- 1Department of Ecological Microbiology, University of Bayreuth, Bayreuth, Germany
- 2Department of Environmental Microbiology, Helmholtz Centre for Environmental Research-UFZ, Leipzig, Germany
- 3German Centre for Integrative Biodiversity Research (iDiv), Leipzig, Germany
- 4Department of Molecular Systems Biology, Helmholtz Centre for Environmental Research-UFZ, Leipzig, Germany
- 5Faculty of Biosciences, Pharmacy and Psychology, Institute of Biochemistry, University of Leipzig, Leipzig, Germany
- 6Department of Chemistry and Bioscience, University of Aalborg, Aalborg, Denmark
- 7IsoLife BV, Wageningen, Netherlands
- 8Microbial Biogeochemistry, RA Landscape Functioning, Leibniz Centre for Agricultural Landscape Research (ZALF), Müncheberg, Germany
Chitin provides a valuable carbon and nitrogen source for soil microorganisms and is a major component of particulate organic matter in agricultural soils. To date, there is no information on interaction and interdependence in chitin-degrading soil microbiomes. Since microbial chitin degradation occurs under both oxic and anoxic conditions and both conditions occur simultaneously in soil, the comparison of the active microbiome members under both conditions can reveal key players for the overall degradation in aerated soil. A time-resolved 16S rRNA stable isotope probing experiment was conducted with soil material from the top soil layer of a wheat-covered field. [13CU]-chitin was largely mineralized within 20 days under oxic conditions. Cellvibrio, Massilia, and several Bacteroidetes families were identified as initially active chitin degraders. Subsequently, Planctomycetes and Verrucomicrobia were labeled by assimilation of 13C carbon either from [13CU]-chitin or from 13C-enriched components of primary chitin degraders. Bacterial predators (e.g., Bdellovibrio and Bacteriovorax) were labeled, too, and non-labeled microeukaryotic predators (Alveolata) increased their relative abundance toward the end of the experiment (70 days), indicating that chitin degraders were subject to predation. Trophic interactions differed substantially under anoxic and oxic conditions. Various fermentation types occurred along with iron respiration. While Acidobacteria and Chloroflexi were the first taxa to be labeled, although at a low 13C level, Firmicutes and uncultured Bacteroidetes were predominantly labeled at a much higher 13C level during the later stages, suggesting that the latter two bacterial taxa were mainly responsible for the degradation of chitin and also provided substrates for iron reducers. Eventually, our study revealed that (1) hitherto unrecognized Bacteria were involved in a chitin-degrading microbial food web of an agricultural soil, (2) trophic interactions were substantially shaped by the oxygen availability, and (3) detectable predation was restricted to oxic conditions. The gained insights into trophic interactions foster our understanding of microbial chitin degradation, which is in turn crucial for an understanding of soil carbon dynamics.
Introduction
Microbiomes in agricultural soils are fed by carbon and energy input from plant-derived organic residues, including root exudates. This input is partially transformed into the fungal and arthropod biomass (Gooday, 1990a,b; Martínez et al., 2009; Kramer et al., 2016). Together with plant residues, fungal and arthropod biomass contributes substantially to the pool of particulate organic matter which is turned over by the soil microbiome (Singh and Gupta, 1977; Scheu, 2002). Structural polymers form a major part of this pool of substrates, of which cellulose, hemicellulose, and chitin are more prone to microbial degradation than lignin. Unraveling agricultural and other soil microbial food webs that are associated with cellulose and complex plant litter degradation has been the goal of previous studies (e.g., El Zahar Haichar et al., 2007; Schellenberger et al., 2010; Kramer et al., 2016). However, the trophic interactions, dynamics, and ecological functions of members of a soil microbiome that degrades chitin await further elucidation. Chitin is the most abundant polysaccharide in terrestrial ecosystems after cellulose (Gooday, 1990a,b). It consists of β-1-4-linked N-acetylglucosamine (GlcNAc) residues and is a structural component of protists, arthropods, and fungi (Gooday, 1990a,b; Martínez et al., 2009). Fungal cell walls contain up to 25% chitin (Blumenthal and Roseman, 1957) and since fungi reach up to 60–90% of the microbial biomass in agricultural soils, they are thus the main source of chitin in such soils (Fernandez and Koide, 2012).
Bacteria have been recognized as the predominant chitin degraders and various species of Actinobacteria, Proteobacteria, and Firmicutes are considered to be important chitinolytic bacteria in soil (Gooday, 1990a,b; Beier and Bertilsson, 2013). However, our understanding of trophic interactions between chitinolytic and other soil microorganisms is solely based on information from pure or co-culture experiments as well as gene marker surveys (Gooday, 1990a,b; Jagmann et al., 2010; Beier and Bertilsson, 2013; Wieczorek et al., 2014), and an experiment that would directly reveal trophic interactions of the soil microbiome members involved in chitin degradation has yet to be completed.
Chitinolytic microorganisms use various and different extracellular enzymes to solubilize chitin, which may allow them to occupy different functional niches in regard to chitin breakdown. The enzymatic hydrolysis of chitin fibers by soil microorganisms is complex and requires the synergetic actions of different enzyme types. Exo- and endochitinases are the most important enzymes in chitin breakdown and hydrolyze chitin into oligomers of N-acetyl-glucosamine (Wild et al., 2018). Many bacterial chitinases are encoded by the gene chiA, which has been used in previous studies to detect chitinolytic microorganisms in the environment (Beier and Bertilsson, 2013; Cretoiu et al., 2013; Wieczorek et al., 2014). In addition, lytic polysaccharide monooxygenases (LPMO) break chitin chains by oxidative cleavage and increase the rate and efficiency of chitin degradation (Vaaje-Kolstad et al., 2010, 2013). N-acetyl-glucosamine dimers are taken up by soil microorganisms and are cleaved by β-N-acetylglucosaminidases to N-acetyl-glucosamine, which is further metabolized as a source of energy, carbon, and nitrogen (Gooday, 1990a; Keyhani and Roseman, 1999).
The aforementioned metabolic steps of microbial chitin breakdown also occur in agricultural soils of arable land which is considered to be largely oxic (Wieczorek et al., 2014). Nonetheless, anoxia occurs in microzones of such soils, as the oxygen distribution is heterogeneous and dynamic (Wagner et al., 1996; Or et al., 2007). Thus, contrasting energy-conserving microbial metabolisms occur at close proximity to each other and contribute to the degradation of biopolymers in agricultural soils (Schellenberger et al., 2010; Kramer et al., 2016). Hence, the availability of oxygen in a soil of arable agricultural land is a key environmental factor that determines the activity of different biopolymer-degrading microbial species.
In a previous study, we detected potential chitinolytic microorganisms with the gene marker chiA (encoding GH18 – glycoside hydrolase family – chitinases), which only allowed for a taxonomically limited identification of chitinolytic microorganisms and did not allow for the identification of trophically linked, non-chitinolytic microorganisms (Wieczorek et al., 2014). Therefore, the objective of the current study was to resolve the carbon flow path through the microbiome and to resolve the trophic interactions in an agricultural soil sample under oxic and anoxic conditions using an RNA-based stable isotope labeling approach with fungal chitin and soil material from a wheat-covered field in South Germany.
Materials and Methods
Sampling Site and Soil Properties
The sampling site is located on the research farm “Klostergut Scheyern” near Munich, Germany (48°30.0´N, 11°20.7´E). The upper 20-cm layer of aerated agricultural soil was sampled in April 2012 and processed within a week. The mean annual precipitation was 803 mm, with a mean annual temperature of 7.4°C (Wieczorek et al., 2014). The soil type was a Dystric Cambisol (FAO soil classification system) (Wieczorek et al., 2014). The C/N ratio was 6.9 ± 0.1. Soil pH (measured in water) was 6.6 ± 0.1, and the gravimetric water content was 21.9% (±1.0%). Ammonium, nitrate, and sulfate concentrations were below the detection limit of 0.1, or were 2.2 ± 0.2, and 1.2 ± 0 0.1 μmol gsoilDW−1, respectively. Total amounts of iron and manganese were 103.4 ± 37.5 and 10.0 ± 6.7 μmol gsoilDW−1, respectively.
Soil Incubations and 13C-Labeling Experiments
Three hundred grams of freshly sampled soil were mixed with 750 ml of either sterile oxic or sterile anoxic water (ion-free distilled water) in rubber-stoppered 2-L flasks to prepare one oxic and one anoxic master slurry. The oxic and anoxic master slurries were flushed for 1 h with sterile air and sterile argon (100%, Rießner, Germany), respectively, and homogenized on an end-over-end shaker overnight at 5°C. Subsequently, the homogenized master slurries were divided into sets of six slurries each containing 110 ml in butyl rubber-stoppered 500-ml flasks. Slurries originating from the oxic master slurry were flushed with sterile air (oxic slurries) and those originating from the anoxic master slurry were flushed with sterile argon (anoxic slurries). Two of the oxic and anoxic slurries were supplemented with either 0.125 g of [13CU]-chitin ([13C]-chitin treatments), 0.125 g of [12CU]-chitin ([12C]-chitin treatments), or kept unsupplemented as control treatments (duplicate analysis). The [13CU]-chitin (>98 atom % 13C) and the [12CU]-chitin (1.1 atom % 13C) were purified from biomass of the fungus Aspergillus niger by IsoLife BV (Wageningen, Netherlands). The supplemented amount of chitin equals 5 mmol (44 mM) of carbon. Oxic and anoxic chitin treatments as well as the respective unsupplemented controls were incubated at 20°C on an end-over-end shaker for 70 days. The gas phase was exchanged in the oxic incubations every few days to keep the oxygen mixing ratios above 10%.
Chemical Analyses
Liquid samples and gas samples were taken with sterile syringes. Liquid samples were centrifuged at 13,000 × g (Himac CT15E, Hitachi Koki Co., Ltd., Tokyo, Japan) for 15 min, and the supernatant was filtered (HPLC nylon filter, pore volume 0.2 μm, Infochroma, Zug, Switzerland). Organic acids and sugars were identified using high-performance liquid chromatography with ion exclusion chromatography (1,090 series II with UV detector, Hewlett Packard, Palo Alto, CA) (Wieczorek et al., 2014). Carbon dioxide, molecular hydrogen, and methane were measured with a gas chromatograph; the pH was measured with a pH electrode; and the ammonium, nitrate, and sulfate concentrations were assessed with ion chromatography, as described previously (Wieczorek et al., 2014).
For the analysis of 12CO2 and 13CO2 in the samples, gas chromatography-mass spectrometry (GC-MS) analysis was performed using a Perkin-Elmer GC Clarus 600 system with a Rtx®-1 capillary column (60 m × 320 μM). For GC-MS detection, an electron ionization system was operated with an ionization energy of 70 eV. Mass spectra were taken from 14 to 70 Da. Helium was used as carrier gas at a constant flow with 300 kPa, and an injection volume of 10 μl (split ratio 10:1) was employed manually by use of gastight syringes. Each sample was measured five times. The total amount of 12CO2 and 13CO2 was analyzed by extraction of the masses 44 and 45 followed by peak integration. The peak areas were corrected with 12CO2 and 13CO2 indoor air values and finally the ratio of the masses 45/44 was calculated. The soil moisture content was determined by weighing sieved soil before and after drying at 105°C for 48 h.
