- 1Crystal and Structural Chemistry, Bijvoet Center for Biomolecular Research, Faculty of Science, Utrecht University, Utrecht, Netherlands
- 2Structural Biology and Protein Biochemistry, Morphic Therapeutic, Waltham, MA, United States
Type-I transmembrane proteins represent a large group of 1,412 proteins in humans with a multitude of functions in cells and tissues. They are characterized by an extracellular, or luminal, N-terminus followed by a single transmembrane helix and a cytosolic C-terminus. The domain composition and structures of the extracellular and intercellular segments differ substantially amongst its members. Most of the type-I transmembrane proteins have roles in cell signaling processes, as ligands or receptors, and in cellular adhesion. The extracellular segment often determines specificity and can control signaling and adhesion. Here we focus on recent structural understanding on how the extracellular segments of several diverse type-I transmembrane proteins engage in interactions and can undergo conformational changes for their function. Interactions at the extracellular side by proteins on the same cell or between cells are enhanced by the transmembrane setting. Extracellular conformational domain rearrangement and structural changes within domains alter the properties of the proteins and are used to regulate signaling events. The combination of structural properties and interactions can support the formation of larger-order assemblies on the membrane surface that are important for cellular adhesion and intercellular signaling.
Introduction
Proteins at the cell surface play an important role in the formation and function of tissues. Transmembrane proteins can receive and transmit signals from the cell outside to the inside and vice versa, and from one cell to the other. In addition, transmembrane and membrane-associated proteins control cell-cell adhesion processes to form tissues and organs. Cell signaling and cell adhesion are dependent on protein-protein interactions at the extracellular side, protein conformations, and conformational changes play important roles in regulating these processes.
Cell-surface expressed proteins with roles in intercellular adhesion and signaling are often part of the type-I transmembrane protein group that constitutes 1,412 members in humans according to Uniprot (www.uniprot.org) (UniProt, 2019). The architecture of type-I transmembrane proteins is defined by an extracellular N-terminus, often consisting of multiple domains, followed by a single transmembrane helix, and a C-terminal intracellular segment. Other than these common features, the proteins display a great diversity in architecture, and it is this structural diversity that underlies the broad range of functionalities that has been assigned to type-I transmembrane proteins. The extracellular segment often plays a role in sensing the outside environment of a cell and in relaying communication between cells where it can act as a receiver or as a transmitter of signals. The dysfunction of cell-surface expressed type-I transmembrane proteins has been associated with a multitude of diseases ranging from developmental pathologies, immune disorders to neurological conditions and cancers. In particular the extracellular segment of these proteins is a target for drug development because of its diversity in structure, allowing specificity, and its accessibility at the outside of the cell providing access to large biologics such as antibodies (Arteaga and Engelman, 2014; Moraga et al., 2015; Large et al., 2019).
Structural biology techniques have provided detailed insights into the molecular mechanisms controlling adhesion and intercellular signaling. Structures of extracellular segments of type-I transmembrane proteins in isolation or in complexes show how these proteins interact in cis and in trans and how they can undergo conformational changes to become activated (Ferguson et al., 2003; Leloup et al., 2017, 2018; Barak et al., 2019). Most of the structural data has been obtained by X-ray diffraction studies from protein crystals, but also from NMR and cryo-electron microscopy experiments. Weak, albeit physiologically relevant, cis and trans interactions are sometimes revealed in structure determination studies that rely on crystals as these interactions can be used by the samples to form the crystal (Seiradake et al., 2010; Harrison et al., 2011; Kong et al., 2016; Pronker et al., 2016).
Here we discuss a diversity of molecular mechanisms that are used by several adhesion and intercellular signaling systems of the type-I transmembrane group of proteins in the control of adhesion and activation of signaling. We focus on the extracellular interactions and conformational changes of these type-I transmembrane proteins and discuss how structural biology techniques have been instrumental in resolving common concepts. The local environment of the proteins, at or between membranes, has an important role in the interactions and dynamics that type-I transmembrane proteins display (Jacobson et al., 2019). The combination of interactions on the same membrane, in cis, and between membranes, in trans, can drive the formation of larger-order assemblies (Seiradake et al., 2010; Harrison et al., 2011; Honig and Shapiro, 2020). The structure and interactions of the extracellular segments of type-I transmembrane proteins are controlled by pre- and post-translational modification that can drive the selectivity and affinity of the proteins in cell adhesion (Pronker et al., 2016; Chandler et al., 2019). Finally, conformational changes and rearrangement of type-I transmembrane proteins in complexes underly their control and activation as receptors in signaling processes (Ferguson et al., 2003; Kong et al., 2016; Leloup et al., 2017; Barak et al., 2019).
Type-I Transmembrane Receptor Extracellular Interactions
Role of Membrane Environment and Physical Constraints
To understand type-I transmembrane protein extracellular interactions, it is important to grasp the distinct molecular environment these molecules operate and evolved in. The physical forces and constraints from the membrane environment have molded topological features and architectures of type-I transmembrane proteins. Biological membranes have been described as highly complex, heterogeneous, and dynamic environments where uniquely distinct signaling and adhesion processes are mediated (Groves and Kuriyan, 2010; Honigmann and Pralle, 2016). Despite a wealth of knowledge on components such as lipids, proteins, and sugars, resolving intricacies of membrane biochemical processes has proven difficult. This is partially due to the experimental intractability of this cellular environment, and the difficulties in producing variable rich and yet well-parametrized models for in silico approaches. Nonetheless, a picture of the physical constraints, kinetics and thermodynamics occurring at membranes are being slowly and steadily elucidated offering insights as to the forces that shaped membrane bound proteins. In the following section we discuss relevant features of membranes that will inform discussion of type-I transmembrane protein extracellular structure and function in signaling and adhesion.
The most evident feature of biological membranes is that they provide two-dimensional (2D) fluid surfaces in which molecules can be inserted anisotropically or adsorbed reversibly (Groves and Kuriyan, 2010; Honigmann and Pralle, 2016) and this setting influences the properties of the associated molecules. The reduced entropy of transmembrane proteins supports interactions with other proteins embedded in the same membrane, because the entropic penalty for complex formation is reduced (Whitty, 2008). Such cis interaction may be very weak when measured in the 3D solution phase but still be relevant in the physiological 2D membrane setting (Pronker et al., 2016). From early study of signaling complexes, it was suggested that membrane as opposed to cytosolic proteins should display altered kinetics given the reduction of dimensionality of diffusible space (Wang et al., 1992; Axelrod and Wang, 1994), but also benefit from increase in probability of encounter dubbed the “local concentration effect” (Kholodenko et al., 2000). Research detailing signaling processes have since established a more nuanced picture whereby spatial temporal dynamics of membrane bound signaling molecules (Jacobson et al., 2019), and their relationship to gradients of intracellular signaling molecules, determine cellular signal interpretation (Groves and Kuriyan, 2010; Kholodenko et al., 2010). Organization of membrane proteins into functional signaling units would seem to depend on fluctuating assemblies dictated by interactions between membrane proteins, membrane lipids, soluble binding partners, and intracellular scaffolds (Kholodenko et al., 2010; Simons and Gerl, 2010).
While membranes provide a two-dimensional surface area, they are also elastic in three dimensions (3D). This deformability of membranes has been suggested to affect signaling processes. By alteration of surface to volume ratios in convex and concave protrusions, membranes may be able to control effective ligand to receptor concentrations (Schmick and Bastiaens, 2014). Membrane structure is influenced by several factors, such as intracellular scaffolding proteins and the cytoskeleton, and at the extracellular side the glycocalyx and extracellular matrix (Jacobson et al., 2019; Shurer et al., 2019). In addition, the membrane chemical composition and physical properties, such as local tension and diffusion, influence the distribution, and activity of membrane proteins (Simons and Gerl, 2010; Shi et al., 2018). Any of these properties can aid the local accumulation of transmembrane proteins. In these settings protein binding sites are likely made possible or the very least enhanced by multivalency induced by biological membranes which may regulate binding specificity and affinity (Jung et al., 2009; Csizmar et al., 2019).
At adhesion sites, where two opposing membranes are interacting through transmembrane proteins, reduced membrane thermal fluctuations (Milstein et al., 2008; Rozycki et al., 2010), and reduced intrinsic protein flexibility (Wu et al., 2011) entropically favor protein clustering. Here, a combination of proteins interacting between opposing membranes in trans and on the same membrane in cis can generate larger-order assemblies. In a crowded environment such as the membrane, for higher-order assemblies to dynamically exist in space and time requires that membrane proteins display diverse moieties to mediate specific interactions. The interactions should range in affinities to enable competitive, cooperative, and allosteric mechanisms that underlie the generation of complex signaling and adhesion patterns. Delving into what is currently known about protein interaction from high throughput approaches, it indeed appears likely that the relevant range of solution-phase affinities for interactions of membrane bound proteins (nM to mM) is broader than that of non-membrane bound proteins (Wright, 2009) and affinities can be very weak. In addition, interactions between type-I transmembrane proteins are regulated dynamically by post-translational modifications such as extracellular glycosylation which adds an additional layer of complexity to transmembrane protein complex formation.
Weak cis Interactions Are Relevant for Signaling
As aforementioned, interactions of proteins found on membranes need not be of particularly high affinity when measured in the solution phase. It is notably becoming apparent through characterization of type-I transmembrane proteins that cis interactions can have particularly low affinities and still be relevant for signaling and adhesion events. Two striking examples in this regard are signaling and adhesion by immunoglobulin superfamily proteins KIT (Yuzawa et al., 2007) and myelin associated glycoprotein (MAG) (Pronker et al., 2016) where structural studies have paved the way to functional understanding of their respective subfamilies (receptor tyrosine kinase (RTK) class III and Siglecs). For both these proteins low affinity cis interactions are critical to mediate biological function.
