
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 22 October 2019
Sec. Food Microbiology
Volume 10 - 2019 | https://doi.org/10.3389/fmicb.2019.02332
 Mariana B. Soares1
Mariana B. Soares1 Valfredo A. Santos-Junior2
Valfredo A. Santos-Junior2 E. R. Tavares Filho2
E. R. Tavares Filho2 Pablo C. B. Lollo2
Pablo C. B. Lollo2 Priscila N. Morato2
Priscila N. Morato2 Jaime Amaya-Farfan2
Jaime Amaya-Farfan2 Eliene P. R. Pereira1,2
Eliene P. R. Pereira1,2 Celso F. Balthazar1,3
Celso F. Balthazar1,3 Adriano G. Cruz3,4
Adriano G. Cruz3,4 Rafael C. R. Martinez1
Rafael C. R. Martinez1 Anderson S. Sant’Ana1*
Anderson S. Sant’Ana1*Dairy product consumption is a common habit in Brazil. These products present a good matrix for probiotic incorporation. Thus, in this study the feasibility of producing a probiotic “requeijão cremoso” incorporated with Bacillus coagulans GBI-30 6086 in three different steps and its metabolic effect in an animal model for 2 weeks has been evaluated. Wistar adult health rats were randomized into one to five groups (n = 8 for each group): Control (C); “requeijão cremoso” without probiotic (RC); probiotic inoculated in the milk before pasteurization at 65°C/30 min (RPP); “requeijão cremoso” inoculated before the fusion step and consequently exposed to 90°C/5 min (RPF); and “requeijão cremoso” inoculated after fusion step, i.e., once the product temperature reached 50°C (RPAF). At the end of treatment, analysis of molecular markers of proteins of stress and antioxidant system, HSP 25, 60, 70 and 90, SOD and catalase were performed in the animals’ muscles by Western Blot technique. The HSP25, HSP90 and catalase levels of C, RPP, RPF, and RPAF were similar, indicating that the homeostasis remained unchanged. The incorporation of B. coagulans GBI-30 6086 in the “requeijão cremoso” was shown to be stable and the microorganism remained viable in all steps tested. The incorporation of the probiotic strain in the fusion stage facilitated the technological process, since it allowed a better homogenization of the product and did not affect the maintenance of the metabolic homeostasis of rats.
Probiotics are well-defined “as living microorganisms which, when administered in adequate amounts, can confer a health benefit to the host” (Hill et al., 2014). Food components are known to interact with probiotic bacteria strains and assist in their colonization, bringing leading beneficial health effects (Desfossés-Foucault et al., 2012; Ranadheera et al., 2012; Jessie-Lau and Chye, 2018; Nambiar et al., 2018; Pérez Montoro et al., 2018; Rabah et al., 2018; da Costa et al., 2019; Majeed et al., 2019). Since food composition is essential for probiotic survival and functionality, it is important to assess the potential health effects of these microorganisms in specific food matrices (Ranadheera et al., 2010). Dairy product consumption is a common habit in Brazil and these foods are considered a good matrix to probiotics (Lollo et al., 2015; Martins et al., 2015, 2018; Dittmann et al., 2017). Therefore, the expansion in the number and diversity of dairy probiotic foods represents an interesting approach to enhance the consumer’s exposure to a variety of probiotics in the diet, consequently aiming to improve host health.
“Requeijão cremoso” comprises a kind of processed cheese broadly manufactured and consumed in Brazil under different formulas and a variety of technologies. The fusion step is key during the production of “requeijão cremoso” as it is aimed to obtain a consistent cheese blend (Cunha et al., 2010; da Cunha et al., 2012). Thus, the use of high temperatures (between 85 and 95°C for up to 5 min) to attain a homogenous cheese emulsion may negatively impact the production of a probiotic “requeijão cremoso” when traditional probiotic strains of Lactobacillus and Bifidobacterium are employed (Oliveira et al., 2016). While probiotic strains of Bifidobacterium and Lactobacillus comprise the most studied and used probiotic microorganisms in processed food products worldwide, especially in dairy foods (Esmerino et al., 2015; Dantas et al., 2016; Felicio et al., 2016; Pereira et al., 2016), these microorganisms are susceptible to high temperatures and would possibly not survive under such processing conditions (Tripathi and Giri, 2014; Oliveira et al., 2018a). An alternative, to overcome this issue and allow the incorporation of probiotics in products such as the “requeijão cremoso,” is the use of probiotic strains of Bacillus (as spores), that can survive severe processing conditions as well as tolerate and resist against the harsh conditions found in the gastrointestinal tract (Cutting, 2011; Soares et al., 2019a, b). The use of probiotic strains of Bacillus can be advantageous due to the long stability of their spores, which will ensure high viability in food products throughout shelf-life (Tripathi and Giri, 2014). An additional advantage of the use of probiotic strains of Bacillus is the possibility of using lower effective doses since the spores present higher survival ability (Blanch et al., 2014).
In fact, probiotic Bacillus strains have been commonly employed in human medicine for the prevention of digestive problems and treatment of urinary tract infections (Nithya and Halami, 2013). Although the use of probiotic strains of Bacillus in food products is still recent (Lee et al., 2017; Jeon et al., 2018; Marcial-Coba et al., 2019; Soares et al., 2019a, b), research has described the health benefits associated with their ingestion (Nyangale et al., 2015; Jäger et al., 2016, 2018; Keller et al., 2017). For instance, probiotic strains of Bacillus were shown to stimulate the immune system (Huang et al., 2013; Sassone-Corsi et al., 2016). Other beneficial effects of probiotic Bacillus strains comprise the enhancements in carbohydrates and protein absorption (Jäger et al., 2018; Keller et al., 2018), microbiota modulation in elderly (Nyangale et al., 2014, Nyangale et al., 2015), enhancement in the recovery from exercises and decrease in injuries of muscle tissues (Jäger et al., 2016), abdominal discomfort and swelling decrease (Hun, 2009, Kalman et al., 2009), inhibitory properties against pathogens (Fitzpatrick et al., 2011; Honda et al., 2011), anti-obesity effects (Choi et al., 2016; Wang et al., 2019), improvement in digestive health (Rhayat et al., 2019) and anti-diarrhea effects (Urdaci et al., 2018).
The regulation of host immunological response is one of the primary ways probiotic exert effects on human beings (Cunningham-Rundles et al., 2000; Galdeano and Perdigón, 2006; Yan and Polk, 2011). The main functions of the intestinal immune system include the immune-inflammatory response suitable to suppress the action of pathogenic microorganisms or promote resistance to various compounds (de Almada et al., 2015). In reality, probiotics can activate the immune response by contact with several cells present in the intestinal mucosa, including monocytes, macrophages, B, T, NK and dendritic cells (Coppola and Gil-Turnes, 2004; Cano et al., 2013; Lollo et al., 2013b). Moreover, probiotics can induce the expression of cellular heat shock proteins (HSP) that provide higher resistance and tolerance toward several aggressive factors. This system of defense-antioxidant proteins play an important role in protecting and repairing damaged cellular proteins (Petrof et al., 2004). Actually, these substances are activated during episodes of increase in body temperature, lack of control of reactive oxygen species (ROS), ischemia, hypoxia and glucose deficiency, among others (Morimoto, 1998; Belter et al., 2004; Jang et al., 2008; Staib et al., 2009; Lollo et al., 2013b). Under these conditions, structure, integrity and functionality of cells are kept by HSPs (Silver et al., 2012). Thus, probiotics can help maintain the homeostasis of the body against different types of stress conditions.
Therefore, the aim of this study was to assess the feasibility of production of a probiotic “requeijão cremoso” added of Bacillus coagulans GBI-30 6086 and to determine the metabolic effect of its acute consumption using an experimental animal model. In this sense, the effect of the consumption of the probiotic “requeijão cremoso” was evaluated using rats through the determination of standard biochemical parameters and molecular markers of stress.
Bacillus coagulans GBI-30 6086 (Ganeden Biotech, Mayfield Heights, United States) is probiotic strain with GRAS (generally recognized as safe) status commercially available in spores’ form. B. coagulans GBI-30 6086 transiently colonizes the intestine without the need for frequent consumption (Maathuis et al., 2010; Orrù et al., 2014; Salvetti et al., 2016). Freeze-dried spores of B. coagulans GBI-30 6086 were added to samples in order to achieve 108–109 spores per portion of the food product (30 g), i.e., approximately 107–108 spores/g.
The “requeijão cremoso” used in the experiments presented the following composition: 40% (w/w) of fresh cheese mass, 37% (w/w) of milk cream with 35% of fat (Atilate, Itatiba, Brazil), 20% (v/w) distilled water, 1.5% (w/w) of emulsifying salt Joha S2 (ICL Food Specialties, São Bernardo do Campo, Brazil) and 1.5% (w/w) of NaCl (Dinâmica, São Paulo, Brazil). The food product was prepared as previously described by Oliveira et al. (2018b). Samples of the “requeijão cremoso” were kept at 6°C throughout the in vivo test period.
The enumeration of B. coagulans GBI-30 6086 spores in “requeijão cremoso” samples was carried out at the beginning and the end of the study. For this, samples of “requeijão cremoso” were heat shocked at 80°C/10 min. Subsequently, samples were diluted (1:10) in 1% peptone water, following serial decimal dilutions. Then, aliquots of 1 mL were pour plated into Petri dishes containing formulated Agar Glucose Yeast Extract – GYE [BC – Yeast Extract (Oxoid, Basingstoke, United Kingdom) (5 g/L); D-glucose (5 g/L); Peptone (Acumedia, Lansing, United States) (5 g/L); Potassium Phosphate Monobasic (0.5 g/L); Dibasic Potassium Phosphate (0.5 g/L); Magnesium Sulfate (0.3 g/L); Bacteriological Agar (Inlab, São Luis, Brazil) (15 g/L); Sodium Chloride (10 mg/mL); Zinc Sulfate (1.6 mg/mL); and Mineral Solution (1 ml/L – Manganese Sulfate (16 mg/mL); Cobalt Sulfate 7⋅H2O (1.6 mg/ml); Copper Sulfate 5⋅H2O (1.6 mg/mL); Iron Sulfate 7⋅H2O (18 mg/mL)]. All components added to GYE agar were from Dinâmica, São Paulo, Brazil, unless otherwise stated. Plates were incubated at 40°C/48 h as recommended by the supplier of B. coagulans GBI-30 6086.
