- 1Department of Pediatrics, The University of Alabama at Birmingham, Birmingham, AL, United States
- 2Department of Medicine, The University of Alabama at Birmingham, Birmingham, AL, United States
Rationale: Mycoplasmas represent important etiologic agents of many human diseases. Due to increasing antimicrobial resistance and slow rate of novel discovery, unconventional methods of drug discovery are necessary. Copper ions are utilized in host microbial killing, and bacteria must regulate intracellular Cu concentrations to avoid toxicity. We hypothesized that human mollicutes may have susceptibility to Cu-induced toxicity, and compounds that augment copper-dependent killing.
Methods: Mycoplasma pneumoniae (Mpn), Ureaplasma parvum (Up), Ureaplasma urealyticum (Uu), and Mycoplasma hominis (Mh) were exposed to CuSO4 to determine minimal inhibitory concentrations (MICs). Once inhibitory concentrations had been determined, bacteria were treated with an FDA-approved drug disulfiram (DSF), glyoxal bis(4-methyl-3-thiosemicarbazone) (GTSM), and 2,9-dimethyl-1,10-phenanthroline (neocuproine), with or without Cu2+, to determine compound MICs.
Results: Ureaplasma species and Mh were able to tolerate 30–60 μM CuSO4, while Mpn tolerated over 10-fold higher concentrations (>1 mM). GTSM inhibited growth of all four organisms, but was unaffected by Cu2+ addition. Inhibition by GTSM was reduced by addition of the cell-impermeant Cu chelator, bathocuproine disulfonate (BCS). Neocuproine exhibited Cu-dependent growth inhibition of all organisms. DSF exhibited Cu-dependent growth inhibition against Mh at low micromolar concentrations, and at intermediate concentrations for Mpn.
Conclusion: MICs for CuSO4 differ widely among human mollicutes, with higher MICs for Mpn compared to Mh, Uu, and Up. DSF and Neocuproine exhibit Cu-dependent inhibition of mollicutes with copper concentrations between 25 and 50 μM. GTSM has copper-dependent anti-microbial activity at low levels of copper. Drug enhanced copper toxicity is a promising avenue for novel therapeutic development research with Mycoplasma and Ureaplasma species.
Introduction
Mollicutes are a class of bacteria that lack a cell wall and are important etiologic agents of many human diseases. Global antibiotic resistance rates in mollicutes differ based on bacterial species, geographical location, and patient population, but rising antibiotic resistance is a concern for some species and antibiotic classes. The trends toward increasing antimicrobial resistance worldwide underscore the need for novel antimicrobial compound discovery. The importance of copper in macrophage-mediated microbial killing has suggested that copper-transporting compounds might prove useful as antimicrobial agents (White et al., 2009).
Screening compounds for copper-dependent killing activity has resulted in the identification of new compounds with copper-dependent antimicrobial activity against Mycobacterium tuberculosis and Staphylococcus aureus (Dalecki et al., 2016; Shah et al., 2016). Previous work has been described examining the effect of antimicrobial compounds on copper-induced stress in Mycoplasma gallisepticum (Smit et al., 1982; Gaisser et al., 1987a,b; de Zwart et al., 1991), but to date studies in this area have not been conducted with human mycoplasmas or ureaplasmas. In the current study, we sought to determine whether compounds with Cu-binding activity exhibit antimicrobial activity against human mollicutes.
Materials and Methods
Drugs and Compounds
Copper sulfate, iron sulfate heptahydrate (FeSO4), zinc sulfate heptahydrate (ZnSO4), and manganese dichloride (MnCl2) were purchased from Sigma-Aldrich, dissolved in water as 100 mM stock solutions and stored at 4°C. The FeSO4 solution was always prepared fresh prior to use. Bathocuproinedisulfonic acid was purchased from Fisher Scientific and stored as a 100 mM stock solution in water at −80°C. Stocks of test compounds were prepared in DMSO and stored at −80°C as follows: Neo (Sigma-Aldrich) 10 mM, DSF (Sigma-Aldrich) 40 mM, bathocuproine (Sigma-Aldrich) 1 mM, and GTSM 10 mM. GTSM was a kind gift from Dr. Stefan Bossmann, who synthesized the compound in his laboratory at Kansas State University following published protocols (Haeili et al., 2014).
Bacterial Strains and Culture
Bacterial strains for each species were utilized for in vitro analyses throughout the course of this work. Clinical isolates were the generous gift of Dr. Ken B. Waites at the University of Alabama at Birmingham Diagnostic Mycoplasma Laboratory. For Mycoplasma pneumoniae, strains M129 (ATCC 29342), FH (ATCC 15531), UAB PO1 (Clinical isolate), and 54524 (Clinical isolate, strain also known as UAB JZY) were used. Ureaplasma parvum and Ureaplasma urealyticum isolates were: Up3 (serovar 3, ATCC 700970), Uu10 (serovar 10, ATCC 33699), and Uu9 (serovar 9, ATCC 33175) (Xiao et al., 2014). Mycoplasma hominis isolates included the type strain PG21 (ATCC 23114), and 55391 (clinical isolate).
Bacteria were cultured in SP4 or 10B broth, and SP4 or A8 agar prepared by the UAB Diagnostic Mycoplasma Laboratory, as described previously (Waites et al., 2012b; Xiao et al., 2014; Totten et al., 2017). In brief, bacterial isolates were thawed from frozen stocks at known concentrations prior to assay conditions, and then diluted as per laboratory standards for human mollicutes MIC assays (Waites et al., 2012a). Bacterial CFU/mL concentrations ranged from 104 to 105 for tested species to ensure accurate susceptibility testing. Growth of all mollicutes species was carried out prepared as previously described. Mpn strains were grown in SP4 broth at 37°C for 5–7 days and examined for yellow-orange color change. Mh isolates were additionally grown in SP4 broth, sealed with adhesive plate sealers and incubated at 37°C for 1–3 days and examined for color change. Ureaplasma spp. were cultured in 10B broth, sealed with adhesive plate sealers, and grown at 37°C for 1–2 days prior to media color change. CFU/mL concentrations were additionally examined for each plate to ensure accurate CFU range on solid media (SP4 agar, or A8 agar) as described previously (Xiao et al., 2014; Totten et al., 2017). Growth index measurements (Feng et al., 2018) were carried out on a microplate reader (Cytation 3, Biotek) by calculating the absorption ratio as follows for each species: Mpn (A430/A560) and Mh/Ureaplasma spp. (A560/A430).
