
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 29 May 2018
Sec. Parkinson’s Disease and Aging-related Movement Disorders
Volume 10 - 2018 | https://doi.org/10.3389/fnagi.2018.00157
There is a high misdiagnosis rate between Parkinson’s disease (PD) and atypical parkinsonian disorders (APD), such as progressive supranuclear palsy (PSP), the second most common parkinsonian syndrome. In our earlier studies, we identified and replicated RNA blood biomarkers in several independent cohorts, however, replication in a cohort that includes PSP patients has not yet been performed. To this end, we evaluated the diagnostic potential of nine previously identified RNA biomarkers using quantitative PCR assays in 138 blood samples at baseline from PD, PSP and healthy controls (HCs) nested in the PD Biomarkers Program. Linear discriminant analysis showed that COPZ1 and PTPN1 distinguished PD from PSP patients with 62.5% accuracy. Five biomarkers, PTPN1, COPZ1, FAXDC2, SLC14A1s and NAMPT were useful for distinguishing PSP from controls with 69% accuracy. Several biomarkers correlated with clinical features in PD patients. SLC14A1-s correlated with Unified Parkinson’s Disease Rating Scale total and part III scores. In addition, COPZ1, PTPN1 and MLST8, correlated with Montreal Cognitive Assessment (MoCA). Interestingly, COPZ1, EFTUD2 and PTPN1 were downregulated in cognitively impaired (CI) compared to normal subjects. Linear discriminant analysis showed that age, PTPN1, COPZ1, FAXDC2, EFTUD2 and MLST8 distinguished CI from normal subjects with 65.9% accuracy. These results suggest that COPZ1 and PTPN1 are useful for distinguishing PD from PSP patients. In addition, the combination of PTPN1, COPZ1, FAXDC2, EFTUD2 and MLST8 is a useful signature for cognitive impairment. Evaluation of these biomarkers in a larger study will be a key to advancing these biomarkers into the clinic.
Parkinson’s disease (PD) is a neurodegenerative disease characterized by the selective loss of dopamine neurons in the substantia nigra pars compacta. Deterioration of the dopaminergic system leads to severe motor symptoms including resting tremor, rigidity, bradykinesia and postural instability. Current treatments for PD afford symptomatic relief, but a disease modifying or neuroprotective agent capable of halting the progression of the disease is not yet available. The lack of a robust biomarker with high sensitivity and specificity has limited the progress towards the development of effective therapeutics for PD. In this context, biomarkers would offer great advantages in clinical trials testing drugs and neuroprotective agents. For example, biomarkers could facilitate the selection and stratification of study participants, monitor disease progression and inform about target selection (Santiago and Potashkin, 2014a; Gwinn et al., 2017).
Distinguishing PD from atypical parkinsonian disorders (APD) is an unmet goal for currently proposed biomarker studies. The overlap in symptoms and pathological features between PD and APD makes these diseases very difficult to distinguish early in the disease process where therapeutic intervention may be more beneficial (Rajput and Rajput, 2014; Santiago and Potashkin, 2014c). Progressive supranuclear palsy (PSP), for example, is frequently misdiagnosed as PD. Deposition of fibrillar aggregates of four-repeat Tau protein in the brainstem and cerebral cortex is a pathological hallmark of PSP (Dickson et al., 2010). Clinical symptoms in PSP patients include prominent hypokinesia, oculo-motor and balance disturbances (Dickson et al., 2010). In contrast, PD is characterized by the accumulation of alpha synuclein (SNCA) in the substantia nigra pars compacta. PD patients exhibit classical motor symptoms that include rigidity, tremor and bradykinesia (Ascherio and Schwarzschild, 2016). To date, diagnosis of PD and PSP patients is based on the assessment of motor symptoms and response to dopaminergic therapy (Ascherio and Schwarzschild, 2016). The problem with this approach is that PSP and PD patients manifest similarities at early stages of the disease and both respond to dopaminergic treatment, which makes the diagnosis very challenging (Dickson et al., 2010). The high misdiagnosis rate between PD and PSP reaching approximately 30% heightens the urgency for the identification of highly specific biomarkers capable of distinguishing these diseases (Rajput and Rajput, 2014; Santiago and Potashkin, 2014c).
Although substantial progress has been made in the discovery of blood biomarkers for PD (Khoo et al., 2012; Ciaramella et al., 2013; Qiang et al., 2013; Santiago and Potashkin, 2013a, 2014a, 2015b, 2017; Santiago et al., 2014, 2016; Alieva et al., 2015; Calligaris et al., 2015; Locascio et al., 2015; Swanson et al., 2015), very few studies have addressed the misdiagnosis problem between PD and PSP. Our first studies identified a splice-variant specific signature capable of distinguishing PD from healthy and APD (Potashkin et al., 2012). This biomarker signature was composed of 13 splice variants including fatty acid hydroxylase domain containing 2 (FAXDC2; C5ORF4), coatomer protein complex subunit zeta 1 (COPZ1), microtubule-actin crosslinking factor 1 (MACF1), wntless WNT ligand secretion mediator (WLS), proteoglycan 3, pro eosinophil major basic protein 2 (PRG3), zinc finger protein 160 (ZNF160), elongation factor Tu GTP binding domain containing 2 (EFTUD2), mitogen-activated protein 4 kinase 1 (MAP4K1), membrane palmitoylated protein 1 (MPP1), pyruvate kinase M2 (PKM2), solute carrier family 14 member 1 (SLC14A1-s), SLC14A1-l and zinc finger protein 134 (ZNF134; Potashkin et al., 2012). Seven out these 13 biomarkers, including FAXDC2 (C5ORF4), COPZ1, MACF1, WLS, PRG3, ZNF160 and EFTUD2, replicated in a second independent cohort of participants that included PD and healthy controls (HCs), but not APD patients (Santiago et al., 2013). Another promising study used a network approach integrating our microarray data (Potashkin et al., 2012) in order to identify protein tyrosine phosphatase, non-receptor type 1 (PTPN1) as a potential biomarker for PSP (Santiago and Potashkin, 2014c). PTPN1 was capable of distinguishing PD from PSP patients with 86% overall diagnostic accuracy. Nonetheless, these markers have not been replicated in an independent set of participants that includes PSP patients. In this study, we tested a subset of nine RNA biomarkers in blood samples obtained from the PD Biomarkers Program (PDBP). We hypothesized that these RNA markers from our previous studies could be helpful for the differential diagnosis between PD and PSP and may be informative of clinical features including disease progression and cognition.
