Commentary: Communication between Viruses Guides Lysis–Lysogeny Decisions
- 1Institute of Science and Technology Austria, Klosterneuburg, Austria
- 2Department of Microbiology, The Ohio State University, Mansfield, OH, United States
A Commentary on
A Host-Produced Quorum-Sensing Autoinducer Controls a Phage Lysis-Lysogeny Decision
by Silpe, J. E., and Bassler, B. L. (2019). Cell 176, 268–280. doi: 10.1016/j.cell.2018.10.059
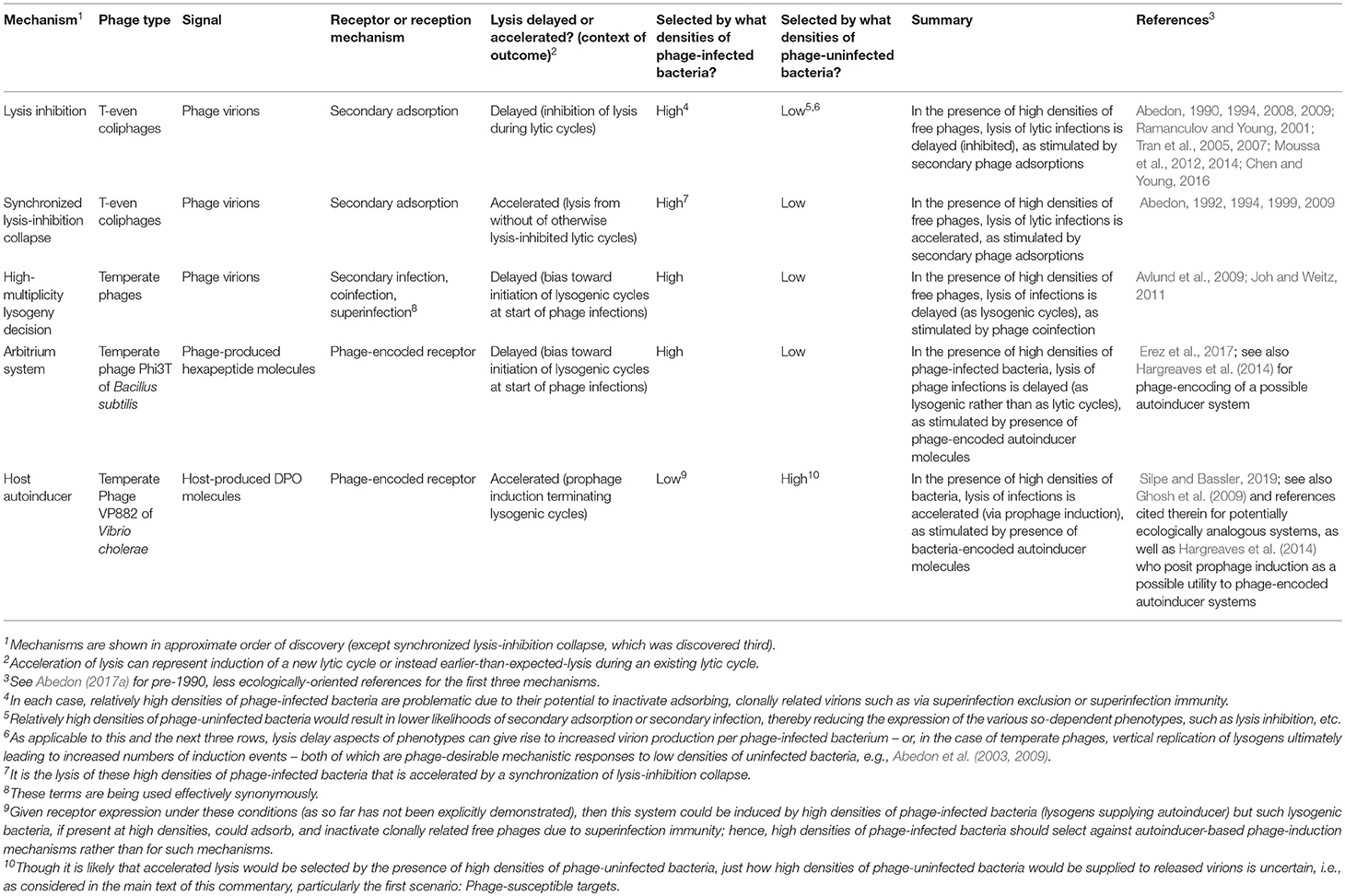
With the recent publication by Silpe and Bassler (2019), considering phage detection of a bacterial quorum-sensing (QS) autoinducer, we now have as many as five examples of phage-associated intercellular communication (Table 1). Each potentially involves ecological inferences by phages as to concentrations of surrounding phage-infected or uninfected bacteria. While the utility of phage detection of bacterial QS molecules may at first glance appear to be straightforward, we suggest in this commentary that the underlying ecological explanation is unlikely to be simple.
