- School of Animal and Veterinary Sciences, The University of Adelaide, Roseworthy, SA, Australia
Determining the viable and non-viable load of foodborne pathogens in animal production can be useful in reducing the number of human outbreaks. In this study, we optimized a PMAxxTM-based qPCR for quantifying viable and non-viable load of Salmonella from soil collected from free range poultry environment. The optimized nucleic acid extraction method resulted in a significantly higher (P < 0.05) yield and quality of DNA from the pure culture and Salmonella inoculated soil samples. The optimized primer for the amplification of the invA gene fragment showed high target specificity and a minimum detection limit of 102 viable Salmonella from soil samples. To test the optimized PMAxxTM-based qPCR assay, soil obtained from a free range farm was inoculated with Salmonella Enteritidis or Salmonella Typhimurium, incubated at 5, 25, and 37°C over 6 weeks. The survivability of Salmonella Typhimurium was significantly higher than Salmonella Enteritidis. Both the serovars showed moisture level dependent survivability, which was significantly higher at 5°C compared with 25°C and 37°C. The PMAxxTM-based qPCR was more sensitive in quantifying the viable load compared to the culture method used in the study. Data obtained in the current study demonstrated that the optimized PMAxxTM-based qPCR is a suitable assay for quantification of a viable and non-viable load of Salmonella from poultry environment. The developed assay has applicability in poultry diagnostics for determining the load of important Salmonella serovars containing invA.
Introduction
Food safety is an important aspect of human health and, therefore; state health departments are routinely tasked to trace back foodborne pathogens for their source of origin. Zoonotic pathogens, such as Salmonella and Campylobacter, often result in human gastroenteritis after the consumption of contaminated poultry products (Kärenlampi et al., 2007; Ford et al., 2018). Due to consumer demand, there has been an increase in free range poultry production over the years. In a free-range poultry production system, birds have access to the range area outside poultry shed. Chickens are the common host for multiple serovars of Salmonella enterica, which naturally colonize their gut. Therefore, infected chickens can shed Salmonella to their environment intermittently. In a free range production system, the infected flock can contaminate range soil and result in the infection of the newly introduced flock. Therefore, it is vital to understand the viable and non-viable load of Salmonella in the poultry environment in relation to changes in temperature.
Soil type and temperature affect the survivability of Salmonella more than moisture level (Underthun et al., 2018). The final distribution of water and bacteria in the soil is influenced by soil texture, pH, temperature, and organic matter (Semenov et al., 2009). Temperature and water activity affect the survivability of Salmonella with the thermodynamic activity of water as the main factor in determining the survival. A study has shown that the incidence of Salmonella increases during warm seasons (Milazzo et al., 2016). Cross-contamination of the range area may pose a continuous threat to the health of the layer flocks. Therefore, understanding the viability of Salmonella in the soil is a crucial factor in devising strategies for its control in poultry production. In Australia, Salmonella Enteritidis is considered as an exotic pathogen in commercial poultry. Recently, there have been several Salmonella Enteritidis outbreaks on free range layer farms in Australia, which has prompted the mass culling of layer flocks. After mass culling, sheds are decontaminated, but it is often a challenge to decontaminate ranging areas.
Quantitative PCR (qPCR) can be used for quantifying pathogen load in clinical samples; however, primers targeting a specific region of the nucleic acids will also amplify DNA obtained from the non-viable load present in the population. Viability assays, such as live/dead cell viability assay, trypan blue, BacTiter-GloTM microbial cell viability assay (Promega, Australia), LIVE/DEAD BacLight bacterial viability kit (ThermoFisher Scientific, Australia) and propidium monoazide (PMA) (Banihashemi et al., 2012; Barbau-Piednoir et al., 2014; Jäger et al., 2018) or ethidium bromide monoazide (Nocker et al., 2006; Wang and Mustapha, 2010) based qPCR can be used for quantifying viable microbial load. PMA is a nucleic acid intercalating dye that is cell impermeant and therefore will diffuse only across the cell membrane of dead microbes. After binding to nucleic acids, upon photolysis, the dye covalently reacts with the microbial DNA/RNA thus inhibiting it from the amplification by PCR. Therefore, PMA-based qPCR can be a reliable assay for differentiating viable cells from non-viable population of microbes. However, given the complex nature of the environmental samples, none of them have been optimized for quantifying the viable and non-viable load of Salmonella from the environment. In the current study, PMAxxTM-based qPCR was optimized for the quantification of Salmonella from poultry environment and in the subsequent Salmonella survivability experiment, its applicability was tested. Therefore, the three main objectives of the current study were: (A) Optimize method for PMAxxTM based viability qPCR for Salmonella quantification from the soil. (B) Understand the effects of temperature on the survivability of Salmonella Typhimurium and Salmonella Enteritidis in soil. (C) Apply the optimized PMAxxTM based qPCR to quantify the viable and non-viable load of Salmonella Typhimurium and Salmonella Enteritidis from soil samples incubated at various temperatures.
Materials and Methods
Pure cultures of Salmonella Typhimurium phage type 9 or Salmonella Enteritidis (phage-type 7A obtained from Elizabeth McArthur Agriculture Institute, NSW, Australia) were streaked onto nutrient agar (NA; ThermoFisher Scientific, Australia) plates and incubated overnight at 37°C. A single colony was subcultured in Luria Bertani (LB; ThermoFisher Scientific, Australia) broth at 37°C in a shaking incubator until the OD600 was approximately 1, which was used for calculating the colony forming unit (CFU) per mL.
DNA Extraction From Pure Culture of Salmonella and Salmonella Inoculated Soil
The Salmonella DNA extraction from pure culture (n = 6) and soil (n = 6) was carried out using the QIAamp DNA Mini Kit (Qiagen, Australia) with some modifications in the protocol. Approximately 109 CFU/mL of Salmonella broth culture was centrifuged at 19,500 × g for 3 min and the pellet was resuspended in 100 μL phosphate buffered saline (PBS) into which 300 μL tissue lysis buffer (ATL) and half spoon of acids washed 106 μm beads (Sigma Aldrich, Australia) were added. The samples were homogenized (Bullet Blender, Next Advance, United States) for 2–3 min. The samples were heated at 70°C for 15 min with occasional mixing and then centrifuged for 1 min at 5,000 × g to remove the beads and undissolved materials. The supernatant was transferred to a 1.5 mL tube that contained 50 μL proteinase K (20 mg/mL). After a brief vortexing, 50 μL lysozyme (100 mg/mL) and 300 μL AL lysis buffer were added. The samples were briefly vortexed and incubated at 70°C for 15 min with occasional inversion. To avoid clump formation, the samples were vortexed at each step of adding the reagents. After the incubation, 300 μL ethanol (100%) was added and the lysates were passed through the spin column by centrifuging for 1 min at 6,000 × g and the process was repeated for the rest of the lysates. The DNA in the spin columns was washed with 500 μL of each of wash buffers I and II and eluted in 50 μL of buffer ATE as per the manufacturer’s protocol. The extracted DNA was assessed for quality and concentration in a Nanodrop 1000 and stored at −20°C for further use.
The DNA extraction method from soil was optimized based on adding 9 mL of PBS to 1 g of soil. Briefly, 1 g of soil was weighed in 15 mL tubes and inoculated with 109 CFU/mL of Salmonella and the samples were mixed thoroughly. The tubes were centrifuged at 900 × g for 1 min to settle down the undissolved materials. The maximum supernatants were transferred to 15 mL tubes and centrifuged at full speed (4500 × g) for 15 min at 4°C. The bacterial pellet layer was gently resuspended in 100 μL of PBS and transferred to 1.5 mL tubes. The DNA was extracted as described previously. In a separate pilot study, to test the efficiency of the bacterial separation method from soil, the bacterial pellet was resuspended in PBS, serially diluted, plated onto xylose-lysine deoxycholate (XLD; ThermoFisher Scientific, Australia) agar media and incubated overnight at 37°C.
Optimization of PMAxxTM Step for Distinguishing Between Viable and Non-viable Load
Salmonella culture in the LB broth was prepared as described previously. The broth culture samples were prepared as viable and non-viable Salmonella. For the non-viable culture preparation, Salmonella was exposed to 95°C for 5 min in a dry heat block. To confirm the non-viable Salmonella, 100 μL of the culture was plated onto XLD and incubated overnight at 37°C. For determining the optimum concentration of PMAxxTM dye (Biotium, United States), 10 μL of the dye solution (2.5, 5, and 10 mM) was added into 1 mL of the Salmonella culture in LB broth (n = 6) or left as a control (n = 6). The PMAxxTM treated samples were incubated in dark for 10 min on a rocker. After the incubation, the samples were exposed to light (500 volts) at a distance of 20 cm for 15 min with intermittent inversion. The samples were centrifuged at 19500 × g for 3 min, the bacterial pellets were suspended in 100 μL PBS and the DNA was extracted as described previously.
