
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 17 June 2020
Sec. Aquatic Microbiology
Volume 11 - 2020 | https://doi.org/10.3389/fmicb.2020.01201
This article is part of the Research TopicCell Death in CyanobacteriaView all 9 articles
There is increasing evidence that programmed cell death (PCD) in cyanobacteria is triggered by oxidative stress and that it contributes to the survival of the cyanobacterial population such as Microcystis aeruginosa. At the same time, microcystins (MCs) released during cell lysis have been implicated in colony formation (enabled by the release of polysaccharides) in M. aeruginosa – a strategy that allows the effect of a stressor, including grazing to be avoided or decreased. This experimental research has explored whether extracts of Daphnia magna and Daphnia cucullata (corresponding to 5, 25, 50, and 100 individuals per liter) reveal the effect on the growth, intracellular reactive oxygen species (ROS) content, lipid peroxidation, PCD, MC-LR release, and bound exopolysaccharide (EPS) level in M. aeruginosa during 7 days of exposure. As demonstrated, extracts of both daphnids induced dose-dependent growth inhibition, increase in ROS levels, lipid peroxidation, and PCD. Moreover, the release of MC-LR and an increase in the bound EPS fraction were observed in treated cultures. Generally, the greatest effects were observed under the influence of D. magna extracts. The study indicates that grazer presence can potentially trigger a series of events in the Microcystis population, with cells undergoing oxidative stress-induced PCD associated with MC release, which in turn increases EPS production by intact cells. As argued, this strategy is likely to have evolved in response to abiotic stressors, since both PCD and synthesis of MC in cyanobacteria predate the metazoan lineage. Nevertheless, it may still provide a benefit for the survival of the MC-producing M. aeruginosa population under grazer pressure.
The interactions between cyanobacteria and zooplankton are of a complex nature. On one hand, the increasing frequency, duration, and intensity of cyanobacterial blooms may positively select for better-adapted zooplankton that may, inter alia, express detoxification mechanisms more efficiently (Wojtal-Frankiewicz et al., 2013; Ger et al., 2014). Maternal exposure to toxigenic cyanobacteria such as Microcystis aeruginosa may induce transgenerational tolerance mechanisms possibly via epigenetic modifications (Ortiz-Rodríguez et al., 2012; Asselman et al., 2017) while the presence of selected sympatric phytoplankton species may decrease the toxic effect of cyanobacteria that are capable of producing metabolites such as microcystins (MCs; Zhang et al., 2009). On the other hand, cyanobacteria display a number of traits that could be considered defensive against grazers such as filamentous or colonial morphology, poor nutritional value, and the production of a wide array of toxins (Rohrlack et al., 2001; Ger et al., 2016).
M. aeruginosa is one of the most commonly identified bloom-forming cyanobacterial species associated with eutrophic freshwaters. It has a nearly worldwide distribution encompassing tropical, subtropical, temperate, and subfrigid climate zones (Harke et al., 2016). Many of its strains are capable of producing MCs and secondary metabolites, whose exact physiological role remains unknown. A number of possibilities in this regard, deriving mostly from experimental observations and correlative field studies, have been put forward and include benthic survival and recruitment, allelopathic interactions, light adaptations, iron acquisition, quorum sensing, oxidative stress protection, nutrient storage, and grazer defense (Omidi et al., 2018; Henao et al., 2019; Hu and Rzymski, 2019). As shown, MCs can particularly inhibit the growth, survival, and reproduction of large-bodied zooplankton species such as generalist grazers belonging to the Daphnia genus (Rohrlack et al., 1999). One should, however, note that phylogenetic evidence based on the comparable levels of sequence divergence and a high degree of congruence between the MC synthetase gene 16S rRNA and rpoC1 suggests the early evolution of MC production in cyanobacteria that predates the metazoan lineage (Rantala et al., 2004). This considered, one could propose that the potential adverse effect of MCs, in the extracellular form or ingested with diet, on zooplankton such as daphnids could have undergone positive selection more recently or may represent an indirect consequence of MC biosynthesis.
It is known that MC is retained intracellularly by intact cells (Rohrlack and Hyenstrand, 2007). This does not necessarily rule out the possibility that its role cannot be manifested extracellularly. As evidenced, an addition of toxin to a culture of M. aeruginosa results in dose-dependent formation and maintenance of its colonies (Schatz et al., 2007; Gan et al., 2012) and also positively correlates with the growth of cyanobacteria (Orr and Jones, 1998). Under normal conditions, MCs can be released during cell lysis and as shown more recently in M. aeruginosa, during programmed cell death (PCD) that is triggered by an increase in the level of intracellular reactive oxygen species (ROS) and further mediated by caspase-like cysteine-dependent proteolytic enzymes (Ross et al., 2006; Klemenčič et al., 2015; Klemenčič and Dolinar, 2016; Hu and Rzymski, 2019). Similarly to multicellular organisms, PCD in cyanobacteria is an active process tightly controlled by a set of specialized molecular machinery (Zheng et al., 2013; Bidle, 2016; Hu and Rzymski, 2019). In Microcystis, it has been demonstrated to represent a general response to a variety of physical and chemical stressors (Ross et al., 2006; He et al., 2016; Hu and Rzymski, 2019).
Considering that MC release from cells is coupled with cellular death, including PCD and that extracellular MCs can trigger colony formation in Microcystis cells (He et al., 2016; Hu and Rzymski, 2019), it would be of interest to understand whether daphnid metabolites can trigger oxidative stress in Microcystis, induce PCD, and subsequently increase the release of MCs to which the survival cells could respond by colony formation which is mediated by extracellular polysaccharides (Sato et al., 2017; Chen et al., 2019). As recently shown, the co-culture of M. aeruginosa and Daphnia magna in a chamber allowing the exchange of metabolites without direct contact of organisms, resulted in stress response in cyanobacteria manifested by increased ROS generation followed by elevated concentration of intracellular and extracellular MC (Bojadzija Savic et al., 2020). These results clearly show that diffusing daphnid metabolites can indeed affect the M. aeuruginosa and calls for further research on coupling of ROS, PCD, MC, and extracellular polysaccharides in this cyanobacterial species.
To address these issues, the present study investigated the effect of two daphnids of different body size, large D. magna, and small Daphnia cucullata, a widespread keystone zooplankton species, on growth, oxidative stress, MC release, PCD, and the content of bound exopolysaccharides (EPS) in M. aeruginosa. The Microcystis cultures were exposed for 7 days to daphnid extracts as opposed to spent Daphnia medium which is often employed to explore the effects of zooplankton on microalgae. The limitation of spent grazer medium is that its chemical composition is difficult to standardize so as to be fully equivalent to cyanobacterial medium (utilized for control samples) and it can also contain allelopathic metabolites released by the microalgae with which cladocerans were fed (Harel et al., 2013; Omidi et al., 2019). The extract concentrations corresponded to a daphnid abundance of 5, 25, 50, and 100 individuals (ind)/L, which is within the range observed in eutrophic lakes (Saunders et al., 1999; Hülsmann and Voigt, 2002; Wojtal-Frankiewicz et al., 2015). This experimental investigation adds to the general understanding of mechanisms behind the interactions between selected cyanobacteria and zooplankton species.
Inoculum of the MC-producing M. aeruginosa SAG 14.85 strain isolated primarily from Little Rideau Lake (Ontario, Canada) was obtained from the Culture Collection of Algae at Goettingen University (Goettingen, Germany). The non-axenic culture was maintained in 250 ml culture flasks containing 100 ml of sterile BG-11 media at 21°C under 80 μmol m−2 s−2 irradiance using cool white fluorescent light with a photoperiod regime of 12 h dark and 12 h light. The use of antibiotics was disregarded since they were documented to produce a number of apoptotic-like hallmarks in bacteria and could potentially interfere with the investigated parameters (Erental et al., 2014; Peeters and de Jonge, 2018).
Individuals of D. magna and D. cucullata were collected with a plankton net (mesh size 100 μm) from small man-made ponds in Western Poland in which they formed mono-species populations. Alive individuals were transported to the laboratory and kept in 5 L containers with filtered (0.45 μm) lake water. Fifty randomly sampled individuals were taken for taxonomical determination. After 24 h, 1,000 individuals of each species were collected with plankton net flushed with distilled water and homogenized mechanically using IKA T 18 homogenizer (IKA, China). The homogenized samples were subjected to ultrasonic treatment on ice (20 kHz, 2 min in two cycles with 60 s break) and filtration through a syringeless 0.22 μm filter to yield a clear stock solution of extract stored in aliquots at −40°C before the experiment.
