- 1Biomedical Research Centrum, University Hospital Hradec Kralove, Hradec Kralove, Czechia
- 2Department of Management, Faculty of Informatics and Management, University of Hradec Kralove, Hradec Kralove, Czechia
- 3Department of Neurology, Faculty of Medicine and University Hospital Hradec Kralove, Charles University in Prague, Hradec Kralove, Czechia
The aim of this review is to summarize the effect of human intestinal microbiome on cognitive impairments and to focus primarily on the impact of diet and eating habits on learning processes. Better understanding of the microbiome could revolutionize the possibilities of therapy for many diseases. The authors performed a literature review of available studies on the research topic describing the influence of human microbiome and diet on cognitive impairment or learning processes found in the world’s acknowledged databases Web of Science, PubMed, Springer, and Scopus. The digestive tube is populated by billions of living microorganisms including viruses, bacteria, protozoa, helminths, and microscopic fungi. In adulthood, under physiological conditions, the intestinal microbiome appears to be relatively steady. However, it is not true that it would not be influenced, both in the positive sense of the word and in the negative one. The basic pillars that maintain a steady microbiome are genetics, lifestyle, diet and eating habits, geography, and age. It is reported that the gastrointestinal tract and the brain communicate with each other through several pathways and one can speak about gut-brain axis. New evidence is published every year about the association of intestinal dysbiosis and neurological/psychiatric diseases. On the other hand, specific diets and eating habits can have a positive effect on a balanced microbiota composition and thus contribute to the enhancement of cognitive functions, which are important for any learning process.
Introduction
The intestinal microbiome is a diverse community of intestinal microorganisms. For a long time, it seemed unlikely that a microbiome could also be responsible for processes outside the digestive tract. However, it has been shown that the composition of intestinal microbiome affects the entire spectrum of physiological processes or the development of the immune system. The optimal microbiome composition thus contributes to overall health (Al-Asmakh and Hedin, 2015; McKenney and Pamer, 2015). An increasing number of research studies demonstrates the enormous importance of the “gut-brain” axis and suggest that triggers for a variety of neurological diseases can be found in the gastrointestinal tract (Turnbaugh et al., 2009; Fasano, 2012; Hsiao et al., 2013; Bauer et al., 2016; Schroeder and Bäckhed, 2016; Dinan and Cryan, 2017).
The microbiome consists of milliard living microorganisms and as such, it has 100 times more genes than the human genome. The microbiome evolves with its human host in a symbiotic relationship. The development of the microbiome as a finely tuned ecosystem depends on a number of factors. The most important are: type of childbirth delivery, eating habits (diet, lifestyle; Daniel et al., 2014; David et al., 2014; Bruce-Keller et al., 2015; Magnusson et al., 2015), it is influenced by infection, stress, genetic predisposition, or person’s age (Yatsunenko et al., 2012; David et al., 2014).
The aim of this review is to summarize the effect of human intestinal microbiome on cognitive impairments and to focus primarily on the impact of diet and eating habits on learning processes. Better understanding of the microbiome could revolutionize the possibilities of therapy for many diseases.
Methods
The authors performed a literature review of available human and in one part also animal studies on the research topic describing the influence of human microbiome and diet on cognitive impairment or learning processes. The research studies were selected on the basis of research topics such as microbiome, neurological disorders, cognitive impairment, dementia, diet, learning processes found in the world’s acknowledged databases Web of Science, PubMed, Springer, and Scopus. The end of the search period is limited by March 2019. Altogether 531 were identified in all these databases. After removing duplicates and titles/abstracts unrelated to the research topic, 178 English-written studies remained. Of these, only 51 articles were relevant to the research topic. These research studies were classified according to their relevancy. The information found in the selected studies on microbiome and cognitive impairment was carefully evaluated and it is described and discussed in the following sections.
Microbiome
The digestive tube is populated by billions of living microorganisms in varying degrees of intensity (Blekhman et al., 2015; Bohórquez and Liddl, 2015; Koppel and Balskus, 2016). Most of them are found in our colon (up to 1014), which is considered an extremely complex ecosystem including viruses, bacteria, protozoa, helminths, and microscopic fungi (Bäckhed et al., 2015). In this review article, only the human intestinal bacterial microbiome is discussed (Ley et al., 2006; Qin et al., 2012).
The density of the microbiome in the digestive tract varies by location (at least in the stomach, most in the colon). Based on rRNA sequencing, 52 bacterial strains are currently defined, seven of which colonize the human intestinal tract. Interestingly, of these seven strains, 97% of the microbiome consists of only four strains—Firmicutes, Actinobacteria, Bacteroidetes, Proteobacteria (Tsai et al., 2019).
It is believed that the fetus in the uterus is free of bacteria, and only when it is born, it is inhabited by various strains of bacteria and viruses. However, according to available data, it is highly dependent on the way in which childbirth is conducted. If the delivery is through natural pathways, the main component of the neonatal intestinal microbiome is the bacteria corresponding to the mother’s vaginal microbioma, i.e., the Lactobacillus and Prevotella strains. While if the labor is performed by the so-called cesarean section, the intestine is preferably colonized by the bacteria of the genera Staphylococcus and Corynebacterium. In addition, it was found that children born by cesarean section are at greater risk of developing autoimmune diseases (Bäckhed et al., 2015). Intestinal microbiome is well adapted to external influences during the first 3 years of development and is also prone to undesirable changes that may be caused by diet or antibiotic use (Yatsunenko et al., 2012; David et al., 2014). Research studies state that it is this period of life that is most important to form a healthy intestinal microbiome (Yang et al., 2016). In animal models, antibiotic administration or drastic changes in dietary habits have been shown to lead to a predisposition to a behavioral disorders, depression, and anxiety at a later age.
