- 1Key Laboratory of Fujian-Taiwan Animal Pathogen Biology, College of Animal Sciences, Fujian Agriculture and Forestry University, Fuzhou, China
- 2State Key Laboratory of Veterinary Etiological Biology, Key Laboratory of Veterinary Parasitology of Gansu Province, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Lanzhou, China
- 3Department of Microbiology and Microbial Engineering, School of Life Sciences, Fudan University, Shanghai, China
- 4College of Animal Science and Veterinary Medicine, Shanxi Agricultural University, Taigu, China
- 5Jiangsu Co-innovation Center for the Prevention and Control of Important Animal Infectious Diseases and Zoonoses, College of Veterinary Medicine, Yangzhou University, Yangzhou, China
Toxoplasmosis, one of the most important health-threatening diseases worldwide, is caused by Toxoplasma gondii, which infects a wide range of warm-blooded animals and humans, leading to enormous health and socioeconomic concerns. T. gondii can establish chronic infection to evade the immune response in hosts. Once a chronic infection has been established, the available treatments cannot efficiently control this stage of T. gondii efficiently. Moreover, the available treatments rely only on a few drugs, such as sulfapyridine and pyrimethamine, that tend to have severe side effects. Given these factors, vaccination has been considered to be the most efficient method to prevent and control this disease. However, there is currently lack of effective vaccine available for use to prevent toxoplasmosis apart form Toxovax®, the only available vaccine, which is used in sheep to prevent abortion. To address this problem, we knocked out the NPT1 gene of the type I T. gondii strain using the CRISPR-Cas9 system, constructed a live-attenuated vaccine and evaluated its protective efficacy in a mouse model. Immunization of mice with RH:ΔNPT1 induced a high level of Toxoplasma-specific IgG1, IgG2a and total IgG 42 days after immunization. There was a significant increase in the levels of cytokines in the splenocyte suspensions of RH:ΔNPT1-infected mice, and a mixed Th1/Th2 response was induced in the mice. Remarkably, after heterologous challenges with tachyzoites of the RH, PYS and Pru strains and cysts of the Pru strain by different infection routes, the immunized animals were protected from toxoplasmosis with a 100% survival rate, in both acute and chronic infection. In addition, compared with control mice, the Pru cyst load was clearly reduced in the brains of RH:ΔNPT1-infected immunization-mice. Our study demonstrated that the RH:ΔNPT1 strain was able to evoke strong anti-Toxoplasma immune responses and provide effective protection against parasite strains with different levels of virulence, suggesting that the RH:ΔNPT1 strain may represent a promising live-attenuated vaccine against toxoplasmosis, which is worthy of further evaluation in food-producing animals and in definitive feline host.
Introduction
Toxoplasma gondii is an obligatory intracellular opportunistic parasitic protozoan that can infect nearly all warm-blooded animals and humans and has a worldwide distribution (Montoya and Liesenfeld, 2004; Elmore et al., 2010). T. gondii causes subclinical or asymptomatic infections in immunocompetent humans and animals and is also responsible for causing serious cases of encephalitis, ophthalmopathy, abortion, and stillbirth, especially in individuals with immunodeficiency or immunosuppression. Thus, this disease poses a serious threat to public health and causes great economic losses of animal husbandry (Petersen et al., 2001; Elbez-Rubinstein et al., 2009).
It has been reported that T. gondii can evade the immune response and establish chronic infections in most hosts, and the presently available treatments, such as sulfapyridine and pyrimethamine, do not work very well for chronic infections, leaving the survivors at a high risk of developing several neuropsychiatric disorders (Alday and Doggett, 2017; Lang et al., 2018). Although drug treatment is effective on the tachyzoite stage, it can also induce severe side effects and promote the development of drug-resistant strains (Doliwa et al., 2013). Given these factors, vaccination has been considered to be an optimal approach for prevention and control of toxoplasmosis. Therefore, developing an effective vaccine would have a large impact on public health and livestock husbandry (Zhang et al., 2015).
The progress of vaccine development has always been limited in some respects, including the complicated life cycle of the pathogen, knowledge of effective target genes and availability of appropriate technology for vaccine development, and thus there is currently lack of effective vaccine available to prevent this disease (Wang et al., 2019).
Although considerable research about the development of an anti-T. gondii vaccine has been carried out using various approaches with some degree of success, such as protein vaccines, DNA vaccines and inactivated vaccines, unfortunately none of these vaccines so far has provided full protection for experimental animals (Gurunathan et al., 2000; Kur et al., 2009; Zhang et al., 2013, 2015). In light of the existing anti-T. gondii vaccines, live attenuated vaccines have more advantages in terms of protective efficacy because they enable the vaccinated mice to develop stronger cellular and humoral responses against toxoplasmosis. Moreover, the only currently available commercial vaccine of anti-T. gondii, Toxovax®, is based on a live-attenuated T. gondii S48 strain, and it shows sufficient protection among sheep and goats (Buxton and Innes, 1995). This vaccine appears to demonstrate the requirements for a successful anti-T. gondii vaccine, namely, that it combines long-term effective protection with biosafety (the potential possibility of residual virus and transmission). However, Toxovax® has some problems that need to be improved, such as its toxicity, stability and exact mechanism of action.
In several studies, researchers have attempted to use different gene-editing approaches to construct mutant strains in order to improve the protective efficiency of live-attenuated vaccine strains of T. gondii. Among these studies, the RH-Mic1-3KO, RH:ΔGRA17 and ME49:Δldh strains displayed better performance in inducing protection against chronic and acute toxoplasmosis infection in a mouse model (Mévélec et al., 2010; Wang et al., 2017; Xia et al., 2018).
Recently, TgNPT1 was demonstrated to be a member of the Novel Putative Transporters (NPTs), which play an important role in the uptake of cationic amino acids by T. gondii. In ΔNPT1 parasites, if the conditions of the parasite’s growth has a high ratio of [Arg]:[Lys], the TgNPT1-independent of cationic amino acids transport pathway(s) would uptake sufficient arginine to maintain growth. However, when this balance of [Arg]:[Lys] is destroyed, for example when the growth conditions in mammalian cells, the mutant parasite growth is restricted by the level of arginine being lower than that required for survival (Rajendran et al., 2016). In view of the importance of TgNPT1 in the survival and virulence of T. gondii, we speculate that the mutant strain with a deletion of the NPT1 gene maybe have the potential to be an attractive candidate for an anti-T. gondii vaccine, and whether RH:ΔNPT1 can be used as an anti-T. gondii vaccine needs to be further verified by animal experiments.
In this study, female Kunming mice were immunized with a live attenuated T. gondii RH ΔNPT1 mutant strain to investigate the vaccine’s efficiency by challenging the mice with heterologous strains with different degrees of virulence. The efficacy of immunization with the RH:ΔNPT1 strain was evaluated by the humoral and cellular immune responses after immunization and the mouse survival rate and cysts burden after acute or chronic infection; in addition, the potential related immune mechanisms of the protection were also addressed.
Materials and Methods
Mice
Six-week-old female Kunming mice of specific-pathogen-free (SPF) grade were obtained from the Laboratory Animal Center, Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Lanzhou, Gansu Province, China. As Kunming mice are susceptibility to acute and latent infection with T. gondii, they were selected to establish an animal model of infection. According to the regulations of the Administration of Affairs Concerning Experimental Animals, all experiments using animals provided care under standard conditions.
Parasites
Tachyzoites of T. gondii type I (RH), type ToxoDB#9 (PYS), type II (Pru), and RH:ΔNPT1 mutant strains used in this study were maintained by serial passage in confluent monolayers of human foreskin fibroblasts (HFFs), which were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) or RPMI-1640 supplemented with 10% fetal bovine serum (FBS). RH:ΔNPT1 mutant tachyzoites were obtained by deleting the TgNPT1 gene of the T. gondii RH strain. Freshly egressed tachyzoites were harvested according to methods described in previous studies and then counted using a hemocytometer (Wang et al., 2016). T. gondii Pru cysts were maintained by continuous passage in Kunming mice and isolated from the mouse brain homogenates of infected PRU strains using a previously described strategy (Zhang et al., 2018).
Preparation of Soluble Tachyzoite Antigens (STAg)
To prepare soluble tachyzoite antigens (STAg), the tachyzoites of the T. gondii RH strain were purified as previously described (Li et al., 2014). The suspension of tachyzoites was collected and then washed with PBS. Subsequently, the precipitate was subjected to freeze-thaw cycles followed by sonication and centrifugation at 14,000 g for 30 min at 4°C. The supernatant containing the STAg was collected and stored at −80°C.
