- 1Department of Haematology, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China
- 2Department of Laboratory Medicine, The Second Affiliated Hospital of Shantou University Medical College, Shantou, China
- 3Department of Dermato-Venereology, Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- 4School of Food Science and Engineering, Guangdong Province Key Laboratory for Green Processing of Natural Products and Product Safety, South China University of Technology, Guangzhou, China
- 5Guangdong Zhongqing Font Biochemical Science and Technology Co. Ltd., Maoming, China
- 6Research Center for Translational Medicine, The Second Affiliated Hospital, Medical College of Shantou University, Shantou, China
- 7Department of Civil and Environmental Engineering, A. James Clark School of Engineering, University of Maryland, College Park, MD, United States
As a common foodborne pathogen, Escherichia coli O157:H7 produces toxins causing serious diseases. However, traditional methods failed in detecting E. coli O157:H7 cells in the viable but non-culturable (VBNC) state, which poses a threat to food safety. This study aimed at investigating the formation, control, and detection of the VBNC state of E. coli O157:H7. Three factors including medium, salt, and acid concentrations were selected as a single variation. Orthogonal experiments were designed with three factors and four levels, and 16 experimental schemes were used. The formation of the VBNC state was examined by agar plate counting and LIVE/DEAD® BacLightTM bacterial viability kit with fluorescence microscopy. According to the effects of environmental conditions on the formation of the VBNC state of E. coli O157:H7, the inhibition on VBNC state formation was investigated. In addition, E. coli in the VBNC state in food samples (crystal cake) was detected by propidium monoazide–polymerase chain reaction (PMA-PCR) assays. Acetic acid concentration showed the most impact on VBNC formation of E. coli O157:H7, followed by medium and salt concentration. The addition of 1.0% acetic acid could directly kill E. coli O157:H7 and eliminate its VBNC formation. In crystal cake, 25, 50, or 100% medium with 1.0% acetic acid could inhibit VBNC state formation and kill E. coli O157:H7 within 3 days. The VBNC cell number was reduced by adding 1.0% acetic acid. PMA-PCR assay could be used to detect E. coli VBNC cells in crystal cake with detection limit at 104 CFU/ml. The understanding on the inducing and inhibitory conditions for the VBNC state of E. coli O157:H7 in a typical food system, as well as the development of an efficient VBNC cell detection method might aid in the control of VBNC E. coli O157:H7 cells in the food industry.
Highlights
– The induction of the VBNC state of Escherichia coli by nutritional conditions, acetic acid concentration, and salt concentration was investigated.
– In crystal cake, the VBNC state of E. coli could be inhibited by adding 1.0% acetic acid in volume fraction at 4 or −20°C.
– Propidium monoazide–polymerase chain reaction (PMA-PCR) assays could be applied in detection of the VBNC state of E. coli.
Introduction
Escherichia coli is one of the major bacterial contaminants associated with foodborne infections worldwide (Zhao et al., 2010b; Sayad et al., 2016; Bao et al., 2017a, b; Xie et al., 2017a; Liu et al., 2018a, b; Renuka et al., 2018; Xu et al., 2020). Since food safety accident caused by E. coli was first reported in the United States in 1982, various reports have been posted in China, the United Kingdom, Japan, Australia, and other countries. In recent years, E. coli has gradually developed into a health and safety issue of worldwide concern (Xu et al., 2007, 2008a, 2008b, 2011b, 2016a, 2016b; You et al., 2012; Lin et al., 2016; Miao et al., 2016, 2017a, 2017b, 2018; Zhao et al., 2018a, b). The high incidence rate of E. coli in food products underlies the urgent need of appropriate strategies for early detection (Zhao et al., 2010b, c, 2011, 2014 2018; Wang et al., 2011; Xu et al., 2011c, 2012b; Lin et al., 2017; Miao et al., 2017c; Liu et al., 2019; Miao et al., 2019). E. coli O157:H7, capable of producing Shiga toxin or Shiga-like toxin, can cause hemorrhagic colitis and hemolytic uremic syndrome in human beings (Neil et al., 2012). Shiga toxin causes various diseases including bloody diarrhea and severe hemolytic uremic syndrome, leading to kidney failure. E. coli O157:H7 is a typical foodborne pathogen, colonizing in food systems and drinking water (Ercumen et al., 2017; Bourely et al., 2018; Zeinhom et al., 2018).
The standard detection method for foodborne pathogens is agar plate counting (Xu et al., 2008a, b, 2009; 2010; 2011a; 2012a; 2016c; 2017a; 2017b; 2018; Zhong et al., 2013; Zhang et al., 2015; Bao et al., 2017c; Xie et al., 2017b; Jia et al., 2018; Wen et al., 2020). However, this method fails in detecting viable but non-culturable (VBNC) state cells that were first reported in 1982 (Xu et al., 1982). Up to now, 85 types of microorganisms have been found to enter the VBNC state, including 18 non-pathogenic bacteria and 67 pathogenic bacteria (Ramamurthy et al., 2014). Studies have shown that E. coli has the ability to enter the VBNC state (Yaron and Matthews, 2002; Zhang et al., 2015; Liu et al., 2017b; Afari et al., 2019). VBNC cells differ from normal cells in both physiology and morphology. However, VBNC cells acquire high ATP concentrations (Lindback et al., 2010) and metabolic activity based on the expression of certain genes (Yaron and Matthews, 2002).
Bacteria in the VBNC state fail to form colonies in routine detection agar plates under certain environmental stresses. However, these cells have food spoilage and pathogenic capacity (Liu et al., 2017b). Factors including adverse nutrition levels, temperatures, and osmotic pressures, which are frequently encountered during food processing and storage environments, have been reported to induce bacteria entering the VBNC state. With the failure in detection by routine method and the capability in causing food safety problems, the VBNC state of E. coli O157:H7 is a major concern in the food industry (Liu et al., 2017b).
Crystal cake is a traditional Chinese snack that has the characteristics of rich nutrition. Its rich nutrients and sufficient water make it a natural medium for various pathogenic bacteria and spoilage bacteria, including E. coli. In this study, crystal cake was applied as a representative food system to study the formation, control, and detection of the VBNC state of E. coli O157:H7 under food processing and storage conditions.
Materials and Methods
Cultivation of E. coli O157:H7
Escherichia coli O157:H7 ATCC25922 was stored at -80°C in Luria-Bertani (LB) broth containing 20% (v/v) glycerol. It was streaked on LB agar plate and grown at 37°C for 24 h. Then, a single colony was inoculated into 2 ml of LB broth and incubated at 37°C for 12 h using a shaker incubator set at 150 r/min. The bacteria suspension was diluted 1:100 in fresh medium and cultured for 4 h to the log phase upon further experiments.
