- 1State Key Laboratory for Infectious Disease Prevention and Control, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China
- 2Pathogenic Biology Institute, University of South China, Hengyang, China
- 3Department of Bioinformatics, College of Life Sciences, Fujian Agriculture and Forestry University, Fuzhou, China
- 4School of Laboratory Medicine and Life Sciences, Wenzhou Medical University, Wenzhou, China
With the increasing incidence of drug-resistant tuberculosis (DR-TB), determining a rapid and accurate drug susceptibility testing (DST) method to identify ethambutol (EMB) resistance in Mycobacterium tuberculosis has become essential for patient management in China. Herein, we evaluated the correlation between three phenotypic DST methods, namely, proportion method (PM), MGIT 960 system, and microplate alamar Blue assay (MABA), and DNA sequencing of embAB in 118 M. tuberculosis isolates from China. When the results of the phenotypic DST methods were compared with those of DNA sequencing, the overall agreement and kappa values of the PM, MGIT 960 system, and MABA were 81.4% and 0.61, 77.1% and 0.55, and 84.7% and 0.67, respectively. The agreement for EMB resistance between MABA and PM was significantly higher than that between the MGIT 960 system and PM (P = 0.02). Moreover, among the isolates with detectable embAB mutations, 97.2% (70/72 isolates) harbored mutations in embB. The analysis of embB mutations predicted EMB resistance with 81.3% sensitivity, 86.8% specificity, and 83.1% accuracy. Thus, MABA may be a better phenotypic DST method for detecting EMB resistance. DNA sequencing of embB may be useful for the early identification of EMB resistance and the consequent optimization of the treatment regimen.
Introduction
Despite a decreasing trend in the incidence of tuberculosis (TB) in recent years, TB remains a major public health threat in China; in 2018, the World Health Organization (WHO) estimated 900,000 new cases and 37,000 deaths (World Health Organization [WHO], 2018). According to the latest national baseline survey, 5.7% of new cases and 26.5% of previously treated cases are drug-resistant TB (DR-TB) (Zhao et al., 2012). DR-TB, particularly multidrug-resistant TB (MDR-TB), generally requires lengthy, more costly, and more toxic treatment regimens, which are associated with higher risks of treatment failure and disease relapse. Rapid and accurate detection of drug resistance is thus crucial for timely optimizing treatment regimens and reducing treatment failure to prevent the transmission of DR-TB and the development of MDR-TB.
Ethambutol (EMB) is an essential first-line anti-TB drug that is routinely recommended in combination with other drugs for treating TB and DR-TB. It also serves as a key drug in second-line regimens for MDR-TB when drug susceptibility testing (DST) results are available. EMB acts against bacterium through inhibiting membrane-associated arabinosyl transferases, encoded by the embCAB operon (embC, embA, and embB) (Telenti et al., 1997; Safi et al., 2013), which are necessary for biosynthesis of arabinogalactan in the cell wall. Previous studies showed that the majority of EMB-resistant isolates carry mutations within embB, primarily at codon 306 (embB306) (Zhang et al., 2013; Brossier et al., 2015), suggesting that the mutations might be used as promising diagnostic markers for the rapid detection of EMB resistance. Furthermore, certain mutations within the upstream region (UR) of embA are associated with EMB resistance (Cui et al., 2014; Brossier et al., 2015; Zhao et al., 2015). However, the presence of these mutations in embAB was also observed in EMB-susceptible isolates (Shi et al., 2011; Zhao et al., 2015; Li et al., 2016; Sun et al., 2018). Besides, there are no mutations within the particular genes responsible for EMB resistance in some EMB-resistant isolates, implying the involvement of other resistance mechanisms (Sharma and Bisht, 2017; Sharma et al., 2018). As per the literature, significant discordance exists between the phenotypic and genotypic methods for EMB susceptibility testing (Kim, 2005; Garrigo et al., 2007; Cheng et al., 2014; Zhang et al., 2014). Hence, it is necessary to evaluate concordance across phenotypic and genotypic methods for EMB resistance testing. In the present study, we compared the correlation between three phenotypic EMB susceptibility testing methods and DNA sequencing of embAB mutations in 118 Mycobacterium tuberculosis (M. tuberculosis) isolates from China.