Autoinducer-Motivated Prophage Induction
In a fascinating study, Silpe and Bassler (2019) show that a temperate vibriophage can both detect and respond to a host-produced QS autoinducer, 3,5-dimethylpyrazin-2-ol (DPO). This cue helps to determine the phage's lifestyle via a mechanism that is potentially ecologically similar to observations provided by Ghosh et al. (2009). DPO normally represses Vibrio cholerae biofilm formation, resulting in biofilm dispersion (Papenfort et al., 2017). Silpe and Bassler (2019) found that VP882, a linear-plasmid prophage, encodes a host-homologous DPO receptor, VqmAPhage, which responds to extracellular DPO by stimulating production of Qtip, an antirepressor of the lysogenic repressor, CI. Resulting CI sequestration activates the phage's lytic pathway and thereby production and release of new VP882 virions. Qtip production also leads to induction of closely related vibriophages that encode non-homologous antirepressor analogs. What remains unclear, as indicated by the authors, is under which circumstances the phage QS receptor is expressed and also, our emphasis here, under what circumstances might DPO detection be beneficial to a phage.
Ecological Scenarios
Bacterial quorums represent high densities of bacteria (Waters and Bassler, 2005), as could be beneficial to phages undergoing lytic cycles (first scenario, below), though not necessarily always (Saucedo-Mora et al., 2017). DPO-motivated prophage induction, however, might offer more subtle benefits, and below we consider six scenarios of varying phage utility. Local bacterial populations are assumed to consist at least initially of individual, clonal microcolonies (Costerton et al., 1985; Kreft, 2004; Nadell and Bassler, 2011; van Gestel et al., 2015).
Phage-Susceptible Targets
VP882 lysogens might be found among non-VP882 infected, phage-susceptible, DPO-supplying bacteria. Here prophage induction presumably would be directly beneficial to the phage. From Silpe and Bassler (2019), “This strategy likely maximizes phage infection of the next V. cholerae cell.” For example, DPO could bias VP882 vibriophages newly infecting a V. cholerae microcolony toward sooner lytic cycles, or a spatially distinct VP882 lysogen microcolony might be situated in close association with a VP882-uninfected V. cholerae microcolony.
Phage-Resistant Targets
VP882 lysogen microcolonies might be found among bacteria which are not VP882 susceptible, e.g., as due to any of various bacterial phage-resistance strategies (Hyman and Abedon, 2010; Labrie et al., 2010). Induction of a lytic cycle in this case would not be of obvious local benefit to the phage. Indeed, induction could be particularly harmful to VP882 if local, resistant bacteria were still adsorbable, but not infectible.
Sister-Lysogen Targets
Due to superinfection immunity (Blasdel and Abedon, 2017), clonally related, fellow lysogens would be adsorbable but not infectable (Ghosh et al., 2009). Unless prophage presence were to dampen DPO production, then resulting induction among sister lysogens could result in virion release into environments consisting of high densities of virion-inactivating bacteria. This scenario is perhaps more plausible than Scenario 1, given the founding of microcolonies by single lysogens, ones not necessarily surrounded by phage-sensitive V. cholerae strains. Prophage induction could still result in virion dissemination to new cells that are found at more distant locations, though with the utility of this dissemination being despite dangers of released virion exposure to virion-inactivating, neighboring lysogens. The following three scenarios build on this scenario.