Induction of the VBNC State
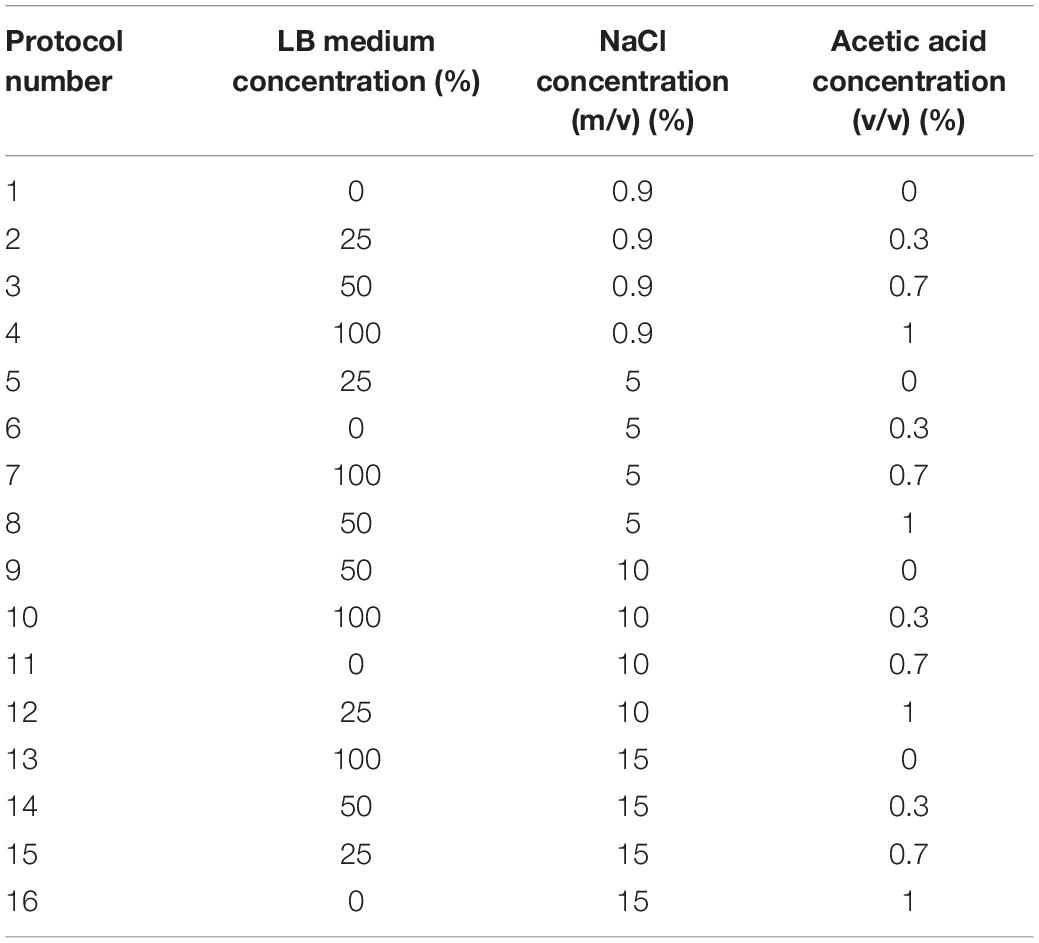
According to the optimal culture conditions of E. coli O157:H7, and the principle of reverse adjustment, conditions that are unfavorable to bacterial growth were selected as candidate factors to induce the VBNC state. Three factors including nutritional state, salt concentration, and acidity were taken as a single variable. To investigate possible VBNC induction conditions, three factors and four levels of orthogonal experiments were designed, and 16 protocols (Table 1) were applied. Log phase cells were centrifuged for 10 min at 5000 r/min and the pellet was washed once with 1 × phosphate buffer solution and then resuspended in the induced solution. The initial concentration of the bacterial solution was diluted to approximately 107 CFU/ml. To avoid the effects of repeated freezing and thawing, the bacterial suspension under each condition was mixed and divided into multiple 1.5-ml centrifuge tubes, followed by induction in refrigerators at 4 and −20°C, respectively.
Determination of Culturable Cell Number During Storage
The culturable cell number in each of the VBNC inducing bacterial culture was determined every 3 days by a plate counting method. Briefly, the culture of E. coli O157:H7 was serial diluted in 0.9% NaCl, spread on LB agar plates, and incubated at 37°C for 24 h. When the culturable number is 1 CFU/ml, the cells were considered non-culturable and possibly enter the VBNC state (Piao et al., 2019).
Determination of Viable Cell Number
To determine if the non-culturable cells are in the VBNC state after exposure to the respective treatment conditions, the LIVE/DEAD® BacLightTM bacterial viability kit (Thermo Fisher Scientific, China) was used. Five hundred microliters of the non-culturable bacterial cell sample was centrifuged at 5000 r/min for 15 min, washed with saline twice, and resuspended in saline. Subsequently, 1.5 μl of SYTO 9 dye and 1.5 μl of propidium iodide were added to the sample, followed by mixing and 30 min incubation in the dark. After incubation, 5-μl cells were captured between a slide and a coverslip and used for fluorescence microscopy. The appearance of green cells indicates the existence of VBNC cells.
Inhibition of VBNC State by Acidity and Nutritional Conditions
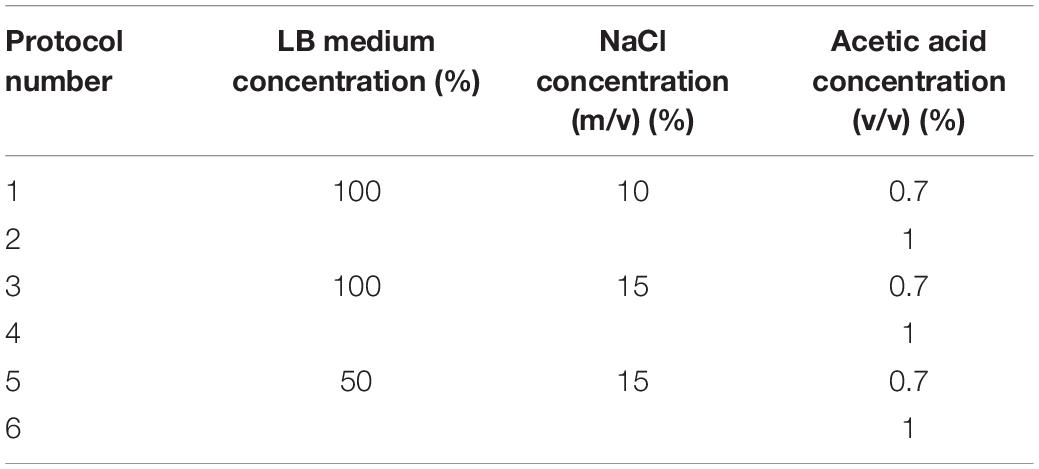
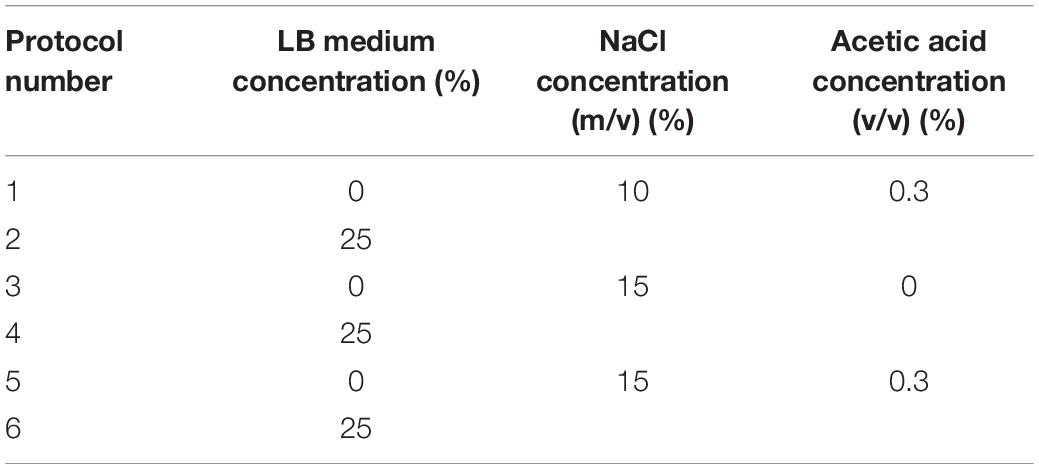
According to the VBNC state induction process, appropriate conditions were selected to inhibit the formation of the VBNC state. The configured medium was resuspended, and the initial concentration of E. coli O157:H7 was adjusted to 107 CFU/ml. The bacterial solution with a volume of 30 ml was stored at 4 and −20°C, respectively. The number of cultivable bacteria was measured by plate counting every 3 days (Tables 2, 3).
Elimination of the VBNC State in Crystal Cake
According to the National Food Safety standards (GB4789.3-2016) in China, 25 g of crystal cake was added to 225 ml of saline, and the concentration of this medium was determined to be 100, 25, and 50% of the food sample culture medium and is configured by dilution and used after autoclaving. Subsequently, E. coli O157:H7 was cultured to the logarithmic phase and centrifuged at 4°C, the supernatant was discarded, and the suspension was resuspended three times with physiological saline. The bacterial cells were resuspended using the sterilized food sample medium, and the concentrations of E. coli O157:H7 were adjusted to 107 CFU/ml, respectively, and a sterile acetic acid solution was added to fix the volume fraction of acetic acid to 1.0%. The induction solution was stored at 4°C, and the culturable number and activity were detected by a plate method combined with a LIVE/DEAD® BacLightTM bacterial viability kit.
