
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 09 November 2018
Sec. Neurocognitive Aging and Behavior
Volume 10 - 2018 | https://doi.org/10.3389/fnagi.2018.00360
This article is part of the Research Topic Sleep and Aging View all 7 articles
Objectives: The aims of the current study are to (1) report the frequency of specific sleep disturbance symptoms in Mild Cognitive Impairment (MCI) and cognitive healthy older persons; (2) examine whether overall poor sleep and specific sleep disturbance symptoms are more common in persons with MCI compared to cognitive healthy older controls and; (3) examine the association between sleep disturbances and performance in general and specific cognitive domains in persons with MCI and separately in cognitive healthy older persons.
Methods: Data were collected at the Fondazione Ospedale San Camillo Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Venice, Italy as part of the European VPH-DARE@IT project. We included 69 persons with MCI (mean age 75.7; SD = 7.7) and 72 sex-matched cognitively healthy controls (mean age 71.8; SD = 7.0). Participants underwent extensive neuropsychological assessment and evaluation of subjective sleep performance with the Sleep Continuity in Alzheimer’s Disease Scale(SCADS).
Results: A fifth of MCI patients (21.7%, n = 15) had poor sleep compared to 15.3% (n = 11) of cognitively healthy controls. MCI patients had a 3.2 higher odds of having poor sleep compared to cognitively healthy controls after adjustment for age, education, sex, and general cognitive functioning (Odds Ratio (OR) = 3.2; 95% Confidence Interval (CI) = 1.1–9.2). Persons who reported waking up twice or more during the night had higher odds of being MCI compared to those who never wake or wake only once (OR = 2.6; 95% CI = 1.1–6.1). In MCI patients, poor sleep was associated with better general cognitive functioning and short-term working memory, whereas in cognitive healthy older persons poor sleep was associated with impairment in episodic memory performance and executive functioning.
Discussion: Our results confirm previous studies showing that sleep disturbances are common in MCI, and this may be due to an ongoing neurodegenerative process rather than a symptom of cognitive impairment. Future research with objective sleep measurements are needed in MCI as well as interventions to improve sleep with the aim of preventing cognitive decline.
Sleep health covers a variety of dimensions, with many different definitions in the literature that generally cover a range of aspects including sleep satisfaction, timing, efficiency, and duration (Buysse, 2014). Sleep disorders are common in aging and increase with advancing age (Gadie et al., 2017). Research has shown that poor sleep is related to poorer general cognitive functioning as well as impairment in specific cognitive domains (Gadie et al., 2017). A meta-analysis concluded that persons with symptoms of insomnia show impairment in cognitive functioning in episodic memory, working memory and executive functioning (Fortier-Brochu et al., 2012). Interestingly, research also suggests that a cumulative index of sleep problems, rather than specific symptoms of poor sleep, is the biggest risk factor for poorer cognitive performance (Gadie et al., 2017). However, evidence is conflicting, with some studies reporting no association between sleep disturbance and cognition (Mecca et al., 2018), and some even reporting slightly better cognitive functioning in those with sleep problems (Kyle et al., 2017).
In addition to concurrent cognitive impairment, sleep quality has also been linked with subsequent cognitive decline over time (Virta et al., 2013; Blackwell et al., 2014) and specific aging disorders such as dementia. At least a quarter of Alzheimer’s disease (AD) patients exhibit severe sleep dysfunction (Moran et al., 2005). It is thought that sleep disorders start to occur in the preclinical phases of AD, as they have been found to predict incident dementia (Ju et al., 2013, 2014; Lim et al., 2013; Sterniczuk et al., 2013; Diem et al., 2016). Poor sleep quality has been found to be associated with Mild Cognitive Impairment (MCI) (Dlugaj et al., 2014), which often represents a prodromal phase of preclinical dementia. A third of MCI patients exhibit nighttime behaviors (Di Iulio et al., 2010), and specific symptoms have been objectively observed in these individuals, such as waking after sleep onset (Naismith et al., 2014). However, results are conflicting, with some studies reporting no link between sleep disturbances and cognitive decline (Mecca et al., 2018).
The overall objective of the current study is to contribute to this ongoing discussion and provide further data to examine the role of sleep disturbances in normal aging and MCI. We aimed to look in detail at different specific symptoms of sleep disturbances using the Sleep Continuity in Alzheimer’s Disease Scale (SCADS) (Manni et al., 2013), which is a validated Italian tool for the early identification and longitudinal monitoring of sleep disturbances in dementia. The specific aims of the current study were to: (1) report the frequency of specific sleep disturbance symptoms in MCI and cognitive healthy older persons; (2) examine whether overall poor sleep and specific sleep disturbance symptoms are more common in persons with MCI compared to cognitively healthy older controls and; (3) examine the association between sleep disturbances and performance in general and specific cognitive domains in persons with MCI, and separately in cognitive healthy older persons.
Data were collected at the Fondazione Ospedale San Camillo Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Lido, Venice, Italy as part of the European VPH-DARE@IT project1. The study was approved by the joint ethics committee of the Health Authority Venice 12 and San Camillo IRCCS (Protocol number 2014.08). All participants gave informed consent prior to participation in the study.
MCI patients were recruited from the outpatient memory service at the hospital. Eligible patients amongst consecutive referrals to this service were enrolled if they met clinical criteria for MCI (Petersen et al., 1999, 2001; Albert et al., 2011) and met the following exclusion criteria: diagnostic entities of clinical concern (brain tumor, hydrocephalous, lesions indicative of multiple sclerosis, large cysts, and excessive leukoaraiosis), chronic or acute cerebrovascular disease as main etiology, history of transient ischemic attacks, presence of uncontrolled brain seizures, peptic ulcer, cardiovascular disease, sick-sinus syndrome, neuropathy with conduction difficulties, proof of abnormal levels of folates, vitamin B12 or thyroid stimulating hormone, significant neuropsychiatric diagnoses such as Major Depression, Anxiety or Psychosis, treatment with medication for research purposes or with toxic effects to internal organs (e.g., drugs that could potentially be toxic to the liver or heart, anti-epileptic drugs, or psychoactive medications such as amphetamine or ketamine). Participants with significant disabilities or with MRI indication of a major diagnostic category of non-neurodegenerative nature, which could otherwise explain cognitive symptoms, were not considered for recruitment. Sleep apnea and presence of neuropsychiatric sleep disturbances were not used as exclusion criteria.
