- 1South Texas Center for Emerging Infectious Diseases, Department of Biology, The University of Texas at San Antonio, San Antonio, TX, United States
- 2Cockrell School of Engineering, The University of Texas at Austin, Austin, TX, United States
Candida auris is an emergent multidrug-resistant pathogenic yeast with an unprecedented ability for a fungal organism to easily spread between patients in clinical settings, leading to major outbreaks in healthcare facilities. The formation of biofilms by C. auris contributes to infection and its environmental persistence. Most antifungals and sanitizing procedures are not effective against C. auris, but antimicrobial nanomaterials could represent a viable alternative to combat the infections caused by this emerging pathogen. We have previously described an easy and inexpensive method to synthesize silver nanoparticles (AgNPs) in non-specialized laboratories. Here, we have assessed the antimicrobial activity of the resulting AgNPs on C. auris planktonic and biofilm growth phases. AgNPs displayed a strong antimicrobial activity against all the stages of all C. auris strains tested, representative of four different clades. Under planktonic conditions, minimal inhibitory concentration (MIC) values of AgNPs against the different strains were <0.5 μg ml−1; whereas calculated IC50 values for inhibition of biofilms formation were <2 μg ml−1 for all, but one of the C. auris strains tested. AgNPs were also active against preformed biofilms formed by all different C. auris strains, with IC50 values ranging from 1.2 to 6.2 μg ml−1. Overall, our results indicate potent activity of AgNPs against strains of C. auris, both under planktonic and biofilm growing conditions, and indicate that AgNPs may contribute to the control of infections caused by this emerging nosocomial threat.
Introduction
Candida auris is an emergent multidrug-resistant yeast that has been reported worldwide since its detection in Japan in 2009 (Centers for Disease Control and Prevention, 2019). It has been determined that there are four geographic clades of this pathogen, including South Asian (clade I), East Asian (clade II), South African (clade III), and South American (clade IV), which interestingly seemed to have emerged independently in different regions of the world at the same time (Jeffery-Smith et al., 2017; Rhodes and Fisher, 2019), with a potential fifth clade recently identified in Iran (Chow et al., 2019). C. auris is described as an ovoid-shaped non-dimorphic yeast that rarely forms pseudohyphae and exhibits two growing typical phenotypes: aggregative and non-aggregative (Ku et al., 2018; Forsberg et al., 2019). C. auris spreads in healthcare settings, posing a risk for hospital patients due to its high mortality rate invasive infections and its healthcare-associated outbreaks (Sears and Schwartz, 2017; Forsberg et al., 2019). It easily contaminates surfaces and medical instrumentation within healthcare facilities for long periods, which poses a risk factor in healthcare facilities worldwide (Sears and Schwartz, 2017). C. auris is considered as an urgent threat by the Centers for Disease Control and Prevention (CDC), according to their “Antibiotic Resistance Threats in the United States, 2019” (CDC, 2019a).
Currently, the prevention and treatment of C. auris are challenging due to several factors. This yeast is known for its resistance to the main classes of clinically-used antifungal agents, and it is usually found as resistant to multiple drugs; also, its antifungal resistance profile is different in each strain (CDC, 2019b), which negatively impact treatment’s effectivity. Additionally, it is commonly misidentified in clinical laboratories, often leading to inappropriate treatments. Furthermore, it is able to form biofilms, C. auris biofilms, besides being intrinsically resistant to all antifungal agents (Sherry et al., 2017), can also withstand exposure to harsh setting conditions, such as high temperature and salinity concentration, and can survive in plastic surfaces up to 2 weeks (Welsh et al., 2017). This yeast is highly resistant to current sanitation processes and treatments, such as UV light and quaternary ammonium compounds (Ku et al., 2018), which defy our capacity to control its propagation.
Therefore, new treatments are needed to prevent and control C. auris growth and dissemination. Nanotechnology can provide new cost-effective antimicrobial nanomaterials (nanoantibiotics) that work as disinfectants and antimicrobial drugs. In particular, silver nanoparticles (AgNPs) exhibit good antimicrobial properties with a wide range of action against a broad range of microorganisms, including several Candida species (Raghunath and Perumal, 2017; Vazquez-Muñoz et al., 2017). Additionally, nanoantibiotics can overcome the microbial drug-resistance to antibiotics (Rudramurthy et al., 2016; Vazquez-Muñoz et al., 2019b). However, to date, only one report from our group has described the effect of AgNPs (synthesized using a different method) against a single isolate of C. auris in suspension and on functionalized medical and environmental surfaces (Lara et al., 2020). This study demonstrated that AgNPs effectively inhibit the C. auris biofilm formation. Additionally, a non-nanostructured silver commercial formulation [0.01% silver nitrate (AgNO3) with 11% hydrogen peroxide] was shown to be effective against C. auris (Biswal et al., 2017).
We have recently reported on a modified facile, inexpensive synthetic method to generate AgNPs in non-specialized laboratories and described their antibacterial and antifungal properties. We hypothesize that AgNPs display strong antifungal activity against multiple strains of C. auris, regardless of their clade, antibiotic-resistant profile, or morphological traits. Therefore, the objective of this study was to assess the antimicrobial activity of AgNPs synthesized using our newly described method, on different C. auris strains, for which we have evaluated the antimicrobial activity of nanoantibiotics against 10 C. auris strains from the CDC panel, representing the four different major clades, both under planktonic and biofilm growing conditions.
Materials and Methods
Reagents
Roswell Park Memorial Institute (RPMI) 1640 culture medium, phosphate saline buffer (PBS), 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide salt (XTT) (0.5 g L−1, in PBS), and menadione (for 3 μM final concentration) (Pierce et al., 2008) were acquired from Sigma-Aldrich (MO). Osmium tetroxide (OsO4; 4% solution) and glutaraldehyde (2.5% solution) were acquired from Ted Pella, Inc. Solutions of the different reagents were prepared in Milli-Q water.
Silver Nanoparticles
AgNPs coated with polyvinylpyrrolidone (PVP) were synthesized by a chemical reduction protocol, reported previously by our group (Vazquez-Muñoz et al., 2019a). The synthesis method uses a simple and fast chemical reduction process that involves the addition of PVP to a warmed AgNO3 solution, followed by sodium borohydride. The AgNPs obtained have an aspect ratio close to 1, an average size of 6.18 ± 5 nm and a zeta potential score of −16.2 mV. The negatively-charged, small, spheroid AgNPs displayed the strong antimicrobial activity against Staphylococcus aureus and Candida albicans (Vazquez-Muñoz et al., 2019a). This easy-to-replicate-synthesis method was specifically developed so that it can be readily implemented in non-specialized facilities and laboratories.
Strains and Culture Conditions
C. auris strains were acquired from the CDC antimicrobial resistance (AR) Isolate Bank stock (CDC, 2019b).The following AR bank strains were used: clade I (#0382, #0387, #0388, #0389, and #0390), clade II (#0381), clade III (#0383 and #0384), and clade IV (#0385 and #0386). Frozen glycerol stocks of the microbial cells were subcultured onto Yeast extract-Peptone-Dextrose (YPD) (BD Difco, MD, USA) agar plates, for 48 h at 37°C. Then, C. auris was cultured into YPD liquid media overnight at 30°C, in an orbital shaker. Cells from these cultures were prepared for the susceptibility tests, as described in the following sections.
Antifungal Susceptibility Testing Under Planktonic Conditions
The antimicrobial activity of the nanoparticles on the C. auris planktonic cells was determined by following the guidelines from the CLSI M27 protocol (CLSI, 2017) for Candida species, with minor modifications. Briefly, the yeast cells were washed twice in PBS, counted in a Neubauer chamber, and adjusted for a final concentration of 103 cells ml−1 in RPMI culture media. Then, 50 μl of the C. auris strains was inoculated in 96 multi-well round-bottom plates (Corning Inc., Corning, NY, USA). AgNPs were prepared in a two-fold dilution series in RPMI, and then 50 μl of the dilution series was added to the plates with the yeast, for final AgNPs concentration range from 0.5 to 256 μg ml−1. Plates were incubated at 35°C for 48 h. The minimal inhibitory concentration (MIC) was set as the concentration in the well at which no microbial growth – turbidity or microbial pellet formation – were observed, as suggested by the CLSI guidelines. The minimal fungicidal concentration (MFC) was also established, as follows: after reading the MIC in each plate, 10 μl from each well containing the untreated and treated microbial cells was reinoculated in YPD agar plates and incubated for 24 h at 37°C. The MFC was set as the lowest concentration of nanoparticles for which growth of ≤2 colony-forming units (CFUs) was observed upon plating, corresponding to the killing of 99.9% of the initial inoculum (Cantón et al., 2009). To ensure reproducibility, the experiment was independently performed by two people, on separate days, using different batches of AgNPs and C. auris cultures. Experiments were performed using duplicates of the plates, which contained triplicates of each condition.