Activation of KIT by cytokine stem cell factor (SCF) is critical to proper developmental time course in haematopoiesis, melanogenesis, and spermatogenesis. The study of activation of KIT by SCF illustrates well-requirements for signaling in a crowded 2D environment, since these receptors undergo ligand induced oligomerization which exploits cooperativity derived from colocalization to the membrane (Whitty, 2008) (Figure 1). Crystallographic structures have shown that dimeric SCF binds in a shallow groove formed by the membrane distal domains 1–3 of KIT. This heteromeric interaction promotes engagement of a lower affinity homomeric interaction site present at membrane proximal domains 4 and 5 in KIT (Liu et al., 2007; Yuzawa et al., 2007). The cis interaction occurring between KIT molecules first went unobserved in analytical ultracentrifugation sedimentation experiments of KIT extracellular segments in complex with SCF (Lemmon et al., 1997). Since, studies of full length KIT by negative stain electron microscopy (Opatowsky et al., 2014), and effects of oncogenic mutations on KIT activation have substantiated a mechanism driven by membrane derived membrane cooperativity whereby weak homotopic interactions zipper up dimer molecules into an activated state (Reshetnyak et al., 2015). Furthermore, the fingerprint motif for dimerization through salt bridges between the D4 domains of KIT as observed in crystal structures (Yuzawa et al., 2007) has been established as a common feature of RTK class III (Elegheert et al., 2011; Verstraete and Savvides, 2012; Felix et al., 2013, 2015) and V (Yang et al., 2010; Markovic-Mueller et al., 2017) receptors. Interestingly, it has been established that for other RTK III family members, homotopic contacts can be altogether stronger than those observed in KIT and form for example between unliganded colony-stimulating factor 1 receptor (CSF-1R) molecules (Elegheert et al., 2011). For other members, such as for Flt3, homotopic contacts are absent (Verstraete et al., 2011), illustrating how evolution may lead to divergent molecular interactions to regulate signaling.
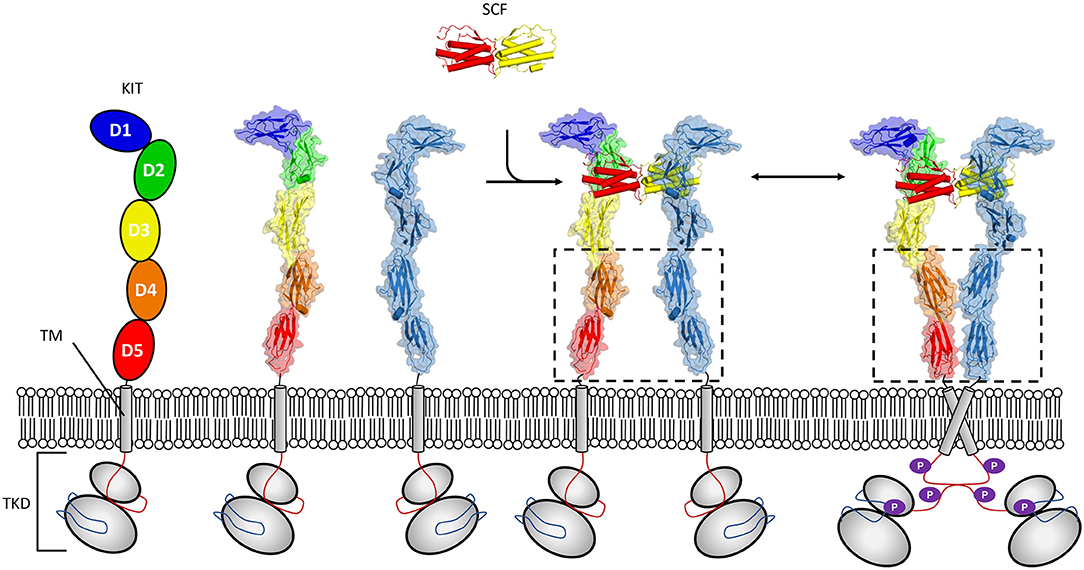
Figure 1. Mechanism of KIT activation by SCF. The receptor tyrosine kinases class III (RTK-IIIs) kit receptor is characterized by an ectodomain (pdb 2ec8) composed of five Ig-like domains a single membrane-spanning helix, and a conserved tyrosine kinase domain (TKD). Membrane distal domains 1–3 form a shallow groove accessible to SCF solution dimer. Binding of dimeric SCF promotes kit dimerization, facilitating conformational reorganization where weak homo cis interactions mediated by domains 4 and 5 contribute to establishing and/or maintaining induced kinase activity (pdb 2ew9).
The cis dimerization of MAG represents a second example in which a very weak solution-state interaction with a Kd of 380 μM is still relevant in a functional setting (Pronker et al., 2016). MAG is a type-I transmembrane protein expressed on the surface of cells that myelinate axons in the nervous system (Quarles, 2007). The MAG extracellular segment consists of five Ig domains and the membrane proximal Ig4 and Ig5 dimerize in an anti-parallel fashion (i.e., Ig4 interacts with Ig5 of the second molecule and vice versa). The dimerization of the MAG extracellular segment was first observed in the packing of crystals for structure determination and subsequently verified in solution experiments (Pronker et al., 2016; Myllykoski et al., 2018). The detailed information from the structural studies allowed to engineer the interface to generate two MAG variants, one that does not dimerize and one that dimerizes with higher affinity. Cell-based assays showed that the dimerization of MAG through domains Ig4 and Ig5 is required for signaling of MAG as a ligand into neuronal cells (Pronker et al., 2016).
Tuning Cross-Reactive Ligand Signaling by Heteromeric Signaling Receptor Complex Formation
To provide specified output to ligand induced signaling, cell surface receptors have generally evolved highly specific molecular interactions that determine ligand selectivity (Wang et al., 2009). Yet, in complex biological systems, evolutionary pressure can also craft signaling systems with high redundancy and diversity of ligands and receptors, hallmarks of system robustness which guarantee fail safes for critical pathways (Kitano, 2004). This is well-illustrated by what is observed in innate and adaptive immunity where signaling systems display poly-specific ligands that bind both to homologous and shared receptors to form signaling complexes. A subgroup of type-I transmembrane receptors, class I cytokine receptors have been extensively characterized and mostly signal through formation of heteromeric complexes (Wang et al., 2009; Morris et al., 2018). They are responsible for cell proliferation and fate decisions of immune and hematopoietic cells. Mechanistically heteromeric complexes exploit surface-capture effects to bind various ligands with ranging affinities leading to observable redundancy and competition in signaling which in turn enable a panoply of biological responses from various target cells.
Class I cytokine receptors are composed of multiple type-I transmembrane protein chains with distinguishing conserved features and motifs. Most notably, their extracellular segments share a characteristic cytokine-binding homology region (CHR) (Bazan, 1990; Boulay et al., 2003; Verstraete et al., 2014) composed of two fibronectin type III domains (FnIII). Within the first, N-terminal, FnIII domain in the CHR region two conserved disulphide bonds are found, whereas a conserved “WSXWS” motif is found in the second FnIII domain that may have a role in folding of the protein (Bazan, 1990; Yawata et al., 1993; Boulay et al., 2003; Verstraete et al., 2014). For most receptors, cytokine binding is mediated by the joint region between FnIII domains composed of the short interdomain linker and domain interstrand loops as first outlined in the structure of the human growth hormone receptor (de Vos et al., 1992; Yawata et al., 1993; Wang et al., 2009; Morris et al., 2018). Nonetheless, various receptors also use additional domains to mediate their function. Intracellularly, these proteins have sequence motifs to allow recruitment of JAK and STAT proteins (Wang et al., 2009; Morris et al., 2018). Signaling mechanisms involving either pre-formed or ligand-induced receptor assemblies have been suggested for class I cytokine receptors (Wang et al., 2009; Kent, 2020), for heteromeric receptor assemblies the prevalent view is however that precise chain stoichiometries are dictated by specific cytokine binding. For the majority of heteromeric complexes a higher affinity ligand binding “alpha chain” is thought to recognize a specific cytokine before assembling with a “shared” chain to initiate signal transduction (Wang et al., 2009; Morris et al., 2018). Three major “shared” chains outline subgroups of heteromeric class I cytokine receptors, gp130 (Boulanger et al., 2003; Skiniotis et al., 2005), γc (Wang et al., 2005; Stauber et al., 2006), and βc (Hansen et al., 2008); with other “shared” chains also used but not as prevalently (LaPorte et al., 2008; Bloch et al., 2018).
Interestingly, some of the major structural insights regarding class I cytokine receptors came from the fact that these structures form various higher-order assemblies out of ligand-bound heteromeric receptors. So while various IL4/13 (LaPorte et al., 2008) and γc (Wang et al., 2005; Stauber et al., 2006) “shared” chain assemblies form heterodimers and trimers, gp130 (Boulanger et al., 2003; Skiniotis et al., 2005), and βc (Hansen et al., 2008) have additional surfaces between receptor subunits that drive ligand bound complex into forming hexameric and dodecameric assemblies relevant for signaling. Throughout, sequential assembly has been established rigorously using an array of biophysical and biochemical methods, including isothermal titration calorimetry (ITC), surface plasmon resonance (SPR), and cellular activity assays, bringing about the consensus of principles established thus far (Wang et al., 2009; Morris et al., 2018). Recent developments in the field on more unique receptors have further shone a light on even more nuanced biology of these receptors proposing allosteric regulation (Verstraete et al., 2014, 2017) and conformational selection mechanisms (Bloch et al., 2018) as driving forces for varying affinities to free and bound ligand for “shared” chains. Adding more details to our understanding of the surface-capture mechanisms of these type-I transmembrane receptors. Recent work dissecting further competition and assembly mechanisms between natural (LaPorte et al., 2008), but also synthetic cytokines (Mitra et al., 2015), or using other engineered approaches (Verstraete et al., 2017) may now pave the way to dissect cytokine signaling for therapeutic approaches and steadily bridge the gap between structural and systems-level biology of cytokine signaling.
Trans Interactions: Regulating Molecular Adhesion and Recognition
Extracellular domains of membrane proteins mediating adhesion have been shaped by competing biophysical and biological constraints. On one hand, they require certain lengths, flexibilities, and affinities to satisfy conditions to establish and maintain adhesion events. While their specificities should also be finely tuneable in spatial and temporal dimensions to establish molecular recognition. Nature's remarkable solutions to this dilemma, are coming to light through breakthrough structural studies of these proteins' extracellular segments. Here, we will focus our discussion of trans interactions to examples drawn from the DSCAM, sidekick, and L1 family of proteins to illustrate how distinct ectodomain size, competing surfaces, and pre- and post-translational modifications influence adhesion and signaling of type-I transmembrane proteins.
