- 1Institute of Neurology of the IRCCS Fondazione Policlinico Gemelli, Catholic University of Rome, Rome, Italy
- 2Department of Clinical and Behavioral Neurology, IRCCS Fondazione Santa Lucia, Rome, Italy
Background: Two main models have been advanced to explain the asymmetries observed in the representation and processing of emotions. The first model, labeled “the right hemisphere hypothesis,” assumes a general dominance of the right hemisphere for all emotions, regardless of affective valence. The second model, named “the valence hypothesis,” assumes an opposite dominance of the left hemisphere for positive emotions and the right hemisphere for negative emotions. Patients with frontotemporal lobar degeneration (FTLD) could contribute to clarifying this issue, because disorders of emotional and social behavior are very common in FTLD and because atrophy, which affects the antero-ventral part of the frontal and temporal lobes, can be clearly asymmetric in the early stages of this disease.
Objective: The main scope of the present review therefore consists of evaluating if results of investigations conducted on emotional and behavioral disorders of patients with right and left FTLD, support the “right hemisphere” or the “valence” hypothesis.
Method: A thorough review of behavioral and emotional disorders in FTLD patients, found that 177 possible studies, but only 32 papers met the requested criteria for inclusion in our review.
Results: Almost all (25 out of 26) studies were relevant with respect to the “right hemisphere hypothesis” and supported the assumption of a general dominance of the right hemisphere for emotional functions, whereas only one of the six investigations were relevant with respect to the “valence hypothesis” and were in part consistent with this hypothesis, though these are also open to interpretation in terms of the “right hemisphere” hypothesis.
Conclusions: This study, therefore, clearly supports the model of a general dominance of the right hemisphere for all emotions, regardless of affective valence.
Introduction
Even if most authors acknowledge that important asymmetries exist in the representation and processing of emotions, the nature of these asymmetries remains controversial. Two main models concerning the relationship between emotions and brain laterality have been advanced. The first model, labeled “the right hemisphere hypothesis” (Gainotti, 1972, 2012; Borod et al., 1998; Mandal and Ambady, 2004), posits a general dominance of the right hemisphere for all emotions, regardless of affective valence. This model was proposed by Gainotti (1972) on the basis of clinical observations made in patients with right and left hemisphere lesions, because (aphasic) left brain-damaged patients typically showed “catastrophic reactions” in their frustrating attempts of verbal expression, whereas patients with severe right hemisphere lesions typically showed an “indifference reaction” in their defects and disabilities. Since this “indifference reaction” included a denial or a lack of concern for the disability and for failures during the neuropsychological examination and a tendency for patients to joke in a fatuous, ironic or sarcastic manner, Gainotti (1972) proposed that this pattern of behavior was much more abnormal than the catastrophic reactions of left brain-damaged patients. It was considered that abnormal emotional reactions were due to the major involvement of the right hemisphere in emotional functions, in agreement with the relationship that exist between language disturbances of left brain-damaged patients and the dominance of the left hemisphere for language. In the following years, this interpretation, assuming a general dominance of the right hemisphere for emotional functions, was confirmed by experimental and clinical investigations which studied the non-verbal communicative aspects of emotions in normal subjects and in patients with unilateral brain lesions. Most of these studies showed, in fact, a general dominance of the right hemisphere, both in comprehension and in facial and vocal expression of positive and negative emotions (see Borod et al., 1998; Mandal and Ambady, 2004 and Gainotti, 2018 for reviews).
The second model, “the valence hypothesis” (Ahern and Schwartz, 1979; Reuter-Lorenz and Davidson, 1981; Rodway et al., 2003), assumes an opposite dominance of the left hemisphere for positive emotions and of the right hemisphere for negative emotions. This model is based on results obtained on a limited number of investigations, which studied the non-verbal communicative aspects of emotions in normal subjects and in patients with unilateral brain lesions. Thus, Reuter-Lorenz and Davidson (1981), Reuter-Lorenz et al. (1983), and Natale et al. (1983), studying emotional comprehension with a tachistoscopic lateralized presentation of emotional faces to the right and left visual field, found a right hemisphere advantage for negative emotions and a left hemisphere advantage for positive emotions. In a similar vein, Sackeim and Gur (1978) and Sackeim and Grega (1987), using composite photographs of posed positive or negative emotions (created using two left or two right half faces), found a left-sided facial asymmetry in the expression of negative but not of positive emotions (see Gainotti et al., 1993; Mandal and Ambady, 2004 and Killgore and Yurgelun-Todd, 2007 for detailed critical reviews).
A variant of this model, proposed by Davidson (1983, 1992) and labeled “the approach-withdrawal” hypothesis, maintains that brain asymmetries observed during positive and negative emotions are not related to the valence of the emotional stimuli, but to the motivational system engaged by those stimuli. According to this model, the left prefrontal cortex (PFC) might be involved in a system facilitating an approach to appetitive stimuli, whereas the right PFC might be involved in a system facilitating withdrawal from aversive stimuli. A different set of data has been used to support, on one hand, the “right hemisphere hypothesis” and the “valence hypothesis” and, on the other hand, the “approach-withdrawal” hypothesis. Data supporting the “right hemisphere hypothesis” and the “valence hypothesis,” respectively, have been obtained by studying the comprehension and expression of positive and negative emotions at the facial or vocal level in normal subjects and in patients with focal unilateral brain damage. On the contrary, most data supporting “the approach-withdrawal” hypothesis have been obtained by studying EEG asymmetries at the level of the frontal lobes during positive and negative emotions or situations involving approach or avoidance/withdrawal components. However, the use of frontal EEG asymmetries to assess emotion or motivation has recently been criticized on the basis of methodological and conceptual reasons. For instance, Miller et al. (2013) have noticed that treating the left- and right- frontal lobes as the units of analysis can be misleading because hemodynamic and electromagnetic neuroimaging studies suggest considerable functional differentiation in specialization and activation of subregions of the frontal cortex, including their connectivity to each other and to other regions. Important methodological objections have also been advanced by Allen et al. (2018) and Reznik and Allen (2018), who have shown that there is no sufficient evidence to support the relationship between frontal EEG asymmetries and emotional or motivational systems. In contemporary clinical science, studies aiming to check these models, with neurological patients, have become much less prevalent than investigations using functional imaging, but results of these studies have failed to clearly support both the “right hemisphere” and the “valence” hypothesis or the “approach-withdrawal” hypothesis (see Wager et al., 2003 and Fusar-Poli et al., 2009 for meta-analytic reviews). These rather disappointing results must, however, be considered with caution, because constraints and limits imposed by functional imaging methodologies constitute a far from ideal environment for studying full-blown emotional and social processes. Thus, Levenson et al. (2014) noticed that, even if it were possible to induce strong emotions in the scanner, huge signal artifacts would be produced by the associated muscular activity in the body and face. Therefore, most emotional and social research conducted in the scanner fall into the realm of social and emotional cognition, where the focus is on how we think and make judgments about social and emotional processes, rather than on real emotions as they unfold in real time. In patients with brain damage, on the contrary, different kinds of emotional behavior can be observed under controlled conditions in the laboratory or in more naturalistic conditions in the patient's natural environment. In recent years the interest of clinical researchers has shifted from patients with focal brain damage to patients with degenerative brain diseases, and in particular to patients with frontotemporal lobar degeneration (FTLD), because disorders of emotional and social behavior are very common in these patients and because atrophy, which affects the antero-ventral parts of the frontal and temporal lobes can be clearly asymmetric in the early stages of this disease. FTLD is the pathological substrate associate with Frontotemporal dementia (FTD), which is, indeed, a clinically heterogeneous disease in which both frontally predominant (fvFTD) and temporally predominant (tvFTD) subtypes have been described. Typically, tvFTD has been defined by the presence of deficits in language and semantic knowledge and is for this reason often labeled Semantic Dementia (SD). On the other hand, fvFTD has been defined by its behavioral features, and is for this reason often labeled “behavioral variant Frontotemporal Dementia” (bvFTD) even if many studies (e.g., Bozeat et al., 2000; Snowden et al., 2001; Rosen et al., 2002a; Liu et al., 2004; Rascovsky et al., 2011) have shown that both of these anatomo-clinical syndromes may be associated with behavioral disturbances. Furthermore, several authors (e.g., Snowden et al., 1996, 2004; Thompson et al., 2003; Seeley et al., 2005, 2008) have shown that in the early stages of FTD the atrophy affecting the frontal and (even more) the anterior temporal lobes (ATLs) can be clearly asymmetric. Since many other studies (e.g., Miller et al., 1993; Edwards-Lee et al., 1997; Mychack et al., 2001; Perry et al., 2001; Seeley et al., 2005; Irish et al., 2013; Binney et al., 2016) have shown that emotional and behavioral disorders may be on the foreground when atrophy prevails in the right frontal or temporal lobes, we believe that a careful review of these studies could help to clarify the nature of the relationships that exist between emotions and brain laterality. A further source of information on this subject was represented by investigations conducted in patients with FTLD, in which correlations had been found between specific emotional disorders and atrophy or dysfunction of equally specific lateralized cortical or subcortical structures. The main scope of the present review therefore consists of evaluating if the results of investigations conducted in patients with FTD, clearly support the “right hemisphere” or the “valence” hypothesis or if it cannot clarify the nature of asymmetries that exist in the representation and processing of emotions.