For the in vivo experimental model, forty male Rattus novergicus, Wistar lineage (free of pathogens, 21 days old) acquired from the Multidisciplinary Center for Biological Research of the University of Campinas (SP, Brazil) were used. Rats were maintained (±22°C, 45–60% RH, 12 h of inverted light cycle) in separate growth enclosures. Animals were provided with feedstuff (Labina, Purina, Brazil) and water ad libitum up until they reach adulthood (about 8 weeks of life and ∼300 g of weight). This weight guaranteed an adequate volume of gavage without causing unnecessary stress to animals. The performance of the experiments was approved by the Ethical Committee in the Use of Animals of the University of Campinas (CEUA/UNICAMP) – Protocol # 3444-1.
The experimental design was used to evaluate the effects of a 2-week consumption of the “requeijão cremoso” with B. coagulans GBI-30 6086 in health adult rats (Figure 1). Animals were randomly allocated into five groups (n = 8 per group): Control (C); “requeijão cremoso” without probiotic (RC); B. coagulans GBI-30 6086 inoculated in the milk before pasteurization at 65°C/30 min (RPP); “requeijão cremoso” inoculated with B. coagulans GBI-30 6086 before the fusion step and consequently exposed to 90°C/5 min (RPF); and “requeijão cremoso” inoculated with B. coagulans GBI-30 6086 after fusion step, i.e., once the product temperature reached 50°C (RAPF).
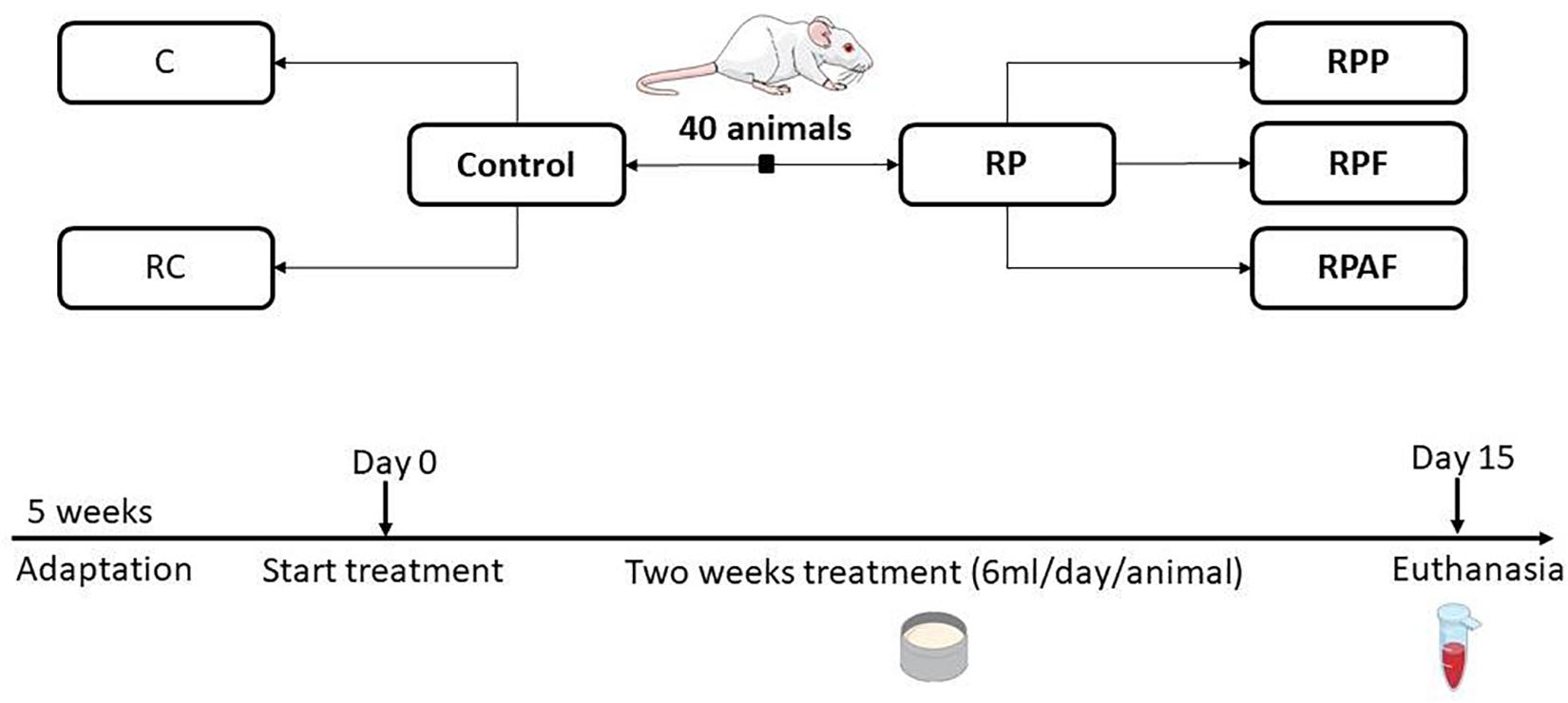
Figure 1. Animals groups and experimental design used in the study. Animals were randomly assigned according to the following groups: Control (C, n = 8); Control “requeijão cremoso” cheese (RC, n = 8); Probiotic “requeijão cremoso” containing spores of B. coagulans GBI-30 6086 inoculated in the milk pasteurization step (RPP, n = 8); Probiotic “requeijão cremoso” containing spores of B. coagulans GBI-30 6086 inoculated during fusion stage (RPF, n = 8); Probiotic “requeijão cremoso” containing spores of B. coagulans GBI-30 6086 inoculated after fusion stage (RPAF, n = 8). Animals were maintained under adaptation until reaching the adult stage (5 weeks) and then treated with control or probiotic product for 2 weeks. Euthanasia took place on the 15th day after 8 h of fasting.
Commercial feed (Labina, Purina, Brazil) was the basis for feeding for all groups throughout the trial (Table 1). Water and chow were offered ad libitum. Treatment intervention and control were administered to animals through an orogastric tube during 2 weeks, as follows: animals were fed daily with 6 mL of the corresponding treatment, divided in two doses of 3 mL per day. After treatment, animals were sacrificed and blood, tissues and organs samples were taken for the performance of biochemical, hematological and molecular analyses.
For the evaluation of the centesimal composition of commercial and experimental diets, values of ash content, moisture, and crude protein (Kjeldahl) were determined according to AOAC methodology (AOAC, 2007) and the number of lipids was obtained by the Bligh and Dyer method (Bligh and Dyer, 1959); carbohydrates, in turn, were indirectly estimated by difference.
Rats were weighed twice a week, and food consumption control was performed by means of the difference between the amount of feed offered and not consumed by the animals.
After animals’ sacrifice, blood samples were taken using BD-Vacutainers (Becton Dickinson, Franklin Lakes, United States). The biological material was maintained at 4°C and centrifuged (Sigma, Germany) at 3,000 g (4°C, 12 min) to attain serum and assess the rates of glucose, total cholesterol, uric acid, high density lipoprotein (HDL), triacylglycerols, total protein, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and creatinine kinase (CK). The biochemical parameters evaluated were determined in duplicate. All spectrophotometric analysis were carried out using Laborclin tools (São Paulo, Brazil) and a Biotech Época microplate reader (BioTek, Winooski, United States).
Blood samples were collected with Vacutainer tubes (4 mL) added of EDTA and K3, and subjected to hematological analysis using an automatic cell counter (Ac. T5diff Hematology Analyzer, Beckman Coulter, High Wycombe, United Kingdom). The intra-test factor of variation for entirely determined parameters was less than 3%.
For the molecular analysis of anti-stress and antioxidant proteins system, right and left sore muscles were collected. Analysis of the molecular markers were performed using the Western blot technique, and protein quantification was done according to the Lowry method (Lowry et al., 1951). Briefly, 100 mg of frozen soleus muscle were homogenized in Triton buffer (100 mM Tris, pH 7.4, 1% Triton X-100) added of NaF (100 mM), sodium pyrophosphate (100 mM), Na3VO4 (10 mM), EDTA (10 mM), aprotinin (0.1 mg/mL) and PMSF (2 mM) as detailed in Lollo et al. (2013b). For the immunoblotting step, SDS-PAGE method was performed using soleus muscle tissues homogenates. Subsequently, the Western Blot Biocom Western system was used to transfer proteins bands to a nitrocellulose membrane (Bridge of Weir, United Kingdom), which were incubated with suitable primary and secondary antibodies for the determination of each target protein and parameter of interest, to know: HSP25 (Enzo Life Sciences, #ADI-SPA-801), HSP60 (Enzo Life Sciences, #ADI-SPA-806), HSP70 (Enzo Life Sciences, #ADI-SPA-810), HSP90 (Enzo Life Sciences, #ADI-SPA-831), SOD (Abcam, #AB51254), and Catalase (Abcam, #AB1877). Bands obtained were observed by chemiluminescence [UVITEC Cambridge (Model Alliance LD2)], and band intensity (semi-quantitative or quantitative evaluation) was determined with the ImageJ software (v. 1.44 for Windows).
The Kolmogorov–Smirnov test was used to check if data obtained presented a normal distribution. Body weight, hematological and biochemical parameters were analyzed by ANOVA, followed by Tukey’s test. For checking significant differences regarding proteins of defense and antioxidant systems (Western Blot technique), the Ducan post hoc test was applied. For all tests performed, the significance level was set at 5% (p < 0.05). GraphPad Prism 7.0 software was used to carry out all statistical analyses (GraphPad Software Inc., San Diego, United States).