Construction of Plasmids and Depletion of NPT1 in the RH Strain
A CRISPR-Cas9 method was engineered to knockout the NPT1 gene of the T. gondii RH strain. Briefly, to generate a vector of pSAG1:CAS9-U6:sgNPT1, single guide (sgRNA) and specific primers (listed in Table 1) were employed to modified the pSAG1:CAS9-U6:sgURPT. Specific primers (listed in Table 1) were designed to amplify the DHFR* resistance cassette. To construct the knockouts, the pSAG1:CAS9-U6:sgNPT1 and DHFR* resistance cassette were transfected into the tachyzoites of the RH strain as described elsewhere (Wang et al., 2016). Then, the transfected parasites were selected with 3 μM pyrimethamine and the successful integration was confirmed by PCR and RT-PCR analysis using detection primers (KO-NPT1-F and KO-NPT1-R, see Table 1). The GRA17 gene was used as positive control to test the quality of cDNA product extracted from different stains, and its primers (GRA17-F and GRA17-R) were listed in the Table 1. The resultant parasite strain was termed RH:ΔNPT1.
Growth and Virulence of Mutants and WT Strains
Growth assays of the RH:ΔNPT1 mutant and WT tachyzoites in vitro were conducted in 6 well plates containing monolayers of HFF cells within the same or different culture media (DMEM, RPMI-1640 and DMEM + 400 μM arginine medium). The plaque assay was also used to determine whether the NPT1 gene was deleted in RH strain. The cells were infected with tachyzoites of RH:ΔNPT1 mutant (∼200 tachyzoites/well) and incubated for 3 h to allow the parasites to enter the host cells. And then washed twice with sterile phosphate-buffered saline to remove unbound parasites. After the fresh medium of DMEM or RPMI-1640 was added to the palate, it was incubated for 7 days at 37°C in 5% CO2 environment. The infected HFF cells were fixed with 4% paraformaldehyde in PBS for 15 min, and then incubated with crystal violet staining solution for 10 min at ambient temperature. The size of plaques was determined using inverted microscope as previously described (Wang et al., 2017). To determine the virulence in vivo and the immunizing dose, fresh RH or RH:ΔNPT1 tachyzoites were intraperitoneally injected into mice and then their physical health and mortality status was recorded daily.
Vaccination of Mice
All of the Kunming mice were randomly divided into two groups (the naive and RH:ΔNPT1 groups, 46 mice per group). The mice were intraperitoneally injected with parasites at a single dose of 1 × 106 RH:ΔNPT1 tachyzoites or mock-vaccinated with 200 μl of PBS. For the immunized mice, the potent immune protection efficiency of the RH:ΔNPT1 was evaluated by challenging the mice with T. gondii infection from different stages of the T. gondii life cycle.
Examination of Cytokine Production
Spleens were collected from six mice from each group, either immunized with RH:ΔNPT1 tachyzoites or PBS, at 42 days post-immunization. Splenocytes from each group were collected as previously described (Li et al., 2014), and their concentrations were adjusted to 3 × 106 cells per milliliter. Then, to measure the cytokine levels, the obtained suspensions were stimulated with 10 μg/ml T. gondii soluble tachyzoite antigen (STAg). Culture supernatants were collected and the secreted cytokines levels were measured at different time points by ELISA; the levels of interleukin-2 (IL-2) and IL-4 were evaluated at 24 h post-incubation (hpi), IL-10 at 72 hpi, and IL-12 and IFN-γ at 96 hpi. Each supernatant sample was examined in triplicate using a mouse cytokine kit (eBioscience Bender MedSystems GmbH, Austria) according to the manufacturer’s instructions.
Measurement of Anti-Toxoplasma IgG Antibodies
For the detection of total antibody production and the subclasses of IgG, sera samples were collected from the mouse tail vein after 14, 28, and 42 days post-immunization and stored at −80°C. We coated 96-well microtiter plates with 100 μg (10 μg/ml) of STAg and incubated them at 4°C overnight. Subsequently, every well was washed and blocked as previously described (Xu et al., 2015). After the blood serum samples were diluted 25-fold in PBS, they were added to each well. Then, the plate was incubated at 37°C for 60 min. To detect the bound IgG, IgG1 and IgG2a antibodies, the wells were washed in PBS, and 100 μl of horseradish-peroxidase-conjugated goat anti-mouse IgG (1:250 diluted with PBS), anti-mouse IgG1 (1:500 diluted with PBS), or IgG2a (1:500 diluted with PBS) were added to the wells. Subsequently, a stop solution was added. For quantitation of IgG, IgG1 and IgG2a, their degrees of absorbance were captured by an iMark Microplate Absorbance Reader (Zhu et al., 2017; Wang et al., 2018).
Protection Against Acute Infection
To determine whether immune protection was activated by immunization with the RH:ΔNPT1 strain, distinct genotype strains of T. gondii were employed to infect the immunized mice. 10 mice of each group was challenged with 1 × 103 tachyzoites of RH or PYS at 42 days post-immunization. The ability of protection against acute infection was determined by the survival rates of all mice (vaccinated and non-vaccinated mice) after infection with strains exhibiting different degrees of virulence. To better understand the effectiveness of immunoprotection, the infected mice were observed and monitored for disease progression for 35 days.
Protection Against Chronic Infection
Kunming mice were immunized with RH:ΔNPT1 and then challenged with 20 or 100 cysts of the Pru strain, which was performed as previously described (Zhang et al., 2018). Non-immunized mice were used as controls. And each group contained 10 mice. At 35 days post-challenge, the mice were euthanized. Subsequently, the brains of these animals were collected and ground into homogenate suspensions. The degree of cyst burden in the brain was determined by optical microscopic observation based on counting the number of cysts on 15 slides prepared from each brain homogenate, as previously described (Wang et al., 2018).
Statistical Analysis
Treatment groups with three or more independent experiments were analyzed for significant differences using Student’s t-test or one-way analysis. The significance level was set to 0.05; if values of P < 0.05, differences were defined as statistically significant. In the different mouse groups, mouse mortality was expressed by plotting survival curves, and significant differences were determined using the Mantel–Cox log-rank test.
Results
Disruption of the NPT1 Gene in the T. gondii RH Strain
To delete the NPT1 gene, the CRISPR-Cas9 plasmids were transduced into tachyzoites of the T. gondii RH strain by inserting the selectable maker dihydrofolate reductase (DHFR*) into the NPT1 gene. Then, the knockouts, the pyrimethamine-resistant clones, were successfully generated (Figure 1A). Following selection of the parasite clones, the efficiency of knockout was verified by PCR with specific primers (Figure 1B), and Sanger sequencing of the mutants was performed to affirm that the NPT1 gene had been knocked out. Meanwhile, the RT-PCR were performed used GRA17-F, GRA17-R, KO-NPT1-Fw and KO-NPT1-R (Figure 1C), which also verified that the gene of NPT1 was successful knockout. As previously reported, the knockouts of the RH strain we obtained had the characteristics of normal growth and proliferation in RPMI 1640 medium and DMEM containing the concentration of arginine present in RPMI-1640 (400 μM), but its growth capability was remarkably attenuated in the DMEM medium (Figure 1D) due to the DMEM medium containing much lower concentrations of arginine compared with RPMI-1640. The WT strain exhibited comparable growth in both environments. The above results revealed that the NPT1 gene was successfully disrupted in the RH strain.
