- 1Laboratory of Aquaculture & Artemia Reference Center, Department of Animal Sciences and Aquatic Ecology, Faculty of Bioscience Engineering, Ghent University, Ghent, Belgium
- 2Department of Animal Nutrition and Management, Faculty of Veterinary Medicine and Animal Sciences, Swedish University of Agricultural Sciences, Uppsala, Sweden
Vibrio campbellii is one of the major bacterial pathogens for animals reared in aquaculture, affecting both vertebrates and invertebrates, and causes significant economic losses. It is now evident that the expressions of virulence factors in this pathogen are regulated by the density of the bacterial population. This type of regulation, termed quorum sensing (QS), is mediated by extracellular signal molecules called autoinducers. In this study, the impact of sodium ascorbate (NaAs) on the virulence of V. campbellii was investigated under both in vitro and in vivo conditions, to develop a natural anti-infective strategy to contain V. campbellii infection in aquacultured animals. Results showed that NaAs significantly decreased swimming motility, biofilm production, and the production of virulence enzymes, such as lipase, caseinase, phospholipase, and hemolysin in V. campbellii. Consistent with this, pretreatment of V. campbellii with NaAs before inoculation into the rearing water resulted in significantly increased survival of gnotobiotic brine shrimp larvae, when compared to larvae challenged with untreated V. campbellii. Furthermore, NaAs could interfere with QS-regulated bioluminescence in V. campbellii, suggesting the QS-inhibitory activity largely determines the protective effect of NaAs toward the brine shrimp. In essence, due to the potent anti-virulence effects observed in in vitro studies and the clinical brine shrimp-V. campbellii infection model, NaAs constitute a promising novel strategy for the control of V. campbellii infections in aquaculture.
Introduction
Vibrio campbellii is a Gram-negative luminous bacterium that lives in a broad range of aquatic environments. It has been regarded as a serious pathogen affecting numerous vertebrates and invertebrates, which leads to significant losses to the aquaculture industry (Defoirdt et al., 2008; Yang et al., 2015). Recent studies have shed some light on the pathogenicity mechanisms of this bacterial strain. In general, the infectious steps of bacterial pathogens include adhesion and incubation in the host, avoidance of host defenses, causing diseases and mortality, and then exit (Donnenberg, 2000). These steps involve the expression of virulence factors that allow the pathogens to infect and damage the host. For Vibrio campbellii, the ability to adhere to host surface and form biofilms, produce extracellular products (E) and lipopolysaccharide (LPS), and interact with bacteriophage and bacteriocin-like substance (BLIS) are its most crucial virulence determinants (Austin and Zhang, 2006; Darshanee Ruwandeepika et al., 2012).
Since virulence factors are often costly metabolic products, their expression usually is under tight control to avoid energy waste. One of the regulatory mechanisms controlling the production of virulence factors in V. campbellii is quorum-sensing (QS), cell-to-cell communication with secreted signaling molecules called autoinducers (Defoirdt et al., 2008). The external concentration of autoinducer varies according to the cell population density of the bacteria in the environment. When the autoinducer concentration reached the threshold, the bacteria could initiate a signal transduction cascade that culminates in a change in the behavior, such as controlling luminescence, biofilm formation, and toxin production, in response to environmental cues (Schauder and Bassler, 2001). The model describing the QS circuit employed in V. campbellii is presented in Figure 1. V. campbellii is a model bacterium in QS research and has been found to contain a three-channel QS system that uses three kinds of signaling molecules: campbellii autoinducer 1 (HAI-1) (Cao and Meighen, 1989), autoinducer 2 (AI-2) (Chen et al., 2002), and cholerae autoinducer 1 (CAI-1) (Higgins et al., 2007). Previous work in our lab had shown that the three-channel QS system is required for the full virulence of V. campbellii (Defoirdt and Sorgeloos, 2012; Pande et al., 2013).
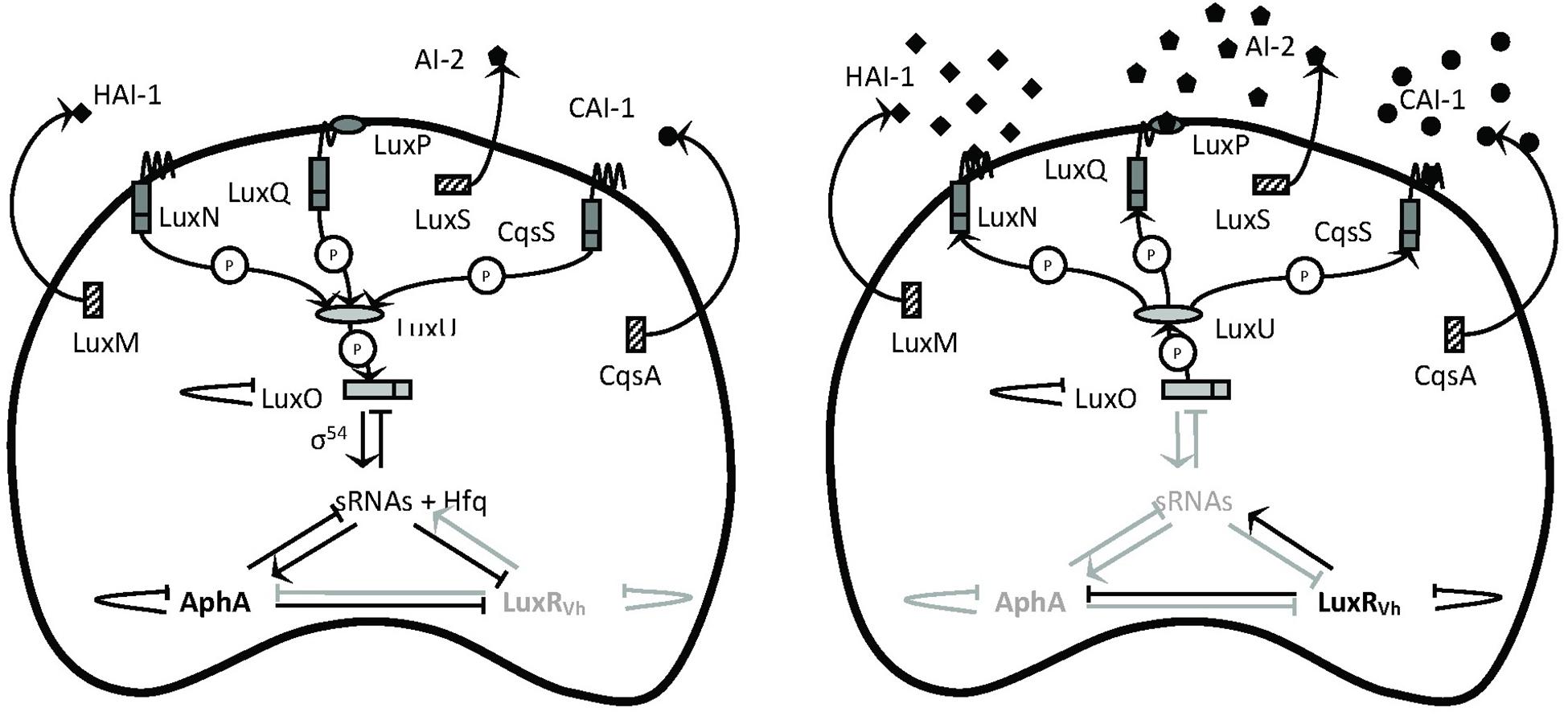
Figure 1. Schematic representation of the quorum-sensing circuit of Vibrio campbellii. The LuxM, LuxS, and CqsA enzymes synthesize the autoinducers HAI-1, AI-2, and CAI-1, respectively (Winzer et al., 2002). These autoinducers are detected at the cell surface by the LuxN, LuxPQ, and CqsS two-component hybrid sensor kinase/response regulator proteins, respectively. In complex of AI-2 by LuxQ requires the interacts with the periplasmic protein LuxP to send the AI-2 signal (Bassler et al., 1993). In the absence of autoinducers (left, at low cell density), the receptors autophosphorylate and transfer phosphate to LuxO via LuxU (Freeman and Bassler, 1999). Phosphorylation activates LuxO, which together with σ54 activates the production of five small regulatory RNAs (sRNAs). The sRNAs, together with the chaperone Hfq, promote translation of the master regulator AphA and inhibit translation of the master regulator LuxRVh (Tu and Bassler, 2007). In the presence of high concentrations of the autoinducers (right, at high cell density), the receptor proteins switch from kinases to phosphatases, which results in dephosphorylation of LuxO. Dephosphorylated LuxO is inactive and therefore, the sRNAs are not formed, AphA is not translated, and LuxRVh is translated. AphA and LuxR are transcriptional regulators that (either individually or together) affect the transcription of many target genes. P means phosphotransfer. Modified with permission from Defoirdt et al. (2008) and Yang et al. (2017).