The experiments were designed to evaluate whether Daphnia extracts can affect the growth, intracellular ROS content, lipid peroxidation, the release of MC, EPS level, and PCD in M. aeuruginosa. In each experiment, M. aeruginosa was harvested at the late log growth phase and incubated at a density of 1 × 104 cells ml−1 in 50-ml culture flasks containing 25-ml of fresh BG-11 media. The stock solution of D. magna or D. cucullata extracts (1,000 ind/L) was used to achieve a target concentration corresponding to extracts from 100, 50, 25, and 5 ind/L in the investigated culture samples. These concentrations reflected the abundance of Daphnia sp., observed in eutrophic lakes (Saunders et al., 1999; Hülsmann and Voigt, 2002; Wojtal-Frankiewicz et al., 2015). A control sample was comprised of cyanobacterial cells incubated at the same density and in the same BG-11 volume but without the addition of extract. Cultures for analyses of growth, extracellular MC concentration, lipid peroxidation, EPS, and PCD were grown for 7 days at 21°C under 80 μmol m−2 s−2 irradiance using cool white fluorescent light with a photoperiod regime of 12 h dark and 12 h light. Each culture was shaken twice a day manually. Because growth and PCD were directly associated parameters, their kinetics was studied at baseline and 1, 3, 5, and 7 days following the incubation. The concentration of extracellular MC, lipid peroxidation level, and EPS content were studied at day 7 of the experiment. The experiments to measure growth kinetics, extracellular MC, lipid peroxidation, and EPS were conducted in a separate run from PCD measurements. The experiments for PCD were conducted separately for each time-point in order to avoid a significant decrease in medium volume. Cultures for intracellular ROS monitoring were incubated in a similar manner for 1 h and the kinetics of reaction were measured at 0, 5, 15, 30, 45, and 60 min. This assay was performed to determine whether daphnid extracts may contain molecules capable of inducing oxidative stress which could later lead to growth retardation and biochemical alterations. Each experiment was carried out in five independent replicates.
The growth kinetics in exposed and control cultures were monitored with OD750 (Moheimani et al., 2013) at baseline and 1, 3, 5, and 7 days following incubation.
The intracellular ROS levels in M. aeruginosa were evaluated using an assay with the cell-permeant 2′7′-dichlorofluorescein diacetate (DCFDA; Abcam, Cambridge, UK), a fluorogenic probe which is deacetylated by cellular esterases to a non-fluorescent polar 2′7′-dichloro-dihydrofluorescein, which is readily oxidized by hydroxyl and peroxyl radicals and other ROS to a highly fluorescent dichlorofluorescein (DCF; Komosa et al., 2017). Microcystis cultures were collected by centrifugation and incubated with 25 μM DCFDA for 30 min in the dark, washed twice by centrifugation with fresh BG-11 medium to remove the excess of the probe, and then incubated with D. magna extracts as described in the “Experimental design” subsection. A 250 μl subset of each culture was transferred to a 96-well plate; the fluorescence of DCF was measured kinetically at 0, 5, 15, 30, 45, and 60 min following exposure to extracts using a Synergy HTX Multi-Mode Microplate Reader (BioTek, USA) at an excitation of 495 nm and emission of 528 nm. The results were normalized over the autofluorescence emission read at 680 nm (Thiel et al., 2017).
Lipid peroxidation was analyzed using a TBARS Assay Kit (Cayman Chemical, United States) by means of malondialdehyde (MDA) content (Poniedziałek et al., 2018). Following the incubation, cells were collected from 5 ml subsample of each culture by centrifugation, washed twice with BG-11 medium and incubated for 30 min at 21°C with gentle shaking on an orbital shaker with a cell-lysis buffer based on 1% Triton X-100 (Cayman Chemical, United States) supplemented with butylated hydroxytoluene (BHT) to prevent artificial lipid peroxidation. Following the incubation, samples were centrifuged (1,600 × g, 10 min, 4°C) to remove insoluble material. The protein content in supernatants (5 μl) was quantified with a Quick Start™ Bradford Protein Assay Kit (Bio-Rad) following the microassay procedure and using bovine serum albumin as a protein standard. The 100 μl of supernatants was transferred to a microcentrifuge tube and supplemented with 800 μl of thiobarbituric acid (TBA) to generate an MDA-TBA adduct. To accelerate this process, samples were incubated at 95°C for 60 min, placed on an ice bath for 10 min to inhibit the reaction and centrifuged (1,600 × g, 10 min, 4°C). The final product was measured colorimetrically at 532 nm using a Synergy HTX Multi-Mode Microplate Reader. The absorbance values were compared to a calibration curve prepared using the MDA standard (Cayman Chemical, USA) and calculated as nmol mL−1.
The level of PCD in M. aeruginosa exposed to D. magna and D. cucullata extracts was evaluated using a Caspase-3/7 Fluorescence Assay Kit (Cayman Chemical, United States) (Hu and Rzymski, 2019). This assay employs a specific substrate, N-Ac-DED-N′-MC-R110, which upon the specific proteolytic cleavage of the amino acid sequence Asp-Glu-Val-Asp by active caspase-7 and/or caspase-7 generates a fluorescent rhodamine-110. The 5 ml subsample of incubated cells were washed twice with the provided Assay Buffer (800 × g, 5 min), incubated with a cell-lysis buffer based on 1% Triton X-100 (Cayman Chemical, United States) for 30 min on an orbital shaker at 21°C, washed again and mixed with caspase-3/7 substrate solution. The fluorescence signal was recorded with a Synergy HTX Multi-Mode Microplate Reader (BioTek, USA) at an excitation of 495 nm and an emission of 528 nm. The results were calculated as the percentage of fluorescence intensity of the control sample.
Subsamples (5 ml) of the cyanobacteria culture were filtered through GF/C filters (Whatman, UK) to separate cyanobacterial cells from water and to determine three most common MCs variants in M. aeruginosa: MC-LR, MC-YR, and MC-RR (Li et al., 2017; Pham and Utsumi, 2018). The filtrated samples were evaporated to dryness in an SC110A SpeedVac® Plus, Thermo Savant (Holbrook, NY, USA) and reconstituted at a volume of 500 μl of 75% aqueous methanol before extracellular MCs analyses.
Chromatographic separation was performed using an Agilent (Waldbronn, Germany) 1100 series HPLC system consisting of a degasser, a quaternary pump, a column compartment thermostat set at 40°C, and a diode array detector operated at 200–300 nm on a Merck (Darmstadt, Germany) Purospher STAR RP-18e column (55 mm × 4 mm I.D. with 3 μm particles) protected by a 4 mm × 4 mm guard column. The mobile phase consisted of water (solvent A) and acetonitile (solvent B), both containing 0.05% trifluoroacetic acid. The flow rate for MCs analyses was 1.0 ml min−1 with the following linear gradient program: 0 min, 25% B; 5 min, 70% B; 6 min, 70% B; 6.1 min, 25% B; and stop time, 9 min. The injection volume was 20 μl. The concentration of MC-LR in the samples was analyzed with a use of standard stock solutions by comparing the retention time and the UV spectrum (200–300 nm with an absorption maximum at 238 nm). The detection limit was 10 ng/L.
The bound fraction of EPS was analyzed according to the method proposed by Yang et al. (2008) and Li et al. (2013). This fraction was chosen as it can directly contribute to the aggregation of phytoplankton cells (Xu et al., 2014; Liu et al., 2018). Following the incubation, 5 ml subsample of each culture was centrifuged (10,000 g, 15 min), supernatants were discarded, pellets were resuspended in distilled water and pH was adjusted to 10. The resuspended pellets were incubated for 4 h at 45°C in a water bath and centrifuged again (10,000 g, 15 min). The resulting supernatant, a representative of a bound extracellular polysaccharide fraction, was collected, and the concentration of polysaccharides was determined with a Total Carbohydrate Assay Kit (Sigma-Aldrich, St. Louis, MO, USA) based on the phenol-sulfuric acid method and spectrophotometric detection of chromagen at 490 nm. The absorbance values were compared to a calibration curve prepared using the glucose standard and calculated as microgram per gram of protein following protein content quantification with a Quick Start™ Bradford Protein Assay Kit (Bio-Rad) as described for the lipid peroxidation assay.
The statistical analyses of the results were performed using STATISTICA 13.0 (StatSoft, Tulsa, OK, USA). Because not all the data met the assumption of Gaussian distribution, the non-parametric methods were applied. The difference between the treated samples and the control was compared using the Mann-Whitney U test. The difference between more than two groups of samples was assessed using Kruskal-Wallis ANOVA with Dunn’s post-hoc test. The relationships between the studied parameters, and between these parameters, time of exposure and extract concentrations, were determined with Spearman’s correlation coefficient (Rs). A value of p < 0.05 was considered statistically significant.
Generally, the growth of cultures exposed to D. magna and D. cucullata extracts was inhibited in a dose-dependent manner (Rs = −0.98 and −0.90, respectively, for day 7; p < 0.05) with 57 and 52% decrease at day 7 following the treatment of 100 ind/L extracts, respectively, when compared to the control. Following exposure to daphnid extracts, an increase in growth was seen at every measured time-point except for 100 ind/L extract of D. magna after 1 day of incubation. In the case of D. magna, cyanobacterial growth was, however, significantly retarded following the exposure to 25–100 ind/L extracts while for D. cucullata – 50 and 100 ind/L. However, no complete inhibition or negative growth was noted – at day 7 all cultures revealed an increase as compared to a baseline level (Figure 1).