RNA Stable Isotope Probing
To reduce sequencing effort and costs for the chitin, substrate duplicated microcosms were conducted and per time point and oxygen treatment only two SIP gradients were analyzed (one for the 12C control and one for 13C treatment). Nonetheless, RNA was extracted two times from duplicated slurries, i.e., two for 12C and two for 13C treatment, were equimolarly pooled and loaded on one gradient. Thus, for both oxygen treatments, from eight flasks, RNA was extracted at each time point. All as 13C-labeled identified OTUs were labeled at several time points. Although SIP gradients were not replicated at each time point, the dataset of labeled OTUs were replicated over time.
Nucleic acids were extracted from 0.4 g of soil slurry (Wieczorek et al., 2014), and RNA was obtained after digestion of DNA with DNase I (Fermentas, St. Leon-Roth, Germany). RNA SIP was performed according to published protocols (Whiteley et al., 2007; Schellenberger et al., 2010). Per analyzed time point of a treatment (Figures 2, 3), 200–500 ng of RNA was added to the gradient solution (buoyant density 1.79 g ml−1). Isopycnic centrifugation was performed to separate “heavy” potentially 13C-labeled RNA from “lighter” 12C-labeled RNA. RNA samples from oxic and anoxic chitin treatments were centrifuged in two separate runs using the same gradient solution to exclude gradient heterogeneity. Each gradient was separated into 10 fractions, and the buoyant density of each fraction was measured at 25°C (Supplementary Figure S1; Schellenberger et al., 2010). RNA was precipitated with ethanol, sodium acetate, and glycogen (Schellenberger et al., 2010) and resuspended in DNAse/RNAse free water (Gibco® Invitrogen, Germany). The RNA in each fraction was quantified with the Quant-iT RiboGreen RNA Assay Kit (Invitrogen, Germany) (Supplementary Figure S2). RNA from fractions with buoyant densities between 1.81 and 1.83 g ml−1 (“heavy” fractions) and RNA from fractions with buoyant densities between 1.76 and 1.77 g ml−1 (“light” fractions) were stored at −80°C and used for high-throughput sequencing of gene markers.
High-Throughput Sequencing of Gene Markers
For testing if after the DNase digestion, DNA was completely removed, all RNA samples were subjected to PCR with primers and conditions used for amplifying cDNA. None of these PCRs revealed a product on a standard agarose gel. Thus, we concluded that those RNA samples were free of amplifiable DNA and proceeded with reverse transcription. Reverse transcription of RNA was performed with random hexamer primers (SuperScript III First-Strand Synthesis Supermix, Invitrogen, Karlsruhe, Germany) according to the manufacturer’s protocol. T4 GP32 protein (EURx Ltd., Gdansk, Poland) was added to a final concentration of 10 μg ml−1. Bacterial 16S rRNA gene transcripts were amplified from cDNA with primers 341F and 805R (Supplementary Tables S1, S2). A two-step amplification protocol was used to minimize the potential primer biases (Berry et al., 2011). In addition, 6-nucleotide (MID) instead of 10-nucleotide barcodes were used (Supplementary Figure S3, Supplementary Tables S3, S4). The first amplification round with untagged primers (25 cycles) was followed by a second amplification round using primers carrying adaptors, key, and MID (10 cycles). Amplicons were gel-purified, quantified, and pyrosequenced as previously described (Stacheter et al., 2013). Archaeal 16S rRNA gene, eukaryotic 18S rRNA gene, and chiA gene transcripts were amplified from cDNA using specific primers and amplification protocols (Supplementary Tables S1, S2), and the amplicons were sequenced by LGC Genomics GmbH with a MiSeq sequencer and V3 reagents (Illumina, Germany).
Analysis of Pyrosequencing-Derived Data Analysis
Sequences were trimmed and quality filtered using ACACIA, such that erroneous homopolymers were corrected and low-quality reads were discarded from the dataset (Bragg et al., 2012). Prior to clustering, potential chimeras were filtered out (UCHIME algorithm implemented in USEARCH with the RDP Gold database release from 2016 for high-quality 16S rRNA gene reference sequences; Edgar et al., 2011). Information on the sequence numbers after each step is given in Supplementary Table S5. Sequences with a minimum length of 400 bp were analyzed using Jaguc2 (Nebel et al., 2011). In brief, Jaguc2 operates with average linkage clustering and pairwise alignments for calling operational taxonomic units (OTUs). Thus, this method is rather insensitive to sequencing errors such as insertions and deletions. Family-level OTUs were called with an average sequence similarity threshold of 87.5% (Yarza et al., 2010). Rarefaction analysis indicated that the sequencing effort was sufficient for the phylogenetic resolution as presented in the study (Supplementary Figure S4). OTUs were phylogenetically affiliated by local nucleotide BLAST using Jaguc2 against the latest SILVA SSU database release (SILVA 128; Yilmaz et al., 2014).
Analysis of Illumina Sequencing-Derived Data
One million read pairs with a minimum length of 300 bp were provided by the commercial sequencing service in the *.fastq data format from all samples together. Paired-end forward and reverse reads were combined using BBMerge 34.481 by the LGC Genomics GmbH and used for further analysis. Random subsamples (4,000 sequences each for archaeal 16S rRNA gene transcript and eukaryotic 18S rRNA gene transcript libraries; 2000 sequences each for chiA libraries) were taken using USEARCH as the computing time of Jaguc2 for the clustering increases exponentially (Nebel et al., 2011). A minimum of 1,221 reads per dataset remained for further analysis. Prior clustering barcodes as used in the pyrosequencing analysis were added to the fastq files in silico. After the addition of barcode sequences, sample files were merged into two fastq files, one file for the oxic and one for anoxic samples, respectively. Sequences were trimmed to 446 bp. Sequences with a minimum length of 400 bp were analyzed using Jaguc2. Two separate clustering runs were performed for oxic and anoxic treatments. Family-level OTUs were called with an average sequence similarity threshold of 87.5% for archaeal 16S rRNA gene transcript and eukaryotic 18S rRNA gene transcript sequences and 80% for chiA gene transcripts.
Identification of 13C-Labeled Taxa by the Comparative Amplicon Pyrosequencing-Based Stable Isotope Probing Approach
Labeled bacterial 16S rRNA gene transcripts were identified following a modified comparative amplicon pyrosequencing-based stable isotope probing (CAP-SIP) approach modified compared to the one introduced by Dallinger and Horn (2014). Mean differences (±standard deviation) of the relative abundances per OTU in amplicon libraries derived from “heavy” fractions of [13C]- and [12C]-treatments at t0 were 0.04 (±0.19%) and 0.05% (±0.39%) for the oxic and anoxic treatments, respectively, indicating that artificial variations (caused by fractionation, amplicon generation, pyrosequencing, etc.) were minimal. These mean differences plus three times the respective standard deviations were calculated as a significance threshold (T: 0.61 and 1.22% for oxic and anoxic treatments, respectively). The threshold suggests a correct-detection rate of differences in 99.73% of the cases (Westgard et al., 1981). OTUs that met the criteria 1–3 and in most cases also criterion 4 were scored labeled at a certain time point tx: (1) Ra − Rb > T (Ra and Rb are the relative abundances of an OTU in the “heavy” fractions of the [13C]- and [12C]-treatment at tx, respectively); (2) Ra − Rc > T (Rc is the relative abundance of an OTU in the “light” fractions of the [13C]-treatment at tx); (3) Ra − Rd > T (Rd is the relative abundance of an OTU in the “heavy” fractions of the [13C]-treatment at t0); (4) Rcs > T [Rcs = (3 × Ra − Rb − Rc − Rd)/3, i.e., the average relative CAP-SIP abundance (hereafter referred to as Rcs score)]. Raw relative abundances and Rcs scores of labeled OTUs are listed in Supplementary Tables S6, S7.
Nucleotide Sequence Accession Numbers
All sequences obtained in the study (project number PRJEB14831) have been deposited under the sample accession numbers ERS1346811-ERS1346814 (18S rRNA reads of Eukaryota), ERS1262549-ERS1262552 (16S rRNA reads of Archaea), ERS1257899-ERS1258026 (16S rRNA reads of Bacteria), and ERS1346815-ERS1346846 (chiA reads) in the ENA archive of the European Bioinformatics Institute. Representative 16S rRNA sequences of labeled OTUs and representative transcript sequences of chiA OTUs were deposited in the ENA archive with accession numbers LT907870 to LT907902 and LT907849 to LT907862, respectively.
Results and Discussion
Chitin Degradation Under Oxic Conditions
CO2 production was stimulated after a short lag phase of about 3 days in chitin-supplemented treatments compared to unsupplemented controls (Figure 1A). This lag phase may reflect the exoenzymatic hydrolysis of the chitin prior to uptake and mineralization of the water-soluble breakdown products (Zhang and Lynd, 2004). Nevertheless, the stimulatory effect on the CO2 production indicated that chitinolytic microorganisms were prone to respond quickly to supplementation of fresh chitin. 13CO2 accumulation leveled off after 21 days (Supplementary Figure S5), suggesting that the supplemented [13CU]-chitin was largely consumed. At this time point, 54% of the chitin-derived 13C was recovered as 13CO2 (Table 1). A fraction of the residual 13C was likely assimilated during the synthesis of intra- and extracellular organic molecules (e.g., RNA or proteins) (Gooday, 1990a).