This study aimed to evaluate the production of a probiotic “requeijão cremoso” incorporated with B. coagulans GBI-30 6086 and to assess the metabolic effect of its consumption in an animal model. The main findings of this research show that the step of incorporation of B. coagulans GBI-30 6086 into the “requeijão cremoso” processed cheese did not affect the maintenance of the metabolic homeostasis of rats. The final product showed stable probiotic counts and better homogenization regardless of the probiotic incorporation step.
As seen in Table 2, “requeijão cremoso” with and without probiotic presented similar nutritional composition. Cheeses are well-known as efficient matrices for carrying probiotic bacteria because high levels of lipids and the proteins protect strains throughout the gut (Cruz et al., 2009; Lollo et al., 2015; Martins et al., 2018).
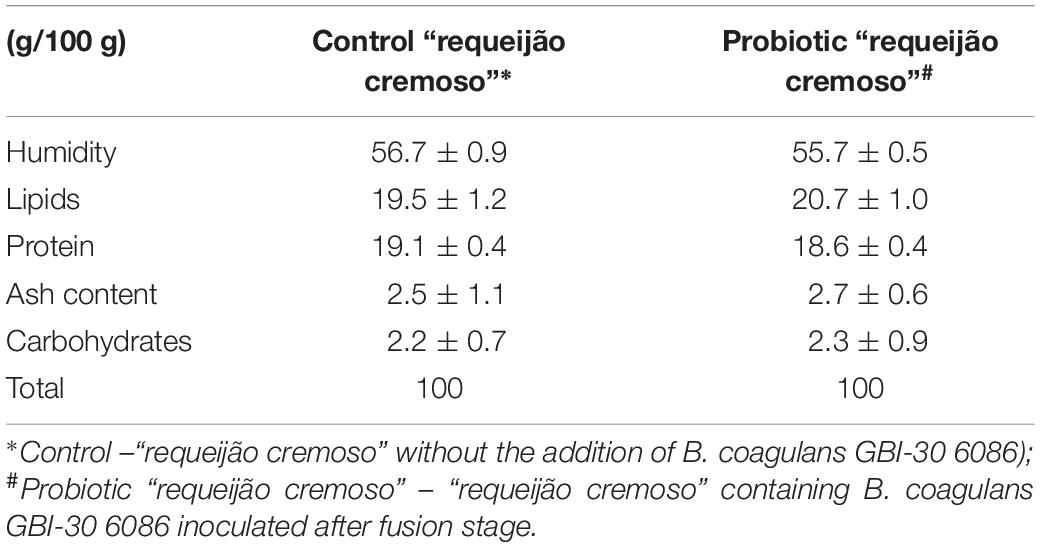
Table 2. Average values (±standard deviation) of different groups of substances evaluated for centesimal composition determined in commercial and experimental diets offered to animals monitored in the study.
Initial counts of B. coagulans GBI-30 6086 observed in the “requeijão cremoso” on the first day of the experiment were 7.8 × 105; 5.5 × 107 and 1.4 × 107 spores/g for RPP, RPF and RPAF, respectively. After 2 weeks, the counts of B. coagulans GBI-30 6086 determined in the food product were, respectively, 2.0 × 104, 2.8 × 107 and 1.2 × 107 spores/g for RPP, RPF and RPAF. The high resistance of spores is demonstrated by the fact that the decrease in their counts was less or equal to 1 log. This result shows that the microorganism remained viable (stable spores’ populations) during the entire experimental period. All animals presented body weight gain throughout the 2 week period of tests (Figure 2). Animals fed with “requeijão cremoso” (RC, RP, RF, and RPF) consumed less amounts of commercial feed compared to control group (C). This fact may have occurred due to a greater satiety of the animals that received the “requeijão cremoso” since it has high viscosity and could have led to an extended period for gastric emptying (Mourão and Bressan, 2009). Also, the “requeijão cremoso” is rich in proteins and lipids, approximately 40% in total, which may also have contributed to the satiety of the animals (Pedrosa et al., 2009). Therefore, differences observed in the amount of food intake cannot be attributed to nutritional deficiency.
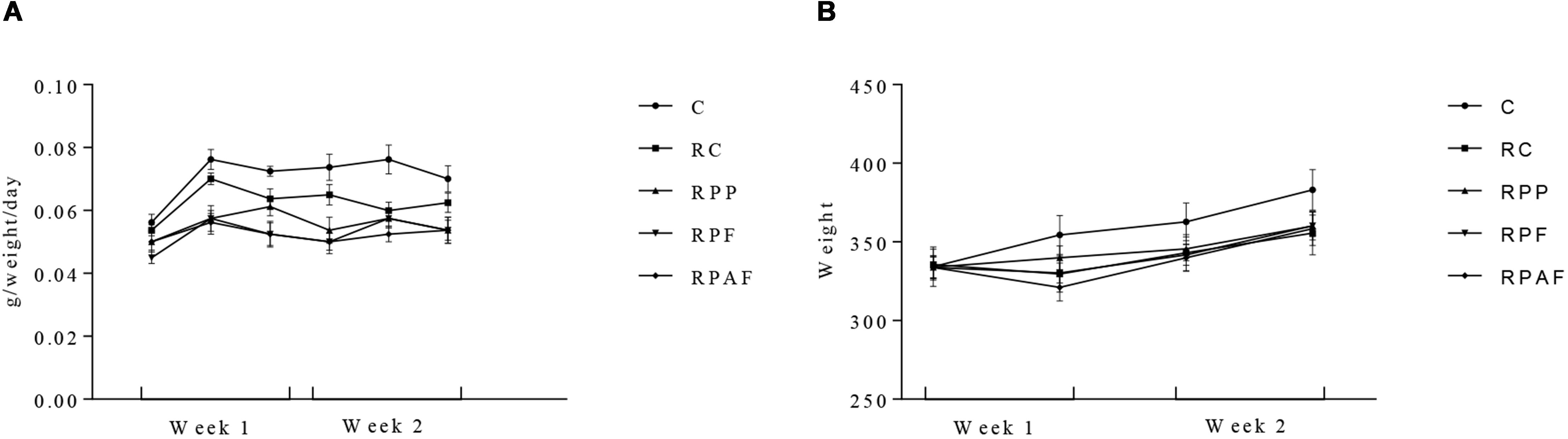
Figure 2. Body weight gain (A) and commercial diet intake (B) in 40 male Wistar rats, randomly assigned to five experimental groups over 2 weeks. Control (C); Control “requeijão cremoso” cheese (RC); Probiotic “requeijão cremoso” containing spores of B. coagulans GBI-30 6086 inoculated in milk pasteurization step (RPP); Probiotic “requeijão cremoso” containing spores of B. coagulans GBI-30 6086 inoculated during fusion stage (RPF); Probiotic “requeijão cremoso” containing spores of B. coagulans GBI-30 6086 inoculated after fusion stage (RPAF).
Figure 3 shows the results obtained for the quantification of different anti-stress and antioxidant proteins in the five groups of animals studied. Levels of stress proteins determined were kept stable in all animal groups tested, indicating a balance in the homeostasis of the cellular metabolism (Figure 3).
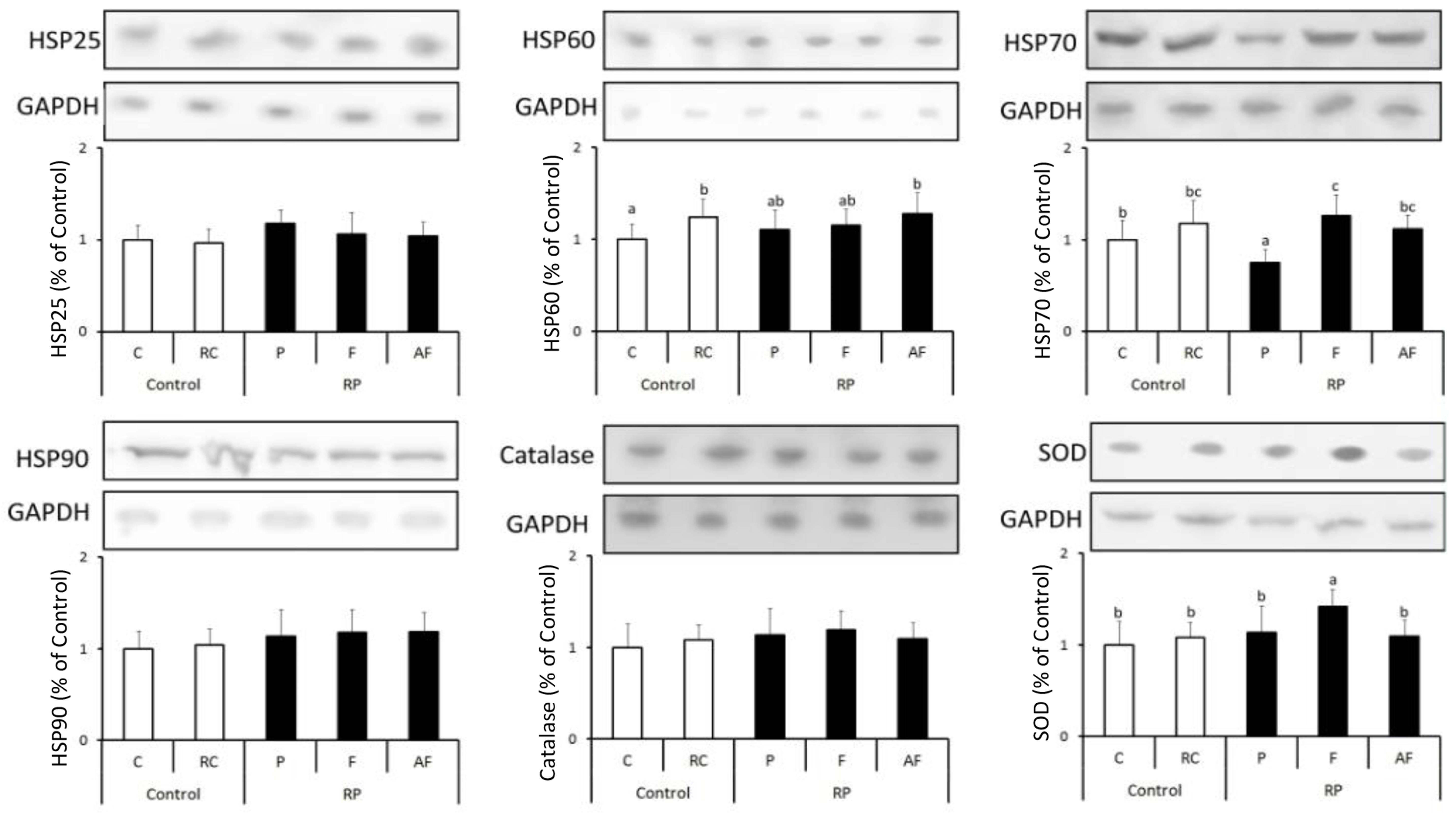
Figure 3. Means and standard deviations of the expression of HSP25, HSP60, HSP70, HSP90, Catalase and SOD determined using the Western blot technique. Control (C); Control “requeijão cremoso” (RC); Probiotic “requeijão cremoso” containing spores of B. coagulans GBI-30 6086 inoculated in milk pasteurization step (RPP); Probiotic “requeijão cremoso” containing spores of B. coagulans GBI-30 6086 inoculated during fusion stage (RPF); Probiotic “requeijão cremoso” containing spores of B. coagulans GBI-30 6086 inoculated after fusion stage (RPAF). Different letters represent a significant difference (p < 0.05) between the groups.