Healthy controls were recruited amongst carers and spouses of the MCI patients and through word of mouth amongst the residents of the island of Lido in Venice. The same relevant exclusion criteria used for patients were also applied for enrolment of controls.
We included 141 persons aged ≥52 years (range 52–89); 69 persons with MCI and 72 sex-matched cognitively healthy controls. Characteristics of the sample are reported in Table 1.
An extensive neuropsychological battery was administered to all participants by a neuropsychologist, including Italian versions of the Mini-Mental State Examination (MMSE) (Measso et al., 1993), Raven’s colored progressive matrices (Basso et al., 1987), Phonemic/Semantic fluency tests (Novelli et al., 1986b), Digit Cancelation test (Orsini et al., 1987), Similarity subtest from the Wechsler Adult Intelligence Scale (Wechsler, 1981), Token test (De Renzi and Faglioni, 1978), Rey complex figure test (Caffarra et al., 2002a), Stroop test (Caffarra et al., 2002b), Corsi block test (Orsini et al., 1987), immediate and delayed word recall (Novelli et al., 1986a), Paired Associates (Novelli et al., 1986a), and the Confrontation Naming test (Novelli et al., 1986b). Test scores were corrected for age, education, and sex as necessary.
In addition to the neuropsychological testing, all participants underwent a clinical examination by a neurologist. MCI was diagnosed according to Petersen et al’s criteria (Petersen et al., 1999, 2001; Albert et al., 2011). Diagnostic status was reached by multidisciplinary consensus based on clinical, neuropsychological and neuroimaging evidence, see Lassila et al. (2018) for a description of the neuroimaging methods.
Sleeping behaviors were assessed during clinical evaluation. We used the SCADS scale (Manni et al., 2013, 2015), which has been validated in Italian patients with cognitive impairment. The scale includes nine self-reported questions regarding length and quality of sleep, waking during the night, and difficulties in getting to sleep; please see Figure 1 for all questions. For each question participants rated the quality of sleep according to four different responses. For the current analyses all questions were recoded so that score 1 indicates no problem and score 4 indicates very poor. Individual questions as well as the total score were investigated in the current analysis. This scale differs from the Neuropsychiatric Inventory because it assesses nine sleep-related symptom separately, thus allowing a more comprehensive insight into specific nighttime behaviors. However, it relies on self-report from the patient rather than a caregiver.
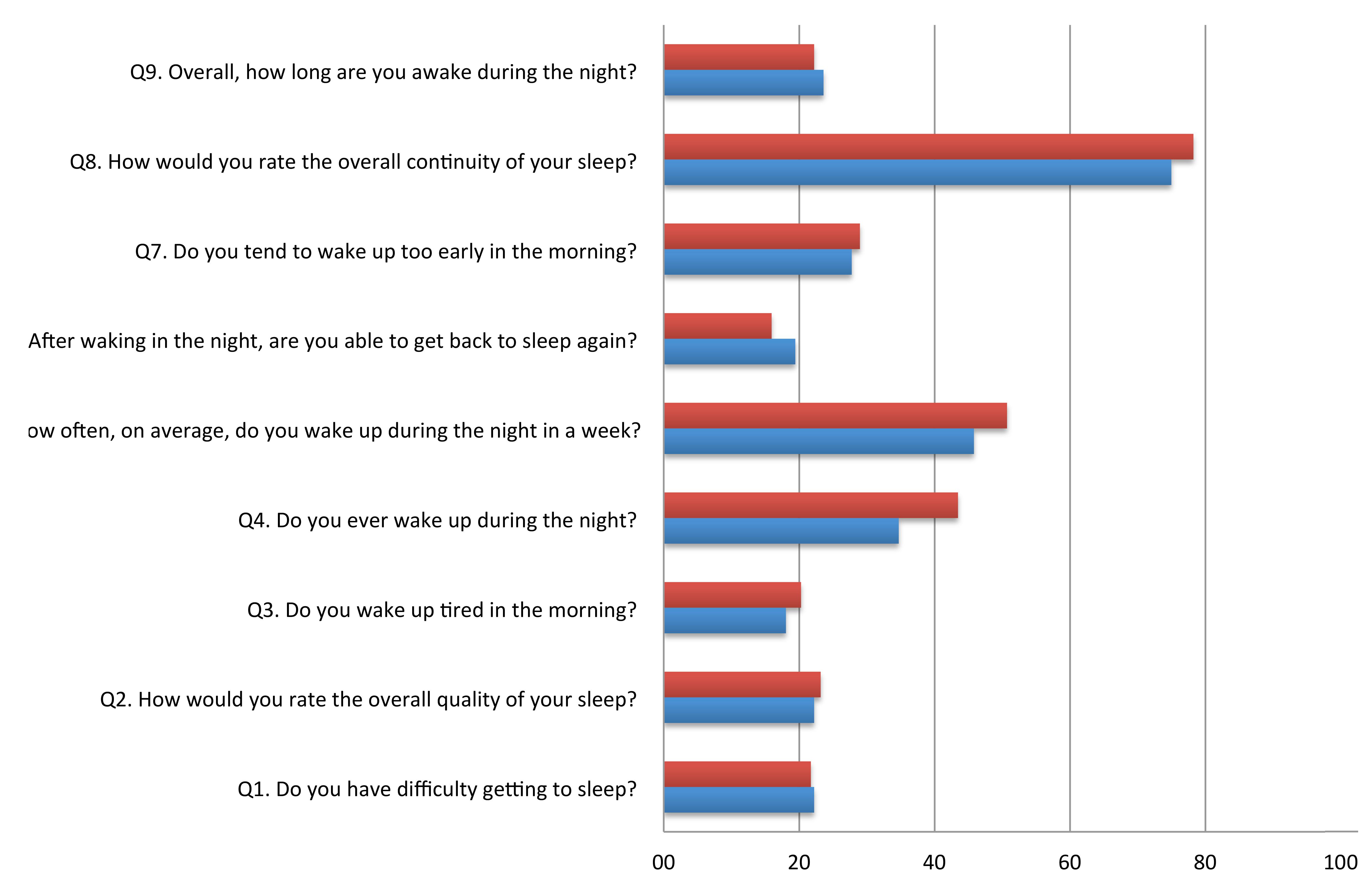
FIGURE 1. Proportion of poor scores (>2) on each item of the Sleep Continuity in Alzheimer’s Disease Scale (SCADS) sleep scale in MCI and cognitively health controls.
