
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 06 February 2020
Sec. Microbial Physiology and Metabolism
Volume 11 - 2020 | https://doi.org/10.3389/fmicb.2020.00113
Bacterial cells respond to environmental stresses by modulating their gene expression and adjusting their proteome. In Staphylococcus aureus, selective degradation by ClpP protease eliminates damaged proteins and regulates the abundance of functional proteins such as many important stress-induced transcriptional regulators. Degradation by ClpP requires the unfolding activity of partner Clp ATPases, such as ClpX and ClpC, and assistance of substrate-specific adaptor proteins such as YjbH and TrfA. Herein, we demonstrated that YjbH is aggregated in response to growth stress stimuli, such as oxidative and antibiotic stresses. In consequence, its function as an adaptor protein is compromised. YjbH controls the degradation of the stress-induced transcriptional regulator, Spx. Aggregated YjbH cannot assist Spx degradation, which results in Spx accumulation. We discovered that depending on the stress stimulus, Spx can be soluble or insoluble, and, consequently, transcriptionally active or inactive. Therefore, Spx accumulation and solubility are key components governing activation of Spx-dependent genes. Spx positively regulates expression of a ClpCP adaptor protein TrfA. TrfA in turn is required for degradation of MazE antitoxin, the unstable component of the MazEF toxin-antitoxin system, that neutralizes the endoribonuclease activity of MazF toxin. Bacterial toxin-antitoxin systems are associated with dormancy and tolerance to antibiotics that are related to chronic and relapsing infections, and it is at present a key unresolved problem in medicine. MazF activity was linked to growth stasis, yet the precise environmental signals that trigger MazE degradation and MazF activation are poorly understood. Here we propose a model where YjbH serves as a sensor of environmental stresses for downstream regulation of MazEF activity. YjbH aggregation, soluble Spx, and TrfA, coordinately control MazE antitoxin levels and consequently MazF toxin activity. This model implies that certain stress conditions culminate in modulation of MazF activity resulting in growth stasis during in vivo infections.
Bacterial antibiotic resistance has been recognized as a worldwide problem with still few solutions. It may occur as a result of mutations in bacterial populations selected during antibiotic treatment. However, another important aspect of antimicrobial resistance is bacterial dormancy.
The first type of dormant bacteria tolerant to antibiotics was described in Staphylococcus aureus strains long ago (Bigger, 1944). Antibiotic treatment of a susceptible bacterial population kills the majority of the cells but induces the formation of non-dividing dormant bacteria that survive antibiotic challenge. Dormant bacteria are not genetically resistant and after removal of antibiotics, they can regrow and evolve into a susceptible population. The mechanism of entry into antibiotic tolerant state with later regrowth after antibiotic removal, may explain the clinical chronic and relapsing infections. This emphasizes the urgent need to understand the molecular pathways that lead to bacterial dormancy.
Toxin-antitoxin systems (TAS) are stress-inducible functional complexes where toxin component binds an antitoxin (Yamaguchi et al., 2011; Page and Peti, 2016). Toxin activity is inhibited by the antitoxin, that is typically an unstable protein susceptible to degradation by proteases. Antitoxin degradation leads to toxin activation that down-regulates central processes in the cell and may result in cell dormancy (Coussens and Daines, 2016). Different bacterial species enter into dormancy through activation of TAS that will interfere with replication (Maki et al., 1992; Aakre et al., 2013; Harms et al., 2015), inhibition of ribosomes (Castro-Roa et al., 2013; Van Melderen and Wood, 2017), cell wall synthesis (Mutschler et al., 2011), and cell division (Masuda et al., 2012; Mok et al., 2015).
One of the best characterized TAS in S. aureus is MazEF, a type II TAS (Schuster and Bertram, 2016). It is found also in other clinically important bacteria (Mittenhuber, 1999; Nguyen et al., 2011; Schifano et al., 2013; Cho et al., 2017). Several studies were conducted to characterize the MazEF locus by studying its transcriptional activation and function (Donegan and Cheung, 2009; Fu et al., 2009; Zhu et al., 2009; Zorzini et al., 2011, 2014; Miyamoto et al., 2018). MazEF is composed of MazF toxin and its activity is modulated by the MazE antitoxin (Figure 1). Under normal growth conditions, high MazE level ensures formation of toxin-antitoxin complex and consequently, MazF inactivity (Fu et al., 2007). MazE is cleaved by the ClpCP degradation module, where ClpC is a chaperone with unfolding activity and ClpP is a protease. MazE degradation is assisted by the adaptor protein TrfA, providing ClpCP specificity and facilitating MazE recognition (Donegan et al., 2010, 2014). We previously showed that transcription of trfA is positively regulated by the transcriptional activator Spx (Jousselin et al., 2013). In Bacillus subtilis, Spx is a central regulator of the stress response. It binds to the alpha subunits of RNA polymerase, and regulates positively and negatively expression of many genes (Schafer et al., 2019). Spx protein levels are regulated by the proteolytic system ClpXP and assisted by the adaptor protein YjbH (Garg et al., 2009; Renzoni et al., 2011; Engman et al., 2012) (Figure 1). It has been reported that Geobacillus thermodenitrificans YjbH directly interacts with C-terminal end of B. subtilis Spx to accelerate Spx proteolysis by ClpX (Chan et al., 2012, 2014). Later the crystal structure of YjbH from Geobacillus kaustrophilus, a functional homologs of YjbH from S. aureus, bound to B. subtilis Spx was published (Awad et al., 2019). In B. subtilis it was demonstrated that YjbH is aggregated in response to environmental stresses, and it was proposed that via aggregation YjbH may control Spx levels (Engman and von Wachenfeldt, 2015). However, in S. aureus the regulation and properties of Spx and YjbH are poorly understood.
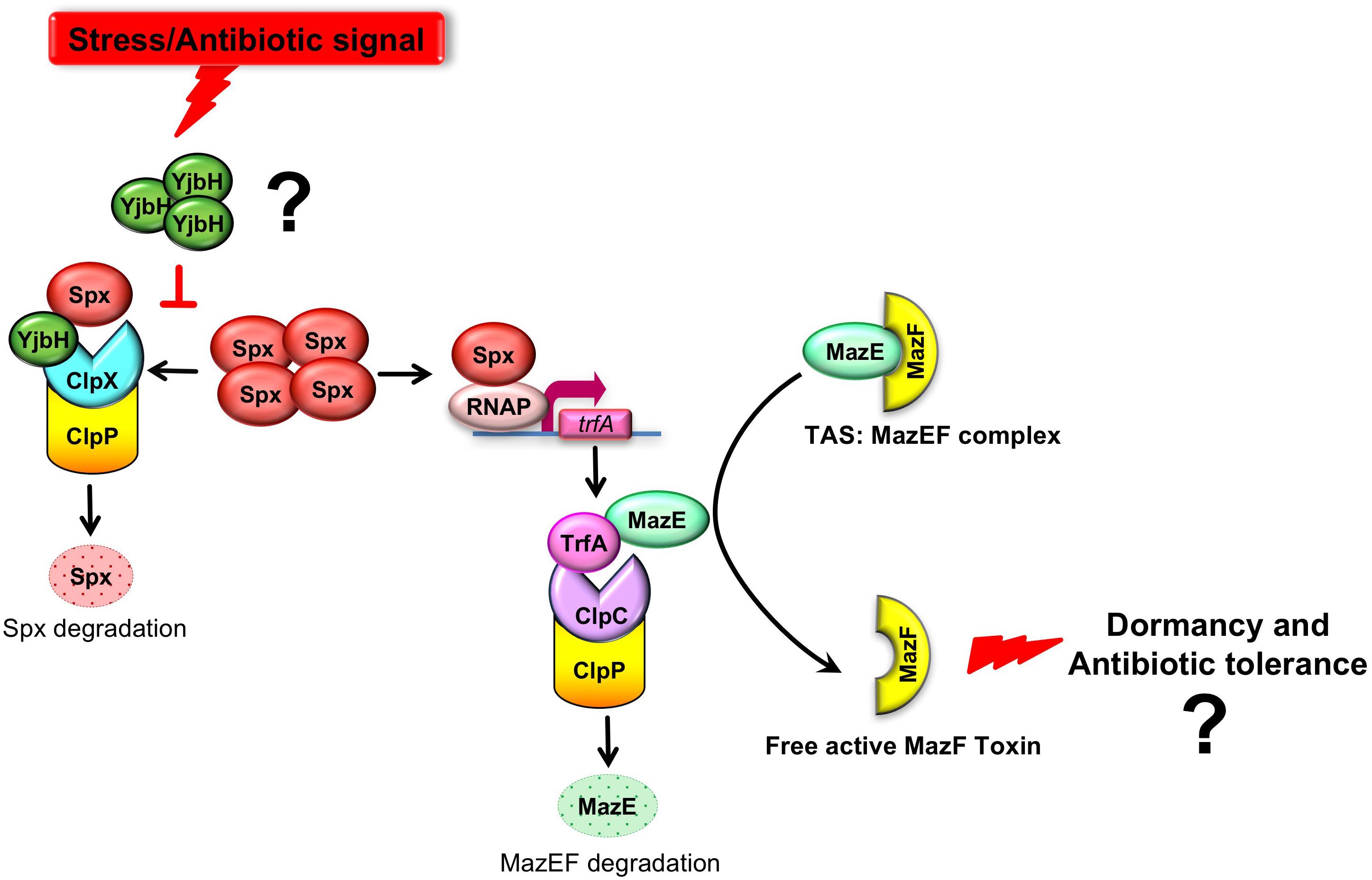
Figure 1. A model where YjbH serves as a sensor of environmental stress and downstream regulation MazEF activity. MazEF complex is composed of MazF toxin and MazE antitoxin which binds MazF and neutralizes MazF activity. MazE is cleaved by the ClpCP degradation module, where ClpC is a chaperone with unfolding activity and ClpP is a protease. MazE degradation is assisted by the adaptor protein TrfA, providing ClpCP specificity and facilitating MazE recognition. The trfA transcription is regulated by the redox sensitive transcriptional factor, Spx. In turn, Spx proteolysis is controlled by ClpXP proteolytic system and requires YjbH adaptor protein. We hypothesized (indicated by question mark) that in S. aureus YjbH aggregates and modulation of YjbH aggregation affects MazEF TAS through the YjbH-Spx-TrfA cascade in response to environmental stresses.
