- 1Department of Ophthalmology, Qingdao Municipal Hospital, Qingdao, China
- 2Department of Pediatrics, Qingdao University Affiliated Hospital, Qingdao, China
- 3Department of Ophthalmology, Shanghai Jiaotong University Affiliated Shanghai First People’s Hospital, Shanghai, China
The aging retinal pigment epithelium and oxidative stress, mediated by reactive oxygen species (ROS) accumulation, have been implicated in the mechanisms of age-related macular degeneration (AMD). The expression level of the adapter protein p66shc, a key protein that regulates cellular oxidative stress, is relatively low under normal conditions because of the effects of silent mating type information regulation 2 homolog 1 (SIRT1) on the binding of fully deacetylated histone H3’ to the p66shc promoter region, thus inhibiting p66shc transcription and expression. The equilibrium between SIRT1 and p66shc is disrupted in the presence of various stresses, including AMD. As a major target gene, SIRT1 is regulated by microRNA-34a (miR-34a), and overexpression of miR-34a results in significant inhibition of post-transcriptional expression of SIRT1. Furthermore, our recent studies demonstrated that miR-34a is significantly upregulated, accompanied by reduced tolerance to oxidative stress in hydrogen peroxide-induced prematurely senescent ARPE-19 cells. Moreover, the expression of SIRT1 is decreased, whereas that of p66shc is increased in these cells. Accordingly, miR-34a may play a key role in age-related susceptibility to oxidative stress in ARPE-19 cells by targeting the SIRT1/p66shc pathway, leading to AMD. In this review article, we discuss the functions of miR-34a in modulating the SIRT1/p66shc pathway in age-related conditions, including AMD.
Introduction
Age-related macular degeneration (AMD), a leading cause of vision impairment in the elderly, is a multifactorial disease that involves age, gene variants of complement regulatory proteins, and smoking. With the increased aging of society, the number of patients with AMD is increasing, making this condition an important public health problem worldwide (Lim et al., 2012). AMD accounts for 54% of blindness in Caucasian Americana (Congdon et al., 2004), and a systematic review of data from an epidemiological survey of AMD prevalence found that the prevalence of any AMD ranged from 2.44% in people ages 45–49 years to 18.98% in people ages 85–89 years (Song et al., 2017). In a global meta-analysis, the overall prevalence of any AMD was 8.69% (Wong et al., 2014), and approximately 1.7 million people in the United States were affected by AMD, causing severe visual impairment in adults older than 65 years (Abbasi, 2018). Approximately 25.0% of eyes considered normal based on dilated eye examinations by primary eye care physicians (Neely et al., 2017); thus, the incidence of AMD is higher. The characteristic lesions of AMD are drusen, which are visible clinically in both the macula and retinal periphery (Mitchell et al., 2018). As AMD progresses, it can develop into two distinct forms of late AMD: “dry,” atrophic AMD, characterized by retinal pigment epithelium (RPE) senescence and geographic RPE loss, and “wet,” neovascular AMD, characterized by abnormal growth of choroidal new vessels (Zhu et al., 2009). Intraretinal or subretinal leakage, hemorrhage, and RPE detachments may occur with neovascular AMD, resulting in a rapid decline in vision. However, RPE atrophy or geographic atrophy may occur with late phase of dry AMD, resulting in a gradual vision decrease (Hallak et al., 2019).
Although the development of anti-vascular endothelial growth factor (VEGF) drugs has revolutionized the treatment of wet AMD (Martin et al., 2011), some patients show poor responses to these drugs. Additionally, there are few effective treatments for dry AMD. Therefore, current studies have focused on exploring the pathogenesis of AMD and identifying targets for early intervention and treatment of AMD.
Although the exact pathogenesis of AMD is not fully understood, a number of metabolic abnormalities have been shown to be associated with the development of this disease (Ding et al., 2009). For example, oxidative stress and oxidative stress-induced inflammation were found to initiate AMD in one study (Hollyfield et al., 2008).
In this review article, we provide a discussion of recent literature describing the mechanisms of AMD development, with a focus on RPE senescence, silent mating type information regulation 2 homolog 1 (SIRT1) signaling, and microRNA (miRNA) activity.
