.
The inclusion and exclusion criteria for MCI patients are available at: http://adni.loni.usc.edu/methods/documents/ (ADNI 1 Procedures Manual). Inclusion criteria was as follows: memory complaint by subject or study partner that is verified by a study partner; abnormal memory function documented by scoring below the education-adjusted cutoff on the logical memory II subscale (delayed paragraph recall) from the Wechsler memory scale-revised (the maximum score is 25): (a) less than or equal to 8 for 16 or more years of education, (b) less than or equal to 4 for 8–15 years of education, (c) less than or equal to 2 for 0–7 years of education; mini-mental state exam score between 24 and 30 (inclusive) (Exceptions may be made for subjects with less than 8 years of education at the discretion of the project director); clinical dementia rating = 0.5 Memory Box score must be at least 0.5; General cognition and functional performance sufficiently preserved such that a diagnosis of AD cannot be made by the site physician at the time of the screening visit.”
The data of Mild cognitive impairment patients from 55 to 90 years old with the same fMRI scanning parameters (TR = 3000 ms, TE = 30 ms, phases = 140 with eyes opened) was used in the present study. Eligibility criteria included MCI at any stage.
Seed-to-voxel correlational analysis were calculated in MATLAB by applying the functional connectivity (CONN) toolbox v17.C4. Similar to our previous studies (Tao et al., 2016, 2019; Liu et al., 2019a, b), the images were preprocessed with slice timing, realigned, co-registered to subjects’ respective structural images, normalized, and smoothed with a 6 mm full width at half maximum kernel. Segmentation of gray matter, white matter, and cerebrospinal fluid for the removal of temporal confounding factors was employed. Band-pass filtering was performed with a frequency window of 0.01 to 0.089 Hz.
To eliminate correlations caused by head motion and artifacts, we identified outlier time points in the motion parameters and global signal intensity using ART5. We treated images as outliers if the composite movement from a preceding image exceeded 0.5 mm or if the global mean intensity was greater than three standard deviations from the mean image intensity. In addition, the 12 motion regressions (three rotation and three translation parameters plus six first-order temporal derivatives) were used in the preprocessing of BOLD time courses. Outliers were included as regressors in the first level general linear model along with motion parameters (Tao et al., 2016; Wang et al., 2017).
Based on the results of the meta-analysis, we chose the key peak MNI coordinates from the brain map (see details in section “Results” and Table 2) as ROIs. To ensure only voxels that were part of the original Neurosynth map were included, and to maintain regional specificity within the mentioned peak coordinates, the mask was derived by taking the overlap of the original forward inference Neurosynth map and a 6 mm radius spherical mask centered on the identified peak coordinates. This methodology is similar to that of a previous study conducted by Goldstein-Piekarski et al. (2018). The ROIs were further defined and refined by WFU-Pick Atlas software (Maldjian et al., 2003, 2004) to ensure that the regions were within the specific structure identified. The BOLD time course from these ROIs was then extracted, and Pearson’s correlation coefficients were computed between that time course and the time courses of all other voxels in the brain. Correlation coefficients were Fisher transformed into Z scores.
Before the rsFC group analysis, we created a brain cortex mask to exclude sub-cortex brain regions from the AAL template using WFU-Pick Atlas software. The mask included bilateral pre and postcentral, superior and middle frontal, superior and inferior and middle occipital, superior and inferior parietal, supramarginal, angular, superior temporal, superior temporal pole, middle temporal, middle temporal pole, inferior temporal, oper inferior frontal, oper Rolandic, tri inferior frontal, superior medial frontal, calcarine, orbital middle and superior and inferior frontal, orbital medial frontal, supplementary motor area, paracentral lobule, precuneus, and cuneus.
For every seed in the subject-level correlation maps, the residual BOLD time course was extracted for each subject, and Pearson’s correlation coefficients were computed between ROIs and all other brain voxels. The resulting correlation coefficients were subsequently transformed into Z scores to increase normality and thus conform to the assumptions of generalized linear models. In the group-level seed-to-voxel analysis, all subject-level seed maps of seed-to-voxel connectivity were included in a one sample t-test to obtain a group-level correlation map (positive and negative separated; Z values greater than or less than zero). A threshold of voxel-wise p < 0.001 uncorrected and cluster-level p < 0.05 family-wise error (FWE) corrected were applied within the cortex mask in data analysis.