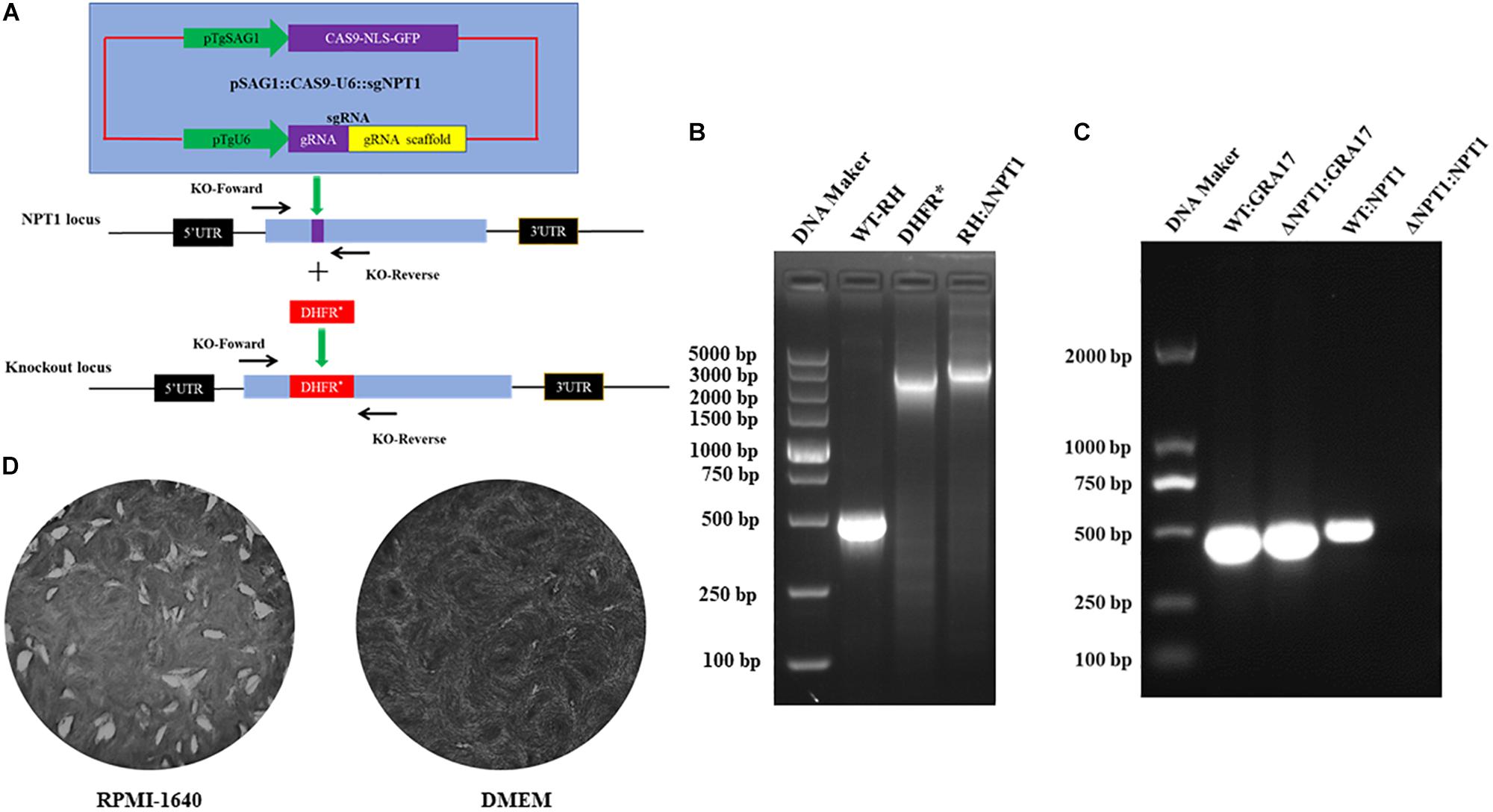
Figure 1. Employment of CRISPR/Cas9 technology to knockout the NPT1 gene in the T. gondii RH strain. (A) Schematic illustration of the CRISPR-Cas9 method to disrupt the gene by insertion of the pyrimethamine-resistant DHFR*. (B) KO-NPT1-F and KO-NPT1-R were used to amplify the target fragment, which can amplify the coding region in wild-type parasites and a larger fragment in the pyrimethamine-resistant clones. Diagnostic PCR confirming the selection maker DHFR* cassette’s successful integration into the NPT1 gene (RH:ΔNPT1). (C) RT-PCR were used to amplify the target fragment, which can amplify the coding region in the wild-type parasites, but did not amplify the targeting aim gene in the NPT1-deficiency clones. The GRA17 gene fragment as the positive control of RT-PCR detected were amplified in wild-type and RH:ΔNPT1 strain. Diagnostic PCR confirming the selection maker DHFR* cassette successful integration into the NPT1 gene (RH:ΔNPT1). (D) Growth analysis of RH:ΔNPT1-infected human foreskin fibroblast cells. RH:ΔNPT1 was normal growth and developed plaque in the RPMI-1640 medium, but its growth capability was remarkably attenuated in the DMEM medium, indicating the successful deletion of the NPT1 gene.
Virulence of the Wild-Type and Mutant Strains
To gain insight into whether RH:ΔNPT1 was able to be used as an attenuated vaccine, we assessed the virulence of the RH:ΔNPT1 strain in mice. We observed clearly different survival rates of mice after treatment with the wild-type or mutated strains. Mice were infected with doses of 500, 1 × 103, 1 × 104, or 1 × 106 parasites of the RH:ΔNPT1 strain, and all of the mice survived the challenge. Furthermore, the mice exhibited no signs of toxoplasmosis, even after infection with high doses of RH:ΔNPT1 (up to 1 × 106) (Figure 2). Infected-WT RH mice succumbed to infection within 8 days after a dose of 1 × 103 tachyzoites. These results suggested that the virulence of RH:ΔNPT1 was significantly attenuated compared with the WT, indicating that it might be usable as a live-attenuated vaccine against toxoplasmosis. Based on the health condition and its immune response elicited by different infected does of immunized mice, the vaccinated dose of the RH:ΔNPT1 strain we selected is 1 × 106.
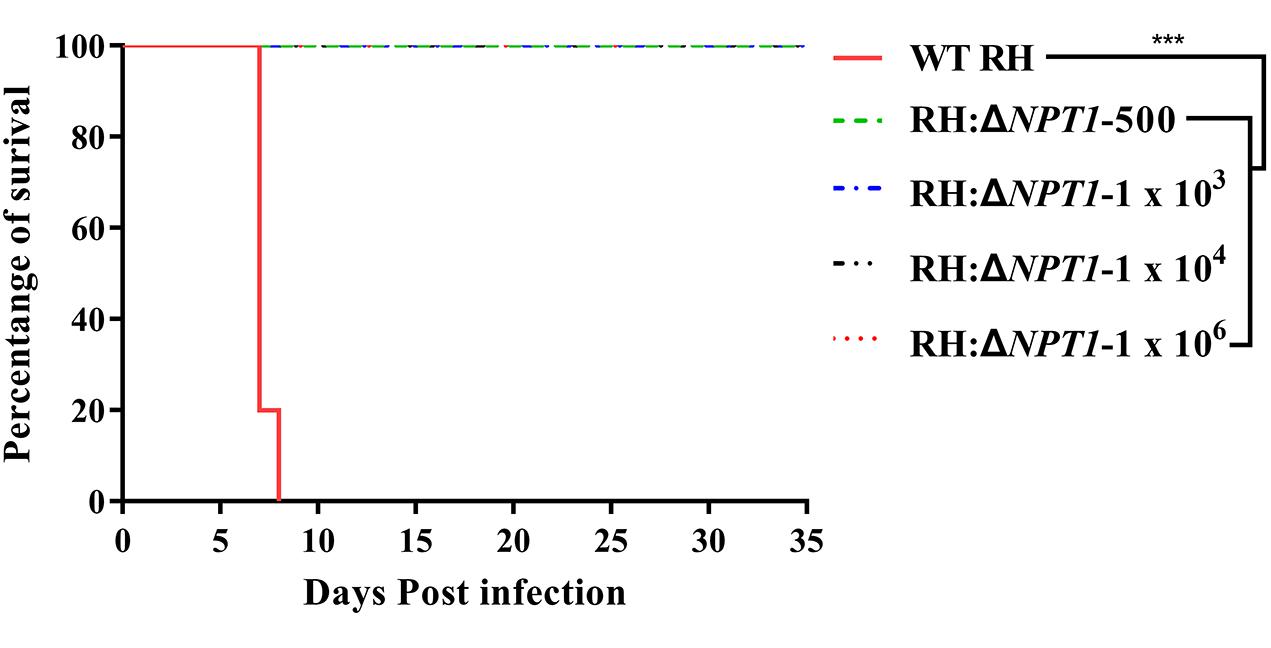
Figure 2. Survival curves of Kunming mice infected with the wild-type T. gondii (RH) strain or the RH:ΔNPT1 strain. The different groups of Kunming mice were challenged with 1 × 103 tachyzoites of the wild-type strain and doses of 500, 1 × 103, 1 × 104, 1 × 106 parasites of the RH:ΔNPT1 strain. Each group included 10 mice, and the survival time was recorded for 35 days after the challenge (n = 10; ∗∗∗P < 0.001; n.s., not significant; Mantel-Cox-test).
Humoral Immune Responses Elicited by RH:ΔNPT1
To elucidate the potential protection efficiency of this attenuated strain, we measured the level of specific anti-T. gondii IgG and IgG isotypes antibodies in the serum of immunized Kunming mice. To establish the experimental model, the mice were vaccinated with 1 × 106 tachyzoites via intraperitoneal injection, and then sera samples were collected from the naive and vaccinated mice. Samples were subjected to ELISA assays to measure their IgG levels at 0, 14, 28, and 42 days post-immunization. In this study, there was a gradual increase in the level of anti-T. gondii IgG antibodies after immunization versus naive mice (Figure 3A). Moreover, the titers of IgG reached a peak at 42 days post-immunization. These results suggested that RH:ΔNPT1 induced a high-level humoral immune response in the immunized mice.