Methods
In this review, to check the above mentioned models, we considered all studies found in the literature that investigated the influence of asymmetrical atrophies on emotional and behavioral disorders in patients with FTLD in the last 25 years (from 1993 to 2018). This time interval was chosen because, even if there had been many earlier papers evaluating the “right hemisphere” hypothesis in other neurological diseases (e.g., Coffey, 1987; Gainotti and Caltagirone, 1989; Borod et al., 1998), the first investigations dealing with the relationships between emotional/behavioral disorders and laterality of atrophy in FTLD were published 25 years ago (Miller et al., 1993). In the earliest studies authors focused on behavioral disorders and the assessment of emotional disturbances was based more on a clinical evaluation than on specific neuropsychological tests. On the contrary, in more recent investigation's specific emotional disorders were studied with specific sophisticated testing procedures. Since data from several studies suggested that strong interconnections exist between behavioral and emotional disorders, both kinds of studies were included in our review, even if a separate analysis of these data was also undertaken. With this aim in mind, we performed searches using PubMed and Medline for studies including diagnostic keywords (“degenerative diseases,” “semantic dementia,” “primary progressive aphasia,” and “FTD”) in conjunction with relevant content keywords related to “behavioral and emotional disorders,” “positive and negative emotions,” and “lesion laterality.” The initial search yielded 168 studies across the databases and 55 additional publications were identified from references of the obtained articles. After removal of 46 duplicates, papers to be included in the review were selected within the 177 remaining publications.
Inclusion Criteria
The following criteria were used for the selection of papers to be included in the review: (a) the presence of data allowing the evaluation of the relationships between emotional and behavioral disorders and the prevalent lesion of the right or left frontal or temporal lobes; (b) the use of precise clinical definitions or neuropsychological measures of the emotional/behavioral disorders considered in the study, in conjunction with (c) a comparison of results obtained on these measures by patients showing a prevalence of left-sided or right-sided lesions (atrophies or FDG-PET hypometabolism) or (d) a study of the correlation between specific emotional disorders and atrophy or hypometabolism of equally specific left-sided or right-sided structures. Most studies screened according to the inclusion criteria were discarded because they had not considered laterality as an important variable (N = 96), whereas some were excluded because of insufficient diagnostic criteria (n = 27) or of methodological inconsistencies (N = 22). Only 32 studies met both the first two and the third or the fourth criterion and are reported in Table 1. Most of these investigations (N = 26) were mainly relevant with respect to the “right hemisphere hypothesis,” whereas few (N = 6) were considered as relevant with respect to the “valence hypothesis.” Some investigations that reported interesting observations, but met only in part the requested criteria, were not included in Table 1, but were considered in the Discussion section.

Table 1. Synthetical description of investigations which have studied emotional and behavioral disorders in patients with frontal or temporal variant of Fronto-Temporal Degeneration, evaluating a prevalence of left-sided or right-sided atrophy in patients with emotional disorders or a correlation between specific emotional disorders and atrophy or dysfunction of lateralized cortical or subcortical structures.
Results
A short descriptive survey of the 32 studies included in our review is reported in Table 1.
Descriptive data reported in Table 1 provide an idea of the complexity and heterogeneity of the research lines considered in our review. These investigations, in fact, though all relevant in relation to the subject of our review, investigated a large array of clinical syndromes as well as behavioral disorders (e.g., papers 1–3, 6–8, 11, and 13–14), resulting (at least in part) from emotional disturbances or emotional disorders per se. Furthermore, even true emotional disorders involved two different categories of emotions. In some cases, basic emotions were considered (e.g., papers 5, 10, 12, 21, 24, 27, and 29), which are regarded as innate and provide high survival values (Ekman, 1984, 1992). In other cases, they considered “complex,” “social,” and “self-conscious emotions” (e.g., 9, 15–18, 22–25, 28, and 30–31), which are subtended by social norms (Ekman, 1984, 1992) and require an appreciation of the self in a social context (Tangney, 1999).
A more analytical tabulation of patients (number of participants), emotional and behavioral disorders (clinical diagnosis, tests undertaken and neuroanatomical assessment) and study methodology (group comparison or correlational analysis) of papers: (a) dealing with behavioral disorders; (b) relevant with respect to the “right hemisphere hypothesis” and (c) specifically concerning the “valence hypothesis” is therefore, reported in Table 2.