Antibiofilm Activity Assays
The antibiofilm activity of AgNPs was evaluated in both the biofilm formation phase and on the preformed biofilm, as previously reported by our group (Pierce et al., 2008). For inhibition of biofilm formation, overnight cultures of C. auris yeast cells were washed twice in PBS and adjusted to 2 × 106 cells ml−1 in RPMI culture media. Fifty microliter of the adjusted cell suspension was transferred to 96 multi-well flat-bottom plates (Corning Inc., Corning, NY, USA). Then, 50 μl of AgNPs prepared in a two-fold dilution series was added into multi-well plates, for a final concentration range from 0.5 to 256 μg ml−1, with appropriate positive and negative controls. The plates were then incubated at 37°C for 24 h to allow for biofilm formation. We also tested the activity against preformed biofilms. Briefly, cells from overnight liquid cultures were washed twice in PBS and adjusted to 1 × 106 cells ml−1 in RPMI. Then, 100 μl of the microbial suspension was inoculated into 96-multi-well plates, and then incubated for 24 h at 37°C. After incubation, the preformed biofilms were washed twice in PBS. Then, 100 μl of AgNPs in two-fold dilutions series (prepared in RPMI culture medium and resulting in final concentration ranging from 512 to 1 μl ml−1) was transferred to the wells of the microtiter plates with the preformed biofilms. Finally, the plates were incubated at 37°C for an additional 24 h.
The AgNPs anti-biofilm activity was determined using the XTT colorimetric method (Pierce et al., 2008) for both inhibition of biofilm formation and the preformed biofilm stages. Briefly, at the end of the procedure, biofilms were washed twice with PBS, and then 100 μl XTT/menadione was added to each well containing treated and untreated biofilms and in the empty wells (blank). Plates were protected from light and incubated at 37°C for 2 h. XTT absorbance was measured at λ = 490 nm in a Benchmark microplate reader (Bio-Rad, Inc.). From the collected data, we generated dose-response curves to assess the IC50 values – the drug concentration required to reduce the biofilm activity by 50% – , by fitting the normalized results to the variable slope Hill equation (for assessing the nonlinear dose-response relationship) using Prism 8 (GraphPad Software, Inc.). To verify the reproducibility of the antibiofilm activity, the experiment was independently repeated by two different people. AgNPs from different rounds of syntheses were tested using two replicates of multi-well plates, each with three replicates of the treatments.
Ultrastructural Analysis
We assessed the effect of AgNPs on the biofilm structure in all C. auris strains from the four clades, using the biofilm inhibition assays. The biofilms were treated with sublethal (yet still inhibitory) concentrations of AgNPs. Treated and untreated (control) biofilms structural analysis was performed using optical and scanning electron microscopy. Biofilms were washed twice with PBS, and then fixed with a 2.5% glutaraldehyde solution for 3 h at 4°C. For the optical microscopy observations, the glutaraldehyde-fixed biofilms were observed under a 400× magnification using the bright field mode, in an inverted optical microscope (Fisher Scientific). For the scanning electron microscopy analysis, the glutaraldehyde-fixed biofilms were postfixed and stained with a 1% OsO4 solution, for 2.5 h at 4°C. Then, the biofilms were dehydrated in an ascending concentration ethanol series, from 30 to 100%. Finally, the ethanol was completely removed, and the dried samples were coated with gold, with 25 mA current for 3 min, in a sputter coater SC7620 (Quorum Technologies). The gold-coated biofilms were observed in a TM4000Plus scanning electron microscope (SEM) (Hitachi Inc.), with magnifications 500 and 2,500×, at a voltage of 10 KeV in the high vacuum mode. The samples were prepared in duplicates, and different fields of both replicates from each sample were observed.
Results
Silver Nanoparticles Inhibit the Planktonic Growth of C. auris
AgNPs exerted the strong antimicrobial activity against all the C. auris strains growing under planktonic conditions. Against 9 out of 10 strains, AgNPs MIC values were <0.5 μg ml−1, and the MFC values were only slightly higher, ranging from 1 to 2 μg ml−1 for all strains, except for AR #0381 strain, which had an elevated MFC of 32 μg ml−1. AgNPs MIC and MFC values against each strain are summarized in Table 1.
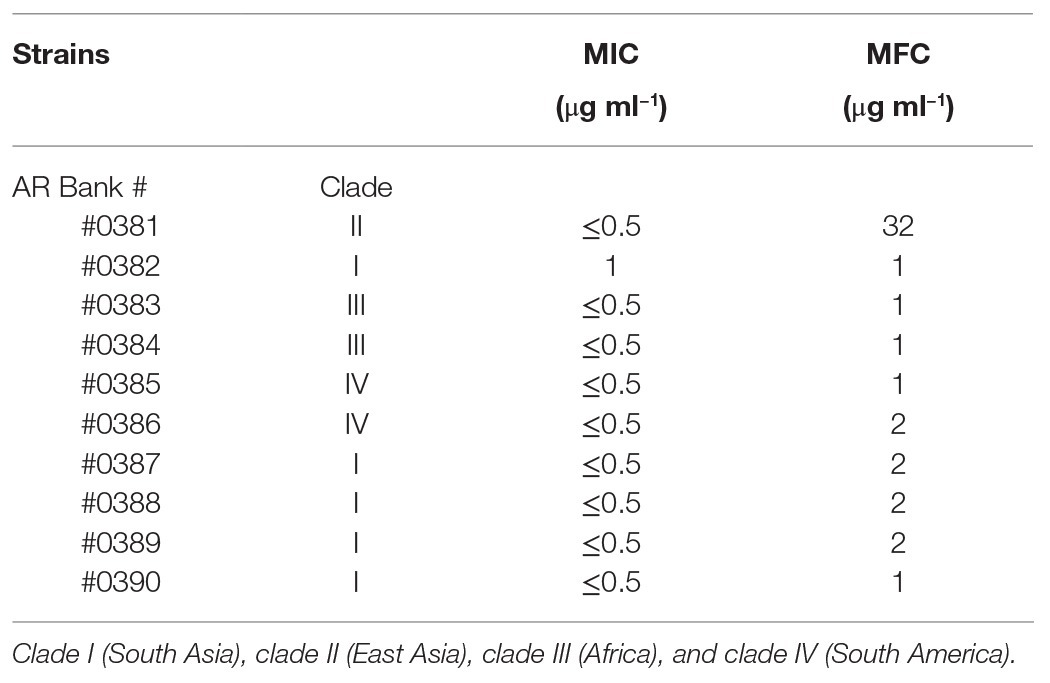
Table 1. Minimal inhibitory concentration (MIC)/minimal fungicidal concentration (MFC) values of silver nanoparticles (AgNPs) against Candida auris strains under planktonic growth conditions.
Silver Nanoparticles Inhibit C. auris Biofilm Formation
AgNPs exhibited a strong activity to prevent biofilm formation in C. auris, regardless of the clade. Figure 1 shows the biofilm-inhibitory effect against representative isolates from each clade, including strains AR #0381 (clade I), #0383 (clade III), #0386 (clade IV), and #0390 (clade II). The AgNPs antibiofilm activity against all 10 strains tested is shown in Supplementary Figure S1A. The calculated IC50 values were ranged from 0.5 to 4.9 μg ml−1 (Table 2), and for 9 out of 10 strains, the IC50 values were <2 μg ml−1. These results indicate that AgNPs exert a potent activity for the prevention of biofilm formation by the different C. auris strains.
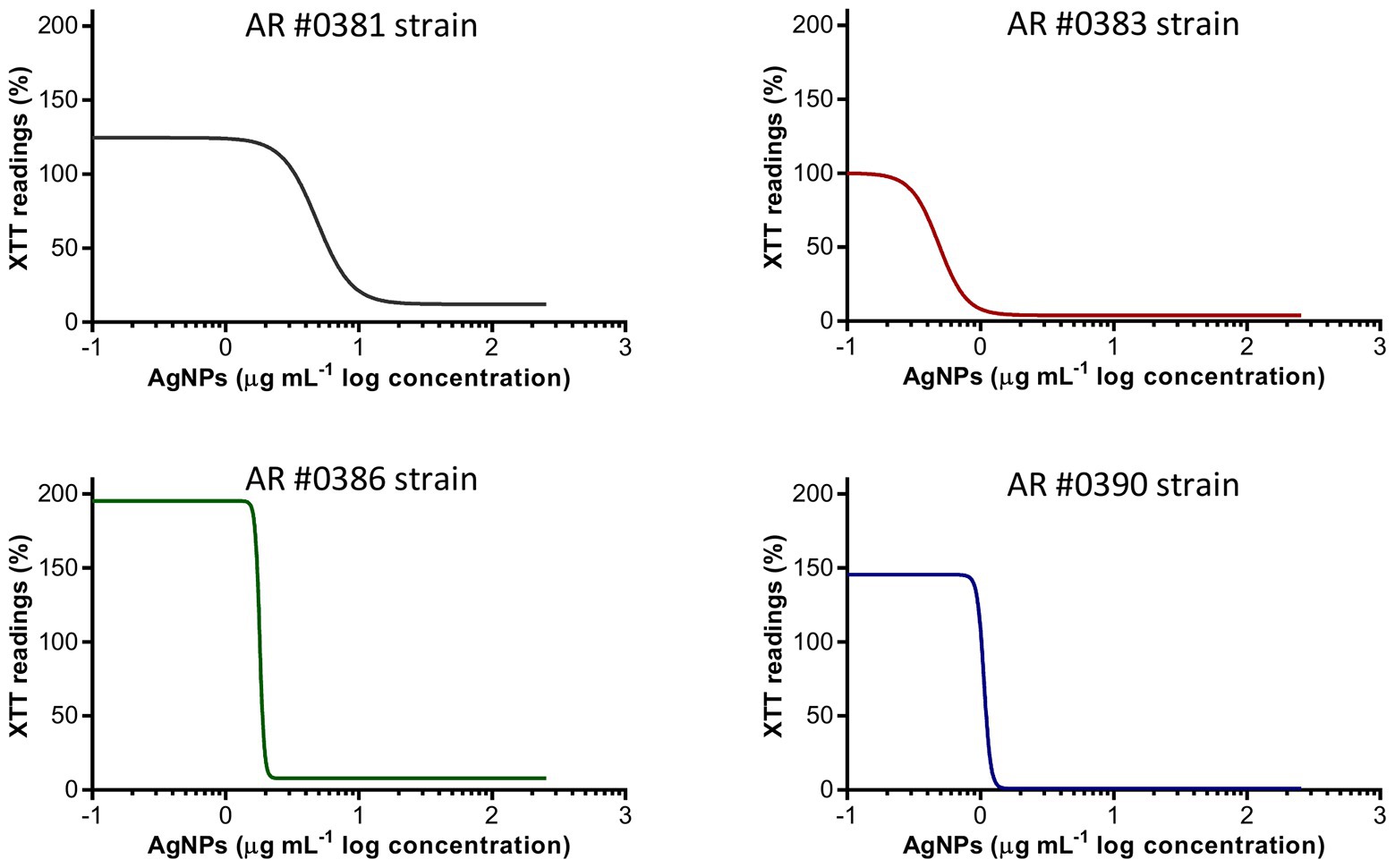
Figure 1. Silver nanoparticles (AgNPs) inhibit the biofilm formation on C. auris. The dose-response curves show that AgNPs display potent inhibitory activity (expressed as XTT) readings against the C. auris AR #0381 (clade I), #0383 (clade III), #0386 (clade IV), and #0390 (clade II) strains during the biofilm formation phase.