Materials and Methods
M. tuberculosis Isolates and DST
In total, 118 M. tuberculosis isolates collected from 118 unrelated patients with pulmonary TB were contained in this study. DST of TB isolates to antitubercular drugs isoniazid (INH), rifampin (RIF), streptomycin (SM), and EMB was performed on Lowenstein–Jensen (L-J) proportion method (PM) according to the WHO guideline, with the critical concentrations being 0.2 μg/ml for INH, 40 μg/ml for RIF, 4.0 μg/ml for SM, and 2.0 μg/ml for EMB (World Health Organization [WHO], 2009). EMB susceptibility testing was also performed with all isolates using the MGIT 960 system and microplate alamarBlue assay (MABA). The MGIT 960 system was carried out according to the manufacturer’s instructions, with the critical concentration of 5.0 μg/ml (Garrigo et al., 2007). MABA was performed as previously described (Leonard et al., 2008). Briefly, the isolate in the log phase was adjusted with saline to McFarland standard 1 and diluted 1:25 in 7H9 broth containing 10% OADC (BD, Franklin Lakes, NJ, United States). Then, 100 μl bacterial suspension was used to inoculate each well of the 96-well plate. EMB was serially diluted twofold in 100 μl 7H9 broth, and the final concentrations were 32, 16, 8, 4, 2, 1, and 0.5 μg/ml. The final volume of each well was 200 μl, containing 100 μl of EMB solution and 100 μl of bacterial suspension. Furthermore, EMB-free (inoculum-only) controls were used to determine the addition time for alamarBlue. Plates were sealed and incubated at 37°C for 1 week. The indicator (25 μl alamarBlue mixed with 25 μl 10% Tween-80) was added to the EMB-containing group when the drug-free control showed a color change (blue to pink). The minimum inhibitory concentration (MIC) value was defined as the lowest EMB concentration that inhibited bacterial growth and prevented a color change. The breakpoint MIC for EMB was taken as 4 μg/ml (Leonard et al., 2008).
DNA Amplification and Sequencing
The hot regions conferring EMB resistance, including embA UR (145 bp upstream to codon 164) and embB (codons 159–518), of all isolates were amplified using the following primers: emb-S1, AACCTAGGAACGGTGACT, and emb-A1, CAACCTGTGGCTTCTTCT; emb-S2, AACT TCGTCGGGCTCAAG, and emb-A2, TAACGCAGGTTC TCGGTATA (Sun et al., 2018). The PCR program included an initial denaturation at 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 40 s, annealing at 58°C for 40 s, and extension at 72°C for 1 min and a final extension at 72°C for 5 min. All amplified products were then sequenced. The sequence data were aligned to the H37Rv reference genome (GenBank accession number NC_000962) using BioEdit 7.05.3.
Data Analysis
