
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 21 January 2020
Sec. Virology
Volume 10 - 2019 | https://doi.org/10.3389/fmicb.2019.03085
This article is part of the Research Topic Host And Pathogen Mechanisms Underpinning Viral Ecology And Emerging Infections View all 34 articles
 Shaoju Qian1
Shaoju Qian1 Zitong Gao1
Zitong Gao1 Rui Cao1
Rui Cao1 Kang Yang1
Kang Yang1 Yijie Cui1
Yijie Cui1 Shaowen Li1,2,3
Shaowen Li1,2,3 Xianrong Meng1,2,3
Xianrong Meng1,2,3 Qigai He1,2,3
Qigai He1,2,3 Zili Li1,2,3*
Zili Li1,2,3*Transmissible gastroenteritis virus (TGEV) is a porcine intestinal coronavirus that causes fatal severe watery diarrhea in piglets. The neonatal Fc receptor (FcRn) is the only IgG transport receptor, its expression on mucosal surfaces is triggered upon viral stimulation, which significantly enhances mucosal immunity. We utilized TGEV as a model pathogen to explore the role of FcRn in resisting viral invasion in overall intestinal mucosal immunity. TGEV induced FcRn expression by activating NF-κB signaling in porcine small intestinal epithelial (IPEC-J2) cells, however, the underlying mechanisms are unclear. First, using small interfering RNAs, we found that TGEV up-regulated FcRn expression via TLR3, TLR9 and RIG-I. Moreover, TGEV induced IL-1β, IL-6, IL-8, TGF-β, and TNF-α production. TGF-β-stimulated IPEC-J2 cells highly up-regulated FcRn expression, while treatment with a JNK-specific inhibitor down-regulated the expression. TGEV nucleocapsid (N) protein also enhanced FcRn promoter activity via the NF-κB signaling pathway and its central region (aa 128–252) was essential for FcRn activation. Additionally, N protein-mediated FcRn up-regulation promotes IgG transcytosis. Thus, TGEV N protein and TGF-β up-regulated FcRn expression, further clarifying the molecular mechanism of up-regulation of FcRn expression by TGEV.
Transmissible gastroenteritis virus (TGEV) infection mainly causes severe watery diarrhea, vomiting, dehydration, and mortality in suckling piglets less than 2 weeks old, as well as colossal economic losses in the worldwide pig industry (Zhao et al., 2014). TGEV has a 28.5-kb single-stranded, positive-sense RNA genome, including at least nine open reading frames (ORFs): ORF1a, ORF1b, Structural proteins, nucleocapsid (N) protein, membrane (M) glycoprotein, spike (S) glycoprotein, a small envelope (E) glycoprotein, and accessory proteins 3a, 3b, and 7 (Escors et al., 2003). ORF1a and ORF1b, located in the 5′ two-thirds of the viral genome, are cleaved into 16 non-structural proteins (nsp1 to nsp16) by the nsp3 and nsp5 proteases. These nsps are responsible for viral replication, viral transcription, and the antagonization of host innate immune responses.
Transmissible gastroenteritis virus replication occurs in the small intestinal epithelial cells, while viral entry and release are restricted to the apical surface of polarized epithelial cells. Neonatal Fc receptor (FcRn) is a specific receptor for the immunoglobulin IgG, which is expressed in many cells, including epithelial cells, macrophages, and dendritic cells (Ye et al., 2008). FcRn transports of IgG in the female reproductive tract plays an important role in combating infection (Li et al., 2011). In addition to IgG, FcRn binds albumin, which regulates liver damage (Pyzik et al., 2017). It has been found that FcRn can enhance HIV-I endocytosis in transmucosal epithelial cells (Gupta et al., 2013). It has also been documented that the use of fusion proteins of the Fc fragment as immunogenic antigens can improve the impact of vaccines. Intranasally inoculated fusion protein, HSV-2 gD, HIV Gag fused with the Fc region of IgG, and targeted FcRn can all induce systemic and mucosal immunity to genital infections (Lu et al., 2011; Ye et al., 2011). Most studies on FcRn have focused on the function of FcRn in humans and mice; so far, only a few relevant studies about pathogenic infection and FcRn expression regulation have been reported. In our previous study, TGEV induced FcRn expression via the NF-κB pathway in IPEC-J2 cells (Guo et al., 2016b), but the regulatory mechanisms underlying this remain unclear; thus, we need to further explore the involvement of pattern recognition receptors (PRRs), inflammatory factors, and virus-coding proteins in the regulation of FcRn.
In the innate immune system, PRRs recognize pathogen-associated molecular patterns (PAMPs) as the first step in the host’s resistance to infection. After recognition of PAMPs, these receptors interact with their corresponding binding molecules to trigger downstream signaling events that activate NF-κB and IFN regulatory factors that induce several types of antiviral cytokines (Thompson and Locarnini, 2007). TGEV infection activates transcriptional activator 1 (JAK-STAT1) signaling pathways according to a quantitative proteomics study in PK-15 cells (An et al., 2014). TGEV infection has been reported to activate the mitogen-activated protein kinase (MAPK) pathway and destroy epithelial barrier integrity in IPEC-J2 cells (Zhao et al., 2014). TGEV N, nsp2 and nsp14 proteins all induce NF-κB activation (Zhou et al., 2017; Wang et al., 2018; Zhang et al., 2018), but the regulatory mechanism of FcRn is still unknown. Most studies on TGEV have focused on the induction and activation of NF-κB and IFN, but host cell biology may affect cell function. However, the detailed mucosal immunity mechanism during TGEV pathogenesis remains unknown.
Viral invasion always triggers an inflammatory response, which is the key mediator of host response to microbial pathogens (McDonnell et al., 2016). TGEV infection has been shown to induce the production of IFNs and pro-inflammatory cytokines in vitro and in vivo (Cruz et al., 2013). A direct correlation between dsRNA antiviral response induction and TGEV virulence has been demonstrated (Cruz et al., 2011). Moreover, the inflammatory factors produced will also contribute to the production of a strong immune response. TNF-α and IL-1β can activate NF-κB to up-regulate the expression of human FcRn, which enhances FcRn-mediated IgG transport (Liu et al., 2007). TGEV infection was also found to induce EMT via TGF-β in IPEC-J2 cells (Xia et al., 2017). The aim of this study was to identify the TGEV-encoded proteins involved in inducing FcRn production.
IPEC-J2 cells donated by Xiaoping Li from Huazhong Agricultural University (Hubei Province, Wuhan, China) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone, United States) containing 10% fetal bovine serum (FBS; Gibco, United States) and 1% penicillin/streptomycin at 37°C in a 5% CO2 atmosphere. The isolated TGEV strain WH-1 (GenBank HQ462571) was propagated in IPEC-J2 cells. In our laboratory, AffiniPure rabbit anti-cytoplasmic tails of porcine FcRn (anti-FcRn-CT) polyclonal antibodies were prepared (Guo et al., 2016a). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit and goat anti-mouse IgG were purchased from Abclona (China). Monoclonal antibody (mAb) against GAPDH in mice was purchased from Abclona (China). Mouse mAbs against HA, IκBα, NF-κB, p65 and rabbit mAbs against phospho-NF-κB and p65 were obtained from Cell Signaling Technology (United States).
Luciferase reporter plasmids FcRn-Luc, NF-κB-Luc, pR-TK, p65-EGFP, and p65-Tag2B were prepared in our laboratory and have been described previously. Genes encoding TGEV proteins were amplified from the genomic RNA of the TGEV strain WH-1 and then cloned into the expression vector pCAGGS-HA (Guo et al., 2016b) (Table 1). pCAGGS-N was used as a template to amplify several deletion mutants of the virus N gene. These mutants were then cloned into the pCAGGS-HA (Table 2). Small interfering RNA (siRNA) molecules targeting TLR2, TLR3, TLR4, TLR8, TLR9, RIG-I, MyD88, TRIF, and negative controls were obtained from Shanghai GenePharma (Table 3). MAPK inhibitors SB203580, SP600125, U0126 and DMSO were purchased from Sigma-Aldrich (United States).
Total RNA was prepared using TRIzol@ reagent (Invitrogen). RT-qPCR was performed using SYBR Green PCR Master Mix (Takara, Osaka, Japan) in the Bio-Rad Sequence Detection System (Bio-Rad). Each transcript from each sample was determined three times. The delta threshold cycle (2–ΔΔCT) method was used to calculate the fold-change compared to the normal gene expression. The specific pig gene primers (Table 4) were designed using Primer Express software (version 3.0; Applied Biological Systems, Carlsbad, CA, United States) as previously described with some modifications (Temeeyasen et al., 2018).