It has been reported that MazF toxin overexpression in S. aureus leads to growth stasis or growth arrest (Fu et al., 2009), raising the question whether MazF may be a potential regulator of bacterial dormancy and antibiotic tolerance. Several studies identified genome-wide targets of MazF trying to clarify its role in growth stasis (Fu et al., 2009; Zhu et al., 2009; Schuster et al., 2015; Culviner and Laub, 2018; Sierra et al., 2019). However, the link of MazEF to bacterial dormancy is still to be determined. The described metabolic effects of MazF have been observed under artificial overexpression of MazF. Presently, it is still unknown which environmental conditions, mechanism of sensing, and signal transmission lead to active and free MazF toxin.
We provide evidences that in S. aureus YjbH aggregates in response to different environmental stresses. Both YjbH aggregation and the stress conditions affect the levels, solubility, and functional state of transcriptional factor Spx and consequently its downstream targets, such as TrfA. We hypothesized the different environmental stimuli may regulated MazEF TAS through YjbH aggregation, soluble Spx, and TrfA (Figure 1).
All bacteria strains and plasmids used in this work are listed in Table 1. Most S. aureus genetic constructs were created in HG003 strain background (Herbert et al., 2010; Sassi et al., 2014). S. aureus bacterial cultures were grown on Mueller Hinton Broth (MHB) media until OD600 of 0.5–0.7 at 37°C with shaking. Bacteria containing plasmids with chloramphenicol or tetracycline resistance were grown in the presence of 15 μg/ml of chloramphenicol and 3 μg/ml of tetracycline, respectively.
Plasmid pOP172 was obtained from GENEWIZ. Synthetic codon optimized S. aureus YjbH gene with HA3 tag (HA3-YjbH) was synthesized and cloned into pUC57 vector resulting in pOP172. To create pOP173 plasmid, HA3-YjbH fragment was KpnI/PstI digested from pOP172 and cloned into pMK4-pHU vector (Andrey et al., 2010). S. aureus strains carrying sfGFP alone or sfGFP-YjbH under control of IPTG inducible promoter were kindly provided by Claes von Wachenfeld (Lund University). The pRAB11-MazF plasmid (pAR1884), expressing mazF gene under control of anhydrotetracycline (ATc) inducible promoter was constructed by amplification of HG003 mazF gene using primers carrying Bgl2 and EcoR1 restriction sites. Amplified product was cut with Bgl2 and EcoR1 and ligated the into pRAB11 vector (Helle et al., 2011). To induce the expression of MazF, 0.2 μM of ATc was added to the cell cultures and incubated for 10–180 min at 37°C with shaking.
Cultures of 25 ml with OD600 of 0.7 were collected and washed three times with 1 ml of Phosphate-buffered saline (PBS) buffer. Cells were lysed in the presence of 400 μl of lysis buffer 1 (LB 1) [PBS, 200 μg/ml lysostaphin, 200 μg/ml DNAse I, protease inhibitors (Roche)] for 20 min at 37°C, chilled on ice, and sonicated 10 times with 30-s cycles using Cell Disrupter B-30 (Branson). Extracts were clarified by centrifugation for 10 min at 14000 × g 4°C. Total protein concentration was measured in supernatants (SN) by the Bradford protein assay. This method permitted to obtain of about 1 mg of total protein with a concentration of about 2.5–3.0 mg/ml. Samples were mixed with Laemmli Sample Buffer (SB) and analyzed by SDS polyacrylamide gel electrophoresis (SDS-PAGE) with following Coomassie Blue staining or western blot.
The protocol for isolation of aggregated proteins from S. aureus was developed on the base of method described for yeast (Panasenko and Collart, 2012). Cells were grown on MHB until OD600 of 0.5–0.8. Control bacterial culture was left at 37°C without treatment. Other cultures were incubated at 37°C for 30 min with 5 mM of diamide, 10% ethanol or with antibiotics [oxacillin (10–40 μg/ml), vancomycin (10–40 μg/ml), kanamycin (50–400 μg/ml), tetracycline (60 μg/ml), erythromycin (20 μg/ml)]. For heat shock (HS), cells were incubated at 53°C for 30 min. After cultures treatment, 15 OD600 units were harvested at 4000 g for 5 min, and washed with 1 ml of PBS. Pellets were resuspended in 0.3 ml (20 μl per 1 OD unit) of lysis buffer 2 (LB2) [20 mM Na-phosphate, pH 7.6, 10 mM DTT, 1 mM EDTA, 0.1% Tween 20, 1 mM PMSF, protease inhibitor cocktail (Roche), 200 μg/ml DNAse, 200 μg/ml lysostaphin] and incubated at 37°C for 20 min. Chilled samples were sonicated 10 times with 40-s cycles, using Cell Disrupter B-30 (Branson), and centrifuged for 20 min at 200 × g at 4°C. Total protein concentration was measured in supernatants by the Bradford protein assay. Whole cell extracts were adjusted to identical protein concentration of 1.0 mg/ml. 30 μl of supernatants were boiled with 10 μl of 4× SB (total proteins). Anti-Pbp2 antibody was used as a control for the equal protein amount in whole cell extracts. Equal amount of whole cell extracts (200 μl) were centrifuged at 16000 × g for 20 min at 4°C to pellet the aggregated proteins. After removing supernatants (soluble fractions), insoluble proteins were washed twice with washing buffer (20 mM Na-phosphate, pH 7.6, 2% of NP-40, 1 mM PMSF, protease inhibitor cocktail (Roche), sonicated (10 s at duty cycle 40%), and centrifuged at 16000 × g for 20 min at 4°C. Insoluble (aggregated) proteins were boiled in 50 μl of SB. 60 μl of soluble fractions were boiled with 20 μl of 4× SB. 15 μl of samples were separated by gradient (4–12%) SDS-PAGE, and analyzed by Coomassie Blue staining or western blot.
Cells expressing either sfGFP-YjbH or only sfGFP under IPTG inducible promoter were grown on MHB with 3 μg/ml of tetracycline overnight. Cultures were diluted 1/100 in MHB with 3 μg/ml of tetracycline and with 1 mM of IPTG and grown 37°C for 5 h. Cells were incubated at 37°C for 30 min with 5 mM of diamide, or with antibiotics [oxacillin (20–40 μg/ml), tetracycline (20–60 μg/ml), kanamycin (200–400 μg/ml)]. For heat shock (HS) cells were incubated at 53°C for 30 min. Control culture was left at 37°C without treatment. 1.5 ml of cultures were chilled and centrifuged at 4°C 10000 × g for 1 min. Pellets were resuspended in 30 μl of PBS and 7 μl of cell suspension was loaded on 1% agar, and analyzed with fluorescent microscope.
RNAs were purified using RNeasy Plus Mini Kit (Qiagen) and QIAshredder Kit (Qiagen). DNA was removed using QIAGEN DNase Kit (Qiagen). TaqMan real-time quantitative polymerase chain reaction (RTqPCR) was performed with Platinum Quantitative RT-PCR ThermoScript One-Step System (Invitrogen). All RNAs were tested for the absence of DNA contaminations. Primers and MGB Double-Dye probes for trfA, rsbW, 16S RNA, and gyrB (Table 2) were designed using Primer Express software (version 1.5; Applied Biosystems), obtained from Eurogentec and used in a concentration 0.05–0.1 μM. For each pair of primers, primer efficiency was calculated and primers and probes were used in concentrations that give the same primer efficiency with housekeeping gene (16S RNA or gyrB). To quantify RTqPCR data the 2^(−ΔΔCT) method has been used (Rao et al., 2013), where fold change of target gene expression in a target (treated) samples relative to a reference (non-treated) samples was normalized to a reference gene (16S RNA or gyrB). Thus, the relative gene expression in non-treated samples was set to 1. The errors for the ΔΔCT were obtained by least square error propagation of the standard deviation for the individual RTqPCR measurements performed in triplicates.
The induction of the MazF expression by ATc was performed for 10 min. Total RNAs were purified as described above in biological duplicates. Ribosomal RNAs were depleted with RiboZero kit (Illumina). Libraries were created using the Illumina TruSeq stranded mRNA kit. 1st strand cDNAs were synthesized with random primer. Libraries were sequenced at Fasteris SA. Results of RNA-seq were normalized for sequencing depth [normalization estimated by edgeR (Robinson and Oshlack, 2010; Robinson et al., 2010)], and by the length of the gene, in kilobases and presented in RPKM (Reads Per Kilobase Million).
Anti-HA (anti-influenza hemagglutinin; Sigma) antibodies were used at the dilution 1:3000. Anti-Spx was a gift from Dorte Frees (University of Copenhagen) (Stahlhut et al., 2017) and used in dilution 1:3000. Anti-MazE and anti-MazF antibodies were a gift from Patrick Viollier (University of Geneva) and used in dilution 1:500. Anti-Pbp2 antibodies were a gift from Mariana Gomes de Pinho (ITQB NOVA, University of Lisbon) and used in dilution 1:8000.
MazE antitoxin is regulated by an upstream pathway activated by yet unknown stimuli and unknown mechanisms (Figure 1). We hypothesized that YjbH adaptor protein may be involved in stimulus sensing. In B. subtilis it was demonstrated that YjbH is aggregated upon heat shock and diamide-induced oxidative stress, and this aggregation is accompanied by an increase in level of transcriptional regulator Spx (Larsson et al., 2007; Engman and von Wachenfeldt, 2015). We first asked whether YjbH in S. aureus is also prone to aggregation and if so, in which conditions. We used cells expressing IPTG inducible sfGFP alone or sfGFP fused to YjbH (sfGFP-YjbH). After IPTG-induction of sfGFP constructs, bacterial cells were subject to treatment with heat shock, diamide and ribosome-targeting antibiotic, kanamycin, and observed with the fluorescent microscopy (Figure 2). A bacterial strain carrying sfGFP alone show a fluorescent signal distributed homogeneously in all cells before and after tested conditions (Figure 2, upper panel, GFP). In non-treated cells, expressing sfGFP-YjbH fusion, fluorescent signal was also distributed homogeneously. In contrast, distinct fluorescent foci were visible after 30 min of treatment with diamide, heat shock, and kanamycin (Figure 2, lower panel, GFP-YjbH). The same foci were detected after treatment with the cell wall antibiotic, oxacillin, and ribosome-targeting antibiotic, tetracycline (data are not shown).