In adulthood, under physiological conditions, the intestinal microbiome can be said to be relatively steady in terms of both quantity and diversity. However, it is not true that it would not be influenced, both in the positive sense of the word and in the negative one. The basic pillars that maintain a steady microbiome of the individual are: genetics, lifestyle, diet and eating habbits, geography, and age (de La Serre et al., 2010; Yatsunenko et al., 2012; David et al., 2014; Noble et al., 2014, 2017a). Reduced diversity or insufficient microbiome is associated with many diseases (Lozupone et al., 2012; Lloyd-Price et al., 2016; Oh et al., 2016).
Microbiome-Gut-Brain Axis
The term “gut-brain axis” generally appears in the literature, which includes afferent and efferent neural connections, endocrine, immune and metabolic signals. This means that the gut not only receives regulatory signals from the central nervous system (CNS), but it is also able to send signals to the brain, and the brain receives them. Based on the information published so far, it is very likely that this concept can be extended to the “microbiome-gut-brain” axis, as even the intestinal microbiome can affect the brain (Bercik et al., 2011; Davari et al., 2013; Hsiao et al., 2013; Bruce-Keller et al., 2015). Both the peripheral nervous system modulation and the CNS may be included among the intestine microbial abilities and their metabolites, i.e., influencing the brain development and brain function. This two-way communication system maintains normal organism homeostasis. Changes in microbiota leading to dysregulation of the gastrointestinal system, central/autonomic nervous system, or immune system may be one of the causes of various diseases (Turnbaugh et al., 2009; Fasano, 2012; Hsiao et al., 2013; Bauer et al., 2016; Schroeder and Bäckhed, 2016; Dinan and Cryan, 2017). The brain and intestine are connected by several physiological pathways, including neuronal, endocrine, immune and metabolic pathway.
Neuronal Pathway
GIT regulation takes place at four levels. At the local level, via the enteric nervous system (ENS) containing mainly sensory and motor neurons; at the level of prevertebral ganglia that pass information from the ENS to the CNS (Furness et al., 2014). Another level is the CNS, which, after receiving signals from the periphery and evaluating them, sends instructions to effector cells in the GIT. The last level is specific brain nuclei. The vagus nerve is the direct nerve junction of the CNS and ENS. If bacterial products (endotoxins, inflammatory cytokines, TNF-α) through the ENS stimulate the vagus nerve, affecting the CNS, which is very important for learning and memory-related processes (Mulak and Bonaz, 2004; Sudo et al., 2004; Bravo et al., 2011; Gareau et al., 2011; Forsythe et al., 2014).
Endocrine Pathway
Enteroendocrine cells are dispersed in the intestinal epithelium. If desired, these cells secrete hormones and other signal peptides. Secretion occurs in response to various luminal stimuli. For example, bacterial by-products have the ability to stimulate enteroendocrine cells. It has been found that if the composition of the intestinal microbiota changes, there will be changes in neuropeptides and neurotransmitters. Possible neuroactive molecules activated in the intestine include, for example, serotonin, GABA, catecholamines, melatonin, acetylcholine, histamine, dopamine (Barrett et al., 2012). These molecules can regulate inflammation, affect stress and anxiety reactions, emotions and mood, play a role in learning and creating memory tracks (O’Mahony et al., 2015). A brief review of the function of serotonin, according to the location of its receptors in the CNS/ENS, and in view of its affectivity and cognition (Table 1).
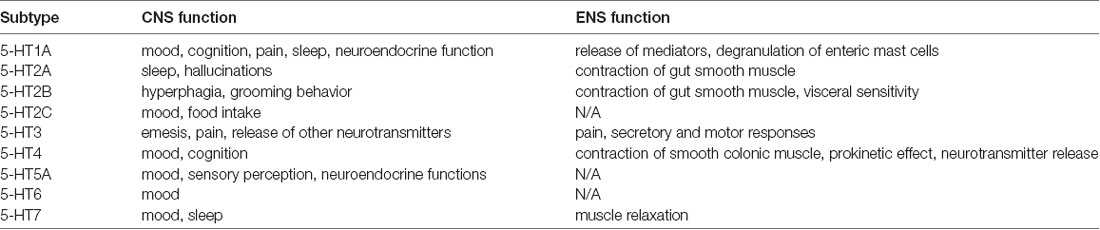
Table 1. Serotonin receptor subtypes through gut-brain axis (adjusted according to O’Mahony et al., 2015).