Data were analyzed with SPSS 16.0 statistical software (SPSS Inc., Chicago, IL, United States). Statistical analysis was performed using the chi-square test or Fisher’s exact test as appropriate, and probability levels < 0.05 were considered as statistically significant. Agreement between different testing results was evaluated with the kappa statistic. The kappa values of < 0.2, 0.2–0.4, 0.41–0.6, 0.61–0.8, and 0.81–1.0 indicated poor agreement, fair agreement, moderate agreement, good agreement, and excellent agreement, respectively (Altman, 1999).
Results
Based on DST by PM, 105, 99, 69, and 72 isolates were resistant to INH, RIF, SM, and EMB, respectively. Overall, 92 isolates were MDR, and 47 isolates were resistant to all four first-line drugs. Eight isolates were poly-drug resistant while five isolates were sensitive to all four drugs.
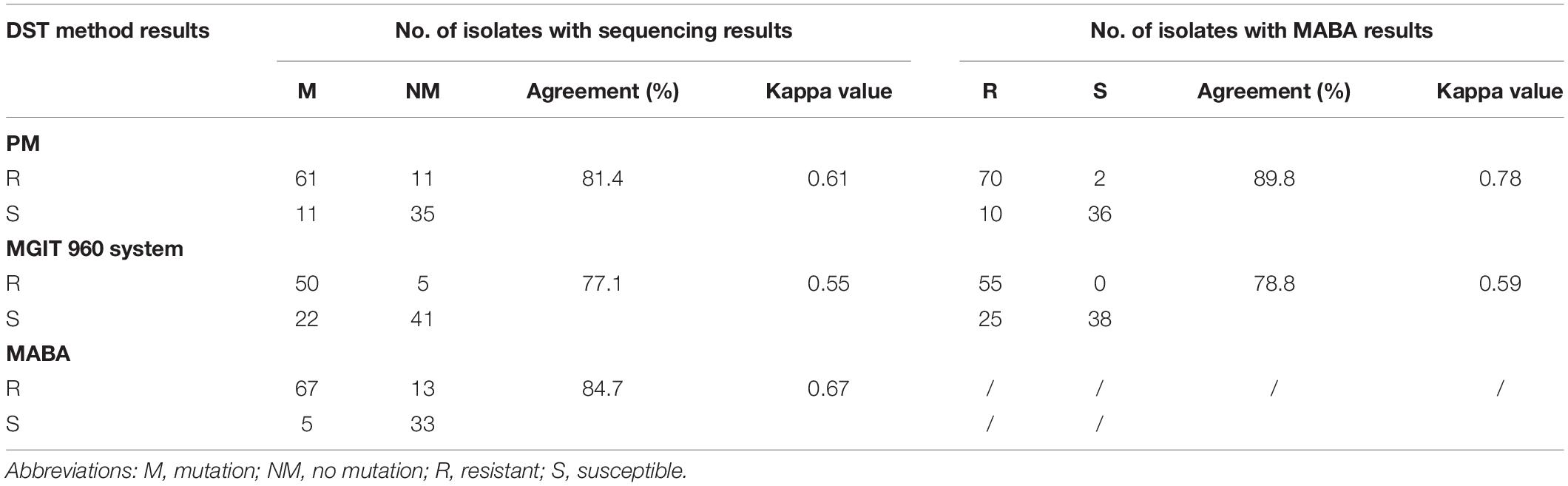
Of the 118 isolates, 72, 55, and 80 were identified to be EMB resistant by the PM, MGIT 960 system, and MABA, respectively (Table 1). For the 72 isolates determined as being EMB resistant by the PM, 61 (84.7%, 61/72 isolates) harbored at least one mutation within embAB. By contrast, 11 (23.9%) of 46 phenotypically susceptible isolates harbored embAB mutations. Further, 50 (90.1%) of the 55 isolates identified to be EMB resistant by the MGIT 960 system showed embAB mutations, with the mutations also occurring in 22 of 63 (34.9%) EMB-susceptible isolates. Finally, 67 (83.8%) of the 80 isolates determined to be EMB resistant by MABA carried embAB mutations; five (13.2%) of 38 EMB-susceptible isolates also showed embAB mutations. When results of the three phenotypic DST methods were compared with those of DNA sequencing results, the overall agreement and kappa values were 81.4% and 0.61, 77.1% and 0.55, and 84.7% and 0.67 for the PM, MGIT 960 system, and MABA, respectively. Table 1 summarizes the results of MABA in comparison with those of the PM and MGIT 960 system. The concordance rate for EMB resistance between MABA and PM was significantly higher than that between MGIT 960 and PM (P = 0.02), with overall agreement/kappa values of 89.8%/0.78 and 78.8%/0.59, respectively.
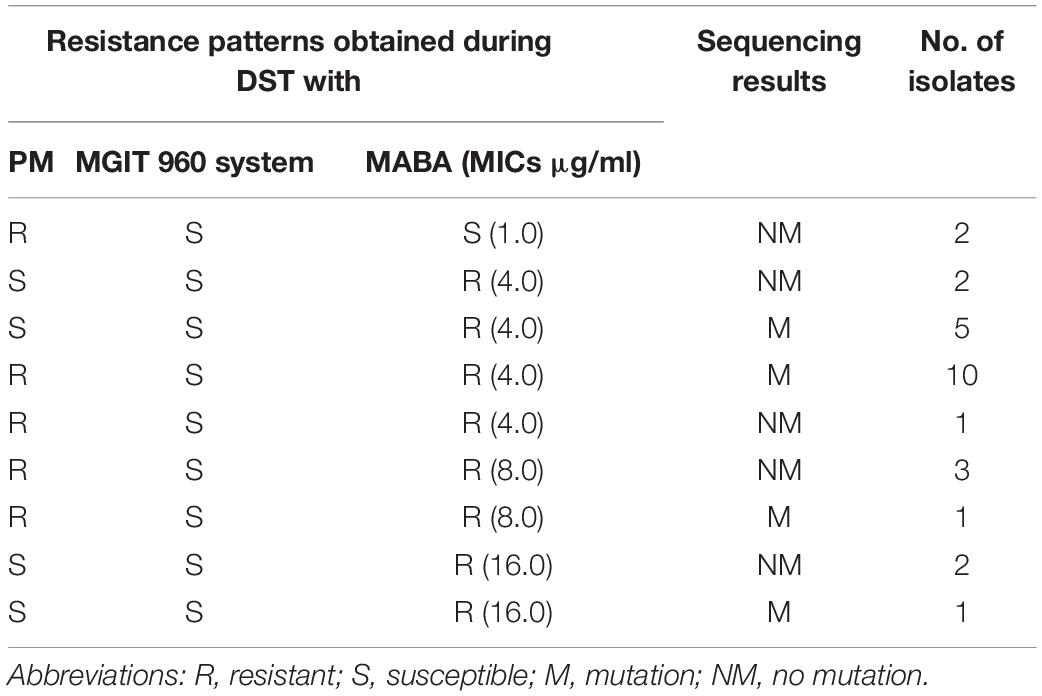
All isolates displaying discordant results between the MGIT 960 system/PM and MABA were further analyzed (Table 2). Twenty-five isolates were determined to be susceptible by the MGIT 960 system but resistant by MABA, including 17 isolates (68.0%, 17/25 isolates) with embAB mutations. Of them, 18 (72.0%, 18/25 isolates) had MICs of 4 μg/ml, four (16.0%, 4/25 isolates) had MICs of 8 μg/ml, and three (12.0%, 3/25 isolates) had MICs of 16 μg/ml. In addition, two isolates without embAB mutations were determined to be resistant by PM but susceptible by MABA, and they had MICs of 1 μg/ml. In contrast, 10 isolates, including six isolates (60.0%, 6/10 isolates) harboring embAB mutations, were identified to be susceptible by PM but resistant by MABA; of them, seven isolates (70.0%, 7/10 isolates) had MICs of 4 μg/ml, and three isolates (30.0%, 3/10 isolates) had MICs of 16 μg/ml.
