- 1State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, China
- 2Department of Operative Dentistry and Endodontics, West China Hospital of Stomatology, Sichuan University, Chengdu, China
Periodontal disease is one of the most common diseases of the oral cavity affecting up to 90% of the worldwide population. Smoking has been identified as a major risk factor in the development and progression of periodontal disease. It is essential to assess the influence of smoking on subgingival microflora that is the principal etiological factor of the disease to clarify the contribution of smoking to periodontal disease. Therefore, this article reviews the current research findings regarding the impact of smoking on subgingival microflora and discusses several potential mechanisms. Cultivation-based and targeted molecular approaches yield controversial results in determining the presence or absence of smoking-induced differences in the prevalence or levels of certain periodontal pathogens, such as the “red complex.” However, substantial changes in the subgingival microflora of smokers, regardless of their periodontal condition (clinical health, gingivitis, or periodontitis), have been demonstrated in recent microbiome studies. Available literature suggests that smoking facilitates early acquisition and colonization of periodontal pathogens, resulting in an “at-risk-for-harm” subgingival microbial community in the healthy periodontium. In periodontal diseases, the subgingival microflora in smokers is characterized by a pathogen-enriched community with lower resilience compared to that in non-smokers, which increases the difficulty of treatment. Biological changes in key pathogens, such as Porphyromonas gingivalis, together with the ineffective host immune response for clearance, might contribute to alterations in the subgingival microflora in smokers. Nonetheless, further studies are necessary to provide solid evidence for the underlying mechanisms.
Introduction
Smoking remains a highly prevalent addiction in many populations worldwide despite the increasing awareness of its harmful effects on general health (World Health Organization [WHO], 2018). The number of smokers is >1.1 billion (1 out of 7) globally now, and over 8 million people die annually because of smoking1. As one of the five leading risk factors for the global burden of the disease, smoking is responsible for various diseases, including cancer, cardiovascular disease, chronic obstructive pulmonary disease, and periodontal disease (GBD 2016 Risk Factors Collaborators, 2017).
Periodontal disease, also known as gum disease, comprises a range of polymicrobial infectious disorders (such as gingivitis and periodontitis) that affect the tooth-supporting tissues (the gingiva, alveolar bone, and periodontal ligament). It is the most common cause of tooth loss and also contributes to systemic diseases. Smoking has been recognized as a major risk factor for periodontal disease, affecting the prevalence, severity, progression, and treatment response of the disease, second only to the dental plaque. Epidemiological studies have presented a significantly higher risk for periodontal disease in smokers compared to non-smokers, and the increased risk is proportional to the duration and rate of smoking (Do et al., 2008; Bergstrom, 2014; Eke et al., 2016). Smokers exhibit a clinically distinct predisposition to periodontal disease, with deeper pockets, more extensive and severe attachment loss, greater levels of bone destruction, and a higher rate of tooth loss (Ylostalo et al., 2004; Baljoon et al., 2005; Johnson and Guthmiller, 2007). In addition, smoking exerts an adverse influence on the clinical treatment outcomes of non-surgical and surgical therapies as well as the long-term success of implant placement (Heasman et al., 2006; Patel et al., 2012).
Considering the well-established deleterious effects of smoking on periodontal health, it is of great importance to understand the underlying mechanisms, which remain largely unclear. Its widely accepted that both periodontal microflora and host response play critical roles in the initiation and progression of periodontal disease (Kinane et al., 2017). Considerable attention has been focused on the effects of smoking on host response in previous studies, which demonstrate that smoking increases the host’s susceptibility and risk of infection by inducing immune dysfunction (Ryder, 2007; Lee et al., 2012). However, it is still necessary to carry out a more detailed assessment of the effects of smoking on subgingival microflora that causes the infectious disease. A previous review article investigated the correlation between smoking and oral and nasopharyngeal bacterial flora, and demonstrated the adverse effects of smoking on the colonization of potential pathogens and the increased frequency of upper respiratory tract infections (Brook, 2011). This review aims to present current research findings of the impact of smoking on subgingival microflora and discuss possible mechanisms by which smoking interferes with the microflora.
Smoking and Subgingival Microflora
In vitro Effects of Smoking on Key Periodontal Pathogens
Model Pathogens and Proxies for Smoking Exposure
The microbial etiology of periodontal disease has been the focus of attention over the years, and various hypotheses have been proposed (Hajishengallis and Lamont, 2012). Notwithstanding, Porphyromonas gingivalis (P. gingivalis), a black-pigmented Gram-negative asaccharolytic bacterium from the phylum Bacteroidetes, has long been considered as an important pathogen involved in the initiation and progression of periodontal disease. P. gingivalis was shown to have a profound effect on both the amount and composition of the oral microbiota, even at a low abundance by acting as a potential community activist (Hajishengallis et al., 2011; Darveau et al., 2012). With a variety of virulence factors (such as the well-known gingipains), P. gingivalis can manipulate the host immune response by different strategies, finally leading to periodontal disease (Zenobia and Hajishengallis, 2015; Sochalska and Potempa, 2017). Therefore, the in vitro effects of smoking have mostly been investigated using P. gingivalis as a model pathogen (Zhou et al., 2007; Bagaitkar et al., 2009; Bondy-Carey et al., 2013).
The smoke generated upon the burning of tobacco is a complex, dynamic, and reactive mixture of over 5000 chemicals, with cytotoxic, mutagenic, carcinogenic, or antigenic properties (Talhout et al., 2011). Nicotine, a potent parasympathomimetic alkaloid, is the most well-known constituent with a highly addictive nature. It is considered as a major contributor to the development of dependence and is responsible for the widespread use and difficulty of quitting smoking (Pollock et al., 2009). Therefore, nicotine and its major metabolite cotinine have been widely used to investigate the influence of smoking on periodontal microorganisms (Cogo et al., 2009; Baek et al., 2012). Besides, cigarette smoke extract (CSE) and cigarette smoke condensate (CSC) are also representative cigarette smoke solutions for conducting the in vitro biological test, in which the non-nicotine constituents are considered (Zhang et al., 2010; Imamura et al., 2015).
Effects of Smoking on the Growth and Virulence of Key Pathogens
Cogo et al. (2008) tested the effects of nicotine and cotinine on growth of seven oral bacteria species including P. gingivalis at concentrations of 400, 100, 25, 6.25, 1.5, and 0.4 μg/mL, which were in agreement with or higher than the physiological levels of nicotine and cotinine found in saliva and gingival crevicular fluid (McGuire et al., 1989; Chen et al., 2001; Dhar, 2004); however, nicotine and cotinine did not alter the growth patterns of any of the bacteria tested. Similarly, the growth of P. gingivalis, Fusobacterium nucleatum (F. nucleatum), and Filifactor alocis (F. alocis) was also shown to be unaffected by CSE exposure at concentrations of 0.5, 2, and 4 μg/mL nicotine equivalents (Bagaitkar et al., 2009; Zeller et al., 2019). Bacteria are equipped with sophisticated mechanisms for adapting to complex environmental changes and thereby ensuring adequate growth and survival within the host (Brooks et al., 2011). Consistent with this fact, while the growth of periodontal pathogens is not found to be directly influenced by smoking, some changes in the virulence factors of bacteria are observed (Table 1). CSE changed the phenotype of P. gingivalis by up-regulating the gene expression of major fimbrial antigen (FimA), inducing the protein expression of the outer membrane RagA and RagB, suppressing the production of capsular polysaccharides, and neutralizing the proinflammatory response to subsequent TLR2 stimulation (Bagaitkar et al., 2009). Nevertheless, most effects were reversed when CSE-exposed bacteria were sub-cultured in a fresh medium without CSE. Thus, P. gingivalis appears to reversibly respond to CSE as an environmental stress. CSE also influenced the cell-bound Kgp and RgP gingipain production in a strain-specific manner (suppression in P. gingivalis ATCC33277 but augmentation in P. gingivalis W83) (Bondy-Carey et al., 2013). In addition, CSE exposure decreased the proinflammatory capacity (TNF-a, IL-6) of P. gingivalis biofilms (Bagaitkar et al., 2011). Taken together, these studies suggest that periodontal pathogens can withstand the complex mixture of toxins in smoking by altering their virulence factors.
Effects of Smoking on the Microbial Functions of Pathogen–Host Cell Interaction
The effects of smoking on the microbial functions of pathogen–host cell interaction constitute another major part of the in vitro studies (three main research focuses are summarized in Table 2). The adherence and invasion of bacteria to the host cells are important steps in the pathogenesis of infections. Epithelial cells that encompass mucosal surfaces represent the first line of defense against bacterial colonization (Tribble and Lamont, 2010). A few studies have investigated the ability of periodontal pathogens to colonize the epithelial cells after bacterial or cellular exposure to harmful substances from smoking (Teughels et al., 2005; Cogo et al., 2009; Imamura et al., 2015); however, discrepant results were reported. Teughels et al. (2005) tested the susceptibility of primary human gingival epithelial cells to be colonized by P. gingivalis and Actinobacillus actinomycetemcomitans (A. actinomycetemcomitans) after pre-exposing the cells to 1 mg/mL cotinine or nicotine. A species-dependent effect of nicotine and cotinine was observed. Whereas a moderate increase in attachment to cells was found in A. actinomycetemcomitans, a decreased attachment was recorded in P. gingivalis. On the contrary, the study by Cogo et al. (2009) showed that 100 μg/mL cotinine increased the adherence and invasion of P. gingivalis to epithelial cells (KB cells), when the bacteria were exposed to this substance; the pre-exposure of epithelial cells to cotinine or nicotine did not alter the colonization properties of P. gingivalis. Another study reported that a low concentration (1 μg/mL) of CSC increased the invasion of P. gingivalis to human gingival epithelial cells (Ca9-22) only both bacterial and cellular exposure were performed (Imamura et al., 2015). Therefore, due to the differences in the experiment design between different studies, such as the concentrations of the materials and cells tested, no certain conclusion could be drawn based on current studies regarding the possible correlation between bacterial cell colonization of periodontal pathogens and smoking exposure.