Interestingly, we observed that some strains exhibited a significant increase in the biofilm activity (determined by the XTT readings) when grown in the presence of very low concentrations of AgNPs. This effect was consistently observed in all replicates, although with different degrees of intensity. To the extent of our knowledge, this phenomenon has not been observed in yeasts treated with AgNPs, but it has been previously reported in bacteria (Kumar-Krishnan et al., 2015). Nevertheless, this increase in activity is promptly extinguished at just slightly higher concentrations of AgNPs. Additionally, to assess if the augmented activity was specific to the AgNPs, we evaluated the influence of AgNO3 on the C. auris AR #0390 strain, under the same culture conditions used for the AgNPs susceptibility assays. We observed an increase in the biofilm activity in subinhibitory concentrations of silver ions (Supplementary Figure S2).
Silver Nanoparticles Display Antibiofilm Activity Against Preformed C. auris Biofilms
AgNPs displays potent activity against fully mature, preformed biofilms of C. auris, irrespective of their clade, as observed for representative isolates AR #0381 (clade I), #0383 (clade III), #0386 (clade IV), and #0390 (clade II) (Figure 2). The AgNPs activity on the preformed biofilms from all 10 C. auris strains tested is shown in Supplementary Figure S1B. From the dose-response experiments, the resulting calculated IC50 values of AgNPs against preformed biofilms of the different C. auris strains were ranged from 1.2 to 6.2 μg ml−1 (Table 2), and were <4 μg ml−1 for 9 out of the 10 strains. As with the biofilm-inhibitory assays described before, we also observed an increase in the biofilm activity (determined by the XTT readings) at very low AgNPs concentrations (Figure 2; Supplementary Figure S2).
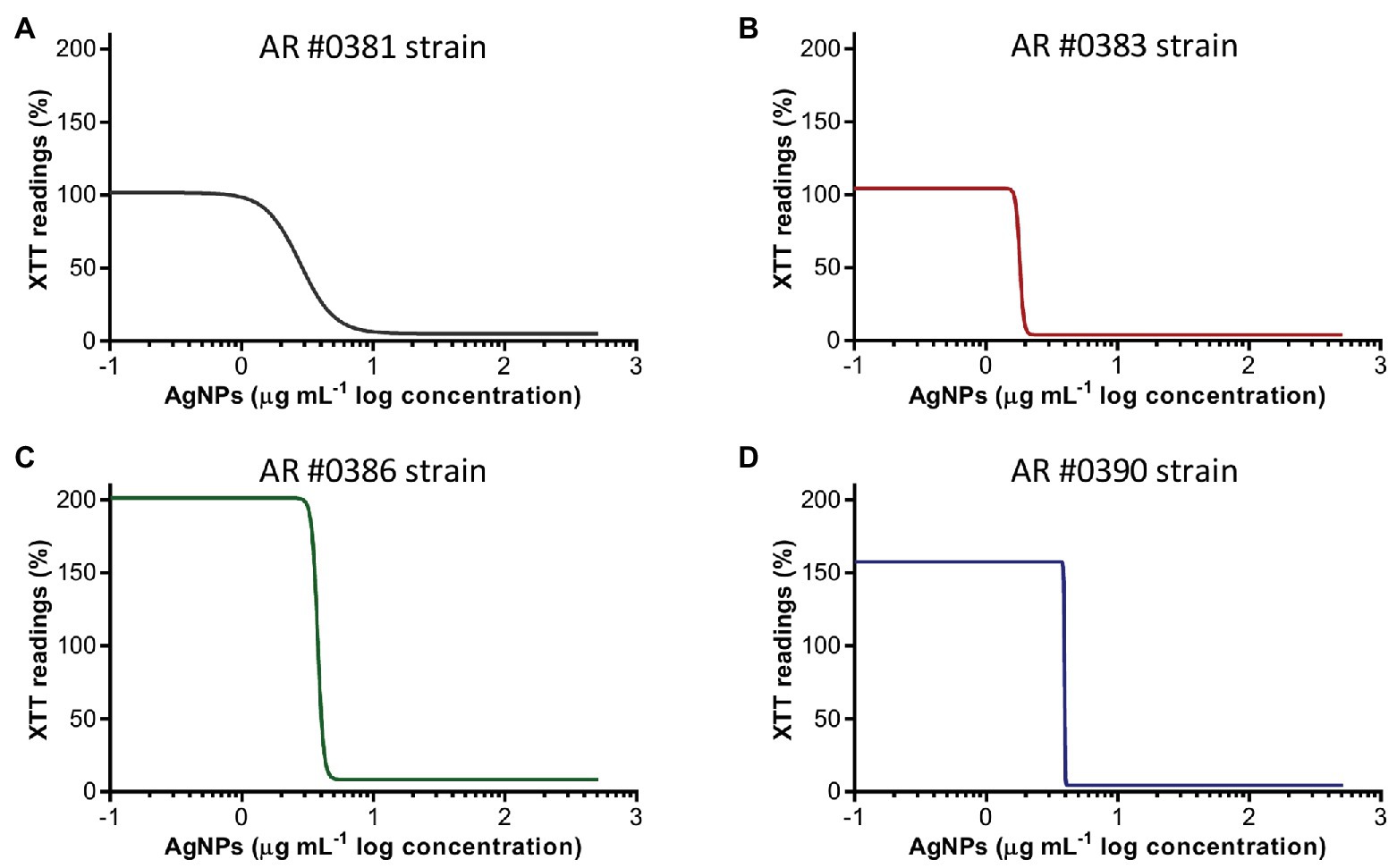
Figure 2. Antibiofilm activity of AgNPs against C. auris preformed biofilms. The dose-response curves show that AgNPs display potent inhibitory activity (expressed as the XTT readings) against preformed biofilms of the C. auris AR #0381 from clade I (A), #0383 from clade III (B), #0386 from clade IV (C), and #0390 from clade II (D) strains.
Alterations of C. auris Biofilm Structure Due to the Inhibitory Activity of AgNPs
Once we had established the activity of AgNPs against C. auris biofilms, we were interested in the visualization of the effects of treatment with these nanoantibiotics exerted on the overall biofilm structure, as well as on individual cells within the biofilms. Thus, in another set of experiments, we grew biofilms of the all different C. auris strains in the presence of subinhibitory concentrations of the AgNPs, with results for a representative strain from each clade shown in Figures 2–5, corresponding to strains #0381 (East Asia clade), #0383 (Africa clade), #0386 (South America clade), and #0390 (South Asia clade), respectively. Optical microscopy revealed that AgNPs decrease the ability of C. auris to form biofilms. As seen in Supplementary Figure S3, in the untreated control samples, biofilms formed by the different strains uniformly covered most of the bottom of the wells in the microtiter plates. In contrast, inhibitory concentrations of AgNPs disrupt the biofilm formation in all C. auris strains, as revealed by the noticeable reduction of the coverage area of biofilms on the bottom of the wells. At higher concentrations of AgNPs, biofilm formation was drastically reduced, with only isolated cells scattered on the bottom of the wells being visible under the microscope.