Samples used in this study were obtained from PDBP, a consortium of clinical sites funded by the National Institute of Neurological Diseases and Stroke (NINDS, National Institutes of Health (NIH), United States). The consortium projects focus on the development of clinical and laboratory-based biomarkers for PD diagnosis, progression and prognosis. RNA samples used in this study were obtained from Penn State Milton S. Hershey Medical Center and University of Florida College of Medicine. The Institutional Review Boards (IRB) of each PDBP center and the Rosalind Franklin University of Medicine and Sciences approved the study protocol. Written informed consent was obtained from all participants before inclusion in the study. PD patients were recruited and evaluated by movement disorder specialists using establish criteria (Rosenthal et al., 2016). Disease severity was assessed using the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III and Hoehn & Yahr scale. Inclusion and exclusion criteria were the following: PD patients had a history of adequate response to dopaminergic therapy and history of asymmetrical symptom onset. HC had no history of neurological disorder; PSP patients were over 40 years old with vertical gaze palsy and/or slow vertical gaze/postural instability during first year of diagnosis. Additional inclusion and exclusion criteria have been published elsewhere (Rosenthal et al., 2016). The demographic and clinical characteristics of the study participants selected for this study are listed in Table 1.
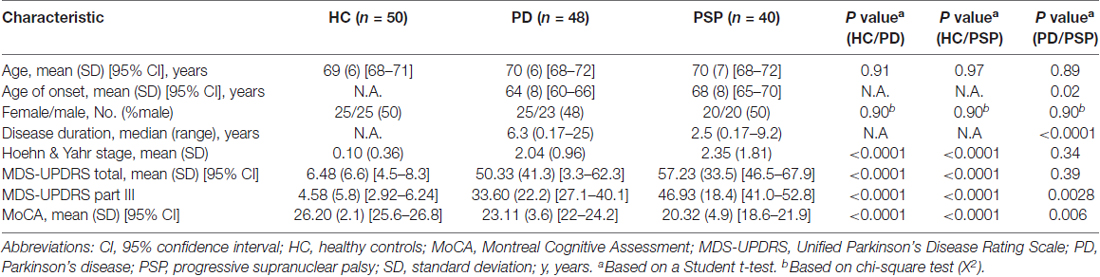
Table 1. Demographic and clinical characteristics of Parkinson’s disease Biomarkers Program (PDBP) participants.
We obtained a total of 138 RNA samples at baseline from age and sex-matched early stage PD, PSP patients and HC from PDBP. Standardized clinical and biospecimen collection procedures were used for all participants. Each biospecimen collection follows the PDBP protocol and mirrors the Alzheimer’s Disease Neuroimaging Initiative (ADNI), BioFIND and PPMI protocols. Collected biosamples are sent to the NINDS Repository (Coriell Laboratories), undergo quality control, and are cataloged. Blinded RNA samples were shipped in dry ice to Rosalind Franklin University of Medicine and Sciences for the studies described herein.
Samples with RNA integrity values >6.0 and absorbance 260/280 between 1.8 and 2.4 were used in this study. 0.4 microgram of RNA was reverse transcribed into cDNA using a mix of random hexamer primers (High Capacity cDNA Synthesis Kit, Life Technologies, Carlsbad, CA, USA). Quantitative polymerase chain reaction assays (qRT-PCR) were performed using ViiA7 real-time PCR system (Thermo Fisher Scientific, Waltham, MA, USA). Each 25 microliters reaction contained Bullseye EvaGreen qPCR 2X Mastermix (MIDSCI, St. Louis, USA) and primers at a concentration of 0.05 mM. Primer sequences are indicated in Table 2. Amplification steps used were as follows: denature at 95°C for 10 min, annealing at 95°C for 15 s extension at 60°C for 45 cycles of amplification and 95°C extension for 15 s Melting curve was performed using the following conditions: 60°C for 1 min following by 5°C/s and 95°C for 15 s qRT-PCR assays were performed in triplicates. The geometric mean of the two reference genes, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and actin beta (ACTB), were used to normalize for input RNA. Expression data was analyzed using the comparative ΔΔCt method. In this method, the amount of target is normalized to an endogenous reference and relative to a calibrator.
Statistical analyses were performed using STATISTICA 12 (StatSoft, OK, USA) and GraphPad Prism version 5 (GraphPad Software Inc., CA, USA). A Student-t-test (unpaired, two tailed) was used to assess the differences between two groups and a chi-square test was used to analyze categorical data. Correlation analysis was performed using the Pearson method. A Bonferroni corrected p-value of 0.005 or less was regarded as significant. A forward stepwise linear discriminant analysis was performed to determine the variables that best discriminated between groups as described previously (Potashkin et al., 2012). Biomarker performance was assessed using a receiver operating characteristic curve (ROC) analysis. All the data used in preparation of this manuscript is publicly available at: https://pdbp.ninds.nih.gov/.