First, we reported sociodemographic variables (age, sex, and years of education) and mean performance on the neuropsychological tests in the whole population (n = 141), and separately in MCI patients (n = 69) and cognitively healthy controls (n = 72). Second, we calculated the mean scores on the 9 SCADS items. Third, SCADS item scores were dichotomized into poor (score of 3–4) vs. good/moderate (score 1–2), and we calculated the frequency of poor sleep in each of the nine items in MCI patients and cognitively healthy controls. The nine scores were then added to create an overall sleep performance score. Persons scoring one standard deviation (SD) above the mean total SCADS score were defined as having poor sleep. Multivariate logistic regression models with 95% CI were used to investigate the association between (i) overall poor sleep and MCI status and (ii) poor sleep on the individuals SCADS questions and MCI status. Finally, we stratified the sample according to MCI status to investigate the association between performance on the individual neuropsychological tests and poor sleep (total SCADS score).
The characteristics of the study population are shown in Table 1; results are shown for the whole population, and stratified according to MCI/control status. The mean age was 73.7 (SD = 7.6) years, with MCI patients being older than cognitively healthy controls (t = 3.149; p = 0.002). There was quite a high level of education in the sample (mean 11.1 years, SD = 4.3), with MCI having a lower mean number of years of education (t = -4.282; p = 0.000). As the sample was matched for sex, the proportion of females was the same in MCI and controls (60.9 vs. 58.3%). Mean scores on the MMSE and domain specific tasks of cognitive functioning are also provided in the Table.
Table 2 shows the mean score for each item of the SCADS sleep scale for MCI, controls, and the whole population. Each question ranges from 1 to 4, with a higher score indicating poorer sleep. Figure 1 illustrates the percentage of person with a poor score (3 or 4 out of 4) on each of the SCADS items, stratified according to MCI status. Overall many people rated their continuity of sleep as poor or very poor (75% of controls and 78.3% of MCI), and waking in the night was commonly reported (questions 4 and 5) in both groups. Most people reported being awake only 30 min or less during the night (question 5). Less than a quarter of people reported having regular difficulties getting to sleep (question 1).
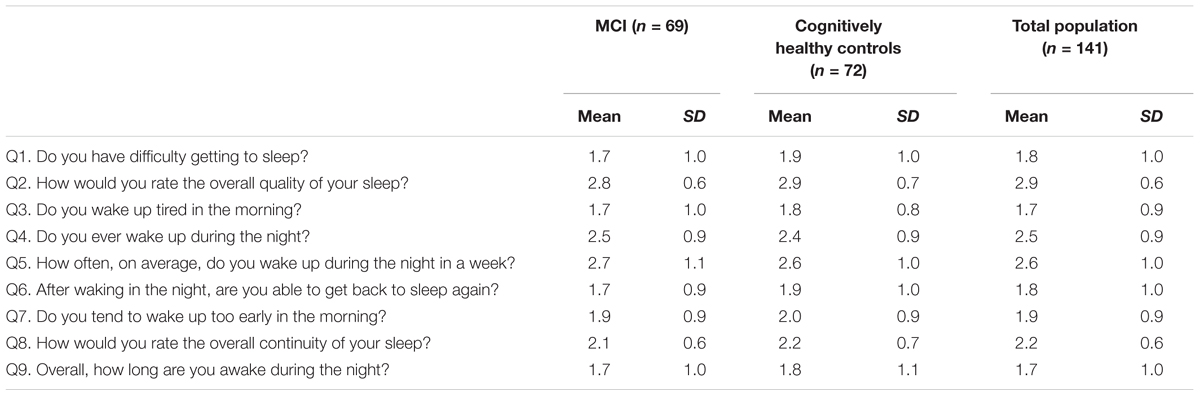
TABLE 2. Mean scores of the Sleep Continuity in Alzheimer’s Disease Scale (SCADS) sleep scale, in persons with MCI and cognitively healthy controls.
Results of the 9 questions were summed to create a total score (max 36 points), and a dichotomous variable was created to indicate poor sleep. The mean total sleep score was 19.1. (SD = 4.3). Persons scoring 1 SD above the mean were classified as having poor sleep. A fifth of MCI patients (21.7%, n = 15) had poor sleep compared to 15.3% (n = 11) of cognitively healthy controls. Table 3 shows the association between poor sleep, demographic variables and MCI status. In the adjusted OR, having MCI was associated with a 3.2 higher odds of having poor sleep compared to cognitively healthy controls (adjusted OR = 3.2; 95% CI = 1.1–9.2).