The samples were harvested with lysis buffer (4% sodium dodecyl sulfate, 3% dithiothreitol, 65 mM Tris–HCl [pH 6.8], and 30% glycerol). Proteins were separated via SDS-PAGE and then electrotransferred onto a polyvinylidene difluoride membrane (Bio-Rad, United States). Western blot analysis was performed using the indicated antibodies following the procedure as described previously (Guo et al., 2016b).
IPEC-J2 cells grown in a six-well plate were infected with TGEV, the supernatant of IPEC-J2 cells was harvested, and IL-1β, IL-6, IL-8, TGF-β, and TNF-α protein levels were measured with a Quantibody Porcine Cytokine Array 1 (Ray Biotech, Norcross, GA, United States) according to the manufacturer’s instructions.
IPEC-J2 cells at 80% confluence were co-transfected with 0.2 μg FcRn-Luc and 0.1 μg pRL-TK using Lipofectamine 2000 (Invitrogen) reagent before treatment with TGEV, specific siRNA molecules, or recombinant plasmids. Cell lysates were collected at the indicated time points over 36 h and measured using a dual-luciferase assay kit (Promega).
The recombinant TGEV N protein were prepared using a baculovirus/insect cell expression system as previously described (Chen et al., 2017). IPEC-J2 cells were added (0, 10, 20, 50, 100 or 200 ng/mL) recombinant TGEV N protein in medium and its FcRn expression detected by RT-qPCR and Western blot.
IgG transport was performed as previously described methods (Guo et al., 2016a). Transepithelial electrical resistance (TEER) was measured with planar electrodes (World Precision Instruments). IPEC-J2 cells were grown on 0.4-μm pore size transwell filter inserts (Corning Costar, United States) to form a monolayer exhibiting TEER > 1000 Ω/cm2 about 6–7 days. Monolayers were stimulated with recombinant TGEV N protein (100 ng/mL) for 12 h. Thereafter, Biotin-IgG (200 μg/mL) were applied to the basolateral or apical DMEM medium and incubated for 3 h at 37°C. Meanwhile, the opposite chamber was incubated in DMEM medium. Three hours later, samples were collected in which apically and basolaterally directed IgG transports were conducted. Subsequently, the bidirectional transport (apical-to-basolateral or basolateral-to-apical) of IgG were measured by Western blot and avidin blot analysis.
All experiments were repeated a minimum of three times. Differences among groups were determined by one-way ANOVA using GraphPad Prism (version 5.0; GraphPad Software). The significance levels for all analyses were set as p < 0.05 (∗) and p < 0.01 (∗∗).
To examine if TGEV infection induces FcRn promoter activation, IPEC-J2 cells were co-transfected with FcRn-luc or pRL-TK. Twenty-four hours later, the cells were incubated with live or UV-inactivated TGEV for 12, 24, 36, and 48 h post-infection (hpi). Enhanced FcRn luciferase activities tended to increase during the progression of TGEV infection from 24 to 48 hpi, and this increase was significantly different compared with mock-infected IPEC-J2 cells (Figure 1A). Therefore, the up-regulation of FcRn is closely related to the replication of TGEV.
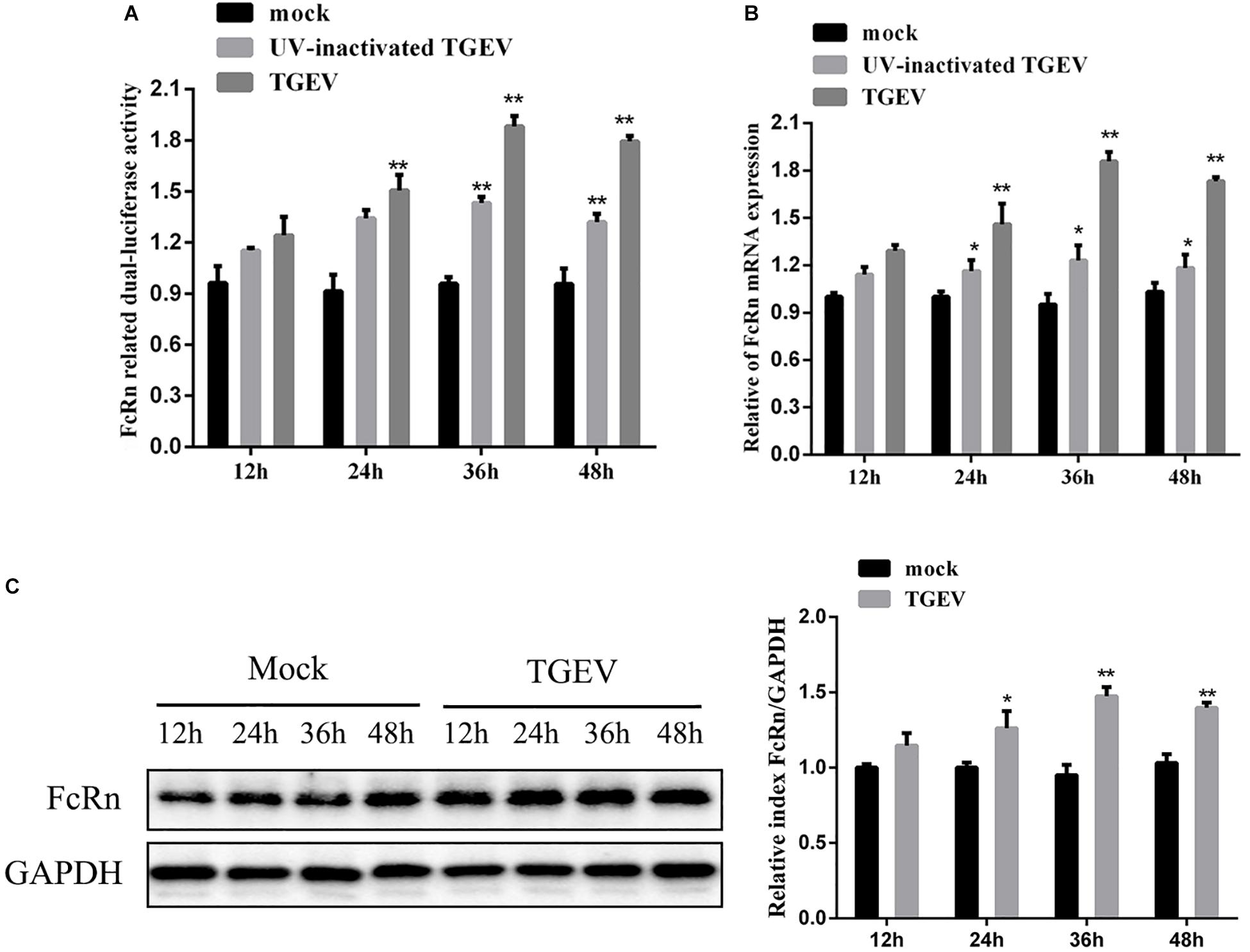
Figure 1. TGEV-induced activation of FcRn depends on viral replication. (A) IPEC-J2 were co-transfected with pRL-TK and FcRn-Luc, followed by alive TGEV or UV-inactivated TGEV (MOI 1). Cells were harvested at 12, 24, 36 or 48 hpi, and the lysates were analyzed by dual-luciferase assay. (B) IPEC-J2 cells were infected with alive TGEV or UV-inactivated TGEV at a MOI of 1. Cells were harvested at 12, 24, 36 or 48 hpi, and the lysates were analyzed by RT-qPCR. (C) IPEC-J2 were infected with alive TGEV at a MOI of 1. Cells were harvested at 12, 24, 36 or 48 hpi, and the lysates were analyzed by Western blot. ∗p < 0.05, ∗∗p < 0.01.
In order to determine whether TGEV induces FcRn expression in IPEC-J2 cells, live or UV-inactivated TGEV at a multiplicity of infection (MOI) of 1 was used to inoculate IPEC-J2 cells, which were then harvested for FcRn analysis at 12, 24, 36, and 48 hpi by RT-qPCR and Western blot (Figures 1B,C). TGEV induced FcRn mRNA expression about two fold higher at 24, 36, and 48 hpi, and the elevation in protein levels correlated with the increased mRNA. However, UV-inactivated viral treatment resulted in only a 1.3-fold up-regulation of FcRn expression compared with that in simulated infected cells, indicating that the replicating virus is more effective than the UV-inactivated virus.