Metal Content Analysis
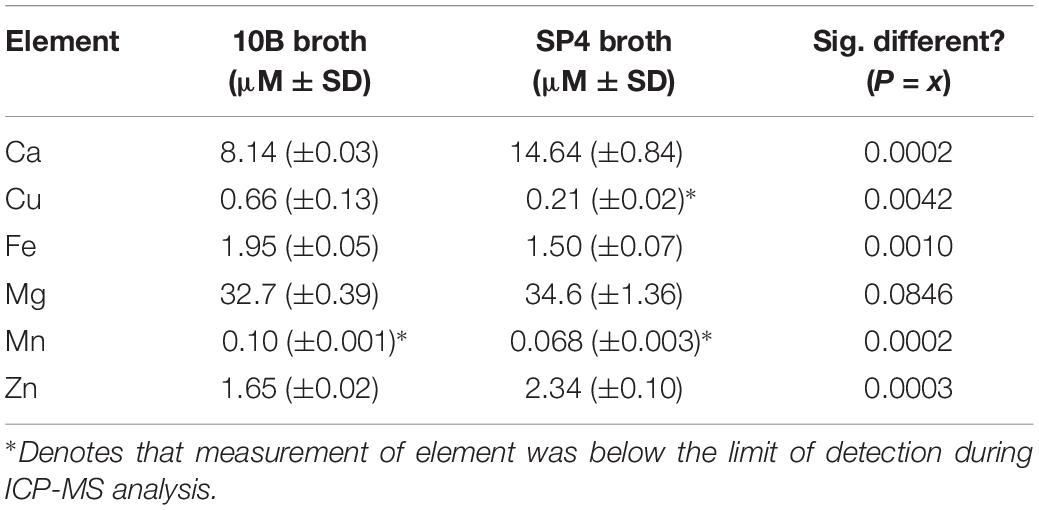
A milliliter of SP4 and 10B was dried overnight in a 65°C oven. The dried pellet was suspended in 500 μL trace metal grade nitric acid (∼70%, Fisher Scientific) and incubated at 65°C for 6 h. The samples were further diluted to 2% nitric acid in LC/MS grade water (Fisher Scientific) prior to analysis. The media was analyzed for the content of six metals by ICP-MS (Agilent) (Cu, Ca, Fe, Mn, Mg, and Zn) and reported as micromolar concentrations. Metal content was determined by comparison to a standard curve (Millipore) with the detection limit of 275 nM copper. The machine was calibrated using Lithium, Scandium, Germanium, and Indium as internal standards (High Purity Standards). Samples were analyzed in triplicate, and error bars represent standard deviations. Data were analyzed using Agilent’s offline data analysis program.
Cu and Compound Microdilutions
For metal toxicity studies, CuSO4 or other transition metals were serially diluted 1:2 from 1 mM to 1.9 μM in SP4 or 10B broth. Bacteria were added to respective wells and incubated as outlined earlier. The assay working concentration for exogenous Cu for each organism was lower than that showing inhibition of bacterial growth in vitro (Table 1).
For compound or drug MICs, broth medium was diluted with or without transition metals at predetermined working concentrations tolerated by each bacterial strain. Compounds for MIC testing were added in serial twofold dilutions up to 10 μM. For MICs in media containing bathocuproine, the compound was diluted to 500 μM final concentration in medium with bacteria for 1 h at 37°C prior to addition to MIC plates. Bacteria were then added to respective wells and their rates of growth measured over time as described above.
Statistical Analysis
Unless otherwise noted, sample means ± SD were utilized for comparison of statistical significance. When data were normally distributed, comparison of more than two experimental groups was performed utilizing One-way ANOVA with Bonferroni pair-wise post-tests or Tukey ad hoc post-test. Where appropriate, two-way ANOVAs were utilized for data groups with more than two independent variables. Log10 transformation was utilized to increase the likelihood of Gaussian distribution on specific data sets. Non-parametric data were analyzed utilizing the Mann–Whitney U test, with a Dunn’s post hoc test for pairwise comparisons. With data sets containing two experimental groups, unpaired t-tests were utilized for analysis. One-tailed tests were utilized to increase statistical significance where prior data had indicated directionality under two-tailed conditions. Data were graphed using Graphpad PRISM v. 8 (Graphpad Software, Inc.). Differences were considered statistically significant when p < 0.05. For significance values the following symbols were used: * for p < 0.05, ∗∗ for p < 0.01, ∗∗∗ for p < 0.001, and **** for p < 0.0001.
Results
MIC for Copper Is Higher for Mpn Than for Urogenital Mollicutes
Prior to examination of susceptibilities of human mollicutes to antimicrobial compounds, elemental analysis was undertaken on samples of the broth media 10B and SP4, which were used to grow Mycoplasma and Ureaplasma spp. in the subsequent experiments. Sterile broth was subjected to elemental analysis by ICP-MS. Elemental analysis carried out for calcium, copper, iron, magnesium, manganese, and zinc showed significant differences between 10B and SP4 (Table 1), but importantly for the studies reported here, the copper concentration was less than 1 μM in both media. 10B had significantly more elemental Cu and Fe and lower amounts of Ca and Zn than SP4. Mn was below the limit of detection (LoD) in both media types, while only SP4 had levels of Cu below the LoD of the ICP-MS (Table 1).
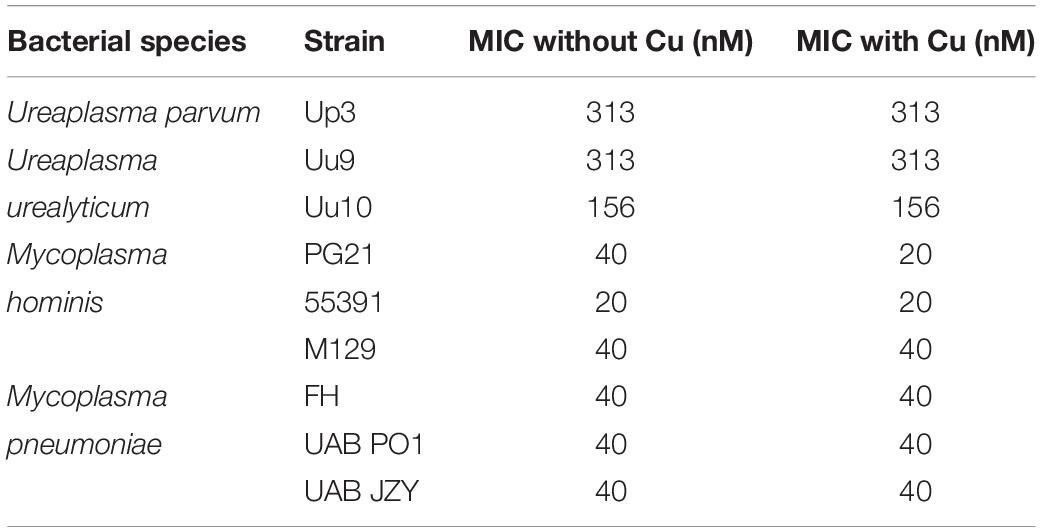
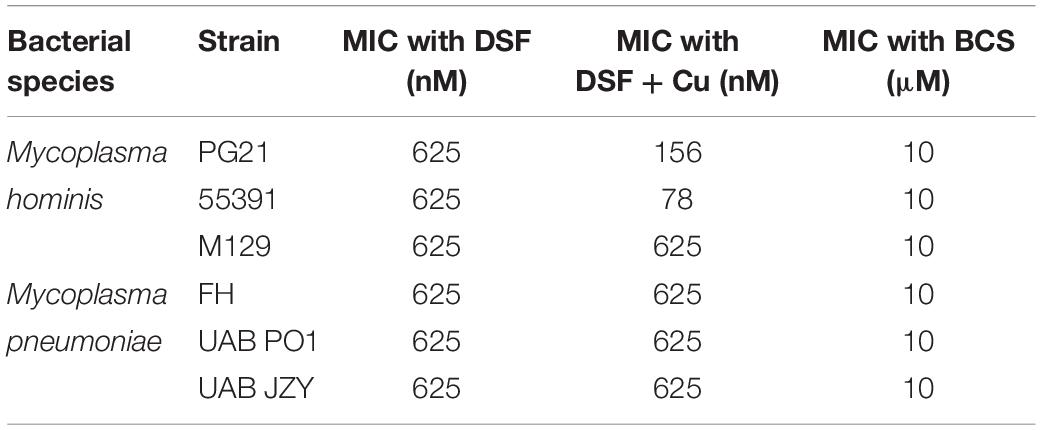
Screening was carried out on Mpn, Mh, Up, and Uu isolates to determine the MICs for copper by adding serial dilutions of CuSO4 to broth cultures. Ureaplasma strains Up serovar 3 (Up3) and Uu serovar 9 (Uu9) were inhibited completely at concentrations above 60–120 μM Cu, but Uu serovar 10 (Uu10) was unable to tolerate levels greater than 30–60 μM (Figure 1A). MICs for Mh strains PG21 and UAB 55391 were comparable, with complete inhibition above 60–120 μM Cu (Figure 1B). Surprisingly, Mpn strains M129 and UAB PO1 (as well as strains FH and UAB JZY, not shown) were all able to tolerate concentrations up to 1 mM CuSO4 (Figure 1C). Due to acidification of the broth with addition of greater than 1 mM CuSO4, testing above this concentration could not be done using the chosen method. Total human serum copper levels average 17 μM (range 7–41), while estimated concentrations in the phagolysosome are predicted to be 10–20-fold (range 100–300 μM) higher than that found in plasma (Twomey et al., 2005; Wagner et al., 2005; White et al., 2009). Based on the MIC results with copper, further testing for an effect of selected compounds with copper was performed using 50 μM exogenous CuSO4 for all strains except Uu10, for which 25 μM was used.