After all whole-brain rsFC results of all ROIs above threshold had been extracted (binary mask), we calculated the sum of all brain masks using DPABI6 to form a correlation map. The intensity of this correlation map represented the number of ROIs, and a higher intensity indicated a greater number of overlapping ROIs in the brain region. The 4 to 6 clusters on the brain surface with the largest peak intensity based on the report, visually confirmed using BrainNet view (Xia et al., 2013) and MRconGL and MRcron_ice toolboxes, were identified as potential stimulation locations.
We also calculated group functional connectivity using the seed that combined all above seeds into one seed. A threshold of voxel-wise p < 0.001 uncorrected and cluster-level p < 0.05 FWE corrected were applied within the cortex mask in data analysis.
Demographic and clinical characteristics of the study group are summarized in Table 1. Of the 45 enrolled MCI patients (24 female), the score of mini mental state examination (MMSE), clinical dementia rating-sum of boxes, and Alzheimer’s disease assessment scale-cognition 12 (ADAS-Cog 12) were 27.64 (1.88), 0.95 (0.78), and 8.68 (3.66), respectively [mean (SD)], with 33.33% APOE4 positive. The average age of MCI patients was 69.32 with a standard deviation of 8.07.
A total of 67 studies were included in the meta-analysis, and 20 clusters had a size greater than 30 continuous voxels. With the BrainNet viewing software, we visually determined that the left orbital IFG (F7) and left angular gyrus (P3) are proximal to the brain surface and can thereby serve as brain stimulation sites directly (Figures 1B,C, Table 2, and Supplementary Figure S1A).
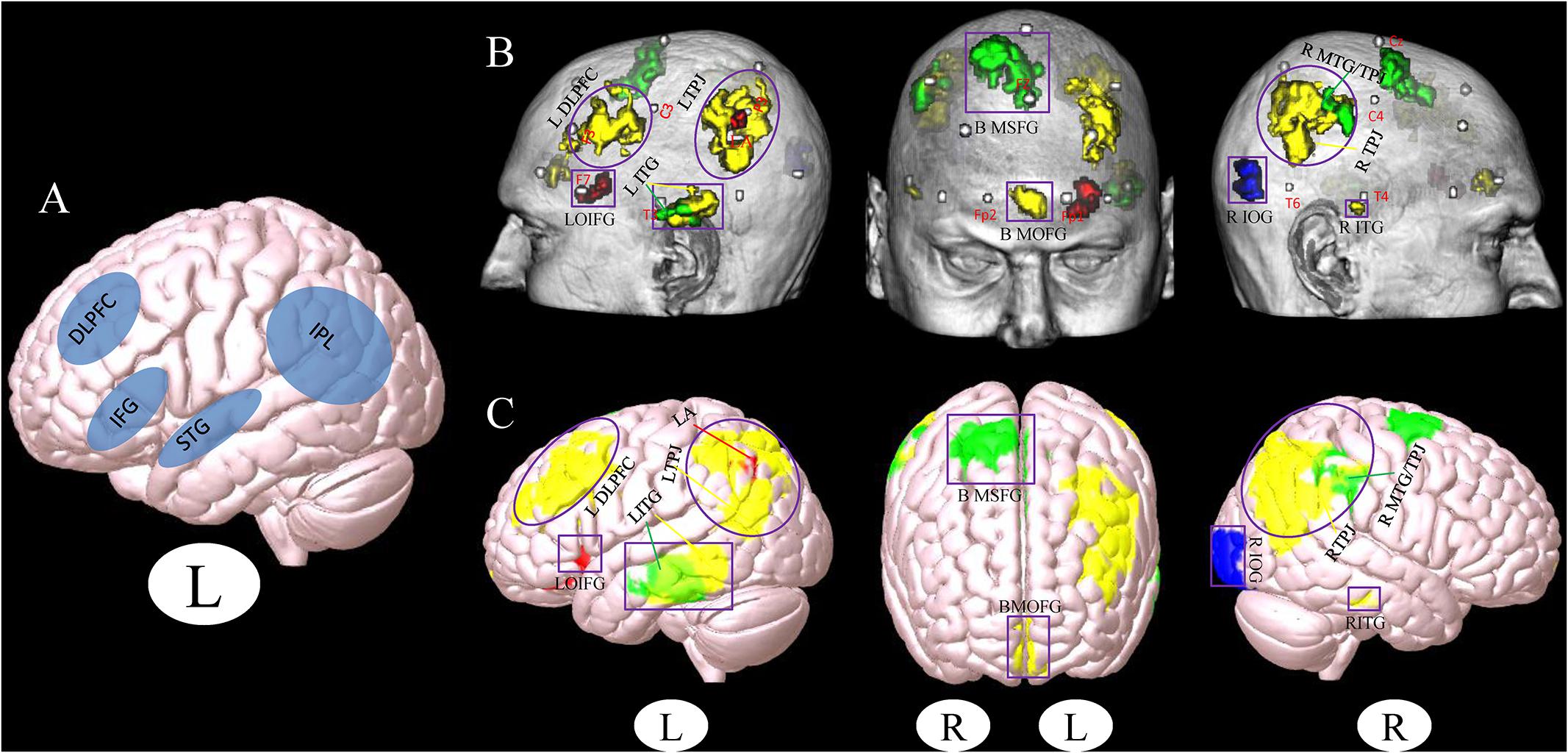
Figure 1. (A) Stimulation location setup from a previous review study (Birba et al., 2017). (B,C) Cluster associated with the brain surface based on the results of meta- and functional connectivity analyses (B), the template of chris_t1.nii.gz was used from Mricro_GL software. (C) The template of mni152.2009.mz 3 was used from Surf_Ice software. Red, results from the meta-analysis; Yellow, results from the positive rsFC; Green, results from the negative rsFC; Blue, results from the combination of all ROIs rsFC; Gray, MNI coordinates of 10–20 scalp positions found by Cutini et al. (2011). Purple box indicates the different brain regions between the results of a previous study (Birba et al., 2017) and our present results. Purple circle indicates the overlapping brain regions between the results of a previous study (Birba et al., 2017) and our present results (L, left and