- 1Department of Pharmacology, Shanghai Tenth People’s Hospital, Tongji University School of Medicine, Shanghai, China
- 2School of Pharmacy, Second Military Medical University, Shanghai, China
In the course of optimizing GPI biosynthesis inhibitors, we designed and synthetized a 2-aminonicotinamide derivative named 11g. After evaluating the antifungal activity of compound 11g in vitro, we investigated the influences of 11g on fungi immunogenicity. In addition, we also took advantage of murine systemic candidiasis model to investigate the protective effects of 11g in vivo. Results show that 11g exhibited potent antifungal activity both in vitro and in vivo. Further study shows that 11g caused the unmasking of fungi β-glucan layer, leading to stronger immune responses in macrophages through Dectin-1. These results suggest that 11g is a very promising antifungal candidate, which assists in eliciting stronger immune responses to help host immune system disposing pathogens. The discovery of 11g might expand the toolbox of fungal infection treatment.
Introduction
Fungi are encountered, ingested, and inhaled by human beings every day. As opportunistic pathogens, their infection spectrum ranges from benignly superficial colonization to systemic fungal infection. As a result of the increasing number of susceptible immunocompromised and immunosuppressed patients, the incidence rate of clinically relevant fungal infections has risen steadily in the past three to four decades (LeibundGut-Landmann et al., 2012; McLellan et al., 2012). Candida species, which caused mostly fungus-related morbidity and mortality, are the fourth leading cause of hospital-acquired bloodstream infections (Cowen et al., 2002). There are between 1.1 and 24 cases of candidemia per 100,000 individuals, killing more than 30% of their victims (Gudlaugsson et al., 2003; Wisplinghoff et al., 2004).
In response to this mounting therapeutic challenge, developing drugs available for human invasive fungal infections has been intensely spurred. Nowadays, there are four major classes of antifungals with different mechanisms widely used in clinic: azoles, polyenes, echinocandins, and fluorinated pyrimidines. However, their own limitations, such as drug resistance, narrow antifungal spectrum, adverse effects, variable pharmacokinetic, and limited types of formulation, etc., restrict their applications in clinic (Martin et al., 2003; Wisplinghoff et al., 2004; Pfaller et al., 2008, 2010; Baddley et al., 2010; Denning and Hope, 2010; Kontoyiannis et al., 2010). To date, systemic fungal infections are not only expensive to treat, but also extremely difficult to eradicate. Therefore, safe and effective antifungal candidates with novel mechanisms are urgently needed for the treatment of serious systemic fungal infections.
The cell wall is clearly of paramount importance to the survival of fungi, which are different from mammalian cells, and it is always taken into account when searching for new antifungal targets. Previous studies have shown that the C. albicans cell wall consists of two layers: The outer layer is enriched with mannoprotein, and the inner layer contains the skeletal polysaccharides β-1, 3-glucan and chitin (Gow et al., 2011). The most abundant cell wall proteins (CWPs) are covalently attached to the meshwork of inner layer through a glycosylphosphatidylinositol (GPI) remnant and β-1, 6-glucan, which are termed GPI-anchored cell wall proteins (GPI-CWPs) (Richard and Plaine, 2007; Chaffin, 2008). It has been reported that GPI-CWPs are responsible for a vast range of functions: They not only help maintain the integrity of fungal cell wall, but also play important roles in adhesion, filamentation, and sensing of the environment. What’s more, they also provide a heavily glycosylated and phosphorylated outer coat to shield fungi from host immune defense (Orlean and Menon, 2007; Klis et al., 2009). Therefore, as a promising antifungal target, GPI has been drawing attention of many researchers in recent years.
To date, several GPI biosynthesis inhibitors, such as BIQ, G884, 10b, and E1210, etc., have been synthetized and regarded as new antifungal agents (Supplementary Figure S1; Tsukahara et al., 2003; Nakamoto et al., 2010; Miyazaki et al., 2011; Mann et al., 2015). In the course of optimizing these compound structures, we designed and synthetized a 2-aminonicotinamide derivative named 11g (Ni et al., 2017). In 2017, our group reported that 11g could decrease the GPI content in the C. albicans cell wall (Ni et al., 2017). In this paper, we further investigated the antifungal effects and mechanisms of 11g, and then tested its protective effect in murine systemic candidiasis model. Our study shows that 11g exhibits a potent and broad-spectrum antifungal activity. In addition, we also demonstrate that 11g has protective effect in vivo through enhancing fungal immunogenicity and spurring host immune responses.
Materials and Methods
Antifungals
11g was synthesized by the Second Military Medical University (Shanghai, China). 11g and fluconazole (Sigma, St. Louis, MO, United States) were dissolved in dimethyl sulfoxide (DMSO; Sigma) and stored in a −40°C freezer until application. On the day of use, stored solutions were diluted with appropriate media to yield DMSO concentrations of 1%.
Strains
All the strains used in this paper were provided by the Second Military Medical University and were maintained in 80% glycerol at −80°C.
Mice
Female 6- to 8-week-old C57BL/6 mice were purchased from Shanghai Laboratory Animal Center of the Chinese Academy of Sciences (Shanghai, China). All mice were kept in barrier conditions at the Experimental Animals Center of Tongji University (Shanghai, China). License Number: SYXK (Shanghai) 2014-0026. All mouse experiments were done according to institutional guidelines and were approved by the Institutional Animal Use and Care Committee of Tongji University.
Minimum Inhibitory Concentration (MIC) Determinations
Assays were performed according to the broth microdilution method detailed by the Clinical and Laboratory Standards Institute (CLSI) in documents M27-A3 (Ma et al., 2015). Briefly, the cell suspensions were diluted with RPMI 1640 medium (Invitrogen, Carlsbad, CA, United States) to obtain inoculum sizes of 1 × 103 cells/mL. The initial concentrations of antifungal agents ranged from 0.0313 to 64 × 10–3 mg/mL. MIC80 values were read after 24 h at 30°C. The results were expressed as the median values of MIC80 from three independent experiments. The MIC80 value was defined as the lowest concentration, at which a prominent decrease (≥80%) in growth turbidity compared to the control (antifungal agent-free) at 620 nm.
Fungal Growth Curve
C. albicans SC5314 was cultured in RPMI 1640 medium containing 11g (0, 0.5, and 1 × 10–3 mg/mL) with an inoculums size of 106 cells/mL. Cells were cultured at 30°C with constant shaking and optical density at 600 nm were measured after incubating for 0, 1, 2, 4, 8, 12, 16, 24, and 32 h (Kesavan et al., 2005). Three independent experiments were performed.
Viability Curve
C. albicans SC5314 was cultured in RPMI 1640 medium containing 11g (0.0625 × 10–3, 0.125 × 10–3, or 0.25 × 10–3 mg/mL) with an inoculum size of 1 × 105 cells/mL. Cells were cultured at 30°C with constant shaking. After 0, 12, 24, 36, and 48 h of incubation, a fraction was obtained from each solution and plated on a Sabouraud dextrose agar (SDA) plate. The numbers of colonies were counted after incubating at 30°C for 48 h. Three independent experiments were performed (Quan et al., 2006).
Filamentation
C. albicans SC5314 was incubated in Sabouraud dextrose broth (SDB) at 25°C for 3-day to obtain a saturated culture. After washing three times with PBS, the cells were then resuspended in RPMI 1640 medium with 11g (0.25 × 10–3 or 0.5 × 10–3 mg/mL) to a concentration of 5 × 105 cells/mL. The cells were pictured after further incubating at 37°C for 3 h.
Staining and Confocal Microscope
Overnight cultures of C. albicans SC5314 were incubated in YPD medium with or without 11g at 30°C, favoring yeast-form growth. For hyphal growth, C. albicans SC5314 (1 × 106 cells/mL) was incubated in Spider medium with or without 11g at 37°C for 3 h. Cells were then washed three times with PBS and then stained with Concanavalin A (ConA; Life Technologies, Carlsbad, CA, United States) to visualize mannan, anti-β-glucan primary antibody (Biosupplies, Bundoora, Australia) followed by Cy3-labeled secondary antibody (Life Technologies) to visualize β-(1, 3)-glucan and CFW (Life Technologies) to visualize chitin (Wheeler and Fink, 2006; Victoria et al., 2010). Microscopy was performed on a Leica confocal microscope (Wetzlar, Gernamy) with a 60 × oil objective.
Cell Surface Hydrophobicity
Overnight cultures of C. albicans SC5314 were incubated in YPD medium with or without 11g (0.0313 × 10–3 mg/mL) at 30°C. Then, C. albicans cells were washed three times with PBS and resuspended in PBS (OD600 = 1.0). Then, cyclohexane (0.75 mL) was added to C. albicans cell suspension (3 mL). After vortex mixing for 3 min, the C. albicans cell suspension was settled at room temperature for 20 min. OD600 of the aqueous phase was measured by NANODROP2000 Ultraviolet spectrophotometer (Thermo Fisher Scientific, Waltham, MA, United States). Relative cell surface hydrophobicity (CSH) = (OD600 of the control – OD600 after octane overlay)/OD600 of the control × 100% (Zhang et al., 2016).
Primary Murine Peritoneal Macrophage Preparation
Primary murine peritoneal macrophages were prepared as described previously (Saijo et al., 2007). Briefly, mice were injected intraperitoneally with 2 mL thioglycollate (3%; Merck, Darmstadt, Germany). Three days later, peritoneal cells were collected by washing with PBS containing 0.5 mM EDTA (BioDee, Beijing, China) and then cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS; Thermo Scientific, Waltham, MA, United States). After 2-day cultivation, adherent cells were used as primary murine peritoneal macrophages.