PMA-PCR Assays
Twenty-five grams of crystal cake was mixed with 225 ml of physiological saline and sterilized, and E. coli in the VBNC state was added. Bacterial suspensions with initial concentrations of 106, 105, 104, 103, 102, and 10 CFU/ml were obtained. Five hundred microliters of the bacteria suspension was added to a 1.5-ml centrifuge tube, and the PMA working solution was added to a final concentration of 5 μg/ml (to distinguish between the VBNC state and dead cells). After mixing, the samples were kept at room temperature for 10 min in the dark. Subsequently, the centrifuge tube was placed on ice, and 15 cm away from a 650 W halogen lamp for 5 min to complete the binding of PMA and DNA. The PMA–DNA was centrifuged at 10,000 r/min for 5 min, and the supernatant was discarded. DNA was then extracted using a bacterial group DNA extraction kit (Dongsheng Biotech, Guangzhou). The extracted DNA was detected by PCR. PCR assay was performed in a 25-μl volume and with 0.6 μM primers (rfbE-F: TTGGCATCGTGTGGACAGGGTAGGACCGCAGAGGAAAG A; rfbE-R: TGGGACAGGTGTGCTACGGTTTCCACGCC AACCAAGATC). The thermal profile for PCR mixtures was 94°C for 5 min, followed by 30 cycles of 94°C for 30 s, 52°C for 60 s, and 72°C for 90 s and a final extension cycle at 72°C for 7 min. The amplified products (5 μl/well) were analyzed by gel electrophoresis in 2% agarose gels and stained with ethidium bromide for 10 min. A negative control was included using sterile water instead of culture or DNA template.
Results and Discussion
Formation of the VBNC State
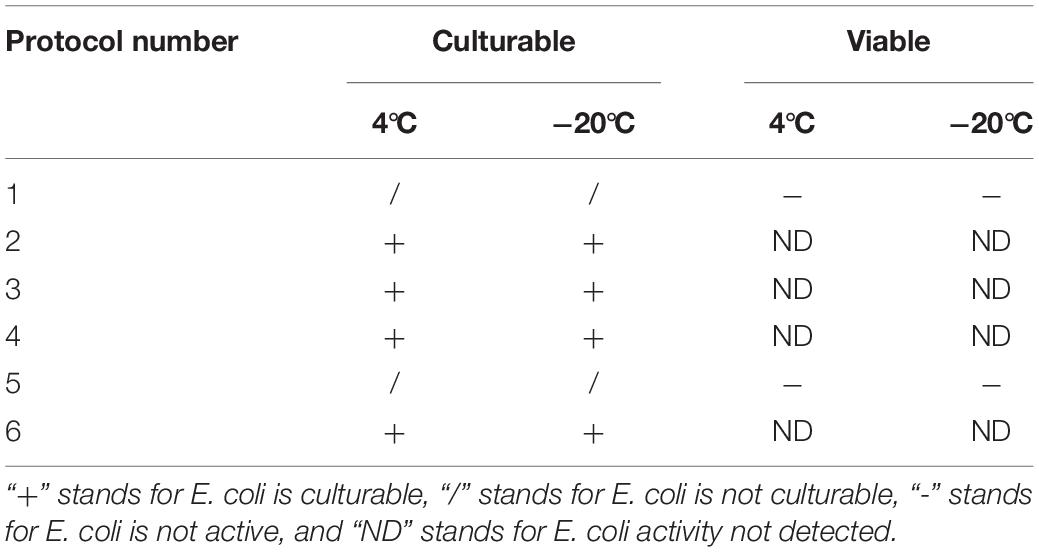
To induce the formation of the VBNC state, 16 induction solutions were applied (Table 1). In induction solution 1, when stored at 4°C, the culturable cell number of E. coli O157:H7 was reduced to 5 × 104 CFU/ml in 66 days. When stored at −20°C, the culturable cell number of E. coli O157:H7 dropped to 0 in 3 days (Figure 1A). In induction solution 2, the number of culturable cells reduced to 0 after 18 days of storage at 4°C, and the number of culturable cells reduced to 0 within 3 days of storage at −20°C (Figure 1B). In induction solution 10, the number of culturable cells reduced to 0 after storing at 4 and −20°C for 39 and 33 days, respectively (Figure 1F). In induction solution 13, the culturable cell number reduced to 0 in 48 and 39 days of storage at 4 and −20°C, respectively (Figure 1G). In induction solution 14, the culturable cell number reduced to 0 in 57 and 66 days when stored at 4 and −20°C, respectively (Figure 1H). In induction solution 5, 7, and 9, although with a downtrend within 66 days, the number of culturable cells did not decrease to 0 (Figures 1C–E). In addition, in induction solutions 3, 4, 6, 8, 11, 12, 15, and 16, the number of culturable cells of E. coli O157:H7 reduced to 0 in 3 days.
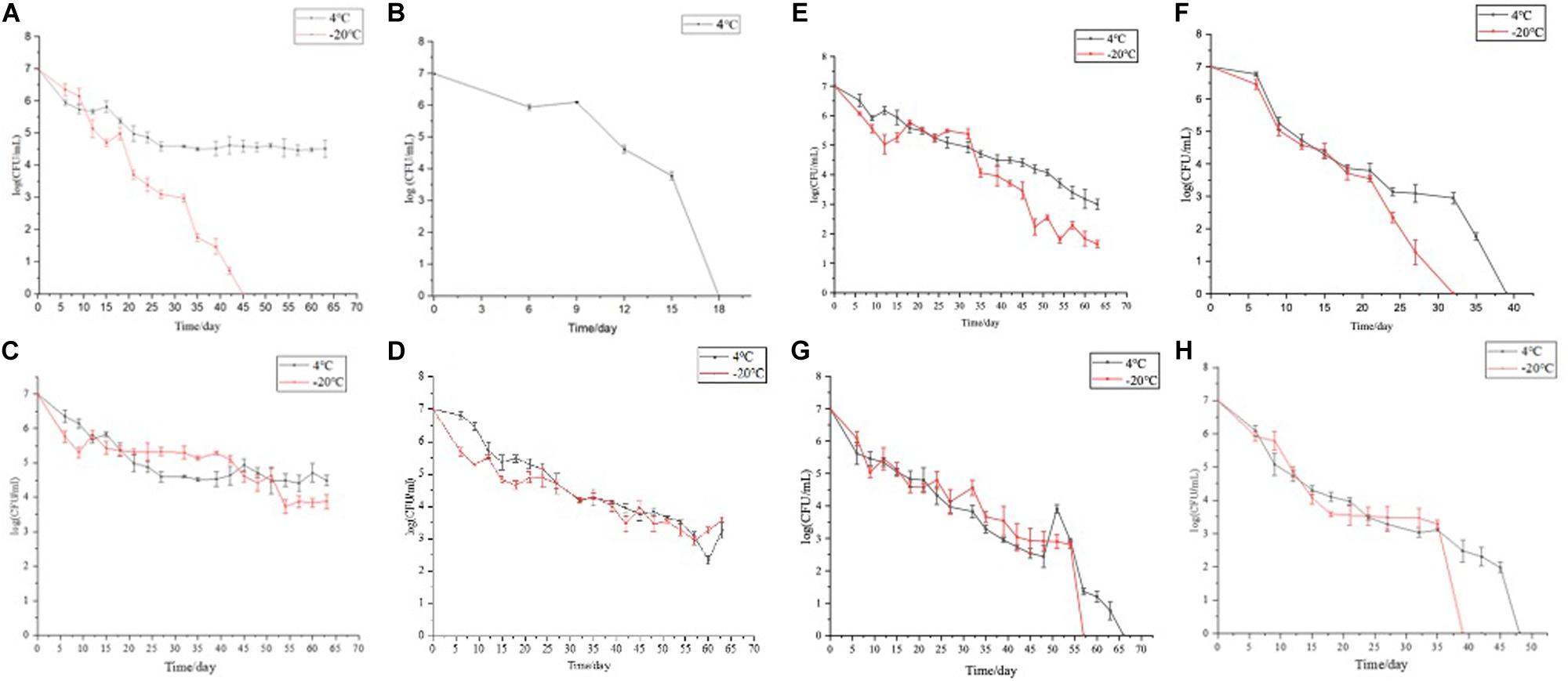
Figure 1. The culturable number of E. coli O157:H7 stored under 16 different conditions (4 and −20°C). (A–H) The cell viability of E. coli O157:H7 when stored at 4 and −20°C under protocols 2, 10, 13, and 14, respectively.
