- 1Institute of Plant and Microbial Biology, Academia Sinica, Taipei, Taiwan
- 2Institute of Biotechnology, National Taiwan University, Taipei, Taiwan
- 3Institute of Biological Chemistry, Academia Sinica, Taipei, Taiwan
- 4Agricultural Biotechnology Research Center, Academia Sinica, Taipei, Taiwan
The type VI secretion system (T6SS) is an effector delivery system used by Gram-negative bacteria to kill other bacteria or eukaryotic hosts to gain fitness. The plant pathogen Agrobacterium tumefaciens utilizes its T6SS to kill other bacteria, such as Escherichia coli. We observed that the A. tumefaciens T6SS-dependent killing outcome differs when using different T6SS-lacking, K-12 E. coli strains as a recipient cell. Thus, we hypothesized that the A. tumefaciens T6SS killing outcome not only relies on the T6SS activity of the attacker cells but also depends on the recipient cells. Here, we developed a high-throughput interbacterial competition platform to test the hypothesis by screening for mutants with reduced killing outcomes caused by A. tumefaciens strain C58. Among the 3,909 strains in the E. coli Keio library screened, 16 mutants with less susceptibility to A. tumefaciens C58 T6SS-dependent killing were identified, and four of them were validated by complementation test. Among the four, the clpP encoding ClpP protease, which is universal and highly conserved in both prokaryotes and eukaryotic organelles, was selected for further characterizations. We demonstrated that ClpP is responsible for enhancing susceptibility to the T6SS killing. Because ClpP protease depends on other adapter proteins such as ClpA and ClpX for substrate recognition, further mutant studies followed by complementation tests were carried out to reveal that ClpP-associated AAA+ ATPase ClpA, but not ClpX, is involved in enhancing susceptibility to A. tumefaciens T6SS killing. Moreover, functional and biochemical studies of various ClpP amino acid substitution variants provided evidence that ClpA–ClpP interaction is critical in enhancing susceptibility to the T6SS killing. This study highlights the importance of recipient factors in determining the outcome of the T6SS killing and shows the universal ClpP protease as a novel recipient factor hijacked by the T6SS of A. tumefaciens.
Introduction
Bacteria have evolved broad strategies in secreting antibiotics or protein toxins to antagonize other bacteria and gain fitness to fight for limited nutrients and space. Among them, the Gram-negative bacteria use a variety of protein secretion systems such as type I secretion system (T1SS) (García-Bayona et al., 2017, 2019), type IV secretion system (T4SS) (Souza et al., 2015; Bayer-Santos et al., 2019), contact-dependent inhibition (CDI; belongs to type V secretion system) (Aoki et al., 2005, 2010), and type VI secretion system (T6SS) (LeRoux et al., 2012; Basler et al., 2013) as antibacterial weapons. Bacteria that produce and deliver protein toxins, the effectors, through secretion systems to kill other bacteria are attacker cells, and the attacked cells are the recipient cells (Costa et al., 2015; Filloux and Sagfors, 2015). Attacker cells also produce cognate immunity proteins to neutralize effectors to prevent self-intoxication (Alteri and Mobley, 2016; Lien and Lai, 2017). A recipient cell is intoxicated if it does not have cognate immunity protein to neutralize the toxicity of its effector.
In the CDI system, non-immunity proteins in the recipient cell also participate in the bacterial competition outcome (Aoki et al., 2008, 2010; Diner et al., 2012; Willett et al., 2015; Jones et al., 2017). For example, the CDI effector CdiA-CTEC93 utilizes recipient’s outer membrane protein BamA and the inner membrane protein AcrB to enter the recipient cell (Aoki et al., 2008). BamA belongs to the BAM complex that functions in outer membrane β-barrel proteins (OMPs) biogenesis. AcrB is an inner membrane protein that belongs to the multidrug efflux pump TolC complex. Another example is the necessity of the recipient O-acetylserine sulfhydrylase A (CysK) to the CDI effector CdiA-CTEC536 (Diner et al., 2012). In the recipient cell, CdiA-CTEC536 binds to CysK to increase its thermostability and its tRNase activity (Johnson et al., 2016). Interestingly, this CysK.CdiA-CTEC536 complex mimics the CysK.CysE complex, which is typically formed during de novo cysteine biogenesis, with a higher binding affinity (Johnson et al., 2016; Jones et al., 2017). Other examples are the recipient elongation factor Tu (EF-Tu) in activating the toxicity of CdiA-CTEC869 and CdiA-CTNC101 (Jones et al., 2017; Michalska et al., 2017), and the involvement of recipient PtsG in CdiA-CT3006 and CdiA-CTNC101 entry (Willett et al., 2015). To summarize, a variety of the non-immunity proteins in the recipient cells affect the CDI antagonizing outcome. As the bacterial secretion systems that serve as an antibacterial weapon share some universal characters, the above phenomenon raised a question of whether non-immunity proteins of the recipient cells also affect the bacterial antagonizing outcome in other secretion systems.
Recently, examples about the involvement of the recipient non-immunity proteins in T6SS competition outcome emerged. The first description is the involvement of the EF-Tu protein of the recipient cell for Tse6 effector-mediated killing by Pseudomonas aeruginosa (Whitney et al., 2015). Although recipient’s EF-Tu was initially proposed to grant access of Tse6 into the recipient cytoplasm (Whitney et al., 2015), a further study demonstrated that Tse6 could penetrate the double bilayer of the EF-Tu-free liposome and exert its toxicity inside it (Quentin et al., 2018). The role of the recipient EF-Tu involved in an interbacterial competition of Tse6 remains elusive. A T6SS study in Serratia marcescens demonstrated that the recipient protein DsbA plays a role in activating S. marcescens T6SS effectors Ssp2 and Ssp4, but not Rhs2 (Mariano et al., 2018). The S. marcescens T6SS kills its Ssp2-sensitive siblings only when the recipient cells harbor dsbA homologs (dsbA1+ dsbA2+). The same results were also observed using Ssp4-sensitive recipient cells, but not Rhs2-sensitive strain as a recipient cell. The above findings highlight the necessity of a recipient factor to facilitate the T6SS attack. However, a systematic screening of the recipient factors that can either promote or reduce the susceptibility of the T6SS attack is still lacking.
This study aimed to explore the recipient genetic factors that affect the T6SS killing outcome using the well-characterized T6SS-possessing plant pathogen Agrobacterium tumefaciens, a causative agent of crown gall disease in many different plants. The A. tumefaciens strain C58 harbors three effector proteins: type VI DNase effector 1 (Tde1), Tde2, and putative type VI amidase effector (Tae). The Tde proteins are the main contributor to A. tumefaciens T6SS-dependent interbacterial competition (Ma et al., 2014). Using the T6SS-lacking Escherichia coli K12 strain as a model recipient cell, we report here a high-throughput, population level, interbacterial competition screening platform for identifying the recipient genetic factors that contribute to A. tumefaciens C58 T6SS’s killing outcome. Among the 3,909 E. coli Keio mutants screened, we confirmed that at least six of them play a role in enhancing susceptibility to A. tumefaciens T6SS attack by an interbacterial competition assay and by complementation in trans. One of the confirmed genes, caseinolytic protease P (clpP), was highlighted in this study owing to its prominent phenotype. A functional ClpP complex consists of a tetradodecameric ClpP and its associated AAA+ ATPase substrate-recognizing partner ClpA or ClpX (Olivares et al., 2015). Further mutant studies showed that clpA, but not clpX, is involved in the outcome of A. tumefaciens T6SS killing. Our data also suggest that the ClpAP complex formation mediates the outcome of T6SS killing. This work not only provides a new screening platform for elucidating factors that are involved in the interbacterial competition but also strengthens the importance of recipient genetic factors in the outcome of the T6SS antibacterial activity.
Materials and Methods
Bacterial Strains, Plasmids, and Growth Conditions
The complete information about the strains and plasmids used in this study is described in Table 1. The E. coli Keio mutants (Baba et al., 2006) and the BW25113 wild type were obtained from the Keio collection from NBRP (NIG, Japan) and used as the recipient cells unless otherwise indicated. A. tumefaciens C58 wild type and the tssL mutants (ΔtssL) were used as the attacker cells. A. tumefaciens was grown at 25°C in 523 medium, and E. coli was grown in lysogeny broth (LB) medium at 37°C unless indicated. The plasmids were maintained in 20 μg/ml of kanamycin (Km), 100 μg/ml of spectinomycin (Sp), and 20 μg/ml of gentamycin for E. coli.
Plasmid Construction
All plasmids (Table 1) were confirmed by sequencing unless otherwise indicated. The complete list of primers used in this study is in Table 2. Plasmid pNptII was created by ligating the XhoI/BamHI-digested nptII PCR product into the same restriction sites of pRL662. The plasmid was transformed into DH10B, and the resulting strain was designated as EML5395. The pRL-rpsL, pRL-galK, pRL-nupG, and pRL-rpsLStr were created by ligating the XhoI/XbaI-digested PCR product into the same restriction sites of pRL662. The plasmid was transformed into DH10B, and the resulting strain was designated as EML5389, EML5390, EML5391, and EML5392. Plasmids pClpP-HA and pClpA-HA were created by ligating SacI/PstI-digested PCR products (clpP and clpA from BW25113 wild type without the stop codon, respectively) into pTrc200HA. The pClpPS111A-HA was created by amplifying fragments using pTRC99C-F plus ClpP-S111A-rv and pTRC99C-R plus ClpP-S111A-fw as primers. The two fragments were then merged and amplified by PCR-Splicing by Overlapping Extension (SOEing) (Heckman and Pease, 2007). The resulting full-length clpP-containing fragment was digested by SacI/PstI and then ligated into pTrc200HA. All other pClpP-HA plasmids with a mutated form of ClpP were created similarly. The plasmid constructs ClpX (ClpX-ΔN-ter), wild-type ClpP-tev-His, and green fluorescent protein (GFP)-ssrA were a kind gift from Dr. Robert T. Sauer (MIT, Cambridge, United States). Site-directed mutagenesis was performed to generate the ClpP variants. All plasmids of pClpP-tev-His with a mutated clpP gene was constructed similar to that of pClpPS111A-HA mentioned above with the differences below: Primer T7 was used instead of pTRC99C-F, and primer T7T was used instead of pTRC99C-R, and the restriction sites used were XbaI/XhoI.