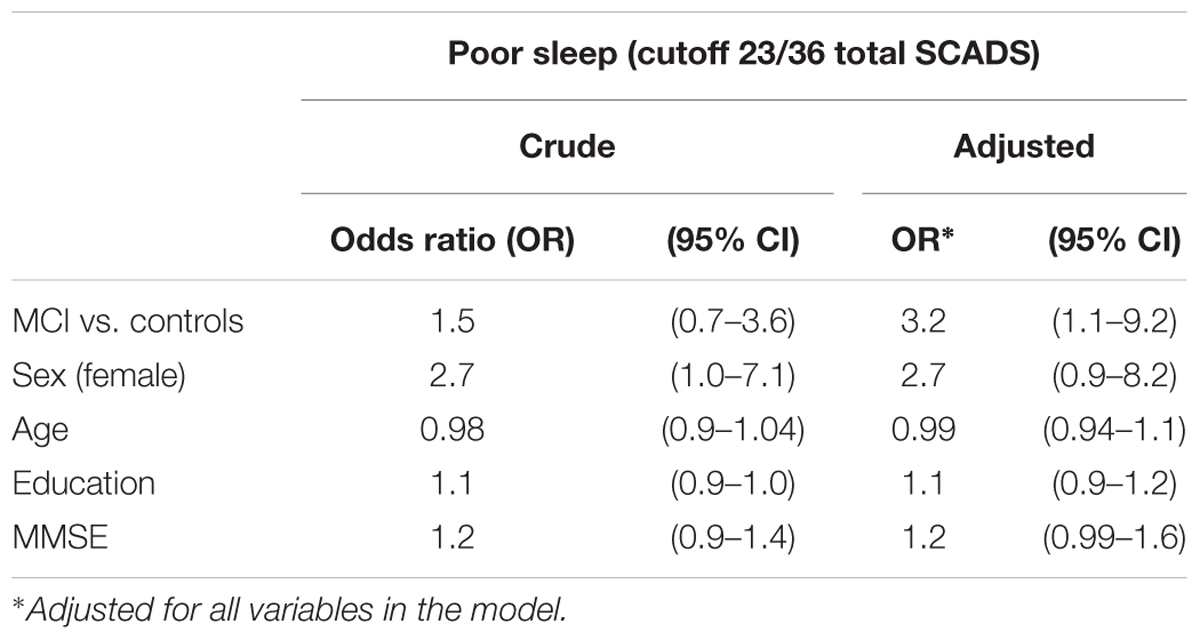
TABLE 3. Logistic regression for the association between poor sleep (total SCADS score), MCI status and sociodemographic factors.
We investigated whether having a poor score (>2) on any of the nine individual items on the SCADS scale was associated with MCI. Only one question was associated with MCI. Almost half of MCI patients woke up twice or more per night (n = 30, 43.5%) compared to a third of controls (n = 25, 24.7%). After adjusting for sex, age, education, and MMSE question 4 (Do you ever wake up during the night?) was associated with MCI status; persons who reported waking up twice or more during the night had a 2.6 higher odds of being MCI compared to those who reported never waking or waking only once (adjusted OR = 2.6; 95% CI = 1.1–6.1).
Finally, we examined whether there was an association between the total sleep score and performance on neuropsychological tests (Table 4). Logistic regression models adjusted for age, sex, and education were run separately in MCI patients and cognitively healthy controls. In cognitively healthy persons, poor sleep was significantly associated with lower performance on the Stroop test (Stroop Time OR = 1.12; 95% CI = 1.04–1.26) and episodic memory total score (OR = 0.89; 95% CI = 0.79–1.00), and there was a borderline significance for episodic memory immediate (p = 0.093) and delayed recall (p = 0.054). In contrast, in MCI patients, poor sleep scores were associated with better cognitive performance on the MMSE (OR = 1.42; 95% CI = 1.03–1.94) and the Corsi block test (OR = 2.98; 95% CI = 1.14–7.76).
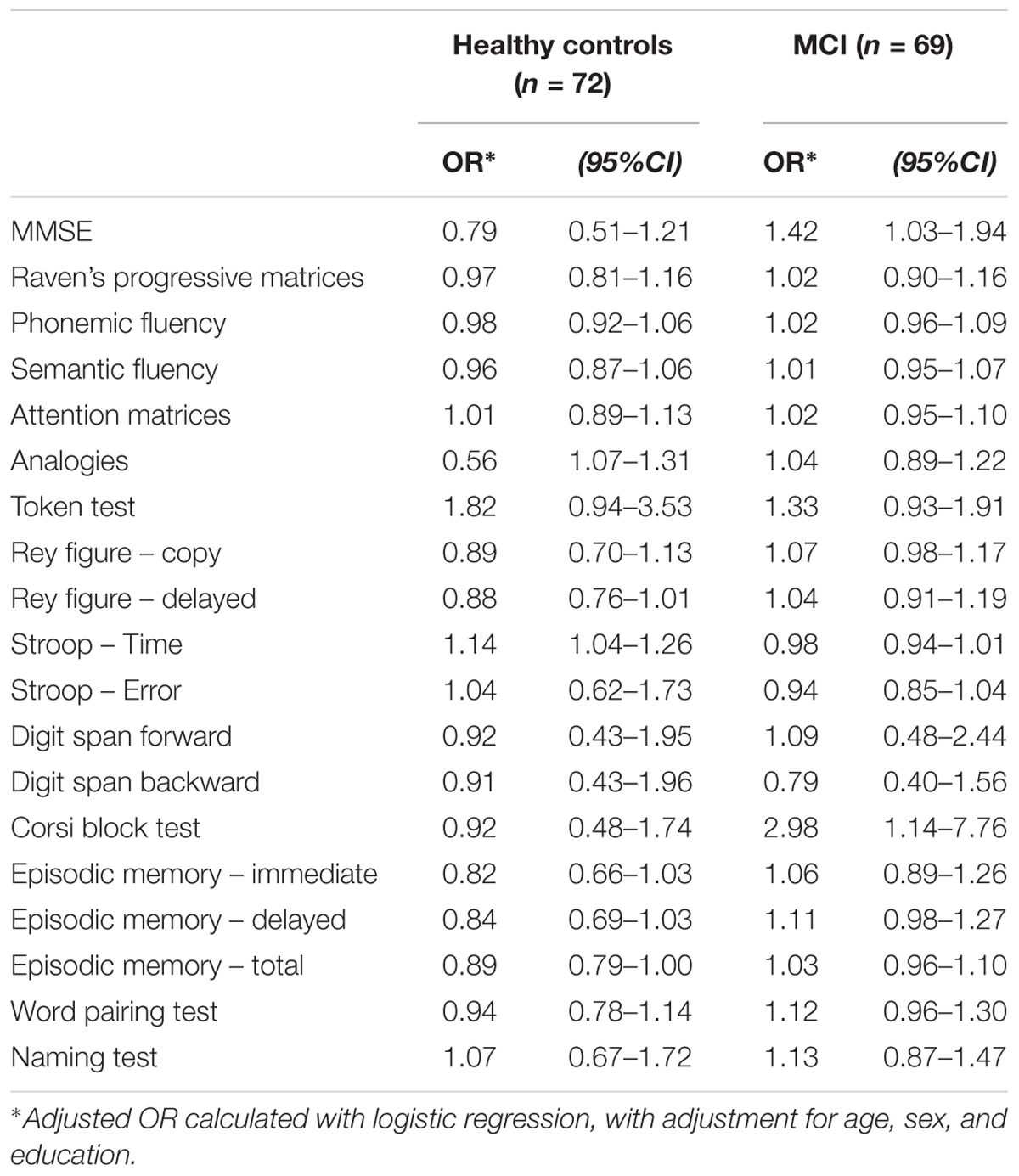
TABLE 4. Association between total sleep score (higher score indicates worse sleep) with neuropsychological tests, stratified by MCI/control status.
Our study demonstrated that persons with MCI are more than three times more likely to have overall poor sleep than cognitive healthy controls, even after adjustment for general cognitive functioning. The specific symptom of waking during the night is more common in MCI patients than controls. We also found that overall self-reported sleep performance is differentially related to cognitive functioning in MCI and controls. In MCI patients, persons with better general cognitive performance (MMSE) and short-term working memory (Corsi block test) have poorer sleep scores. In contrast, sleep disturbances are associated with poorer episodic memory performance and executive functioning in cognitive healthy controls.
Our results are in line with previous studies reporting an association between poor sleep quality and MCI (Dlugaj et al., 2014). Interestingly, the only symptom we found to specifically be related to MCI was waking during the night, which supports previous objective reports that sleep fragmentation occurs in the prodromal phases of AD, predicting subsequent dementia (Lim et al., 2013). Further, results from a sleep laboratory in which patients underwent overnight assessment with dim light melatonin onset assessment showed that MCI patients had greater wake after sleep onset, which was likely due to alterations in the timing of melatonin secretion onset and amount (Naismith et al., 2014). These results back the hypothesis that sleep disorders are related to AD and already begin in the prodromal phase of the disease (Ju et al., 2013, 2014; Lim et al., 2013; Sterniczuk et al., 2013).