Relationship Between RPE Senescence and AMD
Age is the most important risk factor for AMD (Curcio et al., 2009). More than 10% of older people over 80 suffer from advanced AMD (Smith et al., 2001). Indeed, with the increasing age, the organs and tissues of the whole body, including retinal tissue, showed decreased function. The RPE is a highly specialized epithelial cell layer with polarity that interacts with photoreceptors on its apical side and with Bruch’s membrane and the choriocapillaris on its basal side (Datta et al., 2017). The RPE functions in phagocytosis of the photoreceptor outer segment and thereby plays an important role in maintaining the normal physiological function of photoreceptors.
With aging and the gradual accumulation of environmental stresses, RPE cells gradually show dysfunction, including decreased phagocytosis, lipofuscin deposition, and drusen formation. These morphological and functional changes in the outer retina induced by RPE degeneration play an important role in the pathogenesis of AMD.
Notably, oxidative stress induces premature senescence in RPE cells, leading to an imbalance in VEGF and complement factor h, and may be a main player in the induction and progression of AMD (Marazita et al., 2016). As shown in Figure 1, in our preliminary experiments, we found that aged adult human retinal pigment epithelial (ARPE-19) cells produced more reactive oxygen species (ROS) and that cell viability decreased and apoptosis increased significantly after hydrogen peroxide stimulation when compared with the results in young cells, providing support for the age-related susceptibility of ARPE-19 cells to oxidative stress.
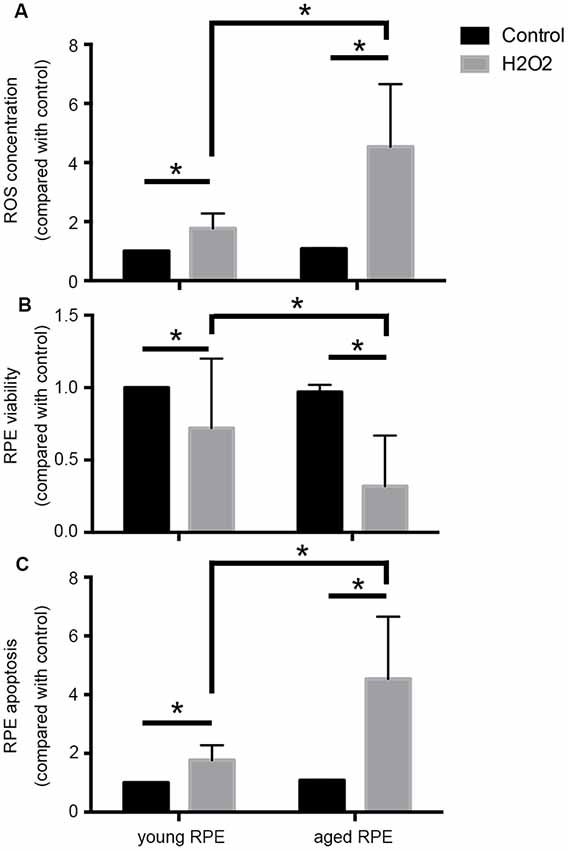
Figure 1. Aging induced susceptibility to oxidative stress in ARPE-19 cells. Aged ARPE-19 cells produced more reactive oxygen species (ROS; A), and cell viability decreased (B), whereas apoptosis increased (C) significantly after hydrogen peroxide stimulation when compared with the effects in young ARPE-19 cells. *p < 0.05 significantly different when compared within two groups.
Relationships Among miR-34a, RPE Senescence, and AMD Pathogenesis
miRNAs are small noncoding RNA molecules (~20–24 nucleotides in length) found in all cell types. These molecules lead to RNA silencing and post-transcriptional regulation of target gene expression, thus affecting cellular function and determining cell fate. Many miRNAs are differentially expressed in the circulation in patients with AMD (Ren et al., 2017). Additionally, significant changes in miNRA expression have been observed in retinal tissues from patients with AMD by bioinformatics and microarray technology. These miRNAs may be involved in the pathological processes of AMD by targeting downstream transcription factors. However, the specific regulatory mechanisms are still unclear (Berber et al., 2017).