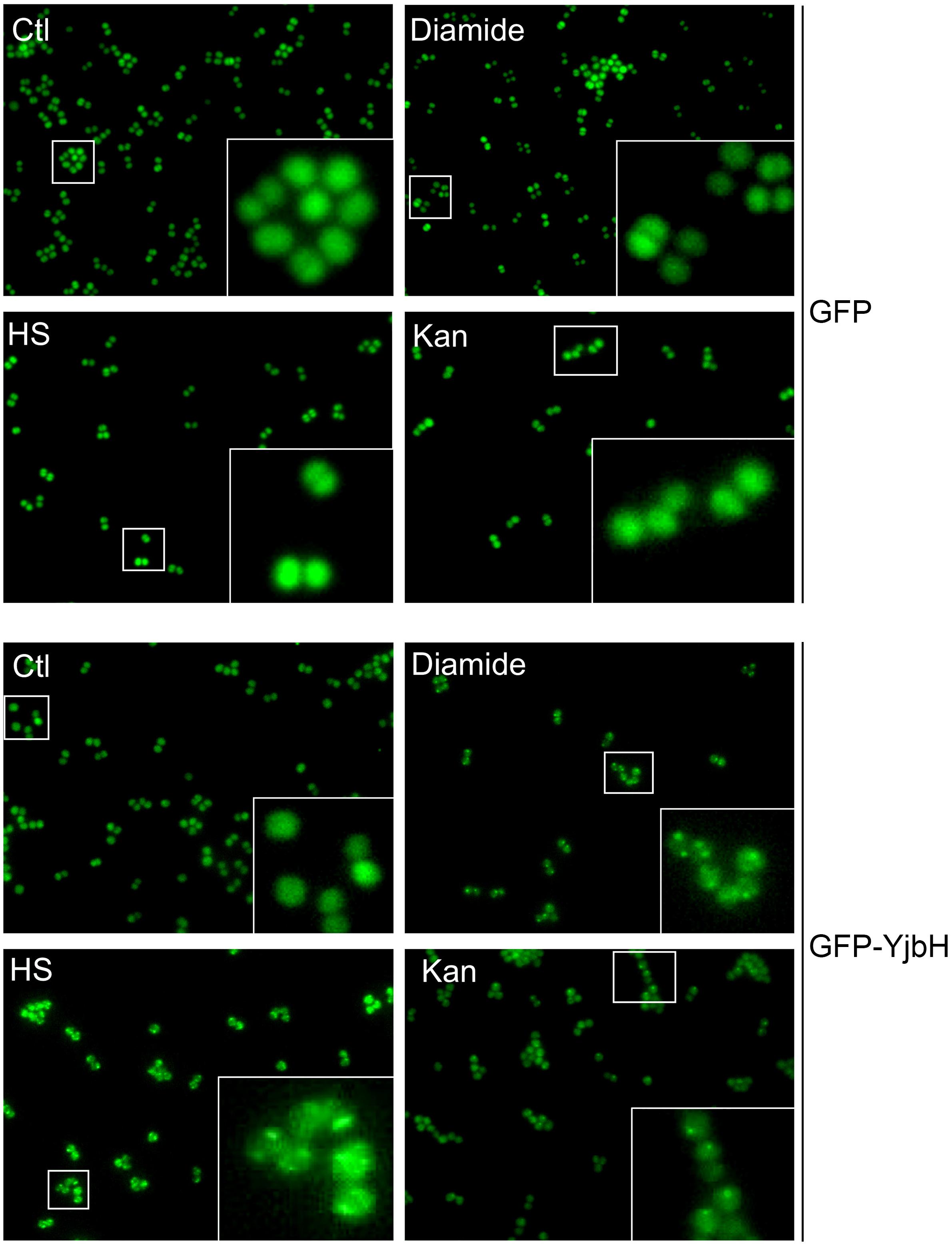
Figure 2. sfGFP-YjbH is accumulated in foci upon heat shock, oxidative stress, and antibiotic treatment. Cells, expressing sfGFP-YjbH were not treated (Ctl), or treated with high temperature (53°C, HS), 5 mM of diamide or 400 μg/ml of ribosome-targeting antibiotic, kanamycin (Kan) and analyzed by fluorescence microscopy (lower panel, GFP-YjbH). Cells, expressing only sfGFP were used as a control and treated in the same manner (upper panel, GFP).
We speculate that visible foci appeared after stress treatment are aggregated sfGFP-YjbH protein, as was described for B. subtilis (Engman and von Wachenfeldt, 2015). To verify this hypothesis, we developed a method to isolate insoluble proteins in S. aureus. We treated wild-type cells with different stress conditions, such as heat shock (53°C), ethanol (10%), diamide (5 mM), and different antibiotics. Whole cell extracts were adjusted to the same protein concentration. Equal amounts of total protein were separated into soluble and insoluble fractions by centrifugation. Precipitated proteins were washed twice by sonication in buffer containing 2% of non-ionic detergent NP-40 to disrupt the membranes. Proteins remained insoluble after that treatment, are likely to be aggregated proteins (Engman and von Wachenfeldt, 2015), therefore we use the term aggregates for them. Insoluble proteins were resuspended in the sample buffer containing 2% of SDS and analyzed by SDS-PAGE and Coomassie Blue staining.
No difference was observed in soluble protein fractions from non-stress or stress conditions (Figure 3A). However, significant difference was in insoluble fractions. Heat shock, ethanol and kanamycin, induced massive protein aggregation compared to non-stressed conditions, suggesting that under these conditions many proteins are altered and prone to aggregation. Oxidative stress induced by diamide, and treatment with cell wall antibiotics oxacillin and vancomycin, did not lead to notable protein aggregation.
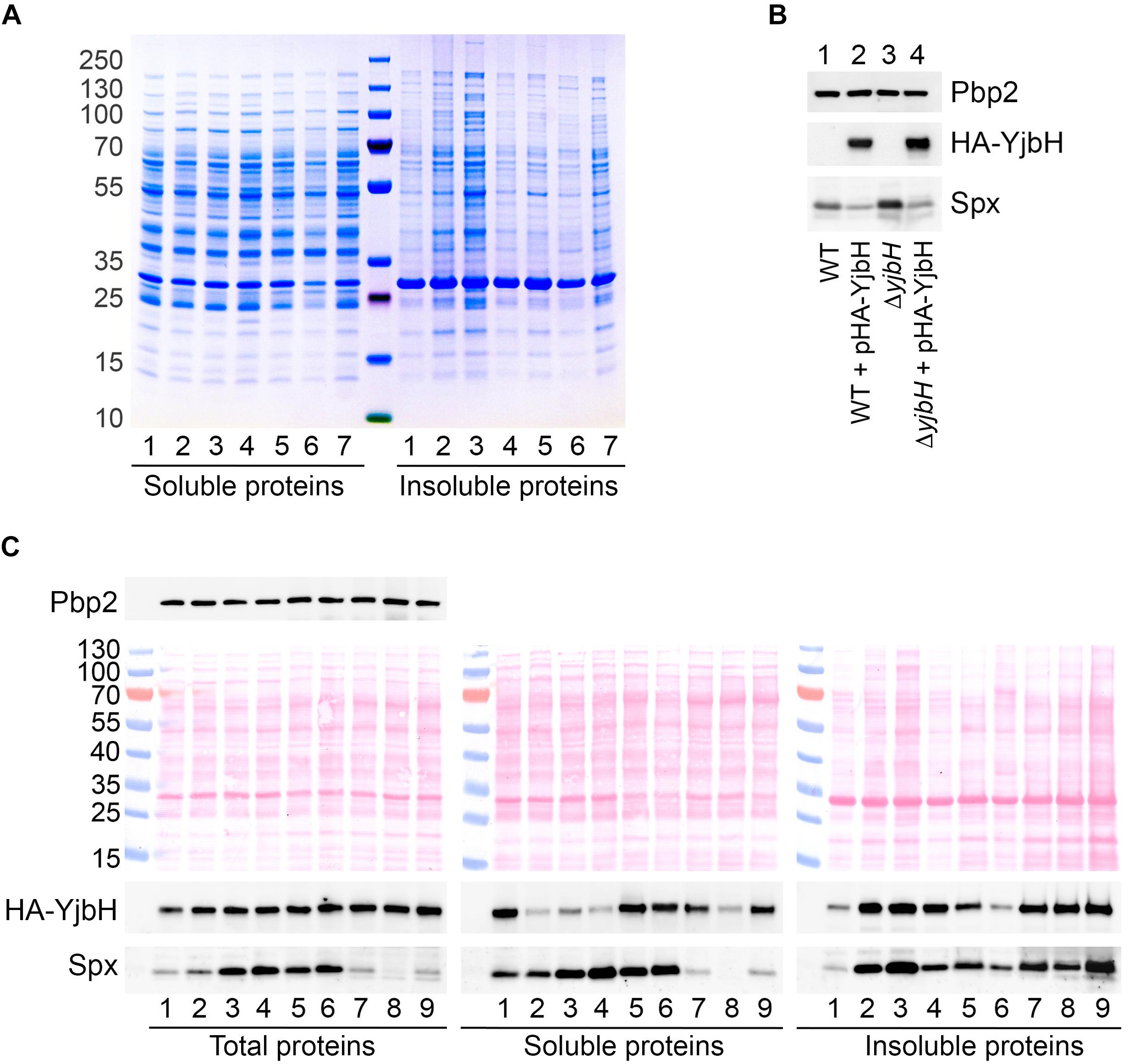
Figure 3. HA-YjbH is aggregated upon heat shock, oxidative stress and antibiotic treatment. (A) Exponentially growing cells (1) were treated with high temperature of 53°C (2), 10% of ethanol (3), 5 mM of diamide (4), 10 μg/ml of oxacillin (5), 10 μg/ml of vancomycin (6), and 50 μg/ml of kanamycin (7). Whole cell extracts were adjusted to the same total protein concentration and fractionated into the soluble and insoluble fractions. Samples were separated by gradient (4–12%) SDS-PAGE, and analyzed by Coomassie Blue staining. (B) Cells, either wild type (WT) or ΔyjbH were transformed with plasmid expressing HA-tagged YjbH (HA-YjbH). Whole cell extracts were prepared and the levels of HA-YjbH and Spx were estimated by western blot with anti-HA and anti-Spx antibodies. Penicillin binding protein 2, Pbp2, was used as a control for equal loading. (C) ΔyjbH cells expressing HA-YjbH were not treated (1) or treated with high temperature (53°C) (2), 10% of ethanol (3), 5 mM of diamide (4), 40 μg/ml of oxacillin (5), 40 μg/ml of vancomycin (6), 400 μg/ml of kanamycin (7), 60 μg/ml of tetracycline (8), and 20 μg/ml of erythromycin (9). Whole cell extracts were adjusted to the equal protein concentration and fractionated into the soluble and insoluble fractions. Proteins were separated by gradient (4–12%) SDS-PAGE and transferred to the nitrocellulose membranes. Membranes were stained with Ponceau S for detection of protein bands and control of loading, and analyzed by western blot with anti-HA, anti-Spx, and anti-Pbp2 antibodies. Pbp2 was used as a control for the equal protein amount in whole cell extracts. Western blots were quantified with ImageJ program and normalized to the signal in untreated control (Supplementary Figure S1).