Phagocytosis Rate and Phagocytosis Index Tests
In this paper, we investigated the phagocytosis rate and phagocytosis index by Wright-Giemsa staining tests. Here, we chose primary murine peritoneal macrophages as effector cells, C. albicans SC5314 and 11g (0.0313 × 10–3 mg/mL)-treated C. albicans SC5314 as target cells. Macrophages were cultured in 48-well plates at 37°C for 48 h. Overnight cultures of C. albicans SC5314 and 11g (0.0313 × 10–3 mg/mL)-treated C. albicans SC5314 were harvested and washed three times with PBS. After discarding the culture medium of macrophages, C. albicans SC5314 or 11g (0.0313 × 10–3 mg/mL)-treated C. albicans SC5314 was added to macrophages [multiplicity of infection (MOI) = 30] and cultured at 37°C for 0.5 h. After discarding the culture medium, 48-well plates were washed by PBS for two times. Cells were stained by rapid Wright-Giemsa staining solution (BBI Life Sciences Corporation, Shanghai, China). Then cells were washed by PBS for three times. Cells were photographed and counted by a microscope (Nicola and Casadevall, 2012). Phagocytosis rate = (number of macrophages phagocytizing fungi in 200 macrophages/200) × 100%; Phagocytosis index = number of fungi phagocytized by 200 macrophages/200.
Fungi Killing Efficiency Tests
Firstly, primary murine peritoneal macrophages were incubated in 24-well plates at 37°C for 48 h. Overnight cultures of C. albicans SC5314 and 11g (0.0313 × 10–3 mg/mL)-treated C. albicans SC5314 were harvested and washed three times with PBS. After discarding the culture medium of macrophages, C. albicans SC5314 or 11g (0.0313 × 10–3 mg/mL)-treated C. albicans SC5314 (MOI = 1) were added to 24-well plates and incubated at 37°C for 6 h. Then, fungi and macrophages suspensions from each well were distributed on the surface of SDA plates and incubated at 30°C for 48 h. Then the CFU of C. albicans SC5314 on each SDA plate was counted. We recorded the group of C. albicans SC5314 as group A, the group of 11g-treated C. albicans SC5314 as group B, the group of C. albicans SC5314 + macrophages as group C, and the group of 11g-treated C. albicans SC5314 + macrophages as group D. The killing efficiency of macrophages to C. albicans SC5314 = (A – C)/A × 100%. The killing efficiency of macrophages to 11g-treated C. albicans SC5314 = (B – D)/B × 100%. Three independent experiments were performed.
Western Blotting
Overnight cultures of C. albicans SC5314 incubated in YPD medium with or without 11g were harvested and washed three times with PBS. The fungi were then exposed to five doses of 100 mjoules/cm2 ultraviolet (UV) radiation in a CL-1000 UV-crosslinker (UVP; Upland, United States) (Victoria et al., 2010). After UV-inactivation, fungi were washed extensively, renormalized by hemocytometry, added to cultures of the primary murine peritoneal macrophages preincubated with or without laminarin (Sigma), and then incubated at 37°C for the indicated time. MOI = 5. Primary murine peritoneal macrophages were harvested, stimulated, and lysed in lysis buffer for total cell lysates. In nuclear extracts immunoblotting experiments, cells were lysed in lysis buffer and extracted by extraction buffer. Both the total cell lysates and nuclear extracts were subjected to SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted with the indicated antibodies (Gorjestani et al., 2012).
Cytokine Detection
After macrophage and UV-inactivated fungus interaction, supernatants of primary murine peritoneal macrophages were harvested, and cytokines concentrations were measured by enzyme-linked immunosorbent assay (ELISA) according to manufacturer’s instructions (eBioscience, San Diego, CA, United States). Three independent experiments were performed.
Cytotoxicity Assay
For cytotoxicity assay, rat cardiac myocytes (H9C2; 8 × 103 cells/well) were cultured in 96-well plates and incubated in DMEM (HyClone, Logan City, United States), supplemented with 10% FBS at 37°C in the presence of 5% CO2. The following day, a CCK-8 assay was performed, as described previously (Huang et al., 2018). Briefly, H9C2 were incubated in DMEM with or without 11g at 37°C for 24 h. After discarding medium with 11g, washing cells two times with PBS, and adding fresh medium with CCK-8 solution, cells were further incubated at 37°C for 1 h. Absorbance at 450 nm was measured by ELISA Plate Reader (Thermo Scientific). Three independent experiments were performed.
Murine Systemic Candidiasis Model and Treatment
C. albicans SC5314 was cultured in YPD medium at 30°C for 12 h. After washing them three times with PBS, cells were resuspended in physiological saline. Infection was induced by injecting a C. albicans suspension into the lateral tail vein (5 × 105 CFU/mouse). 11g (4 mg/kg) or vehicle was administered intraperitoneally 2 h later and was injected once a day for 5 consecutive days (Hata et al., 2011). Survival rates were monitored for 35 days following infection. For measuring fungal burden, serial dilutions of paired kidneys and livers were plated on SDA plates on Day 5 after infection, and further incubated at 30°C for 48 h. For histopathological analysis, paired kidneys were fixed in 10% neutral buffered formalin on Day 5 after infection, and then stained with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) to reveal inflammatory cell infiltration and fungal hyphae. Three independent experiments were performed.
Statistical Analysis
Three biological replicates were performed for all the experiments in this paper, unless otherwise indicated. Statistical analyses were performed using the Prism software, version 5.01 (GraphPad software, San Diego, CA, United States). Log-rank test was used to analyze the difference between the survival curves. The Mann-Whitney U test was used to analyze the difference between the two groups. Two-way ANOVA or one-way ANOVA was used to analyze the difference between multiple groups. A quantitative analysis of mean fluorescent intensity was performed using Image J (Bethesda, MD, United States). A densitometric analysis of Western blot images was performed using Image J. Statistical significance was set at probability (P) value of less than 0.05, 0.01, or 0.001, indicated by ∗, ∗∗, or ∗∗∗, respectively.
Results
MIC80 of 11g in vitro
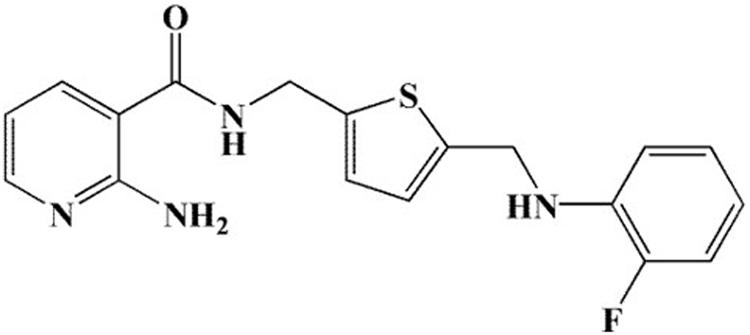
In 2017, our group designed and synthesized a series of novel 2-aminonicotinamide derivatives and reported that compound 11g (Figure 1) is a promising new antifungal agent with potent and broad-spectrum antifungal activity in vitro (Ni et al., 2017). Here, in order to expand the antifungal spectrum of 11g, we investigated the antifungal activity of 11g against 17 clinical isolates of Candida species.
Table 1 shows the MIC80 of 11g against 4 clinical isolates of Fluconazole-susceptible Candida albicans, 8 clinical isolates of Fluconazole-resistant Candida albicans, 2 clinical isolates of Candida krusei, 2 clinical isolates of Candida glabrata, and 1 clinical isolates of Candida tropicalis in vitro. 11g showed potent antifungal activity not only against fluconazole-susceptible C. albicans (MIC80 of 11g ≤ 0.5 × 10–3 mg/mL), but also against fluconazole-resistant C. albicans (MIC80 of 11g ≤ 0.5 × 10–3 mg/mL). In addition, data show that 11g exhibited strong antifungal effect against all the strains tested. Moreover, it should be noted that most of the strains tested here were much more sensitive to 11g than fluconazole. These data further prove that 2-aminonicotinamide derivatives (11g) have potent and broad-spectrum antifungal activity in vitro.
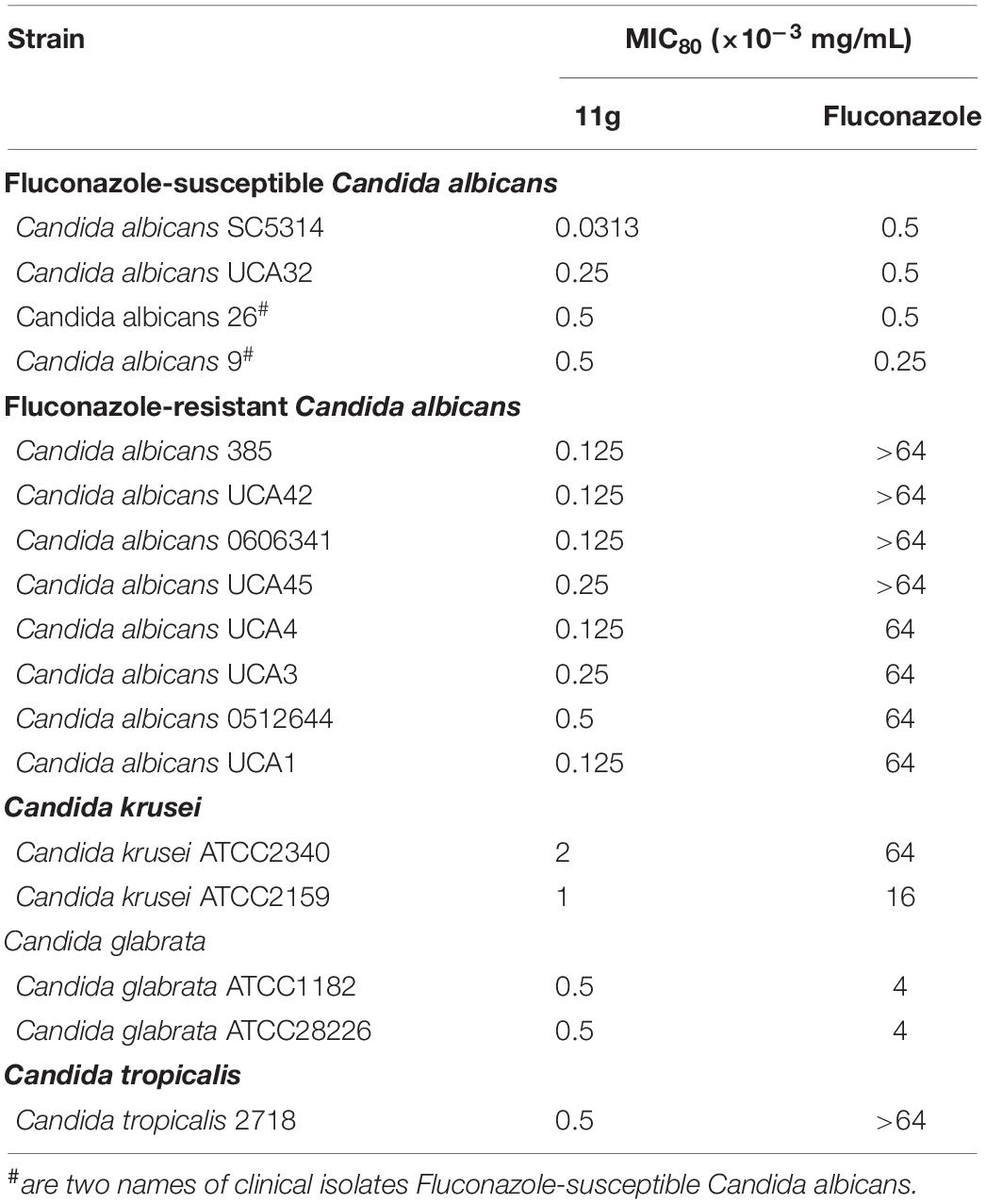
Table 1. In vitro antifungal activities of 11g against Candida albicans, Candida krusei, Candida glabrata, and Candida tropicalis.