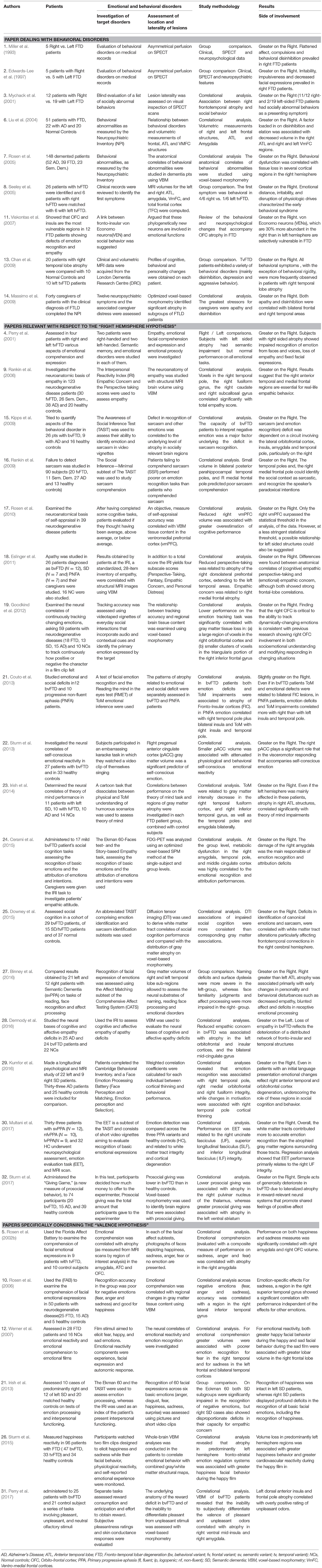
Table 2. More detailed account of papers included in the present review and: (a) dealing with behavioral disorders; (b) relevant to the “right hemisphere hypothesis” and (c) specifically concerning the “valence hypothesis.”
Results obtained in most (N = 26) of these investigations were mainly relevant to the “right hemisphere hypothesis” because they: (1) made a comparison between the prevalence of left-sided or right-sided lesions in patients with emotional or behavioral disorders or (2) showed a significant correlation between emotional disorders and atrophy or hypometabolism of left-sided or right-sided brain structures. Nine of these investigations, namely studies (1), (2), (3), (6), (7), (8), (10), (13), and (14) focused on behavioral disorders, whereas 17 investigations, namely studies (4), (9), (15), (16), (17), (18), (19), (20), (22), (23), (24), (25), (27), (28), (29), (30), and (32) focused on true emotional disorders and were relevant to the “right hemisphere hypothesis.” On the other hand, results obtained in a few (N = 6) of these investigations, namely studies (5), (10), (12), (21), (26), and (31) were relevant to the “valence hypothesis.” They made, indeed, a separate analysis of comprehension or production of positive and negative emotions and compared results obtained on these tasks by FTD patients, with a prevalence of left or right lesions (study 21) or correlated these patterns of emotional disorders with specific left-sided or right-sided structures (studies 5, 10, 12, 26, and 31).
Results of Investigations Mainly Dealing With Behavioral Disorders
Data reported in Table 2 show that all investigations dealing with behavioral disorders (at least in part resulting from emotional disturbances) were consistent with the assumption of a general dominance of the right hemisphere for emotional functions. In fact, all these investigations demonstrated a prevalence of behavioral disorders in FTD patients with atrophy or hypometabolism of right-sided neural structures (studies 1, 2, 3, 8, and 13) or a significant correlation between behavioral disorders and atrophy or hypometabolism of right fronto-temporal structures (studies 6, 7, and 14). This was true irrespective of the nature of the reported behavioral disorder (which consisted of disinhibition in studies 1, 2, 6, 8, 13, and 16, of apathy in studies 1, 13, and 14 and of aggressive behavior in studies 2, 8, and 13) and of the disease stage that was considered. This behavioral disorder was, in fact, an observed symptom in papers 3 and 8, whereas it was observed in the moderate-to-advanced stages of the disease in the other papers.
Results of Investigations Relevant With Respect to the “Right Hemisphere Hypothesis”
As in the case of investigations that mainly dealt with behavioral disorders, the results of studies on emotional disorders and those which were considered as relevant to the “right hemisphere hypothesis,” supported the assumption of a general dominance of the right hemisphere for emotional functions. Even in this case, in fact, almost all (i.e., 16 out of 17) investigations demonstrated either a greater emotional impairment in FTD patients, showing a prevalent atrophy of right-sided brain structures (studies 4 and 27) or a significant correlation between emotional disorders and atrophy, or hypometabolism of the right fronto-temporal structures (studies 9, 15, 16, 17, 18, 19, 21, 22, 23, 24, 25, 29, 30, and 32). In correlational study 28 (Dermody et al., 2016) only, the emotional defect (reduced empathic concern) was associated with prevalent atrophy in left-sided structures (the left orbitofrontal and insular cortices) in bvFTD patients.
Therefore, irrespective of the methodology used (group comparison vs. correlational analysis), results of investigations aiming to check the “right hemisphere hypothesis,” strongly supported the assumption of a general dominance of the right hemisphere for emotional functions. Obviously, this does not mean that the left fronto-temporal structures do not play a relevant role in emotional functions. Even when putting aside results obtained by Dermody et al. (2016), in all the other correlational studies, emotional disorders were associated to atrophy of both the right- and left- anterior temporal, insular and orbitofrontal cortices, although the correlations were stronger in right- than in left-sided structures.
Results of Investigations Specifically Concerning the “Valence Hypothesis”
In the study (5), Rosen et al. (2002b) examined the comprehension of facial expressions of emotion in nine individuals with tvFTD and showed that performance on recognition of facial expressions (evaluated with a composite measure of performance on sadness, anger and fear) correlated with atrophy in the right amygdala. The same authors also investigated the relationship between comprehension of specific emotions and regional cerebral volumes and showed that both happiness and sadness measures were significantly correlated with the right amygdala and the right orbito-frontal cortex (OFC) volume. Thus, the correlation between a composite measure of performance on negative emotions (sadness, anger, and fear) and atrophy in the right amygdala, could be consistent with the “valence hypothesis,” but the fact that both happiness and sadness measures were significantly correlated with the right amygdala and OFC volumes, contradicts this model. Equally inconclusive results were obtained in study (10) by Rosen et al. (2006) and in study (12) by Werner et al. (2007). The first study used the Florida Affect Battery (Bowers et al., 1989) to assess recognition of emotions in 50 patients with neurodegenerative disease and correlated performance on recognition of facial expressions with regional changes in gray matter tissue content, using voxel-based morphometry. The second study compared emotional reactivity and emotional comprehension of emotional films designed to elicit fear, happy, and sad emotions in FTD patients and healthy controls. Consistent with previous studies, Rosen et al. (2006) found that the recognition accuracy in neurodegenerative patients was poor for negative emotions (fear, anger, and sadness) and good for happiness. They also found, however, that tissue content in a region in the right lateral inferior temporal gyrus [Brodman's area (BA) 20] extending into the right middle temporal gyrus (BA 21), was correlated with the accuracy of recognition of negative emotions. On the other hand, Werner et al. (2007) reported two data consistent with the “right hemisphere hypothesis.” The first was that the generation of both happy facial expressions to happy films and sad facial expressions to sad films, were associated with greater lobar volume in the right frontal lobe. The second was that regional brain atrophy was associated with poorer emotion recognition for fear in the right temporal and for sadness in the left frontal and bilateral temporal cortices. The fact that accuracy in recognition of negative emotions was positively correlated with tissue content of the right temporal structures, by Rosen et al. (2006), and with left frontal atrophy by Werner et al. (2007) could in part support the “valence hypothesis.” However, inconsistent with this interpretation are results obtained in study (21) by Irish et al. (2013), in study (26) by Sturm et al. (2015) and in study (31) by Perry et al. (2017), that studied positive and negative emotions in different subgroups of FTD patients, using different experimental paradigms. Irish et al. (2013) compared results, obtained by two groups of predominantly right (n = 10) and left (n = 12) SD patients, on the Ekman 60-Faces test of emotion processing (Young et al., 2002). In this test, left SD cases displayed marked difficulties only in the recognition of negative emotions, whereas recognition of happiness was intact. In the same task, right SD patients displayed profound deficits in the recognition of all basic facial emotions, including the recognition of happiness, and scored significantly worse than the left SD patients in the recognition of anger and happiness. These results, which showed that right SD patients scored worse than the left SD patients in the recognition of happiness, were clearly inconsistent with the “valence hypothesis,” because, according to this model, recognition of happiness should be subtended by the left hemisphere. Equally, at variance with the “valence hypothesis” were data obtained by Sturm et al. (2015), who measured happiness reactivity in FTD patients who watched a film clip designed to elicit happiness and by Perry et al. (2017), who administered a series of tasks involving pleasant, unpleasant, and neutral olfactory stimuli, to bvFTD patients. Sturm et al. (2015) showed that, contrary to the assumptions of the “valence hypothesis,” atrophy in predominantly left hemisphere fronto-striatal emotion regulation systems was associated with greater happiness facial behavior during the film designed to elicit happiness. Perry et al. (2017) showed (in agreement with the “right hemisphere hypothesis”), that the inability to subjectively differentiate the valence of pleasant and unpleasant odors, correlated with atrophy in the right ventral mid-insula and the right amygdala. Furthermore, (at variance with the “valence hypothesis”) they showed that positive rating of unpleasant odors correlated with left anterior insula and frontal pole atrophy.
