- 1Department of Psychiatry, Wenzhou Seventh People’s Hospital, Wenzhou, China
- 2Department of Pathology, Hangzhou Normal University, Hangzhou, China
- 3Department of Ultrasound, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 4Independent Researcher, Hangzhou, China
Objective: To investigate whether APOE ε4 affects the association of verbal memory with neurodegeneration presented by the hippocampal volume/intracranial volume ratio (HpVR).
Methods: The study sample included 371 individuals with normal cognition (NC), 725 subjects with amnestic mild cognitive impairment (aMCI), and 251 patients with mild Alzheimer’s disease (AD) from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) who underwent the rey auditory verbal learning test (RAVLT). Multiple linear regression models were conducted to assess the effect of the APOE ε4∗HpVR interaction on RAVLT in all subjects and in each diagnostic group adjusting for age, gender and educational attainment, and global cognition.
Results: In all subjects, there was no significant APOE ε4 × HpVR interaction for immediate recall or delayed recall (p > 0.05). However, in aMCI subjects, there was a significant APOE ε4 × HpVR interaction for delayed recall (p = 0.008), but not immediate recall (p = 0.15). More specifically, the detrimental effect of APOE ε4 on delayed recall altered by HpVR such that this effect was most evident among subjects with small to moderate HpVR, but this disadvantage was absent or even reversed among subjects with larger HpVR. No significant interaction was observed in the NC or AD group.
Conclusion: These findings highlight a potential role of APOE ε4 status in affecting the association of hippocampus size with delayed recall memory in the early stage of AD.
Introduction
The effect of the apolipoprotein E ε4 (APOE ε4) allele on cognitive abilities is complicated. Previous studies demonstrated the diverse roles of APOE ε4 allele in cognitive abilities dependent on different ages. In young populations, the APOE ε4 allele has a beneficial effect on learning and memory ability. However, the APOE ε4 allele is also associated with the decline of learning and memory ability in old subjects (Han and Bondi, 2008; Caselli et al., 2009; Tuminello and Han, 2011). These interesting findings have been conceptualized as the APOE antagonistic pleiotropy hypothesis (Han and Bondi, 2008; Caselli et al., 2009; Tuminello and Han, 2011).
The hippocampus is critical for episodic memory function and its size is considered as an index of the degree of cognitive decline (Kilpatrick et al., 1997; Eldridge et al., 2000; Ystad et al., 2009). A previous study also showed that the APOE ε4 allele contributes to the reduction of hippocampal volume in patients with mild cognitive impairment (MCI) and Alzheimer’s disease (AD) (Khan et al., 2017). Additionally, APOE ε4 was found to reduce hippocampal volumes and impair episodic memory in a dose-dependent manner (APOE ε4 homozygous > APOE ε4 heterozygous > APOE ε4 non-carriers) (Liu et al., 2016). A recent study revealed that hippocampal volume was positively associated with episodic memory in cognitively normal old individuals with APOE ε4 homozygotes (Lim et al., 2017). However, it is unknown whether the APOE ε4 allele also has antagonistic pleiotropic effects on the association of hippocampal volumes with episodic memory across the AD continuum. Our primary goal was to examine whether the APOE ε4 allele modulates the relationship between hippocampal volumes and verbal memory in subjects with NC, MCI, and mild AD from the Alzheimer’s disease neuroimaging initiative (ADNI) dataset.
Materials and Methods
Alzheimer’s Disease Neuroimaging Initiative
Demographic and imaging data used in the preparation of this study were extracted from the ADNI database1 in January 2017. The primary aim of the ADNI has been to test whether clinical and neuropsychological assessment, neuroimaging and other biomarkers can be integrated to predict the progression of MCI and early AD. The ADNI was conducted after institutional review board approval at each site. Written informed consent was obtained from all participants or their authorized representatives. Our study sample included subjects who had hippocampal volumetric, verbal memory and APOE 4ε genotype data available from one visit cycle (n = 1347). In this study, there were 371 subjects with NC, 725 patients with aMCI, and 251 patients with AD.
Diagnosis Criteria
Inclusive and exclusive criteria can be found in detail at http://www.adni-info.org. The NC group had a score of at least 24 on the mini-mental state examination (MMSE) and a score of 0 on the Clinical Dementia Rating (CDR) scale (Petersen et al., 2010). The aMCI group had a score of 24 or higher on the MMSE, a CDR score of 0.5, a subjective memory complaint, objective memory impairment as examined by the Logical Memory II subscale of the Wechsler Memory Scale–Revised (Wechsler, 1987), essentially preserved activities of daily living, and were not demented (Petersen et al., 2010). The AD group met the National Institute of Neurological and Communicative Disorders and Stroke and AD and Related Disorders Association criteria for probable AD, having scores from 20 to 26 on the MMSE and a score of 0.5 or 1 on the CDR (Petersen et al., 2010).
Neuropsychological Outcomes
The MMSE and CDR were used to evaluate global cognitive function and dementia severity, respectively. The rey auditory verbal learning test (RAVLT) (Schmidt, 1996) was applied to measure verbal memory. The immediate recall scores (range, 0–75) and delayed recall scores (range, 0–15) were utilized as our main evaluation indicators.
Structural Magnetic Resonance Imaging
Structural MRI brain scans were obtained using 1.5T MRI scanners with a standardized protocol, which is described in detail at www.loni.ucla.edu/ADNI. Hippocampal volume measures were performed using FreeSurfer software2. In order to correct for subject differences in head size, hippocampal volumes were normalized by individual intracranial volume (formula: hippocampal/intracranial volume × 103). The MRI volumes of brain structures used in this study were extracted from UCSF data in the ADNI dataset3.
APOE Genotyping
The data of APOE genotypes of our study sample were extracted from the ADNI database. Further information can be found at adni.loni.usc.edu. Participants were classified as APOE ε4-negative (-) if they carried no APOE ε4 allele or APOE ε4-positive (+) if they carried at least one APOE ε4 allele.