To prove that visible foci at Figure 2 are indeed insoluble sfGFP-YjbH, we constructed a plasmid expressing HA-tagged YjbH protein expressed from a constitutive HU promoter. The plasmid was transformed into either wild-type cells or cells deleted for yjbH (ΔyjbH). HA-YjbH expression was detected in both strains (Figure 3B). Spx accumulation was observed upon yjbH deletion, as previously described in B. subtilis (Larsson et al., 2007) and in S. aureus (Renzoni et al., 2011). Spx level was decreased upon YjbH plasmid overexpression (Figure 3B). Thus, HA-tagged YjbH protein was indeed functional and compensated the yjbH deletion.
The effect of different environmental stresses on YjbH protein levels was then analyzed. Exponentially growing ΔyjbH bacteria expressing HA-YjbH were subjected to the different stresses, such as heat, ethanol, diamide and antibiotics. Total protein extracts were adjusted to the same protein concentration and fractionated. Fractions of soluble and insoluble proteins were analyzed by SDS-PAGE and western blot for the presence of YjbH and Spx.
In total protein extracts the levels of YjbH were not changed under stress to non-stress conditions (Figure 3C and Supplementary Figure S1, Total proteins). However, the distribution of YjbH in soluble and insoluble fraction was affected (Figure 3C and Supplementary Figure S1, Soluble and Insoluble proteins). In non-stressed conditions YjbH mainly remained soluble (Figure 3C, lane 1). Only a minor fraction of YjbH was insoluble. In contrast, a significant amount of YjbH was insoluble after exposure to heat shock, ethanol, diamide, and ribosome-targeting antibiotics, such as kanamycin, tetracycline, and erythromycin (Figure 3C, lanes 2–4, 7–9). At the same time, less YjbH remained in soluble fraction under these conditions. It was shown earlier, that insoluble YjbH is not membrane associated but rather an aggregate (Engman and von Wachenfeldt, 2015). Cell wall antibiotics, oxacillin and vancomycin, did not cause significant YjbH aggregation and it remained mainly soluble (Figure 3C, lanes 5–6). Taken together, these observations suggest that in S. aureus YjbH is prone to aggregation upon environmental stimuli like it does in B. subtilis. However, not all tested stress conditions induced similar YjbH aggregation. Importantly, YjbH aggregation was observe under oxidative stress caused by diamide that did not result in dramatic increase of general protein aggregation and looked similar to untreated conditions (Figure 3A, right, lanes 1 and 4). Thus, YjbH aggregation may be selective and exploited to regulate certain environmental responses.
In B. subtilis heat and diamide induced YjbH aggregation and consequent Spx stabilization was observed due to decreased proteolysis of Spx by ClpXP (Garg et al., 2009; Engman and von Wachenfeldt, 2015). To analyze if stress conditions resulting in YjbH aggregation in S. aureus leads to accumulation of Spx protein, we followed steady state Spx protein levels by western blot. We observed increased levels of Spx in case of ethanol and diamide treatment (Figure 3C, Total proteins, lanes 3 and 4), when YjbH was aggregated (Figure 3C, Insoluble proteins, lanes 3 and 4). However, upon oxacillin and vancomycin treatment we also observed increased levels of Spx (Figure 3C, Total proteins, lanes 5 and 6) while YjbH remains mainly soluble in these conditions (Figure 3C, Soluble proteins, lanes 5 and 6). At the same time, heat shock and ribosome-targeting antibiotics, that caused aggregation of YjbH, did not lead to increased levels of Spx in total extracts (Figure 3C, Total proteins, lanes 2, 7–9). Thus, not all stress conditions leading to YjbH aggregation, resulted in Spx increased levels. This observation suggests that Spx steady state levels are not exclusively modulated by YjbH.
To understand better the link between YjbH solubility and Spx levels we next analyzed the distribution of Spx between soluble and insoluble fractions during various stresses. We found that even if the total amount of Spx was not induced upon heat shock or ribosome-targeting antibiotics, the majority of protein was found in aggregates and a very low amount was detected in soluble fractions [Figure 3C, compare Soluble and Insoluble fractions (lanes 2, 7–9)]. Under diamide, oxacillin or vancomycin stresses, where increased Spx levels were observed in the total extracts, also higher amounts were found in the soluble fraction compared to the aggregated fraction [Figure 3C, compare Soluble and Insoluble fractions (lanes 4–6)]. These results demonstrate that diamide and cell wall antibiotics, oxacillin and vancomycin, increase the total levels of Spx. Spx mostly remains soluble and, thus, probably functional under these stress conditions. Heat shock and ribosome-targeting antibiotics, kanamycin, tetracycline, and erythromycin, treatments did not increase total levels of Spx, and Spx was found aggregated in probably an inactive form.
Spx is a transcriptional factor, which interacts with the alpha−subunit of the RNA polymerase and induces transcription of many genes, including trfA (Jousselin et al., 2013). In B. subtilis it was shown that oxidation of Spx molecule leads to formation of intramolecular S-S bond between Cys10 and Cys13, that modulates the activity of Spx (Antelmann and Helmann, 2011; Rojas-Tapias and Helmann, 2018). These amino acid residues are conserved in S. aureus. However, it is unknown how Spx oxidation or aggregation may affect its activity in S. aureus. To determine how functional was Spx upon stress conditions we followed its activity by analyzing trfA transcription using RTqPCR. We analyzed expression of trfA transcript in conditions that cause increased Spx protein levels, such as yjbH deletion, and probably modulate Spx activity, such as diamide. RNAs were purified from diamide treated and non-treated cells, and trfA mRNA levels were measured by RTqPCR. In non-treated conditions we observed an increase of trfA transcript levels in the yjbH deletion mutant compared to the wild-type cells (about three times) (Figure 4A). Diamide treatment increases trfA transcription in both wild-type and yjbH deleted strains. However, the difference in trfA transcription in wild type and yjbH deletion mutant on diamide was less, that in untreated conditions (4.2 and 6.2 compare to 3 and 1). Diamide treatment led to higher induction of trfA in wild-type cells compare to ΔyjbH cells (Figure 4A, blue columns 1 and 4.2 compare to orange columns 3 and 6.2). These data are expected, because deletion of yjbH results only in increase of Spx protein levels, while, diamide treatment results in both, increase of Spx protein levels and oxidation of Spx protein, leading to higher levels of active Spx and consequently, higher trfA transcription.
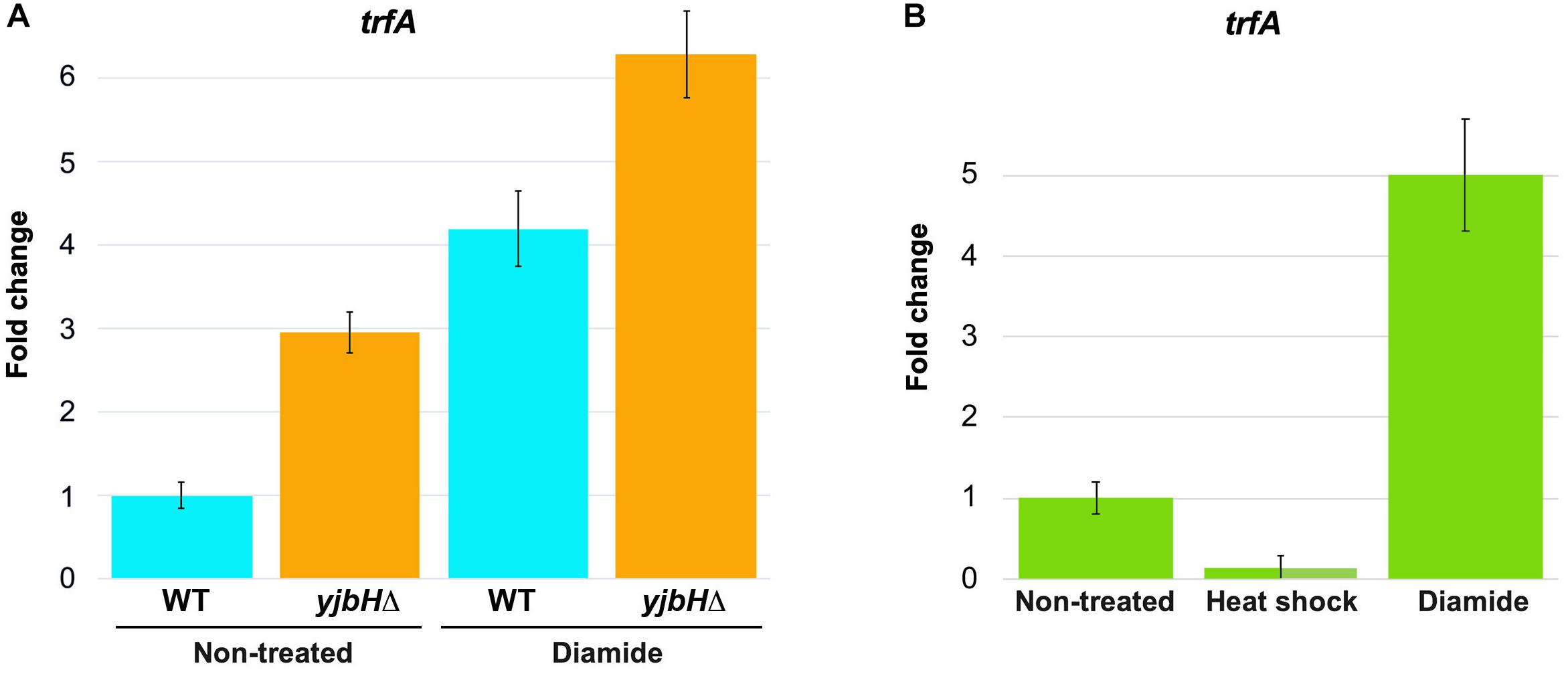
Figure 4. Transcription of trfA correspond to the high protein levels and solubility of Spx. RNAs were purified from cells described in Figures 3B,C. The level of trfA RNA was measured by RTqPCR in non-treated cells and in cells treated for 30 min with either heat shock or oxidative stress induced by diamide. 16S RNA was used as a reference normalizing housekeeping gene. Fold change of trfA gene expression in treated samples relative to non-treated samples was normalized to a reference gene (16S RNA). The relative gene expression in non-treated cells was set to 1. (A) The levels of trfA were increased in the yjbH deletion mutant, where the levels of Spx were increased. (B) Transcription of trfA is decreased after heat shock and increased after oxidative stress, that correspond to solubility of Spx.