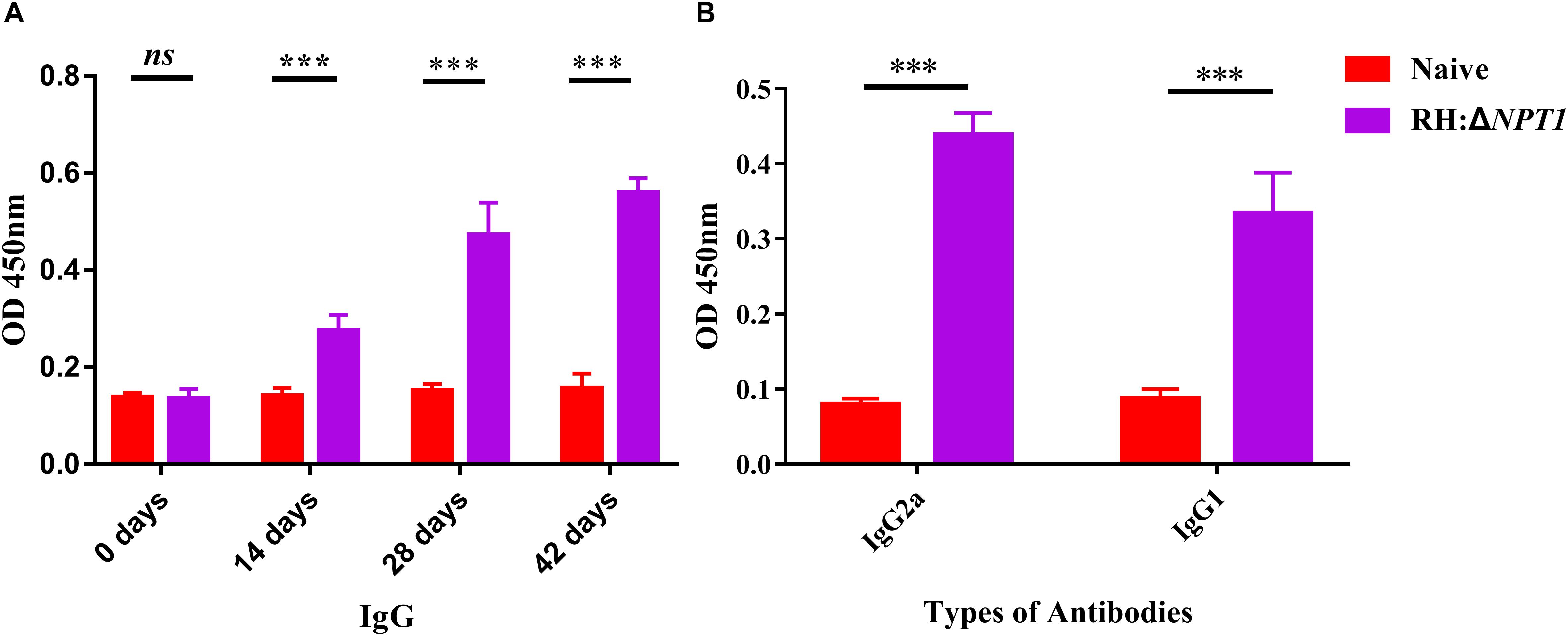
Figure 3. The level of humoral response in the serum of mice immunized with RH:ΔNPT1. (A) Levels of IgG antibody in the sera obtained from RH:ΔNPT1-immunized and non-immunized mice at 0, 14, 28, and 42 days post-vaccination suggest an effective humoral response was elected by the vaccination. (B) Levels of IgG subclass (IgG2a and IgG1) antibodies in the sera were collected at 42 days post-immunization, indicating a Th1 response was induced. The average of six biological replicates and three technical replicates are shown. The results are presented as the mean of OD450 ± SD (n = 6; ∗∗∗P < 0.001; n.s., not significant; ANOVA).
Subsequently, we confirmed that a Th1 or Th2 response was induced by the attenuated vaccine. Serum was collected at 42 days post-immunization to assess the levels of STAg-specific IgG2a and IgG1 isotypes. The serum levels of both IgG2a and IgG1 rose significantly following vaccination, which showed a mixed anti-T. gondii Th1/Th2 response elicited by RH:ΔNPT1 strain infection (Figure 3B). As expected, the augmentation of IgG2a was greater than that of IgG1, which confirmed that a Th1-type response was dominatingly induced.
Cytokine Measurement After Vaccination
To determine whether this clone could induce a sufficient number of cytokines in the immunized mice, the concentration of cytokines, including IFN-γ, IL-2, IL-4, IL-10, and IL-12 in the supernatants of antigen-stimulated splenic cells were determined by ELISA. In the supernatants, significantly higher levels of proinflammatory cytokines (IFN-γ, IL-2, and IL-12), which are essential for protection against intracellular pathogens, were found in the immunized mice relative to the naive mice. IFN-γ was increased the most by immunization (Figures 4A–C). Meanwhile, the production of Th2-associated cytokines (IL-4 and IL-10) by the immunized mice was evidently higher than that in the controls, whereas the augmentation of Th2-associated cytokines were not significantly higher than the Th1-associated cytokines in the vaccinated mice (Figures 4D,E). These findings are consistent with the results that were obtained through the detection of the anti-T. gondii IgG isotype, indicating that immunizing mice with RH:ΔNPT1 could induce a mixed Th1/Th2 response.
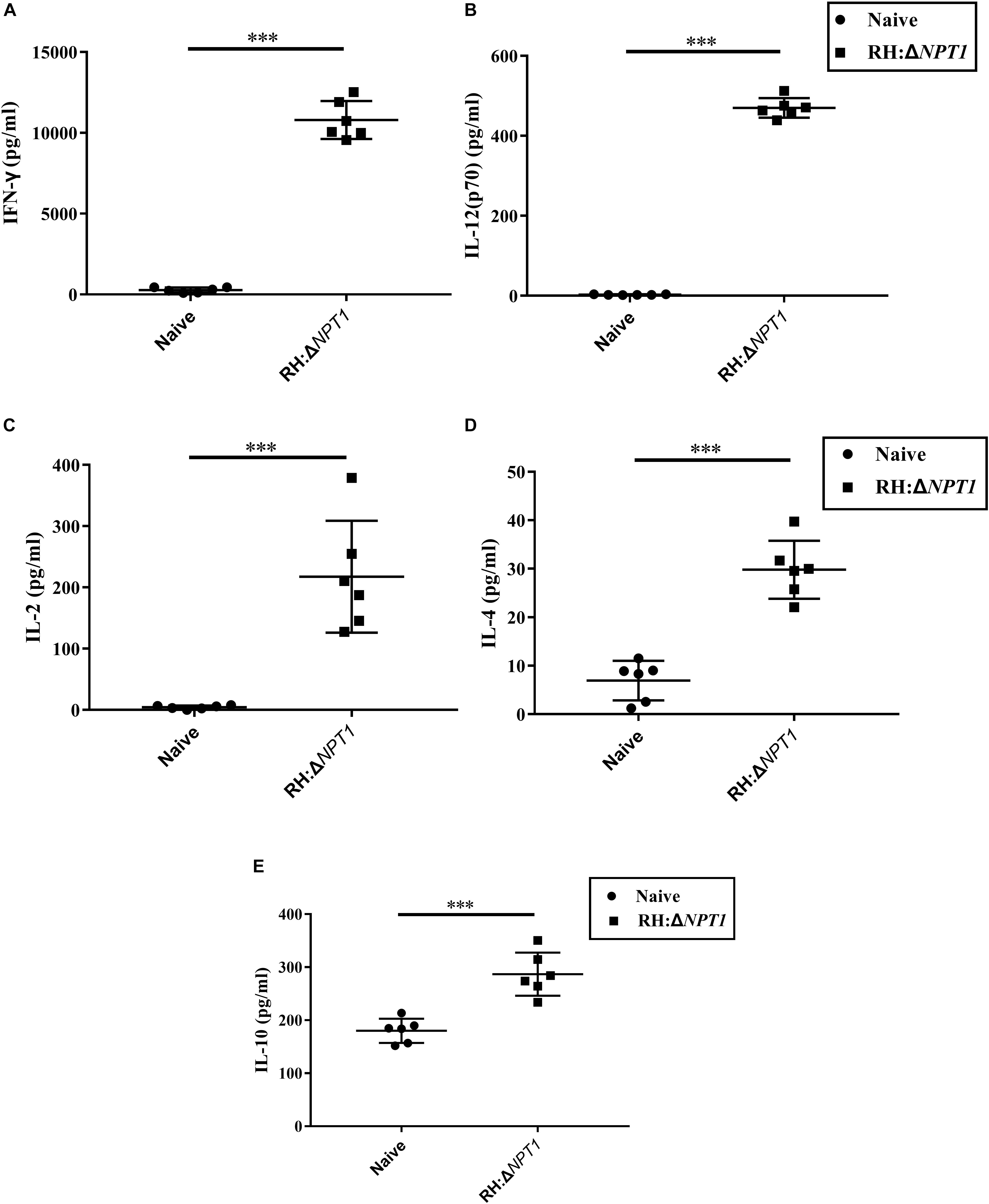
Figure 4. The levels of cytokines in the splenocyte suspension of mice immunized with RH:ΔNPT1. Splenocytes collected from the immunized and non-immunized mice (42 days post-vaccination) were co-incubated with STAg (10 μg/ml). Subsequently the levels of Th1 [IFN-γ (A), IL-12 (B), and IL-2 (C)] and Th2 [IL-4 (D) and IL-10 (E)] in the culture supernatants were analyzed by ELISA. The high levels of cytokines produced by splenocytes culture of immunized mice were significantly higher than naive mice. The results are presented as the mean of OD450 ± SD (n = 6; ∗∗∗P < 0.001; n.s., not significant; ANOVA).