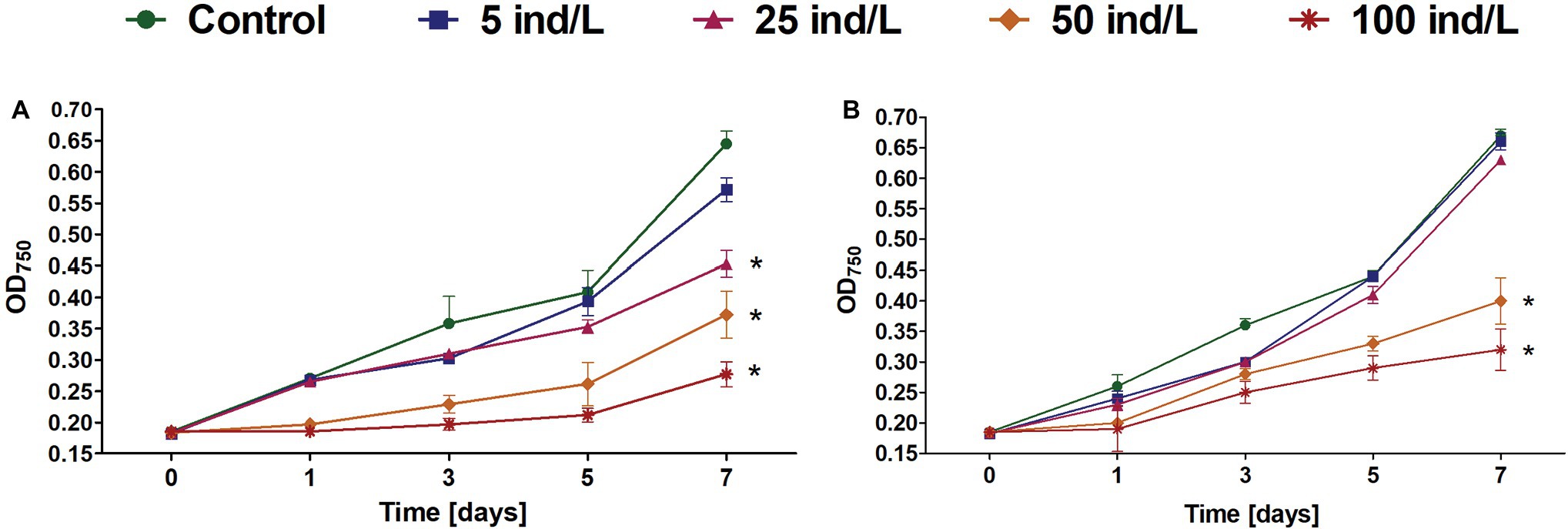
Figure 1. The growth (mean and SD) of Microcystis aeruginosa exposed for 7 days to different concentrations of Daphnia magna (A) and Daphnia cucullata (B) (n = 5). An asterisk indicates a significant difference with the control (p < 0.05; Mann-Whitney U test).
The intracellular content of ROS in M. aeruginosa cultures revealed an increase over 1 h of exposure. The greatest ROS level was observed after 60 min and was significantly higher than in the control in the case of 25–100 ind/L extracts of D. magna and 50–100 ind/L extracts of D. cucullata (Figure 2). Generally, for both daphnids, the concentration-related response was observed at 60 min time intervals (Rs = 0.91 and Rs = 0.87, respectively, p < 0.05).
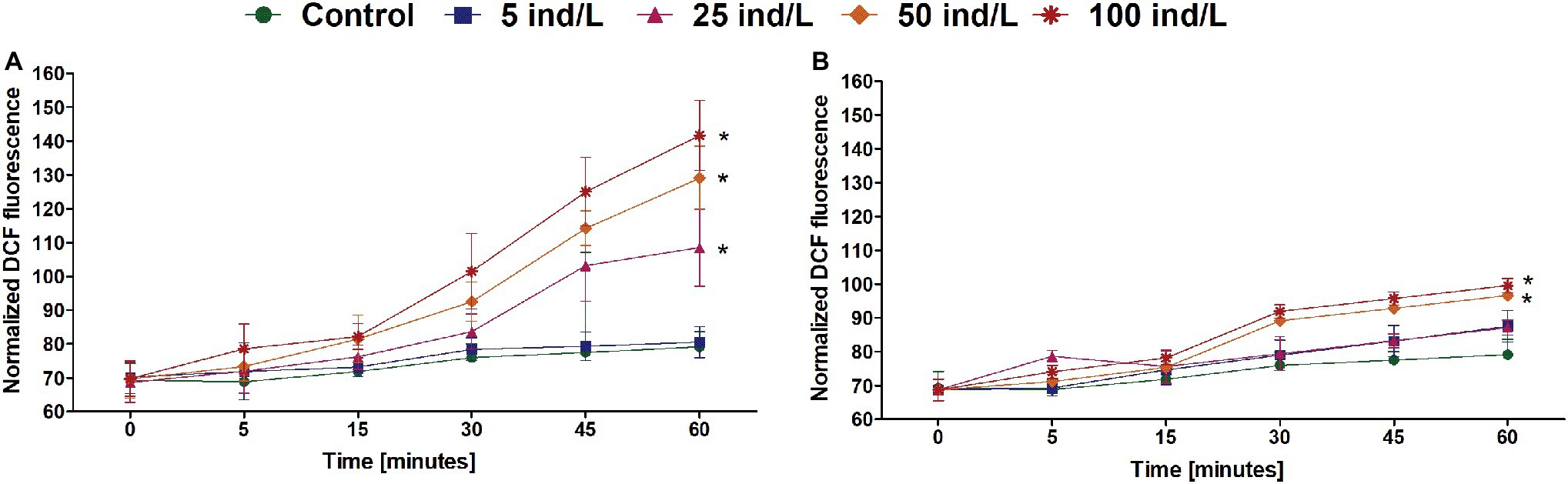
Figure 2. The intracellular reactive oxygen species (ROS) level (mean and SD) of M. aeruginosa exposed for 7 days to different concentrations of D. magna (A) and D. cucullata (B) (n = 5). An asterisk indicates a significant difference with the control (p < 0.05; Mann-Whitney U test).
A concentration-dependent increase in intracellular MDA content, a marker of lipid peroxidation was observed after 7 days of incubation of M. aeruginosa cultures with D. magna and D. cucullata extracts (Rs = 0.93 and Rs = 0.81, respectively, p < 0.05). Compared to the control, a significant increase was noted following treatment with 50 and 100 ind/L extracts of D. magna (by 1.6‐ and 2.4-fold, respectively) and D. cucullata (by 1.4‐ and 2.0-fold, respectively). The observed responses to extracts of these two daphnid species did not differ significantly (Figure 3).
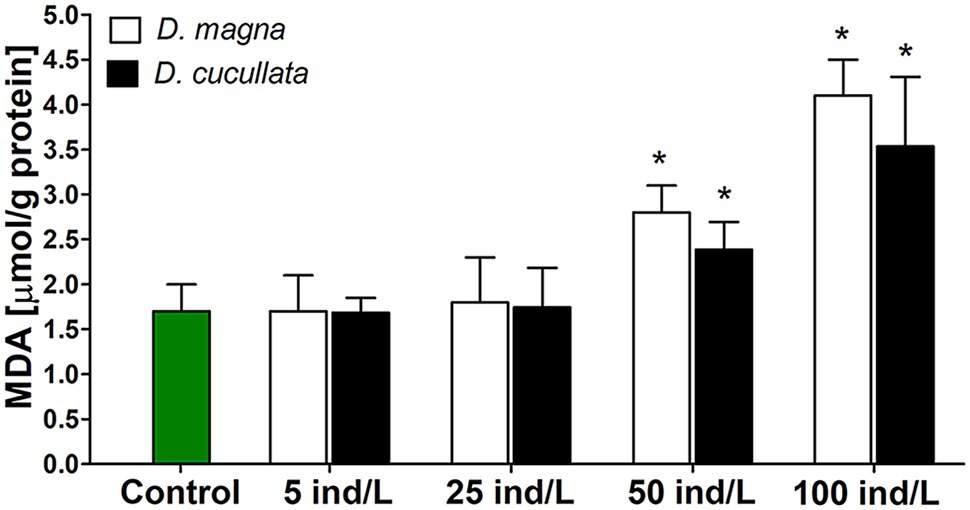
Figure 3. The peroxidation of lipids measured by means of intracellular thiobarbituric acid reactive substance content (mean and SD), mainly represented by malondialdehyde (MDA) in M. aeruginosa exposed for 7 days to different concentrations of D. magna and D. cucullata (n = 5). An asterisk indicates a significant difference with the control (p < 0.05; Mann-Whitney U test).
In general, an increase in the level of PCD in M. aeurignosa cultures was observed in response to daphnid extracts (Figure 1). All employed extracts of D. magna and D. cucullata induced a concentration-dependent increase in PCD at all-time intervals (Rs = 0.82–0.89 for D. magna and Rs = 0.58–0.95 for D. cucullata, p < 0.05). Moreover, the caspase-3-like activity correlated positively with time of exposure to 100 ind/L extracts of D. magna (Rs = 0.84, p < 0.05) and 50 and 100 ind/L extracts of D. cucullata (Rs = 0.91 and Rs = 0.86, respectively, p < 0.05). Compared to the control, a significant increase in the level of PCD was observed following the exposure to 50 and 100 ind/L extracts of D. magna, and this increase was already apparent after the first day of incubation. The maximum caspase-3-like activity, increased by 8 and 21%, respectively, was found after 7 days. In the case of D. cucullata, 50 and 100 ind/L extracts also increased the level of PCD, although this effect became apparent from day 5 of exposure – the maximum caspase-3-like activity at day 7 and was increased by 7 and 15%, respectively, compared to the control (Figure 4).