Effect of 11g on C. albicans Growth and Virulence
Next, we investigated the effect of 11g on the growth and virulence of C. albicans. Fungal growth curves show that 11g at 0.5 × 10–3 and 1 × 10–3 mg/mL could potently inhibit the growth of C. albicans during 32 h culture. In addition, 11g-incubated C. albicans growth in a concentration-dependent manner (Figure 2A). Data show that the doubling time of control group was 6.2 h. However, the doubling times of 11g (0.5 and 1 × 10–3 mg/mL)-treated groups were 8.7 and 9.8 h, respectively, which was significantly longer than that of control group. Results above show that 11g could significantly inhibit the growth of C. albicans SC5314 in vitro. Viability curves show that 11g at 0.0625 × 10–3, 0.125 × 10–3, and 0.25 × 10–3 mg/mL could potently inhibit the viability of C. albicans after 48 h culture. Additionally, 11g inhibited the viability of C. albicans in a concentration-dependent manner (Figure 2B). We further examined the effect of 11g on fungi virulence. It has been reported that the hypha is a virulence factor, which plays an important role in the invasion and infection of C. albicans (Kumamoto and Vinces, 2005). Figures 2C,D shows that 11g (0.25 × 10–3 mg/mL) could significantly shorten the length of the hypha. All these data above suggest that 11g exhibits potent inhibition effect on growth and virulence of C. abicans.
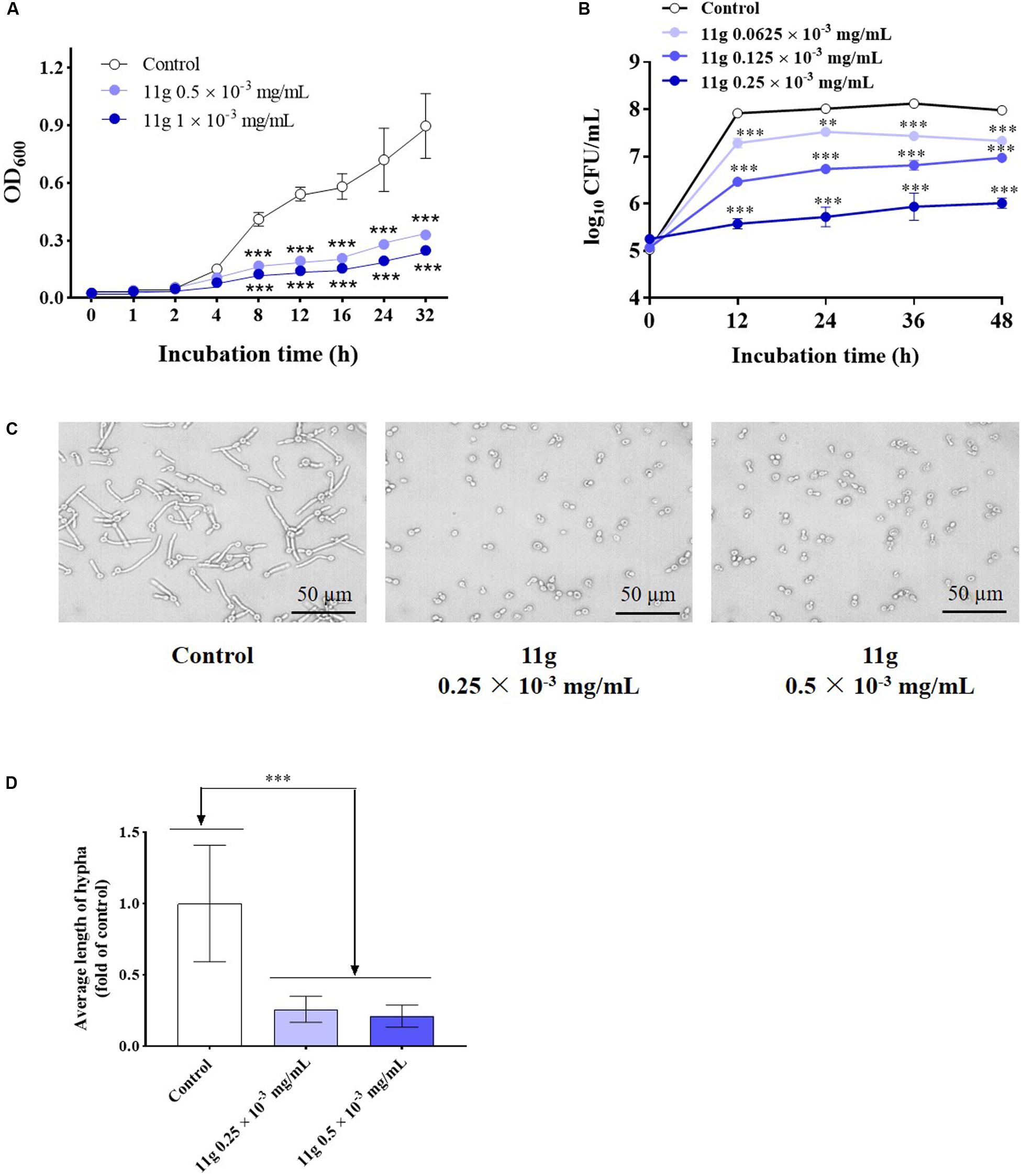
Figure 2. The effect of 11g on the growth and invasive ability of C. albicans in vitro. (A) Fungal growth curves of C. albicans SC5314 incubated with 11g (0, 0.5, and 1 × 10–3 mg/mL; n = 3; mean ± SD; two-way ANOVA with Dunnett test; ***P < 0.001). (B) Viability curves of C. albicans SC5314 incubated with 11g (0.0625 × 10–3, 0.125 × 10–3, and 0.25 × 10–3 mg/mL; n = 3; mean ± SD; two-way ANOVA with Dunnett test; **P < 0.01; ∗∗∗P < 0.001). (C) Microscopic images of C. albicans SC5314 incubated with 11g (0.25 × 10–3 and 0.5 × 10–3 mg/mL) at 37°C for 3 h. (D) A quantitative analysis of hypha length was measured by Image J (n = 50; mean ± SD; one-way ANOVA with Dunnett test; ∗∗∗P < 0.001).
In April 2019, our research group published an article named “Study on the mechanism of NT-89 against Candida albicans based on quantitative proteomics (in Chinese)” (Liu et al., 2019). Here, NT-89 was the former name of 11g. In this study, we firstly extracted the total protein of C. albicans SC5314 and 11g-treated C. albicans SC5314. Then, we discussed the effect of 11g on the protein expression of C. albicans SC5314 by iTRAQ tests. We analyzed the top 20 proteins with reduced relative expression in 11g-treated C. albicans SC5314. Data show that there were 5 GPI-anchored proteins among these 20 proteins, which was consistent with our conjecture that 11g could inhibit the synthesis of GPI (Ni et al., 2017). Among the above 20 proteins, Ywp1p and Pga10p decreased the most. Ywp1p plays a key role in the dispersal in host, adhesion, and biofilm formation (Granger et al., 2005). Pga10p plays a key role in the biofilm formation (Klis et al., 2009). Furthermore, in 11g-treated C. albicans SC5314, the relative expression of some proteins like Hsp family proteins and adhesin-like proteins were also significantly decreased (Liu et al., 2019). Data above indicated that the virulence of 11g-treated C. albicans SC5314 was decreased, which was consistent with the results of hypha formation experiments.
Effect of 11g on C. albicans Cell Wall Polysaccharides
Previous studies have shown that C. albicans cell wall consists of two layers: The outer layer is enriched with mannoprotein, and the inner layer contains the skeletal polysaccharides β-(1, 3)-glucan and chitin (Gow et al., 2011). In 2017, our group investigated the ultrastructure of fungal cell wall by transmission electron microscopy (Ni et al., 2017). We reported that the dense mannoprotein coats became thinner, and GPI content reduced after being incubated with 11g (Ni et al., 2017).