Overall, levels of HSP70 were shown to be similar between groups C and those treated with the probiotic strain. The only exception was related to animals belonging to the group that received the “requeijão cremoso” with B. coagulans GBI-30 6086 added before pasteurization (RPF). In this group, a significant increase (p < 0.05) on HSP70 expression was observed in comparison to C group. Bearing in mind that animals were healthy, a possible justification for these results would be the lower counts of viable spores found in the probiotic “requeijão cremoso” resulting in a lower expression of anti-stress proteins in the RPP group animals. HSP70 is particularly associated with the aid of the antioxidant system, protecting other proteins from possible damages and denaturation (Silver and Noble, 2012; de Moura et al., 2013). According to our results, HSP70 result did not have a concomitant effect on the expression of catalase enzyme. Nonetheless, higher levels of both SOD and HSP70 expression were observed in animals fed with “requeijão cremoso” containing B. coagulans GBI-30 6086 added in the fusion stage (RPF) in comparison to other groups of animals studied. These findings suggest that heat treatment and micro environmental conditions used in the fusion stage may have worked as determinant factors for a greater germination of the spores and, therefore, a higher expression of the enzyme that plays a key role against free radical compounds (Aguiló et al., 2005).
These observations are consistent with the results obtained by the analysis of immune system cells (Tables 3, 4), which counts remained within normal ranges, indicating health condition in animals studied. Finally, a significant increase (p < 0.05) in HSP60 expression was observed by consumption of the “requeijão cremoso” in comparison to C group, although no major differences had been observed with the incorporation of B. coagulans GBI-30 6086.
The quantification of HSPs has a predictive value of animal’s health status since it indicates the homeostatic state of the organism. In fact, for animal health-model studies, the evaluation of HSPs levels can be used to correlate optimal values and beneficial probiotic effects, and also for the determination of intracellular homeostasis parameters.
The ingestion of probiotics, particularly those belonging to genera Bifidobacterium and Lactobacillus can modulate the levels of oxidation of the organism (Jensen et al., 2010). This modulation occurs due to the fact these microorganisms can induce antioxidant reactions, inhibiting the formation of reactive oxygen metabolites in the gut (Amaretti et al., 2013). Hereof, according to an in vitro study published by Jensen et al. (2010), B. coagulans GBI-30 6086 was able to modulate the release of anti-inflammatory by intestinal epithelial cells, highlighting the potential of this strain to exert beneficial effects on human health (Jensen et al., 2010).
Considering that HSPs are stress-indicating proteins, the protective role of probiotics can be mediated, in part, by facilitation of HSP expression under stressful conditions. For instance, if a host is exposed to a certain situation that can disrupt its homeostasis but its GIT is previously colonized by probiotics these beneficial microorganisms can stimulate the production of HSPs, which interact with the immune system leading to the production of defense cells and controlling the inflammatory process (Coppola and Gil-Turnes, 2004; Taverniti and Guglielmetti, 2011; Liu et al., 2014).
It is known that the expression of HSPs is a partial stress-conditioned phenomena (Santos-Junior et al., 2018). Thus, in the present study, since animals tested were only transiently colonized by Bacillus spp. due to a short period of its consumption and also not stressed, this phenomenon could had been attenuated resulting in minor differences observed between treatments (Elshaghabee et al., 2017).
Figure 4 shows no significant alteration (p > 0.05) in lipid profile and glucose levels amongst the groups tested. These findings indicate that the “requeijão cremoso” added of B. coagulans GBI-30 6086 did not induce alterations in the energy metabolism.
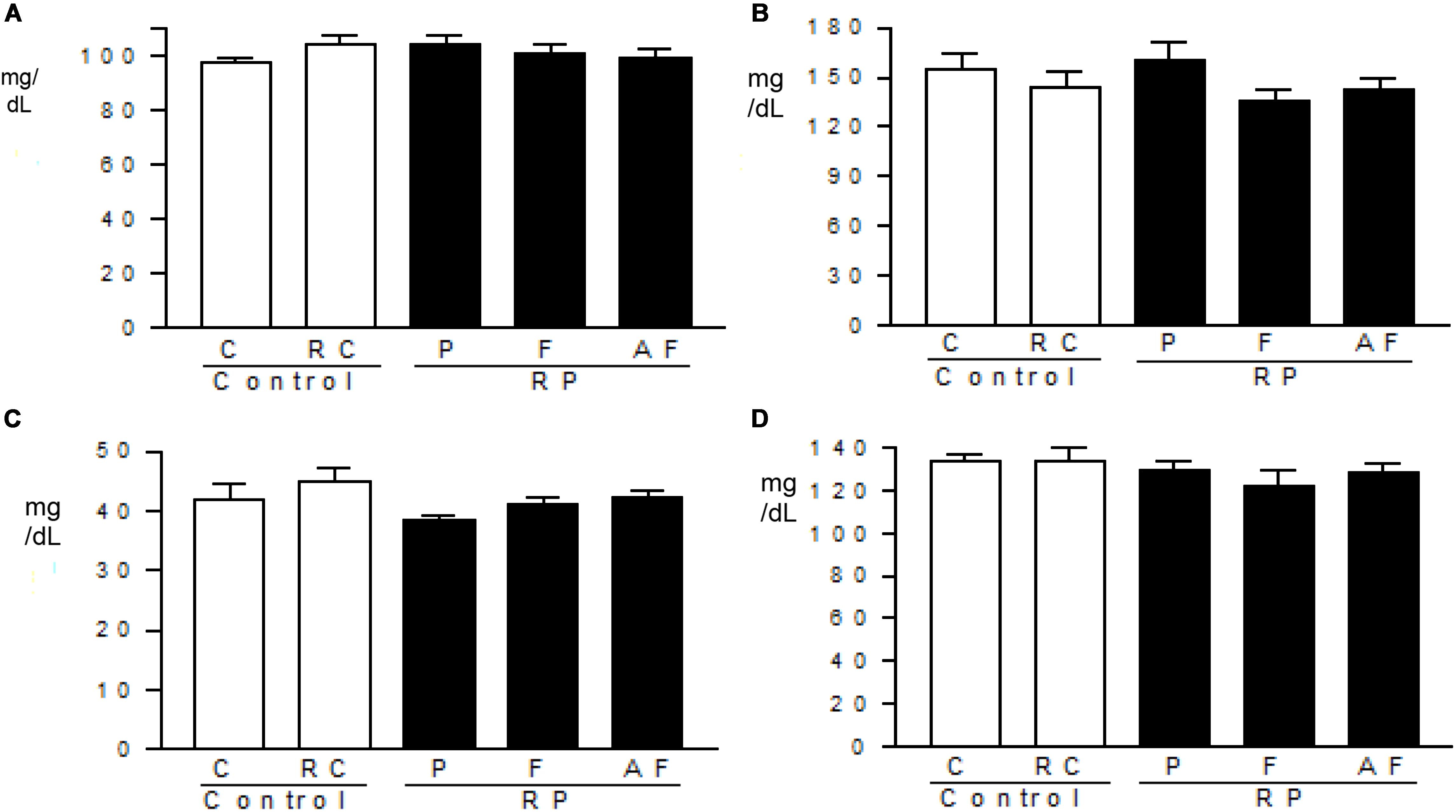
Figure 4. Metabolic profile. Mean values and standard deviations of total cholesterol (A), triglycerides (B), HDL-cholesterol (C) and glucose (D) determined in blood samples. Control (C); Control “requeijão cremoso” (RC); Probiotic “requeijão cremoso” containing spores of B. coagulans GBI-30 6086 inoculated in milk pasteurization step (RPP); Probiotic “requeijão cremoso” containing spores of B. coagulans GBI-30 6086 inoculated during fusion stage (RPF); Probiotic “requeijão cremoso” containing spores of B. coagulans GBI-30 6086 inoculated after fusion stage (RPAF).
The average values determined for total cholesterol (98.9–110.2 mg/dL), HDL-cholesterol (11.4–20.4 mg/dL), triglycerides (110–174.8 mg/dL) and glycemia (150.7–207.5 mg/dL) in blood samples were within the limits of the reference ranges recommended by the Centro de Bioterismo – Faculty of Medicine of the University of São Paulo (FMUSP, 2008). The analysis of the lipid profile is important for the evaluation of the risk of developing chronic illnesses such as dyslipidemia and cardiovascular diseases. Total cholesterol, triglycerides, and HDL are biochemical markers easily altered by diet. Deregulation of these markers can lead to metabolic disorders in the long run (Castro et al., 2004; Zambon et al., 2009).