Interbacterial Competition Assay
The optical densities of the cultured A. tumefaciens and E. coli were measured and adjusted to OD600 equals to 3.0 in 0.9% NaCl (w/v). The recipient E. coli cells were then further diluted to OD600 equals to 0.3 or 0.1, depending on the need of the assay. Afterward, the attacker and the recipient cultures were mixed in equal volume to make the attacker: recipient ratio 10:1 or 30:1, respectively. Ten microliters of the mixed bacterial culture was then spotted onto Agrobacterium Kill-triggering medium (AK medium, 3 g of K2HPO4, 1 g of NaH2PO4, 1 g of NH4Cl, 0.15 g of KCl, and 9.76 g of MES, pH 5.5), solidified by 2% (w/v) agar, and then air-dried to enable contact-dependent competition. The competition plates were cultured at 25°C for 16 h. After the competition, bacteria were recovered using a loop and resuspended into 500 μl of 0.9% NaCl. The recovered bacterial suspension was then serially diluted and plated onto LB supplemented with spectinomycin to select recipient E. coli cells. After overnight culture at 37°C, the recovered colony formation unit (cfu) was counted and recorded. The T6SS-dependent susceptibility index (SI) was defined as the logarithm of the recovered E. coli cfu co-cultured with ΔtssL subtracted by that co-cultured with wild-type A. tumefaciens.
The High-Throughput Interbacterial Competition Platform
Pipetting steps of the screening platform were performed by the pipetting robot EzMate401 (Arise Biotech, Taiwan) unless otherwise specified. Fifty microliters of the cultured attacker A. tumefaciens was pelleted using 8,000 × g for 10 min at 15°C. After the medium was removed, the pellet was washed twice using 0.9% NaCl (w/v) and then adjusted to OD600 equals to 3.0. The OD600-adjusted attacker cells were then dispensed as 300 μl into each well of a 2.2-ml Deepwell microplate (Basic Life, Taiwan). Each well was then added with 10 μl of the cultured recipient E. coli mutants and mixed well to make the attacker:target at 30:1 (v/v). After being mixed, the bacterial mixture was then added onto the competition plate. The competition plate was made by 25 ml of the AK medium with 2% (w/v) agarose solidified in a 96-well lid. The competition plate was then cultured at 25°C for 16 h before recovery. The recovery was performed by stamping a 96-well plate replicator to the competition spots followed by suspending the bacterial cells to a 96-well plate containing 200 μl of 0.9% NaCl in each well. After being mixed, 10 μl of the recovered bacterial suspension was spotted onto LB agar supplemented with kanamycin made in a 96-well lid, cultured at 37°C overnight, and then was observed. In the first screening, only A. tumefaciens C58 wild type was used as the attacker. In the second screening, both wild type and ΔtssL were used as the attackers. For the groups co-cultured with A. tumefaciens C58 wild type, the recovery suspension was either undiluted or diluted to 5 and 25 times before being spotted onto LB agar with kanamycin plate. For the groups co-cultured with A. tumefaciens C58 ΔtssL, the recovery suspensions were either undiluted or diluted to 10 and 100 times before spotted onto LB agar with kanamycin plate. At each stage, the E. coli mutants that formed multiple colonies were identified as the candidates.
Protein Production and Purification
Escherichia coli BL21(DE3) was used as a host to produce all proteins of interests. Cells were cultured in LB medium supplemented with appropriate antibiotics in 1-L flask. When OD600 reached 0.6, the bacterial culture was cooled to 16°C, and IPTG was added (final concentration of 0.5 mM) for the overexpression of the protein. The cells were further allowed to grow for 16 h, followed by centrifugation to pellet them and then resuspended in lysis buffer (50 mM of Tris, pH 8.0, 300 mM of NaCl, 1% Triton X-100, 10 mM of beta-mercaptoethanol, 1 mM of DTT, and 10% glycerol). The cells were lysed by sonication at 4°C (amplitude 10 for 5 s, followed by 15-s breaks; total sonication time was 6 min) (PRO Scientific, United States). The lysates were centrifuged at 20,000 rpm for 30 min at 4°C. The supernatants were collected and loaded onto Ni-NTA column (GE Healthcare, United States) equilibrated with wash buffer (50 mM of Tris, pH 8.0, and 300 mM of NaCl) and eluted by 6 ml of wash buffer containing 250 mM of imidazole. The eluted fractions of the protein were further subjected to size-exclusion chromatography (SEC) by Superdex 200, 16/60 column (GE Life Sciences, United States) in buffer containing 50 mM of Tris, pH 7.5, 100 mM of KCl, 25 mM of MgCl2, 1 mM of DTT, and 10% glycerol. The protein purity was confirmed on 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The samples were flash-frozen and stored in −80°C until further use.
Protein Degradation Assay
Green fluorescent protein (GFP) fluorescence based-degradation assays were carried out in Protein Degradation (PD) buffer (25 mM of HEPES, pH 7.5, 100 mM of KCl, 25 mM of MgCl2, 1 mM of DTT, and 10% glycerol) containing 3 μM of GFP-ssrA as substrate and ATP regeneration system (16 mM of creatine phosphatase and 0.32 mg/ml of creatine kinase) as described previously (Sriramoju et al., 2018). In brief, 0.1 μM of ClpX6 and 0.3 μM of ClpP14 or its variants were mixed at 30°C and allowed to stand for 2 min. The protein degradation reaction was started by addition of ATP to a final concentration of 5 mM. The changes in the fluorescence were measured at 511 nm with an excitation wavelength at 467 nm in a 96-well format using Infinite M1000 PRO plate reader (Tecan, Switzerland).
Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis and Western Blot Analysis
The ΔclpP:kan E. coli strains harboring appropriate plasmid were grown as the same procedure indicated in the Interbacterial Competition Assay. Cells were adjusted to OD600 of 5.0, collected at 5,000 × g for 5 min, and directly resuspended in 1 × SDS sample buffer. The samples were incubated at 96°C for 10 min and then analyzed by SDS-PAGE. Protein samples separated by SDS-PAGE were transferred to an Immobilon-P membrane (Merck Millipore, United States). The monoclonal anti-HA was used at a dilution of 1:10,000 (Yao-Hong Biotech Inc., Taiwan), and the goat–anti-rabbit conjugated to horseradish peroxidase secondary antibody was used at a dilution of 1:10,000 (GeneTex, Taiwan). The Western Lightning ECL Pro (PerkinElmer Life Sciences, United States) was used for color development and visualized by BioSpectrum 600 Imaging System (UVP, United States).
Statistical Analysis and Figure Production
Statistical analyses and figure production were performed using the R program (version 3.5.1) (R Core Team, 2018) and RStudio (version 1.1.456) (RStudio Team, 2015). R packages plyr (version 1.8.4) (Wickham, 2011) and multcompView (version 0.1-7) (Graves et al., 2015) were used for statistical analyses. Figures were produced using the R packages ggplot2 (version 3.0.0) (Wickham, 2016), Hmisc (version 4.1-1) (Harrell et al., 2018), and ggpubr (version 0.2) (Kassambara, 2018). Student’s t-test, one-way analysis of variance (one-way ANOVA), and Tukey’s honestly significant difference test (Tukey HSD test), in which significant difference threshold set as 0.05, were used in all cases.
Results
The Agrobacterium tumefaciens T6SS Killing Outcome Differs Between Different Escherichia coli Strains
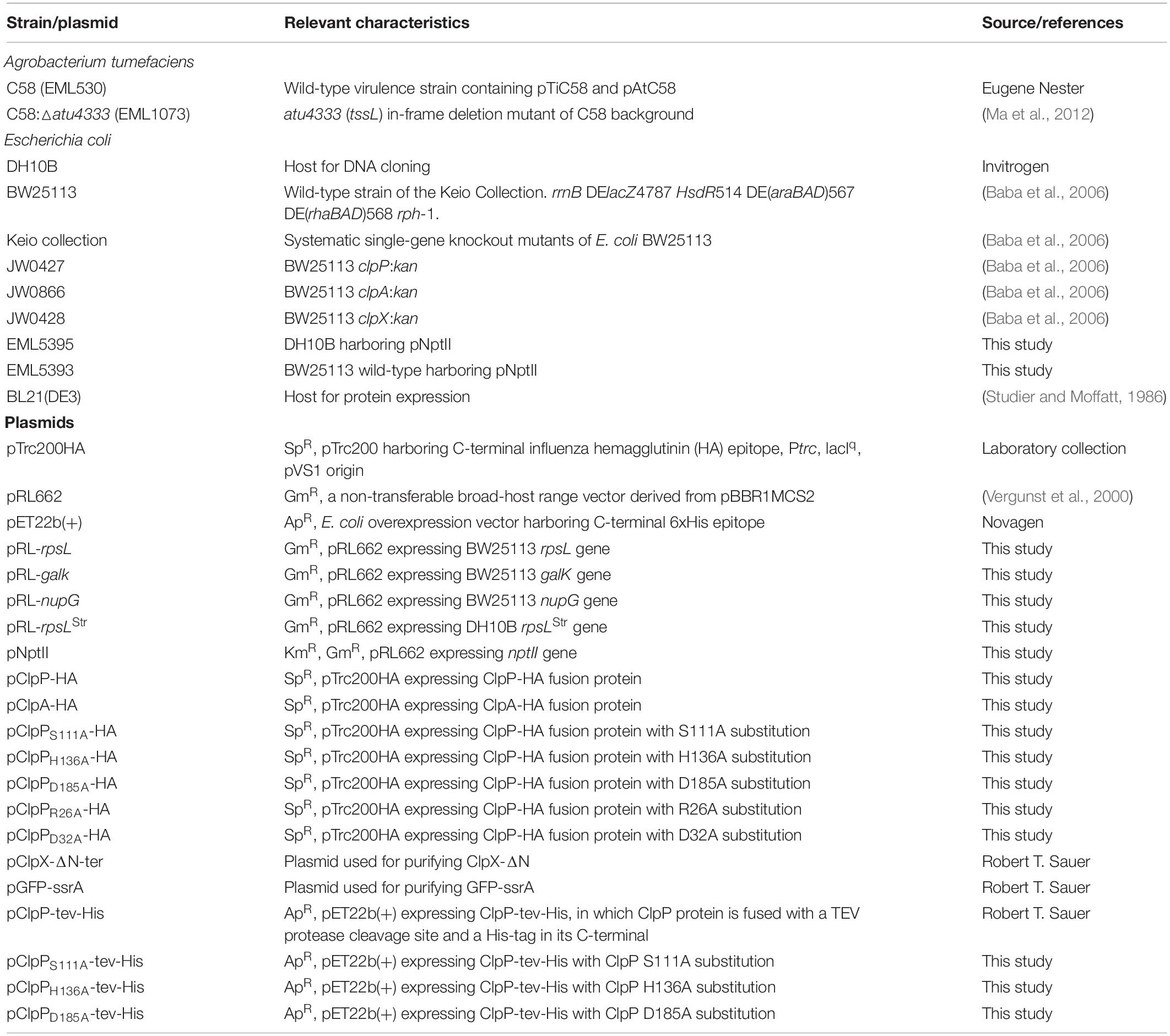
Using an optimized competition condition (AK medium agar that contains basic minerals at pH 5.5), we noticed that when co-cultured with wild-type A. tumefaciens C58, the recovered colony-forming unit (cfu) of E. coli BW25113 was always lower than that of DH10B (Figure 1A). Meanwhile, the recovered cfu of both E. coli strains was the same when co-cultured with ΔtssL A. tumefaciens C58 (hereafter referred to ΔtssL), a T6SS secretion-deficient mutant (Figure 1A). For more intuitive readout, we introduced T6SS-dependent SI, which reflects the strength of the T6SS killing. The SI was defined as the logarithm of the recovered E. coli cfu co-cultured with ΔtssL subtracted by that co-cultured with wild type. The mean SI between A. tumefaciens and BW25113 was significantly higher than that of between A. tumefaciens and DH10B with a P-value of 0.02 (T ≤ t, two-tailed, Figure 1B). This result suggests that some genetic factors of BW25113 may enhance the A. tumefaciens C58 killing outcome in a T6SS-dependent manner.