The DSCAM, sidekick, and L1 cell adhesion protein families are all subfamilies of the immunoglobulin superfamily and contain proteins with ectodomains composed of repeats of immunoglobulin and fibronectin type III domains (Figure 2). Intriguingly, while negative stain electron microscopy data shows the intrinsic conformational flexibility of these proteins (Schurmann et al., 2001; Meijers et al., 2007; Tang et al., 2018), as it appears that these protein families adopt elongated conformations with few domains contributing to homophilic interactions, tomographic electron microscopy data in the context of the membrane environment suggests these proteins can also neatly fit into tight adhesion interfaces (Tang et al., 2018). The specific mechanisms by which such proteins signal from distinct adhesion sites remains to date unclear. Indeed, for these various families intracellular signaling mechanisms involving interactions with scaffolding molecules (Yamagata and Sanes, 2010; Freal et al., 2019), interactions with other signaling molecules (Kiefel et al., 2011), and translocation of intracellular domains (Riedle et al., 2009; Sachse et al., 2019) have been described, nonetheless no unified downstream signaling pathway has been identified. As for how extracellular segments engage signaling, various mechanisms have been proposed. Regular patterns emerging at adhesion sites can act as a driver of signaling by protein-cluster formation (He et al., 2009). Signaling inducing constrained conformations from tight membrane apposition is another mechanism suggested on the basis of the S shaped configuration of the first eight immunoglobulin domains of DSCAM (Sawaya et al., 2008) (Figure 2). Gaining understanding of the elongated architectures of adhesion molecules and their roles in signaling remains a challenge, however progress is being made using combinations of structural techniques as discussed above.
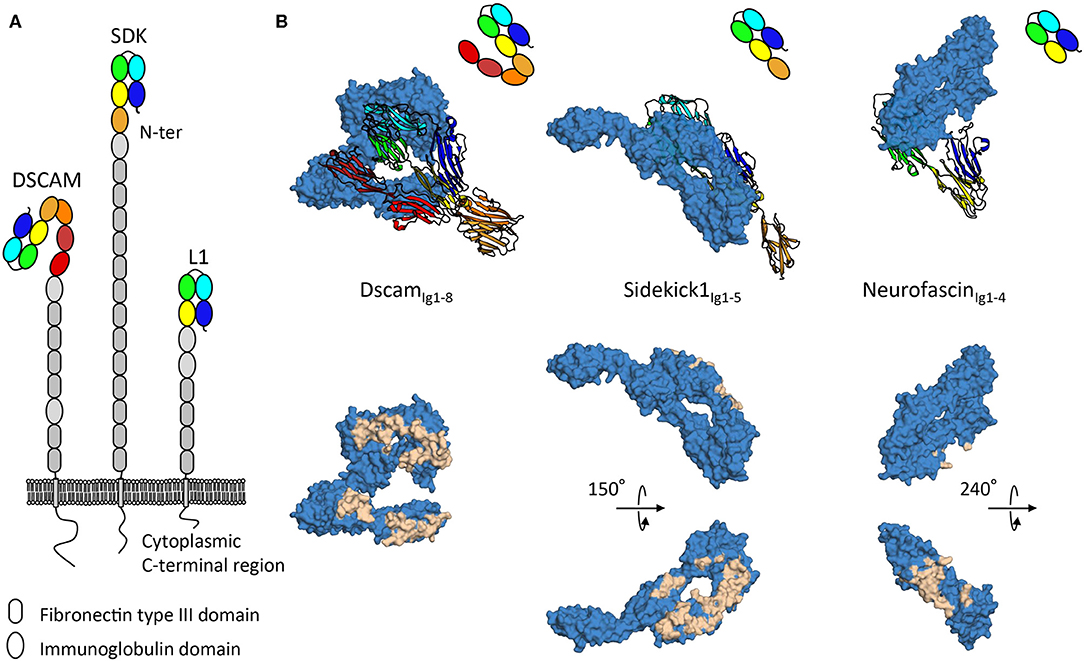
Figure 2. Architecture and homophilic adhesion interfaces of horseshoe containing subfamilies of the immunoglobulin superfamily. (A) Type-I transmembrane extracellular DSCAM, sidekick, and L1 protein families' architecture with modular domain organization comprising Ig-like and fibronectin type III repeats. (B) Crystal structures of homophilic binding modules of DSCAM, sidekick1, and neurofascin from DSCAM, sidekick, and L1 protein families. Top panels show how horseshoes use different faces for interactions. The ribbon copy is held in the same orientation while the space filling copy interacts with distinct faces. Bottom panels show interacting residues plotted on the surface of the space filled copy of the molecules, displaying extensive interfaces on distinct domain faces.
Molecular recognition requires distinct cells be able to selectively establish interactions to transmit signals to one another (de Wit and Ghosh, 2016). From this perspective, defining specific homophilic trans interactions can be viewed as an explicit form of molecular signaling. DSCAM, sidekick, and L1 protein families remarkably all share a distinguishing architectural feature, an N-terminal horseshoe shaped binding supra-module, composed of the first four immunoglobulin domains (Figure 2) (Meijers et al., 2007; Liu et al., 2011; Goodman et al., 2016). This horseshoe feature appears to in large part define homophilic adhesion for molecules in these families, so it is noteworthy that crystallographic structures show that DSCAM, sidekick and L1 protein families use strikingly different faces of their respective horseshoe supra-module to mediate homophilic interactions (Figure 2). This would seem to indicate that, albeit likely sharing a common evolutionary origin, this module in different families has evolved very distinct extensive interaction surfaces which may be in some cases competing or complementary. It is interesting to note that homodimeric proteins have been found to have more interaction partners than non-dimers (Ispolatov et al., 2005), this seems to be well-illustrated by L1 family proteins that form promiscuous interactions with other horseshoe-containing protein families such as the contactin family (Volkmer et al., 1998).
While horseshoe containing families provide an example of the molecular diversity possible at a genomic level for molecular recognition as a form of signaling, pre-and post-translational modifications provide a further layer of complexity to the observed heterogeneity that establishes this form of signaling. Splicing is a particularly important pre-translational modification process for these molecules. For L1 family proteins such as neurofascin, over 50 distinct splice variants defined by various combinations of loop insertions, alternate domain inclusion, and linker length variations have been described that impact biological function at various developmental stages (Hassel et al., 1997; Liu et al., 2011; Kriebel et al., 2012). For Dscam, differences that regulate isoform-specific homophilic binding have been mapped to its 2nd, 3rd, and 7th immunoglobulin domains, changing homophilic binding regions on these domains offering insights into isoform-dependent binding out of thousands of possibilities (Meijers et al., 2007; Sawaya et al., 2008). Post-translational modifications of extracellular proteins, such as glycosylation, modify their surface properties, and influence their biological behavior, regulating diverse biochemical processes from protein folding to protein interactions (Moremen et al., 2012). Given inherent “stickiness” of immunoglobulin domains, glycosylation has in particular been proposed to shield unwanted interactions (Barclay, 2003). What has become more apparent for horseshoe containing proteins is that glycosylation likely plays a more constitutive role as exemplified by the patterning of L1 proteins via N-linked glycans during membrane adhesion assembly (He et al., 2009). Furthermore, they may also provide additional criteria for interaction selectivity as shown by their role in regulating neurofascin—contactin interactions (Bonnon et al., 2007). The astounding heterogeneity coming from subtle structural differences in binding interfaces of type-I transmembrane proteins mediating molecular recognition suggests that detailed structural characterization will be required to fully grasp the nuances of how they mediate their function.
Conformational Changes Regulate Signaling
Conformational Changes Expose Hidden Binding Sites
Proteins use conformational changes to expose or hide binding sites. In multidomain proteins such changes are often accomplished by reorganizing domains as rigid bodies with respect to each other. This inter-domain conformational change mechanism is also used by type-I transmembrane proteins to regulate their function.
One of the most widely studied examples in which conformation-dependent signaling takes place is in the HER family. The HER family consists of EGFR, HER2, HER3, and HER4. HER stands for Human Epidermal growth factor Receptor, the first member of the family discovered. These receptor tyrosine kinases are involved in many processes in development, though they are most well-known for their overexpression being associated with solid tumors (Arienti et al., 2019; Khan et al., 2019; Schettini et al., 2020). The extracellular segment of these type-I transmembrane proteins consists of four domains (I, II, III, and IV). HER proteins exist on the cell surface as tethered, autoinhibited receptors. Except for HER2, for which no ligands have been identified thus far, the members of the HER family undergo a conformational change upon ligand binding. In the tethered conformation, the domain II dimerization arm interacts with domain IV (Ferguson et al., 2003). In the untethered conformation, domain II has pivoted around domain III by about 130° away from the C-terminus of the receptor, so that its dimerization arm is now exposed to the solvent (Figure 3). This untethered receptor is able to homo- or hetero-dimerize through domain II with another family member. Unliganded HER family members such as EGFR can exist on the cell membrane as inactive dimers (Chung et al., 2010; Low-Nam et al., 2011). EGFR homodimer activation is dependent on multiple factors such as interactions of the receptor with the membrane and ligand binding (Arkhipov et al., 2013). In the extended conformation, the ligand can bind in the pocket created by the proximity of domain I and III, thus stabilizing the homodimer (Lu et al., 2010) and inducing a conformational change which brings the two domains IV close by each other. These changes lead to a rearrangement of the transmembrane helixes which result in activation of the intracellular domains (Endres et al., 2013). Indeed, in absence of ligand, the intracellular module is monomeric, and self-inhibited by interactions with the membrane (Endres et al., 2013). Ligand-induced conformational changes modify the configuration of the transmembrane helixes which leads to the asymmetric dimerization of the intracellular domains (Arkhipov et al., 2013), one of which will become the activator and phosphorylate the receiver partner (Figure 3). This transphosphorylation leads to downstream signaling processes that drive cell survival and proliferation (Hubbard and Till, 2000; Lemmon and Schlessinger, 2010).