Statistical Analysis
Socio-demographics and clinical outcomes were compared between diagnostic groups using analysis of variance (ANOVA) for continuous variables and chi-squared for categorical variables in all subjects and within diagnostic groups. The relationship between HpVR and memory outcomes was analyzed by Pearson correlation analysis in all subjects and within diagnostic groups. HpVR and memory outcomes were compared between APOE ε4 carriers and non-carriers using student’s t test in all subjects and within diagnostic groups. The multivariable linear regression was performed to evaluate the independent and interactive relationship of APOE ε4 status and HpVR on verbal memory performance (RAVLT immediate and delayed recall scores) in all subjects and within diagnostic groups. In model 1, we evaluated the independent effects of APOE ε4 status and HpVR. Then, the HpVR × APOE ε4 status interaction was added to model 2, but was eliminated if not significant (p > 0.05). All analyses were adjusted for age, gender, education, MMSE scores and diagnosis (only in the overall sample analysis). The resultant p-values for the associations of the APOE ε4∗ HpVR interaction with memory performance were corrected for multiple comparisons with false discovery rate (FDR) (Benjamini and Hochberg, 1995). IBM SPSS version 20 was used to perform all statistical analyses. A two-tailed P value of less than 0.05 was considered to be statistically significant.
Results
Socio-Demographic Data
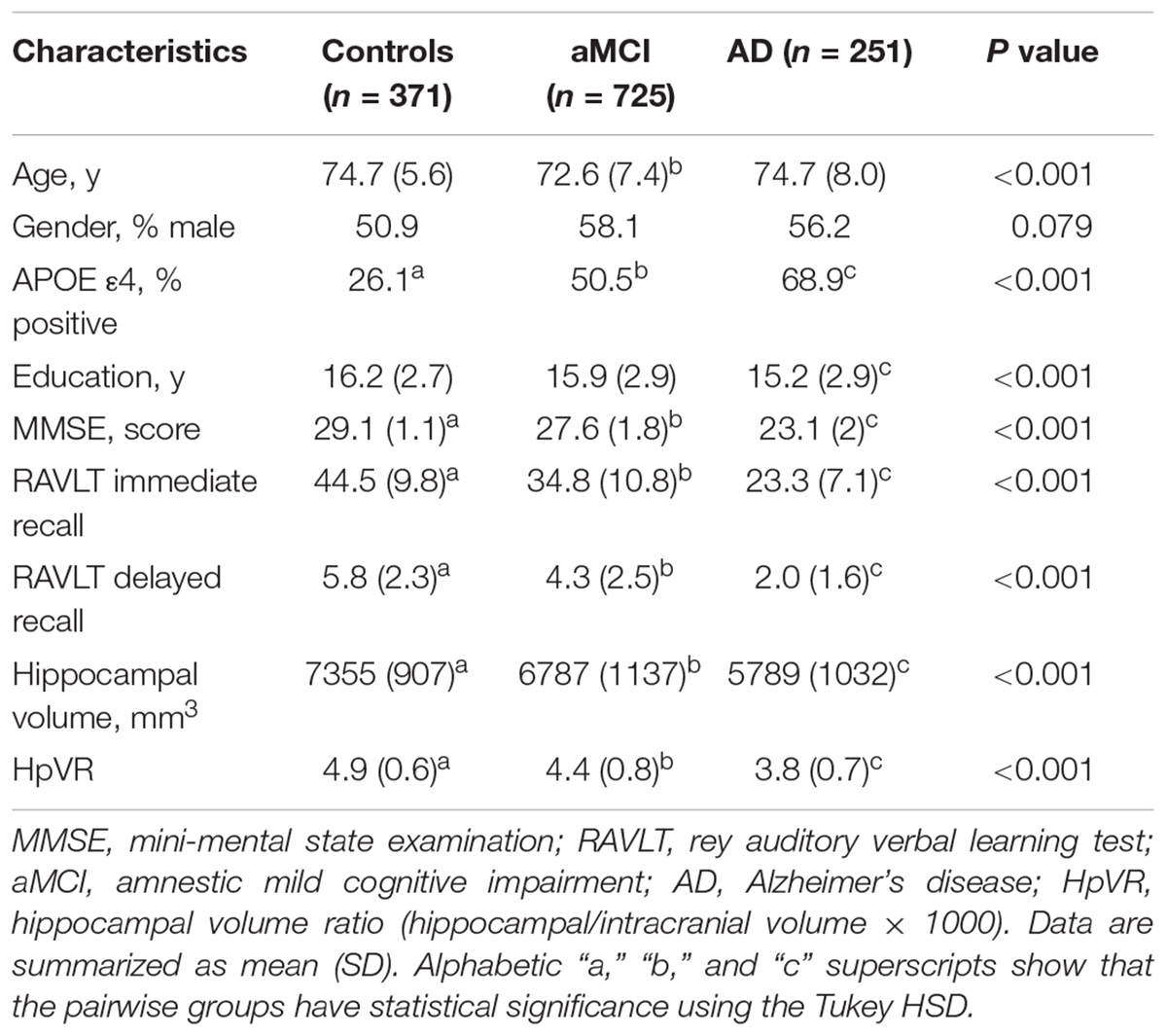
Table 1 listed the socio-demographic characteristics of the subjects. Several demographic and clinical variables differed significantly across the three diagnostic groups (Table 1).
Association of HpVR With RAVLT
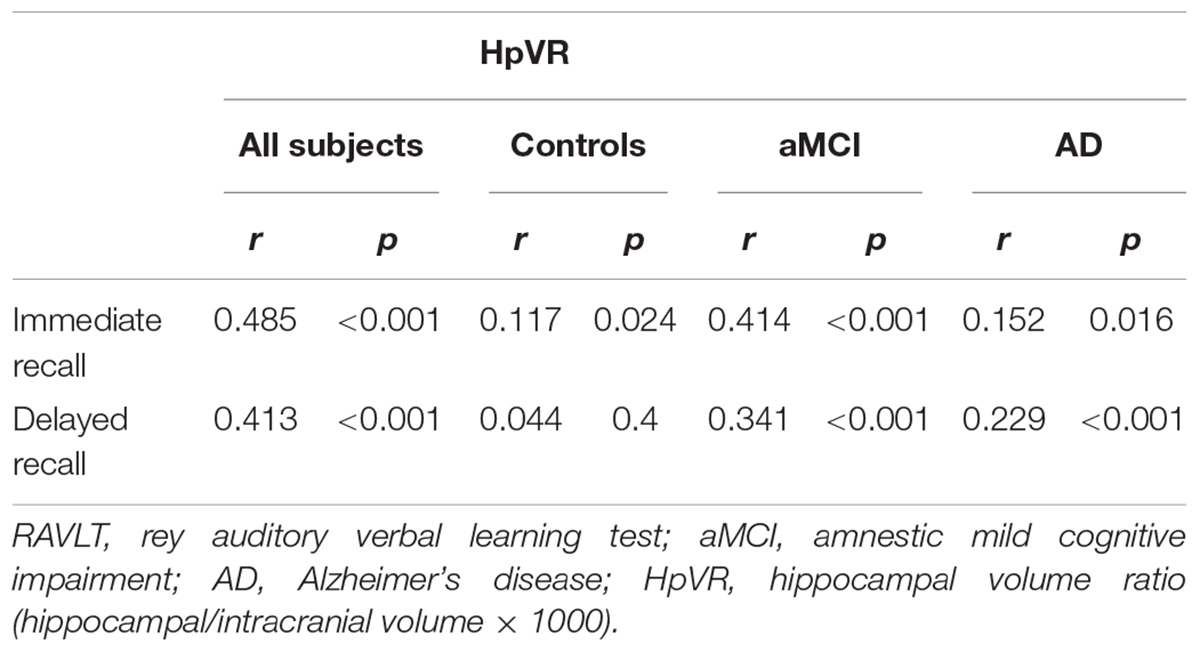
To examine the relationships between HpVR and RAVLT immediate and delayed recall scores, Pearson correlation tests were performed in all subjects and within three diagnostic groups (Table 2). As expected, positive correlations between HpVR and RAVLT immediate (r = 0.485, p < 0.001) and delayed (r = 0.413, p < 0.001) recall scores were found in all subjects. In diagnosis-stratified analyses, a positive correlation between HpVR and immediate recall (r = 0.117, p = 0.024), but not delayed recall (r = 0.044, p = 0.4), was observed in controls. In agreement with findings in the whole sample, HpVR was positively correlated with immediate recall (r = 0.414, p < 0.001), and delayed recall (r = 0.341, p < 0.001) scores in subjects with MCI. Among subjects with AD dementia, HpVR was correlated with immediate recall (r = 0.152, p = 0.016), and delayed recall (r = 0.229, p < 0.001) scores.