As a p53-regulated miRNA, miR-34a was first discovered in studies of cancer. The activation of miR-34a promotes cell apoptosis and inhibits tumorigenesis. Recently, miR-34a has been shown to be closely related to cellular senescence in tissues. Notably, miR-34a is significantly upregulated in aged hearts and can promote the senescence of cardiac myocytes and decrease systolic function by inhibiting the expression of protein phosphatase 1 nuclear-targeting subunit and inducing telomere shortening (Boon et al., 2013). In contrast, miR-34a can also promote cellular senescence to maintain normal physiological function and avoid unlimited cell proliferation. Indeed, miR-34a regulates telomerase activity in hepatocellular carcinoma cells and promotes cellular senescence by targeting the FoxM1/c-Myc pathway (Xu et al., 2015). miR-34a is also a pivotal regulator of aging. For example, some drugs can regulate aging by affecting the expression of miR-34a and activating related signal pathways (Ye et al., 2016; Guo et al., 2017; He et al., 2017). Additionally, there was an age-dependent increase in miR-34a expression in the posterior pole of the mouse eye, and DNA damage in the mitochondria in retinal cells and RPE cells was found to be related to age and upregulation of miR-34a expression (Smit-McBride et al., 2014). Thus, miR-34a may play an important role in the pathophysiological mechanisms of cell senescence and apoptosis in the retina and in RPE cells in the aging eye.
In our previous studies, we evaluated the differential expression of miR-34a in the vitreous humor, collected during pars plana vitrectomy, from young patients without AMD, elderly patients without AMD, and patients with AMD. As shown in Figure 2A, the expression of miR-34a in the vitreous humor of the three groups of patients was increased, and there was a positive correlation between the expression of miR-34a and age (Figure 2B), as demonstrated by Pearson correlation analysis. These results indicated that miR-34a may be involved in RPE aging and the pathogenesis of AMD.
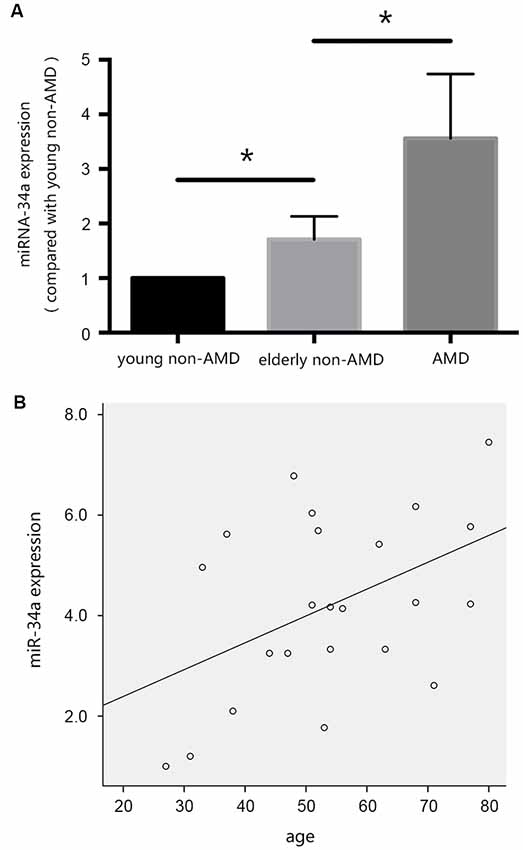
Figure 2. miR-34a may be involved in the pathogenesis of age-related macular degeneration (AMD). (A) The expression of miR-34a in the vitreous humor of the three groups of patients was increased. *p < 0.05 significantly different when compared within two groups. (B) Scatter plot showing the association between miR-34a expression and age. Note that there was a positive relationship between the expression of miR-34a and age (r = 0.457, p = 0.029).
The SIRT1/p66shc Pathway and Resistance to Oxidative Stress
SIRT1, as an NAD-dependent histone deacetylase in mammals (Vaziri et al., 2001), is a homolog of sir2 in lower organisms and plays important roles in various biological activities, such as the cell cycle, apoptosis, DNA repair, and gene silencing. The fundamental mechanisms through which SIRT1 regulates the expression of target genes involve histone H3 deacetylation of the promoter or enhancer (Oppenheimer et al., 2014; Hsu et al., 2016).
p66shc is widely expressed in vertebrates and is a key protein involved in intracellular regulation of oxidative stress and the life cycle. This protein induces cellular oxidative damage by producing a large number of ROS, mainly through the oxidation of cytochrome c in the mitochondria, and by inhibiting the elimination of ROS. Moreover, deletion of the p66shc gene can prolong the life span of mice by 30% via a mechanism involving enhancement of the resistance of mice to oxidative stress (Migliaccio et al., 1999).