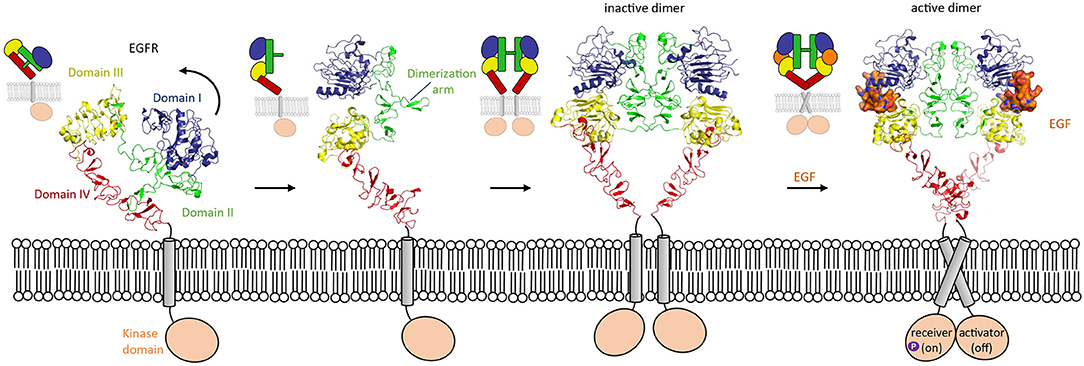
Figure 3. Domain rearrangement of EGFR upon activation by EGF ligand. EGFR (HER-1) undergoes a conformational change from an untethered monomer in which domain II (green) interacts with domain IV (red) (pdb 1nql), to a tethered monomer in equilibrium with an inactive homodimer (model using pdb 1n8z superposed on pdb 1ivo), and finally to an active, EGF-bound (orange) homodimer (pdb 3njp).
Conformational Changes Within Domains Change Protein Surface Properties
In addition to inter-domain changes, intra-domain conformational changes can alter the properties of a protein. The recently described conformational change within the 10-bladed β-propeller of the type-I transmembrane protein sortilin, that belongs to the VPS10 family, represents a striking example of how an intradomain rearrangement can control ligand binding (Januliene et al., 2017a; Leloup et al., 2017). The pH-dependent rotation and translation of blades with respect to each other causes a reorganization of the β-propeller surface in such a way that it induces homodimerization and disrupts the interaction with several ligands.
Several members of the VPS10 family of proteins play important roles in maintaining homeostasis in our tissues. They recognize and bind ligands for internalization into a cell or for trafficking between cell compartments. These ligands also need to be released once their destination has been reached. VPS10 family members can also control neurotrophin signaling when they function by signaling as receptor together with the coreceptor p75 neurotrophic receptor (NTR) (Bothwell, 2019). The VPS10 family consists of five sorting receptors which all contain a VPS10 subunit comprising a large 10-bladed β-propeller flanked by two small stabilizing 10-CC domains. This above-average number of blades probably confers flexibility to the β-propeller, and it enables peptide ligands to bind inside its central tunnel, while larger protein partners interact with the top face of the β-propeller. Three strategies; proteolytic processing, conformational, and oligomeric changes, are employed by the receptors to prevent ill-timed binding of ligands. A common strategy for all VPS10 family members is to block the entrance of the β-propeller tunnel by their propeptide. This propeptide prevents ligand binding in the endoplasmic reticulum and early trans Golgi network (TGN) and is removed in the late TGN by the proprotein convertase furin (Munck Petersen et al., 1999).
The binding of ligands to the β-propeller of Sortilin is pH-dependent. At neutral pH Sortilin is a monomer, and once its propeptide has been removed, it can bind a variety of ligands. However, as a sorting receptor, Sortilin cycles between many different cell compartments, including the TGN (pH 6.0) and early to late endosomes (pH 6.3–5.5). Upon acidification, the β-propeller of Sortilin undergoes a conformational change and its top surface, negatively charged at neutral pH, becomes more neutral (Januliene et al., 2017a; Leloup et al., 2017) (Figure 4A). These conformational and charge changes probably enable ligand release and dimerization of the receptor through its β-propeller top face, which further prevents interactions with ligands.
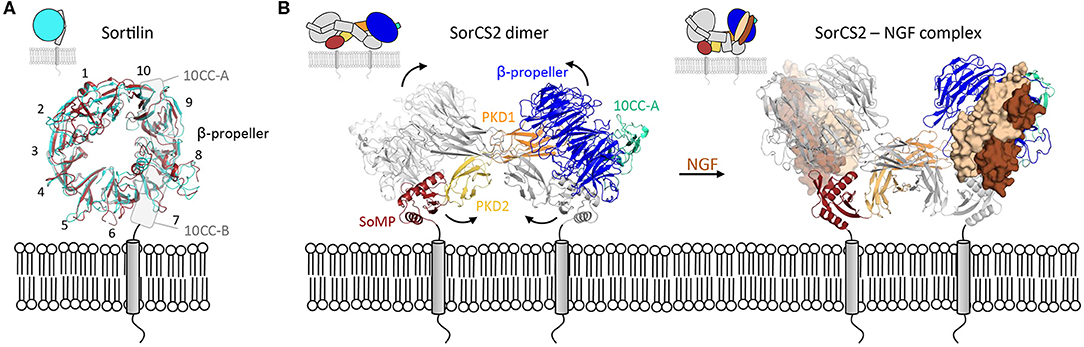
Figure 4. Intra- and inter-domain rearrangements underly the function of VPS10 family members. (A) Substantial conformational changes are apparent within the Sortilin β-propeller in changing from the monomer form (blue, pdb 3f6k) to the dimer form (red, pdb 5nmt). The 10 Sortilin β-propeller blades are numbered. (B) The SorCS2 dimer changes its conformation, predominantly by domain rearrangements, upon ligand binding (unbound, pdb 6ffy and bound, pdb 6fg9).
Except for Sortilin, members of the VPS10 family possess several domains between the VPS10 subunit and the transmembrane helix; two polycystic kidney disease (PKD) domains and a SorCS membrane proximal (SoMP) domain for the three SorCS subfamily members, an epidermal growth factor-like (EGF), 11 low-density lipoprotein receptor type A repeats (LA) and six fibronectin type III (FN3) domains for the fifth family member SorLA. One of the roles of these additional domains is to modulate binding to the VPS10 platform. For example, SorCS2 has been shown to exist in at least two different conformations (Januliene et al., 2017b; Leloup et al., 2018), in one of which ligand binding is rendered impossible by the close proximity of the ligand-binding β-propeller top face to the cell membrane (Figure 4B).
Auto-Inhibition to Prevent Aberrant Signaling
Ligand-induced type-I transmembrane receptor dimerization is the canonical mechanism to trigger cell signaling. Signaling in the absence of ligand is often actively prevented. This inhibition is required because receptors can encounter each other independent of ligand as a function of the local receptor concentration on the cell surface and this chance encounter may induce signaling (Atanasova and Whitty, 2012). In addition, receptors can have weak interaction sites to directly interact with each other to support ligand-induced signaling and this propensity for interaction may result in unwarranted activation. Two auto-inhibition mechanisms, in which the extracellular segment plays an important role, are commonly used by receptors to prevent ligand-independent activation; by adopting an inactive conformation and by forming inactive oligomers (Figures 5, 6). Two signaling systems that are examples for these two mechanisms; an inactive monomer conformation of HER and inactive dimers of PlexinA receptors, are discussed below.
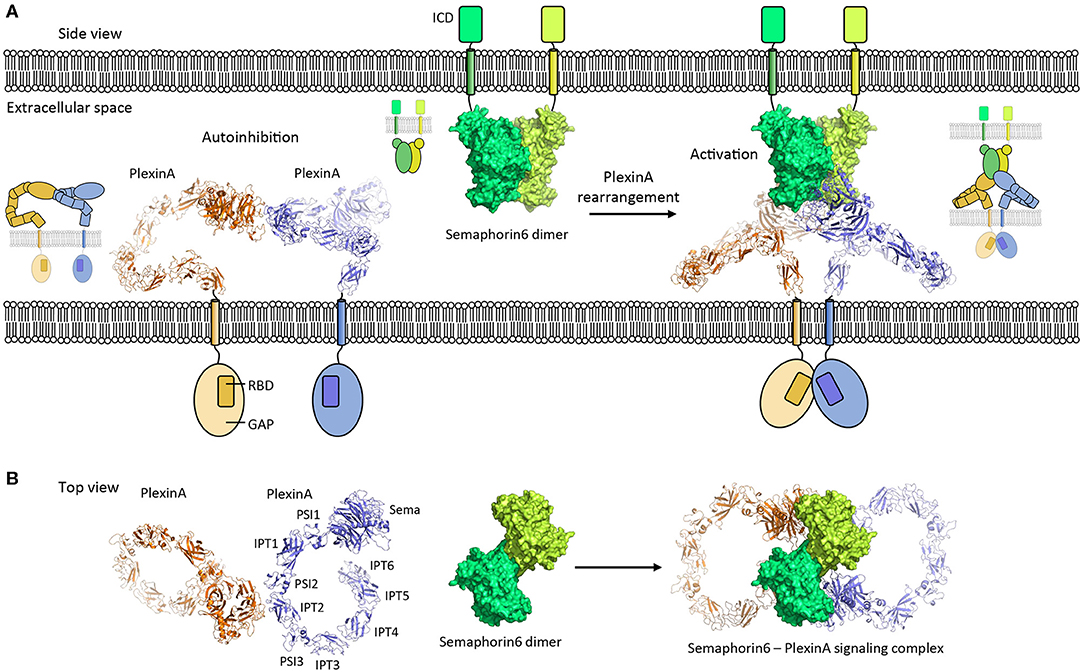
Figure 5. PlexinA autoinhibition model and activation by Semaphorin6 ligand. (A) PlexinA receptors adopt an autoinhibited state by non-symmetric cis dimerization (pdb 5l5k). The transmembrane helixes are separated from each other in this state. Rearrangement of the PlexinA dimer upon Semaphorin6 ligand binding (pdb 3okw) activates the PlexinA receptors by bringing the transmembrane helixes in close proximity (modeled based on 3oky and 5l5k). ICD, intracellular domain; GAP, GTPase activating protein domain; RBD, Rho GTPase binding domain. (B) Top view [i.e., (A) is rotated by 90° along the membrane]. The membranes and cytosolic segments are omitted from the panel. PSI, plexin-semaphorin-integrin; IPT, Ig domain shared by plexins and transcription factors.