Enzyme levels of the hepatic system and biochemical markers of the renal system were not altered by ingestion of the “requeijão cremoso” containing B. coagulans GBI-30 6086 (Figure 5). All treated groups showed similar values in comparison to C group. Levels obtained for hepatic enzymes were lower than the reference range used by FMUSP (2008) (114.0–290.8 U/L for ALT and 129.0–148.1 U/L for AST). Such differences may be related to the genetic variability of the animals, environmental influence or differences in the manipulation of the rats, as pointed out by other investigators (Santos et al., 2010; Lima et al., 2014). Levels of creatine and uric acid determined in the present study were similar to the reference values used by Lima et al. (2014), to know: 0.24–1.20 mg/dL for creatine and 1.00–3.20 mg/dL for uric acid, and no differences were observed between groups.
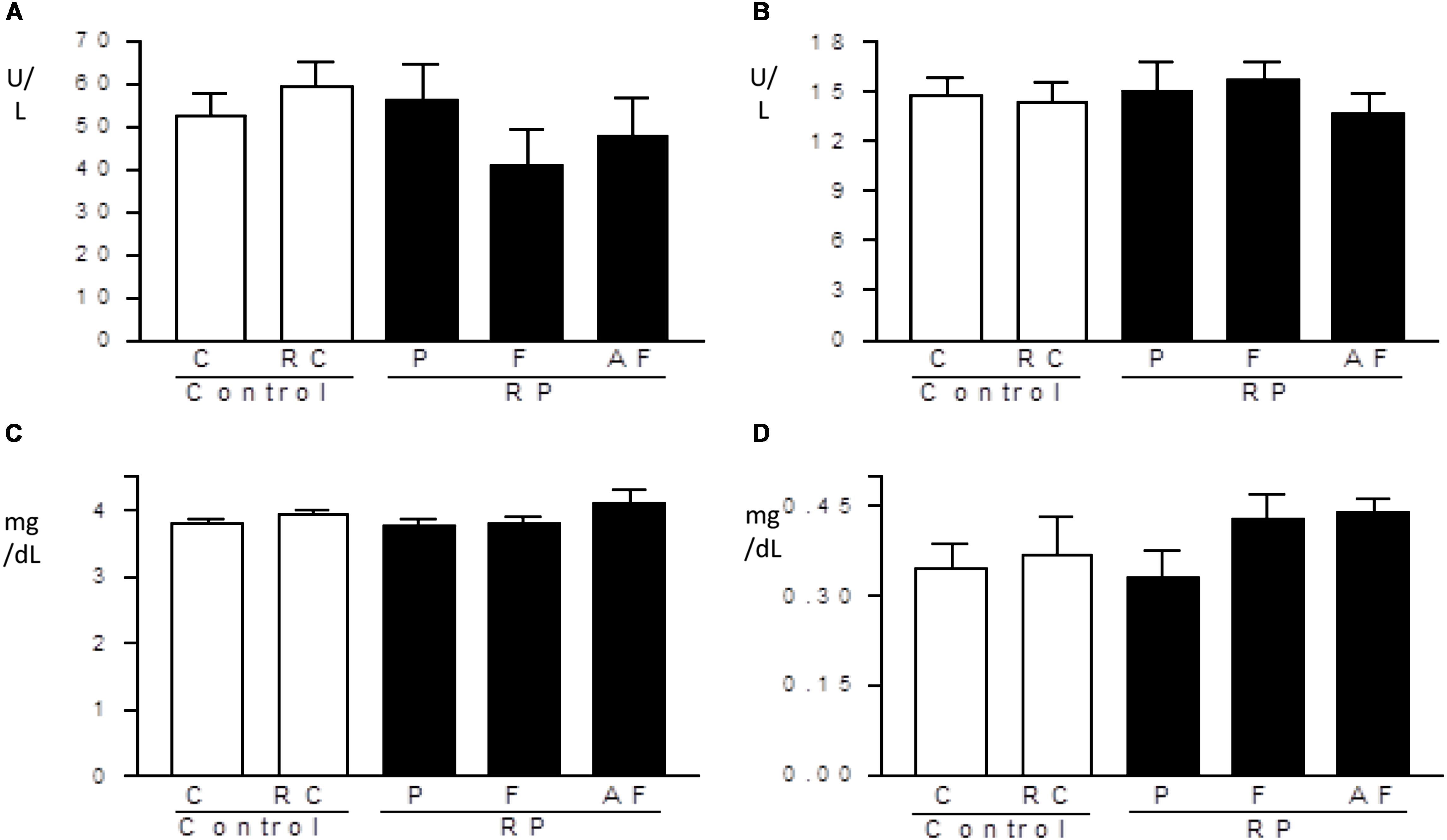
Figure 5. Hepatic and renal profiles. Mean values and standard deviations of AST: aspartate aminotransferase (A), ALT: alanine aminotransferase (B), uric acid (C) and creatinine-kinase (D) determined in blood samples. Control (C); Control “requeijão cremoso” (RC); Probiotic “requeijão cremoso” containing spores of B. coagulans GBI-30 6086 inoculated in milk pasteurization step (RPP); Probiotic “requeijão cremoso” containing spores of B. coagulans GBI-30 6086 inoculated during fusion stage (RPF); Probiotic “requeijão cremoso” containing spores of B. coagulans GBI-30 6086 inoculated after fusion stage (RPAF).
The results obtained for total protein and albumin (Figure 6), are within the respective ranges of reference values adopted by FMUSP laboratory (FMUSP, 2008) (5.5–10.4 g/dL and 2.8–6.1 g/DL, respectively). Hence, the studied groups did not differ (p > 0.05), demonstrating that the nutritional status of the animals was not altered due to the diet. Abnormal total protein values, in turn, indicate nutritional problems, renal or hepatic disease.
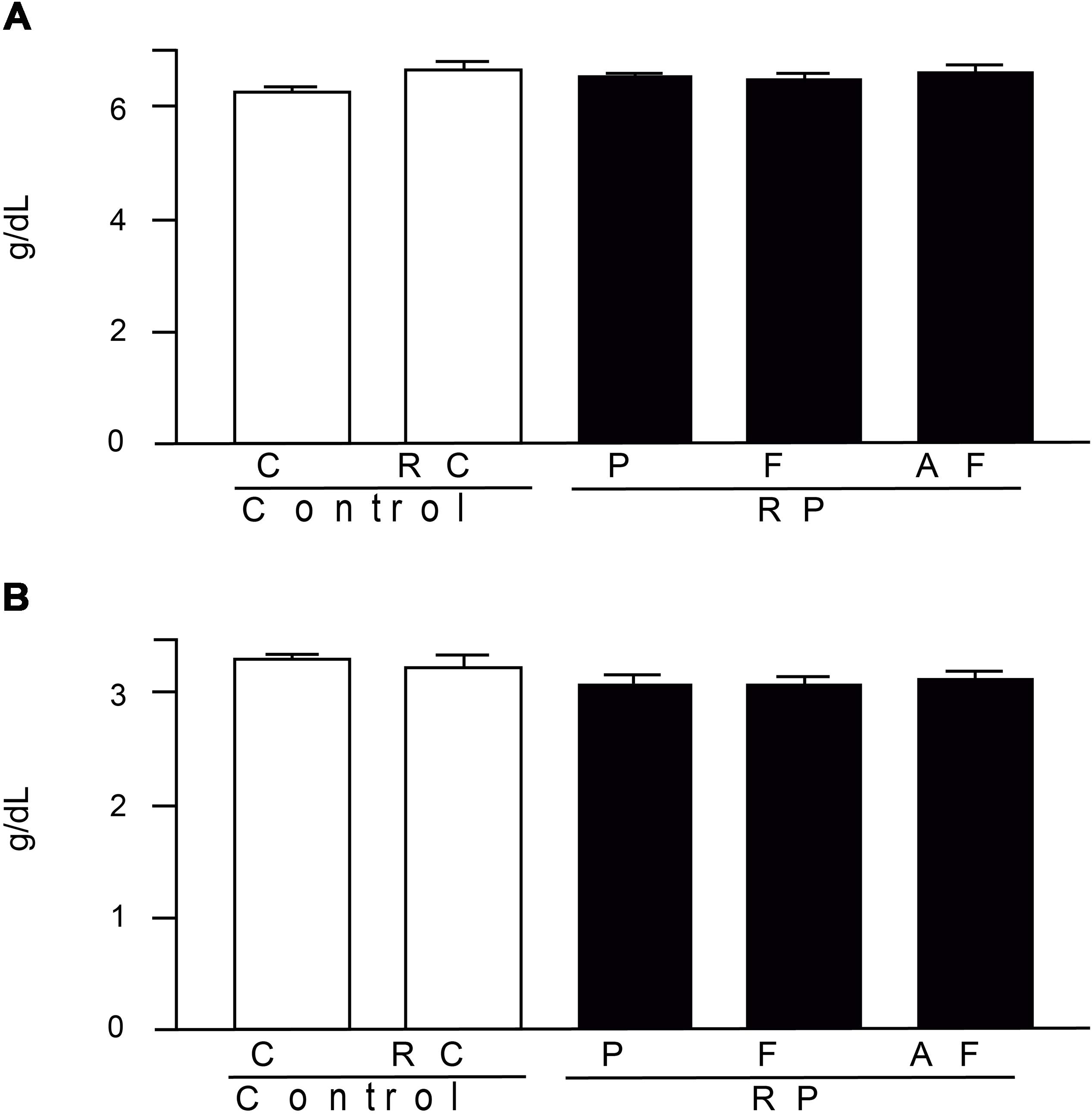
Figure 6. Mean values and standard deviations of total proteins (A) and blood albumin (B) determined in blood samples. Control (C); Control “requeijão cremoso” (RC); Probiotic “requeijão cremoso” containing spores of B. coagulans GBI-30 6086 inoculated in milk pasteurization step (RPP); Probiotic “requeijão cremoso” containing spores of B. coagulans GBI-30 6086 inoculated during fusion stage (RPF); Probiotic “requeijão cremoso” containing spores of B. coagulans GBI-30 6086 inoculated after fusion stage (RPAF).
