- 1Psychiatry, Loma Linda University, Loma Linda, CA, United States
- 2Department of Psychiatry, Patton State Hospital, San Bernardino, CA, United States
The amyloid hypothesis, the assumption that beta-amyloid toxicity is the primary cause of neuronal and synaptic loss, has been the mainstream research concept in Alzheimer's disease for the past two decades. Currently, this model is quietly being replaced by a more holistic, “systemic disease” paradigm which, like the aging process, affects multiple body tissues and organs, including the gut microbiota. It is well-established that inflammation is a hallmark of cellular senescence; however, the infection-senescence link has been less explored. Microbiota-induced senescence is a gradually emerging concept promoted by the discovery of pathogens and their products in Alzheimer's disease brains associated with senescent neurons, glia, and endothelial cells. Infectious agents have previously been associated with Alzheimer's disease, but the cause vs. effect issue could not be resolved. A recent study may have settled this debate as it shows that gingipain, a Porphyromonas gingivalis toxin, can be detected not only in Alzheimer's disease but also in the brains of older individuals deceased prior to developing the illness. In this review, we take the position that gut and other microbes from the body periphery reach the brain by triggering intestinal and blood-brain barrier senescence and disruption. We also surmise that novel Alzheimer's disease findings, including neuronal somatic mosaicism, iron dyshomeostasis, aggressive glial phenotypes, and loss of aerobic glycolysis, can be explained by the infection-senescence model. In addition, we discuss potential cellular senescence targets and therapeutic strategies, including iron chelators, inflammasome inhibitors, senolytic antibiotics, mitophagy inducers, and epigenetic metabolic reprograming.
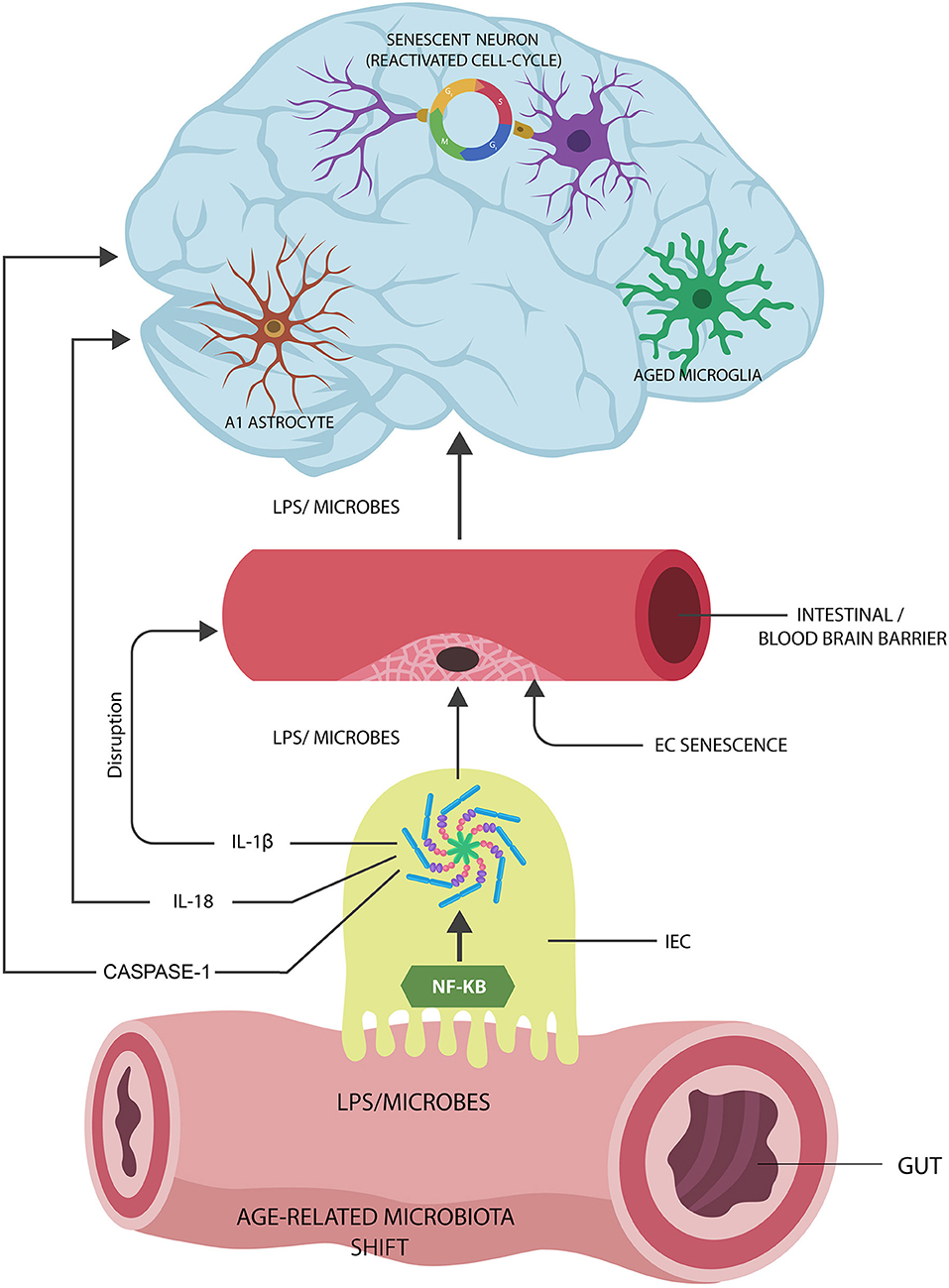
Graphical Abstract. Proposed Alzheimer's disease (AD) pathogenesis: (1) Age-related gut microbiota shift leads to the upregulation of inflammagenic, lipopolysaccharide (LPS)-shedding microbial species. (2) These microorganisms activate nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) and NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasomes in intestinal epithelial cells (IECs), generating interleukin-1β (IL-1β), IL-18, and caspase-1. (3) IL-1β increases the permeability of intestinal and blood-brain barrier, allowing pathogen translocation into the body tissues and organs, including the brain. (4) Microorganisms and LPS induce cellular senescence in neurons, microglia, and astrocyte AD pathology.
Introduction
Alzheimer's disease (AD) is the most common cause of dementia, affecting an estimated 5.5 million people in the US alone (Mayeux and Stern, 2012). Advanced age is a major AD risk factor; therefore, understanding cellular senescence and its impact on endothelial cells (ECs), neurons, glia, and immune cells is an essential prerequisite for elucidating the pathogenesis of this condition (Wiseman et al., 2018).
Brain accumulation of extracellular β-amyloid and intracellular hyperphosphorylated tau are the pathological hallmarks of AD. Both neurons and astrocytes synthesize β-amyloid from amyloid precursor protein (APP), while phagocytic microglia prevent its accumulation by removing it via the triggering receptor expressed on myeloid cells-2 (TREM-2) (discussed in the section “Beta Amyloid: Friend or Foe”).
Aging has been associated with pathological changes in microglia and astrocytes, including loss of neurotrophic properties and gain of toxic functions. These age-related glial alterations may contribute to AD pathology, marked by neuronal loss and memory impairment (discussed at length in the section “Senescent Astrocytes and Microglia”).
The amyloid hypothesis postulates that accumulation and deposition of β-amyloid are the primary causes of AD, which promotes tau aggregation into neurofibrillary tangles (NFTs), ultimately triggering neuronal death (Hardy and Allsop, 1991; Wildsmith et al., 2013). Although never universally accepted, the amyloid hypothesis drove AD research for at least two decades. Lately, however, many researchers and clinicians have questioned this model as amyloid removal failed to improve memory in numerous clinical trials (Fülöp et al., 2018a). With the same token, neuroimaging studies detected significant β-amyloid deposits in 20–30% of healthy older individuals, while in many AD patients, this marker was not observed (Edison et al., 2007; Li et al., 2008; Rodrigue et al., 2013; Higashi et al., 2018). Moreover, β-amyloid was recently characterized as an antimicrobial peptide (AMP), and its accumulation in AD brains may be a reflection of increased microbial burden (Alonso et al., 2018; Fülöp et al., 2018a). AMPs are defensive biomolecules secreted by the innate immune system, including microglia and astrocytes, in response to a variety of microorganisms and malignant cells (Alonso et al., 2018). The β-amyloid-AMP connection is further supported by the observation that central nervous system (CNS) infections were diagnosed in some clinical trials, following the administration of anti-amyloid vaccines (Orgogozo et al., 2003; Brothers et al., 2018; Zhan et al., 2018).
Recent studies have reported co-localization of microorganisms with senescent neurons and glial cells in the brains of both AD patients and healthy older individuals, reviving the infectious hypothesis entertained by Alois Alzheimer himself (De Chiara et al., 2012; Bester et al., 2015; Itzhaki et al., 2016; Alonso et al., 2018; Fulop et al., 2018b; Kritsilis et al., 2018).
CNS infectious agents have been detected previously in AD patients; however, it was difficult to assess if they represented the cause or effect of this condition (Hill et al., 2014). A recent study may have settled this issue as it detected gingipain, a Porphyromonas gingivalis antigen, linked to AD, in the brains of healthy older persons, suggesting that they would have developed the disease if they lived longer (Dominy et al., 2019). As P. gingivalis is a major cause of gum disease and a modifiable AD risk factor, treatment of periodontal infection must be considered a clinical priority.
A new study identified the disruption of the blood-brain barrier (BBB) as an early aging and AD marker, suggesting a portal for microbial brain entry (Montagne et al., 2015; Nation et al., 2019). Moreover, in stroke, microorganisms were shown to directly induce EC senescence and BBB disruption, carving an entry route into the CNS (Muller et al., 2009; Saito et al., 2010; Yamazaki et al., 2016; Aguilera et al., 2018).
Aside from how microbes enter the brain, identifying their source is essential for the development of new treatments. Recent studies have demonstrated elevated levels of microbes and lipopolysaccharide (LPS) in the CNS of both healthy elderly and AD patients, suggesting the gut as their point of origin (Zhao et al., 2017; Kowalski and Mulak, 2019). Interestingly, the gut microbial shift in older individuals is characterized by the increased preponderance of Gram-negative LPS-generating microbes, pointing to the gastrointestinal (GI) tract as the potential source of brain pathogens (Kobayashi et al., 2013; Sato S. et al., 2014; Greiner and Bäckhed, 2016; Odamaki et al., 2016; Yamazaki et al., 2016; Lebrun et al., 2017; Ke et al., 2018). Furthermore, loss of immune tolerance to commensal flora in older individuals and intestinal barrier disruption suggest the gut as the likely reservoir of brain LPS and microbes (Nagpal et al., 2018) (discussed in “The Senescent Intestinal Barrier”).
At the molecular level, cellular senescence has been associated with the activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) and NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasomes (Yamazaki et al., 2016; Zhang W. et al., 2017; Burton and Stolzing, 2018). NLRP3 end products IL-18 and caspase-1 are associated with AD pathogenesis, while interleukin-1β (IL-1β) is an established disruptor of the BBB, linking it to microbial brain access (discussed in detail in “Senescence and Inflammasomes” section). In addition, activated NLRP3 inhibits autophagy and mitophagy (selective mitochondrial autophagy), contributing to inflammaging as the accumulation of senescent cells and damaged organelles triggers inflammation (Argaw et al., 2006; Bossù et al., 2010; Sutinen et al., 2012; Wang et al., 2014; Kim et al., 2016). Conversely, mitophagy enhancers deactivate NLRP3, limiting both cellular senescence and AD pathology (Gurung et al., 2014).
Microbiota-induced brain cells' senescence may explain other novel AD findings, including age-related neuronal genomic variation, aneuploidy, or somatic mosaicism (Argaw et al., 2006; Bossù et al., 2010). Senescent neurons reentering the cell cycle, a hallmark of AD, may account for this phenomenon, especially when apoptosis is inactivated (Paquola et al., 2016; McConnell et al., 2017; Sharma et al., 2017; Bai, 2018; Verheijen et al., 2018) (discussed in “Senescent Neurons and the Cell Cycle” section).
Senescent glial cells, probably including A1 astrocytes, have been associated with AD as they display neurotoxic functions, engaging in the elimination of viable neurons and synapses (Neher et al., 2012; Koellhoffer et al., 2017; Liddelow et al., 2017; Morizawa et al., 2017; Soreq et al., 2017; Boisvert et al., 2018; Bussian et al., 2018; Clarke et al., 2018; Forloni and Balducci, 2018; Jung and Chung, 2018). In contrast, senolysis, elimination of aggressive glia, was associated with enhanced memory in animal models, suggesting a therapeutic strategy (Koellhoffer et al., 2017; Bussian et al., 2018; Forloni and Balducci, 2018).
The infection-senescence link cannot be considered without mentioning the role of iron, a biometal indispensable to both the host and invading pathogens. Iron is well-known for inducing DNA damage and senescence in many cell types, including the ECs, linking it to microbial brain entry (Mollet et al., 2016). The association of AD with iron dysmetabolism is well-documented as, aside from microbial survival, this biometal was linked to tau pathology, reactive oxygen species (ROS), and neuroinflammation (Nakamura et al., 2016; Masaldan et al., 2018; Rao and Adlard, 2018).
Finally, aside from the pathogenetic mechanisms, this article discusses potential AD targets and therapeutic strategies, including inflammasome inhibitors, iron chelators, senolytic antibiotics, mitophagy inducers, and epigenetic reprograming of metabolism.
Beta Amyloid: Friend or Foe?
Amyloid cascade hypothesis, the stipulation that toxic β-amyloid oligomers and fibrils are the primary cause of AD, has been the leading paradigm that drove research in this neurodegenerative disorder for the past three decades. According to this model, β-amyloid induces the formation of NFTs, leading to neuronal and synaptic loss that ultimately impact the memory (Hardy and Higgins, 1992; Morris et al., 2014). Lately, new hypotheses have emerged as numerous anti-amyloid drugs and vaccines failed to improve cognition in clinical trials, and several studies pointed to inconsistencies in the amyloid paradigm (Table 1).
Recent studies have indicated that β-amyloid may function as an AMP released by the host innate immunity in response to invading pathogens (Spitzer et al., 2016; Fülöp et al., 2018a; Gosztyla et al., 2018). This is further supported by the observation that in CNS infections, microglia, and astrocytes secrete a multitude of AMP peptides demonstrated to augment host defenses (Ransohoff and Brown, 2012; Williams et al., 2012; Frost and Li, 2017). Moreover, β-amyloid, released by astrocytes and neurons, presents with antibacterial, fungicidal, and anti-herpes simplex virus, type 1 (HSV1) properties (Lukiw et al., 2010; Bourgade et al., 2016; Frost and Li, 2017; Eimer et al., 2018). This is significant since HSV1, an established disruptor of biological barriers, was found to play a major role in the etiology of both AD and intestinal pathology (Brun et al., 2018; Hogestyn et al., 2018; Itzhaki and Lathe, 2018). Interestingly, a novel study has reported that β-amyloid may work in tandem with a second AMP, probably to augment its microbicidal functions (De Lorenzi et al., 2017). Furthermore, under normal circumstances, β-amyloid may act as an opsonin, attaching to CNS microorganisms and/or their molecules to prepare them for microglial phagocytosis (Figure 1).
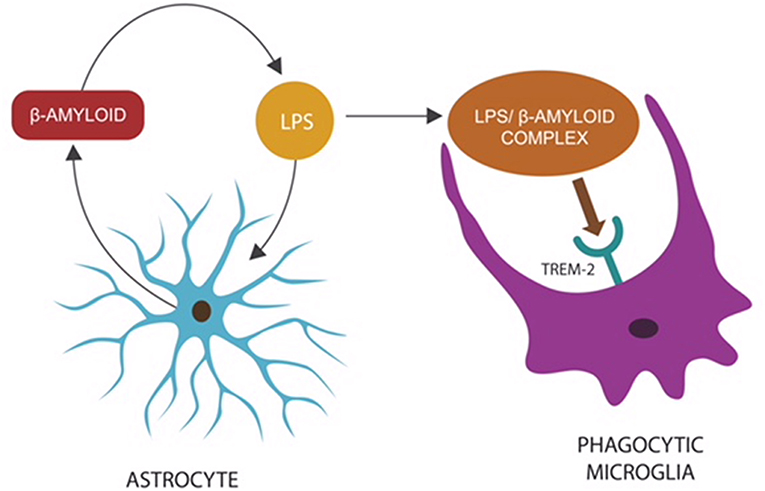
Figure 1. Microbes or LPS that access the CNS comprise “danger” signals, triggering an innate immune response: the release of β-amyloid by astrocytes to opsonize “the intruder,” preparing it for phagocytosis (Zhan et al., 2018). The LPS–β-amyloid complex is subsequently engulfed by microglia, eliminating the “danger.” Microglial TREM-2, a β-amyloid receptor, initiates phagocytosis by binding the entire complex (Zhao et al., 2018). This mechanism may explain the reason TREM-2 genetic variants (with loss of function) present with impaired phagocytosis and β-amyloid accumulation (Guerreiro et al., 2012; Zhan et al., 2018).
Oral and Microbial Tolerance
Other microbial antigens, including bacterial amyloids (also known as curli fibers) and P. gingivalis-released gingipains, may trigger β-amyloid upregulation to opsonize these “danger” molecules (Tükel et al., 2009; Hill et al., 2014; Kumar et al., 2016).
Novel studies have shown that curli fibers derived from gut microbes play a major role in promoting immune tolerance to commensals as well as oral tolerance (immune unresponsiveness to antigens administered by mouth, including food) (Barnhart and Chapman, 2006; Oppong et al., 2015). These curli functions are protective when the microbes are confined to the GI tract but become detrimental after translocation as the systemic immunity (which is not subject to oral tolerance) is activated by curli. Indeed, curli fibers were demonstrated to activate or inhibit innate immune responses, depending on the portal of entry: systemic administration of curli augments, while oral ingestion lowers immune responses (Tursi and Tükel, 2018). As curli fibers promote oral tolerance, their administration by mouth was found to restore the integrity of intestinal barrier, suggesting a potential antitranslocation strategy (Tursi and Tükel, 2018). Indeed, a bioengineered curli was recently utilized as a restorative therapy for intestinal barrier (Duraj-Thatte et al., 2018). Furthermore, like curli, oral administration of LPS derived from Bacteroides vulgatus and Bacteroides dorei was demonstrated to promote tolerance by blocking rather than activating intestinal toll-like receptor 4 (TLR-4), pointing to a mechanism for tolerization (d'Hennezel et al., 2017). Interestingly, live B. vulgatus and B. dorei were recently investigated as therapy for coronary artery disease (CAD), another condition linked to the translocation of gut microbes (Yoshida et al., 2018).
Upon accessing the CNS, curli fibers likely trigger β-amyloid synthesis, an innate immune response, causing the accumulation of this peptide. Others have suggested that curli serve as templates for β-amyloid seeding, resulting in wider CNS depositions (Friedland and Chapman, 2017). We propose that bacterial amyloids are antigens that trigger a defensive response, β-amyloid overproduction, to eliminate “danger” signals.
Aging disrupts both oral and microbial tolerance, leading to immunogenicity and inflammation in response to commensals, disrupting the intestinal barrier, a portal for microbial dissemination (Kato et al., 2003; Santiago et al., 2011).
Taken together, commensal gut microbes live in symbiosis with the host for as long as they are confined to the GI tract where the local immune system maintains a tolerant environment. This symbiosis is dramatically altered upon microorganisms' translocation as gut microbes and their antigens activate systemic immunity. Aging alters both oral and microbial tolerance, disrupting intestinal barrier and enabling microbial translocation. Upon CNS entry, microbes and their molecules induce β-amyloid overproduction. In summary, microbial containment inside the gut lumen is a key objective in the prevention of neurodegeneration, including AD.
Antimicrobial Peptides, Aging, and β-Amyloid
Over the past two decades, AD studies have focused primarily on the detrimental functions of β-amyloid, placing less emphasis on its physiological roles: protection against infections and cancer, BBB repair, and synaptic maintenance (Brothers et al., 2018). The presence of β-amyloid in various tissues and organs of older individuals and AD patients has gained a new significance in the light of this biomolecule functioning as an AMP (Joachim et al., 1989). For example, novel studies have detected microorganisms in older individuals' tissues, including the liver, skeletal muscles, and brain, suggesting that increased microbial burden triggers higher β-amyloid synthesis (Lluch et al., 2015). Furthermore, preclinical studies have reported age-related upregulation of AMPs in senescent tissues, implying that these defense peptides may be directly proportional to the bacterial load (Dinakaran et al., 2014). Interestingly, numerous studies over the past decade linked tissue pathogens to chronic illnesses, including CAD, cancer, stroke, type 2 diabetes mellitus (T2DM), and AD, likely implicating the translocated gut microbes in their etiology (Elkind et al., 2009; Dapito et al., 2012; Sato J. et al., 2014).
Other studies have found that the function of AMPs as antiviral and anticancer agents, suggesting that carcinogenesis and infection are handled in a similar fashion by the immune system (Hoskin and Ramamoorthy, 2007; Suttmann et al., 2008; Pandey et al., 2016). Interestingly, β-amyloid has been shown to display not only anti-HSV1 but also antimalignant properties, further suggesting an adaptive role for this peptide (Bourgade et al., 2015; Mizejewski, 2017).
AMPs have been linked to autophagy, a process involved not only in the clearance of damaged cells and molecules but also in antimicrobial defenses, as they are effective against facultative intracellular pathogens, like P. gingivalis (Muciño et al., 2016).
Another AMP, neuropeptide-like protein 29 (NLP-29), was found to promote the autophagy of damaged dendrites (dendrophagy) in Caenorhabditis elegans, extending the role of AMPs beyond infection and cancer (Lezi et al., 2018). Interestingly, fungi were shown to subvert NLP-29, inducing neuronal senescence, linking them to brain aging (Alonso et al., 2018). This is significant since fungal infections have previously been associated with AD and aging.
Other studies have reported the existence of antiretroviral AMPs, which, like antiretroviral drugs, interfere with the expression of retroviral genes, including Arc (Tencza et al., 1997; Nelson et al., 2003; Kriesel et al., 2017). Cognition-related neuronal gene Arc was demonstrated to migrate from neuron to neuron in a retroviral fashion, possibly linking antiretroviral drugs to cognition (Ashley et al., 2018; Pastuzyn et al., 2018). Interestingly, HSV1 was associated with altered transcription of Arc, linking this virus once again to neuronal senescence and memory loss (Penner et al., 2010; Bi et al., 2018; Acuña-Hinrichsen et al., 2019; Man et al., 2019). This finding is in line with novel epidemiological studies that have connected HSV1 to cellular senescence and AD (Dowd et al., 2017).
Finally, AMPs were found crucial for the integrity of intestinal barrier, suggesting their upregulation as a strategy against bacterial translocation (Robinson et al., 2015). Indeed, a recently synthesized AMP has been shown to neutralize LPS, indicating potential antitranslocation benefits (Li L. H. et al., 2017). In addition, lactoferrin, a recently identified AMP, was found protective of intestinal barrier (Hering et al., 2017).
Senescence and Extracellular Vesicles
Most cells in the human body release extracellular vesicles (EVs) to mediate cellular crosstalk and the exchange of metabolites. Gram-negative microbes also signal with EVs (also called outer membrane vesicles) to facilitate immune evasion (Rodrigues et al., 2018). For example, P. gingivalis emits EVs that trigger pyroptosis in macrophages and microglia, effectively eliminating these key host defenses (Fleetwood et al., 2017). P. gingivalis-derived EVs have been demonstrated to contain antigens, including gingipains and fimbriae, known for disrupting ECs, causing BBB and intestinal barrier damage (Mantri et al., 2014).
Along these lines, novel studies show that the age-related gut microbial shift may be orchestrated via EVs released by microorganisms to alter local immunity and the intestinal barrier (Ahmadi Badi et al., 2017). Other studies have shown that under normal circumstances, the thymus gland releases EVs that act on gut-associated lymphoid tissue (GALT), promoting immunological tolerance to gut microbes (Skogberg et al., 2015). Age-related thymic involution may lower commensals' tolerance, engendering inflammation, and intestinal barrier disruption (Skogberg et al., 2015; Li P. et al., 2016). Interestingly, a recent preclinical study has shown that thymic EVs derived from young donors reversed the inflammaging in older recipients, suggesting that functional restoration of this gland may comprise a senotherapeutic strategy (Wang et al., 2018).
Recent studies have shown that senescent cells release more EVs than their younger counterparts, suggesting a mechanism for molecular waste disposal (Falsone and Falsone, 2015; Takasugi, 2018). For example, senescence-associated secretory phenotype (SASP) has been linked to the accumulation of cytosolic DNA in senescent cells, while DNA export via EVs was shown to inhibit this phenotype (Takahashi et al., 2018). These findings suggest that facilitation of DNA egress from senescent cells may comprise an effective senotherapeutic intervention. In this regard, the antibiotic ciprofloxacin was shown to facilitate DNA export from senescent cells, suggesting anti-SASP properties (Németh et al., 2017). Interestingly, malignant cells also display enhanced DNA export via EVs, suggesting that SASP may be associated with carcinogenesis (Rajagopal and Harikumar, 2018). Conversely, heparin was shown to block recipient cells' uptake of tumor and non-tumor-derived EVs, suggesting a potential strategy (Atai et al., 2013). Indeed, heparin was demonstrated to mimic extracellular DNA, probably interfering with SASP signaling (Jung et al., 2015; Mishra and Horswill, 2017).