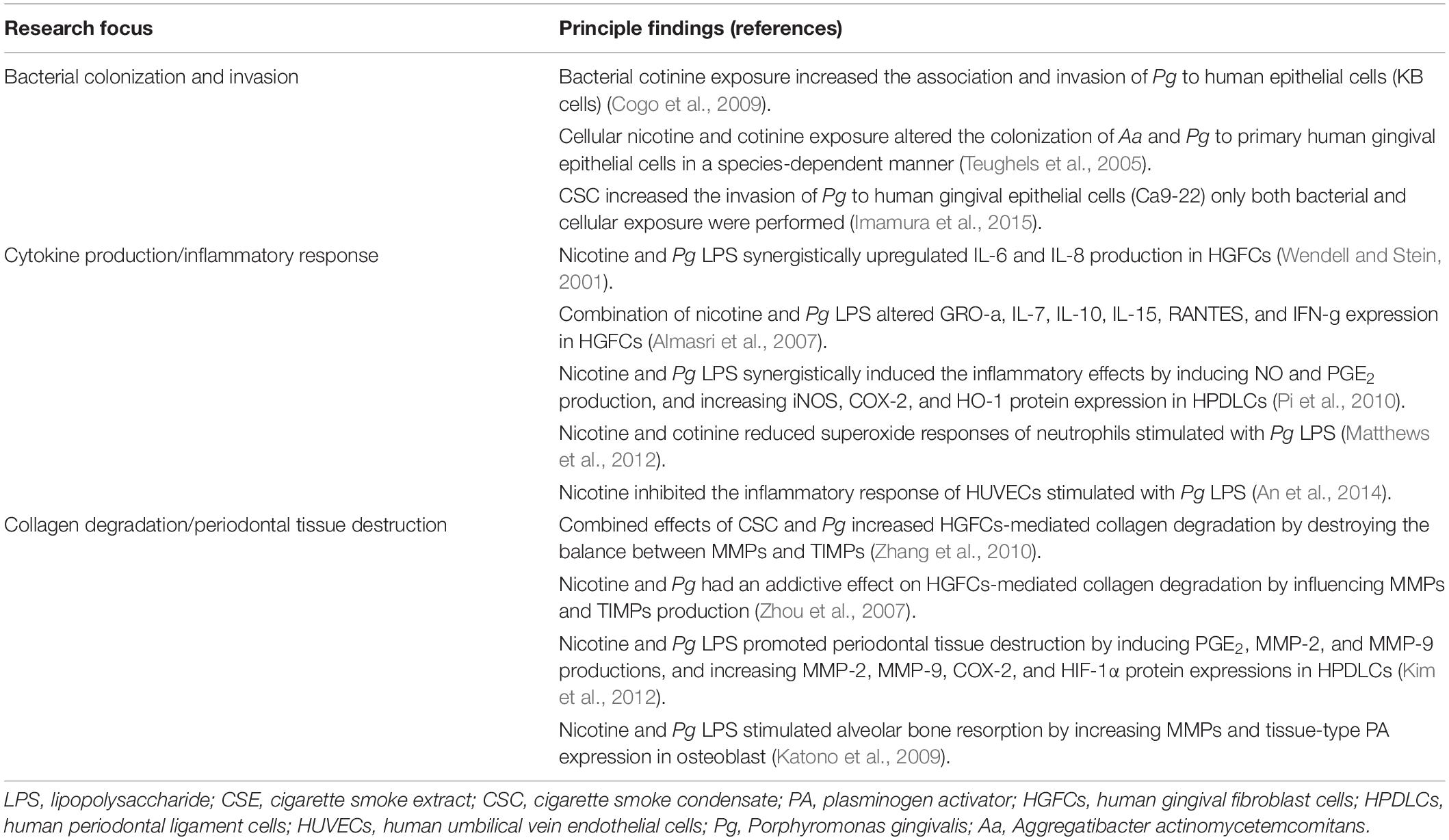
Table 2. Summary of in vitro studies of the effects of smoking on microbial functions for pathogen–host interaction.
Nicotine and P. gingivalis lipopolysaccharide (LPS) were shown to influence the inflammatory response by altering the cytokine production and interfering with immune cell functions (Wendell and Stein, 2001; Almasri et al., 2007; Pi et al., 2010; Matthews et al., 2012). However, such inflammatory effects could be attenuated by carbon monoxide-releasing molecule-3 via the heme oxygenase-1 (HO-1) pathway (Song et al., 2017) or by interfering with the function of resistin (Kang et al., 2015). High concentrations of nicotine also inhibited the inflammatory response of human umbilical vein endothelial cells stimulated by P. gingivalis LPS, which might be associated with decreased gingival bleeding indices in smoking periodontitis patients (An et al., 2014). Similarly, as an explanation to more severe periodontal destruction in smokers compared to non-smokers, the combination of nicotine and P. gingivalis or P. gingivalis LPS increased collagen degradation and bone resorption by tipping the balance between matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) (Zhou et al., 2007; Katono et al., 2009; Zhang et al., 2010; Kim et al., 2012).
Clinical Findings of Subgingival Microflora in Smokers
Given the convincing evidence for differences in the clinical and immunological statuses of the subgingival environment in smokers and non-smokers, it would be reasonable to propose that the subgingival microflora should also exhibit differences between these two types of subject. However, data on the effect of smoking on subgingival microflora are inconsistent in early studies. Some of them found no difference in the subgingival microflora between smokers and non-smokers with different periodontal conditions, concluding that smoking had insignificant effects on the subgingival microflora (Darby et al., 2000; Bostrom et al., 2001; Van der Velden et al., 2003; Apatzidou et al., 2005; Natto et al., 2005; Salvi et al., 2005). In contrast, others reported a higher prevalence of periodontitis and periodontal pathogen counts in smokers, depending on the quantity and duration of cigarette smoking (Shiloah et al., 2000; Haffajee and Socransky, 2001; Gomes et al., 2006). The conflicting findings in these studies might be partly explained by the sensitivity and specificity of the microbiological methods used, including culture (Van der Velden et al., 2003), DNA probes (Shiloah et al., 2000), polymerase chain reaction (PCR) (Darby et al., 2000; Apatzidou et al., 2005), real-time PCR (Gomes et al., 2006), and DNA–DNA hybridization (Bostrom et al., 2001; Haffajee and Socransky, 2001; Natto et al., 2005; Salvi et al., 2005). The difference between smokers and non-smokers in probing depth could also be an explanation as smokers have deeper periodontal pockets than non-smokers, which might confound the effect of smoking on periodontal pathogens (Kigure et al., 1995).
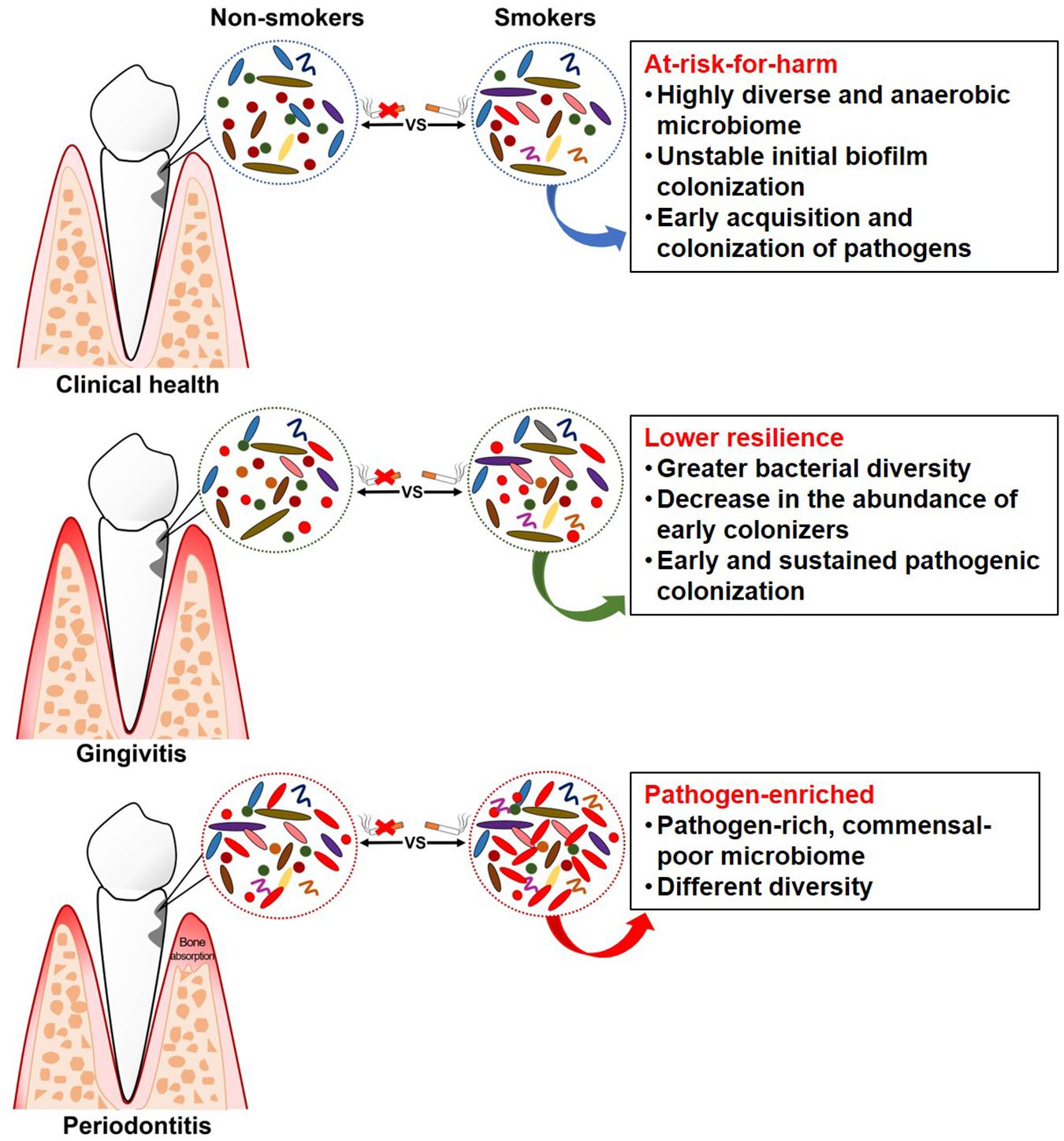
As it is now known that subgingival microflora is far more diverse than what was previously suspected (Griffen et al., 2012), and combined with the limitations of traditional targeted molecular methods (mentioned above) for bacterial identification, it was unknown previously whether smoking can cause qualitative and quantitative changes in the subgingival microflora. A novel technology for microbiome detection by 16S sequencing has been applied to identify the association of smoking and subgingival microflora during the past few years, opening up new horizons for a comprehensive understanding of whether and how these communities are affected. Table 3 presents a summary of the clinical studies (published between 2010 and 2019) on the effects of smoking on subgingival microflora using subgingival plaque samples from subjects with different periodontal conditions. Studies combining systemic conditions (such as diabetes mellitus and pregnancy) (Paropkari et al., 2016; Ganesan et al., 2017; Joaquim et al., 2018) or using granulation tissue from the subgingival area as samples (Coretti et al., 2017; Chowdhry et al., 2018) were also found but were excluded in this review. As shown in Table 3 and Figure 1, while contradictory results were reported by studies employing traditional targeted molecular methods (Kubota et al., 2011; Heikkinen et al., 2012; Guglielmetti et al., 2014; Lanza et al., 2016; Karasneh et al., 2017), altered subgingival microbial communities due to smoking in different periodontal conditions were generally revealed using 16S sequencing (Bizzarro et al., 2013; Mason et al., 2015; Yu et al., 2017).