Effect of APOE ε4 Genotypes on HpVR and RAVLT
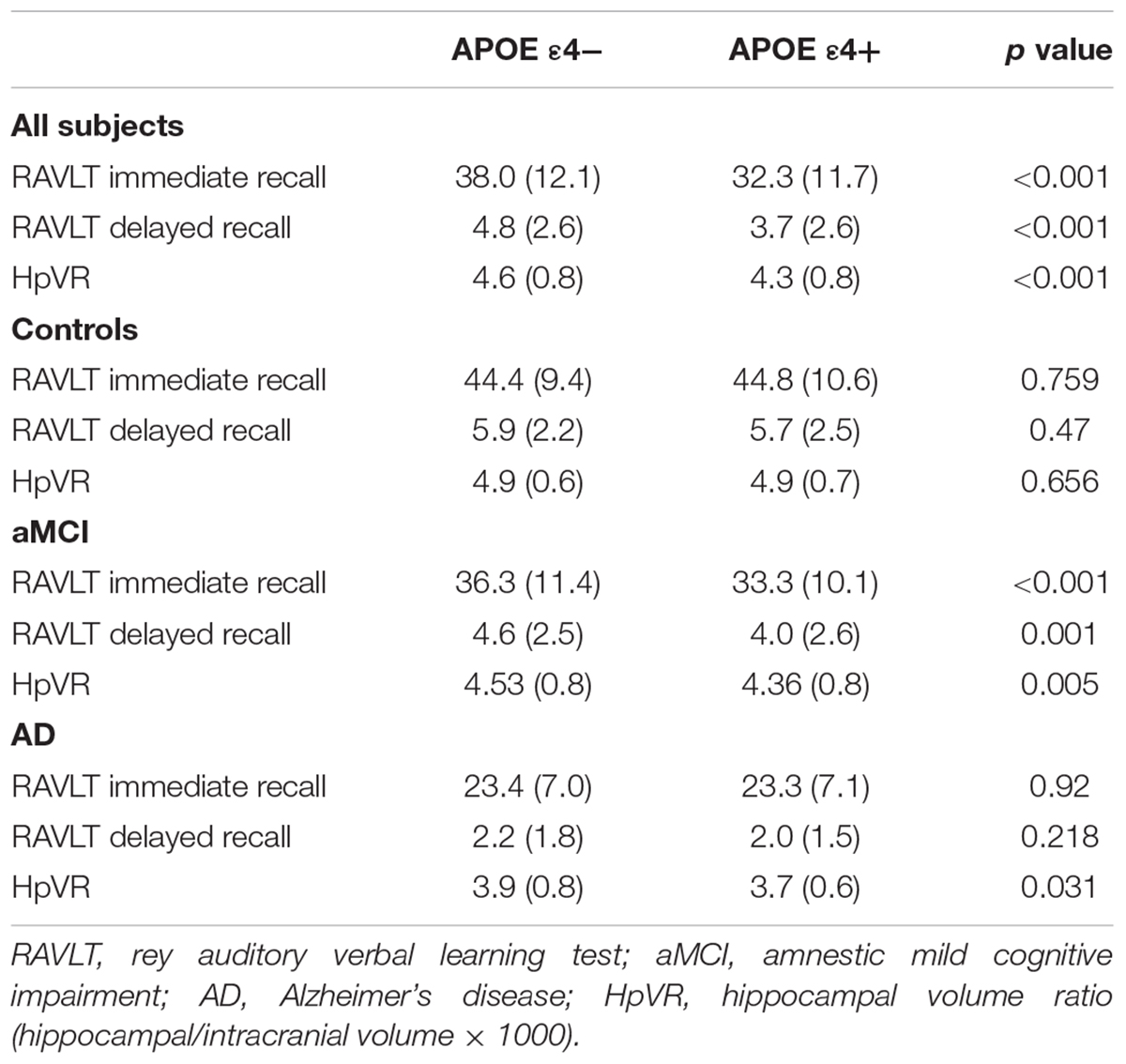
In the overall sample, APOE ε4 carriers had lower RAVLT immediate and delayed recall scores and smaller hippocampal volumes compared to non-carriers (Table 3). In diagnosis-stratified analyses, APOE ε4 carriers had lower RAVLT immediate and delayed recall scores in the MCI group, but not the NC or AD group. Further, compared with non-carriers, APOE ε4 carriers had smaller hippocampal volumes in the MCI and AD groups, but not the NC group.