There were no significant differences in mean age and sex distribution between PD, PSP and HC (Table 1). PD patients had a significantly lower age of onset (64 vs. 68 years old) and a higher disease duration (6.3 vs. 2.5 years) compared to PSP patients. There were no significant differences in Hoehn & Yahr (2.04 vs. 2.35) and MDS-UPDRS total scores (50.33 vs. 57.23) between PD and PSP patients. Analyses of the clinical metrics to assess PD staging and cognition, MDS-UPDRS part III and Montreal Cognitive Assessment (MoCA), showed some differences between PD and PSP patients. For example, PSP patients exhibited significantly higher MDS-UPDRS part III (46.93 vs. 33.60) and lower MoCA scores (20.32 vs. 23.11) compared to PD patients (Table 1).
A subset of nine RNA biomarkers were tested in 50 HC, 48 PD and 40 PSP blood samples at baseline from individuals nested in the PDBP cohort by qRT-PCR assays (Table 2). Seven out of the nine biomarkers including COPZ1, FAXDC2, EFTUD2, SLC14A1s, PTPN1, nicotinamide phosphoribosyl transferase (NAMPT) and polypyrimidine tract binding protein 1 (PTBP1) were tested in our previous studies (Potashkin et al., 2012; Santiago et al., 2013, 2016; Santiago and Potashkin, 2014c, 2015a,b). In this study, we also tested two additional biomarkers, COP9 signalosome subunit 7A (COPS7A) and MTOR associated protein, LST8 homolog (MLST8). These markers were selected from a ranked list of potential candidates identified in a network analysis for PSP (Santiago and Potashkin, 2014c). Several biomarkers showed significant correlations with clinical features. For example, SLC14A1-s mRNA correlated with MDS-UPDRS total (r = −0.18, p = 0.03) and MDS UPDRS part III scores (r = −0.18, p = 0.03). Expression levels of three biomarkers, COPZ1 (r = 0.21, p = 0.01), PTPN1 (r = 0.18, p = 0.03) and MLST8 (r = 0.18, p = 0.01) correlated with MoCA scores.
We next investigated the capacity of each biomarker for distinguishing between the following groups: PD vs. PSP, PD vs. HC, and PSP vs. HC. Analysis of each biomarker alone did not reach significance after adjusting for multiple comparisons (p < 0.005; Supplementary Table S1). Of note, COPZ1 (p = 0.008) and PTPN1 (p = 0.008) trended toward significance when comparing PD vs. HC but failed to reach significance after adjusting for multiple comparisons. We performed a linear discriminant analysis to assess whether combination of biomarkers could be useful for discriminating between PD vs. HC and PSP vs. HC. Linear discriminant analysis showed that PTPN1 and COPZ1 were the only markers retained in the model capable of discriminating PD from HC with 58% overall diagnostic accuracy (Table 3). Evaluation of biomarker performance by receiver operating characteristic curve analysis (ROC) resulted in an area under the curve (AUC) value of 0.56. The same analysis was performed to identify the best group of biomarkers for discriminating PSP from HC. In this analysis, five biomarkers, PTPN1, COPZ1, FAXDC2, SLC14A1s and NAMPT were identified as the best group of markers for distinguishing PSP from HC with an overall diagnostic accuracy of 69% (Table 3). ROC analysis resulted in an AUC value of 0.66.
Similarly, we analyzed which biomarkers in combination could be useful for distinguishing PD from PSP. Linear discriminant analysis showed that two biomarkers, COPZ1 and PTPN1, were retained in the model as useful for discriminating PD from PSP patients. Specifically, PTPN1 and COPZ1 together achieved an overall diagnostic accuracy of 62.5% (Table 3). The remaining variables including age, sex, FAXDC2, NAMPT, SLC14A1s, PTBP1, EFTUD2, COPS7A and MLST8 did not contribute to the discrimination between the groups and were therefore eliminated from the model. ROC analysis resulted in an AUC value of 0.60.
Since some of the biomarkers correlated with MoCA, a standard clinical measure for cognitive performance, we investigated the potential of each biomarker for identifying cognitively impaired (CI) subjects. Cognitive performance was defined using the MoCA cutoff lower than 26 for cognitive impairment as previously described (Santiago and Potashkin, 2015a; Weintraub et al., 2015). There were 51 cognitively normal (CN) and 87 CI participants in this subset of PDBP. Three biomarkers, COPZ1, PTPN1 and EFTUD2 were significantly downregulated in CI compared to CN individuals (Figure 1). Evaluation of biomarker performance by ROC analysis resulted in AUC values of 0.57 for COPZ1, 0.57 for EFTUD2 and 0.64 for PTPN1. Combination of these three markers resulted in AUC value of 0.65. We next performed a linear discriminant analysis to determine which variables best distinguished between CI and CN. This analysis showed that six variables including age, PTPN1, COPZ1, FAXDC2, EFTUD2 and MLST8 were retained in the model as the best discriminant factors (Table 3). Using these six variables CI subjects were distinguished from CN with an overall 65.9% accuracy. Sex, COPS7A, NAMPT, SLC14A1s, and PTBP1 did not contribute to the discrimination between the groups and were therefore eliminated from the model. ROC analysis resulted in AUC value of 0.63.