Optimization of Primers and Construction of Standard Curve
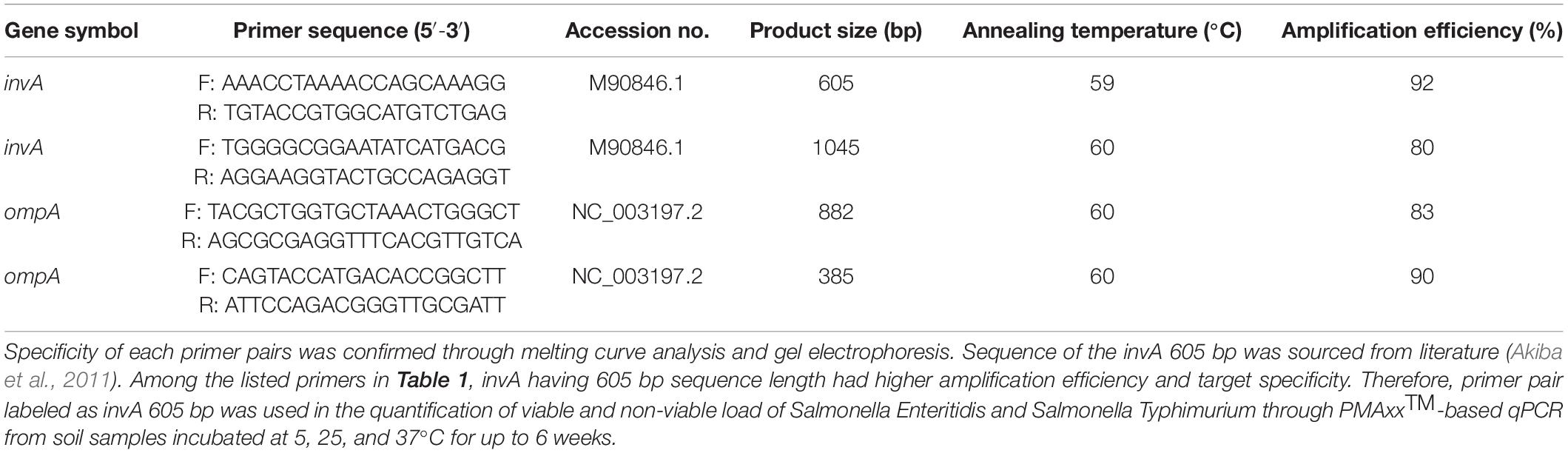
Primers were either designed using the NCBI primer software or sourced from literature. Primers were evaluated for their secondary structure characteristics in web based NetPrimer and Beacon Designer software. Short sequence primers (<300 bp) were avoided to reduce the chances of amplifying genomic DNA obtained from non-viable bacterial population in samples treated with PMAxxTM. To broaden the scope of this study for detection of a range of Salmonella enterica serovars, primer amplifying the fragment of invasion A gene (invA; Table 1) was used for the PMAxxTM-based qPCR. To determine the specificity and amplification efficiency of the invA primer, qPCR was performed on 10-fold serial dilutions (108 to 101) of Salmonella DNA extracted from a pure culture (n = 3 and repeated twice). To determine the minimum detection limit of the invA primer, qPCR was performed on DNA extracted pure culture and from spiked soil samples (n = 3 and repeated twice) with different dilutions of Salmonella (108 to 101). The qPCR products were assessed by melting curve analysis and then visualized on 2% gel electrophoresis to assess the specificity of the primer pair. For the quantification of Salmonella from soil samples, a standard curve was generated from a 10-fold serial dilutions (10–2 to 10–12) of purified qPCR product (using the QIAquick PCR Purification Kit) of invA fragment and used as a reference to determine the load of Salmonella. Primer pairs for amplifying ompA gene were also evaluated for specificity through qPCR conducted on DNA obtained from pure cultures of Salmonella Typhimurium and Escherichia coli (Table 1).
Quantitative PCR
The qPCR was performed with the SensiFAST SYBR Hi-ROX Kit (Bioline, Australia) following the manufacturer’s protocol. The master mix was prepared and applied to reaction wells through Corbett CAS1200 robot (Corbett Life Science, Australia). The final reaction volume of 20 μL contained 10 μL SensiFAST SYBR Hi-Rox mix, 1 μL of each of the forward and reverse primers (10 μM concentration), 6 μL RNase-free water and 2 μL of DNA template. The reaction was performed in duplicates in a Rotor-Gene Disc 100 (Qiagen, Australia) with a Rotor-Gene 6000 Thermocycler (Corbett Life Science, Australia). Each run contained no-template control for ruling out external contaminations. The three-step cycling conditions included an initial denaturation at 95°C for 3 min, then 40 cycles of denaturation at 95°C for 5 s and annealing and extension at 59°C (Table 1) and 72°C for 20 and 30 s, respectively. During qPCR cycles, the fluorescence detection was conducted at the end of each annealing step, and a melting curve analysis step (at a ramp from 55 to 95°C) was included to assess the specificity of the amplification.
Preparation of Soil Samples
Soil from a range area in a free-range production system was collected for assessing the survivability of Salmonella Typhimurium and Salmonella Enteritidis. To mimic the field conditions, the samples were inoculated with 5 × 109 CFU per 100 g of soil with moisture adjusted to around 15%. The moisture level was adjusted by adding autoclaved water into the soil sample containers and measuring the moisture content with a basic Moisture Analyser Model MJ33 (Mettler Toledo, Switzerland). Each treatment group contained three biological replicates and the experiment was repeated twice to confirm the applicability of the optimized method. The Salmonella inoculated soil samples were incubated at 5, 25, and 37°C for 6 weeks and the samples were processed at weekly intervals for quantifying the bacterial load through culture method and PMAxxTM-based qPCR.
The moisture content and water activity of the individual soil samples were measured at each sampling time-point. Moisture content is a measure of the total amount of water in a sample. A basic moisture analyzer that consists of both weighing and heating and is used to determine the moisture content of a sample with the loss on drying principle. For measuring the moisture content, approximately 8 g of soil was spread onto a sample pan and measured by a Basic Moisture Analyzer (Model MJ33, Mettler Toledo, Switzerland) following the below formula:
The values were recorded as loss in total moisture content.
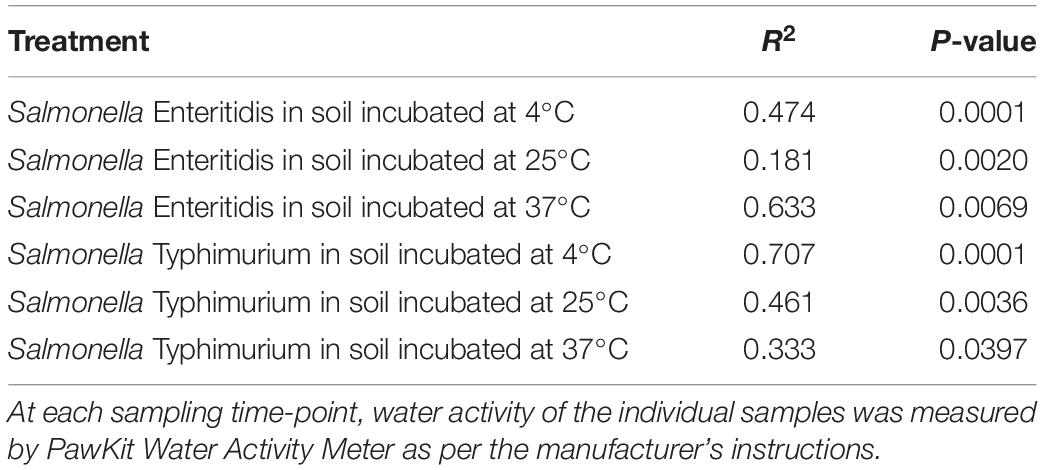
Water activity (aw) is a thermodynamic measurement of the energy of water in a sample. The value indicates how tightly water is bound, structurally or chemically, within a substance. The lower a sample’s water activity, the more tightly bound that water is within the sample. It is measured using a water activity meter (range: 0.00–1.00 aw) that assesses the partial vapor pressure of water in a sample and divides it by the standard state partial vapor pressure of water. To measure the water activity, approximately 3 g of soil was dispensed into a sample cup and measured by PawKit Water Activity Meter (Model P06760; AquaLab, Decagon Devices, United States).
Quantification of Salmonella by Culture Method and PMAxxTM-Based qPCR
In order to understand the applicability of the optimized PMAxxTM-based qPCR in the field conditions, soil samples inoculated with Salmonella Enteritidis or Salmonella Typhimurium, and incubated at different temperatures (5, 25, and 37°C) were processed at weekly intervals for the quantification of Salmonella load through culture method and qPCR. The culture data (obtained by direct plating) were presented as log10 CFU/g, while the PMAxxTM-based qPCR data were presented as log10 Salmonella load for viable and non-viable counts per gram of soil samples.
At each sampling time-point, 1 g of the soil samples was mixed into 9 mL of buffered peptone water (BPW; ThermoFisher Scientific, Australia), serially diluted, plated onto XLD agar media and incubated overnight at 37°C. The Salmonella count was expressed as log10 CFU/g of soil sample. A sub-set of the samples was processed using an enrichment method. For the enrichment method, the BPW samples were incubated overnight at 37°C and a 100 μL was added into 9 mL of Rappaport-Vassiliadis soy peptone broth (RVS; ThermoFisher Scientific, Australia). The RVS samples were incubated overnight at 42°C to allow the selective growth of Salmonella. The incubated RVS samples were streaked on XLD plates for the confirmation of Salmonella in the soil samples. Positive Salmonella samples were scored as 1 and negative as 0 for the determination of the proportion of positive samples.
At each sampling time-point, 1 g of soil from individual samples was weighed into 15 mL tubes containing 9 mL PBS for DNA extraction and PMAxxTM-based qPCR. The soil samples were centrifuged at 900 × g for 1 min at 4°C to remove the undissolved materials. The supernatants were centrifuged at 4500 × g for 15 min at 4°C and the bacterial pellets were resuspended in 1 mL PBS. The samples were treated with PMAxxTM or left as control and processed for DNA extraction and qPCR as described previously. A standard curve was used to quantify the DNA copy number from the cycle quantitation (Cq) values. The qPCR data (log10 DNA copy number) were expressed as viable and non-viable load of Salmonella per gram of soil samples.
Statistical Analysis
Data for Salmonella CFU/g, the proportion of positive samples and viable and non-viable load/g were analyzed in StatView version 5.0.1.0 by taking Salmonella treatment and sampling time-point as main effects. Level of significance was determined at protected least significant difference (PLSD) < 0.05. Mean values ± SD were visualized in GraphPad Prism 8.
Results
Optimization of PMAxxTM-Based qPCR for the Quantification of Viable and Non-viable Load of Salmonella
The optimized method for DNA extraction from pure culture resulted in significantly (P < 0.05) higher yield and pure genomic DNA of Salmonella. Tested in Nanodrop-1000, 1 × 109 CFU/mL of Salmonella broth yielded an average of 101.38 ng/μL DNA with 260/280 and 260/230 ratios of 2.10 and 2.32, respectively (Figure 1). Primer optimization steps showed that primers for the ompA gene were non-specific and amplified the genomic DNA of Escherichia coli (data not provided). Long size sequence primers (∼1045 bp) designed for the amplification of the invA gene were less efficient (80%) compared to the medium size primers (∼605 bp). Therefore, the primers that amplified the 605 bp region of invA were used in the subsequent PMAxxTM -based qPCR for quantifying viable and non-viable load of Salmonella Enteritidis and Salmonella Typhimurium from soil samples incubated at 5, 25, and 37°C for up to 6 weeks. Culture count data obtained from plating the isolated Salmonella from the inoculated soil showed no significant effect of the background materials. These data (4 × 108 CFU/mL) compared well with the dose (109 CFU/mL) used for 1 g of soil inoculation.