Linear Regression Results
In all subjects, there was no significant HpVR by APOE ε4 status interaction for immediate recall (p = 0.26; Table 4) or delayed recall (p = 0.091; Table 4 and Figure 1A). In diagnosis-stratified analyses, the HpVR by APOE ε4 status interaction was significant for delayed recall in an aMCI group (p = 0.008), but not immediate recall (p = 0.15). More specifically, the association between HpVR and delayed recall was stronger in APOE ε4 carriers compared to non-carriers. Figure 1C shows that APOE ε4 carriers outperform non-carriers on delayed recall memory among subjects with large HpVR (right side of the x-axis) but this advantage gradually disappears and reverses to confer memory deficits among subjects with moderate to small HpVR (left side of the x-axis). However, the association of the APOE4∗HpVR interaction with delayed recall scores in the MCI group did not survive FDR correction (p = 0.096). The HpVR by APOE ε4 status interaction for immediate recall and delayed recall was not significant in the control or in the AD dementia group (all p > 0.05, Table 4 and Figures 1B,D). Among controls, no significant difference in immediate (p = 0.8) or delayed recall (p = 0.3) was found between APOE ε4 carriers and non-carriers, and HpVR was not associated with immediate recall (p = 0.7) or delayed recall (p = 0.6) (Table 4). In AD dementia, no significant difference in immediate (p = 0.7) and delayed recall (p = 0.34) was found between APOE ε4 carriers and non-carriers. In AD dementia, larger HpVR was associated with better delayed recall (p = 0.014), but not with immediate recall (p = 0.4).
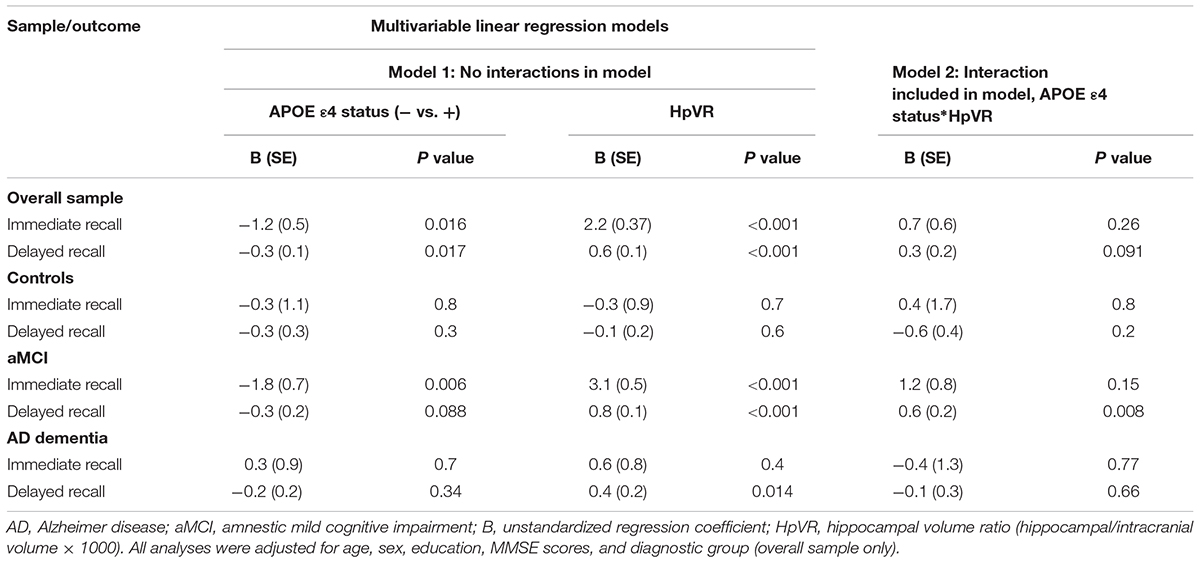
Table 4. Results of multivariable linear regression analyses modeling the independent and interactive effects of APOE ε4 status and HpVR on verbal memory performance.
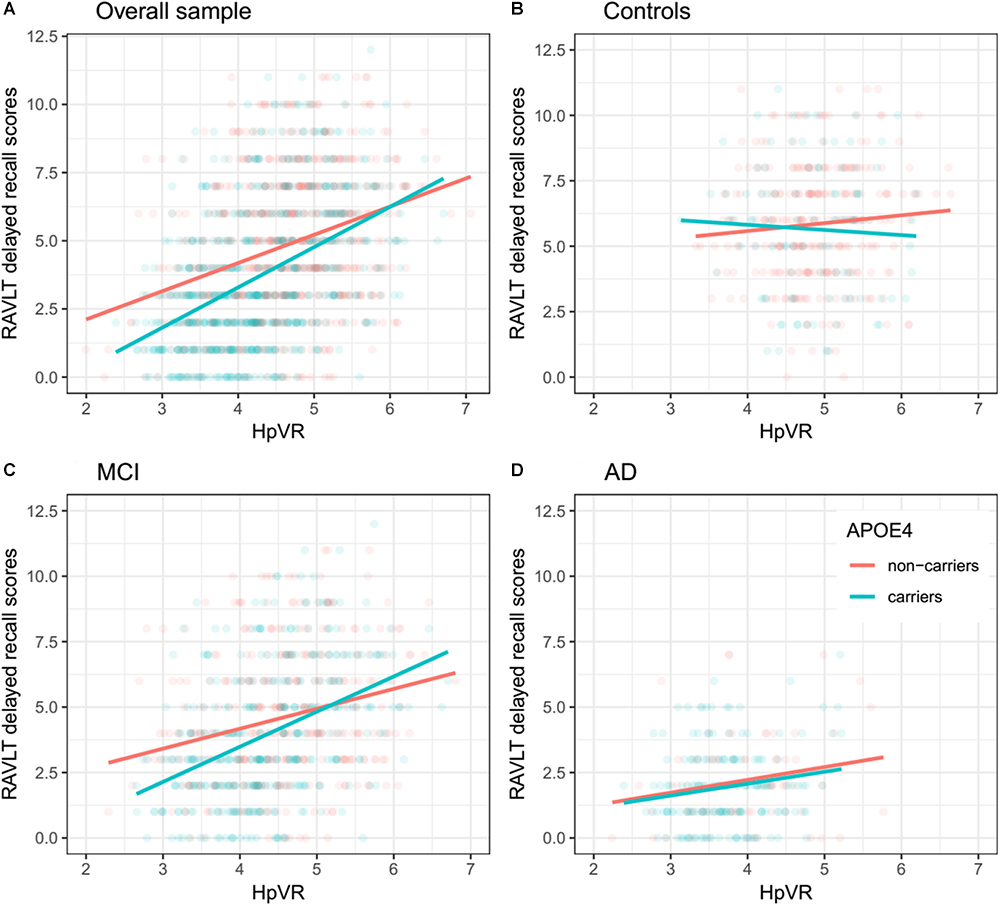
Figure 1. Association between HpVR and rey auditory verbal learning test (RAVLT) delayed recall scores in APOE ε4 carriers and non-carriers. RAVLT delayed recall scores as a function of HpVR (hippocampal/intracranial volume × 103) and APOE ε4 status in the (A) overall sample, (B) controls, (C) amnestic mild cognitive impairment (aMCI), and (D) Alzheimer disease (AD) dementia.