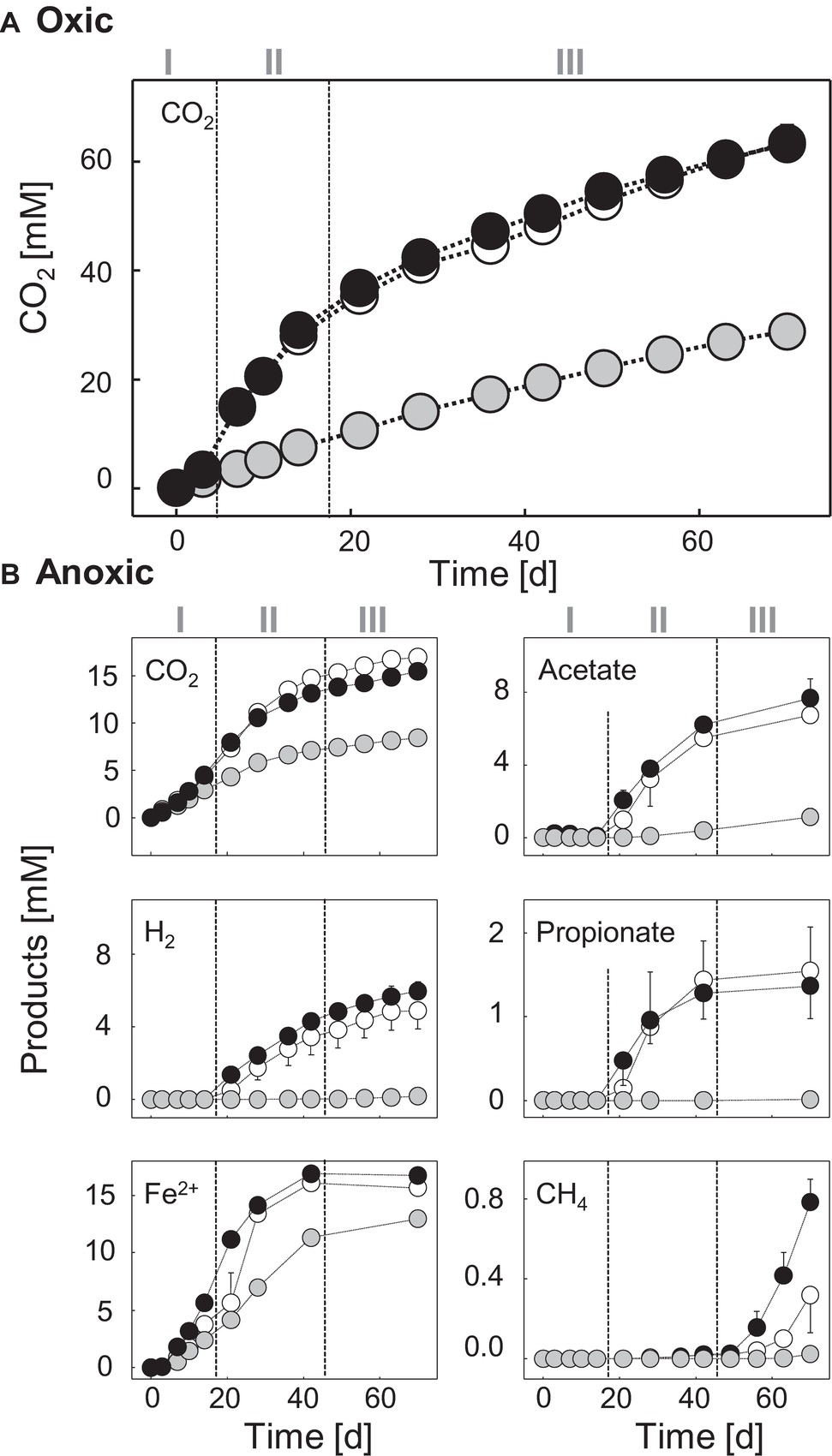
Figure 1. Products in soil slurries supplemented with [13C]-chitin, [12C]-chitin, and unsupplemented controls under oxic (A) and anoxic (B) conditions. ●, [13C]-chitin treatments; ○, [12C]-chitin treatments; ●, unsupplemented controls. Error bars, standard deviations of replicated microcosms (n = 2) are not shown when they were smaller than the symbol. Numerals indicate the different phases during chitin degradation.
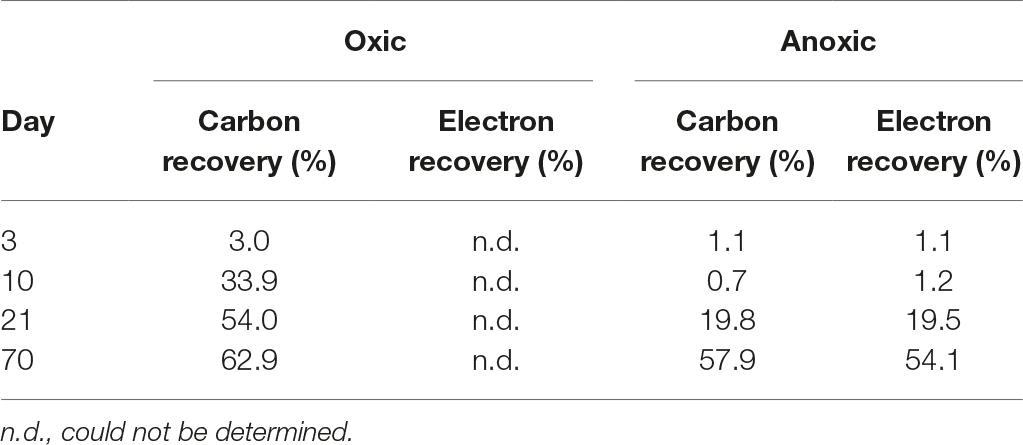
Table 1. Carbon and electron recoveries based on the assumption of the complete degradation of the supplemented [13C]-chitin.
Ammonium can be released during chitin degradation (Wieczorek et al., 2014), and theoretically about 6 mM of ammonium could have been maximally released from the supplemented chitin. However, although most of the CO2 evolved originated from chitin, neither ammonium nor nitrate – as a product of ammonium oxidation catalyzed by nitrifiers – accumulated to considerably higher concentrations in chitin treatments compared to unsupplemented controls (Supplementary Figure S6A). This suggests that chitin-derived ammonium was readily assimilated for the synthesis of nitrogen-containing cell components, such as proteins, nucleic acids, and microbial cell wall components (Gooday, 1990a).
Initial [13C]-Chitin Degraders Under Oxic Conditions
The relative abundances of Gamma- and Betaproteobacteria as well as Bacteroidetes were clearly higher in 16S rRNA amplicon libraries derived from cDNA of heavy fractions of the [13C]- compared to that of the [12C]-chitin treatments after 3 days of incubation (note that the relative abundances prior to the incubation were highly similar in heavy fractions of the [13C]- and [12C]-chitin treatments) (Supplementary Figure S7). These observations suggested that members of the aforementioned taxa were successfully labeled by the incorporation of [13CU]-chitin-derived carbon during synthesis of RNA within a few days.
OTU 310 was not detected prior to the incubation but showed the highest Rcs scores and, thus, was the most dominant of the labeled OTUs after 3 days (Figure 2, Supplementary Table S6). This suggests that OTU 310 was not active at the time point of sampling but was rapidly responding to the supplementation of chitin. Retrieved representative 16S rRNA gene transcript sequences of this OTU were closely related to Cellvibrio (98% identity to Cellvibrio gandavensis [NR_025419.1]), a genus of the Pseudomonadaceae (Gammaproteobacteria) that comprises several chitinolytic species (Humphry et al., 2003). It is likely that OTU 310 represented an important aerobic chitin degrader in the investigated soil, because it was predominantly 13C-labeled and was closely affiliated to a known chitinolytic genus.
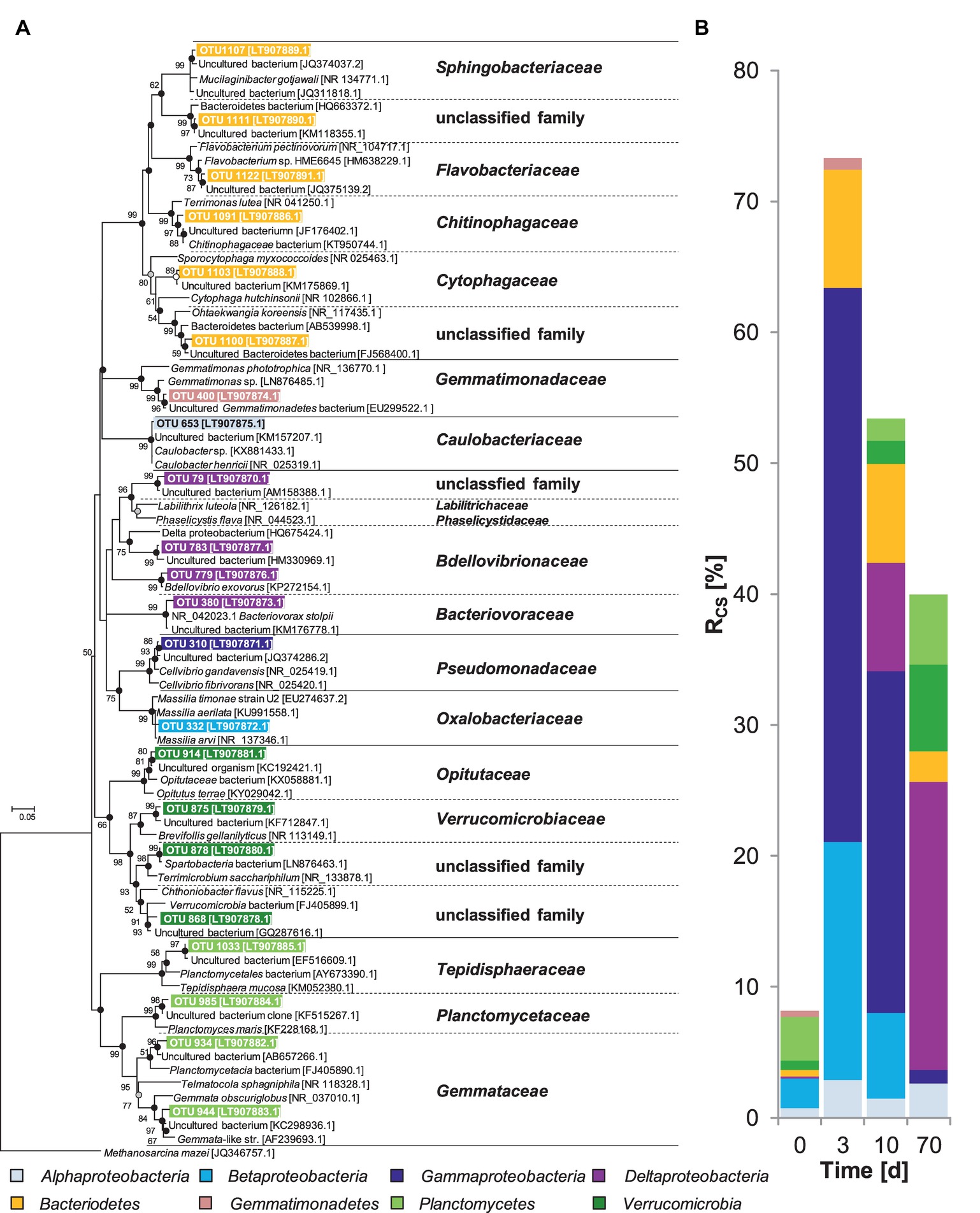
Figure 2. (A) Phylogenetic tree of 16S rRNA cDNA sequences from taxa 13C-labeled by assimilation of [13C]-chitin-derived carbon in soil slurries under oxic conditions and (B) corresponding Rcs scores. At t0, relative abundance values are presented. The consensus tree was calculated with the maximum likelihood method and 1,000 bootstraps. Dots at nodes indicate confirmation of topology by neighbor joining (white circles) and maximum parsimony (grey circles) algorithms. Black circles indicate confirmation by both algorithms. Accession numbers are given in brackets. Scale bar, 5% evolutionary distance. Methanosarcina mazei (JQ346757.1) was used as the out-group. Rcs calculation is described in the “Materials and Methods” section.
The labeled OTU 332 had the second highest Rcs scores after 3 days of incubation (Figure 2, Supplementary Table S6). This OTU was affiliated with Massilia (Betaproteobacteria), a genus that comprises one cultured chitinolytic strain and its growth was stimulated in agricultural soils supplemented with chitin (Faramarzi et al., 2009; Cretoiu et al., 2014). Additionally, a Massilia-affiliated chiA genotype (OTU 4) was strongly 13C-labeled after 3 days (Supplementary Figure S8), which further underscores a contribution of Massilia species to the aerobic chitin degradation in soils.