The biofilms were observed using SEM at low (500×) and high (2,500×) magnifications, to further determine the effect of treatment with AgNPs on the biofilm structure and the cell morphology. C. auris strains from the distinct clades display differences in the cell morphology and the biofilm organization. SEM images confirmed that exposure to inhibitory concentrations of AgNPs decreases the biofilm forming ability of the different C. auris strains (Figures 3–6; Supplementary Figure S4). SEM micrographs showed that untreated biofilms display a uniform distribution with a tight clustering of cells; in contrast, AgNPs-treated biofilms cover a noticeably lesser area, and the cells appear to be less clustered. This finding is similar to that reported recently by Lara et al. (2020), for C. auris strain #0390 when exposed to a different type of AgNPs (Lara et al., 2020).
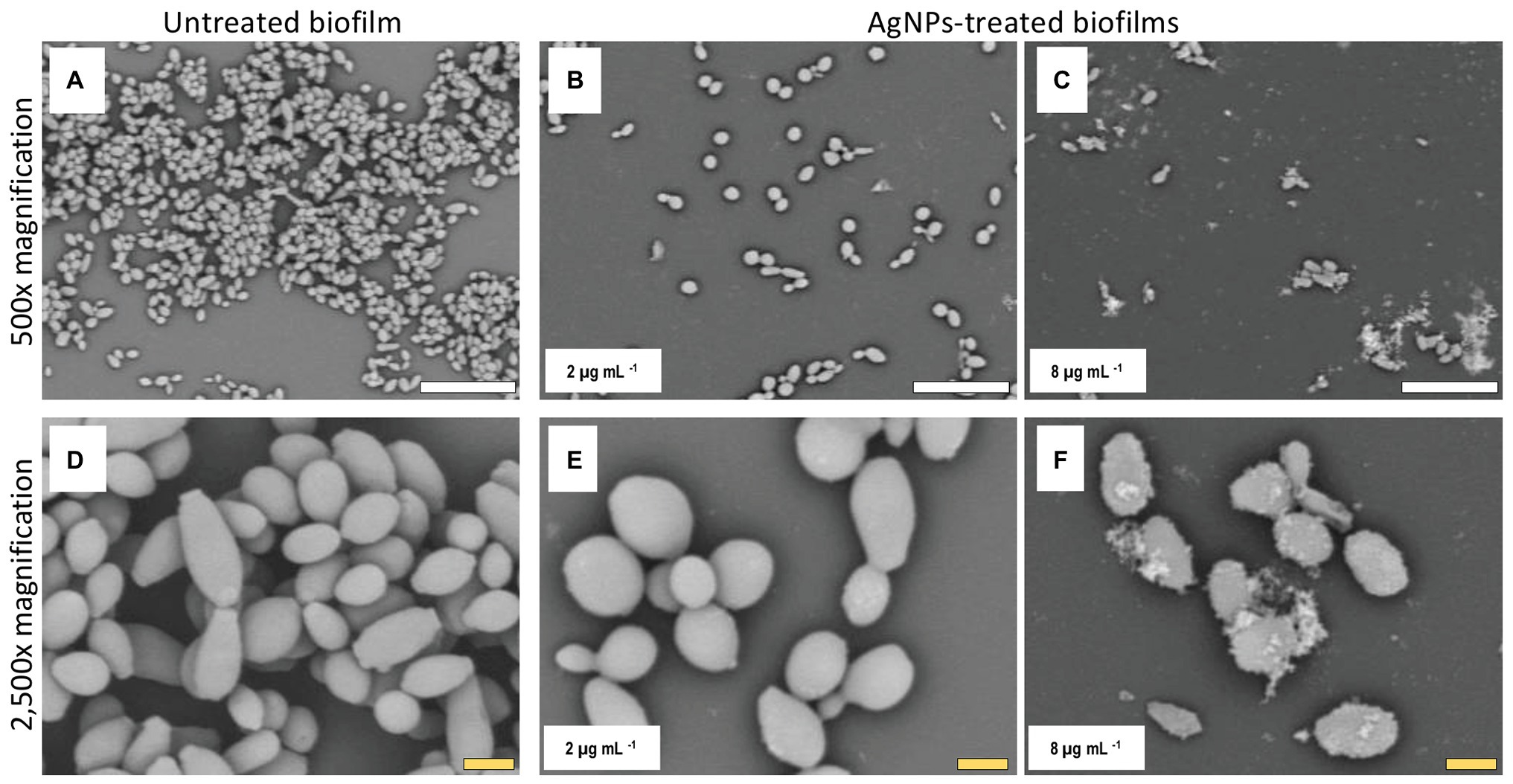
Figure 3. AgNPs affect biofilm structure and cellular morphology of C. auris strain #0381. Scanning electron microscope (SEM) micrographs reveal that untreated biofilms have a larger area of distribution (A) than the AgNPs-treated biofilms (B,C). Also, the shape and size of the cells are affected by the AgNPs (E,F), whereas the untreated biofilms remain unaltered (D). Scale bar: white = 20 μm, yellow = 2 μm.
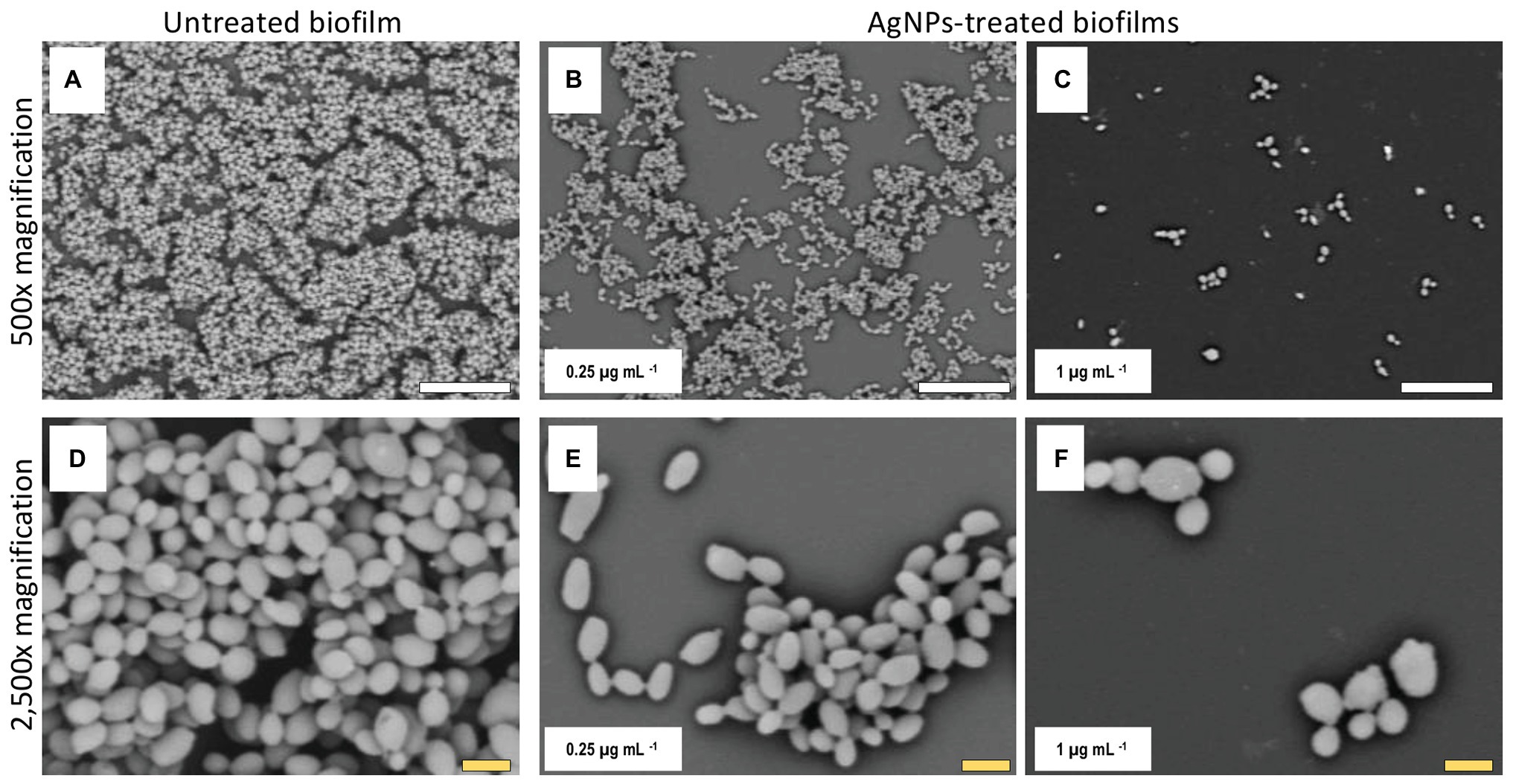
Figure 4. AgNPs reduce biofilm formation of C. auris strain #0383. SEM micrographs show a noticeable reduction in the biofilm formation in the AgNPs-treated biofilms (B,C) when contrasted with the untreated biofilms (A). However, the impact on cell morphology appears to be minimal (E,F), as the cell shape and size of cells within the treated biofilms are similar to those of the untreated control (D). Scale bar: white = 20 μm, yellow = 2 μm.