Resemblance between reference values for animals and humans is due to their physiological similarity. For example, the ideal level for total proteins in humans is in the range of 6–8.3 g/dL, close to those observed in the rodents evaluated in our study (6.29–6.65 g/dL). The uric acid is naturally produced by the body through the breakdown of proteins (purines) mainly obtained from the diet and further eliminated by the kidneys. In humans, the ideal level of uric acid is in the range of 3.4–7 g/dL, a value similar to the ones found in the present study (3.74–4.29 g/dL).
The results of the hematological, metabolic and immune analyses were within health reference values and showed no difference between treatments (p > 0.05). Different results were observed by Lollo et al. (2013a, b). According to these authors, rats submitted to strenuous physical exercise presented impaired functions of the immune system. These functions, however, were re-established after ingestion of probiotic yogurt containing Bifidobacterium longum BL 05 and Lactobacillus acidophilus LA 14. Reference values for hematological parameters vary significantly according to race, lineage, age, sex and health status of the animal, as well as they may undergo changes related to blood manipulation and analysis. The probiotic “requeijão cremoso” should be tested under a highly stressful environment, to verify the similarity of probiotic effect reported by these authors.
Neutrophil is considered a quick and easy marker to quantify stress intensity and indicate inflammatory status (Zahorec, 2001). Increased numbers of neutrophils and lymphocytes in the bloodstream are indicative of inflammatory and infectious states (Lollo et al., 2013b). According to the evaluation of different markers of the defense system, the values observed in the leukogram of the animals studied highlight no major modifications in the levels of stress proteins, indicating that the dietary food matrix tested (“requeijão cremoso”) added of the probiotic strain did not impair either homeostasis or health status of the rats. Therefore, our hematological and biochemical data suggest B. coagulans GBI-30 6086 is safe and did not cause any harm, inflammation or signs suggestive of allergenicity in animals tested during the study.
The incorporation of B. coagulans GBI-30 6086 in the “requeijão cremoso” was successfully achieved and the probiotic strain remained viable in all steps tested. In reality, the incorporation of the spores in the fusion stage facilitated the technological process enabling a better homogenization of the product and could possibly avoid recontamination of the product after heat treatment. Probiotic counts determined in the final product were similar to those observed at different fusion steps throughout the 2-week period of animal experiments. This demonstrates the high resistance of B. coagulans GBI-30 6086 against high temperatures and indicates its great potential to be incorporated in food technological processes. Moreover, the incorporation of the probiotic strain in different steps of the “requeijão cremoso” did not affect the maintenance of the metabolic homeostasis of rats. Thus, new researches could explore the development of new products added of B. coagulans GBI-30 6086 and the evaluation of distinct HSPs and anti-oxidant enzymes. Further efforts can be made to determine the effects of the consumption of B. coagulans GBI-30 6086 in the long term as well as to enhance its functionality by testing other food matrices.
The datasets generated for this study are available on request to the corresponding author.
This study was carried out in accordance with the recommendations of Ethical Principles for Animal Experimentation – Brazilian Association of Laboratory Animal Science (SBCAL) and the federal regulation Law n. 11.794, October 08, 2008, and Decree n. 6.899, July 15, 2009. The protocol was approved by the Animal Research Ethics Committee (CEUA/UNICAMP, protocol no. 3444-1).
MS, AC, PL, and AS were responsible for the study design. MS reviewed the literature, performed both the experiments and data analysis, and prepared the draft of the manuscript. VS-J contributed on the analysis of biochemical, hematological and immunoblotting data, and writing of the manuscript. ET helped on data treatment and writing of the manuscript. PL, JA-F, and PM assisted on the development of the animal experimental protocol and methods used, and helped on data interpretation. JA-F revised the draft manuscript and made suggestions to enhance its readability and scientific content. EP and CB helped on the performance of the experiments, including formulation and preparation of “requeijão cremoso” containing the probiotic microorganism, including all pre-experimental tests needed to ensure a substantial study, and contributed on writing and review of the manuscript. RM co-supervised the work and assisted on preparation and review of the manuscript. AS supervised the work, obtained funds its completion, and contributed on writing and review of the manuscript. All authors critically reviewed the manuscript and approved the final version.
MS acknowledges the support from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for the financial support (Grant #13/21544-9). CB is grateful to FAPESP (Grant #18/24540-8). AS thanks to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ, Grants #302763/2014-7 and #305804/2017-0). This study was financed, in part, by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. AS acknowledges the support from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for the financial support (Grant #2019/21188-4).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Aguiló, A., Tauler, P., Fuentespina, E., Tur, J. A., Córdova, A., and Pons, A. (2005). Antioxidant response to oxidative stress induced by exhaustive exercise. Physiol. Behav. 84, 1–7. doi: 10.1016/j.physbeh.2004.07.034
Amaretti, A., di Nunzio, M., Pompei, A., Raimondi, S., Rossi, M., and Bordoni, A. (2013). Antioxidant properties of potentially probiotic bacteria: in vitro and in vivo activities. Appl. Microbiol. Biotechnol. 97, 809–817. doi: 10.1007/s00253-012-4241-7
AOAC, (2007). Official Methods of Analysis of AOAC International. Rockville, MD: Association of Official Agricultural Chemists International.
Belter, J. G., Carey, H. V., and Garland, T. (2004). Effects of voluntary exercise and genetic selection for high activity levels on HSP72 expression in house mice. J. Appl. Physiol. 96, 1270–1276. doi: 10.1152/japplphysiol.00838.2003
Blanch, A. R., Méndez, J., Castel, S., and Reina, M. (2014). Comparison of procedures for the extraction of supernatants and cytotoxicity tests in vero cells, applied to assess the toxigenic potential of Bacillus spp. and Lactobacillus spp., intended for use as probiotic strains. J. Microbiol. Methods 103, 64–69. doi: 10.1016/j.mimet.2014.05.019
Bligh, E. G., and Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917. doi: 10.1139/o59-099
Cano, P. G., Santacruz, A., Trejo, F. M., and Sanz, Y. (2013). Bifidobacterium CECT 7765 improves metabolic and immunological alterations associated with obesity in high-fat diet-fed mice. Obesity 21, 2310–2321. doi: 10.1002/oby.20330
Castro, L. C. V., Franceschini, S. C. C., Priore, S. E., and Pelúzio, M. C. G. (2004). Nutrição e Doenças Cardiovasculares: Os Marcadores de Risco Em Adultos. Rev. Nutr. 17, 369–377. doi: 10.1590/S1415-52732004000300010
Choi, W. J., Konkit, M., Kim, Y., Kim, M., and Kim, W. (2016). Oral administration of lactococcus chungangensis inhibits 2,4-dinitrochlorobenzene-induced atopic-like dermatitis in NC/Nga mice. J. Dairy Sci. 99, 6889–6901. doi: 10.3168/jds.2016-11301
Coppola, M. M., and Gil-Turnes, C. (2004). Probióticos e Resposta Imune. Ciência Rural 34, 1297–1303. doi: 10.1590/S0103-84782004000400056
Cruz, A. G., Buriti, F. C. A., Souza, C. H. B., Faria, J. A. F., and Saad, S. M. I. (2009). Probiotic cheese: health benefits, technological and stability aspects. Trends Food Sci. Technol. 20, 344–354. doi: 10.1016/j.tifs.2009.05.001
Cunha, C. R., Dias, A. I., and Viotto, W. H. (2010). microstructure, texture, colour and sensory evaluation of a spreadable processed cheese analogue made with vegetable fat. Food Res. Int. 43, 723–729. doi: 10.1016/j.foodres.2009.11.009
Cunningham-Rundles, S., Ahrné, S., Bengmark, S., Johann-Liang, R., Marshall, F., Metakis, L., et al. (2000). Probiotics and immune response. Am. J. Gastroenterol. 95, S22–S25. doi: 10.1016/S0002-9270(99)00813-8
Cutting, S. M. (2011). Bacillus probiotics. Food Microbiol. 28, 214–220. doi: 10.1016/j.fm.2010.03.007
da Costa, W. K. A., Brandão, L. R., Martino, M. E., Garcia, E. F., Alves, A. F., Souza, E. L., et al. (2019). Qualification of tropical fruit-derived lactobacillus plantarum strains as potential probiotics acting on blood glucose and total cholesterol levels in Wistar rats. Food Res. Int. 124, 109–117. doi: 10.1016/j.foodres.2018.08.035
da Cunha, C. R., Alcântara, M. R., and Viotto, W. H. (2012). Effect of the type of emulsifying salt on microstructure and rheological properties of “Requeijão Cremoso” processed cheese spreads. J. Food Sci. 77, E176–E181. doi: 10.1111/j.1750-3841.2012.02797.x
Dantas, A. B., Jesus, V. F., Silva, R., Almada, C. N., Esmerino, E. A., Cappato, L. P., et al. (2016). Manufacture of probiotic minas frescal cheese with Lactobacillus casei Zhang. J. Dairy Sci. 99, 18–30. doi: 10.3168/jds.2015-9880
de Almada, C. N., Almada, C. N., Martinez, R. C. R., and Sant’Ana, A. S. (2015). Characterization of the intestinal microbiota and its interaction with probiotics and health impacts. Appl. Microbiol. Biotechnol. 99, 4175–4199. doi: 10.1007/s00253-015-6582-5
de Moura, C. S., Lollo, P. C. B., Morato, P. N., Carneiro, E. M., and Amaya-Farfan, J. (2013). Whey protein hydrolysate enhances the exercise-induced heat shock protein (HSP70) response in rats. Food Chem. 136, 1350–1357. doi: 10.1016/j.foodchem.2012.09.070
Desfossés-Foucault, É., Dussault-Lepage, V., Le Boucher, C., Savard, P., LaPointe, G., and Roy, D. (2012). Assessment of probiotic viability during Cheddar cheese manufacture and ripening using Propidium monoazide-PCR quantification. Front. Microbiol. 3:350. doi: 10.3389/fmicb.2012.00350
Dittmann, K. K., Chaul, L. T., Lee, S. H. I., Corassin, C. H., Oliveira, C. A. F., De Martinis, E. C. P., et al. (2017). Staphylococcus aureus in some Brazilian dairy industries: changes of contamination and diversity. Front. Microbiol. 8:2049. doi: 10.3389/fmicb.2017.02049
Elshaghabee, F. M. F., Rokana, N., Gulhane, R. D., Sharma, C., and Panwar, H. (2017). Bacillus as potential probiotics: status, concerns, and future perspectives. Front. Microbiol. 8:1490. doi: 10.3389/fmicb.2017.01490
Esmerino, E. A., Paixão, J. A., Cruz, A. G., Garitta, L., Hough, G., and Bolini, H. M. A. (2015). Survival analysis: a consumer-friendly method to estimate the optimum sucrose level in probiotic petit Suisse. J. Dairy Sci. 98, 7544–7551. doi: 10.3168/jds.2015-9651
Felicio, T. L., Esmerino, E. A., Vidal, V. A. S., Cappato, L. P., Garcia, R. K. A., Cavalcanti, R. N., et al. (2016). Physico-chemical changes during storage and sensory acceptance of low sodium probiotic minas cheese added with arginine. Food Chem. 196, 628–637. doi: 10.1016/j.foodchem.2015.09.102
Fitzpatrick, L. R., Small, J. S., Greene, W. H., Karpa, K. D., and Keller, D. (2011). Bacillus coagulans GBI-30 (BC30) improves indices of clostridium difficile-induced colitis in mice. Gut Pathog. 3:16. doi: 10.1186/1757-4749-3-16
Galdeano, C. M., and Perdigón, G. (2006). The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin. Vaccine Immunol. 13, 219–226. doi: 10.1128/CVI.13.2.219-226.2006
Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., et al. (2014). The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514. doi: 10.1038/nrgastro.2014.66
Honda, H., Hoyles, L., Gibson, G. R., Farmer, S., Keller, D., and McCartney, A. L. (2011). Impact of Ganedenbc30 (Bacillus coagulans GBI-30, 6086) on population dynamics of the human gut microbiota in a continuous culture fermentation system. Int. J. Probiotics Prebiotics 6, 65–72.