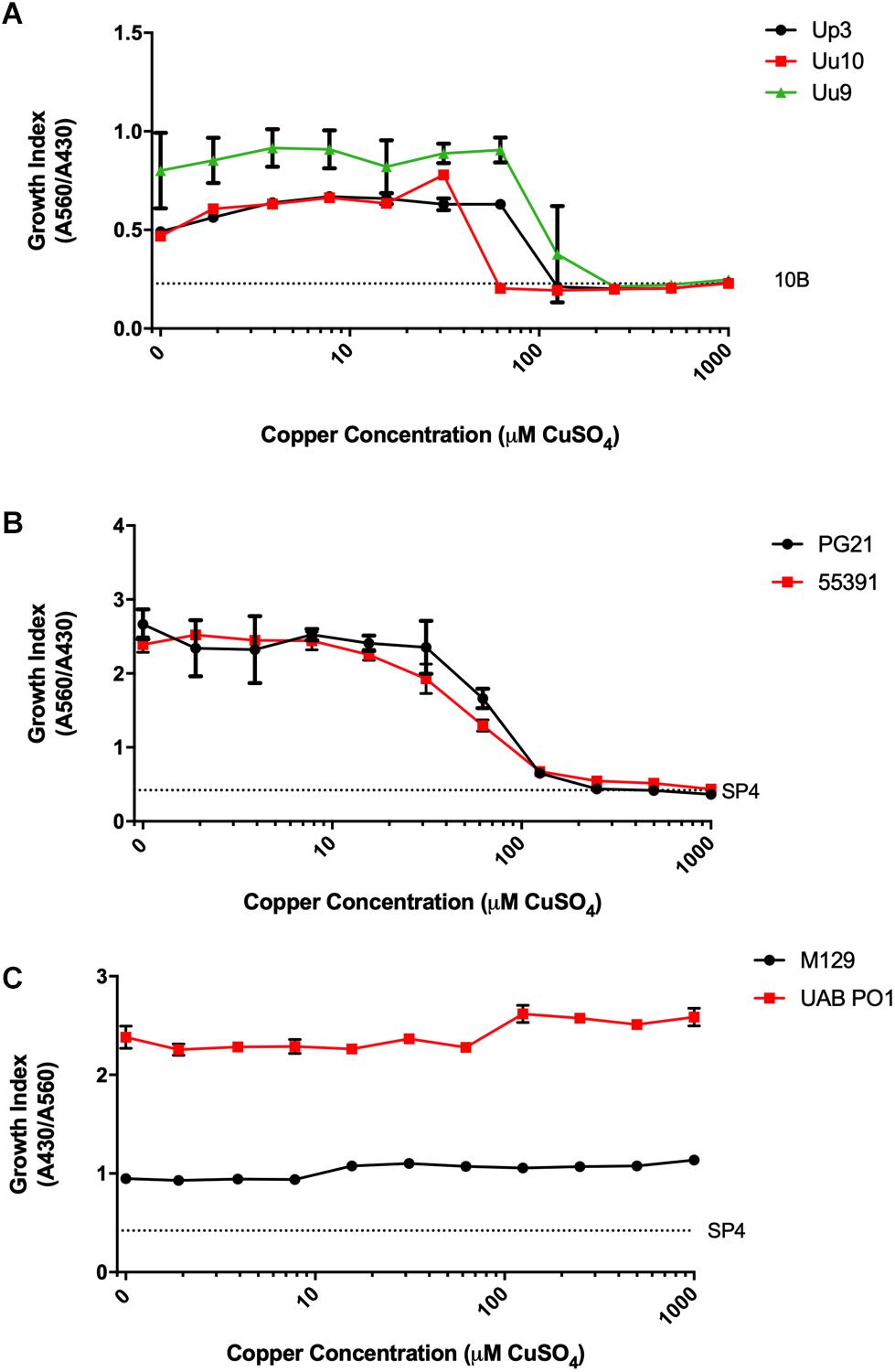
Figure 1. Differential susceptibility of Mollicutes to Cu stress in vitro. Growth of the indicated Mollicute species across three logs of Cu concentrations (A) U. parvum serovar 3, U. urealyticum serovar 9, and U. urealyticum serovar 10; (B) M. hominis strains PG21 and 55391; and (C) Mpn strains M129 and UAB PO1. Graphs depict mean ± SD (n = 3 replicates). Experiments were repeated 2–3 times at minimum.