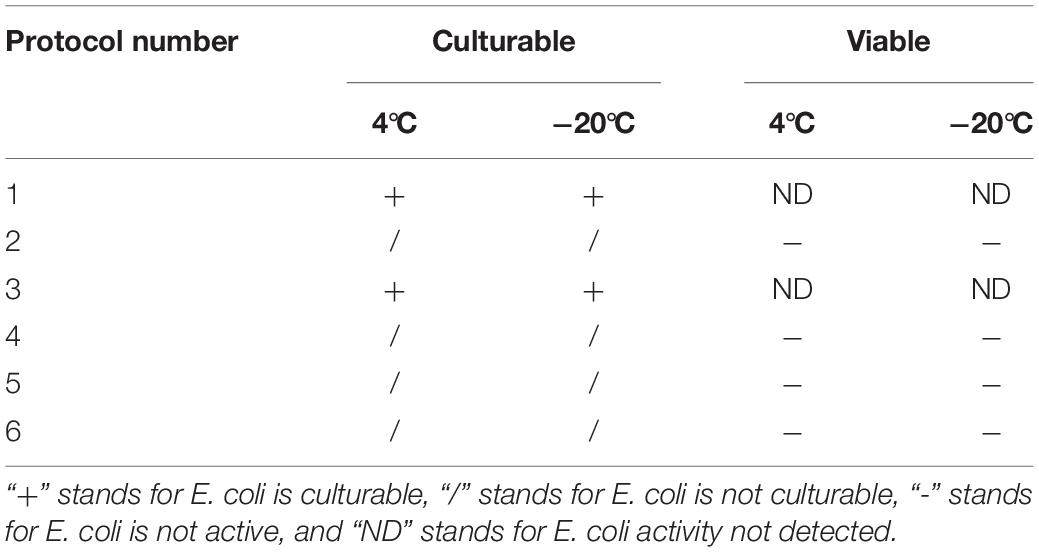
For the induction solutions that were capable of inducing the culturable cell number of E. coli O157:H7 to 0 (Table 4), the existence of viable cells was determined. When the E. coli O157:H7 culture in induction solution 2 was stored at 4°C for 18 days, the cells were all dead, indicating that E. coli O157:H7 did not enter the VBNC state (Figure 2). The fluorescence results of E. coli O157:H7 in induction solutions 1, 10, 13, and 14 showed that both dead and viable cells were detected, indicating that E. coli O157:H7 entered the VBNC state under these conditions.
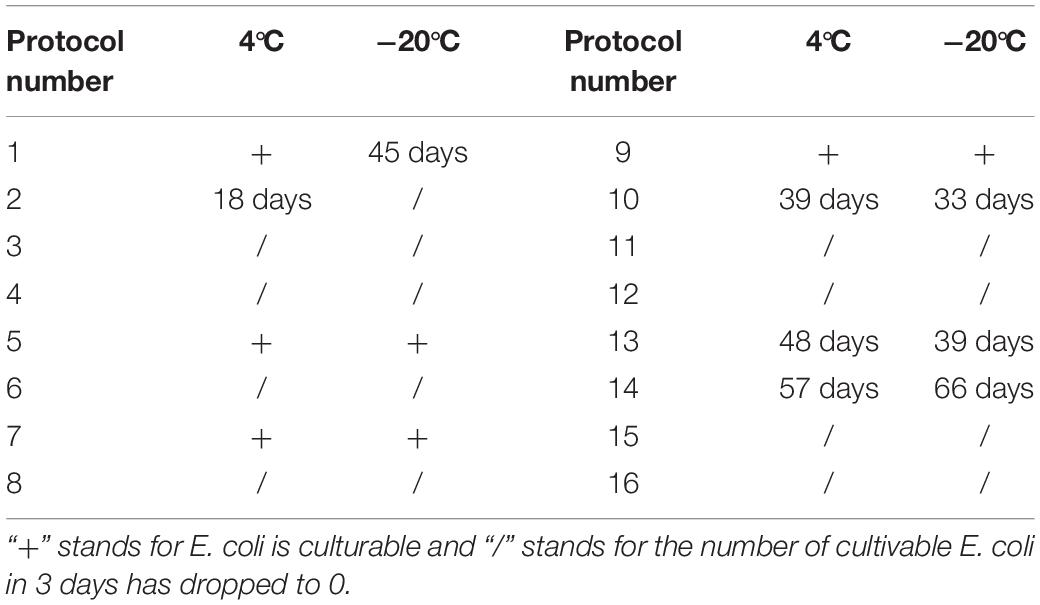
Table 4. The time of culturable number of E. coli O157:H7 decreased to 0 stored at different methods.
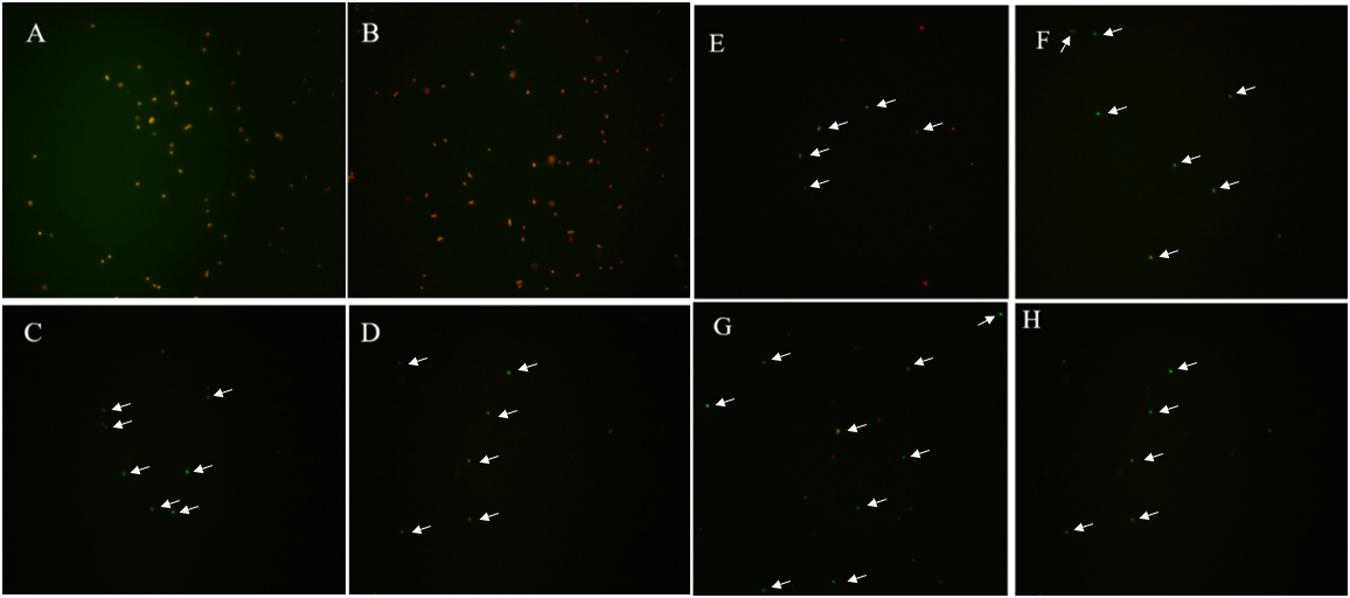
Figure 2. The viability of un-culturable E. coli O157:H7 stored at different conditions with fluorescence. (A–H) E. coli O157:H7 inoculated in the medium configured according to the method 2, 10, 13, 14, and stored at 4 or −20°C, respectively.