Aging and Biological Barriers in Alzheimer's Disease
Cellular senescence is a program of permanent replication arrest which, under normal circumstances, lowers the risk of carcinogenesis. Prompt removal of senescent cells by the immune system prevents their accumulation and the subsequent inflammation (Oppong et al., 2015). Aging has been shown to alter this process, engendering both inflammaging and immunosenescence (Olivieri et al., 2018).
More than five decades ago, Hayflick established that cells divide a limited number of times after which they undergo replicative senescence and apoptosis (Hayflick and Moorhead, 1961). Later on, it was established that the senescence program can be activated prematurely by numerous endogenous or exogenous toxins, including the microbes and their antigens, such as LPS (Nakamura et al., 2016; Calvani et al., 2018; Kritsilis et al., 2018).
Compelling evidence indicates that cellular senescence contributes to organismal aging and the risk of developing age-related diseases, including AD (Jeyapalan and Sedivy, 2008). A growing number of studies have demonstrated that senescent cells' SASP secretome can activate the senescence program in healthy cells, propagating this phenotype throughout the surrounding tissues (Nelson et al., 2012). Conversely, senolysis, senescent cell removal, has been shown to restore homeostasis, ameliorating age-related symptoms (Baar et al., 2017; Kirkland et al., 2017). Accumulation of senescent cells and SASP-derived molecules, due to overproduction or impaired clearance, comprises an early sign of AD (Boccardi et al., 2015; Childs et al., 2015; Kritsilis et al., 2018).
Histologically, senescent cells are enlarged, presenting with β-galactosidase and lipofuscin aggregates. Functionally, they are resistant to apoptosis and metabolically active as evidenced by the intact mammalian target of rapamycin (mTOR) and the SASP secretome. Since senescent cells continue to express mTOR, targeting this molecule may comprise a senotherapeutic strategy for SASP inhibition (Walters and Cox, 2018). For example, rapamycin, an mTOR inhibitor and a natural macrolide antibiotic, was shown to block both cellular senescence and SASP (Wang R. et al., 2017; Wang S. et al., 2017). In addition, as mTOR signaling also modulates ECs synthesis of nitric oxide (NO), a trophic molecule for endothelia, targeting mTOR may restore the integrity of biological barriers, including the BBB (Cheng et al., 2008; Van Skike and Galvan, 2018). Interestingly, P. gingivalis was found to alter mTOR signaling, linking this microbe once again to EC senescence (Stafford et al., 2013). Conversely, azithromycin, an anti-P. gingivalis macrolide antibiotic and mTOR modulator, was found to have senotherapeutic properties, indicating potential benefits in AD (Maezono et al., 2011; Ratzinger et al., 2014; Ozsvari et al., 2018; Weng et al., 2019).
The Senescent Intestinal Barrier
The gut microbial community, consisting of bacteria, fungi, archaea, viruses, and protozoans, live in symbiosis with the human host, contributing to metabolism and immune homeostasis in exchange for nutrients and habitat (Jandhyala et al., 2015). Intestinal epithelial cells (IECs), a one cell layer, separate the host from trillions of microbes and antigens, preventing their translocation outside of the GI tract where systemic immunity is intolerant of them. Aside from IECs, GALT (the GI tract immune system) contributes to the integrity of the intestinal barrier by blocking immunogenicity to beneficial microorganisms, ensuring their containment in the GI tract (Hwang et al., 2012). Loss of tolerance to gut commensals was shown to cause immune activation, barrier disruption, and translocation (Ramanan and Cadwell, 2016). GALT facilitates microbial tolerance by promoting the differentiation of IL-10 secreting B and regulatory T cells (Tregs) (Kelsall and Leon, 2005) (Figure 2). In addition, GI tract lactobacilli, bifidobacteria, and Bacteroides also facilitate microbial acceptance as they promote oral and microbial tolerance (Cebula et al., 2013; Kayama and Takeda, 2014; Nakamoto et al., 2017). Tolerance is believed to be initiated during the early development when GALT receives thymic input, generating a long-lived population of T cells that facilitate microbial tolerance even after the involution of this gland (Cebula et al., 2013).
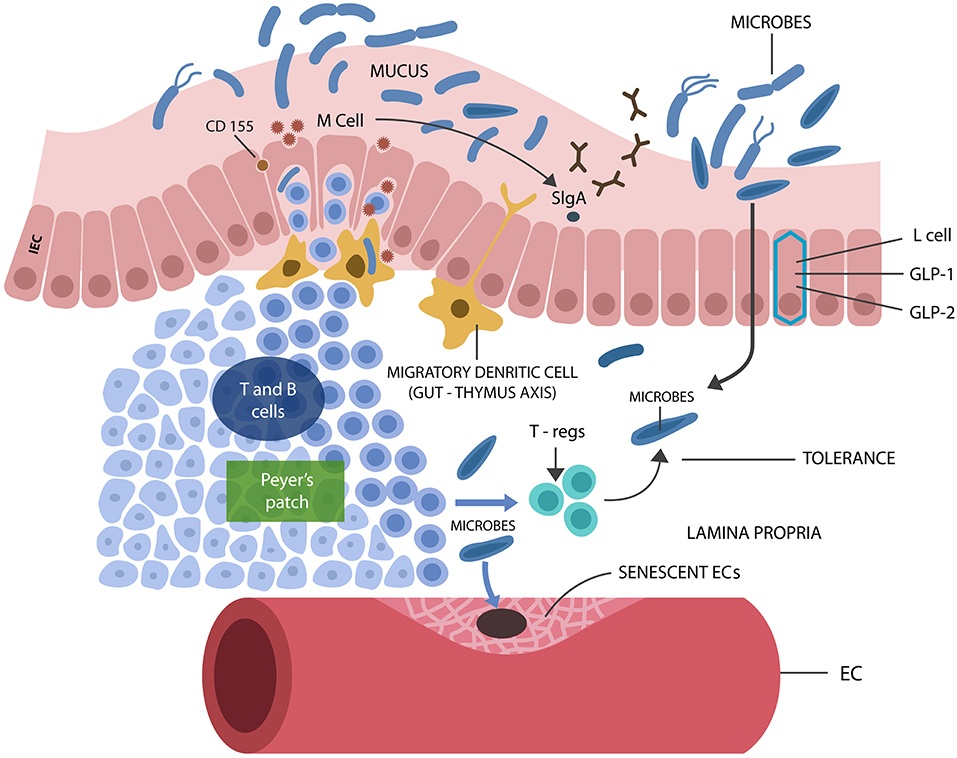
Figure 2. M cells interact with luminal microbes, introducing microbial antigens to the T and B cells, engendering tolerance via Tregs and IL-10 secretion. M cells promote the expression of the mucosal protector, SIgA. L cells synthesize IECs protecting biomolecules GLP-1 and GLP-2. Senescent ECs cause endothelial disruption, allowing pathogens into the circulatory system, from where they find their way into the CNS.
Maintenance of a microbe-friendly GI tract milieu, completely isolated from the systemic immunity, is crucial for averting microbial translocation, a phenomenon that may initiate age-related diseases, including AD. Conversely, restoring the integrity of biological barriers and limiting microbial translocation should be the primary objective of senotherapy. Indeed, recent studies have associated mTOR inhibition with the restoration of intestinal barrier damaged by P. gingivalis (Xu et al., 2015; Nakamoto et al., 2017; Ji et al., 2018; Kato et al., 2018). Inhibitors of mTOR receptors were shown to lower GI tract immunogenicity as a bilateral regulation exists between gut microbes and mTOR, in which the later regulates microbial composition, while the former modulates mTOR expression (Salem et al., 2018). This is significant as it demonstrates that mTOR manipulation may reverse the age-related shift in gut microbiota, lowering the preponderance of pathogenic species and preserving intestinal barrier. On the other hand, microbiota manipulation, for example, via the fecal transplant, may reverse the preponderance of gut LPS-generating species in favor of beneficial microbes, such as Bacteroides, protecting intestinal barrier (Nagpal et al., 2018). Along these lines, a large epidemiological study found that Bacteroides species were less represented in the GI tract of AD patients compared to other microbes, indicating that microbiota manipulation may preempt neurodegeneration (Saji et al., 2019).
Age-related microorganismal shift toward Gram-negative bacteria and LPS induces EC senescence and apoptosis and IEC and GALT damage, disrupting the intestinal barrier (Hoyt et al., 1996; Richter et al., 2012; Nagele et al., 2013; Ke et al., 2018; Sanada et al., 2018; Hou et al., 2019). In addition, several gut microbes were demonstrated to upregulate the host tumor necrotic factor alpha (TNF-α) and interferon-gamma (IFN-γ), increasing intestinal permeability (Al-Sadi and Ma, 2007). Furthermore, these cytokines activate NLRP3, generating IL-1β, a known BBB disruptor (Wang et al., 2014).
Does Aging Start in the Gut?
It was recently reported that GALT dysfunction may occur prior to the systemic immune deterioration, suggesting that immune aging and, perhaps, aging in general could originate in the GI tract with the loss of tolerance to commensals and barrier disruption (Koga et al., 2000; Sato S. et al., 2014).
It was recently demonstrated that glucagon-like peptide-1 (GLP-1) secreted by the gut enteroendocrine (L) cells binds to its receptor, GLP-1R, facilitating immunological tolerance (Yusta et al., 2015). Others have shown that under normal circumstances, LPS upregulates GLP-1, suggesting that this hormone may display AMP-like characteristics (Lebrun et al., 2017). Interestingly, GLP-1R agonists were recently demonstrated to block the conversion of trophic into A1 astrocytes, linking this peptide to CNS homeostasis (Yun et al., 2018). GLP-1R agonists, established therapeutics for T2DM, were previously shown to protect cognition; thus, liraglutide and exenatide are currently in clinical trials for AD and Parkinson's disease (PD), respectively (Kim et al., 2017; Batista et al., 2018; Cummings et al., 2018).
Aside from GLP-1 secretion, L cells sense pathogen-derived molecules, likely suggesting that GLP-1 functions as an AMP (Greiner and Bäckhed, 2016; Lebrun et al., 2017). Aging has been associated with decreased number of L cells, accounting for the loss of GI tract immunological tolerance (Drozdowski and Thomson, 2006; Wu et al., 2018). Aside from L cells, GALT dysfunction may be linked to the loss of membranous (M) cells, known for producing secretory immunoglobulin A (SIgA), an IEC immune protector (Mantis et al., 2011; Kobayashi et al., 2013; Sato S. et al., 2014; Ohno, 2016). Furthermore, aging has been associated with the loss of LPS-binding protein (LBP), another possible AMP, known for its trophic effects on the intestinal barrier (Schmucker et al., 2003; Hamann et al., 2005; Richter et al., 2012).
Another mechanism responsible for tolerance to commensal flora may involve the CD155 poliovirus receptor, expressed by M cells. CD155 binds to T cell co-inhibitory receptor TIGIT (T cell Ig and ITIM domain), initiating the release of IL-10 (Lozano et al., 2013; Ohno, 2016). Dysfunctional TIGIT has been associated with T cell senescence, linking immune aging to the GI tract (Solomon and Garrido-Laguna, 2018). On the other hand, TIGIT blockade, a well-known cancer treatment, activates immunity (Song et al., 2018). This is significant, since T cell co-inhibitory receptors are routinely hijacked by pathogens to lower host immunity and evade detection (Attanasio and Wherry, 2016). For example, P. gingivalis is known for subverting programmed death-1 (PD-1), a co-inhibitory receptor, to escape host immunity (Groeger et al., 2017).
The Senescent Blood-Brain Barrier
ECs pave the interior wall of blood vessels and capillaries, contributing to blood flow, platelet function, and immunity (Ross, 2018). Microorganisms use the host circulatory system to travel around the body, crossing the ECs to enter and exit the bloodstream (Lubkin and Torres, 2016). To facilitate this process, pathogens trigger EC senescence and apoptosis, disrupting biological barriers, including the BBB (Kim, 2008). This action is counteracted by the ECs' secretion of NO, an endothelial protector (Hayashi et al., 2008; Austin et al., 2013). Decreased NO generation was associated with NLRP3 inflammasome activation, aging, and AD (Mao et al., 2013; Sverdlov et al., 2014).
Astrocytic end-feet, ECs, and pericytes comprise the BBB or neurovascular unit (NVU), which feeds neuronal networks, enabling their function (Filosa et al., 2015; Tarantini et al., 2016). Several studies have shown that BBB disruption is an early AD marker, indicating a potential portal for microbial entry into the CNS (Montagne et al., 2015; Nation et al., 2019). A novel study measured platelet-derived growth factor receptor-beta (PDGFRβ), a pericyte marker, and showed that its deficit increased the permeability of BBB, contributing to AD (Nation et al., 2019). In addition, recent AD postmortem studies have associated loss of pericytes with BBB dysfunction in various cortical areas, including the hippocampus (Miners et al., 2017; Schultz et al., 2018).
Pericytes have been reported to play a major role in CNS antimicrobial defenses by secreting microbicidal molecules, including IL-1β, IL-6, and TNF-α (Alcendor et al., 2012; Hurtado-Alvarado et al., 2014; Stark et al., 2018). Several pathogens were demonstrated to evade host immunity by subverting the pericytes, linking these cells to microbes and their portal of entry (Alcendor et al., 2012). For example, a new study has demonstrated that heme-dependent pathogens can damage ECs and pericytes to extract this iron protein from the circulating red blood cells (Choby and Skaar, 2016; Erdei et al., 2018). Along these lines, to acquire heme, P. gingivalis releases gingipain, which attaches to the EC receptor E-selectin, disrupting these cells (Komatsu et al., 2012; Smalley and Olczak, 2017). Other studies have reported that fimbriae, another P. gingivalis antigen, binds EC-expressed complement receptor 3, inducing immune tolerance to enter the CNS undetected (Hajishengallis et al., 2008). This is significant because upregulated complement component C1q and its downstream molecule C3 were linked to AD via A1 astrocytes induction (Wu et al., 2016; Liddelow et al., 2017; Morgan, 2017). ECs are extremely susceptible to microbial disruption as they express the tolerance-inducing complement pathway genes; therefore, when pathogens subvert these cells, they trigger immune unresponsiveness (Walker et al., 2007; Shi et al., 2017).
Aside from P. gingivalis, Helicobacter pylori and Escherichia coli were found to induce EC senescence and apoptosis, linking them to the disruption of biological barriers (Munshi et al., 2002; Krishnan et al., 2012). Moreover, HSV1, connected to both atherosclerosis and AD, was demonstrated to invade ECs, activating glycogen synthase kinase 3 beta (GSK3β), an enzyme previously associated with neurodegeneration (Key et al., 1990; Piacentini et al., 2015; Rybakowski, 2019). Interestingly, lithium, a GSK3β blocker, also presents with anti-HSV1 properties, suggesting a protective effect on endothelia (Amsterdam et al., 1990; Bosche et al., 2016). Indeed, the beneficial effect of lithium in AD may involve endothelial restoration (Bosche et al., 2016; Cummings et al., 2018).
Immune aging or immunosenescence promotion, engendering immune failure, is another mechanism utilized by gut microbes to avoid detection and access the CNS unopposed (Blazkova et al., 2009; Alvarez-Arellano and Maldonado-Bernal, 2014; Aguilera et al., 2018; Costantini et al., 2018).
Taken together, pathogen-induced pericyte and EC senescence and apoptosis along with impaired immune function facilitate microbial translocation into the brain with subsequent AD pathology.
Senescence and Inflammasomes
It has been well-established that inflammation and cellular senescence are closely related, but the role of pathogens in this process has been less emphasized (Balistreri et al., 2013; Secher et al., 2013; Lewinska and Wnuk, 2017; Rybakowski, 2019). At the molecular level, cellular senescence is believed to be initiated by the nuclear translocation of the NF-kB transcription factor, a molecular event that primes NLRP3 inflammasomes (McCool and Miyamoto, 2012; Birch and Passos, 2017) (Figure 3).
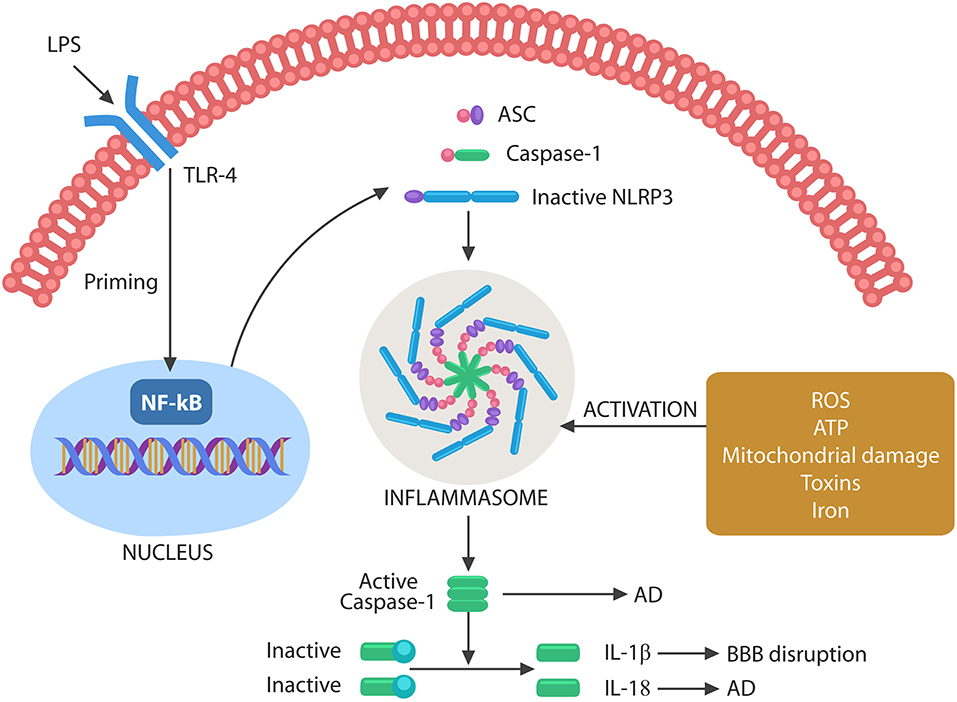
Figure 3. Schematic representation of NLRP3 activation by microbes or their molecules. Microbes or LPS binds to TLR-4, activating the nuclear NF-kB that primes NLRP3. The second step necessary for NLRP3 activation can be composed of various exogenous or endogenous stimuli, including ROS, ATP, DNA, defective mitochondria, iron, and toxins. Assembled inflammasome activates caspase-1, which in turn cleaves immature IL-1β and IL-18 into their active forms. Caspase-1 and IL-18 have been involved in AD pathogenesis, while IL-1β disrupts the BBB, facilitating brain translocation of gut microbes.
Several antibiotics, including minocycline and macrolides, present with both antimicrobial and anti-inflammatory properties as they de-escalate NLRP3 (Pradhan et al., 2016). Recent studies have shown that minocycline also presents with senolytic properties as aside from inhibiting NLRP3, it facilitates senescent cell removal (Labro, 2002; Li J. et al., 2016; Lee et al., 2017).
Other antibiotics with senotherapeutic actions include azithromycin and rifampicin, suggesting that infection, inflammation, and cellular senescence are related phenomena (Golegaonkar et al., 2015; Lendermon et al., 2017; Ozsvari et al., 2018).
Inflammasomes are macromolecular complexes that sense pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) via cytosolic NLRP3 composed of NOD-like receptors, adapter proteins, apoptotic speck containing molecules with a CARD (ASC), and pro-caspase-1 (Uekawa et al., 2004; Broz and Monack, 2011; Wang et al., 2014; Schetters et al., 2018).
Inflammasome assembly requires two steps, a priming event, triggered by NF-kB nuclear translocation, and an activating step, induced by toxins, iron, mitochondrial damage, cytosolic DNA, extracellular ATP, or ROS. Inflammasome assembly activates caspase-1, which in turn cleaves pro-IL-1β and pro-IL-18 into their mature forms (Figure 3). These cytokines have been involved in both BBB disruption and AD (Heneka et al., 2013; Freeman and Ting, 2016; Malik and Kanneganti, 2017).
Pyroptosis is a programmed cell death triggered by infection-induced NLRP3 activation mediated by caspase-1,−4, and−5. Caspase-1 is the product of NLRP3 assembly, while caspase-4 and−5 are LPS activated (Man et al., 2017). Caspases perforate cell membranes via gasdermin D, a pore-forming protein, spilling intracellular content into the ECS, a process that triggers inflammation (Ma et al., 2018). Pyroptosis has been documented in the pathogenesis of neurodegenerative disorders (Walsh et al., 2014; Wang et al., 2014; Ma et al., 2018). In fact, it is believed that pyroptosis and not apoptosis leads to neuronal loss in AD (Bai, 2018; Fali et al., 2018). Moreover, recent studies have reported NLRP3-induced pyroptosis in ECs, likely explaining the disruption of intestinal barrier and BBB during the aging process (Lei et al., 2018; Zhaolin et al., 2018).
Mitochondrial damage was recently recognized as a key NLRP3 activator, emphasizing the role of these organelles in both aging and AD. For example, mitochondrial dysfunction-associated senescence (MiDAS) is an aging phenotype with a specific secretome which, like SASP, can propagate cellular aging throughout the tissues (Gallage and Gil, 2016). Other studies have shown that mitochondrial content, especially mtDNA, in contact with the cytosol activates cellular senescence and SASP, while cytosolic DNA removal inhibits both (Takahashi et al., 2018; Takasugi, 2018). P. gingivalis-induced cellular senescence may involve mtDNA as this microbe has been known for inflicting mitochondrial damage (Bullon et al., 2011). This is in line with a novel study that identified cell free-DNA (cfDNA) as an aging and AD marker, suggesting that cytosolic DNA exported into the ECS may suppress SASP (Takousis et al., 2018; Teo et al., 2018). Together, these studies suggest that enhancing the clearance of cytosolic DNA may facilitate senolysis, lowering the senescent cell burden in tissues and organs (Takousis et al., 2018; Teo et al., 2018).
Senescent Neuron and the Cell Cycle
Accumulating evidence indicates that aging neurons activate a special senescence program, defined as senescence after differentiation (SAD), a phenotype marked by upregulation of β-galactosidase, lipofuscin, SASP, and IL-6 (Jurk et al., 2012; Naylor et al., 2012; Tan et al., 2014). Along these lines, a new study identified senescent neurons in the orexinergic, cholinergic, and dopaminergic tracts of the brainstem and basal forebrain, probably indicating that some neuronal populations undergo senescence earlier than others (Panossian et al., 2011). As opposed to senescent somatic cells which arrest proliferation irreversibly, old neurons may do the opposite, reenter the cell cycle, triggering their own demise (Paquola et al., 2016; McConnell et al., 2017; Verheijen et al., 2018). Indeed, the expression of neuronal cell cycle proteins was detected in both healthy seniors and AD patients, suggesting that these molecules are senescence associated (van Leeuwen and Hoozemans, 2015; Frade and López-Sánchez, 2017). In addition, a novel AD postmortem study has linked neuronal senescence with both aggregated tau and neuronal cell cycle reentry, identifying both as age-related traits (Musi et al., 2018). Moreover, aberrant neuronal cell cycle reentry has been associated with a senescence-linked protein, cyclin-dependent kinase 5 (Cdk5), which phosphorylates both tau and the retinoblastoma protein (pRb) (Mao and Hinds, 2010). The nuclear localization of Cdk5 was found necessary for maintaining neuronal cells in post-mitotic state (Zhang et al., 2008). Conversely, egress of this protein from the nucleus activates the cell cycle (Hamdane et al., 2005; Crews et al., 2011; Hradek et al., 2015; Na et al., 2015). This may be the case in AD, in which microbes and/or LPS may trigger Cdk5 nuclear export and neuronal cell cycle activation (Zhang et al., 2016). In PD animal models, neuronal Cdk5 was found to activate NLRP3, initiating the cell cycle (D'Angelo et al., 2017; Bai, 2018; Wilkaniec et al., 2018). Indeed, to interact with the cytosolic NLRP3, Cdk5 must exit the nucleus, enabling this phenomenon. Conversely, LPS removal via LBP probably promotes Cdk5 nuclear reentry, stabilizing neuronal cells in post-mitotic state (Pretorius et al., 2018).
Iron is a well-known activator of neuronal cell cycle, probably due to DNA damage and NF-kB/NLRP3 activation, suggesting that iron chelators may have senotherapeutic properties (Nakamura et al., 2016; Ashraf et al., 2018; Manickam et al., 2018) (discussed in “Senescence and Iron” section).
It is currently believed that senescent post-mitotic cells, including the neurons, reenter the cell cycle to trigger their own demise. This is thought to take place as these cells lack the molecular machinery to complete mitosis, activating death programs instead (Kruman et al., 2004). For example, in muscular degeneration, adult post-mitotic myocytes were shown to reengage the cell cycle, triggering their own death (Sharma et al., 2017). Conversely, cell cycle inhibitors were recently found neuroprotective in AD organoid models, suggesting a possible therapeutic strategy (Hor et al., 2018).
Most recent studies have suggested that some neuronal populations reentering the cell cycle do not always undergo cell death but remain in the aneuploid state for the rest of their lives (Frade and López-Sánchez, 2017). For example, loss of the p53 tumor suppressor, a DNA repair protein, was associated with neuronal survival in the aneuploid state (Barrio-Alonso et al., 2018).