R, right). LDLPFC, left dorsolateral prefrontal cortex; LTPJ, left temporoparietal junction; LOIFG, left orbital inferior frontal gyrus; LITG, left inferior temporal gyrus; BMOFG, bilateral medial orbital frontal gyrus; BMSFG, bilateral medial superior frontal gyrus; RMTG/TPJ, right middle temporal gyrus and right temporoparietal junction; RITG, right inferior temporal gyrus; RIOG, right inferior occipital gyrus; LA: left angular.
To further investigate potential surface locations of brain stimulation, we selected the peak coordinates from the meta-analysis maps as ROIs (seeds) (Supplementary Figure S2) and performed a rsFC analysis. The 20 seeds of MNI coordinates (X, Y, and Z) are shown in Table 2.
We used the above 20 seeds and applied rsFC analysis separately. The brain surface regions that showed overlap positively correlated with seed regions are listed in Figures 1B,C, Table 3, and Supplementary Figure S1B. These regions include the left inferior temporal gyrus, right inferior temporal gyrus, bilateral medial orbital frontal gyrus, right middle temporal gyrus/temporoparietal junction (TPJ), left middle occipital gyrus/TPJ, and left DLPFC. The left inferior temporal gyrus (T3), right supramarginal gyrus (between C4 and P4), and bilateral medial superior frontal gyrus (T3 and T4) were found to be negatively associated with seed regions applied in this study (Figures 1B,C, Table 3, and Supplementary Figure S1C; Pipeline 2).
When combining all seeds into one large seed, we only found positive rsFC with the right inferior occipital gyrus (between T6 and O2) proximal to the brain surface (Figures 1B,C, Table 3, and Supplementary Figure S1D; Pipeline 3).
In this study, we combined a meta-analysis and rsFC analysis to explore potential stimulation locations at the brain surface in patients with MCI. Our results suggest that the left DLPFC, left inferior orbital frontal gyrus, bilateral inferior temporal gyrus, TPJ, medial superior frontal gyrus, medial orbital prefrontal gyrus, right middle temporal gyrus, and inferior occipital gyrus should be considered as potential brain stimulation sites for MCI.
Location is a critical component in the treatment of MCI via NIBS (Fox and Greicius, 2010; Bergmann et al., 2016). In a previous study, Birba et al. (2017) used a meta-analysis method to test the effects of NIBS techniques on patients with MCI. They found that the DLPFC and IFG are brain stimulation areas commonly used in studies (Figure 1A). These findings are consistent with our results, indicating that these regions should be preferentially selected in the treatment of MCI with NIBS.