Factors Affecting the Survival of E. coli O157:H7 Cells
In the 16 induction solutions, factors including salt, acetic acid, and medium concentrations were applied and analyzed on the effect on the survival of E. coli O157:H7 cells. First, the effect of salt and acid concentrations on the survival of E. coli O157:H7 cells was investigated. In induction solutions 3, 8, 9, and 14, the medium concentration is 50%. In induction solutions 3 (acid: 0.7%, salt: 0.9%) and 8 (acid: 1%, salt: 5%), the culturable cell number of E. coli O157:H7 reduced to 0 in 3 days. However, in induction solutions 9 (acid: 0, salt: 10%) and 14 (acid: 0.3%, salt: 15%), E. coli O157:H7 cells survived for more than 57 days. Especially in induction solution 9, the E. coli O157:H7 cells survived for more than 66 days. In induction solutions 4, 10, and 13, the medium concentration is 100%. In induction solution 4 (acid: 1%, salt: 0.9%), E. coli O157:H7 all died in 3 days and did not enter the VBNC state. In induction solutions 10 (acid: 0.3%, salt: 10%) and 13 (acid: 0, salt: 15%), E. coli O157:H7 cells were able to survive for 33–48 days. These results suggest that the effect of acetic acid concentration on the survival of E. coli O157:H7 is stronger than that of salt concentration.
Second, the effect of acid and medium conditions on the survival of E. coli O157:H7 cells under a certain salt concentration were investigated. In induction solutions 1, 2, 3, and 4, the salt concentration is 0.9%. In induction solutions 1 (medium: 0, acid: 0) and 2 (medium: 25%, acid: 0.3%), E. coli O157:H7 cells survived for more than 66 and 18 days at 4°C, respectively. However, in induction solutions 3 (medium: 50%, acid: 0.7%) and 4 (medium: 100%, acid: 1%), the E. coli O157:H7 cells all died in 3 days. It showed the effect of acid on the survival of E. coli O157:H7 is stronger than that of nutrients. In addition, when the acid concentration is 1% in induction solutions 4, 8, 12, and 16, E. coli O157:H7 cells died in 3 days regardless of the medium and salt concentrations. Especially in induction solution 4 (medium: 100%, acid: 0.9%) with the premium centration of medium and acid, E. coli O157:H7 cells still died in 3 days, indicating that acidity has the most significant effect on the survival of E. coli O157:H7 cells.
Third, under the same acetic acid concentration, the effects of salt and medium concentrations on the survival of E. coli O157:H7 were investigated. In induction solutions 6, 10, and 14, the acetic acid concentration is 0.3%. In induction solution 6 (medium: 0, salt: 5%), E. coli O157:H7 cells died within 3 days. However, in induction solutions 10 (medium: 100%, salt: 10%) and 14 (medium: 50%, salt: 15%), although the salt concentration increased, E. coli O157:H7 cells were able to survive from 33 to 66 days due to the high medium concentration. It indicated that the increase in the medium concentration eliminated the adverse effect of the increase in salt concentration. Among the factors on the survival of E. coli O157:H7, the effect of the medium concentration might be greater than that of the salt concentration.
When the environment changes, porin is crucial for the survival of E. coli. The major external protein omp (ompF, ompC) of E. coli is regulated by envZ (osmotic pressure sensor protein) and ompR. Studies have showed that wild-type, ompF, and ompC mutant strain of E. coli can enter the VBNC state under environmental pressure (pH, osmotic pressure, and starvation stress conditions), but in the envZ mutant strain, they cannot enter the VBNC state. This shows that the envZ mutant strains cannot sense changes in the external environment. When the strains are exposed to adverse conditions, they cannot enter the VBNC state (Pienaar et al., 2016). The expression of (p) ppGpp (guanosine pentaphosphate or guanosine tetraphosphate) synthetic genes relA and spoT are identified to upregulate in the VBNC state of E. coli O157:H7 (Magnusson et al., 2005; Mishra et al., 2012). Compared with normal strains, mutants that failed to synthesize (p) ppGpp lost culturability more quickly, and their ability to enter the VBNC state was significantly reduced. When (p) ppGpp was overexpressed, the number of VBNC cells was significantly increased (Boaretti et al., 2003). When bacteria suffered from amino acid starvation, the expression of RelA or SpoT increased. (p) ppGpp synthesis increased and degradation decreased. In addition, exopolyphosphatase (PPX) was degraded by (p) ppGpp of polyphosphates (Ayrapetyan et al., 2018).
Effects of Acidity and Nutrition on Formation of the VBNC State
Among the factors affecting the survival of E. coli O157:H7, acetic acid concentration plays a major role, followed by medium and salt concentrations. Considering the actual situation in the food system and in the process of food processing, exploring the concentration of acetic acid and culture medium to control the normal state of E. coli O157:H7 and VBNC is meaningful.
When the acetic acid concentration is 1%, the E. coli O157:H7 cells died within 3 days in induction solutions 3 (medium: 100%, salt: 10%), 4 (medium: 100%, salt: 15%), and 6 (medium: 50%, salt: 15%). When the acetic acid concentration is 0.7%, E. coli O157:H7 could survive in induction solutions 1 (medium: 100%, salt: 10%) and 3 (medium: 100%, salt: 15%). In induction solution 5 (medium: 50%, salt: 15%), E. coli O157:H7 cells died within 3 days. When the medium concentration is higher than 50%, treatment with 1.0% acetic acid directly killed E. coli O157:H7 without entering the VBNC state (Table 5).
When the medium concentration is 25%, E. coli O157:H7 cells survived more than 3 days in induction solutions 2 (salt: 10%, acid: 0.3%), 4 (salt: 15%, acid: 0), and 6 (salt: 15%, acid: 0.3). When the medium concentration is 0, in induction solutions 1 (salt: 10%, acid: 0.3%) and 5 (salt: 15%, acid: 0.3%), the number of culturable and viable cells was both 0 in 3 days. In induction solution 3 (salt: 15%, acid: 0), E. coli O157:H7 cells were able to survive for more than 3 days. Under weak acid and high salt conditions, the formation of the VBNC state of E. coli O157:H7 was controlled by changing the nutritional condition (Table 6).
In induction solutions 2, 3, 4, and 6, the culturable cell number of E. coli O157:H7 did not decrease to 0 in 3 days. However, the culturable cell numbers of E. coli O157:H7 reduced to 0 after storing at 4 and −20°C for 3 days in induction solutions 1 and 5, and the cells were not viable. Thus, changing the acid concentration with cold storage might be efficient to control the formation of the VBNC state of E. coli O157:H7.
Elimination of the VBNC State in Crystal Cake
In the crystal cake food system with 100, 50, and 25% nutrient concentrations and 1.0% acetic acid, the culturable cell numbers of E. coli O157:H7 were 0 after storing at 4 and −20°C for 3 days. The results of fluorescence microscopy showed that all the E. coli O157:H7 cells died, suggesting E. coli O157:H7 was not capable of entering into the VBNC state under these conditions (Figure 3). In the food system, without affecting the flavor and quality of the food, the normal and the VBNC state of E. coli O157:H7 was controlled and eliminated by adding 1% acetic acid.
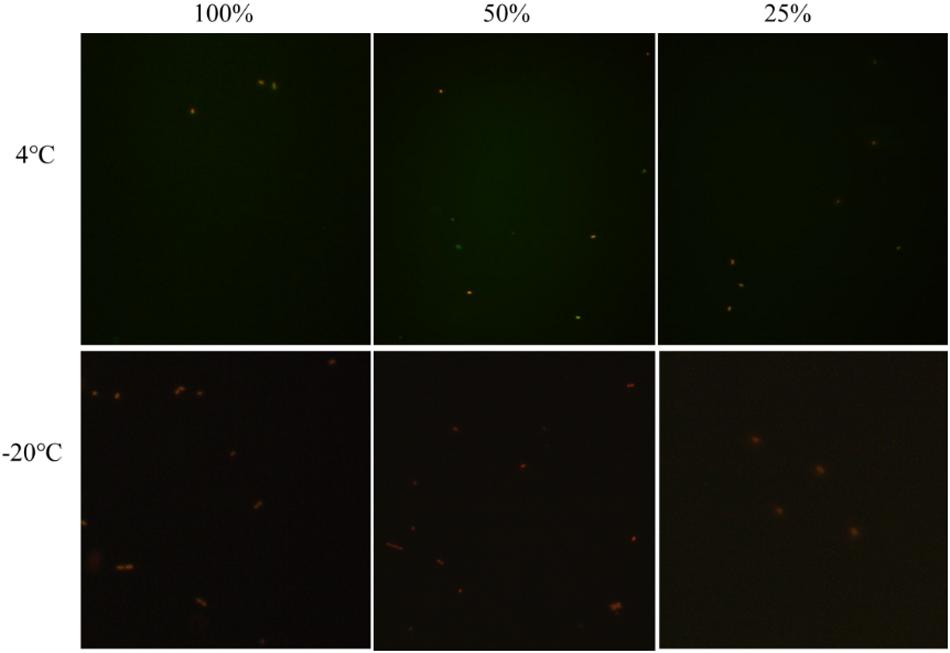
Figure 3. The viability of un-culturable E. coli O157:H7 stored at different conditions with fluorescence.