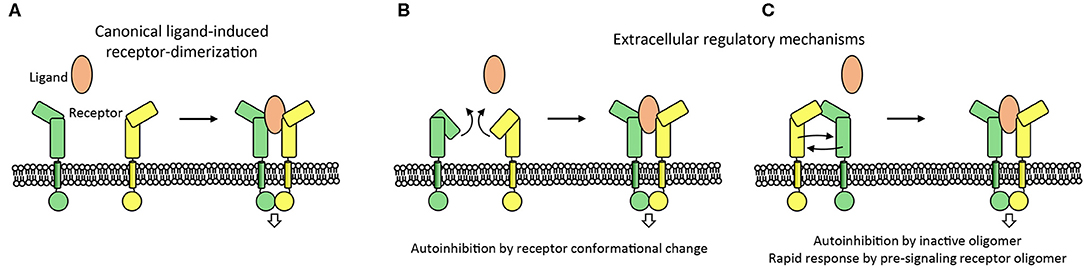
Figure 6. Extracellular mechanisms controlling the activation of cell signaling. (A) Canonical ligand-receptor system is shown in which a ligand molecule brings two receptor intracellular domains into proximity to initiate cell signaling. (B,C) Extracellular regulatory mechanisms that control the initiation of signaling are indicated. Receptor-conformational change or receptor-inactive oligomers can prevent aberrant initiation of signaling. In addition, the oligomerization of receptors before ligand binding may aid the receptor to respond rapidly to ligand binding by receptor reorganization (C).
Regulation of signaling by the conformational-change model in HER family members represents a well-described example of how an auto-inhibited conformation of a receptor prevents activation. In the auto-inhibited “compact” conformation of the HER extracellular segment, dimerization arms in domains II and IV interact and prevent high-affinity binding of the ligand (Cho and Leahy, 2002; Ferguson et al., 2003). This domain II–IV interaction stabilizes the relative orientation of domains I and III in such a way that they cannot form a high-affinity binding site for ligand binding (Figure 3). Possibly, the auto-inhibition is further strengthened by receptor dimers or larger-order clusters on the cell surface that keep the transmembrane helixes and cytosolic segments sufficiently apart to inhibit signaling (Zanetti-Domingues et al., 2018). This form of the receptor is in a dynamic equilibrium with a more extended, albeit less frequent, conformation of the receptor. The extended conformation that contains the high-affinity ligand binding site is stabilized by ligand binding. Exposure of the dimerization arms in the ligand-receptor complex enables receptor dimerization and subsequently signal triggering at the cytosolic side.
PlexinA's receptor signaling is induced by Semaphorin ligands and controls nervous system development and plasticity. The PlexinA extracellular segment has a large unusual ring-like conformation that seems to be required to bring the PlexinA cytosolic segments of two receptors into close proximity in a Semaphorin-induced receptor dimer to trigger signaling (Janssen et al., 2010; Liu et al., 2010; Nogi et al., 2010; Kong et al., 2016). Plexin dimerization induces cytosolic-segment conformational changes that activates the cytosolic Plexin GTPase activating protein (GAP) domain to enable Rap binding and subsequent Rap inactivation by catalyzing its GTP hydrolysis (Wang et al., 2012, 2013). Interestingly, the ring-like conformation is also used by the PlexinA receptor to prevent ligand independent signaling by maintaining a separation between the cytosolic PlexinA segments in a distinct head-to-stalk dimer complex of the extracellular segments (Figure 5). The autoinhibited dimer structure is rearranged upon Semaphorin ligand binding which brings the PlexinA cytosolic segments into close apposition. This activation is achieved without intramolecular conformational changes in the PlexinA extracellular segment and relies solely on rearrangement of PlexinA dimers (Figure 5).
Poised for Signaling
The propensity of cell surface receptors to interact pre-ligand binding may serve a second role. It permits the local concentration of receptors and prepares them to respond rapidly by rearranging into a signaling competent form once ligand is bound (Figure 6C). This mechanism has, for example, been suggested for the PlexinA receptors (Kong et al., 2016), EGFR (Zanetti-Domingues et al., 2018), and for the SorCS2-p75NTR hetero-dimer receptor complex (Deinhardt et al., 2011). The auto-inhibited head-to stalk PlexinA dimer is disrupted when the Semaphorin ligand binds to one of the PlexinA receptors. The second PlexinA receptor then becomes immediately available to form the signaling competent semaphorin-plexin complex consisting of a semaphorin dimer and two PlexinA molecules. In a similar manner the EGFR receptor is pre-organized to rapidly respond to ligand although the receptors also undergo an additional intramolecular conformational change prior to signaling (Zanetti-Domingues et al., 2018). A variation on this theme is provided by the SorCS2 receptor dimer that is pre-associated with the co-receptor p75NTR. Proneurotrophin ligand binding to SorCS2 and p75NTR may separate the two receptors (Leloup et al., 2018) to trigger the dissociation of the guanine nucleotide exchange factor Trio from the cytosolic side of the SorCS2-p75NTR complex and subsequent signaling (Deinhardt et al., 2011). Whether this pre-association mechanism is essential for receptors to respond rapidly to ligand binding has, however, not been established experimentally.
It is interesting to note that whilst also homomeric class I cytokine receptors are believed to be dimers which become activated by a ligand-induced receptor conformational change (Atanasova and Whitty, 2012; Brooks et al., 2014; Waters and Brooks, 2015) and may thus be poised for signaling, it has recently been shown that, at least for three family members, the receptors exist as monomers that are dimerized by their ligands (Wilmes et al., 2020). Using carefully constructed experiments, the authors showed that the thrombopoietin receptor, the Epo receptor, and the growth hormone receptor exist as monomers at physiologically relevant cell-surface densities, and efficiently dimerize and activate upon ligand binding. Several cancer-associated mutations in these receptor systems were shown to aid ligand-independent receptor dimerization, supporting the ligand-induced receptor-dimerization activation model for these receptors (Wilmes et al., 2020). In addition, the authors determined experimentally that ligand-independent receptor dimerization is concentration-dependent and only occurs at very high cell-surface cytokine receptor densities, illustrating the importance of probing ligand-receptor activation mechanisms at physiologic concentrations. Further studies will be required to assess if and under which conditions other class I cytokine receptors are either pre-associated, or dimerized only by their ligands, and in those cases where receptor pre-association occurs whether or not this has a role in preparing the receptor system for signaling.
Concluding Remarks
Here we have discussed several extracellular molecular mechanisms employed by type-I transmembrane proteins in cell signaling and adhesion processes (see also Figure 6). The structures and the interactions of these proteins play a critical role in the activation and control of signaling and adhesion. Structural studies have been essential in revealing the conformations, complexes, and rearrangements that underlie the function of signaling receptors and adhesion proteins. Such insights have recently provided a rationale in the design of modulators that can fine-tune signaling, [e.g., by re-orienting receptors (Moraga et al., 2015), enhancing receptor specificity and stability (Silva et al., 2019), and partially impairing receptor dimerization (Ho et al., 2017)]. These novel modulators hold promise as therapeutic candidates to treat disorders associated with aberrant receptor signaling particularly in cancers, immunotherapy, and regenerative medicine (Ho et al., 2017; Silva et al., 2019).
Several outstanding questions that center on the mechanistic principles underlying the function of type-I transmembrane proteins may be addressed in the near future by structural studies; How are the structures and interactions affected by their physiologic transmembrane setting? What is the influence of protein distribution in space and time on the organization and function of type-I transmembrane proteins? In what way are the extracellular segment and cytosolic portion of type-I transmembrane proteins coupled to organize adhesion and signaling? Currently, there is no detailed structural data that shows the direct coupling of the extracellular part of the protein with its cytosolic part, although several attempts have been made toward this endeavor (Ge et al., 2018; Uchikawa et al., 2019; Kuo et al., 2020). Most likely the transmembrane connection between the two segments, embedded in micelles or nanodisks confers too much flexibility in this setting, precluding structure solution. New techniques, such as cryo-electron tomography (Zeev-Ben-Mordehai et al., 2014) and solid-state nuclear magnetic resonance (Kaplan et al., 2016), are becoming available to study the structure and dynamics of these proteins in a transmembrane setting. Ultimately however, a combination of structural methods and cell-biology techniques is required to fully resolve the mechanistic intricacies of intercellular signaling and adhesion processes in our tissues.
Author Contributions
All authors contributed to the conception and writing of the manuscript.
Funding
BJ was supported by a European Research Council Starting Grant (677500).