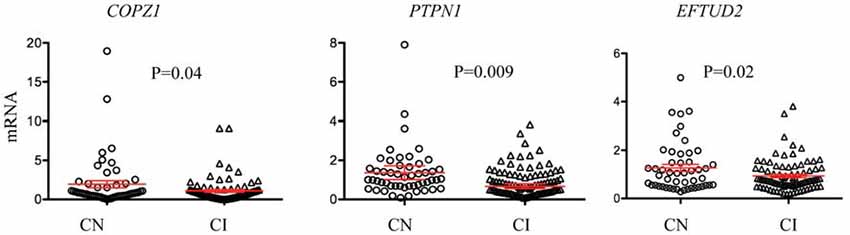
Figure 1. RNA biomarkers associated with cognitive decline. Relative abundance of COPZ1, PTPN1 and EFTUD2 was compared in subjects with normal cognition (CN = 51, circles) to cognitively impaired (CI = 87, triangles). Error bars represent the 95% confidence interval (CI). A p-value of less than 0.05 was regarded as significant based on a Student t-test (two-tailed).
Replication and validation of biomarker studies in PD is essential for the translation of biomarkers into useful diagnostic tools for researchers and clinicians. Several biomarkers have shown promise for identifying early stage PD patients, however, very few studies have been replicated in independent clinical cohorts or addressed the misdiagnosis problem in PD. Specifically, only a handful of studies have evaluated the potential of biomarkers in distinguishing PD from APD (Potashkin et al., 2012; Santiago and Potashkin, 2014c; Hansson et al., 2017). In this study, we evaluated the diagnostic and prognostic potential of previously identified RNA blood biomarkers for PD in samples nested in PDBP. Replication in a well-characterized cohort like PDBP that included PSP patients was important to assess the performance of our biomarkers in distinguishing PD from APD, which remains an unmet need in the field.
The primary objective of this study was to replicate previously identified RNA biomarkers in an independent set of samples that includes PSP patients. We tested a subset of biomarkers including FAXDC2 (C5ORF4), COPZ1, EFTUD2, SLC14A1-s, HNF4A, PTBP1 and PTPN1. The biomarkers evaluated in this study have been implicated in pathways involved in the pathogenesis of PD. For example, FAXDC2 is a member of the fatty acid hydrolase superfamily known to be involved in cholesterol metabolism, which has been shown to contribute to neurodegeneration in PD (Paul et al., 2017). COPZ1 encodes a subunit of the cytoplasmic coatomer complex involved in autophagy and protein trafficking (Santiago and Potashkin, 2015a; Bensellam et al., 2016). EFTUD2 encodes the splicing factor U5–116 kD and it may play a role in RNA splicing during neural development (Lei et al., 2017). In this context, aberrant alternative splicing in blood of PD patients have been reported in numerous studies (Potashkin et al., 2012; Santiago et al., 2013; Alieva et al., 2014). In addition, COPZ1 and EFTUD2 have been associated with cognitive decline in PD patients (Santiago and Potashkin, 2015a). Other biomarkers including hepatocyte nuclear factor 4 alpha (HNF4A), PTBP1 and PTPN1 have been implicated in glucose metabolism and insulin regulation, biological processes that have been extensively associated with the pathogenesis of PD (Knoch et al., 2006; Santiago and Potashkin, 2013b, 2015a). We also tested two additional biomarkers, MLST8 and COPS7A that were identified in our previous study on PSP (Santiago and Potashkin, 2014c). MLST8 is a subunit of the mammalian target rapamycin complexes 1 and 2 (mTORC1, mTORC2) known to regulate mTOR kinase activity (Kim et al., 2003) which has been involved in a neuroprotective mechanism in PD (Malagelada et al., 2010). COPS7A encodes a component of the COP9 signalosome, a protein complex involved in the ubiquitin conjugation pathway, protein misfolding and autophagy (Liu et al., 2016), pathways implicated in the development of PD (Cook et al., 2012).
First, we analyzed the capacity of each biomarker independently to distinguish between groups (PD vs. HC, PD vs. PSP and PD vs. PSP). None of the biomarkers alone reached statistical significance after adjusting for multiple comparisons. Notably, only two biomarkers, COPZ1 and PTPN1 trended toward significance (p = 0.008) but failed to reach significance after adjusting for multiple comparisons. Despite these results, it is important to note that these markers have been replicated in samples from several independent cohorts. For instance, COPZ1 has been replicated in two cohorts of medicated PD patients (Potashkin et al., 2012; Santiago et al., 2013) and in a cohort of drug-naïve PD patients (Santiago and Potashkin, 2015a).
We next investigated whether combination of biomarkers could be useful for distinguishing between groups. To this end, we performed a linear discriminant analysis as previously described (Potashkin et al., 2012; Santiago et al., 2013). Based on the discriminant analysis, two biomarkers, COPZ1 and PTPN1 were capable of distinguishing PD from PSP patients while the remaining biomarkers were excluded from the model. The overall accuracy obtained with these two biomarkers was 62.5%. This accuracy is less than that obtained in our previous studies. For example, our original splice variant signature distinguished PD from APD patients with 95% diagnostic accuracy in samples obtained from the Diagnostic and Prognostic Biomarkers for Parkinson’s Disease (PROBE) study (Potashkin et al., 2012). However, the APD group in PROBE was comprised of multiple system atrophy and PSP patients, whereas the present study only includes PSP patients. Further, PTPN1 alone achieved an overall diagnostic accuracy of 86% in discriminating PD from PSP patients in samples obtained from PROBE (Santiago and Potashkin, 2014c). The discrepancy in the results may be explained by several differences in the clinical studies including, patient population and protocols for sample collection and storage.