Discussion
The outcome of the present review strongly confirms results of previous investigations (e.g., Miller et al., 1993; Edwards-Lee et al., 1997; Seeley et al., 2005; Irish et al., 2013; Binney et al., 2016; Kumfor et al., 2016), which have shown that inappropriate social and emotional behaviors are an early and distinctive marker of the right-sided variants of FTLD. Furthermore, data reported in Table 2 corroborates our decision to consider emotional and behavioral disorders as distinct but highly interconnected phenomena, because a high prevalence of patients with right FTD were observed both in studies based on behavioral abnormalities, and in those evaluating specific aspects of emotional disorders. The former had been assessed clinically (evaluation of medical records) in papers 1. Miller et al. (1993), 2. Edwards-Lee et al. (1997), 3. Mychack et al. (2001), 8. Seeley et al. (2005) and 13. Chan et al. (2009) and with the Neuropsychiatric Inventory (Cummings et al., 1994) in studies 6. Liu et al. (2004), 7. Rosen et al. (2005) and 14. Massimo et al. (2009). The latter, on the contrary, had has often been investigated with specific testing procedures, such as the IRI measure of empathy (Davis, 1983) in studies 9. Rankin et al. (2006), 18. Eslinger et al. (2011) and 28. Dermody et al. (2016); the Awareness of Social Inference Test (TASIT / McDonald et al., 2002) in studies 15. Kipps et al. (2009), 16. Rankin et al. (2009), 19. Goodkind et al. (2012), 25. Downey et al. (2015) and 30. Multani et al. (2017); the TOM cartoons (Lough et al., 2006) in study 23. Irish et al. (2014); the Ekman 60-Faces task (Ek60F /Young et al., 2002) in study 24. Cerami et al. (2015); the Comprehensive Affect Testing System (CATS/ Froming et al., 2006) in study 27. Binney et al. (2016) and the Face and Emotion Processing Battery (Miller et al., 2012) in study 29. Kumfor et al. (2016). However, in spite of these important methodological differences between studies based on behavioral abnormalities, and those evaluating specific aspects of emotional disorders, a similarly high prevalence of right fronto-temporal lesions was found across these investigations. Some authors (e.g., Zahn et al., 2009; Ross and Olson, 2010; Olson et al., 2013) had interpreted these inappropriate social and emotional behaviors within the framework of the “Semantic Hub” model (Rogers et al., 2004; Patterson et al., 2007; Lambon Ralph and Patterson, 2008). However, in contrast with the original version of this model, that viewed the “Hub” as a bilaterally represented unitary mechanism, they had maintained that the left ATL supports general conceptual knowledge, whereas the right ATL subsumes a domain-specific social cognition system. This hypothesis was however questioned by results of a recent review in which Gainotti (2015) showed that the right ATL plays a more important role in behavioral and emotional functions than in higher level social cognition and considered these disorders as due to the disruption of lower level (behavioral and emotional) components than of higher level (social cognitive) aspects.
Discussion of Investigations Relevant With Respect to the “Right Hemisphere Hypothesis”
Based on these premises, the present review aimed to evaluate if results of investigations conducted in patients with FTLD support one of the main models advanced to explain the relationship between emotions and brain laterality, namely the “right hemisphere hypothesis” or the “valence hypothesis.” The first model posits a general dominance of the right hemisphere for all kinds of emotions, while the second assumes an opposite dominance of the left hemisphere for positive emotions and of the right hemisphere for negative emotions. Results of the review clearly support the “right hemisphere hypothesis” more than the “valence hypothesis.” All the group comparisons that investigated the influence of asymmetrical atrophy on emotional and behavioral disorders in FTD patients demonstrated, in fact, a prevalence of these disorders in subjects with atrophy or hypometabolism of right-sided neural structures. Furthermore, results consistent with the “right hemisphere hypothesis” were obtained by all-but-one investigation (i.e., studies 9, 15, 16, 17, 18, 19, 21, 22, 23, 24, 25, 29, 30, and 32) that used a regression analyses to determine potential correlations between behavioral and emotional disturbances and lateralized impairment of specific brain structures. The high prevalence of behavioral abnormalities and of emotional disorders in patients with right FTLD, had been noted by previous authors, who tried to explore the reasons that could explain this association. Thus, Liu et al. (2004) suggested that the prevalence of behavioral disturbances in FTD patients with right-sided lesions may be related to the importance of the right hemisphere for the comprehension of emotional stimuli and an even more articulated interpretation of the links between poor comprehension of emotional facial expressions and behavioral abnormalities was offered by Lough et al. (2006) and Goodkind et al. (2015). These authors acknowledged that the inability of right FTD patients, to read the negative emotional expressions on the faces of personally relevant people, provoked by their socially inappropriate behavior, could contribute to the persistence of these abnormal social conducts. Thus, if a patient's behavior makes family members angry and this anger is correctly recognized, it might motivate the patient to modify his unacceptable conduct, but if this anger is not correctly recognized, improvement of the abnormal social behavior would be less likely. Obviously, if the reviewed data stress a general dominance of the right hemisphere for all kinds of emotions, they do not suggest that the left fronto-temporal structures play an irrelevant role in emotional functions. In fact, in one of the studies included in the present review (Dermody et al., 2016) the emotional defect (reduced empathic concern) in bvFTD patients was associated with prevalent atrophy in left-sided rather than right-sided structures (the left orbitofrontal and insular cortices). In another study (Couto et al., 2013) emotion deficits and Tome impairments correlated more with right- than with left-sided structures (insula and temporal pole) only in progressive non-fluent aphasia (PNFA), but not in bvFTD patients. Overall, in all the other correlational studies, emotional disorders were associated to atrophy of both the right- and left- anterior temporal, insular and orbitofrontal cortices, even though the correlations were stronger with right- than with left-sided structures. The relationship between emotional disorders and atrophy of right fronto-temporal structures is, on the other hand, confirmed by the fact that several studies included in our review (e.g., Rankin et al., 2006; Couto et al., 2013; Irish et al., 2014; Kumfor et al., 2016; Multani et al., 2017) have shown that, even in patients with bilateral or left lateralized forms of FTLD, the emotional aspects of interpersonal functioning correlate with atrophy in right rather than left temporal lobe structures.