Here, in order to further investigate the effect of 11g on fungal cell wall polysaccharides, we stained fungi with fluorescent dyes and then observed cell wall polysaccharides by confocal laser-scanning microscopy. In this paper, we stained fungi with ConA to visualize mannan and anti-β-glucan primary antibody followed by Cy3-labeled secondary antibody to visualize β-(1, 3)-glucan. In 11g-treated yeast-form C. albicans, the fluorescence intensity of ConA is weakened, suggesting mannan content is decreased (Figure 3A); the fluorescence intensity of Cy3 is enhanced, suggesting β-(1, 3)-glucan is exposed (Figure 3B). Consistent with yeast-form cells, the same changes occurred to hyphal mannoprotein coat (Figure 3D) and β-glucan layer (Figure 3E). In addition, we stained fungi with CFW to visualize chitin. CFW could specifically combine with chitin and emitted blue fluorescence at a 355-nm excitation wavelength. In 11g-treated yeast-form C. albicans, the fluorescence intensity of CFW is enhanced, suggesting chitin content has a compensatory increase (Figure 3C). However, there was no significant differences in fluorescence intensity of CFW between 11g-treated hypha and control group (Figure 3F). What’s more, 11g also shortened the length of filaments, which is consistent with the previous results in this paper (Figures 3D–F). Together, these data indicate that 11g can remodel the architecture of fungal cell wall, which is consistent with our previous study (Ni et al., 2017). In addition, we also investigated the effect of 11g on cell surface hydrophobicity. Data show that cell surface hydrophobicity was significantly increased in 11g (0.0313 × 10–3 mg/mL)-treated C. albicans SC5314 (Supplementary Figure S2). Data above indicate that 11g affected the surface property of C. albicans such as hydrophobicity, which confirms the conclusion that 11g led to the remodeling of fungi cell wall.
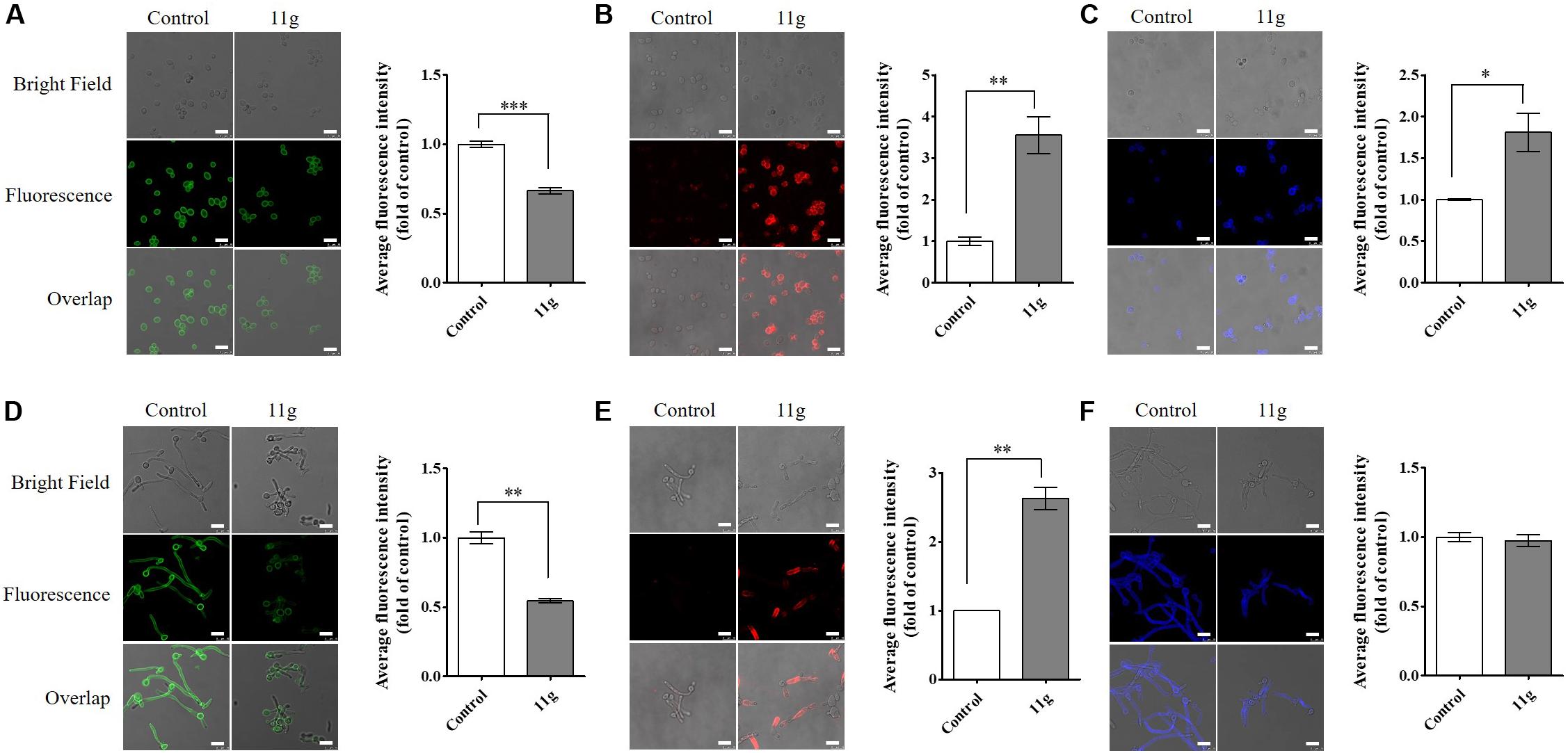
Figure 3. The effect of 11g on cell wall polysaccharides. C. albicans SC5314 was grown in YPD medium (A–C) overnight, favoring yeast-form growth or spider medium (D–F) for 3 h, favoring hyphal growth. Then cultures grown with or without 11g (0.0313 × 10–3 mg/mL) were stained with ConA to visualize mannan (green, A and D), anti-β-glucan primary antibody followed by Cy3-labeled secondary antibody to visualize β-1, 3-glucan (red, B and E), or CFW to visualize chitin (blue, C,F) by a confocal laser-scanning microscope. Scale bars, 10 μm. A quantitative analysis of mean fluorescent intensity was measured by Image J (n = 3; mean ± SD; Mann-Whitney U test; ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001).
The small molecule compound 11g reported in this paper is derived from the structure optimization of the published GPI biosynthesis inhibitors BIQ, G884, 10b, and E1210. It has been reported that all of these four compounds (BIQ, G884, 10b, and E1210) could inhibit the function of Gwt1p, the key enzyme in GPI synthesis pathway in fungi, and finally exhibit antifungal activity (Tsukahara et al., 2003; Nakamoto et al., 2010; Hata et al., 2011; Mann et al., 2015; Zhao et al., 2018, 2019). Therefore, we inferred that Gwt1p might be the target of 11g. In order to verify our conjecture, we constructed GWT1 heterozygous mutant C. albicans strain (GWT1/gwt1△). Results of spot assay show that C. albicans with GWT1/gwt1△ exhibited higher sensitivity to 11g, indicating that Gwt1p might be the target of 11g (data not shown). In the future experiments, the target of 11g will be further verified by GWT1 gene overexpression experiments and gene screening experiments. The research on 11g antifungal target is another story to be reported in later details from our group.
11g Enhances in vitro Innate Immune Responses to C. albicans
Previous studies suggest that β-(1, 3)-glucan is a predominant pathogen-associated molecular patterns (PAMP) in C. albicans cell wall, which is usually masked by the mannoprotein coats (Poulain and Jouault, 2004; Gow et al., 2011). As we found out that 11g can unmask the β-(1, 3)-glucan, we reasoned that 11g can enhance the immunogenicity of C. albicans, leading to the boost of innate immune cell responses (Brown et al., 2003; Gantner et al., 2003; Netea et al., 2008).
To validate our hypothesis, we first investigated the phagocytosis rate and phagocytosis index by Wright-Giemsa staining tests. Here, we chose primary murine peritoneal macrophages as effector cells. C. albicans SC5314 and 11g (0.0313 × 10–3 mg/mL)-treated C. albicans SC5314 as target cells. Data show that there was no significant difference in phagocytosis rate between the two groups. The phagocytosis rate was 100% in both of these two groups, indicating that all macrophages in these two groups exhibited phagocytic behavior. However, the phagocytosis index of macrophages to 11g-treated C. albicans SC5314 was significantly higher than that of C. albicans SC5314, indicating that 11g enhanced the phagocytic function of macrophages to fungi (Supplementary Figure S3). In addition, we investigated the killing efficiency of macrophages to C. albicans SC5314. Data show that the average killing efficiency of macrophages to C. albicans SC5314 and 11g-treated C. albicans SC5314 is 21.2% and 39.9%, respectively. The average killing efficiency of macrophages to 11g-treated C. albicans SC5314 is significantly higher than that of macrophages to C. albicans SC5314, which is consistent with the result of phagocytosis index (Supplementary Figure S4).
Then, we investigated the effect of 11g-treated C. albicans on signaling pathways activation in innate immune cells. Results show that 11g-treated C. albicans can enhance the phosphorylation of Syk and IκBα, as well as the degradation of IκBα in macrophages (Figures 4A–C). Moreover, 11g-treated C. albicans can also promote the nuclear translocation of p65 subunit in macrophages (Figures 4D,E). What’s more, these activations can be significantly inhibited by laminarin, a specific inhibitor of Dectin-1 (Figures 4A–E; Gantner et al., 2005). These results indicate that 11g-treated C. albicans can enhance Dectin-1-dependent NF-κB-signaling activation in macrophages. In this study, we also studied the effect of 11g-treated C. albicans on MAPK-signaling activation in macrophages. Figures 4F–I shows that 11g-treated C. albicans can enhance the phosphorylation of ERK, JNK, and p38 in macrophages. Consistently, these activations can also be significantly inhibited by laminarin. These data suggest that 11g-treated C. albicans can enhance Dectin-1-dependent MAPK-signaling activation in macrophages.