Cu-Binding Compound Neocuproine Exhibits Cu-Dependent Antimicrobial Activity Against Human Mollicutes
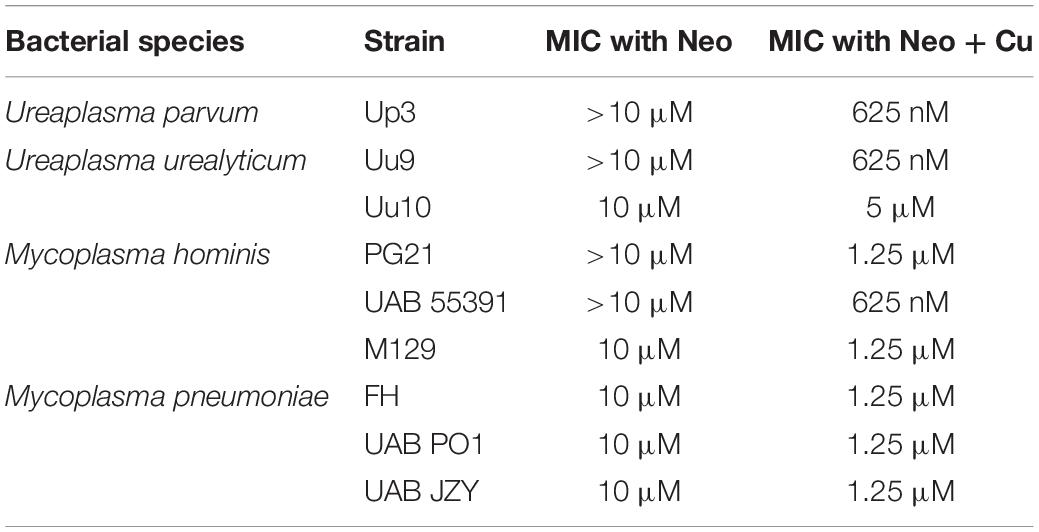
To determine whether compounds with Cu-chelating ability may have antimicrobial activity against human mollicutes, MICs were performed with mollicutes for compounds with and without Cu supplementation. First, we examined the antimicrobial activity of Neo against human mollicutes. Neo activity has previously been examined on a closely related avian mycoplasma, M. gallisepticum, and its antimicrobial activity was shown to be Cu-dependent (Smit et al., 1982; Gaisser et al., 1987a,b; de Zwart et al., 1991). The ureaplasma strains showed differential susceptibility to Neo in vitro (Table 2). Up3 showed no growth inhibition at 10 μM, but had a >30-fold inhibition in the presence of exogenous Cu2+ when compared to 10 μM Neo alone (Figure 2A). Similarly, Uu9 also exhibited a >30-fold inhibition of growth in the presence of Neo and Cu2+ when compared to 10 μM Neo alone (Table 2). Uu10 had a twofold difference in growth inhibition, but this minimal inhibition may be due to a general increased Cu2+ sensitivity (Figure 2B). Screening of Neo on M. hominis strain PG21 showed an MIC of 1.25 μM with Neo and Cu2+ compared to 10 μM with Neo alone (an eightfold difference), and Mh strain 55391 had a MIC of 625 μM with Neo and Cu compared to Neo alone 10 μM, a 16-fold difference (Figures 2C,D and Table 2). The MIC for all four strains of Mpn with Neo and 50 μM Cu2+ was 1.25 μM while the MIC with Neo alone was 10 μM, an eightfold difference (Figure 2E and Table 2). As concentrations of >10 μM were not tested, toxicity of Neo alone on mollicutes’ growth was not determined as part of these studies.
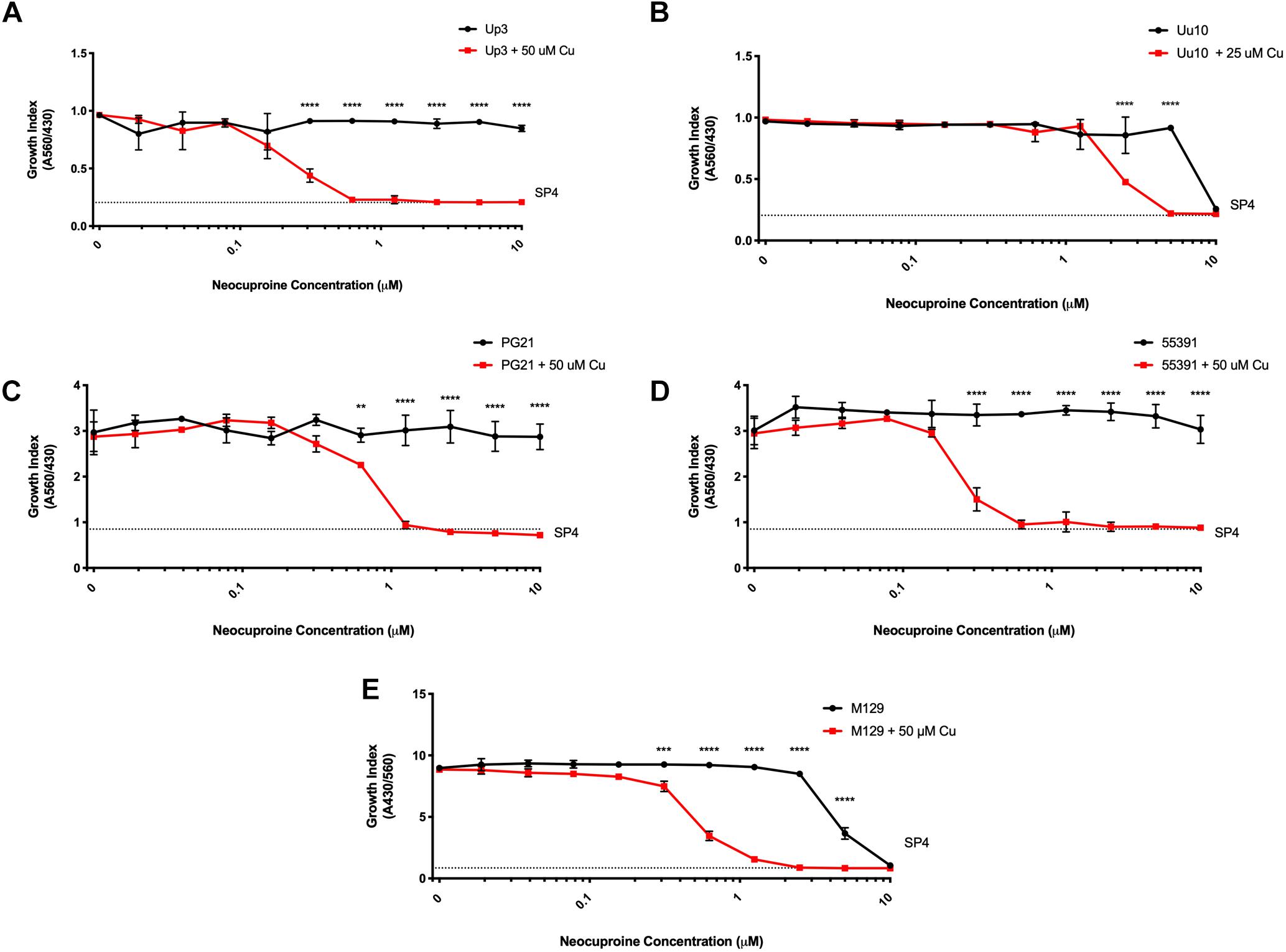
Figure 2. Neocuproine shows Cu-enhanced toxicity in vitro against Ureaplasma and Mycoplasma species. Representative growth indices of Up3 (A), Uu10 (B), Mh strains PG21 (C), UAB 55391 (D), and Mpn strain M129 (E) in the presence of Neo with or without Cu supplementation. Graphs depict group mean ± SD (n = 3 replicates). Experiments were repeated 2–3 times at minimum.
To exclude the potential role of other transition metals in the apparent Cu2+-specific antimicrobial effects of Neo, additional transition metals were tested. Mpn strains were cultured with Neo in the presence or absence of Cu2+, Fe2+, Mn2+, and Zn2+. As was seen previously, a 16-fold difference was present between Neo with Cu2+ compared to the Neo controls without added Cu2+ (Supplementary Figure 1). However, growth of Mpn with Neo in media supplemented with Fe2+, Mn2+, or Zn2+ was not significantly different from the control cultures despite the ability of Neo to form complexes with these metals (Xiao et al., 2011). These data suggest that Neo mediated antimicrobial activity is copper-dependent and independent of other transition metals (Supplementary Figure 1). All strains examined showed MICs of Neo with copper at about 1.25 μM while the MICs of Neo alone or Neo with the other transition metals were about 10 μM (data not shown). These data indicate that the enhanced inhibitory effect of Neo on Mpn growth is Cu2+ specific.