The small intestine of pigs expressed different TLRs, such as TLR1, TLR2, TLR3, TLR4, TLR6, TLR8, TLR9, and TLR10 (Arce et al., 2010). It has been reported that viral nucleic acids are recognized by TLR3, TLR8, and TLR9, whereas viral proteins are recognized by TLR2 and TLR4 (Thompson and Locarnini, 2007). To determine whether TLRs are involved in mediating TGEV-induced FcRn activation, specific siRNAs were synthesized to target binding molecules in the TLR2, TLR3, TLR4, TLR8, TLR9, TRIF, MyD88, and RIG-I pathways. Transient transfection and RT-qPCR assays proved the low knockout efficiency of each siRNA, and the silencing efficiencies of the siRNAs to these receptors were over 50% (Figure 2A). The cells were transfected with each siRNA and then infected with TGEV; then, the changes in FcRn expression were detected by RT-qPCR and Western blot. The results showed that, compared with the cells transfected with NC siRNA, siRNA knockdown of TLR3, TLR9, RIG-I, TRIF, and MyD88 effectively reduced the TGEV-induced up-regulation of FcRn, while FcRn mRNA expression was not inhibited in cells transfected with siRNA targeting TLR2, TLR4, or TLR8 (Figure 2B). Next, IPEC-J2 cells were transfected with TLR-specific siRNA or NC siRNA. After 12 h, the cells were infected with 1 MOI TGEV for an additional 36 h. Compared with NC siRNA, TLR3, TLR9, RIG-I, TRIF, and MyD88-specific siRNAs inhibited TGEV-induced FcRn expression, while no significant change was detected in s TLR2, TLR4 and TLR8-specific siRNAs (Figures 2C,D). These data suggest that TGEV infection activates FcRn via TLR3, TLR9, TRIF, MyD88, and RIG-I.
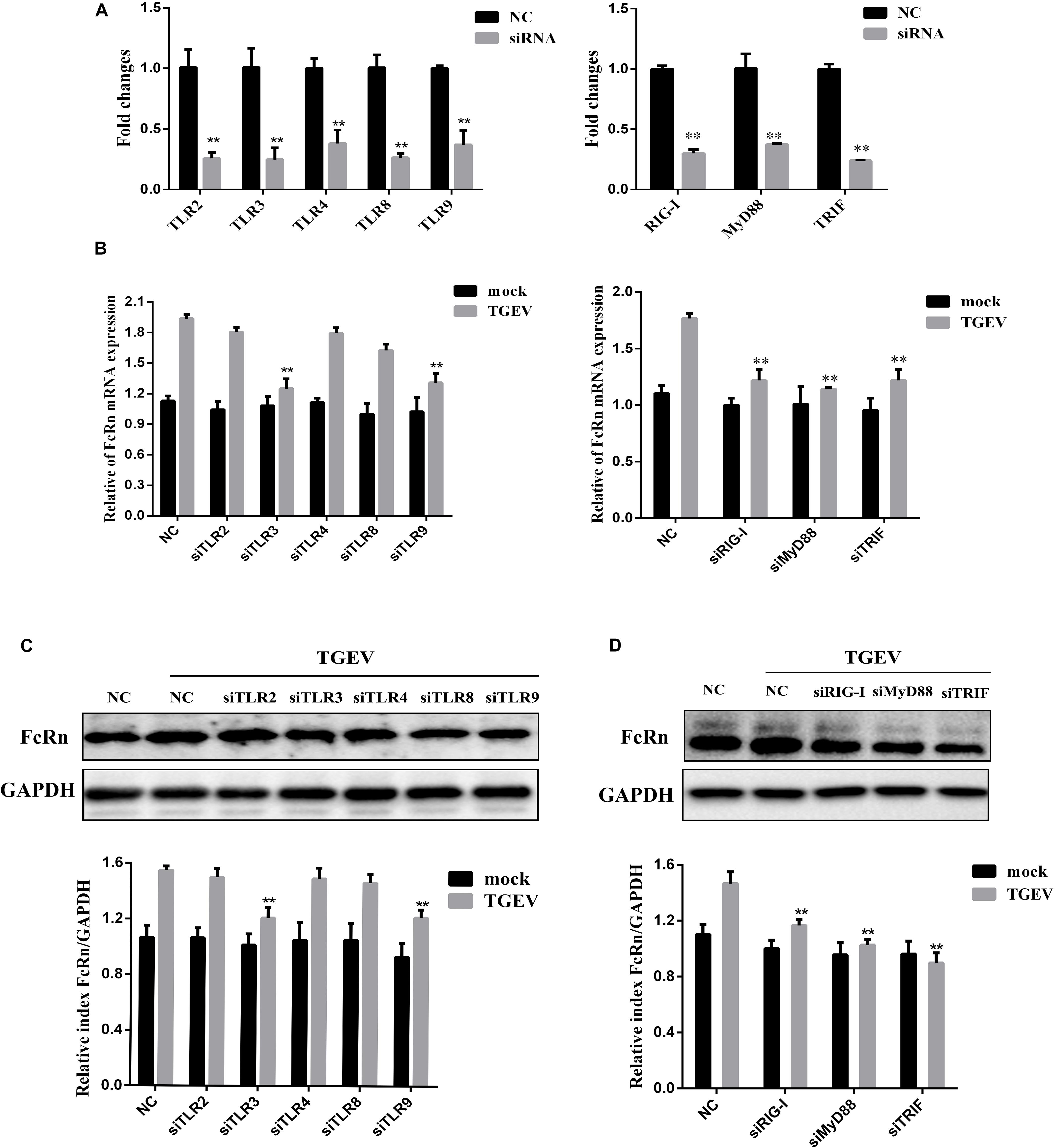
Figure 2. Involvement of TLR signaling cascades in FcRn activation by TGEV. (A) IPEC-J2 cells were transfected with 50 nM specific siRNAs targeting TLR2/3/4/8/9, TRIF, MyD88, RIG-I or NC siRNA, respectively for 24 h, then cells were collected for analysis of mRNA levels using RT-qPCR assays. (B) IPEC-J2 cells were transfected with the siRNAs TLR2/3/4/8/9, TRIF, MyD88, RIG-I or NC siRNA, respectively for 24 h, then with/without TGEV infection (MOI = 1). At 24 hpi, total RNA was extracted and FcRn or GAPDH mRNA expression was analyzed by RT-qPCR. (C,D) IPEC-J2 cells were transfected with the siRNAs TLR2/3/4/8/9, TRIF, MyD88, RIG-I or NC siRNA, respectively for 24 h, then with/without TGEV infection (MOI 1). IPEC-J2 cells were harvested at 36 hpi and measured by Western blot. The right panel represents the quantification of the bands by densitometry, corrected by the amount of GAPDH. ∗∗p < 0.01.
To detect the effect of TGEV infection on inflammatory cytokines, RT-qPCR and a porcine cytokine array were used to detect the expression levels of IL-1β, IL-6, IL-8, TGF-β, and TNF-α in IPEC-J2 cells infected with TGEV at 36 h. TGEV significantly increased the expression levels of five cytokines, especially TGF-β (Figures 3A,B).
