- 1Institute of Food and Drug Inspection, College of Life Science and Agronomy, Zhoukou Normal University, Zhoukou, China
- 2State Key Laboratory of Crop Stress Biology for Arid Areas, Shaanxi Key Laboratory of Agricultural and Environmental Microbiology, College of Life Sciences, Northwest A&F University, Yangling, China
The contact-dependent type VI secretion system (T6SS) in diverse microbes plays crucial roles in both inter-bacterial and bacteria-host interactions. As numerous microorganisms inhabit the intestinal ecosystem at a high density, it is necessary to consider the functions of T6SS in intestinal bacteria. In this mini-review, we discuss T6SS-dependent functions in intestinal microbes, including commensal microbes and enteric pathogens, and list experimentally verified species of intestinal bacteria containing T6SS clusters. Several seminal studies have shown that T6SS plays crucial antibacterial roles in colonization resistance, niche occupancy, activation of host innate immune responses, and modulation of host intestinal mechanics. Some potential roles of T6SS in the intestinal ecosystem, such as targeting of single cell eukaryotic competitors, competition for micronutrients, and stress resistance are also discussed. Considering the distinct activities of T6SS in diverse bacteria residing in the intestine, we suggest that T6SS research in intestinal microbes may be beneficial for the future development of new medicines and clinical treatments.
Introduction
The type VI secretion system (T6SS) is a contact-dependent transmembrane nanomachine that uses a contractile mechanism to inject effectors into adjacent prokaryotic or eukaryotic cells (Hood et al., 2010; Jani and Cotter, 2010; Cianfanelli et al., 2016). A typical T6SS apparatus is composed of 13 core subunits (TssA-TssM) and possesses a structure similar to that of a T4 bacteriophage tail (Boyer et al., 2009; Cascales and Cambillau, 2012; Zoued et al., 2013). In an integral T6SS apparatus, the baseplate complex (TssAEFGK) is anchored to the cell membrane by the membrane complex (TssJLM), providing structural support. The needle sheath (TssBC), tail tube (TssD/Hcp), and spike complex (TssI/VgrG) form the injection apparatus, which is responsible for effector secretion (Cascales and Cambillau, 2012; Silverman et al., 2012). A dynamic T6SS secretion process contains assembly/extension, contraction/puncture, and disassembly of the sheath. Briefly, the membrane complex is formed at the initial stage. Subsequently, baseplate proteins are recruited for sheath/tube anchoring and extension. When attacking, the membrane-puncturing spike pierces target cells through sheath contraction, accompanied by energy consumption and load transportation. Consequently, various effectors are delivered into target cells in a one-step manner (Basler et al., 2012; Brunet et al., 2015; Cianfanelli et al., 2016; Coulthurst, 2019). Although some pathogen-associated T6SSs have been reported to be involved in bacterial pathogenesis (Pukatzki et al., 2007; Aubert et al., 2016; Jiang et al., 2016), the primary function of T6SSs is to compete against rival bacteria in polymicrobial environments (Durand et al., 2014; Cianfanelli et al., 2016). However, other functions of T6SSs, such as resistance to amoeba predation (Zheng et al., 2011), biofilm formation (Gallique et al., 2017), and stress response (Weber et al., 2009; Zhang W. et al., 2013), have also been reported.
The intestinal ecosystem is composed of a densely populated community of multiple microorganisms that may be beneficial or harmful to host health (Walter and Ley, 2011). In intestinal environments where nutrients and space are limited, microbes must use various strategies to coexist or compete with the host and other bacteria. In recent years, the contact-dependent T6SS nanomachine has attracted much attention for its role in interbacterial competition in the mammalian gut (Sana et al., 2017; Verster et al., 2017). T6SS mediated antagonism in the mammalian gut not only benefits the microbiota-mediated colonization resistance by preventing invasion of pathogens, but also assists some pathogens to battle with the resident microbiota to invade an ecosystem and cause disease. Thus, it was stated that there is secret bacterial warfare in the gut, due to the T6SS roles both in the intestinal commensal microbes and enteric pathogens (Sana et al., 2017; Garcia-Bayona and Comstock, 2018). In this review, we have discussed recent studies on intestinal microorganisms with functional T6SS clusters and T6SS-dependent activities related to the intestinal microbial community and host health.
T6SS-Dependent Activities in Intestinal Ecosystem
Interference Competition Mediated by T6SS
Bacterial competition occurs as interference competition (killing of target cells with antibacterial weapons) or exploitation competition (consuming nutrients from the milieu) (Chassaing and Cascales, 2018). As an anti-bacterial weapon, T6SSs were primarily considered to mediate interference competition in the intestinal microbiota. The antibacterial function of T6SSs relies primarily on injection of “anti-bacterial” effectors that target conserved, essential features of the bacterial cell, such as peptidoglycan (Russell et al., 2011), membrane phospholipids (Russell et al., 2013), nucleic acids (Ma L.S. et al., 2014), NAD+ (Whitney et al., 2015), and the critical cell division protein FtsZ (Ting et al., 2018).
Commensal bacteria form a protective barrier that protects the host from bacterial pathogens (Belkaid and Hand, 2014). In healthy adult individuals, Bacteroidales are the most abundant order of bacteria in the intestinal microbiota (Faith et al., 2013). Recently, by performing extensive bioinformatics analyses and creating hidden Markov models for Bacteroidales Tss proteins, Coyne and colleagues identified 130 T6SS loci within 205 human gut Bacteroidales genomes. As Bacteroidales comprise approximately 50% of all colonic bacteria in many people, this suggested that T6SSs are distributed in about 25% of bacteria in the human colon (Coyne et al., 2014; Coyne et al., 2016). Moreover, more than 109 T6SS-firing events were determined per minute per gram of colonic contents, further supporting the importance of this weapon in shaping gut microbiota composition (Wexler et al., 2016). T6SS loci of the human gut Bacteroidales species segregate into three distinct genetic architectures (GA), termed GA1-GA3. GA1 and GA2 loci are present on conserved integrative conjugative elements (ICE) and are transferred between diverse human gut Bacteroidales strains via ICE. GA3 loci are not contained on conserved ICE and are confined to Bacteroides fragilis (Coyne et al., 2014, 2016). The GA3 T6SSs of B. fragilis antagonizes human gut Bacteroidales species in vivo using previously undescribed effectors, likely to create a locally protected niche in the human gut (Wexler et al., 2016). The interbacterial interactions among symbiotic Bacteroidales species could be predicted according to the presence or absence of strain-specific effector/immunity (E/I) repertoires. Further, some of these strains may avoid contact-dependent killing by accumulating immunity genes to neutralize antibacterial effectors that they do not encode to persist in the gut (Wexler et al., 2016). Importantly, symbiotic non-toxigenic B. fragilis strains could restrict enteric colonization by an enterotoxigenic B. fragilis strain, dependent on a functional T6S, to protect the host against intestinal inflammatory disease, suggesting a novel role of T6SS in colonization resistance (Hecht et al., 2016; Casterline et al., 2017).
Type VI secretion systems are widely distributed and have been proposed to be present in about 25% of all sequenced Gram-negative bacteria (Boyer et al., 2009; Salomon and Orth, 2015), including enteric pathogenic microorganisms, e.g., Vibrio cholerae, Salmonella enterica, Shigella sonnei, and Yersinia pseudotuberculosis. Thus, T6SS mediated antagonism in the mammalian gut both benefits microbiota-mediated colonization resistance by preventing pathogen invasion and facilitates some pathogens to battle resident microbiota to invade the ecosystem and cause disease. Some enteric pathogens could utilize T6SS-mediated antibacterial weapons to kill symbionts and establish within the host gut. For example, S. enterica Serovar Typhimurium uses T6SS to kill commensal Klebsiella oxytoca and enhance colonization of the mouse gut (Sana et al., 2016). With a functional T6SS, S. sonnei showed an advantage in competing against E. coli and Shigella flexneri both in vitro and in vivo, which may explain the dominance and increasing global prevalence of S. sonnei in developed countries worldwide (Anderson et al., 2017). Constitutive T6SS expression provides V. cholerae with an advantage in intra-specific and inter-specific competition, such that T6SS-dependent toxicity toward other bacteria could enhance V. cholerae survival in the environment and/or during colonization of a host (MacIntyre et al., 2010; Unterweger et al., 2012; Fu et al., 2013). Through transcriptome sequencing (RNA-Seq), T6SSs and their associated toxins in the gut symbiont of 28 Snodgrassella alvi strains from diverse Apis and Bombus species were analyzed. T6SS-associated Rhs toxins with antibacterial activity could mediate both intraspecific and interspecific competition among S. alvi strains and other bee gut microbes. Furthermore, extensive recombination and horizontal transfer of toxin/immunity genes between strains in the gut microbiota have resulted in tremendous diversity in their toxin repertoires, which suggest that T6SS-mediated competition may be an important driver of coevolution (Steele et al., 2017). These studies showed that enteric pathogens could use their T6SSs for interbacterial competition in vivo and for niche occupancy.