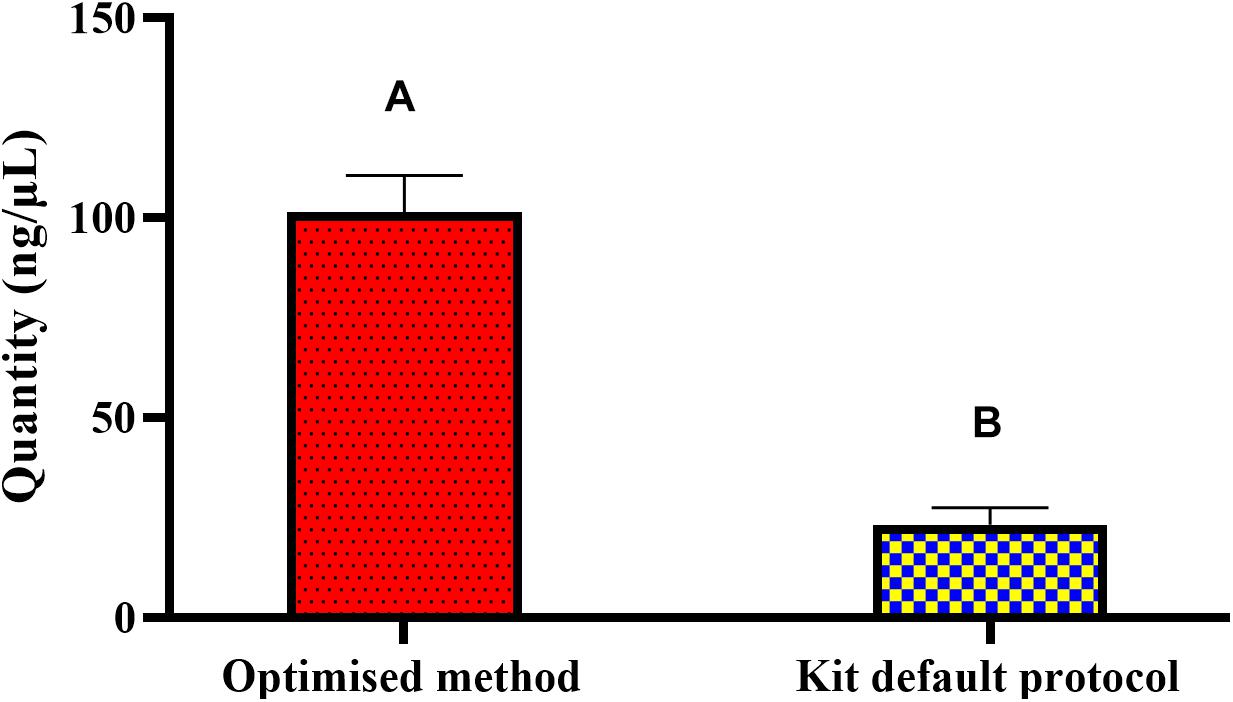
Figure 1. DNA quantity(ng/μL) obtained through the optimized and the standard kit protocol methods from pure culture of Salmonella. The optimized extraction method resulted in significantly higher yield of DNA. Different superscripts (A,B) across the bars show significant difference.
The qPCR results showed that the amplification efficiency of the invA primers in the 10-fold serial dilutions of DNA extracted from Salmonella was 92% with R2 value as 0.99 (Figure 2A). The melting curve analysis of the amplicon showed a single peak, thereby confirming the specificity of the invA primers in the DNA obtained from Salmonella spiked soil samples (Figure 2B). The gel results confirmed that the minimum detection limit of invA in pure culture and soil samples spiked with 10-fold serial dilutions of viable Salmonella subsequently treated with PMAxxTM was 101 and 102 Salmonella cells, respectively (data not shown).
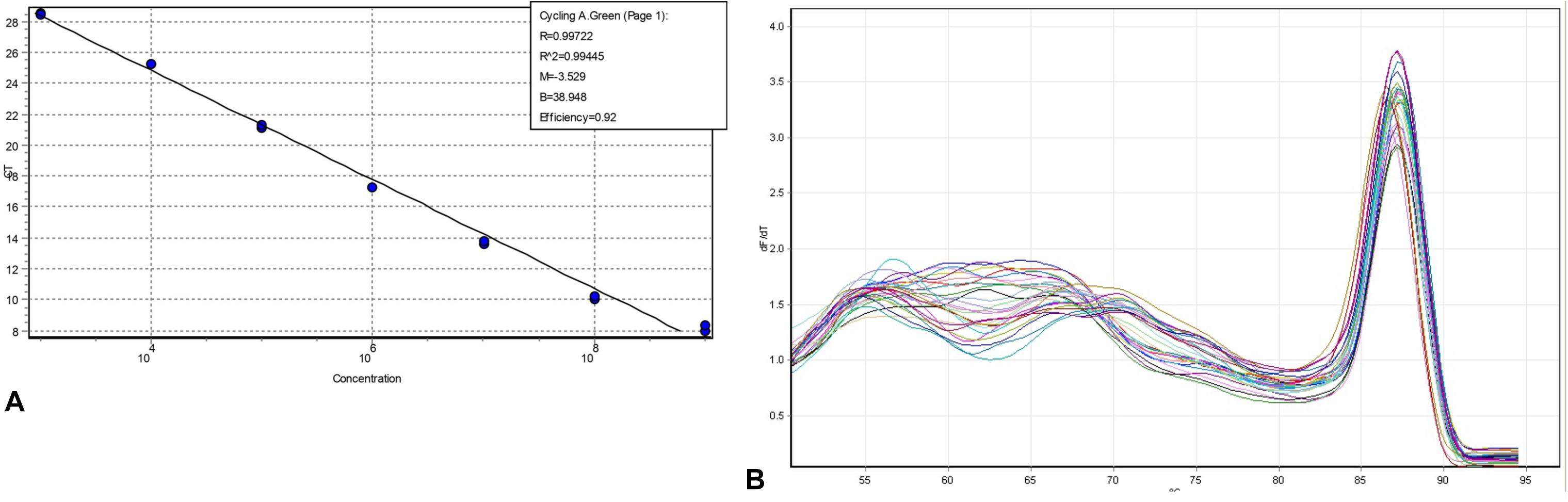
Figure 2. Amplification efficiency and melting curve analyses of invA determined in 10-fold serial dilutions of DNA. (A) PCR performed on Salmonella DNA extracted from a pure culture with 1 × 109 CFU/mL. (B) Melting curve of invA (605 bp) in DNA obtained from 10-fold serially diluted (108 to 101 CFU/g) Salmonella spiked soil samples.
To assess the inhibitory effects of PMAxxTM on the target DNA amplification, qPCR was performed on DNA obtained from viable and non-viable Salmonella culture treated with 3 different concentrations of PMAxxTM. The qPCR data (expressed as log10 Salmonella load) showed that the PMAxxTM concentration did not significantly (P > 0.05) inhibit the amplification of DNA obtained from the viable Salmonella (Figure 3). However, PMAxxTM concentrations significantly (P < 0.05) inhibited the amplification of DNA obtained from the non-viable culture of Salmonella (Figure 3). These data showed that 5 μL of 2.5 mM PMAxxTM per 500 μL of Salmonella culture was sufficient for use in PMAxxTM-based qPCR assay to discriminate between the viable and non-viable Salmonella load. The significantly higher Salmonella load in the control samples of the non-viable group confirmed that PMAxxTM penetrated across the cell membrane of compromised cells only.
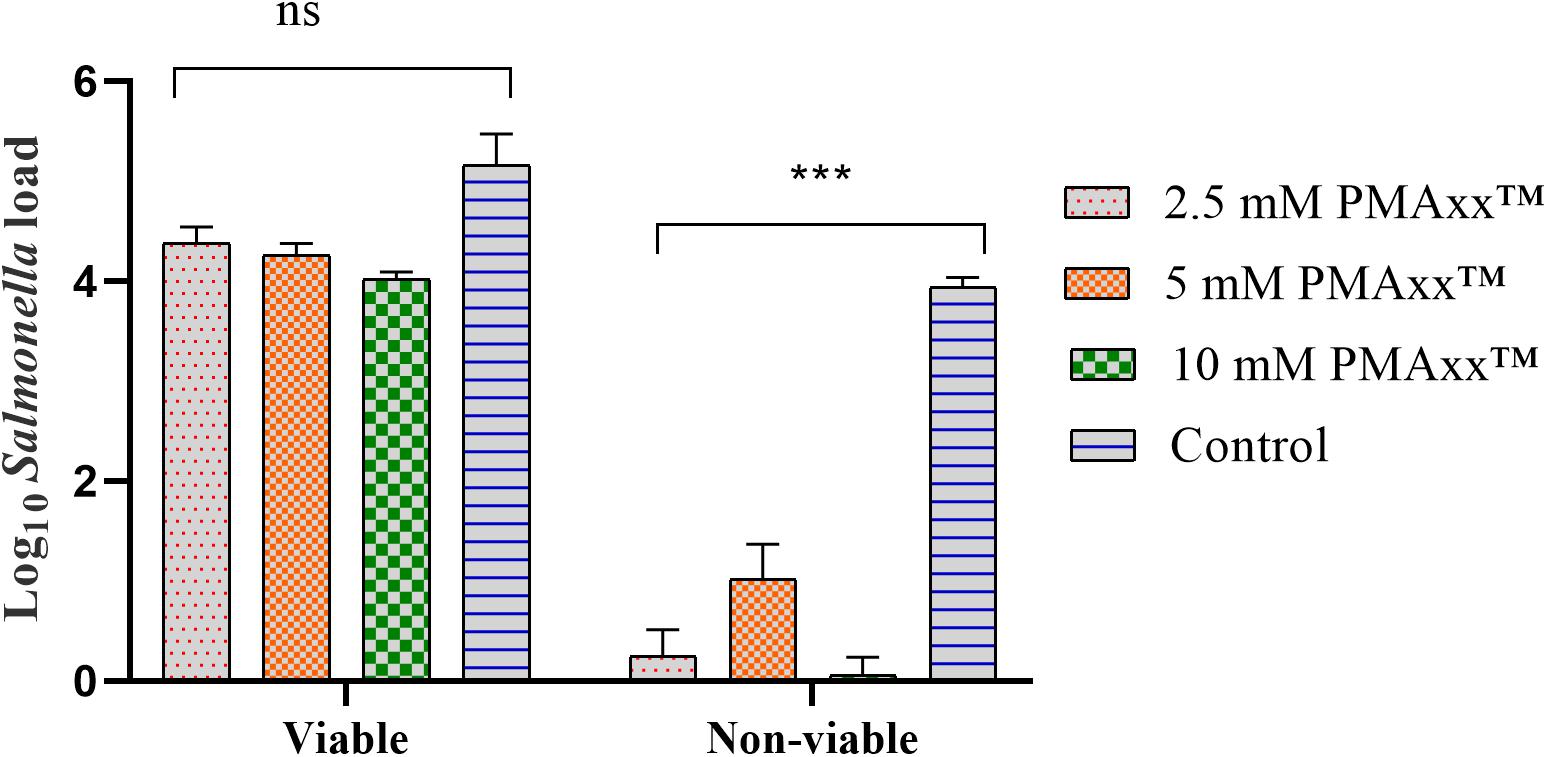
Figure 3. Effects of different concentrations (2.5, 5, and 10 mM) of PMAxxTM on the amplification of target DNA obtained from the viable and non-viable culture of Salmonella. The data showed that PMAxxTM efficiently inhibited the amplification of the target DNA obtained only from the non-viable culture of Salmonella. This trial was repeated three times and an average of the data was presented. For obtaining non-viable cells, Salmonella in LB broth was heated at 95°C for 5 min, and a 100 μL plated on XLD agar and incubated overnight at 37°C confirmed that the heat treatment was effective in killing 100% of Salmonella in the broth. Asterisks (∗∗∗) denote significant difference at P < 0.0001, while ns denotes non-significant difference.