In further support of the important role of the DLPFC in patients with MCI, Yang et al. (2009) found that the BOLD response of the DLPFC is weaker in patients with MCI when compared with normally aging individuals. This finding suggests that a functional abnormality of the DLPFC may be an early indicator of the disease. In addition, results from neuroimaging studies suggest that the IFG plays an important role in cognitive functions such as memory processing (Thompson-Schill et al., 1997). For instance, Bell reported that, compared with amnestic MCI patients, patients with MCI-multiple cognitive domain had significantly reduced volume of the right IFG (Bell-McGinty et al., 2005). Lin found that IFG activities may protect the memory function of individuals with MCI (Lin et al., 2017). These studies demonstrate the functional alterations of the DLPFC and inferior prefrontal gyrus in MCI and provide support for using these regions as treatment targets.
The temporal gyrus/TPJ was an overlapping brain region of both the meta-analysis and rsFC analysis, suggesting that it may be another important stimulation site for patients with MCI. A previous study reported that, compared with healthy elderly subjects, amnesiac MCI (aMCI) patients showed increased ALFF values in the left TPJ (Xi et al., 2013). The TPJ has been implicated across a broad range of cognitive areas, such as attention, social cognition, decision making, and episodic memory reconsolidation (Simon et al., 2017). Olichney reported that there was a significant suppression in amplitude of the late positive potential in the TPJ during a 3-choice vigilance task in individuals with MCI, and this change in amplitude may be a potential biomarker for early dementia (Olichney et al., 2011). Further, a study from Griffith et al. (2010) reported that the volume of the angular gyrus, a brain area within the TPJ, can predict cognitive function in MCI patients. Herholz also found that non-pharmacological interventions like recreational activity can yield an increase in cortical thickness of the right angular gyrus in patients with early stage AD (Herholz et al., 2013).
Our results suggest that the bilateral orbital prefrontal gyrus, inferior temporal gyrus, medial superior frontal gyrus, and right inferior occipital gyrus are potential stimulation sites based on rsFC analysis. Previous studies have suggested that the orbitofrontal cortex is important in cognitive processes such as learning, decision making (Wilson et al., 2014), and maintaining appropriate social behavior (Jonker et al., 2015). Sacuiu showed that cortical atrophy in the frontal lobe, including the orbital prefrontal gyrus, can hasten the conversion of MCI to AD (Sacuiu et al., 2016).
In addition, studies suggest that the inferior temporal gyrus plays an important role in verbal fluency processing (Scheff et al., 2011) and serial visual recognition (Baylis and Rolls, 1987). For instance, investigators found that synaptic loss in the inferior temporal gyrus is a prominent pathological defect in the early stages of AD, and individuals with aMCI have significantly fewer synapses compared to those with no cognitive impairment (Scheff et al., 2011). The current literature suggests that increased activity of the superior frontal gyrus may be closely related to attentional shift between object features (Nagahama et al., 1999), cognitive control, working memory, and execution (Zhang et al., 2012).
Moreover, the medial superior frontal is an important node within the default mode network (DMN). Zhou et al. (2015) found several important nodes within the DMN that are involved in MCI, including the medial superior frontal gyrus. Huang et al. (2016) suggested that the DMN may indeed be a crucial target for interventions of MCI. Fink et al. (1996) found that the left inferior occipital cortex was activated in a local directed attention task, and Belleville suggested that attentional control deficits occur in patients with MCI (Belleville et al., 2007). Taken together, these studies provide further support for the aforementioned brain regions as targets for MCI treatment with NIBS.
It is worth noting that identifying these locations may also help to advance other interventions such as scalp acupuncture. Unlike traditional acupuncture, where needles are inserted into acupuncture points, scalp acupuncture stimulates the area of scalp corresponding to brain regions believed to be involved in the pathology of the disorders (Hao and Hao, 2012). Thus, results obtained from this study may facilitate the development of acupuncture and therapeutic modalities such as transcutaneous electrical nerve stimulation for the treatment of MCI.
Further, the aim of this preliminary study was to investigate potential stimulation locations for NIBS treatment using a unique approach that combined meta- and functional connectivity analyses. However, different stimulation techniques may have different intensities and reach different depth. How to apply and optimize these different treatment modalities to target the brain areas identified in our study is beyond the scope of this manuscript. Investigators should consider the characteristics of different tools when attempting to stimulate these areas.
Our results suggest that the DLPFC, IFG, and TPJ should be preferentially recommended as locations for NIBS treatment of MCI. Furthermore, the bilateral orbital prefrontal gyrus, inferior temporal gyrus, medial superior frontal gyrus, and right inferior occipital gyrus may also be potential brain stimulation sites. Our results ultimately shed light on possible NIBS sites in the treatment of MCI and other cases of age-related cognitive decline.