Analysis of biomarker expression in PD patients compared to HC showed that COPZ1 and PTPN1 were the only two biomarkers selected with the highest discriminant power for distinguishing between groups with a 58% overall diagnostic accuracy. Similarly, we also determined which biomarkers combined could distinguish PSP from HC. Five biomarkers including PTPN1, COPZ1, FAXDC2, SLC14A1s and NAMPT distinguished PSP from HC with a 69% diagnostic accuracy. Although this result is encouraging, the real challenge is not to distinguish PSP from HC but rather finding specific and sensitive biomarkers that facilitate the differential diagnosis between PD and PSP. Collectively, these results suggest that these markers alone or in combination do not possess the ideal diagnostic capacity required in a robust biomarker for distinguishing PD from PSP. Combination of multiple biomarkers including, protein, RNA, imaging and other clinical tests will be required to build a diagnostic model for PD. In this regard, blood neurofilament light chain (NfL) protein distinguished PD from APD patients including PSP, MSA and corticobasal syndrome with high accuracy, 91% specificity and 82% sensitivity (Hansson et al., 2017). In addition, imaging techniques have achieved high sensitivity and specificity in distinguishing PD from APD. For example, free-water derived from diffusion magnetic resonance imaging (MRI) has been useful for detecting differences in the substantia nigra of PD patients compared to PSP, MSA and HC (Planetta et al., 2016; Ofori et al., 2017). Thus, combining COPZ1 and PTPN1 mRNAs with protein markers like NfL and/or imaging markers could improve the diagnostic accuracy of PD and PSP patients. In addition to these markers, there are other interesting molecular signatures that have shown promise in distinguishing PD from HC and other neurodegenerative diseases that could be tested in samples from PSP patients. For example, a molecular signature in blood composed of five genes, p19 S-phase kinase-associated protein 1A (SKP1A), huntingtin interacting protein-2 (HIP2), aldehyde dehydrogenase family 1 subfamily A1 (ALDH1A1), 19 S proteasomal protein (PSMC4) and heat shock 70-kDa protein 8 (HSPA8), distinguished early-stage and de novo PD from HC and Alzheimer’s disease patients with 90.3% sensitivity and 89.1% specificity (Molochnikov et al., 2012). Transcriptomic profiling of blood employing RNA sequencing is a robust method for finding additional candidate biomarkers. In this context, RNA sequencing analysis revealed gene expression changes in blood of PD patients before and after deep brain stimulation treatment (Soreq et al., 2013, 2015a,b). These high-throughput technologies combined with bioinformatic analyses including weighted gene coexpression networks have been useful for identifying changes in gene expression of both protein-coding and small and long regulatory RNAs (lncRNAs) in neurodegenerative diseases (Guffanti et al., 2014; Santiago and Potashkin, 2014b). For instance, RNA sequencing analysis revealed over 3000 lncRNAs coexpressed in both brain and blood of PD patients (Soreq et al., 2014). Therefore, a comprehensive analysis of the blood transcriptome using RNA sequencing and network analyses will be key for identifying biologically relevant biomarkers for PSP. Further, weighted gene co-expression networks analysis in both brain and blood datasets will be useful for elucidating potential mechanisms of disease pathogenesis in PSP (Guffanti et al., 2014; Soreq et al., 2014).
Cognitive impairment is a disabling non-motor symptom frequently observed in PD patients. The decline in cognitive performance has been documented in de novo and untreated PD patients suggesting cognitive impairment may be one of the earliest manifestations in the development of PD (Santiago and Potashkin, 2015a; Weintraub et al., 2015). Identifying predictors and biomarkers of cognitive impairment is expected to be valuable in patient classification, stratification and personalized treatment (Mollenhauer et al., 2014). In this study, three biomarkers, COPZ1, PTPN1 and MLST8 correlated with MoCA, although the correlation values were low. Further analysis showed that three biomarkers COPZ1, EFTUD2 and PTPN1 were significantly downregulated in CI subjects compared to normal cognition suggesting these biomarkers may be useful to assess cognitive performance in patients. Evaluation of biomarker performance by ROC analysis resulted in AUC values of 0.57 for COPZ1, 0.57 for EFTUD2 and 0.64 for PTPN1 indicating a modest diagnostic accuracy. Combination of these three markers together resulted in AUC value of 0.65. The AUC value for PTPN1 alone resulted in 0.64, therefore combination of the three markers did not improve substantially the diagnostic accuracy. We next sought to build a diagnostic model for cognitive impairment. Discriminant analysis showed that the best predictors of cognitive decline were EFTUD2, COPZ1, PTPN1, FAXDC2, MLST8 and age. This signature distinguished cognitive impairment from CN subjects with 66% accuracy. In this regard, two of these biomarkers, EFTUD2 and PTBP1 correlated with MoCA scores in drug naïve PD patients obtained from the Parkinson’s Progression Markers Initiative (PPMI; Santiago and Potashkin, 2015a). Further analysis showed that relative expression of both EFTUD2 and PTBP1 was significantly downregulated in PD patients with cognitive impairment compared to PD patients with normal cognition (Santiago and Potashkin, 2015a). Thus, these results confirm the association of EFTUD2 with cognitive impairment in a second independent cohort. Evaluation of the other markers included in the signature in a larger and well-characterized cohort of participants is warranted. In addition, combination of these RNA markers with protein biomarkers previously identified as predictors of cognitive performance like epidermal growth factor (EGF) and insulin like growth factor 1 (IGF-1) may improve the diagnostic accuracy and patient stratification (Chen-Plotkin et al., 2011; Pellecchia et al., 2014). In addition, given the presence of cognitive impairment in dementia with Lewy bodies, vascular dementia, vascular parkinsonism and PD dementia (Wen et al., 2017; Jellinger and Korczyn, 2018), it will be important to assess the utility of these markers in distinguishing between the different dementia subtypes.