Neuronal cells have recently been reported to present with variable DNA content from one cell to another (somatic mosaicism), especially during the early development and old age (Paquola et al., 2016; McConnell et al., 2017; Sharma et al., 2017; Caneus et al., 2018; Leija-Salazar et al., 2018; Verheijen et al., 2018; Villela et al., 2018). This finding led to the development of a new field, defined as the brain somatic mosaicism (Paquola et al., 2016; McConnell et al., 2017). We speculate that this phenomenon is the result of senescent neurons reengaging the cell cycle and surviving in aneuploid states. In AD, neuronal somatic mosaicism may be reflected in the aneuploidy-induced APP gene variants (Bushman et al., 2015). Interestingly, a recent study has suggested that APP variants are generated via RNA retro-insertion into the DNA, suggesting that antiretroviral drugs may be beneficial for AD (Lee et al., 2018). Others have argued that patients with HIV-associated neurocognitive disorders (HANDs) rarely experience improved memory while in treatment with antiretrovirals (McArthur et al., 2010; Vance et al., 2013). These contradictory findings indicate that more studies are needed to clarify the role of these agents in AD.
Finally, two questions beg for answers: Are aneuploid neurons viable and does it make sense to facilitate their survival?
Novel studies in regenerative medicine reported that facilitating cell cycle completion in senescent cardiomyocytes prevented their apoptosis (Anversa and Leri, 2013; Hesse et al., 2017; Locatelli et al., 2018). Helping neurons survive the cell cycle engagement may comprise a therapeutic strategy in AD, but only if aneuploid cells are functional (Frade and López-Sánchez, 2017). Conversely, preventing neurons from engaging the cell cycle, a more straightforward approach, may be accomplished by blocking the nuclear export of Cdk5 or suppressing this protein in the cytosol with Cdk5 blockers or lithium (Zhang et al., 2008; Carvalho et al., 2013).
Senescent Astrocytes and Microglia
Astrocytes are the most numerous brain cells and their end-feet, ECs and pericytes comprise the BBB. Recent studies report that astrocytes are innate immune cells that, along with microglia, play a key role in the phagocytic removal of molecular waste, dead, or dying cells (Farina et al., 2007; Ransohoff and Brown, 2012; Morizawa et al., 2017). In addition, astrocytes generate AMPs, including β-amyloid, that may opsonize pathogens, facilitating their removal (Figure 1).
Preclinical studies have reported that astrocytes undergo both replicative and stress-induced senescence characterized by SASP, p16INK4a, and p21CIP1 markers; however, the difference between senescent and reactive astrocytes is not entirely clear at this time (Hou et al., 2018; Kritsilis et al., 2018; Maciel-Barón et al., 2018; Perez-Nievas and Serrano-Pozo, 2018). Recent studies seem to indicate that these phenotypes may be closely related or even identical as upregulated inflammatory and synapse-eliminating genes were found in both senescent and reactive astrocytes (Crowe et al., 2016; Boisvert et al., 2018). Along these lines, the aggressive A1 astrocytes may be senescent as they also upregulate inflammatory genes and eliminate healthy synapses (Liddelow et al., 2017; Morizawa et al., 2017; Clarke et al., 2018; Vilalta and Brown, 2018). In support of this hypothesis comes the recent finding that senescence-upregulated cytokines, TNF-α and IL-1, induce the A1 phenotype (Cartier et al., 2014; Altieri et al., 2017; Liddelow et al., 2017; Li P. et al., 2017; Yun et al., 2018).
Microglia are CNS innate immune cells, which, like macrophages at the body periphery, are vigilant and motile, characteristics that help them scrutinize the brain parenchyma, searching for “danger signals.” Microglia respond to invading pathogens by releasing pro-inflammatory cytokines which can trigger astrocytic senescence and reactivity (Cartier et al., 2014). In addition, under normal circumstances, microglia engulf senescent or dead cells, preventing their accumulation and the subsequent inflammation (Jung and Chung, 2018). Aging and immunosenescence were shown to alter microglial phagocytic function, generating inflammaging triggered by the accumulation of molecular waste and cellular corpses (Neumann et al., 2009; Koellhoffer et al., 2017).
Dystrophic microglia with growth arrest and senescent markers have been demonstrated in AD patients, but the difference between the reactive and dystrophic phenotype is unclear at this time (Flanary et al., 2007; Mosher and Wyss-Coray, 2014). Several studies have reported that although senescent microglia may lose their neuroprotective functions, their ability to mount inflammatory responses is preserved and even enhanced (Sierra et al., 2007; Davies et al., 2017). For example, senescent microglia have been shown to upregulate their TLRs, triggering exaggerated inflammation in response to minimal LPS stimulation. On the other hand, continuous LPS presence in the microglial environment induces immunosenescence with deficient phagocytosis (Yu et al., 2012). Recently, “dark,” hypervigilant microglia have been reported, likely representing senescent cells with aberrant phagocytic function (Bisht et al., 2016). Indeed, several studies report that in the presence of LPS, senescent microglia and astrocytes became neurotoxic, engaging in the phagocytosis of healthy neurons and synapses (von Bernhardi et al., 2015; Lana et al., 2017). Moreover, preclinical studies have shown that LPS-exposed microglia promote extracellular trafficking of hyperphosphorylated tau, a phenomenon inhibited by IL-10 (Liu et al., 2016; Magalhães et al., 2017; Hopp et al., 2018; Kametani and Hasegawa, 2018). Furthermore, microglial NLRP3 and its end products, IL-18, caspase-1, and IL-1β, have been associated with cellular senescence and AD (Griffin et al., 2006; Ojala et al., 2009; Cabral and de Lima, 2017). Conversely, caspase-1 inhibition ameliorates AD symptoms in animal models, suggesting a novel target (Yu et al., 2009; Cabral and de Lima, 2017; Flores et al., 2018).
Taken together, senescent microglia, incapable of proper immunosurveillance and phagocytosis, contribute to the accumulation of molecular waste, dead or dying cells, inducing inflammaging and immunosenescence. Astrocytes may respond to these microenvironmental changes by converting to the A1 phenotype marked by aberrant elimination of healthy synapses and neurons, a possible pathogenetic mechanism of AD.
Senescence and Aerobic Glycolysis: Got Lactate?
In the nineteenth century, Otto Warburg noticed that cancer cells converted glucose to lactate even in the presence of oxygen, a metabolic modality defined as aerobic glycolysis (AG). Compared with healthy cells, which oxidize glucose in the mitochondrion via oxidative phosphorylation (OXPHOS), cancer cells prefer cytosolic AG that generates excessive amounts of lactate (Potter et al., 2016). These observations beg the question: Why do cancer cells need lactate?
Recent findings helped solve this dilemma by revealing that cancer, like microorganisms, escapes detection by reprograming host immune cells to AG, a metabolic modality associated with immune tolerance (Roland et al., 2014; San-Millán and Brooks, 2016). In addition, lactate generates an acidic microenvironment which inhibits the host immune system (Romero-Garcia et al., 2016). Furthermore, lactate upregulates snail, a tumorigenic protein (encoded by the SNAI2 gene) which inhibits host cellular senescence, a key antitumor defense (Li X. et al., 2018).
Novel studies found that AG is the metabolic preference not only of cancer cells but also of many healthy tissues, including the brain (Demetrius et al., 2015; Yellen, 2018). Under normal circumstances, 10–12% of brain glucose is catabolized via AG despite oxygen availability (Goyal et al., 2017). Furthermore, the brain regions most dependent on AG are those involved in rapid activation and information processing, such as cognition, memory, and alertness (Dienel and Cruz, 2016).
Along similar lines, it was recently reported that immune cells and ECs preferentially utilize AG, especially when exposed to LPS or pathogens, suggesting that for rapidly proliferating cells, the slower OXPHOS may be an inadequate energy modality (Jones and Bianchi, 2015; Boitsova et al., 2018; Escoll and Buchrieser, 2018; Liu R. et al., 2018; Salmond, 2018). On the other hand, senescent cells rely almost exclusively on OXPHOS, indicating that loss of AG is an aging biomarker (Wen et al., 2012; Li et al., 2013; Goyal et al., 2017). The molecular mechanism of age-related AG loss is incompletely understood; however, under normal circumstances, lactate is synthesized by astrocytes, a neurotrophic function that may be lost in senescent cells (Riske et al., 2016). It has been established that lactate interacts with its receptor GPR81 (also called HCAR1) to generate rapid ATP surges required for neuronal activation (Bergersen and Gjedde, 2012; Díaz-García et al., 2017). Unlike AG, OXPHOS may be incapable of supplying the neurons with large amounts of energy on short notice (Díaz-García et al., 2017).
Aside from the CNS, lactate-GPR81 signaling plays a key role in the GI tract, where it maintains the integrity of intestinal barrier by positively regulating IL-10 (Ranganathan et al., 2018). Aging alters the lactate-GPR81 axis, disrupting both commensals tolerance and the intestinal barrier. In addition, age-related loss of gut Lactobacillus species, a major source of intestinal lactate, may impair GPR81 signaling, increasing intestinal permeability and facilitating microbial translocation (Walter, 2008). Moreover, as lactate-GPR81 interaction blocks the NLRP3 activation, agonists at these receptors may present with senotherapeutic properties (Hoque et al., 2014; Errea et al., 2016; Nolt et al., 2018).
In AD, due to compromised lactate-GPR81 signaling, AG may be unavailable, rendering neuronal cells totally dependent on mitochondrial OXPHOS. However, as the aging process also impairs mitochondria, OXPHOS becomes unreliable, triggering an energy crisis (Fong et al., 2016). Furthermore, the compensatory mechanisms, including mitochondrial fission, fusion, and mitophagy, are also compromised in AD, further lowering OXPHOS and deepening the crisis (Santos et al., 2010; Fang et al., 2016; Kerr et al., 2017).
Immunosenescence and Inflammaging
Immune system aging is closely linked to gut microbes and the loss of AG. Aging affects both the innate and adaptive immunity, but some cells are more affected than others (Burton and Stolzing, 2018). For example, AG-relying effector T cells are more impacted by age than the OXPHOS-preferring memory T cells (Carlos et al., 2018). As a result, antigens are remembered in old age, but they may trigger poor immune activation as evidenced by older individuals' weak response to vaccines (Lord, 2013).
Age-related immune alterations are captured by two words, inflammaging, denoting excessive innate immune activation, and immunosenescence, referring to the depletion of adaptive immune cells (Ventura et al., 2017; Fülöp et al., 2018a). The innate immune changes affect macrophages and natural killer (NK) cells at the body periphery as well as microglia and astrocytes in the CNS (Solana et al., 2018).
The NF-kB/NLRP3 axis was shown to regulate immune aging via proinflammatory cytokines IL-6, TNF-α, IL-1β, and IL-18 (Heneka et al., 2013; Couturier et al., 2016; Rea et al., 2018). Moreover, peripheral infections and inflammation were linked to microglial senescence, suggesting that interventions at the body periphery may influence central immunity (Netea and van der Meer, 2017; Cao and Zheng, 2018; Wendeln et al., 2018). Furthermore, infection with various pathogens, including cytomegalovirus (CMV), human immunodeficiency virus (HIV), HSV1, and Toxoplasma gondii, was implicated in immunosenescence and inflammaging, connecting these phenomena to microbes and their molecules (Solana et al., 2018). This is in line with the immune risk phenotype (IRP), a morbidity marker described in the elderly with CMV infection (Olsson et al., 2000).
Immunosenescence, marked by the depletion of adaptive immune cells, reflects thymic involution, a process starting in childhood and progressing at a rate of 3% per year throughout the adult life (Gui et al., 2012). The gradual loss of thymic function is manifested by a decrease in naïve T cells, increased number of memory cells, and downregulation of T cell receptors (TCRs) (Deleidi et al., 2015). Novel preclinical studies have linked thymic involution to the activation of the NF-κB/NLRP3 axis, while caspase-1 inhibitors were shown to restore thymic lymphopoiesis in elderly (Youm et al., 2012; Wen et al., 2018). Interestingly, viruses, bacteria, fungi, and parasites were demonstrated to infect the thymus directly, probably inducing senescence and premature atrophy (Nunes-Alves et al., 2013). This is significant as it links thymic involution to the loss of intestinal Tregs, impaired barrier function, and microbial translocation. Indeed, a thymus-gut axis was described during the early development when dendritic cells migrate from the GI tract to “educate” the thymus in commensals tolerance (Lathrop et al., 2011; Jain and Seed, 2016) Figure 2 (also discussed in “The senescent intestinal barrier”).
Age-related thymic involution was also associated with the loss of gut IL-10-secreting B cells, which, like Tregs, contribute to the microbial immune tolerance (Ghosh et al., 2013; van der Geest et al., 2016; Ip et al., 2017). Conversely, restoration of thymic function in older individuals or hormonal replacement may reverse immunosenescence, suggesting a novel therapeutic strategy. Indeed, preclinical studies reported that administration of EVs loaded with thymosin alpha 1 and melatonin restored the thymic function in older animals (Molinero et al., 2000; King and Tuthill, 2016; Wang et al., 2018).
Senescence and Iron
Age-related iron dysmetabolism, a phenomenon well-documented in AD, is closely connected to cellular senescence and the loss of AG (Kelleher and Soiza, 2013; Ward et al., 2014; Lane et al., 2015). Iron is known for inducing DNA damage and EC senescence that increases BBB permeability and the risk of microbial translocation (Won et al., 2011; Mollet et al., 2016) (Figure 4). Moreover, iron was demonstrated to activate NLRP3 inflammasomes, linking this biometal to inflammation, dysfunctional mitochondria, and impaired mitophagy (Allen et al., 2013; Xiong et al., 2014; Nakamura et al., 2016). A component of iron–sulfur clusters (ISCs) and heme, iron has been demonstrated to alter mitochondrial glucose metabolism in response to pathogens (Horowitz and Greenamyre, 2010). For example, to deny microbes the access to glucose during infections, heme binds TLR-4, inducing hypoglycemia (Figueiredo et al., 2007; Weis et al., 2017). In AD however, hypoglycemia may be a double-edged sword as it may deepen the cellular energy crisis (Fong et al., 2016). This may explain the link between P. gingivalis, a heme-dependent pathogen, and T2DM, as well as the association of both with AD (Deshpande et al., 2010). In its attempt to extract heme, P. gingivalis, a facultative intracellular microbe, may damage not only cell membranes but also the mitochondrion, triggering a bioenergetic crisis and NLRP3-induced cellular senescence (Bullon et al., 2011). Moreover, age-related brain LPS elevation may trigger intracellular iron migration, an innate immune response to withhold iron from pathogens (Abreu et al., 2018; Ashraf et al., 2018). However, intracellular iron in proximity to redox biomolecules increases the risk of ROS generation, a known trigger of cellular senescence (Lopes et al., 2008; Streit and Xue, 2012). Conversely, the natural iron chelator lactoferrin binds LPS, deactivating NLRP3 (Drago-Serrano et al., 2012; Kruzel et al., 2017; Sfera et al., 2018). Interestingly, lactoferrin was recently identified as an AMP with anti-P. gingivalis properties, suggesting a therapeutic benefit in AD (Drago-Serrano et al., 2012; Kruzel et al., 2017).
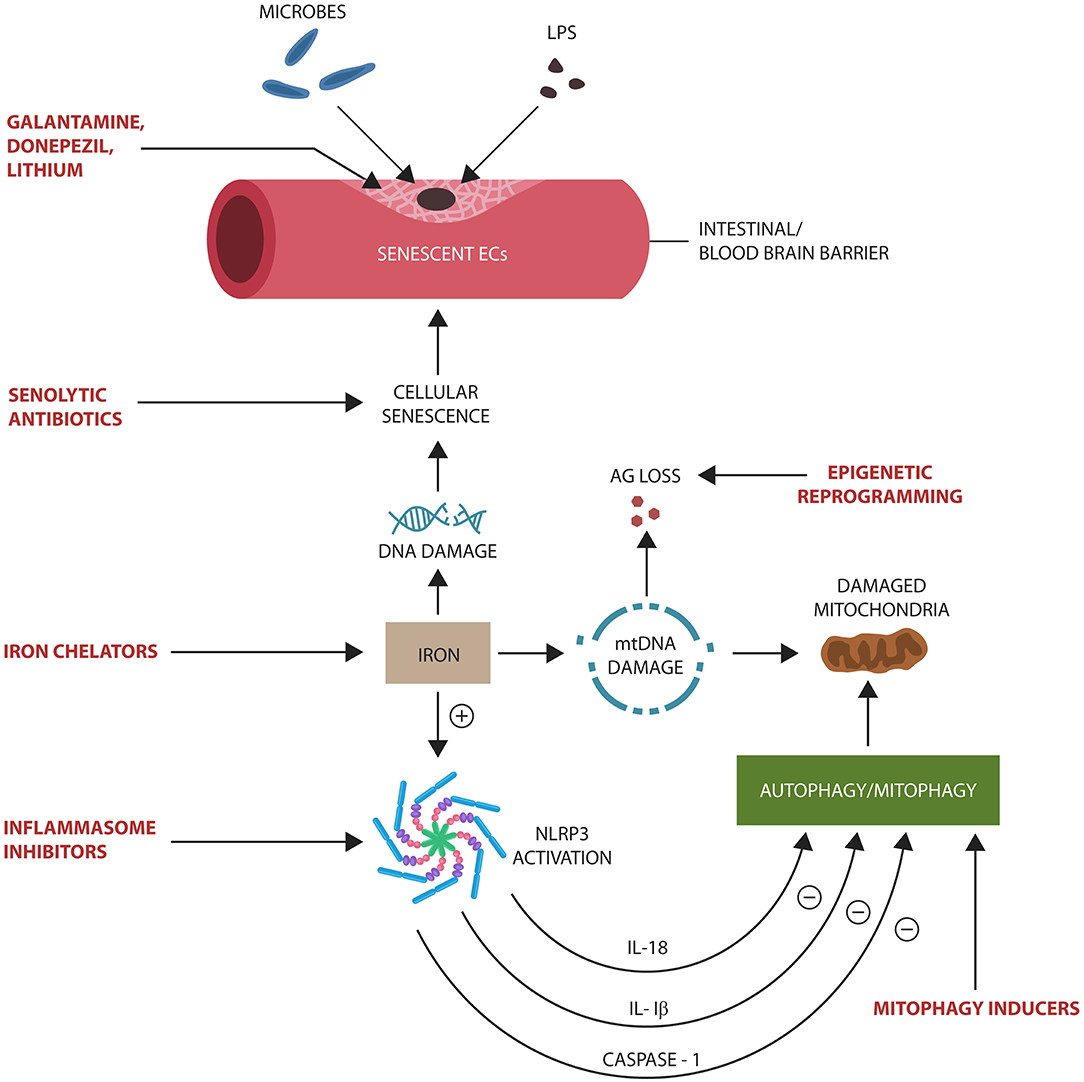
Figure 4. Potential senotherapeutic interventions in AD and the steps at which they may operate. NLRP3 end products, IL-1β, IL-18, and caspase-1, inhibit autophagy and mitophagy, contributing to the accumulation of senescent cells and damaged mitochondria. These, in turn, alter biological barriers, enabling microbial translocation and AG loss. Excess iron induces DNA and mtDNA damage, activating NLRP3 with subsequent cellular senescence.
The aging process, associated with intracellular iron retention, DNA damage, and impaired genomic repair, is a phenomenon we previously defined as ferrosenescence (Sfera et al., 2018). Along these lines, the levels of iron storage protein, ferritin, was found to be a reliable senescence marker, supporting the concept of ferrosenescence (Masaldan et al., 2018). This is in line with a novel hypothesis, suggesting that age-arelated increase in free iron pool resuscitates dormant microbes in the brain parenchyma (Pretorius et al., 2018). Furthermore, intracellular iron can promote Cdk5 nuclear export, tau hyperphosphorylation, and neuronal cell cycle activation (Engmann and Giese, 2009). On the other hand, iron chelation with deferoxamine was shown to have the opposite effect on tau, probably by facilitating Cdk5 nuclear reentry (Guo et al., 2013; Liu J. L. et al., 2018).
Senotherapeutics: Targeting Senescence in Alzheimer's Disease
Senotherapeutics are pharmacological compounds, aiming at restoring senescent cells to non-senescent status or to trigger their apoptosis and clearance (Olivieri et al., 2018). These agents can be classified into senolytics that selectively eliminate senescent cells and senomorphics that delay or reverse senescence. Recent preclinical studies have shown that senotherapeutics can influence the course of age-related diseases, including AD (Kim and Kim, 2019). The agents described below include novel compounds and repurposed drugs with potential senotherapeutic properties.
Repurposed Galantamine, Donepezil, Lithium, and Fluoxetine
Galantamine and donepezil are cholinesterase inhibitors widely used in the treatment of AD. They function by inhibiting acetylcholine (ACh)-degrading enzymes and increasing the bioavailability of this neurotransmitter in brain cholinergic tracts. Both drugs were recently demonstrated to protect intestinal barrier and BBB, displaying potential senotherapeutic properties (Nakao et al., 2008; Zhang T. et al., 2015; Zhang Y. et al., 2017; Wazea et al., 2018). ACh-producing intestinal T cells have been reported to promote commensal microbes' immune tolerance, protecting against inflammation and barrier disruption (Dhawan et al., 2016). A recent study showed that alpha 7 nicotinic ACh receptor (α7nAChR) agonists function by deactivating NLRP3 in monocytes and microglia, promoting tolerance to commensals (Ke et al., 2017). Moreover, vagal nerve stimulation and transcranial direct current stimulation (tDCS) may present with senotherapeutic properties as they enhance cholinergic signaling (Chang et al., 2018).
Lithium, a drug used in the treatment of bipolar disorder, was reported to inhibit both mTOR and GSK3β, protecting the ECs of intestinal barrier and BBB (Motoi et al., 2014; Bosche et al., 2016; Steinbach et al., 2017; Martin et al., 2018). In addition, lithium modulates Cdk5, probably stabilizing neuronal cells in post-mitotic state (Jordà et al., 2005).
Fluoxetine, a selective serotonin reuptake inhibitor (SSRI) utilized in the treatment of major depressive disorder was demonstrated to inhibit NLRP3 and SASP, suggesting senotherapeutic properties (Diniz et al., 2016; Du et al., 2016). Indeed, a novel study reported that SSRIs, as a group, decrease the risk of conversion from mild cognitive impairment (MCI) to AD, likely by lowering cellular senescence (Bartels et al., 2018).
Mitophagy as a Senotherapeutic Strategy
Recent studies have associated defective mitochondria with cellular senescence as defects of these organelles activate NLRP3 (Liu Q. et al., 2018). On the other hand, elimination of defective mitochondria, mitophagy, delays senescence and lowers inflammation. Preclinical studies linked mitophagy enhancement to improved cognition, while accumulation of defective mitochondria was associated with AD pathology (Cai and Tammineni, 2016; Kerr et al., 2017).
Mitophagy as a therapeutic intervention was studied the most in PD in which defective mitochondria are cleared via phosphatase and tensin homolog (PTEN)-induced kinase 1 (PINK1) and the E3 ubiquitin ligase parkin (PARK2) (Pickrell and Youle, 2015). Disruption of this autophagic pathway is a well-established pathogenetic mechanism in PD that may also play a role in AD (Martín-Maestro et al., 2016).
Another mitophagy system, associated with AD and traumatic brain injury (TBI), involves the inner mitochondrial membrane phospholipid, cardiolipin (Chu, 2018; Chao et al., 2019). Externalization of cardiolipin to the mitochondrial surface was shown to activate neuronal mitophagy in rodents (Chu et al., 2013).
Mitophagy-inducing agents currently available include Mito-CP (3-carboxyl proxyl nitroxide), Mito-Metformin, and MitoTam (mitochondria-targeted tamoxifen) (Boyle et al., 2018; Hubackova et al., 2019). These compounds were demonstrated to activate mitophagy by various mechanisms, including depletion of ATP or adenine nucleotide translocase-2 (ANT2) (Singh et al., 2009; Zhang C. et al., 2015). Interestingly, several antibiotics, including quinolones, aminoglycosides, and β-lactams, were found to damage mitochondria, inducing cellular senescence (Kalghatgi et al., 2013; Stefano et al., 2017). Conversely, tetracycline derivatives, doxycycline, and minocycline were associated with the activation of mitophagy in ECs, suggesting protective effects for biological barriers (Dong et al., 2015; Xing et al., 2017).
Histone Deacetylase Inhibitors as Senotherapeutics
Histone deacetylases (HDACs) are enzymes involved in the epigenetic regulation of gene expression via histone proteins. HDACs have been involved in the pathogenesis of AD, and some HDAC inhibitors (HDACi) may present with cognition-enhancing properties (Xu et al., 2011). HDAC 1 and 2 inhibitors, including valproic acid (VPA), have been demonstrated to correct defective microglial phagocytosis, facilitating the elimination of molecular waste and dead cells (Datta et al., 2018). VPA, a drug utilized in the treatment of epilepsy and bipolar disorder, was recently shown to possess anti-HSV-1 actions, indicating a potential benefit in AD (Crespillo et al., 2016). In addition, this compound prevents LPS-induced ECs damage, protecting intestinal barrier and BBB (Chuang et al., 2014; Kasotakis et al., 2017).