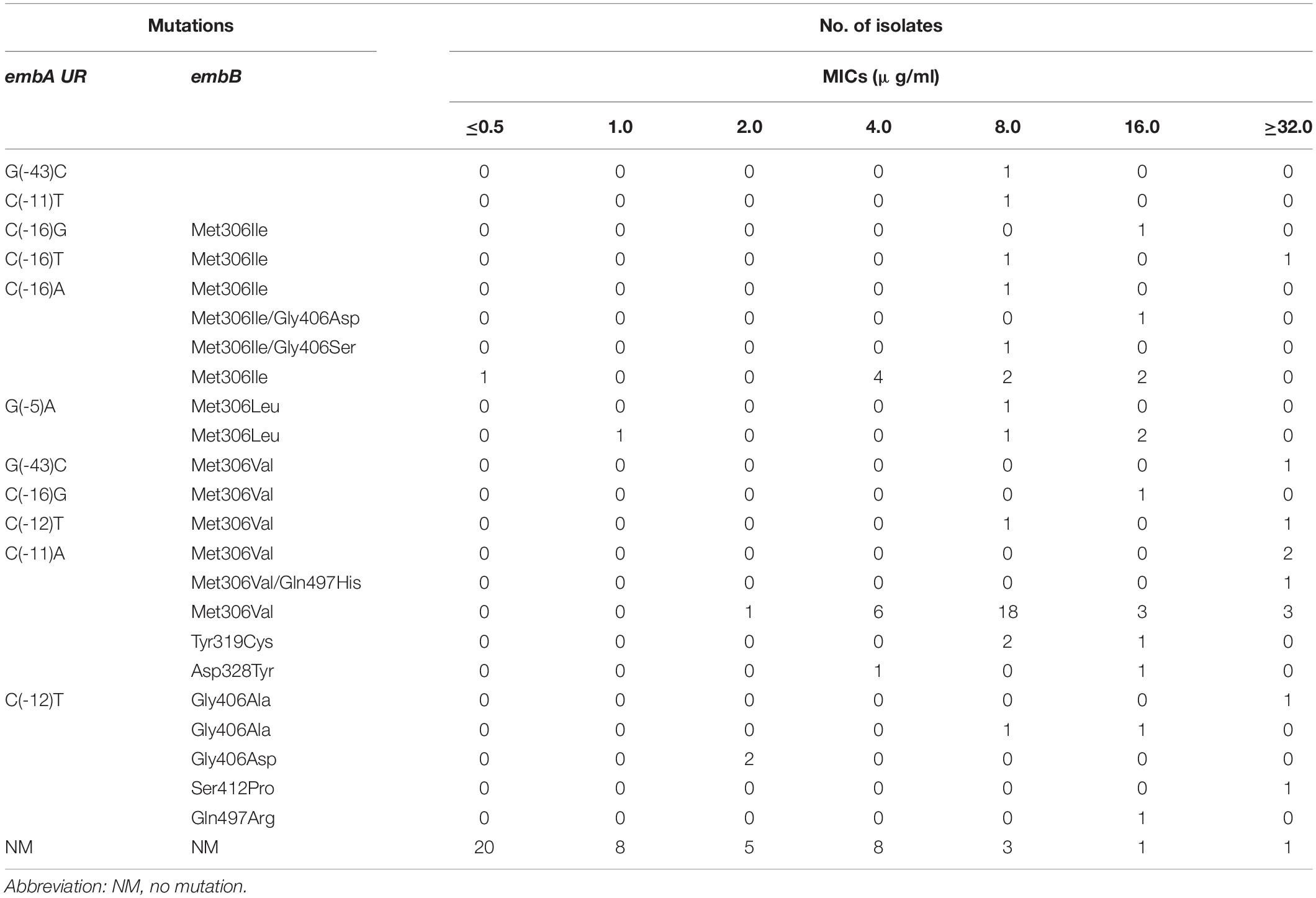
Of the isolates with detectable embAB mutations, 97.2% (70/72 isolates) had mutations within embB (Table 3). All embB mutations existed within codons 306–497. An amino acid replacement at codon 306 was the most frequent and occurred in 58 isolates (82.9%, 58/70 isolates). Met306 was noted to be replaced by Val (38 isolates), Ile (15 isolates), and Leu (five isolates). Of all the isolates with the embB306 mutation, three showed MICs of < 4 μg/ml (0.5, 1.0, and 2.0 μg/ml). The next most prevalent mutated codon was 406. Mutations at this codon were observed in seven isolates, two of which had MICs lower than 4 μg/ml (2 μg/ml). Other mutations were detected at codons 319 (three isolates), 328 (two isolates), 497 (two isolates), and 412 (one isolate). In addition, 14 EMB-resistant isolates displayed nucleotide changes within embA UR, including at −43 (two isolates), −16 (five isolates), −12 (three isolates), −11 (three isolates), and −5 bp sites (one isolate). Notably, most mutations within embA UR were accompanied by other mutations in embB. Only two isolates carried single embA UR mutations at −43 and −11 bp, with MICs of 8 μg/ml.