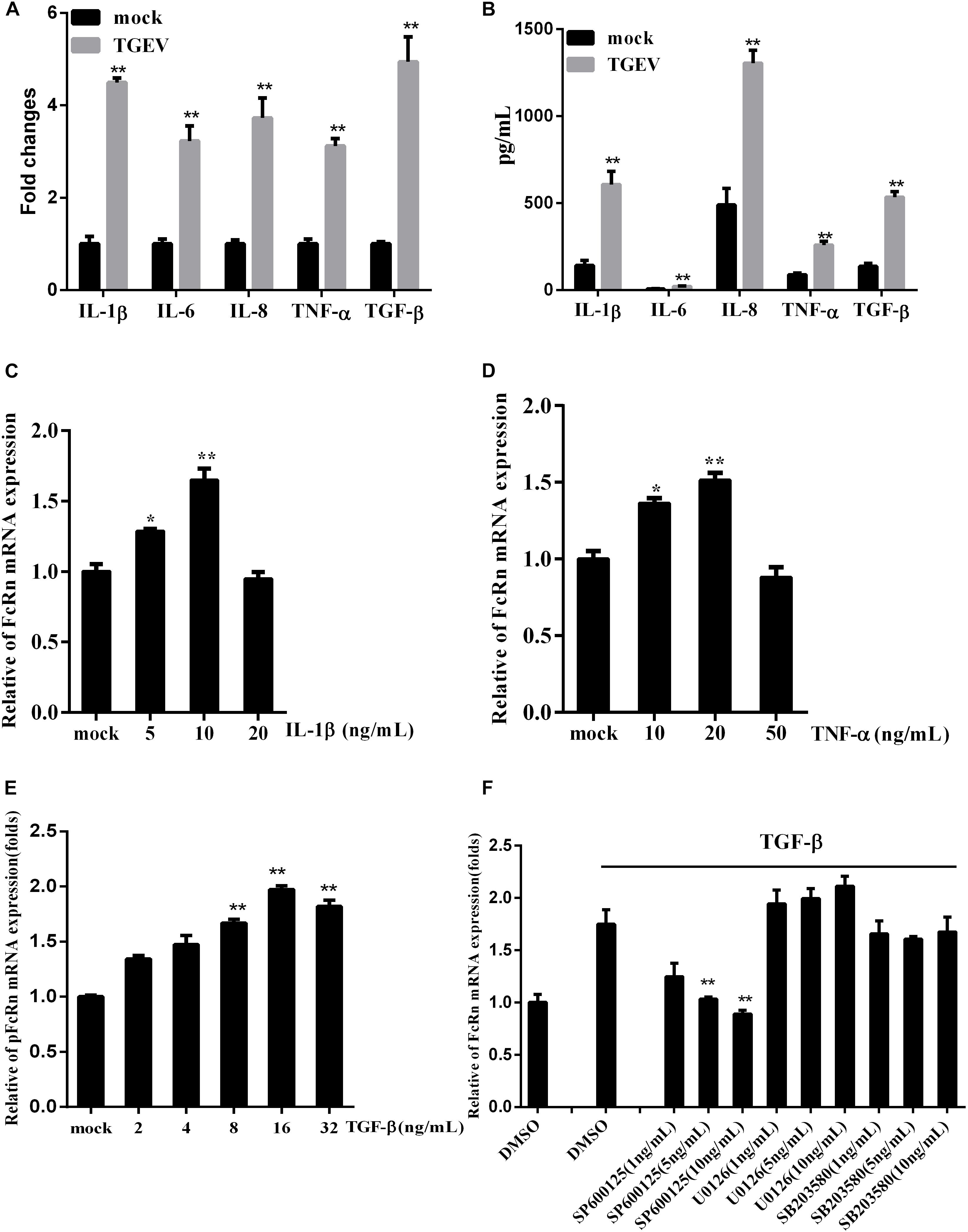
Figure 3. Regulation of FcRn mRNA on IPEC-J2 cells stimulated by TGF-β. (A,B) The relative mRNA and protein levels of IL-1β, IL-6, IL-8, TNF-α, and TGF-β in IPEC-J2 infected with TGEV for 36 hpi by RT-PCR and porcine cytokine array. (C–E) IPEC-J2 cells were stimulated with TNF-α, IL-1β, TGF-β for 2 h, and the levels of FcRn mRNA were detected using RT-qPCR. (F) IPEC-J2 cells were pretreated with SB202190 (1, 5, and 10 ng/mL), SP600125 (1, 5, and 10 ng/mL), or U0126 (1, 5, and 10 ng/mL) or DMSO (control) for 2 h, followed by with/without TGF-β for 2 h and the levels of FcRn mRNA were detected using RT-qPCR. ∗p < 0.05, ∗∗p < 0.01.
We treated IPEC-J2 cells with TNF-α and IL-1β in different concentrations. TNF-α and IL-1β induced FcRn expression in a dose-dependent manner, increasing mRNA levels 1.5–1.7-fold after 2 h (Figures 3C,D). Interestingly, we found that TGF-β can also up-regulate FcRn, increasing the mRNA level 1.9-fold after 2 h (Figure 3E). Meanwhile, IPEC-J2 cells were treated with SB202190 (p38 inhibitor), SP600125 (JNK1/2 inhibitor) and U0126 (ERK1/2 inhibitor), followed by TGF-β incubation. Treatment with SP600125 sharply reduced FcRn expression, indicating that TGF-β up-regulated FcRn expressions via the c-Jun amino-terminal kinase (JNK)-MAPK pathway in IPEC-J2 cells (Figure 3F).
Because NF-κB is a necessary transcription factor for FcRn production, we examined the effects of all TGEV-encoded proteins on NF-κB promoter activity. TGEV encodes 16 non-structural proteins (nsp1–16), four structural proteins (S, E, M, and N), and three helper proteins (ORF7, ORF3a, and ORF3b), which were constructed recombinant plasmids by using the pCAGGS-HA vector. However, efficient expression occurred only for the plasmids encoding N, E, 3a, nsp1, nsp2, nsp5, nsp7, nsp8, nsp9, nsp10, nsp13, and nsp14 (Figure 4C). IPEC-J2 cells were co-transfected with NF-κB-Luc or FcRn, pRL-TK and different TGEV protein expression vectors. We found that TGEV N, nsp2 and nsp14 can all activate NF-κB (Figure 4A), and TGEV N, 3a, nsp1 and nsp5 can up-regulate FcRn by about 1.5–2-fold and N was the most significant inducer produced by FcRn (Figure 4B).
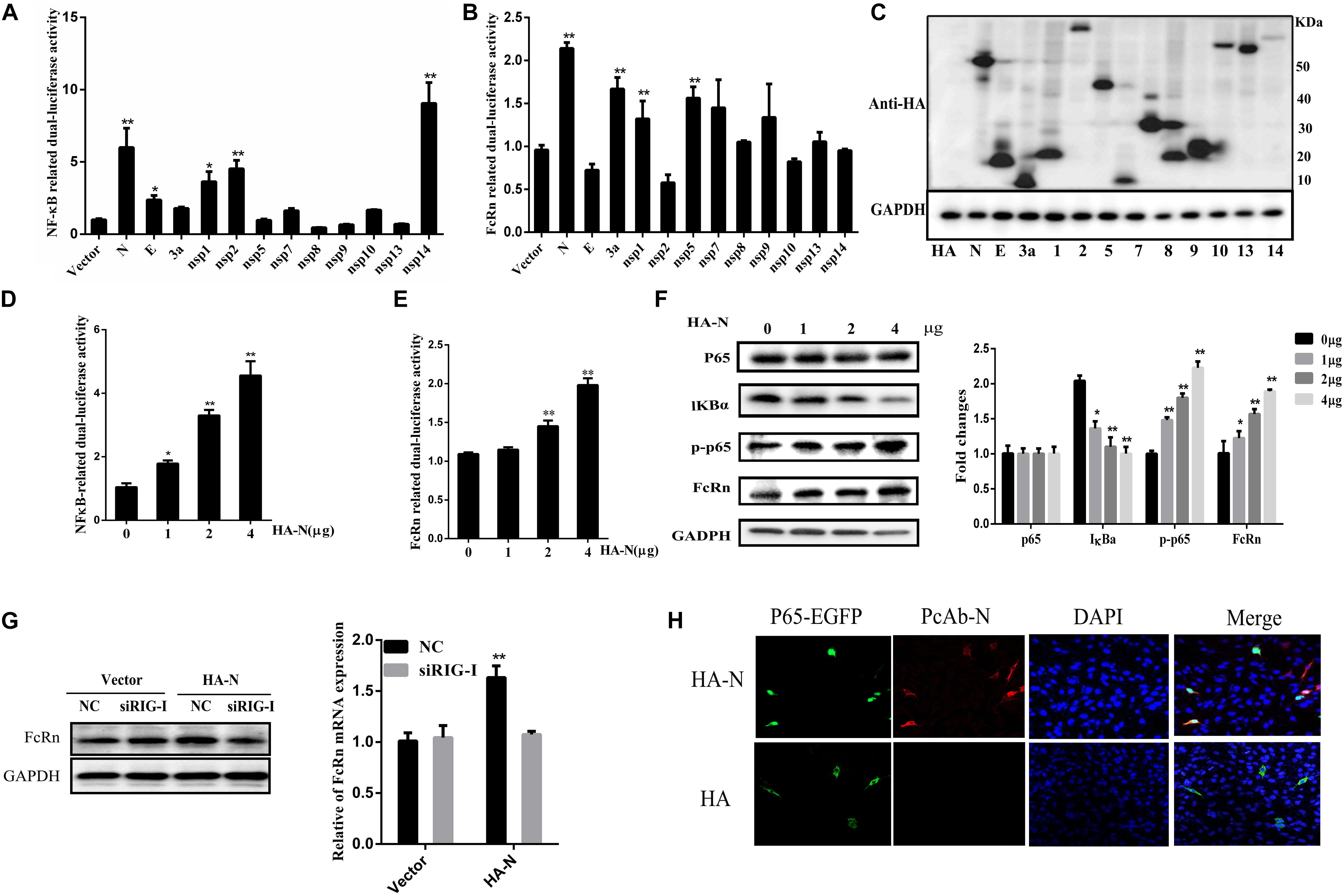
Figure 4. Transmissible gastroenteritis virus N induces FcRn and NF-κB activation. (A,B) IPEC-J2 cells were co-transfected with pRL-TK and FcRn-Luc or NF-κB-Luc together with each expression plasmid of TGEV proteins. (C) Lysates of transfected cells were subjected to Western blot with anti-HA antibody to detect viral protein expression. (D,E) IPEC-J2 were co-transfected with pRL-TK, FcRn-Luc or NF-κB-Luc, and TGEV N expression plasmids (1, 2 or 4 μg) for 36 h. The cell lysates were analyzed by dual-luciferase assay. (F) FcRn, IκBα, p65 and phosphorylated p65 (p-p65), and GAPDH was assayed by Western blot analysis. (G) IPEC-J2 cells were transfected with siRIG or NC siRNA for 24 h and subsequently retransfected with TGEV N-expression plasmids (4 μg) for an additional 24 h. FcRn expression were detected by Western blot analysis. The right panel represents the quantification of the bands by densitometry, corrected by the amount of GAPDH. (H) IPEC-J2 cells were co-transfected with the p65-EGFP fusion protein vector and an empty vector (HA) or the TGEV N expression vector (HA-N) for 24 h. NF-κB p65 (green), TGEV N (red), and the nuclei (blue) were visualized by confocal microscopy. ∗p < 0.05, ∗∗p < 0.01.