Discussion of Investigations Specifically Concerning the “Valence Hypothesis”
If results of investigations concerning the “right hemisphere hypothesis” had clearly supported that model, results of investigations concerning the “valence hypothesis” were, at best, inconclusive. As a matter of fact, only two of these investigations [namely the Rosen et al. (2006) and the Werner et al. (2007) studies] seemed to corroborate at least in part the “valence hypothesis,” because accuracy in recognition of negative emotions was positively correlated with tissue content of right temporal structures in the Rosen et al. (2006) study and with left frontal atrophy in the Werner et al. (2007) paper. In the Rosen et al. (2006) study, however, no lateralization was found with respect to positive emotions, which, according to the “valence hypothesis” should be subserved by left hemisphere structures. Similarly, in the Werner et al. (2007) paper the generation of both happy facial expressions to happy films and of sad facial expressions to sad films, were associated with greater lobar volume in the right frontal lobe (a finding more consistent with the “right hemisphere” than with the “valence hypothesis”). Furthermore, results in disagreement with the “valence hypothesis” were obtained by Rosen et al. (2002b), Irish et al. (2013), Sturm et al. (2015) and Perry et al. (2017). Rosen et al. (2002b) showed that the comprehension of facial expressions of happiness was significantly correlated with the right amygdala and the right orbito-frontal cortex (OFC) volume. Irish et al. (2013) proved that right SD patients scored significantly worse than left SD patients in the recognition of happy faces. Sturm et al. (2015) showed that the projection of a film clip designed to elicit happiness provoked greater happiness facial behavior in patients with left fronto-temporal atrophy and Perry et al. (2017) revealed that, in bvFTD patients, the tendency to give positive ratings of unpleasant odors correlated with atrophy of the left dorsal anterior insula and of the left frontal pole. On the basis of these results, and of data showing that happiness is the simplest facial emotional expression (Ekman, 2007), it is therefore possible to explain results obtained by Rosen et al. (2006) not only in terms of the “valence” but also of the “right hemisphere” hypothesis. In the Rosen et al. (2006) study, in fact, as in many similar investigations conducted on patients with degenerative brain diseases (e.g., Rosen et al., 2002b, 2004; Irish et al., 2013; Bora et al., 2016), performance on the recognition of facial expressions was poorer for negative emotions (fear, anger, and sadness) than for happiness. It is, therefore, possible that the correlation between right temporal structures and the recognition of negative emotions could be due to the greater right hemisphere involvement in the most difficult part of the task than to the (negative) valence of the emotions to be recognized. Rosen et al. (2006) have also noted that if participants recognize that a photograph portrays a negative emotion, they still have to figure out which of several negative emotions is being expressed. In contrast, if they recognize that a photograph is depicting a positive emotion, then it must be happiness. This explains the oft-reported findings that patients have more difficulty recognizing negative emotions than positive emotions. We can, therefore, conclude that results of the present review are more consistent with the “right hemisphere” than with the “valence hypothesis,” because the first model is supported by the outcome of all investigations considered as relevant with respect to the “right hemisphere” hypothesis and of most studies considered as relevant with respect to the “valence hypothesis.”
These conclusions are in accordance with both those of previous papers carried out on other neurological diseases (e.g., Gainotti, 1972; Coffey, 1987; Gainotti and Caltagirone, 1989; Borod et al., 1998; Mandal and Ambady, 2004) that have also supported the “right hemisphere hypothesis,” and with those of a recent paper, in which Gainotti (2018) has tried to rework the “right hemisphere hypothesis” in terms of brain-behavior relationships. As for previous papers, I will only mention here a review paper by Coffey (1987) and a research paper by Borod et al. (1998). Coffey (1987) surveyed clinical and experimental studies conducted with brain-damaged patients, normal subjects, and psychiatric patients and concluded that the right hemisphere is uniquely specialized for the perception, experience, and expression of emotion. Borod et al. (1998) examined emotional comprehension in right and left brain-damaged (BD) stroke patients and in normal controls across three communication channels (facial, prosodic, and lexical) and contrasted the “right-hemisphere” with the “valence hypothesis.” Right BD patients were significantly impaired relative to left BD patients and normal controls across channels and valences, supporting the RH hypothesis. As to the question of what these results could mean for brain-behavior relationships, Gainotti (2018) reviewed results of recent investigations which have ascertained a critical role: (a) of the right amygdala in the unconscious processing of emotional information; (b) of the right ventromedial prefrontal cortex in functions of emotional control, and (c) of the right anterior insula in the conscious experience of emotions. All these data therefore support and provide an updated, congruent version of early models assuming a general dominance of the right hemisphere for emotional functions.
Limitations of the Study
The main limitation of this review probably consists in the heterogeneity of studies included in our survey, because the sources of evidence utilized were different with respect to the content and the methodology used in different studies. As for content related differences, they concerned the different syndromes (e.g., fv FTD vs. tvFTD or bvFTD vs. SD) belonging to the spectrum of FTLD diseases considered in different studies and the (behavioral or properly emotional) nature of the disorders investigated. With respect to the different syndromes considered, it must be noted that the location of disease may be variable in different patients, because lesions are generally bilateral, and there is varying involvement of the frontal and temporal lobes (Kumfor et al., 2016). As for the nature of the disorders investigated, even within studies dealing with true emotional disorders, two main sources of heterogeneity can be underlined. The first refers to the fact that some studies investigated basic emotions, considered as innate and with high survival values (Ekman, 1984, 1992) and in other cases “complex,” “social,” and “self-conscious emotions” subtended by social norms (Ekman, 1984, 1992) and requiring an appreciation of the self in a social context (Tangney, 1999). The second source of heterogeneity refers to the fact that some studies made a global assessment of emotional disorders, whereas other studies made a distinction between negative and positive emotions. With respect to the methodology, the review included: (a) studies based on the comparison between left-sided and right-sided atrophy in FTD patients, with emotional or behavioral disorders and (b) studies based on the assessment of the correlations between specific emotional disorders and atrophy or hypometabolism of equally specific left-sided or right-sided structures. Even if these sources of heterogeneity did not allow for a meta-analysis of data gathered in patients with FTD or other degenerative brain diseases, results of the present review can be considered as substantial proof to support (at the present state of our knowledge) the “right hemisphere” rather than the “valence hypothesis.”