Danger and Opportunity of Dispersal
Upon dispersal, i.e., as is associated with DPO detection, biofilm bacteria can be especially metabolically active and additionally, by breaking free of microcolonies, potentially more vulnerable to lytic phages (Sauer et al., 2002; Abedon, 2017b; Vidakovic et al., 2018). DPO thereby could in part serve as a “danger” signal to a prophage, one analogous to detection of DNA damage (Ptashne, 2004), but signaling instead increased lysogen vulnerability to unrelated lytic phages. At the same time, improved bacterial metabolism at the point of biofilm dispersal could be capable of supporting particularly vigorous induced VP882 lytic cycles. Indeed, depending on the timing of VP882 induction, resulting lysis might not occur until following lysogen dispersal away from the danger of adsorbable fellow lysogens.
Bet Hedging (<<100% Induction)
Induction despite sister lysogens, if limited in extent, could be viewed as a bet-hedging strategy (Avlund et al., 2009; Maslov and Sneppen, 2015; Xue and Leibler, 2018). DPO exposure, perhaps given varying DPO or VqmAPhage-expression levels across microcolonies, thereby could give rise to phage populations existing as robust combinations of both virions and lysogens, each possessing distinct dispersal strategies and vulnerabilities. Thus, VP882 virions are less vulnerable to unrelated phages, but more vulnerable to VP882 lysogens. VP882 lysogens instead are more vulnerable to unrelated phages, invulnerable to fellow VP882 lysogens, and better able (via longer infections) to take advantage of host motility to disperse to new locations.
Near 100% Induction
If lysogens are locally exposed to equivalent levels of DPO, then this might trigger en masse lysis of sister lysogens, reducing their potential to inactivate released virions (similarly, see synchronized lysis-inhibition collapse, Table 1). This too could be bet-hedging, should somewhat smaller numbers of uninduced lysogens remain with reduced vulnerability to unrelated phages, having entered a so-called “numerical refuge” (Chao et al., 1977).
Conclusion
Better understanding DPO-motivated prophage induction will require determining the in situ timing of VqmAPhage-receptor expression and its ecological context, e.g., such as the spatial distribution of its or DPO's presence within phage VP882 lysogen microcolonies. Regardless of specifics, integrating host-derived signals presumably increases the environmental information obtained by a prophage. Perhaps, then, the timing and context of VqmAPhage expression can predispose DPO-motivated prophage induction toward circumstances which are more favorable to virion release than might be seen within dense populations of sister lysogens.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
CI is the recipient of a DOC fellowship (Doctoral Fellowship Programme of the Austrian Academy of Sciences).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thank you to Călin Guet and the reviewers for helpful comments on the manuscript.
References
Abedon, S. T. (1990). Selection for lysis inhibition in bacteriophage. J. Theor. Biol. 146, 501–511. doi: 10.1016/S0022-5193(05)80375-3
Abedon, S. T. (1992). Lysis of lysis inhibited bacteriophage T4-infected cells. J. Bacteriol. 174, 8073–8080. doi: 10.1128/jb.174.24.8073-8080.1992
Abedon, S. T. (1994). “Lysis and the interaction between free phages and infected cells,” in The Molecular Biology of Bacteriophage T4, eds J. D. Karam, E. Kutter, K. Carlson, and B. Guttman (Washington, DC: ASM Press), 397–405.
Abedon, S. T. (1999). Bacteriophage T4 resistance to lysis-inhibition collapse. Genet. Res. 74, 1–11. doi: 10.1017/S0016672399003833
Abedon, S. T. (2008). “Phage population growth: constraints, games, adaptation,” in Bacteriophage Ecology, ed S. T. Abedon (Cambridge, UK, Cambridge University Press), 64–93.
Abedon, S. T. (2009). “Bacteriophage intraspecific cooperation and defection,” in Contemporary Trends in Bacteriophage Research, ed H. T. Adams (Hauppauge, NY, Nova Science Publishers), 191–215.