GTSM and DSF Show Cu-Specific Antimicrobial Effects on Mycoplasma and Ureaplasma spp.
Glyoxal bis(4-methyl-3-thiosemicarbazone) is a compound previously shown to have copper-dependent antimicrobial activity against M. tuberculosis (Speer et al., 2013; Neyrolles et al., 2015). With respect to the ureaplasmas, Uu9 and Up3 showed growth inhibition at 313 nM GTSM, while strain Uu10 showed growth inhibition at 156 nM (Figures 3A,B and Table 3). This inhibition appeared to be independent of exogenous Cu supplementation. Interestingly, there was potent inhibition of Mh strains PG21 and 55391 with GTSM with an MIC of 40 and 20 nM, respectively, without Cu supplementation (Figures 3C,D and Table 3). Only PG21 showed a twofold difference with GTSM MICs, decreasing the MIC to 20 nM. All Mpn strains tested with GTSM showed inhibition of bacterial growth with a MIC of 40 nM. Growth inhibition did not appear to be altered with Cu2+ supplementation, suggesting that exogenous Cu2+ may not be necessary for GTSM to exhibit antibacterial activity in highly complex media (Figure 3E and Table 3).
Figure 3. Glyoxal bis(4-methyl-3-thiosemicarbazone) shows Cu-enhanced toxicity in vitro against Ureaplasma and Mycoplasma species. Representative growth indices in the presence of GTSM with or without Cu supplementation of Up3 (A) and Uu10 (B), Mh strains PG21 (C), UAB 55391 (D), and Mpn strain M129 (E). Growth index of Mpn strain M129 in the presence of GTSM with or without bathocuproine (F). Graphs depict group mean ± SD (n = 3 replicates). Experiments were repeated 2–3 times at minimum.
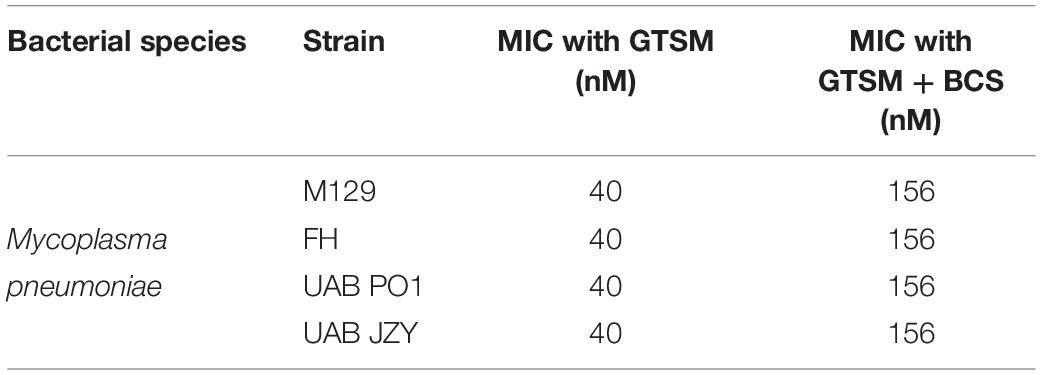
To determine whether GTSM inhibited mycoplasma and ureaplasma growth at least partly by forming complexes with the low level of Cu2+ in the growth media, BCS, a membrane impermeable copper-binding compound, was utilized to sequester free Cu2+ ions in the growth medium. MICs for GTSM were carried out on Mpn in the presence or absence of BCS, without exogenous Cu2+ supplementation. GTSM activity was decreased in the presence of BCS, altering MICs from 40 to 156 nM (Figure 3F and Table 4) against all Mpn strains examined. These increased MICs suggest that the trace amounts of Cu2+ in standard medium are sufficient to boost the growth inhibitory activity of GTSM, and also suggests that GTSM may also act in a copper-independent manner at higher concentrations.
Having shown that membrane-permeable copper-binding compounds can exhibit growth inhibitory activity against human mollicutes, we examined the FDA-approved drug DSF for similar activity, as it has been shown previously to have copper-dependent antimicrobial activity against other bacterial species (Dalecki et al., 2015). DSF was examined for growth inhibitory activity against the Mh type strain PG21 and a MDR clinical isolate (55391) with or without exogenous Cu supplementation. Both strains showed MICs at 625 nM DSF without Cu2+ addition to media (Figures 4A,C). With addition of 50 μM Cu2+ in the presence of DSF, the MIC for PG21 to DSF showed a fourfold decrease to 156 nM (Figure 4A and Table 5). Strain 55391 showed an eightfold difference in DSF susceptibility with Cu2+ addition, with a MIC of approximately 78 nM (Figure 4C).
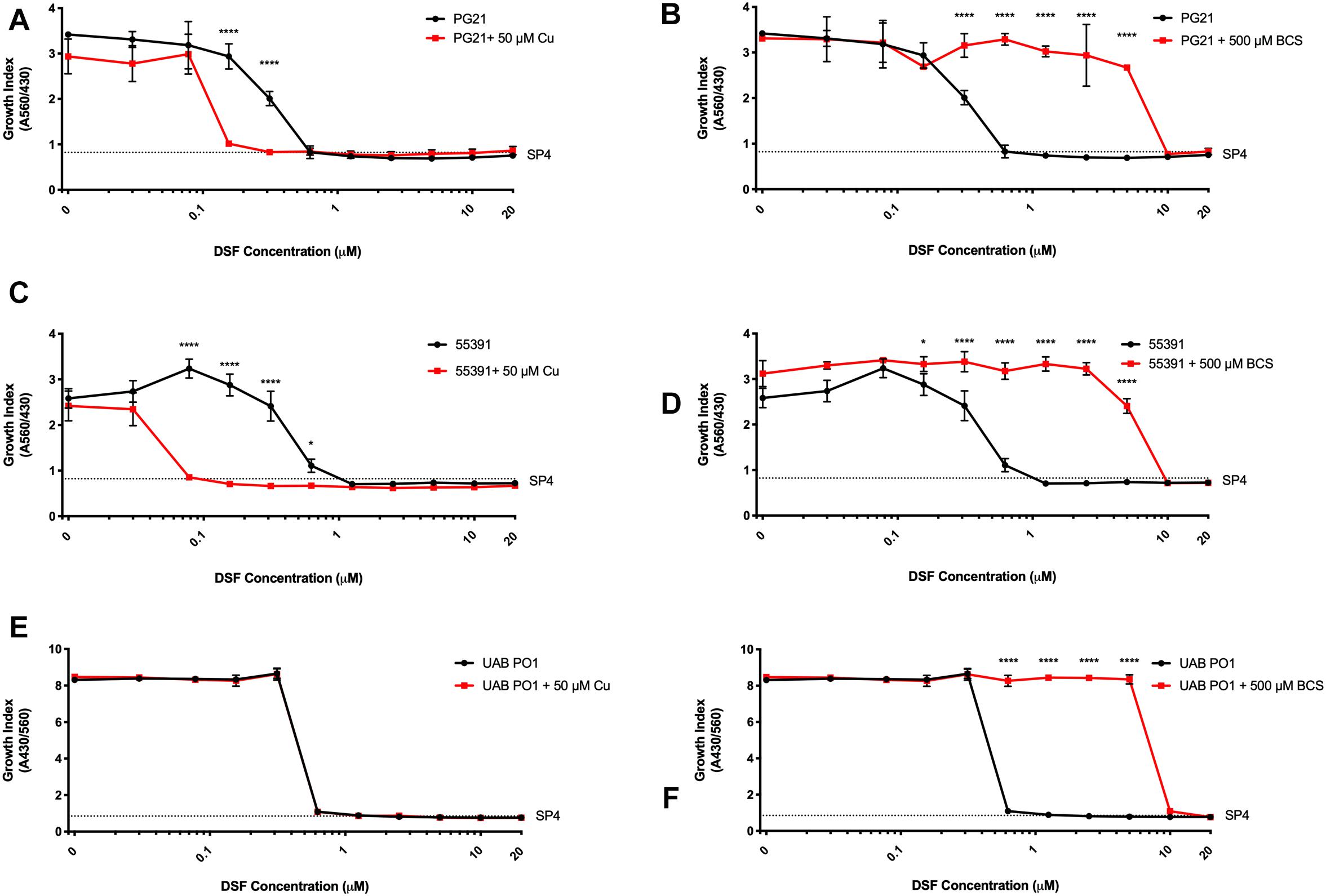
Figure 4. Disulfiram shows toxicity against Mycoplasma species in vitro. Representative growth indexes of M. hominis PG21 (A) in the presence of DSF, with or without Cu supplementation (50 μM), and BCS resue (500 μM) (B). Representative growth indexes of UAB 55391 (C) in the presence of DSF, with and without Cu supplementation (50 μM), and BCS resume (500 μM) (D). Representative growth index of Mpn strain M129 (E) in the presence of DSF with or without Cu (50 μM), and BCS rescue (500 μM) (F). Graphs depict group mean ± SD (n = 3 replicates). Experiments were repeated 2–3 times at minimum.