Disrupting the QS signaling pathways has been proposed as a suitable strategy for the development of new antimicrobial and anti-virulence therapies. This strategy is highly attractive because it is unlikely to pose a selective pressure for the development of bacterial resistance (Rasko and Sperandio, 2010). Specifically, using the enzymes that degrade the autoinducers, a process called quorum quenching, and library screening for the analogs of autoinducers are the most used strategies to inhibit the organism-specific cell to cell signaling. To date, several QS inhibitors have been described. Amongst all, the halogenated furanones produced by the marine algae Delisea pulchra are the most studied QS inhibitors (Rasmussen et al., 2000; Manefield et al., 2001). These compounds, which are structurally similar to AHLs, were reported to disrupt QS in many Gram-negative bacteria by decreasing the DNA-binding activity of the QS master regulator LuxR (Manefield et al., 2002; Smith et al., 2003; Defoirdt et al., 2007). Unfortunately, these halogenated furanones are toxic per se to higher organisms (Wang et al., 2016), which limited their practical applications. However, their proven ability to control bacterial infections in animal models is of considerable importance, since it demonstrates that QS is a useful and promising target for antibacterial agents. Over the past years, several naturally occurring furanones have been explored as an alternative for the corresponding halogenated furanones. One of these compounds, the ascorbic acid [5-(1,2-dihydroxyethyl)-3,4-dihydroxy-2(5H)-furanone] or mineral ascorbates (sodium ascorbate), has been identified as interesting QS analogs because they tend to be relatively less invasive and highly effective in reducing the QS and pathogenic potential of many bacteria like Pseudomonas aeruginosa and Clostridium perfringens (Novak and Fratamico, 2004; El-Mowafy et al., 2014). Although these compounds show a high degree of QS interfering effects in the bacterial pathogens of humans, they were evaluated neither for their effect on QS-regulated virulence factors in aquaculture pathogens of commercial importance nor for their antibacterial activity in vivo. This study aimed at investigating the effect of NaAs on the virulence of V. campbellii in vitro and in vivo in a highly controlled model system with gnotobiotic brine shrimp (Artemia franciscana) larvae, and the results of this study could provide a novel strategy for the control of V. campbellii infections in aquaculture.
Materials and Methods
Bacterial Strains, Culture Media, and Reagents
Vibrio campbellii BAA-1116 wild type strain1, mutant strain JAF548 pAKlux1, and Aeromonas spp. strain LVS3 were used in this study. LVS3, after autoclaving, was used as feed for brine shrimp. The mutant strain contains a point mutation in LuxO, which renders the QS system inactive. And the plasmid pAKlux1 contains the Photorhabdus luminescens bioluminescence operon under the control of a constitutive promoter. Hence, the bioluminescence is independent of the three-channel QS system in this strain. All strains were preserved at −80°C in Marine Broth 2216 (Difco Laboratories, Detroit, MI, United States) with 25% sterile glycerol. The bacterial strains were initially grown at 28°C for 24 h on Marine Agar (Difco Laboratories, Detroit, MI, United States) and then to log phase in Marine Broth by incubation at 28°C with continuous shaking before use. Bacterial cell densities were determined spectrophotometrically (BioMérieux, Marcy L’Etoile, France) at 600 nm according to the McFarland standard, assuming that an optical density of 1 corresponding to 1.2 × 109 cells ml–1. NaAs was purchased from VWR international (Leuven, Belgium).
Bacterial Growth Assays
For the bacterial growth assays, V. campbellii was grown overnight in Marine Broth at 28°C. After that, the culture was diluted to an OD600 of 0.1 in fresh Marine Broth, without NaAs and with NaAs (5 and 10 mg ml–1). The cultures were grown in 10 ml volumes in sterile Erlenmeyer flasks at 28°C for 12 h, and the turbidity at 600 nm was monitored every hour using a Tecan Infinite 200 microplate reader (Tecan, Mechelen, Belgium). Growth curves were determined for three independent cultures.
Bioluminescence Assays
Bioluminescence assays were performed as described previously (Defoirdt and Sorgeloos, 2012). In brief, V. campbellii WT and mutant strains were grown overnight and diluted to an OD600 of 0.1. NaAs were added at 5 and 10 mg ml–1, respectively, the culture without NaAs was treated as control. The cultures were further incubated at 28°C with continuous shaking, and bioluminescence was measured after 1 h with a Tecan Infinite 200 microplate reader (Tecan, Mechelen, Belgium).
Specific Quorum Sensing-Inhibitory Activity AQSI
The specific quorum sensing-inhibitory activity of NaAs at a given concentration was calculated as described previously (Yang et al., 2015):
With
% Inhibition QS–regulated: percentage inhibition of QS regulated bioluminescence in wild type V. campbellii BAA-1116.
% Inhibition QS–independent: percentage inhibition of QS independent bioluminescence of V. campbellii JAF548 pAKlux1.
For inhibition values below 1%, a value of 1% is used in the calculations, and the AQSI values are reported as > “calculated AQSI.” Compounds are considered as false positives if AQSI is lower than 2 at all concentrations tested, whereas they are considered as specific QS inhibitors if they cause significant inhibition of QS-regulated bioluminescence and if AQSI is higher than 10 at one of the concentrations tested.
Swimming Motility Assay
The swimming motility assay was performed on soft agar (Marine agar plates containing 0.2% agar) as described previously by Rui et al. (2008). Freshly prepared and filtered NaAs (at 0, 5, and 10 mg ml–1, respectively) were added to the agar after autoclaving and immediately before pouring the agar into Petri plates. V. campbellii was grown overnight in Marine Broth, and 2 μl aliquots (OD600 = 1.0) were inoculated into the center of the soft agar plates. Plates were incubated for 24 h at 28°C, after which the diameters of the motility halos were measured. All assays were done with freshly prepared media in five replicates.
Lipase and Phospholipase Assays
The lipase and phospholipase activities were measured by a modification of the method described by Austin et al. (2005). Agar plates for lipase and phospholipase assays were prepared by supplementing Marine Agar with 1% Tween 80 (Sigma-Aldrich, Belgium) and 1% egg yolk emulsion (Sigma-Aldrich, Belgium), each sterilized separately at 121°C for 5 min before mixing. The development of opalescent zones around the colonies was observed and the diameter of the zones was measured after 2–4 days of incubation at 28°C.
Caseinase and Hemolysin Assays
The caseinase and hemolysin assays were performed as described previously with some modifications (Zhang and Austin, 2000; Austin et al., 2005). The caseinase assay plates were prepared by mixing double strength Marine Agar with a 4% skim milk powder suspension (Oxoid, Hampshire, United Kingdom), each sterilized separately at 121°C for 5 min. Clearing zones surrounding the bacterial colonies were measured after 2 days of incubation. Hemolytic assay plates were prepared by supplementing Marine Agar with 5% defibrinated sheep blood (Oxoid, Hampshire, United Kingdom) and clearing zones were measured after 2 days of incubation.
Determination of Biofilm Levels
Biofilm formation was quantified by crystal violet staining, as described previously (Stepanovic et al., 2007). In brief, an overnight culture of V. campbellii was diluted to an OD600 of 0.1 in Marine Broth with or without NaAs, and 200 μl aliquots of these suspensions were pipetted into the wells of a polystyrene 96 well plate. Then the bacteria were allowed to adhere and grow without agitation for 24 h at 28°C. After that, the cultures were removed, and the wells were washed three times with 300 μl sterile physiological saline to remove all non-adherent bacteria. The remaining attached bacteria were fixed with 150 μl of 99% methanol per well for 20 min, after which the methanol was removed, and plates were air-dried. Then, biofilms were stained for 15 min with 150 μl per well of a 0.1% crystal violet solution (Pro-Lab Diagnostics, Richmond Hill, ON, Canada). Excess stain was rinsed off by placing the plate under running tap water, and washing was continued until the washings were free of the stain. After the plates were air-dried, the dye bound to the adherent cells was resolubilized with 150 μl of 99.8% ethanol per well, and absorbance was measured at 570 nm. Sterile medium served as a negative control, and the reported values are blank-corrected (OD570 of the blank was 0.1742 ± 0.006).