There are several limitations to our study. First, while we provide several potential stimulation sites for NIBS treatment, we cannot predict the specific effects of such stimulation. For instance, we do not know whether the effect of the stimulation will be excitatory or inhibitory in nature. Another limitation is that the results of the connectivity analysis were strictly functional in the present study (i.e., rsFC). Future studies combining anatomical and functional connectivity analyses may yield stronger evidence for stimulation sites. Furthermore, our present study provided only a starting point for brain stimulation location selection via meta- and rsFC analysis methods. Determining the best brain region for NIBS remains a challenge. One option is to apply stimulation to multiple regions simultaneously. Stimulation paradigms are beyond the scope of this manuscript, and future studies are needed to explore these paradigms based on the specific NIBS modalities and, most importantly, the condition of the individual. In addition, due to the database limitation, we included patients within the age range of 55–90. Future studies with a smaller age range should be conducted to reduce the effects of standard deviation of age.
All datasets generated for this study are included in the manuscript and/or the Supplementary Files.
Data used in preparation of this article were obtained from the Alzheimer’s disease ADNI database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
JK designed the experiments. JL, BZ, and JK analyzed and interpreted the data. JL and GW prepared the manuscript. All authors prepared the manuscript, and read and approved the final manuscript.
JK was supported by grants R01 AT008563, R33 AT009310, R33AT009341, R34DA046635, and R21AT008707 from NIH. JL was supported by the Science and Technology Platform Construction project of Fujian Science and Technology Department (Grant No. 2015Y2001). Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI was funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association, Alzheimer’s Drug Discovery Foundation, Araclon Biotech, BioClinica, Inc., Biogen; Bristol-Myers Squibb Company, CereSpir, Inc., Cogstate, Eisai Inc., Elan Pharmaceuticals, Inc., Eli Lilly and Company, EuroImmun, F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc., Fujirebio, GE Healthcare, IXICO Ltd., Janssen Alzheimer Immunotherapy Research & Development, LLC., Johnson & Johnson Pharmaceutical Research & Development LLC., Lumosity, Lundbeck, Merck & Co., Inc., Meso Scale Diagnostics, LLC., NeuroRx Research, Neurotrack Technologies, Novartis Pharmaceuticals Corporation, Pfizer Inc., Piramal Imaging, Servier, Takeda Pharmaceutical Company, and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
JK has a disclosure to report (holding equity in a startup company, MNT, and pending patents to develop a new brain stimulation device) but declares no conflict of interest.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2019.00228/full#supplementary-material
FIGURE S1 | (A) Results from the meta-analysis. (B) Results from the positive rsFC based on Pipeline 2 analysis. (C) Results from the negative rsFC based on Pipeline 2 analysis. (D) Results from the combination of all ROIs based on Pipeline 3 analysis.
FIGURE S2 | ROIs used for the functional connectivity analysis.
Baylis, G. C., and Rolls, E. T. (1987). Responses of neurons in the inferior temporal cortex in short term and serial recognition memory tasks. Exp. Brain Res. 65, 614–622. doi: 10.1007/BF00235984
Belleville, S., Chertkow, H., and Gauthier, S. (2007). Working memory and control of attention in persons with Alzheimer’s disease and mild cognitive impairment. Neuropsychology 21, 458–459. doi: 10.1037/0894-4105.21.4.458
Bell-McGinty, S., Lopez, O. L., Meltzer, C. C., Scanlon, J. M., Whyte, E. M., DeKosky, S. T., et al. (2005). Differential cortical atrophy in subgroups of mild cognitive impairment. Arch. Neurol. 62, 1393–1397. doi: 10.1001/archneur.62.9.1393
Bergmann, T. O., Karabanov, A., Hartwigsen, G., Thielscher, A., and Siebner, H. R. (2016). Combining non-invasive transcranial brain stimulation with neuroimaging and electrophysiology: current approaches and future perspectives. NeuroImage 140, 4–19. doi: 10.1016/j.neuroimage.2016.02.012
Birba, A., Ibáñez, A., Sedeño, L., Ferrari, J., García, A. M., and Zimerman, M. (2017). Non-invasive brain stimulation: a new strategy in mild cognitive impairment? Front. Aging Neurosci. 9:16. doi: 10.3389/fnagi.2017.00016