To show that DSF was utilizing Cu2+ to increase MICs in Mh strains, BCS was utilized without exogenous Cu2+ supplementation. Upon addition of BCS to DSF-containing cultures, MIC values for both PG21 and 55391 shifted from 625 nM to 10 μM DSF (Table 5 and Figures 4B,D). DSF, in addition to intermediate μM MIC values against Mh, showed MIC values of 0.625 μM against Mpn strains tested (Figure 4E and Table 5), which increased to 10 μM with addition of BCS (Figure 4F). These data suggest that DSF, an FDA-approved, copper-binding drug, exhibits copper-dependent antimicrobial activity against Mh.
Discussion
Nutritional immunity is a relatively new field which has arisen upon observations of utilization of specific transition metals by phagocytes against infective microbes. While metal starvation is one scheme for immunologic sequestration of nutrients required for bacteria growth (Fe2+ and Mn2+), use of metals such as copper in the phagolysosome has been described as one mechanism of cellular antimicrobial activity (Didier et al., 2010; Samanovic et al., 2012; Parrow et al., 2013; Neyrolles et al., 2015). During the process of phagolysosome destruction of infectious microbes, ATP7A, a copper-transporting ATPase, pumps Cu1+ ions into the phagolysosome, thereby creating an environment saturated with transition metals. In conjunction with other host defenses, this contributes to eventual destruction and clearance of microbes in vivo (White et al., 2009; Ladomersky et al., 2017). Thus, Cu1+ ions are important during microbial killing by eukaryotic cells, and not unexpectedly resistance mechanisms also exist that permit bacterial endurance of transition metal exposure, demonstrating the co-evolution of traits for host defense and pathogenesis. P-Type ATPases exist in bacterial species, alongside a large number of transporters, chaperone proteins, and myriad means of Cu chelation (Rensing and Grass, 2003; Shafeeq et al., 2011; Wolschendorf et al., 2011; Johnson et al., 2015). Of the many documented copper resistance mechanisms in related prokaryotic species currently the only annotated genes present in human mollicutes are P-type ATPases (i.e., MgtA) (Alex et al., 2017). Further work will be required to identify the genes required for resistance by different species of mollicutes to copper-mediated toxicity.
Little work has been carried out with respect to antimicrobial effects of transition metals on human mollicutes. Previously published work with S. aureus and M. tuberculosis has demonstrated that compounds that chelate copper can exhibit antibacterial effects (Dalecki et al., 2016; Shah et al., 2016). Previous work in another laboratory during the 1980s examined the effect of Neo on Cu stress with M. gallisepticum (Smit et al., 1982; Gaisser et al., 1987b). The mechanism of action for Neo was determined to be the result of copper toxicity, and not that of the ligand itself (Smit et al., 1982). This was also found for DSF and its activity against M. tuberculosis where the drug was described as a Trojan horse, disguising copper during transition into the bacterial cell and thus preventing exclusion (Dalecki et al., 2015). These data showed potential benefit for drug discovery against an avian pathogen. Sensitivity to transition metals, namely Mn2+, has been reported in U. urealyticum, prior to the separation of U. parvum from the same species (biovar) classification (Robertson and Chen, 1984). Interestingly, all Up isolates (serovars 1, 3, 6, and 14) had a transient growth inhibition with Mn addition, but this was permanent for Uu isolates (serovars 2, 4, 5, 7, 8, 9, 10, 11, and 12) and could not be rescued with the addition of Cu. One probable explanation for the failure of Cu2+ to rescue Mn2+-mediated growth inhibition on Ureaplasma spp. is the relatively low MIC for Cu2+, which our work has explored. As compared to the other examined Mollicutes spp., Mpn was comparatively more resistant to copper-augmented stress with Neo and DSF. The increased resistance of Mpn to Cu2+ ion stress compared to the examined spp., in general, may explain these differences, although more extensive work should be carried out to determine if this is true for additional mycoplasma and Ureaplasma spp.
This is the first report to detail the toxicity of copper against human mycoplasmas and ureaplasmas as well as documenting copper-dependent antimicrobial activity of three different copper-binding compounds. We found a high Cu2+ MIC for isolates of the lung pathogen Mpn and an increased Cu2+ sensitivity in the urogenital organisms Mh and Ureaplasma isolates. The antimicrobial effects of copper seen with the type strains of Mpn, Mh, Uu and Up with the compounds tested in this study were similar with the drug-resistant isolates Uu serovar 9 (tetM positive), and Mh 55391 (resistant to macrolides, tetracyclines, and fluoroquinolones). We speculate that this difference may be due in part to their “normal” anatomic niche, whereby respiratory pathogens must be more resistant to Cu2+ due to increased numbers of macrophages in the respiratory tract. Other respiratory bacteria (i.e., M. tuberculosis and Streptococcus pneumoniae) have been reported have multiple resistance mechanisms to Cu2+, and loss of these response factors during acute phagocyte-derived copper exposure can decrease both virulence and longevity of infections (Shafeeq et al., 2011; Wolschendorf et al., 2011; Johnson et al., 2015). However, contrary to this hypothesis of a role for anatomic niche in copper resistance, the cop operon, such as that found in S. pneumoniae, is present in urogenital pathogens such as uropathogenic Escherichia coli (Rensing and Grass, 2003). Additionally, copper can be found in urine as a host effector molecule to decrease bacterial infiltration into the urinary tract (Hyre et al., 2017). Relatedly, mollicutes typically found in the urogenital tract have also been reported in oropharyngeal and respiratory specimens (Waites et al., 2005; Bharat et al., 2015; Tyner et al., 2016; Atkinson et al., 2018). These conflicting findings suggest that the reason(s) for these observed differences in copper-resistance will require further study.