Besides bacterial competitors, T6SS was also found to target some single cell eukaryotic competitors, including amoebae and fungi. In fact, T6SS was first identified by screening V. cholerae mutants that failed to kill the social amoeba Dictyostelium discoideum, and the lipid-binding effector VasX was found to be required for T6SS-mediated amoeba killing, possibly through plasma membrane perturbations (Pukatzki et al., 2006; Miyata et al., 2011; Zheng et al., 2011). T6SSs in Pseudomonas syringae and Serratia marcescens were reported to be required for competition against other single cell eukaryotic organisms, i.e., yeast and fungi (Haapalainen et al., 2012; Trunk et al., 2018). The first anti-fungal T6SS effectors, Tfe1 and Tfe2, were identified in S. marcescens. Tfe1 causes plasma membrane depolarization without formation of a specific pore, while Tfe2 disrupts nutrient uptake and amino acid metabolism, and induces autophagy (Trunk et al., 2018). Since single cell eukaryotes are important components of the intestinal microbiota (Coyne and Comstock, 2019), it is not surprising that intestinal bacteria might deploy anti-eukaryotic T6SSs to compete for nutrients and space against co-habiting microbial eukaryotes.
Exploitation Competition Meditated by T6SS
As essential micronutrients are involved in a wide range of cellular processes (e.g., DNA replication, respiration, and energy generation), transit of these metal ions is a crucial component of host-microbe interactions, including those in the gastrointestinal tract (Sorbara and Pamer, 2019). For example, E. coli mutants defective in catecholate siderophore production show impaired murine gut colonization (Pi et al., 2012). Without a high-affinity zinc transporter, Campylobacter jejuni is unable to replicate or colonize the gastrointestinal tract, as quantities of zinc in the gastrointestinal tract are reduced by the host (Gielda and DiRita, 2012). Thus, competition for micronutrients in the gut is intense.
Type VI secretion system has been reported to be involved in acquisition of essential micronutrients, such as zinc, manganese, and iron, indicating its novel function in increasing bacterial fitness through competition for essential nutrients. In Pseudomonas aeruginosa, the H3-T6SS secreted effector TseF facilitates iron acquisition by interacting with the iron-chelating molecule PQS (Pseudomonas quinolone signal) (Lin et al., 2017). In Burkholderia thailandensis, T6SS4 secretes zinc- and manganese-scavenging proteins to fulfill the increased cellular demand for these metal ions under oxidative stress (Si et al., 2017a,b). Similarly, in the enteric pathogen Y. pseudotuberculosis, T6SS4 functions to combat host nutritional immunity and multiple adverse stresses by translocating a zinc binding effector YezP (Wang et al., 2015). Distinct from the extensively studied contact-dependent “anti-bacterial” and “anti-eukaryotic” T6SSs, the “metal ion transporting” T6SS secretes metal-binding proteins into the extracellular milieu, independent of cell-cell contact (Si et al., 2017a). Thus, T6SS confers survival advantages to bacterium in niches with multiple bacterial species not only by delivering “anti-bacterial” toxins to kill competing cells for interference competition, but also by enhancing its ability to acquire essential micronutrients for exploitation competition. It will be interesting to determine the physiologic consequence of these metal acquisition T6SSs in the intestinal ecosystem in the future.
Interestingly, expression of Y. pseudotuberculosis T6SS4 was found to be nutrient status-dependently regulated by the stringent response factor RelA and the nutrient-dependent regulator RovM (Song et al., 2015; Yang et al., 2019). Further research is needed to reveal whether T6SS is involved in competition for other nutrients besides metal ions.
Activation of the Innate Immune Response and Virulence
Although it is well-known that enteric pathogens could utilize the antibacterial T6SS weapon to eradicate competing microbes in vivo, little is known about whether the T6SS-dependent interactions with commensal bacteria have additional effects on virulence. Recently, Zhao et al. (2018) discovered that the V. cholerae T6SS could compete against commensal E. coli strains in the mouse gut to facilitate colonization. Importantly, they found that this microbial antagonistic interaction could improve fitness of V. cholera by activating host innate immune responses and improving expression of bacterial virulence genes (i.e., cholera toxin) (Zhao et al., 2018). The authors suggested that the innate immune response was induced by bacterial debris derived from lysed commensals, which together with up-regulated cholera toxin (CT), resulted in elevated disease symptoms and increased fitness of V. cholerae as a pathogen. Fast et al. (2018) used the Drosophila melanogaster model of cholera to define the contribution of T6SS to V. cholerae pathogenesis. They found that interactions between T6SS and the gut commensal Acetobacter pasteurianus intensified disease symptoms and accelerated host death. The disease severity was attenuated by inactivation of T6SS, or removal of A. pasteurianus. Interestingly, mutation of the Immune Deficiency (IMD) pathway relieved T6SS dependent lethality, implicating innate defenses in T6SS-mediated host death (Fast et al., 2018). These studies established that interactions between T6SS and commensal bacteria activate innate immune responses and promote V. cholera pathogenesis.
In addition, a few T6SS toxic effectors result in metabolic disorders or diseases in eukaryotic host cells. VgrG-1 in V. cholerae was responsible for T6SS-dependent cytotoxicity of macrophages through covalent cross-linking of host cell actin (Pukatzki et al., 2007; Ma and Mekalanos, 2010). In the diarrheal isolate Aeromonas hydrophila SSU, T6SS-secreted VgrG1 exhibited actin ADP-ribosylating activity, which can induce a rounded phenotype in HeLa cells, resulting in apoptosis (Suarez et al., 2010). A non-VgrG T6SS effector in Edwardsiella tarda, EvpP, inhibited NLRP3 inflammasome activation through repression of the Ca2+-dependent MAPK-Jnk pathway (Chen et al., 2017). T6SS effectors with phospholipase activity could cause damage to the cell membrane and accelerate the infection process (Dong et al., 2013; Russell et al., 2013). Notably, a few bacterial pathogens with the T6SS apparatus may spread through the digestive tract but cause diseases outside the gastrointestinal system. For example, in the newborn meningitis E. coli (NMEC) K1 strain, the Hcps of T6SS interacts with human brain microvascular endothelial cells (HBMEC) in a coordinated manner, first binding, then invading, and finally causing apoptosis of HBMEC (Zhou et al., 2012). The T6SS-secreted proteins VgrG1 and VgrG2 in Helicobacter hepaticus increases cellular innate pro-inflammatory responses, resulting in liver disease and intestinal inflammation (Bartonickova et al., 2013).
Modulation of Host Intestinal Mechanics
Recently, Logan and colleagues used a combination of microbial genetics, in vitro experiments, and quantitative in vivo imaging in zebrafish to determine the role of V. cholerae T6SS in gut colonization. They found that V. cholerae can expel resident microbiota of the genus Aeromonas in a T6SS-dependent manner. Unexpectedly, T6SS acted primarily to increase the strength of gut contractions, rather than killing the bacterial competitor. Coupling of T6SS activity to host contractions depended on an actin cross-linking domain (ACD) from the T6SS apparatus. When the ACD domain was deleted, V. cholerae could no longer induce enhanced host contractions, and dense Aeromonas communities remained in the gut. Deleting the ACD domain did not affect the ability of V. cholerae to kill A. veronii in vitro or enter and occupy the host intestine. These findings reveal a novel strategy by which enteric pathogens can modulate host intestinal mechanics to redefine gut communities and highlights the role of the host fluid-mechanical environment in shaping gut population dynamics (Logan et al., 2018). Whether this is a common tactic for gut-colonizing bacteria to invade the intestinal ecosystem especially in human needs to be verified in the future.
Stress Resistance
Within the intestinal lumen, enteric pathogens encounter severe stresses, such as bile salts, antimicrobial peptides, free fatty acids, enhanced osmolarity, and oxidative stress (Fang et al., 2016). To survive these adverse environments, bacteria have developed various delicate mechanisms. As a versatile molecular machine, T6SS has been found to play crucial roles in the bacterial stress response and contribute to cell survival under multiple environmental challenges. Vibrio anguillarum T6SS is regulated by the general stress response regulator RpoS and is involved in its resistance to hydrogen peroxide, ethanol, and low pH (Weber et al., 2009). Upon activation by RpoS and the global oxidative stress regulator OxyR, enterohaemorrhagic Escherichia coli (EHEC) uses its T6SS to secrete a Mn-containing catalase, KatN, thus providing a higher resistance to reactive oxygen species (ROS) produced by the host and enhancing survival levels in the host (Wan et al., 2017). Y. pseudotuberculosis T6SS4 was required for bacterial survival under osmotic, oxidative, and acidic stress and for resistance to bile salts, and its expression was regulated by various stress response regulators, such as the stationary growth phase stress σ factor RpoS, the global oxidative stress regulator OxyR, and the acid/osmotic regulator OmpR (Gueguen et al., 2013; Zhang W. et al., 2013; Guan et al., 2015; Wang et al., 2015). In V. cholerae O1, activation of T6SS has been shown to be dependent on the osmolarity of the milieu (Ishikawa et al., 2012). In addition, in the intestine, V. cholerae T6SS genes are overexpressed under mucin (intestinal host factors) and microbiota-modified bile salt conditions (Bachmann et al., 2015). Bile salts can also activate expression of T6SS genes in Salmonella typhimurium (Sana et al., 2016) and C. jejuni (Lertpiriyapong et al., 2012). These studies on gene expression regulation suggest that T6SS is expressed in the intestine and could provide resistance to multiple stresses, increasing bacterial adaptability or survival in the intestinal ecosystem.