Biswal, B. B., Van Kylen, J., and Hyde, J. S. (1997). Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 10, 165–170. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<165::aid-nbm454>3.0.co;2-7
Brunoni, A. R., Boggio, P. S., De Raedt, R., Bensenor, I. M., Lotufo, P. A., Namur, V., et al. (2014). Cognitive control therapy and transcranial direct current stimulation for depression: a randomized, double-blinded, controlled trial. J. Affective Disord. 162, 43–49. doi: 10.1016/j.jad.2014.03.026
Bruscoli, M., and Lovestone, S. (2004). Is MCI really just early dementia? A systematic review of conversion studies. Int. Psychogeriatr. 19, 129–140. doi: 10.1017/s1041610204000092
Cai, S., Chong, T., Peng, Y., Shen, W., Li, J., von Deneen, K. M., et al. (2017). Altered functional brain networks in amnestic mild cognitive impairment: a resting-state fMRI study. Brain Imaging Behav. 11, 619–631. doi: 10.1007/s11682-016-9539-0
Cappon, D., Jahanshahi, M., and Bisiacchi, P. (2016). Value and efficacy of transcranial direct current stimulation in the cognitive rehabilitation: a critical review since 2000. Front. Neurosci. 10:157. doi: 10.3389/fnins.2016.00157
Cutini, S., Scatturin, P., and Zorzi, M. (2011). A new method based on ICBM152 head surface for probe placement in multichannel fNIRS. NeuroImage 54, 919–927. doi: 10.1016/j.neuroimage.2010.09.030
Elder, G. J., and Taylor, J. P. (2014). Transcranial magnetic stimulation and transcranial direct current stimulation: treatments for cognitive and neuropsychiatric symptoms in the neurodegenerative dementias? Alzheimers Res. Ther. 6:74. doi: 10.1186/s13195-014-0074-1
Fink, G. R., Halligan, P. W., Marshall, J. C., Frith, C. D., Frackowiak, R. S. J., and Dolan, R. J. (1996). Where in the brain does visual attention select the forest and the trees? Nature 382, 626–629. doi: 10.1038/382626a0
Fox, M. D., Buckner, R. L., White, M. P., Greicius, M. D., and Pascual-Leone, A. (2012). Efficacy of TMS targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol. Psychiatry 72, 595–603. doi: 10.1016/j.biopsych.2012.04.028
Fox, M. D., and Greicius, M. (2010). Clinical applications of resting state functional connectivity. Front. Syst. Neurosci. 4:19. doi: 10.3389/fnsys.2010.00019
Friston, K. J., Frith, C. D., Liddle, P. F., and Frackowiak, R. S. J. (1993). Functional connectivity: the principal-component analysis of large (PET) data sets. J. Cereb. Blood Flow Metab. 13, 5–14. doi: 10.1038/jcbfm.1993.4
Goldstein-Piekarski, A. N., Staveland, B. R., Ball, T. M., Yesavage, J., Korgaonkar, M. S., and Williams, L. M. (2018). Intrinsic functional connectivity predicts remission on antidepressants: a randomized controlled trial to identify clinically applicable imaging biomarkers. Transl. Psychiatry 8:57. doi: 10.1038/s41398-018-0100-3
Griffith, R., Stewart, C., Stoeckel, L., Okonkwo, O., Den, J., Martin, R., et al. (2010). Magnetic resonance imaging volume of the angular gyri predicts financial skill deficits in people with amnestic mild cognitive impairment. J. Am. Geriatr. Soc. 58, 265–274. doi: 10.1111/j.1532-5415.2009.02679.x
Hao, J. J., and Hao, L. L. (2012). Review of clinical applications of scalp acupuncture for paralysis: an excerpt from chinese scalp acupuncture. Glob. Adv. Health Med. 1, 102–121. doi: 10.7453/gahmj.2012.1.1.017
Herholz, S. C., Herholz, R. S., and Herholz, K. (2013). Non-pharmacological interventions and neuroplasticity in early stage Alzheimer’s disease. Expert Rev. Neurother. 13, 1235–1245. doi: 10.1586/14737175.2013.845086
Huang, P., Fang, R., Li, B. Y., and Chen, S. D. (2016). Exercise-related changes of networks in aging and mild cognitive impairment brain. Front. Aging Neurosci. 8:47. doi: 10.3389/fnagi.2016.00047
Jonker, F. A., Jonker, C., Scheltens, P., and Scherder, E. J. A. (2015). The role of the orbitofrontal cortex in cognition and behavior. Rev. Neurosci. 26, 1–11. doi: 10.1515/revneuro-2014-43
Lin, F., Ren, P., Lo, R. Y., Chapman, B. P., Jacobs, A., Baran, T. M., et al. (2017). Insula and inferior frontal gyrus’ activities protect memory performance against Alzheimer’s disease pathology in old age. J. Alzheimers Dis. 55, 669–678. doi: 10.3233/JAD-160715
Liu, J., Chen, L., Chen, X., Hu, K., Tu, Y., Lin, M., et al. (2019a). Modulation effects of different exercise modalities on the functional connectivity of the periaqueductal grey and ventral tegmental area in patients with knee osteoarthritis – a randomized multi-modal MRI study. Br. J. Anesth. doi: 10.1016/j.bja.2019.06.017 [Epub ahead of print].