Protection Against Acute Infection
Immunoprotection by the attenuated strain was estimated by challenging the mice with 1 × 103 tachyzoites from strains of different degrees of virulence at 6 weeks post-immunization. All mice, including immunized and non-immunized, were observed, including their healthy condition and survival time after challenge. The non-immunized mice infected with PYS and RH parasites exhibited symptoms of toxoplasmosis and died within 10 days post-infection. In contrast, the survival rates of RH:ΔNPT1-vaccinated mice challenged with PYS or RH parasites was 100%, and no obvious symptoms were exhibited over the course of 35 days (Figure 5). Therefore, RH:ΔNPT1 is able to provide efficient protection for the immunized mice against the lethal challenge of PYS or RH parasites.
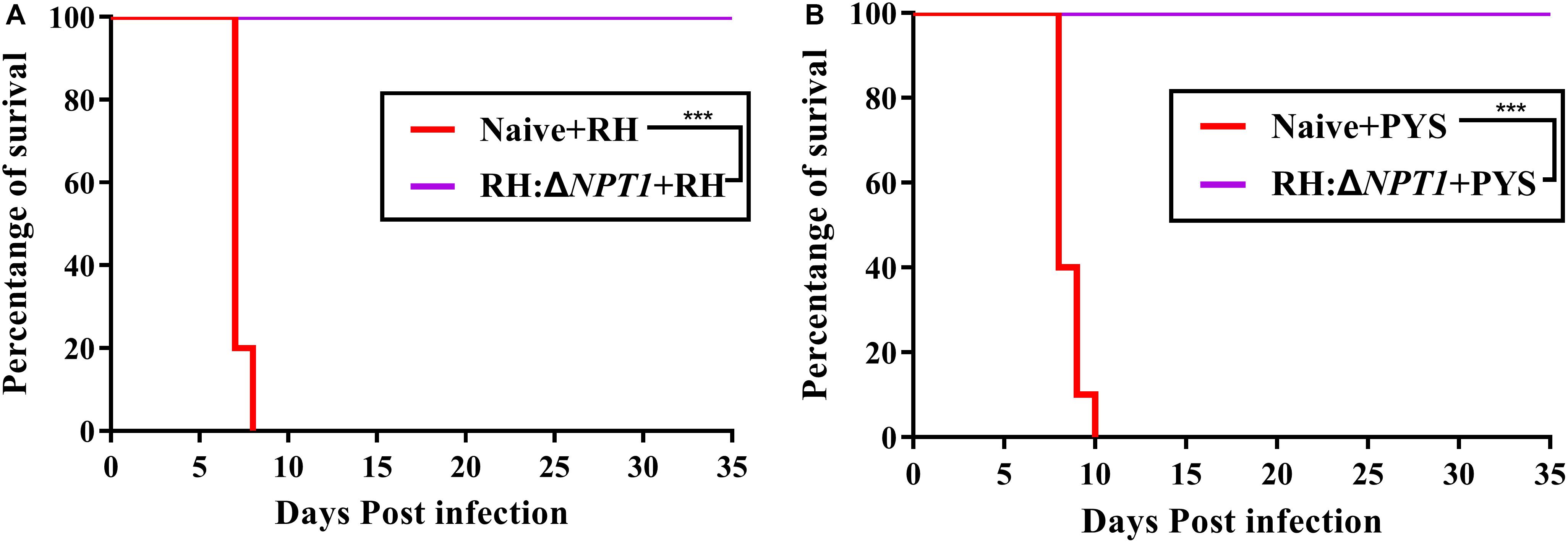
Figure 5. RH:ΔNPT1 vaccination protected against acute T. gondii infection. At 42 days post-immunization, mice were challenged with 1 × 103 tachyzoites of the RH (A) and PYS (B) strains by intraperitoneal injection. The survival rates of the immunized and non-immunized mice were monitored for 35 days. Survival curves showed the immunized mice had a high survival rate compared with the positive control (non-immunized mice) (n = 10; ∗∗∗P < 0.001; n.s., not significant; Mantel–Cox-test).
Protection Against Chronic Infection
To further examine whether the RH:ΔNPT1 parasites could offer effective protection to mice against chronic infection, we monitored disease progression and cyst burdens in the brains of RH:ΔNPT1 strain-immunized or naive mice. Throughout the experimental period, the infected-RH:ΔNPT1 mice that were orally infected with 100 cysts all survived, but the non-infected mice succumbed within 20 days (Figure 6A). When challenged with 20 cysts, the brain cyst burden in the immunized mice (50 ± 28 cysts/brain) was significantly reduced (P < 0.05) compared to that in non-immunized mice (3300 ± 453 cysts/brain) (Figure 6C). Some of the controls died after infection with 20 cysts of the T. gondii Pru strain (Figure 6B). Interestingly, the immunized mice challenged with 100 cysts not only survived until the experiment was completed but also exhibited fewer cysts in the brain, similar to that in the group infected with 100 cysts (data not shown). Taken together, these results showed the RH:ΔNPT1 strain is able to provide excellent protection for the immunized mice against T. gondii chronic infection.