Notably, SIRT1 can target the promoter region of p66shc (−508 to −250 bp), resulting in a decrease in the acetylation of histone H3 bound to this region. p66shc transcription is therefore inhibited, reducing tissue injury caused by oxidative stress. In acute ethanol-induced liver injury, the expression of p66shc was negatively correlated with changes in SIRT1 expression and decreased SIRT1 expression by RNA interference or nicotine significantly increased the expression of p66Shc (Tian et al., 2016). Therefore, the SIRT1/p66shc pathway may play important roles in blocking the effects of oxidative stress.
Many studies have shown that activation of SIRT1 may delay senescence in various tissues (Han et al., 2016; Guo et al., 2017; Hekmatimoghaddam et al., 2017), whereas inhibition of SIRT1 activity can cause premature senescence (Volonte et al., 2015), resulting in damage to cells caused by decreased tolerance of senescent cells to stress (Chuang et al., 2017). Additionally, the effects of SIRT1 on senescence are partly due to the regulation of miR-34a (Yang et al., 2013), although these effects have not been reported in retinal tissues and RPE cells. miR-34a was found to specifically bind with SIRT1 mRNA in the 3′ untranslated region by bioinformatics prediction websites, such as pitcar, targetscan, Miranda, and microRNA.org.
In our preliminary experiments, we established an RPE premature senescence model by hydrogen peroxide stimulation as described previously (Aryan et al., 2016). ARPE19 cells were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA). At passage 11, cells were seeded in 24-well plates at a density of 5 × 104 cells/well, and were routinely cultured in 95% air and 5% CO2 at 37°C in DMEM/F12 medium containing 10% FBS, 50 units/mL penicillin, and 50 μg/mL streptomycin. After 24 h of incubation, the growth medium was removed and replaced with serum-free medium (SFM). The medium of selected wells was changed to SFM + 0.3 mmol/L hydrogen peroxide 24 h later. After 90 min of hydrogen peroxide treatment, the medium in selected wells was changed to 1 ml of DMEM + 10% FBS for the subsequent study on cell senescence. The effect of hydrogen peroxide on retinal pigment epithelial cell senescence was investigated by S-Beta Galactosidase staining 24 h after treatment with hydrogen peroxide. We found that when compared with young RPE cells, the expression of SIRT1 decreased, whereas the expression of p66shc increased in aged RPE cells, consistent with the increase in miR-34a expression (Figure 3A). A reverse correlation between the expression of SIRT1 and miR-34a (Figure 3B) and a positive correlation between the expression of p66shc and miR-34a (Figure 3C) were found by Pearson correlation analysis, suggesting that miR-34a may directly inhibit the expression of SIRT1 in aged RPE cells, resulting in a decrease in the inhibitory effect on downstream p66shc and an increase in the expression of p66shc.