In addition, we also examined the effect of the left-HpVR/Right-HpVR∗APOE ε4 interaction on RAVLT immediate and delayed recall scores (see Figures 2, 3 and Tables 5, 6). Similarly, a significant left-HpVR∗APOE ε4 interaction for delayed recall among MCI subjects was observed (p = 0.004), while the association did not survive FDR correction (p = 0.096). Further, there was a significant right-HpVR∗APOE ε4 interaction for delayed recall among MCI subjects (p = 0.034), but the association did not survive FDR correction (p = 0.27).
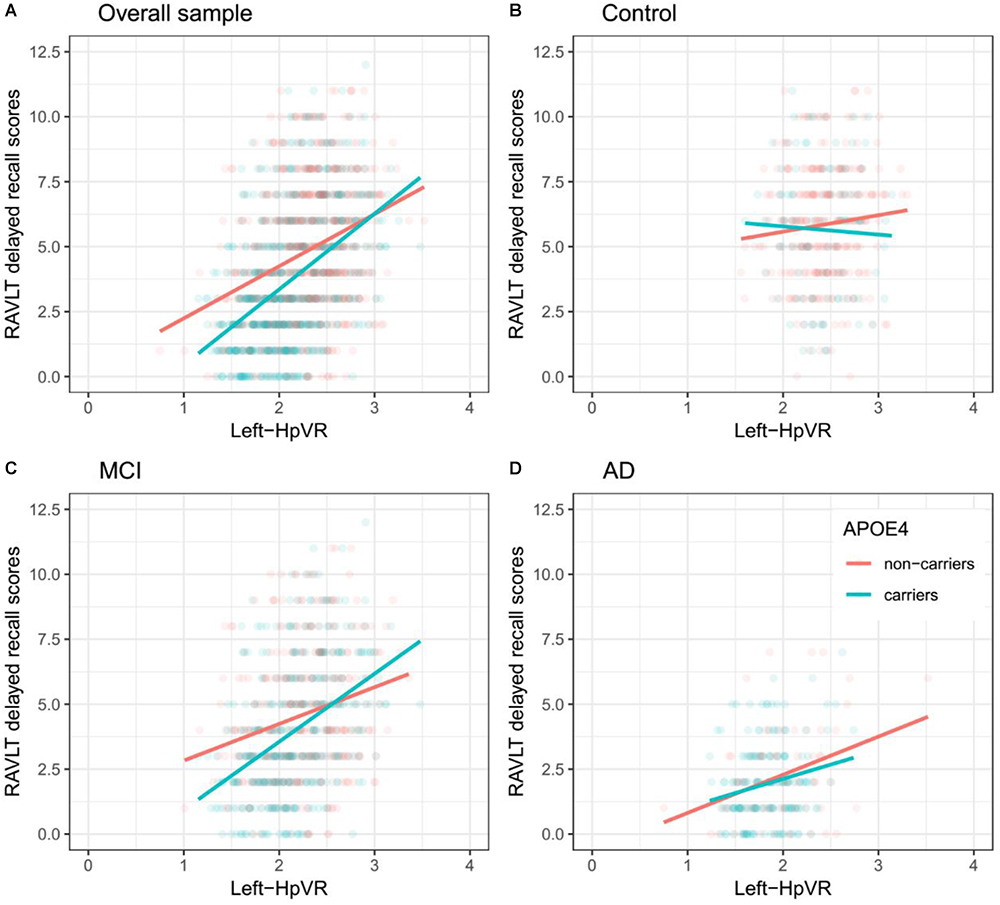
Figure 2. Association between left-HpVR and RAVLT delayed recall scores in APOE ε4 carriers and non-carriers. RAVLT delayed recall scores as a function of Left-HpVR (hippocampal/intracranial volume × 103) and APOE ε4 status in the (A) overall sample, (B) controls, (C) aMCI, and (D) AD dementia.
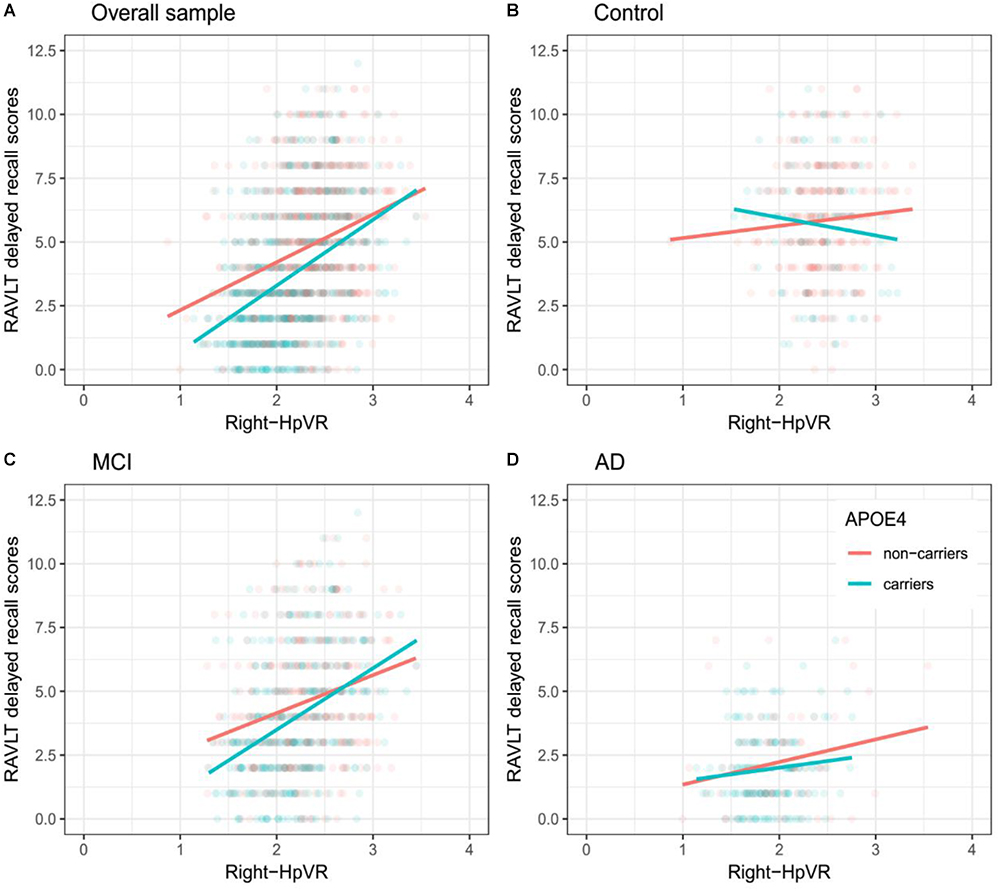
Figure 3. Association between right-HpVR and RAVLT delayed recall scores in APOE ε4 carriers and non-carriers. RAVLT delayed recall scores as a function of Right-HpVR (hippocampal/intracranial volume × 103) and APOE ε4 status in the (A) overall sample, (B) controls, (C) aMCI, and (D) AD dementia.