Conflict of Interest
NL was employed by the company Morphic Therapeutic.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Arienti, C., Pignatta, S., and Tesei, A. (2019). Epidermal growth factor receptor family and its role in gastric cancer. Front. Oncol. 9:1308. doi: 10.3389/fonc.2019.01308
Arkhipov, A., Shan, Y., Das, R., Endres, N. F., Eastwood, M. P., Wemmer, D. E., et al. (2013). Architecture and membrane interactions of the EGF receptor. Cell 152, 557–569. doi: 10.1016/j.cell.2012.12.030
Arteaga, C. L., and Engelman, J. A. (2014). ERBB receptors: from oncogene discovery to basic science to mechanism-based cancer therapeutics. Cancer Cell 25, 282–303. doi: 10.1016/j.ccr.2014.02.025
Atanasova, M., and Whitty, A. (2012). Understanding cytokine and growth factor receptor activation mechanisms. Crit. Rev. Biochem. Mol. Biol. 47, 502–530. doi: 10.3109/10409238.2012.729561
Axelrod, D., and Wang, M. D. (1994). Reduction-of-dimensionality kinetics at reaction-limited cell-surface receptors. Biophys. J. 66, 588–600. doi: 10.1016/S0006-3495(94)80834-3
Barak, R. Yom-Tov, G., Guez-Haddad, J., Gasri-Plotnitsky, L., Maimon, R., Cohen-Berkman, M., et al. (2019). Structural principles in robo activation and auto-inhibition. Cell 177, 272–285 e16. doi: 10.1016/j.cell.2019.02.004
Barclay, A. N. (2003). Membrane proteins with immunoglobulin-like domains - a master superfamily of interaction molecules. Semin. Immunol. 15, 215–223. doi: 10.1016/S1044-5323(03)00047-2
Bazan, J. F. (1990). Structural design and molecular evolution of a cytokine receptor superfamily. Proc. Natl. Acad. Sci. U. S. A. 87, 6934–6938. doi: 10.1073/pnas.87.18.6934
Bloch, Y., Bouchareychas, L., Merceron, R., Skladanowska, K., Van den Bossche, L., Detry, S., et al. (2018). Structural activation of pro-inflammatory human cytokine IL-23 by cognate IL-23 receptor enables recruitment of the shared receptor IL-12Rbeta1. Immunity 48, 45–58 e6. doi: 10.1016/j.immuni.2017.12.008
Bonnon, C., Bel, C., Goutebroze, L., Maigret, B., Girault, J. A., and Faivre-Sarrailh, C. (2007). PGY repeats and N-glycans govern the trafficking of paranodin and its selective association with contactin and neurofascin-155. Mol. Biol. Cell 18, 229–41. doi: 10.1091/mbc.e06-06-0570
Bothwell, M. (2019). Recent advances in understanding context-dependent mechanisms controlling neurotrophin signaling and function. F1000Res 8:1. doi: 10.12688/f1000research.19174.1
Boulanger, M. J., Chow, D. C., Brevnova, E. E., and Garcia, K. C. (2003). Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science 300, 2101–2104. doi: 10.1126/science.1083901
Boulay, J. L., O'Shea, J. J., and Paul, W. E. (2003). Molecular phylogeny within type I cytokines and their cognate receptors. Immunity 19, 159–163. doi: 10.1016/S1074-7613(03)00211-5
Brooks, A. J., Dai, W., O'Mara, M. L., Abankwa, D., Chhabra, Y., Pelekanos, R. A., et al. (2014). Mechanism of activation of protein kinase JAK2 by the growth hormone receptor. Science 344:1249783. doi: 10.1126/science.1249783
Chandler, K. B., Leon, D. R., Kuang, J., Meyer, R. D., Rahimi, N., and Costello, C. E. (2019). N-Glycosylation regulates ligand-dependent activation and signaling of vascular endothelial growth factor receptor 2 (VEGFR2). J. Biol. Chem. 294, 13117–13130. doi: 10.1074/jbc.RA119.008643
Cho, H. S., and Leahy, D. J. (2002). Structure of the extracellular region of HER3 reveals an interdomain tether. Science 297, 1330–1333. doi: 10.1126/science.1074611
Chung, I., Akita, R., Vandlen, R., Toomre, D., Schlessinger, J., and Mellman, I. (2010). Spatial control of EGF receptor activation by reversible dimerization on living cells. Nature 464, 783–787. doi: 10.1038/nature08827
Csizmar, C. M., Petersburg, J. R., Perry, T. J., Rozumalski, L., Hackel, B. J., and Wagner, C. R. (2019). Multivalent ligand binding to cell membrane antigens: defining the interplay of affinity, valency, and expression density. J. Am. Chem. Soc. 141, 251–261. doi: 10.1021/jacs.8b09198
de Vos, A. M., Ultsch, M., and Kossiakoff, A. A. (1992). Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science 255, 306–312. doi: 10.1126/science.1549776
de Wit, J., and Ghosh, A. (2016). Specification of synaptic connectivity by cell surface interactions. Nat. Rev. Neurosci. 17, 22–35. doi: 10.1038/nrn.2015.3
Deinhardt, K., Kim, T., Spellman, D. S., Mains, R. E., Eipper, B. A., Neubert, T. A., et al. (2011). Neuronal growth cone retraction relies on proneurotrophin receptor signaling through Rac. Sci. Signal 4:ra82. doi: 10.1126/scisignal.2002060
Elegheert, J., Desfosses, A., Shkumatov, A. V., Wu, X., Bracke, N., Verstraete, K., et al. (2011). Extracellular complexes of the hematopoietic human and mouse CSF-1 receptor are driven by common assembly principles. Structure 19, 1762–1772. doi: 10.1016/j.str.2011.10.012
Endres, N. F., Das, R., Smith, A. W., Arkhipov, A., Kovacs, E., Huang, Y., et al. (2013). Conformational coupling across the plasma membrane in activation of the EGF receptor. Cell 152, 543–556. doi: 10.1016/j.cell.2012.12.032
Felix, J., De Munck, S., Verstraete, K., Meuris, L., Callewaert, N., Elegheert, J., et al. (2015). Structure and assembly mechanism of the signaling complex mediated by human CSF-1. Structure 23, 1621–1631. doi: 10.1016/j.str.2015.06.019
Felix, J., Elegheert, J., Gutsche, I., Alexander Shkumatov, V., Wen, Y., Bracke, N., et al. (2013). Human IL-34 and CSF-1 establish structurally similar extracellular assemblies with their common hematopoietic receptor. Structure 21, 528–539. doi: 10.1016/j.str.2013.01.018
Ferguson, K. M., Berger, M. B., Mendrola, J. M., Cho, H. S., Leahy, D. J., and Lemmon, M. A. (2003). EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization. Mol. Cell 11, 507–517. doi: 10.1016/S1097-2765(03)00047-9
Freal, A., Rai, D., Tas, R. P., Pan, X., Katrukha, E. A., van de Willige, D., et al. (2019). Feedback-driven assembly of the axon initial segment. Neuron 104, 305–321 e8. doi: 10.1016/j.neuron.2019.07.029
Ge, J., Elferich, J., Goehring, A., Zhao, H., Schuck, P., and Gouaux, E. (2018). Structure of mouse protocadherin 15 of the stereocilia tip link in complex with LHFPL5. Elife 7:34. doi: 10.7554/eLife.38770.034
Goodman, K. M., Yamagata, M., Jin, X., Mannepalli, S., Katsamba, P. S., Ahlsen, G., et al. (2016). Molecular basis of sidekick-mediated cell-cell adhesion and specificity. Elife 5:21. doi: 10.7554/eLife.19058.021
Groves, J. T., and Kuriyan, J. (2010). Molecular mechanisms in signal transduction at the membrane. Nat. Struct. Mol. Biol. 17, 659–665. doi: 10.1038/nsmb.1844
Hansen, G., Hercus, T. R., McClure, B. J., Stomski, F. C., Dottore, M., Powell, J., et al. (2008). The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation. Cell 134, 496–507. doi: 10.1016/j.cell.2008.05.053
Harrison, O. J., Jin, X., Hong, S., Bahna, F., Ahlsen, G., Brasch, J., et al. (2011). The extracellular architecture of adherens junctions revealed by crystal structures of type I cadherins. Structure 19, 244–256. doi: 10.1016/j.str.2010.11.016
Hassel, B., Rathjen, F. G., and Volkmer, H. (1997). Organization of the neurofascin gene and analysis of developmentally regulated alternative splicing. J. Biol. Chem. 272, 28742–28749. doi: 10.1074/jbc.272.45.28742
He, Y. N., Jensen, G. J., and Bjorkman, P. J. (2009). Cryo-electron tomography of homophilic adhesion mediated by the neural cell adhesion molecule L1. Structure 17, 460–471. doi: 10.1016/j.str.2009.01.009
Ho, C. C. M., Chhabra, A., Starkl, P., Schnorr, P. J., Wilmes, S., Moraga, I., et al. (2017). Decoupling the functional pleiotropy of stem cell factor by tuning c-Kit signaling. Cell 168, 1041–1052 e18. doi: 10.1016/j.cell.2017.02.011
Honig, B., and Shapiro, L. (2020). Adhesion protein structure, molecular affinities, and principles of cell-cell recognition. Cell 181, 520–535. doi: 10.1016/j.cell.2020.04.010
Honigmann, A., and Pralle, A. (2016). Compartmentalization of the cell membrane. J. Mol. Biol. 428, 4739–4748. doi: 10.1016/j.jmb.2016.09.022
Hubbard, S. R., and Till, J. H. (2000). Protein tyrosine kinase structure and function. Ann. Rev. Biochem. 69, 373–398. doi: 10.1146/annurev.biochem.69.1.373
Ispolatov, I., Yuryev, A., Mazo, I., and Maslov, S. (2005). Binding properties and evolution of homodimers in protein-protein interaction networks. Nucl. Acids Res. 33, 3629–3635. doi: 10.1093/nar/gki678
Jacobson, K., Liu, P., and Lagerholm, B. C. (2019). The lateral organization and mobility of plasma membrane components. Cell 177, 806–819. doi: 10.1016/j.cell.2019.04.018
Janssen, B. J., Robinson, R. A., Perez-Branguli, F., Bell, C. H., Mitchell, K. J., Siebold, C., et al. (2010). Structural basis of semaphorin-plexin signalling. Nature 467, 1118–1122. doi: 10.1038/nature09468