There are some limitations in our study. SP4 and 10B may be some of the richest microbial culture media currently used, and contain ∼15% serum (Waites et al., 2012a). Thus, our testing was carried out using media containing poorly defined biologic components (e.g., serum, peptone, tryptone, and yeast extract), increasing the potential for finding false positives and false negatives on copper-dependent drug compounds. We did, however, measure the total copper levels in the media and found them to be much lower than the concentrations chosen for testing. This study furthermore does not attempt to determine whether mechanisms of resistance could be uncovered with prolonged exposure of these compounds in vitro. Due to a lack of reliable metrics for consistent MIC analysis of Mycoplasma genitalium, and lack of recent clinical isolates, this organism was excluded from these analyses. This organism may be sufficient to explain the hypotheses pertaining to anatomical site-specific Cu2+ resistance, but further work is required. Finally, these observations are entirely limited in scope to in vitro MIC work, and extensive testing in vivo will be required before usage in patients is possible.
In conclusion, this study has demonstrated differing ranges of Cu2+ toxicity between Mpn, Mh, Uu, and Up. Neo, GTSM, and DSF exhibit interesting, copper-dependent antimicrobial activity against mycoplasmas and ureaplasmas that merits further investigation.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
AT, LX, FW, and TA contributed to the design and implementation of the study. AT, CC, and AD performed the experimental studies and analysis. All authors aided in interpretation and outcomes of experimental analyses, contributed to the revisions and editing of this manuscript, and read and approved the final version of the manuscript for submission. AT and TA wrote the first draft of the manuscript. CC, AD, LX, and FW extensively edited the subsequent drafts of the manuscript.
Funding
This research was completed with internal funding from the Departments of Pediatrics and Medicine, University of Alabama at Birmingham. This research received additional public funding from the National Institutes of Health grant R01AI121364 awarded to FW. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Dr. Kevin Dybvig, Dr. James Daubenspeck, Mrs. Donna Crabb, Mrs. Melanie Fecanin, and Dr. Warren Simmons, for their support and technical assistance in these assays. Thanks are also offered to Dr. Ken B. Waites and Dr. Sixto Leal for the generous gift of clinical isolates, as well as suggestions for manuscript edits for this work. The authors would also like to express their gratitude to Mr. Jordan Lingo and other members of the Wolschendorf Laboratory for their guidance throughout these studies.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.01720/full#supplementary-material
Abbreviations
BCS, bathocuproine disulfonate; CuSO4, copper sulfate; DSF, disulfiram [bis(diethylthiocarbamoyl) disulfide]; FDA, US Food and Drug Administration; GTSM, glyoxal bis(4-methyl-3-thiosemicarbazone); ICP-MS, inductively coupled plasma mass spectrometry; MDR, multidrug resistant; Mh, Mycoplasma hominis; Mpn, Mycoplasma pneumoniae; Neo, neocuproine (2,9-dimethyl-1,10-phenanthroline); Up3, Ureaplasma parvum serovar 3; Uu9/Uu10, Ureaplasma urealyticum serovars 9/10.
References
Alex, G., Dalecki, C. L. C., and Wolschendorf, F. (2017). Copper and antibiotics: discovery, modes of action, and opportunities for medicinal applications. Adv. Microb. Physiol. 70, 193–260. doi: 10.1016/bs.ampbs.2017.01.007
Atkinson, T. P., Centor, R. M., Xiao, L., Wang, F., Cui, X., Van Der Pol, W., et al. (2018). Analysis of the tonsillar microbiome in young adults with sore throat reveals a high relative abundance of Fusobacterium necrophorum with low diversity. PLoS One 13:e0189423. doi: 10.1371/journal.pone.0189423
Bharat, A., Cunningham, S. A., Scott Budinger, G. R., Kreisel, D., DeWet, C. J., Gelman, A. E., et al. (2015). Disseminated ureaplasma infection as a cause of fatal hyperammonemia in humans. Sci. Transl. Med. 7:284re3. doi: 10.1126/scitranslmed.aaa8419
Dalecki, A. G., Haeili, M., Shah, S., Speer, A., Niederweis, M., Kutsch, O., et al. (2015). Disulfiram and copper ions kill Mycobacterium tuberculosis in a synergistic manner. Antimicrob. Agents Chemother. 59, 4835–4844. doi: 10.1128/AAC.00692-15
Dalecki, A. G., Malalasekera, A. P., Schaaf, K., Kutsch, O., Bossmann, S. H., and Wolschendorf, F. (2016). Combinatorial phenotypic screen uncovers unrecognized family of extended thiourea inhibitors with copper-dependent anti-staphylococcal activity. Metallomics 8, 412–421. doi: 10.1039/c6mt00003g
de Zwart, M. A., Bastiaans, H. M., van der Goot, H., and Timmerman, H. (1991). Synthesis and copper-dependent antimycoplasmal activity of amides and amidines derived from 2-amino-1,10-phenanthroline. J. Med. Chem. 34, 1193–1201. doi: 10.1021/jm00107a045
Didier, E. S., Bowers, L. C., Martin, A. D., Kuroda, M. J., Khan, I. A., and Didier, P. J. (2010). Reactive nitrogen and oxygen species, and iron sequestration contribute to macrophage-mediated control of Encephalitozoon cuniculi (Phylum Microsporidia) infection in vitro and in vivo. Microbes Infect. 12, 1244–1251. doi: 10.1016/j.micinf.2010.09.010
Feng, M., Schaff, A. C., Cuadra Aruguete, S. A., Riggs, H. E., Distelhorst, S. L., and Balish, M. F. (2018). Development of Mycoplasma pneumoniae biofilms in vitro and the limited role of motility. Int. J. Med. Microbiol. 308, 324–334. doi: 10.1016/j.ijmm.2018.01.007
Gaisser, H. D., de Vries, J., van der Goot, H., and Timmerman, H. (1987a). Inhibition of NADH oxidase and lactate dehydrogenase of Mycoplasma gallisepticum by copper complexes of 2,2’-bipyridyl analogues. Biochem. Pharmacol. 36, 3237–3241. doi: 10.1016/0006-2952(87)90639-3
Gaisser, H. D., van der Goot, H., Stouthamer, A. H., and Timmerman, H. (1987b). Investigation into the mechanism of copper uptake by Mycoplasma gallisepticum in the presence of 2,9-dimethyl-1,10-phenanthroline. Pharm. Weekbl. Sci. 9, 315–320. doi: 10.1007/bf01956511
Haeili, M., Moore, C., Davis, C. J., Cochran, J. B., Shah, S., Shrestha, T. B., et al. (2014). Copper complexation screen reveals compounds with potent antibiotic properties against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 58, 3727–3736. doi: 10.1128/AAC.02316-13
Hyre, A. N., Kavanagh, K., Kock, N. D., Donati, G. L., and Subashchandrabose, S. (2017). Copper is a host effector mobilized to urine during urinary tract infection to impair bacterial colonization. Infect. Immun. 85, e01041-16. doi: 10.1128/IAI.01041-16
Johnson, M. D., Kehl-Fie, T. E., Klein, R., Kelly, J., Burnham, C., Mann, B., et al. (2015). “Role of copper efflux in pneumococcal pathogenesis and resistance to macrophage-mediated immune clearance. Infect. Immun. 83, 1684–1694. doi: 10.1128/IAI.03015-14
Ladomersky, E., Khan, A., Shanbhag, V., Cavet, J. S., Chan, J., Weisman, G. A., et al. (2017). Host and pathogen copper-transporting P-Type ATPases function antagonistically during Salmonella infection. Infect. Immun. 85:e0035-17. doi: 10.1128/IAI.00351-17
Neyrolles, O., Wolschendorf, F., Mitra, A., and Niederweis, M. (2015). Mycobacteria, metals, and the macrophage. Immunol. Rev. 264, 249–263. doi: 10.1111/imr.12265
Parrow, N. L., Fleming, R. E., and Minnick, M. F. (2013). Sequestration and scavenging of iron in infection. Infect. Immun. 81, 3503–3514. doi: 10.1128/IAI.00602-13
Rensing, C., and Grass, G. (2003). Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol. Rev. 27, 197–213. doi: 10.1016/s0168-6445(03)00049-4
Robertson, J. A., and Chen, M. H. (1984). Effects of manganese on the growth and morphology of Ureaplasma urealyticum. J. Clin. Microbiol. 19, 857–864.