Immune Pathway
An important role of intestinal mucosa is to mediate adaptive immunity because it gets into primary contact with a large number of specific antigens. These specific antigens are PAMPs (pathogen-associated molecular pattern molecules). These are lipids, lipopolysaccharides and lipoproteins, which are part of the bacterial wall. PAMPs are recognized by pattern recognition receptors (PRRs). Activated PRRs provide increased cytokine and interferon production through antigen presenting cells. PRRs include, for example, transmembrane TLRs (Toll-like receptors). TLRs are localized in cells that are part of innate immunity (macrophages, epithelial cells, adipocytes). However, we also find them in cells of acquired immunity (B-lymphocytes, T-lymphocytes or dendritic cells). TLRs-mediated signaling results in the induction of dendritic cells and subsequent cytokine production (Thomas et al., 2017; Caputi and Giron, 2018).
Metabolic Pathway
Short-chain fatty acids (SCFAs), acetate, propionate, and butyrate are among the substances that are produced by the gut microbiome and can affect CNS functions. SCFAs have anti-inflammatory functions that induce due to binding to a specific G protein-coupled receptor (Macfarlane and Macfarlane, 2011). In studies conducted in mice, butyrate was found to regulate energy homeostasis, stimulate leptin production in adipocytes, and cause secretion of several neuropeptides. Butyrate also has anti-inflammatory effects. SCFAs increase the release of serotonin, i.e., they affect behavior and mood, as confirmed by in vitro studies.
Microbiome and Cognitive Impairment
New evidence is published every year about the association of intestinal microbiome, dysbiosis and neurological/psychiatric diseases. The facts relate to both the risk of developing the disease and its progression. Very often, neuro(auto)immune, neurodegenerative or psychiatric diseases are mentioned, such as multiple sclerosis, neuromyelitis optics, Parkinson’s disease (PD), Alzheimer’s disease (AD), amyotrophic lateral sclerosis, anxiety, schizophrenia, autism. Cognitive impairment may occur to a greater or lesser extent with these diseases. While the effect of dieting on cognitive function (see below) has been studied for a long time, the effect of microbiome on cognition is still partially shrouded in mystery (Gareau et al., 2011; Bajaj et al., 2012; Desbonnet et al., 2015; Dinan et al., 2015; Fröhlich et al., 2016; Parashar and Udayabanu, 2016).
Research studies have increasingly put emphasis on the influence of microorganisms on host behavior and its cognitive function. Experiments on germ-free animal models show the appearance of behavioral disorders and reduced cognitive functions. The first animal study has shown that anxiety-like behavior can be achieved by modulating the microbiome. Infection with Citrobacter rodentium in combination with acute stress has resulted in memory failure in mice. Interestingly, in control mice, this dysfunction was prevented by the prophylactic administration of probiotics prior to the infection itself (Gareau et al., 2011; Liang et al., 2015). There is evidence that donor animal-like behavior was demonstrated in the target animal when transferring the fecal transplantation from the donor animal to the target animal (Bercik et al., 2011).
For cognitive processes such as memory and learning, the proper functioning of the HPA axis is absolutely necessary. It has been reported that microorganisms (specifically Bifidocaterium) can modulate this axis—under stress, sterile mouse corticosteroid levels and adrenal cortex hormones are much higher than those of conventional microbial mice. Other agents very important for learning and memory processes are brain derived neurotrophic factor (BDNF), NMDA-receptors (N-methyl-D-aspartate), and c-fos (Wu et al., 2008; Stefanko et al., 2009; Intlekofer et al., 2013). These molecules are reduced in sterile mice. The effect of serotonin on cognition and socialization is well known and therefore the effect of kynurenine pathway metabolites is not so surprising. A well-studied example of the effect of dysbiosis on cognitive function is liver encephalopathy (that can lead to dementia), which is somewhat positively affected by oral antibiotic therapy (Bajaj et al., 2012; Ahluwalia et al., 2016). On the contrary, there exist studies that have demonstrated that the administration of antibiotics in mice causes cognitive impairments. Experiments were performed in mice immediately after weaning and in adult animals. The reduction in the number of microorganisms and their reduced diversity due to antibiotic therapy has always been associated with a decrease in hippocampal BDNF expression. However, it is not known whether the effects of antibiotics on cognitive function are only transient. It is clear from the above how the intestinal microbiome is a fragile community of microorganisms (Fröhlich et al., 2016).
A recently cross-sectional study conducted in Japan showed a comparison of the gut microbiome between demented and non-demented patients and demonstrated two major clusters of microbial taxa. The results were surprising: the number of Bacteroides (enterotype I) was lower and the number of “other” bacteria (enterotype III) was higher in demented than non-demented patients. Multivariable analyses revealed that lower prevalence of Bacteroides and a higher prevalence of “other” bacteria were associated with higher odds ratios than the traditional dementia biomarkers ApoE ε4, SLI and high VSRAD score (Saji et al., 2019).
Interesting facts are brought by research studies on the influence of the use of dairy probiotic cultures among healthy volunteers. They have seen a change in activity in the brain regions responsible for cognitive function, these areas being under serotonergic innervation. Thus, the administration of serotonin and tryptophan precursor for modulating learning processes might be significant (Tillisch et al., 2013).