Survivability of Salmonella Assessed by Culture Method
The culture based method showed that the overall survivability of Salmonella was significantly higher for Salmonella Typhimurium compared with Salmonella Enteritidis (Figure 4A) despite the non-significant difference in moisture level and water activity between the two treatment groups (Figures 4C,D). Samples that were negative by direct plating and processed by the enrichment method showed higher survivability for Salmonella Typhimurium strain compared with Salmonella Enteritidis strain in soil samples incubated at 25 and 37°C (Figure 4B).
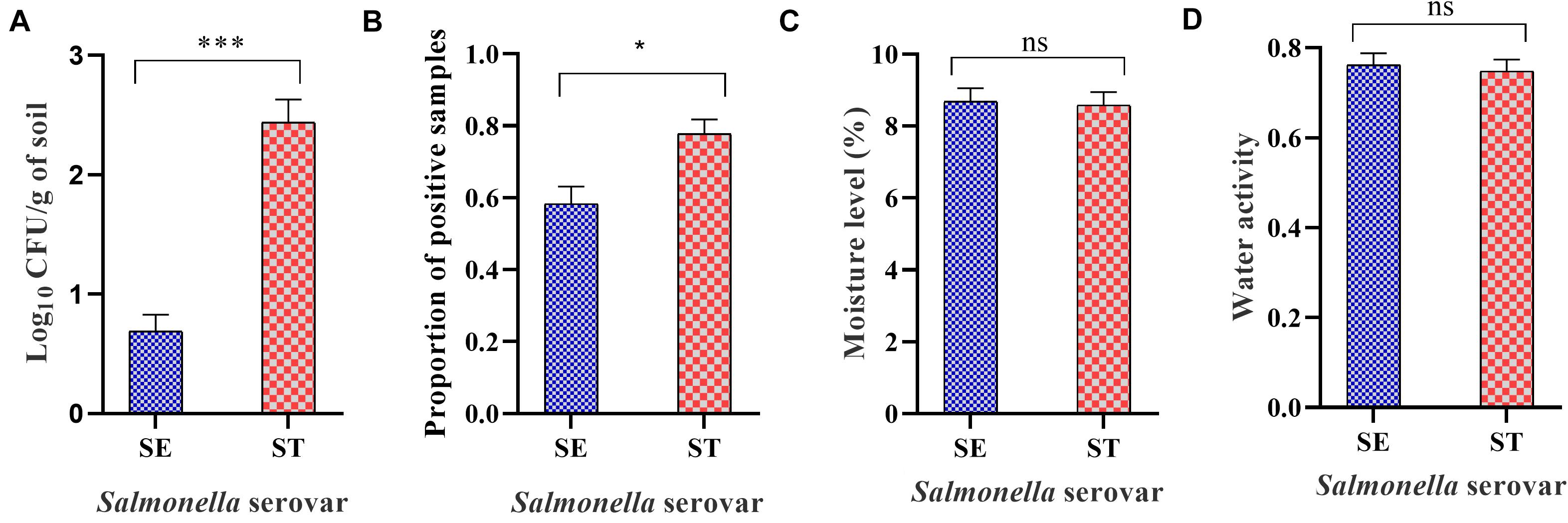
Figure 4. Overall load of Salmonella Enteritidis (SE) and Salmonella Typhimurium (ST) in soil incubated at different temperatures and sampled at weekly intervals. The samples were processed through direct plating for Salmonella load determination. Samples that turned negative through the direct plating were enriched for the qualitative assessment of Salmonella. (A) Mean log10 CFU/g of SE and ST in soil over 6-week of incubation time period. (B) Mean proportion of Salmonella positive samples of SE and ST in soil incubated at different temperatures. (C) Mean moisture level of soil inoculated with SE or ST (D) Mean water activity (aw) of soil inoculated with SE or ST. Asterisks (∗∗∗) and (∗) denote significant difference at P < 0.0001 and P < 0.01, respectively, while ns denotes non-significant difference.
The survivability of Salmonella Enteritidis and Salmonella Typhimurium (log10 CFU) in soil was significantly affected by storage temperature, moisture level and water activity (Figures 5A–D). Salmonella Typhimurium survived at a significantly higher level than Salmonella Enteritidis at 5, 25, and 37°C (Figure 5A). Throughout the experiment, the survivability of both the serovars decreased significantly, whereas Salmonella Enteritidis could not be quantified by culture method from soil samples incubated at the 25 and 37°C from week 2 onward (Figure 5A). Furthermore, both the Salmonella Enteritidis and Salmonella Typhimurium in soil samples incubated at 5°C survived better than the samples incubated at 25 and 37°C. Therefore, the data confirmed that the Salmonella Typhimurium strain had a better capability to resist to temperature and moisture driven changes compared to the Salmonella Enteritidis strain.
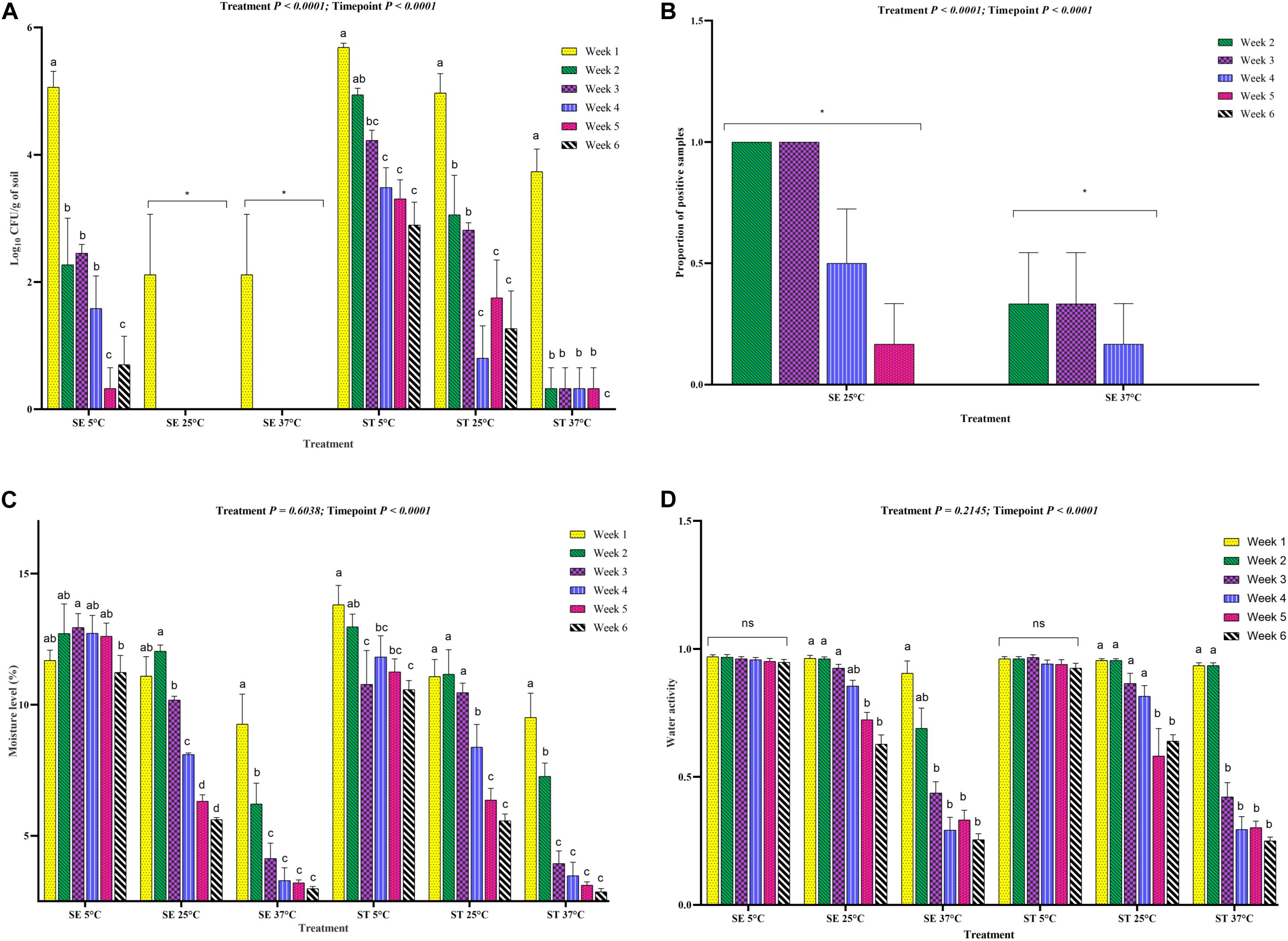
Figure 5. Load of Salmonella Enteritidis (SE) and Salmonella Typhimurium (ST) in soil incubated at three different temperatures for 6 weeks. (A) Log10 CFU/g of soil of SE and ST affected by storage temperatures (5, 25, and 37°C). (B) Proportion of positive soil samples for SE and ST determined through the enrichment method. Only, the samples that did not show any Salmonella growth after direct plating were enriched. (C) Moisture level of the Salmonella inoculated soil samples incubated at 5, 25, and 37°C. (D) Water activity of the Salmonella inoculated soil samples incubated at 5, 25, and 37°C. The Salmonella inoculated soil samples were processed for Salmonella load through culture, Salmonella detection through enrichment method and moisture and water activity determination at weekly intervals for up to 6 weeks of incubation time period. Asterisk (∗) denotes significant difference at P < 0.01, while ns denotes non-significant difference. Different superscripts (a,b,c,d) show significant difference within each treatment group at each sampling timepoint.