Aside from VPA, other HDACis currently in clinical use include trichostatin A, sodium butyrate, and suberoylanilide hydroxamic acid (SAHA or vorinostat). SAHA is both an HDAC 6 inhibitor and an iron chelator, suggesting senotherapeutic properties (Hwang et al., 2015). SAHA is currently approved for the treatment of advanced primary cutaneous T cell lymphoma, but it also possesses anti-P. gingivalis properties, suggesting a therapeutic role in AD and periodontal disease (Yoshioka et al., 2003; Mann et al., 2007). Moreover, VPA and SAHA were recently found efficacious against Mycobacterium tuberculosis, an intracellular pathogen, suggesting efficacy against facultative intracellular microbes, including P. gingivalis (Rao et al., 2018).
It was recently reported that sirtuin 6 (SIRT6), a protein presenting with HDAC-like senotherapeutic properties, inhibits NF-κB and EC senescence, suggesting AD therapeutic benefits (Lappas, 2012; Zhao et al., 2016).
Iron Chelators in Cellular Senescence and Alzheimer's Disease
Iron is a pro-growth nutrient that accumulates in senescent cells, contributing to genomic instability and ROS generation (Killilea et al., 2003). A major component of the aging marker lipofuscin, iron is a driver of cellular senescence via mTOR activation and inhibition of mitophagy (Terman and Brunk, 1998; Höhn et al., 2010; Bayeva et al., 2012). Iron chelators, such as deferoxamine, are mTOR inhibitors demonstrated to lower the markers of senescence (Ohyashiki et al., 2009; Inoue et al., 2018). For example, intranasal administration of deferoxamine was found beneficial in animal models of AD, PD, and stroke (Fine et al., 2017). Moreover, as pathogens and host innate immune cells share the same iron pool, iron chelators deny this biometal to both, lowering microbial survival and ROS formation (Thompson et al., 2012). For this reason, iron chelator nanoparticles have been studied as AD therapeutics (Liu et al., 2010).
Another iron chelator with senotherapeutic properties, α-lipoic acid, is a BBB-crossing mitochondrial molecule with beneficial effects in AD (Baeeri et al., 2019; Camiolo et al., 2019). Preclinical studies linked this compound to mTOR inhibition and protection against brain ischemia (Gao et al., 2018). Other recent studies associated α-lipoic acid with intestinal barrier and BBB protection, indicating antitranslocation properties (Schreibelt et al., 2006; Varasteh et al., 2017).
The natural iron chelator lactoferrin, recently identified as an AMP, was found protective of ECs and biological barriers (Krylov et al., 2007; Wu et al., 2014). Inhibiting mTOR signaling and decreasing the iron pool, lactoferrin may be of potential therapeutic benefit in AD (Jenssen and Hancock, 2009; Zhang et al., 2014; van Splunter et al., 2018).
Inflammasome Inhibitors and Alzheimer's Disease
NLRP3 inhibitors are novel senotherapeutic agents that delay EC senescence and microbial translocation, suggesting beneficial effects in both AD and chronic inflammation (Yi, 2017; Yin et al., 2017; McAllister et al., 2018; Qi et al., 2018). Here, we focus primarily on NLRP3 inhibitors associated with the restoration of biological barriers.
MCC950, a diarylsulphonylurea inhibitor, lowers pyroptosis by selectively blocking NLRP3 inflammasomes, restoring the integrity of intestinal barrier (Fan et al., 2018; Perera et al., 2018). MCC950 also inhibits IL-1β, restoring BBB integrity (Lang et al., 2018). Interestingly, in PD, Cdk5 was shown to activate NLRP3, suggesting that inflammasome inhibitors may lower the detrimental effects of this kinase on neurons, preventing senescence and cell cycle engagement (Zhang et al., 2016). Indeed, to activate cytosolic NLRP3, Cdk5 must exit the nucleus, an event that triggers the neuronal cell cycle. Moreover, MCC950 has been shown to prevent immunosenescence of innate immune cells by blocking P. gingivalis-induced pyroptosis (Fleetwood et al., 2017).
INF 39, an acrylate NLRP3 inhibitor, was shown to decrease bowel inflammation in animal models by downregulating IL-1β, suggesting a therapeutic role against microbial translocation (Cocco et al., 2017; Pellegrini et al., 2018).
Milk fat globule membranes (MFGM) were reported to lower bacterial translocation in animal models by inhibiting NLRP3 and increasing the expression of intestinal tight junctions, proteins opposing microbial translocation (Li Y. et al., 2018).
Short-chain fatty acids (SCFAs) were found trophic for IECs, restoring the integrity of intestinal barrier by functioning as energy sources and NLRP3 deactivators (Feng et al., 2018).
Statins were described as protective of ECs in both intestinal barrier and BBB via NLRP3 inhibition, reviving the debate about the benefit of these drugs in AD (Schreibelt et al., 2006; Krylov et al., 2007; Varasteh et al., 2017).
Conclusions
Commensal gut microbes live in symbiosis with the human host as long as they reside in the GI tract where they can be kept under control. Cellular senescence alters the integrity of biological barriers, allowing translocation and dissemination of gut microorganisms throughout the body tissues, including the brain. Operating “behind enemy lines,” pathogens can gain control of host immune defenses and metabolism, triggering senescence and neurodegenerative pathology.
Senotherapeutics inhibit cellular senescence program, restoring the integrity of biological barriers. Moreover, the recent association of chronic P. gingivalis infection with both cellular senescence and AD emphasizes the importance of promptly treating periodontal disease.
Aging, a major risk factor of AD, is associated with senescent cell accumulation and SASP-induced pathology. In the CNS, senescent brain cells may display aberrant traits, including neuronal cell cycle activation and phagocytosis of viable neurons and synapses by aggressive glial cells. Since the molecular underpinnings of senescence, NF-kB-linked NLRP3 assembly, is modifiable, age-related neurodegenerative disorders could be epigenetically, pharmacologically, and immunometabolically influenced not only from within the CNS but also from the body periphery.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abreu, R., Quinn, F., and Giri, P. K. (2018). Role of the hepcidin–ferroportin axis in pathogen-mediated intracellular iron sequestration in human phagocytic cells. Blood Adv. 2, 1089–1100. doi: 10.1182/bloodadvances.2017015255
Acuña-Hinrichsen, F., Muñoz, M., Hott, M., Martin, C., Mancilla, E., Salazar, P., et al. (2019). Herpes simplex virus type 1 enhances expression of the synaptic protein Arc for its own benefit. Front. Cell. Neurosci. 12:505. doi: 10.3389/fncel.2018.00505
Aguilera, M. O., Delgui, L. R., Romano, P. S., and Colombo, M. I. (2018). Chronic infections: a possible scenario for autophagy and senescence cross-talk. Cells 7, 162. doi: 10.3390/cells7100162
Ahmadi Badi, S., Moshiri, A., Fateh, A., Rahimi Jamnani, F., Sarshar, M., Vaziri, F., et al. (2017). Microbiota-derived extracellular vesicles as new systemic regulators. Front. Microbiol. 8:1610. doi: 10.3389/fmicb.2017.01610
Alcendor, D. J., Charest, A. M., Zhu, W. Q., Vigil, H. E., and Knobel, S. M. (2012). Infection and upregulation of proinflammatory cytokines in human brain vascular pericytes by human cytomegalovirus. J. Neuroinflammation 9:95. doi: 10.1186/1742-2094-9-95
Allen, G. F., Toth, R., James, J., and Ganley, I. G. (2013). Loss of iron triggers PINK1/Parkin-independent mitophagy. EMBO Rep. 14, 1127–1135. doi: 10.1038/embor.2013.168
Alonso, R., Pisa, D., Fernández-Fernández, A. M., and Carrasco, L. (2018). Infection of fungi and bacteria in brain tissue from elderly persons and patients with Alzheimer's disease. Front. Aging Neurosci. 10:159. doi: 10.3389/fnagi.2018.00159
Al-Sadi, R. M., and Ma, T. Y. (2007). IL-1beta causes an increase in intestinal epithelial tight junction permeability. J. Immunol. 178, 4641–4649. doi: 10.4049/jimmunol.178.7.4641
Altieri, P., Murialdo, R., Barisione, C., Lazzarini, E., Garibaldi, S., Fabbi, P., et al. (2017). 5-Fluorouracil causes endothelial cell senescence: potential protective role of glucagon-like peptide 1. Br. J. Pharmacol. 174, 3713–3726. doi: 10.1111/bph.13725
Alvarez-Arellano, L., and Maldonado-Bernal, C. (2014). Helicobacter pylori and neurological diseases: married by the laws of inflammation. World J. Gastrointest. Pathophysiol. 5, 400–404. doi: 10.4291/wjgp.v5.i4.400
Amsterdam, J. D., Maislin, G., and Rybakowski, J. (1990). A possible antiviral action of lithium carbonate in herpes simplex virus infections. Biol. Psychiatry 27, 447–453. doi: 10.1016/0006-3223(90)90555-G
Anversa, P., and Leri, A. (2013). Innate regeneration in the aging heart: healing from within. Mayo Clin. Proc. 88, 871–883. doi: 10.1016/j.mayocp.2013.04.001
Argaw, A. T., Zhang, Y., Snyder, B. J., Zhao, M. L., Kopp, N., Lee, S. C., et al. (2006). IL-1beta regulates blood–brain barrier permeability via reactivation of the hypoxia–angiogenesis program. J. Immunol. 177, 5574–5584. doi: 10.4049/jimmunol.177.8.5574
Ashley, J., Cordy, B., Lucia, D., Fradkin, L. G., Budnik, V., and Thomson, T. (2018). Retrovirus-like Gag protein Arc1 binds RNA and traffics across synaptic boutons. Cell 172, 262–274.e11. doi: 10.1016/j.cell.2017.12.022
Ashraf, A., Clark, M., and So, P. W. (2018). The aging of iron man. Front. Aging Neurosci. 10 :65. doi: 10.3389/fnagi.2018.00065
Atai, N. A., Balaj, L., van Veen, H., Breakefield, X. O., Jarzyna, P. A., Van Noorden, C. J., et al. (2013). Heparin blocks transfer of extracellular vesicles between donor and recipient cells. J. Neurooncol. 115, 343–351. doi: 10.1007/s11060-013-1235-y
Attanasio, J., and Wherry, E. J. (2016). Costimulatory and coinhibitory receptor pathways in infectious disease. Immunity 44, 1052–1068. doi: 10.1016/j.immuni.2016.04.022
Austin, S. A., Santhanam, A. V., Hinton, D. J., Choi, D. S., and Katusic, Z. S. (2013). Endothelial nitric oxide deficiency promotes Alzheimer's disease pathology. J. Neurochem. 127, 691–700. doi: 10.1111/jnc.12334
Baar, M. P., Brandt, R. M. C., Putavet, D. A., Klein, J. D. D., Derks, K. W. J., Bourgeois, B. R. M., et al. (2017). Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 169, 132–147.e16. doi: 10.1016/j.cell.2017.02.031
Baeeri, M., Bahadar, H., Rahimifard, M., Navaei-Nigjeh, M., Khorasani, R., Rezvanfar, M. A., et al. (2019). α-Lipoic acid prevents senescence, cell cycle arrest, and inflammatory cues in fibroblasts by inhibiting oxidative stress. Pharmacol. Res. 141, 214–223. doi: 10.1016/j.phrs.2019.01.003
Bai, B. (2018). U1 snRNP alteration and neuronal cell cycle reentry in Alzheimer disease. Front. Aging Neurosci. 10:75. doi: 10.3389/fnagi.2018.00075
Balistreri, C. R., Candore, G., Accardi, G., Colonna-Romano, G., and Lio, D. (2013). NF-κB pathway activators as potential ageing biomarkers: targets for new therapeutic strategies. Immun. Ageing 10:24. doi: 10.1186/1742-4933-10-24
Barnhart, M. M., and Chapman, M. R. (2006). Curli biogenesis and function. Annu. Rev. Microbiol. 60, 131–147. doi: 10.1146/annurev.micro.60.080805.142106
Barrio-Alonso, E., Hernández-Vivanco, A., Walton, C. C., Perea, G., and Frade, J. M. (2018). Cell cycle reentry triggers hyperploidization and synaptic dysfunction followed by delayed cell death in differentiated cortical neurons. Sci. Rep. 8:14316. doi: 10.1038/s41598-018-32708-4
Bartels, C., Wagner, M., Wolfsgruber, S., Ehrenreich, H., and Schneider, A. (2018). Impact of SSRI therapy on risk of conversion from mild cognitive impairment to Alzheimer's dementia in individuals with previous depression. Am. J. Psychiatry 175, 232–241. doi: 10.1176/appi.ajp.2017.17040404
Batista, A. F., Forny-Germano, L., Clarke, J. R., Lyra E Silva, N. M., Brito-Moreira, J., Boehnke, S. E., et al. (2018). The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer's disease. J. Pathol. 245, 85–100. doi: 10.1002/path.5056
Bayeva, M., Khechaduri, A., Puig, S., Chang, H. C., Patial, S., Blackshear, P. J., et al. (2012). mTOR regulates cellular iron homeostasis through tristetraprolin. Cell Metab. 16, 645–657. doi: 10.1016/j.cmet.2012.10.001
Bergersen, L. H., and Gjedde, A. (2012). Is lactate a volume transmitter of metabolic states of the brain? Front. Neuroenergetics 4:5. doi: 10.3389/fnene.2012.00005
Bester, J., Soma, P., Kell, D. B., and Pretorius, E. (2015). Viscoelastic and ultrastructural characteristics of whole blood and plasma in Alzheimer-type dementia, and the possible role of bacterial lipopolysaccharides (LPS). Oncotarget 6, 35284–35303. doi: 10.18632/oncotarget.6074
Bi, R., Kong, L. L., Xu, M., Li, G. D., Zhang, D. F., et al. (2018). The Arc gene confers genetic susceptibility to Alzheimer's disease in Han Chinese. Mol. Neurobiol. 55, 1217–1226. doi: 10.1007/s12035-017-0397-6
Birch, J., and Passos, J. F. (2017). Targeting the SASP to combat ageing: mitochondria as possible intracellular allies? Bioessays 39:1600235. doi: 10.1002/bies.201600235
Bisht, K., Sharma, K. P., Lecours, C., Sánchez, M. G., El Hajj, H., Milior, G., et al. (2016). Dark microglia: a new phenotype predominantly associated with pathological states. Glia 64, 826–839. doi: 10.1002/glia.22966
Blazkova, H., Krejcikova, K., Moudry, P., Frisan, T., Hodny, Z., and Bartek, J. (2009). Bacterial intoxication evokes cellular senescence with persistent DNA damage and cytokine signalling. J. Cell. Mol. Med. 14, 357–367. doi: 10.1111/j.1582-4934.2009.00862.x
Boccardi, V., Pelini, L., Ercolani, S., Ruggiero, C., and Mecocci, P. (2015). From cellular senescence to Alzheimer's disease: the role of telomere shortening. Ageing Res. Rev. 22, 1–8. doi: 10.1016/j.arr.2015.04.003
Boisvert, M. M., Erikson, G. A., Shokhirev, M. N., and Allen, N. J. (2018). The aging astrocyte transcriptome from multiple regions of the mouse brain. Cell Rep. 22, 269–285. doi: 10.1016/j.celrep.2017.12.039
Boitsova, E. B., Morgun, A. V., Osipova, E. D., Pozhilenkova, E. A., Martinova, G. P., Frolova, O. V., et al. (2018). The inhibitory effect of LPS on the expression of GPR81 lactate receptor in blood–brain barrier model in vitro. J. Neuroinflammation 15, 196. doi: 10.1186/s12974-018-1233-2
Bosche, B., Molcanyi, M., Rej, S., Doeppner, T. R., Obermann, M., Müller, D. J., et al. (2016). Low-dose lithium stabilizes human endothelial barrier by decreasing MLC phosphorylation and universally augments cholinergic vasorelaxation capacity in a direct manner. Front. Physiol. 7:593. doi: 10.3389/fphys.2016.00593
Bossù, P., Ciaramella, A., Salani, F., Vanni, D., Palladino, I., Caltagirone, C., et al. (2010). Interleukin-18, from neuroinflammation to Alzheimer's disease. Curr. Pharm. Des. 16, 4213–4224. doi: 10.2174/138161210794519147
Bourgade, K., Garneau, H., Giroux, G., Le Page, A. Y., Bocti, C., Dupuis, G., et al. (2015). β-Amyloid peptides display protective activity against the human Alzheimer's disease-associated herpes simplex virus-1. Biogerontology 16, 85–98. doi: 10.1007/s10522-014-9538-8
Bourgade, K., Le Page, A., Bocti, C., Witkowski, J. M., Dupuis, G., Frost, E. H., et al. (2016). Protective effect of amyloid-β peptides against herpes simplex virus-1 infection in a neuronal cell culture model. J. Alzheimers. Dis. 50, 1227–1241. doi: 10.3233/JAD-150652
Boyle, K. A., Van Wickle, J., Hill, R. B., Marchese, A., Kalyanaraman, B., and Dwinell, M. B. (2018). Mitochondria-targeted drugs stimulate mitophagy and abrogate colon cancer cell proliferation. J. Biol. Chem. 293, 14891–14904. doi: 10.1074/jbc.RA117.001469
Brothers, H. M., Gosztyla, M. L., and Robinson, S. R. (2018). The physiological roles of amyloid-β peptide hint at new ways to treat Alzheimer's disease. Front. Aging Neurosci. 10:118. doi: 10.3389/fnagi.2018.00118
Broz, P., and Monack, D. M. (2011). Molecular mechanisms of inflammasome activation during microbial infections. Immunol. Rev. 243, 174–190. doi: 10.1111/j.1600-065X.2011.01041.x
Brun, P., Qesari, M., Marconi, P. C., Kotsafti, A., Porzionato, A., Macchi, V., et al. (2018). Herpes simplex virus type 1 infects enteric neurons and triggers gut dysfunction via macrophage recruitment. Front. Cell. Infect. Microbiol. 8:74. doi: 10.3389/fcimb.2018.00074
Bullon, P., Cordero, M. D., Quiles, J. L., Morillo, J. M., del Carmen Ramirez-Tortosa, M., and Battino, M. (2011). Mitochondrial dysfunction promoted by Porphyromonas gingivalis lipopolysaccharide as a possible link between cardiovascular disease and periodontitis. Free Radic. Biol. Med. 50, 1336–1343. doi: 10.1016/j.freeradbiomed.2011.02.018
Burton, D. G. A., and Stolzing, A. (2018). Cellular senescence: immunosurveillance and future immunotherapy. Ageing Res. Rev. 43, 17–25. doi: 10.1016/j.arr.2018.02.001
Bushman, D. M., Kaeser, G. E., Siddoway, B., Westra, J. W., Rivera, R. R., Rehen, S. K., et al. (2015). Genomic mosaicism with increased amyloid precursor protein (APP) gene copy number in single neurons from sporadic Alzheimer's disease brains. Elife 4:e05116. doi: 10.7554/eLife.05116
Bussian, T. J., Aziz, A., Meyer, C. F., Swenson, B. L., van Deursen, J. M., and Baker, D. J. (2018). Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature. 562, 578–582. doi: 10.1038/s41586-018-0543-y
Cabral, B., and de Lima, O. (2017). Microglial NLRP3 activity in Alzheimer's disease. Brain Disord. Treat. 3:019. doi: 10.23937/2469-5866/1510019
Cai, Q., and Tammineni, P. (2016). Alterations in mitochondrial quality control in Alzheimer's disease. Front. Cell. Neurosci. 10:24. doi: 10.3389/fncel.2016.00024
Calvani, R., Picca, A., Lo Monaco, M. R., Landi, F., Bernabei, R., and Marzetti, E. (2018). Of microbes and minds: a narrative review on the second brain aging. Front. Med. 5:53. doi: 10.3389/fmed.2018.00053
Camiolo, G., Tibullo, D., Giallongo, C., Romano, A., Parrinello, N. L., Musumeci, G., et al. (2019). α-Lipoic acid reduces iron-induced toxicity and oxidative stress in a model of iron overload. Int. J. Mol. Sci. 20, E609. doi: 10.3390/ijms20030609
Caneus, J., Granic, A., Rademakers, R., Dickson, D. W., Coughlan, C. M., Chial, H. J., et al. (2018). Mitotic defects lead to neuronal aneuploidy and apoptosis in frontotemporal lobar degeneration caused by MAPT mutations. Mol. Biol. Cell 29, 575–586. doi: 10.1091/mbc.E17-01-0031
Cao, W., and Zheng, H. (2018). Peripheral immune system in aging and Alzheimer's disease. Mol. Neurodegener. 13, 51. doi: 10.1186/s13024-018-0290-4
Carlos, A. R., Weis, S., and Soares, M. P. (2018). Cross-talk between iron and glucose metabolism in the establishment of disease tolerance. Front. Immunol. 9:2498. doi: 10.3389/fimmu.2018.02498
Cartier, N., Lewis, C. A., Zhang, R., and Rossi, F. M. V. (2014). The role of microglia in human disease: therapeutic tool or target? Acta Neuropathol. 128, 363–380. doi: 10.1007/s00401-014-1330-y
Carvalho, M., De Paula, V., and Forlenza, O. (2013). Effect of chronic treatment with lithium on protein cyclin-dependent kinase 5 (CDK5) and tau protein in a primary culture of cortical neurons. Alzheimers Dement. 9, P718. doi: 10.1016/j.jalz.2013.05.1430
Cebula, A., Seweryn, M., Rempala, G. A., Pabla, S. S., McIndoe, R. A., Denning, T. L., et al. (2013). Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature. 497, 258–262. doi: 10.1038/nature12079
Chang, C. H., Lane, H. Y., and Lin, C. H. (2018). Brain stimulation in Alzheimer's disease. Front. Psychiatry 9:201. doi: 10.3389/fpsyt.2018.00201
Chao, H., Lin, C., Zuo, Q., Liu, Y., Xiao, M., Xu, X., et al. (2019). Cardiolipin-dependent mitophagy guides outcome after traumatic brain injury. J. Neurosci. 39, 1930–1943. doi: 10.1523/JNEUROSCI.3415-17.2018
Cheng, C., Tempel, D., Oostlander, A., Helderman, F., Gijsen, F., Wentzel, J., et al. (2008). Rapamycin modulates the eNOS vs. shear stress relationship. Cardiovasc. Res. 78, 123–129. doi: 10.1093/cvr/cvm103
Childs, B. G., Durik, M., Baker, D. J., and van Deursen, J. M. (2015). Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat. Med. 21, 1424–1435. doi: 10.1038/nm.4000
Choby, J. E., and Skaar, E. P. (2016). Heme synthesis and acquisition in bacterial pathogens. J. Mol. Biol. 428, 3408–3428. doi: 10.1016/j.jmb.2016.03.018
Chu, C. T. (2018). Multiple pathways for mitophagy: a neurodegenerative conundrum for Parkinson's disease. Neurosci. Lett. 697, 66–71. doi: 10.1016/j.neulet.2018.04.004
Chu, C. T., Ji, J., Dagda, R. K., Jiang, J. F., Tyurina, Y. Y., Kapralov, A. A., et al. (2013). Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat. Cell Biol. 15, 1197–1205. doi: 10.1038/ncb2837
Chuang, Y. F., Yang, H. Y., Ko, T. L., Hsu, Y. F., Sheu, J. R., Ou, G., et al. (2014). Valproic acid suppresses lipopolysaccharide-induced cyclooxygenase-2 expression via MKP-1 in murine brain microvascular endothelial cells. Biochem. Pharmacol. 88, 372–383. doi: 10.1016/j.bcp.2014.02.004
Clarke, L. E., Liddelow, S. A., Chakraborty, C., Münch, A. E., Heiman, M., and Barres, B. A. (2018). Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci. U.S.A. 115, E1896–E1905. doi: 10.1073/pnas.1800165115
Cocco, M., Pellegrini, C., Martínez-Banaclocha, H., Giorgis, M., Marini, E., Costale, A., et al. (2017). Development of an acrylate derivative targeting the NLRP3 inflammasome for the treatment of inflammatory bowel disease. J. Med. Chem. 60, 3656–3671. doi: 10.1021/acs.jmedchem.6b01624
Costantini, E., D'Angelo, C., and Reale, M. (2018). The role of immunosenescence in neurodegenerative diseases. Mediators Inflamm. 2018:6039171. doi: 10.1155/2018/6039171
Couturier, J., Stancu, I. C., Schakman, O., Pierrot, N., Huaux, F., Kienlen-Campard, P., et al. (2016). Activation of phagocytic activity in astrocytes by reduced expression of the inflammasome component ASC and its implication in a mouse model of Alzheimer disease. J. Neuroinflammation 13:20. doi: 10.1186/s12974-016-0477-y
Crespillo, A. J., Praena, B., Bello-Morales, R., Lerma, L., Vázquez-Calvo, A., Martín-Acebes, M. A., et al. (2016). Inhibition of herpes virus infection in oligodendrocyte cultured cells by valproic acid. Virus Res. 214, 71–79. doi: 10.1016/j.virusres.2016.01.009