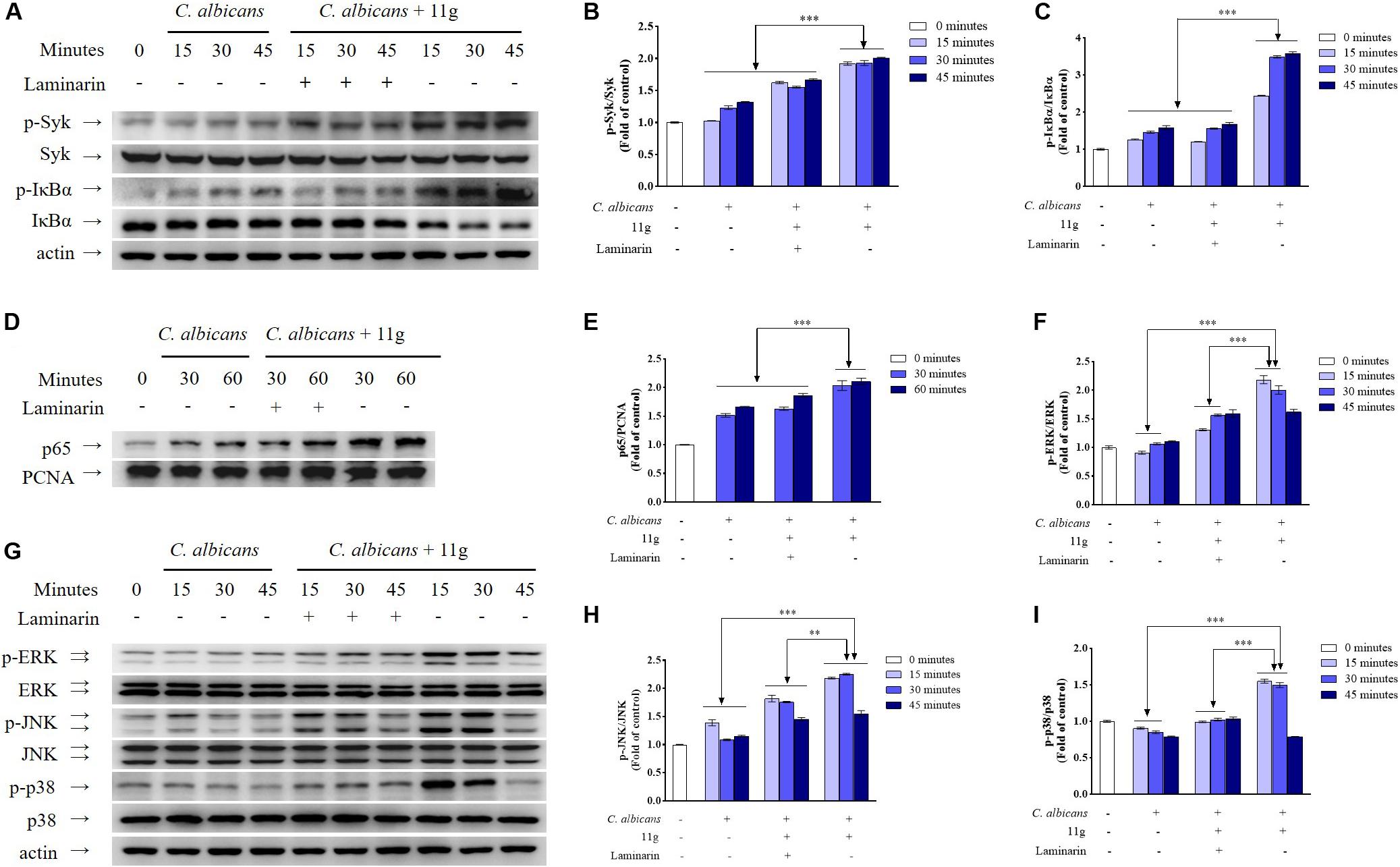
Figure 4. 11g-treated C. albicans-induced NF-κB and MAPK activation in innate immune cells. Primary murine peritoneal macrophages were incubated with or without laminarin (500 × 10–3 mg/mL) at 37°C for 30 min. The macrophages were then stimulated with 11g (0.0313 × 10–3 mg/mL) or vehicle-treated C. albicans (MOI = 5) for the indicated times. The total cell lysates (A,F) and nuclear extracts (D) were analyzed by immunoblotting using the indicated antibodies. A densitometric analysis (B,C for A; E for D; G–I for F) was measured by Image J (n = 3; mean ± SD; two-way ANOVA with Tukey test; ∗∗P < 0.01; ∗∗∗P < 0.001).
Next, we investigated the effect of 11g-treated C. albicans on cytokines secretion in macrophages. We found that 11g-treated C. albicans could increase the secretion of TNF-α (Figure 5A), IL-10 (Figure 5B), IL-12/23 p40 (Figure 5C), and IL-6 (Figure 5D) in macrophages after 6 h post-infection. In addition, these activations were significantly inhibited by laminarin. These data suggest that 11g-treated C. albicans can elicit Dectin-1-dependent cytokines secretion in macrophages.
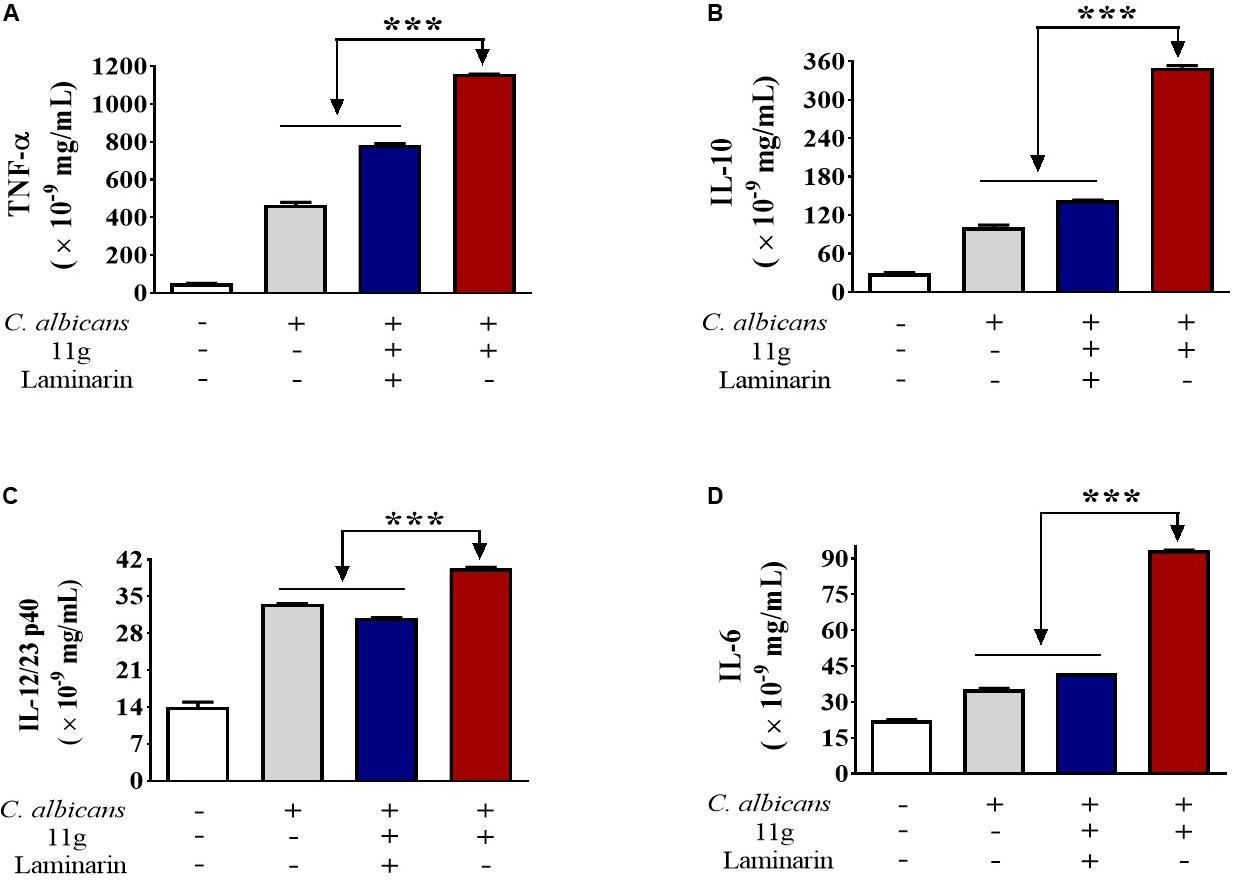
Figure 5. 11g-treated C. albicans induced cytokines secretion in innate immune cells. Primary murine peritoneal macrophages were incubated with or without laminarin (500 × 10–3 mg/mL) at 4 °C for 30 min. The macrophages were then stimulated with 11g (0.0313 × 10–3 mg/mL) or vehicle-treated C. albicans (MOI = 5) for 6 h. Concentrations of TNF-α (A), IL-10 (B), IL-12/23 p40 (C), and IL-6 (D) in supernatant were measured by ELISA (n = 3; mean ± SD; one-way ANOVA with Bonferroni post-test; ***P < 0.001).
Cytotoxicity of 11g in vitro
In order to infer the safety of 11g, we investigated the mammalian cytotoxicity of 11g in vitro (Figure 6). The concentrations of 11g incubated with H9C2 were at 1×, 5×, 25×, and 125 × MIC80 (MIC80 = 0.0313 × 10–3 mg/mL), respectively. Results show that 11g exhibited no significant effect on cell viability of H9C2 at all the concentrations tested, which means the cytotoxicity of 11g is low in vitro.
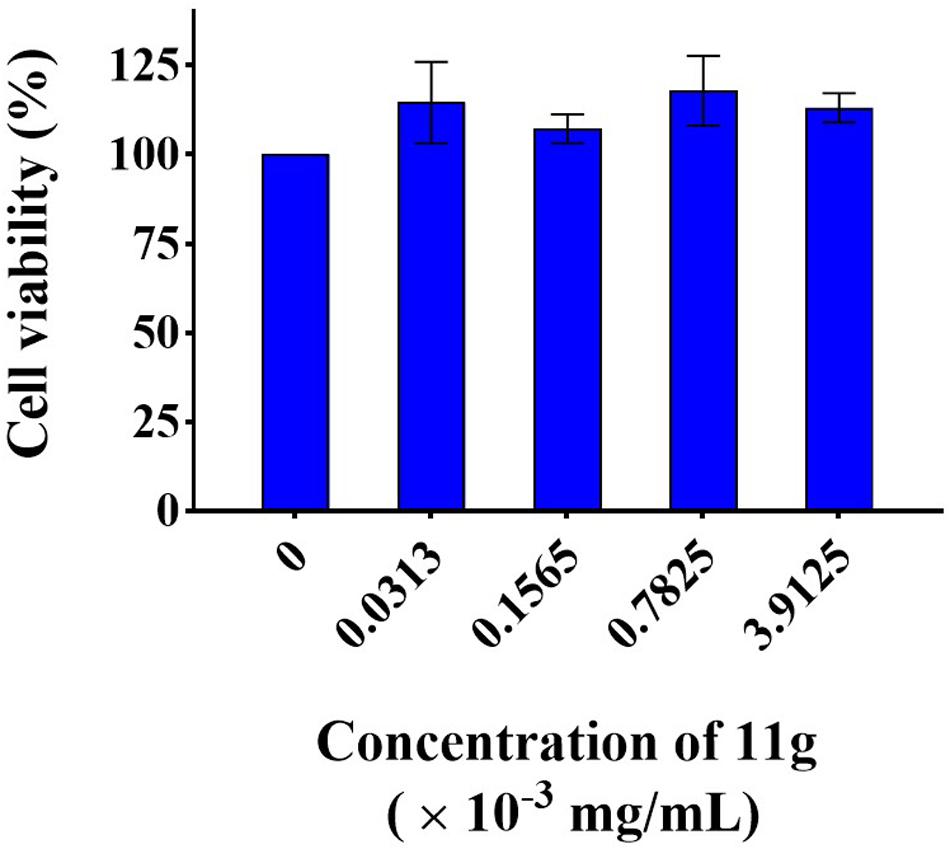
Figure 6. Cytotoxicity of 11g to H9C2. Cells were treated with 11g for 24 h, after which relative viable cell number was measured by CCK-8 assay (n = 3; mean ± SD; one-way ANOVA with Bonferroni post-test).