To determine if there was relatively low viable Salmonella in the samples with no growth after direct plating, samples were further tested by the enrichment method. The results showed that Salmonella Enteritidis was not detectable in the week 5, and 6 soil samples incubated at 37°C, while Salmonella Typhimurium was detected until week 5 of incubation (Figure 5B). At 25°C, Salmonella Typhimurium was quantified from the soil samples until week 6 of incubation (Figure 5A). Therefore, the data confirmed that the culturability was driven by water activity and moisture level in the soil with a higher loss in moisture at 25 and 37°C (Figures 5C,D).
To investigate how moisture level (%) and water activity influenced the survivability of different Salmonella serovars, regression analysis between the moisture level and log10 CFU/g of soil samples was performed. Overall, for both the Salmonella Enteritidis and Salmonella Typhimurium treatment groups at all temperatures, the log10 CFU/g decreased with a decline in the moisture level (Figures 6A–F) and water activity (Table 2). For the samples incubated at 25 and 37°C, the decline of log10 CFU/g was highly associated with the decrease in the moisture level, while lower consistency of results was recorded at 5°C. Especially for Salmonella Enteritidis samples incubated at 5°C, there was a weak correlation between the log10 CFU/g and the moisture level.
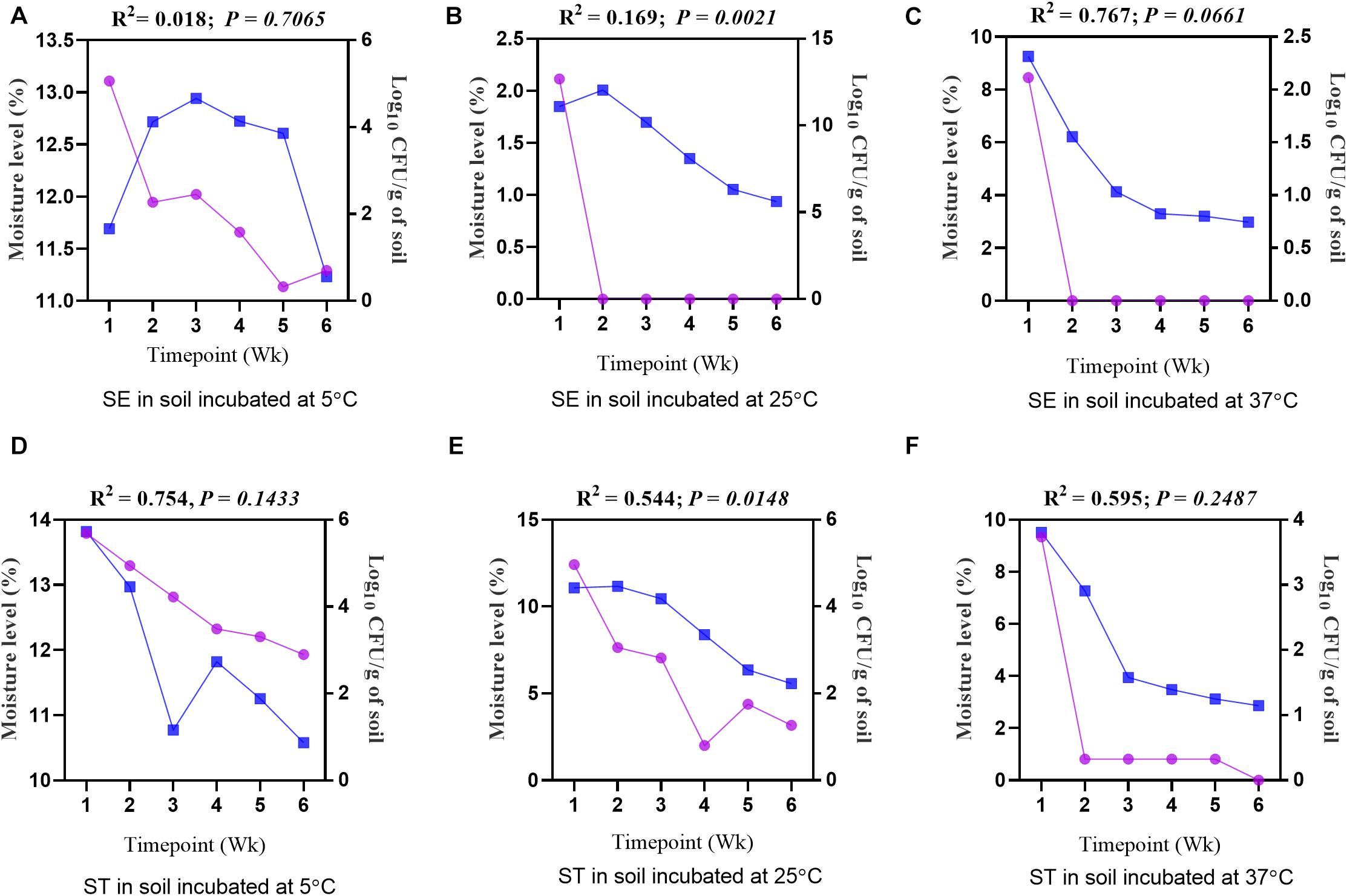
Figure 6. Regression analysis showing a positive correlation between the moisture level (%) and the load of Salmonella Enteritidis (SE) and Salmonella Typhimurium (ST) in soil incubated at 5, 25, and 37°C. (A) Soil inoculated with SE and incubated at 5°C. (B) Soil inoculated with SE and incubated at 25°C. (C) Soil inoculated with SE and incubated at 37°C. (D) Soil inoculated with ST and incubated at 5°C. (E) Soil inoculated with ST and incubated at 25°C. (F) Soil inoculated with ST and incubated at 37°C. Blue lines show moisture level (%), while purple lines show log10 CFU/g of soil.
Survivability of Salmonella Assessed by PMAxxTM -Based qPCR
The optimized PMAxxTM -based qPCR was used to assess the survivability of Salmonella serovars in soil samples incubated at three different temperatures. The overall viable and non-viable load in the Salmonella Enteritidis inoculated soil was significantly lower than Salmonella Typhimurium (Figure 7A). The viable load was significantly higher in soil samples incubated at 5°C compared to the samples incubated at 25 and 37°C (Figure 7B) due to moisture level driven changes.
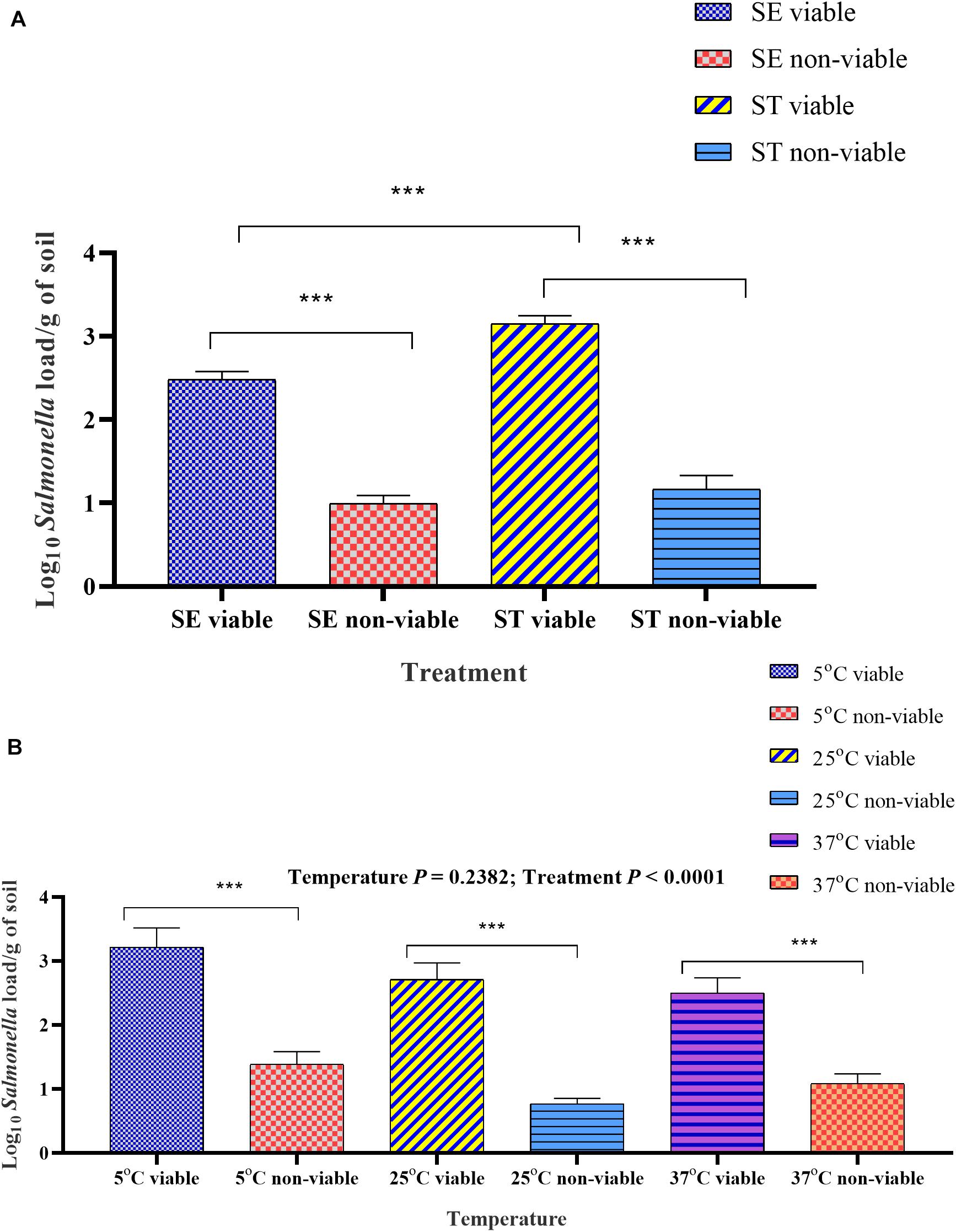
Figure 7. Overall viable and non-viable load quantified by PMAxxTM -based qPCR from Salmonella inoculated soil affected by temperature. (A) Mean log10 viable and non-viable load of Salmonella Enteritidis (SE) and Salmonella Typhimurium (ST) in soil samples incubated at 5, 25, and 37°C and processed at weekly intervals over 6-week of incubation time period. (B) Mean log10 viable and non-viable load of SE and ST affected by temperature. Samples were treated either with PMAxxTM or left as control before the extraction of DNA. The DNA copy number was calculated through the standard curve and the data were presented as log10 Salmonella load. Asterisks (∗∗∗) denote significant difference at P < 0.0001.