Crews, L., Patrick, C., Adame, A., Rockenstein, E., and Masliah, E. (2011). Modulation of aberrant CDK5 signaling rescues impaired neurogenesis in models of Alzheimer's disease. Cell Death Dis. 2, e120. doi: 10.1038/cddis.2011.2
Crowe, E. P., Tuzer, F., Gregory, B. D., Donahue, G., Gosai, S. J., Cohen, J., et al. (2016). Changes in the transcriptome of human astrocytes accompanying oxidative stress-induced senescence. Front. Aging Neurosci. 8:208. doi: 10.3389/fnagi.2016.00208
Cummings, J., Lee, G., Ritter, A., and Zhong, K. (2018). Alzheimer's disease drug development pipeline: 2018. Alzheimers Dement. 4, 195–214. doi: 10.1016/j.trci.2018.03.009
D'Angelo, B., Astarita, C., Boffo, S., Massaro-Giordano, M., Antonella Ianuzzi, C., Caporaso, A., et al. (2017). LPS-induced inflammatory response triggers cell cycle reactivation in murine neuronal cells through retinoblastoma proteins induction. Cell Cycle 16, 2330–2336. doi: 10.1080/15384101.2017.1363943
Dapito, D. H., Mencin, A., Gwak, G. Y., Pradere, J. P., Jang, M. K., Mederacke, I., et al. (2012). Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 21, 504–516. doi: 10.1016/j.ccr.2012.02.007
Datta, M., Staszewski, O., Raschi, E., Frosch, M., Hagemeyer, N., Tay, T. L., et al. (2018). Histone deacetylases 1 and 2 regulate microglia function during development, homeostasis, and neurodegeneration in a context-dependent manner. Immunity 48, 514–529.e6. doi: 10.1016/j.immuni.2018.02.016
Davies, D. S., Ma, J., Jegathees, T., and Goldsbury, C. (2017). Microglia show altered morphology and reduced arborization in human brain during aging and Alzheimer's disease. 253. Brain Pathol. 27, 795–808. doi: 10.1111/bpa.12456
De Chiara, G., Marcocci, M. E., Sgarbanti, R., Civitelli, L., Ripoli, C., Piacentini, R., et al. (2012). Infectious agents and neurodegeneration. Mol. Neurobiol. 46, 614–638. doi: 10.1007/s12035-012-8320-7
De Lorenzi, E., Chiari, M., Colombo, R., Cretich, M., Sola, L., Vanna, R., et al. (2017). Evidence that the human innate immune peptide LL-37 may be a binding partner of amyloid-β and inhibitor of fibril assembly. J. Alzheimers Dis. 59, 1213–1226. doi: 10.3233/JAD-170223
Deleidi, M., Jäggle, M., and Rubino, G. (2015). Immune aging, dysmetabolism, and inflammation in neurological diseases. Front. Neurosci. 9:172. doi: 10.3389/fnins.2015.00172
Demetrius, L. A., Magistretti, P. J., and Pellerin, L. (2015). Alzheimer's disease: the amyloid hypothesis and the Inverse Warburg effect. Front. Physiol. 5:522. doi: 10.3389/fphys.2014.00522
Deshpande, K., Jain, A., Sharma, R., Prashar, S., and Jain, R. (2010). Diabetes and periodontitis. J. Indian Soc. Periodontol. 14, 207–212. doi: 10.4103/0972-124X.76917
Dhawan, S., De Palma, G., Willemze, R. A., Hilbers, F. W., Verseijden, C., Luyer, M. D., et al. (2016). Acetylcholine-producing T cells in the intestine regulate antimicrobial peptide expression and microbial diversity. Am. J. Physiol. Gastrointest. Liver Physiol. 311, G920–G933. doi: 10.1152/ajpgi.00114.2016
d'Hennezel, E., Abubucker, S., Murphy, L. O., and Cullen, T. W. (2017). Total lipopolysaccharide from the human gut microbiome silences toll-like receptor signaling. mSystems 2:e00046–e00017. doi: 10.1128/mSystems.00046-17
Díaz-García, C. M., Mongeon, R., Lahmann, C., Koveal, D., Zucker, H., and Yellen, G. (2017). Neuronal stimulation triggers neuronal glycolysis and not lactate uptake. Cell Metab. 26, 361–374.e4. doi: 10.1016/j.cmet.2017.06.021
Dienel, G. A., and Cruz, N. F. (2016). Aerobic glycolysis during brain activation: adrenergic regulation and influence of norepinephrine on astrocytic metabolism. J. Neurochem. 138, 14–52. doi: 10.1111/jnc.13630
Dinakaran, V., Rathinavel, A., Pushpanathan, M., Sivakumar, R., Gunasekaran, P., and Rajendhran, J. (2014). Elevated levels of circulating DNA in cardiovascular disease patients: metagenomic profiling of microbiome in the circulation. PLoS ONE 9:e105221. doi: 10.1371/journal.pone.0105221
Diniz, B. S., Reynolds, C. F., Sibille, E., Lin, C. W., Tseng, G., Lotrich, F., et al. (2016). Enhanced molecular aging in late-life depression: the senescent-associated secretory phenotype. Am. J. Geriatr. Psychiatry 25, 64–72. doi: 10.1016/j.jagp.2016.08.018
Dominy, S. S., Lynch, C., Ermini, F., Benedyk, M., Marczyk, A., Konradi, A., et al. (2019). Porphyromonas gingivalis in Alzheimer's disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv 5:eaau3333. doi: 10.1126/sciadv.aau3333
Dong, W., Xiao, S., Cheng, M., Ye, X., and Zheng, G. (2015). Minocycline induces protective autophagy in vascular endothelial cells exposed to an in vitro model of ischemia/reperfusion-induced injury. Biomed. Rep. 4, 173–177. doi: 10.3892/br.2015.554
Dowd, J. B., Bosch, J. A., Steptoe, A., Jayabalasingham, B., Lin, J., Yolken, R., et al. (2017). Persistent herpesvirus infections and telomere attrition over 3 years in the Whitehall II cohort. J. Infect. Dis. 216, 565–572. doi: 10.1093/infdis/jix255
Drago-Serrano, M. E., de la Garza-Amaya, M., Luna, J. S., and Campos-Rodríguez, R. (2012). Lactoferri–lipopolysaccharide (LPS) binding as key to antibacterial and antiendotoxic effects. Int. Immunopharmacol. 12, 1–9. doi: 10.1016/j.intimp.2011.11.002
Drozdowski, L., and Thomson, A. B. (2006). Aging and the intestine. World J. Gastroenterol. 12, 7578–7584. doi: 10.3748/wjg.v12.i47.7578
Du, R. H., Tan, J., Sun, X. Y., Lu, M., Ding, J. H., and Hu, G. (2016). Fluoxetine inhibits NLRP3 inflammasome activation: implication in depression. Int. J. Neuropsychopharmacol. 19:pyw037. doi: 10.1093/ijnp/pyw037
Duraj-Thatte, A. M., Praveschotinunt, P., Nash, T. R., Ward, F. R., and Joshi, N. S. (2018). Modulating bacterial and gut mucosal interactions with engineered biofilm matrix proteins. Sci. Rep. 8:3475. doi: 10.1038/s41598-018-21834-8
Edison, P., Archer, H. A., Hinz, R., Hammers, A., Pavese, N., Tai, Y. F., et al. (2007). Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology 68, 501–508. doi: 10.1212/01.wnl.0000244749.20056.d4
Eimer, W. A., Vijaya Kumar, D. K., Navalpur Shanmugam, N. K., et al. (2018). Alzheimer's disease-associated β-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron 99, 56–63.e3. doi: 10.1016/j.neuron.2018.06.030
Elkind, M. S., Ramakrishnan, P., Moon, Y. P., Boden-Albala, B., Liu, K. M., Spitalnik, S. L., et al. (2009). Infectious burden and risk of stroke: the Northern Manhattan Study. Arch. Neurol. 67, 33–38. doi: 10.1001/archneurol.2009.271
Engmann, O., and Giese, K. P. (2009). Crosstalk between Cdk5 and GSK3beta: implications for Alzheimer's disease. Front. Mol. Neurosci. 2:2. doi: 10.3389/neuro.02.002.2009
Erdei, J., Tóth, A., Balogh, E., Nyakundi, B. B., Bányai, E., Ryffel, B., et al. (2018). Induction of NLRP3 inflammasome activation by heme in human endothelial cells. Oxid. Med. Cell. Longev. 2018:4310816. doi: 10.1155/2018/4310816
Errea, A., Cayet, D., Marchetti, P., Tang, C., Kluza, J., Offermanns, S., et al. (2016). Lactate inhibits the pro-inflammatory response and metabolic reprogramming in murine macrophages in a GPR81-independent manner. PLoS ONE 11:e0163694. doi: 10.1371/journal.pone.0163694
Escoll, P., and Buchrieser, C. (2018). Metabolic reprogramming of host cells upon bacterial infection: why shift to a Warburg-like metabolism? FEBS J. 285, 2146–2160. doi: 10.1111/febs.14446
Fali, T., Fabre-Mersseman, V., Yamamoto, T., et al. (2018). Elderly human hematopoietic progenitor cells express cellular senescence markers and are more susceptible to pyroptosis[published online ahead of print, 2018 Jul 12]. JCI Insight 3:e95319. doi: 10.1172/jci.insight.95319
Falsone, A., and Falsone, S. F. (2015). Legal but lethal: functional protein aggregation at the verge of toxicity. Front. Cell. Neurosci. 9:45. doi: 10.3389/fncel.2015.00045
Fan, Y., Du, L., Fu, Q., Zhou, Z., Zhang, J., Li, G., et al. (2018). Inhibiting the NLRP3 inflammasome with MCC950 ameliorates isoflurane-induced pyroptosis and cognitive impairment in aged mice. Front. Cell. Neurosci. 12:426. doi: 10.3389/fncel.2018.00426
Fang, E. F., Kassahun, H., Croteau, D. L., Scheibye-Knudsen, M., Marosi, K., Lu, H., et al. (2016). NAD+ replenishment improves lifespan and healthspan in ataxia telangiectasia models via mitophagy and DNA repair. Cell Metab. 24, 566–581. doi: 10.1016/j.cmet.2016.09.004
Farina, C., Aloisi, F., and Meinl, E. (2007). Astrocytes are active players in cerebral innate immunity. Trends Immunol. 28, 138–145. doi: 10.1016/j.it.2007.01.005
Feng, Y., Wang, Y., Wang, P., Huang, Y., and Wang, F. (2018). Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cell. Physiol. Biochem. 49, 190–205. doi: 10.1159/000492853
Figueiredo, R. T., Fernandez, P. L., Mourao-Sa, D. S., Porto, B. N., Dutra, F. F., Alves, L. S., et al. (2007). Characterization of heme as activator of Toll-like receptor 4. J. Biol. Chem. 282, 282–289. doi: 10.1074/jbc.M610737200
Filosa, J. A., Morrison, H. W., Iddings, J. A., Du, W., and Kim, K. J. (2015). Beyond neurovascular coupling, role of astrocytes in the regulation of vascular tone. Neuroscience 323, 96–109. doi: 10.1016/j.neuroscience.2015.03.064
Fine, J. M., Forsberg, A. C., Stroebel, B. M., Faltesek, K. A., Verden, D. R., Hamel, K. A., et al. (2017). Intranasal deferoxamine affects memory loss, oxidation, and the insulin pathway in the streptozotocin rat model of Alzheimer's disease. J. Neurol. Sci. 380, 164–171. doi: 10.1016/j.jns.2017.07.028
Flanary, B. E., Sammons, N. W., Nguyen, C., Walker, D., and Streit, W. J. (2007). Evidence that aging and amyloid promote microglial cell senescence. Rejuvenation Res. 10, 61–74. doi: 10.1089/rej.2006.9096
Fleetwood, A. J., Lee, M. K. S., Singleton, W., Achuthan, A., Lee, M. C., O'Brien-Simpson, N. M., et al. (2017). Metabolic remodeling, inflammasome activation, and pyroptosis in macrophages stimulated by Porphyromonas gingivalis and its outer membrane vesicles. Front. Cell. Infect. Microbiol. 7:351. doi: 10.3389/fcimb.2017.00351
Flores, J., Noël, A., Foveau, B., Lynham, J., Lecrux, C., and LeBlanc, A. C. (2018). Caspase-1 inhibition alleviates cognitive impairment and neuropathology in an Alzheimer's disease mouse model. Nat. Commun. 9, 3916. doi: 10.1038/s41467-018-06449-x
Fong, S., Teo, E., Ng, L. F., Chen, C. B., Lakshmanan, L. N., Tsoi, S. Y., et al. (2016). Energy crisis precedes global metabolic failure in a novel Caenorhabditis elegans Alzheimer disease model. Sci. Rep. 6:33781. doi: 10.1038/srep33781
Forloni, G., and Balducci, C. (2018). Alzheimer's disease, oligomers, and inflammation. J. Alzheimers Dis. 62, 1261–1276. doi: 10.3233/JAD-170819
Frade, J. M., and López-Sánchez, N. (2017). Neuronal tetraploidy in Alzheimer and aging. Aging 9, 2014–2015. doi: 10.18632/aging.101312
Freeman, L. C., and Ting, J. P. (2016). The pathogenic role of the inflammasome in neurodegenerative diseases. J. Neurochem. 136(Suppl. 1), 29–38. doi: 10.1111/jnc.13217
Friedland, R. P., and Chapman, M. R. (2017). The role of microbial amyloid in neurodegeneration. PLoS Pathog. 13:e1006654. doi: 10.1371/journal.ppat.1006654
Frost, G. R., and Li, Y. M. (2017). The role of astrocytes in amyloid production and Alzheimer's disease. Open Biol. 7:170228. doi: 10.1098/rsob.170228
Fülöp, T., Itzhaki, R. F., Balin, B. J., Miklossy, J., and Barron, A. E. (2018a). Role of microbes in the development of Alzheimer's disease: state of the art—an international symposium presented at the 2017 IAGG Congress in San Francisco. Front. Genet. 9:362. doi: 10.3389/fgene.2018.00362
Fulop, T., Witkowski, J. M., Bourgade, K., et al. (2018b). Can an infection hypothesis explain the beta amyloid hypothesis of Alzheimer's disease? Front. Aging Neurosci. 10:224. doi: 10.3389/fnagi.2018.00224
Gallage, S., and Gil, J. (2016). Mitochondrial dysfunction meets senescence. Trends Biochem. Sci. 41, 207–209. doi: 10.1016/j.tibs.2016.01.005
Gao, X., Chen, W., Li, J., Shen, C., Zhou, P., Che, X., et al. (2018). The protective effect of alpha-lipoic acid against brain ischemia and reperfusion injury via mTOR signaling pathway in rats. Neurosci. Lett. 671, 108–113. doi: 10.1016/j.neulet.2018.02.012
Ghosh, S., Wu, M. D., Shaftel, S. S., Kyrkanides, S., LaFerla, F. M., Olschowka, J. A., et al. (2013). Sustained interleukin-1β overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer's mouse model. J. Neurosci. 33, 5053–5064. doi: 10.1523/JNEUROSCI.4361-12.2013
Golegaonkar, S., Tabrez, S. S., Pandit, A., Sethurathinam, S., Jagadeeshaprasad, M. G., Bansode, S., et al. (2015). Rifampicin reduces advanced glycation end products and activates DAF-16 to increase lifespan in Caenorhabditis elegans. Aging Cell 14, 463–473. doi: 10.1111/acel.12327
Gosztyla, M. L., Brothers, H. M., and Robinson, S. R. (2018). Alzheimer's amyloid-β is an antimicrobial peptide: a review of the evidence. J. Alzheimers Dis. 62, 1495–1506. doi: 10.3233/JAD-171133
Goyal, M. S., Vlassenko, A. G., Blazey, T. M., Su, Y., Couture, L. E., Durbin, T. J., et al. (2017). Loss of brain aerobic glycolysis in normal human aging. Cell Metab. 26, 353–360.e3. doi: 10.1016/j.cmet.2017.07.010
Greiner, T. U., and Bäckhed, F. (2016). Microbial regulation of GLP-1 and L-cell biology. Mol. Metab. 5, 753–758. doi: 10.1016/j.molmet.2016.05.012
Griffin, W. S., Liu, L., Li, Y., Mrak, R. E., and Barger, S. W. (2006). Interleukin-1 mediates Alzheimer and Lewy body pathologies. J. Neuroinflammation 3:5. doi: 10.1186/1742-2094-3-5
Groeger, S., Jarzina, F., Mamat, U., and Meyle, J. (2017). Induction of B7-H1 receptor by bacterial cells fractions of Porphyromonas gingivalis on human oral epithelial cells: B7-H1 induction by Porphyromonas gingivalis fractions. Immunobiology 222, 137–147. doi: 10.1016/j.imbio.2016.10.011
Guerreiro, R., Wojtas, A., Bras, J., Carrasquillo, M., Rogaeva, E., Majounie, E., et al. (2012). TREM2 variants in Alzheimer's disease. N. Engl. J. Med. 368, 117–127. doi: 10.1056/NEJMoa1211851
Gui, J., Mustachio, L. M., Su, D. M., and Craig, R. W. (2012). Thymus size and age-related thymic involution: early programming, sexual dimorphism, progenitors and stroma. Aging Dis. 3, 280–290.
Guo, C., Wang, P., Zhong, M. L., Wang, T., Huang, X. S., Li, J. Y., et al. (2013). Deferoxamine inhibits iron induced hippocampal tau phosphorylation in the Alzheimer transgenic mouse brain. Neurochem. Int. 62, 165–172. doi: 10.1016/j.neuint.2012.12.005
Gurung, P., Lukens, J. R., and Kanneganti, T. D. (2014). Mitochondria: diversity in the regulation of the NLRP3 inflammasome. Trends Mol. Med. 21, 193–201. doi: 10.1016/j.molmed.2014.11.008
Hajishengallis, G., Wang, M., Liang, S., Shakhatreh, M. A., James, D., Nishiyama, S., et al. (2008). Subversion of innate immunity by periodontopathic bacteria via exploitation of complement receptor-3. Adv. Exp. Med. Biol. 632, 203–219. doi: 10.1007/978-0-387-78952-1_15
Hamann, L., Alexander, C., Stamme, C., Zähringer, U., and Schumann, R. R. (2005). Acute-phase concentrations of lipopolysaccharide (LPS)-binding protein inhibit innate immune cell activation by different LPS chemotypes via different mechanisms. Infect. Immun. 73, 193–200. doi: 10.1128/IAI.73.1.193-200.2005
Hamdane, M., Bretteville, A., Sambo, A. V., Schindowski, K., Bégard, S., Delacourte, A., et al. (2005). p25/Cdk5-mediated retinoblastoma phosphorylation is an early event in neuronal cell death. J. Cell. Sci. 118 (Pt 6), 1291–1298. doi: 10.1242/jcs.01724
Hardy, J., and Allsop, D. (1991). Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol. Sci. 12, 383–388. doi: 10.1016/0165-6147(91)90609-V
Hardy, J. A., and Higgins, G. A. (1992). Alzheimer's disease: the amyloid cascade hypothesis. Science 256, 184–185. doi: 10.1126/science.1566067
Hayashi, T., Yano, K., Matsui-Hirai, H., Yokoo, H., Hattori, Y., and Iguchi, A. (2008). Nitric oxide and endothelial cellular senescence. Pharmacol. Ther. 120, 333–339. doi: 10.1016/j.pharmthera.2008.09.002
Hayflick, L., and Moorhead, P. S. (1961). The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621. doi: 10.1016/0014-4827(61)90192-6
Heneka, M. T., Kummer, M. P., Stutz, A., Delekate, A., Schwartz, S., Vieira-Saecker, A., et al. (2013). NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature 493, 674–678. doi: 10.1038/nature11729
Hering, N. A., Luettig, J., Krug, S. M., Wiegand, S., Gross, G., van Tol, E. A., et al. (2017). Lactoferrin protects against intestinal inflammation and bacteria-induced barrier dysfunction in vitro. Ann. N. Y. Acad. Sci. 1405, 177–188. doi: 10.1111/nyas.13405
Hesse, M., Welz, A., and Fleischmann, B. K. (2017). Heart regeneration and the cardiomyocyte cell cycle. Pflugers Arch. 470, 241–248. doi: 10.1007/s00424-017-2061-4
Higashi, T., Nishii, R., Kagawa, S., Kishibe, Y., Takahashi, M., Okina, T., et al. (2018). 18F-FPYBF-2, a new F-18-labelled amyloid imaging PET tracer: first experience in 61 volunteers and 55 patients with dementia. Ann. Nucl. Med. 32, 206–216. doi: 10.1007/s12149-018-1236-1
Hill, J. M., Clement, C., Pogue, A. I., Bhattacharjee, S., Zhao, Y., and Lukiw, W. J. (2014). Pathogenic microbes, the microbiome, and Alzheimer's disease (AD). Front. Aging Neurosci. 6:127. doi: 10.3389/fnagi.2014.00127
Hogestyn, J. M., Mock, D. J., and Mayer-Proschel, M. (2018). Contributions of neurotropic human herpesviruses herpes simplex virus 1 and human herpesvirus 6 to neurodegenerative disease pathology. Neural Regen. Res. 13, 211–221. doi: 10.4103/1673-5374.226380
Höhn, A., Jung, T., Grimm, S., and Grune, T. (2010). Lipofuscin-bound iron is a major intracellular source of oxidants: role in senescent cells. Free Radic. Biol. Med. 48, 1100–1108. doi: 10.1016/j.freeradbiomed.2010.01.030
Hopp, S. C., Lin, Y., Oakley, D., Roe, A. D., DeVos, S. L., Hanlon, D., et al. (2018). The role of microglia in processing and spreading of bioactive tau seeds in Alzheimer's disease. J. Neuroinflammation 15:269. doi: 10.1186/s12974-018-1309-z
Hoque, R., Farooq, A., Ghani, A., Gorelick, F., and Mehal, W. Z. (2014). Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology 146, 1763–1774. doi: 10.1053/j.gastro.2014.03.014
Hor, J. H., Soh, E. S., Tan, L. Y., Lim, V. J. W., Santosa, M. M., Winanto, et al. (2018). Cell cycle inhibitors protect motor neurons in an organoid model of spinal muscular atrophy. Cell Death Dis. 9:1100. doi: 10.1038/s41419-018-1081-0
Horowitz, M. P., and Greenamyre, J. T. (2010). Mitochondrial iron metabolism and its role in neurodegeneration. J. Alzheimers Dis. 20(Suppl. 2) S551–S568. doi: 10.3233/JAD-2010-100354
Hoskin, D. W., and Ramamoorthy, A. (2007). Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta 1778, 357–375. doi: 10.1016/j.bbamem.2007.11.008
Hou, J., Cui, C., Kim, S., Sung, C., and Choi, C. (2018). Ginsenoside F1 suppresses astrocytic senescence-associated secretory phenotype. Chem. Biol. Interact. 283, 75–83. doi: 10.1016/j.cbi.2018.02.002
Hou, X., Yang, S., and Yin, J. (2019). Blocking the REDD1/TXNIP axis ameliorates LPS-induced vascular endothelial cell injury through repressing oxidative stress and apoptosis. Am. J. Physiol. Cell Physiol. 316, C104–C110. doi: 10.1152/ajpcell.00313.2018
Hoyt, D. G., Mannix, R. J., Gerritsen, M. E., Watkins, S. C., Lazo, J. S., and Pitt, B. R. (1996). Integrins inhibit LPS-induced DNA strand breakage in cultured lung endothelial cells. Am. J. Physiol. 270 (4 Pt 1), L689–L694. doi: 10.1152/ajplung.1996.270.4.L689
Hradek, A. C., Lee, H. P., Siedlak, S. L., Torres, S. L., Jung, W., Han, A. H., et al. (2015). Distinct chronology of neuronal cell cycle re-entry and tau pathology in the 3xTg-AD mouse model and Alzheimer's disease patients. J. Alzheimers Dis. 43, 57–65. doi: 10.3233/JAD-141083
Hubackova, S., Davidova, E., Rohlenova, K., Stursa, J., Werner, L., Andera, L., et al. (2019). Selective elimination of senescent cells by mitochondrial targeting is regulated by ANT2. Cell Death Differ. 26, 276–290. doi: 10.1038/s41418-018-0118-3
Huber, C. M., Yee, C., May, T., Dhanala, A., and Mitchell, C. S. (2017). Cognitive decline in preclinical Alzheimer's disease: amyloid-beta versus tauopathy. J. Alzheimers Dis. 61, 265–281. doi: 10.3233/JAD-170490
Hurtado-Alvarado, G., Cabañas-Morales, A. M., and Gómez-Gónzalez, B. (2014). Pericytes: brain–immune interface modulators. Front. Integr. Neurosci. 7:80. doi: 10.3389/fnint.2013.00080
Hwang, I., Lee, E., Jeon, S. A., and Yu, J. W. (2015). Histone deacetylase 6 negatively regulates NLRP3 inflammasome activation. Biochem. Biophys. Res. Commun. 467, 973–978. doi: 10.1016/j.bbrc.2015.10.033
Hwang, J. S., Im, C. R., and Im, S. H. (2012). Immune disorders and its correlation with gut microbiome. Immune Netw. 12, 129–138. doi: 10.4110/in.2012.12.4.129
Ingelsson, M., Fukumoto, H., Newell, K. L., Growdon, J. H., Hedley-Whyte, E. T., Frosch, M. P., et al. (2004). Early Abeta accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology 62, 925–931. doi: 10.1212/01.WNL.0000115115.98960.37
Inoue, H., Hanawa, N., Katsumata-Tsuboi, R., Katsumata, S. I., Takahashi, N., and Uehara, M. (2018). Down-regulation of senescence marker protein 30 by iron-specific chelator deferoxamine drives cell senescence. Biosci. Biotechnol. Biochem. 82, 900–903. doi: 10.1080/09168451.2018.1440190
Ip, W. K. E., Hoshi, N., Shouval, D. S., Snapper, S., and Medzhitov, R. (2017). Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 356, 513–519. doi: 10.1126/science.aal3535
Itzhaki, R. F., and Lathe, R. (2018). Herpes viruses and senile dementia: first population evidence for a causal link. J. Alzheimers Dis. 64, 363–366. doi: 10.3233/JAD-180266
Itzhaki, R. F., Lathe, R., Balin, B. J., Ball, M. J., Bearer, E. L., Braak, H., et al. (2016). Microbes and Alzheimer's disease. J. Alzheimers Dis. 51, 979–984. doi: 10.3233/JAD-160152
Jain, N., and Seed, B. (2016). Intestinal microbiota influence postnatal thymic T cell development. J. Immunol. 196 (1 Suppl.), 67.6.