Several less abundant OTUs were labeled after 3 days (Figure 2, Supplementary Table S6) and affiliated with the chitinolytic families Sphingobacteriaceae, Flavobacteriaceae, Chitinophagaceae, and Cytophagaceae of the Bacteroidetes phylum (Nakagawa, 2011; Yoon et al., 2012; McBride, 2014; Rosenberg, 2014). Bacteroidetes were also stimulated by supplemental chitin in studies with a different agricultural soil and in aquatic systems (Beier and Bertilsson, 2011; Cretoiu et al., 2014). Recently, it has been hypothesized that Bacteroidetes utilize alternative enzymatic mechanisms to solubilize biopolymers besides glycosidic hydrolases, the so-called “polysaccharide-utilizing loci” (PULs) (Naas et al., 2014; Berlemont and Martiny, 2015). PULs’ function in the synergistic solubilization and breakdown of chitin might be the degradation of solubilized chitin oligosaccharides (Martens et al., 2014). Our data eventually imply that members of known chitinolytic bacterial families Gammaproteobacteria, Betaproteobacteria, and Bacteroidetes conducted the initial chitin degradation under oxic conditions.
Potential Secondary Chitin Degraders, Satellite Microbes, and Bacteriovorus Predators Under Oxic Conditions
Rcs scores for the Gammaproteobacteria, Betaproteobacteria, and Bacteroidetes decreased whereas those of the Deltaproteobacteria, Planctomycetes, and Verrucomicrobia increased after day 3 (Figure 2, Supplementary Table S6). The observed shift of labeled taxa is likely caused by different metabolic capabilities and functions of these taxa. In this regard, taxa that got labeled early most likely represent chitin degraders with high metabolic rates that thrive best under high substrate conditions (Lele and Watve, 2014; Lipson, 2015), and taxa that got labeled later during the incubation might represent either slow-growing chitin degraders potentially adapted to low substrate availability or were only indirectly involved in the mineralization of chitin-derived carbon (see discussion below).
In our experiment, Planctomycetes (Gemmataceae, Planctomycetaceae, and Tepidisphaeraceae) and Verrucomicrobia (Opitutaceae, Verrucomicrobiaceae, and unclassified Verrucomicrobia families) assimilated 13C. However, information about the physiology of Planctomycetes and Verrucomicrobia is limited to few cultured species. Some Planctomycetes utilize N-acetyl-glucosamine, the hydrolysis product of chitin (Rabus et al., 2002). Moreover, genomes of planctomycetes contain the chiA gene (Kulichevskaya et al., 2008; Guo et al., 2012; Ravin et al., 2018) and Planctomycetes-like chiA transcripts have been detected in our study (Supplementary Figure S8). Planctomycetes may also exhibit cellulolytic activity, and utilization of cellulose has been confirmed in a previous SIP study (Schellenberger et al., 2010; Kulichevskaya et al., 2012). Collectively, Planctomycetes are capable of biopolymer degradation, harbor enzymes necessary for chitin hydrolysis, utilize N-acetyl-glucosamine, and were labeled in our study, indicating that they might have been involved in chitin degradation in this experiment. However, the determination of chitinolytic capabilities among the Planctomycetes warrants further study. Similar to the Planctomycetes, Verrucomicrobia got 13C-labeled at days 10 and 70 in our experiments (Figure 2), suggesting that they were somehow involved in the mineralization of chitin-derived carbon. Only one study has suggested that members of the Verrucomicrobia might have chitinolytic activities (Chin et al., 1999), and further genome data suggest that they have metabolic potentials for polysaccharide degradation (Martinez-Garcia et al., 2012; Bai et al., 2016). Instead of being directly involved in chitin degradation, Planctomycetes and Verrucomicrobia may have been labeled due to the utilization of labeled carbon from exopolysaccharides (EPS) (Wang et al., 2015). Among taxa being clearly 13C-labeled in our study, the potential to produce EPS has been reported for the taxa Caulobacteriaceae (Alphaproteobacteria) and Pseudomonadaceae (Gammaproteobacteria) (Ravenscroft et al., 1991; Ueda and Saneoka, 2015). Planctomycetes and Verrucomicrobia could have also thrived on labeled bacterial cell envelope fragments that likely accumulated because of cell rupture of primary chitin degraders by predatory Deltaproteobacteria (Koval et al., 2013; see discussion below). A role as consecutive degraders of EPS and biofilm-associated polysaccharides is supported by the fact that members of both phyla have a broad repertoire of carbohydrate-active enzymes (Martinez-Garcia et al., 2012; Berlemont and Martiny, 2015; Bai et al., 2016).
The OTU 653 exhibited low but constant Rcs scores of 2–3% at all sampling days (Supplementary Table S6). It is closely related to Caulobacter henricii (Caulobacteriaceae) (Figure 2). No cultivated member of Caulobacteriaceae has been described as chitinolytic but several isolates can grow on chitin hydrolysis products in co-culture with chitinolytic partners (Eisenbeis et al., 2008; Abraham et al., 2014). Hence, we conclude that Caulobacteriaceae may have functioned as so-called “satellite microbes” that utilize hydrolysis products with saving metabolic investments for the synthesis of chitinolytic enzymes. By scavenging excess of chitin hydrolysis products, they might have accelerated the chitin degradation (Beier and Bertilsson, 2011; Bayer et al., 2013).
Labeled OTUs of the Deltaproteobacteria (OTUs 779, 783, 380, and 79) were affiliated with the Bdellovibrionaceae, Bacteriovoracaceae, and an uncultured family within the order Myxococcales (Figure 2, Supplementary Table S6). All of these taxa comprise well-known bacterial predators (Sockett, 2009; Berleman et al., 2014; Johnke et al., 2014). Thus, the detected Deltaproteobacteria in our experiment were more likely labeled by preying on labeled chitin degraders rather than by feeding on [13CU]-chitin and its hydrolysis products.
In addition to predation by bacteria, we had some evidence of predation by microeukaryotes. Alveolata, which were preying on cellulolytic bacteria in microcosms of the same soil (Chatzinotas et al., 2013), increased in relative abundances in 18S rRNA gene transcript amplicon libraries during the incubation (Supplementary Figure S9). However, a 13C-labeling of eukaryotic taxa could not be proven, since no 18S rRNA amplicons were obtained from the cDNA of heavy fractions from the [13C]-chitin treatments. Thus, eukaryotes might have played a role in the food web, but their 13C incorporation might have been not high enough for 13C-label detection in our experiment.
Chitin Degradation Under Anoxic Conditions
The anaerobic degradation of chitin was separated into an initial phase (day 0 to day 14), an intermediary phase (days 14–42), and a final phase (days 42–70) based on the process data. During the initial phase, CO2 production was slightly stimulated in the chitin treatments compared with the unsupplemented controls (Figure 1B), and the production of [13CU]-chitin-derived 13CO2 was detectable on day 7 for the first time (Supplementary Figure S5). At the same time, the accumulation of ferrous iron started, whereas fermentation products were not detectable prior to day 21 (Figure 1B). Thus, chitin degradation obviously started soon after substrate supplementation under anoxic conditions and iron reducers likely contributed to early anaerobic chitin mineralization either by the degradation of carbohydrates derived from chitin-hydrolysis or by the scavenging of chitin fermentation products.
Molecular hydrogen (H2), acetate, and propionate accumulated in chitin treatments but not in unsupplemented controls after day 14 (Figure 1B). This suggests that chitin was degraded by fermenters and that the capacity for the production of these fermentation products exceeded a potential consumption by iron reducers during the secondary phase. Other fermentation products were detected in traces (butyrate, isobutyrate; Supplementary Figure S6B) or could not be detected (lactate, succinate, formate, and ethanol). These compounds were either not formed or effectively scavenged (e.g., by iron reducers).
In the final phase, the accumulation of fermentation products (including 13CO2) continued, albeit at a slower rate, until the end of the experiment, suggesting that residual chitin was present once the experiment stopped (Figure 1B, Supplementary Figure S5). The concentration of ferrous iron did not increase further in the final phase, most probably because of a depletion of the pool of ferric iron available. The formation of methane after 42 days is in accordance with the finding that in oxic soils, methanogens can be activated by long periods of anoxia (Küsel and Drake, 1994; Angel et al., 2012). However, judged by the low methane concentrations, methanogenesis was only a minor process in the anaerobic mineralization of chitin. Minor concentrations of ammonium accumulated in the anoxic chitin treatments, indicating that most of the chitin-derived ammonium was consumed, likely through assimilatory pathways (Supplementary Figure S6A). Hydrolytic products of chitin (i.e., N-acetylglucosamine and chitobiose) were not detected in either the oxic or the anoxic treatments, suggesting an efficient microbial consumption that resulted in low steady-state concentrations of these hydrolysis products (below the detection limit of 30 μM).
Iron Reducers During Early Chitin Degradation Under Anoxic Conditions
During the initial phase, ferrous iron but no fermentation products accumulated, pointing toward a contribution of iron reducers to chitin mineralization (Figure 1B). However, it cannot be concluded from the process data whether the iron reducers were (1) truly chitinolytic (i.e., excreted chitin-degrading exoenzymes), (2) satellite microbes that fed on sugars derived from chitin hydrolysis, or (3) scavengers of chitin-derived fermentation products. The latter is likely for OTU 120, which was labeled at day 10 and affiliated to the Geobacteraceae, a family that comprises iron reducers that use various fermentation products but not sugars as electron donor (Figure 3, Supplementary Table S7; Garrity et al., 2005). Such fermentation products may have been provided by chitinolytic fermenters potentially represented by the OTUs 410 and 203 that were labeled at day 10. OTU 410 was closely related to Candidatus “Koribacter versatilis,” a member of the Acidobacteriaceae that harbors genes encoding for chitin degradation (Ward et al., 2009). For other members of the Acidobacteria, biopolymer hydrolysis (incl. chitin) and fermentation of chitin hydrolysis products have been proven (Pankratov et al., 2012; Foesel et al., 2013; Kulichevskaya et al., 2014; Belova et al., 2018). OTU 203 was affiliated with Paenibacillus, a genus that comprises several species that can ferment chitin (Lee et al., 2004). Interestingly, some members of the Acidobacteria and Paenibacillus can use sugars as electron donors during iron reduction (Kulichevskaya et al., 2014; Li et al., 2014) and thus it might be possible that the labeled Acidobacteria and Paenibacillus phylotypes coupled chitin mineralization to iron reduction. However, both OTUs were also labeled at the end of the experiment when the pool of available ferric iron was depleted, indicating that these taxa were not restricted to iron reduction.