Abedon, S. T. (2017a). Commentary: communication between viruses guides lysis-lysogeny decisions. Front. Microbiol. 8:983. doi: 10.3389/fmicb.2017.00983
Abedon, S. T. (2017b). Phage “delay” towards enhancing bacterial escape from biofilms: a more comprehensive way of viewing resistance to bacteriophages. AIMS Microbiol. 3, 186–226. doi: 10.3934/microbiol.2017.2.186
Abedon, S. T., Duffy, S., and Turner, P. E. (2009). “Bacteriophage ecology,” in Encyclopedia of Microbiology, ed M. Schaecter (Oxford, Elsevier), 42–57.
Abedon, S. T., Hyman, P., and Thomas, C. (2003). Experimental examination of bacteriophage latent-period evolution as a response to bacterial availability. Appl. Environ. Microbiol. 69, 7499–7506. doi: 10.1128/AEM.69.12.7499-7506.2003
Avlund, M., Dodd, I. B., Semsey, S., Sneppen, K., and Krishna, S. (2009). Why do phage play dice? J. Virol. 83, 11416–11420. doi: 10.1128/JVI.01057-09
Blasdel, B. G., and Abedon, S. T. (2017). “Superinfection immunity,” in Reference Module in Life Sciences, ed B. D. Roitberg (Elsevier). doi: 10.1016/B978-0-12-809633-8.90021-9
Chao, L., Levin, B. R., and Stewart, F. M. (1977). A complex community in a simple habitat: an experimental study with bacteria and phage. Ecology 58, 369–378. doi: 10.2307/1935611
Chen, Y., and Young, R. (2016). The last r locus unveiled: T4 RIII is a cytoplasmic antiholin. J. Bacteriol. 198, 2448–2457. doi: 10.1128/JB.00294-16
Costerton, J. W., Marrie, T. J., and Cheng, K.-J. (1985). “Phenomena of bacterial adhesion,” in Bacterial Adhesion: Mechanisms and Physiological Significance, eds D. C. Savage and M. Fletcher (New York, NY: Plenum Press), 3–43.
Erez, Z., Steinberger-Levy, I., Shamir, M., Doron, S., Stokar-Avihail, A., Peleg, Y., et al. (2017). Communication between viruses guides lysis-lysogeny decisions. Nature 541, 488–493. doi: 10.1038/nature21049
Ghosh, D., Roy, K., Williamson, K. E., Srinivasiah, S., Wommack, K. E., and Radosevich, M. (2009). Acyl-homoserine lactones can induce virus production in lysogenic bacteria: an alternative paradigm for prophage induction. Appl. Environ. Microbiol. 75, 7142–7152. doi: 10.1128/AEM.00950-09
Hargreaves, K. R., Kropinski, A. M., and Clokie, M. R. (2014). What does the talking?: quorum sensing signalling genes discovered in a bacteriophage genome. PLoS ONE 9:e85131. doi: 10.1371/journal.pone.0085131
Hyman, P., and Abedon, S. T. (2010). Bacteriophage host range and bacterial resistance. Adv. Appl. Microbiol. 70, 217–248. doi: 10.1016/S0065-2164(10)70007-1
Joh, R. I., and Weitz, J. S. (2011). To lyse or not to lyse: transient-mediated stochastic fate determination in cells infected by bacteriophages. PLoS Comput. Biol. 7:e1002006. doi: 10.1371/journal.pcbi.1002006
Kreft, J. U. (2004). Biofilms promote altruism. Microbiology 150, 2751–2760. doi: 10.1099/mic.0.26829-0
Labrie, S. J., Samson, J. E., and Moineau, S. (2010). Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8, 317–327. doi: 10.1038/nrmicro2315
Maslov, S., and Sneppen, K. (2015). Well-temperate phage: optimal bet-hedging against local environmental collapses. Sci. Rep. 5:10523. doi: 10.1038/srep10523
Moussa, S. H., Kuznetsov, V., Tran, T. A., Sacchettini, J. C., and Young, R. (2012). Protein determinants of phage T4 lysis inhibition. Protein Sci. 21, 571–582. doi: 10.1002/pro.2042