Jandhyala, S. M., Talukdar, R., Subramanyam, C., Vuyyuru, H., Sasikala, M., and Nageshwar Reddy, D. (2015). Role of the normal gut microbiota. World J. Gastroenterol. 21, 8787–8803. doi: 10.3748/wjg.v21.i29.8787
Jenssen, H., and Hancock, R. E. (2009). Antimicrobial properties of lactoferrin. Biochimie 91, 19–29. doi: 10.1016/j.biochi.2008.05.015
Jeyapalan, J. C., and Sedivy, J. M. (2008). Cellular senescence and organismal aging. Mech. Ageing Dev. 129, 467–474. doi: 10.1016/j.mad.2008.04.001
Ji, Y., Luo, X., Yang, Y., Dai, Z., Wu, G., and Wu, Z. (2018). Endoplasmic reticulum stress-induced apoptosis in intestinal epithelial cells: a feed-back regulation by mechanistic target of rapamycin complex 1 (mTORC1). J. Anim. Sci. Biotechnol. 9:38. doi: 10.1186/s40104-018-0253-1
Joachim, C. L., Mori, H., and Selkoe, D. J. (1989). Amyloid beta-protein deposition in tissues other than brain in Alzheimer's disease. Nature 341, 226–230. doi: 10.1038/341226a0
Jones, W., and Bianchi, K. (2015). Aerobic glycolysis: beyond proliferation. Front. Immunol. 6:227. doi: 10.3389/fimmu.2015.00227
Jordà, E. G., Verdaguer, E., Canudas, A. M., Jiménez, A., Garcia de Arriba, S., Allgaier, C., et al. (2005). Implication of cyclin-dependent kinase 5 in the neuroprotective properties of lithium. Neuroscience 134, 1001–1011. doi: 10.1016/j.neuroscience.2005.04.061
Jung, S. H., Lee, H. C., Yu, D. M., Kim, B. C., Park, S. M., Lee, Y. S., et al. (2015). Heparan sulfation is essential for the prevention of cellular senescence. Cell Death Differ. 23, 417–429. doi: 10.1038/cdd.2015.107
Jung, Y. J., and Chung, W. S. (2018). Phagocytic roles of glial cells in healthy and diseased brains. Biomol. Ther. 26, 350–357. doi: 10.4062/biomolther.2017.133
Jurk, D., Wang, C., Miwa, S., Maddick, M., Korolchuk, V., Tsolou, A., et al. (2012). Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell 11, 996–1004. doi: 10.1111/j.1474-9726.2012.00870.x
Kalghatgi, S., Spina, C. S., Costello, J. C., Liesa, M., Morones-Ramirez, J. R., Slomovic, S., et al. (2013). Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in mammalian cells. Sci. Transl. Med. 5:192ra85. doi: 10.1126/scitranslmed.3006055
Kametani, F., and Hasegawa, M. (2018). Reconsideration of amyloid hypothesis and tau hypothesis in Alzheimer's disease. Front. Neurosci. 12:25. doi: 10.3389/fnins.2018.00025
Kasotakis, G., Galvan, M., King, E., Sarkar, B., Stucchi, A., Mizgerd, J. P., et al. (2017). Valproic acid mitigates the inflammatory response and prevents acute respiratory distress syndrome in a murine model of Escherichia coli pneumonia at the expense of bacterial clearance. J. Trauma Acute Care Surg. 82, 758–765. doi: 10.1097/TA.0000000000001389
Kato, H., Fujihashi, K., Kato, R., Dohi, T., Fujihashi, K., Hagiwara, Y., et al. (2003). Lack of oral tolerance in aging is due to sequential loss of Peyer's patch cell interactions. Int. Immunol. 15, 145–158. doi: 10.1093/intimm/dxg011
Kato, T., Yamazaki, K., Nakajima, M., Date, Y., Kikuchi, J., Hase, K., et al. (2018). Oral administration of Porphyromonas gingivalis alters the gut microbiome and serum metabolome. mSphere 3:e00460–e00418. doi: 10.1128/mSphere.00460-18
Kayama, H., and Takeda, K. (2014). Polysaccharide A of Bacteroides fragilis: actions on dendritic cells and T cells. Mol. Cell 54, 206–207. doi: 10.1016/j.molcel.2014.04.002
Ke, P., Shao, B. Z., Xu, Z. Q., Chen, X. W., Wei, W., and Liu, C. (2017). Activating α7 nicotinic acetylcholine receptor inhibits NLRP3 inflammasome through regulation of β-arrestin-1. CNS Neurosci. Ther. 23, 875–884. doi: 10.1111/cns.12758
Ke, Y., Li, D., Zhao, M., Liu, C., Liu, J., Zeng, A., et al. (2018). Gut flora-dependent metabolite trimethylamine-N-oxide accelerates endothelial cell senescence and vascular aging through oxidative stress. Free Radic. Biol. Med. 116, 88–100. doi: 10.1016/j.freeradbiomed.2018.01.007
Kelleher, R. J., and Soiza, R. L. (2013). Evidence of endothelial dysfunction in the development of Alzheimer's disease: is Alzheimer's a vascular disorder? Am. J. Cardiovasc. Dis. 3, 197–226.
Kelsall, B. L., and Leon, F. (2005). Involvement of intestinal dendritic cells in oral tolerance, immunity to pathogens, and inflammatory bowel disease. Immunol. Rev. 206, 132–148. doi: 10.1111/j.0105-2896.2005.00292.x
Kerr, J. S., Adriaanse, B. A., Greig, N. H., Mattson, M. P., Cader, M. Z., Bohr, V. A., et al. (2017). Mitophagy and Alzheimer's disease: cellular and molecular mechanisms. Trends Neurosci. 40, 151–166. doi: 10.1016/j.tins.2017.01.002
Key, N. S., Vercellotti, G. M., Winkelmann, J. C., Moldow, C. F., Goodman, J. L., Esmon, N. L., et al. (1990). Infection of vascular endothelial cells with herpes simplex virus enhances tissue factor activity and reduces thrombomodulin expression. Proc. Natl. Acad. Sci. U.S.A. 87, 7095–7099. doi: 10.1073/pnas.87.18.7095
Killilea, D. W., Atamna, H., Liao, C., and Ames, B. N. (2003). Iron accumulation during cellular senescence in human fibroblasts in vitro. Antioxid. Redox Signal. 5, 507–516. doi: 10.1089/152308603770310158
Kim, D. S., Choi, H. I., Wang, Y., Luo, Y., Hoffer, B. J., and Greig, N. H. (2017). A new treatment strategy for Parkinson's disease through the gut–brain axis: the glucagon-like peptide-1 receptor pathway. Cell Transplant. 26, 1560–1571. doi: 10.1177/0963689717721234
Kim, E. C., and Kim, J. R. (2019). Senotherapeutics: emerging strategy for healthy aging and age-related disease. BMB Rep. 52, 47–55. doi: 10.5483/BMBRep.2019.52.1.293
Kim, J., Chakrabarty, P., Hanna, A., March, A., Dickson, D. W., Borchelt, D. R., et al. (2013). Normal cognition in transgenic BRI2-Aβ mice. Mol. Neurodegener. 8:15. doi: 10.1186/1750-1326-8-15
Kim, K. S. (2008). Mechanisms of microbial traversal of the blood–brain barrier. Nat. Rev. Microbiol. 6, 625–634. doi: 10.1038/nrmicro1952
Kim, M. J., Yoon, J. H., and Ryu, J. H. (2016). Mitophagy: a balance regulator of NLRP3 inflammasome activation. BMB Rep. 49, 529–535. doi: 10.5483/BMBRep.2016.49.10.115
King, R., and Tuthill, C. (2016). Immune modulation with thymosin alpha 1 treatment. Vitam. Horm. 102, 151–178. doi: 10.1016/bs.vh.2016.04.003
Kirkland, J. L., Tchkonia, T., Zhu, Y., Niedernhofer, L. J., and Robbins, P. D. (2017). The clinical potential of senolytic drugs. J. Am. Geriatr. Soc. 65, 2297–2301. doi: 10.1111/jgs.14969
Kobayashi, A., Donaldson, D. S., Erridge, C., Kanaya, T., Williams, I. R., Ohno, H., et al. (2013). The functional maturation of M cells is dramatically reduced in the Peyer's patches of aged mice. Mucosal Immunol. 6, 1027–1037. doi: 10.1038/mi.2012.141
Koellhoffer, E. C., McCullough, L. D., and Ritzel, R. M. (2017). Old maids: aging and its impact on microglia function. Int. J. Mol. Sci. 18:769. doi: 10.3390/ijms18040769
Koga, T., McGhee, J. R., Kato, H., Kato, R., Kiyono, H., and Fujihashi, K. (2000). Evidence for early aging in the mucosal immune system. J. Immunol. 165, 5352–5359. doi: 10.4049/jimmunol.165.9.5352
Komatsu, T., Nagano, K., Sugiura, S., Hagiwara, M., Tanigawa, N., Abiko, Y., et al. (2012). E-selectin mediates Porphyromonas gingivalis adherence to human endothelial cells. Infect. Immun. 80, 2570–2576. doi: 10.1128/IAI.06098-11
Kowalski, K., and Mulak, A. (2019). Brain–gut–microbiota axis in Alzheimer's disease. J. Neurogastroenterol. Motil. 25, 48–60. doi: 10.5056/jnm18087
Kriesel, J. D., Bhetariya, P. J., Chan, B. K., Wilson, T., and Fischer, K. F. (2017). Enrichment of retroviral sequences in brain tissue from patients with severe demyelinating diseases. J. Emerg. Dis. Virol. 3. doi: 10.16966/2473-1846.132
Krishnan, S., Fernandez, G. E., Sacks, D. B., and Prasadarao, N. V. (2012). IQGAP1 mediates the disruption of adherens junctions to promote Escherichia coli K1 invasion of brain endothelial cells. Cell. Microbiol. 14, 1415–1433. doi: 10.1111/j.1462-5822.2012.01805.x
Kritsilis, M. V., Rizou, S., Koutsoudaki, P. N., Evangelou, K., Gorgoulis, V. G., and Papadopoulos, D. (2018). Ageing, cellular senescence and neurodegenerative disease. Int. J. Mol. Sci. 19:2937. doi: 10.3390/ijms19102937
Kruman, I. I., Wersto, R. P., Cardozo-Pelaez, F., Smilenov, L., Chan, S. L., Chrest, F. J., et al. (2004). Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron 41, 549–561. doi: 10.1016/S0896-6273(04)00017-0
Kruzel, M. L., Zimecki, M., and Actor, J. K. (2017). Lactoferrin in a context of inflammation-induced pathology. Front. Immunol. 8:1438. doi: 10.3389/fimmu.2017.01438
Krylov, A. V., Kisseleva, E. P., Aleshina, G. M., Shamova, O. V., and Kokryakov, V. N. (2007). Effects of defensin and lactoferrin on functional activity of endothelial cells in vitro. Bull. Exp. Biol. Med. 144, 331–334. doi: 10.1007/s10517-007-0325-2
Kumar, D. K., Choi, S. H., Washicosky, K. J., Eimer, W. A., Tucker, S., Ghofrani, J., et al. (2016). Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease. Sci. Transl. Med. 8:340ra72. doi: 10.1126/scitranslmed.aaf1059
Lana, D., Ugolini, F., Nosi, D., Wenk, G. L., and Giovannini, M. G. (2017). Alterations in the Interplay between neurons, astrocytes and microglia in the rat dentate gyrus in experimental models of neurodegeneration. Front. Aging Neurosci. 9:296. doi: 10.3389/fnagi.2017.00296
Lane, D. J., Bae, D. H., Merlot, A. M., Sahni, S., and Richardson, D. R. (2015). Duodenal cytochrome b (DCYTB) in iron metabolism: an update on function and regulation. Nutrients 7, 2274–2296. doi: 10.3390/nu7042274
Lang, Y., Chu, F., Shen, D., Zhang, W., Zheng, C., Zhu, J., et al. (2018). Role of inflammasomes in neuroimmune and neurodegenerative diseases: a systematic review. Mediators Inflamm. 2018:1549549. doi: 10.1155/2018/1549549
Lappas, M. (2012). Anti-inflammatory properties of sirtuin 6 in human umbilical vein endothelial cells. Mediators Inflamm. 2012:597514. doi: 10.1155/2012/597514
Lathrop, S. K., Bloom, S. M., Rao, S. M., Nutsch, K., Lio, C. W., Santacruz, N., et al. (2011). Peripheral education of the immune system by colonic commensal microbiota. Nature 478, 250–254. doi: 10.1038/nature10434
Lebrun, L. J., Lenaerts, K., Kiers, D., Pais de Barros, J. P., Le Guern, N., Plesnik, J., et al. (2017). Enteroendocrine L cells sense LPS after gut barrier injury to enhance GLP-1 secretion. Cell Rep. 21, 1160–1168. doi: 10.1016/j.celrep.2017.10.008
Lee, G. J., Lim, J. J., and Hyun, S. (2017). Minocycline treatment increases resistance to oxidative stress and extends lifespan in Drosophila via FOXO. Oncotarget 8, 87878–87890. doi: 10.18632/oncotarget.21224
Lee, M. H., Siddoway, B., Kaeser, G. E., Segota, I., Rivera, R., and Romanow, W. J. (2018). Somatic APP gene recombination in Alzheimer's disease and normal neurons. Nature 563, 639–645. doi: 10.1038/s41586-018-0718-6
Lei, Q., Yi, T., and Chen, C. (2018). NF-κB–Gasdermin D (GSDMD) axis couples oxidative stress and NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome-mediated cardiomyocyte pyroptosis following myocardial infarction. Med. Sci. Monit. 24, 6044–6052. doi: 10.12659/MSM.908529
Leija-Salazar, M., Piette, C., and Proukakis, C. (2018). Review: somatic mutations in neurodegeneration. Neuropathol. Appl. Neurobiol. 44, 267–285. doi: 10.1111/nan.12465
Lendermon, E. A., Coon, T. A., Bednash, J. S., Weathington, N. M., McDyer, J. F., and Mallampalli, R. K. (2017). Azithromycin decreases NALP3 mRNA stability in monocytes to limit inflammasome-dependent inflammation. Respir. Res. 18, 131. doi: 10.1186/s12931-017-0608-8
Lewinska, A., and Wnuk, M. (2017). Helicobacter pylori-induced premature senescence of extragastric cells may contribute to chronic skin diseases. Biogerontology 18, 293–299. doi: 10.1007/s10522-017-9676-x
Lezi, E., Zhou, T., Koh, S., Chuang, M., Sharma, R., Pujol, N., et al. (2018). An antimicrobial peptide and its neuronal receptor regulate dendrite degeneration in aging and infection. Neuron 97, 125–138.e5. doi: 10.1016/j.neuron.2017.12.001
Li, J., Chen, J., Mo, H., Chen, J., Qian, C., Yan, F., et al. (2016). Minocycline protects against NLRP3 inflammasome-induced inflammation and P53-associated apoptosis in early brain injury after subarachnoid hemorrhage. Mol. Neurobiol. 53, 2668–2678. doi: 10.1007/s12035-015-9318-8
Li, L. H., Ju, T. C., Hsieh, C. Y., Dong, W. C., Chen, W. T., Hua, K. F., et al. (2017). A synthetic cationic antimicrobial peptide inhibits inflammatory response and the NLRP3 inflammasome by neutralizing LPS and ATP. PLoS ONE 12:e0182057. doi: 10.1371/journal.pone.0182057
Li, M., Durbin, K. R., Sweet, S. M., Tipton, J. D., Zheng, Y., and Kelleher, N. L. (2013). Oncogene-induced cellular senescence elicits an anti-Warburg effect. Proteomics 13, 2585–2596. doi: 10.1002/pmic.201200298
Li, P., Gan, Y., Xu, Y., Song, L., Wang, L., Ouyang, B., et al. (2017). The inflammatory cytokine TNF-α promotes the premature senescence of rat nucleus pulposus cells via the PI3K/Akt signaling pathway. Sci. Rep. 7:42938. doi: 10.1038/srep42938
Li, P., Liu, C., Yu, Z., and Wu, M. (2016). New insights into regulatory T cells: exosome- and non-coding RNA-mediated regulation of homeostasis and resident Treg cells. Front. Immunol. 7:574. doi: 10.3389/fimmu.2016.00574
Li, X., Zhang, Z., Zhang, Y., Cao, Y., Wei, H., and Wu, Z. (2018). Upregulation of lactate-inducible snail protein suppresses oncogene-mediated senescence through p16INK4a inactivation. J. Exp. Clin. Cancer Res. 37, 39. doi: 10.1186/s13046-018-0701-y
Li, Y., Rinne, J. O., Mosconi, L., Pirraglia, E., Rusinek, H., DeSanti, S., et al. (2008). Regional analysis of FDG and PIB-PET images in normal aging, mild cognitive impairment, and Alzheimer's disease. Eur. J. Nucl. Med. Mol. Imaging 35, 2169–2181. doi: 10.1007/s00259-008-0833-y
Li, Y., Wu, J., Niu, Y., Chen, H., Tang, Q., Zhong, Y., et al. (2018). Milk fat globule membrane inhibits NLRP3 inflammasome activation and enhances intestinal barrier function in a rat model of short bowel. JPEN J. Parenter. Enteral Nutr. doi: 10.1002/jpen.1435. [Epub ahead of print].
Liddelow, S. A., Guttenplan, K. A., Clarke, L. E., Bennett, F. C., Bohlen, C. J., Schirmer, L., et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. doi: 10.1038/nature21029
Liu, G., Men, P., Perry, G., and Smith, M. A. (2010). Nanoparticle and iron chelators as a potential novel Alzheimer therapy. Methods Mol. Biol. 610, 123–144. doi: 10.1007/978-1-60327-029-8_8
Liu, J., Wang, D., Li, S. Q., Yu, Y., and Ye, R. D. (2016). Suppression of LPS-induced tau hyperphosphorylation by serum amyloid A. J. Neuroinflammation 13:28. doi: 10.1186/s12974-016-0493-y
Liu, J. L., Fan, Y. G., Yang, Z. S., Wang, Z. Y., and Guo, C. (2018). Iron and Alzheimer's disease: from pathogenesis to therapeutic implications. Front. Neurosci. 12:632. doi: 10.3389/fnins.2018.00632
Liu, Q., Zhang, D., Hu, D., Zhou, X., and Zhou, Y. (2018). The role of mitochondria in NLRP3 inflammasome activation. Mol. Immunol. 103, 115–124. doi: 10.1016/j.molimm.2018.09.010
Liu, R., Luo, Q., You, W., and Jin, M. (2018). MicroRNA-106 attenuates hyperglycemia-induced vascular endothelial cell dysfunction by targeting HMGB1. Gene 677, 142–148. doi: 10.1016/j.gene.2018.07.063
Lluch, J., Servant, F., Païss,é, S., Valle, C., Valière, S., Kuchly, C., et al. (2015). The characterization of novel tissue microbiota using an optimized 16S metagenomic sequencing pipeline. PLoS ONE 10:e0142334. doi: 10.1371/journal.pone.0142334
Locatelli, P., Giménez, C. S., Vega, M. U., Crottogini, A., and Belaich, M. N. (2018). Targeting the cardiomyocyte cell cycle for heart regeneration. Curr. Drug Targets 20, 241–254. doi: 10.2174/1389450119666180801122551
Lopes, K. O., Sparks, D. L., and Streit, W. J. (2008). Microglial dystrophy in the aged and Alzheimer's disease brain is associated with ferritin immunoreactivity. Glia 56, 1048–1060. doi: 10.1002/glia.20678
Lord, J. M. (2013). The effect of ageing of the immune system on vaccination responses. Hum. Vaccin. Immunother. 9, 1364–1367. doi: 10.4161/hv.24696
Lozano, E., Joller, N., Cao, Y., Kuchroo, V. K., and Hafler, D. A. (2013). The CD226/CD155 interaction regulates the proinflammatory (Th1/Th17)/anti-inflammatory (Th2) balance in humans. J. Immunol. 191, 3673–3680. doi: 10.4049/jimmunol.1300945
Lubkin, A., and Torres, V. J. (2016). Bacteria and endothelial cells: a toxic relationship. Curr. Opin. Microbiol. 35, 58–63. doi: 10.1016/j.mib.2016.11.008
Lukiw, W. J., Cui, J. G., Yuan, L. Y., Bhattacharjee, P., Corkern, M., Clement, C., et al. (2010). Acyclovir or Aβ42 peptides attenuate HSV-1-induced miRNA-146a levels in human primary brain cells. Neuroreport 21, 922–927. doi: 10.1097/WNR.0b013e32833da51a
Ma, Y., Jiang, J., Gao, Y., Shi, T., Zhu, X., Zhang, K., et al. (2018). Research progress of the relationship between pyroptosis and disease. Am. J. Transl. Res. 10, 2213–2219.