Next, we tested if solubility affects functional state of Spx. We analyzed expression of trfA under heat and oxidative stress, where we observed different solubility of Spx. We found, that trfA mRNA levels were decreased upon heat shock stress while increased upon oxidative stress (Figure 4B). These observations corroborate our hypothesis that Spx is aggregated and less functional after heat shock, but not after diamide treatment where Spx was found soluble.
The correlations between YjbH aggregation, protein levels of active/inactive Spx and trfA transcription after heat or oxidative stresses, predict a different end-point effect on MazE antitoxin levels and MazF toxin activity. We, therefore, analyzed YjbH, Spx and MazE protein levels after diamide or heat shock treatment at different time points. Soluble and insoluble proteins were isolated from cell extracts adjusted to the same total protein concentrations.
In total protein extracts the levels of YjbH were similar before and after both stress conditions (Figure 5, Total proteins). YjbH was mostly found in the soluble fractions before stress (time 0). After 10 min of diamide treatment YjbH aggregated and, concomitantly, Spx was stabilized (Figure 5, left panel, diamide, Spx in Total proteins). As diamide treatment induces trfA transcription through increased amounts of functional Spx (Figure 4B), we expected an increase of MazE proteolysis. Indeed, after 10 min of diamide treatment we observed a dramatic reduction of MazE protein levels suggesting an increase of trfA-dependent MazE proteolysis by ClpCP (Figure 5, left panel, diamide, MazE in Total proteins). At the same time upon diamide treatment the level of MazF was increased, compared to untreated cells (Figure 5, left panel, diamide, MazF in Total proteins).
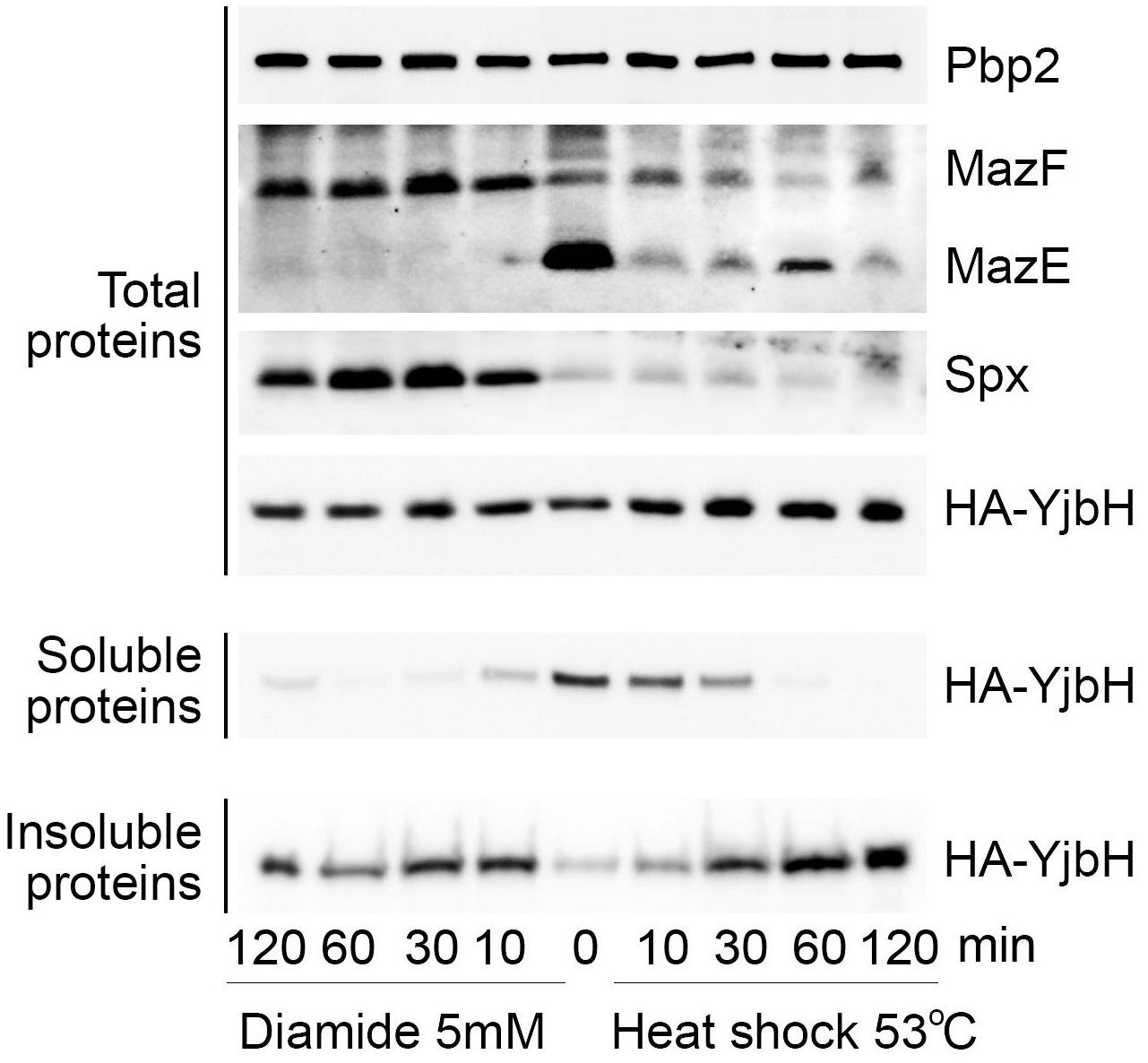
Figure 5. Upon heat shock and oxidative stresses MazE levels correlate with YjbH aggregation and Spx solubility. ΔyjbH cells expressing HA-YjbH were treated with high temperature (53°C, right), or with 5 mM of diamide (left) for different times, as indicated. Non-treated cells are marked by time 0. Whole cell extracts (Total protein) were adjusted to the same protein concentration and soluble and insoluble proteins were isolated. Samples were separated by gradient (4–12%) SDS-PAGE and analyzed by western blot with anti-HA, anti-Spx, anti-MazE, and anti-MazF antibodies. Pbp2 was used as a control for the equal protein amount in whole cell extracts.
After heat shock YjbH remained soluble for longer than upon diamide stress, but finally aggregated after 60 min of treatment (Figure 5, right panel, YjbH in Soluble and Insoluble proteins). Despite YjbH aggregation, Spx levels remained low and unchanged during all times of heat shock treatment compared to non-stressed conditions (Figure 5, right panel, heat shock, Spx in Total proteins). In agreement with inactive Spx that was found mostly aggregated after heat shock (Figure 3C), we observed a consequent reduced transcription of trfA gene (Figure 4B). A decreased trfA transcription suggests stabilization of MazE antitoxin, as reduction of TrfA will reduce MazE proteolysis. However, we did not observe stabilization of MazE proteolysis after heat shock. Levels of MazE protein were reduced compared to non-stress conditions, but not as much as after diamide treatment (Figure 5, right panel, MazE in Total proteins). These observations suggest that other potential factors must therefore be affected by heat shock and that could explain reduced MazE protein levels without an increase of TrfA.
The decreased MazE levels upon diamide or heat shock stress predict not only stabilized MazF, but also a higher MazF endoribonuclease activity compared to non-stressed condition. To investigate MazF activity we created a plasmid expressing MazF under control of inducible promoter. We transformed this plasmid in the strain deleted for mazEF TAS to model the situation when MazF is not inhibited by MazE. MazF protein was visible after 10 min of induction (Figure 6A). To investigate MazF endoribonuclease activity we analyzed mRNA levels of rsbW gene, one of the previously described MazF endoribonuclease targets (Schuster et al., 2015). We expect a higher cleavage or decreased mRNA levels of rsbW upon MazF toxin expression or conditions increasing MazF activity. The level of rsbW transcript was slightly increased in ΔmazEF cells compared to wild-type cells, and it was dramatically decreased when mazF transcription was induced in ΔmazEF cells (Figure 6B). In-depth analysis of reads covering rsbW transcript, clearly showed a reduction of reads mapping the identified MazF cleavage motif (UACAU) present in rsbW gene (Figure 6C). These results corroborate previous observations that rsbW mRNA is a target of MazF toxin (Schuster et al., 2015) and show that rsbW transcript levels are decreased upon MazF overexpression.