Other Potential Functions
Recently, it was suggested that T6SS in V. cholerae serves as a predatory weapon and fosters horizontal gene transfer. For incorporation of DNA released by lysed cells into a competent predator cell, natural transformation and evolution can occur (Borgeaud et al., 2015). Thus, T6SS may promote co-evolution of bacterial microflora within their environments, including intestinal ecosystems.
Compelling data showed that H. hepaticus interacts with intestinal epithelial cells (IECs) and employs T6SS to limit within-host growth and virulence (Chow and Mazmanian, 2010). This feature was regarded as T6SS function of anti-virulence (Jani and Cotter, 2010). Nevertheless, this phenomenon is rarely seen.
To summarize, we constructed a schematic diagram showing the functions of T6SS in the intestinal ecosystem (Figure 1).
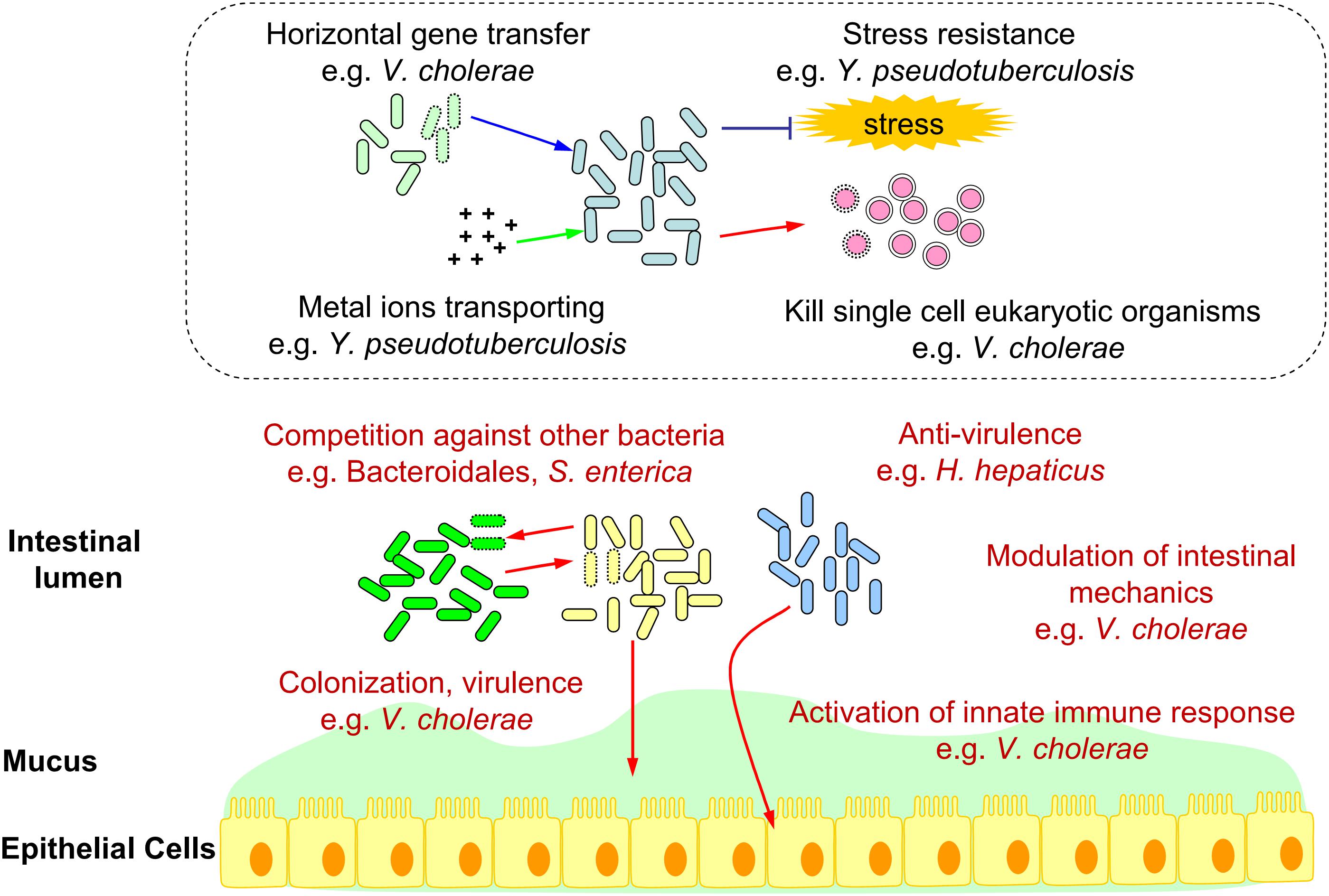
Figure 1. Schematic diagram of bacterial T6SS functions in the intestinal ecosystem. T6SS functions verified in the gut (e.g., Competition against other bacteria, Colonization, Virulence, Anti-virulence, Modulation of intestinal mechanics, and Activation of innate immune response.) were marked in red. Deduced T6SS functions in the gut (e.g., Horizontal gene transfer, Metal ions transporting, Stress resistance, and Kill single cell eukaryotic organisms.) based on in vitro analysis were shown in the dotted box.
Commensal Intestinal Microbes With T6SS Clusters
Bacteroidetes
Bacteroidetes, a phylum of Gram-negative bacteria, is highly abundant in the gastrointestinal tracts of humans and other mammals (Lozupone et al., 2012; Russell et al., 2014; Wexler and Goodman, 2017). Despite Bacteroidales being predominant in the mammalian gut, T6SSs in Bacteroidetes species were not identified until 2014, perhaps because the T6SS in Bacteroidetes does not have sufficient sequence similarity with core T6SS proteins of Proteobacteria for protein-protein comparisons (e.g., BLASTP) or protein-profile comparisons (e.g., Pfam, COG) (Russell et al., 2014; Coyne et al., 2016). Studies have revealed that the T6SS loci are segregated into three distinct genetic architectures (GA1-GA3), among which the T6SS loci GA1 and GA2 are contained on highly similar integrative conjugative element (ICE) (Coyne et al., 2016). Among the numerous mechanisms that compete in the extremely dense ecosystem of the gut, T6SSs are probably very prevalent antagonistic systems in gut Bacteroidales (Wilson et al., 2015; Chatzidaki-Livanis et al., 2016; Coyne et al., 2016; Coyne and Comstock, 2019), as the Bacteroides T6SS genes are widespread in human gut metagenomes (Verster et al., 2017). Given that T6SSs in Bacteroidetes mediate inter-bacterial antagonism against pathogens and in view of the observed stability of Bacteroidetes in the healthy human intestine, it predicts that T6SS in the stable Bacteroidetes community contributes to intestinal homeostasis. We believe that further research on T6SSs in Bacteroidales species will add to our understanding of microbial stability in the gut and to our ability to diagnose and treat gut microbial infections.
Enteric Pathogens With T6SS Clusters
Vibrio cholerae
Vibrio cholerae is a Gram-negative pathogen consisting of over 200 serogroups that usually cause diarrhoeal diseases ranging from cholera to mild gastroenteritis after ingestion of contaminated food or water containing them (Kitaoka et al., 2011; Unterweger et al., 2014). All V. cholerae strains examined to date contain T6SS gene clusters (Unterweger et al., 2012). This microorganism provided several important and initial discoveries about T6SS, including the definition of the contact-dependent secretion model (Pukatzki et al., 2006; Basler et al., 2012), identification of T6SS-dependent effector-immunity pairs (Dong et al., 2013; Russell et al., 2013; Altindis et al., 2015), and induction of intestinal inflammation through actin cross-linking in host cells (Ma and Mekalanos, 2010). Recently, in vivo analysis presented new insight on the pathogenic mechanism of V. cholerae with T6SS. T6SS in V. cholerae acts on commensal bacteria A. pasteurianus to accelerate death of the Drosophila host (Fast et al., 2018). Another study revealed the roles of V. cholerae T6SS in antagonism against host commensal microbiota in vivo over their niche, which facilitated bacterial colonization of the mice gut (Zhao et al., 2018). Besides killing host gut bacterial symbionts, V. cholerae T6SS could modulate host intestinal mechanics, enhancing intestinal movements that led to expulsion of resident microbiota by the host (Logan et al., 2018).
Vibrio parahaemolyticus
Research indicated that vpT6SS2 contributed to adhesion of V. parahaemolyticus to host cells (Yu et al., 2012). In addition, the vpT6SS2 effector could induce autophagy in macrophage cells without causing apparent cytotoxicity (Yu et al., 2015).