Maciel-Barón, L. Á., Morales-Rosales, S. L., Silva-Palacios, A., Rodríguez-Barrera, R. H., García-Álvarez, J. A., Luna-López, A., et al. (2018). The secretory phenotype of senescent astrocytes isolated from Wistar newborn rats changes with anti-inflammatory drugs, but does not have a short-term effect on neuronal mitochondrial potential. Biogerontology 19, 415–433. doi: 10.1007/s10522-018-9767-3
Maezono, H., Noiri, Y., Asahi, Y., Yamaguchi, M., Yamamoto, R., Izutani, N., et al. (2011). Antibiofilm effects of azithromycin and erythromycin on Porphyromonas gingivalis. Antimicrob. Agents Chemother. 55, 5887–5892. doi: 10.1128/AAC.05169-11
Magalhães, C. A., Carvalho, M. D. G., Sousa, L. P., Caramelli, P., and Gomes, K. B. (2017). Alzheimer's disease and cytokine IL-10 gene polymorphisms: is there an association? Arq. Neuropsiquiatr. 75, 649–656. doi: 10.1590/0004-282x20170110
Malik, A., and Kanneganti, T. D. (2017). Inflammasome activation and assembly at a glance. J. Cell Sci. 130, 3955–3963. doi: 10.1242/jcs.207365
Man, A., Slevin, M., Petcu, E., and Fraefel, C. (2019). The cyclin-dependent kinase 5 inhibitor peptide inhibits herpes simplex virus type 1 replication. Sci. Rep. 9, 1260. doi: 10.1038/s41598-018-37989-3
Man, S. M., Karki, R., and Kanneganti, T. D. (2017). Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 277, 61–75. doi: 10.1111/imr.12534
Manickam, V., Dhakshinamoorthy, V., and Perumal, E. (2018). Iron oxide nanoparticles induces cell cycle-dependent neuronal apoptosis in mice. J. Mol. Neurosci. 64, 352–362. doi: 10.1007/s12031-018-1030-5
Mann, B. S., Johnson, J. R., Cohen, M. H., Justice, R., and Pazdur, R. (2007). FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist 12, 1247–1252. doi: 10.1634/theoncologist.12-10-1247
Mantis, N. J., Rol, N., and Corthésy, B. (2011). Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 4, 603–611. doi: 10.1038/mi.2011.41
Mantri, C. K., Chen, C. H., Dong, X., Goodwin, J. S., Pratap, S., Paromov, V., et al. (2014). Fimbriae-mediated outer membrane vesicle production and invasion of Porphyromonas gingivalis. Microbiologyopen 4, 53–65. doi: 10.1002/mbo3.221
Mao, D., and Hinds, P. W. (2010). p35 is required for CDK5 activation in cellular senescence. J. Biol. Chem. 285, 14671–14680. doi: 10.1074/jbc.M109.066118
Mao, K., Chen, S., Chen, M., Ma, Y., Wang, Y., Huang, B., et al. (2013). Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell Res. 23, 201–212. doi: 10.1038/cr.2013.6
Martin, S. A., Souder, D. C., Miller, K. N., Clark, J. P., Sagar, A. K., Eliceiri, K. W., et al. (2018). GSK3β regulates brain energy metabolism. Cell Rep. 23, 1922–1931.e4. doi: 10.1016/j.celrep.2018.04.045
Martín-Maestro, P., Gargini, R., Perry, G., Avila, J., and García-Escudero, V. (2016). PARK2 enhancement is able to compensate mitophagy alterations found in sporadic Alzheimer's disease. Hum. Mol. Genet. 25, 792–806. doi: 10.1093/hmg/ddv616
Masaldan, S., Clatworthy, S. A. S., Gamell, C., Meggyesy, P. M., Rigopoulos, A. T., Haupt, S., et al. (2018). Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. Redox Biol. 14, 100–115. doi: 10.1016/j.redox.2017.08.015
Mayeux, R., and Stern, Y. (2012). Epidemiology of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2:a006239. doi: 10.1101/cshperspect.a006239
McAllister, M. J., Chemaly, M., Eakin, A. J., Gibson, D. S., and McGilligan, V. E. (2018). NLRP3 as a potentially novel biomarker for the management of osteoarthritis. Osteoarthr. Cartil. 26, 612–619. doi: 10.1016/j.joca.2018.02.901
McArthur, J. C., Steiner, J., Sacktor, N., and Nath, A. (2010). Human immunodeficiency virus-associated neurocognitive disorders: mind the gap. Ann. Neurol. 67, 699–714. doi: 10.1002/ana.22053
McConnell, M. J., Moran, J. V., Abyzov, A., Akbarian, S., Bae, T., Cortes-Ciriano, I., et al. (2017). Intersection of diverse neuronal genomes and neuropsychiatric disease: the Brain Somatic Mosaicism Network. Science 356:eaal1641. doi: 10.1126/science.aal1641
McCool, K. W., and Miyamoto, S. (2012). DNA damage-dependent NF-κB activation: NEMO turns nuclear signaling inside out. Immunol. Rev. 246, 311–326. doi: 10.1111/j.1600-065X.2012.01101.x
Miners, J. S., Schulz, I., and Love, S. (2017). Differing associations between Aβ accumulation, hypoperfusion, blood–brain barrier dysfunction and loss of PDGFRB pericyte marker in the precuneus and parietal white matter in Alzheimer's disease. J. Cereb. Blood Flow Metab. 38, 103–115. doi: 10.1177/0271678X17690761
Mishra, S., and Horswill, A. R. (2017). Heparin mimics extracellular DNA in binding to cell surface-localized proteins and promoting Staphylococcus aureus biofilm formation. mSphere 2:e00135–e00117. doi: 10.1128/mSphere.00135-17
Mizejewski, G. J. (2017). Breast cancer and amyloid bodies: is there a role for amyloidosis in cancer-cell dormancy? Breast Cancer 9, 287–291. doi: 10.2147/BCTT.S131394
Molinero, P., Soutto, M., Benot, S., Hmadcha, A., and Guerrero, J. M. (2000). Melatonin is responsible for the nocturnal increase observed in serum and thymus of thymosin alpha1 and thymulin concentrations: observations in rats and humans. J. Neuroimmunol. 103, 180–188. doi: 10.1016/S0165-5728(99)00237-4
Mollet, I. G., Patel, D., Govani, F. S., Giess, A., Paschalaki, K., Periyasamy, M., et al. (2016). Low dose iron treatments induce a DNA damage response in human endothelial cells within minutes. PLoS ONE 11:e0147990. doi: 10.1371/journal.pone.0147990
Montagne, A., Barnes, S. R., Sweeney, M. D., Halliday, M. R., Sagare, A. P., Zhao, Z., et al. (2015). Blood–brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302. doi: 10.1016/j.neuron.2014.12.032
Morgan, B. P. (2017). Complement in the pathogenesis of Alzheimer's disease. Semin. Immunopathol. 40, 113–124. doi: 10.1007/s00281-017-0662-9
Morizawa, Y. M., Hirayama, Y., Ohno, N., Shibata, S., Shigetomi, E., Sui, Y., et al. (2017). Reactive astrocytes function as phagocytes after brain ischemia via ABCA1-mediated pathway. Nat. Commun. 8:28. doi: 10.1038/s41467-017-01594-1
Morris, G. P., Clark, I. A., and Vissel, B. (2014). Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer's disease. Acta Neuropathol. Commun. 2:135. doi: 10.1186/s40478-014-0135-5
Mosher, K. I., and Wyss-Coray, T. (2014). Microglial dysfunction in brain aging and Alzheimer's disease. Biochem. Pharmacol. 88, 594–604. doi: 10.1016/j.bcp.2014.01.008
Motoi, Y., Shimada, K., Ishiguro, K., and Hattori, N. (2014). Lithium and autophagy. ACS Chem. Neurosci. 5, 434–442. doi: 10.1021/cn500056q
Muciño, G., Castro-Obregón, S., Hernandez-Pando, R., and Del Rio, G. (2016). Autophagy as a target for therapeutic uses of multifunctional peptides. IUBMB Life 68, 259–267. doi: 10.1002/iub.1483
Muller, M., Li, Z., and Maitz, P. K. (2009). Pseudomonas pyocyanin inhibits wound repair by inducing premature cellular senescence: role for p38 mitogen-activated protein kinase. Burns 35, 500–508. doi: 10.1016/j.burns.2008.11.010
Munshi, N., Fernandis, A. Z., Cherla, R. P., Park, I. W., and Ganju, R. K. (2002). Lipopolysaccharide-induced apoptosis of endothelial cells and its inhibition by vascular endothelial growth factor. J. Immunol. 168, 5860–5866. doi: 10.4049/jimmunol.168.11.5860
Musi, N., Valentine, J. M., Sickora, K. R., Baeuerle, E., Thompson, C. S., Shen, Q., et al. (2018). Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell 17:e12840. doi: 10.1111/acel.12840
Na, Y. R., Jung, D., Gu, G. J., Jang, A. R., Suh, Y. H., and Seok, S. H. (2015). The early synthesis of p35 and activation of CDK5 in LPS-stimulated macrophages suppresses interleukin-10 production. Sci. Signal. 8:ra121. doi: 10.1126/scisignal.aab3156
Nagele, E. P., Han, M., Acharya, N. K., DeMarshall, C., Kosciuk, M. C., and Nagele, R. G. (2013). Natural IgG autoantibodies are abundant and ubiquitous in human sera, and their number is influenced by age, gender, and disease. PLoS ONE 8:e60726. doi: 10.1371/journal.pone.0060726
Nagpal, R., Mainali, R., Ahmadi, S., Wang, S., Singh, R., Kavanagh, K., et al. (2018). Gut microbiome and aging: physiological and mechanistic insights. Nutr Healthy Aging 4, 267–285. doi: 10.3233/NHA-170030
Nakamoto, N., Amiya, T., Aoki, R., Taniki, N., Koda, Y., Miyamoto, K., et al. (2017). Commensal Lactobacillus controls immune tolerance during acute liver injury in mice. Cell Rep. 21, 1215–1226. doi: 10.1016/j.celrep.2017.10.022
Nakamura, K., Kawakami, T., Yamamoto, N., Tomizawa, M., Fujiwara, T., Ishii, T., et al. (2016). Activation of the NLRP3 inflammasome by cellular labile iron. Exp. Hematol. 44, 116–124. doi: 10.1016/j.exphem.2015.11.002
Nakao, A., Kaczorowski, D. J., Zuckerbraun, B. S., Lei, J., Faleo, G., Deguchi, K., et al. (2008). Galantamine and carbon monoxide protect brain microvascular endothelial cells by heme oxygenase-1 induction. Biochem. Biophys. Res. Commun. 367, 674–679. doi: 10.1016/j.bbrc.2007.12.152
Nation, D. A., Sweeney, M. D., Montagne, A., Sagare, A. P., D'Orazio, L. M., Pachicano, M., et al. (2019). Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 25, 270–276. doi: 10.1038/s41591-018-0297-y
Naylor, R. M., Baker, D. J., and van Deursen, J. M. (2012). Senescent cells: a novel therapeutic target for aging and age-related diseases. Clin. Pharmacol. Ther. 93, 105–116. doi: 10.1038/clpt.2012.193
Neher, J. J., Neniskyte, U., and Brown, G. C. (2012). Primary phagocytosis of neurons by inflamed microglia: potential roles in neurodegeneration. Front. Pharmacol. 3:27. doi: 10.3389/fphar.2012.00027
Nelson, G., Wordsworth, J., Wang, C., Jurk, D., Lawless, C., Martin-Ruiz, C., et al. (2012). A senescent cell bystander effect: senescence-induced senescence. Aging Cell 11, 345–349. doi: 10.1111/j.1474-9726.2012.00795.x
Nelson, P. N., Carnegie, P. R., Martin, J., Ejtehadi, H. D., Hooley, P., Roden, D., et al. (2003). Demystified. Human endogenous retroviruses. Mol. Pathol. 56, 11–18. doi: 10.1136/mp.56.1.11
Németh, A., Orgovan, N., Sódar, B. W., Osteikoetxea, X., Pálóczi, K., Szabó-Taylor, K. É., et al. (2017). Antibiotic-induced release of small extracellular vesicles (exosomes) with surface-associated DNA. Sci. Rep. 7:8202. doi: 10.1038/s41598-017-08392-1
Netea, M. G., and van der Meer, J. W. (2017). Trained immunity: an ancient way of remembering. Cell Host Microbe 21, 297–300. doi: 10.1016/j.chom.2017.02.003
Neumann, H., Kotter, M. R., and Franklin, R. J. (2009). Debris clearance by microglia: an essential link between degeneration and regeneration. Brain 132, 288–295. doi: 10.1093/brain/awn109
Nolt, B., Tu, F., Wang, X., Ha, T., Winter, R., Williams, D. L., et al. (2018). Lactate and Immunosuppression in sepsis. Shock 49, 120–125. doi: 10.1097/SHK.0000000000000958
Nunes-Alves, C., Nobrega, C., Behar, S. M., and Correia-Neves, M. (2013). Tolerance has its limits: how the thymus copes with infection. Trends Immunol. 34, 502–510. doi: 10.1016/j.it.2013.06.004
Odamaki, T., Kato, K., Sugahara, H., Hashikura, N., Takahashi, S., Xiao, J. Z., et al. (2016). Age-related changes in gut microbiota composition from newborn to centenarian: a cross-sectional study. BMC Microbiol. 16:90. doi: 10.1186/s12866-016-0708-5
Ohno, H. (2016). JB special review—crosstalk between the Intestinal immune system and gut commensal microbiota. J. Biochem. 159, 151–160. doi: 10.1093/jb/mvv121
Ohyashiki, J. H., Kobayashi, C., Hamamura, R., Okabe, S., Tauchi, T., and Ohyashiki, K. (2009). The oral iron chelator deferasirox represses signaling through the mTOR in myeloid leukemia cells by enhancing expression of REDD1. Cancer Sci. 100, 970–977. doi: 10.1111/j.1349-7006.2009.01131.x
Ojala, J., Alafuzoff, I., Herukka, S. K., van Groen, T., Tanila, H., and Pirttil,ä, T. (2009). Expression of interleukin-18 is increased in the brains of Alzheimer's disease patients. Neurobiol. Aging 30, 198–209. doi: 10.1016/j.neurobiolaging.2007.06.006
Olivieri, F., Prattichizzo, F., Grillari, J., and Balistreri, C. R. (2018). Cellular senescence and inflammaging in age-related diseases. Mediators Inflamm. 2018:9076485. doi: 10.1155/2018/9076485
Olsson, J., Wikby, A., Johansson, B., Löfgren, S., Nilsson, B. O., and Ferguson, F. G. (2000). Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech. Ageing Dev. 121, 187–201. doi: 10.1016/S0047-6374(00)00210-4
Oppong, G. O., Rapsinski, G. J., Tursi, S. A., Biesecker, S. G., Klein-Szanto, A. J., Goulian, M., et al. (2015). Biofilm-associated bacterial amyloids dampen inflammation in the gut: oral treatment with curli fibres reduces the severity of hapten-induced colitis in mice. NPJ Biofilms Microbiomes 1, 15019. doi: 10.1038/npjbiofilms.2015.19
Orgogozo, J. M., Gilman, S., Dartigues, J. F., Laurent, B., Puel, M., Kirby, L. C., et al. (2003). Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 61, 46–54. doi: 10.1212/01.WNL.0000073623.84147.A8
Ozsvari, B., Nuttall, J. R., Sotgia, F., and Lisanti, M. P. (2018). Azithromycin and roxithromycin define a new family of “senolytic” drugs that target senescent human fibroblasts. Aging 10, 3294–3307. doi: 10.18632/aging.101633
Pandey, P., Sliker, B., Peters, H. L., Tuli, A., Herskovitz, J., Smits, K., et al. (2016). Amyloid precursor protein and amyloid precursor-like protein 2 in cancer. Oncotarget 7, 19430–19444. doi: 10.18632/oncotarget.7103
Panossian, L., Fenik, P., Zhu, Y., Zhan, G., McBurney, M. W., and Veasey, S. (2011). SIRT1 regulation of wakefulness and senescence-like phenotype in wake neurons. J. Neurosci. 31, 4025–4036. doi: 10.1523/JNEUROSCI.5166-10.2011
Paquola, A. C. M., Erwin, J. A., and Gage, F. H. (2016). Insights into the role of somatic mosaicism in the brain. Curr. Opin. Syst. Biol. 1, 90–94. doi: 10.1016/j.coisb.2016.12.004
Pastuzyn, E. D., Day, C. E., Kearns, R. B., Kyrke-Smith, M., Taibi, A.V., McCormick, J., et al. (2018). The neuronal gene arc encodes a repurposed retrotransposon gag protein that mediates intercellular RNA transfer. Cell 172, 275–288.e18. doi: 10.1016/j.cell.2017.12.024
Pellegrini, C., Fornai, M., Colucci, R., Benvenuti, L., D'Antongiovanni, V., Natale, G., et al. (2018). A comparative study on the efficacy of NLRP3 inflammasome signaling inhibitors in a pre-clinical model of bowel inflammation. Front. Pharmacol. 9:1405. doi: 10.3389/fphar.2018.01405
Penner, M. R., Roth, T. L., Chawla, M. K., Hoang, L. T., Roth, E. D., Lubin, F. D., et al. (2010). Age-related changes in Arc transcription and DNA methylation within the hippocampus. Neurobiol. Aging 32, 2198–2210. doi: 10.1016/j.neurobiolaging.2010.01.009
Perera, A. P., Fernando, R., Shinde, T., Gundamaraju, R., Southam, B., Sohal, S. S., et al. (2018). MCC950, a specific small molecule inhibitor of NLRP3 inflammasome attenuates colonic inflammation in spontaneous colitis mice. Sci. Rep. 8:8618. doi: 10.1038/s41598-018-26775-w
Perez-Nievas, B. G., and Serrano-Pozo, A. (2018). Deciphering the astrocyte reaction in Alzheimer's disease. Front. Aging Neurosci. 10:114. doi: 10.3389/fnagi.2018.00114
Piacentini, R., Li Puma, D. D., Ripoli, C., Marcocci, M. E., De Chiara, G., Garaci, E., et al. (2015). Herpes simplex virus type-1 infection induces synaptic dysfunction in cultured cortical neurons via GSK-3 activation and intraneuronal amyloid-β protein accumulation. Sci. Rep. 5:15444. doi: 10.1038/srep15444
Pickrell, A. M., and Youle, R. J. (2015). The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron 85, 257–273. doi: 10.1016/j.neuron.2014.12.007
Potter, M., Newport, E., and Morten, K. J. (2016). The Warburg effect: 80 years on. Biochem. Soc. Trans. 44, 1499–1505. doi: 10.1042/BST20160094
Pradhan, S., Madke, B., Kabra, P., and Singh, A. L. (2016). Anti-inflammatory and immunomodulatory effects of antibiotics and their use in dermatology. Indian J. Dermatol. 61, 469–481. doi: 10.4103/0019-5154.190105
Pretorius, E., Bester, J., Page, M. J., and Kell, D. B. (2018). The potential of LPS-binding protein to reverse amyloid formation in plasma fibrin of individuals with Alzheimer-type dementia. Front. Aging Neurosci. 10:257. doi: 10.3389/fnagi.2018.00257
Qi, Y., Klyubin, I., Cuello, A. C., and Rowan, M. J. (2018). NLRP3-dependent synaptic plasticity deficit in an Alzheimer's disease amyloidosis model in vivo. Neurobiol. Dis. 114, 24–30. doi: 10.1016/j.nbd.2018.02.016
Rajagopal, C., and Harikumar, K. B. (2018). The origin and functions of exosomes in cancer. Front. Oncol. 8:66. doi: 10.3389/fonc.2018.00066
Ramanan, D., and Cadwell, K. (2016). Intrinsic defense mechanisms of the intestinal epithelium. Cell Host Microbe 19, 434–441. doi: 10.1016/j.chom.2016.03.003
Ranganathan, P., Shanmugam, A., Swafford, D., Suryawanshi, A., Bhattacharjee, P., Hussein, M. S., et al. (2018). GPR81, a cell-surface receptor for lactate, regulates intestinal homeostasis and protects mice from experimental colitis. J. Immunol. 200, 1781–1789. doi: 10.4049/jimmunol.1700604
Ransohoff, R. M., and Brown, M. A. (2012). Innate immunity in the central nervous system. J. Clin. Invest. 122, 1164–1171. doi: 10.1172/JCI58644
Rao, M., Valentini, D., Zumla, A., and Maeurer, M. (2018). Evaluation of the efficacy of valproic acid and suberoylanilide hydroxamic acid (vorinostat) in enhancing the effects of first-line tuberculosis drugs against intracellular Mycobacterium tuberculosis. Int. J. Infect. Dis. 69, 78–84. doi: 10.1016/j.ijid.2018.02.021
Rao, S. S., and Adlard, P. A. (2018). Untangling tau and iron: exploring the interaction between iron and tau in neurodegeneration. Front. Mol. Neurosci. 11:276. doi: 10.3389/fnmol.2018.00276
Ratzinger, F., Haslacher, H., Poeppl, W., Hoermann, G., Kovarik, J. J., Jutz, S., et al. (2014). Azithromycin suppresses CD4(+) T-cell activation by direct modulation of mTOR activity. Sci. Rep. 4:7438. doi: 10.1038/srep07438
Rea, I. M., Gibson, D. S., McGilligan, V., McNerlan, S. E., Alexander, H. D., and Ross, O. A. (2018). Age and age-related diseases: role of inflammation triggers and cytokines. Front. Immunol. 9:586. doi: 10.3389/fimmu.2018.00586
Richter, J. M., Schanbacher, B. L., Huang, H., Xue, J., Bauer, J. A., and Giannone, P. J. (2012). LPS-binding protein enables intestinal epithelial restitution despite LPS exposure. J. Pediatr. Gastroenterol. Nutr. 54, 639–644. doi: 10.1097/MPG.0b013e31823a895a
Riske, L., Thomas, R. K., Baker, G. B., and Dursun, S. M. (2016). Lactate in the brain: an update on its relevance to brain energy, neurons, glia and panic disorder. Ther. Adv. Psychopharmacol. 7, 85–89. doi: 10.1177/2045125316675579
Robinson, K., Deng, Z., Hou, Y., and Zhang, G. (2015). Regulation of the intestinal barrier function by host defense peptides. Front. Vet. Sci. 2:57. doi: 10.3389/fvets.2015.00057
Rodrigue, K. M., Rieck, J. R., Kennedy, K. M., Devous, M. D., Diaz-Arrastia, R., and Park, D. C. (2013). Risk factors for β-amyloid deposition in healthy aging: vascular and genetic effects. JAMA Neurol. 70, 600–606. doi: 10.1001/jamaneurol.2013.1342
Rodrigues, M., Fan, J., Lyon, C., Wan, M., and Hu, Y. (2018). Role of extracellular vesicles in viral and bacterial infections: pathogenesis, diagnostics, and therapeutics. Theranostics 8, 2709–2721. doi: 10.7150/thno.20576
Roland, C. L., Arumugam, T., Deng, D., Liu, S. H., Philip, B., Gomez, S., et al. (2014). Cell surface lactate receptor GPR81 is crucial for cancer cell survival. Cancer Res. 74, 5301–5310. doi: 10.1158/0008-5472.CAN-14-0319
Romero-Garcia, S., Moreno-Altamirano, M. M., Prado-Garcia, H., and Sánchez-García, F. J. (2016). Lactate contribution to the tumor microenvironment: mechanisms, effects on immune cells and therapeutic relevance. Front. Immunol. 7:52. doi: 10.3389/fimmu.2016.00052
Ross, M. D. (2018). Endothelial regenerative capacity and aging: influence of diet, exercise and obesity. Curr. Cardiol. Rev. 14, 233–244. doi: 10.2174/1573403X14666180726112303
Rybakowski, J. K. (2019). Commentary: corroboration of a major role for herpes simplex virus type 1 in Alzheimer's disease. Front. Aging Neurosci. 10:433. doi: 10.3389/fnagi.2018.00433
Saito, Y., Murata-Kamiya, N., Hirayama, T., Ohba, Y., and Hatakeyama, M. (2010). Conversion of Helicobacter pylori CagA from senescence inducer to oncogenic driver through polarity-dependent regulation of p21. J. Exp. Med. 207, 2157–2174. doi: 10.1084/jem.20100602
Saji, N., Niida, S., Murotani, K., Hisada, T., Tsuduki, T., Sugimoto, T., et al. (2019). Analysis of the relationship between the gut microbiome and dementia: a cross-sectional study conducted in Japan. Sci. Rep. 9:1008. doi: 10.1038/s41598-018-38218-7
Salem, I., Ramser, A., Isham, N., and Ghannoum, M. A. (2018). The gut microbiome as a major regulator of the gut–skin axis. Front. Microbiol. 9:1459. doi: 10.3389/fmicb.2018.01459
Salmond, R. J. (2018). mTOR regulation of glycolytic metabolism in T cells. Front. Cell. Dev. Biol. 6 :122. doi: 10.3389/fcell.2018.00122
Sanada, F., Taniyama, Y., Muratsu, J., Otsu, R., Shimizu, H., Rakugi, H., et al. (2018). Source of chronic inflammation in aging. Front. Cardiovasc. Med. 5:12. doi: 10.3389/fcvm.2018.00012
San-Millán, I., and Brooks, G. A. (2016). Reexamining cancer metabolism: lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 38, 119–133. doi: 10.1093/carcin/bgw127
Santiago, A. F., Alves, A. C., Oliveira, R. P., Fernandes, R. M., Paula-Silva, J., Assis, F. A., et al. (2011). Aging correlates with reduction in regulatory-type cytokines and T cells in the gut mucosa. Immunobiology 216, 1085–1093. doi: 10.1016/j.imbio.2011.05.007
Santos, R. X., Correia, S. C., Wang, X., Perry, G., Smith, M. A., Moreira, P. I., et al. (2010). A synergistic dysfunction of mitochondrial fission/fusion dynamics and mitophagy in Alzheimer's disease. J. Alzheimers Dis. 20(Suppl. 2), S401–S412. doi: 10.3233/JAD-2010-100666
Sato, J., Kanazawa, A., Ikeda, F., Yoshihara, T., Goto, H., Abe, H., et al. (2014). Gut dysbiosis and detection of “live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabetes Care 37, 2343–2350. doi: 10.2337/dc13-2817
Sato, S., Kiyono, H., and Fujihashi, K. (2014). Mucosal immunosenescence in the gastrointestinal tract: a mini-review. Gerontology 61, 336–342. doi: 10.1159/000368897
Schetters, S. T. T., Gomez-Nicola, D., Garcia-Vallejo, J. J., and Van Kooyk, Y. (2018). Neuroinflammation: microglia and T cells get ready to tango. Front. Immunol. 8:1905. doi: 10.3389/fimmu.2017.01905
Schmucker, D. L., Owen, R. L., Outenreath, R., and Thoreux, K. (2003). Basis for the age-related decline in intestinal mucosal immunity. Clin. Dev. Immunol. 10, 167–172. doi: 10.1080/10446670310001642168