In the context of inappropriate eating habits, excess energy intake and lifestyle without enough physical activity, one can talk about the obesity epidemic in the western world. The findings of the research studies have shown that obesity and intestinal dysbiosis go hand in hand. In addition, in these studies, it was suggested that the intestinal microbiotic composition also manifested itself in results of cognitive and other tests. Persons with a higher incidence of actinobacteria (non-obese) were found to have better results at the speed of movement, better attention, and better cognition scores. Mechanistic pathway is not yet studied, however, there is a link between diet, microbiome, inflammation and resulting cognitive dysfunction in obese patients (Pistell et al., 2010; Puig et al., 2012; Herculano et al., 2013; Tillisch et al., 2013; Camer et al., 2015).
Diets and Cognitive Dysfunction
Research studies show that diet and eating habits have both long-term and short-term effects on people’s cognitive functions (Mahoney et al., 2005; Prado and Dewey, 2012). Diet and proper eating habits influence person’s cognition already in prenatal age since diet plays a key role in the maturation of vital organs and the establishment of neuronal connections (Moody et al., 2017). Research indicates that diseases (e.g., AD or autism) connected with cognitive impairments and learning disabilities have their etiology in an early life (Moody et al., 2017). Thus, maternal diet can have a long-term effect on child’s cognitive development. Particularly two extremes are inappropriate; malnutrition or food deficit and excessive intake of saturated fat and refined carbohydrates (Mahoney et al., 2005; Prado and Dewey, 2012).
Malnutrition is typical of the so-called developing countries such as India, where researchers discovered among 8-year-old children suffering from chronic malnutrition that 19% of them find it difficult to read simple sentences like “I like dogs” or “The sun is hot,” 12.5% make a mistake when they are asked to write a simple sentence and 7% make mistakes while responding to simple mathematics such as eight minus three (Angre, 2019). In addition, Wang C. et al. (2016) in their study among 1,366 Chinese adults born between 1950 and 1964 found out that malnutrition is associated with overall and specific cognitive decline, affecting selective attention and response inhibition particularly. Nevertheless, food deficiency is also connected with socio-economic status even in affluent countries where children from deprived backgrounds receive food of low quality, with fewer micronutrients (e.g., iron or iodine), fewer calories, but with higher fat (Darmon and Drewnowski, 2008). As Ross (2019) state food consumption is vital to the brain to make the right amount of amino acids and choline—important precursor molecules to make the brain function normally. Furthermore, researchers emphasize the importance of habitual breakfast for school children since such breakfast has a positive effect on pupils’ academic performance, mainly on task-performance behavior in the classroom (Adolphus et al., 2013).
On the contrary, the excessive intake of saturated fat and refined carbohydrates is typical of western diet (Reichelt et al., 2017), which also has a negative impact on cognitive functioning since high fat and sugar change intestine bacteria colonies and increase intestinal permeability and lower blood brain barrier. This develops a vulnerability to the influx of toxins from circulation to the brain, which results in cognitive dysfunction (Noble et al., 2017b).
Research reveals that diets, especially therapeutic for some pathological conditions such as irritable bowel syndrome or neurological disorders, are also effective in enhancing cognitive functions (Lichtwark et al., 2014; Reddel et al., 2019). This concerns mainly specific dietary regimens such as low-fermentable, oligo-, di-, monosaccharides and polyols (low-FODMAPs) and gluten-free (GFD). They seem to be useful for recovery and maintenance of a eubiotic gut microbiota, particularly if supported with probiotics (Reddel et al., 2019).
Discussion and Conclusion
Neurological diseases are a global problem and the professional public expects them to grow. The rapid lifestyle, the enormous consumption of antibiotics and other eradicating microbial processes that have so far coexisted with humans for millennia, can be summarized as westernization. Westernization is a huge challenge for medical research. The changing intestinal microbiome, its association with neurological diseases, and the association with prolonged life expectancy are beginning to play a significant role for society.
A number of animal model studies have been reported to indicate that changes in the intestinal microbiota can be linked to gut-brain axis effect and influencing nutrient absorption, energy distribution or immunity. The opposite situation is with experiments on humans. There is an insufficient number of adequately designed controlled trials leading to a difficult assessment if the microbiome composition can influence cognition and learning processes. Considering the results from animal studies, one can reasonably believe that microbiome could affect gut-brain axis and influence cognition in humans. This means that positive qualitative and quantitative changes in the human gut microbiota might interfere with the onset and development of cognitive impairment and improve learning processes.
More and more studies are focused on TLR receptors and their association with neurological disorders and the microbiome-gut-brain axis. Increasing evidence attributes positive effects to probiotic restoration of disrupted intestinal barriers, stimulation of the human mucosal immune system, and prevention of growth of pathogenic microorganisms. TLR ligands derived from probiotics appear to have a positive effect on inflammatory production of anti-inflammatory cytokines (Thomas et al., 2017; Caputi and Giron, 2018).
On the basis of published studies, the use of probiotics can be particularly recommended to support cognitive functions. Most work has been done on animals, but the portability of results to humans seems realistic. B. longum, B. breve, B. infantis, L. helveticus, L. rhamnosus, L. plantarum, and L. casei were most effective at improving CNS function (anxiety, depressive, affective, stress, memory; Tsai et al., 2019; Wang H. et al., 2016).