Axenic Brine Shrimp Hatching
Axenic Artemia were obtained following decapsulation and hatching (Han et al., 2019). Briefly, 0.2 mg of Artemia cysts originating from the Great Salt Lake, Utah, United States (EG Type, batch 21452, INVE Aquaculture, Dendermonde, Belgium) were hydrated in 18 ml of distilled water for 1 h. Sterile cysts and nauplii were obtained via decapsulation using 660 μl NaOH (32%) and 10 ml NaOCl (50%). During the reaction, 0.22 μm filtered aeration was provided. All manipulations were carried out under a laminar flow hood and all tools were autoclaved at 121°C for 20 min. The decapsulation was stopped after about 2 min by adding 14 ml Na2S2O3 at 10 g l–1. The aeration was then terminated and the decapsulated cysts were washed with filtered (0.2 μm) and autoclaved artificial seawater (FASW) containing 35 g l–1 of instant ocean synthetic sea salt (Aquarium Systems, Sarrebourg, France). The cysts were suspended in 50 ml falcon tubes containing 30 ml FASW and incubated for 28 h on a rotor at 4 rpm at 28°C with constant illumination (2000 lux). The emerged nauplii at the instar II stage (at which time they start ingesting bacteria) were collected.
Brine Shrimp Challenge Tests
The impacts of NaAs on the virulence of V. campbellii were determined in a standardized challenge test with gnotobiotic brine shrimp larvae. Unless mentioned otherwise, V. campbellii was incubated with or without NaAs at 0, 5, and 10 mg ml–1 concentrations for 6 h. Afterward, the cultures were washed with phosphate-buffered saline (pH 7.4) before inoculation into the brine shrimp rearing water at 107 CFU ml–1. A suspension of autoclaved LVS3 bacteria in filtered and autoclaved seawater was added as feed at the start of the challenge test at 107 cells ml–1. Brine shrimp cultures, to which only autoclaved LVS3 bacteria were added as feed, were used as controls. The survival of the larvae was counted 48 h after the addition of the pathogens. Each treatment was carried out in five replicates and each experiment was repeated once to verify the reproducibility. In each test, the sterility of the control treatments was checked at the end of the challenge by inoculating 1 ml of rearing water to 9 ml of Marine Broth and incubating the mixture for 2 days at 28°C.
RNA Isolation, cDNA Synthesis, and Reverse Transcription Quantitative PCR
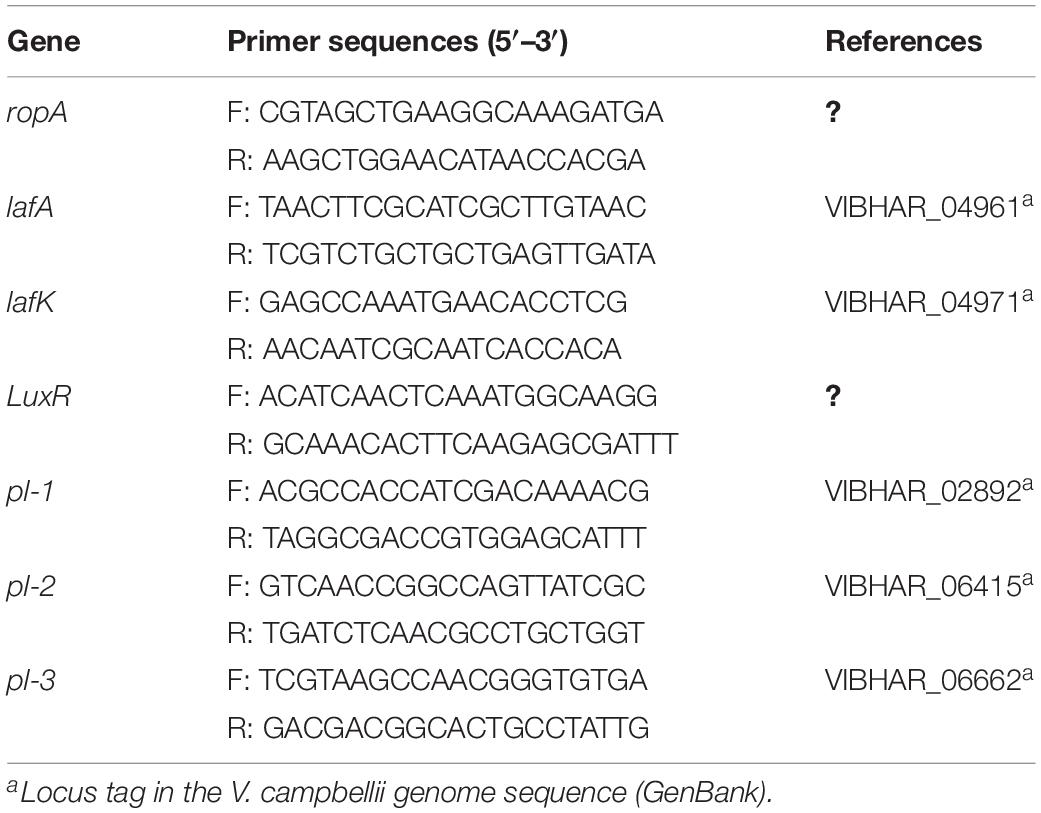
Vibrio campbellii strains were grown in triplicate in Marine Broth with or without 10 g ml–1 NaAs at 28°C for 24 h. Total RNA was extracted in 6 h (exponential phase) and 12 h (early stationary phase) from both groups, using RNeasy Plus Mini Kit (Qiagen, Germany) following the manufacturer’s manual. The genomic DNA was eliminated with a gDNA eliminator spin in the kit when isolating the RNA. The quality and quantity of RNA were confirmed by NanoDrop 2000 (Thermo Scientific, United States). Then an aliquot of total RNA was used to synthesize the first-strand cDNA using RevertAid H Minus First Strand cDNA Synthesis Kit (Thermo Scientific, United States) with an oligo(dT) primer. The reverse transcription quantitative PCR (RT-qPCR) assay was performed on Step One Plus Real-Time System (Applied Biosystems, United States) using Maxima SYBR Green/ROX qPCR Master Mix (2X) (Thermo Scientific, United States) in a total volume of 20 μl, containing 10 μl of 2X SYBR green master mix, 2 μl of forward and reverse primers (300 nM), and 8 μl of template cDNA. The thermal cycling consisted of an initial denaturation at 95°C for 10 min followed by 40 cycles of denaturation at 95 for 15 s and primer annealing and elongation at 60°C for 1 min. A dissociation curve analysis was performed to check for the amplification of untargeted fragments. Data acquisition was performed with the qbase plus software version 3.0 (Biogazelle, Belgium). Gene-specific primers for RT-qPCR analysis (designed by cross-exon strategy and using the software Primer Premier version 5.0) are shown in Table 1. The RNA polymerase A submit (ropA) was used as a reference gene (Defoirdt et al., 2007).
Statistical Analysis
Survival data were arcsine transformed to satisfy normality and homoscedasticity requirements as necessary. Data on survival, virulence factor, and bacterial growth were then subjected to one-way analysis of variances followed by Duncan’s multiple range tests using the statistical software Statistical Package for the Social Sciences version 22 to determine significant differences among treatments.
Results
NaAs Does Not Affect Bacterial Growth but Inhibits the QS System of V. campbellii
The growth of V. campbellii treated with 5 and 10 mg ml–1 NaAs (neutral pH form) was measured to verify whether indicated concentrations of this compound are bactericidal toward the strain. As shown in Figure 2A, up to 10 mg ml–1 NaAs did not significantly change the growth rate of V. campbellii as that of untreated cultures, and the early stationary stage was reached at approximately 12 h in both treated and untreated cultures. Meanwhile, NaAs was could significantly (P < 0.01) inhibit the production of bioluminescence in wild type V. campbellii at both concentrations tested (Figure 2B). To check whether the effect of NaAs on bioluminescence was due to interference with the QS, the impact of NaAs on the bioluminescence mutant strain JAF548 pAKlux1 (the bioluminescence of this strain is independent of the three-channel QS system) was measured (Defoirdt and Sorgeloos, 2012). NaAs has no effect on the bioluminescence of JAF548 pAKlux1 at the concentrations tested (Figure 2B). Consistent with this, the mRNA levels of QS master regulator LuxR were significantly decreased after NaAs treatment in V. campbellii (Table 2).