The PMAxxTM -based qPCR data for both the Salmonella Enteritidis and Salmonella Typhimurium treatment groups showed that the survivability of Salmonella decreased throughout incubation period (Figures 8A,B). The data at weekly intervals and incubation specific temperature showed some discrepancy in terms of higher load at week 2 compared with week 1 possibly due to the unequal distribution of Salmonella in the soil samples. The viable load of Salmonella Enteritidis and Salmonella Typhimurium in the samples incubated at 5°C was significantly higher than the samples incubated at 25 and 37°C (Figures 8A,B) due to moisture level driven changes. The viable load decreased with the increase in storage temperature and sample incubation period.
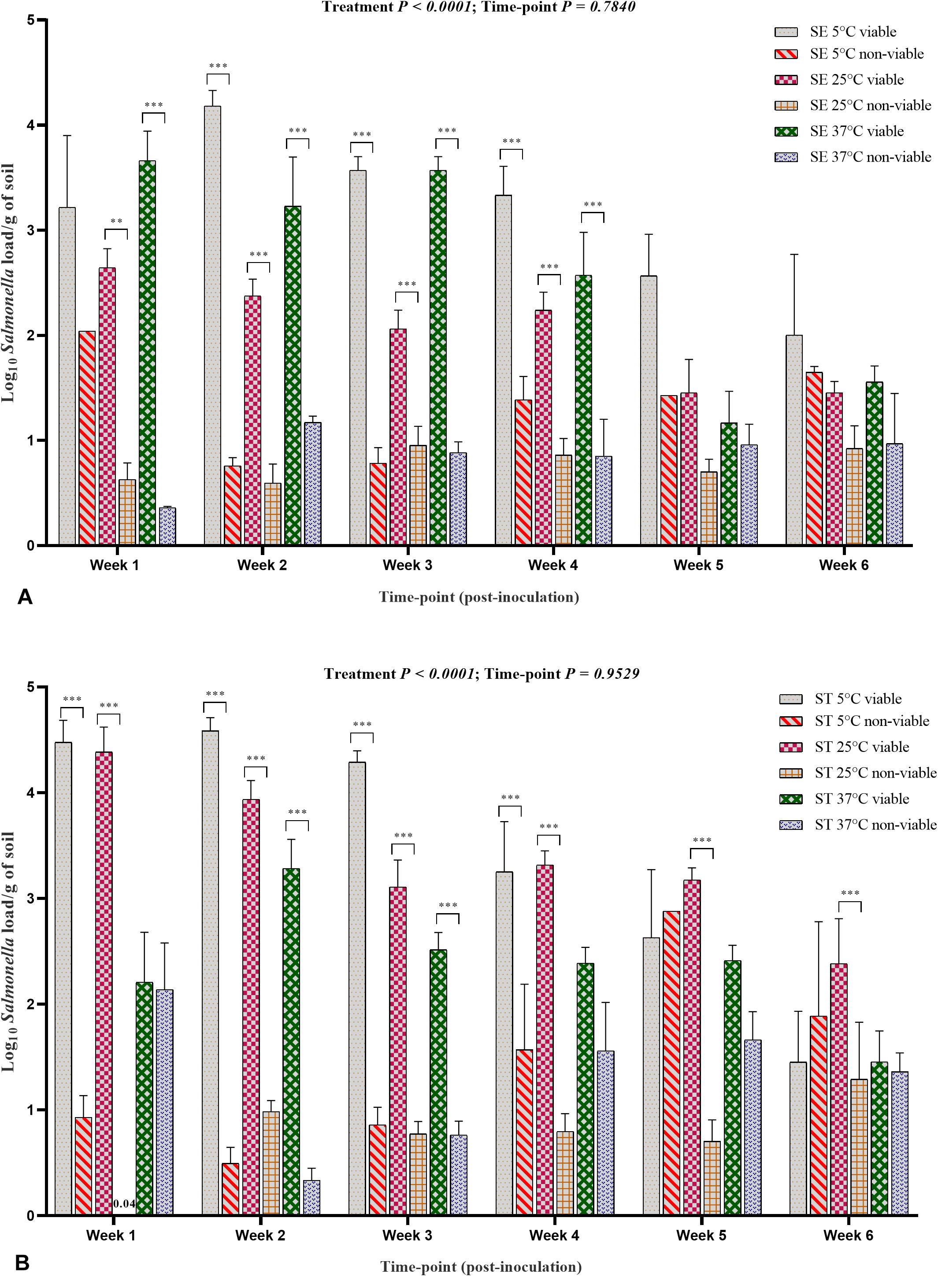
Figure 8. Viable and non-viable load of Salmonella Enteritidis (SE) and Salmonella Typhimurium (ST) in soil samples. (A) SE load. (B) ST load. The samples were incubated at 5, 25, and 37°C and processed at weekly intervals for PMAxxTM -based qPCR. Asterisks (∗∗∗) show significant difference (P < 0.0001) between the viable and non-viable load of Salmonella affected by each incubation temperature at each sampling timepoint.
The CFU and viable load data obtained from different treatment groups were compared to understand a correlation between the culture method and the optimized PMAxxTM -based qPCR. There was a significant positive correlation between the CFU and viable load data (Figures 9A–F). The decline in CFU was positively correlated with the qPCR data. The PMAxxTM -based qPCR was more sensitive in quantifying the viable load of Salmonella serovars as compared to the culture method used in the study. For both the Salmonella Enteritidis and Salmonella Typhimurium samples incubated at 5°C, the correlation between CFU and the viable load was significantly stronger compared with the samples incubated at 25 and 37°C due to higher moisture content.
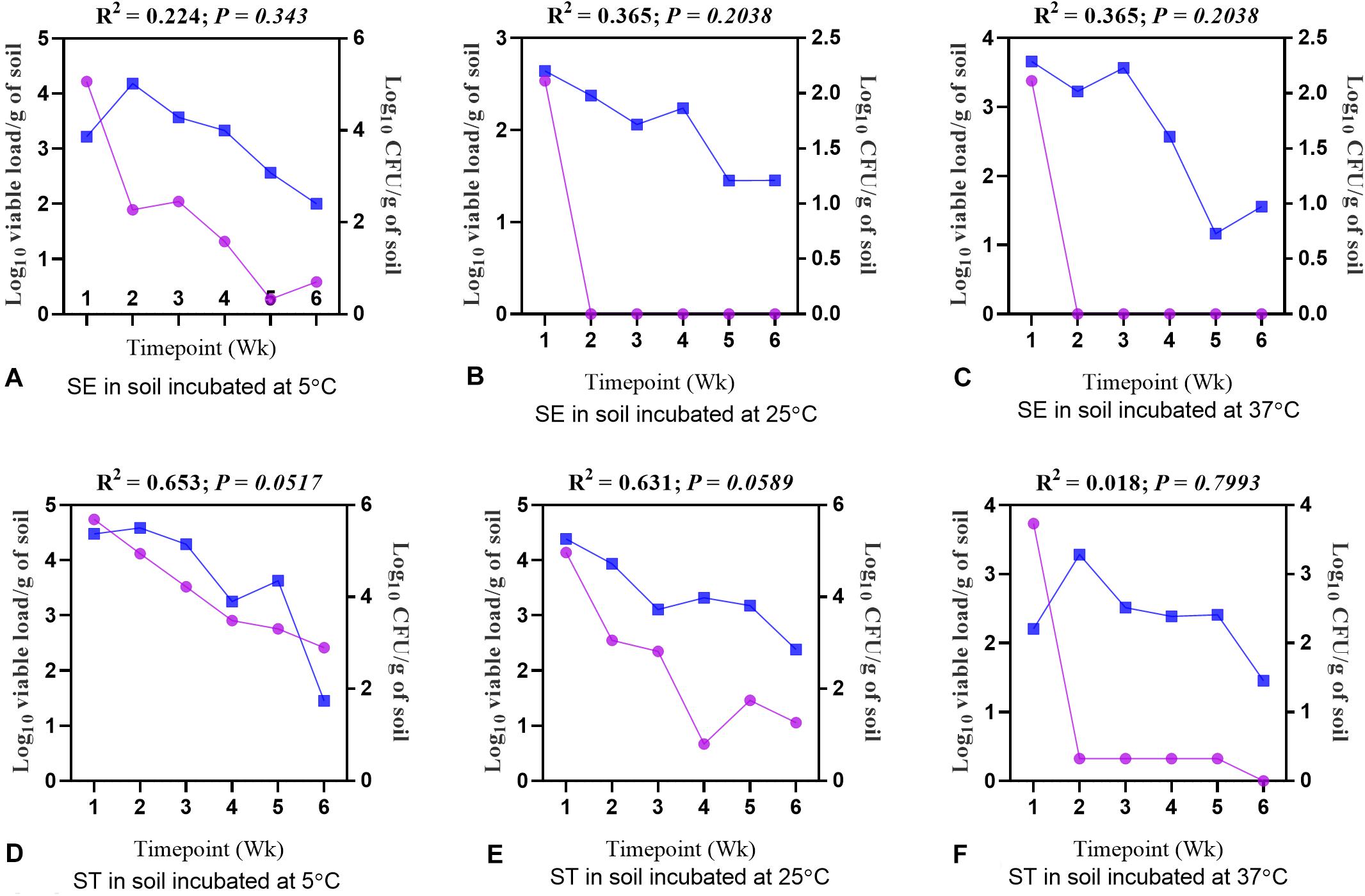
Figure 9. Correlation between log10 CFU/g and log10 viable load of Salmonella. (A) Soil inoculated with Salmonella Enteritidis (SE) and incubated at 5°C. (B) Soil inoculated with SE and incubated at 25°C. (C) Soil inoculated with SE and incubated at 37°C. (D) Soil inoculated with Salmonella Typhimurium (ST) and incubated at 5°C. (E) Soil inoculated with ST and incubated at 25°C. (F) Soil inoculated with ST and incubated at 37°C. Blue lines represent log10 viable load, while purple lines represent log10 CFU per gram of soil.