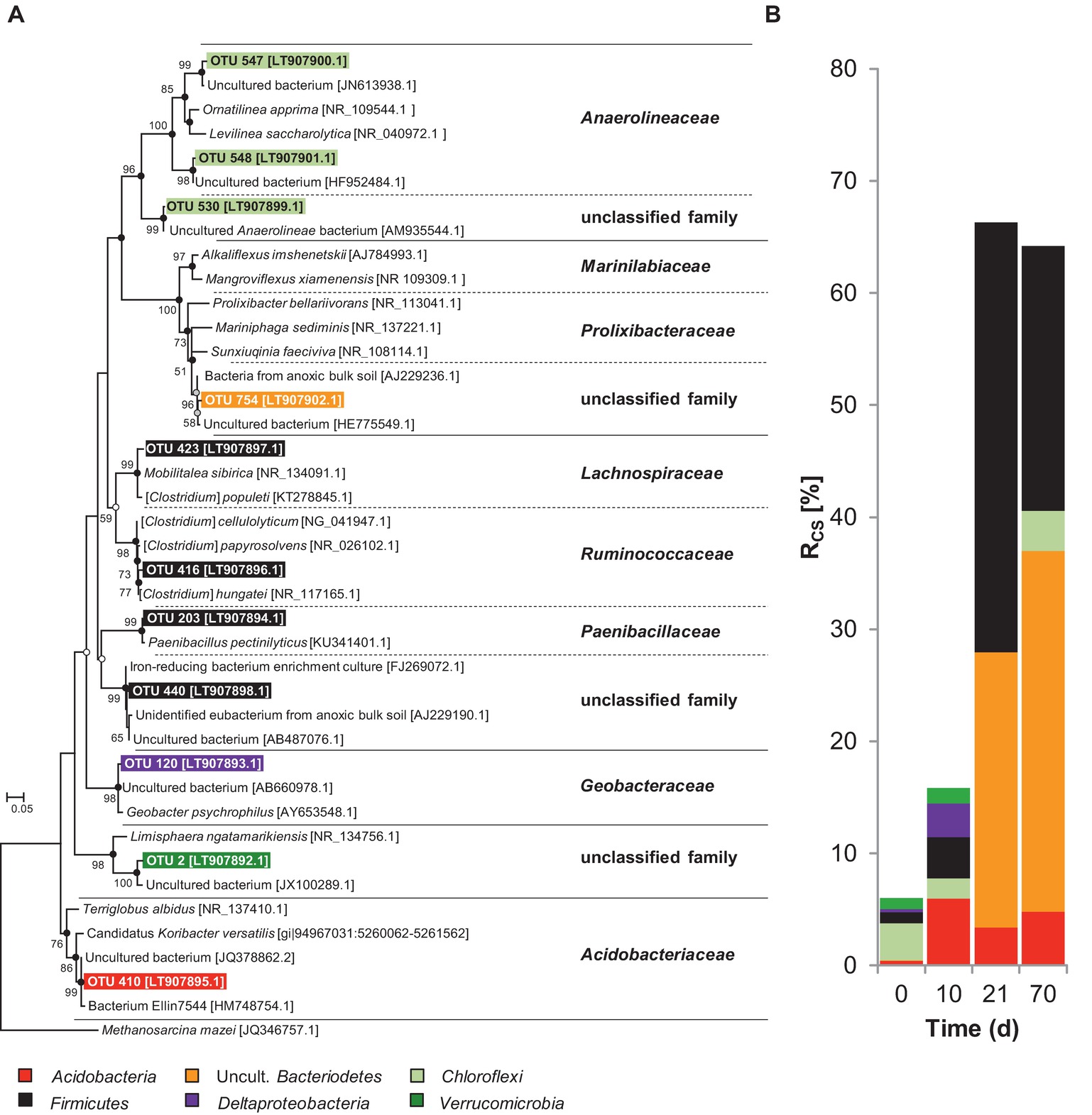
Figure 3. (A) Phylogenetic tree of 16S rRNA cDNA sequences from taxa labeled by assimilation of [13C]-chitin-derived carbon in soil slurries under anoxic conditions and (B) corresponding Rcs scores. At t0, relative abundance values are presented. The consensus tree was calculated with the maximum likelihood method and 1,000 bootstraps. Dots at nodes indicate confirmation of topology by neighbor joining (white circles) and maximum parsimony (grey circles) algorithms. Black circles indicate confirmation by both algorithms. Accession numbers are given in brackets. Scale bar, 5% evolutionary distance. Methanosarcina mazei (JQ346757.1) was used as the out-group. Rcs calculation is described in the “Materials and Methods” section.
Obligate Anaerobes Take Over After Prolonged Periods of Anoxia
After the initial phase, typical fermentation products accumulated (Figure 1) and the cumulative Rcs scores of all labeled taxa increased from 15.9% at day 10 to 66.4% at day 21 and remained constant afterward (64% after 70 days) (Figure 3, Supplementary Table S7). This suggests that chitin was readily fermented and chitin-derived 13C was increasingly assimilated. Interestingly, of the taxa that were labeled at day 10 (initial phase), only the Paenibacillaceae increased in abundance over time whereas the other initially labeled OTUs had lower Rcs scores or were not labeled anymore at day 21 or day 70 (Supplementary Table S7). In contrast, uncultured Bacteroidetes (OTU 754), Lachnospiraceae (OTU 423), and Ruminococcaceae (OTU 416) that were not labeled at day 10 made up for the dominant labeled taxa at days 21 and 70. All cultured isolates of Lachnospiraceae and Ruminococcaceae are obligate anaerobes (Rainey, 2009a,b), and the Bacteroidetes-affiliated OTU 754 was closely related (98% Blastn identity) to two obligate anaerobic isolates [strains PB90-2 (AJ229236) and XB45 (AJ229237)] from flooded paddy rice soils (Chin et al., 1999). Thus, our results point toward a shift in the anaerobic chitin-degrading microbial community after prolonged periods of anoxia most likely from facultative aerobes to obligate anaerobes.
The 13C incorporation by Firmicutes, i.e., Ruminococcaceae and Lachnospiraceae, agrees with pure culture-based observations that members of both families can degrade both chitin and cellulose (Evvyernie et al., 2000; Reguera and Leschine, 2001). Noteworthy, a Firmicutes-like chiA transcript (55% protein identity with Clostridium beijerinckii) was labeled (Supplementary Figure S10). In agreement with this finding, glycosyl hydrolase transcripts involved in chitin breakdown have been reported to be predominantly expressed by the Firmicutes in paddy soil slurries supplemented with rice straw (Wegner and Liesack, 2016). Taken together, the results emphasize the significant role of Firmicutes in the anaerobic degradation of chitin. Along with fermentation, ferric iron reduction was still ongoing until day 42. As Geobacteriaceae were not among the labeled phylotypes in the second half of the experiment, other iron-reducing taxa must have been active. The Paenibacillus OTUs were labeled during the whole experiment. Paenibacillaceae are known to be chitinolytic and capable of ferric iron reduction (Lee et al., 2004; Raza and Shen, 2010; Li et al., 2014). Thus, we think that Paenibacillaceae were partly responsible for the anaerobic mineralization of chitin and the observed ferric iron reduction in the second half of the experiment. At the end of the experiment, uncultured Bacteroidetes was the most abundant labeled phylum followed by Firmicutes, Acidobacteria, and Chloroflexi suggesting these were the primary chitin degraders in the second half of the experiment (Figure 3, Supplementary Table S7). Consistently, this phase of anaerobic chitin degradation was characterized by a substantial decrease of the ferric iron reduction rate.
Functions of Archaea in Chitin Degradation
In agreement with the observed methane formation toward the end of the anoxic incubation (Figure 1), methanogenic Archaea (Methanomicrobia and Methanobacteria) were detected (Supplementary Figure S11). However, their relative abundances in libraries prepared from pooled PCR products derived from light fractions of [13C]- and [12C]-chitin treatments were low (Supplementary Figure S11) and no PCR products were obtained from the cDNA of heavy fractions with Archaea-specific primers. Nonetheless, in the amplicon libraries obtained with Bacteria-specific primers (Supplementary Figure S7), archaeal 16S rRNA gene sequences [98% Blastn identity with Methanobacterium flexile (NR_116276.1)] were present in the heavy fractions of 13C-treatments whereas no sequences were detected in the heavy fractions of 12C-chitin treatments. Thus, we conclude that methanogenic Archaea have assimilated some [13CU]-chitin-derived carbon by consumption of labeled metabolic products (i.e., CO2 and/or acetate) produced by obligate anaerobic chitin degraders and satellite microbes. However, note that the labeling was primed by a rather artificially prolonged anoxia and thus, conclusions regarding ecological functions of Archaea in chitin degradation based in soil on our data remain questionable.
Weakly 13C-Labeled Bacterial Taxa
Gemmatimonadetes were labeled under oxic conditions whereas Chloroflexi were labeled under anoxic conditions albeit with low Rcs scores (Figures 2, 3, Supplementary Tables S6, S7). Our experimental data and literature knowledge are not conclusive enough to resolve their functions and trophic role in chitin degradation. Nonetheless, we conclude that based on their consistent low Rsc scores, Gemmatimonadetes and Chloroflexi were of minor importance.
General Trends of Collective Rcs Scores
The sum of Rcs scores of all [13C]-labeled taxa under oxic conditions was highest after 3 days and decreased over time (Figure 2). This finding is in accordance with the observed rapid degradation of chitin (Figure 1A, Table 1). Under anoxic conditions, the trend in Rcs scores was opposite (Figure 3) but fitting well to the observed slower degradation of chitin and the increasing metabolic activity over time (Figure 1B).
Final Conclusions
The data collected in this study were combined to reconstruct a hypothetical model of the processes and linked microbial taxa during the aerobic and anaerobic degradation of chitin in the investigated soil (Figure 4). To minimize sequencing effort and costs for the chitin substrate, our experiment was duplicated and SIP gradients were done from pooled RNA from both duplicates per time point and oxygen treatment. Previous studies revealed low technical variability (Morawe et al., 2017; Chaignaud et al., 2018). All labeled OTUs occurred at two or more time points. Hence, the conclusion that they were 13C-labeled is based on at least two replicated observations over time. Chitin was likely enzymatically solubilized by endo- and exo-chitinases, and potentially also by the activity of LPMOs and PULs. Few key chitinolytic taxa, i.e., Pseudomonas and Massilia, were responsible for most of the chitin degradation under oxic conditions. Furthermore, several family taxa of Bacteroidetes with known chitinolytic representatives incorporated substantial amounts of [13CU]-chitin-derived carbon, underlining the ecological importance of Bacteroidetes for degradation of complex polysaccharides in soils. The role of PUL-harboring Bacteroidetes in the synergistic breakdown of chitin might be the degradation of solubilized chitin oligosaccharides as has previously suggested (Martens et al., 2014). Chitin hydrolysis products were not detected indicating that polymer hydrolysis was rate limiting. In addition to primary chitin degraders, “satellite microbes” were potentially active in keeping the concentrations of chitin hydrolysis products at low levels. The growth of primary consumers and “satellite microbes” allowed for predation by bacteriovorus Bacteriovoraceae, Bdellovibrionaceae, and uncultured Myxococcales that incorporated 13C from their prey cells. How Planctomycetes and Verrucomicrobia incorporated 13C is difficult to answer based on our data. However, we think that their main ecological function was degradation of complex heteropolysaccharides in EPS and cell walls (Kulichevskaya et al., 2007; Wang et al., 2015). Nonetheless, there is increasing evidence that members of aerobic Planctomycetes are chitinolytic microorganisms in wetland soils (Ivanova et al., 2018; Dedysh and Ivanova, 2019). Hence, they might have been less relevant but active chitin degraders also in our experiment.