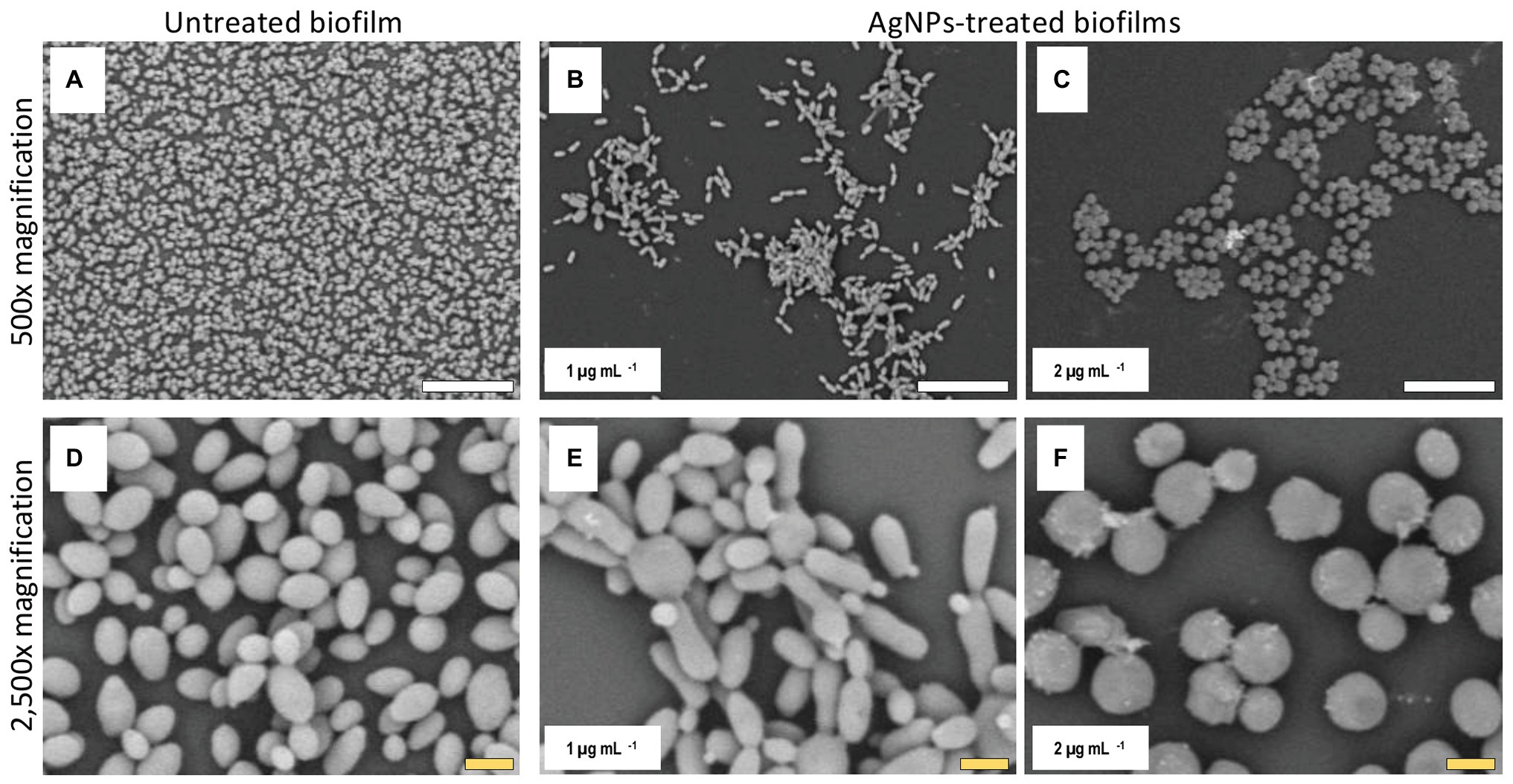
Figure 5. AgNPs affect biofilm structure and cellular morphology of C. auris strain #0386. SEM micrographs show that biofilms and individual cell morphology are drastically affected by the AgNPs. Treated biofilms (B,C) show an evident decrease in coverage area as compared to untreated biofilms (A), whereas the cell morphology is changed by treatment with AgNPs (E,F) as compared to cells in untreated biofilms (D). Scale bar: white = 20 μm, yellow = 2 μm.
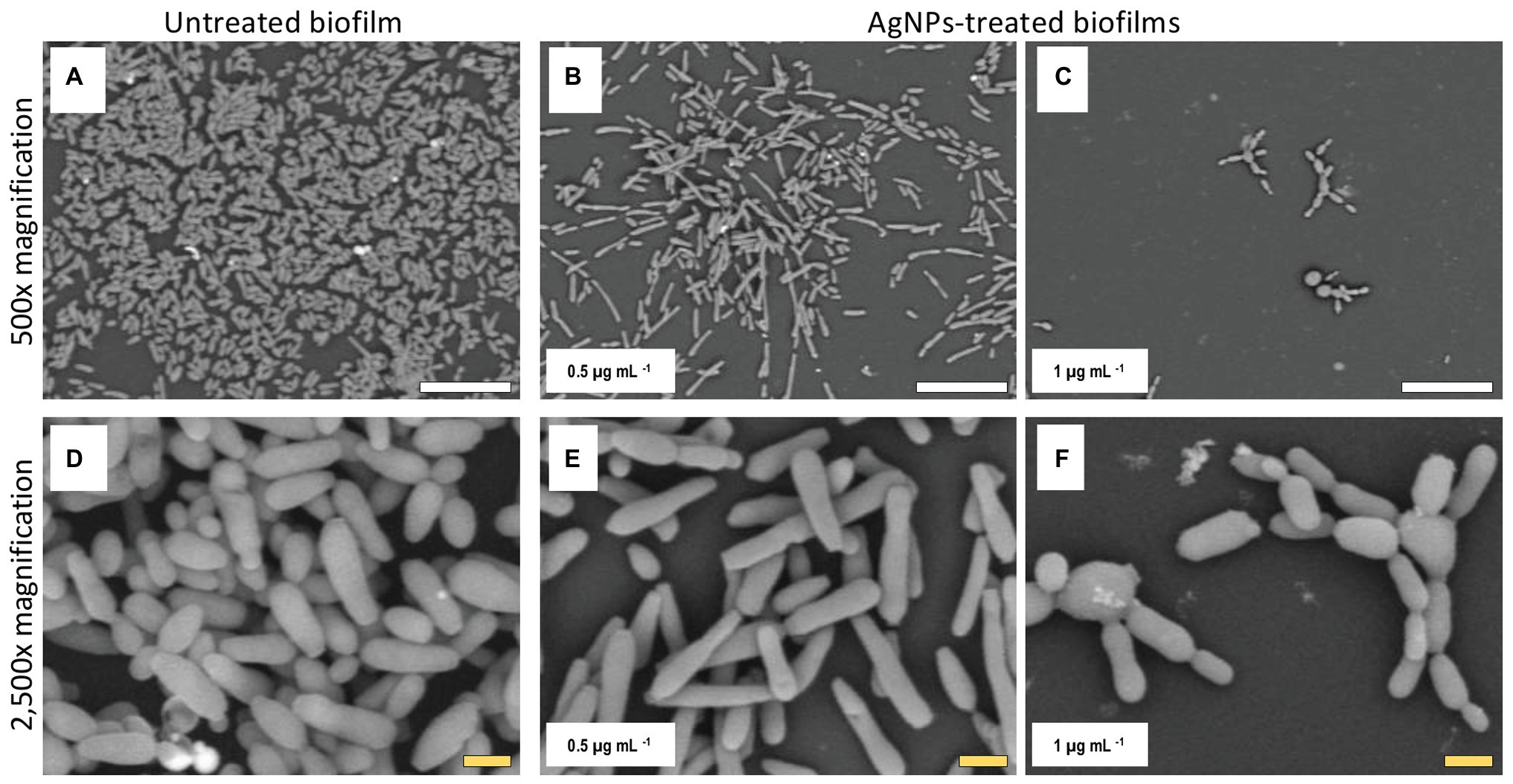
Figure 6. AgNPs affect biofilm structure and cellular morphology of C. auris strain #0390. The SEM micrographs of C. auris #0390 strain reveal that untreated biofilms (A) display a larger area of coverage than the AgNPs-treated biofilms (B,C). Moreover, untreated cells display pseudohyphae-like shape (D), which is also observed at the lowest concentration of AgNPs (E), but higher concentrations of AgNPs induce an aberrant morphology on cells and reduce the cell separation process (F) as compared to cells in untreated biofilms (D). Scale bar: white = 20 μm, yellow = 2 μm.