Discussion
The main objective of this study was to optimize a PMAxxTM -based qPCR for quantification of viable and non-viable Salmonella from environmental samples and to test the optimized assay on the artificially contaminated samples. The findings showed that the optimized qPCR can be accurately used for differentiating viable and non-viable Salmonella in soil samples collected from the free-range egg farm environment.
The QIAamp Fast DNA Stool Mini Kit (Qiagen, Australia) was used for the extraction of Salmonella DNA from pure cultures and Salmonella inoculated soil samples. As the manufacturer’s protocol did not yield high quality and quantity of DNA and was not applicable for the inclusion of the PMA step hence, the optimized method for DNA extraction was used in this study. The optimized method in the current study was based on microbe separation from the samples through gradient centrifugation. The qPCR results showed no effects of background materials in soil samples on amplification efficiency. This showed that the optimized method developed in this study removed the background materials during bacterial pelleting from soil samples. The invA primers used during this study showed that the primer pair was highly specific for target amplification with high efficiency (92%). The data also showed that its minimum detection limit was 102 Salmonella per gram of soil. Primer optimization for the amplification of ompA gene showed that these primers were non-specific by amplifying DNA obtained from Escherichia coli.
PMA has been used in distinguishing viable from non-viable cells of bacteria in pure culture (Nocker et al., 2006; Li and Chen, 2013) and from food/water matrices (Dinu and Bach, 2013; Kibbee and Örmeci, 2017; Li et al., 2017; Jäger et al., 2018). Similarly, PMA has also been used to differentiate live viruses from the dead population (Bellehumeur et al., 2015). However, the method has not been optimized for complex materials such as soil. Also, previous literature presented data as Cq values rather than viable and non-viable load (Li and Chen, 2013; Li et al., 2015). Therefore, the method optimized during this study is accurate and rapid that can be used to test the presence of viable Salmonella from complex soil matrices.
In the current study, the data showed that 10 μL of 2.5 mM PMAxxTM into 1 mL of the Salmonella culture was sufficient to differentiate viable cells from the non-viable cells. The DNA extracted from the non-viable bacteria was minimally amplified by qPCR. Studies have reported different concentrations of PMA. For example, 10 μL of 2.5 mM PMA in 0.5 mL of culture samples was used (Liang et al., 2011). However, in the current study, there was no significant difference in the load of Salmonella obtained from the viable culture treated with 2.5, 5, and 10 mM PMAxxTM. The concentration used in the current study is the recommended concentration from the manufacturer of the improved version of PMA, which is called PMAxxTM. If used in the high throughput diagnostic assay platforms, the lower concentration of PMAxxTM can save significant costs required for veterinary diagnostics. Data in the current study showed that the PMAxxTM concentration had no significant inhibitory effects on the amplification of DNA obtained from viable Salmonella.
In the subsequent applicability steps of the PMAxxTM -based qPCR, the CFU and qPCR data showed that the survivability of Salmonella Typhimurium and Salmonella Enteritidis was driven by incubation temperature, moisture level and water activity. The data showed that Salmonella Typhimurium had significantly higher capability to survive in soil compared with the Salmonella Enteritidis in the conditions applied during this study. However, it is worth to note that survivability of different strains of the same serovars of Salmonella may vary in different conditions. Significantly higher survivability at 5°C showed that both the Salmonella Enteritidis and Salmonella Typhimurium can remain viable for longer periods at 5°C in soil. The significant role of moisture and water activity in the survivability of Salmonella shows that the survival of Salmonella in the soil in dry and hot summer might be lower due to hot temperatures and low moisture levels. The decrease in the moisture level has been proven as a factor responsible for decline in viable count (Marsh et al., 1998). Nevertheless, our data showed that compared to the Salmonella Typhimurium, the Salmonella Enteritidis viable load declined at a significantly higher rate when the moisture level and water activity declined. The findings were consistent when the trial was repeated. A higher proportion of positive samples for Salmonella Typhimurium compared with Salmonella Enteritidis from the enriched samples further proved that the viability of Salmonella Enteritidis strain was more susceptible to changes in temperature, water activity and moisture level. A positive correlation between the CFU and PMAxxTM -based qPCR data obtained in the current study is in agreement with the findings of Shah et al. (2019) who quantified the viable count of Salmonella Newport through a culture method and PMA-qPCR from heat treated poultry amended soil.
A sharp decline in the survivability of both the serovars after 1 week of incubation at 37°C was due to a significantly higher loss of moisture and water activity at this temperature. In the current study, keeping the moisture level around 15% was to simulate real field conditions where the temperature in summer in South Australia can reach up to 45°C with moisture level quite low. A higher moisture content favors the survivability of bacteria (Cools et al., 2001), where Salmonella Newport can survive in soil for longer periods (Shah et al., 2019). The Salmonella Enteritidis strain used in this study was isolated from a commercial egg farm. Recently, several Salmonella Enteritidis infected commercial free range egg flocks were culled in Australia because Salmonella Enteritidis infection in poultry is categorized as exotic and notifiable. Our previous epidemiological investigation suggested that Salmonella Typhimurium was persistent in the range area of free range farms during winter (Gole et al., 2017). After Salmonella Enteritidis infection of the flocks, the poultry sheds are typically cleaned but, it is challenging to clean the ranging area of a free range poultry farm. The persistence of Salmonella Enteritidis or Salmonella Typhimurium in range area can result in reinfection of current flock or infection of the newly introduced flock. The assay developed in this study can be used for the rapid detection of Salmonella Enteritidis and Salmonella Typhimurium from soil samples and can provide useful information to the industry in making decisions on restocking flocks after mass culling. The data on the survival of Salmonella Typhimurium and Salmonella Enteritidis in soil samples are useful for mitigating the risks on farms and also for developing on farm biosecurity measures to avoid future on farm Salmonella outbreaks. The reduction of Salmonella in food animals and in a farm environment is essential to reduce human infection. This study was conducted on soil samples collected from a small number of egg farms located in the South Australia region, therefore; further studies are required to investigate the effects of different soil types on the survival of Salmonella. Soil type and temperature can influence the persistence of Salmonella (Garcia et al., 2010; Underthun et al., 2018). For the persistence of Salmonella in soil, humidity has been identified as an important meteorological factor (Hwang et al., 2020).
Salmonella can form biofilm when exposed to harsh environmental conditions (De Oliveira et al., 2014) such as soil. Biofilm is an assemblage of microorganism associated with the cell surface, which on the one hand can be the attachment to the surface of the materials in the poultry production system or on the other hand, the ability of the biofilm has been confirmed as one of the reasons that the foodborne pathogen can exist in the system continuously (Wang et al., 2013). Our study revealed that when the soil was inoculated with Salmonella and incubated at 5°C, Salmonella survived better compared with the samples incubated at higher temperature due to moisture level and water activity driven changes. This finding is in agreement with the previous report (Garcia et al., 2010). The possible reason might be that the biofilm was formed as a barrier to protect bacteria in a harsh environment (De Oliveira et al., 2014), however; further studies are necessary to confirm this hypothesis. Salmonella can be exposed to extreme temperature fluctuations including freezing and thawing. Freezing and thawing cycle can significantly affect bacterial cell viability and induce viable but non-culturable (VBNC) state with compromised ability to form a biofilm (Rocard et al., 2018). In this experiment, Salmonella Enteritidis and Salmonella Typhimurium remained undetected by culture method from week 5 onward, but the qPCR assay was able to detect the viable bacteria from the soil at week 6. This suggests that the low moisture level and high temperature can also induce the VBNC state of Salmonella.
The PMAxxTM -based qPCR data showed that the PMA penetrated through the compromised cell membrane of Salmonella thereby inhibiting the DNA amplification through qPCR. The positive correlation graphs between the CFU and viable count data at 5, 25, and 37°C for Salmonella Enteritidis and Salmonella Typhimurium showed that the optimized assay correlated well with the culture method. The viable counts obtained through the PMAxxTM -based qPCR from the soil sampled at weekly intervals showed that the optimized qPCR was sensitive in detecting the load of Salmonella compared to the culture method.