To confirm the involvement of N protein in NF-κB or FcRn promoter activation, IPEC-J2 cells were co-transfected with pRL-TK, NF-κB-Luc or FcRn-Luc and N expression vectors were added at different concentrations. Interestingly, N enhanced the activation of NF-κB and FcRn in a dose-dependent manner (Figures 4D,E), following Western blot analysis with antibodies against FcRn, IκBα, phospho-p65, total-p65. The results showed that the expression of N had no significant effects on the total amount of p65; however, the level of phosphorylated p65 (p-p65) and nuclear p65 increased markedly, and the level of IκBα decreased significantly (Figure 4F). As shown in Figure 4G, pre-treatment of cells with siRIG-I impaired FcRn expression by TGEV N protein in comparison with IPEC-J2 cells transfected with NC siRNA. This result showed that RIG-I may be responsible for TGEV N-protein-induced FcRn expression. In this study, we used confocal microscopy to identify that the TGEV N protein has an effect on the nuclear translocation of the NF-κB subunit p65. We observed the control vector-transfected IPEC-J2 cells and p65-EGFP was mainly present in the cytoplasm. Interestingly, the overexpression of TGEV N could lead to p65-EGFP expression in the nucleus in transfected IPEC-J2 cells (Figure 4G). Taken together, these results demonstrated that TGEV N protein can both significantly activate NF-κB and up-regulate the expression of FcRn.
In order to further explore N functional domains, N protein truncations were expressed in this study. According to the Lasergene DNASTAR Protean 9.0 software prediction, the N-protein contains two nuclear export signal (NES) sequences at aa 221–236 (NES-1), aa 330–353 (NES-2), an RNA-binding region at aa 33–159, a specific SR-rich region at aa 156–198 and a ZnF region at aa 137–219 (Figure 5A). TGEV N protein is mainly located at aa 128–252 and this region has high hydrophilicity and flexibility. In order to study the TGEV N protein, the structure of which is essential for FcRn activation, four truncation mutants were cloned into the eukaryotic expression plasmid, pCAGGS-HA, named as N1(1–128), N2(128–382), N3(252–382), and N4(128–252). The relationship between these mutants and FcRn activation was analyzed through luciferase reporter assays and Western blot. As shown in Figure 5, N4, which contains NES1 and the immune dominant area, compared with the total length of the TGEV N protein, increased the FcRn activation and expression (Figures 5B,C). The result showed that the central region (128–252) of N protein is critical for FcRn activation.

Figure 5. Schematic representation of the TGEV N-protein serial-deletion mutant constructs. (A) Schematic representation of the TGEV N-protein serial-deletion mutant constructs. (B) IPEC-J2 cells were co-transfected with pRL-TK, FcRn-Luc, and TGEV N deletion-mutant expression plasmids. The cell lysates were harvested for dual luciferase assays at 24 hpi. (C) IPEC-J2 cells were co-transfected with TGEV N deletion-mutant expression plasmids for 36 h and protein expression in cell lysates was measured by Western blot using anti-HA antibody and anti-FcRn antibody. The right panel represents the quantification of the bands by densitometry, corrected by the amount of GAPDH. ∗p < 0.05, ∗∗p < 0.01.
Transmissible gastroenteritis virus N protein was expressed by Bac-to-Bac baculovirus expression systems, the secreted 49 KDa was verified as we expected by Western blot (Figure 6A). IPEC-J2 cells were stimulated with different doses of recombinant TGEV N protein. We further verified the FcRn up-regulation up to two times by recombinant TGEV N stimulation, as assessed by RT-qPCR and Western blot (Figures 6B,C).
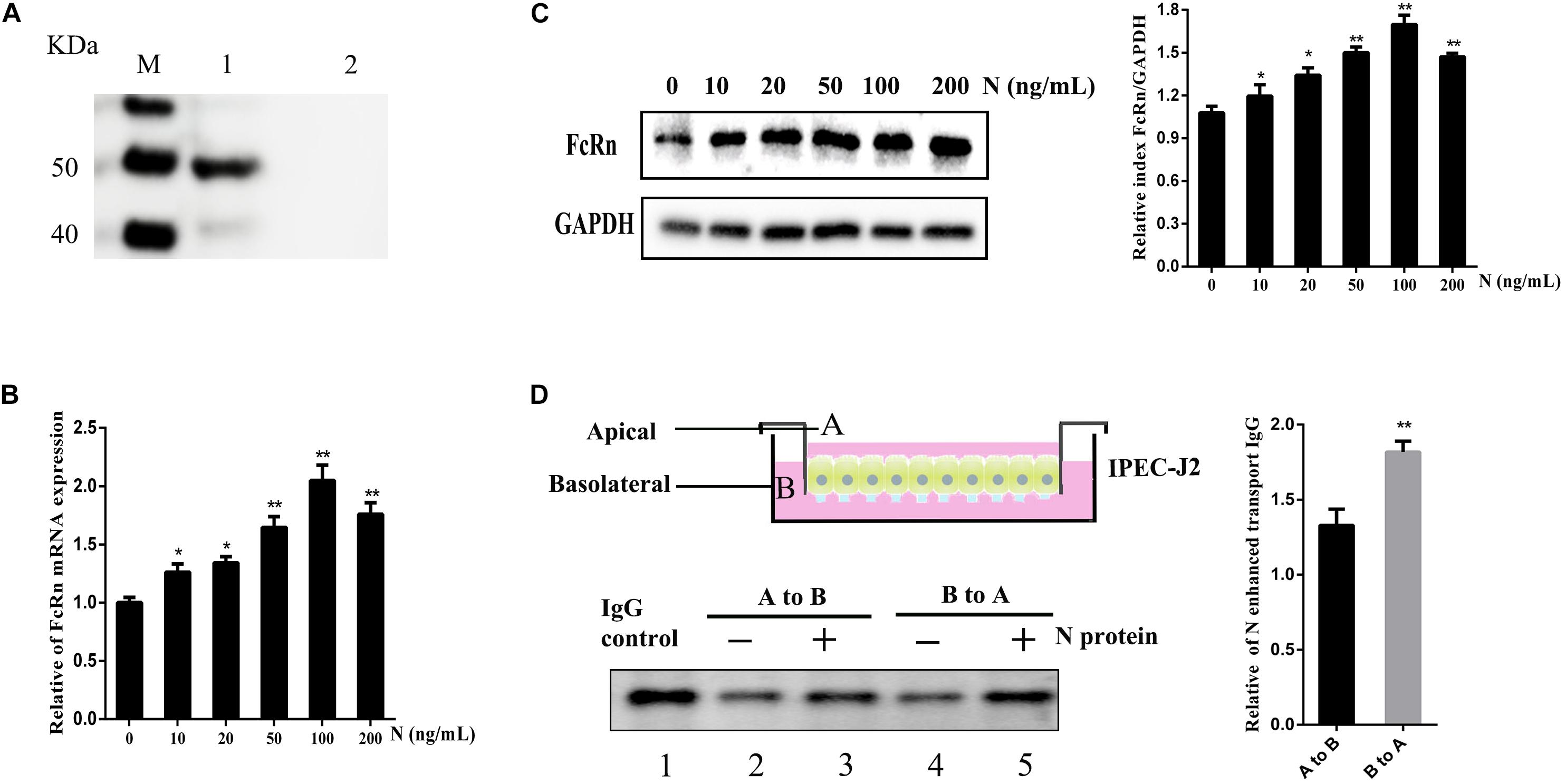
Figure 6. Effects of recombinant TGEV N stimulation on the IgG transcytosis in IPEC-J2 cells. (A) Analysis of recombinant TGEV N protein by Western blot. M: Protein marker. 1: TGEV recombinant N. 2: Negative Control. IPEC-J2 cells were treated with recombinant TGEV N protein (0, 10, 20, 50, 100 or 200 ng/mL) for 12 h. (B,C) FcRn expression levers were detected by RT-qPCR and Western blot. The right panel represents the quantification of the bands by densitometry, corrected by the amount of GAPDH. (D) Transwell system used throughout this study. A, apical; B, basolateral. IPEC-J2 cells were grown in a 12-well transwell plate about 6–7 day until TEER > 1000 Ω/cm2. IPEC-J2 cells were stimulated with or without recombinant TGEV N (100 ng/ml) for 12 h. Cells were loaded with porcine biotin-IgG at 37°C in either the apical (lanes 2 and 3) or basolateral (lanes 4 and 5) chamber. Lane 1 represents an IgG. ∗p < 0.05, ∗∗p < 0.01.