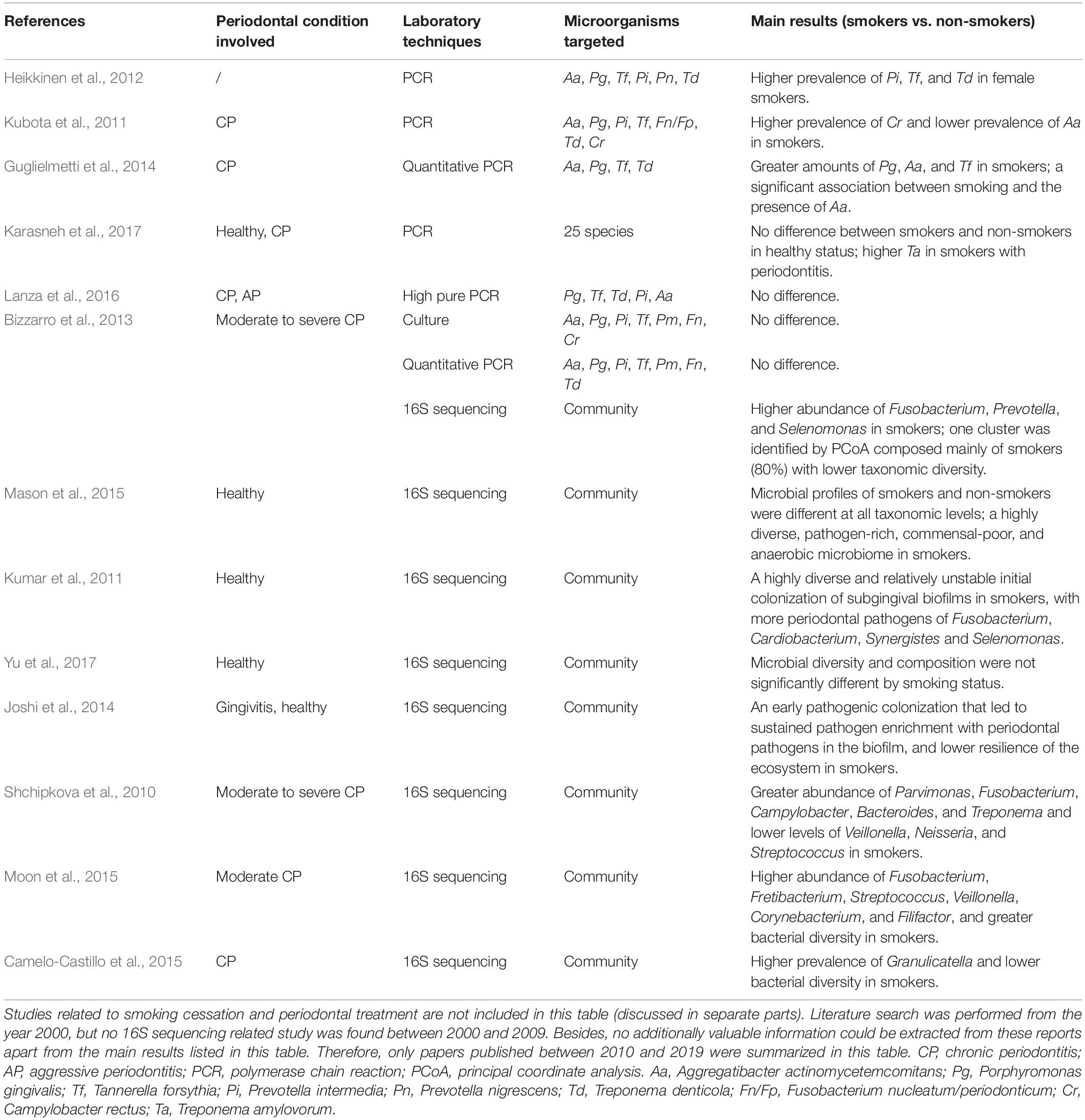
Table 3. Clinical studies in relation to the effects of smoking on subgingival microflora (published between 2010 and 2019).
Clinical Health
Studies have revealed some “unhealthy” signatures in the subgingival microflora of smokers even without any clinical manifestation. Mason et al. (2015) collected the subgingival plaque samples from 200 systemically and periodontally healthy smokers and non-smokers, and the microbial profiles were analyzed by 16S pyrotag sequencing. Two clusters with distinct microbial composition were revealed by principal coordinate analysis (PCoA), which were mainly composed of either smokers or non-smokers. The subgingival microflora of smokers was characterized by a highly diverse, pathogen-rich, commensal-poor, and anaerobic microbiome, which is thought to be more closely related to a disease-associated community. Moreover, periodontally healthy smokers had a highly diverse and relatively unstable initial colonization of subgingival biofilms, and an early acquisition and colonization of periodontal pathogens belonging to the genera Fusobacterium, Cardiobacterium, Synergistes, and Selenomonas (Kumar et al., 2011). Therefore, smoking may play a role in creating an “at-risk-for-harm” subgingival microbial community, making the subgingival environment more prone to periodontal destruction.
Gingivitis
Gingivitis is a necessary precursor to periodontitis, and the experimental gingivitis model provides a controlled and reversible method for examining the dynamic changes in health and disease (Loe et al., 1965). A longitudinal study, which was carried out on subjects with pre-existing gingivitis following a subsequent gingivitis resolution, experimental gingivitis induction, and experimental gingivitis resolution strategy, examined and compared the subgingival microflora in smokers and non-smokers at different clinical stages (Joshi et al., 2014). In this study, smokers demonstrated greater subgingival bacterial diversity than non-smokers during naturally occurring gingivitis and resolution state from disease to health. Smokers also had an earlier onset of clinically visible inflammation compared to non-smokers, which was attributed to the early pathogenic colonization, leading to sustained pathogen enrichment with periodontal pathogens. This is consistent with other findings in smokers, indicating that gingivitis is preceded by a decrease in the abundance of early colonizers, such as the genera Streptococcus and Veillonella, and an increase in the abundance of periodontopathogens, such as the genera Treponema and Selenomonas (Matthews et al., 2013; Peruzzo et al., 2016). The capability of an ecosystem to deal with perturbations while maintaining itself in a steady state in which the core species and key functions are retained is defined as resilience, which plays an important role in susceptibility to disease (Rosier et al., 2018). However, smokers demonstrated higher abundance of pathogenic species, suggesting that the subgingival microflora in smokers had lower resilience as they failed to maintain themselves in the original state when dealing with perturbation, thereby possibly increasing the risk to future disease (Joshi et al., 2014).
Periodontitis
Evidence has steadily accumulated, suggesting a pathogen-enriched community in smokers compared to non-smokers in periodontitis. Bizzarro et al. (2013) compared the subgingival microbiome in smokers and non-smokers with chronic periodontitis, using two traditional targeted techniques and a next-generation sequencing method. While no differences were found in the prevalence and quantity for any of the targeted species (Table 3) between smokers and non-smokers with culture and quantitative PCR, different subgingival microbial profiles were identified by pyrosequencing. Operational taxonomic units (OTUs) belonging to the genera Fusobacterium, Prevotella, and Selenomonas were more abundant in smokers, while OTUs belonging to the genera Peptococcus and Capnocytophaga were more abundant in non-smokers. Differences in bacterial communities between smokers and non-smokers were also detected at different taxonomic levels in other studies (Shchipkova et al., 2010; Camelo-Castillo et al., 2015; Moon et al., 2015), with variations in the types of bacteria identified. At the general level, Fusobacterium, as the most frequently identified bacteria, was more abundant in smokers than non-smokers, which was suggested to be one of the major determinants of subgingival bacterial community shift induced by smoking (Moon et al., 2015). Fusobacterium, especially the F. nucleatum, plays a critical role in the subgingival biofilm due to its “bridging species” role among microorganisms as well as its local immunosuppressive capability (Signat et al., 2011), thus contributing to the progression and severity of periodontal disease. Other bacteria that are consistently associated with periodontal disease include Parvimonas, Treponema, Filifactor, and Bacteroides (Kumar et al., 2005; Shi et al., 2015). However, conflicting results regarding the abundance of two common genera, i.e., Streptococcus and Veillonella, were reported in two studies (Shchipkova et al., 2010; Moon et al., 2015). As Veillonella and Streptococcus species are known to be abundant in health-associated biofilms (Paster et al., 2001; Kumar et al., 2005, 2006), the higher abundance in smokers found in the study by Moon et al. (2015) was speculated to be the result of bacterial interaction.
Considering the microbial diversity, which is thought to be another important parameter for an ecosystem, different results are reported in different studies. Bizzarro et al. (2013) identified one cluster by PCoA, which was mainly composed of smokers (80%) with significantly lower taxonomic diversity, consistent with the findings of a study by Camelo-Castillo et al. (2015). However, some studies have reported a higher diversity in smokers compared to non-smokers (Moon et al., 2015), or no difference in the mean number of species/phylotypes between smokers and non-smokers (Shchipkova et al., 2010). As microbial diversity in a given microbial community not only refers to the species richness but also relates to their evenness, the different conclusions in recent studies might be attributed to the different indices used to evaluate microbial diversity. Furthermore, the bioinformatic data processing method of the obtained sequences can also influence the results (Nearing et al., 2018). Although the important role of key species in disease development has been recognized, the role of microbial diversity is less clear (Karkman et al., 2017). Some studies have shown a higher microbial diversity at more severely affected sites in periodontitis (Griffen et al., 2012), but some others have reported it the other way around (Kirst et al., 2015). Taking these findings together, it seems that no unified conclusion can be drawn currently regarding how smoking-related microbial diversity changes would contribute to periodontitis.