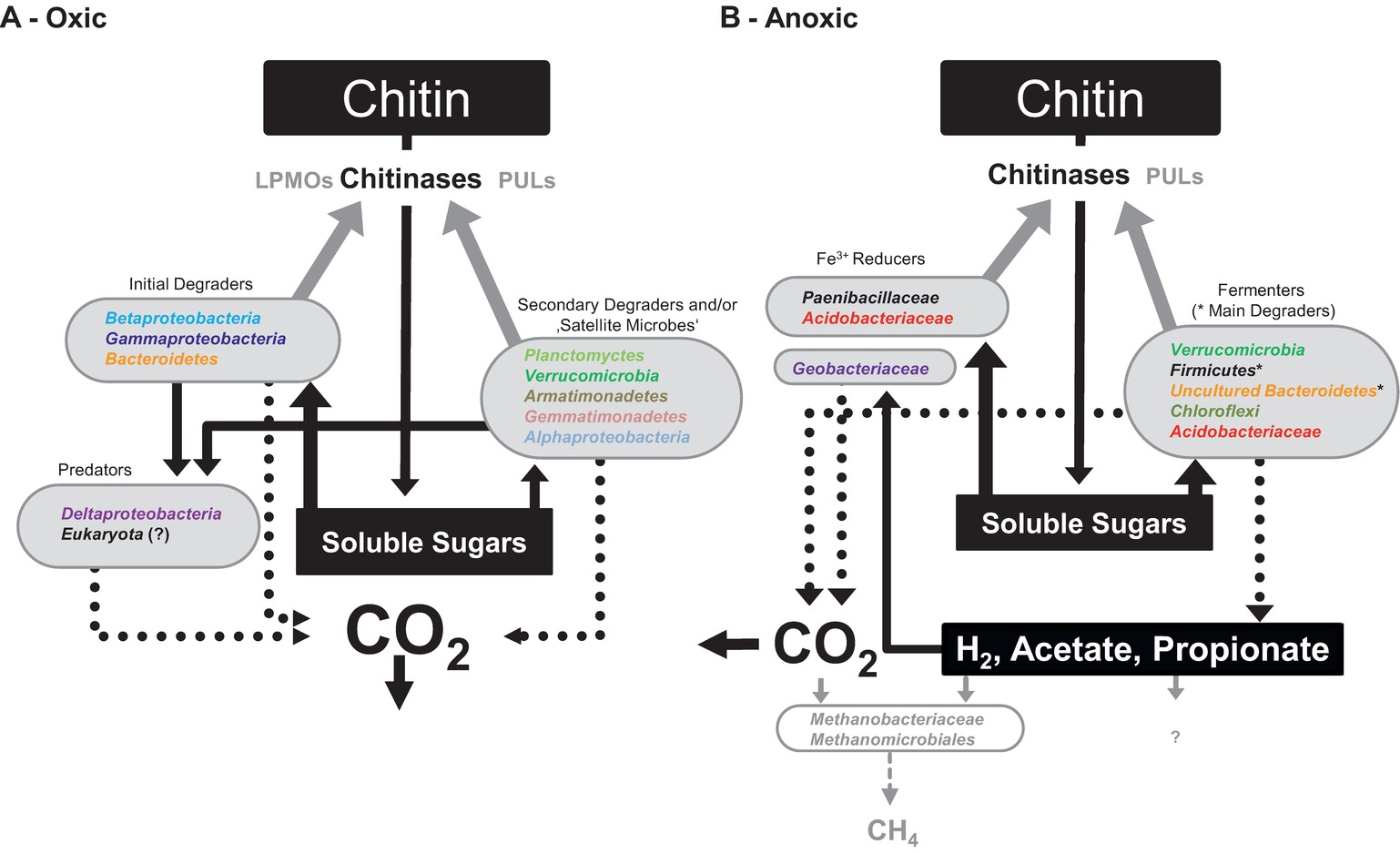
Figure 4. ‘Conceptual model of soil microbiome interactions when degrading chitin under oxic (A) and anoxic (B) conditions based on experimental observations made in our study. Dotted lines represent carbon fluxes and/or synthesis of enzymes. Solid black lines and arrows represent substrate and metabolite transfer to the respective organisms. Grey shaded information represent likely but not experimentally well-proven information. Colors of taxa follow the same color code as used in Figures 2, 3.
Under anoxic conditions, chitin was readily degraded but its degradation was slower compared to that under oxic conditions, which might be partially explained by inactivity of LPMOs as they require oxygen for their enzymatic mechanism (Vaaje-Kolstad et al., 2010, 2013). Overall, different members of the soil microbiome were labeled by the assimilation of [13CU]-chitin-derived carbon under anoxic conditions. The initial anaerobic degradation of chitin was characterized by a low accumulation of fermentation products and a low labeling ratio (i.e., a low collective Rcs score). While Acidobacteria and Chloroflexi were initially labeled, Firmicutes and uncultured Bacteroidetes were predominantly labeled, suggesting that the latter two bacterial phyla were mainly responsible for chitin degradation and provided substrates for iron reducers. The fermentation products H2/CO2 and acetate were likely consumed by hydrogenotrophic methanogens that assimilated additionally acetate for anabolic reactions. No evidence for prey-predator or saprotrophic interactions was observed in the course of the experiment under anoxic conditions. However, literature indicates that anaerobic predatory microeukaryotes occur in soil (Murase and Frenzel, 2007; Murase et al., 2014). Thus, our experiment might have failed to detect them because our approach was insensitive.
Our data indicated (1) that hitherto unrecognized Bacteria were involved in the chitin-degrading food web of an agricultural soil, (2) that trophic interactions of the chitin-degrading microbial food web were substantially shaped by the oxygen availability, and (3) that predation was restricted to oxic conditions. The functional redundancy of the soil microbiome and the catabolic diversity likely enable continued biopolymer degradation independent of oxygen availability. A rapid decomposition of chitin to CO2 as found in the oxic treatments with pure chitin is in line with previous studies addressing chitin degradation in aerated top soil (Fernandez and Koide, 2012), suggesting that chitin is not as recalcitrant as it is sometimes believed to be (Godbold et al., 2006; Langley et al., 2006). The insights we gained into the trophic interactions of a chitin-degrading microbiome improve the understanding of turnover dynamics of chitin in soil. Soil chitin degradation is driven by complex microbiome of which members fulfill certain functional steps and together facilitate chitin mineralization.
Author Contributions
SK designed the study and wrote together with AW and OS the manuscript. AW conducted the experimental work. AC and MB supported the interpretation of data and contributed to the final manuscript version. AG developed a laboratory procedure to produce high-quality [13CU]-chitin from a fungal strain.
Funding
The study has been funded by the project Ko2912/3-2 of the Deutsche Forschungsgemeinschaft. The work of MB was partially funded by DFG CRC Aquadiva.
Conflict of Interest Statement
AG was employed by and director of the company IsoLife BV (Netherlands).The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank M. Schloter (Helmholtz Centre Munich German Center of Environmental Health) for providing Ap soil of a wheat field.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fmicb.2019.01293/full#supplementary-material
Footnotes
References
Abraham, W. R., Rohde, M., and Bennasar, A. (2014). “The family Caulobacteraceae” in The prokaryotes. eds. E. Rosenberg, E. F. DeLong, S. Lory, E. Stackebrandt, and F. Thompson (Heidelberg, Germany: Springer), 179–205.
Angel, R., Claus, P., and Conrad, R. (2012). Methanogenic archaea are globally ubiquitous in aerated soils and become active under wet anoxic conditions. ISME J. 6, 847–862. doi: 10.1038/ismej.2011.141
Bai, Y., Eijsink, V. G., Kielak, A. M., van Veen, J. A., and de Boer, W. (2016). Genomic comparison of chitinolytic enzyme systems from terrestrial and aquatic bacteria. Environ. Microbiol. 18, 38–49. doi: 10.1111/1462-2920.12545
Bayer, E. A., Shoham, Y., and Lamed, R. (2013). “Lignocellulose-decomposing bacteria and their enzyme systems” in The prokaryotes. eds. E. Rosenberg, E. F. DeLong, S. Lory, E. Stackebrandt, and F. Thompson (Heidelberg, Germany: Springer), 215–266.
Beier, S., and Bertilsson, S. (2011). Uncoupling of chitinase activity and uptake of hydrolysis products in freshwater bacterioplankton. Limnol. Oceanogr. 56, 1179–1188. doi: 10.4319/lo.2011.56.4.1179
Beier, S., and Bertilsson, S. (2013). Bacterial chitin degradation-mechanisms and ecophysiological strategies. Front. Microbiol. 4:149. doi: 10.3389/fmicb.2013.00149
Belova, S. E., Ravin, N. V., Pankratov, T. A., Rakitin, A. L., Ivanova, A. A., Beletsky, A. V., et al. (2018). Hydrolytic capabilities as a key to environmental success: chitinolytic and cellulolytic Acidobacteria from acidic sub-arctic soils and boreal peatlands. Front. Microbiol. 9:2775. doi: 10.3389/fmicb.2018.02775
Berleman, J. E., Allen, S., Danielewicz, M. A., Remis, J. P., Gorur, A., Cunha, J., et al. (2014). The lethal cargo of Myxococcus xanthus outer membrane vesicles. Front. Microbiol. 5:474. doi: 10.3389/fmicb.2014.00474
Berlemont, R., and Martiny, A. C. (2015). Genomic potential for polysaccharide deconstruction in bacteria. Appl. Environ. Microbiol. 81, 1513–1519. doi: 10.1128/AEM.03718-14
Berry, D., Ben Mahfoudh, K., Wagner, M., and Loy, A. (2011). Barcoded primers used in multiplex amplicon pyrosequencing bias amplification. Appl. Environ. Microbiol. 77, 7846–7849. doi: 10.1128/AEM.05220-11
Blumenthal, H. J., and Roseman, S. (1957). Quantitative estimation of chitin in fungi. J. Bacteriol. 74, 222–224.
Bragg, L., Stone, G., Imelfort, M., Hugenholtz, P., and Tyson, G. W. (2012). Fast, accurate error-correction of amplicon pyrosequences using Acacia. Nat. Methods 9, 425–426. doi: 10.1038/nmeth.1990
Chaignaud, P., Morawe, M., Besaury, L., Kröber, E., Vuilleumier, S., Bringel, F., et al. (2018). Methanol consumption drives the bacterial chloromethane sink in a forest soil. ISME J. 12, 2681–2693. doi: 10.1038/s41396-018-0228-4
Chatzinotas, A., Schellenberger, S., Glaser, K., and Kolb, S. (2013). Assimilation of cellulose-derived carbon by microeukaryotes in oxic and anoxic slurries of an aerated soil. Appl. Environ. Microbiol. 79, 5777–5781. doi: 10.1128/AEM.01598-13
Chin, K.-J., Hahn, D., Hengstmann, U., Liesack, W., and Janssen, P. H. (1999). Characterization and identification of numerically abundant culturable bacteria from the anoxic bulk soil of rice paddy microcosms. Appl. Environ. Microbiol. 65, 5042–5049.