Moreover, when observed at higher magnification, it was revealed that treatment with AgNPs damages the fungal cell structure. In the control (untreated) samples, cells within the biofilms formed by strains #0381 (Figure 3), #0383 (Figure 4), and #0386 (Figure 5) displayed a typical oval yeast shape, whereas those in biofilms formed by strain #0390 (Figure 6) mostly exhibited a more elongated (almost pseudohyphal) morphology. For all the strains, inhibitory concentrations of AgNPs caused alterations in the shape and size of individual cells within the biofilms with also less cell clustering observed. In the case of C. auris strain #0386, low concentrations of AgNPs induced elongation of the shape in the yeast cells, similar to the pseudohyphae. However, when exposed to a higher concentration of AgNPs, the cell shape becomes spheroid, and no yeast‐ or pseudohyphae-shaped cells were observed (Figure 5). In contrast, in the C. auris strain #0390, low concentrations of AgNPs induce enlargement of the pseudohyphae-shaped cells, growing longer than in the control (Figure 6), and their presence appears to be relatively higher. However, at higher concentrations of AgNPs, the cells of this strain become yeast-shaped again but with aberrant morphology. Also, in several instances, yeast cells remained attached to each other after cell division, leading to the formation of small multibranched chains of cells, typically in groups of less than 10 cells. Supplementary Figure S3 includes SEM observations for the reminder of C. auris strains, with similar effects on biofilm structure and cellular morphology (Supplementary Figure S4).
Discussion
Silver Nanoparticles Inhibit the Planktonic Growth of C. auris
Our results show that AgNPs display potent antifungal activity at very low concentrations in virtually all C. auris strains tested. However, AgNPs MFC was higher in strain AR #0381 (32 μg ml−1). AR 0381 is the only strain from clade II and might have particular biological mechanisms that allow it to withstand the AgNPs killing effect, even when its growth is still prevented at very low concentrations of AgNPs. In a previous report (Vazquez-Muñoz et al., 2019a), similarly synthesized AgNPs exhibited strong antibacterial and antifungal activities. The MIC for S. aureus was 4 μg ml−1, whereas for C. albicans, the MIC was 2 μg ml−1, which is similar to the most common antifungals. It is worth noting that all C. auris strains are more susceptible to the AgNPs than C. albicans tested under similar experimental conditions. Also, the anti-Candidal activity of these AgNPs against planktonic cells of C. auris parallels other studies using different nanoparticles against different Candida species with typical MIC values in the 1–10 μg ml−1 range (Monteiro et al., 2011; Wady et al., 2014; Patra and Baek, 2017; Vazquez-Muñoz et al., 2017). Moreover, all C. auris strains tested here displayed susceptibility to AgNPs, irrespective of their growth characteristics, susceptibility profiles against conventional antifungal, geographical origin (clade), or their ability to form aggregates in planktonic in vitro cultures. Borman’s group showed that some clades (South African) form aggregates when grown in vitro, whereas other clades (South Asian) do not display that ability. Moreover, the aggregating phenotype may be associated with their drug susceptibility (Szekely et al., 2019). This has been observed in other microorganisms, including other Candida species, where the drug-resistant strains and drug-sensitive strains from the same species display a similar susceptibility (MIC value) to AgNPs (Romero-Urbina et al., 2015; Perween et al., 2019). Additionally, our results suggest that AgNPs antimicrobial performance is better than the main antifungals on all the tested C. auris CDC AR strains, according to their antifungal susceptibility profile reported by the CDC (CDC, 2020). Although there are not established MIC breakpoints for the main available antifungals against C. auris, the tentative MIC breakpoints for some of them are the following: fluconazole >32 μg ml−1, amphotericin B >2 μg ml−1, caspofungin >2 μg ml−1, and micafungin >4 μg ml−1 (CDC, 2020). The AgNPs MIC (<0.5 μg ml−1) outperforms even the most potent antifungal drug.
This may be due to the proposed mechanisms of action of AgNPs. The mechanism of action of the antifungal drugs is linked to specific molecular targets that disrupt the cell metabolism or structure, affecting growth. In response to these stresses, specific but relatively small changes at the structural or molecular level may increase their probability to resist the action of antifungals, as previously described for C. auris (Krishnasamy et al., 2018; Chaabane et al., 2019). In contrast, AgNPs cause several simultaneous types of structural and metabolic damages in the Candida cells, such as membrane depolarization (Zamperini et al., 2013), cell wall/membrane disruption (Lara et al., 2015), increase in ROS production (Radhakrishnan et al., 2018), inhibit enzymatic function (Babele et al., 2019), cell arrest (Zamperini et al., 2013), among many others. This massive disruption of cellular structure and function reduces their ability to withstand the AgNPs effects. Therefore, the metabolic/structural differences among the different strains are not significant when the cells from different C. auris strains are exposed to AgNPs.
Silver Nanoparticles Inhibit C. auris Biofilm Formation
C. auris is capable of forming biofilms that improve their adherence to surfaces (Forsberg et al., 2019) and increase their resistance to antifungal drugs (Sherry et al., 2017; Ku et al., 2018). The mechanisms that enhance their resistance are mostly unknown, but some factors are known to help C. auris withstand harsh conditions include the protection by matrix polysaccharides (Dominguez et al., 2019) and the overexpression of efflux pumps (Kean et al., 2018). Thus, the formation of C. auris biofilms represents a current threat to both individual patients and healthcare facilities (Sears and Schwartz, 2017). In previous work, we demonstrated the antimicrobial activity of the AgNPs on the planktonic stage of C. albicans (Vazquez-Muñoz et al., 2019a), although their activity was not assessed on the biofilm stages. In this work, we evaluate the anti-biofilm activity of the AgNPs during the biofilm formation phase and against fully mature, preformed biofilms.
Our results show that AgNPs exert a potent activity for the prevention of biofilm formation by the different C. auris strains, regardless of the clade. Interestingly, these values are only slightly higher than the MIC values obtained under planktonic growth. These values also compare favorably to those described before for conventional antifungals against biofilm formation for some of the same C. auris strains (Dekkerová et al., 2019). We note that the AgNPs IC50 value for the C. auris AR #0390 strain is higher than the value reported for the same strain as reported by Lara et al. (2015) (1.1 vs. 0.06 μg ml−1, respectively), which is most likely related to the different techniques used for the synthesis of these nanoantibiotics resulting in AgNPs with different characteristics. Also, the antibiofilm activity of our AgNPs is comparable to the activity described for AgNPs synthesized using different methods against other Candida species (Lara et al., 2015; Muthamil et al., 2018; Nadhe et al., 2019).
Regarding the increase in the biofilm activity at very low concentrations of AgNPs, this effect on the biofilm activity must be addressed in further studies, to assess any potential disadvantage of low-silver-content products intended against C. auris biofilms. As mentioned above, there are commercially available products containing silver (Biswal et al., 2017), and therefore their viability for combat the biofilms of microbial pathogens must be assessed.
Silver Nanoparticles Display Antibiofilm Activity Against Preformed C. auris Biofilms
It is well-known that once a biofilm is established, Candidal cells within these biofilms display increased susceptibility to most clinically-used antifungal agents (Ramage et al., 2009; Sherry et al., 2017). This is particularly true in the case of C. auris biofilms, which are intrinsically resistant to all the three main classes of antifungals (polyenes, azoles, and echinocandins) as well as to physical and chemical sanitizing methods (Sherry et al., 2017; Ku et al., 2018).
AgNPs displayed potent activity against fully mature, preformed biofilms of all C. auris AR strains, irrespective of their clade, with calculated IC50 values <4 μg ml−1 for 9 out of the 10 strains. Interestingly, these values are similar (typically within one-fold dilution) to those observed for the same strains in the case of biofilm inhibition (compare values in both columns of Table 2). Therefore, in stark contrast with conventional antifungal agents, the AgNPs potency does not seem to be particularly reduced after the biofilm has reached maturity. Moreover, the AgNPs antifungal activity against the preformed biofilms is equivalent or even better to that of conventional antifungals. For the C. auris AR strains #0383, #0386, and #0390, the AgNPs antibiofilm activity is superior to the activity of fluconazole (range from >64 to >1,024 μg ml−1) and caspofungin (>16 μg ml−1). Furthermore, the AgNPs potency parallels the activity of amphotericin B (1–>8 μg ml−1) (Dekkerová et al., 2019; Nadhe et al., 2019). Overall, although multiple mechanisms confer resistance of cells within biofilms against conventional antifungal agents (Srinivasan et al., 2014), our results suggest that these do not equally affect the anti-biofilm activity of AgNPs.
Alterations of C. auris Biofilm Structure Due to the Inhibitory Activity of AgNPs
The observed effects of AgNPs on cellular morphology merit some further discussions. These effects seem to be clade-related, based on our SEM analysis for the 10 strains included in this study. Both strains from clade III (South Africa) were not affected in their cellular morphology. In contrast, in both strains from clade IV (South America), inhibitory concentrations of the AgNPs altered the cell shape, leading to round-shaped cells. Interestingly, in clade I (South Asia), we observed two different effects: in strains AR #0382 and #0387, the cells were altered, from the typical yeast shape to a more elongated, pseudohyphae-like shape and was not uncommon to observe mother-daughter cells attached to each other; whereas cells from strains AR #0388, #0389, and #0390 acquire an aberrant morphology and form small multibranched chains as a result of treatment with AgNPs. In the case of C. auris strain AR #0381, the only one from clade II (East Asia), the cells became aberrant but remained separate from each other. Other studies have shown that AgNPs disrupt the biofilm and the cell ultrastructure in other Candida species, particularly on C albicans (Zamperini et al., 2013; Vazquez-Muñoz et al., 2014; Lara et al., 2015), but the effect on other morphological traits, as the cell wall thickness, extracellular matrix integrity, and intracellular bioaccumulation of cells remained to be further studied.