Salmonella enterica
Experiments suggested that T6SS contributed to pathogenicity, at least in the S. enterica serotypes Gallinarum (Blondel et al., 2013), Enteritidis (Blondel et al., 2010; Troxell, 2018), Typhi (Wang et al., 2011), Dublin (Pezoa et al., 2014), and Typhimurium (Mulder et al., 2012). A recent study provided in vivo evidence that S. typhimurium used T6SS as a weapon to kill commensal bacteria (K. oxytoca) to successfully colonize the mouse gut (Sana et al., 2016).
Yersinia pseudotuberculosis
Studies of Y. pseudotuberculosis revealed roles for T6SS in responses to various stressors and identified corresponding regulators, including OmpR (Gueguen et al., 2013; Zhang W. et al., 2013), OxyR (Wang et al., 2015), ZntR (Wang et al., 2017), RpoS (Guan et al., 2015), RovM (Song et al., 2015), and YpsI/YtbI (Zhang et al., 2011). Notably, T6SS4 in Y. pseudotuberculosis was found to be involved in zinc transportation by secreting a zinc-binding protein YezP to mitigate the impact of detrimental hydroxyl radicals under oxidative stress (Wang et al., 2015). The contribution of T6SS to ion transport was also confirmed in B. thailandensis and P. aeruginosa (Lin et al., 2017; Si et al., 2017a,b).
Escherichia coli
Escherichia coli are model bacteria that have been studied extensively. Although less abundant than Bacteroidales in the gastrointestinal tract, symbiotic gut E. coli play important roles in colonization resistance against enteric pathogens of the proteobacterial phylum (Coyne and Comstock, 2019). To date, more than 150 E. coli strains have been identified. Most are harmless, but several serotypes have been proposed to be pathogenic and may cause significant diarrheal and extraintestinal diseases (Croxen et al., 2013). The current knowledge regarding the prevalence, the assembly, the regulation, and the roles of the T6SS in E. coli has been reviewed (Journet and Cascales, 2016). It has been suggested that T6SS contributes to pathogenesis in E. coli strains. For example, T6SS-dependent Hcp1 in E. coli K1 induced actin cytoskeleton rearrangement, apoptosis, and release of interleukin-6 (IL-6) and IL-8 in human brain microvascular endothelial cells (HBMEC) (Zhou et al., 2012). Further, T6SS in avian pathogenic E. coli (APEC) strains contributes to APEC pathogenesis (Ma et al., 2018). Several structural proteins of T6SS in E. coli have been studied to clarify the specific functions of these T6SS components (Felisberto-Rodrigues et al., 2011; Aschtgen et al., 2012; Durand et al., 2012). However, whether E. coli gut symbionts have T6SSs that function in colonization resistance against enteric pathogens, and how enteric pathogenic E. coli T6SSs function in the intestinal ecosystem still remain enigmatic.
Notably, the T6SS apparatus has a common evolutionary origin with phage tail-associated protein complexes. Yet, the involvement of phages in the evolution of bacterial T6SS remains unclear.
Other Enteric Pathogens
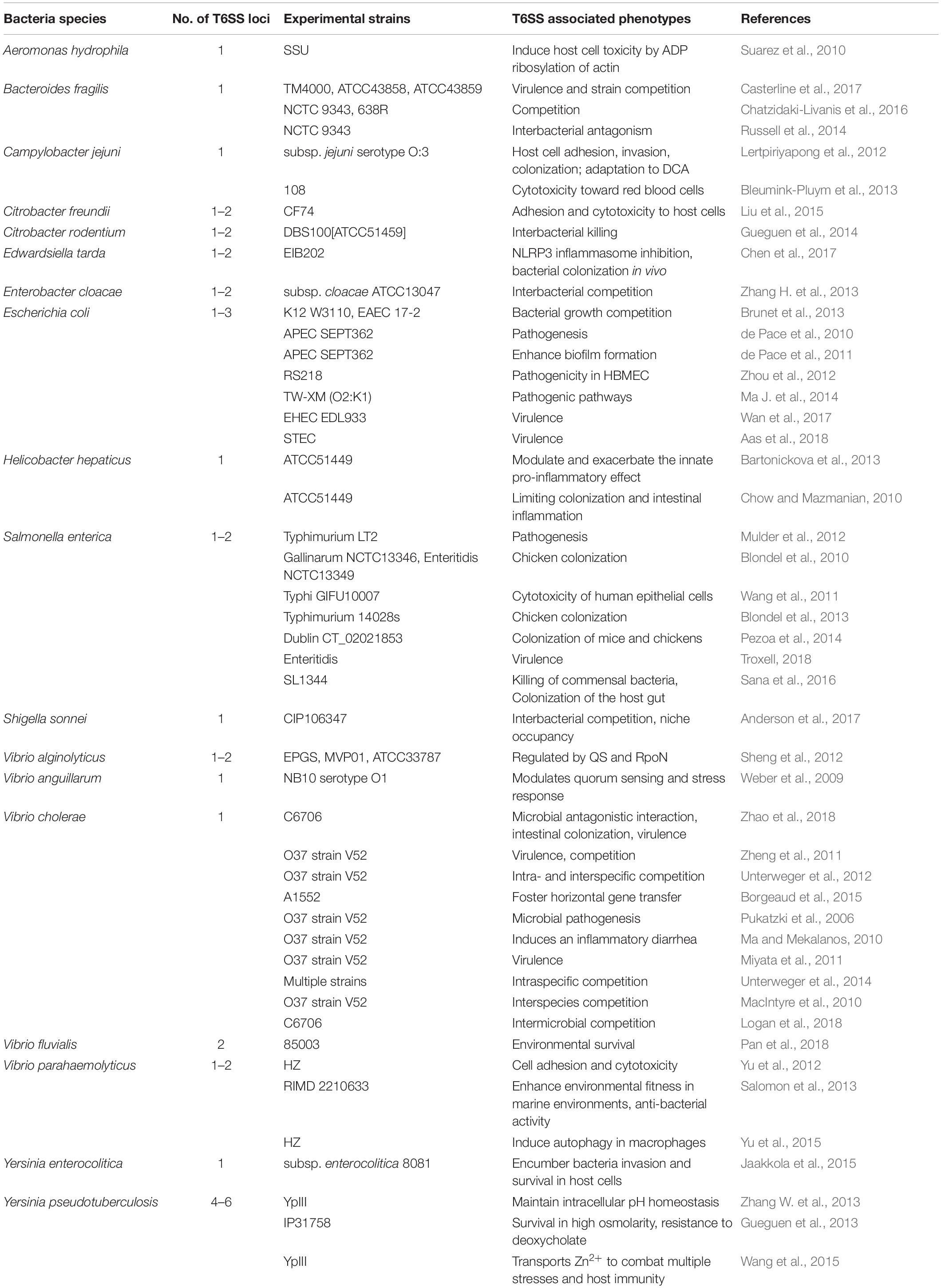
Type VI secretion system in some other enteric pathogens has also been studied. In C. jejuni, T6SS mediates host cell adhesion, invasion, colonization, and adaptation to deoxycholic acid (DCA) (Lertpiriyapong et al., 2012). In Citrobacter freundii, T6SS plays a wide-ranging role in the regulation of the flagellar system and in motility; it is also involved in the adherence and cytotoxicity to host cells (Liu et al., 2015). T6SS2 in Vibrio fluvialis is associated with anti-bacterial activity and contributes to bacteria survival in highly competitive environments (Pan et al., 2018). The T6SS effector VgrG1 from A. hydrophila induces host cell toxicity by ADP ribosylation of actin (Suarez et al., 2010). In E. tarda, the T6SS effector EvpP significantly inhibits NLRP3 inflammasome activation by inhibiting the Ca2+-dependent MAPK-Jnk pathway (Chen et al., 2017). Additionally, functional T6SSs have been verified in the enteric pathogens Enterobacter cloacae (Whitney et al., 2014), Edwardsiella ictaluri (Rogge and Thune, 2011), Yersinia enterocolitica (Jaakkola et al., 2015), and V. anguillarum (Weber et al., 2009). To facilitate retrieval for future research, we have listed the experimentally verified intestinal bacteria that contain functional T6SS loci in Table 1.