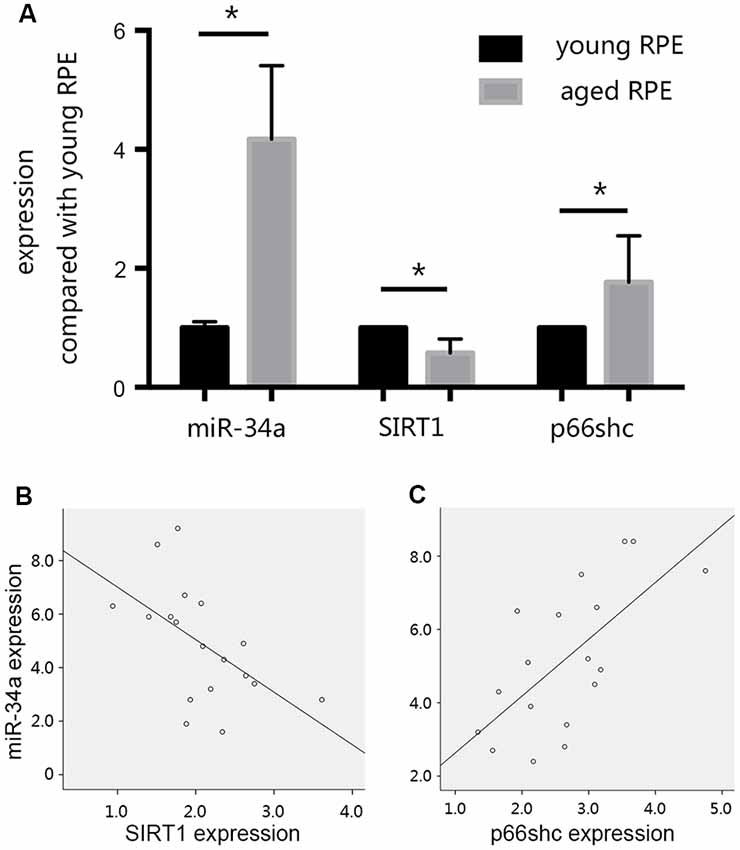
Figure 3. Changes in the expression levels of miR-34a, silent mating type information regulation 2 homolog 1 (SIRT1), and p66shc. (A) The expression of SIRT1 decreased, and the expression of p66shc increased in aged ARPE-19 cells, consistent with the increase in miR-34a expression. *p < 0.05 significantly different when compared within two groups. (B) Scatter plot showing the association between miR-34a expression and SIRT1 expression in ARPE-19 cells. Note that there was an inverse relationship between these targets (r = −0.549, p = 0.018). (C) Scatter plot showing the association between miR-34a expression and p66shc expression in ARPE-19 cells. Note that there was a positive relationship between these targets (r = 0.666, p = 0.003).
A Hypothesis Regarding the Pivotal Role of miR-34a in Age-Related Susceptibility to Oxidative Stress by Targeting the SIRT1/p66shc Pathway
As described above, previous relevant studies and our preliminary experiments have led us to hypothesize that miR-34a may play important roles in age-related susceptibility to oxidative stress.
Throughout life, the RPE cells are constantly challenged by high oxygen tension and exposure to photic stress, particularly in the macular region. As shown in Figure 4, the expression of the pro-apoptotic gene p53 and miR-34a in young RPE cells was relatively low. The low expression of miR-34a reduces its inhibitory effect on the downstream target gene SIRT1, which can target the promoter regions of p66shc and p53, resulting in decreased acetylation of histone H3 bound to these regions. The transcription of p66shc and p53 is therefore inhibited. Thus, the activities of p66shc and p53 in young RPE cells are relatively low, and the cells appear to be resilient to oxidative stress to a certain extent.
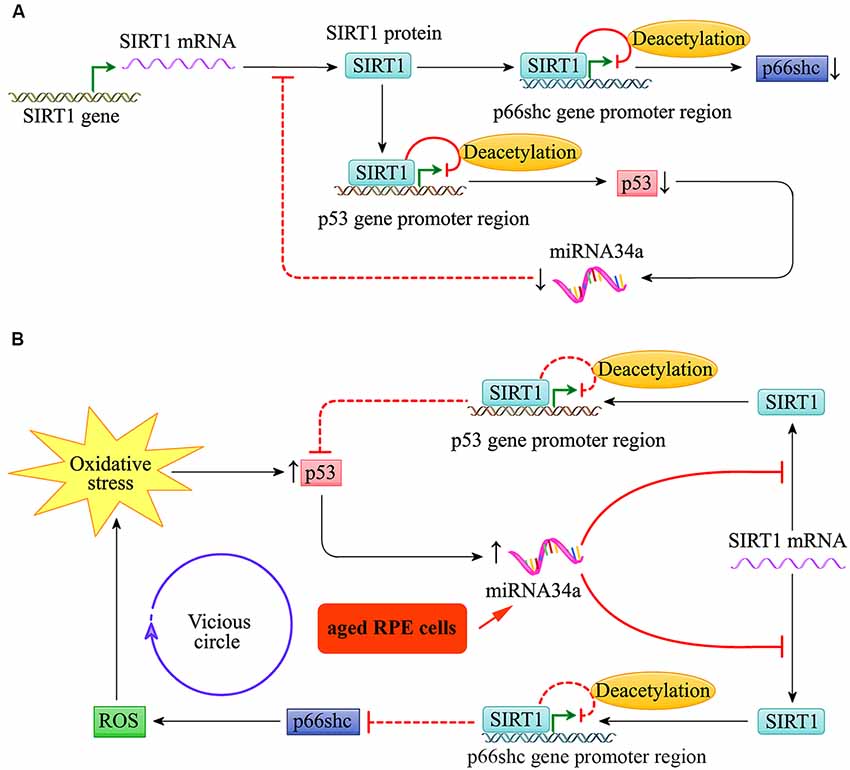
Figure 4. miR-34a regulated the SIRT1/p66shc pathway in young and aged retinal pigment epithelium (RPE) cells. (A) Schematic diagram for miR-34a and the SIRT1/p66shc pathway in young RPE cells. (B) Schematic diagram for miR-34a and the SIRT1/p66shc pathway in aged RPE cells.