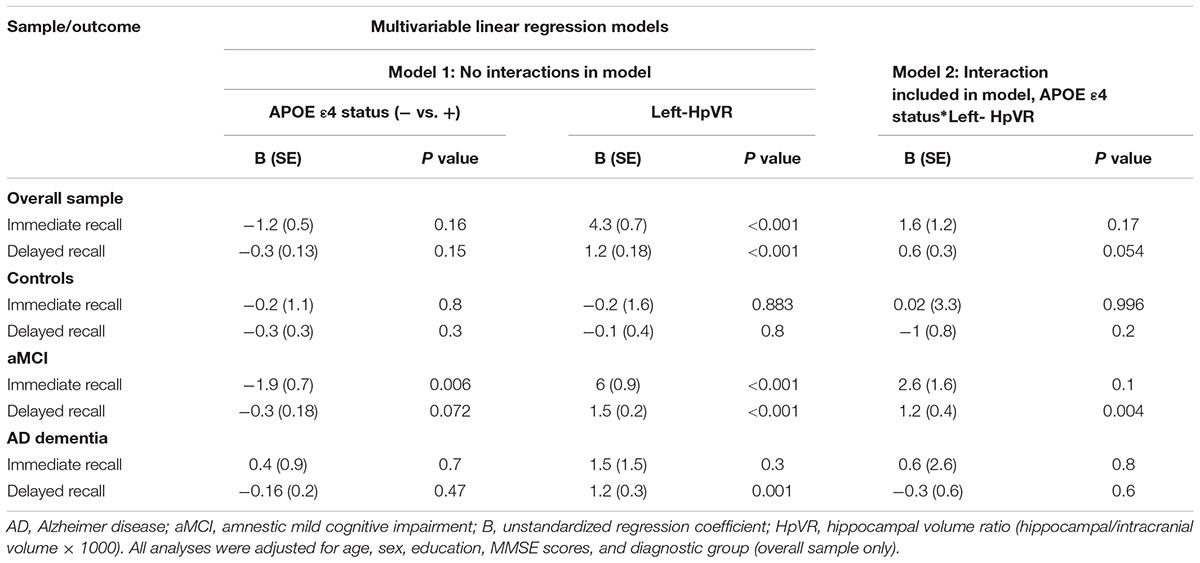
Table 5. Results of multivariable linear regression analyses modeling the independent and interactive effects of APOE ε4 status and left-HpVR on verbal memory performance.
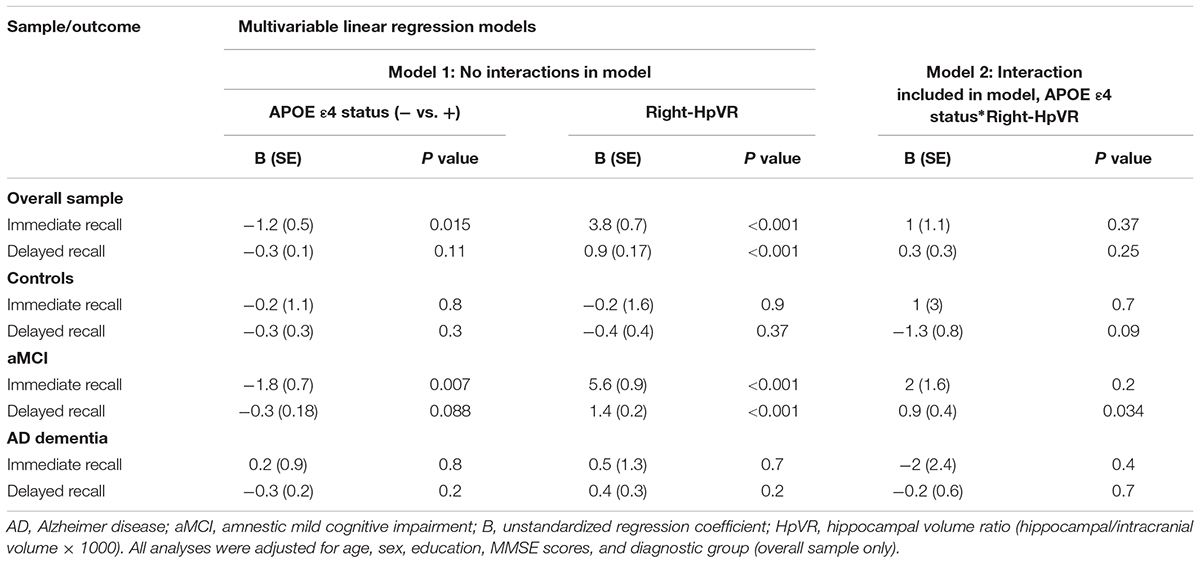
Table 6. Results of multivariable linear regression analyses modeling the independent and interactive effects of APOE ε4 status and Right-HpVR on verbal memory performance.
Discussion
In the present study, we examined the effect of the APOE ε4 allele on the interplay between episodic memory and hippocampal volume in the three diagnostic groups (controls, aMCI, mild AD). Our study found an interesting fact that, depending on different hippocampal volumes, the APOE ε4 allele asserts different effects on delayed recall. Our data suggested that the deleterious effect of APOE ε4 on delayed recall altered by HpVR such that this effect was most evident among aMCI patients with small to moderate HpVR, but this disadvantage was absent or even reversed among aMCI patients with larger HpVR, indicating the dual effects of APOE ε4 on the association of delayed recall memory and hippocampal volumes.