The FcRn protein has been well known to transport IgG bidirectionally in polarized epithelial cells. Therefore, we hypothesized TGEV N protein could alter the role of IgG transcytosis epithelial cells and constructed the transwell system to mimic the porcine intestinal microenvironment using IPEC-J2 cells (Figure 6D). The polarized monolayers (TEER > 1000 Ω/cm2) were stimulated with recombinant TGEV N protein (100 ng/mL) for 12 h. Then, porcine biotin-IgG was added to the apical or basolateral surface of a IPEC-J2 cell monolayer, after 3 h at 37°C we assessed the IgG transported to the opposite basolateral or apical chamber, respectively. The IgG H chain was detected in medium by Western blot (Figure 6D, lane 1). Importantly, IgG transport was enhanced 1.3-fold in the apical to basolateral (Figure 6D, lane 3) direction or 1.8-fold in the basolateral to apical (Figure 6D, lane 5) direction by TGEV N in comparison to the mock-treated monolayer (Figure 6D, lanes 2 and 4).
The secretory IgA and IgG play major roles in mucosal anti-infection immunity and are obtained primarily through the polymeric immunoglobulin receptor (plgR) and FcRn-mediated transport. Reovirus, dsRNA (a ligand for TLR3), LPS (a ligand for TLR4), IFN-γ, IL-4, IL-1β, TNF-α, and TGF-β all regulate pIgR expression (Johansen and Brandtzaeg, 2004; Pal et al., 2005; Schneeman et al., 2005). Therefore, LPS, IL-1β, and TNF-α could up-regulate the expression of FcRn via the NF-κB pathway. NF-κB appears to be a positive regulator of FcRn expression. In our previous study, we identified that TGEV infection up-regulated FcRn via the activation of the NF-κB pathway (Guo et al., 2016b). Consistent with this, the replication of the virus is directly related to FcRn expression.
Transmissible gastroenteritis virus infection up-regulates the expression levels of IL-1β, IL-6, IL-8, TNF-α, and TGF-β, which are important factors in chronic inflammation. The expression of FcRn was up-regulated by the inflammatory cytokine TNF-α and IL-1β in a dose-dependent manner, as determined by RT-qPCR (Figures 3A,B), and this was consistent with that observed in a previous study (Liu et al., 2007). MAPK is a highly conserved serine protein kinase in the cytoplasm that mediates a signaling pathway that plays an important role in the body’s inflammatory response. The MAPK family is mainly divided into three classes: extracellular signal-regulated kinases (ERKs), JNKs, and p38 MAPK kinases. Multiple stimulating factors activate the MAPK pathway, including inflammatory factors, growth factors, and cellular responses (Huang et al., 2004). TGF-β dose-dependently induces pIgR production via the p38/MAPK pathway in human bronchial epithelial cells (Ratajczak et al., 2010). Moreover, TGF-β was said to inhibit pIgR production in bronchial epithelium (Gohy et al., 2014). In this study, we observed that TGF-β up-regulates FcRn via the JNK MAPK pathway, perhaps because TGEV activates the JNK MAPK pathway in porcine cells.
The ability of TGEV proteins to stimulate the FcRn promoter was identified by constructing a series of recombinant expression plasmids. However, efficient expression was obtained only from plasmids encoding the N, E, 3a, nsp1, nsp2, nsp5, nsp7, nsp8, nsp9, nsp10, nsp13, and nsp14. The expression of viral protein was not high or related to cell type and state. Among these proteins, N was the most potent NF-κB activator, and it induced FcRn expression. The proteins 3a, nsp1, and nsp5 also enhance but have little influence on NF-κB promoter activity. While nsp2 and nsp14 are also potential NF-κB activators, consistent with that reported in previous studies (Zhou et al., 2017; Wang et al., 2018). Since the essential and multifunctional N protein is fundamental for immune regulation and pathogenesis, we examined it more closely. Porcine epidemic diarrhea virus (PEDV) N protein mediates NF-κB activation through TLR pathways and has functions in cell growth, cell cycle and ER stress (Xu et al., 2013). In fact, the interaction of TGEV N protein with several host cell proteins has been shown (Zhang et al., 2014, 2015). In addition, overexpression of TGEV N protein induces cell cycle arrest (Ding et al., 2014a). Using domain-specific mutations, we determined that the domain at aa 128–252 of the N protein (immunodominant central region) is critical for FcRn activation; it contains a ZnF structure and a SR-rich region. Both the SR-rich truncated proteins of the N protein of SARS coronavirus and PEDV can effectively promote the activation of the NF-κB signaling pathway (Zhang et al., 2007; Cao et al., 2015a).
Epithelial TLR expression plays an important role in the innate and adaptive immune responses. NF-κB is a branch of the downstream signaling pathways of TLR and RLR, which also play important roles in various immune responses. Diverse coronaviruses can escape host immune responses via NF-κB activation through different pathways. PEDV activates NF-κB signaling through TLRs, while mouse hepatitis virus (MHV) does so through RLRs (Li et al., 2010; Cao et al., 2015a). IPEC-J2 cells express mRNA encoding TLR1, TLR2, TLR3, TLR4, TLR6, TLR8, TLR9, TLR10, IL-1α, IL-6, IL-7, IL-8, IL-18, TNF-α, and GM-CSF (Bowie and Haga, 2005; Burkey et al., 2009; Mariani et al., 2009). Rotavirus infection triggers the production of IL-8, IL-6, and TNF-α in IECs by activating the TLR3 and RIG-I pathways (Broquet et al., 2011). Compared with the NC siRNA, TLR3-, TLR9-, RIG-, MyD88-, and TRIF-specific siRNAs inhibited TGEV-induced FcRn activation, while TLR2-, TLR4-, and TLR8-specific siRNAs did not. We speculate that TGEV activates NF-κB via the TLR3, TLR9, and RIG-I pathways. Our results suggest that TLR3, TLR9, and RIG-I may be used to activate the FcRn signaling pathway when TGEV infects IPEC-J2 cells. Recently, our research has shown that PEDV down-regulate the NF-κB signaling pathway to inhibit FcRn by TLR3 and RIG-I pathway in the early stage of infected piglets (Qian et al., 2019). PEDV and PDCoV N protein inhibited IFN-β by RIG-I pathway (Ding et al., 2014b; Likai et al., 2019), and we found that TGEV N protein up-regulated FcRn by RIG-I pathway. Moreover, TGEV infection induced IFN-β production and PEDV inhibited IFN-β production (An et al., 2014; Cao et al., 2015b). These results may be important causes of PEDV being more pathogenic than TGEV.