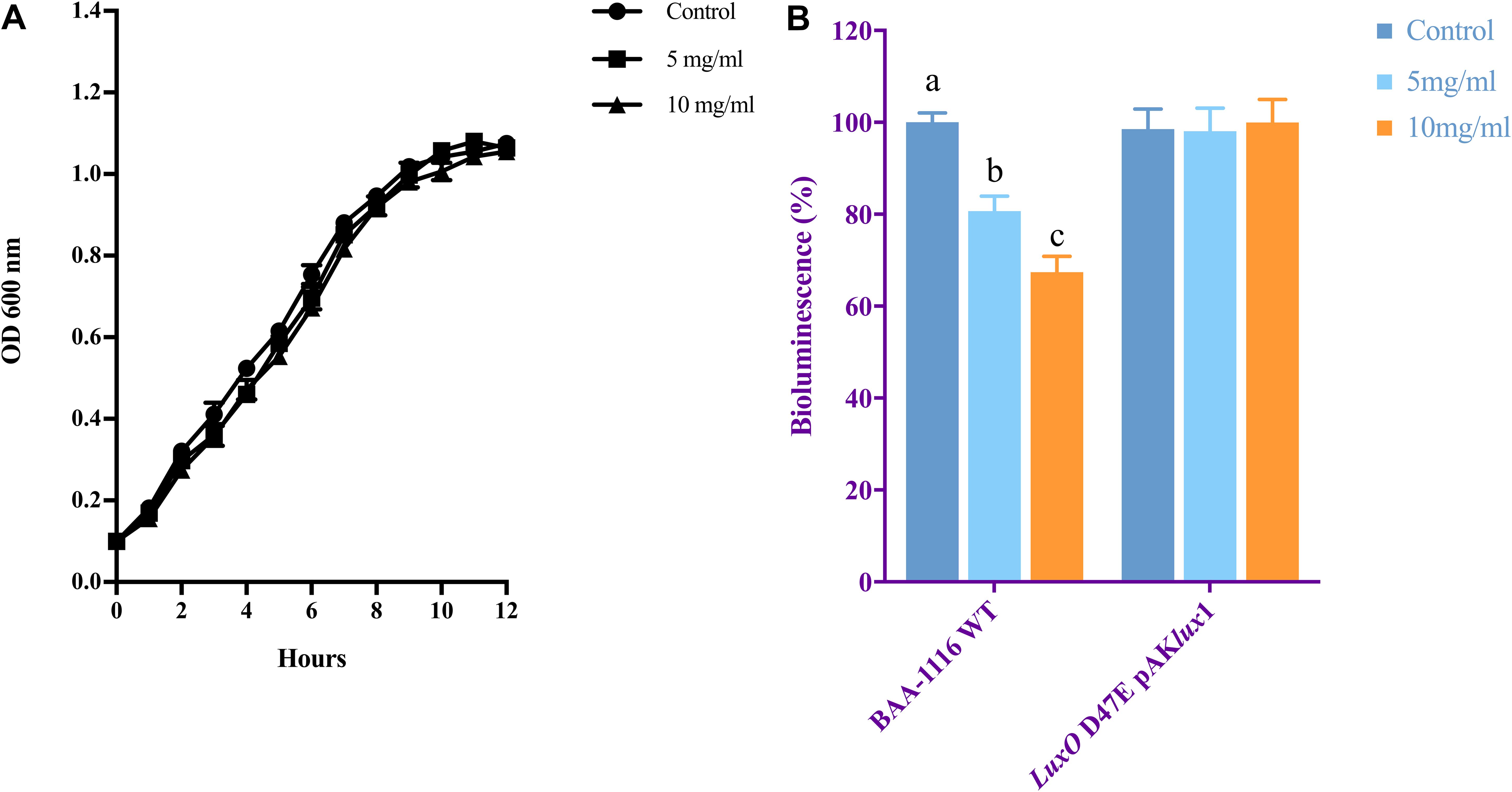
Figure 2. (A) Growth of V. campbellii treated with 5 and 10 mg ml–1 NaAs compared with untreated culture. The growth curves were determined for three independent cultures. (B) Impact of 5 and 10 mg m–1 NaAs on bioluminescence of V. campbellii wild type BAA-1116 and mutant LuxO D47E containing plasmid pAKlux1, in which bioluminescence is independent of quorum sensing. For each strain, bioluminescence in the control treatment was set at 100% and other treatments were normalized accordingly. The error bars represent the standard deviation of three replicates. Different letters indicate significant differences (One-way ANOVA with Duncan’s post hoc test, P < 0.01).
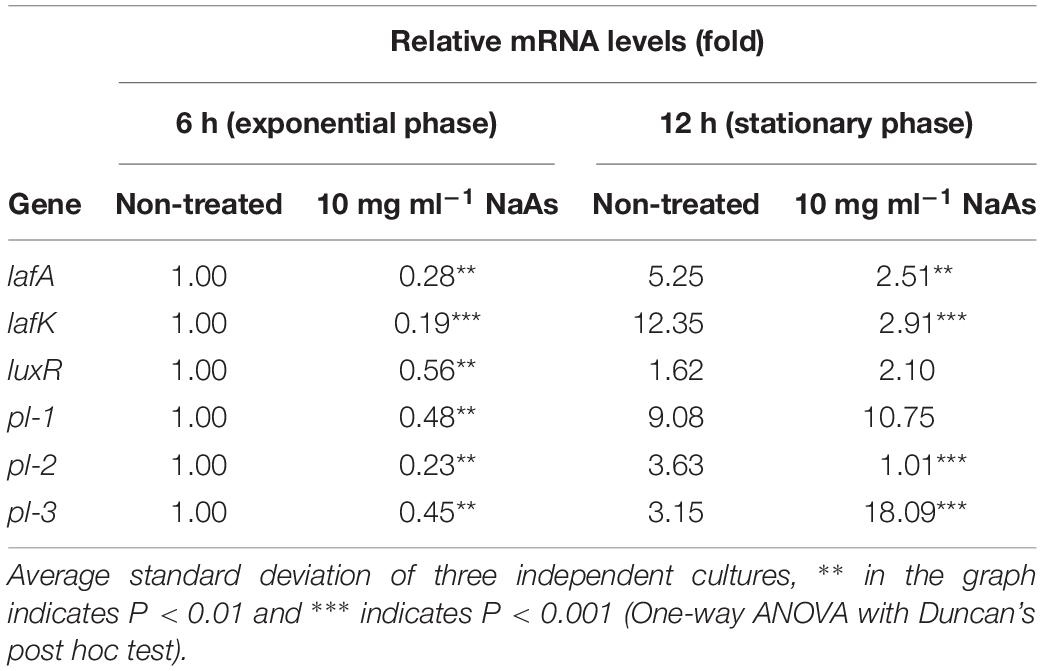
Table 2. Relative mRNA levels of lateral flagellar genes, the quorum sensing master regulator gene and phospholipase genes in Vibrio campbellii during incubation in Marine Broth in the absence and presence of 10 mg ml–1 NaAs..
NaAs Decreases the Swimming Motility and Production of Biofilm
The effect of NaAs on the swimming motility of V. campbellii was studied in the soft agar. NaAs was found to significantly decrease the swimming motility in a concentration-dependent manner, with a 3.4-fold decrease at 5 mg ml–1 and with a 7.2-fold decrease at 10 mg ml–1 (Figure 3). Furthermore, the effect of NaAs on the expression of selected two genes involved in flagellar motility in V. campbellii was investigated. The mRNA levels of the lateral flagellar regulator (lafK) and lateral flagellar flagellin (lafA) were both significantly (P < 0.001) decreased when comparing the NaAs-treated and untreated cells in both exponential phase and early stationary phase (Table 2). Moreover, as shown in Figure 3B, the production of the biofilm level was significantly (P < 0.01) affected when V. campbellii was treated with 10 mg ml–1 of NaAs.