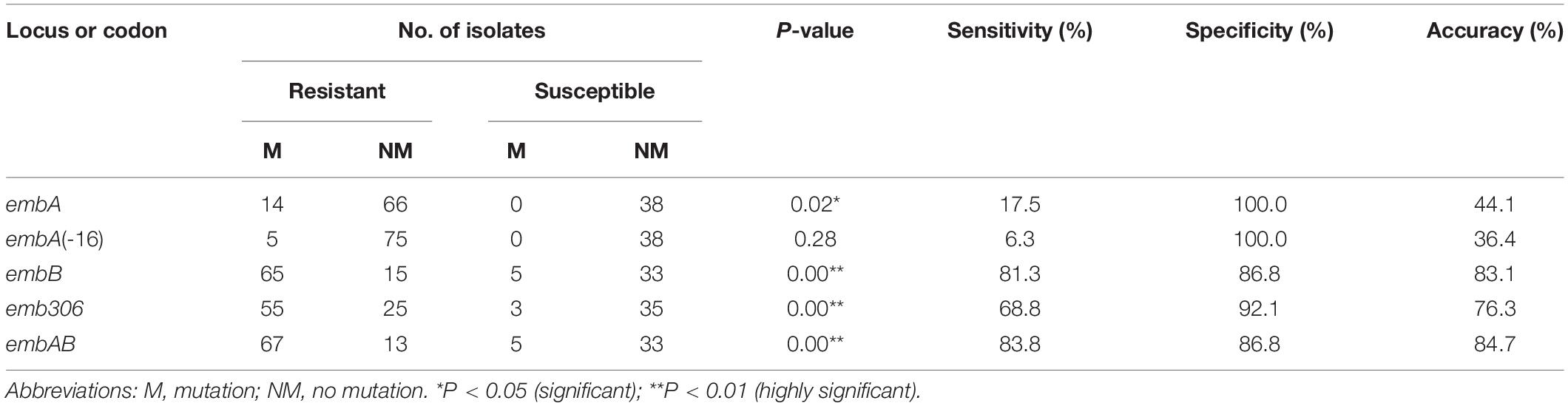
With reference to the results of MABA, the sensitivities, specificities, and accuracies for detection of the EMB resistance via DNA sequencing analysis of the different regions and codons in embAB are summarized in Table 4. Screening embB achieved satisfactory accuracy (83.1%). Inclusion of embA increased the assay accuracy to 84.7%. In addition, analyzing the embB306 mutations predicted EMB resistance with 68.8% sensitivity, 92.1% specificity, and 76.3% accuracy.
Discussion
In China, phenotypic DST based on L-J solid media and the liquid culture system MGIT 960 is routinely performed with all M. tuberculosis isolates. The presence of embAB mutations, particularly embB306, which are believed to be the major cause of EMB resistance in M. tuberculosis, has also been reported in EMB-susceptible isolates (Plinke et al., 2009; Zhao et al., 2015; Li et al., 2016; Sun et al., 2018). Because these routine phenotypic DST methods are notoriously problematic (Garrigo et al., 2007; Cheng et al., 2014; Zhang et al., 2014), presumably inaccurate results may be the major cause for the detection of “EMB-susceptible” isolates harboring embAB mutations (Plinke et al., 2009; Campbell et al., 2011). Identifying false susceptibility to EMB is of considerable importance for the successful treatment of DR-TB, especially MDR-TB, as treatment regimens for these patients should include any active first-line drug for improved outcomes. Thus, there is increasing emphasis on developing a reliable method to detect EMB resistance.
In this study, we found that in comparison with DNA sequencing, the overall agreement values for the PM, MGIT 960 system, and MABA were 81.4, 77.1, and 84.7%, respectively. The kappa values were 0.61 (good agreement), 0.55 (moderate agreement), and 0.67 (good agreement) for these three phenotypic DST methods. As evident, EMB resistance determined by MABA showed the best correlation with DNA sequencing results. We also examined concordance across all three phenotype DST methods by calculating the kappa coefficients. There was a good agreement for EMB between PM and MABA (κ, 0.78). Although PM is the gold standard recommended by the WHO and the current standard method used in China for EMB susceptibility testing of M. tuberculosis isolates, it requires at least 28 days before results can be obtained. As a rapid phenotypic DST based on liquid media, MABA can reduce the turnaround time to less than 14 days, which makes it a better choice for identifying EMB resistance.