Effect of Smoking Cessation on the Subgingival Microflora
Using a quantitative and cultivation-independent method, i.e., terminal restriction fragment length polymorphism for bacterial profiling, Fullmer et al. (2009) analyzed the subgingival plaque samples from smokers and quitters and showed that microbial profiles differed significantly between these two groups at 6- and 12-month intervals after giving up smoking. The microbial community in smokers was similar to baseline, while quitters exhibited significantly divergent profiles. At the bacterial species level, smoking cessation led to a decrease in periodontal pathogens, including Porphyromonas endodontalis, Dialister pneumosintes, Parvimonas micra, F. alocis, and Treponema denticola (T. denticola), in association with an increase in the level of health-associated species Veillonella parvula (Delima et al., 2010). The beneficial effects of smoking cessation on the periodontium are evident. Smoking cessation reduces the risks of the onset and progression of periodontal disease, reduces the risk of tooth loss, and improves the clinical outcomes of periodontal therapy (Dietrich et al., 2015; Leite et al., 2018). From the microbiological point of view, previous studies have also revealed the crucial role of smoking cessation in changing the subgingival microflora, and consequently, the response to periodontal treatment.
Effect of Smoking on Subgingival Microflora in Periodontal Treatment
Since smokers have different etiologies and clinical manifestations of periodontal diseases compared to non-smokers, it is not surprising that they also respond differently to periodontal treatment. The negative influence of smoking on the response to periodontal therapies has been reviewed previously through clinical parameters, such as bleeding on probing (BoP) and probing pocket depth (PD) (Heasman et al., 2006; Nociti et al., 2015). Most recently, some microbiological findings concerning periodontal treatment response in smokers additionally suggest the adverse effects of smoking. Although the clinical parameters improved in both smoking and non-smoking periodontitis patients following non-surgical and/or surgical therapy, a lower reduction and greater post-therapy prevalence of periodontal pathogens, including the well-known “red-complex” and “orange-complex” bacteria, were observed in smokers (van Winkelhoff et al., 2001; Van der Velden et al., 2003; Darby et al., 2005; Meulman et al., 2012; Bunaes et al., 2015). Smokers were also reported to be more susceptible to the re-establishment of a pathogenic subgingival biofilm than non-smokers after scaling and root planning (SRP) because a significant decrease in the pathogenic species was only observed in non-smokers 180 days after treatment (Feres et al., 2015).
It is therefore rational to utilize adjunctive antimicrobial agents, either locally or systemically, in the periodontal treatment of smokers based on the evidence that subgingival pathogens seem to be more difficult to eliminate in smokers following non-surgical and surgical therapy. Machion et al. (2004) showed that locally delivered doxycycline promoted the elimination of Tannerella forsythia (T. forsythia) and P. gingivalis in a greater proportion of sites compared to conventional SRP in smokers with chronic periodontitis. Similarly, SRP alone was ineffective in reducing the counts or proportions of the “red-complex” or “orange-complex” bacteria in current smokers with periodontitis, whereas a combination of minocycline and SRP significantly reduced both (Grossi et al., 2007). The adjunctive use of metronidazole and amoxicillin systemically in the SRP treatment of smokers with chronic periodontitis also led to the most beneficial changes in the subgingival microbial profile by reducing the mean counts and proportions of periodontal pathogens, such as T. forsythia, P. gingivalis, and T. denticola, and increasing the proportions of host-compatible species (Matarazzo et al., 2008; Faveri et al., 2014). The microbiological effect of adjunctive antimicrobial photodynamic therapy on non-surgical periodontal treatment was also investigated; however, no difference was identified (Queiroz et al., 2014).
The use of dental implants has become a popular alternative for the replacement of missing teeth. Several studies have evaluated the influence of smoking on the peri-implant microflora, demonstrating a trend similar to the microbiological findings in the subgingival microflora, indicating that smoking is associated with a higher prevalence of pathogenic species (Tsigarida et al., 2015; Ata-Ali et al., 2016; Eick et al., 2016; Stokman et al., 2017). The most impressive findings were presented by a study using a deep-sequencing method to identify the effect of smoking on the peri-implant microbiome in the health and disease states (Tsigarida et al., 2015). Compared to non-smokers, microbial signatures of health in smokers exhibited a lower diversity and an enrichment in species traditionally regarded as periodontal and/or systemic pathogens, including those belonging to the genera Capnocytophaga, Treponema, Propionibacterium, Pseudomonas, Lactobacillus, and Leptotrichia. Although a core microbiome is identified, which is composed of species most suited to the peri-implant habitat in both smokers and non-smokers, smoking modifies this environment containing 31 different species. The transition of the microbiome from health to mucositis has also been observed to be different in smokers and non-smokers, although peri-implant mucositis is an important event in both groups. In non-smokers, it resembles primary ecological succession, with the acquisition of several species without replacement of pioneer organisms; however, in smokers, further enrichment in pathogenic species and a decrease in diversity are shown.
Potential Mechanisms Contributing to the Negative Impact of Smoking on Subgingival Microflora
Subgingival microflora is a highly diverse and structured community attaching to the tooth surface as a biofilm. A comprehensive understanding of the underlying mechanisms by which smoking influences these biofilms is challenging with currently limited studies, as in addition to the direct effects of smoking on biofilm formation and succession, an indirect effect, mainly from the host immune response, must also be taken into account under the subgingival settings. Furthermore, the complex interplay between these two aspects makes it more complicated. For a brief discussion here on several possible mechanisms (Figure 2), the situation is simplified into two separate parts by the dominant factor discussed.
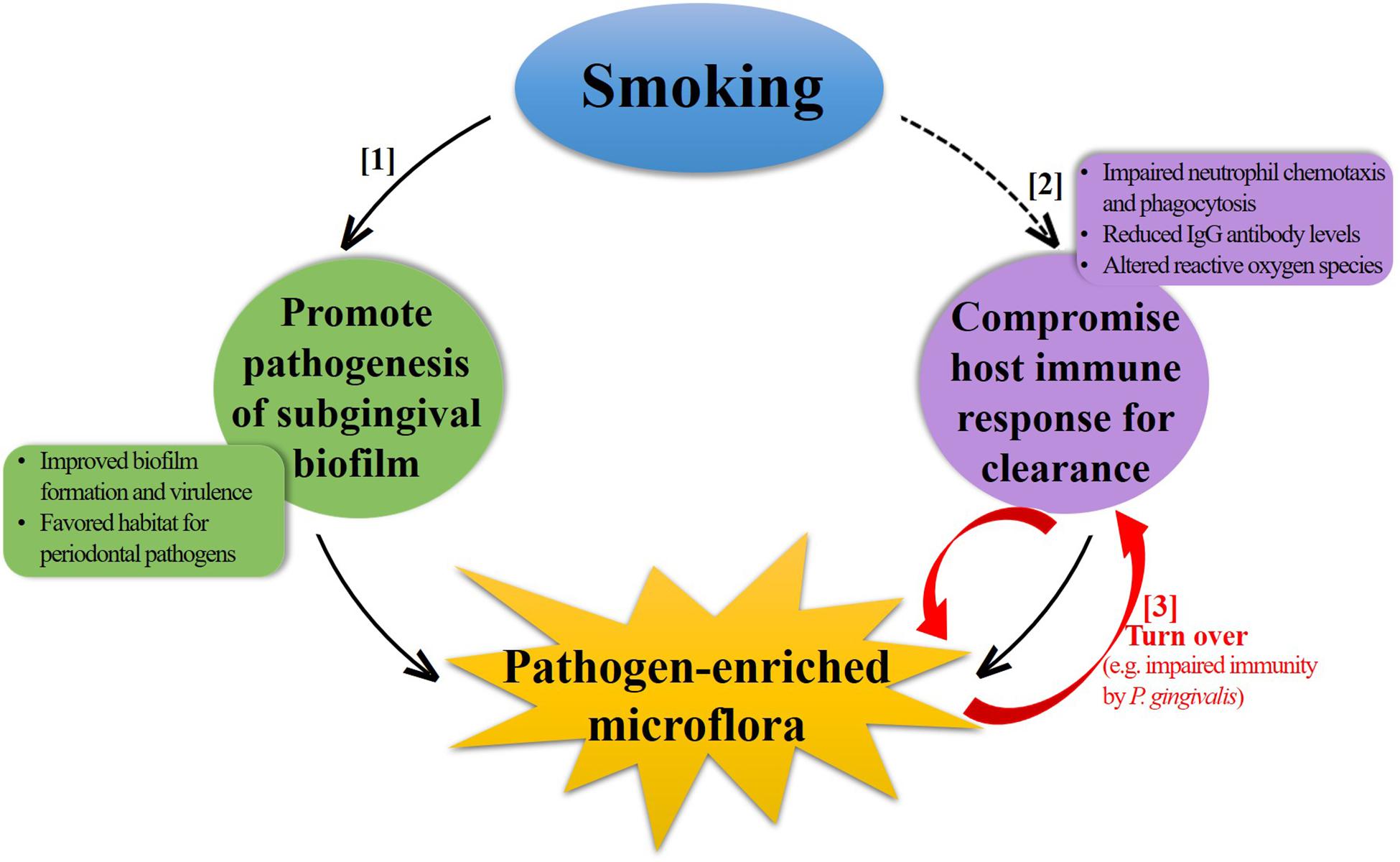
Figure 2. Schematic illustration of potential mechanisms contributing to the negative impact of smoking on subgingival microflora. Smoking can induce a pathogen-enriched subgingival microflora through increasing the pathogenesis of subgingival biofilm ([1]) and decreasing the host immune response for bacterial clearance ([2]). Some periodontal pathogens can also subvert the host immune response in turn, forming a vicious circle ([3]).