Cretoiu, M. S., Kielak, A. M., Schluter, A., and van Elsas, J. D. (2014). Bacterial communities in chitin-amended soil as revealed by 16S rRNA gene based pyrosequencing. Soil Biol. Biochem. 76, 5–11. doi: 10.1016/j.soilbio.2014.04.027
Cretoiu, M. S., Korthals, G. W., Visser, J. H. M., and van Elsas, J. D. (2013). Chitin amendment increases soil suppressiveness toward plant pathogens and modulates the actinobacterial and oxalobacteraceal communities in an experimental agricultural field. Appl. Evniron. Microbiol. 17, 5291–5301. doi: 10.1128/AEM.01361-13
Dallinger, A., and Horn, M. A. (2014). Agricultural soil and drilosphere as reservoirs of new and unusual assimilators of 2,4-dichlorophenol carbon. Environ. Microbiol. 16, 84–100. doi: 10.1111/1462-2920.12209
Dedysh, S. N., and Ivanova, A. A. (2019). Planctomycetes in boreal and subarctic wetlands: diversity patterns and potential ecological functions. FEMS Microbiol. Ecol. 95. doi: 10.1093/femsec/fiy227
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C., and Knight, R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200. doi: 10.1093/bioinformatics/btr381
Eisenbeis, S., Lohmiller, S., Valdebenito, M., Leicht, S., and Braun, V. (2008). NagA-dependent uptake of N-acetyl-glucosamine and N-acetyl-chitin oligosaccharides across the outer membrane of Caulobacter crescentus. J. Bacteriol. 190, 5230–5238. doi: 10.1128/JB.00194-08
El Zahar Haichar, F., Achouak, W., Christen, R., Heulin, T., Marol, C., Marais, M.-F., et al. (2007). Identification of cellulolytic bacteria in soil by stable isotope probing. Environ. Microbiol. 9, 625–634. doi: 10.1111/j.1462-2920.2006.01182.x
Evvyernie, D., Yamazaki, S., Morimoto, K., Karita, S., Kimura, T., Sakka, K., et al. (2000). Identification and characterization of clostridium paraputrificum M-21, a chitinolytic, mesophilic and hydrogen-producing bacterium. J. Biosci. Bioeng. 89, 596–601. doi: 10.1016/S1389-1723(00)80063-8
Faramarzi, M. A., Fazeli, M., Yazdi, M. T., Adrangi, S., Al-Ahmadi, K. J., Tasharrofi, N., et al. (2009). Optimization of cultural conditions for production of chitinase by a soil isolate of Massilia timonae. Biotechnology 8, 93–99. doi: 10.3923/biotech.2009.93.99
Fernandez, C. W., and Koide, R. T. (2012). The role of chitin in the decomposition of ectomycorrhizal fungal litter. Ecology 93, 24–28. doi: 10.1890/11-1346.1
Foesel, B. U., Rohde, M., and Overmann, J. (2013). Blastocatella fastidiosa gen. nov., sp. nov., isolated from semiarid savanna soil - the first described species of Acidobacteria subdivision 4. Syst. Appl. Microbiol. 36, 82–89. doi: 10.1016/j.syapm.2012.11.002
Garrity, G. M., Bell, J. A., and Lilburn, T. (2005). “Family II. Geobacteriaceae fam. nov.” in Bergey’s manual of systematic bacteriology. eds. G. M. Garrity, D. J. Brenner, N. R. Krieg, and J. T. Staley (New York, NY: Springer), 1017–1020.
Godbold, D. L., Hoosbeek, M. R., Lukac, M., Cotrufo, M. F., Janssens, I. A., Ceulemans, R., et al. (2006). Mycorrhizal hyphal turnover as a dominant process for carbon input into soil organic matter. Plant Soil 281, 15–24. doi: 10.1007/s11104-005-3701-6
Gooday, G. W. (1990a). Physiology of microbial degradation of chitin and chitosan. Biodegradation 1, 177–190. doi: 10.1007/BF00058835
Gooday, G. W. (1990b). The ecology of chitin degradation. Adv. Microb. Ecol. 11, 387–430. doi: 10.1007/978-1-4684-7612-5_10
Guo, M., Han, X., Jin, T., Zhou, L., Yang, J., Li, Z., et al. (2012). Genome sequences of three species in the family Planctomycetaceae. J. Bacteriol. 194, 3740–3741. doi: 10.1128/JB.00639-12
Humphry, D. R., Black, G. W., and Cummings, S. P. (2003). Reclassification of ‘Pseudomonas fluorescens subsp. cellulosa’ NCIMB 10462 (Ueda et al. 1952) as Cellvibrio japonicus sp. nov. and revival of Cellvibrio vulgaris sp. nov., nom. rev. and Cellvibrio fulvus sp. nov., nom. rev. Int. J. Syst. Evol. Microbiol. 53, 393–400. doi: 10.1099/ijs.0.02271-0
Ivanova, A. A., Wegner, C.-E., Kim, Y., Liesack, W., and Dedysh, S. N. (2018). Metatranscriptomics reveals the hydrolytic potential of peat-inhabiting Planctomycetes. Antonie Van Leeuwenhoek 111, 801–809. doi: 10.1007/s10482-017-0973-9
Jagmann, N., Brachvogel, H. P., and Philipp, B. (2010). Parasitic growth of Pseudomonas aeruginosa in co-culture with the chitinolytic bacterium Aeromonas hydrophila. Environ. Microbiol. 12, 1787–1802. doi: 10.1111/j.1462-2920.2010.02271.x
Johnke, J., Cohen, Y., de Leeuw, M., Kushmaro, A., Jurkevitch, E., and Chatzinotas, A. (2014). Multiple micro-predators controlling bacterial communities in the environment. Curr. Opin. Biotechnol. 27, 185–190. doi: 10.1016/j.copbio.2014.02.003
Keyhani, N. O., and Roseman, S. (1999). Physiological aspects of chitin catabolism in marine bacteria. Biochim. Biophys. Acta 1473, 108–122. doi: 10.1016/S0304-4165(99)00172-5
Koval, S. F., Hynes, S. H., Flannagan, R. S., Pasternak, Z., Davidov, Y., and Jurkevitch, E. (2013). Bdellovibrio exovorus sp. nov., a novel predator of Caulobacter crescentus. Int. J. Syst. Evol. Microbiol. 63, 146–151. doi: 10.1099/ijs.0.039701-0
Kramer, S., Dibbern, D., Moll, J., Huenninghaus, M., Koller, R., Krueger, D., et al. (2016). Resource Partitioning between bacteria, fungi, and protists in the detritusphere of an agricultural soil. Front. Microbiol. 7:1524. doi: 10.3389/fmicb.2016.01524
Kulichevskaya, I. S., Belova, S. N., Kevbrin, V. V., Dedysh, S. N., and Zavarzin, G. A. (2007). Analysis of the bacterial community developing in the course of Sphagnum moss decomposition. Microbiology 76, 621–629. doi: 10.1134/S0026261707050165
Kulichevskaya, I. S., Ivanova, A. O., Baulina, O. I., Bodelier, P. L. E., Sinninghe Damsté, J. S., and Dedysh, S. N. (2008). Singulisphaera acidiphila gen. nov., Sp. nov., a non-filamentous, Isosphaera-like planctomycete from acidic northern wetlands. Int. J. Syst. Evol. Microbiol. 58, 1186–1193. doi: 10.1099/ijs.0.65593-0
Kulichevskaya, I. S., Serkebaeva, Y. M., Kim, Y., Rijpstra, W. I. C., Damste, J. S. S., Liesack, W., et al. (2012). Telmatocola sphagniphila gen. nov., sp. nov., a novel dendriform planctomycete from northern wetlands. Front. Microbiol. 3:146. doi: 10.3389/fmicb.2012.00146
Kulichevskaya, I. S., Suzina, N. E., Rijpstra, W. I. C., Sinninghe Damsté, J. S., and Dedysh, S. N. (2014). Paludibaculum fermentans gen. nov., Sp. nov., a facultative anaerobe capable of dissimilatory iron reduction from subdivision 3 of the Acidobacteria. Int. J. Syst. Evol. Microbiol. 64, 2857–2864. doi: 10.1099/ijs.0.066175-0
Küsel, K., and Drake, H. L. (1994). Acetate synthesis in soil from a Bavarian beech forest. Appl. Environ. Microbiol. 60, 1370–1373.
Langley, J. A., Chapman, S. K., and Hungate, B. A. (2006). Ectomycorrhizal colonization slows root decomposition: the post-mortem fungal legacy. Ecol. Lett. 9, 955–959. doi: 10.1111/j.1461-0248.2006.00948.x
Lee, J.-S., Pyun, Y.-R., and Bae, K. S. (2004). Transfer of Bacillus ehimensis and Bacillus chitinolyticus to the genus Paenibacillus with emended descriptions of Paenibacillus ehimensis comb. nov. and Paenibacillus chitinolyticus comb. nov. Int. J. Syst. Evol. Microbiol. 54, 929–933. doi: 10.1099/ijs.0.02765-0
Lele, U., and Watve, M. (2014). Bacterial growth rate and growth yield: is there a relationship? Proc. Indian Natn. Sci. Acad. 80, 537–546. doi: 10.16943/ptinsa/2014/v80i3/55129
Li, J., Lu, Q., Liu, T., Zhou, S., Yang, G., and Zhao, Y. (2014). Paenibacillus guangzhouensis sp. nov., an Fe(III)- and humus-reducing bacterium from a forest soil. Int. J. Syst. Evol. Microbiol. 64, 3891–3896. doi: 10.1099/ijs.0.067173-0
Lipson, D. A. (2015). The complex relationship between microbial growth rate and yield and its implications for ecosystem processes. Front. Microbiol. 6:615. doi: 10.3389/fmicb.2015.00615
Martens, E. C., Kelly, A. G., Tauzin, A. S., and Brumer, H. (2014). The devil lies in the details: how variations in polysaccharide fine-structure impact the physiology and evolution of gut microbes. J. Mol. Biol. 426, 3851–3865. doi: 10.1016/j.jmb.2014.06.022
Martínez, J. P., Falomir, M. P., and Gozalbo, D. (2009). “Chitin: a structural biopolysaccharide” in Encyclopedia of life sciences (ELS). (Chichester, UK: John Wiley & Sons, Ltd).
Martinez-Garcia, M., Brazel, D. M., Swan, B. K., Arnosti, C., Chain, P. S., Reitenga, K. G., et al. (2012). Capturing single cell genomes of active polysaccharide degraders: an unexpected contribution of Verrucomicrobia. PLoS One 7:e35314. doi: 10.1371/journal.pone.0035314
McBride, M. J. (2014). “The family Flavobacteriaceae” in The prokaryotes. eds. E. Rosenberg, E. F. DeLong, S. Lory, E. Stackebrandt, and F. Thompson (Heidelberg, Germany: Springer), 643–676.