Samanovic, M. I., Ding, C., Thiele, D. J., and Darwin, K. H. (2012). Copper in microbial pathogenesis: meddling with the metal. Cell Host Microbe 11, 106–115. doi: 10.1016/j.chom.2012.01.009
Shafeeq, S., Yesilkaya, H., Kloosterman, T. G., Narayanan, G., Wandel, M., Andrew, P. W., et al. (2011). The cop operon is required for copper homeostasis and contributes to virulence in Streptococcus pneumoniae. Mol. 81, 1255–1270. doi: 10.1111/j.1365-2958.2011.07758.x
Shah, S., Dalecki, A. G., Malalasekera, A. P., Crawford, C. L., Michalek, S. M., Kutsch, O., et al. (2016). 8-Hydroxyquinolines are boosting agents of copper-related toxicity in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 60, 5765–5776. doi: 10.1128/AAC.00325-16
Smit, H., van der Goot, H., Nauta, W. T., Timmerman, H., de Bolster, M. W., Stouthamer, A. H., et al. (1982). Mechanism of action of the copper(I) complex of 2,9-dimethyl-1,10-phenanthroline on Mycoplasma gallisepticum. Antimicrob. Agents Chemother. 21, 881–886. doi: 10.1128/aac.21.6.881
Speer, A., Shrestha, T. B., Bossmann, S. H., Basaraba, R. J., Harber, G. J., Michalek, S. M., et al. (2013). Copper-boosting compounds: a novel concept for antimycobacterial drug discovery. Antimicrob. Agents Chemother. 57, 1089–1091. doi: 10.1128/AAC.01781-12
Totten, A. H., Xiao, L., Crabb, D. M., Ratliff, A. E., Dybvig, K., Waites, K. B., et al. (2017). Shaken or stirred: comparison of methods for dispersion of Mycoplasma pneumoniae aggregates for persistence in vivo. J. Microbiol. Methods 132, 56–62. doi: 10.1016/j.mimet.2016.11.011
Twomey, P. J., Viljoen, A., House, I. M., Reynolds, T. M., and Wierzbicki, A. S. (2005). Relationship between serum copper, ceruloplasmin, and non-ceruloplasmin-bound copper in routine clinical practice. Clin. Chem. 51, 1558–1559. doi: 10.1373/clinchem.2005.052688
Tyner, H. L., Virk, A., Nassr, A., and Razonable, R. (2016). Mycoplasma hominis vertebral spine infection: case report and a review of infections of bone and joints. J. Infect. Chemother. 22, 755–758. doi: 10.1016/j.jiac.2016.04.008
Wagner, D., Maser, J., Lai, B., Cai, Z., Barry, C. E. III, Honer Zu Bentrup, K., et al. (2005). Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell’s endosomal system. J. Immunol. 174, 1491–1500. doi: 10.4049/jimmunol.174.3.1491
Waites, K. B., Duffy, L. B., Bebear, C. M., Matlow, A., Talkington, D. F., Kenny, G. E., et al. (2012a). Standardized methods and quality control limits for agar and broth microdilution susceptibility testing of Mycoplasma pneumoniae, Mycoplasma hominis, and Ureaplasma urealyticum. J. Clin. Microbiol. 50, 3542–3547. doi: 10.1128/JCM.01439-12
Waites, K. B., Xiao, L., Paralanov, V., Viscardi, R. M., and Glass, J. I. (2012b). Molecular methods for the detection of Mycoplasma and Ureaplasma infections in humans: a paper from the 2011 william beaumont hospital symposium on molecular pathology. J. Mol. Diagn. 14, 437–450. doi: 10.1016/j.jmoldx.2012.06.001
Waites, K. B., Katz, B., and Schelonka, R. L. (2005). Mycoplasmas and ureaplasmas as neonatal pathogens. Clin. Microbiol. Rev. 18, 757–789. doi: 10.1128/cmr.18.4.757-789.2005
White, C., Lee, J., Kambe, T., Fritsche, K., and Petris, M. J. (2009). A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity. J. Biol. Chem. 284, 33949–33956. doi: 10.1074/jbc.M109.070201
Wolschendorf, F., Ackart, D., Shrestha, T. B., Hascall-Dove, L., Nolan, S., Lamichhane, G., et al. (2011). Copper resistance is essential for virulence of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 108, 1621–1626. doi: 10.1073/pnas.1009261108
Xiao, L., Crabb, D. M., Dai, Y., Chen, Y., Waites, K. B., and Atkinson, T. P. (2014). Suppression of antimicrobial peptide expression by Ureaplasma species. Infect. Immun. 82, 1657–1665. doi: 10.1128/IAI.01231-13
Xiao, Z., Brose, J., Schimo, S., Ackland, S. M., La Fontaine, S., and Wedd, A. G. (2011). Unification of the copper(I) binding affinities of the metallo-chaperones Atx1, Atox1, and related proteins: detection probes and affinity standards. J. Biol. Chem. 286, 11047–11055. doi: 10.1074/jbc.M110.213074
Keywords: mollicutes, copper, drug discovery, disulfiram, GTSM, neocuproine, Mycoplasma, Ureaplasma
Citation: Totten AH, Crawford CL, Dalecki AG, Xiao L, Wolschendorf F and Atkinson TP (2019) Differential Susceptibility of Mycoplasma and Ureaplasma Species to Compound-Enhanced Copper Toxicity. Front. Microbiol. 10:1720. doi: 10.3389/fmicb.2019.01720
Received: 05 March 2019; Accepted: 12 July 2019;
Published: 30 July 2019.
Edited by:
Glenn Francis Browning, The University of Melbourne, AustraliaReviewed by:
Sargurunathan Subashchandrabose, Texas A&M University, United StatesMitchell F. Balish, Miami University, United States
Copyright © 2019 Totten, Crawford, Dalecki, Xiao, Wolschendorf and Atkinson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arthur H. Totten, YXJ0aHVydG90dGVuQHVhYm1jLmVkdQ==; Thomas P. Atkinson, UEF0a2luc29uQHBlZHMudWFiLmVkdQ==
 Arthur H. Totten
Arthur H. Totten Cameron L. Crawford
Cameron L. Crawford Alex G. Dalecki
Alex G. Dalecki Li Xiao2
Li Xiao2 Frank Wolschendorf
Frank Wolschendorf Thomas P. Atkinson
Thomas P. Atkinson