Promote Pathogenesis of Subgingival Biofilm (Figure 2, [1])
Biofilm formation in the oral cavity is a gradated process, consisting of four stages, of which the initial bacterial adhesion and colonization are crucial steps (Hao et al., 2018). In this regard, smoking can enhance biofilm formation by some key species. The in vitro effects of smoking on key pathogens (as reviewed in the section “In vitro Effects of Smoking on Key Periodontal Pathogens”) showed that exposure to CSE can alter the expression of several virulence factors of P. gingivalis, facilitating biofilm formation process by upregulating the expression of FimA and several outer membrane proteins (RagA, RagB, and a putative lipoprotein co-transcribed with the minor fimbrial antigen), and suppressing the expression of capsular polysaccharide (Bagaitkar et al., 2009, 2010). FimA is the major fimbrial antigen of P. gingivalis that plays a critical role in bacterial colonization and invasion to the periodontium through adhering to salivary proteins, extracellular matrix, eukaryotic cells, and bacteria of either the same or other species (Bostanci and Belibasakis, 2012). The observed upregulation of FimA not only increased the biofilm formation of P. gingivalis but also promoted the interaction between P. gingivalis and Streptococcus gordonii (an early biofilm colonizer) by improving its binding to glyceraldehyde-3-phosphate dehydrogenase, thus enhancing dual-species biofilm formation (Bagaitkar et al., 2011). Simultaneously, the upregulation of FimA also reduced the host response to P. gingivalis by abrogating the proinflammatory response to subsequent TLR2 stimulation (Bagaitkar et al., 2010), and therefore increasing bacterial survival. As P. gingivalis has a prominent role in orchestrating the virulence of the biofilm and the consequent inflammatory response, earning itself the characteristics of a “keystone” periodontal pathogen (Hajishengallis et al., 2012), a change in the virulence of P. gingivalis might exert a community-wide influence to promote the shift of subgingival biofilm to a dysbiotic state, predisposing the individual to periodontal destruction.
Fusobacterium, especially F. nucleatum, plays a key role in physical interactions between Gram-positive and Gram-negative species and is considered as an intermediate colonizer, bridging the attachment of commensals that colonize the tooth and epithelial surfaces with true pathogens (Signat et al., 2011). Thus, the frequently detected higher Fusobacterium amount in the subgingival microflora in smokers (Shchipkova et al., 2010; Bizzarro et al., 2013; Moon et al., 2015) might allow it to transport periodontal pathogenic bacteria, leading to a pathogen-enriched biofilm formation.
In addition to enhancing biofilm formation, some other phenomena might promote the pathogenesis of subgingival biofilm by creating a suitable environment for periodontal pathogens. For example, Hanioka et al. (2000) reported significantly low oxygen tension within the periodontal pockets in smokers, which might favor the growth of anaerobic periodontal pathogens even in shallow pockets; this was supported by the clinical findings that smoking creates a favorable habitat for bacteria, such as P. gingivalis, Prevotella intermedia, and A. actinomycetemcomitans at shallow sites (≤5 mm) (Eggert et al., 2001). Shah et al. (2017) showed that smoke-induced transcriptional shifts in commensal biofilms triggered a proinflammatory response and created a cytokine-rich, pro-oxidant, and anaerobic environment, leading to the early commensal death and creation of pathogen-enriched biofilms in smokers.
Compromise Host Immune Response (Figure 2, [2])
Smoking compromises various aspects of the innate and adaptive host immune responses, as summarized in previous reviews of mechanisms of smoking-related periodontal destruction (Palmer et al., 2005; Ryder, 2007; Johannsen et al., 2014). The general observation is that smoking stimulates the inflammatory response and impairs protective response, thus accelerating the periodontal destruction. The changes associated with the immune response in subgingival environment can also have an impact on the microbial community. Neutrophils are the primary leukocytes, which are critical for the defense against bacterial invasion by phagocytosis in human body. However, both in vitro and in vivo studies have shown that smoking can impair the chemotaxis and phagocytosis of neutrophils in the periodontium (Guntsch et al., 2006; Zappacosta et al., 2011), leading to defective clearance of bacteria and thereby increasing the colonization. Furthermore, smoking has been shown to inversely correlate with the levels of serum immunoglobulin (Ig) G antibodies specific for some periodontal pathogens (Graswinckel et al., 2004; Tebloeva et al., 2014). Vlachojannis et al. (2010) assessed the levels of IgG antibody to multiple periodontal bacteria in a large population of US adults and found that current smokers were less likely to have higher antibody titers for periodontal pathogens, such as P. gingivalis, Campylobacter rectus, and Prevotella nigrescens after adjusting important confounding factors. The reduced level of IgG antibody can impair the host immune response and exert a “protective” effect on these periodontal pathogens. In addition, the generation of reactive oxygen species, which play an important role in intracellular bacterial killing, can be affected by smoking, possibly resulting in a decreased innate immune response to periodontal pathogens (Matthews et al., 2011). From another perspective, P. gingivalis can modulate innate host defense function, such as immune subversion of IL-8 secretion, complement activity, or TLR4 activation by several virulence factors, resulting in impaired host defense (Darveau et al., 2012). In this sense, it seems that a vicious circle (Figure 2, [3]) forms between pathogen-enriched subgingival microflora and the host immune response due to smoking, which might worsen the situation even further in terms of health.
Conclusion
As discussed in this review, smoking exposure represents an environmental stress to which periodontal pathogens, such as P. gingivalis, can adapt by altering their gene and protein expressions. These changes may alter the virulence of bacteria and host–pathogen interactions, and finally contribute to the development of periodontal disease. Although early studies based on traditional targeted molecular methods yield conflicting findings concerning the effects of smoking on subgingival microflora associated with periodontal disease, microbial composition differences in smokers, compared to non-smokers, have been shown by the newly applied 16S sequencing technology, regardless of the periodontal condition. Smoking can create an “at-risk-for-harm” subgingival microbial community in the healthy periodontium, lower the resilience of the subgingival ecosystem in gingivitis, and promote a pathogen-enriched subgingival microflora in periodontitis, thus playing a role in the clinically observed increased susceptibility and severity of periodontal destruction in smokers. The pathogen-enriched subgingival microflora responds poorly to the periodontal treatment, whereas smoking cessation alters the subgingival biofilm, suggesting a mechanism for improved periodontal health associated with smoking cessation.
Although the negative impact of smoking on subgingival microflora is certain, the underlying mechanism is unclear and open for debate. In this review, the possible mechanisms by which smoking creates pathogen-enriched biofilms were discussed, including the promotion of pathogenesis of subgingival biofilms via key pathogens and the ineffective host immune response for clearance. However, we are currently just at the beginning when it comes to an understanding of the underlying mechanisms. Further studies are required to elucidate the mechanisms of the effect of smoking on subgingival microflora for the prevention of periodontal destruction.
Author Contributions
YJ drafted the manuscript. XZ, LC, and ML edited and added the valuable insights into the manuscript. All authors approved the final manuscript and agreed to be accountable for all aspects of the work.
Funding
This study was supported by the National Key Research Program of China (2017YFC0840100 and 2017YFC0840107 to LC); the National Natural Science Foundation of China (Grant Nos. 81430011 to XZ, 81400501 to ML, and 81870759 to LC); and the Innovative Research Team Program of Sichuan Province to LC.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
Almasri, A., Wisithphrom, K., Windsor, L. J., and Olson, B. (2007). Nicotine and lipopolysaccharide affect cytokine expression from gingival fibroblasts. J. Periodontol. 78, 533–541. doi: 10.1902/jop.2007.060296
An, N., Andrukhov, O., Tang, Y., Falkensammer, F., Bantleon, H. P., Ouyang, X., et al. (2014). Effect of nicotine and Porphyromonas gingivalis lipopolysaccharide on endothelial cells in vitro. PLoS One 9:e96942. doi: 10.1371/journal.pone.0096942
Apatzidou, D. A., Riggio, M. P., and Kinane, D. F. (2005). Impact of smoking on the clinical, microbiological and immunological parameters of adult patients with periodontitis. J. Clin. Periodontol. 32, 973–983. doi: 10.1111/j.1600-051x.2005.00788.x