Tryptophan resp. the serotonin system plays an irreplaceable role in the gut-brain axis. Given the diversity of serotonin functions throughout the human body, it does not seem realistic yet to treat individual diseases caused by the serotonin deregulation. It is both a heterogeneity of the disease and a low level of exploration of the area. Because preclinical data strongly favor this neuromediator, the connection of microbiome-gut-serotonin-CNS to researchers seems to be a major challenge, but studies are now in the process of adding that systems such as GABA are completely unexplored and open to cognition (O’Mahony et al., 2015).
Moreover, specific diets (low-FODMAPs and GFD) and eating habits can have a positive effect on a balanced microbiota composition and thus contribute to the enhancement of cognitive functions, important for any learning process.
Probably the biggest controversy can bring contradictions in the results of individual studies. Some articles describe sensational discoveries, others exploring virtually the same, finding changes barely statistically detectable. It is not uncommon to find contradictory results. The reasons may be different. Cognition disorders and learning difficulties are often multi-etiological and the same cognitive impairment in one patient may be caused by other causes, whether genetic, metabolic, or immunological, to the other individual. Another fact is that cognition disorders can be masked, for example, by training to improve test results; cognition and learning can also disrupt mood disorder already altered by possible psychotherapy or life circumstances. False negative results can be caused by an inappropriate selection of control objects (for example, when examining microbiomes of family members as controls). Other patient groups (e.g., due to possible microbiological contamination in the hospital environment) may also be an inappropriate control. The choice of the subjects themselves should also be carried out wisely, especially in the case of small numbers. Some of the microbiome sequencing studies operate with a small number of subjects, and in addition, from the same geographical area. Therefore, it cannot be ruled out that in other areas, countries, cultures with other dietary and hygiene habits, dysbiosis at the microbiome level may be manifested differently. For a complete list of problems in understanding the analysis, it is worth noting that stool samples, which most studies explore, do not have to show the real representation of species in the gut. For example, there are mucinophilic bacteria, mutualists, and possibly pathogens, who are only minimally involved in excretion and thus invasive methods are needed to investigate them.
In conclusion, the authors are convinced that by changing the lifestyle, including changes in dietary habits, can positively affect the cognitive brain functions and learning-related processes, especially by supporting the physiological mechanisms discussed in this mini-review. In addition, recent research (Toman et al., 2018) shows that other interventions such as physical activities or cognitive training are also an integral part of cognitive processes.
Author Contributions
MN, BK, and MV equally contributed to the drafting, analyses and final version of the whole manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by MH CZ—DRO (UHHK, 00179906) by the grant projects of the Ministry of Health of the Czech Republic (FN HK 00179906) and of the Charles University in Prague, Czech Republic (PROGRES Q40). Supported by the project: PERSONMED—Center for the Development of Personalized Medicine in Age-Related Diseases, Reg. Nr. CZ.02.1.01/0.0/0.0/17_048/0007441, co-financed by ERDF and state budget of the Czech Republic, and by the SPEV project 2104/2019, run at the Faculty of Informatics and Management of the University of Hradec Kralove, Czech Republic.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Josef Toman for his help with data collection.
References
Adolphus, K., Lawton, C. L., and Dye, L. (2013). The effects of breakfast on behavior and academic performance in children and adolescents. Front. Hum. Neurosci. 7:425. doi: 10.3389/fnhum.2013.00425
Ahluwalia, V., Betrapally, N. S., Hylemon, P. B., White, M. B., Gillevet, P. M., Unser, A. B., et al. (2016). Impaired gut-liver-brain axis in patients with cirrhosis. Sci. Rep. 6:26800. doi: 10.1038/srep26800
Al-Asmakh, M., and Hedin, L. (2015). Microbiota and the control of blood-tissue barriers. Tissue Barriers 3:e1039691. doi: 10.1080/21688370.2015.1039691
Angre, K. (2019). How malnutrition impacts learning. Available online at: https://www.ndtv.com/india-news/how-malnutrition-impacts-learning-523729. Accessed March 10, 2019.