Huang, Q., Xu, X., Mao, Y. L., Huang, Y., Rajput, I. R., and Li, W. F. (2013). Effects of Bacillus subtilis B10 spores on viability and biological functions of murine macrophages. Anim. Sci. J. 84, 247–252. doi: 10.1111/j.1740-0929.2012.01064.x
Hun, L. (2009). Original research: Bacillus coagulans significantly improved abdominal pain and bloating in patients with IBS. Postgrad. Med. 121, 119–124. doi: 10.3810/pgm.2009.03.1984
Jäger, R., Purpura, M., Farmer, S., Cash, H. A., and Keller, D. (2018). Probiotic bacillus coagulans GBI-30, 6086 improves protein absorption and utilization. Probiotics Antimicrob. Proteins 10, 611–615. doi: 10.1007/s12602-017-9354-y
Jäger, R., Shields, K. A., Lowery, R. P., Souza, E. O., Partl, J. M., Hollmer, C., et al. (2016). Probiotic Bacillus coagulans GBI-30, 6086 reduces exercise-induced muscle damage and increases recovery. PeerJ 4:e2276. doi: 10.7717/peerj.2276
Jang, H. J., Kwak, J. H., Cho, E. Y., We, Y. M., Lee, Y. H., Kim, S. C., et al. (2008). Glutamine induces heat-shock protein-70 and glutathione expression and attenuates ischemic damage in rat islets. Transplant. Proc. 40, 2581–2584. doi: 10.1016/j.transproceed.2008.08.075
Jensen, G. S., Benson, K. F., Carter, S. G., and Endres, J. R. (2010). GanedenBC30TM cell wall and metabolites: anti-inflammatory and immune modulating effects in vitro. BMC Immunol. 11:15. doi: 10.1186/1471-2172-11-5
Jeon, H. L., Yang, S. J., Son, S. H., Kim, W. S., Lee, N. K., and Paik, H. D. (2018). Evaluation of probiotic Bacillus subtilis P229 isolated from Cheonggukjang and its application in soybean fermentation. LWT 97, 94–99. doi: 10.1016/j.lwt.2018.06.054
Jessie-Lau, L. Y., and Chye, F. Y. (2018). Antagonistic effects of Lactobacillus plantarum 0612 on the adhesion of selected foodborne enteropathogens in various colonic environments. Food Control 91, 237–247. doi: 10.1016/j.foodcont.2018.04.001
Kalman, D. S., Schwartz, H. I., Alvarez, P., Feldman, S., Pezzullo, J. C., and Krieger, D. R. (2009). A prospective, randomized, double-blind, placebo-controlled parallel-group dual site trial to evaluate the effects of a bacillus coagulans-based product on functional intestinal gas symptoms. BMC Gastroenterol. 9:85. doi: 10.1186/1471-230X-9-85
Keller, M. K., Brandsborg, E., Holmstrøm, K., and Twetman, S. (2018). Effect of tablets containing probiotic candidate strains on gingival inflammation and composition of the salivary microbiome: a randomised controlled trial. Benef. Microbes 9, 487–494. doi: 10.3920/BM2017.0104
Keller, D., Dinter, R. V., Cash, H., Farmer, S., and Venema, K. (2017). Bacillus coagulans GBI-30, 6086 increases plant protein digestion in a dynamic, computer-controlled in vitro model of the small intestine (TIM-1). Benef. Microbes 8, 491–496. doi: 10.3920/BM2016.0196
Lee, S., Lee, J., Jin, Y. I., Jeong, J. C., Chang, Y. H., Lee, Y., et al. (2017). Probiotic characteristics of Bacillus strains isolated from korean traditional soy sauce. LWT Food Sci. Technol. 79, 518–524. doi: 10.1016/j.lwt.2016.08.040
Lima, C. M., Lima, A. K., Melo, M. G. D., Dória, G. A. A., Leite, B. L. S., Serafini, M. R., et al. (2014). Valores de Referência Hematológicos e Bioquímicos de Ratos (Rattus Novergicus Linhagem Wistar) Provenientes Do Biotério Da Universidade Tiradentes. Scientia Plena 10, 1–9.
Liu, H., Dicksved, J., Lundh, T., and Lindberg, J. E. (2014). Heat shock proteins: intestinal gatekeepers that are influenced by dietary components and the gut microbiota. Pathogens 3, 187–210. doi: 10.3390/pathogens3010187
Lollo, P. C. B., Morato, P. N., Moura, C. S., Almada, C. N., Felicio, T. L., Esmerino, E. A., et al. (2015). Hypertension parameters are attenuated by the continuous consumption of probiotic minas cheese. Food Res. Int. 76, 611–617. doi: 10.1016/j.foodres.2015.07.015
Lollo, P. C. B., Moura, C. S., Morato, P. N., and Amaya-Farfan, J. (2013a). Differential response of heat shock proteins to uphill and downhill exercise in heart, skeletal muscle, lung and kidney tissues. J. Sports Sci. Med. 12, 461–466.
Lollo, P. C. B., Moura, C. S., Morato, P. N., Cruz, A. G., Castro, W. F., Betim, C. B., et al. (2013b). Probiotic yogurt offers higher immune-protection than probiotic whey beverage. Food Res. Int. 54, 118–124. doi: 10.1016/j.foodres.2013.06.003
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951). Protein measurement with the folin-phenol reagent. J. Biol. Cem. 193, 265–275.