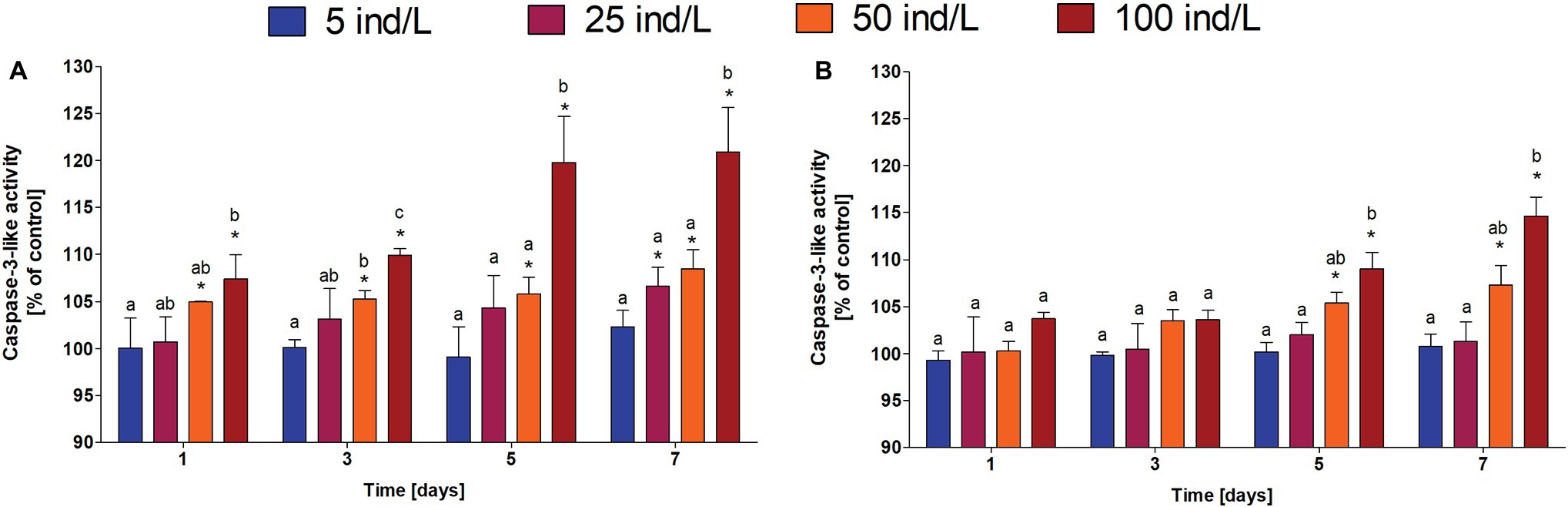
Figure 4. The caspase-3-like activity (mean and SD), a marker of PCD, in M. aeruginosa exposed for 7 days to different concentrations of D. magna (A) and D. cucullata (B) (n = 5). Different letters indicate statistically significant differences between studied groups for each time interval (p < 0.05; post-hoc Dunn’s test following Kruskal-Wallis ANOVA).
From three analyzed MC variants, MC-YR and MC-RR were below detection limit (10 ng/L). The exposure of M. aeruginosa cultures to daphnid extracts revealed an increase in extracellular MC concentration in a dose-dependent manner (D. magna: Rs = 0.87, p < 0.05; D. cucullata: Rs = 0.86, p < 0.05). However, the observed toxic levels tended to be higher following exposure to the D. magna extract. Compared to the control, cultures exposed to 25, 50, and 100 ind/L extracts exhibited a significant increase in extracellular MC concentration by 49, 169, and 204%, respectively. Significant changes were also observed following exposure to 50 and 100 ind/L extracts of D. cucullata and resulted in 51 and 87% increase in toxin levels, respectively, (Figure 5A).
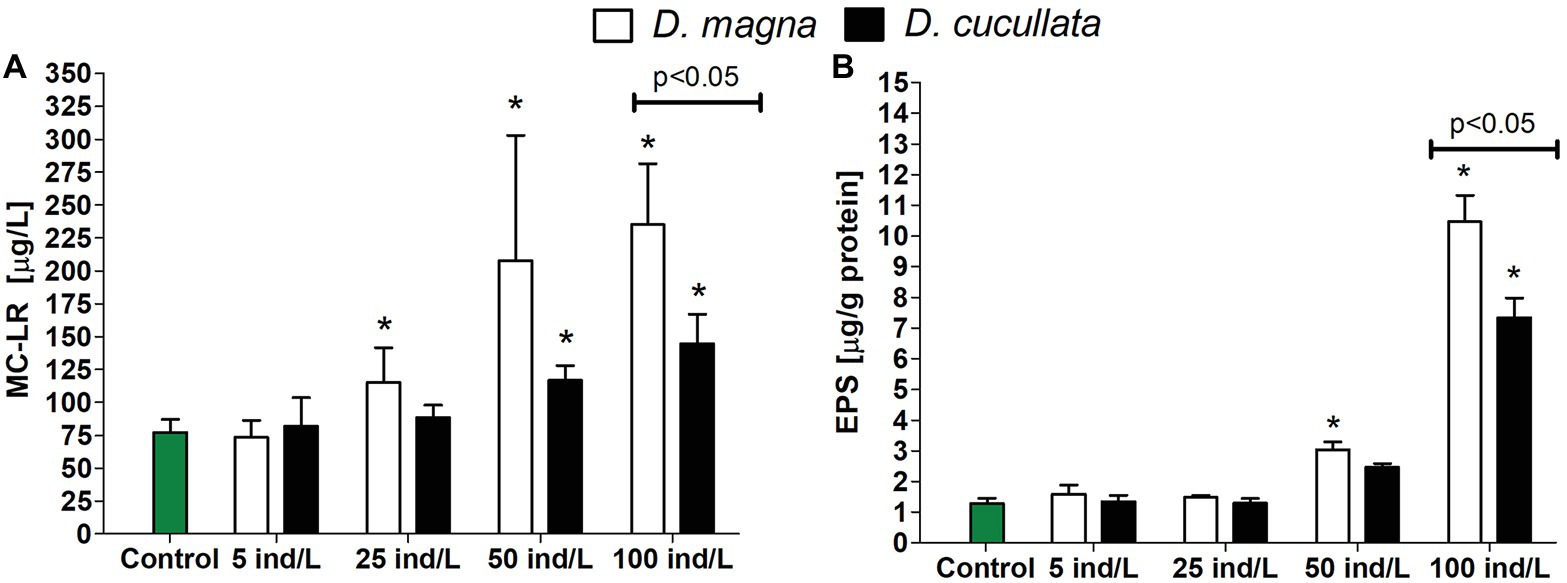
Figure 5. The extracellular concentration (mean and SD) of microcystin-LR (A) and level of bound exopolysaccharide (EPS) fraction (B) in M. aeruginosa exposed for 7 days to different concentrations of D. magna and D. cucullata (n = 5). An asterisk indicates a significant difference with the control (p < 0.05; Mann-Whitney U test).
The level of the bound EPS fraction in M. aeruginosa cultures exposed to extracts of both daphnids increased in a concentration-dependent manner (Rs = 0.89–0.90, p < 0.05). The greatest effect was observed following exposure to 100 ind/L extracts of D. magna and D. cucullata, although cultures treated with the former exhibited a higher level of bound EPS – its content increased 10‐ and 7-fold, respectively, (Figure 5B).
All parameters studied after 7 days of M. aeruginosa exposure to daphnid extracts remained in a significant relationship. For both D. magna and D. cucullata, negative correlations of culture growth and MDA concentration, level of PCD, extracellular MC, and bound EPS level were found. The level of MDA was positively correlated with the level of PCD and the content of bound EPS. Both PCD and MC were positively correlated with EPS content. A summary of these correlations is presented in Table 1. Additionally, intracellular ROS content measured after 60 min of exposure to extracts of D. magna and D. cucullata revealed a positive correlation with MDA content determined after 7 days (Rs = 0.93 and Rs = 0.76, respectively, p < 0.05) as well as with the level of PCD after 1 day (Rs = 0.93 and Rs = 0.76, respectively, p < 0.05) and 7 days of exposure (Rs = 0.85 and Rs = 0.84, respectively, p < 0.05).