Bäckhed, F., Roswall, J., Peng, Y., Feng, Q., Jia, H., Kovatcheva-Datchary, P., et al. (2015). Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 690–703. doi: 10.1016/j.chom.2015.04.004
Bajaj, J. S., Ridlon, J. M., Hylemon, P. B., Thacker, L. R., Heuman, D. M., Smith, S., et al. (2012). Linkage of gut microbiome with cognition in hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G168–G175. doi: 10.1152/ajpgi.00190.2011
Barrett, E., Ross, R. P., O’Toole, P. W., Fitzgerald, G. F., and Stanton, C. (2012). γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 113, 411–417. doi: 10.1111/j.1365-2672.2012.05344.x
Bauer, K. C., Huus, K. E., and Finlay, B. B. (2016). Microbes and the mind: emerging hallmarks of the gut microbiota-brain axis. Cell. Microbiol. 18, 632–644. doi: 10.1111/cmi.12585
Bercik, P., Denou, E., Collins, J., Jackson, W., Lu, J., Jury, J., et al. (2011). The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141, 599–609. doi: 10.1053/j.gastro.2011.04.052
Blekhman, R., Goodrich, J. K., Huang, K., Sun, Q., Bukowski, R., Bell, J. T., et al. (2015). Host genetic variation impacts microbiome composition across human body sites. Genome Biol. 16:191. doi: 10.1186/s13059-015-0759-1
Bohórquez, D. V., and Liddl, R. A. (2015). The gut connectome: making sense of what you eat. J. Clin. Invest. 125, 888–890. doi: 10.1172/JCI81121
Bravo, J. A., Forsythe, P., Chew, M. V., Escaravage, E., Savignac, H. M., Dinan, T. G., et al. (2011). Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U S A 108, 16050–16055. doi: 10.1073/pnas.1102999108
Bruce-Keller, A. J., Salbaum, J. M., Luo, M., Blanchard, E., Taylor, C. M., Welsh, D. A., et al. (2015). Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol. Psychiatry 77, 607–615. doi: 10.1016/j.biopsych.2014.07.012
Camer, D., Yu, Y., Szabo, A., Fernandez, F., Dinh, C. H. L., and Huang, X. F. (2015). Bardoxolone methyl prevents high-fat diet-induced alterations in prefrontal cortex signalling molecules involved in recognition memory. Prog. Neuropsychopharmacol. Biol. Psychiatry 59, 68–75. doi: 10.1016/j.pnpbp.2015.01.004
Caputi, V., and Giron, M. C. (2018). Microbiome-gut-brain axis and toll-like receptors in Parkinson’s disease. Int. J. Mol. Sci. 19:E1689. doi: 10.3390/ijms19061689
Daniel, H., Gholami, A. M., Berry, D., Desmarchelier, C., Hahne, H., Loh, G., et al. (2014). High-fat diet alters gut microbiota physiology in mice. ISME J. 8, 295–308. doi: 10.1038/ismej.2013.155
Darmon, N., and Drewnowski, A. (2008). Does social class predict diet quality? Am. J. Clin. Nutr. 87, 1107–1117. doi: 10.1093/ajcn/87.5.1107
Davari, S., Talaei, S. A., Alaei, H., and Salami, M. (2013). Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: behavioral and electrophysiological proofs for microbiome-gut-brain axis. Neuroscience 240, 287–296. doi: 10.1016/j.neuroscience.2013.02.055
David, L. A., Maurice, C. F., Carmody, R. N., Gootenberg, D. B., Button, J. E., Wolfe, B. E., et al. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563. doi: 10.1038/nature12820
de La Serre, C. B., Ellis, C. L., Lee, J., Hartman, A. L., Rutledge, J. C., and Raybould, H. E. (2010). Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G440–G448. doi: 10.1152/ajpgi.00098.2010
Desbonnet, L., Clarke, G., Traplin, A., O’Sullivan, O., Crispie, F., Moloney, R. D., et al. (2015). Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav. Immun. 48, 165–173. doi: 10.1016/j.bbi.2015.04.004
Dinan, T. G., Stilling, R. M., Stanton, C., and Cryan, J. F. (2015). Collective unconscious: how gut microbes shape human behavior. J. Psychiatr. Res. 63, 1–9. doi: 10.1016/j.jpsychires.2015.02.021
Dinan, T. G., and Cryan, J. F. (2017). Gut instintcs: microbiota as a key regulator of brain development, ageing and neurodegeneration. J. physiol. 595, 489–503. doi: 10.1113/JP273106
Fasano, A. (2012). Leaky gut and autoimmune diseases. Clin. Rev. Allergy Immunol. 42, 71–78. doi: 10.1007/s12016-011-8291-x
Forsythe, P., Bienenstock, J., and Kunze, W. A. (2014). Vagal pathways for microbiome-brain-gut axis communication. Adv. Exp. Med. Biol. 817, 115–133. doi: 10.1007/978-1-4939-0897-4_5
Fröhlich, E. E., Farzi, A., Mayerhofer, R., Reichmann, F., Jačan, A., Wagner, B., et al. (2016). Cognitive impairment by antibiotic-induced gut dysbiosis: analysis of gut microbiota-brain communication. Brain Behav. Immun. 56, 140–155. doi: 10.1016/j.bbi.2016.02.020
Furness, J. B., Callaghan, B. P., Rivera, L. R., and Cho, H. J. (2014). The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv. Exp. Med. Biol. 817, 39–71. doi: 10.1007/978-1-4939-0897-4_3
Gareau, M. G., Wine, E., Rodrigues, D. M., Cho, J. H., Whary, M. T., Philpott, D. J., et al. (2011). Bacterial infection causes stress-induced memory dysfunction in mice. Gut 60, 307–317. doi: 10.1136/gut.2009.202515