Protective Effect of 11g Against Fungal Infection in vivo
To investigate the protective effect of 11g in vivo, mice were intravenously infected with C. albicans to prepare systemic candidiasis, and then treated with 11g (4 mg/kg) for 5 consecutive days. Results of survival rates show that mice treated with vehicle all died within 22 days post-infection. The median survival of mice in control group was attained at 14 days and at 24 days in 11g group (Figure 7A). These results suggest that 11g exhibited significantly protective effect against systemic candidiasis. Data of fungal burden of paired kidneys show that the median value of fungal burden is significantly lower in 11g group than in control group (Figure 7B). At the same time, we tested fungal burden in livers either. However, there was no significant differences in fungal burden between 11g group and control group (Figure 7C). Histopathological analysis of kidneys shows that there were less inflammatory cells and fungal hyphae in 11g group than in control group (Figures 7D,E). These data suggest that 11g has potent protective effect against fungal infection in vivo.
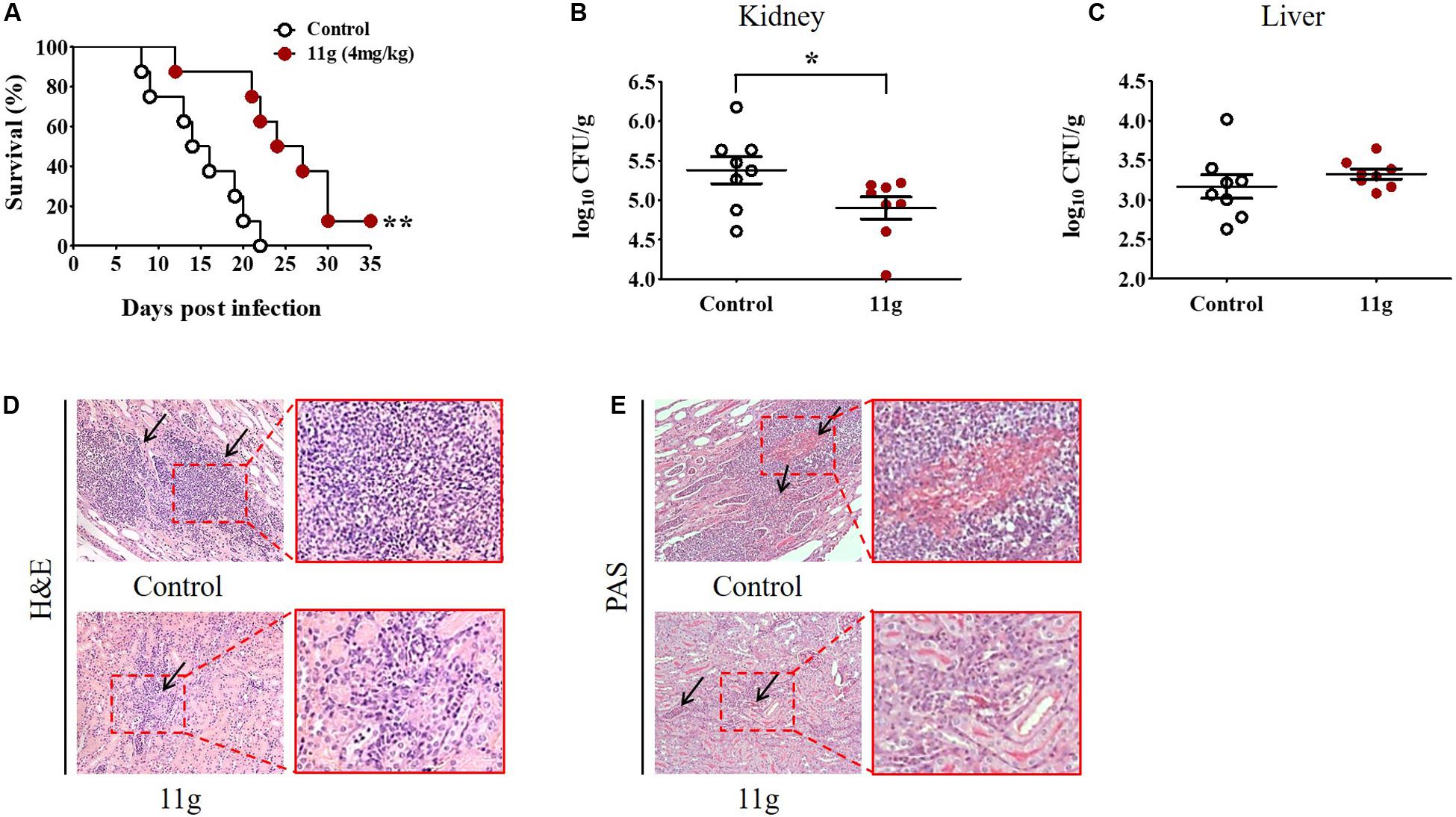
Figure 7. 11g exerts protective effects in mice with systemic candidiasis. Mice (n = 8 per group) were intravenously infected with 5 × 105 CFU of C. albicans SC5314. Then 11g (4 mg/kg) was administered intraperitoneally 2 h later. Antifungal agents were injected once a day for 5 consecutive days. (A) Survival rates were monitored for 35 days after infection (n = 8; log-rank test; **P < 0.01). (B,C) Fungal burdens of paired kidneys (B) and livers (C) were measured on day 5 post-infection. Fungal burden was determined as log10 CFU/g (n = 8; mean ± SD; Mann-Whitney U test; *P < 0.05). (D,E) Histopathological analysis of kidneys from 11g treated group. (D) H&E-stained sections. (E) PAS-stained sections. Black arrows in (D) indicate inflammatory cell infiltration and in (E) indicate hyphae. Magnification = 200×. Data are representative of three independent experiments.
In this paper, 11g (4 mg/kg) was used to treat mice with systemic candidiasis. 11g showed significant inhibition effect on fungal burden in kidneys, but not in livers. We inferred that this might be due to the low experimental concentration of 11g used in this paper. Therefore, we increased the experimental concentration of 11g in the follow-up experiment. Higher concentrations (8, 16, and 32 mg/kg) of 11g were used to treat mice with systemic candidiasis. Data show that the mice survival rate increased with the increase of the dose concentration. In the fourth week after infection, the survival rate of 11g (8 mg/kg) treatment group increased to 50%, and the survival rate of 11g (16 or 32 mg/kg) treatment group increased to 75% (data not shown). The fungal burden of different organs in 11g-treated mice (at higher concentrations, such as: 8, 16, and 32 mg/kg) with systemic candidiasis will be investigated in the future. A detailed report about the therapeutic effect of 11g (higher concentrations, such as: 8, 16, and 32 mg/kg) will be discussed in the next paper by our group.
Discussion
In recently years, a series of GPI biosynthesis inhibitors have been reported by researchers (Tsukahara et al., 2003; Nakamoto et al., 2010; Miyazaki et al., 2011; Mann et al., 2015). In 2003, Tsukahara et al. reported a GPI-anchored mannoproteins inhibitor (BIQ), which targets GWT1 gene product (Tsukahara et al., 2003). On the basis of optimizing BIQ, Eisai Co. Ltd reported two novel 2-aminopyridine derivatives (10b and E1210) in 2010 and 2011, which have been showed as effective GWT1 protein inhibitors (Nakamoto et al., 2010; Miyazaki et al., 2011). Three years later, another GPI inhibitor agent named G884 was reported by Mann et al. (2015), which proved to have an effective inhibition effect on Gwt1-mediated acylation. In the course of further studies on the structure–activity relationship of these GPI biosynthesis inhibitors, our group designed and synthesized a series of 2-aminonicotinamide derivatives (Ni et al., 2017). Among them, 11g is the most promising antifungal agent, which arouses our interest in further research.
It has been reported that the C. albicans cell wall consists two layers (Gow et al., 2011). Skeletal polysaccharides β-(1, 3)-glucan and chitin are located in the inner layer and are covered by mannoprotein (outer layer) (Gow et al., 2011). Most CWPs are GPI-CWPs. Most often, they are covalently attached to the inner layer through a GPI remnant and β-(1, 6)-glucan polymers (Richard and Plaine, 2007; Chaffin, 2008). Therefore, GPI absence would remove the attachment sites of GPI-CWPs, leading to the destruction of mannoprotein layer and the exposure of β-glucan.