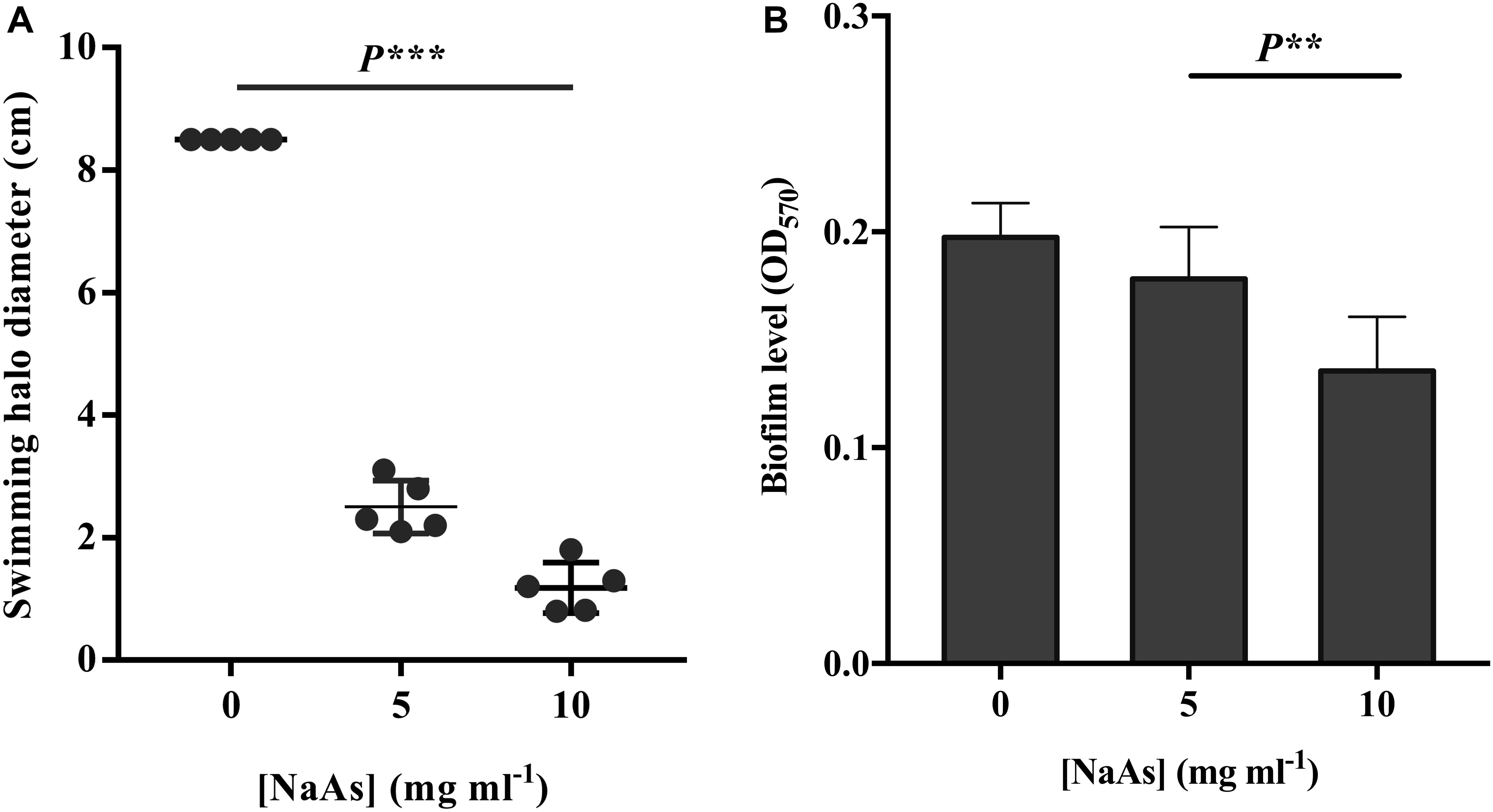
Figure 3. (A) Impact of NaAs on swimming motility of V. campbellii on soft agar plates. (B) Impact of NaAs on the biofilm levels on polystyrene 96-well plates. The error bar represents the standard deviation of five replicates for swimming motility and six independent experiments for biofilm. P∗∗ in the graph indicates P < 0.01 and P∗∗∗ indicates P < 0.001 (One-way ANOVA with Duncan’s post hoc test).
NaAs Decreases the Virulence of V. campbellii Toward Gnotobiotic Brine Shrimp Larvae
A standard challenge test with gnotobiotic brine shrimp larvae was performed to check the impact of NaAs on the virulence of V. campbellii toward the brine shrimp. To eliminate the effect of NaAs on the larvae, V. campbellii was incubated in the presence of NaAs at 5 and 10 mg ml–1, respectively, after which the cultures were washed to remove NaAs before adding in the rearing water. A significantly (P < 0.01) increased survival of brine shrimp challenged with NaAs-pretreated V. campbellii was observed when compared with untreated strain, indicating that the virulence of V. campbellii was significantly decreased after NaAs pretreatment at both the indicated doses (Table 3).

Table 3. Survival of gnotobiotic brine shrimp larvae after 2 days of challenge with V. campbellii BAA-1116 (average ± standard deviation of four replicates).
NaAs Decreases the Production of Virulence Factors in V. campbellii
To determine the effect of NaAs on the production of virulence factors in V. campbellii, an in vitro study was carried out by incubating V. campbellii with the two doses of NaAs by plating the culture on specific agar as described above. As shown in Figure 4, a significant decrease in the activity of lipase, caseinase, hemolysin, and phospholipase were recorded due to exposure of V. campbellii to NaAs (5 and 10 mg ml–1) compared to the untreated control. The expression of three phospholipase genes was checked after V. campbellii treated with 10 mg ml–1 NaAs in both the exponential phase and early stationary phase. As shown in Table 2, the expression of three phospholipase genes was significantly (P < 0.01) decreased in the exponential phase compared to the untreated control. In the early stationary phase, the pl-2 gene had the same tendency, but the expression of the pl-3 gene was significantly (P < 0.01) increased.
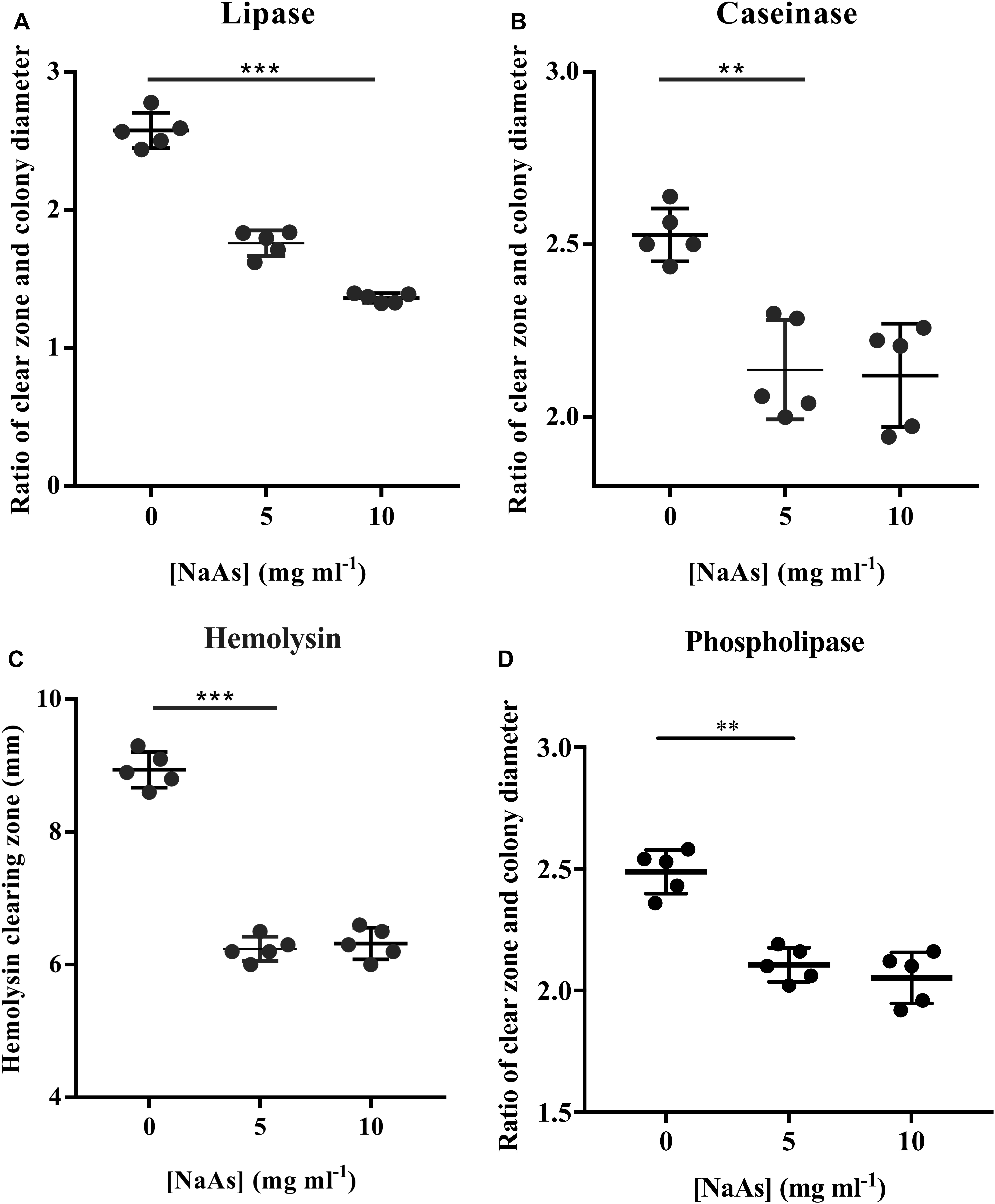
Figure 4. Activities of (A) Lipase, (B) Caseinase, (C) Hemolysin, and (D) Phospholipase of V. campbellii incubated with NaAs. The activities of the virulent determinants were expressed as the ratio of clear zone (mm) and colony diameter (mm). Results are expressed as mean ± standard error of three replicates. P∗∗ in the graph indicates P < 0.01 and P∗∗∗ indicates P < 0.001 (One-way ANOVA with Duncan’s post hoc test).