Herculano, B., Tamura, M., Ohba, A., Shimatani, M., Kutsuna, N., and Hisatsune, T. (2013). β-alanyl-L-histidine rescues cognitive deficits caused by feeding a high fat diet in a transgenic mouse model of Alzheimer’s disease. J. Alzheimers Dis. 33, 983–997. doi: 10.3233/JAD-2012-121324
Hsiao, E. Y., McBride, S. W., Hsien, S., Sharon, G., Hyde, E. R., McCue, T., et al. (2013). Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463. doi: 10.1016/j.cell.2013.11.024
Intlekofer, K. A., Berchtold, N. C., Malvaez, M., Carlos, A. J., McQuown, S. C., Cunningham, M. J., et al. (2013). Exercise and sodium butyrate transform a subthreshold learning event into long-term memory via a brain-derived neurotrophic factor-dependent mechanism. Neuropsychopharmacology 38, 2027–2034. doi: 10.1038/npp.2013.104
Koppel, N., and Balskus, E. P. (2016). Exploring and understanding the biochemical diversity of the human microbiota. Cell Chem. Biol. 23, 18–30. doi: 10.1016/j.chembiol.2015.12.008
Ley, R. E., Peterson, D. A., and Gordon, J. I. (2006). Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124, 837–848. doi: 10.1016/j.cell.2006.02.017
Liang, S., Wang, T., Hu, X., Luo, J., Li, W., Wu, X., et al. (2015). Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 310, 561–577. doi: 10.1016/j.neuroscience.2015.09.033
Lichtwark, I. T., Newnham, E. D., Robinson, S. R., Shepherd, S. J., Hosking, P., Gibson, P. R., et al. (2014). Cognitive impairment in coeliac disease improves on a gluten-free diet and correlates with histological and serological indices of disease severity. Aliment. Pharmacol. Ther. 40, 160–170. doi: 10.1111/apt.12809
Lloyd-Price, J., Abu-Ali, G., and Huttenhower, C. (2016). The healthy human microbiome. Genome Med. 8:51. doi: 10.1186/s13073-016-0307-y
Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K., and Knight, R. (2012). Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230. doi: 10.1038/nature11550
Macfarlane, G. T., and Macfarlane, S. (2011). Fermentation in the human large intestine: its physiologic consequences and the potential contribution of prebiotics. J. Clin. Gastroenterol. 45, S120–S127. doi: 10.1097/mcg.0b013e31822fecfe
Magnusson, K. R., Hauck, L., Jeffrey, B. M., Elias, V., Humphrey, A., Nath, R., et al. (2015). Relationships between diet-related changes in the gut microbiome and cognitive flexibility. Neuroscience 300, 128–140. doi: 10.1016/j.neuroscience.2015.05.016
Mahoney, C., Taylor, H., and Kanarek, R. (2005). “The acute effects of meals on cognitive performance,” in Nutritional Neuroscience, eds H. Lieberman, R. Kanarek and C. Prasad (Boca Raton, FL: CRC Press), 73–91.
McKenney, P. T., and Pamer, E. G. (2015). From hype to hope: the gut microbiota in enteric infectious disease. Cell 163, 1326–1332. doi: 10.1016/j.cell.2015.11.032
Moody, L., Chen, H., and pan, Y. X. (2017). Early-life nutritional programming of cognition-the fundamental role of epigenetic mechanisms in mediating the relation between early-life environment and learning and memory process. Adv. Nutr. 8, 337–356. doi: 10.3945/an.116.014209
Mulak, A., and Bonaz, B. (2004). Irritable bowel syndrome: a model of the brain-gut interactions. Med. Sci. Monit. 10, RA55–RA62. doi: 10.1016/j.jpsychores.2004.04.089
Noble, J. M., Scarmeas, N., Celenti, R. S., Elkind, M. S. V., Wright, C. B., Schupf, N., et al. (2014). Serum IgG antibody levels to periodontal microbiota are associated with incident Alzheimer disease. PLoS ONE 9:e114959. doi: 10.1371/journal.pone.0114959
Noble, E. E., Hsu, T. M., Jones, R. B., Fodor, A. A., Goran, M. I., and Kanoski, S. E. (2017a). Early-life sugar consumption affects the rat microbiome independently of obesity. J. Nutr. 147, 20–28. doi: 10.3945/jn.116.238816
Noble, E. E., Hsu, T. M., and Kanoski, S. E. (2017b). Gut to brain dysbiosis: mechanisms linking western diet consumption, the microbiome and cognitive impairment. Front. Behav. Neurosci. 11:9. doi: 10.3389/fnbeh.2017.00009
Oh, J., Byrd, A. L., Park, M., NISC Comparative Sequencing Program, Kong, H. H., and Segre, J. A. (2016). Temporal stability of the human skin microbiome. Cell 165, 854–866. doi: 10.1016/j.cell.2016.04.008
O’Mahony, S. M., Clarke, G., Borre, Y. E., Dinan, T. G., and Cryan, J. F. (2015). Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 277, 32–48. doi: 10.1016/j.bbr.2014.07.027
Parashar, A., and Udayabanu, M. (2016). Gut microbiota regulates key modulators of social behavior. Eur. Neuropsychopharmacol. 26, 78–91. doi: 10.1016/j.euroneuro.2015.11.002
Pistell, P. J., Morrison, C. D., Gupta, S., Knight, A. G., Keller, J. N., Ingram, D. K., et al. (2010). Cognitive impairment following high fat diet consumption is associated with brain inflammation. J. Neuroimmunol. 219, 25–32. doi: 10.1016/j.jneuroim.2009.11.010
Prado, E., and Dewey, K. (2012). Nutrition and Brain Development in Early Life. AandT Technical Brief Issue 4. Washington, DC: Alive and Thrive.