Ata-Ali, J., Flichy-Fernandez, A. J., Alegre-Domingo, T., Ata-Ali, F., and Penarrocha-Diago, M. (2016). Impact of heavy smoking on the clinical, microbiological and immunological parameters of patients with dental implants: a prospective cross-sectional study. J. Investig. Clin. Dent. 7, 401–409. doi: 10.1111/jicd.12176
Baek, O., Zhu, W., Kim, H. C., and Lee, S. W. (2012). Effects of nicotine on the growth and protein expression of Porphyromonas gingivalis. J. Microbiol. 50, 143–148. doi: 10.1007/s12275-012-1212-8
Bagaitkar, J., Daep, C. A., Patel, C. K., Renaud, D. E., Demuth, D. R., and Scott, D. A. (2011). Tobacco smoke augments Porphyromonas gingivalis-Streptococcus gordonii biofilm formation. PLoS One 6:e27386. doi: 10.1371/journal.pone.0027386
Bagaitkar, J., Demuth, D. R., Daep, C. A., Renaud, D. E., Pierce, D. L., and Scott, D. A. (2010). Tobacco upregulates P. gingivalis fimbrial proteins which induce TLR2 hyposensitivity. PLoS One 5:e9323. doi: 10.1371/journal.pone.0009323
Bagaitkar, J., Williams, L. R., Renaud, D. E., Bemakanakere, M. R., Martin, M., Scott, D. A., et al. (2009). Tobacco-induced alterations to Porphyromonas gingivalis-host interactions. Environ. Microbiol. 11, 1242–1253. doi: 10.1111/j.1462-2920.2008.01852.x
Baljoon, M., Natto, S., and Bergstrom, J. (2005). Long-term effect of smoking on vertical periodontal bone loss. J. Clin. Periodontol. 32, 789–797. doi: 10.1111/j.1600-051x.2005.00765.x
Bergstrom, J. (2014). Smoking rate and periodontal disease prevalence: 40-year trends in Sweden 1970-2010. J. Clin. Periodontol. 41, 952–957. doi: 10.1111/jcpe.12293
Bizzarro, S., Loos, B. G., Laine, M. L., Crielaard, W., and Zaura, E. (2013). Subgingival microbiome in smokers and non-smokers in periodontitis: an exploratory study using traditional targeted techniques and a next-generation sequencing. J. Clin. Periodontol. 40, 483–492. doi: 10.1111/jcpe.12087
Bondy-Carey, J. L., Galicia, J., Bagaitkar, J., Potempa, J. S., Potempa, B., Kinane, D. F., et al. (2013). Neutrophils alter epithelial response to Porphyromonas gingivalis in a gingival crevice model. Mol. Oral Microbiol. 28, 102–113. doi: 10.1111/omi.12008
Bostanci, N., and Belibasakis, G. N. (2012). Porphyromonas gingivalis: an invasive and evasive opportunistic oral pathogen. FEMS Microbiol. Lett. 333, 1–9. doi: 10.1111/j.1574-6968.2012.02579.x
Bostrom, L., Bergstrom, J., Dahlen, G., and Linder, L. E. (2001). Smoking and subgingival microflora in periodontal disease. J. Clin. Periodontol. 28, 212–219. doi: 10.1034/j.1600-051x.2001.028003212.x
Brook, I. (2011). The impact of smoking on oral and nasopharyngeal bacterial flora. J. Dent. Res. 90, 704–710. doi: 10.1177/0022034510391794
Brooks, A. N., Turkarslan, S., Beer, K. D., Lo, F. Y., and Baliga, N. S. (2011). Adaptation of cells to new environments. Wiley Interdiscip. Rev. Syst. Biol. Med. 3, 544–561. doi: 10.1002/wsbm.136
Bunaes, D. F., Lie, S. A., Enersen, M., Aastrom, A. N., Mustafa, K., and Leknes, K. N. (2015). Site-specific treatment outcome in smokers following non-surgical and surgical periodontal therapy. J. Clin. Periodontol. 42, 933–942. doi: 10.1111/jcpe.12462
Camelo-Castillo, A. J., Mira, A., Pico, A., Nibali, L., Henderson, B., Donos, N., et al. (2015). Subgingival microbiota in health compared to periodontitis and the influence of smoking. Front. Microbiol. 6:119. doi: 10.3389/fmicb.2015.00119
Chen, X., Wolff, L., Aeppli, D., Guo, Z., Luan, W., Baelum, V., et al. (2001). Cigarette smoking, salivary/gingival crevicular fluid cotinine and periodontal status. A 10-year longitudinal study. J. Clin. Periodontol. 28, 331–339. doi: 10.1034/j.1600-051x.2001.028004331.x
Chowdhry, R., Singh, N., Sahu, D. K., Tripathi, R. K., Mishra, A., Singh, A., et al. (2018). 16S rRNA long-read sequencing of the granulation tissue from nonsmokers and smokers-severe chronic periodontitis patients. Biomed. Res. Int. 2018:4832912. doi: 10.1155/2018/4832912
Cogo, K., Calvi, B. M., Mariano, F. S., Franco, G. C., Goncalves, R. B., and Groppo, F. C. (2009). The effects of nicotine and cotinine on Porphyromonas gingivalis colonisation of epithelial cells. Arch. Oral Biol. 54, 1061–1067. doi: 10.1016/j.archoralbio.2009.08.001
Cogo, K., Montan, M. F., Bergamaschi Cde, C., D Andrade, E., Rosalen, P. L., and Groppo, F. C. (2008). In vitro evaluation of the effect of nicotine, cotinine, and caffeine on oral microorganisms. Can. J. Microbiol. 54, 501–508. doi: 10.1139/w08-032
Coretti, L., Cuomo, M., Florio, E., Palumbo, D., Keller, S., Pero, R., et al. (2017). Subgingival dysbiosis in smoker and nonsmoker patients with chronic periodontitis. Mol. Med. Rep. 15, 2007–2014. doi: 10.3892/mmr.2017.6269
Darby, I. B., Hodge, P. J., Riggio, M. P., and Kinane, D. F. (2000). Microbial comparison of smoker and non-smoker adult and early-onset periodontitis patients by polymerase chain reaction. J. Clin. Periodontol. 27, 417–424. doi: 10.1034/j.1600-051x.2000.027006417.x
Darby, I. B., Hodge, P. J., Riggio, M. P., and Kinane, D. F. (2005). Clinical and microbiological effect of scaling and root planing in smoker and non-smoker chronic and aggressive periodontitis patients. J. Clin. Periodontol. 32, 200–206. doi: 10.1111/j.1600-051x.2005.00644.x
Darveau, R. P., Hajishengallis, G., and Curtis, M. A. (2012). Porphyromonas gingivalis as a potential community activist for disease. J. Dent. Res. 91, 816–820. doi: 10.1177/0022034512453589
Delima, S. L., Mcbride, R. K., Preshaw, P. M., Heasman, P. A., and Kumar, P. S. (2010). Response of subgingival bacteria to smoking cessation. J. Clin. Microbiol. 48, 2344–2349. doi: 10.1128/JCM.01821-09
Dhar, P. (2004). Measuring tobacco smoke exposure: quantifying nicotine/cotinine concentration in biological samples by colorimetry, chromatography and immunoassay methods. J. Pharm. Biomed. Anal. 35, 155–168. doi: 10.1016/j.jpba.2004.01.009
Dietrich, T., Walter, C., Oluwagbemigun, K., Bergmann, M., Pischon, T., Pischon, N., et al. (2015). Smoking, smoking cessation, and risk of tooth loss: the EPIC-potsdam study. J. Dent. Res. 94, 1369–1375. doi: 10.1177/0022034515598961
Do, L. G., Slade, G. D., Roberts-Thomson, K. F., and Sanders, A. E. (2008). Smoking-attributable periodontal disease in the Australian adult population. J. Clin. Periodontol. 35, 398–404. doi: 10.1111/j.1600-051X.2008.01223.x
Eggert, F. M., Mcleod, M. H., and Flowerdew, G. (2001). Effects of smoking and treatment status on periodontal bacteria: evidence that smoking influences control of periodontal bacteria at the mucosal surface of the gingival crevice. J. Periodontol. 72, 1210–1220. doi: 10.1902/jop.2000.72.9.1210
Eick, S., Ramseier, C. A., Rothenberger, K., Bragger, U., Buser, D., and Salvi, G. E. (2016). Microbiota at teeth and implants in partially edentulous patients. A 10-year retrospective study. Clin. Oral Implants Res. 27, 218–225. doi: 10.1111/clr.12588
Eke, P. I., Wei, L., Thornton-Evans, G. O., Borrell, L. N., Borgnakke, W. S., Dye, B., et al. (2016). Risk indicators for periodontitis in US Adults: NHANES 2009 to 2012. J. Periodontol. 87, 1174–1185. doi: 10.1902/jop.2016.160013
Faveri, M., Rebello, A., De Oliveira Dias, R., Borges-Junior, I., Duarte, P. M., Figueiredo, L. C., et al. (2014). Clinical and microbiologic effects of adjunctive metronidazole plus amoxicillin in the treatment of generalized chronic periodontitis: smokers versus non-smokers. J. Periodontol. 85, 581–591. doi: 10.1902/jop.2013.130278
Feres, M., Bernal, M., Matarazzo, F., Faveri, M., Duarte, P. M., and Figueiredo, L. C. (2015). Subgingival bacterial recolonization after scaling and root planing in smokers with chronic periodontitis. Aust. Dent. J. 60, 225–232. doi: 10.1111/adj.12225
Fullmer, S. C., Preshaw, P. M., Heasman, P. A., and Kumar, P. S. (2009). Smoking cessation alters subgingival microbial recolonization. J. Dent. Res. 88, 524–528. doi: 10.1177/0022034509338676
Ganesan, S. M., Joshi, V., Fellows, M., Dabdoub, S. M., Nagaraja, H. N., O’donnell, B., et al. (2017). A tale of two risks: smoking, diabetes and the subgingival microbiome. ISME J. 11, 2075–2089. doi: 10.1038/ismej.2017.73
GBD 2016 Risk Factors Collaborators (2017). Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1345–1422. doi: 10.1016/S0140-6736(17)32366-8
Gomes, S. C., Piccinin, F. B., Oppermann, R. V., Susin, C., Nonnenmacher, C. I., Mutters, R., et al. (2006). Periodontal status in smokers and never-smokers: clinical findings and real-time polymerase chain reaction quantification of putative periodontal pathogens. J. Periodontol. 77, 1483–1490. doi: 10.1902/jop.2006.060026
Graswinckel, J. E., Van Der Velden, U., Van Winkelhoff, A. J., Hoek, F. J., and Loos, B. G. (2004). Plasma antibody levels in periodontitis patients and controls. J. Clin. Periodontol. 31, 562–568. doi: 10.1111/j.1600-051x.2004.00522.x
Griffen, A. L., Beall, C. J., Campbell, J. H., Firestone, N. D., Kumar, P. S., Yang, Z. K., et al. (2012). Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 6, 1176–1185. doi: 10.1038/ismej.2011.191
Grossi, S. G., Goodson, J. M., Gunsolley, J. C., Otomo-Corgel, J., Bland, P. S., Doherty, F., et al. (2007). Mechanical therapy with adjunctive minocycline microspheres reduces red-complex bacteria in smokers. J. Periodontol. 78, 1741–1750. doi: 10.1902/jop.2007.070118
Guglielmetti, M. R., Rosa, E. F., Lourenção, D. S., Inoue, G., Gomes, E. F., De Micheli, G., et al. (2014). Detection and quantification of periodontal pathogens in smokers and never-smokers with chronic periodontitis by real-time polymerase chain reaction. J. Periodontol. 85, 1450–1457. doi: 10.1902/jop.2014.140048