Much recent evidence has focused on establishing the biological mechanisms through which sleep problems are related to neurodegeneration and cognitive decline. One explanation may be due to degeneration at the subcortical level. Degeneration of the hypothalamus occurs in AD (Iacono and Sandyk, 1987; Vercruysse et al., 2018), and this area is important for sleep regulation (Ono and Yamanaka, 2017). There is also evidence of a link between sleep disturbance and subcortical infarcts (Lim et al., 2016). Sleep disturbances in AD are also influenced by tau AD pathology (Holth et al., 2017), and there is also some suggestion that hypothalamus dysfunction may be due to reduced acetylcholine (Iacono and Sandyk, 1987).
There is a link between sleep disturbances and beta-amyloid deposition as measured by PET (Spira et al., 2013) and it has been proposed that the relationship may be bi-directional with poor sleep contributing to amyloid deposition but also sleep being disrupted as a result of amyloid plaque formation (Ju et al., 2014). Mice studies have shown that neurotoxic waste products such as beta-amyloid are cleared during sleep (Xie et al., 2013). It has also been suggested that sleep and cognitive decline may be related through stress and hormonal dysregulation (Maggio et al., 2013). Further, circadian rhythms contribute to memory formation possibly through molecular oscillator pathways such as the cAMP signaling cascade, see Gerstner and Yin (2010) for a review.
Our results showed that persons with MCI are more than three times more likely to have sleep problems than cognitively healthy controls even after adjustment for MMSE. This suggests that the sleeping problems are not related to a drop in cognitive functioning per se, but rather the sleep problems may reflect an ongoing neurodegenerative process, the mechanisms of which are discussed above.
Interestingly, in our sample, poor sleep was associated with better cognitive functioning in the MCI patients, in terms of general cognitive functioning and short term working memory. These results are somewhat surprising; although there are previous studies that have reported that persons with sleep problems such as insomnia have slightly better cognitive functioning (Kyle et al., 2017). There are two potential explanations for these results. First, as the SCADS is a self-rated scale, it is possible that MCI patients with a higher MMSE score are in a milder phase of the syndrome and are able to better report their sleep performance with more accuracy. Second, these milder MCI cases may be more aware of their prognosis and experience stress and worry about an impending dementia, which consequently affects their sleep. Sleep quality is strongly associated with both anxiety and depression (Gadie et al., 2017), which are also common symptoms in MCI (Di Iulio et al., 2010; Palmer et al., 2010). It is also worth noting that MCI is a heterogeneous syndrome, which does not always progress to AD and, thus, there may be other underlying etiologies that cause the cognitive impairment but do not cause sleep disturbances.