Maathuis, A. J., Keller, D., and Farmer, S. (2010). Survival and metabolic activity of the GanedenBC 30 Strain of Bacillus coagulans in a dynamic in vitro model of the stomach and small intestine. Benef. Microbes 1, 31–36. doi: 10.3920/BM2009.0009
Majeed, M., Majeed, S., Nagabhushanam, K., Arumugam, S., Beede, K., and Ali, F. (2019). Evaluation of the in Vitro cholesterol-lowering activity of the probiotic strain Bacillus coagulans MTCC 5856. Int. J. Food Sci. Technol. 54, 212–220. doi: 10.1111/ijfs.13926
Marcial-Coba, M. S., Pjaca, A. S., Andersen, C. J., Knøchel, S., and Nielsen, D. S. (2019). Dried date paste as carrier of the proposed probiotic Bacillus coagulans BC4 and viability assessment during storage and simulated gastric passage. LWT 99, 197–201. doi: 10.1016/j.lwt.2018.09.052
Martins, A. A., Santos-Junior, V. A., Filho, E. R. T., Silva, H. L. A., Ferreira, M. V. S., Graça, J. S., et al. (2018). Probiotic prato cheese consumption attenuates development of renal calculi in animal model of urolithiasis. J. Funct. Foods 49, 378–383. doi: 10.1016/j.jff.2018.08.041
Martins, M. L. B., Kac, G., Silva, R. A. M., Bettiol, H., Barbieri, M. A., Cardoso, V. C., et al. (2015). Dairy consumption is associated with a lower prevalence of metabolic syndrome among young adults from Ribeirão Preto, Brazil. Nutrition 31, 716–721. doi: 10.1016/j.nut.2014.12.017
Morimoto, R. I. (1998). Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 12, 3788–3796. doi: 10.1101/gad.12.24.3788
Mourão, D. M., and Bressan, J. (2009). Influência de Alimentos Líquidos e Sólidos No Controle Do Apetite. Rev. Nutr. 22, 537–547. doi: 10.1590/S1415-52732009000400009
Nambiar, R. B., Sellamuthu, P. S., and Perumal, A. B. (2018). Development of milk chocolate supplemented with microencapsulated Lactobacillus plantarum HM47 and to determine the safety in a Swiss albino mice model. Food Control 94, 300–306. doi: 10.1016/j.foodcont.2018.07.024
Nithya, V., and Halami, P. M. (2013). Evaluation of the probiotic characteristics of Bacillus species isolated from different food sources. Ann. Microbiol. 63, 129–137. doi: 10.1007/s13213-012-0453-4
Nyangale, E. P., Farmer, S., Cash, H. A., Keller, D., Chernoff, D., and Gibson, G. R. (2015). Bacillus coagulans GBI-30, 6086 modulates Faecalibacterium prausnitzii in older men and women. J. Nutr. 145, 1446–1452. doi: 10.3945/jn.114.199802
Nyangale, E. P., Farmer, S., Keller, D., Chernoff, D., and Gibson, G. R. (2014). Effect of prebiotics on the fecal microbiota of elderly volunteers after dietary supplementation of bacillus coagulans GBI-30, 6086. Anaerobe 30, 75–81. doi: 10.1016/j.anaerobe.2014.09.002
Oliveira, R. B. A., Baptista, R. C., Chincha, A. A. I. A., Conceição, D. A., Nascimento, J. S., Costa, L. E. O., et al. (2018a). Thermal inactivation kinetics of Paenibacillus sanguinis 2301083PRC and Clostridium sporogenes JCM1416MGA in full and low fat “requeijão cremoso.”. Food Control 84, 395–402. doi: 10.1016/j.foodcont.2017.08.030
Oliveira, R. B. A., Lopes, L. S., Baptista, R. C., Chincha, A. A. I. A., Portela, J. B., Nascimento, J. S., et al. (2018b). Occurrence, populations, diversity, and growth potential of spore-forming bacteria in “requeijão cremoso”. LWT 89, 24–31. doi: 10.1016/j.lwt.2017.10.029
Oliveira, R. B. A., Margalho, L. P., Nascimento, J. S., Costa, L. E. O., Portela, J. B., Cruz, A. G., et al. (2016). Processed cheese contamination by spore-forming bacteria: a review of sources, routes, fate during processing and control. Trends Food Sci. Technol. 57, 11–19. doi: 10.1016/j.tifs.2016.09.008
Orrù, L., Salvetti, E., Cattivelli, L., Lamontanara, A., Michelotti, V., Capozzi, V., et al. (2014). Draft genome sequence of Bacillus coagulans GBI-30, 6086, a widely used spore-forming probiotic strain. Genome Announc. 2:e1080-14. doi: 10.1128/genomeA.01080-14
Pedrosa, R. G., Donato Junior, J., and Tirapegui, J. (2009). Dieta Rica Em Proteína Na Redução Do Peso Corporal. Rev. Nutr. 22, 105–111. doi: 10.1590/S1415-52732009000100010
Pereira, E. P. R., Faria, J. A. F., Cavalcanti, R. N., Garcia, R. K. A., Silva, R., Esmerino, E. A., et al. (2016). Oxidative stress in probiotic petit suisse: is the jabuticaba skin extract a potential option? Food Res. Int. 81, 149–156. doi: 10.1016/j.foodres.2015.12.034
Pérez Montoro, B., Benomar, N., Gómez, N. C., Said, E., Horvatovich, P., Knapp, C. W., et al. (2018). Proteomic analysis of lactobacillus pentosus for the identification of potential markers involved in acid resistance and their influence on other probiotic features. Food Microbiol. 72, 31–38. doi: 10.1016/j.fm.2017.11.006
Petrof, E. O., Kojima, K., Ropeleski, M. J., Musch, M. W., Tao, Y., Simone, C., et al. (2004). Probiotics inhibit nuclear factor-K B and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology 127, 1474–1487. doi: 10.1053/j.gastro.2004.09.001
Rabah, H., Ferret-Bernard, S., Huang, S., Le Normand, L., Cousin, F. J., Gaucher, F., et al. (2018). The cheese matrix modulates the immunomodulatory properties of Propionibacterium freudenreichii CIRM-BIA 129 in healthy piglets. Front. Microbiol. 9:2584. doi: 10.3389/fmicb.2018.02584
Ranadheera, C. S., Baines, S. K., and Adams, M. C. (2010). Importance of food in probiotic efficacy. Food Res. Int. 43, 1–7. doi: 10.1016/j.foodres.2009.09.009
Ranadheera, C. S., Evans, C. A., Adams, M. C., and Baines, S. K. (2012). In vitro analysis of gastrointestinal tolerance and intestinal cell adhesion of probiotics in goat’s milk ice cream and yogurt. Food Res. Int. 49, 619–625. doi: 10.1016/j.foodres.2012.09.007
Rhayat, L., Maresca, M., Nicoletti, C., Perrier, J., Brinch, K. S., Christian, S., et al. (2019). Effect of Bacillus subtilis strains on intestinal barrier function and inflammatory response. Front. Immunol. 10:564. doi: 10.3389/fimmu.2019.00564
Salvetti, E., Orrù, L., Capozzi, V., Martina, A., Lamontanara, A., Keller, D., et al. (2016). Integrate genome-based assessment of safety for probiotic strains: Bacillus coagulans GBI-30, 6086 as a case study. Appl. Microbiol. Biotechnol. 100:4595. doi: 10.1007/s00253-016-7416-9
Santos, M. R. V., Souza, V. H., Menezes, I. A. C., Bitencurt, J. L., Resende-Neto, J. M., Barreto, A. S., et al. (2010). Parâmetros Bioquímicos, Fisiológicos e Morfológicos de Ratos (Rattus Novergicus Linhagem Wistar) Produzidos Pelo Biotério Central Da Universidade Federal de Sergipe. Sci. Plena 6:106101.
Santos-Junior, V. A., Lollo, P. C. B., Cantero, M. A., Moura, C. S., Amaya-Farfan, J., and Morato, P. N. (2018). Heat shock proteins: protection and potential biomarkers for ischemic injury of cardiomyocytes after surgery. Brazilian J. Cardiovasc. Surg. 33, 291–302. doi: 10.21470/1678-9741-2017-169
Sassone-Corsi, M., Nuccio, S. P., Liu, H., Hernandez, D., Vu, C. T., Takahashi, A. A., et al. (2016). Microcins Mediate Competition among Enterobacteriaceae in the Inflamed Gut. Nature 540, 280–283. doi: 10.1038/nature20557
Silver, J. T., and Noble, E. G. (2012). Regulation of survival gene Hsp70. Cell Stress Chaperones 17, 1–9. doi: 10.1007/s12192-011-0290-6
Silver, J. T., Kowalchuk, H., and Noble, E. G. (2012). Hsp70 mRNA temporal localization in rat skeletal myofibers and blood vessels post-exercise. Cell Stress Chaperones 17, 109–120. doi: 10.1007/s12192-011-0291-5
Soares, M. B., Almada, C. N., Almada, C. N., Martinez, R. C. R., Pereira, E. P. R., Balthazar, C. F., et al. (2019a). The resistance of Bacillus, Bifidobacterium, and Lactobacillus strains with claimed probiotic properties in different food matrices exposed to simulated gastrointestinal tract conditions. Food Res. Int. 125:108542. doi: 10.1016/j.foodres.2019.108542
Soares, M. B., Martinez, R. C. R., Pereira, E. P. R., Balthazar, C. F., Cruz, A. G., Ranadheera, C. S., et al. (2019b). Behavior of different Bacillus strains with claimed probiotic properties throughout processed cheese (“requeijão cremoso”) manufacturing and storage. Int. J. Food Microbiol. 307:108288. doi: 10.1016/j.ijfoodmicro.2019.108288
Staib, J. L., Tümer, N., and Powers, S. K. (2009). Increased temperature and protein oxidation lead to HSP72 MRNA and protein accumulation in the in vivo exercised rat heart. Exp. Physiol. 94, 71–80. doi: 10.1113/expphysiol.2008.044685
Taverniti, V., and Guglielmetti, S. (2011). The immunomodulatory properties of probiotic microorganisms beyond their viability (Ghost Probiotics: Proposal of Paraprobiotic Concept). Genes Nutr. 6, 261–274. doi: 10.1007/s12263-011-0218-x
Tripathi, M. K., and Giri, S. K. (2014). Probiotic functional foods: survival of probiotics during processing and storage. J. Funct. Foods 9, 225–241. doi: 10.1016/j.jff.2014.04.030
Urdaci, M. C., Lefevre, M., Lafforgue, G., Cartier, C., Rodriguez, B., and Fioramonti, J. (2018). Antidiarrheal action of Bacillus subtilis CU1 CNCM I-2745 and Lactobacillus plantarum CNCM I-4547 in mice. Front. Microbiol. 9:1537. doi: 10.3389/fmicb.2018.01537
Wang, Y., Wu, Y., Wang, B., Xu, H., Mei, X., Xu, X., et al. (2019). Bacillus amyloliquefaciens SC06 protects mice against high-fat diet-induced obesity and liver injury via regulating host metabolism and gut microbiota. Front. Microbiol. 10:1161. doi: 10.3389/fmicb.2019.01161
Yan, F., and Polk, D. B. (2011). Probiotics and immune health. Curr. Opin. Gastroenterol. 27, 496–501. doi: 10.1097/MOG.0b013e32834baa4d
Zahorec, R. (2001). Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically Ill. Bratisl. Lek. Listy 102, 5–14.
Keywords: dairy foods, functional foods, diet, dairy consumption, anti-stress system
Citation: Soares MB, Santos-Junior VA, Tavares Filho ER, Lollo PCB, Morato PN, Amaya-Farfan J, Pereira EPR, Balthazar CF, Cruz AG, Martinez RCR and Sant’Ana AS (2019) The Step of Incorporation of Bacillus coagulans GBI-30 6086 Into “requeijão cremoso” Processed Cheese Does Not Affect Metabolic Homeostasis of Rats. Front. Microbiol. 10:2332. doi: 10.3389/fmicb.2019.02332
Received: 14 April 2019; Accepted: 24 September 2019;
Published: 22 October 2019.
Edited by:
Vittorio Capozzi, University of Foggia, ItalyReviewed by:
Alejandra de Moreno de LeBlanc, CONICET Centro de Referencia para Lactobacilos (CERELA), ArgentinaCopyright © 2019 Soares, Santos-Junior, Tavares Filho, Lollo, Morato, Amaya-Farfan, Pereira, Balthazar, Cruz, Martinez and Sant’Ana. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anderson S. Sant’Ana, YW5kQHVuaWNhbXAuYnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.