AgNPs are among the most used nanomaterials for health-related and cosmetic applications worldwide (Vance et al., 2015). AgNPs have been used to control skin infections in vivo in wound dressings to treat burn wounds and are used in healthcare and cosmetic applications (Sim et al., 2018). Our results suggest that AgNPs may be used as sanitizers and potentially in future uses to control skin colonization and contribute to control of the nosocomial spread of this emerging pathogen. However, the potential toxicity of these specific nanoantibiotics is still to be addressed before their direct applications in human patients. Research regarding the toxicity and effects in vivo of AgNPs is still a challenging topic, due to the complex interactions of silver with the living tissue, although there is a long use of silver in history (Klasen, 2000a,b). Recently, different studies have been aiming to find a balance between potent antimicrobial properties and toxicity, as AgNPs have several properties of clinical interest (Zivic et al., 2018). However, AgNPs may be applied, where no direct contact with humans is needed, particularly for sanitizing surfaces. Overall, our results indicate that AgNps display potent antimicrobial activity against all C. auris strains tested, both under planktonic and biofilm growing conditions. Furthermore, the antimicrobial activity is irrespective of their clade or geographical origin and regardless of their susceptibility and resistance patterns against clinically-used antifungals.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Author Contributions
All authors contributed to the study design and execution, data collection and analysis, and the preparation of the manuscript. All authors have approved the final version of the manuscript.
Funding
This research was funded by the Mexican Council of Science and Technology of Mexico (CONACYT, RV-M postdoctoral scholarship). Support in the laboratory was provided by the Margaret Batts Tobin Foundation, San Antonio, TX, USA. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript, and the content is solely the responsibility of the authors.
Conflict of Interest
The authors declare that there the research as performed in the absence of any commercial relationship that may be a potential conflict of interest.
Acknowledgments
We thank the South Texas Center for Emerging Infectious Diseases (STCEID) for the purchase of the SEM microscope used in these studies.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.01673/full#supplementary-material.
SUPPLEMENTARY FIGURE S1 | Antibiofilm activity of AgNPs against the different C. auris strains. The dose-response curve shows that AgNPs display potent inhibitory activity against all the different C. auris strains included in this study, including inhibition of biofilm formation (panel A) and against preformed biofilms (panel B).
SUPPLEMENTARY FIGURE S2 | Silver compounds exhibit a paradoxical effect on C. auris biofilms at low concentrations. Incubation in the presence of low subinhibitory concentrations of AgNO3 and AgNPs lead to an increase in biofilm activity (XTT readings) for biofilms formed by C. auris strain #0390 strain. In contrast to silver ions, this effect rapidly disappears in the case of AgNPs, turning into potent inhibitory activity at still relatively low concentrations.
SUPPLEMENTARY FIGURE S3 | Supplementary Figure S3AgNPs reduce the biofilm formation in C. auris. Optical microscopy images reveal that subinhibitory concentrations AgNPs reduce the ability of C. auris to form biofilms – as seen in the reduced area of biofilm surface coverage – when compared with their respective untreated controls.
SUPPLEMENTARY FIGURE S4 | Supplementary Figure S4Ultrastructure analysis of the C. auris biofilms. SEM images reveal that subinhibitory concentrations of AgNPs reduce the biofilm formation on all C. auris strains. Also, AgNPs negatively alter the shape and size in some C. auris strains (#0382, #0385, #0387, #0388, and #0389 strains). The effect on morphology is clade-related. Scale bar = 2 μm.
Abbreviations
AgNPs, Silver nanoparticles; MIC, Minimal inhibitory concentration; MFC, Minimal fungicidal concentration; IC50, Inhibitory concentration that reduces the microbial activity (XTT reading) by 50%; SEM, Scanning electron microscopy; CDC, Centers for disease control and prevention.
References
Babele, P. K., Singh, A. K., and Srivastava, A. (2019). Bio-inspired silver nanoparticles impose metabolic and epigenetic toxicity to Saccharomyces cerevisiae. Front. Pharmacol. 10:1016. doi: 10.3389/fphar.2019.01016
Biswal, M., Rudramurthy, S. M., Jain, N., Shamanth, A. S., Sharma, D., and Jain, K. (2017). Controlling a possible outbreak of Candida auris infection: lessons learnt from multiple interventions. J. Hosp. Infect. 97, 363–370. doi: 10.1016/j.jhin.2017.09.009
Cantón, E., Espinel-Ingroff, A., and Pemán, J. (2009). Trends in antifungal susceptibility testing using CLSI reference and commercial methods. Expert Rev. Anti-infect. Ther. 7, 107–119. doi: 10.1586/14787210.7.1.107
CDC (2019a). Antibiotic resistance threats in the United States, 2019. Atlanta, GA. Available at: www.cdc.gov/DrugResistance/Biggest-Threats.html. (Accessed February 19, 2020).
CDC (2019b). FDA-CDC antimicrobial resistance isolate bank. CDC website. Available at: https://wwwn.cdc.gov/arisolatebank (Accessed September 3, 2019).
CDC (2020). Antifungal susceptibility testing and interpretation Candida auris. Fungal Dis. Available at: https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html (Accessed January 15, 2020).
Centers for Disease Control and Prevention (2019). Candida auris CDC. CDC Website. Available at: https://www.cdc.gov/fungal/candida-auris/index.html (Accessed August 29, 2019).
Chaabane, F., Graf, A., Jequier, L., and Coste, A. T. (2019). Review on antifungal resistance mechanisms in the emerging pathogen Candida auris. Front. Microbiol. 10:2788. doi: 10.3389/fmicb.2019.02788
Chow, N. A., De Groot, T., Badali, H., Abastabar, M., Chiller, T. M., and Meis, J. F. (2019). Potential fifth clade of Candida auris, Iran, 2018. Emerg. Infect. Dis. 25, 1780–1781. doi: 10.3201/eid2509.190686
CLSI (2017). “Chapter 3. Antifungal broth dilutions susceptibility testing process for yeast” in M27: Reference method for Broth dilution antifungal susceptibility testing of yeasts. 4th Edn. ed. B. D. Alexander (Wayne, PA: Clinical Laboratory Standards Institute).
Dekkerová, J., Lopez-Ribot, J. L., and Bujdáková, H. (2019). Activity of anti-CR3-RP polyclonal antibody against biofilms formed by Candida auris, a multidrug-resistant emerging fungal pathogen. Eur. J. Clin. Microbiol. Infect. Dis. 38, 101–108. doi: 10.1007/s10096-018-3400-x
Dominguez, E. G., Zarnowski, R., Choy, H. L., Zhao, M., Sanchez, H., Nett, J. E., et al. (2019). Conserved role for biofilm matrix polysaccharides in Candida auris drug resistance. mSphere 4, e00680–e00718. doi: 10.1128/mSphereDirect.00680-18
Forsberg, K., Woodworth, K., Walters, M., Berkow, E. L., Jackson, B., Chiller, T., et al. (2019). Candida auris: the recent emergence of a multidrug-resistant fungal pathogen. Med. Mycol. 57, 1–12. doi: 10.1093/mmy/myy054
Jeffery-Smith, A., Taori, S. K., Schelenz, S., Jeffery, K., Johnson, E. M., Borman, A., et al. (2017). Candida auris: a review of the literature. Clin. Microbiol. Rev. 31, e00029–e000317. doi: 10.1128/CMR.00029-17
Kean, R., Delaney, C., Sherry, L., Borman, A., Johnson, E. M., Richardson, M. D., et al. (2018). Transcriptome assembly and profiling of Candida auris reveals novel insights into biofilm-mediated resistance. mSphere 3, 1–12. doi: 10.1128/mSphere.00334-18
Klasen, H. J. (2000a). A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 26, 131–138. doi: 10.1016/S0305-4179(99)00116-3
Klasen, H. J. (2000b). Historical review of the use of silver in the treatment of burns. I. Early uses. Burns 26, 117–130. doi: 10.1016/S0305-4179(99)00108-4
Krishnasamy, L., Krishnakumar, S., Kumaramanickavel, G., and Saikumar, C. (2018). Molecular mechanisms of antifungal drug resistance in Candida species. J. Clin. Diagn. Res. 12, DE01–DE06. doi: 10.7860/JCDR/2018/36218.11961