Our study also found that in cognitively healthy participants poor sleep was related to worse performance in episodic memory and executive functioning, in agreement with previous findings (Wilckens et al., 2014; Bernstein et al., 2018); low sleep efficiency can lead to loss of attention, resulting in more errors on executive tasks like the Stroop test. It has also been hypothesized that stress hormones have a role in sleep disruption and cognition during aging (Maggio et al., 2013). Another hypothesis is that poor sleep is related to impaired cognitive functioning via a third pathway, such as neuropsychiatric symptoms. Studies suggest that night-time behaviors often cluster with other affective symptoms, particularly depression and anxiety, and that up to two-thirds of non-demented persons with affective syndromes characterized by depression, anxiety and nighttime behaviors take psychotropic medications (Cravello et al., 2011), which may also affect cognitive functioning. Unfortunately, in the current study we were not able to investigate the potential confounding effect of prescription drugs or neuropsychiatric symptoms.
There are several strengths of our study. Both cases and controls underwent the same rigorous evaluation for MCI diagnosis and extensive neuropsychological assessment. We also used a sleep scale that has been validated in an Italian population and is designed to address sleep problems related to cognitive decline and dementia. However, several limitations should also be discussed. First, as the study is cross-sectional we cannot determine any causal relationships between cognitive functioning and sleep disturbances. Second, the sleep scale was self-reported and we cannot exclude that a reporting bias may have occurred due to cognitive impairment in the MCI patients. Further, although difficult to measure in a large sample, an objective measure of sleep performance would provide more accurate results. Third, although cases and controls were matched by sex, and we made adjustments for sociodemographics, there may be other unmeasured confounding that may affect the relationships investigated, particularly psychiatric factors such as depression and anxiety or medication use. Another important limitation is that we were not able to establish whether waking during the night was due to obstructive sleep apnea, which is known to cause cognitive deficits and increase the risk of MCI and dementia (Gagnon et al., 2014). Finally, although statistically significant, the reported OR on the association between MCI and poor sleep should be interpreted with caution due to the lower CI being close to 1.
Our results support previous findings in the literature that suggest that sleep problems are common in MCI, which may be relevant for clinicians to consider during clinical evaluation. There is much discussion about whether specific interventions to improve sleep will help to prevent AD (Spira and Gottesman, 2017), but as there is no evidence that interventions will have a successful preventative effect and may be difficult to implement on a large scale, there are currently no guidelines regarding this issue. Future research is needed, with specially designed clinical trials on pharmaceutical and non-pharmaceutical interventions focused on different outcomes, including prevention of AD but also cognitive impairment in general in older adults. Considering the potential side effects of pharmaceutical drugs, lifestyle interventions such as exercise and other non-pharmaceutical interventions may be of particular interest. A recent review concluded that there is insufficient evidence on the role of cognitive behavioral therapy for insomnia for improving cognitive performance (Herbert et al., 2018). One issue in the current literature is the wide range of sleep disorders and symptoms and variation in defining the problems, from insomnia, sleep-disordered breathing, and inefficient sleeping, to delayed sleeping. Objective measures of sleep performance such as actigraphy, bed sensors, and eyelid movement sensors (Van de Water et al., 2011) are needed on a wider scale in MCI patients to better understand the role of sleeping in this syndrome.
Our results show that sleep disturbances are impaired in MCI, possibly due to ongoing neurodegenerative processes, and that in cognitive healthy controls poor sleep is associated with poor episodic memory and executive functioning.
FB, MM, FM, and AV collected data and contributed to critical revision of the manuscript. KP, MM, FM, and AV contributed to hypothesis and study design. KP analyzed the data and wrote the manuscript. KP, MM, and AV interpreted the result.
This study was supported by the European Union Seventh Framework Programme (FP7/2007 – 2013) under grant agreement no. 601055, VPH-DARE@IT to AV. This is a summary of independent research carried out at the NIHR Sheffield Biomedical Research Centre (Translational Neuroscience). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 270–279. doi: 10.1016/j.jalz.2011.03.008
Basso, A., Capitani, E., and Laiacona, M. (1987). Raven’s coloured progressive matrices: normative values on 305 adult normal controls. Funct. Neurol. 2, 189–194.
Bernstein, J. P. K., Calamia, M., and Keller, J. N. (2018). Multiple self-reported sleep measures are differentially associated with cognitive performance in community-dwelling nondemented elderly. Neuropsychology 32, 220–229. doi: 10.1037/neu0000407
Blackwell, T., Yaffe, K., Laffan, A., Ancoli-Israel, S., Redline, S., Ensrud, K. E., et al. (2014). Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: the MrOS sleep study. Sleep 37, 655–663. doi: 10.5665/sleep.3562
Buysse, D. J. (2014). Sleep health: can we define it? Does it matter? Sleep 37, 9–17. doi: 10.5665/sleep.3298
Caffarra, P., Vezzadini, G., Dieci, F., Zonato, F., and Venneri, A. (2002a). Rey-Osterrieth complex figure: normative values in an Italian population sample. Neurol. Sci. 22, 443–447. doi: 10.1007/s100720200003
Caffarra, P., Vezzadini, G., Dieci, F., Zonato, F., and Venneri, A. (2002b). Una versione abbreviata del test di Stroop. Dati normative nella popolazione italiana. Nuova Rivista Neurol. 12, 111–115.