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ahern, G. L., and Schwartz, G. E. (1979). Differential lateralization for positive versus negative emotion. Neuropsychologia 17, 693–698. doi: 10.1016/0028-3932(79)90045-9
Allen, J. J. B., Keune, P. M., Schönenberg, M., and Nusslock, R. (2018). Frontal EEG alpha asymmetry and emotion: from neural underpinnings and methodological considerations to psychopathology and social cognition. Psychophysiology 55:e13028. doi: 10.1111/psyp.13028
Binney, R. J., Henry, M. L., Babiak, M., Pressman, P. S., Santos-Santos, M. A., Narvid, J., et al. (2016). Reading words and other people: A comparison of exception word, familiar face and affect processing in the left and right temporal variants of primary progressive aphasia. Cortex 82, 147–163. doi: 10.1016/j.cortex.2016.05.014
Bora, E., Velakoulis, D., and Walterfang, M. (2016). Meta-analysis of facial emotion recognition in behavioral variant frontotemporal dementia: comparison with alzheimer disease and healthy controls. J. Geriatr. Psychiatry Neurol. 29, 205–211. doi: 10.1177/0891988716640375
Borod, J. C., Cicero, B. A., Obler, L. K., Welkowitz, J., Erhan, H. M., Santschi, C., et al. (1998). Right hemisphere emotional perception: evidence across multiple channels. Neuropsychology 12, 446–458. doi: 10.1037/0894-4105.12.3.446
Bowers, D., Blonder, L. X., and Heilman, K. M. (1989). The Florida Affect Battery, Revised. Gainsville, FL: The Center for Neuropsychological Studies, University of Florida.
Bozeat, S., Gregory, C. A., Lambon Ralph, M. A., and Hodges, J. R. (2000). Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer's disease? J. Neurol. Neurosurg. Psychiatry 69, 178–186. doi: 10.1136/jnnp.69.2.178
Cerami, C., Dodich, A., Iannaccone, S., Marcone, A., Lettieri, G., Crespi, C., et al. (2015). Right limbic FDG-PET hypometabolism correlates with emotion recognition and attribution in probable behavioral variant of frontotemporal dementia patients. PLoS ONE 10:e0141672. doi: 10.1371/journal.pone.0141672
Chan, D., Anderson, V., Pijnenburg, Y., Whitwell, J., Barnes, J., Scahill, R., et al. (2009). The clinical profile of right temporal lobe atrophy. Brain 132, 1287–1298. doi: 10.1093/brain/awp037
Coffey, C. E. (1987). Cerebral laterality and emotion: the neurology of depression. Compr. Psychiatry 28, 197–219. doi: 10.1016/0010-440X(87)90027-7
Couto, B., Manes, F., Montañés, P., Matallana, D., Reyes, P., Velasquez, M., et al. (2013). Structural neuroimaging of social cognition in progressive non-fluent aphasia and behavioral variant of frontotemporal dementia. Front. Hum. Neurosci. 7:467. doi: 10.3389/fnhum.2013.00467
Cummings, J., Mega, M., Gray, K., Rosenberg-Thompson, S., Carusi, D. A., and Gornbein, J. (1994). The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology 44, 2308–2314. doi: 10.1212/WNL.44.12.2308
Davidson, R. J. (1983). “Hemispheric specialization for cognition and affect,” in Physiological Correlates of Human Behavior. eds A. Gale and J. Edwards (London, Academic Press), 203–216.
Davidson, R. J. (1992). Anterior cerebral asymmetry and the nature of emotion. Brain Cogn. 20, 125–151. doi: 10.1016/0278-2626(92)90065-T
Davis, M. H. (1983). Measuring individual differences in empathy: evidence for a multidimensional approach. J. Pers. Soc. Psychol. 44, 113–126. doi: 10.1037/0022-3514.44.1.113
Dermody, N., Wong, S., Ahmed, R., Piguet, O., Hodges, J. R., and Irish, M. (2016). Uncovering the neural bases of cognitive and affective empathy deficits in Alzheimer's disease and the behavioral-variant of frontotemporal dementia. J Alzheimers Dis. 53, 801–816. doi: 10.3233/JAD-160175
Downey, L. E., Mahoney, C. J., Buckley, A. H., Golden, H. L., Henley, S. M., Schmitz, N., et al. (2015). White matter tract signatures of impaired social cognition in frontotemporal lobar degeneration. Neuroimage Clin. 23, 640–651. doi: 10.1016/j.nicl.2015.06.005
Edwards-Lee, T., Miller, B. L., Benson, D. F., Cummings, J. L., Russell, G. L., Boone, K., et al. (1997). The temporal variant of frontotemporal dementia. Brain 120, 1027–1040. doi: 10.1093/brain/120.6.1027
Ekman, P. (1984). “Expression and the nature of emotion,” in Approachs to Emotion, eds K. Scherer and P. Ekman (Hillsdale, NJ: Erlbaum), 319–343.
Ekman, P. (1992). An argument for basic emotions. Cogn Emot. 6, 169–200. doi: 10.1080/02699939208411068
Ekman, P. (2007). Emotions Revealed: Recognizing Faces and Feelings to Improve Communication and Emotional Life. New York, NY: Henry Holt and Company.
Eslinger, P. J., Moore, P., Anderson, C., and Grossman, M. (2011). Social cognition, executive functioning, and neuroimaging correlates of empathic deficits in frontotemporal dementia. J. Neuropsychiatry Clin. Neurosci. 23, 74–82. doi: 10.1176/appi.neuropsych.23.1.74
Froming, K., Levy, M., Schaffer, S., and Ekman, P. (2006). The Comprehensive Affect Testing System. Sharpsburg, PA: Psychology Software, Inc.
Fusar-Poli, P., Piacentino, A., Carletti, F., Allen, P., Landi, P., Abbamonte, M., et al. (2009). Laterality effect on emotional faces processing: ALE meta-analysis of evidence. Neurosci. Lett. 452, 262–267. doi: 10.1016/j.neulet.2009.01.065
Gainotti, G. (1972). Emotional behavior and hemispheric side of the lesion. Cortex 8, 41–55. doi: 10.1016/S0010-9452(72)80026-1
Gainotti, G. (2012). Unconscious processing of emotions and the right hemisphere. Neuropsychologia 50, 205–218. doi: 10.1016/j.neuropsychologia.2011.12.005
Gainotti, G. (2015). Is the difference between right and left ATLs due to the distinction between general and social cognition or between verbal and non-verbal representations? Neurosci Biobehav. Rev. 51, 296–312. doi: 10.1016/j.neubiorev.2015.02.004
Gainotti, G. (2018). Emotions and the right hemisphere: can new data clarify old models? Neuroscientist. [Epub ahead of print]. doi: 10.1177/1073858418785342.