Puig, K. L., Floden, A. M., Adhikari, R., Golovko, M. Y., and Combs, C. K. (2012). Amyloid precursor protein and proinflammatory changes are regulated in brain and adipose tissue in a murine model of high fat diet-induced obesity. PLoS One 7:e30378. doi: 10.1371/journal.pone.0030378
Qin, J., Li, Y., Cai, Z., Li, S., Zhu, J., Zhang, F., et al. (2012). A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 490, 55–60. doi: 10.1038/nature11450
Reddel, S., Putignani, L., and Del Chierico, F. (2019). The impact of low-FODMAPs, gluten-free and ketogenic diets on gut microbiota modulation in pathological conditions. Nutrients 11:373. doi: 10.3390/nu11020373
Reichelt, A. C., Westbrook, R. F., and Morris, M. J. (2017). Editorial: impact of diet on learning, memory and cognition. Front. Behav. Neurosci. 11:96. doi: 10.3389/fnbeh.2017.00096
Ross, A. (2019). Nutrition and its effects on academic performance. Available online at: https://www.nmu.edu/sites/DrupalEducation/files/UserFiles/Files/Pre-Drupal/SiteSections/Students/GradPapers/Projects/Ross_Amy_MP.pdf. Accessed on March 15, 2019.
Saji, N., Niida, S., Murotani, K., Hisada, T., Tsuduki, T., Sugimoto, T., et al. (2019). Analysis of the relationship between the gut microbiome and dementia: a cross-sectional study conducted in Japan. Sci. Rep. 9:1008. doi: 10.1038/s41598-018-38218-7
Schroeder, B. O., and Bäckhed, F. (2016). Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 22, 1079–1089. doi: 10.1038/nm.4185
Stefanko, D. P., Barrett, R. M., Ly, A. R., Reolon, G. K., and Wood, M. A. (2009). Modulation of long-term memory for object recognition via HDAC inhibition. Proc. Natl. Acad. Sci. U S A 106, 9447–9452. doi: 10.1073/pnas.0903964106
Sudo, N., Chida, Y., Aiba, Y., Sonoda, J., Oyama, N., Yu, X. N., et al. (2004). Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 558, 263–275. doi: 10.1113/jphysiol.2004.063388
Thomas, S., Izard, J., Walsh, E., Batich, K., Chongsathidkiet, P., Clarke, G., et al. (2017). The host microbiome regulates and maintains human health: a primer and perspective for non-microbiologists. Cancer Res. 77, 1783–1812. doi: 10.1158/0008-5472.can-16-2929
Tillisch, K., Labus, J., Kilpatrick, L., Jiang, Z., Stains, J., Ebrat, B., et al. (2013). Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology 144, 1394–1401. doi: 10.1053/j.gastro.2013.02.043
Toman, J., Klímová, B., and Vališ, M. (2018). Multidomain lifestyle intervention strategies for the delay of cognitive impairment in healthy aging. Nutrients 10:E1560. doi: 10.3390/nu10101560
Tsai, Y. L., Lin, T. L., Chang, C. J., Wu, T. R., Lai, W. F., Lu, C. C., et al. (2019). Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 26:3. doi: 10.1186/s12929-018-0493-6
Turnbaugh, P. J., Hamady, M., Yatsunenko, T., Cantarel, B. L., Duncan, A., Ley, R. E., et al. (2009). A core gut microbiome in obese and lean twins. Nature 457, 480–484. doi: 10.1038/nature07540
Wang, C., An, Y., Yu, H., Feng, L., Liu, Q., Lu, Y., et al. (2016). Association between exposure to the Chinese famine in different stages of early life and decline in cognitive functioning in adulthood. Front. Behav. Neurosci. 10:146. doi: 10.3389/fnbeh.2016.00146
Wang, H., Lee, I. S., Braun, C., and Enck, P. (2016). Effect of probiotics on central nervous system functions in animals and humans: a systematic review. J. Neurogastroenterol. Motil. 22, 589–605. doi: 10.5056/jnm16018
Wu, X., Chen, P. S., Dallas, S., Wilson, B., Block, M. L., Wang, C. C., et al. (2008). Histone deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons. Int. J. Neuropsychopharmacol. 11, 1123–1134. doi: 10.1017/s1461145708009024
Yang, I., Corwin, E. J., Brennan, P. A., Jordan, S., Murphy, J. R., and Dunlop, A. (2016). The infant microbiome: implications for infant health and neurocognitive development. Nurs. Res. 65, 76–88. doi: 10.1097/nnr.0000000000000133
Keywords: microbiome, diet, cognition, learning, SCFA, antibiotics, neurological disorders
Citation: Novotný M, Klimova B and Valis M (2019) Microbiome and Cognitive Impairment: Can Any Diets Influence Learning Processes in a Positive Way? Front. Aging Neurosci. 11:170. doi: 10.3389/fnagi.2019.00170
Received: 29 April 2019; Accepted: 17 June 2019;
Published: 28 June 2019.
Edited by:
Paula I. Moreira, University of Coimbra, PortugalReviewed by:
Naoki Saji, National Center for Geriatrics and Gerontology (NCGG), JapanKathy R. Magnusson, Oregon State University, United States
Copyright © 2019 Novotný, Klimova and Valis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Blanka Klimova, YmxhbmthLmtsaW1vdmFAdWhrLmN6
 Michal Novotný
Michal Novotný Blanka Klimova
Blanka Klimova Martin Valis
Martin Valis