Cravello, L., Palmer, K., de Girolamo, G., Caltagirone, C., and Spalletta, G. (2011). Neuropsychiatric symptoms and syndromes in institutionalized elderly people without dementia. Int. Psychogeriatr. 23, 425–434. doi: 10.1017/s1041610210001304
De Renzi, E., and Faglioni, P. (1978). Normative data and screening power of a shortened version of the Token Test. Cortex 14, 41–49. doi: 10.1016/S0010-9452(78)80006-9
Di Iulio, F., Palmer, K., Blundo, C., Casini, A. R., Gianni, W., Caltagirone, C., et al. (2010). Occurrence of neuropsychiatric symptoms and psychiatric disorders in mild Alzheimer’s disease and mild cognitive impairment subtypes. Int. Psychogeriatr. 22, 629–640. doi: 10.1017/s1041610210000281
Diem, S. J., Blackwell, T. L., Stone, K. L., Yaffe, K., Tranah, G., Cauley, J. A., et al. (2016). Measures of sleep-wake patterns and risk of mild cognitive impairment or dementia in older women. Am. J. Geriatr. Psychiatry 24, 248–258. doi: 10.1016/j.jagp.2015.12.002
Dlugaj, M., Weinreich, G., Weimar, C., Stang, A., Dragano, N., Wessendorf, T. E., et al. (2014). Sleep-disordered breathing, sleep quality, and mild cognitive impairment in the general population. J. Alzheimers Dis. 41, 479–497. doi: 10.3233/jad-132132
Fortier-Brochu,É, Beaulieu-Bonneau, S., Ivers, H., and Morin, C. M. (2012). Insomnia and daytime cognitive performance: a meta-analysis. Sleep Med. Rev. 16, 83–94. doi: 10.1016/j.smrv.2011.03.008
Gadie, A., Shafto, M., Leng, Y., and Kievit, R. A. (2017). How are age-related differences in sleep quality associated with health outcomes? An epidemiological investigation in a UK cohort of 2406 adults. BMJ Open 7:e014920. doi: 10.1136/bmjopen-2016-014920
Gagnon, K., Baril, A. A., Gagnon, J. F., Fortin, M., Decary, A., Lafond, C., et al. (2014). Cognitive impairment in obstructive sleep apnea. Pathol. Biol. 62, 233–240. doi: 10.1016/j.patbio.2014.05.015
Gerstner, J. R., and Yin, J. C. (2010). Circadian rhythms and memory formation. Nat. Rev. Neurosci. 11, 577–588. doi: 10.1038/nrn2881
Herbert, V., Kyle, S. D., and Pratt, D. (2018). Does cognitive behavioural therapy for insomnia improve cognitive performance? A systematic review and narrative synthesis. Sleep Med. Rev. 39, 37–51. doi: 10.1016/j.smrv.2017.07.001
Holth, J., Patel, T., and Holtzman, D. M. (2017). Sleep in Alzheimer’s Disease - beyond amyloid. Neurobiol. Sleep Circ. Rhythms 2, 4–14. doi: 10.1016/j.nbscr.2016.08.002
Iacono, R. P., and Sandyk, R. (1987). Alzheimer’s disease and the pivotal role of the hypothalamus and the intrinsic opioid system. Int. J. Neurosci. 32, 710–714. doi: 10.3109/00207458709043326
Ju, Y. E., Lucey, B. P., and Holtzman, D. M. (2014). Sleep and Alzheimer disease pathology–a bidirectional relationship. Nat. Rev. Neurol. 10, 115–119. doi: 10.1038/nrneurol.2013.269
Ju, Y. E., McLeland, J. S., Toedebusch, C. D., Xiong, C., Fagan, A. M., Duntley, S. P., et al. (2013). Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 70, 587–593. doi: 10.1001/jamaneurol.2013.2334
Kyle, S. D., Sexton, C. E., Feige, B., Luik, A. I., Lane, J., Saxena, R., et al. (2017). Sleep and cognitive performance: cross-sectional associations in the UK Biobank. Sleep Med. 38, 85–91. doi: 10.1016/j.sleep.2017.07.001
Lassila, T., Marco, L. Y. D., Mitolo, M., Iaia, V., Levedianos, G., Venneri, A., et al. (2018). Screening for cognitive impairment by model-assisted cerebral blood flow estimation. IEEE Trans. Biomed. Eng. 65, 1654–1661. doi: 10.1109/TBME.2017.2759511
Lim, A. S., Kowgier, M., Yu, L., Buchman, A. S., and Bennett, D. A. (2013). Sleep fragmentation and the risk of incident Alzheimer’s Disease and cognitive decline in older persons. Sleep 36, 1027–1032. doi: 10.5665/sleep.2802
Lim, A. S. P., Yu, L., Schneider, J. A., Bennett, D. A., and Buchman, A. S. (2016). Sleep fragmentation, cerebral arteriolosclerosis, and brain infarct pathology in community-dwelling older people. Stroke 47, 516–518. doi: 10.1161/strokeaha.115.011608
Maggio, M., Colizzi, E., Fisichella, A., Valenti, G., Ceresini, G., Dall’Aglio, E., et al. (2013). Stress hormones, sleep deprivation and cognition in older adults. Maturitas 76, 22–44. doi: 10.1016/j.maturitas.2013.06.006
Manni, R., Sinforiani, E., Terzaghi, M., Rezzani, C., and Zucchella, C. (2015). Sleep continuity scale in Alzheimer’s disease (SCADS): application in daily clinical practice in an Italian center for dementia. Neurol. Sci. 36, 469–471. doi: 10.1007/s10072-014-1975-2
Manni, R., Sinforiani, E., Zucchella, C., Terzaghi, M., and Rezzani, C. (2013). A sleep continuity scale in Alzheimer’s disease: validation and relationship with cognitive and functional deterioration. Neurol. Sci. 34, 701–705. doi: 10.1007/s10072-012-1118-6
Measso, G., Cavarzeran, F., Zappalà, G., Lebowitz, B. D., Crook, T. H., Pirozzolo, F. J., et al. (1993). The mini mental state examination: normative study of an italian random sample. Dev. Neuropsychol. 9, 77–85. doi: 10.1080/87565649109540545
Mecca, A. P., Michalak, H. R., McDonald, J. W., Kemp, E. C., Pugh, E. A., Becker, M. L., et al. (2018). Sleep disturbance and the risk of cognitive decline or clinical conversion in the ADNI cohort. Dement. Geriatr. Cogn. Disord. 45, 232–242. doi: 10.1159/000488671
Moran, M., Lynch, C. A., Walsh, C., Coen, R., Coakley, D., and Lawlor, B. A. (2005). Sleep disturbance in mild to moderate Alzheimer’s disease. Sleep Med. 6, 347–352. doi: 10.1016/j.sleep.2004.12.005
Naismith, S. L., Hickie, I. B., Terpening, Z., Rajaratnam, S. M., Hodges, J. R., Bolitho, S., et al. (2014). Circadian misalignment and sleep disruption in mild cognitive impairment. J. Alzheimers Dis. 38, 857–866. doi: 10.3233/jad-131217
Novelli, G., Papagno, C., Capitani, E., Laiacona, M., Cappa, S. F., and Vallar, G. (1986a). Tre test clinici di memoria verbale a lungo termine. Taratura susoggetti normali. Arch Psicol. Neurol. Psichiatr. 47, 278–296.