Gainotti, G., and Caltagirone, C. (1989). Emotions and the Dual Brain. Heidelberg: Springer. doi: 10.1007/978-3-642-73396-3
Gainotti, G., Caltagirone, C., and Zoccolotti, P. (1993). Left/right and cortical/subcortical dichotomies in the neuropsychological study of human emotions. Cogn. Emot. 7, 71–93. doi: 10.1080/02699939308409178
Goodkind, M. S., Sollberger, M., Gyurak, A., Rosen, H. J., Rankin, K. P., Miller, B., et al. (2012). Tracking emotional valence: the role of the orbitofrontal cortex. Hum. Brain Mapp. 33, 753–762. doi: 10.1002/hbm.21251
Goodkind, M. S., Sturm, V. E., Ascher, E. A., Shdo, S. M., Miller, B. L., Rankin, K. P., et al. (2015). Emotion recognition in frontotemporal dementia and Alzheimer's disease: a new film-based assessment. Emotion 15, 416–427. doi: 10.1037/a0039261
Irish, M., Hodges, J. R., and Piguet, O. (2014). Right anterior temporal lobe dysfunction underlies theory of mind impairments in semantic dementia. Brain 137, 1241–1253. doi: 10.1093/brain/awu003
Irish, M., Kumfor, F., Hodges, J. R., and Piguet, O. (2013). A tale of two hemispheres: contrasting socioemotional dysfunction in right- versus left-lateralised semantic dementia. Dement. Neuropsychol. 7, 88–95. doi: 10.1590/S1980-57642013DN70100014
Killgore, W. D., and Yurgelun-Todd, D. A. (2007). The right-hemisphere and valence hypotheses: could they both be right (and sometimes left)? Soc. Cogn. Affect. Neurosci. 2, 240–250. doi: 10.1093/scan/nsm020
Kipps, C. M., Nestor, P. J., Acosta-Cabronero, J., Arnold, R., and Hodges, J. R. (2009). Understanding social dysfunction in the behavioural variant of frontotemporal dementia: the role of emotion and sarcasm processing. Brain 132, 592–603. doi: 10.1093/brain/awn314
Kumfor, F., Landin-Romero, R., Devenney, E., Hutchings, R., Grasso, R., Hodges, J. R., et al. (2016). On the right side? A longitudinal study of left- versus right-lateralized semantic dementia. Brain 139, 986–998. doi: 10.1093/brain/awv387
Lambon Ralph, M. A., and Patterson, K. (2008). Generalization and differentiation in semantic memory insights from semantic dementia. Ann. N. Y. Acad. Sci. 1124, 61–76. doi: 10.1196/annals.1440.006
Levenson, R. W., Sturm, V. E., and Haase, C. M. (2014). Emotional and behavioral symptoms in neurodegenerative disease: a model for studying the neural bases of psychopathology. Annu. Rev. Clin. Psychol. 10, 581–606. doi: 10.1146/annurev-clinpsy-032813-153653
Liu, W., Miller, B. L., Kramer, J. H., Rankin, K., Wyss-Coray, C., Gearhart, R., et al. (2004). Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology 62, 742–748. doi: 10.1212/01.WNL.0000113729.77161.C9
Lough, S., Kipps, C., Treise, C., Watson, P., Blair, J., and Hodges, J. (2006). Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsychologia 44, 950–958. doi: 10.1016/j.neuropsychologia.2005.08.009
Mandal, M. K., and Ambady, N. (2004). Laterality of facial expressions of emotion: universal and culture-specific influences. Behav. Neurol. 15, 23–34. doi: 10.1155/2004/786529
Massimo, L., Powers, C., Moore, P., Vesely, L., Avants, B., Gee, J., et al. (2009). Neuroanatomy of apathy and disinhibition in frontotemporal lobar degeneration. Dement. Geriatr. Cogn. Disord. 27, 96–104. doi: 10.1159/000194658
McDonald, S., Flanagan, S., and Rollins, J. (2002). The Awareness of Social Inference Test. Bury St Edmunds: Thames Valley Test Company.
Miller, B. L., Chang, L., Mena, I., Boone, K., and Lesser, I. M. (1993). Progressive right frontotemporal degeneration: clinical, neuropsychological and SPECT characteristics. Dementia 4, 204–213.
Miller, G. A., Crocker, L. D., Spielberg, J. M., Infantolino, Z. P., and Heller, W. (2013). Issues in localization of brain function: the case of lateralized frontal cortex in cognition, emotion, and psychopathology. Front. Integr. Neurosci. 7:2. doi: 10.3389/fnint.2013.00002
Miller, L. A., Hsieh, S., Lah, S., Savage, S., Hodges, J. R., and Piguet, O. (2012). One size does not fit all: face emotion processing impairments in semantic dementia, behavioural-variant frontotemporal dementia and Alzheimer's disease are mediated by distinct cognitive deficits. Behav. Neurol. 25, 53–60. doi: 10.1155/2012/683052
Multani, N., Galantucci, S., Wilson, S. M., Shany-Ur, T., Poorzand, P., Growdon, M. E., et al. (2017). Emotion detection deficits and changes in personality traits linked to loss of white matter integrity in primary progressive aphasia. NeuroImage Clin. 16, 447–454. doi: 10.1016/j.nicl.2017.08.020
Mychack, P., Kramer, J. H., Boone, K. B., and Miller, B. L. (2001). The influence of right frontotemporal dysfunction on social behavior in frontotemporal dementia. Neurology 56(11 Suppl. 4), S11–15. doi: 10.1212/WNL.56.suppl_4.S11
Natale, M., Gur, R. E., and Gur, R. C. (1983). Hemispheric asymmetries in processing emotional expressions. Neuropsychologia 21, 555–565. doi: 10.1016/0028-3932(83)90011-8
Olson, I. R., McCoy, D., Klobusicky, E., and Ross, L. A. (2013). Social cognition and the anterior temporal lobes: a review and theoretical framework. Soc Cogn Affect Neurosci. 8, 123–133. doi: 10.1093/scan/nss119
Patterson, K., Nestor, P. J., and Rogers, T. T. (2007). Where do you know what you know? The representation of semantic knowledge in the human brain. Nat. Rev. Neurosci. 8, 976–987. doi: 10.1038/nrn2277
Perry, D. C., Datta, S., Sturm, V. E., Wood, K. A., Zakrzewski, J., Seeley, W. W., et al. (2017). Reward deficits in behavioural variant frontotemporal dementia include insensitivity to negative stimuli. Brain 140, 3346–3356. doi: 10.1093/brain/awx259
Perry, R. J., Rosen, H. R., Kramer, J. H., Beer, J. S., Levenson, R. L., and Miller, B. L. (2001). Hemispheric dominance for emotions, empathy and social behaviour: evidence from right and left handers with frontotemporal dementia. Neurocase 7, 145–160. doi: 10.1093/neucas/7.2.145
Rankin, K. P., Gorno-Tempini, M. L., Allison, S. C., Stanley, C. M., Glenn, S., Weiner, M. W., et al. (2006). Structural anatomy of empathy in neurodegenerative disease. Brain 129, 2945–2956. doi: 10.1093/brain/awl254
Rankin, K. P., Salazar, A., Gorno-Tempini, M. L., Sollberger, M., Wilson, S. M., Pavlic, D., et al. (2009). Detecting sarcasm from paralinguistic cues: anatomic and cognitive correlates in neurodegenerative disease. Neuroimage 47, 2005–2015. doi: 10.1016/j.neuroimage.2009.05.077
Rascovsky, K., Hodges, J. R., Knopman, D., Mendez, M. F., Kramer, J. H., Neuhaus, J., et al. (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477. doi: 10.1093/brain/awr179
Reuter-Lorenz, P., and Davidson, R. J. (1981). Differential contribution of the two cerebral hemispheres to the perception of happy and sad faces, Neuropsychologia 19, 609–613. doi: 10.1016/0028-3932(81)90030-0