Transgenic mice that overexpress hFcRn can improve the antigen presentation ability of dendritic cells and enhance the humoral immune response of the body (Végh et al., 2012). FcRn not only transports IgG, but also transports antigen-antibody complexes, which are taken up by antigen-presenting cells (APCs) (Rath et al., 2013). When the pathogen stimulates the mucosal epithelium, the expression of FcRn may be regulated. Simian/human immunodeficiency virus (SHIV) infection decreases FcRn and pIgR expression, which may also be the mechanism responsible for its pathogenesis in rhesus monkeys (Wang and Yang, 2016). On the other hand, TGEV up-regulates FcRn expression, which is potentially what provides the mucosal protection. To observe and measure IgG transcytosis in vitro, we applied the transwell system to mimic the porcine intestinal microenvironment, using IPEC-J2 cells to construct the transwell model, our previous research showed that FcRn not only transports specific antibodies across the mucosal epithelium in vitro, but also reduces the yield of TGEV (Guo et al., 2016a). We speculated that the up-regulation of FcRn by N protein may mediates more TGEV-specific antibody transcytosis across the mucosal epithelium, thereby enhancing the host’s anti-infective ability. On the other hand, N protein-enhanced FcRn mediates more IgG immune complex transcytosis, followed by antigen uptake by specialized APCs to activate adaptive immune responses. Overall, we elucidated the immune mechanism of TGEV induced FcRn expression and provided a scientific and theoretical basis for the prevention and control of TGEV infection.
All datasets generated for this study are included in the article/supplementary material.
SQ wrote the manuscript. SQ and ZL conceived and initiated the study. SQ, ZG, RC, and KY extracted the dataset. SQ, YC, SL, XM, QH, and ZL performed the analysis. All authors reviewed the manuscript.
This work was supported by the National Natural Science Foundation of China (31572500 to ZL).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
An, K., Fang, L., Luo, R., Wang, D., Xie, L., Yang, J., et al. (2014). Quantitative proteomic analysis reveals that transmissible gastroenteritis virus activates the JAK-STAT1 signaling pathway. J. Proteome Res. 13, 5376–5390. doi: 10.1021/pr500173p
Arce, C., Ramirez-Boo, M., Lucena, C., and Garrido, J. J. (2010). Innate immune activation of swine intestinal epithelial cell lines (IPEC-J2 and IPI-2I) in response to LPS from Salmonella typhimurium. Comp. Immunol. Microbiol. Infect. Dis. 33, 161–174. doi: 10.1016/j.cimid.2008.08.003
Bowie, A. G., and Haga, I. R. (2005). The role of Toll-like receptors in the host response to viruses. Mol. Immunol. 42, 859–867. doi: 10.1016/j.molimm.2004.11.007
Broquet, A. H., Hirata, Y., McAllister, C. S., and Kagnoff, M. F. (2011). RIG-I/MDA5/MAVS are required to signal a protective IFN response in rotavirus-infected intestinal epithelium. J. Immunol. 186, 1618–1626. doi: 10.4049/jimmunol.1002862
Burkey, T. E., Skjolaas, K. A., Dritz, S. S., and Minton, J. E. (2009). Expression of porcine Toll-like receptor 2, 4 and 9 gene transcripts in the presence of lipopolysaccharide and Salmonella enterica serovars Typhimurium and Choleraesuis. Vet. Immunol. Immunopathol. 130, 96–101. doi: 10.1016/j.vetimm.2008.12.027
Cao, L., Ge, X., Gao, Y., Ren, Y., Ren, X., and Li, G. (2015a). Porcine epidemic diarrhea virus infection induces NF-kappaB activation through the TLR2, TLR3 and TLR9 pathways in porcine intestinal epithelial cells. J. Gen. Virol. 96, 1757–1767. doi: 10.1099/vir.0.000133
Cao, L., Ge, X., Gao, Y., Herrler, G., Ren, Y., Ren, X., et al. (2015b). Porcine epidemic diarrhea virus inhibits dsRNA-induced interferon-beta production in porcine intestinal epithelial cells by blockade of the RIG-I-mediated pathway. Virol. J. 12:127. doi: 10.1186/s12985-015-0345-x
Chen, X., Zhang, Q., Bai, J., Zhao, Y., Wang, X., Wang, H., et al. (2017). The nucleocapsid protein and nonstructural protein 10 of highly pathogenic porcine reproductive and respiratory syndrome virus enhance CD83 production via NF-kappaB and Sp1 signaling pathways. J. Virol. 91:JVI.00986-17. doi: 10.1128/JVI.00986-17
Cruz, J. L., Becares, M., Sola, I., Oliveros, J. C., Enjuanes, L., and Zuniga, S. (2013). Alphacoronavirus protein 7 modulates host innate immune response. J. Virol. 87, 9754–9767. doi: 10.1128/JVI.01032-13
Cruz, J. L., Sola, I., Becares, M., Alberca, B., Plana, J., Enjuanes, L., et al. (2011). Coronavirus gene 7 counteracts host defenses and modulates virus virulence. PLoS Pathog. 7:e1002090. doi: 10.1371/journal.ppat.1002090
Ding, L., Huang, Y., Du, Q., Dong, F., Zhao, X., Zhang, W., et al. (2014a). TGEV nucleocapsid protein induces cell cycle arrest and apoptosis through activation of p53 signaling. Biochem. Biophys. Res. Commun. 445, 497–503. doi: 10.1016/j.bbrc.2014.02.039
Ding, Z., Fang, L., Jing, H., Zeng, S., Wang, D., Liu, L., et al. (2014b). Porcine epidemic diarrhea virus nucleocapsid protein antagonizes beta interferon production by sequestering the interaction between IRF3 and TBK1. J. Virol. 88, 8936–8945. doi: 10.1128/JVI.00700-14
Escors, D., Izeta, A., Capiscol, C., and Enjuanes, L. (2003). Transmissible gastroenteritis coronavirus packaging signal is located at the 5′ end of the virus genome. J. Virol. 77, 7890–7902. doi: 10.1128/jvi.77.14.7890-7902.2003
Gohy, S. T., Detry, B. R., Lecocq, M., Bouzin, C., Weynand, B. A., Amatngalim, G. D., et al. (2014). Polymeric immunoglobulin receptor down-regulation in chronic obstructive pulmonary disease. Persistence in the cultured epithelium and role of transforming growth factor-beta. Am. J. Respir. Crit. Care Med. 190, 509–521. doi: 10.1164/rccm.201311-1971OC
Guo, J., Li, F., He, Q., Jin, H., Liu, M., Li, S., et al. (2016a). Neonatal Fc receptor-mediated IgG transport across porcine intestinal epithelial cells: potentially provide the mucosal protection. DNA Cell Biol. 35, 301–309. doi: 10.1089/dna.2015.3165
Guo, J., Li, F., Qian, S., Bi, D., He, Q., Jin, H., et al. (2016b). TGEV infection up-regulates FcRn expression via activation of NF-kappaB signaling. Sci. Rep. 6:32154. doi: 10.1038/srep32154
Gupta, S., Gach, J. S., Becerra, J. C., Phan, T. B., Pudney, J., Moldoveanu, Z., et al. (2013). The Neonatal Fc receptor (FcRn) enhances human immunodeficiency virus type 1 (HIV-1) transcytosis across epithelial cells. PLoS Pathog. 9:e1003776. doi: 10.1371/journal.ppat.1003776
Huang, C., Jacobson, K., and Schaller, M. D. (2004). MAP kinases and cell migration. J. Cell Sci. 117, 4619–4628. doi: 10.1242/jcs.01481
Johansen, F. E., and Brandtzaeg, P. (2004). Transcriptional regulation of the mucosal IgA system. Trends Immunol. 25, 150–157. doi: 10.1016/j.it.2004.01.001
Li, J., Liu, Y., and Zhang, X. (2010). Murine coronavirus induces type I interferon in oligodendrocytes through recognition by RIG-I and MDA5. J. Virol. 84, 6472–6482. doi: 10.1128/JVI.00016-10
Li, Z., Palaniyandi, S., Zeng, R., Tuo, W., Roopenian, D. C., and Zhu, X. (2011). Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc. Natl. Acad. Sci. U.S.A. 108, 4388–4393. doi: 10.1073/pnas.1012861108
Likai, J., Shasha, L., Wenxian, Z., Jingjiao, M., Jianhe, S., Hengan, W., et al. (2019). Porcine deltacoronavirus nucleocapsid protein suppressed IFN-beta production by interfering porcine RIG-I dsRNA-binding and K63-linked polyubiquitination. Front. Immunol. 10:1024. doi: 10.3389/fimmu.2019.01024
Liu, X., Ye, L., Christianson, G. J., Yang, J. Q., Roopenian, D. C., and Zhu, X. (2007). NF-kappaB signaling regulates functional expression of the MHC class I-related neonatal Fc receptor for IgG via intronic binding sequences. J. Immunol. 179, 2999–3011. doi: 10.4049/jimmunol.179.5.2999
Lu, L., Palaniyandi, S., Zeng, R., Bai, Y., Liu, X., Wang, Y., et al. (2011). A neonatal Fc receptor-targeted mucosal vaccine strategy effectively induces HIV-1 antigen-specific immunity to genital infection. J. Virol. 85, 10542–10553. doi: 10.1128/JVI.05441-11
Mariani, V., Palermo, S., Fiorentini, S., Lanubile, A., and Giuffra, E. (2009). Gene expression study of two widely used pig intestinal epithelial cell lines: IPEC-J2 and IPI-2I. Vet. Immunol. Immunopathol. 131, 278–284. doi: 10.1016/j.vetimm.2009.04.006
McDonnell, K., Nienaber, V., Andersen, J. T., Mizoguchi, A., Blumberg, L., Purohit, S., et al. (2016). Viral evasion strategies in type I IFN signaling - a summary of recent developments. Proc. Natl. Acad. Sci. U.S.A. 7:498.