Concluding Remarks
Although the composition and function of the intestinal microbiota has been well documented, the underlying mechanisms of its assembly remain poorly understood. T6SS is a contact-dependent molecular weapon primarily used for interbacterial competition. Considering that T6SS loci exist in 50% of intestinal Bacteroidetes, which are the dominant microflora in the gastrointestinal system, and in some other commensal intestinal microbes and enteric pathogens, T6SS might be widely used in the intestinal ecosystem to shape microbiota composition. Actually, several seminal studies have greatly advanced our understanding of the importance of T6SS in the intestine. These studies showed that the T6SS antibacterial weapons are not only used by pathogens to colonize their hosts, but also by gut commensals to prevent pathogen colonization. Interestingly, two studies in V. cholera further indicated that microbial antagonistic interactions mediated by T6SS could elevate disease symptoms by activating host innate immune responses (Fast et al., 2018; Zhao et al., 2018). Moreover, using a zebrafish model, Logan et al. (2018) revealed that the T6SS of V. cholera could modulate host intestinal mechanics to redefine the gut microbial composition. These findings provide new insights into the mechanisms used by enteric pathogens for gut colonization. As for the gut commensals, Verster et al. (2017) investigate the prevalence and roles of the T6SS in the human gut microbiome by using metagenomic analyses. In addition to the compatibility with species GA1 and GA2, GA3 is associated with increased Bacteroides abundance in infant microbiomes, and confers an advantage in Bacteroides-rich ecosystems. Thus, it revealed the prevalence and potential role of T6SS-dependent competition in shaping human gut microbial composition (Verster et al., 2017).
It is noteworthy that sometimes a few invertebrate models and small animal models (e.g., Drosophila, zebrafish) were used to analyze T6SS functions in the intestinal ecosystem (Fast et al., 2018; Logan et al., 2018). These results should be further verified in mammalian models. In addition, further in vivo studies are urgently needed to confirm whether anti-fungal, stress-resistant and metal ion-acquiring T6SSs are functional in the intestinal ecosystem. Studies of T6SS in the intestinal ecosystem are just beginning and have a long way to go. As T6SS in several bacterial pathogens contributes to virulence against the host, the T6SS apparatus could be exploited as a therapeutic drug target. Based on this possibility, a novel natural antimicrobial was identified that can reduce the pathogenicity of Campylobacter T6SS in vitro and also decrease its colonization in vivo (Sima et al., 2018). These findings suggest that studies on T6SS functions in the intestinal ecosystem may provide a theoretical basis for the development of new medicines for various pathogenic infections in the future.
Author Contributions
CC and XY collected and assessed the references. CC and XS contributed in the proposal and guidelines of the review. CC and XY wrote this manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (Nos. 31725003 and 31670053) and the Open Project Program of the State Key Laboratory of Pathogen and Biosecurity (No. SKLPBS1825).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aas, C. G., Drablos, F., Haugum, K., and Afset, J. E. (2018). Comparative transcriptome profiling reveals a potential role of type VI secretion system and fimbriae in virulence of Non-O157 shiga toxin-producing Escherichia coli. Front. Microbiol. 9:1416. doi: 10.3389/fmicb.2018.01416
Altindis, E., Dong, T., Catalano, C., and Mekalanos, J. (2015). Secretome analysis of Vibrio cholerae type VI secretion system reveals a new effector-immunity pair. mBio 6:e00075. doi: 10.1128/mBio.00075-15
Anderson, M. C., Vonaesch, P., Saffarian, A., Marteyn, B. S., and Sansonetti, P. J. (2017). Shigella sonnei encodes a functional T6SS used for interbacterial competition and niche occupancy. Cell Host Microbe 21, 769.e3–776.e3. doi: 10.1016/j.chom.2017.05.004
Aschtgen, M. S., Zoued, A., Lloubes, R., Journet, L., and Cascales, E. (2012). The C-tail anchored TssL subunit, an essential protein of the enteroaggregative Escherichia coli Sci-1 Type VI secretion system, is inserted by YidC. Microbiologyopen 1, 71–82. doi: 10.1002/mbo3.9
Aubert, D. F., Xu, H., Yang, J., Shi, X., Gao, W., Li, L., et al. (2016). A Burkholderia type VI effector deamidates Rho GTPases to activate the pyrin inflammasome and trigger inflammation. Cell Host Microbe 19, 664–674. doi: 10.1016/j.chom.2016.04.004
Bachmann, V., Kostiuk, B., Unterweger, D., Diaz-Satizabal, L., Ogg, S., and Pukatzki, S. (2015). Bile salts modulate the mucin-activated type VI secretion system of pandemic Vibrio cholerae. PLoS Negl. Trop. Dis. 9:e0004031. doi: 10.1371/journal.pntd.0004031
Bartonickova, L., Sterzenbach, T., Nell, S., Kops, F., Schulze, J., Venzke, A., et al. (2013). Hcp and VgrG1 are secreted components of the Helicobacter hepaticus type VI secretion system and VgrG1 increases the bacterial colitogenic potential. Cell Microbiol. 15, 992–1011. doi: 10.1111/cmi.12094
Basler, M., Pilhofer, M., Henderson, G. P., Jensen, G. J., and Mekalanos, J. J. (2012). Type VI secretion requires a dynamic contractile phage tail-like structure. Nature 483, 182–186. doi: 10.1038/nature10846
Belkaid, Y., and Hand, T. W. (2014). Role of the microbiota in immunity and inflammation. Cell 157, 121–141. doi: 10.1016/j.cell.2014.03.011
Bleumink-Pluym, N. M., van Alphen, L. B., Bouwman, L. I., Wosten, M. M., and van Putten, J. P. (2013). Identification of a functional type VI secretion system in Campylobacter jejuni conferring capsule polysaccharide sensitive cytotoxicity. PLoS Pathog. 9:e1003393. doi: 10.1371/journal.ppat.1003393
Blondel, C. J., Jimenez, J. C., Leiva, L. E., Alvarez, S. A., Pinto, B. I., Contreras, F., et al. (2013). The type VI secretion system encoded in Salmonella pathogenicity island 19 is required for Salmonella enterica serotype Gallinarum survival within infected macrophages. Infect. Immun. 81, 1207–1220. doi: 10.1128/IAI.01165-12
Blondel, C. J., Yang, H. J., Castro, B., Chiang, S., Toro, C. S., Zaldivar, M., et al. (2010). Contribution of the type VI secretion system encoded in SPI-19 to chicken colonization by Salmonella enterica serotypes Gallinarum and Enteritidis. PLoS One 5:e11724. doi: 10.1371/journal.pone.0011724
Borgeaud, S., Metzger, L. C., Scrignari, T., and Blokesch, M. (2015). The type VI secretion system of Vibrio cholerae fosters horizontal gene transfer. Science 347, 63–67. doi: 10.1126/science.1260064
Boyer, F., Fichant, G., Berthod, J., Vandenbrouck, Y., and Attree, I. (2009). Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: what can be learned from available microbial genomic resources? BMC Genomics 10:104. doi: 10.1186/1471-2164-10-104
Brunet, Y. R., Espinosa, L., Harchouni, S., Mignot, T., and Cascales, E. (2013). Imaging type VI secretion-mediated bacterial killing. Cell Rep. 3, 36–41. doi: 10.1016/j.celrep.2012.11.027
Brunet, Y. R., Zoued, A., Boyer, F., Douzi, B., and Cascales, E. (2015). The Type VI secretion TssEFGK-VgrG phage-like baseplate is recruited to the TssJLM membrane complex via multiple contacts and serves as assembly platform for tail tube/sheath polymerization. PLoS Genet. 11:e1005545. doi: 10.1371/journal.pgen.1005545
Cascales, E., and Cambillau, C. (2012). Structural biology of type VI secretion systems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 1102–1111. doi: 10.1098/rstb.2011.0209
Casterline, B. W., Hecht, A. L., Choi, V. M., and Bubeck Wardenburg, J. (2017). The Bacteroides fragilis pathogenicity island links virulence and strain competition. Gut Microbes 8, 374–383. doi: 10.1080/19490976.2017.1290758