Liu, J., Tao, J., Liu, W., Huang, J., Xue, X., Li, M., et al. (2019b). Different modulation effects of tai chi chuan and baduanjin on resting state functional connectivity of the default mode network in older adults. Soc. Cogn. Affect. Neurosci. 14, 217–224. doi: 10.1093/scan/nsz001
Maldjian, J. A., Laurienti, P. J., and Burdette, J. H. (2004). Precentral gyrus discrepancy in electronic versions of the talairach atlas. NeuroImage 21, 450–455. doi: 10.1016/j.neuroimage.2003.09.032
Maldjian, J. A., Laurienti, P. J., Kraft, R. A., and Burdette, J. H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage 19, 1233–1239. doi: 10.1016/S1053-8119(03)00169-1
Melrose, R. J., Jimenez, A. M., Riskin-Jones, H., Weissberger, G., Veliz, J., Hasratian, A. S., et al. (2018). Alterations to task positive and task negative networks during executive functioning in mild cognitive impairment. NeuroImage 19, 970–981. doi: 10.1016/j.nicl.2018.06.014
Nagahama, Y., Okada, T., Katsumi, Y., Hayashi, T., Yamauchi, H., Sawamoto, N., et al. (1999). Transient neural activity in the medial superior frontal gyrus and precuneus time locked with attention shift between object features. NeuroImage 10, 193–199. doi: 10.1006/nimg.1999.0451
Nardone, R., Tezzon, F., Höller, Y., Golaszewski, S., Trinka, E., and Brigo, F. (2014). Transcranial magnetic stimulation (TMS)/repetitive TMS in mild cognitive impairment and Alzheimer’s disease. Acta Neurol. Scand. 129, 351–366. doi: 10.1111/ane.12223
Olichney, J. M., Yang, J. C., Taylor, J., and Kutas, M. (2011). Cognitive event-related potentials: biomarkers of synaptic dysfunction across the stages of Alzheimer’s disease. Adv. Alzheimers Dis. 2, 421–434.
Padala, P. R., Padala, K. P., Lensing, S. Y., Jackson, A. N., Hunter, C. R., Parkes, C. M., et al. (2018). Repetitive transcranial magnetic stimulation for apathy in mild cognitive impairment: a double-blind, randomized, sham-controlled, cross-over pilot study. Psychiatry Res. 261, 312–318. doi: 10.1016/j.psychres.2017.12.063
Petersen, R. C., Lopez, O., Armstrong, M. J., Getchius, T. S. D., Ganguli, M., Gloss, D., et al. (2018). Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the american academy of neurology. Neurology 90, 126–135. doi: 10.1212/WNL.0000000000004826
Pievani, M., Pini, L., Ferrari, C., Pizzini, F. B., Boscolo Galazzo, I., Cobelli, C., et al. (2017). Coordinate-based meta-analysis of the default mode and salience network for target identification in non-invasive brain stimulation of Alzheimer’s disease and behavioral variant frontotemporal dementia networks. J. Alzheimers Dis. 57, 825–843. doi: 10.3233/JAD-161105
Rajji, T. K. (2019). Impaired brain plasticity as a potential therapeutic target for treatment and prevention of dementia. Expert Opin. Ther. Targets 23, 21–28. doi: 10.1080/14728222.2019.1550074
Sacuiu, S., Insel, P. S., Mueller, S., Tosun, D., Mattsson, N., Jack, C. R., et al. (2016). Chronic depressive symptomatology in mild cognitive impairment is associated with frontal atrophy rate which hastens conversion to Alzheimer dementia. Am. J. Geriatr. Psychiatry 24, 126–135. doi: 10.1016/j.jagp.2015.03.006
Scheff, S. W., Price, D. A., Schmitt, F. A., Scheff, M. A., and Mufson, E. J. (2011). Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer disease. J. Alzheimers Dis. 24, 547–557. doi: 10.3233/JAD-2011-101782