Schreibelt, G., Musters, R. J., Reijerkerk, A., de Groot, L. R., van der Pol, S. M., Hendrikx, E. M., et al. (2006). Lipoic acid affects cellular migration into the central nervous system and stabilizes blood–brain barrier integrity. J. Immunol. 177, 2630–2637. doi: 10.4049/jimmunol.177.4.2630
Schultz, N., Brännström, K., Byman, E., Moussaud, S., Nielsen, H. M., Bank, N. B., et al. (2018). Amyloid-α 1–40 is associated with alterations in NG2+ pericyte population ex vivo and in vitro. Aging Cell 17:e12728. doi: 10.1111/acel.12728
Secher, T., Samba-Louaka, A., Oswald, E., and Nougayrède, J. P. (2013). Escherichia coli producing colibactin triggers premature and transmissible senescence in mammalian cells. PLoS ONE 8:e77157. doi: 10.1371/journal.pone.0077157
Sfera, A., Bullock, K., Price, A., Inderias, L., and Osorio, C. (2018). Ferrosenescence: the iron age of neurodegeneration? Mech. Ageing Dev. 174, 63–75. doi: 10.1016/j.mad.2017.11.012
Sharma, R., Kumar, D., Jha, N. K., Jha, S. K., Ambasta, R. K., and Kumar, P. (2017). Re-expression of cell cycle markers in aged neurons and muscles: whether cells should divide or die? Biochim. Biophys. Acta 1863, 324–336. doi: 10.1016/j.bbadis.2016.09.010
Shi, Q., Chowdhury, S., Ma, R., Le, K. X., Hong, S., Caldarone, B. J., et al. (2017). Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci. Transl. Med. 9:eaaf6295. doi: 10.1126/scitranslmed.aaf6295
Sierra, A., Gottfried-Blackmore, A. C., McEwen, B. S., and Bulloch, K. (2007). Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 55, 412–424. doi: 10.1002/glia.20468
Singh, P., Suman, S., Chandna, S., and Das, T. K. (2009). Possible role of amyloid-beta, adenine nucleotide translocase and cyclophilin-D interaction in mitochondrial dysfunction of Alzheimer's disease. Bioinformation. 3, 440–445. doi: 10.6026/97320630003440
Skogberg, G., Telemo, E., and Ekwall, O. (2015). Exosomes in the thymus: antigen transfer and vesicles. Front. Immunol. 6:366. doi: 10.3389/fimmu.2015.00366
Smalley, J. W., and Olczak, T. (2017). Heme acquisition mechanisms of Porphyromonas gingivalis—strategies used in a polymicrobial community in a heme-limited host environment. Mol. Oral Microbiol. 32, 1–23. doi: 10.1111/omi.12149
Solana, C., Tarazona, R., and Solana, R. (2018). Immunosenescence of natural killer cells, inflammation, and Alzheimer's disease. Int. J. Alzheimers Dis. 2018:3128758. doi: 10.1155/2018/3128758
Solomon, B. L., and Garrido-Laguna, I. (2018). TIGIT: a novel immunotherapy target moving from bench to bedside. Cancer Immunol. Immunother. 67, 1659–1667. doi: 10.1007/s00262-018-2246-5
Song, Y., Wang, B., Song, R., Hao, Y., Wang, D., Li, Y., et al. (2018). T-cell Immunoglobulin and ITIM domain contributes to CD8+ T-cell immunosenescence. Aging Cell 17:e12716. doi: 10.1111/acel.12716
Soreq, L., Rose, J., Soreq, E., Hardy, J., et al. (2017). Major shifts in glial regional identity are a transcriptional hallmark of human brain aging. Cell Rep. 18, 557–570. doi: 10.1016/j.celrep.2016.12.011
Spitzer, P., Condic, M., Herrmann, M., Oberstein, T. J., Scharin-Mehlmann, M., Gilbert, D. F., et al. (2016). Amyloidogenic amyloid-β-peptide variants induce microbial agglutination and exert antimicrobial activity. Sci. Rep. 6:32228. doi: 10.1038/srep32228
Stafford, P., Higham, J., Pinnock, A., Murdoch, C., Douglas, C. W., Stafford, G. P., et al. (2013). Gingipain-dependent degradation of mammalian target of rapamycin pathway proteins by the periodontal pathogen Porphyromonas gingivalis during invasion. Mol. Oral Microbiol. 28, 366–378. doi: 10.1111/omi.12030
Stark, K., Pekayvaz, K., and Massberg, S. (2018). Role of pericytes in vascular immunosurveillance. Front. Biosci. 23, 767–781. doi: 10.2741/4615
Stefano, G. B., Samuel, J., and Kream, R. M. (2017). Antibiotics may trigger mitochondrial dysfunction inducing psychiatric disorders. Med. Sci. Monit. 23, 101–106. doi: 10.12659/MSM.899478
Steinbach, G., Hockenbery, D. M., Huls, G., Furlong, T., Myerson, D., Loeb, K. R., et al. (2017). Pilot study of lithium to restore intestinal barrier function in severe graft-versus-host disease. PLoS ONE 12:e0183284. doi: 10.1371/journal.pone.0183284
Streit, W. J., and Xue, Q. S. (2012). Alzheimer's disease, neuroprotection, and CNS immunosenescence. Front. Pharmacol. 3:138. doi: 10.3389/fphar.2012.00138
Sutinen, E. M., Pirttil,ä, T., Anderson, G., Salminen, A., and Ojala, J. O. (2012). Pro-inflammatory interleukin-18 increases Alzheimer's disease-associated amyloid-β production in human neuron-like cells. J. Neuroinflammation 9 :199. doi: 10.1186/1742-2094-9-199
Suttmann, H., Retz, M., Paulsen, F., Harder, J., Zwergel, U., Kamradt, J., et al. (2008). Antimicrobial peptides of the Cecropin-family show potent antitumor activity against bladder cancer cells. BMC Urol. 8:5. doi: 10.1186/1471-2490-8-5
Sverdlov, A. L., Ngo, D. T., Chan, W. P., Chirkov, Y. Y., and Horowitz, J. D. (2014). Aging of the nitric oxide system: are we as old as our NO? J. Am. Heart Assoc. 3:e000973. doi: 10.1161/JAHA.114.000973
Takahashi, A., Loo, T. M., Okada, R., Kamachi, F., Watanabe, Y., Wakita, M., et al. (2018). Downregulation of cytoplasmic DNases is implicated in cytoplasmic DNA accumulation and SASP in senescent cells. Nat. Commun. 9, 1249. doi: 10.1038/s41467-018-03555-8
Takasugi, M. (2018). Emerging roles of extracellular vesicles in cellular senescence and aging. Aging Cell 17:e12734. doi: 10.1111/acel.12734
Takousis, P., Devonshire, A. S., Redshaw, N., von Baumgarten, L., Whale, A. S., and Gerwyn, M. J. (2018). Cell-free DNA in cerebrospinal fluid: evaluating a new biomarker for Alzheimer's disease. Alzheimers. Dement. 14, 777. doi: 10.1016/j.jalz.2018.06.952
Tan, F. C., Hutchison, E. R., Eitan, E., and Mattson, M. P. (2014). Are there roles for brain cell senescence in aging and neurodegenerative disorders? Biogerontology 15, 643–660. doi: 10.1007/s10522-014-9532-1
Tarantini, S., Tran, C. H. T., Gordon, G. R., Ungvari, Z., and Csiszar, A. (2016). Impaired neurovascular coupling in aging and Alzheimer's disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp. Gerontol. 94, 52–58. doi: 10.1016/j.exger.2016.11.004
Tencza, S. B., Douglass, J. P., Creighton, D. J., Montelaro, R. C., and Mietzner, T. A. (1997). Novel antimicrobial peptides derived from human immunodeficiency virus type 1 and other lentivirus transmembrane proteins. Antimicrob. Agents Chemother. 41, 2394–2398. doi: 10.1128/AAC.41.11.2394
Teo, Y. V., Capri, M., Morsiani, C., Pizza, G., Faria, A. M. C., Franceschi, C., et al. (2018). Cell-free DNA as a biomarker of aging. Aging Cell 18:e12890. doi: 10.1111/acel.12890
Terman, A., and Brunk, U. T. (1998). Lipofuscin: mechanisms of formation and increase with age. APMIS 106, 265–276. doi: 10.1111/j.1699-0463.1998.tb01346.x
Thompson, M. G., Corey, B. W., Si, Y., Craft, D. W., and Zurawski, D. V. (2012). Antibacterial activities of iron chelators against common nosocomial pathogens. Antimicrob. Agents Chemother. 56, 5419–5421. doi: 10.1128/AAC.01197-12
Tükel, C., Wilson, R. P., Nishimori, J. H., Pezeshki, M., Chromy, B. A., and Bäumler, A. J. (2009). Responses to amyloids of microbial and host origin are mediated through toll-like receptor 2. Cell Host Microbe 6, 45–53. doi: 10.1016/j.chom.2009.05.020
Tursi, S. A., and Tükel, Ç. (2018). Curli-containing enteric biofilms inside and out: matrix composition, immune recognition, and disease implications. Microbiol. Mol. Biol. Rev. 82:e00028–e00018. doi: 10.1128/MMBR.00028-18
Uekawa, N., Nishikimi, A., Isobe, K., Iwakura, Y., and Maruyama, M. (2004). Involvement of IL-1 family proteins in p38 linked cellular senescence of mouse embryonic fibroblasts. FEBS Lett. 575, 30–34. doi: 10.1016/j.febslet.2004.08.033
van der Geest, K. S., Lorencetti, P. G., Abdulahad, W. H., Horst, G., Huitema, M., Roozendaal, C., et al. (2016). Aging-dependent decline of IL-10 producing B cells coincides with production of antinuclear antibodies but not rheumatoid factors. Exp. Gerontol. 75, 24–29. doi: 10.1016/j.exger.2015.12.009
van Leeuwen, L. A., and Hoozemans, J. J. (2015). Physiological and pathophysiological functions of cell cycle proteins in post-mitotic neurons: implications for Alzheimer's disease. Acta Neuropathol. 129, 511–525. doi: 10.1007/s00401-015-1382-7
Van Skike, C. E., and Galvan, V. (2018). A perfect sTORm: the role of the mammalian target of rapamycin (mTOR) in cerebrovascular dysfunction of Alzheimer's disease: a mini-review. Gerontology 64, 205–211. doi: 10.1159/000485381
van Splunter, M., Perdijk, O., Fick-Brinkhof, H., Feitsma, A. L., Floris-Vollenbroek, E. G., Meijer, B., et al. (2018). Bovine lactoferrin enhances TLR7-mediated responses in plasmacytoid dendritic cells in elderly women: results from a nutritional intervention study with bovine lactoferrin, GOS and vitamin D. Front. Immunol. 9:2677. doi: 10.3389/fimmu.2018.02677
Vance, D. E., Fazeli, P. L., Moneyham, L., Keltner, N. L., and Raper, J. L. (2013). Assessing and treating forgetfulness and cognitive problems in adults with HIV. J. Assoc. Nurses AIDS Care 24, S40–60. doi: 10.1016/j.jana.2012.03.006
Varasteh, S., Fink-Gremmels, J., Garssen, J., and Braber, S. (2017). α-Lipoic acid prevents the intestinal epithelial monolayer damage under heat stress conditions: model experiments in Caco-2 cells. Eur. J. Nutr. 57, 1577–1589. doi: 10.1007/s00394-017-1442-y
Ventura, M. T., Casciaro, M., Gangemi, S., and Buquicchio, R. (2017). Immunosenescence in aging: between immune cells depletion and cytokines up-regulation. Clin. Mol. Allergy 15:21. doi: 10.1186/s12948-017-0077-0
Verheijen, B. M., Vermulst, M., and van Leeuwen, F. W. (2018). Somatic mutations in neurons during aging and neurodegeneration. Acta Neuropathol. 135, 811–826. doi: 10.1007/s00401-018-1850-y
Vilalta, A., and Brown, G. C. (2018). Neurophagy, the phagocytosis of live neurons and synapses by glia, contributes to brain development and disease. FEBS J. 285, 3566–3575. doi: 10.1111/febs.14323
Villela, D., Suemoto, C. K., Leite, R., Pasqualucci, C. A., Grinberg, L. T., Pearson, P., et al. (2018). Increased DNA copy number variation mosaicism in elderly human brain. Neural Plast. 2018:2406170. doi: 10.1155/2018/2406170
von Bernhardi, R., Eugenín-von Bernhardi, L., and Eugenín, J. (2015). Microglial cell dysregulation in brain aging and neurodegeneration. Front. Aging Neurosci. 7:124. doi: 10.3389/fnagi.2015.00124
Walker, D. G., Dalsing-Hernandez, J. E., and Lue, L. F. (2007). Human postmortem brain-derived cerebrovascular smooth muscle cells express all genes of the classical complement pathway: a potential mechanism for vascular damage in cerebral amyloid angiopathy and Alzheimer's disease. Microvasc. Res. 75, 411–419. doi: 10.1016/j.mvr.2007.10.004
Walsh, J. G., Muruve, D. A., and Power, C. (2014). Inflammasomes in the CNS. Nat. Rev. Neurosci. 15, 84–97. doi: 10.1038/nrn3638
Walter, J. (2008). Ecological role of lactobacilli in the gastrointestinal tract: implications for fundamental and biomedical research. Appl. Environ. Microbiol. 74, 4985–4996. doi: 10.1128/AEM.00753-08
Walters, H. E., and Cox, L. S. (2018). mTORC inhibitors as broad-spectrum therapeutics for age-related diseases. Int. J. Mol. Sci. 19, 2325. doi: 10.3390/ijms19082325
Wang, R., Yu, Z., Sunchu, B., Shoaf, J., Dang, I., Zhao, S., et al. (2017). Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2-independent mechanism. Aging Cell 16, 564–574. doi: 10.1111/acel.12587
Wang, S., Xie, X., Lei, T., Zhang, K., Lai, B., Zhang, Z., et al. (2017). Statins attenuate activation of the NLRP3 inflammasome by oxidized LDL or TNFα in vascular endothelial cells through a PXR-dependent mechanism. Mol. Pharmacol. 92, 256–264. doi: 10.1124/mol.116.108100
Wang, W., Wang, L., Ruan, L., Oh, J., Dong, X., Zhuge, Q., et al. (2018). Extracellular vesicles extracted from young donor serum attenuate inflammaging via partially rejuvenating aged T-cell immunotolerance. FASEB J. doi: 10.1096/fj.201800059R. [Epub ahead of print].
Wang, Y., Jin, S., Sonobe, Y., Cheng, Y., Horiuchi, H., Parajuli, B., et al. (2014). Interleukin-1β induces blood–brain barrier disruption by downregulating Sonic hedgehog in astrocytes. PLoS ONE 9:e110024. doi: 10.1371/journal.pone.0110024
Ward, R. J., Zucca, F. A., Duyn, J. H., Crichton, R. R., and Zecca, L. (2014). The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 13, 1045–1060. doi: 10.1016/S1474-4422(14)70117-6
Wazea, S. A., Wadie, W., Bahgat, A. K., and El-Abhar, H. S. (2018). Galantamine anti-colitic effect: role of alpha-7 nicotinic acetylcholine receptor in modulating Jak/STAT3, NF-κB/HMGB1/RAGE and p-AKT/Bcl-2 pathways. Sci. Rep. 8:5110. doi: 10.1038/s41598-018-23359-6
Weis, S., Carlos, A. R., Moita, M. R., Singh, S., Blankenhaus, B., Cardoso, S., et al. (2017). Metabolic adaptation establishes disease tolerance to sepsis. Cell 169, 169–75.e14. doi: 10.1016/j.cell.2017.05.031
Wen, H., Ting, J. P., and O'Neill, L. A. (2012). A role for the NLRP3 inflammasome in metabolic diseases—did Warburg miss inflammation? Nat. Immunol. 13, 352–357. doi: 10.1038/ni.2228
Wen, L., Zhang, Q. S., Heng, Y., Chen, Y., Wang, S., Yuan, Y. H., et al. (2018). NLRP3 inflammasome activation in the thymus of MPTP-induced Parkinsonian mouse model. Toxicol. Lett. 288, 1–8. doi: 10.1016/j.toxlet.2018.02.003
Wendeln, A. C., Degenhardt, K., Kaurani, L., Gertig, M., Ulas, T., Jain, G., et al. (2018). Innate immune memory in the brain shapes neurological disease hallmarks. Nature 556, 332–338. doi: 10.1038/s41586-018-0023-4
Weng, D., Wu, Q., Chen, X. Q., Du, Y. K., Chen, T., Li, H., et al. (2019). Azithromycin treats diffuse panbronchiolitis by targeting T cells via inhibition of mTOR pathway. Biomed. Pharmacother. 110, 440–448. doi: 10.1016/j.biopha.2018.11.090
Wildsmith, K. R., Holley, M., Savage, J. C., Skerrett, R., and Landreth, G. E. (2013). Evidence for impaired amyloid β clearance in Alzheimer's disease. Alzheimers Res. Ther. 5:33. doi: 10.1186/alzrt187
Wilkaniec, A., Gassowska-Dobrowolska, M., Strawski, M., Adamczyk, A., and Czapski, G. A. (2018). Inhibition of cyclin-dependent kinase 5 affects early neuroinflammatory signalling in murine model of amyloid beta toxicity. J. Neuroinflammation 15:1. doi: 10.1186/s12974-017-1027-y
Williams, W. M., Castellani, R. J., Weinberg, A., Perry, G., and Smith, M. A. (2012). Do β-defensins and other antimicrobial peptides play a role in neuroimmune function and neurodegeneration? Sci. World J. 2012:905785. doi: 10.1100/2012/905785
Wiseman, F. K., Pulford, L. J., Barkus, C., Liao, F., Portelius, E., Webb, R., et al. (2018). Trisomy of human chromosome 21 enhances amyloid-β deposition independently of an extra copy of APP. Brain 141, 2457–2474. doi: 10.1093/brain/awy159
Won, S. M., Lee, J. H., Park, U. J., Gwag, J., Gwag, B. J., and Lee, Y. B. (2011). Iron mediates endothelial cell damage and blood–brain barrier opening in the hippocampus after transient forebrain ischemia in rats. Exp. Mol. Med. 43, 121–128. doi: 10.3858/emm.2011.43.2.020
Wu, F., Zou, Q., Ding, X., Shi, D., Zhu, X., Hu, W., et al. (2016). Complement component C3a plays a critical role in endothelial activation and leukocyte recruitment into the brain. J. Neuroinflammation 13:23. doi: 10.1186/s12974-016-0485-y
Wu, J., Chen, J., Wu, W., Shi, J., Zhong, Y., van Tol, E. A., et al. (2014). Enteral supplementation of bovine lactoferrin improves gut barrier function in rats after massive bowel resection. Br. J. Nutr. 112, 486–492. doi: 10.1017/S000711451400107X
Wu, J., Ren, W., Li, L., Luo, M., Xu, K., Shen, J., et al. (2018). Effect of aging and glucagon-like peptide 2 on intestinal microbiota in SD rats. Aging Dis. 9, 566–577. doi: 10.14336/AD.2017.1001
Xing, Y., Liqi, Z., Jian, L., Qinghua, Y., and Qian, Y. (2017). Doxycycline induces mitophagy and suppresses production of interferon-β in IPEC-J2 cells. Front. Cell. Infect. Microbiol. 7:21. doi: 10.3389/fcimb.2017.00021
Xiong, S., Mu, T., Wang, G., and Jiang, X. (2014). Mitochondria-mediated apoptosis in mammals. Protein Cell 5, 737–749. doi: 10.1007/s13238-014-0089-1
Xu, G., Li, Z., Ding, L., Tang, H., Guo, S., Liang, H., et al. (2015). Intestinal mTOR regulates GLP-1 production in mouse L cells. Diabetologia 58, 1887–1897. doi: 10.1007/s00125-015-3632-6
Xu, K., Dai, X. L., Huang, H. C., and Jiang, Z. F. (2011). Targeting HDACs: a promising therapy for Alzheimer's disease. Oxid. Med. Cell. Longev. 2011:143269. doi: 10.1155/2011/143269
Yamazaki, Y., Baker, D. J., Tachibana, M., Liu, C. C., van Deursen, J. M., Brott, T. G., et al. (2016). Vascular cell senescence contributes to blood–brain barrier breakdown. Stroke 47, 1068–1077. doi: 10.1161/STROKEAHA.115.010835
Yellen, G. (2018). Fueling thought: management of glycolysis and oxidative phosphorylation in neuronal metabolism. J. Cell Biol. 217, 2235–2246. doi: 10.1083/jcb.201803152
Yi, Y. S. (2017). Role of inflammasomes in inflammatory autoimmune rheumatic diseases. Korean J. Physiol. Pharmacol. 22, 1–15. doi: 10.4196/kjpp.2018.22.1.1
Yin, Y., Zhou, Z., Liu, W., Chang, Q., Sun, G., and Dai, Y. (2017). Vascular endothelial cells senescence is associated with NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome activation via reactive oxygen species (ROS)/thioredoxin-interacting protein (TXNIP) pathway. Int. J. Biochem. Cell Biol. 84, 22–34. doi: 10.1016/j.biocel.2017.01.001
Yoshida, N., Emoto, T., Yamashita, T., Watanabe, H., Hayashi, T., Tabata, T., et al. (2018). Bacteroides vulgatus and Bacteroides dorei reduce gut microbial lipopolysaccharide production and inhibit atherosclerosis. Circulation 138, 2486–2498. doi: 10.1161/CIRCULATIONAHA.118.033714
Yoshioka, M., Yokoyama, N., Masuda, K., Honna, T., Hinode, D., Nakamura, R., et al. (2003). Effect of hydroxamic acid-based matrix metalloproteinase inhibitors on human gingival cells and Porphyromonas gingivalis. J Periodontol 74, 1219–1224. doi: 10.1902/jop.2003.74.8.1219
Youm, Y. H., Kanneganti, T. D., Vandanmagsar, B., Zhu, X., Ravussin, A., Adijiang, A., et al. (2012). The Nlrp3 inflammasome promotes age-related thymic demise and immunosenescence. Cell Rep. 1, 56–68. doi: 10.1016/j.celrep.2011.11.005
Yu, H. M., Zhao, Y. M., Luo, X. G., Feng, Y., Ren, Y., Shang, H., et al. (2012). Repeated lipopolysaccharide stimulation induces cellular senescence in BV2 cells. Neuroimmunomodulation 19, 131–136. doi: 10.1159/000330254
Yu, J. T., Tan, L., Song, J. H., Sun, Y. P., Chen, W., Miao, D., et al. (2009). Interleukin-18 promoter polymorphisms and risk of late onset Alzheimer's disease. Brain Res. 1253, 169–175. doi: 10.1016/j.brainres.2008.11.083
Yun, S. P., Kam, T. I., Panicker, N., Kim, S., Oh, Y., Park, J. S., et al. (2018). Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson's disease. Nat. Med. 24, 931–938. doi: 10.1038/s41591-018-0051-5
Yusta, B., Baggio, L. L., Koehler, J., Holland, D., Cao, X., Pinnell, L. J., et al. (2015). GLP-1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte (IEL) GLP-1R. Diabetes 64, 2537–2549. doi: 10.2337/db14-1577
Zhan, X., Stamova, B., and Sharp, F. R. (2018). Lipopolysaccharide associates with amyloid plaques, neurons and oligodendrocytes in Alzheimer's disease brain: a review. Front. Aging Neurosci. 10:42. doi: 10.3389/fnagi.2018.00042
Zhang, C., Rissman, R. A., and Feng, J. (2015). Characterization of ATP alternations in an Alzheimer's disease transgenic mouse model. J. Alzheimers Dis. 44, 375–378. doi: 10.3233/JAD-141890
Zhang, J., Cicero, S. A., Wang, L., Romito-Digiacomo, R. R., Yang, Y., and Herrup, K. (2008). Nuclear localization of Cdk5 is a key determinant in the postmitotic state of neurons. Proc. Natl. Acad. Sci. U.S.A. 105, 8772–8777. doi: 10.1073/pnas.0711355105
Zhang, P., Shao, X. Y., Qi, G. J., Chen, Q., Bu, L. L., Chen, L. J., et al. (2016). Cdk5-dependent activation of neuronal inflammasomes in Parkinson's disease. Mov. Disord. 31, 366–376. doi: 10.1002/mds.26488
Zhang, T., Tian, F., Wang, J., Zhou, S., Dong, X., Guo, K., et al. (2015). Donepezil attenuates high glucose-accelerated senescence in human umbilical vein endothelial cells through SIRT1 activation. Cell Stress Chaperones 20, 787–792. doi: 10.1007/s12192-015-0601-4
Zhang, W., Zhang, Y., Guo, X., Zeng, Z., Wu, J., Liu, Y., et al. (2017). Sirt1 protects endothelial cells against LPS-induced barrier dysfunction. Oxid. Med. Cell. Longev. 2017:4082102. doi: 10.1155/2017/4082102
Zhang, Y., Nicolau, A., Lima, C. F., and Rodrigues, L. R. (2014). Bovine lactoferrin induces cell cycle arrest and inhibits mTOR signaling in breast cancer cells. Nutr. Cancer 66, 1371–1385. doi: 10.1080/01635581.2014.956260
Zhang, Y., Zhao, L., Wu, Z., Chen, X., and Ma, T. (2017). Galantamine alleviates senescence of U87 cells induced by beta-amyloid through decreasing ROS production. Neurosci Lett. 653, 183–188. doi: 10.1016/j.neulet.2017.05.055
Zhao, G., Wang, H., Xu, C., Wang, P., Chen, J., Wang, P., et al. (2016). SIRT6 delays cellular senescence by promoting p27Kip1 ubiquitin-proteasome degradation. Aging 8, 2308–2323. doi: 10.18632/aging.101038
Zhao, Y., Cong, L., and Lukiw, W. J. (2017). Lipopolysaccharide (LPS) accumulates in neocortical neurons of Alzheimer's disease (AD) brain and impairs transcription in human neuronal–glial primary co-cultures. Front. Aging Neurosci. 9 :407. doi: 10.3389/fnagi.2017.00407
Zhao, Y., Wu, X., Li, X., Jiang, L. L., Gui, X., Liu, Y., et al. (2018). TREM2 Is a Receptor for β-amyloid that mediates microglial function. Neuron 97, 1023–1031.e7. doi: 10.1016/j.neuron.2018.01.031
Keywords: microbiome, amyloid hypothesis, infection, senescence, inflammation
Citation: Osorio C, Kanukuntla T, Diaz E, Jafri N, Cummings M and Sfera A (2019) The Post-amyloid Era in Alzheimer's Disease: Trust Your Gut Feeling. Front. Aging Neurosci. 11:143. doi: 10.3389/fnagi.2019.00143
Received: 28 February 2019; Accepted: 29 May 2019;
Published: 26 June 2019.
Edited by:
Anne Eckert, University Psychiatric Clinic Basel, SwitzerlandReviewed by:
Ramesh Kandimalla, Texas Tech University Health Sciences Center, United StatesMorgan Newman, University of Adelaide, Australia
Copyright © 2019 Osorio, Kanukuntla, Diaz, Jafri, Cummings and Sfera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adonis Sfera, adonis.sfera@PSH.ca.gov
 Carolina Osorio1
Carolina Osorio1 Eddie Diaz
Eddie Diaz Adonis Sfera
Adonis Sfera