Twenty-seven isolates exhibited discordant results between the PM/MGIT 960 system and MABA. Of them, 18 isolates (66.7%) had MICs of 4.0 μg/ml, which was the breakpoint MIC definition of EMB resistance. Furthermore, there were four (14.8%) isolates with MICs of 8.0 μg/ml, which is close to the breakpoint MIC. EMB is a slow-acting anti-TB drug, and culture-based methods can be problematic because of the bacteriostatic nature of EMB. The storage conditions of the drug and the type of medium used also influence the drug activity (Piersimoni et al., 2006). The MIC range of EMB-susceptible and EMB-resistant strains was relatively narrow (Madison et al., 2002; Cheng et al., 2014), and the critical breakpoints of different phenotypic DST methods were different (Madison et al., 2002; Banu et al., 2014; Cheng et al., 2014). Additionally, the presence of microcolonies on solid media and the proximity of EMB MICs of clinical isolates near the critical concentration could make determination of resistance difficult (Parsons et al., 2004; Plinke et al., 2009). Significant discrepancies were encountered when comparing the results obtained in different phenotypic DST methods (Plinke et al., 2009; Cheng et al., 2014; Ahmad et al., 2016). Hence, there were reports suggesting that molecular tests for detecting EMB resistance warranted accurate EMB susceptibility results (Lacoma et al., 2015; Ahmad et al., 2016).
With reference to results of MABA, 67 (83.8%) of 80 EMB-resistant isolates contained at least one mutation in embAB. This result validated that embAB mutations are associated with EMB resistance (Brossier et al., 2015; Zhao et al., 2015). The majority of embAB mutations were concentrated in embB. Of them, embB306 was the most frequent and was detected in 68.8% (55/80 isolates) isolates. The embB306 mutation was also detected in EMB-susceptible isolates. However, the mutation frequency in EMB-resistant isolates 68.8% (55/80 isolates) was significantly higher than that in EMB-susceptible isolates (7.9%, 3/38 isolates). A previous study indicated these mutations were likely to be important, in that they represent the first step toward acquiring EMB resistance (Safi et al., 2013), indicating the need to gain insight into the molecular basis of EMB resistance. It is also desirable to have a better understanding of treatment outcomes and treatment failure in patients infected with strains carrying embB mutations. Despite the fact that 14 EMB-resistant isolates harbored embA mutations in this study, 12 had additional mutations in embB. These multiple mutations were shown to be correlated with high-level EMB resistance (Sun et al., 2018).
Although 83.8% EMB-resistant isolates were identified with DNA sequencing of embAB, the inclusion of embA in molecular diagnoses only marginally increased the testing sensitivity by 2.5%. The best strategy at present for molecular diagnostics is selective targeting of embB (81.3% sensitivity, 86.8% specificity, and 83.1% accuracy). Analyzing the embB306 mutation could predict EMB resistance with 68.8% sensitivity, 92.1% specificity, and 76.3% accuracy. Thus, the effects of this mutation on detecting EMB resistance were relatively limited.
There were still some EMB-resistant isolates determined by phenotypic DST methods (PM, MGIT, and MABA), which lacked embAB mutations. This implied that these strains probably harbored mutations outside embAB (Safi et al., 2013; He et al., 2015; Tulyaprawat et al., 2019) or that the resistance may have been caused by other mechanisms, such as overexpressed efflux pumps and loss of porins (Sharma and Bisht, 2017; Sharma et al., 2018). Careful interpretation of a negative DNA sequencing result (no mutation) is thus necessary in the accurate management of suspected DR-TB.
This study also contained several limitations. (i) The number of isolates included was relatively limited. Additional research containing more isolates will be required to evaluate the effectiveness of the MABA and DNA sequencing. (ii) DNA sequencing is unable to identify EMB-resistant isolates that do not carry mutations in embAB. (iii) The association between embB306 mutation and other drug resistance was not evaluated.
Conclusion
Our results indicated that MABA is suitable for detecting EMB resistance, showing good concordance with traditional PM methods and embAB mutations. DNA sequencing to screen embB may be useful for the rapid identification of EMB resistance. However, we do not suggest phenotypic DST methods to be replaced with DNA sequencing in China. It may be used as an initial diagnosis tool for the early identification of EMB resistance and consequently optimizing the treatment regimens.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LZ and K-LW conceived and designed the study. LZ, ML, HL, and SL analyzed the data. ML, RC, YL, GL, ZL, and XZ performed the laboratory experiments. ML, RC, YL, and HL contributed to the data collection and analysis. LZ, K-LW, and SL wrote the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the projects from National Key Program of Mega Infectious Diseases (Grant No. 2018ZX10302302) and National Basic Research Program of China (973 Program, Grant No. 2015CB554202). The funder had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ahmad, S., Mokaddas, E., Al-Mutairi, N., Eldeen, H. S., and Mohammadi, S. (2016). Discordance across phenotypic and molecular methods for drug susceptibility testing of drug-resistant Mycobacterium tuberculosis isolates in a low TB incidence country. PLoS One 11:e0153563. doi: 10.1371/journal.pone.0153563
Altman, D. G. (1999). “Inter-rater agreement,” in Practical Statistics for Medical Research, ed. D. G. Altman (London: Chapman & Hall), 403–409.