The foodborne microorganisms in the VBNC state have food spoilage ability. Compared with normal state, VBNC state cells have reduced metabolic activity and increased resistance of the cell wall to external environmental stress, including high concentrations of antibiotics, heavy metal ions, high temperature, high salt, and higher acidity (Ayrapetyan and Oliver, 2016; Shekar et al., 2017; Schottroff et al., 2018). Due to the presence of resistance, E. coli O157:H7 in the VBNC state is more difficult to be completely eliminated. Under favorable conditions, E. coli O157:H7 in the VBNC state might be able to overcome this inactive state and become active again, thus being able to reproduce in the food system. During the processing of crystal cake, the surface of the processing equipment and the inside of the pipeline should be cleaned in time to ensure no residual nutrients, thereby inhibiting the survival of E. coli O157:H7. In addition, combined with a certain concentration of acetic acid treatment equipment surface (1.0% acetic acid), it ensures the elimination of E. coli O157:H7 and its VBNC state and avoid potential food safety risks.
Detection of the VBNC State of E. coli in Crystal Cake
The risk of food safety issues can be partially reduced by the efficient detection of the VBNC state of E. coli in food system. Propidium monoazide (PMA) is a photoreactive DNA-binding dye. Dead microorganisms lose their capability to keep their membranes intact, which leaves the “naked” DNA in the cytosol ready to react with PMA. DNA of living organisms are not exposed to the PMA, as they have a complete cell membrane. DNA extraction and PCR amplification of PMA-treated samples can effectively detect bacteria in the VBNC state. The detection limit was determined by detecting different concentrations of E. coli O157:H7 in the VBNC state. In this study, the detection limit of the VBNC state E. coli O157 in the crystal cake food system using PMA–polymerase chain reaction (PMA-PCR) was 104 CFU/ml, which can effectively avoid the potential threat brought by VBNC state bacteria.
Conclusion
The effect of external environmental conditions on the formation of the VBNC state of E. coli O157:H7 was as follows: acidity > nutritional state > salt concentration. When the nutrient concentration is higher than 50%, E. coli O157:H7 was killed by adding 1.0% acetic acid. In the crystal cake system, when the nutrient concentration is 25, 50, and 100%, E. coli O157:H7 cells died in 3 days by adding 1.0% acetic acid with no VBNC cells identified. In the food system, the VBNC state formation of E. coli O157:H7 was inhibited by adding 1.0% acetic acid. In addition, PMA-PCR assays can be utilized in the detection of the VBNC state of E. coli in the food system.
Data Availability Statement
All datasets presented in this study are included in the article/supplementary material.
Author Contributions
JL and KW conceived the study and participated in its design and coordination. YML and T-YH performed the experimental work. CY and LC analyzed the data. YL and JL prepared and revised this manuscript. All authors reviewed and approved the final manuscript.
Funding
Fundamental Research Funds for the Central Universities (D2191310).
Conflict of Interest
The author YL was employed by Guangdong Zhongqing Font Biochemical Science and Technology Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Afari, G. K., Liu, H., and Hung, Y. (2019). The effect of produce washing using electrolyzed water on the induction of the viable but non-culturable (VBNC) state in Listeria monocytogenes and Escherichia coli O157:H7. LWT Food Sci. Technol. 110, 275–282. doi: 10.1016/j.lwt.2019.04.089
Ayrapetyan, M., and Oliver, J. D. (2016). The viable but non-culturable state and its relevance in food safety. Curr. Opin. Food Sci. 8, 127–133. doi: 10.1016/j.cofs.2016.04.010
Ayrapetyan, M., Williams, T., and Oliver, J. D. (2018). Relationship between the viable but nonculturable state and antibiotic persister cells. J. Bacteriol. 200:e0249-18.
Bao, X., Jia, X., Chen, L., Peters, B. M., Lin, C., Chen, D., et al. (2017a). Effect of polymyxin resistance (pmr) on biofilm formation of Cronobacter sakazakii. Microb. Pathogen. 106, 16–19. doi: 10.1016/j.micpath.2016.12.012
Bao, X., Yang, L., Chen, L., Li, B., Li, L., Li, Y., et al. (2017b). Analysis on pathogenic and virulent characteristics of the Cronobacter sakazakii strain BAA-894 by whole genome sequencing and its demonstration in basic biology science. Microb. Pathogen. 109, 280–286. doi: 10.1016/j.micpath.2017.05.030
Bao, X., Yang, L., Chen, L., Li, B., Li, L., Li, Y., et al. (2017c). Virulent and pathogenic features on the Cronobacter sakazakii polymyxin resistant pmr mutant strain s-3. Microb. Pathogen. 110, 359–364. doi: 10.1016/j.micpath.2017.07.022
Boaretti, M., Lleo, M. M., Bonato, B., Signoretto, C., and Canepari, P. (2003). Involvement of rpoS in the survival of Escherichia coli in the viable but non-culturable state. Environ. Microbiol. 5, 986–996. doi: 10.1046/j.1462-2920.2003.00497.x
Bourely, C., Chauvin, C., Jouy, E., Cazeau, G., Jarrige, N., Leblond, A., et al. (2018). Comparative epidemiology of E. coli resistance to third-generation cephalosporins in diseased food-producing animals. Vet. Microbiol. 223, 72–78. doi: 10.1016/j.vetmic.2018.07.025
Ercumen, A., Pickering, A. J., Kwong, L. H., Arnold, B. F., Parvez, S. M., Alam, M., et al. (2017). Animal feces contribute to domestic fecal contamination: evidence from E-coli measured in water, hands, food, flies, and soil in Bangladesh. Environ. Sci. Technol. 51, 8725–8734. doi: 10.1021/acs.est.7b01710
Jia, X., Hua, J., Liu, L., Xu, Z., and Li, Y. (2018). Phenotypic characterization of pathogenic Cronobacter spp. strains. Microb. Pathogen. 121, 232–237. doi: 10.1016/j.micpath.2018.05.033
Lin, S., Li, L., Li, B., Zhao, X., Lin, C., Deng, Y., et al. (2016). Development and evaluation of quantitative detection of n-epsilon-carboxymethyl-lysine in Staphylococcus aureus biofilm by LC-MS method. Basic Clin. Pharmacol. 118, 33–33.
Lin, S., Yang, L., Chen, G., Li, B., Chen, D., Li, L., et al. (2017). Pathogenic features and characteristics of food borne pathogens biofilm: biomass, viability and matrix. Microb. Pathogen. 111, 285–291. doi: 10.1016/j.micpath.2017.08.005
Lindback, T., Rottenberg, M. E., Roche, S. M., and Rorvik, L. M. (2010). The ability to enter into an avirulent viable but non-culturable (VBNC) form is widespread among Listeria monocytogenes isolates from salmon, patients and environment. Vet. Res. 41:8.