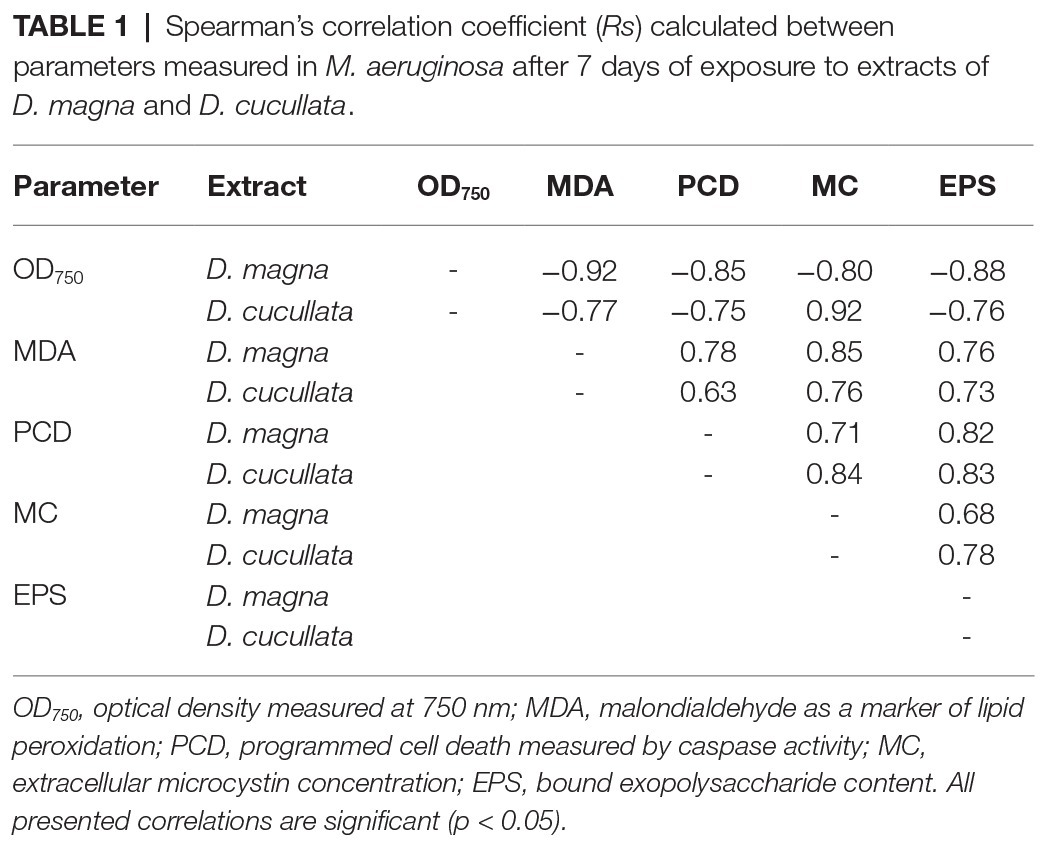
Table 1. Spearman’s correlation coefficient (Rs) calculated between parameters measured in M. aeruginosa after 7 days of exposure to extracts of D. magna and D. cucullata.
The present study explored the effect of daphnid metabolites on the physiological responses of M. aeruginosa. As shown, extracts of both D. magna and D. cucullata not only increased ROS content in cyanobacterial cells but also led, at higher concentrations, to oxidative stress, as indicated by an elevated level of MDA, a major product of lipid peroxidation (Ayala et al., 2014). It appears that ROS are the universal mediators of the cyanobacterial PCD as well as in other prokaryotic microorganisms (Hu and Rzymski, 2019). Their content in Microcystis can be increased under the various abiotic stressors which act as direct oxidants or trigger the internal generation of ROS (Ross et al., 2006; Ding et al., 2013; Hu and Rzymski, 2019). So far, the PCD in Microcystis has been shown to be induced by exogenous oxidants (e.g., H2O2; Ross et al., 2006; Ding et al., 2012; Zhou et al., 2018), mesohaline conditions (Ross et al., 2019), darkness (Bouchard and Purdie, 2011), high concentration of ascorbic acid (Chen et al., 2017), aldehydes (e.g., cinnamaldehyde; Hu et al., 2011), herbicides (fenoxaprop-p-ethyl, glyphosate, and methyl viologen; Ross et al., 2006; Wu et al., 2016; Du et al., 2017; Ye et al., 2019), allelochemicals (pyrogallic acid and phenolic compounds; He et al., 2016; Lu et al., 2017), bacterial pigments (e.g., prodigiosin; Yang et al., 2017), high pH, depletion of CO2 (Sigee et al., 2007), and ultraviolet radiation (Ding et al., 2013). The present study is the first to couple ROS production and PCD in Microcystis under the influence of molecules originating from zooplankton organisms.
Contrary to multicellular organisms, the occurrence of PCD in bacteria may appear counterintuitive, although it generally fits well into the concept of kin selection, particularly if one considers that cells of a single bacterial strain are highly similar on a molecular level, and can be often regarded as clones (Lewis, 2000; Hu and Rzymski, 2019). In that sense, the PCD in selected cells can confer a survival advantage to the genes that enable this process and which are present in the remaining population of cells. In the context of the present study, this advantage appears to be associated with MC release and an increase in the content of bound EPS.
In line with previous findings, the level of PCD in M. aeruginosa cultures studied in the present study was correlated with an increase in extracellular MC concentration. MC is known to be a typically intracellular metabolite but its extracellular multifunctional traits, e.g., in the aspects of nutrient uptake (Utkilen and Gjølme, 1995) and cell-cell communication (Rohrlack et al., 2001; Schatz et al., 2007), have also been established. Importantly, extracellular MC has been evidenced to play a role in the formation and maintenance of colonies in Microcystis (Kurmayer et al., 2003; Gan et al., 2012). Colony formation is a crucially important morphological trait of Microcystis as it enables the resistance to damage induced by abiotic stressors such as light or toxic metals (Wu et al., 2007; Zhang et al., 2011) and provides a defense against zooplankton grazing (Yang et al., 2006; Yang and Fanxiang, 2012). In Microcystis, it is enabled by the cohesion of individual cells within a structureless slimy layer known as mucilage, which consists mostly of anthrone-reacting polysaccharide, a type of extracellular polysaccharide (Kessel and Eloff, 1975; Plude et al., 1991). As shown previously, an increase in extracellular MC concentration led to the induction of genes related to polysaccharide synthesis, up-regulation in content of EPS, and an increase in colony size (Gan et al., 2012). Although the present study did not investigate the colony formation and size, the level of bound extracellular polysaccharides was considerably increased in M. aeruginosa cultures after 7 days of exposure to the highest concentration of extracts obtained from both Daphnia species. Moreover, their level correlated with the level of PCD in cultures and the extracellular concentration of MC. Although the previous observations indicate that MC up-regulates the content of bound EPS in intact Microcystis cells, it cannot be fully excluded that in the present study, this content was also, to some extent, increased in cells undergoing PCD.
The findings of the present study suggest that inducement of PCD in Microcystis initiates a series of events that can lead to the potential increase of the survival of the intact cyanobacterial cells and their genes (including those involved in the PCD). The assessment of the PCD threshold required to provide such benefits on the population level would, however, require further studies directly estimating the percentage of intact, necrotic, and apoptotic-like cells (Hu and Rzymski, 2019). One should note that MC can also be released to the environment via typical necrosis, although in such a case, this process is not controlled intracellularly. The PCD mechanism allows the function of the released MC to be tightly controlled, which may likely be advantageous compared to uncontrolled cell lysis (Hu and Rzymski, 2019). If one also considers that MCs have been shown to affect reproductive success adversely and reveal a general toxicity in Daphnia (Rohrlack et al., 2005; Dao et al., 2010; Herrera et al., 2015), the entire mechanism resulting in toxin release may benefit M. aeruginosa in both the short-term (protection against ongoing grazing) and long-term (decrease of grazer density). Importantly, the entire process may not necessarily require the induction of PCD but could also result from the consumption of M. aeruginosa cells and excretion of the internalized toxin.
It is unlikely that the observed physiological reactions of Microcystis to daphnid extracts represent a grazer-specific defense mechanism selected in response to zooplankton pressure. This is because PCD and MC production have both ancient evolutionary roots, and predate the metazoan lineage with metacaspases originating ∼3 billion years ago (Rantala et al., 2004; Asplund-Samuelsson et al., 2012). It is more probable that PCD coupled with MC release may evolve primarily as a response to abiotic stressors such as ultraviolet radiation and other factors generating increased intracellular ROS levels, which appear to be a key component of the PCD process. As shown in the present study, daphnid extracts were capable of inducing oxidative stress and therefore enabling other potentially associated events (PCD, MC, and bound EPS release) to occur. It is thus plausible that at some point in cyanobacterial evolution, the presence of grazers became a part of a much broader, universal defense mechanism. If one considers the great advantages it can provide for the survival of genes, a rise of grazers may further push its positive selection. Interestingly, previous research has shown that there is an inter-strain variability in response to daphnid infochemicals with some strain revealing a marked increase in MC production as well as a higher percentage of cells recruited into colonies while some others exhibit a weak or no response in this regard (van Gremberghe et al., 2009). The basis behind this observation requires further investigation, although it can be hypothesized that under different conditions, e.g., zooplankton pressure, different functional traits in M. aeurinosa have been selected.