Additionally, as shown in Figure 4, the expression of miR-34a in the RPE increases with cell aging, and its effect on repression of SIRT1 translation is enhanced. Then, the SIRT1/p66shc pathway is suppressed, and histone H3 deacetylation of p66Shc and p53 by SIRT1 is inhibited. The upregulation of p66shc induces oxidative stress by restoring excessive ROS and further induces the upregulation of miR-34a via the transcription factor p53. This results in a vicious circle of amplification for oxidative stress and leads to reduced resistance to oxidative stress in aged RPE cells.
Conclusion
miR-34a may be involved in regulating susceptibility to oxidative stress by targeting the SIRT1/p66shc pathway during senescence in the RPE and the development of AMD. Targeting the inhibition of miR-34a may have implications in gene therapy for the early prevention and treatment of AMD. To test this hypothesis, further studies in mice with miR-34a knockout or overexpression are needed.
Ethics Statement
The study was approved by Ethic Committee of Shanghai Jiaotong University Affiliated Shanghai First People’s Hospital, and patients provided written informed consent for participation in the study.
Author Contributions
XW and NT conceived and designed the study. NT, RJ and ZZ performed the experiments and reviewed the literatures. NT and RJ wrote the article. XW, NT, RJ and ZZ reviewed and edited the manuscript. All authors read and approved the manuscript.
Funding
This study was supported by National Natural Science Foundation of China (No. 81801381, No. 81701587, No. 81674027).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
References
Abbasi, J. (2018). Stem cell implants for age-related macular degeneration. JAMA 319:2263. doi: 10.1001/jama.2018.7447
Aryan, N., Betts-Obregon, B. S., Perry, G., and Tsin, A. T. (2016). Oxidative stress induces senescence in cultured RPE cells. Open Neurol. J. 10, 83–87. doi: 10.2174/1874205x01610010083
Berber, P., Grassmann, F., Kiel, C., and Weber, B. H. (2017). An eye on age-related macular degeneration: the role of microRNAs in disease pathology. Mol. Diagn. Ther. 21, 31–43. doi: 10.1007/s40291-016-0234-z
Boon, R. A., Iekushi, K., Lechner, S., Seeger, T., Fischer, A., Heydt, S., et al. (2013). MicroRNA-34a regulates cardiac ageing and function. Nature 495, 107–110. doi: 10.1038/nature11919
CATT Research Group, Martin, D. F., Maguire, M. G., Ying, G. S., Grunwald, J. E., Fine, S. L., et al. (2011). Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 364, 1897–1908. doi: 10.1056/NEJMoa1102673
Chuang, P. Y., Cai, W., Li, X., Fang, L., Xu, J., Yacoub, R., et al. (2017). Reduction in podocyte SIRT1 accelerates kidney injury in aging mice. Am. J. Physiol. Renal Physiol. 313, F621–F628. doi: 10.1152/ajprenal.00255.2017
Congdon, N., O’Colmain, B., Klaver, C. C., Klein, R., Muñoz, B., Friedman, D. S., et al. (2004). Causes and prevalence of visual impairment among adults in the United States. Arch. Ophthalmol. 122, 477–485. doi: 10.1001/archopht.122.4.477
Curcio, C. A., Johnson, M., Huang, J. D., and Rudolf, M. (2009). Aging, age-related macular degeneration, and the response-to-retention of apolipoprotein B-containing lipoproteins. Prog. Retin. Eye Res. 28, 393–422. doi: 10.1016/j.preteyeres.2009.08.001
Datta, S., Cano, M., Ebrahimi, K., Wang, L., and Handa, J. T. (2017). The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 60, 201–218. doi: 10.1016/j.preteyeres.2017.03.002
Ding, X., Patel, M., and Chan, C. C. (2009). Molecular pathology of age-related macular degeneration. Prog. Retin. Eye Res. 28, 1–18. doi: 10.1016/j.preteyeres.2008.10.001
Guo, Y., Li, P., Gao, L., Zhang, J., Yang, Z., Bledsoe, G., et al. (2017). Kallistatin reduces vascular senescence and aging by regulating microRNA-34a-SIRT1 pathway. Aging Cell 16, 837–846. doi: 10.1111/acel.12615
Hallak, J. A., de Sisternes, L., Osborne, A., Yaspan, B., Rubin, D. L., and Leng, T. (2019). Imaging, genetic and demographic factors associated with conversion to neovascular Age-Related Macular Degeneration: secondary analysis of a randomized clinical trial. JAMA Ophthalmol. doi: 10.1001/jamaophthalmol.2019.0868 [Epub ahead of print].