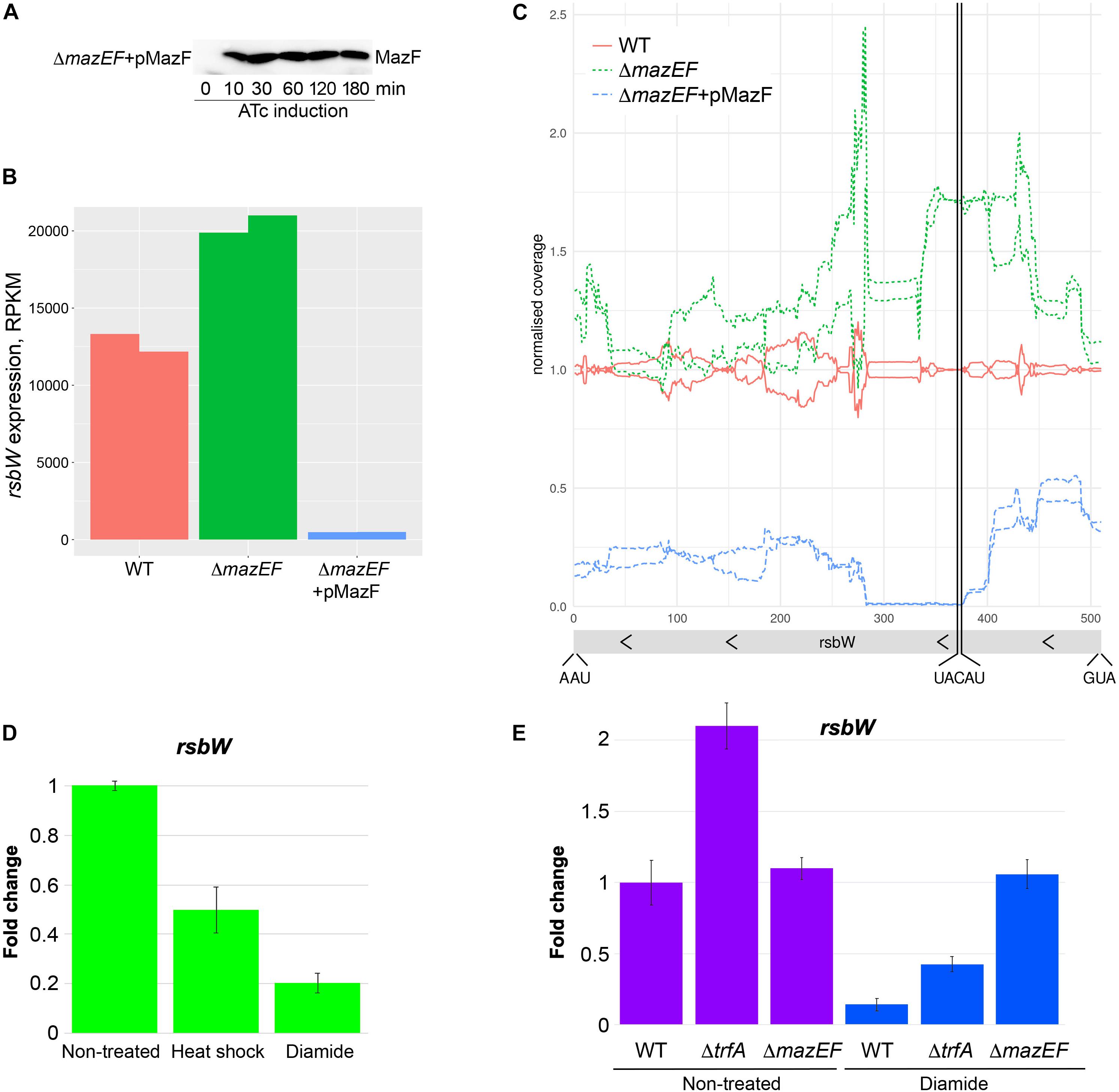
Figure 6. MazF cleaves rsbW at UACAU cleavage site and MazF activity correlates with YjbH aggregation and Spx solubility. (A) Western blot analysis of MazF protein produced after anhydrotetracycline (ATc) induction. The mazEF deleted strain was transformed with the pRAB11-MazF plasmid (indicated as pMazF), expressing mazF gene under control of ATc inducible promoter. Cultures were collected before ATc induction (time 0). Then ATc was added and cultures were collected after 10, 30, 60, 120, and 180 min. Protein were extracted and analyzed by SDS-PAGE and western blot with anti-MazF antibody. (B) Effect of MazF on rsbW level. Wild-type or ΔmazEF cells expressing either empty vector (WT and ΔmazEF) or mazF gene under control of ATc promoter (ΔmazEF + pMazF) were collected after 10 min of ATc induction. Total RNAs were purified and analyzed by RNA-seq. The RPKM values (Reads Per Kilobase Million) normalized by edgeR after excluding plasmid genes are show in duplicates. (C) In-depth analysis of rsbW gene expression by RNA-seq. Cells were treated as in panel (B). RNA-seq coverage along the rsbW region relative to the average wild type is shown. The raw RNASeq coverage was normalized to the total number of reads over the chromosome region and then divided by the coverage of the wild-type samples. The start codon, stop codon and, MazF cleavage site are indicated. (D) RNAs were purified from cells described in Figure 5. The level of rsbW RNA was measured by RTqPCR in non-treated cells and in cells treated for 30 min with either heat shock or oxidative stress induced by diamide. gyrB was used as a reference normalizing housekeeping gene. Fold change of rsbW gene expression in treated samples relative to non-treated samples was normalized to a reference gene (gyrB). The relative gene expression in non-treated cells was set to 1. (E) The level of rsbW RNA was measured by RTqPCR in wild-type, ΔtrfA or ΔmazEF cells treated or not with diamide for 30 min, as described in panel (D). The relative gene expression in non-treated wild-type cells was set to 1.
To analyze if diamide or heat shock stress affect MazF endoribonuclease activity, we measure levels of rsbW mRNA target by RTqPCR using a probe hybridizing exactly on the MazF cleavage motif. For control and normalization, we used gyrB gene that was previously shown to be a non-target of MazF (Fu et al., 2009). We observed two-fold and a five-fold reduction of rsbW mRNA levels upon heat shock or diamide treatment, respectively (Figure 6D).
Taken together, these results show that decreased levels of MazE antitoxin upon diamide and heat shock resulted in modulation of MazF toxin activity. The slight decrease of rsbW mRNA cleavage, despite the reduced levels of MazE upon heat shock, can be explained by residual MazE sufficient to maintain MazF inactive. In contrast, MazE levels upon diamide are absent or highly reduced thus resulting in liberated highly active MazF toxin.
To show that decreased levels of MazE protein upon diamide treatment (Figure 5), affecting MazF activity, is dependent on trfA adaptor, we analyzed rsbW levels in trfA deleted strain with or without diamide treatment (Figure 6E). As expected, the deletion of trfA resulted in increased levels of rsbW in both conditions, confirming the role of TrfA in MazE degradation and, consequently, MazF activation. We further analyzed whether the deletion of mazEF affects rsbW levels in cells treated or not with diamide. Deletion of mazEF in non-treated conditions did not dramatically change rsbW levels, being in agreement with data obtained by RNA-seq (Figure 6B). These results were expected as MazF activity is inhibited by MazE in wild type or not present in ΔmazEF mutant. However, upon diamide treatment we observed strong stabilization of rsbW in ΔmazEF mutant, that may be explained by some additional effects of diamide on rsbW expression by unknown mechanisms.
MazE antitoxin level ensures formation of at least a stoichiometric toxin-antitoxin complex and consequently, MazF inactivity. Under unknown conditions, the MazF endoribonuclease activity can increase and result in destruction of mRNA molecules leading to growth stasis (reviewed in Sierra et al., 2019). The clinical importance of MazF was recently highlighted by studies showing the role of MazF expression in chronic S. aureus infections (Ma et al., 2019). Using a murine abscess model of infection, MazF expression was shown to inhibit biofilm formation and to increase antibiotic tolerance allowing transition of S. aureus from acute to chronic infections (Ma et al., 2019). However, it is still unknown how S. aureus induces MazF expression and activity under in vivo infection conditions. To understand the possible mechanisms of MazF-activation, we investigated how MazF is regulated under different growth conditions.
In S. aureus, evidence suggests that the dormancy-related MazEF TAS is controlled by two different upstream proteolytic systems (ClpCP and ClpXP) involving two adaptor proteins, TrfA and YjbH, respectively. As YjbH adaptor was shown in B. subtilis to be prone to aggregation (Engman and von Wachenfeldt, 2015), we postulated that modulation of YjbH activity through aggregation can be a key step to control MazEF-dependent hdormancy (Figure 1). Indeed, we showed that upon various environmental stress stimulus S. aureus YjbH also aggregates and induces downstream effects regulating MazEF TAS through Spx/TrfA pathway. We showed that diamide induced oxidative stress, causes an increase of MazF toxin activity. Specifically, we uncovered a diamide-stress modulation of MazF activity through an upstream pathway that involves YjbH aggregation, accumulation of active/soluble Spx and increase transcription of the ClpCP adaptor protein TrfA. Increase of trfA transcription upon antibiotic treatment is accompanied by an increase of TrfA protein levels (Jousselin et al., 2013). And the decreased protein levels of TrfA are correlated with the increased protein levels of MazE (Donegan et al., 2014). In agreement with these previous studies, we showed, that the TrfA increase leads to decreased levels of MazE antitoxin and higher amounts of active MazF toxin. These results show that at least oxidative stress increases MazF activity, a stress condition certainly found during S. aureus eukaryotic cell infection.
Despite a decrease in MazE antitoxin levels upon heat shock there was only a slight decrease in MazF activity. Since, the level of MazF was also decreased upon heat shock, we suggested that the low amount of MazE antitoxin present was sufficient to maintain low level of MazF inactive. Interestingly, heat shock reduction of MazE levels was achieved by upstream changes that do not affect Spx accumulation in contrast to diamide stress. Heat shock did not induce accumulation of Spx protein but instead resulted in higher amounts of aggregated and inactive Spx, in accordance with decreased transcription of trfA. A potential decreased activity of ClpCP proteolytic systems at heat shock may explain TrfA stabilization that assists MazE degradation.
We observed that not all conditions leading to YjbH aggregation resulted in Spx accumulation. This finding can be explained by stimuli-specific changes in other proteolytic partners involved in Spx degradation. Indeed, Spx accumulation upon diamide stress in B. subtilis is dependent on decreased activity of ClpP protease and aggregation of both ClpX and YjbH proteins (Garg et al., 2009; Engman and von Wachenfeldt, 2015). Moreover, it has recently been observed that depending on the stimuli, different regulatory events occur to increase and activate Spx (Rojas-Tapias and Helmann, 2018). While activation of Spx regulon under oxidative stress is accompanied by decreased proteolysis and decreased oxidation state of Spx itself, activation of Spx regulon under cell wall stress requires Spx transcriptional increase and Spx reduced state (Rojas-Tapias and Helmann, 2018).
Our observations suggest that a similar stimuli-dependent effect is present in S. aureus. We observed an increased accumulation of Spx protein both under diamide and vancomycin stress, however, achieved through different regulatory events. Diamide induces YjbH aggregation and no effect on Spx transcription was observed (our unpublished observation). In contrast, vancomycin does not induce YjbH aggregation but induces Spx transcription (our unpublished observation). Interestingly, both conditions result in production of an active Spx because transcription of Spx targets, such as trfA, is increased (Jousselin et al., 2013).