Pal, K., Kaetzel, C. S., Brundage, K., Cunningham, C. A., and Cuff, C. F. (2005). Regulation of polymeric immunoglobulin receptor expression by reovirus. J. Gen. Virol. 86, 2347–2357. doi: 10.1099/vir.0.80690-0
Pyzik, M., Rath, T., Kuo, T. T., Win, S., Baker, K., Hubbard, J. J., et al. (2017). Hepatic FcRn regulates albumin homeostasis and susceptibility to liver injury. Proc. Natl. Acad. Sci. U.S.A. 114, E2862–E2871. doi: 10.1073/pnas.1618291114
Qian, S., Zhang, W., Jia, X., Sun, Z., Zhang, Y., Xiao, Y., et al. (2019). Isolation and identification of porcine epidemic diarrhea virus and its effect on host natural immune response. Front. Microbiol. 10:2272. doi: 10.3389/fmicb.2019.02272
Ratajczak, C., Guisset, A., Detry, B., Sibille, Y., and Pilette, C. (2010). Dual effect of neutrophils on pIgR/secretory component in human bronchial epithelial cells: role of TGF-beta. J. Biomed. Biotechnol. 2010:428618. doi: 10.1155/2010/428618
Rath, T., Kuo, T. T., Baker, K., Qiao, S. W., Kobayashi, K., Yoshida, M., et al. (2013). The immunologic functions of the neonatal Fc receptor for IgG. J. Clin. Immunol. 33(Suppl. 1), S9–S17. doi: 10.1007/s10875-012-9768-y
Schneeman, T. A., Bruno, M. E., Schjerven, H., Johansen, F. E., Chady, L., and Kaetzel, C. S. (2005). Regulation of the polymeric Ig receptor by signaling through TLRs 3 and 4: linking innate and adaptive immune responses. J. Immunol. 175, 376–384. doi: 10.4049/jimmunol.175.1.376
Temeeyasen, G., Sinha, A., Gimenez-Lirola, L. G., Zhang, J. Q., and Piñeyro, P. E. (2018). Differential gene modulation of pattern-recognition receptor TLR and RIG-I-like and downstream mediators on intestinal mucosa of pigs infected with PEDV non S-INDEL and PEDV S-INDEL strains. Virology 517, 188–198. doi: 10.1016/j.virol.2017.11.024
Thompson, A. J., and Locarnini, S. A. (2007). Toll-like receptors, RIG-I-like RNA helicases and the antiviral innate immune response. Immunol. Cell Biol. 85, 435–445. doi: 10.1038/sj.icb.7100100
Végh, A., Farkas, A., Kövesdi, D., Papp, K., Cervenak, J., Schneider, Z., et al. (2012). FcRn overexpression in transgenic mice results in augmented APC activity and robust immune response with increased diversity of induced antibodies. PLoS One 7:e36286. doi: 10.1371/journal.pone.0036286
Wang, L., Qiao, X., Zhang, S., Qin, Y., Guo, T., Hao, Z., et al. (2018). Porcine transmissible gastroenteritis virus nonstructural protein 2 contributes to inflammation via NF-kappaB activation. Virulence 9, 1685–1698. doi: 10.1080/21505594.2018.1536632
Wang, Y., and Yang, G. B. (2016). Alteration of polymeric immunoglobulin receptor and neonatal Fc receptor expression in the Gut Mucosa of immunodeficiency virus-infected rhesus macaques. Scand. J. Immunol. 83, 235–243. doi: 10.1111/sji.12416
Xia, L., Dai, L., Yu, Q., and Yang, Q. (2017). Persistent transmissible gastroenteritis virus infection enhances enterotoxigenic Escherichia coli K88 adhesion by promoting epithelial-mesenchymal transition in intestinal epithelial cells. J. Virol. 91:JVI.01256-17. doi: 10.1128/JVI.01256-17
Xu, X., Zhang, H., Zhang, Q., Huang, Y., Dong, J., Liang, Y., et al. (2013). Porcine epidemic diarrhea virus N protein prolongs S-phase cell cycle, induces endoplasmic reticulum stress, and up-regulates interleukin-8 expression. Vet. Microbiol. 164, 212–221. doi: 10.1016/j.vetmic.2013.01.034
Ye, L., Tuo, W., Liu, X., Simister, N. E., and Zhu, X. (2008). Identification and characterization of an alternatively spliced variant of the MHC class I-related porcine neonatal Fc receptor for IgG. Dev. Comp. Immunol. 32, 966–979. doi: 10.1016/j.dci.2008.01.008
Ye, L., Zeng, R., Bai, Y., Roopenian, D. C., and Zhu, X. (2011). Efficient mucosal vaccination mediated by the neonatal Fc receptor. Nat. Biotechnol. 29, 158–163. doi: 10.1038/nbt.1742
Zhang, Q., Xu, Y., Chang, R., Tong, D., and Xu, X. (2018). Transmissible gastroenteritis virus N protein causes endoplasmic reticulum stress, up-regulates interleukin-8 expression and its subcellular localization in the porcine intestinal epithelial cell. Res. Vet. Sci. 119, 109–115. doi: 10.1016/j.rvsc.2018.06.008
Zhang, X., Shi, H., Chen, J., Shi, D., Dong, H., and Feng, L. (2015). Identification of the interaction between vimentin and nucleocapsid protein of transmissible gastroenteritis virus. Virus Res. 200, 56–63. doi: 10.1016/j.virusres.2014.12.013
Zhang, X., Shi, H., Chen, J., Shi, D., Li, C., and Feng, L. (2014). EF1A interacting with nucleocapsid protein of transmissible gastroenteritis coronavirus and plays a role in virus replication. Vet. Microbiol. 172, 443–448. doi: 10.1016/j.vetmic.2014.05.034
Zhang, X., Wu, K., Wang, D., Yue, X., Song, D., Zhu, Y., et al. (2007). Nucleocapsid protein of SARS-CoV activates interleukin-6 expression through cellular transcription factor NF-kappaB. Virology 365, 324–335. doi: 10.1016/j.virol.2007.04.009
Zhao, S., Gao, J., Zhu, L., and Yang, Q. (2014). Transmissible gastroenteritis virus and porcine epidemic diarrhoea virus infection induces dramatic changes in the tight junctions and microfilaments of polarized IPEC-J2 cells. Virus Res. 192, 34–45. doi: 10.1016/j.virusres.2014.08.014
Keywords: transmissible gastroenteritis virus, nucleocapsid protein, TGF-β, neonatal Fc receptor, toll-like receptor, NF-κB
Citation: Qian S, Gao Z, Cao R, Yang K, Cui Y, Li S, Meng X, He Q and Li Z (2020) Transmissible Gastroenteritis Virus Infection Up-Regulates FcRn Expression via Nucleocapsid Protein and Secretion of TGF-β in Porcine Intestinal Epithelial Cells. Front. Microbiol. 10:3085. doi: 10.3389/fmicb.2019.03085
Received: 14 October 2019; Accepted: 20 December 2019;
Published: 21 January 2020.
Edited by:
Ian M. Jones, University of Reading, United KingdomReviewed by:
Pinghuang Liu, Harbin Veterinary Research Institute (CAAS), ChinaCopyright © 2020 Qian, Gao, Cao, Yang, Cui, Li, Meng, He and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zili Li, bGl6aWxpQG1haWwuaHphdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.