Although extracts of both daphnids revealed a similar trend of response in exposed M. aeruginosa, its magnitude was greater in the case of D. magna than in D. cucullata. The former is a representative of a large species of Daphnia genus with an adult body length in the 2.3–6.0 mm range as opposed to the small D. cucullata with a length ranging from 0.5 to 1.4 mm (Green, 1954; Peter and Lampert, 1989). Smaller daphnids have a lower feeding rate and therefore exhibit a lower grazing pressure on Microcystis. However, it is also likely that the difference in response strength between the two species may also result, at least to some extent, from the lower quantity of metabolites extracted from the same number of D. cucullata individuals than in the case of D. magna. Nevertheless, the findings of the present study suggest that both species contain molecules that may serve as infochemicals for M. aeruginosa. It can be therefore hypothesized that such a response of cyanobacterial cells is generalized and can be initiated by the presence of any other daphnid species.
The present study is experimental and therefore a number of limitations have to be pointed out. Firstly, the Microcystis cultures were exposed to homogenized extracts of cladocerans to avoid the exposure to chemical compounds that are unrelated directly to daphnid presence and which may alter the study results. For example, spent Daphnia medium, even though its nutrient levels can be standardized, may contain metabolites originating from microalgae used to feed daphnids which in turn can cause severe cell lysis in Microcystis (Harel et al., 2013; Omidi et al., 2019; Qiu et al., 2019). One should, however, note that in situ only a fraction of daphnid molecules is released and could operate as infochemicals for cyanobacteria by triggering the defense mechanisms. It is yet to be directly studied whether these infochemicals can induce oxidative stress and subsequently initiate the PCD and MC release. This scenario is plausible, however, since the presence of Daphnia and the addition of spent daphnid medium have been already demonstrated to up-regulate MC production in Microcystis (van Gremberghe et al., 2009; Pineda-Mendoza et al., 2014; Pérez-Morales et al., 2015). Importantly, the use of spent medium or daphnid extracts allows only to study the effect of grazers on cyanobacteria while in situ a mutual interaction can occur, and possibly the array of molecules released by daphnids may be altered by the cyanobacteria presence. One should also note that the anti-grazing defense in Microcystis, including its non-MC producing strains, may also be attributed to other metabolites such as cyanopeptolin A or microcyclamide 7806A whose roles are much less studied (Sadler and von Elert, 2014; Bojadzija Savic et al., 2019, 2020). The present study employed only one strain of M. aeruginosa which was capable of MC-production. Further research addressing a similar issue in non-MC producing strain would provide a better understanding whether observed responses are specific to MC or if they could be attributed to other secondary metabolites, such as cyanopeptolin or microcyclamide already shown to be produced in the presence of grazers (Bojadzija Savic et al., 2020). The demonstrated relationship between the studied parameters gives a general overview of the potential coupling of oxidative stress, PCD, MC, and EPS release, although one should note that correlation does not necessarily imply the causation. Last but not least, the findings of the present study should also be confirmed on the molecular level. They also raise interesting questions regarding the role of MC and coupling of their production with PCD in filamentous toxin producers such as Planktothrix agardhii, which do not form colonies (Tonk et al., 2005; Wejnerowski et al., 2018). Under stress stimuli, including grazer presence, these cyanobacteria can undergo morphological changes (e.g., increase in width; Cerbin et al., 2013), but the role of ROS, PCD, and MC in these potential adaptive defense mechanisms is yet to be explored.
The present experimental study reveals a potential cascade of events in Microcystis cells under the presence of daphnid-derived molecules, which suggest that this cyanobacterium may have a specific potential to sense the presence of grazers and initiate a response that is advantageous for population survival. This response appears to be related to ROS generation and related oxidative stress, the conditions which appear to induce PCD in part of the cells constituting a Microcystis population. Consequently, MC is released to the extracellular environment and affects the surviving cells by inducing the release of EPS, known to be involved in colony formation and maintenance – a strategy that can decrease grazer pressure. The study adds to the general understanding of survival traits of Microcystis and the role that PCD may play in its ecological success.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
PR, PK, and BP conceptualized the study. PR, BP, and TJ did the analysis. All authors wrote, reviewed and edited the manuscript.
This study was supported by the National Research Center grant no. 2018/02/X/NZ8/02249 NCN MINIATURA-2.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Asplund-Samuelsson, J., Bergman, B., and Larsson, J. (2012). Prokaryotic caspase homologs: phylogenetic patterns and functional characteristics reveal considerable diversity. PLoS One 7:e49888. doi: 10.1371/journal.pone.0049888
Asselman, J., De Coninck, D. I. M., Beert, E., Janssen, C. R., Orsini, L., Pfrender, M. E., et al. (2017). Bisulfite sequencing with Daphnia highlights a role for epigenetics in regulating stress response to Microcystis through preferential differential methylation of serine and threonine amino acids. Environ. Sci. Technol. 51, 924–931. doi: 10.1021/acs.est.6b03870
Ayala, A., Muñoz, M. F., and Argüelles, S. (2014). Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 360438–360438. doi: 10.1155/2014/360438
Bidle, K. D. (2016). Programmed cell death in unicellular phytoplankton. Curr. Biol. 26, R594–R607. doi: 10.1016/j.cub.2016.05.056
Bojadzija Savic, G., Bormans, M., Edwards, C., Lawton, L., Briand, E., and Wiegand, C. (2020). Cross talk: two way allelopathic interactions between toxic Microcystis and Daphnia. Harmful Algae 94:101803. doi: 10.1016/j.hal.2020.101803
Bojadzija Savic, G., Edwards, C., Briand, E., Lawton, L., Wiegand, C., and Bormans, M. (2019). Daphnia magna exudates impact physiological and metabolic changes in Microcystis aeruginosa. Toxins 11:421. doi: 10.3390/toxins11070421
Bouchard, J. N., and Purdie, D. A. (2011). Effect of elevated temperature, darkness, and hydrogen peroxide treatment on oxidative stress and cell death in the bloom-forming toxic cyanobacterium Microcystis aeruginosa. J. Phycol. 47, 1316–1325. doi: 10.1111/j.1529-8817.2011.01074.x
Cerbin, S., Wejnerowski, Ł., and Dziuba, M. (2013). Aphanizomenon gracile increases in width in the presence of Daphnia. A defence mechanism against grazing? J. Limnol. 72:e41. doi: 10.4081/jlimnol.2013.e41
Chen, Y., Li, J., Wei, J., Kawan, A., Wang, L., and Zhang, X. (2017). Vitamin C modulates Microcystis aeruginosa death and toxin release by induced Fenton reaction. J. Hazard. Mater. 321, 888–895. doi: 10.1016/j.jhazmat.2016.10.010
Chen, M., Tian, L. -L., Ren, C. -Y., Xu, C. -Y., Wang, Y. -Y., and Li, L. (2019). Extracellular polysaccharide synthesis in a bloom-forming strain of Microcystis aeruginosa: implications for colonization and buoyancy. Sci. Rep. 9:1251. doi: 10.1038/s41598-018-37398-6
Dao, T. S., Do-Hong, L. -C., and Wiegand, C. (2010). Chronic effects of cyanobacterial toxins on Daphnia magna and their offspring. Toxicon 55, 1244–1254. doi: 10.1016/j.toxicon.2010.01.014
Ding, Y., Gan, N., Li, J., Sedmak, B., and Song, L. (2012). Hydrogen peroxide induces apoptotic-like cell death in Microcystis aeruginosa (chroococcales, cyanobacteria) in a dose-dependent manner. Phycologia 51, 567–575. doi: 10.2216/11-107.1
Ding, Y., Song, L., and Sedmak, B. (2013). UVB radiation as a potential selective factor favoring microcystin producing bloom forming cyanobacteria. PLoS One 8:e73919. doi: 10.1371/journal.pone.0083771
Du, Y., Ye, J., Wu, L., Yang, C., Wang, L., and Hu, X. (2017). Physiological effects and toxin release in Microcystis aeruginosa and Microcystis viridis exposed to herbicide fenoxaprop-p-ethyl. Environ. Sci. Pollut. Res. 24, 7752–7763. doi: 10.1007/s11356-017-8474-y
Erental, A., Kalderon, Z., Saada, A., Smith, Y., and Engelberg-Kulka, H. (2014). Apoptosis-like death, an extreme SOS response in Escherichia coli. MBio 5:e01426-14. doi: 10.1128/mBio.01426-14
Gan, N., Xiao, Y., Zhu, L., Wu, Z., Liu, J., Hu, C., et al. (2012). The role of microcystins in maintaining colonies of bloom-forming Microcystis spp. Environ. Microbiol. 14, 730–742. doi: 10.1111/j.1462-2920.2011.02624.x
Ger, K. A., Hansson, L. -A., and Lürling, M. (2014). Understanding cyanobacteria-zooplankton interactions in a more eutrophic world. Freshw. Biol. 59, 1783–1798. doi: 10.1111/fwb.12393
Ger, K. A., Urrutia-Cordero, P., Frost, P. C., Hansson, L. -A., Sarnelle, O., Wilson, A. E., et al. (2016). The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful Algae 54, 128–144. doi: 10.1016/j.hal.2015.12.005
Green, J. (1954). Size and reproduction in Daphnia magna (crustacea: cladocera). Proc. Zool. Soc. London 124, 535–545.