In the published paper “Study on the mechanism of NT-89 against Candida albicans based on quantitative proteomics (in Chinese),” we analyzed the effect of 11g on the protein expression of C. albicans SC5314 by iTRAQ tests (Liu et al., 2019). Data show that the expression of some GPI-anchored proteins (such as RHD3) were significantly decreased in 11g-treated C. albicans SC5314. RHD3 is a GPI-anchored cell wall protein in C. albicans SC5314. It has been reported that rhd3 mutants display a significant reduction of cell wall mannan, which is consistent with the results of confocal experiments in this paper (de Boer et al., 2010). At the same time, the expression of some proteins (such as PMI1 and PMT1) was significantly increased in 11g-treated C. albicans SC5314. PMI1 is a phosphomannose isomerase, which is involved in biosynthesis of the cell wall (Smith et al., 1995); PMT1 is a protein mannosyltransferase responsible for the initiation of O-mannosylation, which plays an important role in maintaining the integrity of cell wall (Prill et al., 2005). Based on the above data, we inferred that 11g could affect the structure of mannose layer in cell wall by inhibiting the GPI biosynthesis pathway. At the same time, this effect triggered the feedback regulation in fungi, leading to the repair of mannose layer and the expression enhancement of some mannose proteins in cell wall. Therefore, 11g could result in the remodeling of fungi cell wall and the exposure of partial glucan. The interaction of mannose and glucan enhanced the immunogenicity of 11g-treated C. albicans SC5314.
It is known that β-glucan is a predominant PAMP in fungal cell wall. The innate immune system can clear fungi after recognizing β-glucan in host-pathogen interaction (Poulain and Jouault, 2004). However, the truth is that a fungus often escapes from the host immune recognition by camouflaging the majority of its β-glucan layer (Wheeler and Fink, 2006). Here, as an effective antifungal agent, 11g could enhance fungal immunogenicity by exposing the β-glucan layer. Results show that 11g-treated C. albicans activated the NF-κB- and MAPK-signaling cascades, and also elicited specific cytokine (TNF-α, IL-10, IL-12/23 p40, and IL-6) secretions from macrophages. Previous studies show that TNF-α or IL-12 could facilitate Th1 cells differentiation and activation; IL-6 or IL-23 could facilitate Th17 cells differentiation and activation (Aggarwal et al., 2003; Trinchieri, 2003). The activation of Th1 and Th17 cells would trigger inflammatory responses, leading to the recruitment of humoral and cellular immune factors (LeibundGut-Landmann et al., 2012; Cassone, 2013). IL-10 could facilitate CD4+CD25+Treg cell proliferation, and trigger immunosuppression to modulate immune responses (Netea et al., 2004; Sutmuller et al., 2006). Additionally, our results also show that all the immune recognition activities induced by 11g were significantly inhibited by laminarin, suggesting these immune recognition activities were mainly Dectin-1-dependent, which is consistent with previous reports (Brown et al., 2002; Gantner et al., 2005; LeibundGut-Landmann et al., 2012).
It has been reported that laminarin is a specific inhibitor of Dectin-1(Gantner et al., 2005). Compared with the data of C. albicans SC5314 + 11g group, the activation of NF-κB- and MAPK-signaling pathway was significantly inhibited in C. albicans SC5314 + 11g + laminarin group. At the same time, the release of inflammatory cytokines (TNF-α, IL-10, IL-12/23 p40, and IL-6) was significantly decreased in C. albicans SC5314 + 11g + laminarin group, too. These data suggest that 11g-treated C. albicans SC5314 could activate Dectin-1-dependent NF-κB- and MAPK-signaling pathway and further elicit Dectin-1-dependent cytokines secretion in macrophages. However, compared with C. albicans SC5314 group, the release of inflammatory factors partially increased in C. albicans SC5314 + 11g + laminarin group. These data suggest that there existed some other signaling pathways resulting in the release of inflammatory factors in C. albicans SC5314 + 11g + laminarin group. Therefore, Dectin-1-dependent signaling pathway is one of the main signaling pathways activated by 11g-treated C. albicans in macrophages. Also, it should be noted that some mannan (a PAMP) still remained on the surface of fungi after 11g treatment. Therefore, we cannot exclude the possibility that other pattern-recognition receptors (PRRs) participated in the immune recognition process.
Mannan is a PAMP. It seems to directly contradict with the viewpoint that fungi reduce its immunogenicity by covering mannan on β-glucan layer. A possible explanation is that mannan could prevent the recognition of Dectin-1 to β-glucan, as well as the interaction between mannan-TLR and β-glucan-Dectin-1 pathways. It has been shown that mannan-TLR and β-glucan-Dectin-1 pathways exhibit potent synergistic effect on immune response. Therefore, although mannan could be recognized by immune cells, it cannot cause the very efficient activation of host immune system by itself (Netea et al., 2008).
In summary, our study shows that 11g is a potent antifungal agent both in vitro and in vivo. Further study shows that 11g could enhance the immunogenicity of fungi by unmasking β-glucan layer, activating the Dectin-1-dependent protective immune responses. Thus, 11g is a very promising antifungal drug, which is expected to be a candidate to expand the toolbox of fungal infection treatment. Further studies are expected to investigate the molecular target of 11g on GPI synthesis or transport process. In addition, Dectin-1 knockout mice will be used to further verify the mechanism of Dectin-1-dependent protective immune responses induced by 11g-treated C. albicans.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The animal study was reviewed and approved by Institutional Animal Use and Care Committee of Tongji University.
Author Contributions
All authors contributed toward data analysis, drafting and revising the manuscript, and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Science Foundation of China (81573473, 81673280, and 81330083).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.01324/full#supplementary-material
Abbreviations
ConA, concanavalin A; CSH, cell surface hydrophobicity; CWPs, cell wall proteins; DMSO, dimethyl sulfoxide; ELISA, enzyme-linked immunosorbent assay; FBS, fetal bovine serum; GPI, glycosylphosphatidylinositol; GPI-CWPs, GPI-anchored cell wall proteins; H&E, hematoxylin and eosin; MIC, minimum inhibitory concentration; MOI, multiplicity of infection; PAMP, pathogen-associated molecular patterns; PAS, periodic acid-schiff; PRRs, pattern-recognition receptors; SDA, sabouraud dextrose agar; SDB, sabouraud dextrose broth; UV, ultraviolet; YPD, yeast peptone dextrose.
References
Aggarwal, S., Ghilardi, N., Xie, M. H., de Sauvage, F. J., and Gurney, A. L. (2003). Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278, 1910–1914. doi: 10.1074/jbc.M207577200
Baddley, J. W., Andes, D. R., Marr, K. A., Kontoyiannis, D. P., Alexander, B. D., Kauffman, C. A., et al. (2010). Factors associated with mortality in transplant patients with invasive aspergillosis. Clin. Infect. Dis. 50, 1559–1567. doi: 10.1086/652768
Brown, G. D., Herre, J., Williams, D. L., Willment, J. A., Marshall, A. S., and Gordon, S. (2003). Dectin-1 mediates the biological effects of beta-glucans. J. Exp. Med. 197, 1119–1124. doi: 10.1084/jem.20021890
Brown, G. D., Taylor, P. R., Reid, D. M., Willment, J. A., Williams, D. L., Martinez-Pomares, L., et al. (2002). Dectin-1 is a major beta-glucan receptor on macrophages. J. Exp. Med. 196, 407–412. doi: 10.1084/jem.20020470
Cassone, A. (2013). Development of vaccines for Candida albicans: fighting a skilled transformer. Nat. Rev. Microbiol. 11, 884–891. doi: 10.1038/nrmicro3156
Chaffin, W. L. (2008). Candida albicans cell wall proteins. Microbiol. Mol. Biol. Rev. 72, 495–544. doi: 10.1128/mmbr.00032-07
Cowen, L. E., Anderson, J. B., and Kohn, L. M. (2002). Evolution of drug resistance in Candida albicans. Annu. Rev. Microbiol. 56, 139–165. doi: 10.1146/annurev.micro.56.012302.160907
de Boer, A. D., de Groot, P. W., Weindl, G., Schaller, M., Riedel, D., Diez-Orejas, R., et al. (2010). The Candida albicans cell wall protein Rhd3/Pga29 is abundant in the yeast form and contributes to virulence. Yeast 27, 611–624. doi: 10.1002/yea.1790
Denning, D. W., and Hope, W. W. (2010). Therapy for fungal diseases: opportunities and priorities. Trends Microbiol. 18, 195–204. doi: 10.1016/j.tim.2010.02.004
Gantner, B. N., Simmons, R. M., Canavera, S. J., Akira, S., and Underhill, D. M. (2003). Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197, 1107–1117. doi: 10.1084/jem.20021787
Gantner, B. N., Simmons, R. M., and Underhill, D. M. (2005). Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 24, 1277–1286. doi: 10.1038/sj.emboj.7600594
Gorjestani, S., Darnay, B. G., and Lin, X. (2012). Tumor necrosis factor receptor-associated factor 6 (TRAF6) and TGFbeta-activated kinase 1 (TAK1) play essential roles in the C-type lectin receptor signaling in response to Candida albicans infection. J. Biol. Chem. 287, 44143–44150. doi: 10.1074/jbc.M112.414276
Gow, N. A., van de Veerdonk, F. L., Brown, A. J., and Netea, M. G. (2011). Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat. Rev. Microbiol. 10, 112–122. doi: 10.1038/nrmicro2711
Granger, B. L., Flenniken, M. L., Davis, D. A., Mitchell, A. P., and Cutler, J. E. (2005). Yeast wall protein 1 of Candida albicans. Microbiology 151(Pt 5), 1631–1644. doi: 10.1099/mic.0.27663-0
Gudlaugsson, O., Gillespie, S., Lee, K., Vande Berg, J., Hu, J., Messer, S., et al. (2003). Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 37, 1172–1177. doi: 10.1086/378745
Hata, K., Horii, T., Miyazaki, M., Watanabe, N. A., Okubo, M., Sonoda, J., et al. (2011). Efficacy of oral E1210, a new broad-spectrum antifungal with a novel mechanism of action, in murine models of Candidiasis, Aspergillosis, and fusariosis. Antimicrob. Agents Chemother. 55, 4543–4551. doi: 10.1128/aac.00366-11
Huang, X., Fan, C., Zhu, H., Le, W., Cui, S., Chen, X., et al. (2018). Glypican-1-antibody-conjugated Gd-Au nanoclusters for FI/MRI dual-modal targeted detection of pancreatic cancer. Int. J. Nanomedicine 13, 2585–2599. doi: 10.2147/ijn.s158559
Kesavan, C., Raghunathan, M., and Ganesan, N. (2005). Morphological and growth altering effects of Cisplatin in C. albicans using fluorescence microscopy. Ann. Clin. Microbiol. Antimicrob. 4:7. doi: 10.1186/1476-0711-4-7
Klis, F. M., Sosinska, G. J., de Groot, P. W., and Brul, S. (2009). Covalently linked cell wall proteins of Candida albicans and their role in fitness and virulence. FEMS Yeast Res. 9, 1013–1028. doi: 10.1111/j.1567-1364.2009.00541.x
Kontoyiannis, D. P., Marr, K. A., Park, B. J., Alexander, B. D., Anaissie, E. J., Walsh, T. J., et al. (2010). Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the transplant-associated infection surveillance network (TRANSNET) database. Clin. Infect Dis. 50, 1091–1100. doi: 10.1086/651263
Kumamoto, C. A., and Vinces, M. D. (2005). Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell Microbiol. 7, 1546–1554. doi: 10.1111/j.1462-5822.2005.00616.x
LeibundGut-Landmann, S., Wuthrich, M., and Hohl, T. M. (2012). Immunity to fungi. Curr. Opin. Immunol. 24, 449–458. doi: 10.1016/j.coi.2012.04.007
Liu, Y., Yan, L., and Jiang, Y. (2019). Study on the mechanism of NT-89 against Candida albicans based on quantitative proteomics. Chin. J. Mycol. 14, 70–77.