There are several important aspects in this study design that need to be considered when interpreting the results from this study and its discrepancies with our previous studies. For example, PDBP differs from PPMI in that PD patients enrolled in the latter were de novo patients not taking PD medications at the time of enrollment. Dopaminergic treatment can certainly affect gene expression and introduce bias since control groups are exposed to different medications. To ensure consistency and allow the successful replication and validation of biomarker studies, standardized protocols for RNA extraction, sample processing and storage must be followed among all clinical sites.
Given the complex heterogeneity in PD clinical subtypes it has become evident that a single biomarker may not be useful as a diagnostic tool for PD and APD. Multimodal biomarker approaches including molecular markers, imaging and clinical tests may be the route to improve the diagnosis and the clinical management of PD patients. Further, the different PD clinical subtypes and comorbidities associated with PD should be considered in the design of future biomarker studies (Santiago et al., 2017). Replication and validation of biomarkers studies in independent cohorts as well as the dissemination of positive and negative results should be encouraged among researchers in order to advance the development of diagnostic strategies for PD.
JS and JP: conceived and designed the experiments. JS and VB: performed experiments and wrote the article. JS, VB and JP: analyzed the data.
This study was funded by the National Institute of Neurological Disorders and Stroke (NINDS) Grant No. U01NS097037 to JP. Data and biospecimens used in preparation of this manuscript were obtained from the PD Biomarkers Program (PDBP) Consortium, part of the National Institute of Neurological Disorders and Stroke at the National Institutes of Health. Investigators include: Roger Albin, Roy Alcalay, Alberto Ascherio, DuBois Bowman, Alice Chen-Plotkin, Ted Dawson, Richard Dewey, Dwight German, Xuemei Huang, Judith Potashkin, Rachel Saunders-Pullman, Liana Rosenthal, Clemens Scherzer, David Vaillancourt, Vladislav Petyuk, David Walt, Andy West and Jing Zhang. The PDBP Investigators have not participated in reviewing the data analysis or content of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to the participants and clinicians who participated in the PDBP study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2018.00157/full#supplementary-material
Alieva, A., Filatova, E. V., Karabanov, A. V., Illarioshkin, S. N., Slominsky, P. A., and Shadrina, M. I. (2015). Potential biomarkers of the earliest clinical stages of Parkinson’s disease. Parkinsons Dis. 2015:294396. doi: 10.1155/2015/294396
Alieva, A., Shadrina, M. I., Filatova, E. V., Karabanov, A. V., Illarioshkin, S. N., Limborska, S. A., et al. (2014). Involvement of endocytosis and alternative splicing in the formation of the pathological process in the early stages of Parkinson’s disease. Biomed Res. Int. 2014:718732. doi: 10.1155/2014/718732
Ascherio, A., and Schwarzschild, M. A. (2016). The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 15, 1257–1272. doi: 10.1016/S1474-4422(16)30230-7
Bensellam, M., Maxwell, E. L., Chan, J. Y., Luzuriaga, J., West, P. K., Jonas, J. C., et al. (2016). Hypoxia reduces ER-to-Golgi protein trafficking and increases cell death by inhibiting the adaptive unfolded protein response in mouse β cells. Diabetologia 59, 1492–1502. doi: 10.1007/s00125-016-3947-y
Calligaris, R., Banica, M., Roncaglia, P., Robotti, E., Finaurini, S., Vlachouli, C., et al. (2015). Blood transcriptomics of drug-naive sporadic Parkinson’s disease patients. BMC Genomics 16:876. doi: 10.1186/s12864-015-2058-3
Chen-Plotkin, A. S., Hu, W. T., Siderowf, A., Weintraub, D., Goldmann Gross, R., Hurtig, H. I., et al. (2011). Plasma epidermal growth factor levels predict cognitive decline in Parkinson disease. Ann. Neurol. 69, 655–663. doi: 10.1002/ana.22271
Ciaramella, A., Salani, F., Bizzoni, F., Pontieri, F. E., Stefani, A., Pierantozzi, M., et al. (2013). Blood dendritic cell frequency declines in idiopathic Parkinson’s disease and is associated with motor symptom severity. PLoS One 8:e65352. doi: 10.1371/journal.pone.0065352
Cook, C., Stetler, C., and Petrucelli, L. (2012). Disruption of protein quality control in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2:a009423. doi: 10.1101/cshperspect.a009423
Dickson, D. W., Ahmed, Z., Algom, A. A., Tsuboi, Y., and Josephs, K. A. (2010). Neuropathology of variants of progressive supranuclear palsy. Curr. Opin. Neurol. 23, 394–400. doi: 10.1097/WCO.0b013e32833be924