Banu, S., Rahman, S. M., Khan, M. S., Ferdous, S. S., Ahmed, S., Gratz, J., et al. (2014). Discordance across several methods for drug susceptibility testing of drug-resistant Mycobacterium tuberculosis isolates in a single laboratory. J. Clin. Microbiol. 52, 156–163. doi: 10.1128/JCM.02378-13
Brossier, F., Sougakoff, W., Bernard, C., Petrou, M., Adeyema, K., Pham, A., et al. (2015). Molecular analysis of the embCAB locus and embR gene involved in ethambutol resistance in clinical isolates of Mycobacterium tuberculosis in France. Antimicrob. Agents Chemother. 59, 4800–4808. doi: 10.1128/AAC.00150-15
Campbell, P. J., Morlock, G. P., Sikes, R. D., Dalton, T. L., Metchock, B., Starks, A. M., et al. (2011). Molecular detection of mutations associated with first- and second-line drug resistance compared with conventional drug susceptibility testing of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 55, 2032–2041. doi: 10.1128/AAC.01550-10
Cheng, S., Cui, Z., Li, Y., and Hu, Z. (2014). Diagnostic accuracy of a molecular drug susceptibility testing method for the antituberculosis drug ethambutol: a systematic review and meta-analysis. J. Clin. Microbiol. 52, 2913–2924.
Cui, Z., Li, Y., Cheng, S., Yang, H., Lu, J., Hu, Z., et al. (2014). Mutations in the embC-embA intergenic region contribute to Mycobacterium tuberculosis resistance to ethambutol. Antimicrob. Agents Chemother. 58, 6837–6843. doi: 10.1128/AAC.03285-14
Garrigo, M., Aragon, L. M., Alcaide, F., Borrell, S., Cardeñosa, E., Galán, J. J., et al. (2007). Multicenter laboratory evaluation of the MB/BacT Mycobacterium detection system and the BACTEC MGIT 960 system in comparison with the BACTEC 460TB system for susceptibility testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 45, 1766–1770.
He, L., Wang, X., Cui, P., Jin, J., Chen, J., Zhang, W., et al. (2015). ubiA (Rv3806c) encoding DPPR synthase involved in cell wall synthesis is associated with ethambutol resistance in Mycobacterium tuberculosis. Tuberculosis 95, 149–154. doi: 10.1016/j.tube.2014.12.002
Kim, S. J. (2005). Drug-susceptibility testing in tuberculosis: methods and reliability of results. Eur. Respir. J. 25, 564–569.
Lacoma, A., Molina-Moya, B., Prat, C., Pimkina, E., Diaz, J., Dudnyk, A., et al. (2015). Pyrosequencing for rapid detection of Mycobacterium tuberculosis second-line drugs and ethambutol resistance. Diagn. Microbiol. Infect. Dis. 83, 263–269. doi: 10.1016/j.diagmicrobio.2015.07.004
Leonard, B., Coronel, J., Siedner, M., Grandjean, L., Caviedes, L., Navarro, P., et al. (2008). Inter- and intra-assay reproducibility of microplate Alamar blue assay results for isoniazid, rifampicin, ethambutol, streptomycin, ciprofloxacin, and capreomycin drug susceptibility testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 46, 3526–3529. doi: 10.1128/JCM.02083-07
Li, Y., Wang, Y., Zhang, Z., Gao, H., Wang, H., Cao, J., et al. (2016). Association between embB Codon 306 mutations, phenotypic resistance profiles, and genotypic characterization in clinical Mycobacterium tuberculosis Isolates from Hebei, China. Antimicrob. Agents Chemother. 60, 7295–7302.
Madison, B., Robinson-Dunn, B., George, I., Gross, W., Lipman, H., Metchock, B., et al. (2002). Multicenter evaluation of ethambutol susceptibility testing of Mycobacterium tuberculosis by agar proportion and radiometric methods. J. Clin. Microbiol. 40, 3976–3979.
Parsons, L. M., Somoskovi, A., Urbanczik, R., and Salfinger, M. (2004). Laboratory diagnostic aspects of drug resistant tuberculosis. Front. Biosci. 9:2086–2105. doi: 10.2741/1290
Piersimoni, C., Olivieri, A., Benacchio, L., and Scarparo, C. (2006). Current perspectives on drug susceptibility testing of Mycobacterium tuberculosis complex: the automated nonradiometric systems. J. Clin. Microbiol. 44, 20–28.
Plinke, C., Cox, H. S., Kalon, S., Doshetov, D., Rüsch-Gerdes, S., Niemann, S., et al. (2009). Tuberculosis ethambutol resistance: concordance between phenotypic and genotypic test results. Tuberculosis 89, 448–452.
