
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 12 August 2020
Sec. Microbiotechnology
Volume 11 - 2020 | https://doi.org/10.3389/fmicb.2020.01989
This article is part of the Research TopicExploring the Role and Function of the Microbiota in Terrestrial Anaerobic Environments and their Potential Biotechnological ApplicationView all 8 articles
The objective of this study was to investigate the mechanism of Lactobacillus plantarum (L. plantarum) involved in improving fermentation quality of naturally ensiled alfalfa under poor conditions. High-moisture wilted alfalfa was ensiled without inoculants (CK) or with inoculation of two L. plantarum additives (LPI and LPII). The pH and fermentation products of silage were determined after 30 and 90 days of ensiling. Additionally, the bacterial community compositions were analyzed. The L. plantarum inoculants significantly promoted lactic acid accumulation, and Lactobacillus abundance for both periods. At 90 days, silage in CK exhibited a high pH, a loss in dry matter, and a high concentration of ammoniacal nitrogen. The inoculations of L. plantarum significantly inhibited the growth of Clostridia, and reduced ammoniacal nitrogen concentration in silage (P < 0.05). Thus, inoculation with L. plantarum improved the fermentation quality of alfalfa silage and inhibited the growth of spoilage microorganisms, and further delayed spoilage of alfalfa silage under adverse ensiling conditions.
Ensiling is an anaerobic microbial-based fermentation process, dominated by lactic acid bacteria (LAB), which produce the lactic acid (LA) required for pH decline and inhibition of harmful microorganisms. It has long been a common method for forage preservation (Eikmeyer et al., 2013). Alfalfa, a widely cultivated and economically valuable pasture plant, is an important forage crop used for ensiling worldwide (Dunière et al., 2013). However, it can be hard to ensile owing to its high buffering capacity (BC) and lack of water soluble carbohydrates (WSCs) (Nkosi et al., 2016), especially when the moisture concentration exceeds 70%, resulting in clostridial fermentation (Coblentz and Muck, 2012). Wilting to a dry matter (DM) of 300–400 g/kg fresh weight (FW) is recommended before ensiling for wet grasses and legumes to prevent effluent production according to Dunière et al. (2013). However, high precipitation in some areas can make it difficult to wilt the forage before ensiling. Alfalfa is a perennial herb that is harvested in spring, summer, and autumn in most areas in China other than the north. At the end of spring or early summer, mid-eastern and southern China have abundant rainwater, and the alfalfa ensiled for over 2 or 3 months will reach high temperatures during summer. Sudden rainfall and high air humidity may cause extended wilting periods, which affect characteristics of the ensiling material. Sustained high temperatures during the ensiling process may also affect the stability of fermentation.
The microbial community plays an important role in the fermentation process and is likely to be impacted by multiple factors, such as ensiling conditions, inoculants, and epiphytic microorganisms of the fresh forage (Kasmaei et al., 2017; Guan et al., 2018). The development of PCR-based techniques enables us to define the microbial communities more accurately, and a larger variation in the microbiota in alfalfa silages than in cereal silages has been documented (McAllister et al., 2018). Inoculations of Lactobacillus plantarum could enhance the acidification of silage and adaption to a low pH environment, which contributes to the inhibition of competing microorganisms and effectively improves silage quality. Thus, L. plantarum is the most commonly used bacterial inoculant in forage ensiling studies (Oliveira et al., 2017). During ensiling, L. plantarum enhances LA fermentation and survives and adapts to the silage environment (Guo et al., 2018; Ogunade et al., 2018; Yang et al., 2019). However, the fermentation quality of the silage varies owing to different factors, such as BC, water activity, and presence of epiphytic microbes. The latter can differ owing to several factors, such as forage type, climatic conditions, and irrigation level. Adverse ensiling conditions, such as extremely high temperature, high humidity, and soil incorporation, can result in the poor fermentation of alfalfa silage. Under good conditions, silage without L. plantarum inoculation can produce a good fermentation quality, with a decline in pH, abundant LA accumulation, and sufficient LAB counts. However, few studies have examined the effects of L. plantarum on alfalfa silage fermentation quality when poor fermentation conditions occur (Yang et al., 2020).
The aim of this study was to investigate the mechanism of L. plantarum in improving fermentation quality of naturally ensiled alfalfa under the poor conditions that commonly occur in spring and summer ensiling in China. Yellow River-irrigated alfalfa was harvested during the high precipitation season when the relative humidity exceeded 80%, and ensiled for 90 days after a 1-day wilting with or without L. plantarum inoculation. Particular attention was paid to bacterial communities, fermentation properties, and their interactions in silage.
Fresh alfalfa was harvested at the early bloom stage in Zhengzhou, Henan Province (temperate monsoon climate, 34.76°N, 113.65°E, altitude 110.4 m above sea level), and wilted for 24 h. The wilted material (DM 266 g/kg FW) was chopped into 1–2-cm lengths. For inoculant preparation, L. plantarum YX was isolated from the Yaxin alfalfa ensiling additive (Yaxin Biotechnology Co., Ltd., Taiwan, China) and L. plantarum A345, an alfalfa epiphytic strain, was isolated from Shanxi, China. The L. plantarum strains were cultured in MRS medium at 30°C for 12 h. Then, the culture was centrifuged at 12,000 g for 10 min at 4°C. The precipitate was mixed with distilled water to an OD600 of 0.8.
Approximately 500 g for each of three replicates of the chopped alfalfa were treated with the following: (1) distilled water control (CK); (2) 1 × 106 colony forming units (cfu)/g of L. plantarum YX (LPI); and (3) 1 × 106 cfu/g of L. plantarum A345 (LPII). All replicates were packed into polyethylene plastic bags, vacuumed, sealed with a vacuum sealer (P-290, Shineye, Dongguan, China), and ensiled at ambient temperature (24–40°C) for 30 and 90 days.
The AOAC (1990) was used for DM determination. Subsamples were dried in an oven at 65°C for 48 h and pulverized to pass through a 1-mm screen using a laboratory knife mill (FW100, Taisite Instrument Co., Ltd., Tianjin, China). The WSCs were measured using anthrone colorimetry (Murphy, 1958).
Other subsamples of silage (10 g) were diluted with 90 ml of distilled water and filtered through a 0.45-μm membrane. The pH was measured using a pH meter. The organic acid contents were determined using high-performance liquid chromatography (Waters Alliance e2695, Waters, MA, United States). Carbomix H-NP 10:8% (7.8 mm × 300 mm × 10 μm) was used as the stationary phase, and the column temperature was maintained at 55°C. The injected sample volume was 10 μl. The mobile phase composition was 0.0254% sulfuric acid. The mobile phase was filtered through a 0.45-μm pore size, 47-mm diameter nylon membrane and degassed before use. The flow rate of the mobile phase was 0.6 ml/min. The detection wavelength for samples was 214 nm using a UV detector. The ammoniacal nitrogen (NH3-N) level was determined using Berthelot colorimetry (Broderick and Kang, 1980). The BC was determined using the hydrochloric acid–sodium hydroxide method (Playne and Mcdonald, 1996). The fermentation coefficient of alfalfa silage was predicted using the formula of Addah et al. (2011).
Each of the 21 samples (3 bags of fresh alfalfa and 18 bags of treatments) (10 g) was mixed with 100 mL of sterile phosphate buffer saline by vigorous shaking at 180 r/min for 2 h. The mixture was filtered and the liquor was then centrifuged at 10,000 r/min for 10 min at 4°C. The deposit was resuspended in 1 mL of sterile phosphate buffer saline. The liquor was centrifuged at 12,000 r/min for 10 min at 4°C to collect microbial pellet. Total DNAs were extracted using a Bacterial DNA Kit D3350-02 (Omega Biotek, Norcross, GA, United States). The purity levels and concentrations of DNAs were evaluated by 1% agarose gel electrophoresis. The PCR amplifications of the V3–V4 regions of the bacterial 16SrDNA gene were performed using Primer F (Illumina adapter sequence 1+ CCTACGGGNGGCWGCAG) and Primer R (Illumina adapter sequence 2+ GACTACHVGGGTATCTAATCC). The PCR products were extracted from a 2% agarose gel. The amplicon sequencing of 16SrDNA was conducted using the Miseq platform (Genesky Bio-Tech Co., Ltd., Shanghai, China) after the purification and quantification of the PCR products. All the raw reads were checked using FLASH2, and low quality sequences (quality scores below 20) were discarded according to the QIIME quality control process (version 1.7.0). Operational taxonomic units were clustered using Uparse (Uparse v7.0.10011). The analysis of taxonomy assignment of representative sequences was performed using the Ribosome Database Project (Cole et al., 2009). The sequence data reported in this study had been deposited in the GSA database (Accession No. CRA002694).