Simon, K. C. N. S., Gómez, R. L., Nadel, L., and Scalf, P. E. (2017). Brain correlates of memory reconsolidation: a role for the TPJ. Neurobiol. Learn. Mem. 142, 154–161. doi: 10.1016/j.nlm.2017.03.003
Tao, J., Liu, J., Chen, X., Xia, R., Li, M., Huang, M., et al. (2019). Mind-body exercise improves cognitive function in patients with mild cognitive impairment and centrally modulates the hippocampus/ACC: a multi-modal MRI study. NeuroImage 23:101834. doi: 10.1016/j.nicl.2019.101834
Tao, J., Liu, J., Egorova, N., Chen, L., Sun, S., Xue, H., et al. (2016). Increased hippocampus–medial prefrontal cortex resting state functional connectivity and memory function after tai chi chuan practice in elder adults. Front. Aging Neurosci. 8:25. doi: 10.3389/fnagi.2016.00025
Thompson-Schill, S. L., D’Esposito, M., Aguirre, G. K., and Farah, M. J. (1997). Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc. Natl. Acad. Sci. U.S.A. 94, 14792–14797. doi: 10.1073/pnas.94.26.14792
Wang, Z., Wang, X., Liu, J., Chen, J., Liu, X., Nie, G., et al. (2017). Acupuncture treatment modulates the corticostriatal reward circuitry in major depressive disorder. J. Psychiatr. Res. 84, 18–26. doi: 10.1016/j.jpsychires.2016.09.014
Wilson, R. C., Takahashi, Y. K., Schoenbaum, G., and Niv, Y. (2014). Orbitofrontal cortex as a cognitive map of task space. Neuron 81, 267–278. doi: 10.1016/j.neuron.2013.11.005
Xi, Q., Zhao, X. H., Wang, P. J., Guo, Q. H., and He, Y. (2013). Abnormal intrinsic brain activity in amnestic mild cognitive impairment revealed by amplitude of low-frequency fluctuation: a resting-state functional magnetic resonance imaging study. Chin. Med. J. 126, 2912–2917. doi: 10.3760/cma.j.issn.0366-6999.20130431
Xia, M., Wang, J., and He, Y. (2013). BrainNet viewer: a network visualization tool for human brain connectomics. PLoS One 8:e68910. doi: 10.1371/journal.pone.0068910
Yang, Y. H., Liang, P. P., Lu, S. F., Li, K. C., and Zhong, N. (2009). The role of the DLPFC in inductive reasoning of MCI patients and normal agings: an fMRI study. Sci. Chin. C 52, 789–795. doi: 10.1007/s11427-009-0089-1
Yarkoni, T., Poldrack, R. A., Nichols, T. E., Van Essen, D. C., and Wager, T. D. (2011). Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 8, 665–670. doi: 10.1038/nmeth.1635
Zhang, S., Ide, J. S., and Li, C. S. R. (2012). Resting-state functional connectivity of the medial superior frontal cortex. Cereb. Cortex 22, 99–111. doi: 10.1093/cercor/bhr088
Keywords: mild cognitive impairment, non-invasive brain stimulation, stimulation site, meta-analysis, resting state functional connectivity
Citation: Liu J, Zhang B, Wilson G, Kong J and the Alzheimer’s Disease Neuroimaging Initiative (2019) New Perspective for Non-invasive Brain Stimulation Site Selection in Mild Cognitive Impairment: Based on Meta- and Functional Connectivity Analyses. Front. Aging Neurosci. 11:228. doi: 10.3389/fnagi.2019.00228
Received: 12 April 2019; Accepted: 09 August 2019;
Published: 27 August 2019.
Edited by:
Johannes Boltze, The University of Warwick, United KingdomReviewed by:
Gabriel Gonzalez-Escamilla, Johannes Gutenberg University Mainz, GermanyCopyright © 2019 Liu, Zhang, Wilson, Kong and the Alzheimer’s Disease Neuroimaging Initiative. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Kong, a29uZ2pAbm1yLm1naC5oYXJ2YXJkLmVkdQ==
†Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.