Guffanti, A., Simchovitz, A., and Soreq, H. (2014). Emerging bioinformatics approaches for analysis of NGS-derived coding and non-coding RNAs in neurodegenerative diseases. Front. Cell. Neurosci. 8:89. doi: 10.3389/fncel.2014.00089
Gwinn, K., David, K. K., Swanson-Fischer, C., Albin, R., Hillaire-Clarke, C. S., Sieber, B. A., et al. (2017). Parkinson’s disease biomarkers: perspective from the NINDS Parkinson’s Disease Biomarkers Program. Biomark. Med. 11, 451–473. doi: 10.2217/bmm-2016-0370
Hansson, O., Janelidze, S., Hall, S., Magdalinou, N., Lees, A. J., Andreasson, U., et al. (2017). Blood-based NfL: a biomarker for differential diagnosis of parkinsonian disorder. Neurology 88, 930–937. doi: 10.1212/WNL.0000000000003680
Jellinger, K. A., and Korczyn, A. D. (2018). Are dementia with Lewy bodies and Parkinson’s disease dementia the same disease? BMC Med. 16:34. doi: 10.1186/s12916-018-1016-8
Khoo, S. K., Petillo, D., Kang, U. J., Resau, J. H., Berryhill, B., Linder, J., et al. (2012). Plasma-based circulating MicroRNA biomarkers for Parkinson’s disease. J. Parkinsons Dis. 2, 321–331. doi: 10.3233/JPD-012144
Kim, D. H., Sarbassov, D. D., Ali, S. M., Latek, R. R., Guntur, K. V., Erdjument-Bromage, H., et al. (2003). GβL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol. Cell 11, 895–904. doi: 10.1016/s1097-2765(03)00114-x
Knoch, K. P., Meisterfeld, R., Kersting, S., Bergert, H., Altkrüger, A., Wegbrod, C., et al. (2006). cAMP-dependent phosphorylation of PTB1 promotes the expression of insulin secretory granule proteins in β cells. Cell Metab. 3, 123–134. doi: 10.1016/j.cmet.2005.12.008
Lei, L., Yan, S. Y., Yang, R., Chen, J. Y., Li, Y., Bu, Y., et al. (2017). Spliceosomal protein eftud2 mutation leads to p53-dependent apoptosis in zebrafish neural progenitors. Nucleic Acids Res. 45, 3422–3436. doi: 10.1093/nar/gkw1043
Liu, J., Su, H., and Wang, X. (2016). The COP9 signalosome coerces autophagy and the ubiquitin-proteasome system to police the heart. Autophagy 12, 601–602. doi: 10.1080/15548627.2015.1136773
Locascio, J. J., Eberly, S., Liao, Z., Liu, G., Hoesing, A. N., Duong, K., et al. (2015). Association between α-synuclein blood transcripts and early, neuroimaging-supported Parkinson’s disease. Brain 138, 2659–2671. doi: 10.1093/brain/awv202
Malagelada, C., Jin, Z. H., Jackson-Lewis, V., Przedborski, S., and Greene, L. A. (2010). Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. J. Neurosci. 30, 1166–1175. doi: 10.1523/JNEUROSCI.3944-09.2010
Mollenhauer, B., Rochester, L., Chen-Plotkin, A., and Brooks, D. (2014). What can biomarkers tell us about cognition in Parkinson’s disease? Mov. Disord. 29, 622–633. doi: 10.1002/mds.25846
Molochnikov, L., Rabey, J. M., Dobronevsky, E., Bonucelli, U., Ceravolo, R., Frosini, D., et al. (2012). A molecular signature in blood identifies early Parkinson’s disease. Mol. Neurodegener. 7:26. doi: 10.1186/1750-1326-7-26
Ofori, E., Krismer, F., Burciu, R. G., Pasternak, O., McCracken, J. L., Lewis, M. M., et al. (2017). Free water improves detection of changes in the substantia nigra in parkinsonism: a multisite study. Mov. Disord. 32, 1457–1464. doi: 10.1002/mds.27100
Paul, R., Choudhury, A., Kumar, S., Giri, A., Sandhir, R., and Borah, A. (2017). Cholesterol contributes to dopamine-neuronal loss in MPTP mouse model of Parkinson’s disease: involvement of mitochondrial dysfunctions and oxidative stress. PLoS One 12:e0171285. doi: 10.1371/journal.pone.0171285
Pellecchia, M. T., Santangelo, G., Picillo, M., Pivonello, R., Longo, K., Pivonello, C., et al. (2014). Insulin-like growth factor-1 predicts cognitive functions at 2-year follow-up in early, drug-naive Parkinson’s disease. Eur. J. Neurol. 21, 802–807. doi: 10.1111/ene.12137
Planetta, P. J., Ofori, E., Pasternak, O., Burciu, R. G., Shukla, P., DeSimone, J. C., et al. (2016). Free-water imaging in Parkinson’s disease and atypical parkinsonism. Brain 139, 495–508. doi: 10.1093/brain/awv361
Potashkin, J. A., Santiago, J. A., Ravina, B. M., Watts, A., and Leontovich, A. A. (2012). Biosignatures for Parkinson’s disease and atypical parkinsonian disorders patients. PLoS One 7:e43595. doi: 10.1371/journal.pone.0043595
Qiang, J. K., Wong, Y. C., Siderowf, A., Hurtig, H. I., Xie, S. X., Lee, V. M., et al. (2013). Plasma apolipoprotein A1 as a biomarker for Parkinson disease. Ann. Neurol. 74, 119–127. doi: 10.1002/ana.23872
Rajput, A. H., and Rajput, A. (2014). Accuracy of Parkinson disease diagnosis unchanged in 2 decades. Neurology 83, 386–387. doi: 10.1212/WNL.0000000000000653
Rosenthal, L. S., Drake, D., Alcalay, R. N., Babcock, D., Bowman, F. D., Chen-Plotkin, A., et al. (2016). The NINDS Parkinson’s disease biomarkers program. Mov. Disord. 31, 915–923. doi: 10.1002/mds.26438