The statistical analysis of the fermentation products was performed using IBM SPSS version 21.0 (SPSS Inc., Chicago, IL, United States). The effects of different treatments were evaluated by one-way analysis of variance, with Duncan’s multiple range tests. The alpha diversity of the bacterial communities was calculated using mothur (version 1.9.1). The beta diversity analyses and correlation analyses between bacterial compositions and environmental factors were performed using R software (version 2.15.3).
The wilted alfalfa had pH of 6.06, DM of 266 g/kg FW, WSC concentration of 17.56 g/kg DM, and a high BC of 460 mEq/kg DM. Organic acid and NH3-N were not detected.
Organic acid and NH3-N contents, as well as the pH level, of alfalfa silage inoculated with or without L. plantarum strains are shown in Table 1. The L. plantarum inoculants effectively accelerated the LA fermentation and acetic acid (AA) accumulation, resulting in a lower pH compared with the CK group (P < 0.001) at 30 days; and the enhanced LA accumulation was significantly greater in the LPII group (P < 0.001). For 90-d silage, increase in pH level and NH3-N concentration, reduction in LA and WSC concentration, as well as formation of propionic acid (PA), occurred compared with 30-d silage. Significantly higher pH (P < 0.001) and NH3-N accumulation (P < 0.001), as well as a significant loss in DM (P = 0.017) were apparent in the CK compared with the inoculated groups. Silage in the LPII group had better fermentation quality compared with other groups, indicated by a relatively low pH, high LA and AA concentrations, and inhibition of NH3-N formation.
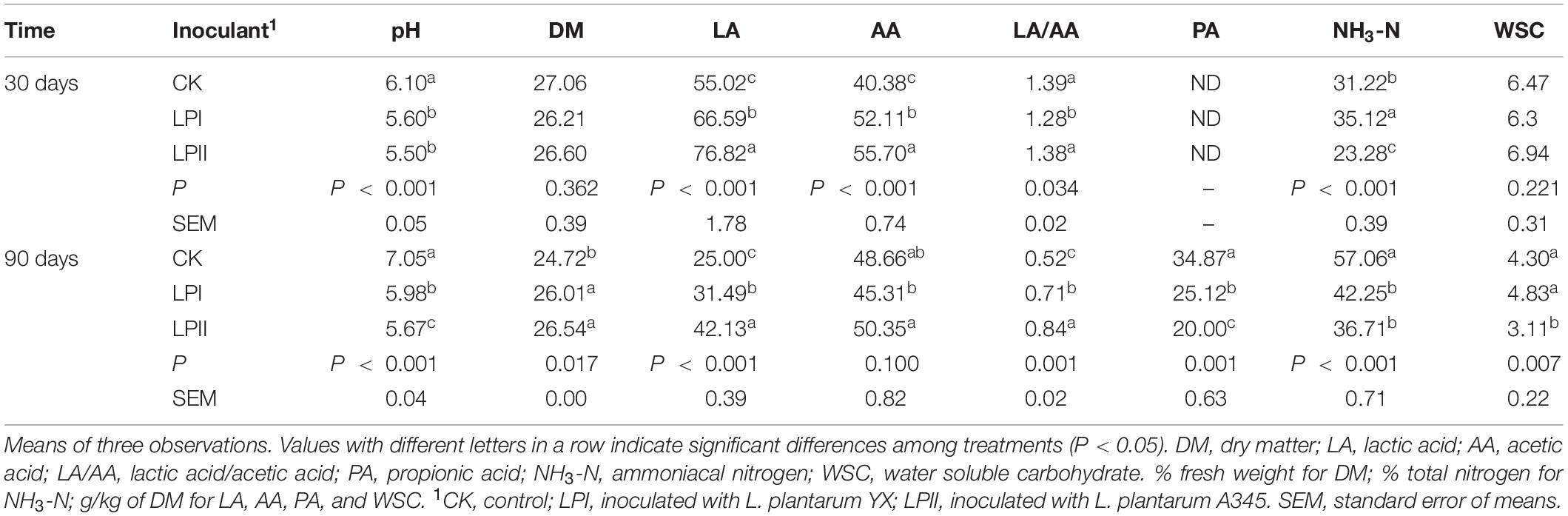
Table 1. Fermentation end-products after different Lactobacillus plantarum treatments during alfalfa ensiling.
High-throughput analysis was used to determine the bacterial diversity in alfalfa silage, and the valid sequences were clustered into 552 operational taxonomic units based on a 97% sequence identity. Richness and diversity indices of the bacterial communities, represented by observed species, the Chao1 and the Shannon indexes, respectively, are shown in Figures 1A–C. The richness of the bacterial community decreased after ensiling for 30 days compared with the fresh material, and then increased at 90 days (P = 0.005). The diversity of the bacterial community decreased after ensiling (P = 0.006). The diversity of the bacterial community in silage in LPII slightly and non-significantly increased at 90 days compared with 30-d silage (P = 0.1).
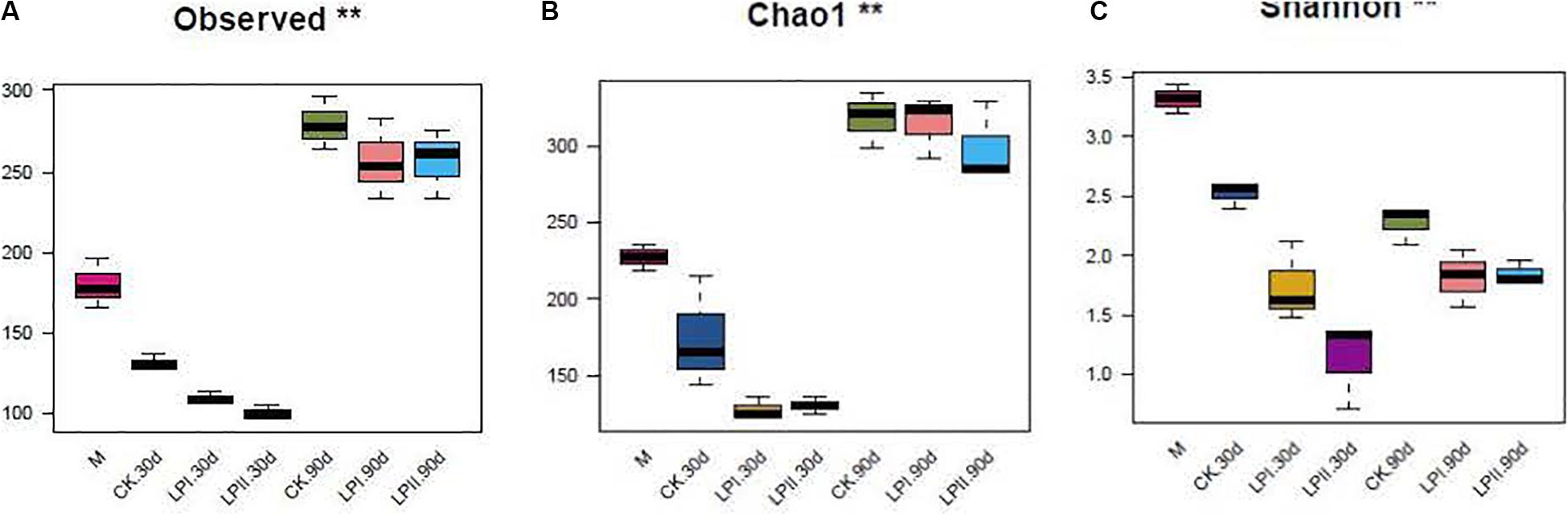
Figure 1. Box-plots of observed bacterial species (A), Chao 1 (B) and Shannon indices (C) of bacterial communities in alfalfa silage. M, alfalfa material before ensiling; CK, control; LPI, inoculated with L. plantarum YX; LPII, inoculated with L. plantarum A345. The numbers following CK, LPI, and LPII stand for ensiled days of silage. Observed, observed bacterial species. *P < 0.05; **P < 0.01.
The principal coordinate analysis (PCoA) clearly reflected the variation within the bacterial community (Figure 2). The clear separation between bacterial communities of the wilted alfalfa and alfalfa silage indicated a shift after ensiling. Divisions in the plots representing silage with and without inoculants for both periods indicated that the distribution of the bacterial community was shifted by L. plantarum inoculations. The distribution of the bacterial communities among the three replications within LPII was more similar compared to those within CK and LPI at 30 days. Although the Shannon indexes were similar between 30-d and 90-d silage (Figure 1C), divisions in the plots representing silage for the two periods indicated a variation within the bacterial community.