Discussion
In this study, an in vivo model system using gnotobiotically grown brine shrimp was developed to determine the impact of NaAs on the QS-regulated virulence factors in an important aquaculture pathogen V. campbellii. Working in the absence of other bacteria is vital for this type of experiment because it might cause bias. As far as we know, it is the first time that this type of experiment was carried out in a well-defined gnotobiotic environment. The results from the present study demonstrated that NaAs could decrease the virulence of V. campbellii toward the brine shrimp host, as manifested by higher survival of brine shrimp larvae exposed to the pathogen pre-treated with NaAs at either 5 or 10 mg ml–1 concentration (Table 3). Our results are in agreement with the findings of our previous study showing that the 20 mg ml–1 of halogenated furanones, (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone, could increase the survival of brine shrimp larvae upon challenge with different pathogens including V. campbellii and Vibrio parahaemolyticus (Defoirdt et al., 2006). As mentioned above, many halogenated furanones are highly toxic to higher organisms (Natrah et al., 2012), and this restricts their application to control vibriosis (the diseases caused by V. campbellii and related vibrio pathogens) in aquaculture animals. In contrast, NaAs is relatively less toxic and well-tolerated in many farmed animals. Additionally, it is cheap, easily available, and has long been used as additives in aquaculture feeds and had proven to cause antioxidant and immune-stimulating effects in different aquaculture animals (Thomas and Holt, 1978; Waagbo et al., 1993).
However, our previous study also demonstrated that high concentration continuous exposure of NaAs (above 1 mg ml–1) is also toxic to brine shrimp larvae after 48 h (Han et al., 2019). Searching the optimal concentration of NaAs that decrease the virulence of the pathogen but has no adverse effect on the host would be interesting and could be applied in practice. For this purpose, the effect of 1 mg ml–1 NaAs (critical concentration of NaAs for toxicity in the brine shrimp culture system) on the bioluminescence of V. campbellii BAA-1116 wild type strain and mutant strain JAF548 pAKlux1 was measured (Supplementary Figure S1). The study showed that the non-toxic dose of NaAs could also lead to the quorum quenching effects, although the inhibition effect showed the dose-dependent manner.
Contrary to the classical antibiotics, disrupting the QS pathways of bacteria inhibits virulence rather than bacterial growth, minimizing the possibility of imposing selective pressure on the pathogen for generating resistance within the bacteria. In our study, treatment of V. campbellii with 5 or 10 mg ml–1 of NaAs showed no significant change in the growth rate when compared with untreated control. Furthermore, the density of V. campbellii in the brine shrimp rearing water after 1 and 2 days of the challenge was measured (Supplementary Figure S2) to verify whether the higher survival of brine shrimp larvae that were challenged with NaAs-pretreated V. campbellii when compared with untreated V. campbellii was due to the bactericidal effect of the compound. The results showed that NaAs did not exhibit bactericidal ability within the doses tested, and brine shrimp larvae in all the experimental groups were exposed to the same density of V. campbellii. The results demonstrated that the observed increase in the survival of Artemia was not due to the loss of bacterial cells. However, high concentrations of vitamin C was demonstrated to induce lethal oxidative stress via Fenton’s reaction which could sterilize the cultures of Mycobacterium tuberculosis (Ascorbic Acid, 4 nM) (Vilchèze et al., 2013) and Bacillus subtilis (Sodium ascorbate, 30 nM) (Pandit et al., 2017).
In V. campbellii, the density-dependent expression of the luciferase structural operon luxCDABE is tightly controlled by the QS system (Miller and Bassler, 2001). In this study, using the mutant strain JAF548 pAKlux1 as the control, the AQSI value was proposed to evaluate the specific QS-disrupting activity. The AQSI value obtained for NaAs were 20 and 33 at 5, and 10 mg ml–1, respectively (Figure 2B) which indicated that NaAs at these doses could cause significant inhibition of the QS-regulated bioluminescence, which is in agreement with the most specific quorum sensing inhibitor revealed previously (e.g., thiophenone TF203, AQSI = 17 at 0.25 μM) (Yang et al., 2015). Consistent with this, the mRNA levels of quorum sensing master regulator LuxR were significantly decreased. Our results revealed that the downregulation of the LuxR and the QS-regulated phenotype (bioluminescence) has a positive correlation with the decreased virulence of V. campbellii, suggesting the QS-inhibitory activity largely determines the protective effect of NaAs toward the brine shrimp.
To determine the underlying virulence mechanisms in V. campbellii, several important virulence factors, including extracellular products (ECPs), swimming motility, and biofilm formation, were examined. The bacterial motility is an important virulence factor in many pathogens. It is essential for the pathogen during the initial phases of infection of higher organisms, including attachments, swarming, and biofilm formation (McCarter, 2001). The previous study showed that V. campbellii possesses dual flagellar systems, a single polar flagellum for swimming in the liquid environment while numerous lateral flagella enable the bacteria to swarm over a surface or move in other environments (McCarter, 2004). Work in our lab proved that QS positively regulates flagellar motility in V. campbellii, and the QS master regulator LuxR deletion mutant KM669 showed significantly lower motility. Moreover, the study revealed that the addition of the motility inhibitor phenamil (50 μM) could completely inhibit the swimming motility of V. campbellii and significantly decreased the virulence of this bacteria toward gnotobiotic brine shrimp (Yang and Defoirdt, 2015). Extending these findings, our study showed that the swimming motility of V. campbellii treated with NaAs was significantly impaired relative to untreated bacteria and decreased motility by NaAs has also been reported in other important pathogenic species such as P. aeruginosa (Abbas et al., 2012; El-Mowafy et al., 2014). The mRNA levels of the lateral flagellar regulator (lafK) and lateral flagellar flagellin (lafA) were both significantly decreased, compared with the NaAs treated and untreated cells together with the observation the decrease of the biofilm production level. Flagella are required for biofilm development at an early stage, and mutants lacking the flagellum have a decreased biofilm formation ability (Watnick and Kolter, 1999). However, the expression of the flagellar filament (flaA) would decrease when the bacteria cell in a mature biofilm (Moorthy and Watnick, 2004). The regulation of motility during biofilm formation in Vibrio species is rather complex and needs further investigation (Guttenplan and Kearns, 2013; Rossi et al., 2018).
The expression of the QS master regulator LuxR also significantly decreased and the previous study has also demonstrated that QS-deficient strain P. aeruginosa has less swimming motility and form thin and disperse biofilms (Heydorn et al., 2002). A recent study also revealed that a low concentration of vitamin C could inhibit Bacillus subtilis biofilm formation by deduction of extracellular polymeric substances (EPS), which is the most abundant component of the biofilm matrix and protect bacterial biofilms from hostile environmental situations (Pandit et al., 2017). Another possible explanation is that Vitamin C could inhibit the synthesis of (p)ppGpp, which is regarded as the key molecules in the stress response pathway and targeting (p)ppGpp can also inhibit biofilm production (Syal et al., 2017). Our data indicated that QS positively regulates flagellar motility and biofilm production, and NaAs can decrease the process of the colonization of the V. campbellii community by interference with the QS signaling pathways.