Januliene, D., Andersen, J. L., Nielsen, J. A., Quistgaard, E. M., Hansen, M., Strandbygaard, D., et al. (2017a). Acidic environment induces dimerization and ligand binding site collapse in the Vps10p domain of sortilin. Structure 25, 1809–1819 e3. doi: 10.1016/j.str.2017.09.015
Januliene, D., Manavalan, A., Ovesen, P. L., Pedersen, K. M., Thirup, S., Nykjaer, A., et al. (2017b). Hidden twins: SorCS neuroreceptors form stable dimers. J. Mol. Biol. 429, 2907–2917. doi: 10.1016/j.jmb.2017.08.006
Jung, H., Robison, A. D., and Cremer, P. S. (2009). Multivalent ligand-receptor binding on supported lipid bilayers. J. Struct. Biol. 168, 90–94. doi: 10.1016/j.jsb.2009.05.010
Kaplan, M., Narasimhan, S., de Heus, C., Mance, D., van Doorn, S., Houben, K., et al. (2016). EGFR dynamics change during activation in native membranes as revealed by NMR. Cell 167, 1241–1251 e11. doi: 10.1016/j.cell.2016.10.038
Kent, D. (2020). There and back again: a cytokine receptor's tail. Hemasphere 4, e349–e349. doi: 10.1097/HS9.0000000000000349
Khan, K., Valeri, N., Dearman, C., Rao, S., Watkins, D., Starling, N., et al. (2019). Targeting EGFR pathway in metastatic colorectal cancer- tumour heterogeniety and convergent evolution. Crit. Rev. Oncol. Hematol. 143, 153–163. doi: 10.1016/j.critrevonc.2019.09.001
Kholodenko, B. N., Hancock, J. F., and Kolch, W. (2010). Signalling ballet in space and time. Nat. Rev. Mol. Cell Biol. 11, 414–426. doi: 10.1038/nrm2901
Kholodenko, B. N., Hoek, J. B., and Westerhoff, H. V. (2000). Why cytoplasmic signalling proteins should be recruited to cell membranes. Trends Cell Biol. 10, 173–178. doi: 10.1016/S0962-8924(00)01741-4
Kiefel, H., Pfeifer, M., Bondong, S., Hazin, J., and Altevogt, P. (2011). Linking L1CAM-mediated signaling to NF-kappaB activation. Trends Mol. Med. 17, 178–187. doi: 10.1016/j.molmed.2010.11.005
Kong, Y., Janssen, B. J., Malinauskas, T., Vangoor, V. R., Coles, C. H., Kaufmann, R., et al. (2016). Structural basis for plexin activation and regulation. Neuron 91, 548–560. doi: 10.1016/j.neuron.2016.06.018
Kriebel, M., Wuchter, J., Trinks, S., and Volkmer, H. (2012). Neurofascin: a switch between neuronal plasticity and stability. Int. J. Biochem. Cell Biol. 44, 694–697. doi: 10.1016/j.biocel.2012.01.012
Kuo, Y. C., Chen, H., Shang, G., Uchikawa, E., Tian, H., Bai, X. C., et al. (2020). Cryo-EM structure of the PlexinC1/A39R complex reveals inter-domain interactions critical for ligand-induced activation. Nat. Commun. 11:1953. doi: 10.1038/s41467-020-15862-0
LaPorte, S. L., Juo, Z. S., Vaclavikova, J., Colf, L. A., Qi, X., Heller, N. M., et al. (2008). Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell 132, 259–272. doi: 10.1016/j.cell.2007.12.030
Large, D. E., Soucy, J. R., Hebert, J., and Auguste, D. T. (2019). Advances in receptor-mediated, tumor-targeted drug delivery. Adv. Therapeutics 2:91. doi: 10.1002/adtp.201800091
Leloup, N., Chataigner, L. M. P., and Janssen, B. J. C. (2018). Structural insights into SorCS2-Nerve Growth Factor complex formation. Nat. Commun. 9:2979. doi: 10.1038/s41467-018-05405-z
Leloup, N., Lossl, P., Meijer, D. H., Brennich, M., Heck, A. J. R., Thies-Weesie, D. M. E, et al. (2017). Low pH-induced conformational change and dimerization of sortilin triggers endocytosed ligand release. Nat. Commun. 8:1708. doi: 10.1038/s41467-017-01485-5
Lemmon, M. A., Pinchasi, D., Zhou, M., Lax, I., and Schlessinger, J. (1997). Kit receptor dimerization is driven by bivalent binding of stem cell factor. J. Biol. Chem. 272, 6311–6317. doi: 10.1074/jbc.272.10.6311
Lemmon, M. A., and Schlessinger, J. (2010). Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134. doi: 10.1016/j.cell.2010.06.011
Liu, H., Chen, X., Focia, P. J., and He, X. (2007). Structural basis for stem cell factor-KIT signaling and activation of class III receptor tyrosine kinases. EMBO J. 26, 891–901. doi: 10.1038/sj.emboj.7601545
Liu, H., Focia, P. J., and He, X. (2011). Homophilic adhesion mechanism of neurofascin, a member of the L1 family of neural cell adhesion molecules. J. Biol. Chem. 286, 797–805. doi: 10.1074/jbc.M110.180281
Liu, H., Juo, Z. S., Shim, A. H., Focia, P. J., Chen, X., Garcia, K. C., et al. (2010). Structural basis of semaphorin-plexin recognition and viral mimicry from Sema7A and A39R complexes with PlexinC1. Cell 142, 749–761. doi: 10.1016/j.cell.2010.07.040
Low-Nam, S. T., Lidke, K. A., Cutler, P. J., Roovers, R. C., van Bergen en Henegouwen, P. M., Wilson, B. S., et al. (2011). ErbB1 dimerization is promoted by domain co-confinement and stabilized by ligand binding. Nat. Struct. Mol. Biol. 18, 1244–1249. doi: 10.1038/nsmb.2135
Lu, C., Mi, L. Z., Grey, M. J., Zhu, J., Graef, E., Yokoyama, S., et al. (2010). Structural evidence for loose linkage between ligand binding and kinase activation in the epidermal growth factor receptor. Mol. Cell. Biol. 30, 5432–5443. doi: 10.1128/MCB.00742-10
Markovic-Mueller, S., Stuttfeld, E., Asthana, M., Weinert, T., Bliven, S., Goldie, K. N., et al. (2017). Structure of the full-length VEGFR- and 1 extracellular domain in complex with VEGF-A. Structure 25, 341–352. doi: 10.1016/j.str.2016.12.012
Meijers, R., Puettmann-Holgado, R., Skiniotis, G., Liu, J. H., Walz, T., Wang, J. H., et al. (2007). Structural basis of Dscam isoform specificity. Nature 449, 487–491. doi: 10.1038/nature06147
Milstein, O., Tseng, S. Y., Starr, T., Llodra, J., Nans, A., Liu, M., et al. (2008). Nanoscale increases in CD2-CD48-mediated intermembrane spacing decrease adhesion and reorganize the immunological synapse. J. Biol. Chem. 283, 34414–34422. doi: 10.1074/jbc.M804756200
Mitra, S., Ring, A. M., Amarnath, S., Spangler, J. B., Li, P., Ju, W., et al. (2015). Interleukin-2 activity can be fine tuned with engineered receptor signaling clamps. Immunity 42, 826–838. doi: 10.1016/j.immuni.2015.04.018
Moraga, I., Wernig, G., Wilmes, S., Gryshkova, V., Richter, C. P., Hong, W. J., et al. (2015). Tuning cytokine receptor signaling by re-orienting dimer geometry with surrogate ligands. Cell 160, 1196–1208. doi: 10.1016/j.cell.2015.02.011
Moremen, K. W., Tiemeyer, M., and Nairn, A. V. (2012). Vertebrate protein glycosylation: diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 13, 448–462. doi: 10.1038/nrm3383
Morris, R., Kershaw, N. J., and Babon, J. J. (2018). The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 27, 1984–2009. doi: 10.1002/pro.3519
Munck Petersen, C., Nielsen, M. S., Jacobsen, C., Tauris, J., Jacobsen, L., Gliemann, J., et al. (1999). Propeptide cleavage conditions sortilin/neurotensin receptor-3 for ligand binding. EMBO J. 18, 595–604. doi: 10.1093/emboj/18.3.595
Myllykoski, M., Eichel, M. A., Jung, R. B., Kelm, S., Werner, H. B., and Kursula, P. (2018). High-affinity heterotetramer formation between the large myelin-associated glycoprotein and the dynein light chain DYNLL1. J. Neurochem. 147, 764–783. doi: 10.1111/jnc.14598
Nogi, T., Yasui, N., Mihara, E., Matsunaga, Y., Noda, M., Yamashita, N., et al. (2010). Structural basis for semaphorin signalling through the plexin receptor. Nature 467, 1123–1127. doi: 10.1038/nature09473
Opatowsky, Y., Lax, I., Tome, F., Bleichert, F., Unger, V. M., and Schlessinger, J. (2014). Structure, domain organization, and different conformational states of stem cell factor-induced intact KIT dimers. Proc. Natl. Acad. Sci. U. S. A. 111, 1772–1777. doi: 10.1073/pnas.1323254111
Pronker, M. F., Lemstra, S., Snijder, J., Heck, A. J., Thies-Weesie, D. M., Pasterkamp, R. J., et al. (2016). Structural basis of myelin-associated glycoprotein adhesion and signalling. Nat. Commun. 7:13584. doi: 10.1038/ncomms13584
Quarles, R. H. (2007). Myelin-associated glycoprotein (MAG): past, present and beyond. J. Neurochem. 100, 1431–1448. doi: 10.1111/j.1471-4159.2006.04319.x
Reshetnyak, A. V., Opatowsky, Y., Boggon, T. J., Folta-Stogniew, E., Tome, F., Lax, I., et al. (2015). The strength and cooperativity of KIT ectodomain contacts determine normal ligand-dependent stimulation or oncogenic activation in cancer. Mol. Cell 57, 191–201. doi: 10.1016/j.molcel.2014.11.021
Riedle, S., Kiefel, H., Gast, D., Bondong, S., Wolterink, S., Gutwein, P., et al. (2009). Nuclear translocation and signalling of L1-CAM in human carcinoma cells requires ADAM10 and presenilin/gamma-secretase activity. Biochem. J. 420, 391–402. doi: 10.1042/BJ20081625
Rozycki, B., Lipowsky, R., and Weikl, T. R. (2010). Segregation of receptor-ligand complexes in cell adhesion zones: phase diagrams and the role of thermal membrane roughness. New J. Phys. 12:3. doi: 10.1088/1367-2630/12/9/095003