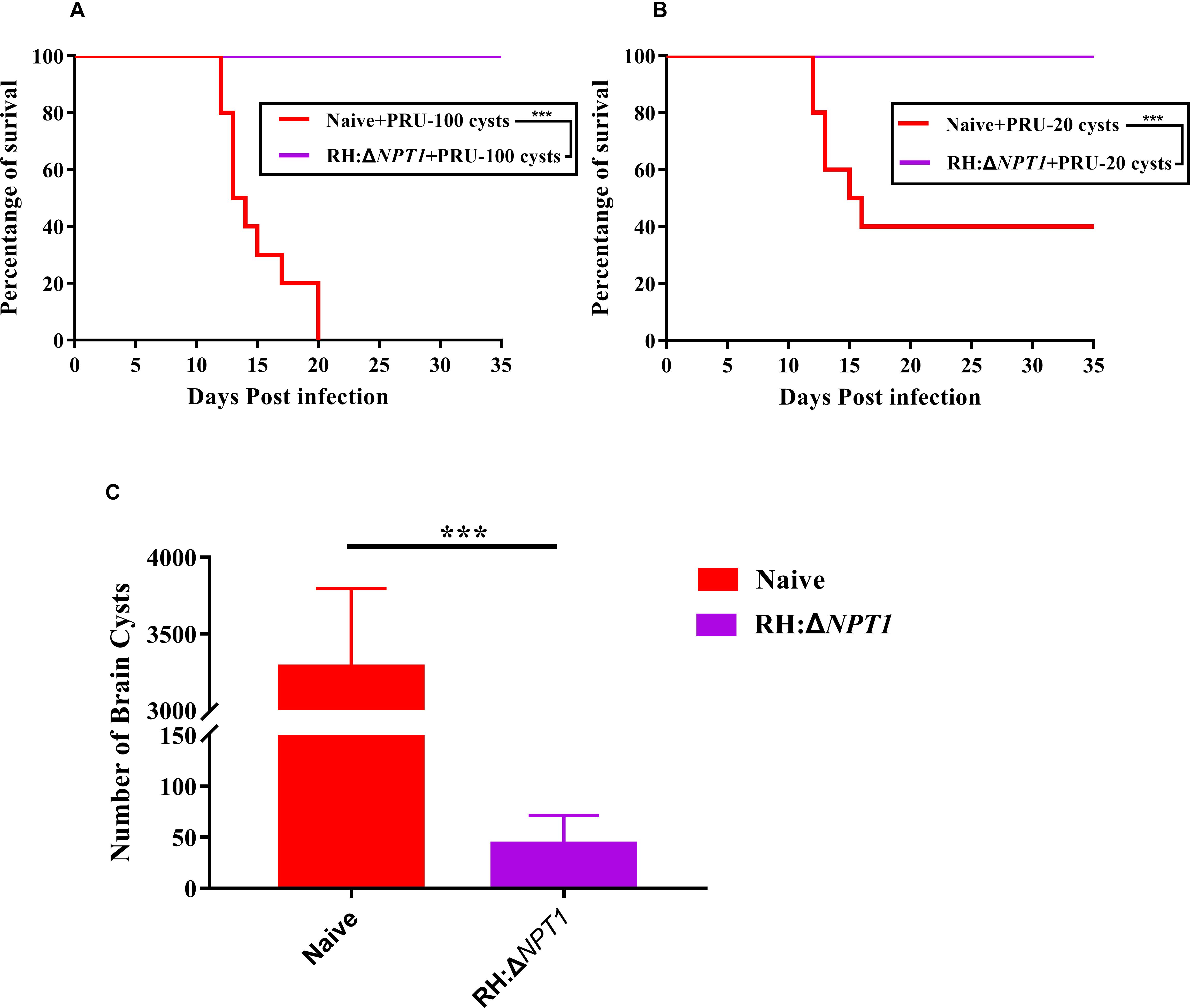
Figure 6. RH:ΔNPT1 vaccination promotes survival and reduced cysts loads in the brain. (A,B) Survival curves derived from mice infected with 20 or 100 cysts of the Pru strain. Compared with non-immunized mice, all of the vaccinated mice survived the challenge (n = 10; ∗∗∗P < 0.001; n.s., not significant; Mantel–Cox-test). (C) Protection efficiency of RH:ΔNPT1 against chronic infection was determined by cyst burden assays. Compared with the non-immunized mice, cyst burden in the brains of immunized mice were significantly reduced after the mice were infected with 20 cysts of the Pru strain. Significance was determined by ANOVA (n = 6; ∗∗∗P < 0.001; n.s., not significant).
Discussion
In light of the fact that T. gondii causes serious public health problems and enormous economic losses, safer and more efficient approaches need to be developed to control this ubiquitous pathogen (Dubey, 2010; Zhu et al., 2017). Previous studies have suggested that DNA vaccines, subunit vaccines, inactivated vaccines and secreted antigens could induce a certain level of humoral and cellular responses and prolong the lifespan of vaccinated mice, but none of these vaccines could provide complete protection against further infection (Innes et al., 2011; Ezz Eldin et al., 2015; Yang et al., 2017; Zheng et al., 2017). Although many researchers have performed a tremendous amount of work to pursue effective drugs and better vaccines, a mature product is unlikely to be available in the near future. However, these attempts appear to show that a live-attenuated vaccine may be the best choice. The incomplete strain S48 (Toxovax®) has shown promise in reducing the rates of abortion caused by T. gondii in sheep. Although this vaccine has some limitations that impede its large-scale application, the success of this attenuated strain displays a more sophisticated requests than other vaccines, and this vaccine should be further validated by experiments to assess its virulence, stability, immunoprotection, and oocyst formation ability (Wastling et al., 1994).
Using the efficient CRISPR-Cas9 strategy to edit genes of T. gondii has clearly accelerated the development of attenuated vaccines. This method has been used in the construction of RH:ΔGRA17, Pru:ΔCDPK2 and ME49:Δldh mutants to explore the possibility of whether the ablation of critical genes in tachyzoites of T. gondii could create an ideal vaccine. In this regard, these attempts were successful because the mouse model exhibited an effective protection after vaccination when the mice were faced with a lethal challenge (Shen et al., 2014; Wang et al., 2017, 2018; Xia et al., 2018). Previous studies have shown that NPT1 is a selective arginine transporter that plays an important role in uptake of cationic amino acids and thus is essential for the survival and virulence of T. gondii (Rajendran et al., 2016). When the NPT1 gene was knocked out in the wild-type T. gondii, the mutant strain failed to kill the infected mice, even after infection with 1 × 106 parasites (Rajendran et al., 2016). Based on this observation, we speculated that NPT1-defective mutants of a T. gondii RH strain might be a live attenuated vaccine candidate to prevent toxoplasmosis. To verify this speculation, we constructed the RH:ΔNPT1 strain by using the CRISPR-Cas9 method and obtained an effective vaccine candidate against toxoplasmosis.
The RH:ΔNPT1 strain exhibited some characteristics that meet the prerequisite of an ideal live vaccine. One striking characteristic is that the parasites lacking NPT1 developed normally within RPMI 1640 medium but could not proliferate in DMEM medium, analogous to what has been described in the drug screening functions of the other edited strains. Acquiring the RH:ΔNPT1 strain without the need for a drug-screening step makes it easier to produce. If the construction of the RH:ΔNPT1 strain is adopted for use in inserting other genes, this screening method will avoid the occurrence of drug-resistant strains.
The RH strain of T. gondii lack the potential for production of bradyzoite and tissue cyst forming (Asgari et al., 2013), which is an important factor for an ideal attenuated vaccine to leave no cysts in the immunized groups in order to avoid the threat of parasite dissemination via vaccination. For this reason, we decided to start with the RH strain, which probably overcomes some limitations presented by the strains Pru and ME49 (for example, no cyst formation or better immunogenicity), including lack of the risk of transmittance during vaccination or the possibility of a back mutation (which might happen when a cat is vaccinated). Thus, all of these advantages suggest that the RH:ΔNPT1 strain is more secure than others to use when developing a vaccine.
RH:ΔNPT1 did not cause any deaths even when the inoculation dose reached 1 × 106 tachyzoites/mouse, which demonstrated that this vaccine has a high biosafety among mice with a normal immunity (Gigley et al., 2009; Fox and Bzik, 2015; Wang et al., 2017, 2018; Xia et al., 2018). However, further research is still required to test whether this attenuated strain can be safely administered to immunocompromised mice.
Specific IgGs are a critical factor in protecting host cells from infection (Sayles et al., 2000; Chen et al., 2017). By detecting levels of specific IgGs in the serum from the vaccinated mice, we demonstrated that RH:ΔNPT1 could elicit a high level of anti-T. gondii IgG antibodies. Moreover, the RH:ΔNPT1-vaccinated mice also exhibited a sequential increase in IgG1 and IgG2a, indicating that a Th1/Th2-type mixed immune response was elicited and maintained by immunization. It has been previously reported that IgG2a is more efficient in clearing tachyzoites of T. gondii than IgG1 in rodent models (Wang et al., 2018). Thus, in order to better elucidate the humoral immune responses provoked by RH:ΔNPT1, we measured the ratio of IgG1/IgG2a by ELISA. We found the level of IgG2a was higher than that of IgG1, suggesting that a prominent Th1 type humoral response was successfully elicited in the vaccinated mice 42 days post-vaccination. If this attenuated strain is considered for use as an ideal commercial vaccine, further research into its ability to provoke long-term immune protection is necessary, similar to the studies conducted on the 1-1 strain (Gigley et al., 2009).
When mice are infected with T. gondii, an IFN-γ-dependent cell-mediated immune response is the central contributor to limiting tachyzoite proliferation, while a humoral immune response is induced to a relatively lesser extent (Sasai et al., 2018). This expected trend was observed with this strain. RH:ΔNPT1-vaccinated mice produced high levels of Th1-associated cytokines (IFN-γ, IL-2 and IL-12), which can trigger multiple intracellular mechanisms to control and kill tachyzoites by activating cytotoxic lymphocytes and macrophages, leading to effective protection against T. gondii acute infection (Khan et al., 1994; Gazzinelli et al., 1996; Sa et al., 2013). We also found that the levels of Th2-biased cytokines (IL-4 and IL-10) were increased. IL-10 and IL-4 are important negative regulators of inflammatory responses during the course of infection with T. gondii, which are sufficient to provoke a Th1 immune response to avoid host mortality associated with the development of severe immunopathology (Gazzinelli et al., 1996; Roberts et al., 1996; Suzuki et al., 2000). IL-4 can modify intracellular replication and prevent cyst formation in the brain, and thus, the high level of IL-4 may shed light on the reduced number of brain cysts in chronic infection (Suzuki et al., 1996).