Morawe, M., Hoeke, H., Wissenbach, D. K., Lentendu, G., Wubet, T., Kröber, E., et al. (2017). Acidotolerant bacteria and fungi as a sink of methanol-derived carbon in a deciduous forest soil. Front. Microbiol. 8:1361. doi: 10.3389/fmicb.2017.01361
Murase, J., and Frenzel, P. (2007). A methane-driven microbial food web in a wetland rice soil. Environ. Microbiol. 9, 3025–3034. doi: 10.1111/j.1462-2920.2007.01414.x
Murase, J., Takenouchi, Y., Iwasaki, K., and Kimura, M. (2014). Microeukaryotic community and oxygen response in rice field soil revealed using a combined rRNA-gene and rRNA-based approach. Microbes Environ. 29, 74–81. doi: 10.1264/jsme2.ME13128
Naas, A. E., Mackenzie, A. K., Mravec, J., Schückel, J., Willats, W. G. T., Eijsink, V. G. H., et al. (2014). Do rumen Bacteroidetes utilize an alternative mechanism for cellulose degradation? MBio 5, e01401–e01414. doi: 10.1128/mBio.01401-14
Nakagawa, Y. (2011). “Family I. Cytophagaceae fam. nov.” in Bergey’s manual of systematic bacteriology. eds. N. R. Krieg, J. T. Staley, D. R. Brown, B. P. Hedlund, B. J. Paster, and N. L. Ward, et al. (New York, NY: Springer), 371–423.
Nebel, M. E., Wild, S., Holzhauser, M., Huttenberger, L., Reitzig, R., Sperber, M., et al. (2011). Jaguc – a software package for environmental diversity analyses. J. Bioinforma. Comput. Biol. 9, 749–773. doi: 10.1142/S0219720011005781
Or, D., Smets, B. F., Wraith, J. M., Dechesne, A., and Freidman, S. P. (2007). Physical constraints affecting bacterial habitats and activity in unsaturated porous media – a review. Adv. Water Resour. 30, 1505–1527. doi: 10.1016/j.advwatres.2006.05.025
Pankratov, T. A., Kirsanova, L. A., Kaparullina, E. N., Kevbrin, V. V., and Dedysh, S. N. (2012). Telmatobacter bradus gen. nov., sp. nov., a cellulolytic facultative anaerobe from subdivision 1 of the Acidobacteria, and emended description of Acidobacterium capsulatum Kishimoto et al. 1991. Int. J. Syst. Evol. Microbiol. 62, 430–437. doi: 10.1099/ijs.0.029629-0
Rabus, R., Gade, D., Helbig, R., Bauer, M., Glöckner, F. O., Kube, M., et al. (2002). Analysis of N-acetylglucosamine metabolism in the marine bacterium Pirellula sp. strain 1 by a proteomic approach. Proteomics 2, 649–655. doi: 10.1002/1615-9861(200206)2:6<649::AID-PROT649>3.0.CO;2-R
Rainey, F. A. (2009a). “Family V. Lachnospiraceae fam. nov.” in Bergey’s manual of systematic bacteriology. eds. P. De Vos, G. M. Garrity, D. Jones, N. R. Krieg, W. Ludwig, and F. A. Rainey, et al. (New York, NY: Springer), 921–968.
Rainey, F. A. (2009b). “Family VIII. Ruminococcaceae fam. nov.” in Bergey’s manual of systematic bacteriology. eds. P. De Vos, G. M. Garrity, D. Jones, N. R. Krieg, W. Ludwig, and F. A. Rainey, et al. (New York, NY: Springer), 1016–1043.
Ravenscroft, N., Walker, S. G., Dutton, G. G., and Smit, J. (1991). Identification, isolation, and structural studies of extracellular polysaccharides produced by Caulobacter crescentus. J. Bacteriol. 173, 5677–5684. doi: 10.1128/jb.173.18.5677-5684.1991
Ravin, N. V., Rakitin, A. L., Ivanova, A. A., Beletsky, A. V., Kulichevskaya, I. S., Mardanov, A. V., et al. (2018). Genome analysis of Fimbriiglobus ruber SP5T, a planctomycete with confirmed chitinolytic capability. Appl. Environ. Microbiol. 84:e02645-17. doi: 10.1128/AEM.02645-17
Raza, W., and Shen, Q. (2010). Growth, Fe3+ reductase activity, and siderophore production by Paenibacillus polymyxa SQR-21 under differential iron conditions. Curr. Microbiol. 61, 390–395. doi: 10.1007/s00284-010-9624-3
Reguera, G., and Leschine, S. B. (2001). Chitin degradation by cellulolytic anaerobes and facultative aerobes from soils and sediments. FEMS Microbiol. Lett. 204, 367–374. doi: 10.1111/j.1574-6968.2001.tb10912.x
Rosenberg, E. (2014). “The family Chitinophagaceae” in The prokaryotes. eds. E. Rosenberg, E. F. DeLong, S. Lory, E. Stackebrandt, and F. Thompson (Heidelberg, Germany: Springer), 493–495.
Schellenberger, S., Kolb, S., and Drake, H. L. (2010). Metabolic responses of novel cellulolytic and saccharolytic agricultural soil Bacteria to oxygen. Environ. Microbiol. 12, 845–861. doi: 10.1111/j.1462-2920.2009.02128.x
Scheu, S. (2002). The soil food web: structure and perspectives. Eur. J. Soil Biol. 38, 11–20. doi: 10.1016/S1164-5563(01)01117-7
Singh, J. S., and Gupta, S. R. (1977). Plant decomposition and soil respiration in terrestrial ecosystems. Bot. Rev. 43, 449–528. doi: 10.1007/BF02860844
Sockett, R. E. (2009). Predatory lifestyle of Bdellovibrio bacteriovorus. Annu. Rev. Microbiol. 63, 523–539. doi: 10.1146/annurev.micro.091208.073346
Stacheter, A., Noll, M., Lee, C. K., Selzer, M., Glowik, B., Ebertsch, L., et al. (2013). Methanol oxidation by temperate soils and environmental determinants of associated methylotrophs. ISME J. 7, 1051–1064. doi: 10.1038/ismej.2012.167
Ueda, A., and Saneoka, H. (2015). Characterization of the ability to form biofilms by plant-associated Pseudomonas species. Curr. Microbiol. 70, 506–513. doi: 10.1007/s00284-014-0749-7
Vaaje-Kolstad, G., Horn, S. J., Sørlie, M., and Eijsink, V. G. H. (2013). The chitinolytic machinery of Serratia marcescens – a model system for enzymatic degradation of recalcitrant polysaccharides. FEBS J. 280, 3028–3049. doi: 10.1111/febs.12181
Vaaje-Kolstad, G., Westereng, B., Horn, S. J., Liu, Z., Zhai, H., Sorlie, M., et al. (2010). An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science 330, 219–222. doi: 10.1126/science.1192231
Wagner, C., Griesshammer, A., and Drake, H. L. (1996). Acetogenic capacities and the anaerobic turnover of carbon in a Kansas prairie soil. Appl. Environ. Microbiol. 62, 494–500.
Wang, X., Sharp, C. E., Jones, G. M., Grasby, S. E., Brady, A. L., and Dunfield, P. F. (2015). Stable-isotope probing identifies uncultured planctomycetes as primary degraders of a complex heteropolysaccharide in soil. Appl. Environ. Microbiol. 81, 4607–4615. doi: 10.1128/AEM.00055-15
Ward, N. L., Challacombe, J. F., Janssen, P. H., Hentrissat, B., Coutinho, B., Wu, M., et al. (2009). Three genomes from the phylum Acidobacteria provide insight into the lifestyles of these microorganisms in soils. Appl. Environ. Microbiol. 75, 2046–2056. doi: 10.1128/AEM.02294-08
Wegner, C. E., and Liesack, W. (2016). Microbial community dynamics during the early stages of plant polymer breakdown in paddy soil. Environ. Microbiol. 18, 2825–2842. doi: 10.1111/1462-2920.12815
Westgard, J. O., Barry, P. L., Hunt, M. R., and Groth, T. (1981). A multi-rule Shewhart chart for quality control in clinical chemistry. Clin. Chem. 27, 493–501.
Whiteley, A. S., Thomson, B., Lueders, T., and Manefield, M. (2007). RNA stable-isotope probing. Nat. Protoc. 2, 838–844. doi: 10.1038/nprot.2007.115
Wieczorek, A. S., Hetz, S. A., and Kolb, S. (2014). Microbial responses to chitin and chitosan in oxic and anoxic agricultural soil slurries. Biogeosciences 11, 3339–3352. doi: 10.5194/bg-11-3339-2014
Wild, B., Li, J., Pihlblad, J., Bengtson, P., and Rütting, T. (2018). Decoupling of priming and microbial N mining during a short-term soil incubation. Soil Biol. Biochem. 129, 71–79. doi: 10.1016/j.soilbio.2018.11.014
Yarza, P., Ludwig, W., Euzéby, J., Amann, R., Schleifer, K., Glöckner, F. O., et al. (2010). Update of the all-species living tree project based on 16S and 23S rRNA sequence analyses. Syst. Appl. Microbiol. 33, 291–299. doi: 10.1016/j.syapm.2010.08.001
Yilmaz, P., Parfrey, L. W., Yarza, P., Gerken, J., Pruesse, E., Quast, C., et al. (2014). The SILVA and “all-species living tree project (LTP)” taxonomic frameworks. Nucl. Acids Res. 42, D643–D648. doi: 10.1093/nar/gkt1209
Yoon, J. H., Kang, S. J., Park, S., and Oh, T. K. (2012). Mucilaginibacter litoreus sp. nov., isolated from marine sand. Int. J. Syst. Evol. Microbiol. 62, 2822–2827. doi: 10.1099/ijs.0.034900-0
Keywords: soil, microbiome, stable isotope probing, agriculture, soil carbon, food web, Ap horizon
Citation: Wieczorek AS, Schmidt O, Chatzinotas A, von Bergen M, Gorissen A and Kolb S (2019) Ecological Functions of Agricultural Soil Bacteria and Microeukaryotes in Chitin Degradation: A Case Study. Front. Microbiol. 10:1293. doi: 10.3389/fmicb.2019.01293
Edited by:
Svetlana N. Dedysh, Winogradsky Institute of Microbiology (RAS), RussiaReviewed by:
Usman Irshad, COMSATS University Islamabad, PakistanCarl-Eric Wegner, Friedrich Schiller University Jena, Germany
Michael Pester, German Collection of Microorganisms and Cell Cultures GmbH (DSMZ), Germany
Copyright © 2019 Wieczorek, Schmidt, Chatzinotas, von Bergen, Gorissen and Kolb. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steffen Kolb, a29sYkB6YWxmLmRl
†Present address: Steffen Kolb, Faculty of Life Sciences, Albrecht Daniel Thaer Institute, Humboldt University of Berlin, Berlin, Germany
 Adam S. Wieczorek
Adam S. Wieczorek Oliver Schmidt
Oliver Schmidt Antonis Chatzinotas
Antonis Chatzinotas Martin von Bergen4,5,6
Martin von Bergen4,5,6 Antonie Gorissen
Antonie Gorissen Steffen Kolb
Steffen Kolb