Antagonistic pleiotropy (Williams, 1957) posits that certain genes or alleles may affect fitness (for instance, survival, and reproduction) differentially at different ages. Recently, the antagonistic pleiotropy hypothesis of APOE has been proposed by some researchers based on findings that the impact of the APOE ε4 allele on cognitive functioning, episodic memory in particular, may be beneficial at younger ages, while it appears detrimental in later life (Han and Bondi, 2008; Caselli et al., 2009; Tuminello and Han, 2011; Jochemsen et al., 2012). However, concomitant underlying mechanisms of these changes remain unclear. Increasing evidence suggested that APOE ε4 carriers may primarily recruit greater compensatory resources to maintain equivalent or superior levels of memory performance as APOE ε4 non-carriers. Nevertheless, once AD pathological burdens sufficiently accumulate, APOEε4 carriers’ compensatory recruitment will fail to sustain memory performance and memory decline ensues (Han and Bondi, 2008). For instance, one study (Bookheimer et al., 2000) using fMRI techniques suggested that APOE ε4 carriers, in non-demented older people, showed greater activation in the hippocampus and other brain regions during the encoding portion of a memory task, providing evidence for APOEε4 carriers’ compensatory recruitment. In other words, APOE ε4 carriers may more greatly activate memory-related brain regions to sustain the same or better level of cognitive performance as APOE ε4 non-carriers. Dickerson et al. (2005) also reported findings supportive of APOEε4 carriers’ compensatory recruitment in three diagnostic groups, including cognitively normal older people and patients with MCI or AD. They found that APOEε4 carriers, irrespective of diagnosis, showed increased hippocampal activity during the encoding portion of a face-name task. Similarly, a more recent study suggested that APOEε4 carriers recruited additional neural resources to successfully complete a challenging working memory task (Scheller et al., 2017). However, once the compensatory recruitment of neural resources fails, memory may begin to decline (Han and Bondi, 2008; Tuminello and Han, 2011). Most cross-sectional studies have supported the idea that APOE ε4 carriers in older people performed more poorly than non-carriers in episodic memory tasks (Honea et al., 2009; Adamson et al., 2010; Kukolja et al., 2010), while not universally the case (Welsh-Bohmer et al., 2009). Furthermore, one important study by Caselli et al. (2009) longitudinally followed individuals ranging from 21 to 97 years of age. They found that memory decline in APOEε4 carriers started before 60 years of age and showed a steeper rate of memory decline compared to non-carriers, indicating that the detrimental effects of APOE ε4 on episodic memory may become evident around the age of 60 years. Similarly, based on our findings (Figure 1C), APOE ε4 carriers in the aMCI group, despite similar hippocampal volumes, outperformed non-carriers on episodic memory among subjects with large HpVR (HpVR > approximately 5.2, referring to the compensatory recruitment state). Once AD pathological burdens sufficiently accrue (such as hippocampal atrophy), this APOE ε4 advantage was not evident and gradually reversed to confer memory deficits among subjects with moderate to small HpVR (HpVR < approximately 5.2, referring to the failure of compensatory recruitment). Our data indicated that APOE ε4 carriers as an at-risk population for AD may benefit from drug or non-drug interventions that are tailored to the levels of hippocampal atrophy. After acquisition of APOE ε4 genotype and hippocampal volume data, APOE ε4 carriers could be further targeted by some interventions to maintain memory performance at this paramount turning point.
Our findings have several important implications. The dual effects of APOE ε4 on the association of delayed recall memory with hippocampal volumes are clinically critical because delayed recall memory is used to diagnose aMCI and AD dementia (Albert et al., 2011; McKhann et al., 2011; Dubois et al., 2014) and norms of episodic memory tests are currently not APOE ε4-adjusted. Therefore, this may lead to some misdiagnoses if we do not take APOE ε4 status into consideration. For instance, due to our findings (Figure 1C), a true aMCI diagnosis may be more likely to be delayed in APOE ε4 carriers than non-carriers in individuals with HpVR > approximately 5.2, because beneficial effects of APOE ε4 on episodic memory in these subjects may mask underlying AD neuropathology, particularly in the earlier stages of disease.
There are several potential limitations in the present study. First, the cross-sectional design prevents us from determining temporality in the association between episodic memory and HpVR. In this cross-section analysis, we also could not measure rates of memory decline in APOE ε4 carriers vs. non-carriers. Longitudinal studies are needed to more accurately test the hypothesis that APOE ε4 status may modulate the relationship between hippocampus volumes and episodic memory. Second, the APOE antagonistic pleiotropy hypothesis proposes that the APOE ε4 allele is linked to better cognitive functioning in young adulthood and then it reverses to confer cognitive deficits in older age. However, in the current study, the lack of younger people limits our test of the APOE antagonistic pleiotropy hypothesis. Third, the HpVR by APOE ε4 interaction for memory testing was not significant in the control or in the AD dementia group. One potential explanation may be due to the fact that the variability of memory scores and hippocampal volumes may be low in controls, and memory scores and hippocampal volumes may have reached a plateau in AD patients. Finally, after FDR correction, a marginally significant two-way APOE ε4∗HpVR interaction for delayed recall in MCI subjects was observed (p = 0.096), and thus this finding requires replication.
In summary, our study highlights the potential role of APOE ε4 in affecting the relationship between hippocampus volumes and delayed recall memory in MCI patients.
Data Availability
Publicly available datasets were analyzed in this study. This data can be found here: http://adni.loni.usc.edu/.
Ethics Statement
All subjects gave signed, informed consent to participate in the study. Clinical research described in the manuscript was carried out in accordance with Declaration of Helsinki promulgated by the National Institute of Health.
Author Contributions
JZ and XL conceived and designed the study. XW, WZ, and TY performed the research, analyzed the data, and wrote the manuscript.