Guntsch, A., Erler, M., Preshaw, P. M., Sigusch, B. W., Klinger, G., and Glockmann, E. (2006). Effect of smoking on crevicular polymorphonuclear neutrophil function in periodontally healthy subjects. J. Periodontal Res. 41, 184–188. doi: 10.1111/j.1600-0765.2005.00852.x
Haffajee, A. D., and Socransky, S. S. (2001). Relationship of cigarette smoking to the subgingival microbiota. J. Clin. Periodontol. 28, 377–388. doi: 10.1034/j.1600-051x.2001.028005377.x
Hajishengallis, G., Darveau, R. P., and Curtis, M. A. (2012). The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 10, 717–725. doi: 10.1038/nrmicro2873
Hajishengallis, G., and Lamont, R. J. (2012). Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 27, 409–419. doi: 10.1111/j.2041-1014.2012.00663.x
Hajishengallis, G., Liang, S., Payne, M. A., Hashim, A., Jotwani, R., Eskan, M. A., et al. (2011). Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 10, 497–506. doi: 10.1016/j.chom.2011.10.006
Hanioka, T., Tanaka, M., Takaya, K., Matsumori, Y., and Shizukuishi, S. (2000). Pocket oxygen tension in smokers and non-smokers with periodontal disease. J. Periodontol. 71, 550–554. doi: 10.1902/jop.2000.71.4.550
Hao, Y., Huang, X., Zhou, X., Li, M., Ren, B., Peng, X., et al. (2018). Influence of dental prosthesis and restorative materials interface on oral biofilms. Int. J. Mol. Sci. 19:E3157. doi: 10.3390/ijms19103157
Heasman, L., Stacey, F., Preshaw, P. M., Mccracken, G. I., Hepburn, S., and Heasman, P. A. (2006). The effect of smoking on periodontal treatment response: a review of clinical evidence. J. Clin. Periodontol. 33, 241–253. doi: 10.1111/j.1600-051x.2006.00902.x
Heikkinen, A. M., Pitkäniemi, J., Kari, K., Pajukanta, R., Elonheimo, O., Koskenvuo, M., et al. (2012). Effect of teenage smoking on the prevalence of periodontal bacteria. Clin. Oral Investig. 16, 571–580. doi: 10.1007/s00784-011-0521-3
Imamura, K., Kokubu, E., Kita, D., Ota, K., Ishihara, K., and Saito, A. (2015). Cigarette smoke condensate modulates migration of human gingival epithelial cells and their interactions with Porphyromonas gingivalis. J. Periodontal Res. 50, 411–421. doi: 10.1111/jre.12222
Joaquim, C. R., Miranda, T. S., Marins, L. M., Silva, H. D. P., Feres, M., Figueiredo, L. C., et al. (2018). The combined and individual impact of diabetes and smoking on key subgingival periodontal pathogens in patients with chronic periodontitis. J. Periodontal Res. 53, 315–323. doi: 10.1111/jre.12516
Johannsen, A., Susin, C., and Gustafsson, A. (2014). Smoking and inflammation: evidence for a synergistic role in chronic disease. Periodontology 2000, 111–126. doi: 10.1111/j.1600-0757.2012.00456.x
Johnson, G. K., and Guthmiller, J. M. (2007). The impact of cigarette smoking on periodontal disease and treatment. Periodontology 2000, 178–194. doi: 10.1111/j.1600-0757.2007.00212.x
Joshi, V., Matthews, C., Aspiras, M., De Jager, M., Ward, M., and Kumar, P. (2014). Smoking decreases structural and functional resilience in the subgingival ecosystem. J. Clin. Periodontol. 41, 1037–1047. doi: 10.1111/jcpe.12300
Kang, S. K., Park, Y. D., Kang, S. I., Kim, D. K., Kang, K. L., Lee, S. Y., et al. (2015). Role of resistin in the inflammatory response induced by nicotine plus lipopolysaccharide in human periodontal ligament cells in vitro. J. Periodontal Res. 50, 602–613. doi: 10.1111/jre.12240
Karasneh, J. A., Al Habashneh, R. A., Marzouka, N. A. S., and Thornhill, M. H. (2017). Effect of cigarette smoking on subgingival bacteria in healthy subjects and patients with chronic periodontitis. BMC Oral Health 17:64. doi: 10.1186/s12903-017-0359-4
Karkman, A., Lehtimaki, J., and Ruokolainen, L. (2017). The ecology of human microbiota: dynamics and diversity in health and disease. Ann. N. Y. Acad. Sci. 1399, 78–92. doi: 10.1111/nyas.13326
Katono, T., Kawato, T., Tanabe, N., Tanaka, H., Suzuki, N., Kitami, S., et al. (2009). Effects of nicotine and lipopolysaccharide on the expression of matrix metalloproteinases, plasminogen activators, and their inhibitors in human osteoblasts. Arch. Oral Biol. 54, 146–155. doi: 10.1016/j.archoralbio.2008.09.017
Kigure, T., Saito, A., Seida, K., Yamada, S., Ishihara, K., and Okuda, K. (1995). Distribution of Porphyromonas gingivalis and Treponema denticola in human subgingival plaque at different periodontal pocket depths examined by immunohistochemical methods. J. Periodontal Res. 30, 332–341. doi: 10.1111/j.1600-0765.1995.tb01284.x
Kim, Y. S., Shin, S. I., Kang, K. L., Chung, J. H., Herr, Y., Bae, W. J., et al. (2012). Nicotine and lipopolysaccharide stimulate the production of MMPs and prostaglandin E2 by hypoxia-inducible factor-1alpha up-regulation in human periodontal ligament cells. J. Periodontal Res. 47, 719–728. doi: 10.1111/j.1600-0765.2012.01487.x
Kinane, D. F., Stathopoulou, P. G., and Papapanou, P. N. (2017). Periodontal diseases. Nat. Rev. Dis. Primers 3:17038. doi: 10.1038/nrdp.2017.38
Kirst, M. E., Li, E. C., Alfant, B., Chi, Y. Y., Walker, C., Magnusson, I., et al. (2015). Dysbiosis and alterations in predicted functions of the subgingival microbiome in chronic periodontitis. Appl. Environ. Microbiol. 81, 783–793. doi: 10.1128/AEM.02712-14
Kubota, M., Tanno-Nakanishi, M., Yamada, S., Okuda, K., and Ishihara, K. (2011). Effect of smoking on subgingival microflora of patients with periodontitis in Japan. BMC Oral Health 11:1. doi: 10.1186/1472-6831-11-1
Kumar, P. S., Griffen, A. L., Moeschberger, M. L., and Leys, E. J. (2005). Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J. Clin. Microbiol. 43, 3944–3955. doi: 10.1128/jcm.43.8.3944-3955.2005
Kumar, P. S., Leys, E. J., Bryk, J. M., Martinez, F. J., Moeschberger, M. L., and Griffen, A. L. (2006). Changes in periodontal health status are associated with bacterial community shifts as assessed by quantitative 16S cloning and sequencing. J. Clin. Microbiol. 44, 3665–3673. doi: 10.1128/jcm.00317-06
Kumar, P. S., Matthews, C. R., Joshi, V., De Jager, M., and Aspiras, M. (2011). Tobacco smoking affects bacterial acquisition and colonization in oral biofilms. Infect. Immun. 79, 4730–4738. doi: 10.1128/IAI.05371-11
Lanza, E., Magan-Fernandez, A., Bermejo, B., De Rojas, J., Marfil-Alvarez, R., and Mesa, F. (2016). Complementary clinical effects of red complex bacteria on generalized periodontitis in a caucasian population. Oral Dis. 22, 430–437. doi: 10.1111/odi.12471
Lee, J., Taneja, V., and Vassallo, R. (2012). Cigarette smoking and inflammation: cellular and molecular mechanisms. J. Dent. Res. 91, 142–149. doi: 10.1177/0022034511421200
Leite, F. R. M., Nascimento, G. G., Baake, S., Pedersen, L. D., Scheutz, F., and Lopez, R. (2018). Impact of smoking cessation on periodontitis: a systematic review and meta-analysis of prospective longitudinal observational and interventional studies. Nicotine Tob. Res. 21, 1600–1608. doi: 10.1093/ntr/nty147
Loe, H., Theilade, E., and Jensen, S. B. (1965). Experimental gingivitis in man. J. Periodontol. 36, 177–187. doi: 10.1902/jop.1965.36.3.177
Machion, L., Andia, D. C., Saito, D., Klein, M. I., Goncalves, R. B., Casati, M. Z., et al. (2004). Microbiological changes with the use of locally delivered doxycycline in the periodontal treatment of smokers. J. Periodontol. 75, 1600–1604. doi: 10.1902/jop.2004.75.12.1600
Mason, M. R., Preshaw, P. M., Nagaraja, H. N., Dabdoub, S. M., Rahman, A., and Kumar, P. S. (2015). The subgingival microbiome of clinically healthy current and never smokers. ISME J. 9, 268–272. doi: 10.1038/ismej.2014.114
Matarazzo, F., Figueiredo, L. C., Cruz, S. E., Faveri, M., and Feres, M. (2008). Clinical and microbiological benefits of systemic metronidazole and amoxicillin in the treatment of smokers with chronic periodontitis: a randomized placebo-controlled study. J. Clin. Periodontol. 35, 885–896. doi: 10.1111/j.1600-051X.2008.01304.x
Matthews, C. R., Joshi, V., De Jager, M., Aspiras, M., and Kumar, P. S. (2013). Host-bacterial interactions during induction and resolution of experimental gingivitis in current smokers. J. Periodontol. 84, 32–40. doi: 10.1902/jop.2012.110662
Matthews, J. B., Chen, F. M., Milward, M. R., Ling, M. R., and Chapple, I. L. (2012). Neutrophil superoxide production in the presence of cigarette smoke extract, nicotine and cotinine. J. Clin. Periodontol. 39, 626–634. doi: 10.1111/j.1600-051X.2012.01894.x
Matthews, J. B., Chen, F. M., Milward, M. R., Wright, H. J., Carter, K., Mcdonagh, A., et al. (2011). Effect of nicotine, cotinine and cigarette smoke extract on the neutrophil respiratory burst. J. Clin. Periodontol. 38, 208–218. doi: 10.1111/j.1600-051X.2010.01676.x
McGuire, J. R., Mcquade, M. J., Rossmann, J. A., Garnick, J. J., Sutherland, D. E., Scheidt, M. J., et al. (1989). Cotinine in saliva and gingival crevicular fluid of smokers with periodontal disease. J. Periodontol. 60, 176–181. doi: 10.1902/jop.1989.60.4.176
Meulman, T., Casarin, R. C., Peruzzo, D. C., Giorgetti, A. P., Barbagallo, A., Casati, M. Z., et al. (2012). Impact of supragingival therapy on subgingival microbial profile in smokers versus non-smokers with severe chronic periodontitis. J. Oral Microbiol. 4:8640. doi: 10.3402/jom.v4i0.8640
Moon, J. H., Lee, J. H., and Lee, J. Y. (2015). Subgingival microbiome in smokers and non-smokers in Korean chronic periodontitis patients. Mol. Oral Microbiol. 30, 227–241. doi: 10.1111/omi.12086