Chassaing, B., and Cascales, E. (2018). antibacterial weapons: targeted destruction in the microbiota. Trends Microbiol. 26, 329–338. doi: 10.1016/j.tim.2018.01.006
Chatzidaki-Livanis, M., Geva-Zatorsky, N., and Comstock, L. E. (2016). Bacteroides fragilis type VI secretion systems use novel effector and immunity proteins to antagonize human gut Bacteroidales species. Proc. Natl. Acad. Sci. U.S.A. 113, 3627–3632. doi: 10.1073/pnas.1522510113
Chen, H., Yang, D., Han, F., Tan, J., Zhang, L., Xiao, J., et al. (2017). The bacterial T6SS effector EvpP prevents NLRP3 inflammasome activation by inhibiting the Ca2+-dependent MAPK-Jnk pathway. Cell Host Microbe 21, 47–58. doi: 10.1016/j.chom.2016.12.004
Chow, J., and Mazmanian, S. K. (2010). A pathobiont of the microbiota balances host colonization and intestinal inflammation. Cell Host Microbe 7, 265–276. doi: 10.1016/j.chom.2010.03.004
Cianfanelli, F. R., Monlezun, L., and Coulthurst, S. J. (2016). Aim, load, fire: the type VI secretion system, a bacterial nanoweapon. Trends Microbiol. 24, 51–62. doi: 10.1016/j.tim.2015.10.005
Coulthurst, S. (2019). The Type VI secretion system: a versatile bacterial weapon. Microbiology 165, 503–515. doi: 10.1099/mic.0.000789
Coyne, M. J., and Comstock, L. E. (2019). Type VI secretion systems and the gut microbiota. Microbiol Spectr 7:PSIB–0009–2018. doi: 10.1128/microbiolspec.PSIB-0009-2018
Coyne, M. J., Roelofs, K. G., and Comstock, L. E. (2016). Type VI secretion systems of human gut Bacteroidales segregate into three genetic architectures, two of which are contained on mobile genetic elements. BMC Genomics 17:58. doi: 10.1186/s12864-016-2377-z
Coyne, M. J., Zitomersky, N. L., McGuire, A. M., Earl, A. M., and Comstock, L. E. (2014). Evidence of extensive DNA transfer between bacteroidales species within the human gut. mBio 5, e01305–e01314. doi: 10.1128/mBio.01305-14
Croxen, M. A., Law, R. J., Scholz, R., Keeney, K. M., Wlodarska, M., and Finlay, B. B. (2013). Recent advances in understanding enteric pathogenic Escherichia coli. Clin. Microbiol. Rev. 26, 822–880. doi: 10.1128/CMR.00022-13
de Pace, F., Boldrin de Paiva, J., Nakazato, G., Lancellotti, M., Sircili, M. P., Guedes Stehling, E., et al. (2011). Characterization of IcmF of the type VI secretion system in an avian pathogenic Escherichia coli (APEC) strain. Microbiology 157(Pt 10), 2954–2962. doi: 10.1099/mic.0.050005-0
de Pace, F., Nakazato, G., Pacheco, A., de Paiva, J. B., Sperandio, V., and da Silveira, W. D. (2010). The type VI secretion system plays a role in type 1 fimbria expression and pathogenesis of an avian pathogenic Escherichia coli strain. Infect. Immun. 78, 4990–4998. doi: 10.1128/IAI.00531-10
Dong, T. G., Ho, B. T., Yoder-Himes, D. R., and Mekalanos, J. J. (2013). Identification of T6SS-dependent effector and immunity proteins by Tn-seq in Vibrio cholerae. Proc. Natl. Acad. Sci. U.S.A. 110, 2623–2628. doi: 10.1073/pnas.1222783110
Durand, E., Cambillau, C., Cascales, E., and Journet, L. (2014). VgrG, Tae, Tle, and beyond: the versatile arsenal of Type VI secretion effectors. Trends Microbiol. 22, 498–507. doi: 10.1016/j.tim.2014.06.004
Durand, E., Zoued, A., Spinelli, S., Watson, P. J., Aschtgen, M. S., Journet, L., et al. (2012). Structural characterization and oligomerization of the TssL protein, a component shared by bacterial type VI and type IVb secretion systems. J. Biol. Chem. 287, 14157–14168. doi: 10.1074/jbc.M111.338731
Faith, J. J., Guruge, J. L., Charbonneau, M., Subramanian, S., Seedorf, H., Goodman, A. L., et al. (2013). The long-term stability of the human gut microbiota. Science 341:1237439. doi: 10.1126/science.1237439
Fang, F. C., Frawley, E. R., Tapscott, T., and Vazquez-Torres, A. (2016). Bacterial stress responses during host infection. Cell Host Microbe 20, 133–143. doi: 10.1016/j.chom.2016.07.009
Fast, D., Kostiuk, B., Foley, E., and Pukatzki, S. (2018). Commensal pathogen competition impacts host viability. Proc. Natl. Acad. Sci. U.S.A. 115, 7099–7104. doi: 10.1073/pnas.1802165115
Felisberto-Rodrigues, C., Durand, E., Aschtgen, M. S., Blangy, S., Ortiz-Lombardia, M., Douzi, B., et al. (2011). Towards a structural comprehension of bacterial type VI secretion systems: characterization of the TssJ-TssM complex of an Escherichia coli pathovar. PLoS Pathog. 7:e1002386. doi: 10.1371/journal.ppat.1002386
Fu, Y., Waldor, M. K., and Mekalanos, J. J. (2013). Tn-Seq analysis of Vibrio cholerae intestinal colonization reveals a role for T6SS-mediated antibacterial activity in the host. Cell Host Microbe 14, 652–663. doi: 10.1016/j.chom.2013.11.001
Gallique, M., Decoin, V., Barbey, C., Rosay, T., Feuilloley, M. G., Orange, N., et al. (2017). Contribution of the Pseudomonas fluorescens MFE01 Type VI secretion system to biofilm formation. PLoS One 12:e0170770. doi: 10.1371/journal.pone.0170770
Garcia-Bayona, L., and Comstock, L. E. (2018). Bacterial antagonism in host-associated microbial communities. Science 361:eaat2456. doi: 10.1126/science.aat2456
Gielda, L. M., and DiRita, V. J. (2012). Zinc competition among the intestinal microbiota. mBio 3:e00171-12. doi: 10.1128/mBio.00171-12
Guan, J., Xiao, X., Xu, S., Gao, F., Wang, J., Wang, T., et al. (2015). Roles of RpoS in Yersinia pseudotuberculosis stress survival, motility, biofilm formation and type VI secretion system expression. J. Microbiol. 53, 633–642. doi: 10.1007/s12275-015-0099-6
Gueguen, E., Durand, E., Zhang, X. Y., d’Amalric, Q., Journet, L., and Cascales, E. (2013). Expression of a Yersinia pseudotuberculosis type VI secretion system is responsive to envelope stresses through the OmpR transcriptional activator. PLoS One 8:e66615. doi: 10.1371/journal.pone.0066615
Gueguen, E., Wills, N. M., Atkins, J. F., and Cascales, E. (2014). Transcriptional frameshifting rescues Citrobacter rodentium type VI secretion by the production of two length variants from the prematurely interrupted tssM gene. PLoS Genet. 10:e1004869. doi: 10.1371/journal.pgen.1004869
Haapalainen, M., Mosorin, H., Dorati, F., Wu, R. F., Roine, E., Taira, S., et al. (2012). Hcp2, a secreted protein of the phytopathogen Pseudomonas syringae pv. tomato DC3000, is required for fitness for competition against bacteria and yeasts. J. Bacteriol. 194, 4810–4822. doi: 10.1128/JB.00611-12
Hecht, A. L., Casterline, B. W., Earley, Z. M., Goo, Y. A., Goodlett, D. R., and Bubeck Wardenburg, J. (2016). Strain competition restricts colonization of an enteric pathogen and prevents colitis. EMBO Rep. 17, 1281–1291. doi: 10.15252/embr.201642282
Hood, R. D., Singh, P., Hsu, F., Guvener, T., Carl, M. A., Trinidad, R. R., et al. (2010). A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 7, 25–37. doi: 10.1016/j.chom.2009.12.007
Ishikawa, T., Sabharwal, D., Broms, J., Milton, D. L., Sjostedt, A., Uhlin, B. E., et al. (2012). Pathoadaptive conditional regulation of the type VI secretion system in Vibrio cholerae O1 strains. Infect. Immun. 80, 575–584. doi: 10.1128/IAI.05510-11
Jaakkola, K., Somervuo, P., and Korkeala, H. (2015). Comparative genomic hybridization analysis of Yersinia enterocolitica and Yersinia pseudotuberculosis identifies genetic traits to elucidate their different ecologies. Biomed. Res. Int. 2015:760494. doi: 10.1155/2015/760494
Jani, A. J., and Cotter, P. A. (2010). Type VI secretion: not just for pathogenesis anymore. Cell Host Microbe 8, 2–6. doi: 10.1016/j.chom.2010.06.012
Jiang, F., Wang, X., Wang, B., Chen, L., Zhao, Z., Waterfield, N. R., et al. (2016). The Pseudomonas aeruginosa type VI secretion PGAP1-like effector induces host autophagy by activating endoplasmic reticulum stress. Cell Rep. 16, 1502–1509. doi: 10.1016/j.celrep.2016.07.012
Journet, L., and Cascales, E. (2016). The type VI secretion system in Escherichia coli and related species. EcoSal Plus 7, 1–20. doi: 10.1128/ecosalplus.ESP-0009-2015