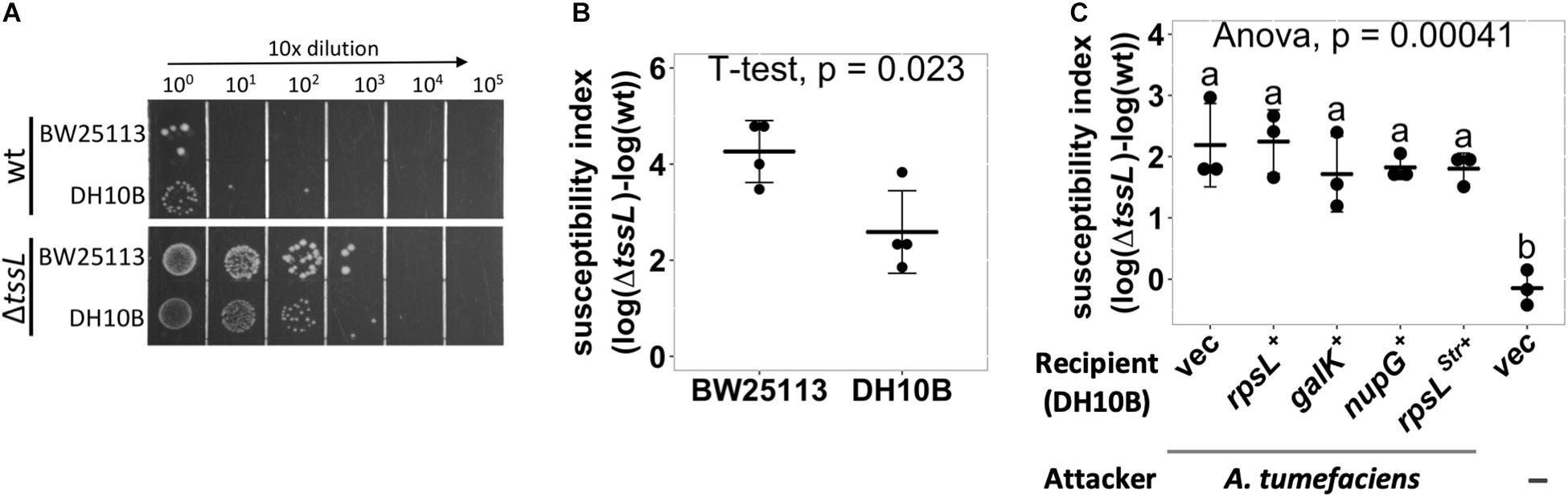
Figure 1. Agrobacterium tumefaciens type VI secretion system (T6SS)-dependent antibacterial activity against Escherichia coli strains. (A,B) A. tumefaciens T6SS antibacterial activity against E. coli strains DH10B and BW25113. A. tumefaciens was co-cultured at a ratio of 30:1 with E. coli DH10B or BW25113, both E. coli strains harboring vector pRL662, on Agrobacterium Kill (AK) agar medium for 16 h. The bacterial mixtures were serially diluted and spotted (A) or quantified by counting cfu (B) on gentamicin-containing lysogeny broth (LB) agar plates to selectively recover E. coli. (C) E. coli DH10B was complemented by either vector only (vec) or derivative expressing rpsL, galK, nupG, or rpsLStr in trans before being subjected to A. tumefaciens T6SS-dependent antibacterial activity assay as described in (B). Susceptibility index (SI) was defined as the subtraction difference of the recovery log(cfu) of that attacked by ΔtssL to that attacked by wild-type A. tumefaciens C58. Data are mean ± SD of three independent experiments calculated by t-test with P < 0.05 for statistical significance (B) or single-factor analysis of variance (ANOVA) and Tukey honestly significant difference (HSD), in which two groups with significant differences are indicated with different letters (a and b) (C).
We tested whether the genes that are functional in BW25113 but not in DH10B could be the cause of the higher SI in BW25113. The galK and nupG genes are functional in BW25113 but are pseudogenes in DH10B. The rpsL has a mutation in DH10B (rpsLStr), which renders the strain resistant to streptomycin, but not in BW25113. The rpsL, galK, or nupG gene from BW25113 was cloned into pRL662 and expressed by constitutive lacZ promoter in DH10B as a recipient for a T6SS interbacterial competition assay (Figure 1C). The DH10B expressing the rpsLStr (overexpressing rpsLStr) was also included. The DH10B harboring empty vector (vec) served as a negative control. A group without attacker was also included to monitor whether the decrease in cfu after the competition solely comes from co-culture with A. tumefaciens attacker. The SIs were not significantly different between DH10B and any of the complemented groups, and each had an SI mean of about 2 (Figure 1C). The above approach was not able to identify the genetic factors that contributed to the enhanced resistance in DH10B, which may imply that precise control of transgene expression or multiple complementation would be required. Therefore, we developed a high-throughput screening method to identify the individual genes that contribute to the enhanced susceptibility of BW25113.
Establishment of a High-Throughput Interbacterial Competition Platform to Identify Recipient Escherichia coli Mutants With Less Susceptibility to Agrobacterium tumefaciens C58 T6SS Killing
We decided to screen the BW25113 single-gene mutant library (Keio collection from NBRP [NIG, Japan]: E. coli) for strains with less susceptibility to A. tumefaciens T6SS-mediated killing. An interbacterial competition assay starts from mixing the attacker and the recipient cells, followed by counting the recovered recipient E. coli on selective media (Figure 2A). This protocol only allowed screening of 10 mutants per day, which was not efficient enough for screening 3,909 strains of the Keio library.
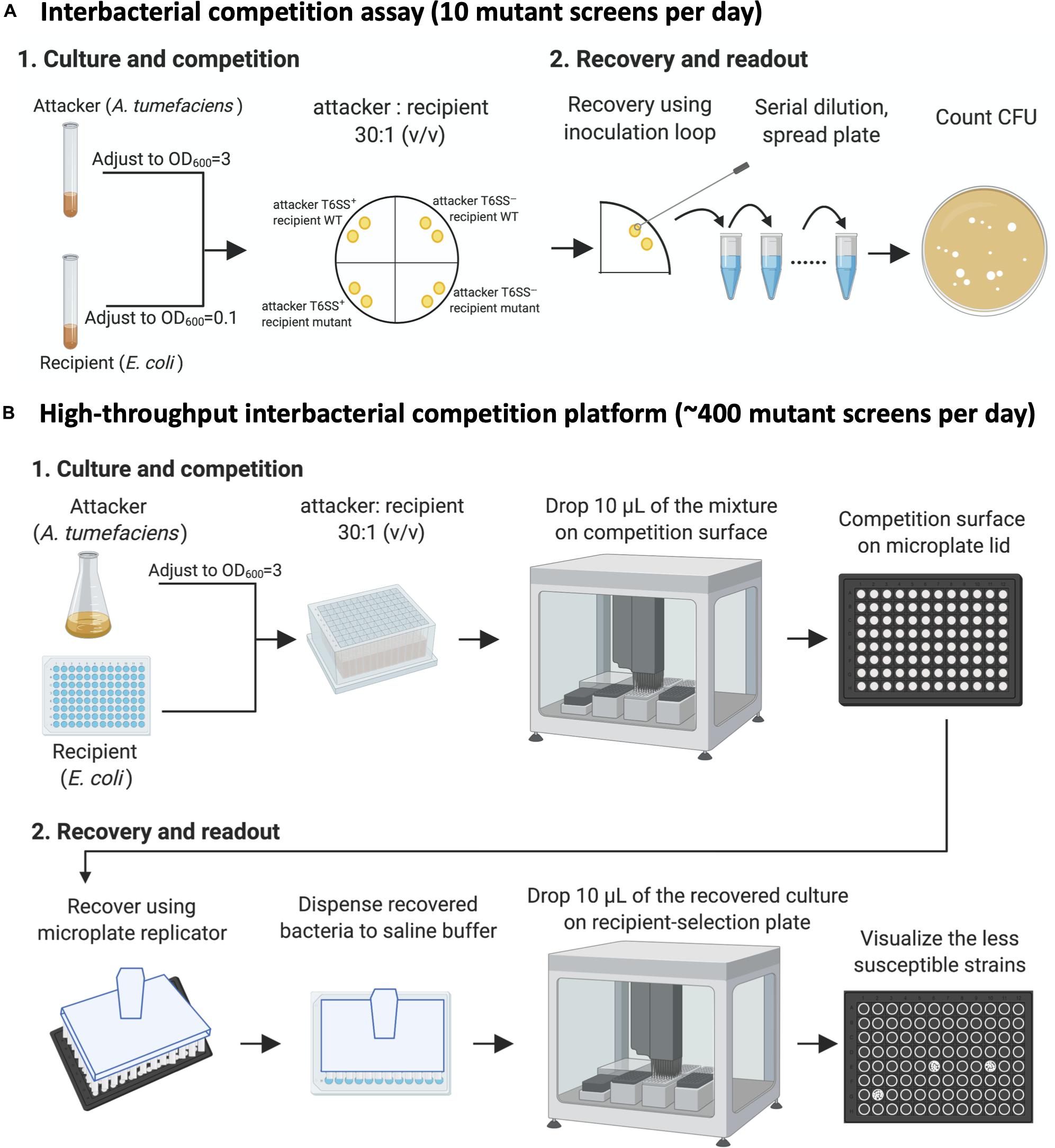
Figure 2. The high-throughput interbacterial competition platform. (A) Interbacterial competition assay. Cultured attacker Agrobacterium tumefaciens and recipient Escherichia coli were mixed and then spotted on the Agrobacterium Kill (AK) agar medium to allow interbacterial competition for 16 h at 25°C followed by recovery of mixed cultures, serially diluted, and then spread onto lysogeny broth (LB) plate supplemented with appropriate antibiotics to select for recipient cells. (B) High-throughput interbacterial competition screening platform. Recipient cells were grown and mixed with attacker A. tumefaciens in a 96-well plate. The bacterial mixture was dropped onto the AK agar medium competition surface using an automated pipetting system. The competition surface was made on a microplate lid. Recovery was performed using microplate replicator. The candidates are the strains that show multiple colonies grown after recovery as opposed to wild-type controls and most strains with no or few colonies. This high-throughput A. tumefaciens type VI secretion system (T6SS) killing platform enables ∼400 mutant screens per day. This figure was created with BioRender (https://biorender.com/).