We argue that adaptor proteins YjbH and TrfA, proteolytic systems ClpXP and ClpCP and, finally, Spx transcriptional regulator are involved in upstream control of MazEF TAS. However, expression and activity of YjbH and TrfA, ClpXP and ClpCP, and Spx can be differently modulated, as it was observed previously upon diamide and heat shock in B. subtilis and S. aureus (Frees et al., 2003, 2004; Zhang and Zuber, 2007; Garg et al., 2009). YjbH can be controlled at both transcriptional and/or post-transcriptional levels. Oxidative stress induced by diamide, activated transcription of yjbH in S. aureus (Engman et al., 2012). However, no other stress stimuli have been tested. At the post-transcriptional level, YjbH activity can be modulated by aggregation and/or through anti-adaptor proteins and/or phosphorylation following different stimuli, as shown in B. subtilis and other bacteria (Kirstein et al., 2007; Elsholz et al., 2011; Engman and von Wachenfeldt, 2015; Rojas-Tapias and Helmann, 2018). Our results show that in S. aureus, YjbH can also aggregate, however, the identification of YjbH anti-adaptors such as YirB in B. subtilis, or adaptor phosphorylation remains to be investigated. Also, no studies have been conducted to investigate potential anti-adaptors or phosphorylation of TrfA, the second adaptor protein governing MazEF TAS.
Finally, some studies have analyzed the effect of different stress stimuli on Spx. First, Spx is transcribed from at least two different promoters and affected by mutation of clpP and clpX genes (Pamp et al., 2006; Jousselin et al., 2013). Conditions inducing Spx protein accumulation reduced Spx transcription, thus, Spx was proposed to be a repressor of its own expression (Pamp et al., 2006; Donegan et al., 2019). Interestingly, Spx transcription is induced by anaerobiosis and not affected by high temperature, diamide or NaCl (Pamp et al., 2006). In agreement with these observations, we did not observe increased Spx transcription under diamide, heat shock, oxacillin or kanamycin stress while a two-fold induction was observed upon vancomycin stress [(Jousselin et al., 2013) (and our unpublished observation)]. Secondly, Spx is not regulated only at the transcriptional level. As no correlation between transcription and Spx protein levels was observed, Spx post-transcriptional regulation was postulated. Indeed, Spx levels can be modulated by proteolysis through ClpXP, and Spx activity can be modulated by its redox state that differs upon different stimuli in B. subtilis (Rojas-Tapias and Helmann, 2018). We observed a stimuli dependent Spx protein aggregation that affected Spx activity, and could conceivably be responsible for downstream effects, such as regulation of MazEF TAS.
In conclusion, the exact mechanisms of sensing and signal transmission that culminate in MazEF TAS activation remain to be elucidated. Our work demonstrates YjbH aggregation in S. aureus in response to different environmental stresses, may presumably lead to modulation of MazF activity. We found oxidative stress as one potential in vivo growth condition regulating MazF activity. Our observation supports the hypothesis that oxidative stress, found upon in vivo infection in Escherichia coli, can trigger activation of MazF endonuclease leading to a reduced metabolic state and, consequently, entry into persistence state (Mok et al., 2015). Our hypothesis was strongly supported by the very interesting recent study where the persister-like phenotype in B. subtilis was connected to increased levels of Spx at specific environmental conditions, such as stationary phase (Schafer et al., 2019).
The datasets generated for this study are available on request to the corresponding author.
OP designed and performed the experiments, constructed the plasmids and strains, and participated in the writing of the manuscript and conception of the work. OK did the fluorescent microscopy. AR participated in the writing of the manuscript and conception of the work. FB did all the RNAseq analysis and the analysis of MazF cleavage site, and participated in the discussion of the results, revision of the manuscript, writing of the revised manuscript, and conception of the work.
This work was supported by the Swiss National Science Foundation grants 310030-169404 awarded to AR, and grants from Helmut Horten Stiftung and from Ernst and Lucie Schmidheiny Foundation awarded to OP.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We kindly thank Claes von Wachenfeld (Lund University) for sfGFP-YjbH plasmids and strains, Dorte Frees for anti-Spx antibody, Patrick Viollier for anti-MazE and anti-MazF antibodies, Friedrich Götz for HG003 mazEF deleted strain, Mariana Gomes de Pinho for anti-Pbp2 antibodies, and William Kelley for critical reading of the manuscript.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.00113/full#supplementary-material
Aakre, C. D., Phung, T. N., Huang, D., and Laub, M. T. (2013). A bacterial toxin inhibits DNA replication elongation through a direct interaction with the beta sliding clamp. Mol. Cell 52, 617–628. doi: 10.1016/j.molcel.2013.10.014
Andrey, D. O., Renzoni, A., Monod, A., Lew, D. P., Cheung, A. L., and Kelley, W. L. (2010). Control of the Staphylococcus aureus toxic shock tst promoter by the global regulator SarA. J. Bacteriol. 192, 6077–6085. doi: 10.1128/JB.00146-10
Antelmann, H., and Helmann, J. D. (2011). Thiol-based redox switches and gene regulation. Antioxid. Redox Signal. 14, 1049–1063. doi: 10.1089/ars.2010.3400
Awad, W., Al-Eryani, Y., Ekstrom, S., Logan, D. T., and von Wachenfeldt, C. (2019). Structural basis for YjbH adaptor-mediated recognition of transcription factor spx. Structure 27, 923–936.e926. doi: 10.1016/j.str.2019.03.009
Bigger, J. W. (1944). Treatment of staphylococcal infections with penicillin by intermittent sterilisation. Lancet 244, 497–500. doi: 10.1016/S0140-6736(00)74210-3
Castro-Roa, D., Garcia-Pino, A., De Gieter, S., van Nuland, N. A. J., Loris, R., and Zenkin, N. (2013). The Fic protein Doc uses an inverted substrate to phosphorylate and inactivate EF-Tu. Nat. Chem. Biol. 9, 811–817. doi: 10.1038/nchembio.1364
Chan, C. M., Garg, S., Lin, A. A., and Zuber, P. (2012). Geobacillus thermodenitrificans YjbH recognizes the C-terminal end of Bacillus subtilis Spx to accelerate Spx proteolysis by ClpXP. Microbiology 158(Pt 5), 1268–1278. doi: 10.1099/mic.0.057661-0
Chan, C. M., Hahn, E., and Zuber, P. (2014). Adaptor bypass mutations of Bacillus subtilis spx suggest a mechanism for YjbH-enhanced proteolysis of the regulator Spx by ClpXP. Mol. Microbiol. 93, 426–438. doi: 10.1111/mmi.12671
Cho, J., Carr, A. N., Whitworth, L., Johnson, B., and Wilson, K. S. (2017). MazEF toxin-antitoxin proteins alter Escherichia coli cell morphology and infrastructure during persister formation and regrowth. Microbiology 163, 308–321. doi: 10.1099/mic.0.000436
Coussens, N. P., and Daines, D. A. (2016). Wake me when it’s over – bacterial toxin-antitoxin proteins and induced dormancy. Exp. Biol. Med. (Maywood) 241, 1332–1342. doi: 10.1177/1535370216651938
Culviner, P. H., and Laub, M. T. (2018). Global analysis of the E. coli toxin MazF reveals widespread cleavage of mRNA and the Inhibition of rRNA maturation and ribosome biogenesis. Mol. Cell 70, 868–880.e10. doi: 10.1016/j.molcel.2018.04.026
Donegan, N. P., and Cheung, A. L. (2009). Regulation of the mazEF toxin-antitoxin module in Staphylococcus aureus and its impact on sigB expression. J. Bacteriol. 191, 2795–2805. doi: 10.1128/JB.01713-08
Donegan, N. P., Manna, A. C., Tseng, C. W., Liu, G. Y., and Cheung, A. L. (2019). CspA regulation of Staphylococcus aureus carotenoid levels and sigma(B) activity is controlled by YjbH and Spx. Mol. Microbiol. 112, 532–551. doi: 10.1111/mmi.14273
Donegan, N. P., Marvin, J. S., and Cheung, A. L. (2014). Role of adaptor TrfA and ClpPC in controlling levels of SsrA-tagged proteins and antitoxins in Staphylococcus aureus. J. Bacteriol. 196, 4140–4151. doi: 10.1128/JB.02222-14
Donegan, N. P., Thompson, E. T., Fu, Z., and Cheung, A. L. (2010). Proteolytic regulation of toxin-antitoxin systems by ClpPC in Staphylococcus aureus. J. Bacteriol. 192, 1416–1422. doi: 10.1128/JB.00233-09
Elsholz, A. K., Hempel, K., Michalik, S., Gronau, K., Becher, D., Hecker, M., et al. (2011). Activity control of the ClpC adaptor McsB in Bacillus subtilis. J. Bacteriol. 193, 3887–3893. doi: 10.1128/JB.00079-11
Engman, J., Rogstam, A., Frees, D., Ingmer, H., and von Wachenfeldt, C. (2012). The YjbH adaptor protein enhances proteolysis of the transcriptional regulator Spx in Staphylococcus aureus. J. Bacteriol. 194, 1186–1194. doi: 10.1128/JB.06414-11
Engman, J., and von Wachenfeldt, C. (2015). Regulated protein aggregation: a mechanism to control the activity of the ClpXP adaptor protein YjbH. Mol. Microbiol. 95, 51–63. doi: 10.1111/mmi.12842
Frees, D., Chastanet, A., Qazi, S., Sorensen, K., Hill, P., Msadek, T., et al. (2004). Clp ATPases are required for stress tolerance, intracellular replication and biofilm formation in Staphylococcus aureus. Mol. Microbiol. 54, 1445–1462. doi: 10.1111/j.1365-2958.2004.04368.x
Frees, D., Qazi, S. N., Hill, P. J., and Ingmer, H. (2003). Alternative roles of ClpX and ClpP in Staphylococcus aureus stress tolerance and virulence. Mol. Microbiol. 48, 1565–1578. doi: 10.1046/j.1365-2958.2003.03524.x
Fu, Z., Donegan, N. P., Memmi, G., and Cheung, A. L. (2007). Characterization of MazFSa, an endoribonuclease from Staphylococcus aureus. J. Bacteriol. 189, 8871–8879. doi: 10.1128/JB.01272-07
Fu, Z., Tamber, S., Memmi, G., Donegan, N. P., and Cheung, A. L. (2009). Overexpression of MazFsa in Staphylococcus aureus induces bacteriostasis by selectively targeting mRNAs for cleavage. J. Bacteriol. 191, 2051–2059. doi: 10.1128/JB.00907-08
Garg, S. K., Kommineni, S., Henslee, L., Zhang, Y., and Zuber, P. (2009). The YjbH protein of Bacillus subtilis enhances ClpXP-catalyzed proteolysis of Spx. J. Bacteriol. 191, 1268–1277. doi: 10.1128/JB.01289-08
Harms, A., Stanger, F. V., Scheu, P. D., de Jong, I. G., Goepfert, A., Glatter, T., et al. (2015). Adenylylation of Gyrase and Topo IV by FicT toxins disrupts bacterial DNA topology. Cell Rep. 12, 1497–1507. doi: 10.1016/j.celrep.2015.07.056
Helle, L., Kull, M., Mayer, S., Marincola, G., Zelder, M. E., Goerke, C., et al. (2011). Vectors for improved Tet repressor-dependent gradual gene induction or silencing in Staphylococcus aureus. Microbiology 157(Pt 12) 3314–3323. doi: 10.1099/mic.0.052548-0
Herbert, S., Ziebandt, A. K., Ohlsen, K., Schafer, T., Hecker, M., Albrecht, D., et al. (2010). Repair of global regulators in Staphylococcus aureus 8325 and comparative analysis with other clinical isolates. Infect. Immun. 78, 2877–2889. doi: 10.1128/IAI.00088-10
Jousselin, A., Kelley, W. L., Barras, C., Lew, D. P., and Renzoni, A. (2013). The Staphylococcus aureus thiol/oxidative stress global regulator Spx controls trfA, a gene implicated in cell wall antibiotic resistance. Antimicrob. Agents Chemother. 57, 3283–3292. doi: 10.1128/AAC.00220-13
Kirstein, J., Dougan, D. A., Gerth, U., Hecker, M., and Turgay, K. (2007). The tyrosine kinase McsB is a regulated adaptor protein for ClpCP. EMBO J. 26, 2061–2070. doi: 10.1038/sj.emboj.7601655
Larsson, J. T., Rogstam, A., and von Wachenfeldt, C. (2007). YjbH is a novel negative effector of the disulphide stress regulator, Spx, in Bacillus subtilis. Mol. Microbiol. 66, 669–684. doi: 10.1111/j.1365-2958.2007.05949.x
Ma, D., Mandell, J. B., Donegan, N. P., Cheung, A. L., Ma, W., Rothenberger, S., et al. (2019). The toxin-antitoxin MazEF drives Staphylococcus aureus biofilm formation, antibiotic tolerance, and chronic infection. mBio 10:e01658-19. doi: 10.1128/mBio.01658-19
Maki, S., Takiguchi, S., Miki, T., and Horiuchi, T. (1992). Modulation of DNA supercoiling activity of Escherichia coli DNA gyrase by F plasmid proteins. Antagonistic actions of LetA (CcdA) and LetD (CcdB) proteins. J. Biol. Chem. 267, 12244–12251.