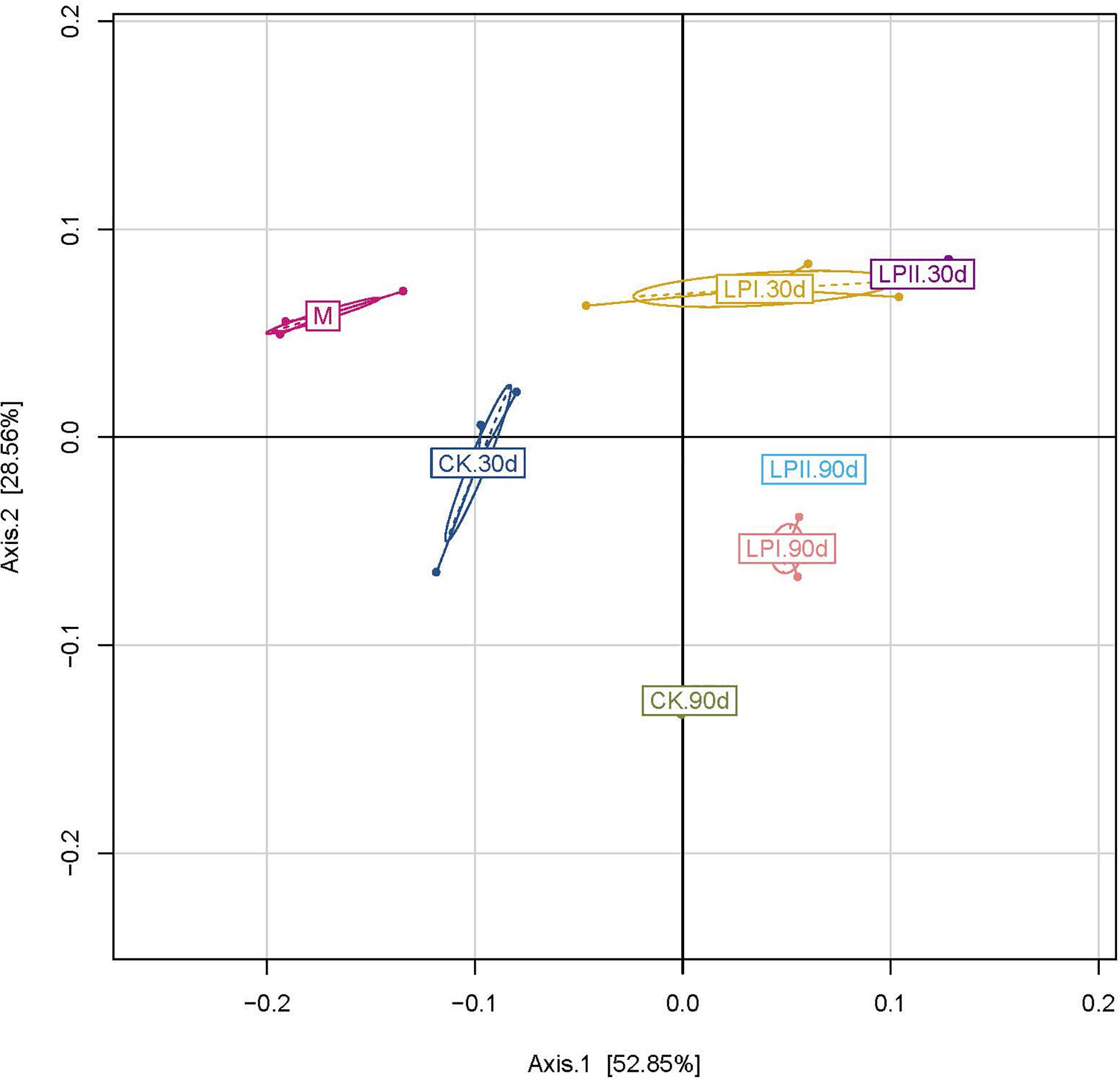
Figure 2. Cluster analysis of bacterial communities in alfalfa silage as assessed by a Principal Coordinate Analysis. M, alfalfa material before ensiling; CK, control; LPI, inoculated with L. plantarum YX; LPII, inoculated with L. plantarum A345. The numbers following CK, LPI, and LPII stand for ensiled days of silage.
Proteobacteria (52.14%) was the predominant phylum in the wilted alfalfa, followed by Firmicutes (30.33%) (Figure 3A). Firmicutes became the predominant phylum after ensiling, except for the CK group at 30 days. Inoculation increased the relative abundance of Firmicutes in the 30-d silage compared with CK, and the relative abundance of Firmicutes was greater in LPII (84.74%) than LPI (64.62%). At the genus level, Pseudomonas (15.35%), Exiguobacterium (12.36%), Massilla (14.15%), and Planococcus (8.00%) dominated the epiphytic bacterial community of alfalfa, while abundance levels of these genera reduced after ensiling. Low abundance of LAB species was exhibited in the alfalfa epiphytic bacterial community, including Lactobacillus (1.36%), Weissella (0.70%), Lactococcus (0.07%), Pediococcus (0.01%), and Leuconostoc (<0.01%). A complex bacterial community composition was exhibited in CK in the 30-d silage, with the prevalent genera being Enterobacter (38.54%), Weissella (11.59%), Garciella (10.42%), and Lactococcus (9.59%) (Figure 3B). However, Lactobacillus, the predominant genus in LPI and LPII (57.82 and 82.19%, respectively), only exhibited a relative abundance of 2.05% in CK at 30 days. Inoculation inhibited the growth of Enterobacter at 30 days, with the lowest relative abundance for LPII (10.20%). The relative abundance of Enterobacter became similar among the three treatments in the 90-d silage (14.96% in CK, 11.60% in LPI, and 14.83% in LPII). Garciella became a prevalent genus at 90 days, with relative abundance of 34.95% in CK, 38.38% in LPI, and 24.04% in LPII. The relative abundance of Lactobacillus in the inoculated silage decreased, and Clostridia including Clostridium_XlVa and Clostridium_sensu_stricto grew in all treatments at 90 days compared with 30-d silage. An inhibitory effect against Clostridium_XlVa was apparent in LPII compared with the other two groups in 90-d silage.
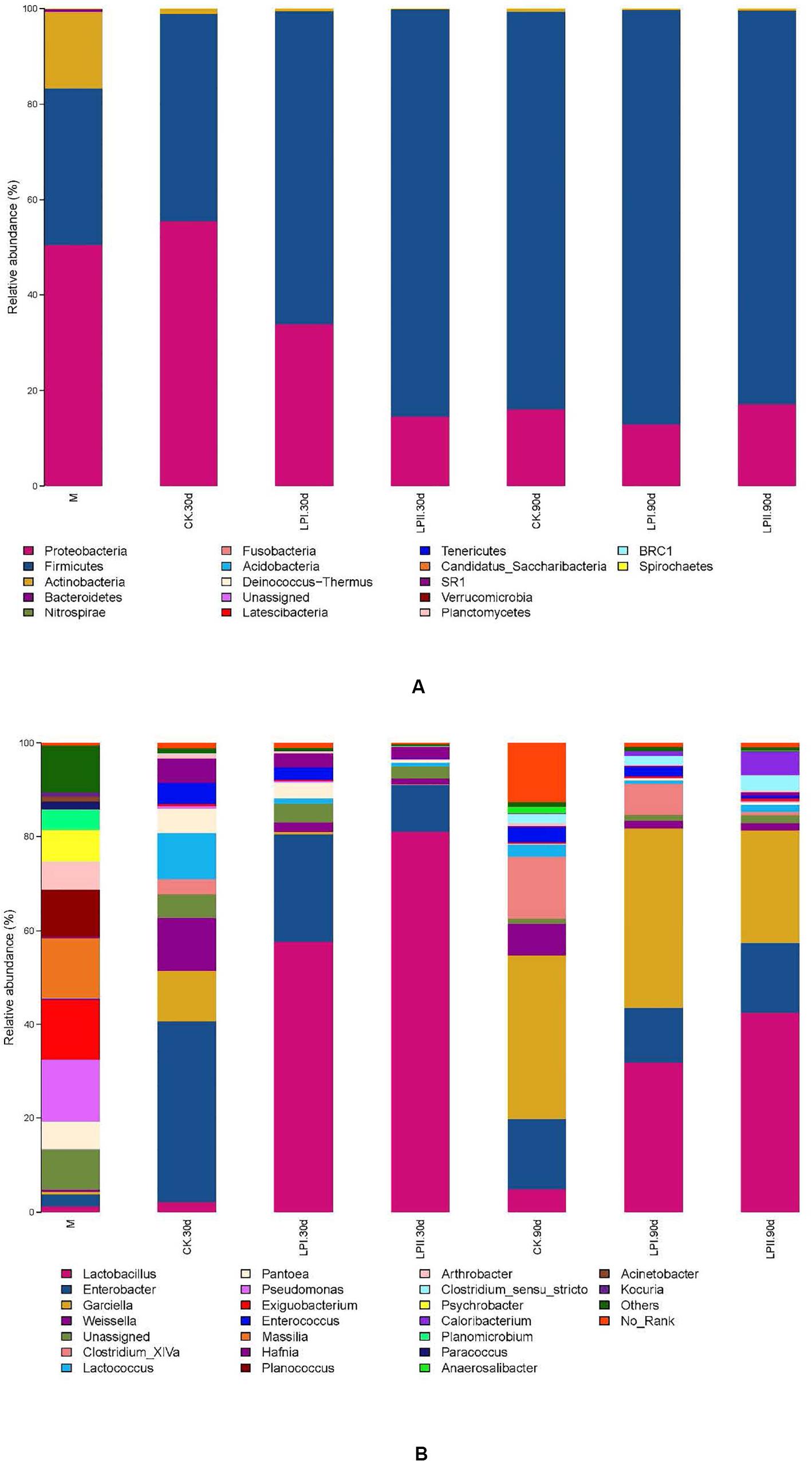
Figure 3. Bacterial community and relative abundances by phylum (A) and genus (B) for alfalfa silage. M, alfalfa material before ensiling; CK, control; LPI, inoculated with L. plantarum YX; LPII, inoculated with L. plantarum A345. The numbers following CK, LPI, and LPII stand for ensiled days of silage.