Harel, M., Weiss, G., Lieman-Hurwitz, J., Gun, J., Lev, O., Lebendiker, M., et al. (2013). Interactions between Scenedesmus and Microcystis may be used to clarify the role of secondary metabolites. Environ. Microbiol. Rep. 5, 97–104. doi: 10.1111/j.1758-2229.2012.00366.x
Harke, M. J., Steffen, M. M., Gobler, C. J., Otten, T. G., Wilhelm, S. W., Wood, S. A., et al. (2016). A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 54, 4–20. doi: 10.1016/j.hal.2015.12.007
He, Y., Zhou, Q. -H., Liu, B. -Y., Cheng, L., Tian, Y., Zhang, Y. -Y., et al. (2016). Programmed cell death in the cyanobacterium Microcystis aeruginosa induced by allelopathic effect of submerged macrophyte Myriophyllum spicatum in co-culture system. J. Appl. Phycol. 28, 2805–2814. doi: 10.1007/s10811-016-0814-7
Henao, E., Rzymski, P., and Waters, M. N. (2019). A review on the study of cyanotoxins in paleolimnological research: current knowledge and future needs. Toxins 12:6. doi: 10.3390/toxins12010006
Herrera, N. A., Echeverri, L. F., and Ferrão-Filho, A. S. (2015). Effects of phytoplankton extracts containing the toxin microcystin-LR on the survival and reproduction of cladocerans. Toxicon 95, 38–45. doi: 10.1016/j.toxicon.2014.12.016
Hu, C., and Rzymski, P. (2019). Programmed cell death-like and accompanying release of microcystin in freshwater bloom-forming cyanobacterium Microcystis: from identification to ecological relevance. Toxins 11:706. doi: 10.3390/toxins11120706
Hu, L. B., Zhou, W., Yang, J. D., Chen, J., Yin, Y. F., and Shi, Z. Q. (2011). Cinnamaldehyde induces PCD-like death of Microcystis aeruginosa via reactive oxygen species. Water Air Soil Pollut. 217, 105–113. doi: 10.1007/s11270-010-0571-1
Hülsmann, S., and Voigt, H. (2002). Life history of Daphnia galeata in a hypertrophic reservoir and consequences of non-consumptive mortality for the initiation of a midsummer decline. Freshw. Biol. 47, 2313–2324. doi: 10.1046/j.1365-2427.2002.00991.x
Kessel, M., and Eloff, J. N. (1975). The ultrastructure and development of the colonial sheath of Microcystis marginata. Arch. Microbiol. 106, 209–214. doi: 10.1007/BF00446525
Klemenčič, M., and Dolinar, M. (2016). Orthocaspase and toxin-antitoxin loci rubbing shoulders in the genome of Microcystis aeruginosa PCC 7806. Curr. Genet. 62, 669–675. doi: 10.1007/s00294-016-0582-6
Klemenčič, M., Novinec, M., and Dolinar, M. (2015). Orthocaspases are proteolytically active prokaryotic caspase homologues: the case of Microcystis aeruginosa. Mol. Microbiol. 98, 142–150. doi: 10.1111/mmi.13110
Komosa, A., Rzymski, P., Perek, B., Ropacka-Lesiak, M., Lesiak, M., Siller-Matula, J. M., et al. (2017). Platelets redox balance assessment: current evidence and methodological considerations. Vasc. Pharmacol. 93-95, 6–13. doi: 10.1016/j.vph.2017.06.002
Kurmayer, R., Christiansen, G., and Chorus, I. (2003). The abundance of microcystin-producing genotypes correlates positively with colony size in Microcystis sp., and determines its microcystin net production in Lake Wannsee. Appl. Environ. Microbiol. 69, 787–795. doi: 10.1128/AEM.69.2.787-795.2003
Lewis, K. (2000). Programmed death in bacteria. Microbiol. Mol. Biol. Rev. 64, 503–514. doi: 10.1128/MMBR.64.3.503-514.2000
Li, J., Li, R., and Li, J. (2017). Current research scenario for microcystins biodegradation—a review on fundamental knowledge, application prospects and challenges. Sci. Total Environ. 595, 615–632. doi: 10.1016/j.scitotenv.2017.03.285
Li, M., Zhu, W., Gao, L., and Lu, L. (2013). Changes in extracellular polysaccharide content and morphology of Microcystis aeruginosa at different specific growth rates. J. Appl. Phycol. 25, 1023–1030. doi: 10.1007/s10811-012-9937-7
Liu, L., Huang, Q., and Qin, B. (2018). Characteristics and roles of Microcystis extracellular polymeric substances (EPS) in cyanobacterial blooms: a short review. J. Freshw. Ecol. 33, 183–193. doi: 10.1080/02705060.2017.1391722
Lu, Z., Sha, J., Tian, Y., Zhang, X., Liu, B., and Wu, Z. (2017). Polyphenolic allelochemical pyrogallic acid induces caspase-3(like)-dependent programmed cell death in the cyanobacterium Microcystis aeruginosa. Algal Res. 21, 148–155. doi: 10.1016/j.algal.2016.11.007
Moheimani, N. R., Borowitzka, M. A., Isdepsky, A., and Sing, S. F. (2013). “Standard methods for measuring growth of algae and their composition” in Algae for biofuels and energy. eds. M. A. Borowitzka and N. R. Moheimani (Dordrecht, Netherlands: Springer), 265–284.
Omidi, A., Esterhuizen-Londt, M., and Pflugmacher, S. (2018). Still challenging: the ecological function of the cyanobacterial toxin microcystin—what we know so far. Toxin Rev. 37, 87–105. doi: 10.1080/15569543.2017.1326059
Omidi, A., Esterhuizen-Londt, M., and Pflugmacher, S. (2019). Interspecies interactions between Microcystis aeruginosa PCC 7806 and Desmodesmus subspicatus SAG 86.81 in a co-cultivation system at various growth phases. Environ. Int. 131:105052. doi: 10.1016/j.envint.2019.105052
Orr, P. T., and Jones, G. J. (1998). Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnol. Oceanogr. 43, 1604–1614. doi: 10.4319/lo.1998.43.7.1604
Ortiz-Rodríguez, R., Dao, T. S., and Wiegand, C. (2012). Transgenerational effects of microcystin-LR on Daphnia magna. J. Exp. Biol. 215, 2795–2805. doi: 10.1242/jeb.069211
Peeters, S. H., and De Jonge, M. I. (2018). For the greater good: programmed cell death in bacterial communities. Microbiol. Res. 207, 161–169. doi: 10.1016/j.micres.2017.11.016
Pérez-Morales, A., Sarma, S. S. S., and Nandini, S. (2015). Microcystins production in Microcystis induced by Daphnia pulex (Cladocera) and Brachionus calyciflorus (Rotifera). Hidrobiológica 25, 411–415.