Therefore, we developed a high-throughput interbacterial competition platform that enables 96 population-level, interbacterial competition simultaneously (Figure 2B). The recipient Keio E. coli strains were cultured in the 96-well, and the attacker A. tumefaciens was cultured in a flask. After the culture, the attacker was adjusted to OD600 equals to 3.0 and then dispensed to a 2.2-ml deep-well plate. The recipient cells were added into the attacker-containing plates in a volume ratio of 30 to 1. Ten microliters of the attacker-recipient mixtures were dropped on the competition surface made by agar solidified on a 96-well lid. A microplate replicator was used to stamp on the competition spots to recover the bacterial cells of each competition group. The recovered bacteria were suspended in the saline buffer (0.9% NaCl), mixed, and then spotted on the recipient-selection surface made by agar solidified on a microplate lid. The competition condition was set at the strength that enables A. tumefaciens to kill almost all BW25113 wild-type recipients so that only a few or no cells would survive. This setup made recognizing the resistant mutants simple – the ones with the multiple colonies are the candidates (Figure 2B).
All the 3,909 strains in the Keio were screened using A. tumefaciens C58 wild type as the attacker. In each screening, at least two wild type E. coli BW25113 replicates were incorporated and screened in parallel as parental controls. The Keio mutants that formed colonies in this stage were selected, and 196 strains showed enhanced resistant to A. tumefaciens C58 attack. The 196 strains were subjected to second screening using both wild type and ΔtssL as the attackers. At this stage, we incorporated a grading system: Grade I mutants were at least 25 times less susceptible to C58 T6SS-dependent killing, whereas grade II mutants were at least 10 times less susceptible. Six grade I mutants and 10 grade II mutants were identified.
Confirmation of the Escherichia coli Mutants With Less Susceptibility to Agrobacterium tumefaciens C58 Type VI Secretion System Killing
The enhanced resistance of the six grade I mutants were further verified by an interbacterial competition assay and by complementation tests. For complementation, wild-type genes from BW25113 were cloned into plasmid pTrc200HA plasmid and expressed by trc promoter. Five out of six showed lower susceptibility to A. tumefaciens T6SS attack than that of BW25113 wild type (Table 3 and Supplementary Figure S1). These are clpP, gltA, ydhS, ydaE, and cbpA mutants. The yeaX mutant, on the other hand, did not differ when compared with the wild type. The cbpA mutant showed a milder phenotype and could not be complemented in trans under the condition tested (Table 3 and Supplementary Figure S1). As cbpA is the first gene in its operon, the failure in complementation could be due to the requirement of other gene(s) in the operon. Nevertheless, the verification performed above showed that the high-throughput interbacterial competition platform was reliable in identifying the recipient genetic factors that participate in T6SS killing.
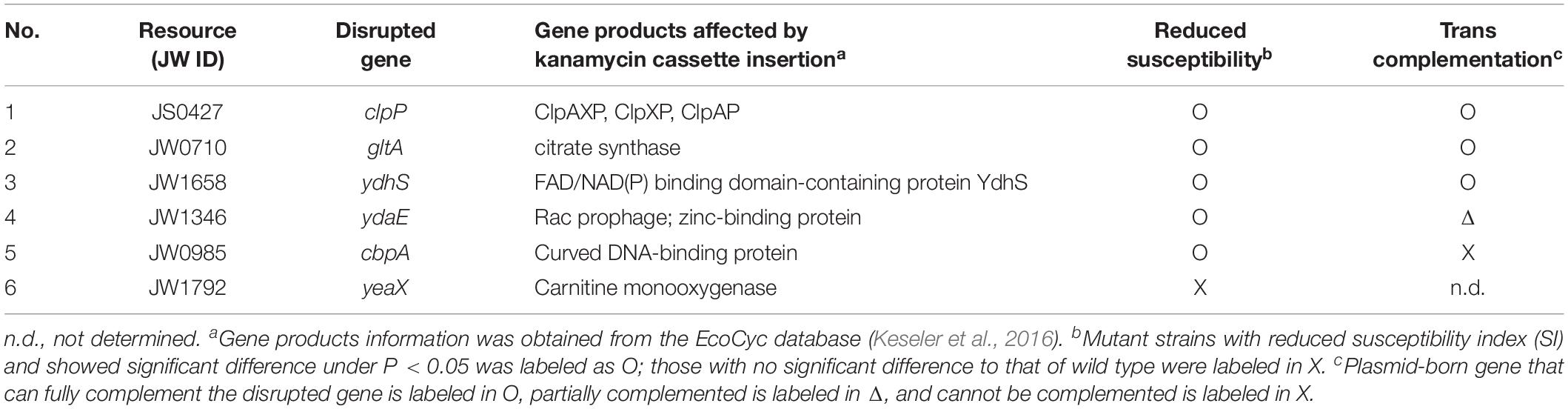
Table 3. Escherichia coli strains that showed reduced susceptibility to Agrobacterium tumefaciens T6SS attack.
The ClpP Protein Plays a Role in Enhancing Susceptibility to Agrobacterium tumefaciens Type VI Secretion System Killing
Because known recipient cell factors affecting antibacterial activity are often conserved components, we selected ΔclpP:kan (labeled as ΔclpP) for further studies. ClpP is a highly conserved, housekeeping AAA+ serine protease that exists in prokaryotes, plastids, and mitochondria (Alexopoulos et al., 2012; Bhandari et al., 2018; Mahmoud and Chien, 2018). We performed a quantitative interbacterial competition assay using A. tumefaciens as the attacker and the BW25113 wild type, ΔclpP, or complemented strain clpP+ as the recipient cells (Figure 3A). The initial cfu of the E. coli at 0 h was about 106 in all groups (one-way ANOVA with P = 0.88), indicating that any E. coli cfu difference at 16 h was not due to initial bacteria titer difference. The cfu among different recipient E. coli strains was not significantly different at 16 h when using A. tumefaciens ΔtssL (one-way ANOVA with P = 0.67), indicating that co-culture with T6SS-deficient strain will not cause recipient titer to differ. On the other hand, the recovered cfu of ΔclpP was about 104, whereas it was about 5 × 102 in BW25113 wild type and in clpP+ after 16-h competition using wild-type A. tumefaciens (Figure 3A). The mean SI of the BW25113 wild type to A. tumefaciens C58 is significantly higher than that of ΔclpP (one-way ANOVA with P = 0.02, Figure 3B). The less susceptible phenotype of the ΔclpP can be fully complemented in trans (clpP+) (P = 0.96 compared with BW25113 wild type). These results confirmed that clpP contributes to enhancing susceptibility to T6SS antibacterial activity of A. tumefaciens C58.
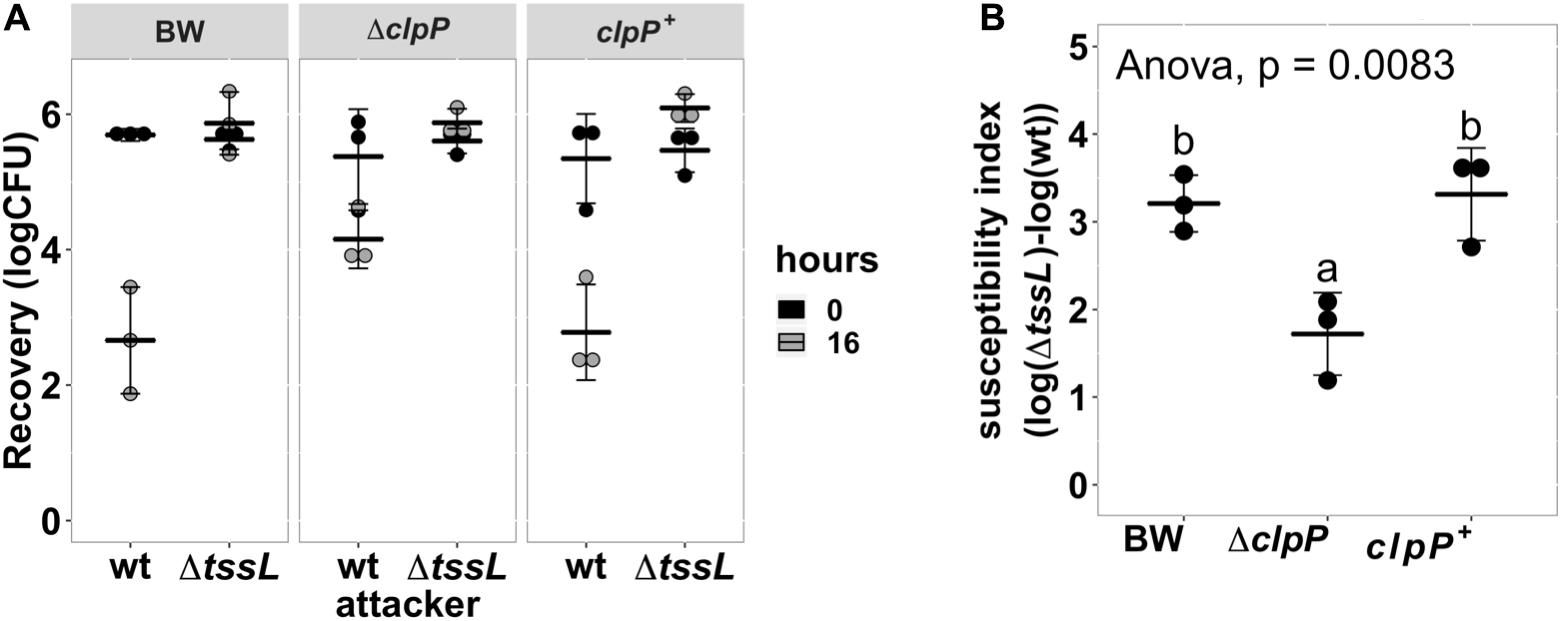
Figure 3. Agrobacterium tumefaciens susceptibility to type VI secretion system (T6SS)-dependent antibacterial activity was reduced in Escherichia coli clpP:kan and can be fully complemented in trans. (A) Recovery of surviving E. coli cells at 0 h and 16 h after being co-cultured with either A. tumefaciens wild type C58 (wt) or ΔtssL at a ratio of 30:1. (B) The susceptibility index (SI) of E. coli BW25113 wild type (BW), ΔclpP, and ΔclpP complemented with clpP expressed on plasmid (clpP+) was calculated from the recovery rate shown in (A). Statistical analysis involved single-factor analysis of variance (ANOVA) and Tukey honestly significant difference (HSD). Data are mean ± SD of three independent experiments, and two groups with significant differences are indicated with different letters (a and b) (P < 0.05 for statistical significance).