Han, X., Niu, J., Zhao, Y., Kong, Q., Tong, T., and Han, L. (2016). HDAC4 stabilizes SIRT1 via sumoylation SIRT1 to delay cellular senescence. Clin. Exp. Pharmacol. Physiol. 43, 41–46. doi: 10.1111/1440-1681.12496
He, X., Yang, A., McDonald, D. G., Riemer, E. C., Vanek, K. N., Schulte, B. A., et al. (2017). MiR-34a modulates ionizing radiation-induced senescence in lung cancer cells. Oncotarget 8, 69797–69807. doi: 10.18632/oncotarget.19267
Hekmatimoghaddam, S., Dehghani Firoozabadi, A., Zare-Khormizi, M. R., and Pourrajab, F. (2017). Sirt1 and Parp1 as epigenome safeguards and microRNAs as SASP-associated signals, in cellular senescence and aging. Ageing Res. Rev. 40, 120–141. doi: 10.1016/j.arr.2017.10.001
Hollyfield, J. G., Bonilha, V. L., Rayborn, M. E., Yang, X., Shadrach, K. G., Lu, L., et al. (2008). Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat. Med. 14, 194–198. doi: 10.1038/nm1709
Hsu, W. W., Wu, B., and Liu, W. R. (2016). Sirtuins 1 and 2 are universal histone deacetylases. ACS Chem. Biol. 11, 792–799. doi: 10.1021/acschembio.5b00886
Lim, L. S., Mitchell, P., Seddon, J. M., Holz, F. G., and Wong, T. Y. (2012). Age-related macular degeneration. Lancet 379, 1728–1738. doi: 10.1016/S0140-6736(12)60282-7
Marazita, M. C., Dugour, A., Marquioni-Ramella, M. D., Figueroa, J. M., and Suburo, A. M. (2016). Oxidative stress-induced premature senescence dysregulates VEGF and CFH expression in retinal pigment epithelial cells: implications for age-related macular degeneration. Redox Biol. 7, 78–87. doi: 10.1016/j.redox.2015.11.011
Migliaccio, E., Giorgio, M., Mele, S., Pelicci, G., Reboldi, P., Pandolfi, P. P., et al. (1999). The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402, 309–313. doi: 10.1038/46311
Mitchell, P., Liew, G., Gopinath, B., and Wong, T. Y. (2018). Age-related macular degeneration. Lancet 392, 1147–1159. doi: 10.1016/S0140-6736(18)31550-2
Neely, D. C., Bray, K. J., Huisingh, C. E., Clark, M. E., McGwin, G. Jr., and Owsley, C. (2017). Prevalence of undiagnosed age-related macular degeneration in primary eye care. JAMA Ophthalmol. 135, 570–575. doi: 10.1001/jamaophthalmol.2017.0830
Oppenheimer, H., Kumar, A., Meir, H., Schwartz, I., Zini, A., Haze, A., et al. (2014). Set7/9 impacts COL2A1 expression through binding and repression of SirT1 histone deacetylation. J. Bone Miner. Res. 29, 348–360. doi: 10.1002/jbmr.2052
Ren, C., Liu, Q., Wei, Q., Cai, W., He, M., Du, Y., et al. (2017). Circulating miRNAs as potential biomarkers of age-related macular degeneration. Cell. Physiol. Biochem. 41, 1413–1423. doi: 10.1159/000467941
Smith, W., Assink, J., Klein, R., Mitchell, P., Klaver, C. C., Klein, B. E., et al. (2001). Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology 108, 697–704. doi: 10.1016/S0161-6420(00)00580-7
Smit-McBride, Z., Forward, K. I., Nguyen, A. T., Bordbari, M. H., Oltjen, S. L., and Hjelmeland, L. M. (2014). Age-dependent increase in miRNA-34a expression in the posterior pole of the mouse eye. Mol. Vis. 20, 1569–1578.