Ma, C., Du, F., Yan, L., He, G., He, J., Wang, C., et al. (2015). Potent activities of roemerine against Candida albicans and the underlying mechanisms. Molecules 20, 17913–17928. doi: 10.3390/molecules201017913
Mann, P. A., McLellan, C. A., Koseoglu, S., Si, Q., Kuzmin, E., Flattery, A., et al. (2015). Chemical genomics-based antifungal drug discovery: targeting glycosylphosphatidylinositol (GPI) precursor biosynthesis. ACS Infect. Dis. 1, 59–72. doi: 10.1021/id5000212
Martin, G. S., Mannino, D. M., Eaton, S., and Moss, M. (2003). The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348, 1546–1554. doi: 10.1056/NEJMoa022139
McLellan, C. A., Whitesell, L., King, O. D., Lancaster, A. K., Mazitschek, R., and Lindquist, S. (2012). Inhibiting GPI anchor biosynthesis in fungi stresses the endoplasmic reticulum and enhances immunogenicity. ACS Chem. Biol. 7, 1520–1528. doi: 10.1021/cb300235m
Miyazaki, M., Horii, T., Hata, K., Watanabe, N. A., Nakamoto, K., Tanaka, K., et al. (2011). In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob. Agents Chemother. 55, 4652–4658. doi: 10.1128/aac.00291-11
Nakamoto, K., Tsukada, I., Tanaka, K., Matsukura, M., Haneda, T., Inoue, S., et al. (2010). Synthesis and evaluation of novel antifungal agents-quinoline and pyridine amide derivatives. Bioorg. Med. Chem. Lett. 20, 4624–4626. doi: 10.1016/j.bmcl.2010.06.005
Netea, M. G., Brown, G. D., Kullberg, B. J., and Gow, N. A. (2008). An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 6, 67–78. doi: 10.1038/nrmicro1815
Netea, M. G., Sutmuller, R., Hermann, C., Van der Graaf, C. A., Van der Meer, J. W., van Krieken, J. H., et al. (2004). Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J. Immunol. 172, 3712–3718. doi: 10.4049/jimmunol.172.6.3712
Ni, T., Li, R., Xie, F., Zhao, J., Huang, X., An, M., et al. (2017). Synthesis and biological evaluation of novel 2-aminonicotinamide derivatives as antifungal agents. ChemMedChem 12, 319–326. doi: 10.1002/cmdc.201600545
Nicola, A. M., and Casadevall, A. (2012). In vitro measurement of phagocytosis and killing of Cryptococcus neoformans by macrophages. Methods Mol. Biol. 844, 189–197. doi: 10.1007/978-1-61779-527-5_14
Orlean, P., and Menon, A. K. (2007). Thematic review series: lipid posttranslational modifications. GPI anchoring of protein in yeast and mammalian cells, or: how we learned to stop worrying and love glycophospholipids. J. Lipid Res. 48, 993–1011. doi: 10.1194/jlr.R700002-JLR200
Pfaller, M. A., Diekema, D. J., Gibbs, D. L., Newell, V. A., Ellis, D., Tullio, V., et al. (2010). Results from the ARTEMIS DISK global antifungal surveillance study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida Species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J. Clin. Microbiol. 48, 1366–1377. doi: 10.1128/jcm.02117-09
Pfaller, M. A., Messer, S. A., Boyken, L., Rice, C., Tendolkar, S., Hollis, R. J., et al. (2008). In vitro survey of triazole cross-resistance among more than 700 clinical isolates of Aspergillus species. J. Clin. Microbiol. 46, 2568–2572. doi: 10.1128/jcm.00535-08
Poulain, D., and Jouault, T. (2004). Candida albicans cell wall glycans, host receptors and responses: elements for a decisive crosstalk. Curr. Opin. Microbiol. 7, 342–349. doi: 10.1016/j.mib.2004.06.011
Prill, S. K., Klinkert, B., Timpel, C., Gale, C. A., Schroppel, K., and Ernst, J. F. (2005). PMT family of Candida albicans: five protein mannosyltransferase isoforms affect growth, morphogenesis and antifungal resistance. Mol. Microbiol. 55, 546–560. doi: 10.1111/j.1365-2958.2004.04401.x
Quan, H., Cao, Y. Y., Xu, Z., Zhao, J. X., Gao, P. H., Qin, X. F., et al. (2006). Potent in vitro synergism of fluconazole and berberine chloride against clinical isolates of Candida albicans resistant to fluconazole. Antimicrob. Agents Chemother. 50, 1096–1099. doi: 10.1128/aac.50.3.1096-1099.2006
Richard, M. L., and Plaine, A. (2007). Comprehensive analysis of glycosylphosphatidylinositol-anchored proteins in Candida albicans. Eukaryot Cell 6, 119–133. doi: 10.1128/ec.00297-06
Saijo, S., Fujikado, N., Furuta, T., Chung, S. H., Kotaki, H., Seki, K., et al. (2007). Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat. Immunol. 8, 39–46. doi: 10.1038/ni1425
Smith, D. J., Proudfoot, A. E., Detiani, M., Wells, T. N., and Payton, M. A. (1995). Cloning and heterologous expression of the Candida albicans gene PMI 1 encoding phosphomannose isomerase. Yeast 11, 301–310. doi: 10.1002/yea.320110402
Sutmuller, R. P., den Brok, M. H., Kramer, M., Bennink, E. J., Toonen, L. W., Kullberg, B. J., et al. (2006). Toll-like receptor 2 controls expansion and function of regulatory T cells. J. Clin. Invest. 116, 485–494. doi: 10.1172/jci25439
Trinchieri, G. (2003). Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3, 133–146. doi: 10.1038/nri1001
Tsukahara, K., Hata, K., Nakamoto, K., Sagane, K., Watanabe, N. A., Kuromitsu, J., et al. (2003). Medicinal genetics approach towards identifying the molecular target of a novel inhibitor of fungal cell wall assembly. Mol. Microbiol. 48, 1029–1042. doi: 10.1046/j.1365-2958.2003.03481.x
Victoria, G. S., Kumar, P., and Komath, S. S. (2010). The Candida albicans homologue of PIG-P, CaGpi19p: gene dosage and role in growth and filamentation. Microbiology 156(Pt 10), 3041–3051. doi: 10.1099/mic.0.039628-0
Wheeler, R. T., and Fink, G. R. (2006). A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog. 2:e35. doi: 10.1371/journal.ppat.0020035
Wisplinghoff, H., Bischoff, T., Tallent, S. M., Seifert, H., Wenzel, R. P., and Edmond, M. B. (2004). Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect Dis. 39, 309–317. doi: 10.1086/421946
Zhang, S. Q., Zou, Z., Shen, H., Shen, S. S., Miao, Q., Huang, X., et al. (2016). Mnn10 maintains pathogenicity in Candida albicans by extending alpha-1,6-mannose backbone to evade host dectin-1 mediated antifungal immunity. PLoS Pathog. 12:e1005617. doi: 10.1371/journal.ppat.1005617
Zhao, M., Lepak, A. J., Marchillo, K., Vanhecker, J., Sanchez, H., Ambrose, P. G., et al. (2019). APX001 pharmacokinetic/pharmacodynamic target determination against aspergillus fumigatus in an in vivo model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 63:e2372-18. doi: 10.1128/aac.02372-18
Keywords: Candida albicans, antifungal agent, β-glucan, dectin-1, immunogenicity
Citation: Huang X, Liu Y, Ni T, Li L, Yan L, An M, Zhang D and Jiang Y (2020) 11g, a Potent Antifungal Candidate, Enhances Candida albicans Immunogenicity by Unmasking β-Glucan in Fungal Cell Wall. Front. Microbiol. 11:1324. doi: 10.3389/fmicb.2020.01324
Received: 29 October 2019; Accepted: 25 May 2020;
Published: 30 June 2020.
Edited by:
Miguel Cacho Teixeira, University of Lisbon, PortugalReviewed by:
Hector Mora Montes, University of Guanajuato, MexicoTeresa Zelante, University of Perugia, Italy
Carol Munro, University of Aberdeen, United Kingdom
Copyright © 2020 Huang, Liu, Ni, Li, Yan, An, Zhang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dazhi Zhang, emR6aGFuZ195amh4QHNtbXUuZWR1LmNu; Yuanying Jiang, amlhbmd5eUB0b25namkuZWR1LmNu
†These authors have contributed equally to this work
‡Present address: Xin Huang, Shanghai East Hospital, The Institute for Biomedical Engineering and Nano Science, Tongji University School of Medicine, Shanghai, China
 Xin Huang
Xin Huang Yu Liu
Yu Liu Tingjunhong Ni
Tingjunhong Ni Liping Li1
Liping Li1