Funding
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI was funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
- ^http://adni.loni.usc.edu
- ^http://surfer.nmr.mgh.harvard.edu
- ^https://ida.loni.usc.edu/pages/access/studyData.jsp
References
Adamson, M. M., Landy, K. M., Duong, S., Fox-Bosetti, S., Ashford, J. W., Murphy, G. M., et al. (2010). Apolipoprotein E epsilon4 influences on episodic recall and brain structures in aging pilots. Neurobiol. Aging 31, 1059–1063. doi: 10.1016/j.neurobiolaging.2008.07.017
Albert, M. S., Dekosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., et al. (2011). The diagnosis of mild cognitive impairment due to alzheimer’s disease: recommendations from the national institute on aging-alzheimer’s association workgroups on diagnostic guidelines for alzheimer’s disease. Alzheimers Dement. 7, 270–279. doi: 10.1016/j.jalz.2011.03.008
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 57, 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
Bookheimer, S. Y., Strojwas, M. H., Cohen, M. S., Saunders, A. M., Pericak-Vance, M. A., Mazziotta, J. C., et al. (2000). Patterns of brain activation in people at risk for alzheimer’s disease. N. Engl. J. Med. 343, 450–456. doi: 10.1056/NEJM200008173430701
Caselli, R. J., Dueck, A. C., Osborne, D., Sabbagh, M. N., Connor, D. J., Ahern, G. L., et al. (2009). Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N. Engl. J. Med. 361, 255–263. doi: 10.1056/NEJMoa0809437
Dickerson, B. C., Salat, D. H., Greve, D. N., Chua, E. F., Rand-Giovannetti, E., Rentz, D. M., et al. (2005). Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 65, 404–411. doi: 10.1212/01.wnl.0000171450.97464.49
Dubois, B., Feldman, H. H., Jacova, C., Hampel, H., Molinuevo, J. L., Blennow, K., et al. (2014). Advancing research diagnostic criteria for alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 13, 614–629. doi: 10.1016/S1474-4422(14)70090-0
Eldridge, L. L., Knowlton, B. J., Furmanski, C. S., Bookheimer, S. Y., and Engel, S. A. (2000). Remembering episodes: a selective role for the hippocampus during retrieval. Nat. Neurosci. 3, 1149–1152. doi: 10.1038/80671
Han, S. D., and Bondi, M. W. (2008). Revision of the apolipoprotein E compensatory mechanism recruitment hypothesis. Alzheimers Dement. 4, 251–254. doi: 10.1016/j.jalz.2008.02.006
Honea, R. A., Vidoni, E., Harsha, A., and Burns, J. M. (2009). Impact of APOE on the healthy aging brain: a voxel-based MRI and DTI study. J. Alzheimers Dis. 18, 553–564. doi: 10.3233/JAD-2009-1163
Jochemsen, H. M., Muller, M., Van Der Graaf, Y., and Geerlings, M. I. (2012). APOE epsilon4 differentially influences change in memory performance depending on age. The smart-MR study. Neurobiol. Aging 33:832.e15-22. doi: 10.1016/j.neurobiolaging.2011.07.016
Khan, W., Giampietro, V., Banaschewski, T., Barker, G. J., Bokde, A. L., Buchel, C., et al. (2017). A multi-cohort study of ApoE varepsilon4 and amyloid-beta effects on the hippocampus in alzheimer’s disease. J. Alzheimers Dis. 56, 1159–1174. doi: 10.3233/JAD-161097
Kilpatrick, C., Murrie, V., Cook, M., Andrewes, D., Desmond, P., and Hopper, J. (1997). Degree of left hippocampal atrophy correlates with severity of neuropsychological deficits. Seizure 6, 213–218. doi: 10.1016/S1059-1311(97)80008-8
Kukolja, J., Thiel, C. M., Eggermann, T., Zerres, K., and Fink, G. R. (2010). Medial temporal lobe dysfunction during encoding and retrieval of episodic memory in non-demented APOE epsilon4 carriers. Neuroscience 168, 487–497. doi: 10.1016/j.neuroscience.2010.03.044
Lim, Y. Y., Williamson, R., Laws, S. M., Villemagne, V. L., Bourgeat, P., Fowler, C., et al. (2017). Effect of APOE genotype on amyloid deposition, brain volume, and memory in cognitively normal older individuals. J. Alzheimers Dis. 58, 1293–1302. doi: 10.3233/JAD-170072
Liu, Y., Tan, L., Wang, H. F., Liu, Y., Hao, X. K., Tan, C. C., et al. (2016). Multiple effect of APOE genotype on clinical and neuroimaging biomarkers across alzheimer’s disease spectrum. Mol. Neurobiol. 53, 4539–4547. doi: 10.1007/s12035-015-9388-7
McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R Jr, Kawas, C. H., et al. (2011). The diagnosis of dementia due to alzheimer’s disease: recommendations from the national institute on aging-alzheimer’s association workgroups on diagnostic guidelines for alzheimer’s disease. Alzheimers Dement. 7, 263–269. doi: 10.1016/j.jalz.2011.03.005
Petersen, R. C., Aisen, P. S., Beckett, L. A., Donohue, M. C., Gamst, A. C., Harvey, D. J., et al. (2010). Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology 74, 201–209. doi: 10.1212/WNL.0b013e3181cb3e25
Scheller, E., Peter, J., Schumacher, L. V., Lahr, J., Mader, I., Kaller, C. P., et al. (2017). APOE moderates compensatory recruitment of neuronal resources during working memory processing in healthy older adults. Neurobiol. Aging 56, 127–137. doi: 10.1016/j.neurobiolaging.2017.04.015
Schmidt, M. (1996). Rey Auditory Verbal Learning Test A Handbook. Los Angeles, CA: Western Psychological Services.
Tuminello, E. R., and Han, S. D. (2011). The apolipoprotein e antagonistic pleiotropy hypothesis: review and recommendations. Int. J. Alzheimers Dis. 2011:726197. doi: 10.4061/2011/726197
Welsh-Bohmer, K. A., Ostbye, T., Sanders, L., Pieper, C. F., Hayden, K. M., Tschanz, J. T., et al. (2009). Neuropsychological performance in advanced age: influences of demographic factors and apolipoprotein E: findings from the cache county memory study. Clin. Neuropsychol. 23, 77–99. doi: 10.1080/13854040801894730
Williams, G. C. (1957). Pleiotropy, natural selection, and the evolution of senescence. Evolution 11, 398–411. doi: 10.1111/j.1558-5646.1957.tb02911.x
Keywords: Alzheimer’s disease, amnestic mild cognitive impairment, hippocampus, episodic memory, APOE ε4
Citation: Wang X, Zhou W, Ye T, Lin X, Zhang J and the Alzheimer’s Disease Neuroimaging Initiative (2019) The Relationship Between Hippocampal Volumes and Delayed Recall Is Modified by APOE ε4 in Mild Cognitive Impairment. Front. Aging Neurosci. 11:36. doi: 10.3389/fnagi.2019.00036
Received: 11 December 2018; Accepted: 06 February 2019;
Published: 26 February 2019.
Edited by:
Panteleimon Giannakopoulos, Université de Genève, SwitzerlandReviewed by:
Ramesh Kandimalla, Texas Tech University Health Sciences Center, United StatesSven Haller, Affidea CDRC, Switzerland
Copyright © 2019 Wang, Zhou, Ye, Lin, Zhang and the Alzheimer’s Disease Neuroimaging Initiative. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaodong Lin, MTMzMjU3Nzk3MThAMTYzLmNvbQ== Jie Zhang, amF5emhhbmcxMDE0QGdtYWlsLmNvbQ==
†Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
 Xiwu Wang
Xiwu Wang Wenjun Zhou2
Wenjun Zhou2 Jie Zhang
Jie Zhang