Song, P., Du, Y., Chan, K. Y., Theodoratou, E., and Rudan, I. (2017). The national and subnational prevalence and burden of age-related macular degeneration in China. J. Glob. Health 7:020703. doi: 10.7189/jogh.07.020703
Tian, X., Hu, Y., Li, M., Xia, K., Yin, J., Chen, J., et al. (2016). Carnosic acid attenuates acute ethanol-induced liver injury via a SIRT1/p66Shc-mediated mitochondrial pathway. Can. J. Physiol. Pharmacol. 94, 416–425. doi: 10.1139/cjpp-2015-0276
Vaziri, H., Dessain, S. K., Ng Eaton, E., Imai, S. I., Frye, R. A., Pandita, T. K., et al. (2001). hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107, 149–159. doi: 10.1016/S0092-8674(01)00527-X
Volonte, D., Zou, H., Bartholomew, J. N., Liu, Z., Morel, P. A., and Galbiati, F. (2015). Oxidative stress-induced inhibition of Sirt1 by caveolin-1 promotes p53-dependent premature senescence and stimulates the secretion of interleukin 6 (IL-6). J. Biol. Chem. 290, 4202–4214. doi: 10.1074/jbc.m114.598268
Wong, W. L., Su, X., Li, X., Cheung, C. M., Klein, R., Cheng, C. Y., et al. (2014). Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob. Health 2, e106–e116. doi: 10.1016/s2214-109x(13)70145-1
Xu, X., Chen, W., Miao, R., Zhou, Y., Wang, Z., Zhang, L., et al. (2015). miR-34a induces cellular senescence via modulation of telomerase activity in human hepatocellular carcinoma by targeting FoxM1/c-Myc pathway. Oncotarget 6, 3988–4004. doi: 10.18632/oncotarget.2905
Yang, M. M., Fan, X. J., Du, W. T., Ren, P. L., and Liu, J. (2013). Research on regulation of cell senescence by miRNA-34a. Sichuan Da Xue Xue Bao Yi Xue Ban 44, 1–5.
Ye, Z., Fang, J., Dai, S., Wang, Y., Fu, Z., Feng, W., et al. (2016). MicroRNA-34a induces a senescence-like change via the down-regulation of SIRT1 and up-regulation of p53 protein in human esophageal squamous cancer cells with a wild-type p53 gene background. Cancer Lett. 370, 216–221. doi: 10.1016/j.canlet.2015.10.023
Keywords: microRNA-34a, silent mating type information regulation 2 homolog 1, p66shc, age-related macular degeneration, oxidative stress, premature senescence
Citation: Tong N, Jin R, Zhou Z and Wu X (2019) Involvement of microRNA-34a in Age-Related Susceptibility to Oxidative Stress in ARPE-19 Cells by Targeting the Silent Mating Type Information Regulation 2 Homolog 1/p66shc Pathway: Implications for Age-Related Macular Degeneration. Front. Aging Neurosci. 11:137. doi: 10.3389/fnagi.2019.00137
Received: 25 November 2018; Accepted: 22 May 2019;
Published: 13 June 2019.
Edited by:
Anne Eckert, University Psychiatric Clinic Basel, SwitzerlandReviewed by:
Zhigang Liu, Northwest A&F University, ChinaBhaskar Roy, University of Alabama at Birmingham, United States
Copyright © 2019 Tong, Jin, Zhou and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingwei Wu, ZXlld3h3QDEyNi5jb20=
† These authors have contributed equally to this work
 Nianting Tong
Nianting Tong Rong Jin2†
Rong Jin2† Xingwei Wu
Xingwei Wu