Kitaoka, M., Miyata, S. T., Unterweger, D., and Pukatzki, S. (2011). Antibiotic resistance mechanisms of Vibrio cholerae. J. Med. Microbiol. 60(Pt 4), 397–407. doi: 10.1099/jmm.0.023051-0
Lertpiriyapong, K., Gamazon, E. R., Feng, Y., Park, D. S., Pang, J., Botka, G., et al. (2012). Campylobacter jejuni type VI secretion system: roles in adaptation to deoxycholic acid, host cell adherence, invasion, and in vivo colonization. PLoS One 7:e42842. doi: 10.1371/journal.pone.0042842
Lin, J., Zhang, W., Cheng, J., Yang, X., Zhu, K., Wang, Y., et al. (2017). A Pseudomonas T6SS effector recruits PQS-containing outer membrane vesicles for iron acquisition. Nat. Commun. 8:14888. doi: 10.1038/ncomms14888
Liu, L., Hao, S., Lan, R., Wang, G., Xiao, D., Sun, H., et al. (2015). The type VI secretion system modulates flagellar gene expression and secretion in Citrobacter freundii and contributes to adhesion and cytotoxicity to host cells. Infect. Immun. 83, 2596–2604. doi: 10.1128/IAI.03071-14
Logan, S. L., Thomas, J., Yan, J., Baker, R. P., Shields, D. S., Xavier, J. B., et al. (2018). The Vibrio cholerae type VI secretion system can modulate host intestinal mechanics to displace gut bacterial symbionts. Proc. Natl. Acad. Sci. U.S.A. 115, E3779–E3787. doi: 10.1073/pnas.1720133115
Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K., and Knight, R. (2012). Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230. doi: 10.1038/nature11550
Ma, A. T., and Mekalanos, J. J. (2010). In vivo actin cross-linking induced by Vibrio cholerae type VI secretion system is associated with intestinal inflammation. Proc. Natl. Acad. Sci. U.S.A. 107, 4365–4370. doi: 10.1073/pnas.0915156107
Ma, J., Bao, Y., Sun, M., Dong, W., Pan, Z., Zhang, W., et al. (2014). Two functional type VI secretion systems in avian pathogenic Escherichia coli are involved in different pathogenic pathways. Infect. Immun. 82, 3867–3879. doi: 10.1128/IAI.01769-14
Ma, L. S., Hachani, A., Lin, J. S., Filloux, A., and Lai, E. M. (2014). Agrobacterium tumefaciens deploys a superfamily of type VI secretion DNase effectors as weapons for interbacterial competition in planta. Cell Host Microbe 16, 94–104. doi: 10.1016/j.chom.2014.06.002
Ma, J., Sun, M., Pan, Z., Song, W., Lu, C., and Yao, H. (2018). Three Hcp homologs with divergent extended loop regions exhibit different functions in avian pathogenic Escherichia coli. Emerg. Microbes Infect. 7:49. doi: 10.1038/s41426-018-0042-0
MacIntyre, D. L., Miyata, S. T., Kitaoka, M., and Pukatzki, S. (2010). The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc. Natl. Acad. Sci. U.S.A. 107, 19520–19524. doi: 10.1073/pnas.1012931107
Miyata, S. T., Kitaoka, M., Brooks, T. M., McAuley, S. B., and Pukatzki, S. (2011). Vibrio cholerae requires the type VI secretion system virulence factor VasX to kill Dictyostelium discoideum. Infect. Immun. 79, 2941–2949. doi: 10.1128/IAI.01266-10
Mulder, D. T., Cooper, C. A., and Coombes, B. K. (2012). Type VI secretion system-associated gene clusters contribute to pathogenesis of Salmonella enterica serovar Typhimurium. Infect. Immun. 80, 1996–2007. doi: 10.1128/IAI.06205-11
Pan, J., Zhao, M., Huang, Y., Li, J., Liu, X., Ren, Z., et al. (2018). Integration host factor modulates the expression and function of T6SS2 in Vibrio fluvialis. Front. Microbiol. 9:962. doi: 10.3389/fmicb.2018.00962
Pezoa, D., Blondel, C. J., Silva, C. A., Yang, H. J., Andrews-Polymenis, H., Santiviago, C. A., et al. (2014). Only one of the two type VI secretion systems encoded in the Salmonella enterica serotype Dublin genome is involved in colonization of the avian and murine hosts. Vet. Res. 45:2. doi: 10.1186/1297-9716-45-2
Pi, H., Jones, S. A., Mercer, L. E., Meador, J. P., Caughron, J. E., Jordan, L., et al. (2012). Role of catecholate siderophores in gram-negative bacterial colonization of the mouse gut. PLoS One 7:e50020. doi: 10.1371/journal.pone.0050020
Pukatzki, S., Ma, A. T., Revel, A. T., Sturtevant, D., and Mekalanos, J. J. (2007). Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc. Natl. Acad. Sci. U.S.A. 104, 15508–15513. doi: 10.1073/pnas.0706532104
Pukatzki, S., Ma, A. T., Sturtevant, D., Krastins, B., Sarracino, D., Nelson, W. C., et al. (2006). Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl. Acad. Sci. U.S.A. 103, 1528–1533. doi: 10.1073/pnas.0510322103
Rogge, M. L., and Thune, R. L. (2011). Regulation of the Edwardsiella ictaluri type III secretion system by pH and phosphate concentration through EsrA, EsrB, and EsrC. Appl. Environ. Microbiol. 77, 4293–4302. doi: 10.1128/AEM.00195-11
Russell, A. B., Hood, R. D., Bui, N. K., LeRoux, M., Vollmer, W., and Mougous, J. D. (2011). Type VI secretion delivers bacteriolytic effectors to target cells. Nature 475, 343–347. doi: 10.1038/nature10244
Russell, A. B., LeRoux, M., Hathazi, K., Agnello, D. M., Ishikawa, T., Wiggins, P. A., et al. (2013). Diverse type VI secretion phospholipases are functionally plastic antibacterial effectors. Nature 496, 508–512. doi: 10.1038/nature12074
Russell, A. B., Wexler, A. G., Harding, B. N., Whitney, J. C., Bohn, A. J., Goo, Y. A., et al. (2014). A type VI secretion-related pathway in Bacteroidetes mediates interbacterial antagonism. Cell Host Microbe 16, 227–236. doi: 10.1016/j.chom.2014.07.007
Salomon, D., Gonzalez, H., Updegraff, B. L., and Orth, K. (2013). Vibrio parahaemolyticus type VI secretion system 1 is activated in marine conditions to target bacteria, and is differentially regulated from system 2. PLoS One 8:e61086. doi: 10.1371/journal.pone.0061086
Salomon, D., and Orth, K. (2015). Type VI secretion system. Curr. Biol. 25, R265–R266. doi: 10.1016/j.cub.2015.02.031
Sana, T. G., Flaugnatti, N., Lugo, K. A., Lam, L. H., Jacobson, A., Baylot, V., et al. (2016). Salmonella Typhimurium utilizes a T6SS-mediated antibacterial weapon to establish in the host gut. Proc. Natl. Acad. Sci. U.S.A. 113, E5044–E5051. doi: 10.1073/pnas.1608858113
Sana, T. G., Lugo, K. A., and Monack, D. M. (2017). T6SS: the bacterial “fight club” in the host gut. PLoS Pathog. 13:e1006325. doi: 10.1371/journal.ppat.1006325
Sheng, L., Gu, D., Wang, Q., Liu, Q., and Zhang, Y. (2012). Quorum sensing and alternative sigma factor RpoN regulate type VI secretion system I (T6SSVA1) in fish pathogen Vibrio alginolyticus. Arch. Microbiol. 194, 379–390. doi: 10.1007/s00203-011-0780-z
Si, M., Wang, Y., Zhang, B., Zhao, C., Kang, Y., Bai, H., et al. (2017a). The type VI secretion system engages a redox-regulated dual-functional heme transporter for zinc acquisition. Cell Rep. 20, 949–959. doi: 10.1016/j.celrep.2017.06.081
Si, M., Zhao, C., Burkinshaw, B., Zhang, B., Wei, D., Wang, Y., et al. (2017b). Manganese scavenging and oxidative stress response mediated by type VI secretion system in Burkholderia thailandensis. Proc. Natl. Acad. Sci. U.S.A. 114, E2233–E2242. doi: 10.1073/pnas.1614902114
Silverman, J. M., Brunet, Y. R., Cascales, E., and Mougous, J. D. (2012). Structure and regulation of the type VI secretion system. Annu. Rev. Microbiol. 66, 453–472. doi: 10.1146/annurev-micro-121809-151619
Sima, F., Stratakos, A. C., Ward, P., Linton, M., Kelly, C., Pinkerton, L., et al. (2018). A novel natural antimicrobial can reduce the in vitro and in vivo pathogenicity of T6SS positive Campylobacter jejuni and Campylobacter coli chicken isolates. Front. Microbiol. 9:2139. doi: 10.3389/fmicb.2018.02139
Song, Y., Xiao, X., Li, C., Wang, T., Zhao, R., Zhang, W., et al. (2015). The dual transcriptional regulator RovM regulates the expression of AR3- and T6SS4-dependent acid survival systems in response to nutritional status in Yersinia pseudotuberculosis. Environ. Microbiol. 17, 4631–4645. doi: 10.1111/1462-2920.12996
Sorbara, M. T., and Pamer, E. G. (2019). Interbacterial mechanisms of colonization resistance and the strategies pathogens use to overcome them. Mucosal Immunol. 12, 1–9. doi: 10.1038/s41385-018-0053-0