Effects of ClpP Catalytic Variants in Enhancing Agrobacterium tumefaciens Type VI Secretion System Antibacterial Activity and Protease Activity
A functional ClpP complex consists of a tetradodecameric ClpP (ClpP14) and its associated AAA+ ATPase substrate-recognizing partner ClpA or ClpX, both in a hexameric form (Olivares et al., 2015). The protease catalytic triad of the E. coli ClpP is composed of S111, H136, and D185 (counted from the Met1) (Maurizi et al., 1990; Wang et al., 1997). We tested whether the ClpP protease is essential in enhancing E. coli susceptibility to A. tumefaciens C58 T6SS attack. E. coliΔclpP complemented with pTrc200HA expressing either wild-type or catalytic variants ClpP S111A, H136A, and D185A was used as a recipient strain. All ClpP variants contain a C-terminal HA tag. Two of the catalytic variants, S111A+ and H136A+, failed to complement, whereas surprisingly, the third catalytic variant, D185A+, can fully complement the phenotype (Figure 4A). The difference of ClpP catalytic variants to complement ΔclpP was not due to their protein-expression level as determined by Western blot (Figure 4B). The protein migration of the ClpPS111A and ClpPH136A was slower than that of the ClpPwt and ClpPD185A owing to their inability to remove the N-terminal propeptide (1–14 amino acids) as in ClpPwt and ClpPD185A (Maurizi et al., 1990; Bewley et al., 2006).
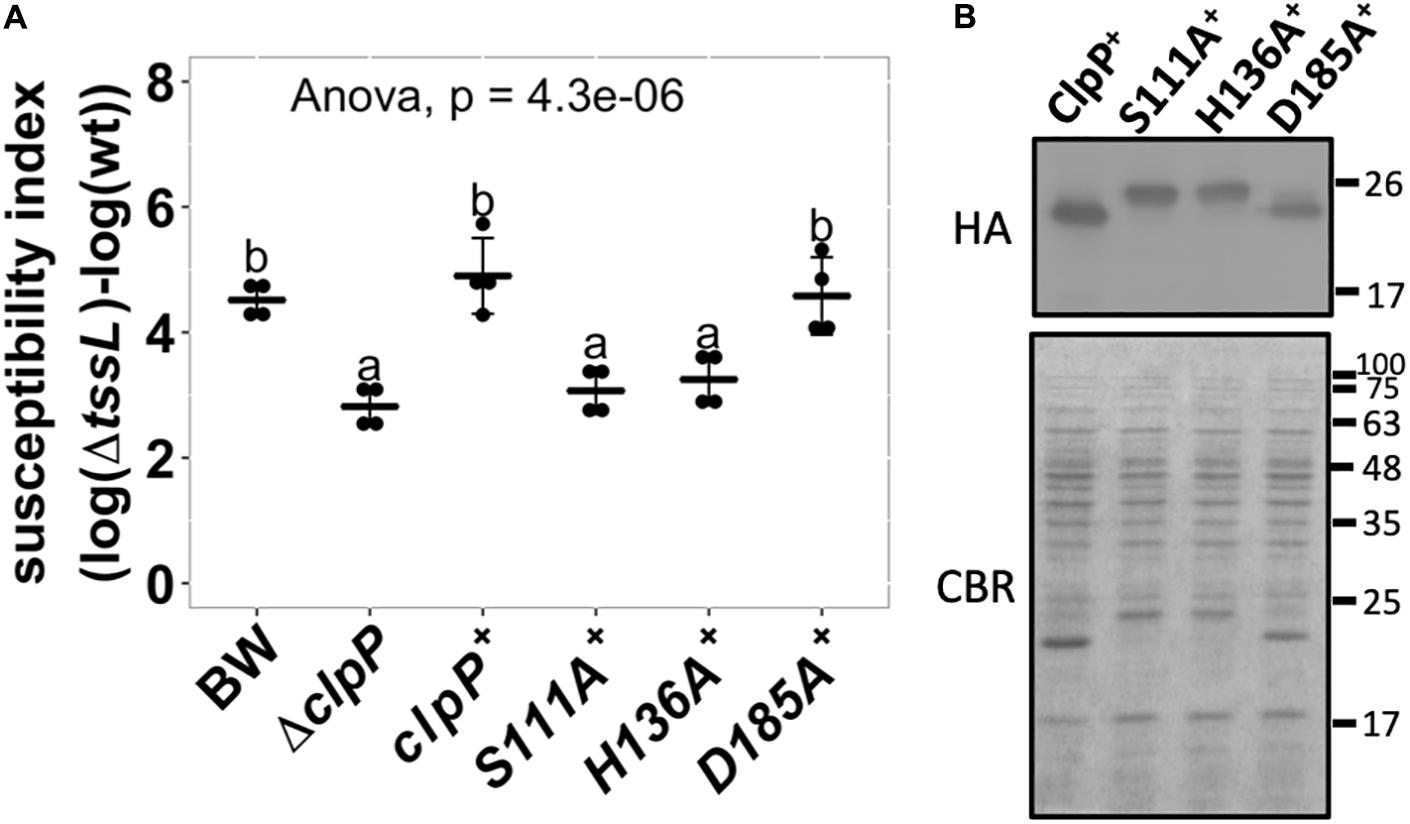
Figure 4. Effects of ClpP protease catalytic variants in enhancing Agrobacterium tumefaciens type VI secretion system (T6SS) antibacterial activity. (A) The susceptibility index calculated from A. tumefaciens interbacterial activity assay against Escherichia coli. The A. tumefaciens C58 wild-type or ΔtssL were co-cultured at a ratio of 10:1 with E. coli BW25113 wild type (BW), ΔclpP, and ΔclpP complemented with clpP and its variants expressed on plasmid. The complemented clpP strains were either wild type (clpP+) or catalytic variants ClpPS111A (S111A+), ClpPH136A (H136A+), and ClpPD185A (D185A+), with C-terminus HA-tag. The susceptibility index (SI) of each E. coli was calculated from the logarithm recovery rate of the ΔtssL co-cultured group minus that of the wild-type co-cultured group. Data are mean ± SD of four biological replicates from two independent experiments. Statistical analysis involved single-factor analysis of variance (ANOVA) and Tukey honestly significant difference (HSD) with P < 0.05 for statistical significance. Two groups with significant differences are indicated with different letters (a and b). (B) The ClpP protein levels of the ΔclpP complemented strains used in (A). The ClpP-expressing E. coli strains were cultured at the same condition used in interbacterial competition assay. Instead of co-culture with A. tumefaciens, protein samples were collected, normalized, and subjected to Western blot analysis of ClpP:HA and its variants. Representative result of three independent experiments is shown.
As ClpPD185A was able to complement the phenotype, we further investigated the ClpP protease activity of the above ClpP variants by a widely adopted ClpP protein degradation assay using GFP-ssrA as the model substrate. Loss of GFP fluorescence is used as a reporter to monitor substrate degradation by ClpXP as a function of time (Weber-Ban et al., 1999; Sriramoju et al., 2018). The results showed that over time, wild-type ClpP effectively degraded GFP-ssrA with a half-life of about 30 min (Figure 5A). Meanwhile, less than a 10% decrease of the GFP-ssrA signal was observed in GFP-ssrA only and wild type without ATP groups, both served as negative controls. The decreasing rates of the GFP-ssrA fluorescence of ClpPS111A, ClpPH136A, and ClpPD185A were significantly slower than those of ClpPWT and showed no significant difference among the three variants at the end of the test (Figures 5A,B). Although ClpPD185A showed no statistically difference in GFP-ssrA degradation compared with ClpPS111A and ClpPH136A at the final time point, it showed significantly lower GFP-ssrA signal to that of the negative control groups (Figure 5B).
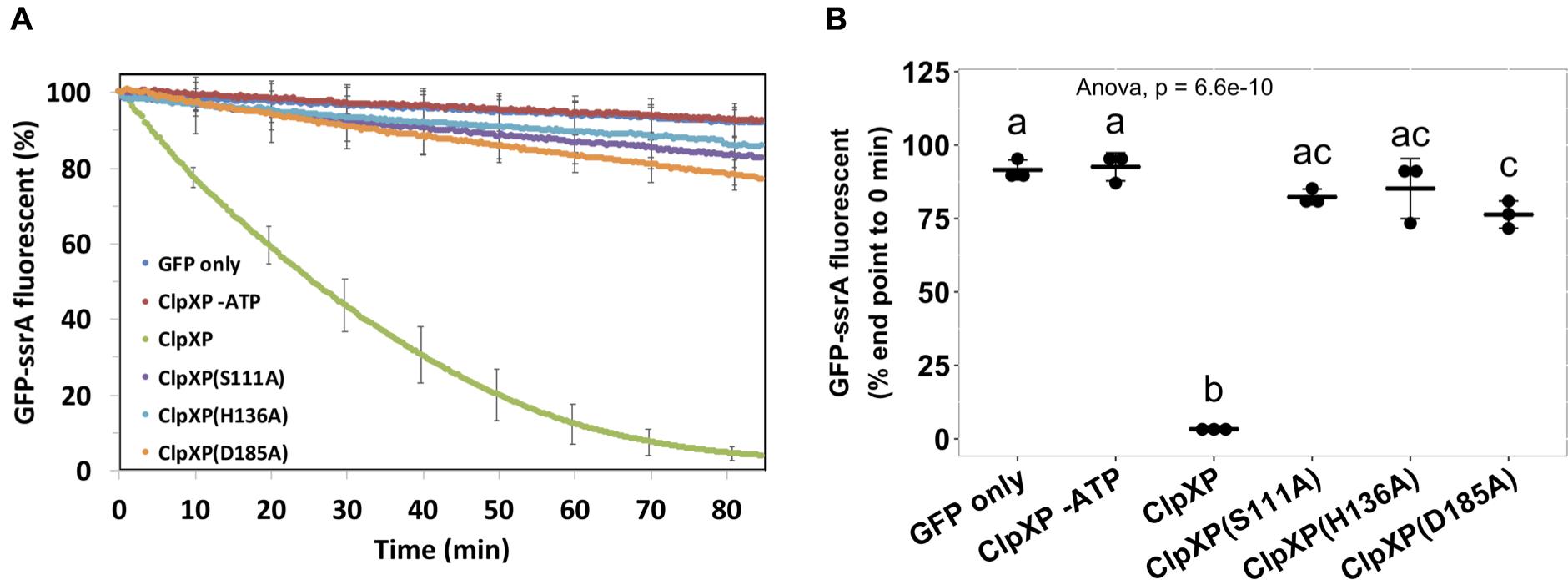
Figure 5. Protease activity assay of the ClpP and its catalytic variants. The wild-type ClpP and its catalytic variants were each pre-assembled with ClpX followed by providing its substrate, the ssrA-tagged green fluorescent protein (GFP). The GFP fluorescent signals were monitored (A) over time, and (B) statistical analysis was measured at the end of the assay. Statistical analysis involved single-factor analysis of variance (ANOVA) and Tukey honestly significant difference (HSD) with P < 0.05 for statistical significance. Two groups with significant differences are indicated with different letters (a and b). Data are mean ± SD of three biological replicates from one representative result of at least two independent experiments.