LefSe was performed to further explore the variations in the bacterial communities among the treatments (Figure 4). Significantly higher abundances of species of Enterobacter, Lactococcus, and Enterococcus were observed in CK at 30 days. Lactobacillus species grew well in LPII in 30-d silage. At 90 days, the relative abundance of some species belonging to spoilage genus Clostridium and pathogenic genus Listeria were significantly higher in the CK group. Although silage in LPII effectively inhibited the growth of Clostridium_XlVa (Figure 3B) (13.42% in CK, 6.67% in LPI, and 0.67% in LPII, respectively), it showed a relatively poorer inhibition against some Clostridia genera with low abundance. Clostridium_sensu_stricto, for instance, a genus belonging to family Clostridiaceae_1, exhibited relative abundances of 1.94, 1.88, and 3.23% in CK, LPI, and LPII in 90-d silage, respectively.
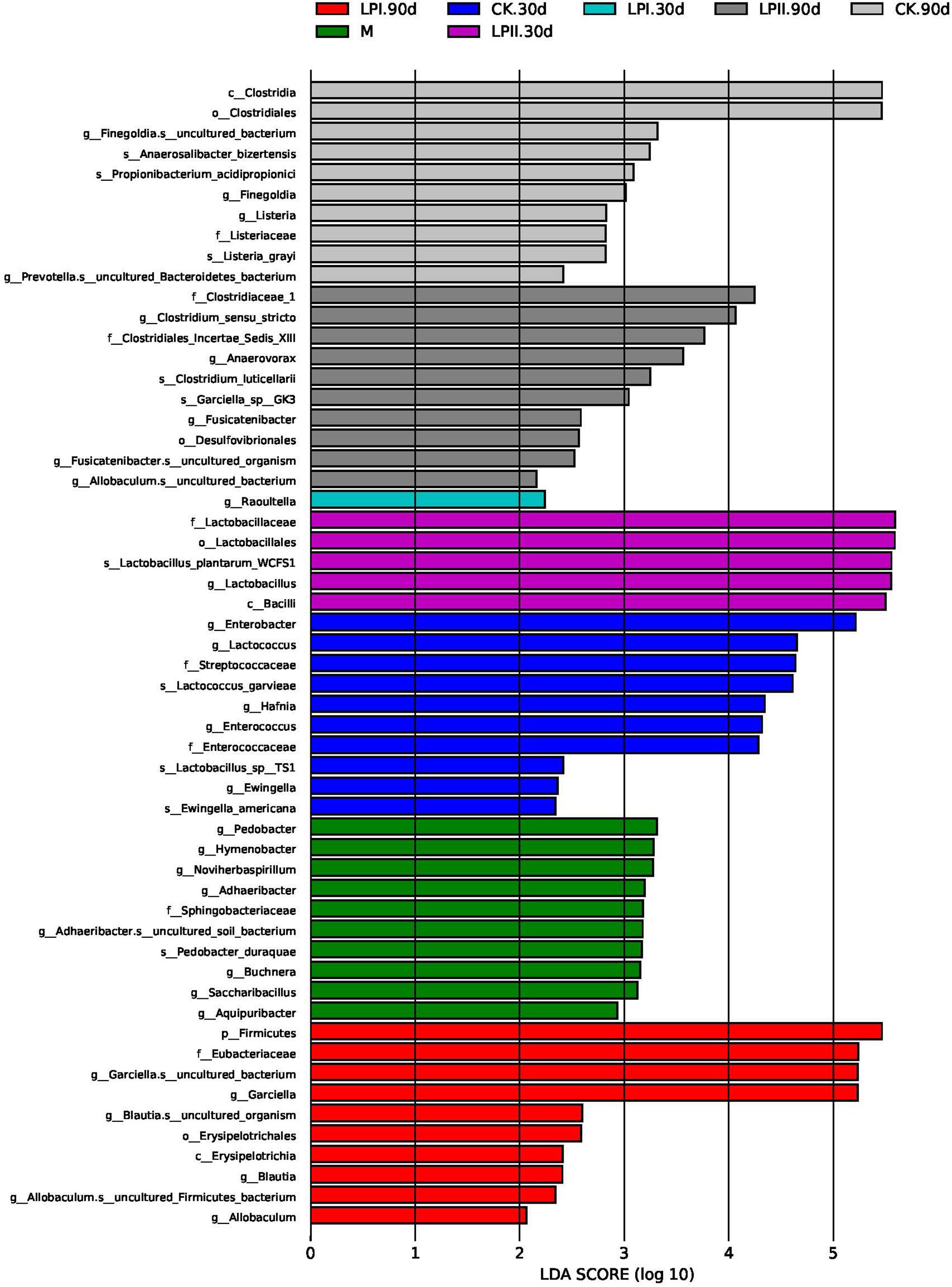
Figure 4. Comparison of microbial variations using LefSe analysis. M, alfalfa material before ensiling; CK, control; LPI, inoculated with L. plantarum YX; LPII, inoculated with L. plantarum A345. The numbers following CK, LPI, and LPII stand for ensiled days of silage.
Mantel tests were performed to reveal the relationships between bacterial community compositions at different taxon levels and silage properties (Table 2). Results indicated significant correlations between fermentation properties and the bacterial community composition (P < 0.05) at different taxon levels, except for pH value and bacterial community composition at phylum level. Spearman’s correlation analysis indicated that the pH value and LA concentration had significant correlations with richness and diversity indices of the bacterial communities (P < 0.05). A strong negative correlation was found between AA concentration and the Shannon index (P < 0.05). Meanwhile, the number of observed species and the Chao1 index of the bacterial community showed positive correlations with PA and NH3-N concentrations, and negative correlations with WSC concentration (P < 0.05).
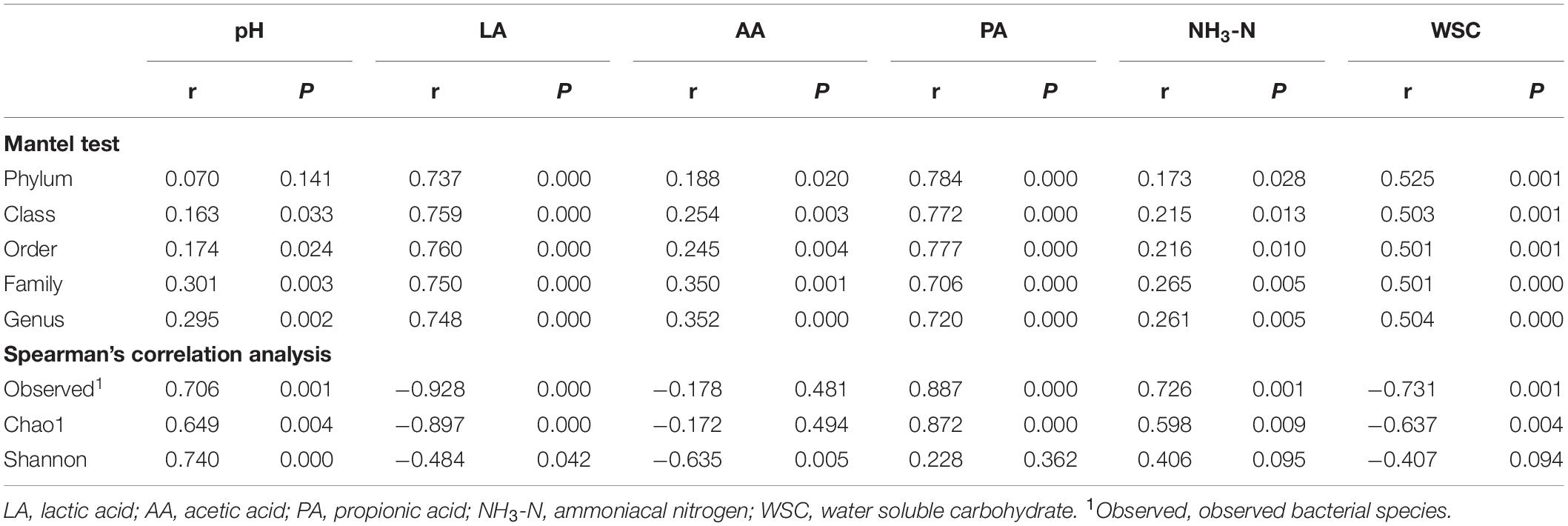
Table 2. Correlation of bacterial community with fermentation properties in alfalfa silage illustrated by Mantel test and Spearman’s correlation analysis.