Ku, T. S. N., Walraven, C. J., and Lee, S. A. (2018). Candida auris: disinfectants and implications for infection control. Front. Microbiol. 9:726. doi: 10.3389/fmicb.2018.00726
Kumar-Krishnan, S., Prokhorov, E., Hernández-Iturriaga, M., Mota-Morales, J. D., Vázquez-Lepe, M., Kovalenko, Y., et al. (2015). Chitosan/silver nanocomposites: synergistic antibacterial action of silver nanoparticles and silver ions. Eur. Polym. J. 67, 242–251. doi: 10.1016/j.eurpolymj.2015.03.066
Lara, H. H., Ixtepan-Turrent, L., Jose Yacaman, M., and Lopez-Ribot, J. (2020). Inhibition of Candida auris biofilm formation on medical and environmental surfaces by silver nanoparticles. ACS Appl. Mater. Interfaces 12, 21183–21191. doi: 10.1021/acsami.9b20708
Lara, H. H., Romero-Urbina, D. G., Pierce, C., Lopez-Ribot, J. L., Arellano-Jiménez, M. J., and Jose-Yacaman, M. (2015). Effect of silver nanoparticles on Candida albicans biofilms: an ultrastructural study. J. Nanobiotechnol. 13:91. doi: 10.1186/s12951-015-0147-8
Monteiro, D. R., Gorup, L. F., Silva, S., Negri, M., de Camargo, E. R., Oliveira, R., et al. (2011). Silver colloidal nanoparticles: antifungal effect against adhered cells and biofilms of Candida albicans and Candida glabrata. Biofouling 27, 711–719. doi: 10.1080/08927014.2011.599101
Muthamil, S., Devi, V. A., Balasubramaniam, B., Balamurugan, K., and Pandian, S. K. (2018). Green synthesized silver nanoparticles demonstrating enhanced in vitro and in vivo antibiofilm activity against Candida spp. J. Basic Microbiol. 58, 343–357. doi: 10.1002/jobm.201700529
Nadhe, S. B., Singh, R., Wadhwani, S. A., and Chopade, B. A. (2019). Acinetobacter sp. mediated synthesis of AgNPs, its optimization, characterization and synergistic antifungal activity against C. albicans. J. Appl. Microbiol. 127, 445–458. doi: 10.1111/jam.14305
Patra, J. K., and Baek, K. H. (2017). Antibacterial activity and synergistic antibacterial potential of biosynthesized silver nanoparticles against foodborne pathogenic bacteria along with its anticandidal and antioxidant effects. Front. Microbiol. 8:167. doi: 10.3389/fmicb.2017.00167
Perween, N., Khan, H. M., and Fatima, N. (2019). Silver nanoparticles: an upcoming therapeutic agent for the resistant Candida infections. J. Microbiol. Exp. 7, 49–54. doi: 10.15406/jmen.2019.07.00240
Pierce, C. G., Uppuluri, P., Tristan, A. R., Wormley, F. L., Mowat, E., Ramage, G., et al. (2008). A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 3, 1494–1500. doi: 10.1038/nprot.2008.141
Radhakrishnan, V. S., Mudiam, M. K. R., Kumar, M., Dwivedi, S. P., Singh, S. P., and Prasad, T. (2018). Silver nanoparticles induced alterations in multiple cellular targets, which are critical for drug susceptibilities and pathogenicity in fungal pathogen (Candida albicans). Int. J. Nanomed. 13, 2647–2663. doi: 10.2147/IJN.S150648
Raghunath, A., and Perumal, E. (2017). Metal oxide nanoparticles as antimicrobial agents: a promise for the future. Int. J. Antimicrob. Agents 49, 137–152. doi: 10.1016/j.ijantimicag.2016.11.011
Ramage, G., Mowat, E., Jones, B., Williams, C., and Lopez-Ribot, J. (2009). Our current understanding of fungal biofilms. Crit. Rev. Microbiol. 35, 340–355. doi: 10.3109/10408410903241436
Rhodes, J., and Fisher, M. C. (2019). Global epidemiology of emerging Candida auris. Curr. Opin. Microbiol. 52, 84–89. doi: 10.1016/j.mib.2019.05.008
Romero-Urbina, D. G., Lara, H. H., Velázquez-Salazar, J. J., Arellano-Jiménez, M. J., Larios, E., Srinivasan, A., et al. (2015). Ultrastructural changes in methicillin-resistant Staphylococcus aureus induced by positively charged silver nanoparticles. Beilstein J. Nanotechnol. 6, 2396–2405. doi: 10.3762/bjnano.6.246
Rudramurthy, G. R., Swamy, M. K., Sinniah, U. R., and Ghasemzadeh, A. (2016). Nanoparticles: alternatives against drug-resistant pathogenic microbes. Molecules 21:836. doi: 10.3390/molecules21070836
Sears, D., and Schwartz, B. S. (2017). Candida auris: an emerging multidrug-resistant pathogen. Int. J. Infect. Dis. 63, 95–98. doi: 10.1016/j.ijid.2017.08.017
Sherry, L., Ramage, G., Kean, R., Borman, A., Johnson, E. M., Richardson, M. D., et al. (2017). Biofilm-forming capability of highly virulent, multidrug-resistant Candida auris. Emerg. Infect. Dis. 23, 328–331. doi: 10.3201/eid2302.161320
Sim, W., Barnard, R. T., Blaskovich, M. A. T., and Ziora, Z. M. (2018). Antimicrobial silver in medicinal and consumer applications: a patent review of the past decade (2007–2017). Antibiotics 7:93. doi: 10.3390/antibiotics7040093
Srinivasan, A., Lopez-Ribot, J. L., and Ramasubramanian, A. K. (2014). Overcoming antifungal resistance. Drug Discov. Today Technol. 11, 65–71. doi: 10.1016/j.ddtec.2014.02.005
Szekely, A., Borman, A. M., and Johnsona, E. M. (2019). Candida auris isolates of the Southern Asian and South African lineages exhibit different phenotypic and antifungal susceptibility profiles in vitro. J. Clin. Microbiol. 57, e02055–e02118. doi: 10.1128/JCM.02055-18
Vance, M. E., Kuiken, T., Vejerano, E. P., McGinnis, S. P., Hochella, M. F., and Hull, D. R. (2015). Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 6, 1769–1780. doi: 10.3762/bjnano.6.181
Vazquez-Muñoz, R., Arellano-Jimenez, M. J., Lopez, F. D., and Lopez-Ribot, J. L. (2019a). Protocol optimization for a fast, simple and economical chemical reduction synthesis of antimicrobial silver nanoparticles in non-specialized facilities. BMC. Res. Notes 12:773. doi: 10.1186/s13104-019-4813-z
Vazquez-Muñoz, R., Avalos-Borja, M., and Castro-Longoria, E. (2014). Ultrastructural analysis of Candida albicans when exposed to silver nanoparticles. PLoS One 9:e108876. doi: 10.1371/journal.pone.0108876
Vazquez-Muñoz, R., Borrego, B., Juárez-Moreno, K., García-García, M., Mota Morales, J. D., Bogdanchikova, N., et al. (2017). Toxicity of silver nanoparticles in biological systems: does the complexity of biological systems matter? Toxicol. Lett. 276, 11–20. doi: 10.1016/j.toxlet.2017.05.007
Vazquez-Muñoz, R., Meza-Villezcas, A., Fournier, P. G. J., Soria-Castro, E., Juarez-Moreno, K., Gallego-Hernández, A. L., et al. (2019b). Enhancement of antibiotics antimicrobial activity due to the silver nanoparticles impact on the cell membrane. PLoS One 14:e0224904. doi: 10.1371/journal.pone.0224904
Wady, A. F., Machado, A. L., Foggi, C. C., Zamperini, C. A., Zucolotto, V., Moffa, E. B., et al. (2014). Effect of a silver nanoparticles solution on Staphylococcus aureus and Candida spp. J. Nanomater. 2014, 1–7. doi: 10.1155/2014/545279
Welsh, R. M., Bentz, M. L., Shams, A., Houston, H., Lyons, A., Rose, L. J., et al. (2017). Survival, persistence, and isolation of the emerging multidrug-resistant pathogenic yeast Candida auris on a plastic health care surface. J. Clin. Microbiol. 55, 2996–3005. doi: 10.1128/JCM.00921-17
Zamperini, C. A., André, R. S., Longo, V. M., Mima, E. G., Vergani, C. E., Machado, A. L., et al. (2013). Antifungal applications of Ag-decorated hydroxyapatite nanoparticles. J. Nanomater. 2013, 1–9. doi: 10.1155/2013/174398
Zivic, F., Grujovic, N., Mitrovic, S., Ahad, I. U., and Brabazon, D. (2018). “Characteristics and applications of silver nanoparticles” in Commercialization of nanotechnologies—A case study approach. eds. D. Brabazon, E. Pellicer, F. Zivic, J. Sort, M. D. Baró, and N. Grujovic, et al. (Cham: Springer International Publishing), 227–273. doi: 10.1007/978-3-319-56979-6_10
Keywords: nanoantibiotics, Candida auris, biofilms, metallic nanoparticles, silver nanoparticles, antimicrobial nanomaterials
Citation: Vazquez-Munoz R, Lopez FD and Lopez-Ribot JL Silver Nanoantibiotics Display Strong Antifungal Activity Against the Emergent Multidrug-Resistant Yeast Candida auris Under Both Planktonic and Biofilm Growing Conditions. Front. Microbiol. 11:1673. doi: 10.3389/fmicb.2020.01673
Edited by:
Juliana Campos Junqueira, São Paulo State University, BrazilReviewed by:
Gordon Ramage, University of Glasgow, United KingdomLiliana Scorzoni, São Paulo State University, Brazil
Copyright © Vazquez-Munoz, Lopez and Lopez-Ribot. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberto Vazquez-Munoz, cm9iZXJ0by52YXpxdWV6bXVub3pAdXRzYS5lZHU=; Jose L. Lopez-Ribot, am9zZS5sb3BlenJpYm90QHV0c2EuZWR1
 Roberto Vazquez-Munoz
Roberto Vazquez-Munoz Fernando D. Lopez2
Fernando D. Lopez2 Jose L. Lopez-Ribot
Jose L. Lopez-Ribot