Steele, M. I., Kwong, W. K., Whiteley, M., and Moran, N. A. (2017). Diversification of type VI secretion system toxins reveals ancient antagonism among bee gut microbes. MBio 8:e01630-17. doi: 10.1128/mBio.01630-17
Suarez, G., Sierra, J. C., Erova, T. E., Sha, J., Horneman, A. J., and Chopra, A. K. (2010). A type VI secretion system effector protein, VgrG1, from Aeromonas hydrophila that induces host cell toxicity by ADP ribosylation of actin. J. Bacteriol. 192, 155–168. doi: 10.1128/JB.01260-09
Ting, S. Y., Bosch, D. E., Mangiameli, S. M., Radey, M. C., Huang, S., Park, Y. J., et al. (2018). Bifunctional immunity proteins protect bacteria against FtsZ-targeting ADP-ribosylating toxins. Cell 175, 1380.e14–1392.e14. doi: 10.1016/j.cell.2018.09.037
Troxell, B. (2018). A type 6 secretion system (T6SS) encoded gene within Salmonella enterica serovar Enteritidis contributes to virulence. Virulence 9, 585–587. doi: 10.1080/21505594.2017.1421829
Trunk, K., Peltier, J., Liu, Y. C., Dill, B. D., Walker, L., Gow, N. A. R., et al. (2018). The type VI secretion system deploys antifungal effectors against microbial competitors. Nat. Microbiol. 3, 920–931. doi: 10.1038/s41564-018-0191-x
Unterweger, D., Kitaoka, M., Miyata, S. T., Bachmann, V., Brooks, T. M., Moloney, J., et al. (2012). Constitutive type VI secretion system expression gives Vibrio cholerae intra- and interspecific competitive advantages. PLoS One 7:e48320. doi: 10.1371/journal.pone.0048320
Unterweger, D., Miyata, S. T., Bachmann, V., Brooks, T. M., Mullins, T., Kostiuk, B., et al. (2014). The Vibrio cholerae type VI secretion system employs diverse effector modules for intraspecific competition. Nat. Commun. 5:3549. doi: 10.1038/ncomms4549
Verster, A. J., Ross, B. D., Radey, M. C., Bao, Y., Goodman, A. L., Mougous, J. D., et al. (2017). The landscape of type VI secretion across human gut microbiomes reveals its role in community composition. Cell Host Microbe 22, 411.e14–419.e14. doi: 10.1016/j.chom.2017.08.010
Walter, J., and Ley, R. (2011). The human gut microbiome: ecology and recent evolutionary changes. Annu. Rev. Microbiol. 65, 411–429. doi: 10.1146/annurev-micro-090110-102830
Wan, B., Zhang, Q., Ni, J., Li, S., Wen, D., Li, J., et al. (2017). Type VI secretion system contributes to Enterohemorrhagic Escherichia coli virulence by secreting catalase against host reactive oxygen species (ROS). PLoS Pathog. 13:e1006246. doi: 10.1371/journal.ppat.1006246
Wang, M., Luo, Z., Du, H., Xu, S., Ni, B., Zhang, H., et al. (2011). Molecular characterization of a functional type VI secretion system in Salmonella enterica serovar Typhi. Curr. Microbiol. 63, 22–31. doi: 10.1007/s00284-011-9935-z
Wang, T., Chen, K., Gao, F., Kang, Y., Chaudhry, M. T., Wang, Z., et al. (2017). ZntR positively regulates T6SS4 expression in Yersinia pseudotuberculosis. J. Microbiol. 55, 448–456. doi: 10.1007/s12275-017-6540-2
Wang, T., Si, M., Song, Y., Zhu, W., Gao, F., Wang, Y., et al. (2015). Type VI secretion system transports Zn2+ to combat multiple stresses and host immunity. PLoS Pathog. 11:e1005020. doi: 10.1371/journal.ppat.1005020
Weber, B., Hasic, M., Chen, C., Wai, S. N., and Milton, D. L. (2009). Type VI secretion modulates quorum sensing and stress response in Vibrio anguillarum. Environ. Microbiol. 11, 3018–3028. doi: 10.1111/j.1462-2920.2009.02005.x
Wexler, A. G., Bao, Y., Whitney, J. C., Bobay, L. M., Xavier, J. B., Schofield, W. B., et al. (2016). Human symbionts inject and neutralize antibacterial toxins to persist in the gut. Proc. Natl. Acad. Sci. U.S.A. 113, 3639–3644. doi: 10.1073/pnas.1525637113
Wexler, A. G., and Goodman, A. L. (2017). An insider’s perspective: Bacteroides as a window into the microbiome. Nat. Microbiol. 2:17026. doi: 10.1038/nmicrobiol.2017.26
Whitney, J. C., Beck, C. M., Goo, Y. A., Russell, A. B., Harding, B. N., De Leon, J. A., et al. (2014). Genetically distinct pathways guide effector export through the type VI secretion system. Mol. Microbiol. 92, 529–542. doi: 10.1111/mmi.12571
Whitney, J. C., Quentin, D., Sawai, S., LeRoux, M., Harding, B. N., Ledvina, H. E., et al. (2015). An interbacterial NAD(P)(+) glycohydrolase toxin requires elongation factor Tu for delivery to target cells. Cell 163, 607–619. doi: 10.1016/j.cell.2015.09.027
Wilson, M. M., Anderson, D. E., and Bernstein, H. D. (2015). Analysis of the outer membrane proteome and secretome of Bacteroides fragilis reveals a multiplicity of secretion mechanisms. PLoS One 10:e0117732. doi: 10.1371/journal.pone.0117732
Yang, X., Song, Y., Dai, Q., Zhang, H., Song, L., Wang, Z., et al. (2019). The stringent response factor, RelA, positively regulates T6SS4 expression through the RovM/RovA pathway in Yersinia pseudotuberculosis. Microbiol. Res. 220, 32–41. doi: 10.1016/j.micres.2018.12.002
Yu, Y., Fang, L., Zhang, Y., Sheng, H., and Fang, W. (2015). VgrG2 of type VI secretion system 2 of Vibrio parahaemolyticus induces autophagy in macrophages. Front. Microbiol. 6:168. doi: 10.3389/fmicb.2015.00168
Yu, Y., Yang, H., Li, J., Zhang, P., Wu, B., Zhu, B., et al. (2012). Putative type VI secretion systems of Vibrio parahaemolyticus contribute to adhesion to cultured cell monolayers. Arch. Microbiol. 194, 827–835. doi: 10.1007/s00203-012-0816-z
Zhang, H., Gao, Z. Q., Wei, Y., Xu, J. H., and Dong, Y. H. (2013). Insights into the cross-immunity mechanism within effector families of bacteria type VI secretion system from the structure of StTae4-EcTai4 complex. PLoS One 8:e73782. doi: 10.1371/journal.pone.0073782
Zhang, W., Wang, Y., Song, Y., Wang, T., Xu, S., Peng, Z., et al. (2013). A type VI secretion system regulated by OmpR in Yersinia pseudotuberculosis functions to maintain intracellular pH homeostasis. Environ. Microbiol. 15, 557–569. doi: 10.1111/1462-2920.12005
Zhang, W., Xu, S., Li, J., Shen, X., Wang, Y., and Yuan, Z. (2011). Modulation of a thermoregulated type VI secretion system by AHL-dependent quorum sensing in Yersinia pseudotuberculosis. Arch. Microbiol. 193, 351–363. doi: 10.1007/s00203-011-0680-2
Zhao, W., Caro, F., Robins, W., and Mekalanos, J. J. (2018). Antagonism toward the intestinal microbiota and its effect on Vibrio cholerae virulence. Science 359, 210–213. doi: 10.1126/science.aap8775
Zheng, J., Ho, B., and Mekalanos, J. J. (2011). Genetic analysis of anti-amoebae and anti-bacterial activities of the type VI secretion system in Vibrio cholerae. PLoS One 6:e23876. doi: 10.1371/journal.pone.0023876
Zhou, Y., Tao, J., Yu, H., Ni, J., Zeng, L., Teng, Q., et al. (2012). Hcp family proteins secreted via the type VI secretion system coordinately regulate Escherichia coli K1 interaction with human brain microvascular endothelial cells. Infect. Immun. 80, 1243–1251. doi: 10.1128/IAI.05994-11
Zoued, A., Durand, E., Bebeacua, C., Brunet, Y. R., Douzi, B., Cambillau, C., et al. (2013). TssK is a trimeric cytoplasmic protein interacting with components of both phage-like and membrane anchoring complexes of the type VI secretion system. J. Biol. Chem. 288, 27031–27041. doi: 10.1074/jbc.M113.499772
Keywords: type VI secretion system, intestinal microbiota, enteric pathogen, colonization resistance, competition
Citation: Chen C, Yang X and Shen X (2019) Confirmed and Potential Roles of Bacterial T6SSs in the Intestinal Ecosystem. Front. Microbiol. 10:1484. doi: 10.3389/fmicb.2019.01484
Received: 22 January 2019; Accepted: 14 June 2019;
Published: 28 June 2019.
Edited by:
Eric Cascales, Aix-Marseille Université, FranceReviewed by:
Thibault Géry Sana, École Polytechnique Fédérale de Lausanne, SwitzerlandBenjamin Ross, University of Washington, United States
Copyright © 2019 Chen, Yang and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xihui Shen, eGlodWlzaGVuQG53c3VhZi5lZHUuY24=
†These authors have contributed equally to this work
 Can Chen
Can Chen Xiaobing Yang
Xiaobing Yang Xihui Shen
Xihui Shen