Sachse, S. M., Lievens, S., Ribeiro, L. F., Dascenco, D., Masschaele, D., Horre, K., et al. (2019). Nuclear import of the DSCAM-cytoplasmic domain drives signaling capable of inhibiting synapse formation. EMBO J. 38:669. doi: 10.15252/embj.201899669
Sawaya, M. R., Wojtowicz, W. M., Andre, I., Qian, B., Wu, W., Baker, D., et al. (2008). A double S shape provides the structural basis for the extraordinary binding specificity of Dscam isoforms. Cell 134, 1007–1018. doi: 10.1016/j.cell.2008.07.042
Schettini, F., Pascual, T., Conte, B., Chic, N., Braso-Maristany, F., Galvan, P., et al. (2020). HER2-enriched subtype and pathological complete response in HER2-positive breast cancer: a systematic review and meta-analysis. Cancer Treat. Rev. 84:101965. doi: 10.1016/j.ctrv.2020.101965
Schmick, M., and Bastiaens, P. I. H. (2014). The interdependence of membrane shape and cellular signal processing. Cell 156, 1132–1138. doi: 10.1016/j.cell.2014.02.007
Schurmann, G., Haspel, J., Grumet, M., and Erickson, H. P. (2001). Cell adhesion molecule L1 in folded (Horseshoe) and extended conformations. Mol. Biol. Cell 12, 1765–1773. doi: 10.1091/mbc.12.6.1765
Seiradake, E., Harlos, K., Sutton, G., Aricescu, A. R., and Jones, E. Y. (2010). An extracellular steric seeding mechanism for Eph-ephrin signaling platform assembly. Nat. Struct. Mol. Biol. 17, 398–402. doi: 10.1038/nsmb.1782
Shi, Z., Graber, Z. T., Baumgart, T., Stone, H. A., and Cohen, A. E. (2018). Cell membranes resist flow. Cell 175, 1769–1779 e13. doi: 10.1016/j.cell.2018.09.054
Shurer, C. R., Kuo, J. C., Roberts, L. M., Gandhi, J. G., Colville, M. J., Enoki, T. A., et al. (2019). Physical principles of membrane shape regulation by the glycocalyx. Cell 177, 1757–1770 e21. doi: 10.1016/j.cell.2019.04.017
Silva, D. A., Yu, S., Ulge, U. Y., Spangler, J. B., Jude, K. M., Labao-Almeida, C., et al. (2019). De novo design of potent and selective mimics of IL-2 and IL-15. Nature 565, 186–191. doi: 10.1038/s41586-018-0830-7
Simons, K., and Gerl, M. J. (2010). Revitalizing membrane rafts: new tools and insights. Nat. Rev. Mol. Cell Biol. 11, 688–699. doi: 10.1038/nrm2977
Skiniotis, G., Boulanger, M. J., Garcia, K. C., and Walz, T. (2005). Signaling conformations of the tall cytokine receptor gp130 when in complex with IL-6 and IL-6 receptor. Nat. Struct. Mol. Biol. 12, 545–551. doi: 10.1038/nsmb941
Stauber, D. J., Debler, E. W., Horton, P. A., Smith, K. A., and Wilson, I. A. (2006). Crystal structure of the IL-2 signaling complex: paradigm for a heterotrimeric cytokine receptor. Proc. Natl. Acad. Sci. U. S. A. 103, 2788–2793. doi: 10.1073/pnas.0511161103
Tang, H., Chang, H. S., Dong, Y., Guo, L. Q., Shi, X. Y., Wu, Y. C., et al. (2018). Architecture of cell-cell adhesion mediated by sidekicks. Proc. Natl. Acad. Sci. U. S. A. 115, 9246–9251. doi: 10.1073/pnas.1801810115
Uchikawa, E., Choi, E., Shang, G., Yu, H., and Bai, X. C. (2019). Activation mechanism of the insulin receptor revealed by cryo-EM structure of the fully liganded receptor-ligand complex. Elife 8:30. doi: 10.7554/eLife.48630.030
UniProt, C. (2019). UniProt: a worldwide hub of protein knowledge. Nucl. Acids Res. 47, D506–D515. doi: 10.1093/nar/gky1049
Verstraete, K., Peelman, F., Braun, H., Lopez, J., Van Rompaey, D., Dansercoer, A., et al. (2017). Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. Nat. Commun. 8:14937. doi: 10.1038/ncomms14937
Verstraete, K., and Savvides, S. N. (2012). Extracellular assembly and activation principles of oncogenic class III receptor tyrosine kinases. Nat. Rev. Cancer 12, 753–766. doi: 10.1038/nrc3371
Verstraete, K., van Schie, L., Vyncke, L., Bloch, Y., Tavernier, J., Pauwels, E., et al. (2014). Structural basis of the proinflammatory signaling complex mediated by TSLP. Nat. Struct. Mol. Biol. 21, 375–382. doi: 10.1038/nsmb.2794
Verstraete, K., Vandriessche, G., Januar, M., Elegheert, J., Shkumatov, A. V., Desfosses, A., et al. (2011). Structural insights into the extracellular assembly of the hematopoietic Flt3 signaling complex. Blood 118, 60–68. doi: 10.1182/blood-2011-01-329532
Volkmer, H., Zacharias, U., Norenberg, U., and Rathjen, F. G. (1998). Dissection of complex molecular interactions of neurofascin with axonin-1, F11, and tenascin-R, which promote attachment and neurite formation of tectal cells. J. Cell Biol. 142, 1083–1093. doi: 10.1083/jcb.142.4.1083
Wang, D., Gou, S. Y., and Axelrod, D. (1992). Reaction rate enhancement by surface diffusion of adsorbates. Biophys. Chem. 43, 117–137. doi: 10.1016/0301-4622(92)80027-3
Wang, X., Lupardus, P., Laporte, S. L., and Garcia, K. C. (2009). Structural biology of shared cytokine receptors. Annu. Rev. Immunol. 27, 29–60. doi: 10.1146/annurev.immunol.24.021605.090616
Wang, X., Rickert, M., and Garcia, K. C. (2005). Structure of the quaternary complex of interleukin-2 with its alpha, beta, and gammac receptors. Science 310, 1159–1163. doi: 10.1126/science.1117893
Wang, Y., He, H., Srivastava, N., Vikarunnessa, S., Chen, Y. B., Jiang, J., et al. (2012). Plexins are GTPase-activating proteins for Rap and are activated by induced dimerization. Sci. Signal 5:ra6. doi: 10.1126/scisignal.2002636
Wang, Y., Pascoe, H. G., Brautigam, C. A., He, H., and Zhang, X. (2013). Structural basis for activation and non-canonical catalysis of the Rap GTPase activating protein domain of plexin. Elife 2:e01279. doi: 10.7554/eLife.01279.020
Waters, M. J., and Brooks, A. J. (2015). JAK2 activation by growth hormone and other cytokines. Biochem. J. 466, 1–11. doi: 10.1042/BJ20141293
Whitty, A. (2008). Cooperativity and biological complexity. Nat. Chem. Biol. 4, 435–439. doi: 10.1038/nchembio0808-435
Wilmes, S., Hafer, M., Vuorio, J., Tucker, J. A., Winkelmann, H., Lochte, S., et al. (2020). Mechanism of homodimeric cytokine receptor activation and dysregulation by oncogenic mutations. Science 367, 643–652. doi: 10.1126/science.aaw3242
Wright, G. J. (2009). Signal initiation in biological systems: the properties and detection of transient extracellular protein interactions. Mol. Biosyst. 5, 1405–1412. doi: 10.1039/b903580j
Wu, Y., Vendome, J., Shapiro, L. A, and Ben-Shaul Honig, B. (2011). Transforming binding affinities from three dimensions to two with application to cadherin clustering. Nature 475, 510–513. doi: 10.1038/nature10183
Yamagata, M., and Sanes, J. R. (2010). Synaptic localization and function of Sidekick recognition molecules require MAGI scaffolding proteins. J. Neurosci. 30, 3579–3588. doi: 10.1523/JNEUROSCI.6319-09.2010
Yang, Y., Xie, P., Opatowsky, Y., and Schlessinger, J. (2010). Direct contacts between extracellular membrane-proximal domains are required for VEGF receptor activation and cell signaling. Proc. Natl. Acad. Sci. U. S. A. 107, 1906–1911. doi: 10.1073/pnas.0914052107
Yawata, H., Yasukawa, K., Natsuka, S., Murakami, M., Yamasaki, K., Hibi, M., et al. (1993). Structure-function analysis of human IL-6 receptor: dissociation of amino acid residues required for IL-6-binding and for IL-6 signal transduction through gp130. EMBO J. 12, 1705–1712. doi: 10.1002/j.1460-2075.1993.tb05815.x
Yuzawa, S., Opatowsky, Y., Zhang, Z., Mandiyan, V., Lax, I., and Schlessinger, J. (2007). Structural basis for activation of the receptor tyrosine kinase KIT by stem cell factor. Cell 130, 323–334. doi: 10.1016/j.cell.2007.05.055
Zanetti-Domingues, L. C., Korovesis, D., Needham, S. R., Tynan, C. J., Sagawa, S., Roberts, S. K., et al. (2018). The architecture of EGFR's basal complexes reveals autoinhibition mechanisms in dimers and oligomers. Nat. Commun. 9:4325. doi: 10.1038/s41467-018-06632-0
Keywords: structures, cell signaling, transmembrane, interactions, conformations, rearrangements
Citation: Chataigner LMP, Leloup N and Janssen BJC (2020) Structural Perspectives on Extracellular Recognition and Conformational Changes of Several Type-I Transmembrane Receptors. Front. Mol. Biosci. 7:129. doi: 10.3389/fmolb.2020.00129
Received: 13 March 2020; Accepted: 02 June 2020;
Published: 07 August 2020.
Edited by:
Filippo Prischi, University of Essex, United KingdomReviewed by:
Savvas Nicos Savvides, Ghent University, BelgiumBirthe B. Kragelund, University of Copenhagen, Denmark
Matthew Edwin Call, Walter and Eliza Hall Institute of Medical Research, Australia
Copyright © 2020 Chataigner, Leloup and Janssen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bert J. C. Janssen, Yi5qLmMuamFuc3NlbiYjeDAwMDQwO3V1Lm5s
†These authors have contributed equally to this work
 Lucas M. P. Chataigner
Lucas M. P. Chataigner Nadia Leloup
Nadia Leloup Bert J. C. Janssen
Bert J. C. Janssen