Liu, J., Li, L., Li, B., Peters, B. M., Deng, Y., Xu, Z., et al. (2017a). Study on spoilage capability and VBNC state formation and recovery of Lactobacillus plantarum. Microb. Pathogen. 110, 257–261. doi: 10.1016/j.micpath.2017.06.044
Liu, J., Zhou, R., Li, L., Peters, B. M., Li, B., Lin, C., et al. (2017b). Viable but non-culturable state and toxin gene expression of enterohemorrhagic Escherichia coli 0157 under cryopreservation. Res. Microbiol. 168, 188–193. doi: 10.1016/j.resmic.2016.11.002
Liu, L., Lu, Z., Li, L., Li, B., Zhang, X., and Xu, Z. (2018a). Physical relation and mechanism of ultrasonic bactericidal activity on pathogenic E. coli with WPI. Microb. Pathogen. 117, 73–79. doi: 10.1016/j.micpath.2018.02.007
Liu, L., Xu, R., Li, L., Li, B., Zhang, X., and Xu, Z. (2018b). Correlation and in vitro mechanism of bactericidal activity on E. coli with whey protein isolate during ultrasonic treatment. Microb. Pathogen. 115, 154–158. doi: 10.1016/j.micpath.2017.12.062
Liu, L., Ye, C., Soteyome, T., Zhao, X., Xia, J., Xu, W., et al. (2019). Inhibitory effects of two types of food additives on biofilm formation by foodborne pathogens. Microbiologyopen 8:e00853.
Magnusson, L. U., Farewell, A., and Nystrom, T. (2005). ppGpp: a global regulator in Escherichia coli. Trends. Microbiol. 13, 236–242. doi: 10.1016/j.tim.2005.03.008
Miao, J., Chen, L., Wang, J., Wang, W., Chen, D., Li, L., et al. (2017a). Current methodologies on genotyping for nosocomial pathogen methicillin-resistant Staphylococcus aureus (MRSA). Microb. Pathogen. 107, 17–28. doi: 10.1016/j.micpath.2017.03.010
Miao, J., Chen, L., Wang, J., Wang, W., Chen, D., Li, L., et al. (2017b). Evaluation and application of molecular genotyping on nosocomial pathogen-methicillin-resistant Staphylococcus aureus isolates in Guangzhou representative of Southern China. Microb. Pathogen. 107, 397–403. doi: 10.1016/j.micpath.2017.04.016
Miao, J., Liang, Y., Chen, L., Wang, W., Wang, J., Li, B., et al. (2017c). Formation and development of Staphylococcus biofilm: with focus on food safety. J. Food Saf. 7:e012358.
Miao, J., Lin, S., Soteyome, T., Peters, B. M., Li, Y., Chen, H., et al. (2019). Biofilm formation of Staphylococcus aureus under food heat processing conditions: first report on cml production within biofilm. Sci. Rep. 9:1331.
Miao, J., Peters, B. M., Li, L., Li, B., Zhao, X., Xu, Z., et al. (2016). Evaluation of ERIC-PCR for fingerprinting methicillin-resistant Staphylococcus aureus strains. Basic Clin. Pharmacol. 118, 33–33.
Miao, J., Wang, W., Xu, W., Su, J., Li, L., Li, B., et al. (2018). The fingerprint mapping and genotyping systems application on methicillin-resistant Staphylococcus aureus. Microb. Pathogen. 125, 246–251. doi: 10.1016/j.micpath.2018.09.031
Mishra, A., Taneja, N., and Sharma, M. (2012). Viability kinetics, induction, resuscitation and quantitative real-time polymerase chain reaction analyses of viable but nonculturable Vibrio cholerae O1 in freshwater microcosm. J. Appl. Microbiol. 112, 945–953. doi: 10.1111/j.1365-2672.2012.05255.x
Neil, K. P., Biggerstaff, G., MacDonald, J. K., Trees, E., Medus, C., Musser, K. A., et al. (2012). A novel vehicle for transmission of Escherichia coli O157:H7 to humans: multistate outbreak of E. coli O157:H7 infections associated with consumption of ready-to-bake commercial prepackaged cookie dough–United States, 2009. Clin. Infect. Dis. 54, 511–518. doi: 10.1093/cid/cir831
Piao, M., Li, Y., Wang, Y., Wang, F., Zhen, T., and Deng, Y. (2019). Induction of viable but putatively non-culturable Lactobacillus acetotolerans by thermosonication and its. LWT Food Sci. Technol. 109, 313–318. doi: 10.1016/j.lwt.2019.04.046
Pienaar, J. A., Singh, A., and Barnard, T. G. (2016). The viable but non-culturable state in pathogenic Escherichia coli: a general review. Sci. Direct. 5:e00368.
Ramamurthy, T., Ghosh, A., Pazhani, G. P., and Shinoda, S. (2014). Current perspectives on viable but non-culturable (VBNC) pathogenic bacteria. Front. Public Health 2:103. doi: 10.3389/fpubh.2014.00103
Renuka, R. M., Achuth, J., Chandan, H. R., Venkataramana, M., and Kadirvelu, K. (2018). A fluorescent dual aptasensor for the rapid and sensitive onsite detection of E-coli O157:H7 and its validation in various food matrices. New J. Chem. 42, 10807–10817. doi: 10.1039/c8nj00997j
Sayad, A. A., Ibrahim, F., Uddin, S. M., Pei, K. X., Mohktar, M. S., Madou, M., et al. (2016). A microfluidic lab-on-a-disc integrated loop mediated isothermal amplification for foodborne pathogen detection. Sens. Actua. B Chem. 227, 600–609. doi: 10.1016/j.snb.2015.10.116
Schottroff, F., Froehling, A., Zunabovic, P. M., Krottenthaler, A., and Schlueter, O. (2018). Sublethal injury and viable but non-culturable (VBNC) state in microorganisms during preservation of food and biological materials by non-thermal processes. Front. Microbiol. 9:2773. doi: 10.3389/fpubh.2014.02773
Shekar, A., Babu, L., Ramlal, S., Sripathy, M. H., and Batra, H. (2017). Selective and concurrent detection of viable Salmonella spp., E-coli, Staphylococcus aureus, E-coli 0157:H7, and Shigella spp., in low moisture food products by PMA-mPCR assay with internal amplification control. LWT Food Sci. Technol. 86, 586–593. doi: 10.1016/j.lwt.2017.08.023
Wang, L., Zhao, X., Chu, J., Li, Y., Li, Y., Li, C., et al. (2011). Application of an improved loop-mediated isothermal amplification detection of Vibrio parahaemolyticus from various seafood samples. AFR J. Microbiol. Res. 5, 5765–5771.
Wen, S., Feng, D., Chen, D., Yang, L., and Xu, Z. (2020). Molecular epidemiology and evolution of Haemophilus influenzae. Infect. Genet. Evol. 80:104205. doi: 10.1016/j.meegid.2020.104205
Xie, J., Peters, B. M., Li, B., Li, L., Yu, G., Xu, Z., et al. (2017a). Clinical features and antimicrobial resistance profiles of important Enterobacteriaceae pathogens in Guangzhou representative of Southern China, 2001-2015. Microb. Pathogen. 107, 206–211. doi: 10.1016/j.micpath.2017.03.038
Xie, J., Yang, L., Peters, B. M., Chen, L., and Chen, D. (2017b). A 16-year retrospective surveillance report on the pathogenic features and antimicrobial susceptibility of Pseudomonas aeruginosa isolates from FAHJU in Guangzhou representative of Southern China. Microb. Pathogen. 110, 37–41. doi: 10.1016/j.micpath.2017.06.018
Xu, H. S., Roberts, N., Singleton, F. L., Attwell, R. W., Grimes, D. J., and Colwel, R. R. (1982). Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb. Ecol. 8, 313–323.
Xu, Z., Gui, Z., Zhao, X., Zhang, Y., He, X., Li, W., et al. (2012a). Expression and purification of gp41-gp36 fusion protein and application in serological screening assay of HIV-1 and HIV-2. AFR J. Microbiol. Res. 6, 6295–6299.
Xu, Z., Li, L., Chu, J., Peters, B. M., Harris, M. L., Li, B., et al. (2012b). Development and application of loop-mediated isothermal amplification assays on rapid detection of various types of staphylococci strains. Food Res. Int. 47, 166–173. doi: 10.1016/j.foodres.2011.04.042
Xu, Z., Hou, Y., Peters, B. M., Chen, D., Li, B., Li, L., et al. (2016a). Chromogenic media for MRSA diagnostics. Mol. Biol. Rep. 43, 1205–1212. doi: 10.1007/s11033-016-4062-3
Xu, Z., Hou, Y., Qin, D., Liu, X., Li, B., Li, L., et al. (2016b). Evaluation of current methodologies for rapid identification of methicillin-resistant Staphylococcus aureus Strains. Basic Clin. Pharmacol. 118:33.