Santiago, J. A., Bottero, V., and Potashkin, J. A. (2017). Biological and clinical implications of comorbidities in Parkinson’s disease. Front. Aging Neurosci. 9:394. doi: 10.3389/fnagi.2017.00394
Santiago, J. A., Littlefield, A. M., and Potashkin, J. A. (2016). Integrative transcriptomic meta-analysis of Parkinson’s disease and depression identifies NAMPT as a potential blood biomarker for de novo Parkinson’s disease. Sci. Rep. 6:34579. doi: 10.1038/srep34579
Santiago, J. A., and Potashkin, J. A. (2013a). Integrative network analysis unveils convergent molecular pathways in Parkinson’s disease and diabetes. PLoS One 8:e83940. doi: 10.1371/journal.pone.0083940
Santiago, J. A., and Potashkin, J. A. (2013b). Shared dysregulated pathways lead to Parkinson’s disease and diabetes. Trends Mol. Med. 19, 176–186. doi: 10.1016/j.molmed.2013.01.002
Santiago, J. A., and Potashkin, J. A. (2014a). Current challenges towards the development of a blood test for Parkinson’s disease. Diagnostics 4, 153–164. doi: 10.3390/diagnostics4040153
Santiago, J. A., and Potashkin, J. A. (2014b). A network approach to clinical intervention in neurodegenerative diseases. Trends Mol. Med. 20, 694–703. doi: 10.1016/j.molmed.2014.10.002
Santiago, J. A., and Potashkin, J. A. (2014c). A network approach to diagnostic biomarkers in progressive supranuclear palsy. Mov. Disord. 29, 550–555. doi: 10.1002/mds.25761
Santiago, J. A., and Potashkin, J. A. (2015a). Blood biomarkers associated with cognitive decline in early stage and drug-naive Parkinson’s disease patients. PLoS One 10:e0142582. doi: 10.1371/journal.pone.0142582
Santiago, J. A., and Potashkin, J. A. (2015b). Network-based metaanalysis identifies HNF4A and PTBP1 as longitudinally dynamic biomarkers for Parkinson’s disease. Proc. Natl. Acad. Sci. U S A 112, 2257–2262. doi: 10.1073/pnas.1423573112
Santiago, J. A., and Potashkin, J. A. (2017). Evaluation of RNA blood biomarkers in individuals at risk of Parkinson’s disease. J. Parkinsons Dis. 7, 653–660. doi: 10.3233/JPD-171155
Santiago, J. A., Scherzer, C. R., and Potashkin, J. A. (2013). Specific splice variants are associated with Parkinson’s disease. Mov. Disord. 28, 1724–1727. doi: 10.1002/mds.25635
Santiago, J. A., Scherzer, C. R., and Potashkin, J. A. (2014). Network analysis identifies SOD2 mRNA as a potential biomarker for Parkinson’s disease. PLoS One 9:e109042. doi: 10.1371/journal.pone.0109042
Soreq, L., Guffanti, A., Salomonis, N., Simchovitz, A., Israel, Z., Bergman, H., et al. (2014). Long non-coding RNA and alternative splicing modulations in Parkinson’s leukocytes identified by RNA sequencing. PLoS Comput. Biol. 10:e1003517. doi: 10.1371/journal.pcbi.1003517
Soreq, L., Salomonis, N., Bronstein, M., Greenberg, D. S., Israel, Z., Bergman, H., et al. (2013). Small RNA sequencing-microarray analyses in Parkinson leukocytes reveal deep brain stimulation-induced splicing changes that classify brain region transcriptomes. Front. Mol. Neurosci. 6:10. doi: 10.3389/fnmol.2013.00010
Soreq, L., Salomonis, N., Guffanti, A., Bergman, H., Israel, Z., and Soreq, H. (2015a). Whole transcriptome RNA sequencing data from blood leukocytes derived from Parkinson’s disease patients prior to and following deep brain stimulation treatment. Genom. Data 3, 57–60. doi: 10.1016/j.gdata.2014.11.009
Soreq, L., Salomonis, N., Israel, Z., Bergman, H., and Soreq, H. (2015b). Analyzing alternative splicing data of splice junction arrays from Parkinson patients’ leukocytes before and after deep brain stimulation as compared with control donors. Genom. Data 5, 340–343. doi: 10.1016/j.gdata.2015.07.014
Swanson, C. R., Berlyand, Y., Xie, S. X., Alcalay, R. N., Chahine, L. M., and Chen-Plotkin, A. S. (2015). Plasma apolipoprotein A1 associates with age at onset and motor severity in early Parkinson’s disease patients. Mov. Disord. 30, 1648–1656. doi: 10.1002/mds.26290
Weintraub, D., Simuni, T., Caspell-Garcia, C., Coffey, C., Lasch, S., Siderowf, A., et al. (2015). Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson’s disease. Mov. Disord. 30, 919–927. doi: 10.1002/mds.26170
Keywords: Parkinson’s disease, progressive supranuclear palsy, biomarker, blood, PDBP
Citation: Santiago JA, Bottero V and Potashkin JA (2018) Evaluation of RNA Blood Biomarkers in the Parkinson’s Disease Biomarkers Program. Front. Aging Neurosci. 10:157. doi: 10.3389/fnagi.2018.00157
Received: 01 March 2018; Accepted: 08 May 2018;
Published: 29 May 2018.
Edited by:
Catarina Oliveira, University of Coimbra, PortugalReviewed by:
Elizabeta Blagoja Mukaetova-Ladinska, University of Leicester, United KingdomCopyright © 2018 Santiago, Bottero and Potashkin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Judith A. Potashkin, anVkeS5wb3Rhc2hraW5Acm9zYWxpbmRmcmFua2xpbi5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.