Masuda, H., Tan, Q., Awano, N., Wu, K. P., and Inouye, M. (2012). YeeU enhances the bundling of cytoskeletal polymers of MreB and FtsZ, antagonizing the CbtA (YeeV) toxicity in Escherichia coli. Mol. Microbiol. 84, 979–989. doi: 10.1111/j.1365-2958.2012.08068.x
Mittenhuber, G. (1999). Occurrence of mazEF-like antitoxin/toxin systems in bacteria. J. Mol. Microbiol. Biotechnol. 1, 295–302.
Miyamoto, T., Yokota, A., Ota, Y., Tsuruga, M., Aoi, R., Tsuneda, S., et al. (2018). Nitrosomonas europaea MazF specifically recognises the UGG motif and promotes selective RNA degradation. Front. Microbiol. 9:2386. doi: 10.3389/fmicb.2018.02386
Mok, W. W., Park, J. O., Rabinowitz, J. D., and Brynildsen, M. P. (2015). RNA futile cycling in model persisters derived from MazF accumulation. MBio 6:e01588-15. doi: 10.1128/mBio.01588-15
Mutschler, H., Gebhardt, M., Shoeman, R. L., and Meinhart, A. (2011). A novel mechanism of programmed cell death in bacteria by toxin-antitoxin systems corrupts peptidoglycan synthesis. PLoS Biol. 9:e1001033. doi: 10.1371/journal.pbio.1001033
Nguyen, D., Joshi-Datar, A., Lepine, F., Bauerle, E., Olakanmi, O., Beer, K., et al. (2011). Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science 334, 982–986. doi: 10.1126/science.1211037
Page, R., and Peti, W. (2016). Toxin-antitoxin systems in bacterial growth arrest and persistence. Nat. Chem. Biol. 12, 208–214. doi: 10.1038/nchembio.2044
Pamp, S. J., Frees, D., Engelmann, S., Hecker, M., and Ingmer, H. (2006). Spx is a global effector impacting stress tolerance and biofilm formation in Staphylococcus aureus. J. Bacteriol. 188, 4861–4870. doi: 10.1128/JB.00194-06
Panasenko, O. O., and Collart, M. A. (2012). Presence of Not5 and ubiquitinated Rps7A in polysome fractions depends upon the Not4 E3 ligase. Mol. Microbiol. 83, 640–653. doi: 10.1111/j.1365-2958.2011.07957.x
Rao, X., Huang, X., Zhou, Z., and Lin, X. (2013). An improvement of the 2^(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinforma. Biomath. 3, 71–85.
Renzoni, A., Andrey, D. O., Jousselin, A., Barras, C., Monod, A., Vaudaux, P., et al. (2011). Whole genome sequencing and complete genetic analysis reveals novel pathways to glycopeptide resistance in Staphylococcus aureus. PLoS One 6:e21577. doi: 10.1371/journal.pone.0021577
Robinson, M. D., McCarthy, D. J., and Smyth, G. K. (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. doi: 10.1093/bioinformatics/btp616
Robinson, M. D., and Oshlack, A. (2010). A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11:R25. doi: 10.1186/gb-2010-11-3-r25
Rojas-Tapias, D. F., and Helmann, J. D. (2018). Induction of the Spx regulon by cell wall stress reveals novel regulatory mechanisms in Bacillus subtilis. Mol. Microbiol. 107, 659–674. doi: 10.1111/mmi.13906
Sassi, M., Felden, B., and Augagneur, Y. (2014). Draft genome sequence of Staphylococcus aureus subsp. aureus strain HG003, an NCTC8325 derivative. Genome Announc. 2:e00855-14. doi: 10.1128/genomeA.00855-14
Schafer, H., Heinz, A., Sudzinova, P., Voss, M., Hantke, I., Krasny, L., et al. (2019). Spx, the central regulator of the heat and oxidative stress response in B. subtilis, can repress transcription of translation-related genes. Mol. Microbiol. 111, 514–533. doi: 10.1111/mmi.14171
Schifano, J. M., Edifor, R., Sharp, J. D., Ouyang, M., Konkimalla, A., Husson, R. N., et al. (2013). Mycobacterial toxin MazF-mt6 inhibits translation through cleavage of 23S rRNA at the ribosomal a site. Proc. Natl. Acad. Sci. U.S.A. 110, 8501–8506. doi: 10.1073/pnas.1222031110
Schuster, C. F., and Bertram, R. (2016). Toxin-antitoxin systems of Staphylococcus aureus. Toxins (Basel) 8:140. doi: 10.3390/toxins8050140
Schuster, C. F., Mechler, L., Nolle, N., Krismer, B., Zelder, M. E., Gotz, F., et al. (2015). The mazEF toxin-antitoxin system alters the beta-lactam susceptibility of Staphylococcus aureus. PLoS One 10:e0126118. doi: 10.1371/journal.pone.0126118
Sierra, R., Viollier, P., and Renzoni, A. (2019). Linking toxin-antitoxin systems with phenotypes: a Staphylococcus aureus viewpoint. Biochim. Biophys. Acta Gene Regul. Mech. 1862, 742–751. doi: 10.1016/j.bbagrm.2018.07.009
Stahlhut, S. G., Alqarzaee, A. A., Jensen, C., Fisker, N. S., Pereira, A. R., Pinho, M. G., et al. (2017). The ClpXP protease is dispensable for degradation of unfolded proteins in Staphylococcus aureus. Sci. Rep. 7:11739. doi: 10.1038/s41598-017-12122-y
Van Melderen, L., and Wood, T. K. (2017). Commentary: what is the link between stringent response, endoribonuclease encoding type II toxin-antitoxin systems and persistence? Front. Microbiol. 8:191. doi: 10.3389/fmicb.2017.00191
Yamaguchi, Y., Park, J. H., and Inouye, M. (2011). Toxin-antitoxin systems in bacteria and archaea. Annu. Rev. Genet. 45, 61–79. doi: 10.1146/annurev-genet-110410-132412
Zhang, Y., and Zuber, P. (2007). Requirement of the zinc-binding domain of ClpX for Spx proteolysis in Bacillus subtilis and effects of disulfide stress on ClpXP activity. J. Bacteriol. 189, 7669–7680. doi: 10.1128/JB.00745-07
Zhu, L., Inoue, K., Yoshizumi, S., Kobayashi, H., Zhang, Y., Ouyang, M., et al. (2009). Staphylococcus aureus MazF specifically cleaves a pentad sequence, UACAU, which is unusually abundant in the mRNA for pathogenic adhesive factor SraP. J. Bacteriol. 191, 3248–3255. doi: 10.1128/JB.01815-08
Zorzini, V., Buts, L., Sleutel, M., Garcia-Pino, A., Talavera, A., Haesaerts, S., et al. (2014). Structural and biophysical characterization of Staphylococcus aureus SaMazF shows conservation of functional dynamics. Nucleic Acids Res. 42, 6709–6725. doi: 10.1093/nar/gku266
Keywords: YjbH, aggregation, Staphylococcus aureus, Spx, MazEF, toxin-antitoxin system, dormancy, antibiotic resistance
Citation: Panasenko OO, Bezrukov F, Komarynets O and Renzoni A (2020) YjbH Solubility Controls Spx in Staphylococcus aureus: Implication for MazEF Toxin-Antitoxin System Regulation. Front. Microbiol. 11:113. doi: 10.3389/fmicb.2020.00113
Received: 26 August 2019; Accepted: 17 January 2020;
Published: 06 February 2020.
Edited by:
Satoshi Tsuneda, Waseda University, JapanReviewed by:
Dorte Frees, University of Copenhagen, DenmarkCopyright © 2020 Panasenko, Bezrukov, Komarynets and Renzoni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olesya O. Panasenko, b2xlc3lhLnBhbmFzZW5rb0B1bmlnZS5jaA==; Adriana Renzoni, YWRyaWFuYS5yZW56b25pQGhjdWdlLmNo
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.