Considering the results of the elevated levels of IgG and cytokines in the immunized mice, we concluded that RH:ΔNPT1 could confer effective protection for mice against a lethal challenge. As anticipated, the non-immunized mice were euthanized due to severe illness within 7–10 days post-infection, whereas all immunized mice survived, indicating that vaccination with RH:ΔNPT1 is able to achieve complete protection of mice against challenge with strains of different degrees of virulence. It is worth noting that compared with most existing vaccines, RH:ΔNPT1 can prevent damage in many aspects, such as the death caused by high-dose infection, the incomplete protection caused by low-dose infection, and the formation of cysts (Wang et al., 2018).
To test the protective efficacy of the RH:ΔNPT1 mutant strain against chronic infection, the vaccinated mice were orally infected with 20 or 100 cysts of type II Pru. Consistent with the results of the cytokines, this vaccination severely reduced the parasite cyst burden and potentially prevented cyst formation in the brain of the vaccinated mice as a means of enhancing protection after ingestion of a high dose of type II cysts. However, there was the somewhat unexpected fact that vaccination could not completely prevent cyst formation in the chronic infection experiment, and a few brain cysts were observed in the mice vaccinated with the RH:ΔNPT1 strain. To improve its protective effect against cyst formation after infection with a type II strain, the vaccine needs to be further developed to focus on other strains, such as Pru and ME49, as material to confirm the efficiency of various attenuated vaccines (Wang et al., 2018; Xia et al., 2018). For preparation of a live-attenuation vaccine, we suggest using different endemic strains with distinct capabilities of pathogenicity to confirm the direct role of vaccination in immunity.
Conclusion
In conclusion, in this study, we constructed a RH:ΔNPT1 mutant strain and evaluated its potential as a live attenuated vaccine against T. gondii infection. The results demonstrated that vaccination with the RH:ΔNPT1 mutant strain can elicit high levels of humoral and cellular immune responses and provide effective immune protection against infection in mice. RH:ΔNPT1 not only can completely protect against acute infection from various genotype strains of T. gondii but also can improve the survival rate and reduce the brain cyst burden in response to chronic infection, suggesting that the RH:ΔNPT1 strain is a potential and promising live-attenuated vaccine against toxoplasmosis. Further work should evaluate its protective efficacy in food-producing animals and in the definitive feline host.
Data Availability
All datasets generated for this study are included in the manuscript.
Ethics Statement
This study was approved by the Animal Administration and Ethics Committee of Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences. All mice were cared for and handled in accordance with the regulations of the Good Animal Practice requirements of the Animal Ethics Procedures and Guidelines of the People’s Republic of China for animal experimentation. All efforts were made to minimize animal suffering and to reduce the numbers of animals used in the experiments.
Author Contributions
D-HZ, J-LW, and X-QZ conceived the project, designed the experiments, and critically revised the manuscript. W-BY, J-LW, QG, and KC performed the experiments and analyzed the data. W-BY and J-LW drafted the manuscript. YZ, QL, and Q-LL helped in the implementation of the experiments. All authors reviewed and approved the final version of the manuscript.
Funding
Project support was provided by the National Natural Science Foundation of China (Grant No. 31672549), National Key Research and Development Program of China (Grant No. 2017YFD0501304), the Open Fund of the Key Laboratory of Fujian Province Livestock Epidemic Prevention, Control and Biological Technology (2018KF02), and the Agricultural Science and Technology Innovation Program (ASTIP) (Grant No. CAAS-ASTIP-2016-LVRI-03).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to Meng-Jie Bai, Xiao-Dan Yuan, and Lan-Bi Nie from the Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences for technical assistance.
References
Alday, P. H., and Doggett, J. S. (2017). Drugs in development for toxoplasmosis: advances, challenges, and current status. Drug Des. Dev. Ther. 11, 273–293. doi: 10.2147/DDDT.S60973
Asgari, Q., Keshavarz, H., Shojaee, S., Motazedian, M. H., Mohebali, M., Miri, R., et al. (2013). In Vitro and In Vivo potential of RH strain of Toxoplasma gondii (Type I) in tissue cyst forming. Iran. J. Parasitol. 8, 367–375.
Buxton, D., and Innes, E. A. (1995). A commercial vaccine for ovine toxoplasmosis. Parasitology 110(Suppl.), S11–S16. doi: 10.1017/S003118200000144X
Chen, K., Wang, J. L., Huang, S. Y., Yang, W. B., Zhu, W. N., and Zhu, X. Q. (2017). Immune responses and protection after DNA vaccination against Toxoplasma gondii calcium-dependent protein kinase 2 (TgCDPK2). Parasite 24:41. doi: 10.1051/parasite/2017045
Doliwa, C., Escotte-Binet, S., Aubert, D., Sauvage, V., Velard, F., Schmid, A., et al. (2013). Sulfadiazine resistance in Toxoplasma gondii: no involvement of overexpression or polymorphisms in genes of therapeutic targets and ABC transporters. Parasite 20:19. doi: 10.1051/parasite/2013020
Dubey, J. P. (2010). Toxoplasmosis of Animals and Humans, 2nd Edn. Boca Raton, FL: CRC Press, 1–313.
Elbez-Rubinstein, A., Ajzenberg, D., Dardé, M. L., Cohen, R., Dumètre, A., Year, H., et al. (2009). Congenital toxoplasmosis and reinfection during pregnancy: case report, strain characterization, experimental model of reinfection, and review. J. Infect. Dis. 199, 280–285. doi: 10.1086/595793
Elmore, S. A., Jones, J. L., Conrad, P. A., Patton, S., Lindsay, D. S., and Dubey, J. P. (2010). Toxoplasma gondii: epidemiology, feline clinical aspects, and prevention. Trends Parasitol. 26, 190–196. doi: 10.1016/j.pt.2010.01.009
Ezz Eldin, H. M., Kamel, H. H., Badawy, A. F., and Shash, L. S. (2015). A comparative study between excretory/secretory and autoclaved vaccines against RH strain of Toxoplasma gondii in murine models. J. Parasit. Dis. 39, 526–535. doi: 10.1007/s12639-013-0390-6
Fox, B. A., and Bzik, D. J. (2015). Nonreplicating, cyst-defective type II Toxoplasma gondii vaccine strains stimulate protective immunity against acute and chronic infection. Infect. Immun. 83, 2148–2155. doi: 10.1128/IAI.02756-14
Gazzinelli, R. T., Wysocka, M., Hieny, S., Scharton-Kersten, T., Cheever, A., Kuhn, R., et al. (1996). In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J. Immunol. 157, 798–805.
Gigley, J. P., Fox, B. A., and Bzik, D. J. (2009). Long-term immunity to lethal acute or chronic type II Toxoplasma gondii infection is effectively induced in genetically susceptible C57BL/6 mice by immunization with an attenuated type I vaccine strain. Infect. Immun. 77, 5380–5388. doi: 10.1128/IAI.00649-09
Gurunathan, S., Klinman, D. M., and Seder, R. A. (2000). DNA vaccines: immunology, application, and optimization. Annu. Rev. Immunol. 11, 1983–1990. doi: 10.1080/21645515.2015.1011987
Innes, E. A., Bartley, P. M., Rocchi, M., Benavidas-Silvan, J., Burrells, A., Hotchkiss, E., et al. (2011). Developing vaccines to control protozoan parasites in ruminants: dead or alive? Vet. Parasitol. 180, 155–163. doi: 10.1016/j.vetpar.2011.05.036
Khan, I. A., Matsuura, T., and Kasper, L. H. (1994). Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect. Immun. 62, 1639–1642.