Xu, Z., Liang, Y., Lin, S., Chen, D., Li, B., Li, L., et al. (2016c). Crystal violet and XTT assays on Staphylococcus aureus biofilm quantification. Curr. Microbiol. 73, 474–482. doi: 10.1007/s00284-016-1081-1
Xu, Z., Li, L., Shirtliff, M. E., Alam, M. J., Yamasaki, S., and Shi, L. (2009). Occurrence and characteristics of class 1 and 2 integrons in Pseudomonas aeruginosa isolates from patients in Southern China. J. Clin. Microbiol. 47, 230–234. doi: 10.1128/jcm.02027-08
Xu, Z., Li, L., Shi, L., and Shirtliff, M. E. (2011a). Class 1 integron in staphylococci. Mol. Biol. Rep. 38, 5261–5279. doi: 10.1007/s11033-011-0676-7
Xu, Z., Li, L., Shirtliff, M. E., Peters, B. M., Li, B., Peng, Y., et al. (2011b). Resistance class 1 integron in clinical methicillin-resistant Staphylococcus aureus strains in southern China, 2001-2006. Clin. Microbiol. Infect. 17, 714–718. doi: 10.1111/j.1469-0691.2010.03379.x
Xu, Z., Li, L., Zhao, X., Chu, J., Li, B., Shi, L., et al. (2011c). Development and application of a novel multiplex polymerase chain reaction (PCR) assay for rapid detection of various types of staphylococci strains. AFR. J. Microbiol. Res. 5, 1869–1873.
Xu, Z., Li, L., Shirtliff, M. E., Peters, B. M., Peng, Y., Alam, M. J., et al. (2010). First report of class 2 integron in clinical Enterococcus faecalis and class 1 integron in Enterococcus faecium in South China. Diagn. Micro. Infect. Dis. 68, 315–317. doi: 10.1016/j.diagmicrobio.2010.05.014
Xu, Z., Luo, Y., Mao, Y., Peng, R., Chen, J., Soteyome, T., et al. (2020). Spoilage lactic acid bacteria in the brewing industry. J. Microbiol. Biotech. doi: 10.4014/jmb.1908.08069 [Epub ahead of print].
Xu, Z., Li, L., Alam, M. J., Zhang, L., Yamasaki, S., and Shi, L. (2008a). First confirmation of integron-bearing methicillin-resistant Staphylococcus aureus. Curr. Microbiol. 57, 264–268. doi: 10.1007/s00284-008-9187-8
Xu, Z., Shi, L., Alam, M. J., Li, L., and Yamasaki, S. (2008b). Integron-bearing methicillin-resistant coagulase-negative staphylococci in South China, 2001-2004. FEMS Microbiol. Lett. 278, 223–230. doi: 10.1111/j.1574-6968.2007.00994.x
Xu, Z., Shi, L., Zhang, C., Zhang, L., Li, X., Cao, Y., et al. (2007). Nosocomial infection caused by class 1 integron-carrying Staphylococcus aureus in a hospital in South China. Clin. Microbiol. Infect. 13, 980–984. doi: 10.1111/j.1469-0691.2007.01782.x
Xu, Z., Xie, J., Peters, B. M., Li, B., Li, L., Yu, G., et al. (2017a). Longitudinal surveillance on antibiogram of important Gram-positive pathogens in Southern China, 2001 to 2015. Microb. Pathogen. 103, 80–86. doi: 10.1016/j.micpath.2016.11.013
Xu, Z., Xu, X., Qi, D., Yang, L., Li, B., Li, L., et al. (2017b). Effect of aminoglycosides on the pathogenic characteristics of microbiology. Microb. Pathogen. 113, 357–364. doi: 10.1016/j.micpath.2017.08.053
Xu, Z., Xie, J., Yang, L., Chen, D., Peters, B. M., and Shirtliff, M. E. (2018). Complete sequence of pCY-CTX, a plasmid carrying a phage-like region and an ISEcp1-Mediated Tn2 element from Enterobacter cloacae. Microb. Drug Resist. 24, 307–313. doi: 10.1089/mdr.2017.0146
Yaron, S., and Matthews, K. R. (2002). A reverse transcriptase-polymerase chain reaction assay for detection of viable Escherichia coli O157:H7: investigation of species target genes. J. Appl. Microbiol. 92, 633–640. doi: 10.1046/j.1365-2672.2002.01563.x
You, R., Gui, Z., Xu, Z., Shirtliff, E. M., Yu, G., Zhao, X., et al. (2012). Methicillin-resistance Staphylococcus aureus detection by an improved rapid PCR assay. Afr. J. Microbiol. Res. 6, 7131–7133.
Zeinhom, M. M. A., Wang, Y., Song, Y., Zhu, M., Lin, Y., and Du, D. (2018). A portable smart-phone device for rapid and sensitive detection of E. coli O157:H7 in Yoghurt and Egg. Biosens. Bioelectron. 99, 479–485. doi: 10.1016/j.bios.2017.08.002
Zhang, S., Ye, C., Lin, H., Lv, L., and Yu, X. (2015). UV disinfection induces a vbnc state in Escherichia coli and Pseudomonas aeruginosa. Environ. Sci. Technol. 49, 1721–1728. doi: 10.1021/es505211e
Zhao, X., Li, M., and Xu, Z. (2018a). Detection of foodborne pathogens by surface enhanced raman spectroscopy. Front. Microbiol. 9:1236. doi: 10.3389/fpubh.2014.001236
Zhao, X., Yu, Z., and Xu, Z. (2018b). Study the features of 57 confirmed CRISPR Loci in 38 strains of Staphylococcus aureus. Front. Microbiol. 9:1591. doi: 10.3389/fpubh.2014.001591
Zhao, X., Lin, C., Wang, J., and Oh, D. H. (2014). Advances in rapid detection methods for foodborne pathogens. J. Microbiol. Biotechnol. 24, 297–312.
Zhao, X., Li, Y., Wang, L., You, L., Xu, Z., Li, L., et al. (2010a). Development and application of a loop-mediated isothermal amplification method on rapid detection Escherichia coli O157 strains from food samples. Mol. Biol. Rep. 37, 2183–2188. doi: 10.1007/s11033-009-9700-6
Zhao, X., Wang, L., Chu, J., Li, Y., Li, Y., Xu, Z., et al. (2010b). Development and application of a rapid and simple loop-mediated isothermal amplification method for food-borne Salmonella detection. Food Sci. Biotechnol. 19, 1655–1659. doi: 10.1007/s10068-010-0234-4
Zhao, X., Wang, L., Chu, J., Li, Y., Li, Y., Xu, Z., et al. (2010c). Rapid detection of vibrio parahaemolyticus strains and virulent factors by loop-mediated isothermal amplification assays. Food Sci. Biotechnol. 19, 1191–1197. doi: 10.1007/s10068-010-0170-3
Zhao, X., Wang, L., Li, Y., Xu, Z., Li, L., He, X., et al. (2011). Development and application of a loop-mediated isothermal amplification method on rapid detection of Pseudomonas aeruginosa strains. World J. Microb. Biot. 27, 181–184. doi: 10.1007/s11274-010-0429-0
Keywords: E. coli O157:H7, VBNC, food system, control, detection
Citation: Li Y, Huang T-Y, Ye C, Chen L, Liang Y, Wang K and Liu J (2020) Formation and Control of the Viable but Non-culturable State of Foodborne Pathogen Escherichia coli O157:H7. Front. Microbiol. 11:1202. doi: 10.3389/fmicb.2020.01202
Received: 23 March 2020; Accepted: 12 May 2020;
Published: 16 June 2020.
Edited by:
Xihong Zhao, Wuhan Institute of Technology, ChinaReviewed by:
Hengyi Xu, Nanchang University, ChinaWensen Jiang, Cedars Sinai Medical Center, United States
Yuting Tian, Fujian Agriculture and Forestry University, China
Copyright © 2020 Li, Huang, Ye, Chen, Liang, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junyan Liu, jliu81@uthsc.edu; Kan Wang, 120384746@qq.com
†These authors have contributed equally to this work
 Yanmei Li1†
Yanmei Li1† Junyan Liu
Junyan Liu