Safi, H., Lingaraju, S., Amin, A., Kim, S., Jones, M., Holmes, M., et al. (2013). Evolution of high-level ethambutol-resistant tuberculosis through interacting mutations in decaprenylphosphoryl-beta-D-arabinose biosynthetic and utilization pathway genes. Nat. Genet. 45, 1190–1197. doi: 10.1038/ng.2743
Sharma, D., and Bisht, D. (2017). M. tuberculosis hypothetical proteins and proteins of unknown function: hope for exploring novel resistance mechanisms as well as future target of drug resistance. Front. Microbiol. 8:465. doi: 10.3389/fmicb.2017.00465
Sharma, D., Bisht, D., and Khan, A. U. (2018). Potential alternative strategy against drug resistant tuberculosis: a proteomics prospect. Proteomes 6:26. doi: 10.3390/proteomes6020026
Shi, D., Li, L., Zhao, Y., Jia, Q., Li, H., Coulter, C., et al. (2011). Characteristics of embB mutations in multidrug-resistant Mycobacterium tuberculosis isolates in Henan. China. J. Antimicrob. Chemother. 66, 2240–2247. doi: 10.1093/jac/dkr284
Sun, Q., Xiao, T. Y., Liu, H. C., Zhao, X. Q., Liu, Z. G., Li, Y. N., et al. (2018). Mutations within embCAB are associated with variable level of ethambutol resistance in Mycobacterium tuberculosis Isolates from China. Antimicrob. Agents Chemother. 62, e1279-17. doi: 10.1128/AAC.01279-17
Telenti, A., Philipp, W. J., Sreevatsan, S., Bernasconi, C., Stockbauer, K. E., Wieles, B., et al. (1997). The emb operon, a gene cluster of Mycobacterium tuberculosis involved in resistance to ethambutol. Nat. Med. 3, 567–570.
Tulyaprawat, O., Chaiprasert, A., Chongtrakool, P., Suwannakarn, K., and Ngamskulrungroj, P. (2019). Association of ubiA mutations and high-level of ethambutol resistance among Mycobacterium tuberculosis Thai clinical isolates. Tuberculosis 114, 42–46. doi: 10.1016/j.tube.2018.11.006
World Health Organization [WHO] (2009). Guidelines for Surveillance of Drug Resistance in Tuberculosis, 4th Edn. Geneva: World Health Organization.
Zhang, H., Li, D., Zhao, L., Fleming, J., Lin, N., Wang, T., et al. (2013). Genome sequencing of 161 Mycobacterium tuberculosis isolates from China identifies genes and intergenic regions associated with drug resistance. Nat. Genet. 45, 1255–1260. doi: 10.1038/ng.2735
Zhang, Z., Wang, Y., Pang, Y., and Kam, K. M. (2014). Ethambutol resistance as determined by broth dilution method correlates better than sequencing results with embB mutations in multidrug-resistant Mycobacterium tuberculosis isolates. J. Clin. Microbiol,. 52, 638–641. doi: 10.1128/JCM.02713-13
Zhao, L. L., Sun, Q., Liu, H. C., Wu, X. C., Xiao, T. Y., Zhao, X. Q., et al. (2015). Analysis of embCAB mutations associated with ethambutol resistance in multidrug-resistant Mycobacterium tuberculosis isolates from China. Antimicrob. Agents Chemother. 59, 2045–2050. doi: 10.1128/AAC.04933-14
Keywords: Mycobacterium tuberculosis, ethambutol resistance, phenotypic drug susceptibility testing, DNA sequencing, microplate alamarBlue assay
Citation: Li M, Chen R, Lin S, Lu Y, Liu H, Li G, Liu Z, Zhao X, Zhao L and Wan K-L (2020) Detecting Ethambutol Resistance in Mycobacterium tuberculosis Isolates in China: A Comparison Between Phenotypic Drug Susceptibility Testing Methods and DNA Sequencing of embAB. Front. Microbiol. 11:781. doi: 10.3389/fmicb.2020.00781
Received: 12 December 2019; Accepted: 01 April 2020;
Published: 08 May 2020.
Edited by:
Jesica Mazza-Stalder, CHU de Lausanne (CHUV), SwitzerlandReviewed by:
Divakar Sharma, Indian Institute of Technology Delhi, IndiaShibali Das, Washington University School of Medicine in St. Louis, United States
Copyright © 2020 Li, Chen, Lin, Lu, Liu, Li, Liu, Zhao, Zhao and Wan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-li Zhao, emhhb2xpbGlAaWNkYy5jbg==; Kang-Lin Wan, d2Fua2FuZ2xpbkBpY2RjLmNu
†These authors have contributed equally to this work
 Ma-chao Li
Ma-chao Li Rong Chen1,2†
Rong Chen1,2† Hai-can Liu
Hai-can Liu Gui-lian Li
Gui-lian Li Li-li Zhao
Li-li Zhao