Kur, J., Holec-Gasior, L., and Hiszczynska-Sawicka, E. (2009). Current status of toxoplasmosis vaccine development. Expert Rev. Vaccines 8, 791–808. doi: 10.1586/erv.09.27
Lang, D., Schott, B. H., van Ham, M., Morton, L., Kulikovskaja, L., Herrera-Molina, R., et al. (2018). Chronic Toxoplasma infection is associated with distinct alterations in the synaptic protein composition. J. Neuroinflammation 15:216. doi: 10.1186/s12974-018-1242-1
Li, Z. Y., Chen, J., Petersen, E., Zhou, D. H., Huang, S. Y., Song, H. Q., et al. (2014). Synergy of mIL-21 and mIL-15 in enhancing DNA vaccine efficacy against acute and chronic Toxoplasma gondii infection in mice. Vaccine 32, 3058–3065. doi: 10.1016/j.vaccine.2014.03.042
Mévélec, M. N., Ducournau, C., Bassuny Ismael, A., Olivier, M., Sèche, E., Lebrun, M., et al. (2010). Mic1-3 knockout Toxoplasma gondii is a good candidate for a vaccine against T. gondii-induced abortion in sheep. Vet. Res 41:49. doi: 10.1051/vetres/2010021
Montoya, J. G., and Liesenfeld, O. (2004). Toxoplasmosis. Lancet 363, 1965–1976. doi: 10.1016/S0140-6736(04)16412-X
Petersen, E., Pollak, A., and Reiter-Owona, I. (2001). Recent trends in research on congenital toxoplasmosis. Int. J. Parasitol. 31, 115–144. doi: 10.1016/S0020-7519(00)00140-5
Rajendran, E., Hapuarachchi, S. V., Miller, C. M., Fairweather, S. J., Cai, Y., Smith, N. C., et al. (2016). Cationic amino acid transporters play key roles in the survival and transmission of apicomplexan parasites. Nat. Commun. 8:14455. doi: 10.1038/ncomms14455
Roberts, C. W., Ferguson, D. J., Jebbari, H., Satoskar, A., Bluethmann, H., and Alexander, J. (1996). Different roles for interleukin-4 during the course of Toxoplasma gondii infection. Infect. Immun. 64, 897–904.
Sa, Q., Woodward, J., and Suzuki, Y. (2013). IL-2 produced by CD8+ immune T cells can augment their IFN-γ production independently from their proliferation in the secondary response to an intracellular pathogen. J. Immunol. 190, 2199–2207. doi: 10.4049/jimmunol.1202256
Sasai, M., Pradipta, A., and Yamamoto, M. (2018). Host immune responses to Toxoplasma gondii. Int. Immunol. 30, 113–119. doi: 10.1093/intimm/dxy004
Sayles, P. C., Gibson, G. W., and Johnson, L. L. (2000). B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect. Immun. 68, 1026–1033. doi: 10.1128/iai.68.3.1026-1033.2000
Shen, B., Brown, K. M., Lee, T. D., and Sibley, L. D. (2014). Efficient gene disruption in diverse strains of Toxoplasma gondii using CRISPR/CAS9. mBio 5:e1114. doi: 10.1128/mBio.01114-14
Suzuki, Y., Sher, A., Yap, G., Park, D., Neyer, L. E., Liesenfeld, O., et al. (2000). IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J. Immunol. 164, 5375–5382. doi: 10.4049/jimmunol.164.10.5375
Suzuki, Y., Yang, Q., Yang, S., Nguyen, N., Lim, S., Liesenfeld, O., et al. (1996). IL-4 is protective against development of toxoplasmic encephalitis. J. Immunol. 157, 2564–2569.
Wang, J. L., Elsheikha, H. M., Zhu, W. N., Chen, K., Li, T. T., Yue, D. M., et al. (2017). Immunization with Toxoplasma gondii GRA17 deletion mutant induces partial protection and survival in challenged mice. Front. Immunol. 8:730. doi: 10.3389/fimmu.2017.00730
Wang, J. L., Huang, S. Y., Li, T. T., Chen, K., Ning, H. R., and Zhu, X. Q. (2016). Evaluation of the basic functions of six calcium-dependent protein kinases in Toxoplasma gondii using CRISPR-Cas9 system. Parasitol. Res. 115, 697–702. doi: 10.1007/s00436-015-4791-6
Wang, J. L., Li, T. T., Elsheikha, H. M., Chen, K., Cong, W., Yang, W. B., et al. (2018). Live attenuated Pru:Δcdpk2 strain of Toxoplasma gondii protects against acute, chronic, and congenital toxoplasmosis. J. Infect. Dis. 218, 768–777. doi: 10.1093/infdis/jiy211
Wang, J. L., Zhang, N. Z., Li, T. T., He, J. J., Elsheikha, H. M., and Zhu, X. Q. (2019). Advances in the development of Anti-Toxoplasma gondii vaccines: challenges, opportunities, and perspectives. Trends Parasitol. 35, 239–253. doi: 10.1016/j.pt.2019.01.005
Wastling, J. M., Harkins, D., and Buxton, D. (1994). Western blot analysis of the IgG response of sheep vaccinated with S48 Toxoplasma gondii (Toxovax). Res. Vet. Sci. 57, 384–386. doi: 10.1016/(94)90135-X
Xia, N., Zhou, T., Liang, X., Ye, S., Zhao, P., Yang, J., et al. (2018). A lactate fermentation mutant of toxoplasma stimulates protective immunity against acute and chronic toxoplasmosis. Front. Immunol. 9:1814. doi: 10.3389/fimmu.2018.01814
Xu, Y., Zhang, N. Z., Wang, M., Dong, H., Feng, S. Y., Guo, H. C., et al. (2015). A long-lasting protective immunity against chronic toxoplasmosis in mice induced by recombinant rhoptry proteins encapsulated in poly (lactide-co-glycolide) microparticles. Parasitol. Res. 114, 4195–4203. doi: 10.1007/s00436-015-4652-3
Yang, W. B., Zhou, D. H., Zou, Y., Chen, K., Liu, Q., Wang, J. L., et al. (2017). Vaccination with a DNA vaccine encoding Toxoplasma gondii ROP54 induces protective immunity against toxoplasmosis in mice. Acta Trop. 176, 427–432. doi: 10.1016/j.actatropica.2017.09.007
Zhang, N. Z., Chen, J., Wang, M., Petersen, E., and Zhu, X. Q. (2013). Vaccines against Toxoplasma gondii: new developments and perspectives. Expert Rev. Vaccines 12, 1287–1299. doi: 10.1586/14760584.2013.844652
Zhang, N. Z., Gao, Q., Wang, M., Elsheikha, H. M., Wang, B., Wang, J. L., et al. (2018). Immunization with a DNA vaccine cocktail encoding TgPF, TgROP16, TgROP18, TgMIC6, and TgCDPK3 genes protects mice against chronic toxoplasmosis. Front. Immunol. 9:1505. doi: 10.3389/fimmu.2018.01505
Zhang, N. Z., Wang, M., Xu, Y., Petersen, E., and Zhu, X. Q. (2015). Recent advances in developing vaccines against Toxoplasma gondii: an update. Expert Rev. Vaccines 14, 1609–1621. doi: 10.1586/14760584.2015.1098539
Zheng, B., Ding, J., Chen, X., Yu, H., Lou, D., Tong, Q., et al. (2017). Immuno-efficacy of a T. gondii secreted protein with an altered thrombospondin repeat (TgSPATR) as a novel DNA vaccine candidate against acute toxoplasmosis in BALB/c mice. Front. Microbiol. 8:216. doi: 10.3389/fmicb.2017.00216
Zhu, W. N., Wang, J. L., Chen, K., Yue, D. M., Zhang, X. X., Huang, S. Y., et al. (2017). Evaluation of protective immunity induced by DNA vaccination with genes encoding Toxoplasma gondii GRA17 and GRA23 against acute toxoplasmosis in mice. Exp. Parasitol. 179, 20–27. doi: 10.1016/j.exppara.2017.06.002
Keywords: Toxoplasma gondii, RH:ΔNPT1, immunization, live-attenuated vaccine, mice
Citation: Yang W-B, Wang J-L, Gui Q, Zou Y, Chen K, Liu Q, Liang Q-L, Zhu X-Q and Zhou D-H (2019) Immunization With a Live-Attenuated RH:ΔNPT1 Strain of Toxoplasma gondii Induces Strong Protective Immunity Against Toxoplasmosis in Mice. Front. Microbiol. 10:1875. doi: 10.3389/fmicb.2019.01875
Received: 16 April 2019; Accepted: 29 July 2019;
Published: 13 August 2019.
Edited by:
Lihua Xiao, South China Agricultural University, ChinaReviewed by:
Longxian Zhang, Henan Agricultural University, ChinaMario Alberto Flores-Valdez, CONACYT Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco (CIATEJ), Mexico
Copyright © 2019 Yang, Wang, Gui, Zou, Chen, Liu, Liang, Zhu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin-Lei Wang, d2FuZ2ppbmxlaTkwQDEyNi5jb20=
Dong-Hui Zhou, ZG9uZ2h1aTgyMjAwMkAxNjMuY29t
 Wen-Bin Yang
Wen-Bin Yang Jin-Lei Wang
Jin-Lei Wang Qian Gui3
Qian Gui3 Yang Zou
Yang Zou Kai Chen
Kai Chen Xing-Quan Zhu
Xing-Quan Zhu Dong-Hui Zhou
Dong-Hui Zhou