The production of extracellular virulence products (ECPs) by V. campbellii has been identified as one of the factors responsible for its pathogenesis, and its production is coordinately regulated by QS systems (Austin and Zhang, 2006). The increment of these virulence products may contribute to inflicting host tissue damages, allowing the pathogens to obtain nutrients and to spread through the tissues (Niu et al., 2014). In the present study, our data showed a significant reduction by NaAs of QS-related virulence factors such as lipase, caseinase, hemolysin, and phospholipase (Figure 4). Similarly, the NaAs inhibit the total protease, hemolysin, and elastase activities in P. aeruginosa (El-Mowafy et al., 2014). Furthermore, the expression of three phospholipase genes was checked after treatment with NaAs. The results showed that three phospholipase genes significantly decreased in the exponential phase compared to the untreated control.
Conclusion
The present study addressed that NaAs could function as a quorum sensing inhibitor leading to decreased virulence of V. campbellii without affecting its growth. Moreover, NaAs was found to decrease the QS-regulated bioluminescence, suggesting the protective effect of NaAs toward the brine shrimp was due to its QS-inhibitory activity. Furthermore, NaAs can inhibit the virulence factors production, swimming motility, and biofilm formation in V. campbellii. These results may provide a new anti-virulence strategy of this compound for the future treatment of Vibrio infections in aquaculture.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
PB and BH designed the experiments. BH and XZ performed the experiments and analyzed the data. BH wrote this manuscript. KB, BH, and PB revised the manuscript. All authors approved the final manuscript.
Funding
The authors acknowledge financial support from the China Scholarship Council and the Belgian Science Policy Office (BELSPO) project entitled AquaStress, project number: IUAPVII/64/Sorgeloos.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Professor Bonnie Bassler for kindly providing us with the V. campbellii wild type and quorum sensing mutants.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.01054/full#supplementary-material
Footnotes
- ^ It should be noted here, some works of literature cited in this paper regarding the study of Vibrio harveyi BAA-1116 (BB120), especially for the quorum sensing study, need a change in species designation to Vibrio campbellii BAA-1116, according to the report from Lin et al. (2010).
References
Abbas, H. A., Serry, F. M., and El-Masry, E. M. (2012). Combating Pseudomonas aeruginosa biofilms by potential biofilm inhibitors. Asian J. Pharm. Sci. 2, 66–72.
Austin, B., Austin, D., Sutherland, R., Thompson, F., and Swings, J. (2005). Pathogenicity of vibrios to rainbow trout (Oncorhynchus mykiss, Walbaum) and Artemia nauplii. Environ. Microbiol. 7, 1488–1495. doi: 10.1111/j.1462-2920.2005.00847.x
Austin, B., and Zhang, X. H. (2006). Vibrio harveyi: a significant pathogen of marine vertebrates and invertebrates. Lett. Appl. Microbiol. 43, 119–124. doi: 10.1111/j.1472-765x.2006.01989.x
Bassler, B. L., Wright, M., Showalter, R. E., and Silverman, M. R. (1993). Intercellular signaling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol. Microbiol. 9, 773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x
Cao, J. G., and Meighen, E. A. (1989). Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi. J. Biol. Chem. 264, 21670–21676.
Chen, X., Schauder, S., Potier, N., Van Dorsselaer, A., Pelczer, I., Bassler, B. L., et al. (2002). Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415, 545–549. doi: 10.1038/415545a
Darshanee Ruwandeepika, H. A., Sanjeewa Prasad, U., Jayaweera, T., Paban Bhowmick, P., Karunasagar, I., Bossier, P., et al. (2012). Pathogenesis, virulence factors and virulence regulation of vibrios belonging to the Harveyi clade. Rev. Aquacult. 4, 59–74. doi: 10.1111/j.1753-5131.2012.01061.x
Defoirdt, T., Boon, N., Sorgeloos, P., Verstraete, W., and Bossier, P. (2008). Quorum sensing and quorum quenching in Vibrio harveyi: lessons learned from in vivo work. ISME J. 2, 19–26. doi: 10.1038/ismej.2007.92
Defoirdt, T., Crab, R., Wood, T. K., Sorgeloos, P., Verstraete, W., and Bossier, P. (2006). Quorum sensing-disrupting brominated furanones protect the gnotobiotic brine shrimp Artemia franciscana from pathogenic Vibrio campbellii, Vibrio campbellii, and Vibrio parahaemolyticus isolates. Appl. Environ. Microbiol. 72, 6419–6423. doi: 10.1128/aem.00753-06
Defoirdt, T., Miyamoto, C. M., Wood, T. K., Meighen, E. A., Sorgeloos, P., Verstraete, W., et al. (2007). The natural furanone (5Z)-4-bromo-5-(bromomethylene)-3-butyl-2(5H)-furanone disrupts quorum sensing-regulated gene expression in Vibrio harveyi by decreasing the DNA-binding activity of the transcriptional regulator protein luxR. Environ. Microbiol. 9, 2486–2495. doi: 10.1111/j.1462-2920.2007.01367.x
Defoirdt, T., and Sorgeloos, P. (2012). Monitoring of Vibrio harveyi quorum sensing activity in real time during infection of brine shrimp larvae. ISME J. 6, 2314–2319. doi: 10.1038/ismej.2012.58
Donnenberg, M. S. (2000). Pathogenic strategies of enteric bacteria. Nature 406, 768–774. doi: 10.1038/35021212
El-Mowafy, S., Shaaban, M., and Abd El Galil, K. (2014). Sodium ascorbate as a quorum sensing inhibitor of Pseudomonas aeruginosa. J. Appl. Microbiol. 117, 1388–1399. doi: 10.1111/jam.12631
Freeman, J. A., and Bassler, B. L. (1999). A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol. Microbiol. 31, 665–677. doi: 10.1046/j.1365-2958.1999.01208.x
Guttenplan, S. B., and Kearns, D. B. (2013). Regulation of flagellar motility during biofilm formation. FEMS Microbiol. Rev. 37, 849–871. doi: 10.1111/1574-6976.12018
Han, B., Kaur, V. I., Baruah, K., Nguyen, V. D., and Bossier, P. (2019). High doses of sodium ascorbate act as a prooxidant and protect gnotobiotic brine shrimp larvae (Artemia franciscana) against Vibrio campbellii infection coinciding with heat shock protein 70 activation. Dev. Comp. Immunol. 92, 69–76. doi: 10.1016/j.dci.2018.11.007
Heydorn, A., Ersboll, B., Kato, J., Hentzer, M., Parsek, M. R., Tolker-Nielsen, T., et al. (2002). Statistical analysis of Pseudomonas aeruginosa biofilm development: impact of mutations in genes involved in twitching motility, cell-to-cell signaling, and stationary-phase sigma factor expression. Appl. Environ. Microbiol. 68, 2008–2017. doi: 10.1128/aem.68.4.2008-2017.2002
Higgins, D. A., Pomianek, M. E., Kraml, C. M., Taylor, R. K., Semmelhack, M. F., and Bassler, B. L. (2007). The major Vibrio cholerae autoinducer and its role in virulence factor production. Nature 450, 883–886. doi: 10.1038/nature06284
Lin, B., Wang, Z., Malanoski, A. P., O’grady, E. A., Wimpee, C. F., Vuddhakul, V., et al. (2010). Comparative genomic analyses identify the Vibrio harveyi genome sequenced strains BAA-1116 and HY01 as Vibrio campbellii. Environ. Microbiol. Rep. 2, 81–89. doi: 10.1111/j.1758-2229.2009.00100.x
Manefield, M., Rasmussen, T. B., Henzter, M., Andersen, J. B., Steinberg, P., Kjelleberg, S., et al. (2002). Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 148, 1119–1127. doi: 10.1099/00221287-148-4-1119
Manefield, M., Welch, M., Givskov, M., Salmond, G. P., and Kjelleberg, S. (2001). Halogenated furanones from the red alga, Delisea pulchra, inhibit carbapenem antibiotic synthesis and exoenzyme virulence factor production in the phytopathogen Erwinia carotovora. FEMS Microbiol. Lett. 205, 131–138. doi: 10.1111/j.1574-6968.2001.tb10936.x
McCarter, L. L. (2001). Polar flagellar motility of the Vibrionaceae. Microbiol. Mol. Biol. Rev. 65, 445–462. doi: 10.1128/mmbr.65.3.445-462.2001
McCarter, L. L. (2004). Dual flagellar systems enable motility under different circumstances. J. Mol. Microbiol. Biotechnol. 7, 18–29. doi: 10.1159/000077866
Miller, M. B., and Bassler, B. L. (2001). Quorum sensing in bacteria. Annu. Rev. Microbiol. 55, 165–199.