In conclusion, the PMAxxTM -based qPCR optimized in this study was sensitive in determining a load of Salmonella in soil samples. Both the culture based method and the optimized qPCR data showed that the Salmonella Typhimurium survived better in soil than Salmonella Enteritidis. Future studies would be able to use this optimized assay in conditions, such as the efficacy of sanitizers against Salmonella in poultry production. In poultry and food industries, the quantification of viable Salmonella from feces, dust, egg, meat, food products, or other complex mixture of inorganic materials could be tested through our optimized method.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This study was supported by the School of Animal and Veterinary Sciences, The University of Adelaide.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Help from Sonali Deshmukh and Qixing Lin during soil samples processing for moisture content and water activity measurements and DNA extraction is highly acknowledged. We would like to acknowledge Dr. Cheryl Jenkins, Elizabeth Macarthur Agricultural Institute, NSW Department of Primary Industries for providing Salmonella Enteritidis strain for this study.
References
Akiba, M., Kusumoto, M., and Iwata, T. (2011). Rapid identification of Salmonella enterica serovars, typhimurium, choleraesuis, infantis, hadar, enteritidis, dublin and gallinarum, by multiplex PCR. J. Microbiol. Methods 85, 9–15. doi: 10.1016/j.mimet.2011.02.002
Banihashemi, A., Van Dyke, M. I., and Huck, P. M. (2012). Long-amplicon propidium monoazide-PCR enumeration assay to detect viable Campylobacter and Salmonella. J. Appl. Microbiol. 113, 863–873. doi: 10.1111/j.1365-2672.2012.05382.x
Barbau-Piednoir, E., Mahillon, J., Pillyser, J., Coucke, W., Roosens, N. H., and Botteldoorn, N. (2014). Evaluation of viability-qPCR detection system on viable and dead Salmonella serovar Enteritidis. J. Microbiol. Methods 103, 131–137. doi: 10.1016/j.mimet.2014.06.003
Bellehumeur, C., Boyle, B., Charette, S. J., Harel, J., L’homme, Y., Masson, L., et al. (2015). Propidium monoazide (PMA) and ethidium bromide monoazide (EMA) improve DNA array and high-throughput sequencing of porcine reproductive and respiratory syndrome virus identification. J. Virol. Methods 222, 182–191. doi: 10.1016/j.jviromet.2015.06.014
Cools, D., Merckx, R., Vlassak, K., and Verhaegen, J. (2001). Survival of E. coli and Enterococcus spp. derived from pig slurry in soils of different texture. Appl. Soil Ecol. 17, 53–62. doi: 10.1016/s0929-1393(00)00133-5
De Oliveira, D. C. V., Fernandes Junior, A., Kaneno, R., Silva, M. G., Araujo Junior, J. P., Silva, N. C. C., et al. (2014). Ability of Salmonella spp. to produce biofilm is dependent on temperature and surface material. Foodborne Pathog. Dis. 11, 478–483. doi: 10.1089/fpd.2013.1710
Dinu, L.-D., and Bach, S. (2013). Detection of viable but non-culturable Escherichia coli O157: H7 from vegetable samples using quantitative PCR with propidium monoazide and immunological assays. Food Control 31, 268–273. doi: 10.1016/j.foodcont.2012.10.020
Ford, L., Moffatt, C. R., Fearnley, E., Miller, M., Gregory, J., Sloan-Gardner, T. S., et al. (2018). The epidemiology of Salmonella enterica outbreaks in Australia, 2001–2016. Front. Sustainab. Food Syst. 2:86. doi: 10.3389/fsufs.2018.00086
Garcia, R., Baelum, J., Fredslund, L., Santorum, P., and Jacobsen, C. S. (2010). Influence of temperature and predation on survival of Salmonella enterica serovar Typhimurium and expression of invA in soil and manure-amended soil. Appl. Environ. Microbiol. 76, 5025–5031. doi: 10.1128/aem.00628-10
Gole, V. C., Woodhouse, R., Caraguel, C., Moyle, T., Rault, J. L., Sexton, M., et al. (2017). Dynamics of Salmonella shedding and welfare of hens in free-range egg production systems. Appl. Environ. Microbiol. 83:e03313-16.
Hwang, D., Rothrock, M. J. Jr., Pang, H., Guo, M., and Mishra, A. (2020). Predicting Salmonella prevalence associated with meteorological factors in pastured poultry farms in southeastern United States. Sci. Total Environ. 713:136359. doi: 10.1016/j.scitotenv.2019.136359
Jäger, T., Alexander, J., Kirchen, S., Dötsch, A., Wieland, A., Hiller, C., et al. (2018). Live-dead discrimination analysis, qPCR assessment for opportunistic pathogens, and population analysis at ozone wastewater treatment plants. Environ. Pollut. 232, 571–579. doi: 10.1016/j.envpol.2017.09.089
Kärenlampi, R., Rautelin, H., Schönberg-Norio, D., Paulin, L., and Hänninen, M.-L. (2007). Longitudinal study of Finnish Campylobacter jejuni and C. coli isolates from humans, using multilocus sequence typing, including comparison with epidemiological data and isolates from poultry and cattle. Appl. Environ. Microbiol. 73, 148–155. doi: 10.1128/aem.01488-06
Kibbee, R. J., and Örmeci, B. (2017). Development of a sensitive and false-positive free PMA-qPCR viability assay to quantify VBNC Escherichia coli and evaluate disinfection performance in wastewater effluent. J. Microbiol. Methods 132, 139–147. doi: 10.1016/j.mimet.2016.12.004
Li, B., and Chen, J.-Q. (2013). Development of a sensitive and specific qPCR assay in conjunction with propidium monoazide for enhanced detection of live Salmonella spp. in food. BMC Microbiol. 13:273. doi: 10.1186/1471-2180-13-273
Li, F., Li, F., Chen, B., Zhou, B., Yu, P., Yu, S., et al. (2017). Sextuplex PCR combined with immunomagnetic separation and PMA treatment for rapid detection and specific identification of viable Salmonella spp., Salmonella enterica serovars Paratyphi B, Salmonella Typhimurium, and Salmonella ENTERITIDIS in raw meat. Food Control 73, 587–594. doi: 10.1016/j.foodcont.2016.09.009
Li, H., Xin, H., and Li, S. F. Y. (2015). Multiplex PMA–qPCR Assay with internal amplification control for simultaneous detection of viable Legionella pneumophila, Salmonella typhimurium, and Staphylococcus aureus in environmental waters. Environ. Sci. Technol. 49, 14249–14256. doi: 10.1021/acs.est.5b03583
Liang, N., Dong, J., Luo, L., and Li, Y. (2011). Detection of viable Salmonella in lettuce by propidium monoazide real-time PCR. J. Food Sci. 76, M234–M237.
Marsh, P., Morris, N. Z., and Wellington, E. M. (1998). Quantitative molecular detection of Salmonella typhimurium in soil and demonstration of persistence of an active but non-culturable population. FEMS Microbiol. Ecol. 27, 351–363. doi: 10.1111/j.1574-6941.1998.tb00551.x
Milazzo, A., Giles, L. C., Zhang, Y., Koehler, A. P., Hiller, J. E., and Bi, P. (2016). The effect of temperature on different Salmonella serotypes during warm seasons in a Mediterranean climate city, Adelaide, Australia. Epidemiol. Infect. 144, 1231–1240. doi: 10.1017/s0950268815002587
Nocker, A., Cheung, C.-Y., and Camper, A. K. (2006). Comparison of propidium monoazide with ethidium monoazide for differentiation of live vs. dead bacteria by selective removal of DNA from dead cells. J. Microbiol. Methods 67, 310–320. doi: 10.1016/j.mimet.2006.04.015
Rocard, J. M., Asadishad, B., Samonte, P. R. V., Ghoshal, S., and Tufenkji, N. (2018). Natural freeze-thaw cycles may increase the risk associated with Salmonella contamination in surface and groundwater environments. Water Res. X 1:100005. doi: 10.1016/j.wroa.2018.10.002
Semenov, A. V., Van Overbeek, L., and Van Bruggen, A. H. C. (2009). Percolation and survival of Escherichia coli O157: H7 and Salmonella enterica serovar Typhimurium in soil amended with contaminated dairy manure or slurry. Appl. Environ. Microbiol. 75, 3206–3215. doi: 10.1128/aem.01791-08
Shah, M. K., Bradshaw, R., Nyarko, E., Handy, E. T., East, C., Millner, P. D., et al. (2019). Salmonella enterica in soils amended with heat-treated poultry pellets survived longer than bacteria in unamended soils and more readily transferred to and persisted on spinach. Appl. Environ. Microbiol. 85:e00334-19.
Underthun, K., De, J., Gutierrez, A., Silverberg, R., and Schneider, K. R. (2018). Survival of Salmonella and Escherichia coli in two different soil types at various moisture levels and temperatures. J. Food Prot. 81, 150–157. doi: 10.4315/0362-028x.jfp-17-226
Wang, H., Ding, S., Wang, G., Xu, X., and Zhou, G. (2013). In situ characterization and analysis of Salmonella biofilm formation under meat processing environments using a combined microscopic and spectroscopic approach. Int. J. Food Microbiol. 167, 293–302. doi: 10.1016/j.ijfoodmicro.2013.10.005
Keywords: viability assay, Salmonella contamination, poultry production, PMAxx-based qPCR, Salmonella load
Citation: Zhang J, Khan S and Chousalkar KK (2020) Development of PMAxxTM-Based qPCR for the Quantification of Viable and Non-viable Load of Salmonella From Poultry Environment. Front. Microbiol. 11:581201. doi: 10.3389/fmicb.2020.581201
Received: 08 July 2020; Accepted: 31 August 2020;
Published: 22 September 2020.
Edited by:
David Rodriguez-Lazaro, University of Burgos, SpainReviewed by:
Eduardo Cesar Tondo, Federal University of Rio Grande do Sul, BrazilAlejandro Garrido-Maestu, International Iberian Nanotechnology Laboratory (INL), Portugal
Copyright © 2020 Zhang, Khan and Chousalkar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kapil K. Chousalkar, S2FwaWwuY2hvdXNhbGthckBhZGVsYWlkZS5lZHUuYXU=
 Jiawei Zhang
Jiawei Zhang Samiullah Khan
Samiullah Khan Kapil K. Chousalkar
Kapil K. Chousalkar