Reuter-Lorenz, P., Givis, R. P., and Moscovitch, M. (1983). Hemispheric specialization and the perception of emotion: Evidence from right –handers and from inverted and noninverted left handers, Neuropsychologia 21, 687–692. doi: 10.1016/0028-3932(83)90068-4
Reznik, S. J., and Allen, J. J. B. (2018). Frontal asymmetry as a mediator and moderator of emotion: an updated review. Psychophysiology 55:e12965. doi: 10.1111/psyp.12965
Rodway, P., Wright, L., and Hardie, S. (2003). The valence-specific laterality effect in free viewing conditions: the influence of sex, handedness, and response bias. Brain Cogn. 53, 452–463. doi: 10.1016/S0278-2626(03)00217-3
Rogers, T. T., Lambon Ralph, M. A., Garrard, P., Bozeat, S., McClelland, J. L., Hodges, J. R., et al. (2004). The structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol. Rev. 111, 205–235. doi: 10.1037/0033-295X.111.1.205
Rosen, H. J., Alcantar, O., Rothlind, J., Sturm, V., Kramer, J. H., Weiner, M. W., et al. (2010). Neuroanatomical correlates of cognitive self-appraisal in neurodegenerative disease. Neuroimage 49, 3358–3364. doi: 10.1016/j.neuroimage.2009.11.041
Rosen, H. J., Allison, S. C., Schauer, G. F., Gorno-Tempini, M. L., Weiner, M. W., and Miller, B. L. (2005). Neuroanatomical correlates of behavioural disorders in dementia. Brain 128, 2612–2625. doi: 10.1093/brain/awh628
Rosen, H. J., Gorno-Tempini, M. L., Goldman, W. P., Perry, R. J., Schuff, N., Weiner, M., et al. (2002a). Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 58, 198–208. doi: 10.1212/WNL.58.2.198
Rosen, H. J., Pace-Savitsky, C., Perry, R. J., Kramer, J. H., Miller, B. L., and Levinson, R. W. (2004). Recognition of emotion in the frontal and temporal variants of frontotemporal dementia. Dement. Geriatr. Cogn. Disord. 17, 277–281. doi: 10.1159/000077154
Rosen, H. J., Perry, R. J., Murphy, J., Kramer, J. H., Mychack, P., Schuff, N., et al. (2002b). Emotion comprehension in the temporal variant of frontotemporal dementia. Brain 125, 2286–2295. doi: 10.1093/brain/awf225
Rosen, H. J., Wilson, M. R., Schauer, G. F., Allison, S., Gorno-Tempini, M. L., Pace-Savitsky, C., et al. (2006). Neuroanatomical correlates of impaired recognition of emotion in dementia. Neuropsychologia 44, 365–373. doi: 10.1016/j.neuropsychologia.2005.06.012
Ross, L. A., and Olson, I. R. (2010). Social cognition and the anterior temporal lobes. Neuroimage 49, 3452–3462. doi: 10.1016/j.neuroimage.2009.11.012
Sackeim, H. A., and Grega, D. M. (1987). Perceiver bias in the processing of deliberately asymmetric emotional expressions. Brain Cogn. 6, 464–473. doi: 10.1016/0278-2626(87)90140-0
Sackeim, H. A., and Gur, R. C. (1978). Lateral asymmetry in intensity of emotional expression. Neuropsychologia 16, 473–481. doi: 10.1016/0028-3932(78)90070-2
Seeley, W. W., Bauer, A. M., Miller, B. L., Gorno-Tempini, M. L., Kramer, J. H., Weiner, M., et al. (2005). The natural history of temporal variant frontotemporal dementia. Neurology 64, 1384–1390. doi: 10.1212/01.WNL.0000158425.46019.5C
Seeley, W. W., Crawford, R., Rascovsky, K., Kramer, J. H., Weiner, M., Miller, B. L., et al. (2008). Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch. Neurol. 65, 249–255. doi: 10.1001/archneurol.2007.38
Snowden, J. S., Bathgate, D., Varma, A., Blackshaw, A., Gibbons, Z. C., and Neary, D. (2001). Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J. Neurol. Neurosurg. Psychiatry 70, 323–332. doi: 10.1136/jnnp.70.3.323
Snowden, J. S., Neary, D., and Mann, D. M. A. (1996). Fronto-Temporal Lobar Degeneration: Fronto-Temporal Dementia, Progressive Aphasia, Semantic Dementia. New York, NY: Churchill Livingstone.
Snowden, J. S., Thompson, J. C., and Neary, D. (2004). Knowledge of famous faces and names in semantic dementia. Brain 127, 860–872. doi: 10.1093/brain/awh099
Sturm, V. E., Perry, D. C., Wood, K., Hua, A. Y., Alcantar, O., Datta, S., et al. (2017). Prosocial deficits in behavioral variant frontotemporal dementia relate to reward network atrophy. Brain Behav. 7:e00807. doi: 10.1002/brb3.807
Sturm, V. E., Sollberger, M., Seeley, W. W., Rankin, K. P., Ascher, E. A., Rosen, H. J., et al. (2013). Role of right pregenual anterior cingulate cortex in self-conscious emotional reactivity. Soc. Cogn. Affect. Neurosci. 8, 468–474. doi: 10.1093/scan/nss023
Sturm, V. E., Yokoyama, J. S., Eckart, J. A., Zakrzewski, J., Rosen, H. J., Miller, B. L., et al. (2015). Damage to left frontal regulatory circuits produces greater positive emotional reactivity in frontotemporal dementia. Cortex 64, 55–67. doi: 10.1016/j.cortex.2014.10.002
Tangney, J. P. (1999). “The self-conscious emotions: shame, guilt, embarrassment and pride,” in: Handbook of Cognition and Emotion. eds T. Dalgleish and M. J. Power (New York, NY: Wiley), 541–568. doi: 10.1002/0470013494.ch26
Thompson, S. A., Patterson, K., and Hodges, J. R. (2003). Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology 61, 1196–1203. doi: 10.1212/01.WNL.0000091868.28557.B8
Viskontas, I. V., Possin, K. L., and Miller, B. L. (2007). Symptoms of frontotemporal dementia provide insights into orbitofrontal cortex function and social behavior. Ann. N. Y. Acad. Sci. 1121, 528–545. doi: 10.1196/annals.1401.025
Wager, T. D., Phan, K. L., Liberzon, I., and Taylor, S. F. (2003). Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage 19, 513–531. doi: 10.1016/S1053-8119(03)00078-8
Werner, K. H., Roberts, N. A., Rosen, H. J., Dean, D. L., Kramer, J. H., Weiner, M. W., et al. (2007). Emotional reactivity and emotion recognition in frontotemporal lobar degeneration. Neurology, 69, 148–155. doi: 10.1212/01.wnl.0000265589.32060.d3
Young, A. W., Perrett, D., Calder, A. J., Sprengelmeyer, R., and Ekman, P. (2002). Facial Expressions of Emotion - Stimuli and Tests (FEEST). Bury St Edmunds: Thames Valley Test Company.
Keywords: laterality of emotions, the “right hemisphere” hypothesis, the “valence hypothesis”, asymmetrical forms of frontotemporal dementia, emotional and behavioral disorders, positive and negative emotions
Citation: Gainotti G (2019) The Role of the Right Hemisphere in Emotional and Behavioral Disorders of Patients With Frontotemporal Lobar Degeneration: An Updated Review. Front. Aging Neurosci. 11:55. doi: 10.3389/fnagi.2019.00055
Received: 18 July 2018; Accepted: 22 February 2019;
Published: 19 March 2019.
Edited by:
Lea T. Grinberg, University of California, San Francisco, United StatesReviewed by:
Maria Carmela Tartaglia, University of Toronto, CanadaMario F. Mendez, UCLA David Geffen School of Medicine, United States
Copyright © 2019 Gainotti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guido Gainotti, Z3VpZG8uZ2Fpbm90dGlAdW5pY2F0dC5pdA==
 Guido Gainotti
Guido Gainotti