Peter, H., and Lampert, W. (1989). The effect of Daphnia body size on filtering rate inhibition in the presence of a filamentous cyanobacterium. Limnol. Oceanogr. 34, 1084–1089. doi: 10.4319/lo.1989.34.6.1084
Pham, T. -L., and Utsumi, M. (2018). An overview of the accumulation of microcystins in aquatic ecosystems. J. Environ. Manag. 213, 520–529. doi: 10.1016/j.jenvman.2018.01.077
Pineda-Mendoza, R. M., Zúñiga, G., and Martínez-Jerónimo, F. (2014). Infochemicals released by Daphnia magna fed on Microcystis aeruginosa affect mcyA gene expression. Toxicon 80, 78–86. doi: 10.1016/j.toxicon.2014.01.008
Plude, J. L., Parker, D. L., Schommer, O. J., Timmerman, R. J., Hagstrom, S. A., Joers, J. M., et al. (1991). Chemical characterization of polysaccharide from the slime layer of the cyanobacterium Microcystis flos-aquae C3-40. Appl. Environ. Microbiol. 57, 1696–1700. doi: 10.1128/AEM.57.6.1696-1700.1991
Poniedziałek, B., Rzymski, P., Pięt, M., Gąsecka, M., Stroińska, A., Niedzielski, P., et al. (2018). Relation between polyphenols, malondialdehyde, antioxidant capacity, lactate dehydrogenase and toxic elements in human colostrum milk. Chemosphere 191, 548–554. doi: 10.1016/j.chemosphere.2017.10.098
Qiu, Y., Wang, Z., Liu, F., Liu, J., Tan, K., and Ji, R. (2019). Inhibition of Scenedesmus quadricauda on Microcystis flos-aquae. Appl. Microbiol. Biotechnol. 103, 5907–5916. doi: 10.1007/s00253-019-09809-9
Rantala, A., Fewer, D. P., Hisbergues, M., Rouhiainen, L., Vaitomaa, J., Börner, T., et al. (2004). Phylogenetic evidence for the early evolution of microcystin synthesis. Proc. Natl. Acad. Sci. U. S. A. 101, 568–573. doi: 10.1073/pnas.0304489101
Rohrlack, T., Christoffersen, K., Dittmann, E., Nogueira, I., Vasconcelos, V., and Börner, T. (2005). Ingestion of microcystins by Daphnia: intestinal uptake and toxic effects. Limnol. Oceanogr. 50, 440–448. doi: 10.4319/lo.2005.50.2.0440
Rohrlack, T., Dittmann, E., Börner, T., and Christoffersen, K. (2001). Effects of cell-bound microcystins on survival and feeding of Daphnia spp. Appl. Environ. Microbiol. 67, 3523–3529. doi: 10.1128/AEM.67.8.3523-3529.2001
Rohrlack, T., Dittmann, E., Henning, M., Börner, T., and Kohl, J.-G. (1999). Role of microcystins in poisoning and food ingestion inhibition of Daphnia galeata caused by the cyanobacterium Microcystis aeruginosa. Appl. Environ. Microbiol. 65, 737–739. doi: 10.1128/AEM.65.2.737-739.1999
Rohrlack, T., and Hyenstrand, P. (2007). Fate of intracellular microcystins in the cyanobacterium Microcystis aeruginosa (chroococcales, cyanophyceae). Phycologia 46, 277–283. doi: 10.2216/06-14.1
Ross, C., Santiago-Vázquez, L., and Paul, V. (2006). Toxin release in response to oxidative stress and programmed cell death in the cyanobacterium Microcystis aeruginosa. Aquat. Toxicol. 78, 66–73. doi: 10.1016/j.aquatox.2006.02.007
Ross, C., Warhurst, B. C., Brown, A., Huff, C., and Ochrietor, J. D. (2019). Mesohaline conditions represent the threshold for oxidative stress, cell death and toxin release in the cyanobacterium Microcystis aeruginosa. Aquat. Toxicol. 206, 203–211. doi: 10.1016/j.aquatox.2018.11.019
Sadler, T., and Von Elert, E. (2014). Physiological interaction of Daphnia and Microcystis with regard to cyanobacterial secondary metabolites. Aquat. Toxicol. 156, 96–105. doi: 10.1016/j.aquatox.2014.08.003
Sato, M., Amano, Y., Machida, M., and Imazeki, F. (2017). Colony formation of highly dispersed Microcystis aeruginosa by controlling extracellular polysaccharides and calcium ion concentrations in aquatic solution. Limnology 18, 111–119. doi: 10.1007/s10201-016-0494-7
Saunders, A. P., Porter, G. K., and Taylor, E. B. (1999). Population dynamics of Daphnia spp. and implications for trophic interactions in small, monomictic lake. J. Plankton Res. 21, 1823–1845. doi: 10.1093/plankt/21.10.1823
Schatz, D., Keren, Y., Vardi, A., Sukenik, A., Carmeli, S., Börner, T., et al. (2007). Towards clarification of the biological role of microcystins, a family of cyanobacterial toxins. Environ. Microbiol. 9, 965–970. doi: 10.1111/j.1462-2920.2006.01218.x
Sigee, D. C., Selwyn, A., Gallois, P., and Dean, A. P. (2007). Patterns of cell death in freshwater colonial cyanobacteria during the late summer bloom. Phycologia 46, 284–292. doi: 10.2216/06-69.1
Thiel, K., Vuorio, E., Aro, E. -M., and Kallio, P. T. (2017). The effect of enhanced acetate influx on Synechocystis sp. PCC 6803 metabolism. Microb. Cell Factories 16:21. doi: 10.1186/s12934-017-0640-x
Tonk, L., Visser, P. M., Christiansen, G., Dittmann, E., Snelder, E. O. F. M., Wiedner, C., et al. (2005). The microcystin composition of the cyanobacterium Planktothrix agardhii changes toward a more toxic variant with increasing light intensity. Appl. Environ. Microbiol. 71, 5177–5181. doi: 10.1128/AEM.71.9.5177-5181.2005
Utkilen, H., and Gjølme, N. (1995). Iron-stimulated toxin production in Microcystis aeruginosa. Appl. Environ. Microbiol. 61, 797–800. doi: 10.1128/AEM.61.2.797-800.1995
van Gremberghe, I., Vanormelingen, P., Van Der Gucht, K., Mancheva, A., D’hondt, S., De Meester, L., et al. (2009). Influence of Daphnia infochemicals on functional traits of Microcystis strains (cyanobacteria). Hydrobiologia 635, 147–155. doi: 10.1007/s10750-009-9907-5
Wejnerowski, Ł., Rzymski, P., Kokociński, M., and Meriluoto, J. (2018). The structure and toxicity of winter cyanobacterial bloom in a eutrophic lake of the temperate zone. Ecotoxicology 27, 752–760. doi: 10.1007/s10646-018-1957-x
Wojtal-Frankiewicz, A. B. J., Jurczak, T., Gwoździński, K., Frankiewicz, P., and Wielanek, M. (2013). Microcystin assimilation and detoxification by Daphnia spp. in two ecosystems of different cyanotoxin concentrations. J. Limnol. 72:13. doi: 10.4081/jlimnol.2013.e13
Wojtal-Frankiewicz, A., Kruk, A., Frankiewicz, P., Oleksińska, Z., and Izydorczyk, K. (2015). Long-term patterns in the population dynamics of Daphnia longispina, Leptodora kindtii and cyanobacteria in a shallow reservoir: a self-organising map (SOM) approach. PLoS One 10:e0144109. doi: 10.1371/journal.pone.0144109
Wu, Z. -X., Gan, N. -Q., Huang, Q., and Song, L. -R. (2007). Response of Microcystis to copper stress—do phenotypes of Microcystis make a difference in stress tolerance? Environ. Pollut. 147, 324–330. doi: 10.1016/j.envpol.2006.05.022
Wu, L., Qiu, Z., Zhou, Y., Du, Y., Liu, C., Ye, J., et al. (2016). Physiological effects of the herbicide glyphosate on the cyanobacterium Microcystis aeruginosa. Aquat. Toxicol. 178, 72–79. doi: 10.1016/j.aquatox.2016.07.010
Xu, H., Jiang, H., Yu, G., and Yang, L. (2014). Towards understanding the role of extracellular polymeric substances in cyanobacterial Microcystis aggregation and mucilaginous bloom formation. Chemosphere 117, 815–822. doi: 10.1016/j.chemosphere.2014.10.061
Yang, K., Chen, Q., Zhang, D., Zhang, H., Lei, X., Chen, Z., et al. (2017). The algicidal mechanism of prodigiosin from Hahella sp. KA22 against Microcystis aeruginosa. Sci. Rep. 7:7750. doi: 10.1038/s41598-017-08132-5
Yang, Z., and Fanxiang, K. (2012). Formation of large colonies: a defense mechanism of Microcystis aeruginosa under continuous grazing pressure by flagellate Ochromonas sp. J. Limnol. 71:5. doi: 10.4081/jlimnol.2012.e5
Yang, Z., Kong, F., Shi, X., and Cao, H. (2006). Morphological response of Microcystis aeruginosa to grazing by different sorts of zooplankton. Hydrobiologia 563, 225–230. doi: 10.1007/s10750-005-0008-9
Yang, Z., Kong, F., Shi, X., Zhang, M., Xing, P., and Cao, H. (2008). Changes in the morphology and polysaccharide content of Microcystis aeruginosa (cyanobacteria) during flagellate grazing. J. Phycol. 44, 716–720. doi: 10.1111/j.1529-8817.2008.00502.x
Ye, J., Huang, C., Qiu, Z., Wu, L., and Xu, C. (2019). The growth, apoptosis and oxidative stress in Microcystis viridis exposed to glyphosate. Bull. Environ. Contam. Toxicol. 103, 585–589. doi: 10.1007/s00128-019-02691-1
Zhang, M., Shi, X., Yu, Y., and Kong, F. (2011). The acclimative changes in photochemistry after colony formation of the cyanobacteria Microcystis aeruginosa. J. Phycol. 47, 524–532. doi: 10.1111/j.1529-8817.2011.00987.x
Zhang, X., Warming, T. P., Hu, H. -Y., and Christoffersen, K. S. (2009). Life history responses of Daphnia magna feeding on toxic Microcystis aeruginosa alone and mixed with a mixotrophic Poterioochromonas species. Water Res. 43, 5053–5062. doi: 10.1016/j.watres.2009.08.022
Zheng, W., Rasmussen, U., Zheng, S., Bao, X., Chen, B., Gao, Y., et al. (2013). Multiple modes of cell death discovered in a prokaryotic (cyanobacterial) endosymbiont. PLoS One 8:e66147. doi: 10.1371/journal.pone.0084776
Keywords: Microcystis aeruginosa, microcystins, programmed cell death, Daphnia, cyanobacteria, stress response
Citation: Rzymski P, Klimaszyk P, Jurczak T and Poniedziałek B (2020) Oxidative Stress, Programmed Cell Death and Microcystin Release in Microcystis aeruginosa in Response to Daphnia Grazers. Front. Microbiol. 11:1201. doi: 10.3389/fmicb.2020.01201
Received: 17 April 2020; Accepted: 12 May 2020;
Published: 17 June 2020.
Edited by:
Eva Ortega-Retuerta, UMR7621 Laboratoire d’anographie microbienne (LOMIC), FranceReviewed by:
Myriam Bormans, Centre National de la Recherche Scientifique (CNRS), FranceCopyright © 2020 Rzymski, Klimaszyk, Jurczak and Poniedziałek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Piotr Rzymski, cnp5bXNraXBpb3RyQHVtcC5lZHUucGw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.