Natto, S., Baljoon, M., Dahlen, G., and Bergstrom, J. (2005). Tobacco smoking and periodontal microflora in a Saudi Arabian population. J. Clin. Periodontol. 32, 549–555. doi: 10.1111/j.1600-051x.2005.00710.x
Nearing, J. T., Douglas, G. M., Comeau, A. M., and Langille, M. G. I. (2018). Denoising the denoisers: an independent evaluation of microbiome sequence error-correction approaches. PeerJ 6:e5364. doi: 10.7717/peerj.5364
Nociti, F. H. Jr., Casati, M. Z., and Duarte, P. M. (2015). Current perspective of the impact of smoking on the progression and treatment of periodontitis. Periodontology 2000, 187–210. doi: 10.1111/prd.12063
Palmer, R. M., Wilson, R. F., Hasan, A. S., and Scott, D. A. (2005). Mechanisms of action of environmental factors–tobacco smoking. J. Clin. Periodontol. 32(Suppl. 6), 180–195. doi: 10.1111/j.1600-051x.2005.00786.x
Paropkari, A. D., Leblebicioglu, B., Christian, L. M., and Kumar, P. S. (2016). Smoking, pregnancy and the subgingival microbiome. Sci. Rep. 6:30388. doi: 10.1038/srep30388
Paster, B. J., Boches, S. K., Galvin, J. L., Ericson, R. E., Lau, C. N., Levanos, V. A., et al. (2001). Bacterial diversity in human subgingival plaque. J. Bacteriol. 183, 3770–3783. doi: 10.1128/jb.183.12.3770-3783.2001
Patel, R. A., Wilson, R. F., and Palmer, R. M. (2012). The effect of smoking on periodontal bone regeneration: a systematic review and meta-analysis. J. Periodontol. 83, 143–155. doi: 10.1902/jop.2011.110130
Peruzzo, D. C., Gimenes, J. H., Taiete, T., Casarin, R. C., Feres, M., Sallum, E. A., et al. (2016). Impact of smoking on experimental gingivitis. A clinical, microbiological and immunological prospective study. J. Periodontal Res. 51, 800–811. doi: 10.1111/jre.12363
Pi, S. H., Jeong, G. S., Oh, H. W., Kim, Y. S., Pae, H. O., Chung, H. T., et al. (2010). Heme oxygenase-1 mediates nicotine- and lipopolysaccharide-induced expression of cyclooxygenase-2 and inducible nitric oxide synthase in human periodontal ligament cells. J. Periodontal Res. 45, 177–183. doi: 10.1111/j.1600-0765.2009.01215.x
Pollock, J. D., Koustova, E., Hoffman, A., Shurtleff, D., and Volkow, N. D. (2009). Treatments for nicotine addiction should be a top priority. Lancet 374, 513–514. doi: 10.1016/s0140-6736(09)60352-4
Queiroz, A. C., Suaid, F. A., de Andrade, P. F., Novaes, A. B. Jr., Taba, M. Jr., Palioto, D. B., et al. (2014). Antimicrobial photodynamic therapy associated to nonsurgical periodontal treatment in smokers: microbiological results. J. Photochem. Photobiol. B 141, 170–175. doi: 10.1016/j.jphotobiol.2014.10.017
Rosier, B. T., Marsh, P. D., and Mira, A. (2018). Resilience of the oral microbiota in health: mechanisms that prevent dysbiosis. J. Dent. Res. 97, 371–380. doi: 10.1177/0022034517742139
Ryder, M. I. (2007). The influence of smoking on host responses in periodontal infections. Periodontology 2000, 267–277. doi: 10.1111/j.1600-0757.2006.00163.x
Salvi, G. E., Ramseier, C. A., Kandylaki, M., Sigrist, L., Awedowa, E., and Lang, N. P. (2005). Experimental gingivitis in cigarette smokers: a clinical and microbiological study. J. Clin. Periodontol. 32, 441–447. doi: 10.1111/j.1600-051x.2005.00691.x
Shah, S. A., Ganesan, S. M., Varadharaj, S., Dabdoub, S. M., Walters, J. D., and Kumar, P. S. (2017). The making of a miscreant: tobacco smoke and the creation of pathogen-rich biofilms. NPJ Biofilms Microbiomes 3:26. doi: 10.1038/s41522-017-0033-2
Shchipkova, A. Y., Nagaraja, H. N., and Kumar, P. S. (2010). Subgingival microbial profiles of smokers with periodontitis. J. Dent. Res. 89, 1247–1253. doi: 10.1177/0022034510377203
Shi, B., Chang, M., Martin, J., Mitreva, M., Lux, R., Klokkevold, P., et al. (2015). Dynamic changes in the subgingival microbiome and their potential for diagnosis and prognosis of periodontitis. mBio 6:e1926-14. doi: 10.1128/mBio.01926-14
Shiloah, J., Patters, M. R., and Waring, M. B. (2000). The prevalence of pathogenic periodontal microflora in healthy young adult smokers. J. Periodontol. 71, 562–567. doi: 10.1902/jop.2000.71.4.562
Signat, B., Roques, C., Poulet, P., and Duffaut, D. (2011). Fusobacterium nucleatum in periodontal health and disease. Curr. Issues Mol. Biol. 13, 25–36.
Sochalska, M., and Potempa, J. (2017). Manipulation of neutrophils by Porphyromonas gingivalis in the development of periodontitis. Front. Cell Infect. Microbiol. 7:197. doi: 10.3389/fcimb.2017.00197
Song, L., Li, J., Yuan, X., Liu, W., Chen, Z., Guo, D., et al. (2017). Carbon monoxide-releasing molecule suppresses inflammatory and osteoclastogenic cytokines in nicotine- and lipopolysaccharide-stimulated human periodontal ligament cells via the heme oxygenase-1 pathway. Int. J. Mol. Med. 40, 1591–1601. doi: 10.3892/ijmm.2017.3129
Stokman, M. A., Van Winkelhoff, A. J., Vissink, A., Spijkervet, F. K., and Raghoebar, G. M. (2017). Bacterial colonization of the peri-implant sulcus in dentate patients: a prospective observational study. Clin. Oral Investig. 21, 717–724. doi: 10.1007/s00784-016-1941-x
Talhout, R., Schulz, T., Florek, E., Van Benthem, J., Wester, P., and Opperhuizen, A. (2011). Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health 8, 613–628. doi: 10.3390/ijerph8020613
Tebloeva, L. M., Revazova, Z. E., Fabrikant, K. G., Dmitrieva, L. A., and Gurevich, K. G. (2014). Differences in immune response to Porphyromonas gingivalis. J. Contemp. Dent. Pract. 15, 573–575. doi: 10.5005/jp-journals-10024-1581
Teughels, W., Van Eldere, J., Van Steenberghe, D., Cassiman, J. J., Fives-Taylor, P., and Quirynen, M. (2005). Influence of nicotine and cotinine on epithelial colonization by periodontopathogens. J. Periodontol. 76, 1315–1322. doi: 10.1902/jop.2005.76.8.1315
Tribble, G. D., and Lamont, R. J. (2010). Bacterial invasion of epithelial cells and spreading in periodontal tissue. Periodontology 2000, 68–83. doi: 10.1111/j.1600-0757.2009.00323.x
Tsigarida, A. A., Dabdoub, S. M., Nagaraja, H. N., and Kumar, P. S. (2015). The influence of smoking on the peri-implant microbiome. J. Dent. Res. 94, 1202–1217. doi: 10.1177/0022034515590581
Van der Velden, U., Varoufaki, A., Hutter, J. W., Xu, L., Timmerman, M. F., Van Winkelhoff, A. J., et al. (2003). Effect of smoking and periodontal treatment on the subgingival microflora. J. Clin. Periodontol. 30, 603–610. doi: 10.1034/j.1600-051x.2003.00080.x
van Winkelhoff, A. J., Bosch-Tijhof, C. J., Winkel, E. G., and Van Der Reijden, W. A. (2001). Smoking affects the subgingival microflora in periodontitis. J. Periodontol. 72, 666–671. doi: 10.1902/jop.2001.72.5.666
Vlachojannis, C., Dye, B. A., Herrera-Abreu, M., Pikdoken, L., Lerche-Sehm, J., Pretzl, B., et al. (2010). Determinants of serum IgG responses to periodontal bacteria in a nationally representative sample of US adults. J. Clin. Periodontol. 37, 685–696. doi: 10.1111/j.1600-051X.2010.01592.x
Wendell, K. J., and Stein, S. H. (2001). Regulation of cytokine production in human gingival fibroblasts following treatment with nicotine and lipopolysaccharide. J. Periodontol. 72, 1038–1044. doi: 10.1902/jop.2001.72.8.1038
World Health Organization [WHO] (2018). WHO Global Report on Trends in Prevalence of Tobacco Smoking 2000–2025. Geneva: World Health Organization.
Ylostalo, P., Sakki, T., Laitinen, J., Jarvelin, M. R., and Knuuttila, M. (2004). The relation of tobacco smoking to tooth loss among young adults. Eur. J. Oral Sci. 112, 121–126. doi: 10.1111/j.0909-8836.2004.00111.x
Yu, G., Phillips, S., Gail, M. H., Goedert, J. J., Humphrys, M. S., Ravel, J., et al. (2017). The effect of cigarette smoking on the oral and nasal microbiota. Microbiome 5:3. doi: 10.1186/s40168-016-0226-6
Zappacosta, B., Martorana, G. E., Papini, S., Gervasoni, J., Iavarone, F., Fasanella, S., et al. (2011). Morpho-functional modifications of human neutrophils induced by aqueous cigarette smoke extract: comparison with chemiluminescence activity. Luminescence 26, 331–335. doi: 10.1002/bio.1233
Zeller, I., Malovichko, M. V., Hurst, H. E., Renaud, D. E., and Scott, D. A. (2019). Cigarette smoke reduces short chain fatty acid production by a Porphyromonas gingivalis clinical isolate. J. Periodontal Res. 54, 566–571. doi: 10.1111/jre.12660
Zenobia, C., and Hajishengallis, G. (2015). Porphyromonas gingivalis virulence factors involved in subversion of leukocytes and microbial dysbiosis. Virulence 6, 236–243. doi: 10.1080/21505594.2014.999567
Zhang, W., Song, F., and Windsor, L. J. (2010). Effects of tobacco and P. gingivalis on gingival fibroblasts. J. Dent. Res. 89, 527–531. doi: 10.1177/0022034509358567
Keywords: periodontal disease, smoking, subgingival microflora, nicotine, microbial diversity
Citation: Jiang Y, Zhou X, Cheng L and Li M (2020) The Impact of Smoking on Subgingival Microflora: From Periodontal Health to Disease. Front. Microbiol. 11:66. doi: 10.3389/fmicb.2020.00066
Received: 25 October 2019; Accepted: 13 January 2020;
Published: 29 January 2020.
Edited by:
Giovanna Batoni, University of Pisa, ItalyReviewed by:
Kesavalu Naidu Lakshmyya, University of Florida, United StatesTomoki Maekawa, Niigata University, Japan
Copyright © 2020 Jiang, Zhou, Cheng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Cheng, chenglei@scu.edu.cn; Mingyun Li, limingyun@scu.edu.cn
 Yaling Jiang
Yaling Jiang Xuedong Zhou
Xuedong Zhou Lei Cheng
Lei Cheng Mingyun Li
Mingyun Li