The ClpP-Associated AAA+ ATPase ClpA but Not ClpX Is Involved in Enhancing Susceptibility to Agrobacterium tumefaciens Type VI Secretion System Activity
ClpP is a protein protease dependent on other adapter proteins such as ClpA and ClpX for substrate recognition (Maurizi, 1991; Gottesman et al., 1998). Therefore, we next determined whether the resistant phenotype of ΔclpP is mediated by ClpA or ClpX through the interbacterial competition assay of the deletion mutants ΔclpA:kan (hereafter referred to as ΔclpA) and ΔclpX:kan (hereafter referred to as ΔclpX) as recipients. SI demonstrates that ΔclpA was less susceptible to A. tumefaciens T6SS killing than BW25113 wild-type (P = 0.02), whereas ΔclpX was similar to BW25113 wild type (P = 1.00) (Figure 6A). The decreased A. tumefaciens T6SS killing phenotype of ΔclpA was fully complemented in trans (Figure 6B). No difference could be detected among the growth of the BW25113 wild-type, ΔclpA, ΔclpP, and their respective complemented strains when co-cultured with ΔtssL (P = 0.58) (Figure 6C). Therefore, the killing outcome is caused by Agrobacterium T6SS-mediated interbacterial competition rather than the growth rate of the different recipient strains under the competition condition. This suggested that ClpA could be the adapter that interacts with ClpP leading to the enhanced susceptibility to T6SS attack in BW25113 wild type. In this case, the interaction between ClpA and ClpP should be required for enhancing A. tumefaciens T6SS killing. The interaction between ClpA and ClpP is well studied, and it has been demonstrated that the R26A and D32A variants of ClpP lose their ability to bind to ClpA by 50 and 100%, respectively (Bewley et al., 2006). Therefore, we complemented ClpPR26A and ClpPD32A in ΔclpP to determine whether the two variants could restore the susceptibility. The R26A+ was able to complement (P = 0.96, compared to ClpP+), whereas D32A+ failed to complement and showed no statistical difference in SI than that of ΔclpP (P < 10–4) (Figure 7). These results suggest that the phenotype observed in ΔclpP and in ΔclpA could be associated with ClpA–ClpP interaction. Because the retained N-terminal propeptide does not prevent ClpP–ClpA binding (Maurizi et al., 1990), the inability of unprocessed ClpPS111A and ClpPH136A in enhanced susceptibility is independent of ClpP–ClpA complex formation.
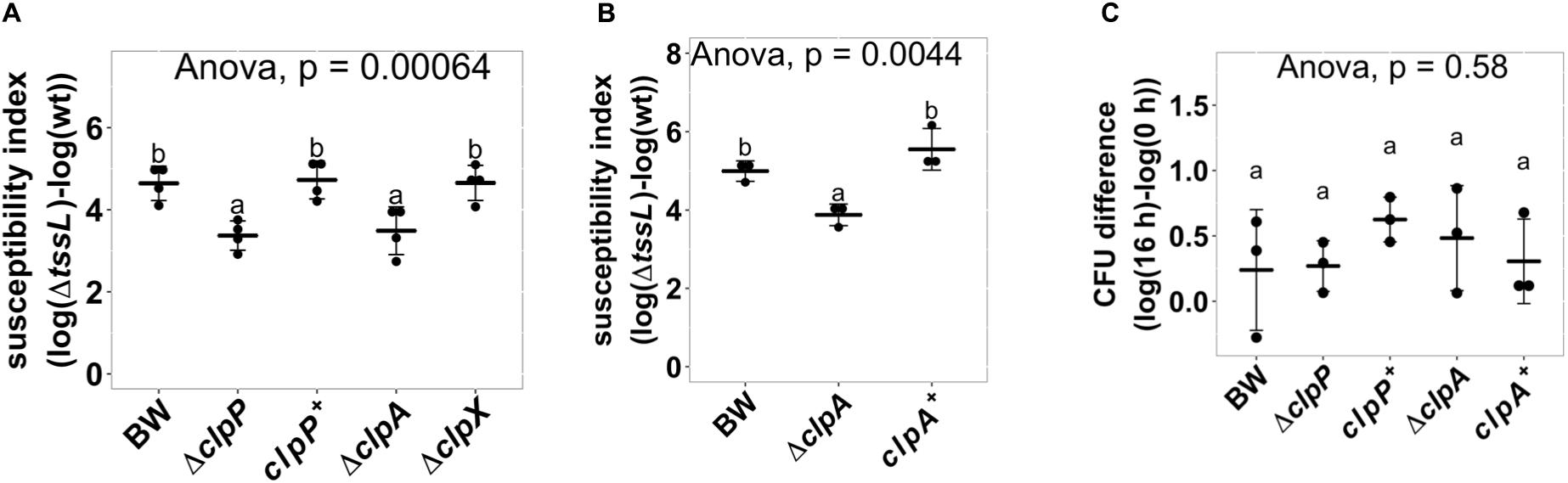
Figure 6. ClpP associated AAA+ ATPase ClpA but not ClpX is involved in enhancing Agrobacterium tumefaciens type VI secretion system (T6SS) antibacterial activity. (A) A. tumefaciens T6SS antibacterial activity against Escherichia coliΔclpP and its complement strain, ΔclpA and ΔclpX. The A. tumefaciens and the E. coli were co-cultured at a ratio of 10:1 on Agrobacterium Kill (AK) agar medium for 16 h. Afterward, the recovery of E. coli strains was quantified, and the susceptibility index was calculated by subtracting the difference of the recovered log(cfu) of that attacked by ΔtssL to that by wild-type A. tumefaciens C58. (B) A. tumefaciens T6SS antibacterial activity assay and the susceptibility index were performed as described in (A) using E. coli wild type (BW), ΔclpA, and ΔclpA complemented with clpA expressed on plasmid (clpA+). (C) Growth of E. coli when co-culturing with the ΔtssL A. tumefaciens. Data in (A–C) are mean ± SD of at least three independent experiments. Statistical analysis involved single-factor analysis of variance (ANOVA) and Tukey honestly significant difference (HSD) with P < 0.05 for statistical significance. Two groups with significant differences are indicated with different letters (a and b).
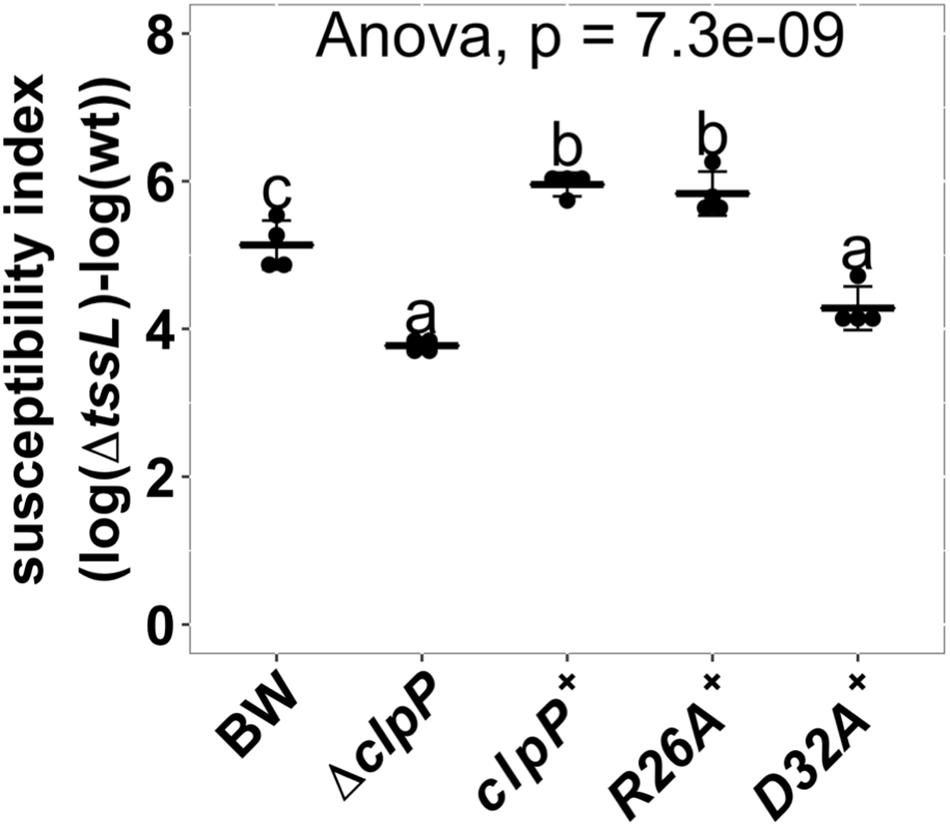
Figure 7. Effects of ClpP variants impaired with ClpA-binding ability in enhancing Agrobacterium tumefaciens type VI secretion system (T6SS) antibacterial activity. Interbacterial competition assay between A. tumefaciens and Escherichia coli wild type, ΔclpP, and ΔclpP complement strains expressing wild-type ClpP (clpP+), ClpAP complex formation mutants ClpPR26A (R26A+), and ClpPD32A (D32A+). The ClpAP complex forming ability is half than that of wild-type ClpP in ClpPR26A and is completely lost in ClpPD32A (Bewley et al., 2006). The T6SS killing data are mean ± SD of four biological replicates from two independent experiments. Statistical analysis involved single-factor analysis of variance (ANOVA) and Tukey honestly significant difference (HSD) with P < 0.05 for statistical significance. Two groups with significant differences are indicated with different letters (a and b).