Spearman’s correlations further illustrated the relationships between bacterial genera and silage properties for different periods (Figures 5A,B). Lactobacillus was positively correlated with LA (r = 0.92 at 30 days and 0.87 at 90 days), and negatively correlated with pH (r = −0.92 at 30 days and −0.88 at 90 days) for both periods. Positive correlations were found between NH3-N and two genera at 30 days (P < 0.05), Rosenbergiella and Bifidobacterium. The NH3-N was negatively correlated with Nocardioides, Methylobacterium, and Prevotella in 30-d silage (P < 0.05). The NH3-N was positively correlated with a series of nitrogen-fermenting genera at 90 days, including Anaerotruncus, Peptoniphilus, and genera belonging to Clostridia (Clostridium_XlVa, Clostridium_XI, and Finegoldia) (P < 0.05). Other genera also showed positive correlations with NH3-N at 90 days, including Weissella, Eremococcus, Brevibacillus, Vagococcus, Anaerococcus, Enterococcus, and Propionibacterium (P < 0.05). Nocardioides and Methylobacterium retained their negative correlations with NH3-N at 90 days. Another genus belonging to Nocardioidaceae, Marmoricola, was also negatively correlated with NH3-N. Negative correlations with NH3-N were also apparent for the acid-producing genus Lactobacillus, nitrate-reducing genera Pantoea and Exiguobacterium, putrescine-fermenting genus Anaerovorax, and other genera, including Planomicrobium, Acinetobacter, Kocuria, and Janibacter, with unknown functions in the ensiling process.
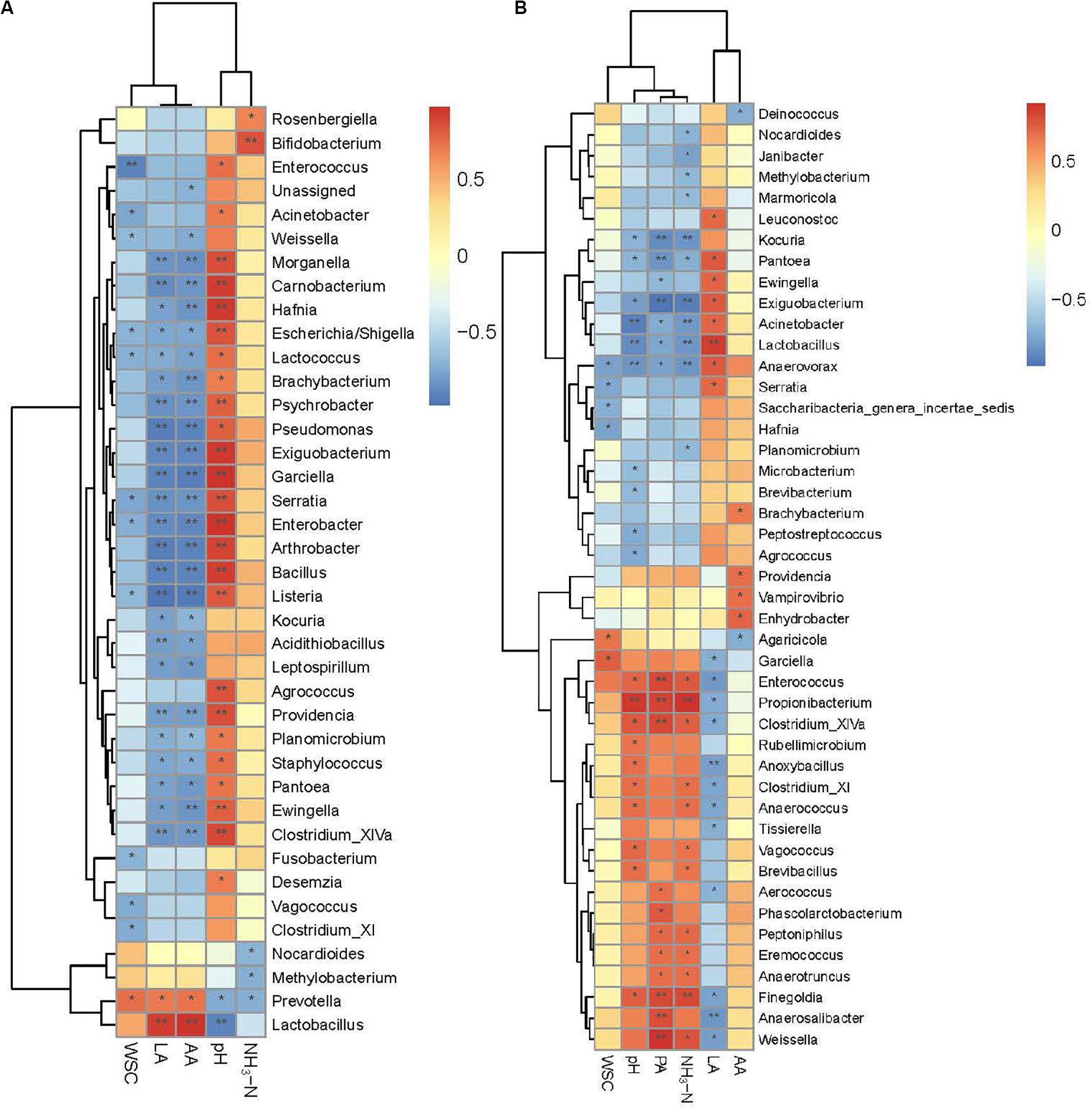
Figure 5. Spearman correlation heatmap of abundance of bacterial genera and fermentation properties in alfalfa ensiled for 30 days (A) and 90 days (B). LA, lactic acid; AA, acetic acid; LA/AA, lactic acid/acetic acid; PA, propionic acid; NH3-N, ammoniacal nitrogen; WSC, water soluble carbohydrate. *P < 0.05; **P < 0.01.
Adverse conditions during harvesting and ensiling could result in spoilage of silage. In this study, Yellow River-irrigated alfalfa was chosen as the ensiling material, and was harvested at the early bloom stage during the high precipitation season. Although the wilting period was extended compared with previous studies (Li X. et al., 2018; Li J. et al., 2018), the DM (266 g/kg FW) was still lower than the recommended content (300–400 g/kg FW) (Yuan et al., 2017). This might due to the high precipitation during the harvest season. Frequent rainfall resulting in high air humidity slowed the moisture loss during wilting. As reported by Agarussi et al. (2019) and Tao et al. (2017), wilting decreased the WSC concentration and crude protein content in fresh alfalfa. The 24-h wilting may have exacerbated the lack of WSC (17.56 g/kg DM) in this study. The WSC of the wilted alfalfa was lower than 50 g/kg DM, which was insufficient for adequate fermentation during ensiling (Ni et al., 2018). The BC was still high after wilting in this study. Frequent rainfall might increase the incorporation of soil in fresh alfalfa during harvest, resulting in an increase in BC (Weinberg and Ashbell, 2003). This might stimulate the epiphytic aerobic microorganisms to remain active for an extended period and so reduce the quantity of WSCs for further LAB fermentation. The low fermentation coefficient (26.9 < 35) also suggested that the material was too hard to ensile naturally (Ke et al., 2015).
Inoculation with L. plantarum had a positive effect on fermentation properties in terms of lower pH and higher LA accumulation. Similar effects were also reported by Wang et al. (2019) and Yang et al. (2019), in which LA fermentation was accelerated by L. plantarum inoculants in Moringa oleifera leaves and in alfalfa silage, respectively. The LPII exhibited enhanced LA fermentation than LPI. This might be explained by better adaption of the alfalfa epiphytic inoculant in LPII. Good adaption to the alfalfa ensiling environment of the inoculant aids in improvement of silage quality (Zhao et al., 2020). The AA concentrations increased with L. plantarum inoculations at 30 days. The AA is a promoter of aerobic stability during the ensiling process (Schmidt and Kung, 2010) and an effective inhibitor of fungi (Le Lay et al., 2016). This enhancement in AA accumulation might help with reduction in microbial richness at early stages of ensiling. Although inoculation enhanced LA fermentation in the 30-d silage, pH in the silage was highly above ideal level (<4.20) (Wang et al., 2017). The increase in pH in 90-d silage also suggested that LA fermentation was insufficient to stabilize the ensiled mass. The insufficient LA fermentation might be due to a lack of available substrate and high BC of the wilted alfalfa.