Moorthy, S., and Watnick, P. I. (2004). Genetic evidence that the Vibrio cholerae monolayer is a distinct stage in biofilm development. Mol. Microbiol. 52, 573–587. doi: 10.1111/j.1365-2958.2004.04000.x
Natrah, F. M., Alam, M. I., Pawar, S., Harzevili, A. S., Nevejan, N., Boon, N., et al. (2012). The impact of quorum sensing on the virulence of Aeromonas hydrophila and Aeromonas salmonicida towards burbot (Lota lota L.) larvae. Vet. Microbiol. 159, 77–82. doi: 10.1016/j.vetmic.2012.03.014
Niu, Y. F., Norouzitallab, P., Baruah, K., Dong, S. L., and Bossier, P. (2014). A plant-based heat shock protein inducing compound modulates host-pathogen interactions between Artemia franciscana and Vibrio campbellii. Aquaculture 430, 120–127. doi: 10.1016/j.aquaculture.2014.04.001
Novak, J. S., and Fratamico, P. M. (2004). Evaluation of ascorbic acid as a quorum-sensing analogue to control growth, sporulation, and enterotoxin production in Clostridium perfringens. J. Food Sci. 69, FMS72–FMS78.
Pande, G. S., Natrah, F. M., Sorgeloos, P., Bossier, P., and Defoirdt, T. (2013). The Vibrio campbellii quorum sensing signals have a different impact on virulence of the bacterium towards different crustacean hosts. Vet. Microbiol. 167, 540–545. doi: 10.1016/j.vetmic.2013.08.021
Pandit, S., Ravikumar, V., Abdel-Haleem, A. M., Derouiche, A., Mokkapati, V., Sihlbom, C., et al. (2017). Low concentrations of Vitamin C reduce the synthesis of extracellular polymers and destabilize bacterial biofilms. Front. Microbiol. 8:2599. doi: 10.3389/fmicb.2017.02599
Rasko, D. A., and Sperandio, V. (2010). Anti-virulence strategies to combat bacteria-mediated disease. Nat. Rev. Drug Discov. 9, 117–128. doi: 10.1038/nrd3013
Rasmussen, T. B., Manefield, M., Andersen, J. B., Eberl, L., Anthoni, U., Christophersen, C., et al. (2000). How Delisea pulchra furanones affect quorum sensing and swarming motility in Serratia liquefaciens MG1. Microbiology 146, 3237–3244. doi: 10.1099/00221287-146-12-3237
Rossi, E., Paroni, M., and Landini, P. (2018). Biofilm and motility in response to environmental and host-related signals in Gram negative opportunistic pathogens. J. Appl. Microbiol. 125, 1587–1602. doi: 10.1111/jam.14089
Rui, H., Liu, Q., Ma, Y., Wang, Q., and Zhang, Y. (2008). Roles of LuxR in regulating extracellular alkaline serine protease A, extracellular polysaccharide and motility of Vibrio alginolyticus. FEMS Microbiol. Lett. 285, 155–162. doi: 10.1111/j.1574-6968.2008.01185.x
Rutherford, S. T., Van Kessel, J. C., Shao, Y., and Bassler, B. L. (2011). AphA and LuxR/HapR reciprocally control quorum sensing in vibrios. Genes Dev. 25, 397–408. doi: 10.1101/gad.2015011
Schauder, S., and Bassler, B. L. (2001). The languages of bacteria. Genes Dev. 15, 1468–1480. doi: 10.1101/gad.899601
Smith, K. M., Bu, Y., and Suga, H. (2003). Library screening for synthetic agonists and antagonists of a Pseudomonas aeruginosa autoinducer. Chem. Biol. 10, 563–571. doi: 10.1016/s1074-5521(03)00107-8
Stepanovic, S., Vukovic, D., Hola, V., Di Bonaventura, G., Djukic, S., Cirkovic, I., et al. (2007). Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by Staphylococci. Apmis 115, 891–899. doi: 10.1111/j.1600-0463.2007.apm_630.x
Syal, K., Bhardwaj, N., and Chatterji, D. (2017). Vitamin C targets (p)ppGpp synthesis leading to stalling of long-term survival and biofilm formation in Mycobacterium smegmatis. FEMS Microbiol. Lett. 364:fnw282. doi: 10.1093/femsle/fnw282
Thomas, W. R., and Holt, P. G. (1978). Vitamin C and immunity: an assessment of the evidence. Clin. Exp. Immunol. 32, 370–379.
Tu, K. C., and Bassler, B. L. (2007). Multiple small RNAs act additively to integrate sensory information and control quorum sensing in Vibrio harveyi. Genes Dev. 21, 221–233. doi: 10.1101/gad.1502407
Vilchèze, C., Hartman, T., Weinrick, B., and Jacobs, W. R. Jr. (2013). Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat. Commun. 4:1881.
Waagbo, R., Glette, J., Raa-Nilsen, E., and Sandnes, K. (1993). Dietary vitamin C, immunity and disease resistance in Atlantic salmon (Salmo salar). Fish Physiol. Biochem. 12, 61–73. doi: 10.1007/bf00004323
Wang, T., Wang, D., Lin, Z., An, Q., Yin, C., and Huang, Q. (2016). Prediction of mixture toxicity from the hormesis of a single chemical: a case study of combinations of antibiotics and quorum-sensing inhibitors with gram-negative bacteria. Chemosphere 150, 159–167. doi: 10.1016/j.chemosphere.2016.02.018
Watnick, P. I., and Kolter, R. (1999). Steps in the development of a Vibrio cholerae El Tor biofilm. Mol. Microbiol. 34, 586–595. doi: 10.1046/j.1365-2958.1999.01624.x
Winzer, K., Hardie, K. R., Burgess, N., Doherty, N., Kirke, D., Holden, M. T., et al. (2002). LuxS: its role in central metabolism and the in vitro synthesis of 4-hydroxy-5-methyl-3(2H)-furanone. Microbiology 148, 909–922. doi: 10.1099/00221287-148-4-909
Yang, Q., and Defoirdt, T. (2015). Quorum sensing positively regulates flagellar motility in pathogenic Vibrio harveyi. Environ. Microbiol. 17, 960–968. doi: 10.1111/1462-2920.12420
Yang, Q., Pande, G. S. J., Wang, Z., Lin, B., Rubin, R. A., Vora, G. J., et al. (2017). Indole signalling and (micro)algal auxins decrease the virulence of Vibrio campbellii, a major pathogen of aquatic organisms. Environ. Microbiol. 19, 1987–2004. doi: 10.1111/1462-2920.13714
Yang, Q., Scheie, A. A., Benneche, T., and Defoirdt, T. (2015). Specific quorum sensing-disrupting activity (AQSI) of thiophenones and their therapeutic potential. Sci. Rep. 5:18033.
Keywords: sodium ascorbate, quorum sensing inhibitor, virulence, Vibrio campbellii, Artemia
Citation: Han B, Zheng X, Baruah K and Bossier P (2020) Sodium Ascorbate as a Quorum-Sensing Inhibitor Leads to Decreased Virulence in Vibrio campbellii. Front. Microbiol. 11:1054. doi: 10.3389/fmicb.2020.01054
Received: 19 December 2019; Accepted: 28 April 2020;
Published: 05 June 2020.
Edited by:
Daniela Ceccarelli, European Commission, BelgiumCopyright © 2020 Han, Zheng, Baruah and Bossier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biao Han, Ymlhby5oYW5AdWdlbnQuYmU=; Ymlhby5oYW5Ac2NudS5lZHUuY24=
 Biao Han
Biao Han Xiaoting Zheng
Xiaoting Zheng Kartik Baruah2
Kartik Baruah2 Peter Bossier
Peter Bossier