Discussion
This study provides evidence that the genetic factors of the recipient cells play an important role in affecting the outcome of the T6SS antibacterial activity. The high throughput interbacterial competition platform developed in this study proved to be an effective method in identifying recipient factors that affect the outcome of A. tumefaciens T6SS antibacterial activity. Further exploration led to the confirmation of at least six genes (clpP, clpA, gltA, ydhS, ydaE, and cbpA) encoding known or putative cytoplasmic proteins (Keseler et al., 2016), whereas CbpA resides both in the cytoplasm and in the nucleoid (Orfanoudaki and Economou, 2014). None of these gene products were localized to the inner membrane, periplasm, outer membrane, or extracellular milieu. This result implies that the process affecting the outcome of A. tumefaciens T6SS killing to E. coli occurs in the cytoplasm, presumably after the injection of the T6SS puncturing apparatus. Previous studies have mainly focused on how attacker T6SS is regulated and sensed (Filloux and Sagfors, 2015; Alteri and Mobley, 2016; Hood et al., 2017). This study provides a new insight that recipient cell genes can also affect the T6SS killing outcome and that it could take place after the injection of the T6SS apparatus into the recipient cytoplasm.
Our data showed that ClpA but not ClpX, together with ClpP, contributes to the susceptibility of the recipient E. coli to A. tumefaciens T6SS killing. The clpX transcript level drops and fades 15 min after the onset of carbon starvation (Li et al., 2000), which is the condition used for our interbacterial competition. Thus, ClpX is probably not available to form the ClpXP complex during Agrobacterium T6SS attacks. The ΔclpA was indeed identified in the first screening but was accidentally misplaced and did not enter the second screening process. Therefore, ΔclpA did not appear in our final candidate list until we obtained the correct strain for confirmation. The results that the three catalytic variants ClpPS111A, ClpPH136A, and ClpPD185A did not significantly differ in their ability to degrade GFP-ssrA substrate suggested that the protease activity may not be the leading cause in enhancing A. tumefaciens T6SS attack. On the other hand, unlike ClpPS111A and ClpPH136A, which do not exhibit significant protease activity as compared with that of the negative controls, ClpPD185A may possess weak protease activity, as the GFP-ssrA fluorescence level is significantly lower than that of the negative controls at the final time point. Thus, the involvement of the ClpP protease activity cannot be completely ruled out as the weak protease activity of ClpPD185A may be sufficient to exhibit its function in enhancing A. tumefaciens T6SS attack. Of note, the ClpP protease activity monitored by the in vitro protease activity assay using either ClpX or ClpA as a protein unfoldase showed a highly similar pattern among 24 ClpP variants (Bewley et al., 2006). As this GFP-ssrA degradation assay is an in vitro system and that it is difficult to monitor the ClpP protease activity of the recipient under competition condition, the role of ClpP protease remains elusive.
Our data also suggest that ClpAP complex is required in enhancing recipient susceptibility during A. tumefaciens T6SS killing on the basis of the results that ClpP variant that loses its ability to form a complex with ClpA did not complement the phenotype whereas those with ClpA binding ability do. This implies that the ClpA–ClpP complex, rather than ClpP alone, is the cause of the enhanced susceptibility to T6SS attack. As ClpP allosterically activates the polypeptide translocation activity of ClpA (Miller et al., 2013), the necessity of the ClpAP complex may depend on the unfoldase activity of ClpA. The detailed mechanism on how recipient ClpAP is involved in T6SS susceptibility enhancement awaits further investigations. One promising future direction would be identifying the potential ClpA substrates and their effects on increasing susceptibility of T6SS attack.
Hijacking a highly conserved and essential molecule of the recipient cell to improve attacker fitness is not uncommon. The examples are CdiA-CTEC93 hijacking essential proteins BamA and AcrB, CdiA-CTEC536 hijacking the recipient CysK, and Ssp2 and Ssp4 hijacking recipient DsbA (Aoki et al., 2008; Diner et al., 2012; Mariano et al., 2018). The ClpP protease, on the other hand, is highly conserved in both prokaryotes and eukaryotic organelles like plastid and mitochondria (Culp and Wright, 2016; Moreno et al., 2017). The ClpP protease cooperates with different AAA+ ATPases in different organisms. It works with ClpA and ClpX in Gram-negative bacteria; with ClpC and ClpE in Gram-positive bacteria; with ClpC1, ClpC2, and ClpD in the chloroplast; and with ClpX in human mitochondria. In all these cases, the ClpP protease seems to play a central role in protein homeostasis. Dysfunction of the system can lead to severe developmental defects, a reduction in the pathogenicity, or lethality (Cole et al., 2015; Nishimura and Van Wijk, 2015; Bhandari et al., 2018). The current result suggests that the ClpP protease system could be another target hijacked by the T6SS attacker to improve its competitive advantage.
To our knowledge, the involvement of the ClpAP complex in enhancing the recipient’s susceptibility to A. tumefaciens T6SS activity has not been described in the contact-dependent competitor elimination systems in Gram-negative bacteria like T1SS, T4SS, CDI, and T6SS. It would be of interest to uncover how and what A. tumefaciens factors hijack this universal and highly conserved ClpP and its associated AAA+ ATPase substrate recognizing partner. The current finding provides additional evidence to support that T6SS can manipulate the essential and highly conserved molecules of recipient cells to achieve better inhibition of the performance (Russell et al., 2014). Elucidating the underlying molecular mechanisms of ClpAP and other recipient factors would be the next direction to understand further how genetic factors can affect the recipient susceptibility to the T6SS attacks.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
H-HL, MY, and E-ML conceived and designed the experiments. H-HL performed most of the experiments. MS contributed to the protease activity assay. MS and S-TH provided the materials and tools for the protease activity assay. S-TH, C-TL, and E-ML supervised the execution of the experiments. H-HL and E-ML, with contributions from MY, MS, S-TH, and C-TL wrote the manuscript. All authors read and approved the final manuscript.
Funding
The funding for this project was provided by the Ministry of Science and Technology of Taiwan (MOST) (Grant Nos. 104-2311-B-001-025-MY3 to E-ML; 107-2628-M-001-005-MY3 to S-TH; and 107-2811-M-001-1574 to MS) and Academia Sinica Investigator Award to E-ML (Grant No. AS-IA-107-L01).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank National BioResource Project (NIG, Japan): E. coli for providing the Keio collection. We also thank Lai lab members for discussion and suggestions. We acknowledge the staffs in the Plant Cell Biology Core Laboratory and the DNA Sequencing Core Laboratory at the Institute of Plant and Microbial Biology, and Biophysics Facility at the Institute of Biological Chemistry, Academia Sinica, Taiwan, and Chia Lee for technical support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.03077/full#supplementary-material
References
Alexopoulos, J. A., Guarné, A., and Ortega, J. (2012). ClpP: a structurally dynamic protease regulated by AAA+ proteins. J. Struct. Biol. 179, 202–210. doi: 10.1016/j.jsb.2012.05.003
Alteri, C. J., and Mobley, H. L. T. (2016). The versatile type VI secretion system. Microbiol. Spectr. 4:26.
Aoki, S. K., Diner, E. J., De Roodenbeke, C. T. K., Burgess, B. R., Poole, S. J., Braaten, B. A., et al. (2010). A widespread family of polymorphic contact-dependent toxin delivery systems in bacteria. Nature 468, 439–442. doi: 10.1038/nature09490
Aoki, S. K., Malinverni, J. C., Jacoby, K., Thomas, B., Pamma, R., Trinh, B. N., et al. (2008). Contact-dependent growth inhibition requires the essential outer membrane protein BamA (YaeT) as the receptor and the inner membrane transport protein AcrB. Mol. Microbiol. 70, 323–340. doi: 10.1111/j.1365-2958.2008.06404.x
Aoki, S. K., Pamma, R., Hernday, A. D., Bickham, J. E., Braaten, B. A., and Low, D. A. (2005). Contact-dependent inhibition of growth in Escherichia coli. Science 309, 1245–1248. doi: 10.1126/science.1115109
Baba, T., Ara, T., Hasegawa, M., Takai, Y., Okumura, Y., Baba, M., et al. (2006). Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008.
Basler, M., Ho, B. T., and Mekalanos, J. J. (2013). Tit-for-tat: type VI secretion system counterattack during bacterial cell-cell interactions. Cell 152, 884–894. doi: 10.1016/j.cell.2013.01.042
Bayer-Santos, E., Cenens, W., Matsuyama, B. Y., Di Sessa, G., Mininel, I. D. V., and Farah, C. S. (2019). The opportunistic pathogen Stenotrophomonas maltophilia utilizes a type IV secretion system for interbacterial killing. PLoS Pathog. 15:e1007651. doi: 10.1371/journal.ppat.1007651
Bewley, M. C., Graziano, V., Griffin, K., and Flanagan, J. M. (2006). The asymmetry in the mature amino-terminus of ClpP facilitates a local symmetry match in ClpAP and ClpXP complexes. J. Struct. Biol. 153, 113–128. doi: 10.1016/j.jsb.2005.09.011
Bhandari, V., Wong, K. S., Zhou, J. L., Mabanglo, M. F., Batey, R. A., and Houry, W. A. (2018). The role of ClpP protease in bacterial pathogenesis and human diseases. ACS Chem. Biol. 13, 1413–1425. doi: 10.1021/acschembio.8b00124
Cole, A., Wang, Z., Coyaud, E., Voisin, V., Gronda, M., Jitkova, Y., et al. (2015). Inhibition of the mitochondrial protease ClpP as a therapeutic strategy for human acute myeloid leukemia. Cancer Cell 27, 864–876. doi: 10.1016/j.ccell.2015.05.004
Costa, T. R. D., Felisberto-Rodrigues, C., Meir, A., Prevost, M. S., Redzej, A., Trokter, M., et al. (2015). Secretion systems in Gram-negative bacteria: structural and mechanistic insights. Nat. Rev. Microb. 13, 343–359. doi: 10.1038/nrmicro3456
Culp, E., and Wright, G. D. (2016). Bacterial proteases, untapped antimicrobial drug targets. J. Antibiot. 70, 366–377. doi: 10.1038/ja.2016.138
Diner, E. J., Beck, C. M., Webb, J. S., Low, D. A., and Hayes, C. S. (2012). Identification of a target cell permissive factor required for contact-dependent growth inhibition (CDI). Genes Dev. 26, 515–525. doi: 10.1101/gad.182345.111
Filloux, A., and Sagfors, A. (2015). “3 - News and views on protein secretion systems,” in The Comprehensive Sourcebook of Bacterial Protein Toxins (Fourth Edition), eds J. Alouf, D. Ladant, and M. R. Popoff, (Boston: Academic Press), 77–108. doi: 10.1016/b978-0-12-800188-2.00003-3
García-Bayona, L., Gozzi, K., and Laub, M. T. (2019). Mechanisms of resistance to the contact-dependent bacteriocin CdzC/D in Caulobacter crescentus. J. Bacteriol. 201:e538-18. doi: 10.1128/JB.00538-18
García-Bayona, L., Guo, M. S., and Laub, M. T. (2017). Contact-dependent killing by Caulobacter crescentus via cell surface-associated, glycine zipper proteins. eLife 6:e24869. doi: 10.7554/eLife.24869
Gottesman, S., Roche, E., Zhou, Y., and Sauer, R. T. (1998). The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes Dev. 12, 1338–1347. doi: 10.1101/gad.12.9.1338
Graves, S., Piepho, H.-P., Selzer, L., and Dorai-Raj, S. (2015). “multcompView: visualizations of paired comparisons,” in R package version 0.1-7.