The LA to AA ratio became much lower at 90 days in all treatments, indicating reduction in LA fermentation in the silage. The PA contents in silage were also out of the acceptable range of 1–10 g/kg DM (Agarussi et al., 2019). The NH3-N is considered a representative of amino acid deamination and decarboxylation (Scherer et al., 2015), which generally reduce the nutritional value of silage. The remarkable increase in pH value and NH3-N concentration, as well as the loss in DM in CK at 90 days indicate potential spoilage of silage. The delay in spoilage with L. plantarum inoculation was shown by the lower pH and NH3-N concentration, higher LA residual, and good DM maintenance. The NH3-N concentration in LPII was significantly lower compared with other treatments, indicating better protein maintenance with the inoculant.
Notably, the NH3-N concentration increased in LPI (P < 0.001), but the LA accumulation and pH decline were greater, compared with the CK group. Effects of Clostridia and plant proteolytic enzymes may be typical causes of NH3-N accumulation (Kung and Shaver, 2001). Tao et al. (2012) reported that most plant proteolytic enzymes in alfalfa silage showed greater activities at pH 5.0–6.0, which could explain the higher NH3-N concentration in LPI. Plant proteolytic enzymes tended to be more active at the pH level in LPI (5.60) than in CK (6.10). Although LPI and LPII had similar pH values (5.60 and 5.50, respectively), LPII showed low NH3-N formation. This might result from the greater acidogenic capability of the inoculant in LPII in terms of higher LA and AA concentrations, which contributed to an increased inhibition of proteolytic microorganisms.
Although richness of bacterial community decreased compared with the wilted alfalfa at the early stage of ensiling, the richness flourished at 90 days. Similar trends were also reported by Zheng et al. (2017) in direct-cut alfalfa silage with and without LAB inoculant and sugar, in which richness of the bacterial community decreased at the early stage of ensiling compared with the alfalfa material, and later increased during the ensiling process. Consistent with the study of Ni K. et al. (2017), diversity of the bacterial community decreased after ensiling. Inoculation slightly reduced diversity of the bacterial community. Although the Shannon indexes were similar between the 30-d and 90-d silage, the PCoA showed variation within the bacterial community. The clear separation between bacterial communities of the wilted alfalfa and alfalfa silage indicated a shift after ensiling, consistent with previous studies (Ni K. K. et al., 2017; Yang et al., 2019). Divisions between the plots representing silage with and without inoculants indicated that the distribution of the bacterial community was shifted by L. plantarum inoculations, which could explain the differences in fermentation quality (Ni et al., 2018). The distribution of the bacterial communities among the three replications within LPII was more similar compared with those within LPI for both periods. The more stable bacterial community distribution in LPII might result from its stronger LA fermentation represented by the highest LA concentration (Table 1) and Lactobacillus abundance (Figure 3B) among the treatments for both periods.
Proteobacteria (52.14%) was the most predominant phylum in the wilted alfalfa, while abundance of Proteobacteria and Firmicutes reversed after being ensiled for 90 days, with Firmicutes dominating the bacterial community. Keshri et al. (2018) concluded that environmental conditions that developed during ensiling favor the selection of species of the phylum Firmicutes that flourish under low pH and anaerobic conditions. The increasing abundance of Firmicutes at 30 days in inoculated silage might indicate an acceleration of the LA fermentation process in the presence of L. plantarum. Acceleration of the LA fermentation with L. plantarum inoculation was also shown at the genus level. The major bacteria involved in LA fermentation of silage belong to the genera Lactobacillus, Pediococcus, Weissella, and Leuconostoc (Pang et al., 2011; Ni et al., 2018). Low abundance of these genera was exhibited in the wilted alfalfa before ensiling, and aerobic microorganisms dominated the bacterial community. This was consistent with the results of Tao et al. (2017), in which a long period of wilting decreased the LAB count and promoted growth of aerobes in alfalfa before ensiling.
A series of Clostridia genera grew in the 90-d silage. Clostridia may lead to excessive protein degradation, DM loss, and butyric acid (BA) production in silage, and further promote the growth of other less acid-tolerant spoilage microorganisms (Wang et al., 2019). Thus, they are undesirable in silage. Clostridiales (64.47%) dominated the bacterial community in CK at 90 days at the order level. Inoculation with L. plantarum effectively inhibited the dominance of Clostridiales (48.27% in LPI and 29.26% in LPII, respectively), which further reduced NH3-N formation, maintained the DM (Table 1), and inhibited less acid-tolerant Listeria pathogens in silage (Figure 4). The increased Clostridiales abundance and pH values in alfalfa silages indicated a secondary fermentation at 90 days (Flythe and Russell, 2004), which led to LA consumption, and accumulation of some weaker volatile fatty acids like AA, PA, and BA. This was consisted with the presence of PA in 90-d silage (Table 1). Although Clostridia is a major producer of BA in silage, presence of BA was not detected in our study. This might result from the activity of BA-utilizing microbes in silage. For instance, relative abundance of Aerococcus, a butyrate-producing saccharolytic genus, increased at 90 days in all the three treatments compared with 30-d silage (P < 0.05). Similarly, relative abundance of Serratia, whose presence is typically associated with 2,3-butanediol production (McDonald et al., 1991), increased at 90 days in LPII (0.03% at 30 days, 0.11% at 90 days). Flythe and Russell (2004) reported that some Clostridia could produce large amounts of AA apart from BA. This might explain the high AA concentration in CK at 90 days. A high abundance of Garciella, an anaerobic and thermophilic bacterium belonging to Clostridiales (Miranda-Tello et al., 2003), was apparent in the 90-d silage. A high abundance level of Garciella was also reported by Zhang et al. (2018) in alfalfa silage ensiled at a high temperature of 40°C. The relatively high temperature during July (with the highest temperature over 40°C during the day time) might be one cause of Garciella growth. Spearman’s correlation analysis showed a weak relationship between Garciella and NH3-N (Figure 5B). In all, the current evidence is insufficient to indicate a certain role of Garciella in the ensiling process.
Although L. plantarum inoculation promoted Lactobacillus abundance in silage, it failed to stabilize the abundance of Lactobacillus at 90 days. The overlong period of wilting before ensiling may have exacerbated the poor stability of the bacterial community. The period of wilting reduced the WSC concentration available for LA fermentation, and led to a lower LAB population before ensiling. Occurrence of continuing relatively high temperature at daytime during summer (38–40°C) may also exacerbated the hardness for LAB dominance under high moisture condition. With the pH values in silage highly above ideal level (4.2), such environmental condition is suitable for the growth of some harmful microorganisms like Enterobacter and Clostridium, which competes with LAB growth.
Recent studies illustrated an interaction of bacterial community and silage properties (McAllister et al., 2018). Mantel tests revealed close correlations of the pH, LA, AA, PA, NH3-N, and WSC with bacterial community composition. The pH and LA affected both the richness and diversity of bacterial community. The concentration of AA remained stable for both periods (Table 1) regardless of the shift in bacterial abundance. This is consistent with its poor relationship with bacterial richness illustrated by Spearman correlations. There was a negative correlation between AA and diversity of the bacterial community, possibly due to an inhibitory effect of AA toward some bacterial species. The PA and NH3-N had positive correlations with bacterial richness, but this did not affect diversity of the bacterial community. A probable explanation is that PA and NH3-N were mainly produced by some specific species in silage, so concentrations of these products mainly depended on the number of their producers rather than the composition of microbes. The WSC was negatively correlated with bacterial abundance. This is predictable, because WSC is consumed with the growth of microbes, as a main nutrient source of microbes in silage.
Spearman’s correlation analysis illustrated some potential spoilage genera in alfalfa silage. Genera that correlated with NH3-N differed between the two periods. This indicates a potential shift in the main promoters of NH3-N formation during ensiling. At 30 days, Rosenbergiella and Bifidobacterium were potential promoters of NH3-N formation. Rosenbergiella is a genus belonging to Enterobacteriaceae. An abundance of Bifidobacterium was also reported in direct-cut alfalfa silage with a high NH3-N concentration (Zheng et al., 2017). At 90 days, presence of Clostridia lead to increasing formation of NH3-N by fermenting amino acids (Flythe and Russell, 2004). Spearman’s correlation analysis illustrates a series of NH3-N producers belonging to Clostridia, including Clostridium_XlVa, Clostridium_XI, and Finegoldia. Another two nitrogen-utilizing genera, Anaerotruncus and Peptoniphilus, were also considered to promote NH3-N formation. There is little evidence concerning the role of Eremococcus in silage fermentation. One recent study reported dominance of Eremococcus in the anodic biofilms in air–cathode microbial fuel cells (Zhang et al., 2016). In air–cathode microbial fuel cells, electroactive microorganisms, mainly in the form of anodic biofilms, accelerate the rate of electrochemical oxidation of complex organic substrates (Borole et al., 2011). This indicates the potential capability of Eremococcus in oxidizing organic materials; however, this study did not show that oxidation by Eremococcus could occur under acidic conditions. Thus, further research is needed to explore its role in the ensiling system. Anaerococcus is a butyrate-producing saccharolytic genus, and so may exhibit synergy with Clostridium during ensiling – this might explain its positive correlation with NH3-N. Some species of Vagococcus were reported to exhibit capability for arginine hydrolysis (Teixeira et al., 1997), which might aggravate protein degradation in silage. In this study, Weissella was outcompeted by Lactobacillus following L. plantarum inoculation, and exhibited low abundance in the inoculated groups. This might explain the positive correlation between Weissella and NH3-N. A higher abundance of Weissella was exhibited in CK, for which the NH3-N concentration was significantly higher than the other treatments. Propionibacterium requires complex nutrition, including multiple vitamins and an abundant nitrogen source to produce PA and AA from glucose and other carbohydrates (Rogers et al., 2006). Accumulation of NH3-N might promote its growth and fermentation metabolism, resulting in the high PA concentrations in the 90-d silage. Brevibacillus was also positively correlated with NH3-N, but its role in ensiling is currently unclear.