Moussa, S. H., Lawler, J. L., and Young, R. (2014). Genetic dissection of T4 lysis. J. Bacteriol. 196, 2201–2209. doi: 10.1128/JB.01548-14
Nadell, C. D., and Bassler, B. L. (2011). A fitness trade-off between local competition and dispersal in Vibrio cholerae biofilms. Proc. Natl. Acad. Sci. U S A. 108, 14181–14185. doi: 10.1073/pnas.1111147108
Papenfort, K., Silpe, J. E., Schramma, K. R., Cong, J. P., Seyedsayamdost, M. R., and Bassler, B. L. (2017). A Vibrio cholerae autoinducer-receptor pair that controls biofilm formation. Nat. Chem. Biol. 13, 551–557. doi: 10.1038/nchembio.2336
Ptashne, M. (2004). Genetic Switch: Phage Lambda Revisited. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
Ramanculov, E., and Young, R. (2001). An ancient player unmasked: T4 rI encodes a t-specific antiholin. Mol. Microbiol. 41, 575–583. doi: 10.1046/j.1365-2958.2001.02491.x
Saucedo-Mora, M. A., Castaneda-Tamez, P., Cazares, A., Perez-Velazquez, J., Hense, B. A., Cazares, D., et al. (2017). Selection of functional quorum sensing systems by lysogenic bacteriophages in Pseudomonas aeruginosa. Front. Microbiol. 8:1669. doi: 10.3389/fmicb.2017.01669
Sauer, K., Camper, A. K., Ehrlich, G. D., Costerton, J. W., and Davies, D. G. (2002). Pseudomonas aeruginosa displays multiple phenotypes during development as a biofilm. J. Bacteriol. 184, 1140–1154. doi: 10.1128/jb.184.4.1140-1154.2002
Silpe, J. E., and Bassler, B. L. (2019). A host-produced quorum-sensing autoinducer controls a phage lysis-lysogeny decision. Cell 176, 268–280. doi: 10.1016/j.cell.2018.10.059
Tran, T. A., Struck, D. K., and Young, R. (2005). Periplasmic domains define holin-antiholin interactions in T4 lysis inhibition. J. Bacteriol. 187, 6631–6640. doi: 10.1128/JB.187.19.6631-6640.2005
Tran, T. A., Struck, D. K., and Young, R. (2007). The T4 RI antiholin has an N-terminal signal anchor release domain that targets it for degradation by DegP. J. Bacteriol. 189, 7618–7625. doi: 10.1128/JB.00854-07
van Gestel, J., Vlamakis, H., and Kolter, R. (2015). Division of labor in biofilms: the ecology of cell differentiation. Microbiol. Spectr. 3, MB-0002–2014. doi: 10.1128/microbiolspec.MB-0002-2014
Vidakovic, L., Singh, P. K., Hartmann, R., Nadell, C. D., and Drescher, K. (2018). Dynamic biofilm architecture confers individual and collective mechanisms of viral protection. Nat. Microbiol. 3, 26–31. doi: 10.1038/s41564-017-0050-1
Waters, C. M., and Bassler, B. L. (2005). Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21, 319–346. doi: 10.1146/annurev.cellbio.21.012704.131001
Keywords: communication, extracellular signaling, ecology, induction, prophage, temperate
Citation: Igler C and Abedon ST (2019) Commentary: A Host-Produced Quorum-Sensing Autoinducer Controls a Phage Lysis-Lysogeny Decision. Front. Microbiol. 10:1171. doi: 10.3389/fmicb.2019.01171
Received: 23 January 2019; Accepted: 07 May 2019;
Published: 03 June 2019.
Edited by:
Manuel Martinez Garcia, University of Alicante, SpainReviewed by:
Benjamin Knowles, Rutgers University, The State University of New Jersey, United StatesMonica Lluesma Gomez, Universidad Miguel Hernández de Elche, Spain
Copyright © 2019 Igler and Abedon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephen T. Abedon, abedon.1@osu.edu
 Claudia Igler
Claudia Igler Stephen T. Abedon
Stephen T. Abedon