Harrell, F. E. Jr., Dupont, C., and Others, M. (2018). Hmisc: Harrell Miscellaneous. Vienna: R Foundation for Statistical Computing.
Heckman, K. L., and Pease, L. R. (2007). Gene splicing and mutagenesis by PCR-driven overlap extension. Nat. Protoc. 2, 924–932. doi: 10.1038/nprot.2007.132
Hood, R. D., Peterson, S. B., and Mougous, J. D. (2017). From striking out to striking gold: discovering that type VI secretion targets bacteria. Cell Host Microbe 21, 286–289. doi: 10.1016/j.chom.2017.02.001
Johnson, P. M., Beck, C. M., Morse, R. P., Garza-Sánchez, F., Low, D. A., Hayes, C. S., et al. (2016). Unraveling the essential role of CysK in CDI toxin activation. Proc. Natl. Acad. Sci. U.S.A. 113, 9792–9797. doi: 10.1073/pnas.1607112113
Jones, A. M., Garza-Sánchez, F., So, J., Hayes, C. S., and Low, D. A. (2017). Activation of contact-dependent antibacterial tRNase toxins by translation elongation factors. Proc. Natl. Acad. Sci. U.S.A. 114, E1951–E1957. doi: 10.1073/pnas.1619273114
Keseler, I. M., Mackie, A., Santos-Zavaleta, A., Billington, R., Bonavides-Martínez, C., Caspi, R., et al. (2016). The EcoCyc database: reflecting new knowledge about Escherichia coli K-12. Nucleic Acids Res. 45, D543–D550. doi: 10.1093/nar/gkw1003
LeRoux, M., De Leon, J. A., Kuwada, N. J., Russell, A. B., Pinto-Santini, D., Hood, R. D., et al. (2012). Quantitative single-cell characterization of bacterial interactions reveals type VI secretion is a double-edged sword. Proc. Natl. Acad. Sci. U.S.A. 109, 19804–19809. doi: 10.1073/pnas.1213963109
Li, C., Tao, Y. P., and Simon, L. D. (2000). Expression of different-size transcripts from the clpP-clpX operon of Escherichia coli during carbon deprivation. J. Bacteriol. 182, 6630–6637. doi: 10.1128/jb.182.23.6630-6637.2000
Lien, Y.-W., and Lai, E.-M. (2017). Type VI secretion effectors: methodologies and biology. Front. Cell Infect. Microbiol. 7:254. doi: 10.3389/fcimb.2017.00254
Ma, L.-S., Hachani, A., Lin, J.-S., Filloux, A., and Lai, E.-M. (2014). Agrobacterium tumefaciens deploys a superfamily of type VI secretion dnase effectors as weapons for interbacterial competition in planta. Cell Host Microbe 16, 94–104. doi: 10.1016/j.chom.2014.06.002
Ma, L.-S., Narberhaus, F., and Lai, E.-M. (2012). IcmF family protein TssM exhibits ATPase activity and energizes type VI secretion. J. Biol. Chem. 287, 15610–15621. doi: 10.1074/jbc.M111.301630
Mahmoud, S. A., and Chien, P. (2018). Regulated proteolysis in bacteria. Annu. Rev. Biochem. 87, 677–696. doi: 10.1146/annurev-biochem-062917-012848
Mariano, G., Monlezun, L., and Coulthurst, S. J. (2018). Dual role for DsbA in attacking and targeted bacterial cells during type VI secretion system-mediated competition. Cell Rep. 22, 774–785. doi: 10.1016/j.celrep.2017.12.075
Maurizi, M. R. (1991). ATP-promoted interaction between Clp A and Clp P in activation of Clp protease from Escherichia coli. Biochem. Soc. Trans. 19, 719–723. doi: 10.1042/bst0190719
Maurizi, M. R., Clark, W. P., Kim, S. H., and Gottesman, S. (1990). Clp P represents a unique family of serine proteases. J. Biol. Chem. 265, 12546–12552.
Michalska, K., Gucinski, G. C., Garza-Sánchez, F., Johnson, P. M., Stols, L. M., Eschenfeldt, W. H., et al. (2017). Structure of a novel antibacterial toxin that exploits elongation factor Tu to cleave specific transfer RNAs. Nucleic Acids Res. 45, 10306–10320. doi: 10.1093/nar/gkx700
Miller, J. M., Lin, J., Li, T., and Lucius, A. L. (2013). E. coli ClpA catalyzed polypeptide translocation is allosterically controlled by the protease ClpP. J. Mol. Biol. 425, 2795–2812. doi: 10.1016/j.jmb.2013.04.019
Moreno, J. C., Tiller, N., Diez, M., Karcher, D., Tillich, M., Schöttler, M. A., et al. (2017). Generation and characterization of a collection of knock-down lines for the chloroplast Clp protease complex in tobacco. J. Exp. Bot. 68, 2199–2218. doi: 10.1093/jxb/erx066
Nishimura, K., and Van Wijk, K. J. (2015). Organization, function and substrates of the essential Clp protease system in plastids. Biochim. Biophys. Acta 1847, 915–930. doi: 10.1016/j.bbabio.2014.11.012
Olivares, A. O., Baker, T. A., and Sauer, R. T. (2015). Mechanistic insights into bacterial AAA+ proteases and protein-remodelling machines. Nat. Rev. Microbiol. 14, 33–44. doi: 10.1038/nrmicro.2015.4
Orfanoudaki, G., and Economou, A. (2014). Proteome-wide subcellular topologies of E. coli polypeptides database (STEPdb). Mol. Cell. Proteomics 13, 3674–3687. doi: 10.1074/mcp.O114.041137
Quentin, D., Ahmad, S., Shanthamoorthy, P., Mougous, J. D., Whitney, J. C., and Raunser, S. (2018). Mechanism of loading and translocation of type VI secretion system effector Tse6. Nat. Microbiol. 3, 1142–1152. doi: 10.1038/s41564-018-0238-z
R Core Team, (2018). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Russell, A. B., Peterson, S. B., and Mougous, J. D. (2014). Type VI secretion system effectors: poisons with a purpose. Nat. Rev. Microbiol. 12, 137–148. doi: 10.1038/nrmicro3185
Souza, D. P., Oka, G. U., Alvarez-Martinez, C. E., Bisson-Filho, A. W., Dunger, G., Hobeika, L., et al. (2015). Bacterial killing via a type IV secretion system. Nat. Commun. 6:6453. doi: 10.1038/ncomms7453
Sriramoju, M. K., Chen, Y., Lee, Y.-T. C., and Hsu, S.-T. D. (2018). Topologically knotted deubiquitinases exhibit unprecedented mechanostability to withstand the proteolysis by an AAA+ protease. Sci. Rep. 8:7076. doi: 10.1038/s41598-018-25470-0
Studier, F. W., and Moffatt, B. A. (1986). Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189, 113–130. doi: 10.1016/0022-2836(86)90385-2
Vergunst, A. C., Schrammeijer, B., Den Dulk-Ras, A., De Vlaam, C. M. T., Regensburg-Tuïnk, T. J. G., and Hooykaas, P. J. (2000). VirB/D4-dependent protein translocation from Agrobacterium into plant cells. Science 290, 979–982. doi: 10.1126/science.290.5493.979
Wang, J., Hartling, J. A., and Flanagan, J. M. (1997). The structure of ClpP at 2.3 Å resolution suggests a model for ATP-dependent proteolysis. Cell 91, 447–456. doi: 10.1016/s0092-8674(00)80431-6
Weber-Ban, E. U., Reid, B. G., Miranker, A. D., and Horwich, A. L. (1999). Global unfolding of a substrate protein by the Hsp100 chaperone ClpA. Nature 401, 90–93. doi: 10.1038/43481
Whitney, J. C., Quentin, D., Sawai, S., Leroux, M., Harding, B. N., Ledvina, H. E., et al. (2015). An interbacterial NAD(P)+ glycohydrolase toxin requires elongation factor Tu for delivery to target cells. Cell 163, 607–619. doi: 10.1016/j.cell.2015.09.027
Keywords: type VI secretion system, antibacterial activity, recipient cells, ClpP, ClpA, Agrobacterium tumefaciens, Escherichia coli
Citation: Lin H-H, Yu M, Sriramoju MK, Hsu S-TD, Liu C-T and Lai E-M (2020) A High-Throughput Interbacterial Competition Screen Identifies ClpAP in Enhancing Recipient Susceptibility to Type VI Secretion System-Mediated Attack by Agrobacterium tumefaciens. Front. Microbiol. 10:3077. doi: 10.3389/fmicb.2019.03077
Received: 07 October 2019; Accepted: 19 December 2019;
Published: 05 February 2020.
Edited by:
Ignacio Arechaga, University of Cantabria, SpainReviewed by:
Weili Liang, National Institute for Communicable Disease Control and Prevention (China CDC), ChinaMeriyem Aktas, Ruhr University Bochum, Germany
Copyright © 2020 Lin, Yu, Sriramoju, Hsu, Liu and Lai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chi-Te Liu, Y2hpdGVsaXVAbnR1LmVkdS50dw==; Erh-Min Lai, ZW1sYWlAZ2F0ZS5zaW5pY2EuZWR1LnR3
 Hsiao-Han Lin
Hsiao-Han Lin Manda Yu
Manda Yu Manoj Kumar Sriramoju
Manoj Kumar Sriramoju Shang-Te Danny Hsu
Shang-Te Danny Hsu Chi-Te Liu
Chi-Te Liu Erh-Min Lai
Erh-Min Lai