The L. plantarum inoculation induced a rapid drop in pH at 30 days, which would inhibit proteolytic bacteria and contribute to true protein preservation (Weinberg and Muck, 1996). This was also illustrated by the inhibitory effect against Clostridiales in the inoculated silage and the negative correlation between Lactobacillus and NH3-N. Apart from Lactobacillus, correlation analysis suggests that some other genera may also contribute to protein preservation. However, the roles of these genera in the ensiling process remain little understood, and more evidence is needed to elucidate their relationships with protein preservation in silage.
In conclusion, high precipitation in spring and summer during harvest and high temperatures during the ensiling process of alfalfa are universal in mid-eastern and southern China, as well as other areas with similar climatic characteristics. Inoculations of L. plantarum significantly promoted pH decline, LA accumulation, and Lactobacillus abundance under such poor fermentation conditions in alfalfa silage. Growth of Clostridia were inhibited after L. plantarum inoculations, which further reduced NH3-N formation in the 90-d silage. Thus, L. plantarum improved the fermentation properties of alfalfa silage under adverse ensiling conditions by enhancing LA fermentation and inhibiting the growth of spoilage microorganisms, and further delayed the spoilage of silage.
This manuscript has been released as a pre-print at ResearchSquare, Applied & Industrial Microbiology.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
FY: conceptualization, formal analysis, writing – original draft, and visualization. YaW: writing – review and editing, supervision, project administration, and funding acquisition. SZ: methodology, validation and investigation. YuW: resources. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (31772672).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AA, acetic acid; BA, butyric acid; BC, buffering capacity; DM, dry matter; FW, fresh weight; LA, lactic acid; LAB, lactic acid bacteria; LDA, linear discriminant analysis; NH3-N, ammoniacal nitrogen; PA, propionic acid; WSC, water soluble carbohydrate.
Addah, W., Baah, J., Groenewegen, P., Okine, E. K., and McAllister, T. A. (2011). Comparison of the fermentation characteristics, aerobic stability and nutritive value of barley and corn silages ensiled with or without a mixed bacterial inoculant. Can. J. Anim. Sci. 91, 133–146. doi: 10.4141/cjas10071
Agarussi, M. C. N., Pereira, O. G., Silva, V. P. D., Leandro, E. S., and Santos, S. A. (2019). Fermentative profile and lactic acid bacterial dynamics in non-wilted and wilted alfalfa silage in tropical conditions. Mol. Biol. Rep. 46, 451–460. doi: 10.1007/s11033-018-4494-z
AOAC (1990). Official Methods of Analysis, 15th Edn, Arlington, VA: Association of Official Analytical Chemists.
Borole, A. P., Reguera, G., Ringeisen, B., Wang, Z.-W., Feng, Y., and Kim, B. H. (2011). Electroactive biofilms: current status and future research needs. Energy Environ. Sci. 4, 4813–4834.
Broderick, G. A., and Kang, J. H. (1980). Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 63, 64–75. doi: 10.3168/jds.s0022-0302(80)82888-8
Coblentz, W. K., and Muck, R. E. (2012). Effects of natural and simulated rainfall on indicators of ensilability and nutritive value for wilting alfalfa forages sampled before preservation as silage. J. Dairy Sci. 95, 6635–6653. doi: 10.3168/jds.2012-5672
Cole, J. R., Wang, Q., Cardenas, E., Fish, J., Chai, B., Farris, R. J., et al. (2009). The ribosomal database project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 37, 141–145.
Dunière, L., Sindou, J., Chaucheyras-Durand, F., Chevallier, I., and Thevenot-Sergentet, D. (2013). Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim. Feed Sci. Tech. 182, 1–15. doi: 10.1016/j.anifeedsci.2013.04.006
Eikmeyer, F. G., Kofinger, P., Poschenel, A., Junemann, S., Zakrzewski, M., Heinl, S., et al. (2013). Metagenome analyses reveal the influence of the inoculant Lactobacillus buchneri CD034 on the microbial community involved in grass ensiling. J. Biotechnol. 167, 334–343. doi: 10.1016/j.jbiotec.2013.07.021
Flythe, M. D., and Russell, J. B. (2004). The effect of pH and a bacteriocin (bovicin HC5) on Clostridium sporogenes MD1, a bacterium that has the ability to degrade amino acids in ensiled plant materials. FEMS Microbiol. Ecol. 47, 215–222. doi: 10.1016/s0168-6496(03)00259-9
Guan, H., Yan, Y. H., Li, X. L., Li, X. M., Shuai, Y., Feng, G. Y., et al. (2018). Microbial communities and natural fermentation of corn silages prepared with farm bunker-silo in Southwest China. Bioresour. Technol. 265, 282–290. doi: 10.1016/j.biortech.2018.06.018
Guo, X. S., Ke, W. C., Ding, W. R., Ding, L. M., Xu, D. M., Wang, W. W., et al. (2018). Profiling of metabolome and bacterial community dynamics in ensiled Medicago sativa inoculated without or with Lactobacillus plantarum or Lactobacillus buchneri. Sci. Rep. 6:28358. doi: 10.1038/srep28358
Kasmaei, K. M., Dicksved, J., Sporndly, R., and Uden, P. (2017). Separating the effects of forage source and field microbiota on silage fermentation quality and aerobic stability. Grass Forage Sci. 72, 281–289. doi: 10.1111/gfs.12238
Ke, W. C., Yang, F. Y., Undersander, D. J., and Guo, X. S. (2015). Fermentation characteristics, aerobic stability, proteolysis and lipid composition of alfalfa silage ensiled with apple or grape pomace. Anim. Feed. Sci. Tech. 202, 12–19. doi: 10.1016/j.anifeedsci.2015.01.009
Keshri, J., Chen, Y. R., Pinto, R., Kroupitski, Y., Weinberg, Z. G., and Sela, S. (2018). Microbiome dynamics during ensiling of corn with and without Lactobacillus plantarum inoculant. Appl. Microbiol. Biot. 102, 4025–4037. doi: 10.1007/s00253-018-8903-y
Kung, L. J., and Shaver, R. (2001). Interpretation and use of silage fermentation analysis reports. Focus Forage 3, 1–5.
Le Lay, C., Coton, E., Le Blay, G., Chobert, J. M., Haertle, T., and Choiset, Y. (2016). Identification and quantification of antifungal compounds produced by lactic acid bacteria and propionibacteria. Int. J. Food Microbiol. 239, 79–85. doi: 10.1016/j.ijfoodmicro.2016.06.020
Li, X., Tian, J., Zhang, Q., Jiang, Y., Wu, Z., and Yu, Z. (2018). Effects of mixing red clover with alfalfa at different ratios on dynamics of proteolysis and protease activities during ensiling. J. Dairy Sci. 101, 1–11.
Li, J., Yuan, X., Desta, S. T., Dong, Z., Mugabe, W., and Shao, T. (2018). Characterization of Enterococcus faecalis JF85 and Enterococcus faecium Y83 isolated from Tibetan yak (Bos grunniens) for ensiling Pennisetum sinese. Bioresour. Technol. 257, 76–83. doi: 10.1016/j.biortech.2018.02.070
McAllister, T. A., Duniere, L., Drouin, P., Xu, S., Wang, Y., Munns, K., et al. (2018). Silage review: using molecular approaches to define the microbial ecology of silage. J. Dairy Sci. 101, 4060–4074. doi: 10.3168/jds.2017-13704
McDonald, P., Henderson, A. R., and Heron, S. J. E. (1991). The Biochemistry of Silage. Hoboken, NJ: Wiley.