Novelli, G., Papagno, C., Capitani, E., Laiacona, M., Cappa, S. F., and Vallar, G. (1986b). Tre test clinici di produzione lessicale. Taratura su soggetti normali. Arch Psicol. Neurol. Psichiatr. 47, 477–506.
Ono, D., and Yamanaka, A. (2017). Hypothalamic regulation of the sleep/wake cycle. Neurosci. Res. 118, 74–81. doi: 10.1016/j.neures.2017.03.013
Orsini, A., Grossi, D., Capitani, E., Laiacona, M., Papagno, C., and Vallar, G. (1987). Verbal and spatial immediate memory span: normative data from 1355 adults and 1112 children. Ital. J. Neurol. Sci. 8, 539–548. doi: 10.1007/BF02333660
Palmer, K., Di Iulio, F., Varsi, A. E., Gianni, W., Sancesario, G., Caltagirone, C., et al. (2010). Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: the role of depression and apathy. J. Alzheimers Dis. 20, 175–183. doi: 10.3233/JAD-2010-1352
Petersen, R. C., Doody, R., Kurz, A., Mohs, R. C., Morris, J. C., Rabins, P. V., et al. (2001). Current concepts in mild cognitive impairment. Arch. Neurol. 58, 1985–1992. doi: 10.1001/archneur.58.12.1985
Petersen, R. C., Smith, G. E., Waring, S. C., Ivnik, R. J., Tangalos, E. G., and Kokmen, E. (1999). Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 56, 303–308. doi: 10.1001/archneur.56.3.303
Spira, A. P., Gamaldo, A. A., An, Y., Wu, M. N., Simonsick, E. M., Bilgel, M., et al. (2013). Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol. 70, 1537–1543. doi: 10.1001/jamaneurol.2013.4258
Spira, A. P., and Gottesman, R. F. (2017). Sleep disturbance: an emerging opportunity for Alzheimer’s disease prevention? Int. Psychogeriatr. 29, 529–531. doi: 10.1017/s1041610216002131
Sterniczuk, R., Theou, O., Rusak, B., and Rockwood, K. (2013). Sleep disturbance is associated with incident dementia and mortality. Curr. Alzheimer Res. 10, 767–775. doi: 10.2174/15672050113109990134
Van de Water, A. T., Holmes, A., and Hurley, D. A. (2011). Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography–a systematic review. J. Sleep Res. 20(1 Pt 2), 183–200. doi: 10.1111/j.1365-2869.2009.00814.x
Vercruysse, P., Vieau, D., Blum, D., Petersén, A., and Dupuis, L. (2018). Hypothalamic alterations in neurodegenerative diseases and their relation to abnormal energy metabolism. Front. Mol. Neurosci. 11:2. doi: 10.3389/fnmol.2018.00002
Virta, J. J., Heikkila, K., Perola, M., Koskenvuo, M., Raiha, I., Rinne, J. O., et al. (2013). Midlife sleep characteristics associated with late life cognitive function. Sleep 36, 1533–1541, 1541A. doi: 10.5665/sleep.3052
Wechsler, D. (1981). WAIS-R : Wechsler Adult Intelligence Scale-revised. New York, NY: Psychological Corporation.
Wilckens, K. A., Woo, S. G., Kirk, A. R., Erickson, K. I., and Wheeler, M. E. (2014). Role of sleep continuity and total sleep time in executive function across the adult lifespan. Psychol Aging 29, 658–665. doi: 10.1037/a0037234
Keywords: sleep, cognition, MCI, cognitive impairment, aging, waking, neuropsychological testing, memory
Citation: Palmer K, Mitolo M, Burgio F, Meneghello F and Venneri A (2018) Sleep Disturbance in Mild Cognitive Impairment and Association With Cognitive Functioning. A Case-Control Study. Front. Aging Neurosci. 10:360. doi: 10.3389/fnagi.2018.00360
Received: 28 August 2018; Accepted: 22 October 2018;
Published: 09 November 2018.
Edited by:
Nirinjini Naidoo, University of Pennsylvania, United StatesReviewed by:
Cynthia Cheng, Thomas Jefferson University, United StatesCopyright © 2018 Palmer, Mitolo, Burgio, Meneghello and Venneri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katie Palmer, a2F0aWUucGFsbWVyQG9zcGVkYWxlc2FuY2FtaWxsby5uZXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.