Miranda-Tello, E., Fardeau, M. L., Sepúlveda, J., Fernández, L., Cayol, J. L., Thomas, P., et al. (2003). Garciella nitratireducens gen. nov., sp. nov., an anaerobic, thermophilic, nitrateand thiosulfate-reducing bacterium isolated from an oilfield separator in the Gulf of Mexico. Int. J. Syst. Evol. Micr. 53, 1509–1514. doi: 10.1099/ijs.0.02662-0
Murphy, R. P. (1958). A method for the extraction of plant samples and the determination of total soluble carbohydrates. J. Sci. Food Agric. 9, 714–717. doi: 10.1002/jsfa.2740091104
Ni, K., Minh, T., Tu, T., Tsuruta, T., Pang, H., and Nishino, N. (2017). Comparative microbiota assessment of wilted Italian ryegrass, whole crop corn, and wilted alfalfa silage using denaturing gradient gel electrophoresis and next-generation sequencing. Appl. Microbiol. Biot. 101, 1385–1394. doi: 10.1007/s00253-016-7900-2
Ni, K. K., Wang, F. F., Zhu, B. G., Yang, J. X., Zhou, G. A., Pan, Y., et al. (2017). Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour. Technol. 238, 706–715. doi: 10.1016/j.biortech.2017.04.055
Ni, K. K., Zhao, J. Y., Zhu, B. G., Su, R. N., Pan, Y., Ma, J. K., et al. (2018). Assessing the fermentation quality and microbial community of the mixed silage of forage soybean with crop corn or sorghum. Bioresour. Technol. 265, 563–567. doi: 10.1016/j.biortech.2018.05.097
Nkosi, B. D., Meeske, R., Langa, T., Motiang, M. D., Modiba, S., Mkhize, N. R., et al. (2016). Effects of ensiling forage soybean (Glycine max (L.) Merr.) with or without bacterial inoculants on the fermentation characteristics, aerobic stability and nutrient digestion of the silage by Damara rams. Small Ruminant Res. 134, 90–96. doi: 10.1016/j.smallrumres.2015.12.001
Ogunade, I. M., Jiang, Y., Cervantes, A. A. P., Kim, D. H., Oliveira, A. S., Vyas, D., et al. (2018). Bacterial diversity and composition of alfalfa silage as analyzed by Illumina MiSeq sequencing: effects of Escherichia coli O157:H7 and silage additives. J. Dairy Sci. 101, 2048–2059. doi: 10.3168/jds.2017-12876
Oliveira, A. S., Weinberg, Z. G., Ogunade, I. M., Cervantes, A. A. P., Arriola, K. G., Jiang, Y., et al. (2017). Meta-analysis of effects of inoculation with homofermentative and facultative heterofermentative lactic acid bacteria on silage fermentation, aerobic stability, and the performance of dairy cows. J. Dairy Sci. 100, 4587–4603. doi: 10.3168/jds.2016-11815
Pang, H. L., Qin, G. Y., Tan, Z. F., Li, Z. W., Wang, Y. P., and Cai, Y. M. (2011). Natural populations of lactic acid bacteria associated with silage fermentation as determined by phenotype, 16S ribosomal RNA and recA gene analysis. Syst. Appl. Microbiol. 34, 235–241. doi: 10.1016/j.syapm.2010.10.003
Playne, M. J., and Mcdonald, P. (1996). The buffering constituents of herbage and of silage. J. Sci. Food Agric. 17, 264–268. doi: 10.1002/jsfa.2740170609
Rogers, P., Chen, J. S., and Zidwick, M. J. (2006). “Organic acid and solvent production,” in The Prokaryotes, ed. M. Dworkin (New York, NY: Springer), 611–615.
Scherer, R., Gerlach, K., and Sudekum, K. H. (2015). Biogenic amines and gamma-amino butyric acid in silages: formation, occurrence and influence on dry matter intake and ruminant production. Anim. Feed. Sci. Tech. 210, 1–16. doi: 10.1016/j.anifeedsci.2015.10.001
Schmidt, R. J., and Kung, L. (2010). The effects of Lactobacillus buchneri with or without a homolactic bacterium on the fermentation and aerobic stability of corn silages made at different locations. J. Dairy Sci. 93, 1616–1624. doi: 10.3168/jds.2009-2555
Tao, L., Guo, X. S., Zhou, H., Undersander, D. J., and Nandety, A. (2012). Short communication: characteristics of proteolytic activities of endo- and exopeptidases in alfalfa herbage and their implications for proteolysis in silage. J. Dairy Sci. 95, 4591–4595. doi: 10.3168/jds.2012-5383
Tao, L., Zhou, H., Zhang, N., Si, B., Tu, Y., Ma, T., et al. (2017). Effects of different source additives and wilt conditions on the pH value, aerobic stability, and carbohydrate and protein fractions of alfalfa silage. Anim. Sci. J. 88, 99–106. doi: 10.1111/asj.12599
Teixeira, L. M., Carvalho, M. G. S., Merquior, V. L. C., Steigerwalt, A. G., and Facklam, R. R. (1997). Phenotypic and genotypic characterization of Vagococcus fluvialis, including strains isolated from human sources. J. Clin. Microbiol. 35, 2778–2781. doi: 10.1128/jcm.35.11.2778-2781.1997
Wang, C., He, L. W., Xing, Y. Q., Zhou, W., Yang, F. Y., Chen, X. Y., et al. (2019). Fermentation quality and microbial community of alfalfa and stylo silage mixed with Moringa oleifera leaves. Bioresour. Technol. 284, 240–247. doi: 10.1016/j.biortech.2019.03.129
Wang, S., Yuan, X., Dong, Z., Li, J., and Shao, T. (2017). Effect of ensiling corn stover with legume herbages in different proportions on fermentation characteristics, nutritive quality and in vitro digestibility on the Tibetan Plateau. Grassl. Sci. 63, 236–244. doi: 10.1111/grs.12173
Weinberg, Z. G., and Ashbell, G. (2003). Engineering aspects of ensiling. Biochem. Eng. J. 13, 181–188. doi: 10.1016/s1369-703x(02)00130-4
Weinberg, Z. G., and Muck, R. E. (1996). New trends in development and use of inoculants for silage. FEMS Microbiol. Rev. 19, 53–68. doi: 10.1111/j.1574-6976.1996.tb00253.x
Yang, F. Y., Wang, Y. P., Zhao, S. S., and Wang, Y. (2020). Lactobacillus plantarum inoculation delays decomposition of alfalfa silage under adverse ensiling conditions by regulating bacterial community composition. ResearchSquare [Preprint], Available online at: https://www.researchsquare.com/article/rs-22777/v1 (accessed April 21, 2020).
Yang, L. L., Yuan, X. J., Li, J. F., Dong, Z. H., and Shao, T. (2019). Dynamics of microbial community and fermentation quality during ensiling of sterile and nonsterile alfalfa with or without Lactobacillus plantarum inoculant. Bioresour. Technol. 275, 280–287. doi: 10.1016/j.biortech.2018.12.067
Yuan, X., Wen, A., Dong, Z., Desta, S. T., and Shao, T. (2017). Effects of formic acid and potassium diformate on the fermentation quality, chemical composition and aerobic stability of alfalfa silage. Grass Forage Sci. 72, 833–839. doi: 10.1111/gfs.12296
Zhang, E., Zhai, W., Luo, Y., Scott, K., Wang, X., and Diao, G. (2016). Acclimatization of microbial consortia to alkaline conditions and enhanced electricity generation. Bioresour. Technol. 211, 736–742. doi: 10.1016/j.biortech.2016.03.115
Zhang, Q., Yu, Z., Wang, X., and Tian, J. (2018). Effects of inoculants and environmental temperature on fermentation quality and bacterial diversity of alfalfa silage. Anim. Sci. J. 89, 1085–1092. doi: 10.1111/asj.12961
Zhao, S. S., Wang, Y. P., Yang, F. Y., Wang, Y., and Zhang, H. (2020). Screening a Lactobacillus plantarum strain for good adaption in alfalfa ensiling and demonstrating its improvement of alfalfa silage quality. J. Appl. Microbiol. 129, 233–242. doi: 10.1111/jam.14604
Keywords: Lactobacillus plantarum, spoilage microorganism, alfalfa silage, adverse ensiling condition, high-throughput sequencing
Citation: Yang F, Wang Y, Zhao S and Wang Y (2020) Lactobacillus plantarum Inoculants Delay Spoilage of High Moisture Alfalfa Silages by Regulating Bacterial Community Composition. Front. Microbiol. 11:1989. doi: 10.3389/fmicb.2020.01989
Received: 28 May 2020; Accepted: 27 July 2020;
Published: 12 August 2020.
Edited by:
Andreas Otto Wagner, University of Innsbruck, AustriaReviewed by:
Zwi G. Weinberg, Agricultural Research Organization (ARO), IsraelCopyright © 2020 Yang, Wang, Zhao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanping Wang, d3lwQHp6dS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.