
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 08 May 2019
Sec. Virology
Volume 10 - 2019 | https://doi.org/10.3389/fmicb.2019.01000
 Xue Yang1
Xue Yang1 Yuwen Lu2*
Yuwen Lu2* Xing Zhao1
Xing Zhao1 Liangliang Jiang3,4
Liangliang Jiang3,4 Shengchun Xu5
Shengchun Xu5 Jiejun Peng2,3
Jiejun Peng2,3 Hongying Zheng2,3
Hongying Zheng2,3 Lin Lin2,3
Lin Lin2,3 Yuanhua Wu1
Yuanhua Wu1 Stuart MacFarlane6
Stuart MacFarlane6 Jianping Chen2,3*
Jianping Chen2,3* Fei Yan2*
Fei Yan2*Histone H2B protein is not only structurally important for chromosomal DNA packaging but is also involved in the regulation of gene expression, including the immune response of plants against pathogens. In this study, we show that the potato virus X (PVX) infection resulted in the reduced expression of H2B at both the mRNA and protein level in Nicotiana benthamiana. Tobacco rattle virus (TRV)-based virus-induced gene silencing (VIGS) was then used to down-regulate the expression of H2B in N. benthamiana and tests showed that the titre of TRV was similar in these plants to that in control treated plants. When these H2B-silenced plants were inoculated with PVX, the virus spread more slowly through the plant and there was a lower titre of PVX compared to non-silenced plants. Abnormal leaf development and stem necrosis were observed in the H2B-silenced plants, which were alleviated in H2B-silenced NahG transgenic plants suggesting the involvement of salicylic acid (SA) in the production of these symptoms. Indeed, quantitative reverse transcription (qRT)-PCR and liquid chromatography tandem mass spectroscopy (LC-MS) results showed that endogenous SA is increased in H2B-silenced N. benthamiana. Thus, downregulation of H2B induced the accumulation of endogenous SA, which was correlated with stem necrosis and a decreased accumulation of PVX in N. benthamiana.
Histones are nuclear proteins that are classified into five major protein groups: histones H2A, H2B, H3, and H4 are known as the core histones, while histones H1/H5 are known as the linker histones (Luger et al., 1997; Bellaïche and Grégoire, 2006). The core histone octamers (two molecules of each protein) act as spools that package eukaryotic chromosomal DNA into structural units called nucleosomes.
In addition to their role in organizing eukaryotic DNA, post-translationally modified H2B proteins can modulate the nucleosome/chromatin structure or DNA accessibility to affect the transcriptional pathways linked to embryonic development and cell differentiation (Zhang, 2003; Shilatifard, 2006; Fleming et al., 2008; Kyriss et al., 2010; Ríos-Uzeda and Wallace, 2014; Rønningen et al., 2015; Sadakierska-Chudy and Filip, 2015). Histones, including H2B, are subject to a range of post-translational modifications including acetylation and ubiquitination. In plants, the development and differentiation of Arabidopsis thaliana stem cells correlates with changes in histone H2B acetylation (Rosa et al., 2014). In rice (Oryza sativa), histone H2B monoubiquitination (H2Bub1) contributes to the regulation of flowering time and yield potential (Du et al., 2016). More recently, studies have revealed that post-translational modifications of H2B are involved in plant pathogen defense and non-host resistance (Isaac et al., 2009; Conn et al., 2011; Seo et al., 2011; Ding and Wang, 2015; Ramirez-Prado et al., 2018). For example, H2B mono-ubiquitination is catalyzed by two RING E3 ubiquitin ligase enzymes, HUB1 and HUB2, and HUB1 has been shown to be necessary for resistance to various necrotrophic fungal pathogens, by regulating ET- and SA-mediated responses in Arabidopsis (Dhawan et al., 2009). Moreover, disrupting tomato H2B mono-ubiquitination by silencing SIHUB1 and SIHUB2 increases susceptibility to Botrytis cinerea, which is related to the balance between salicylic acid (SA)- and jasmonic acid (JA)/ethylene (ET)-mediated signaling pathways (Zhang et al., 2015).
Plant viruses are important plant pathogens, which cause significant agricultural losses world-wide. There are at least two major mechanisms for plants to fight against virus infection. The primary plant defense is thought to be based on RNA silencing, an evolutionarily conserved, sequence-specific, mechanism that targets invasive nucleic acids for enzymatic degradation (Voinnet, 2001; Ding and Voinnet, 2007). In Arabidopsis, four Dicer-like ribonucleases (DCLs), 10 Argonaute proteins (AGOs), five dsRNA-binding proteins (DRBs), and six RNA-directed RNA polymerases (RDRs) have been identified, which participate in at least four different endogenous RNA silencing pathways, to combat virus infection as well as ensuring spatial and temporal regulation of the gene expression throughout the plant life cycle (Qu et al., 2005; Brodersen and Voinnet, 2006; Qu et al., 2008). The second defensive system that contributes to plant defense against viral pathogens is known as plant innate immunity. A well-studied example of this is the involvement of the tobacco N protein in conferring resistance to the tobacco mosaic virus (TMV) by recognizing the virus replicase protein and leading to the initiation of local programmed cell death (PCD) and a priming of resistance in more distant (systemic) regions of the plant (Caplan et al., 2008, 2009). It is well known that phytohormones, particularly salicylic acid (SA) and jasmonic acid (JA), participate in plant innate immunity to viruses. Foliar application of JA followed by SA triggers a strong systemic resistance to TMV (Zhu et al., 2014). Furthermore, exogenous SA is an inducer of systemic acquired resistance (SAR) to the potato virus X (PVX) infection in Lycopersicum esculentum and Solanum tuberosum (Sanchez et al., 2010; Falcioni et al., 2013). However, SA-induced resistance to viruses does not involve factors such as pathogenesis-related (PR) proteins or NPR1 (non-expressor of PR1) that are involved in plant defense to bacteria and fungi (Carr et al., 2010). There is also increasing evidence that phytohormone expression and activity is closely integrated with the activity of some components of the RNA silencing system in plants (Yang et al., 2004; Campos et al., 2014; Alazem et al., 2017).
In this study, we examined H2B accumulation in PVX-infected Nicotiana benthamiana plants and found that PVX infection caused a lowered accumulation of H2B transcripts and protein. Moreover, silencing of the H2B gene by VIGS caused an increase in the level of endogenous SA, and led to a decrease in the spread and titre of PVX in inoculated plants.
Wild type N. benthamiana and NahG transgenic N. benthamiana plants (provided by Dr Yule Liu, Tsinghua University, Beijing, China) were grown under a 16-h day at 22°C and an 8-h night at 18°C. The Agrobacterium strain GV3101 was used and infiltration was performed as described (Liu et al., 2002). Equal volumes of individual agrobacterium cultures (OD600 = 1.0) were mixed before co-infiltration. GFP fluorescence was observed under long-wavelength UV-light (Black Ray model B 100A, Ultra-Violet Products Ltd., Upland, California, United States) and photographed using a Cannon digital camera.
The TRV vectors were kindly provided by Dr Yule Liu, Tsinghua University, Beijing, China (Liu et al., 2002). pTRV containing pYL279a TRV RNA2 was used to express the partial sequence of different plant genes in order to silence them. A fragment of about 300 bp of the N terminal of N. benthamiana H2B gene was inserted into the TRV RNA2 expression vector (creating TRV:H2B) and co-infiltrated into plants in combination with a TRV RNA1 vector as described before (Liu et al., 2002). A control infection consisted of the TRV RNA1 vector infiltrated in combination with an empty TRV2 vector (TRV:00), as previously described (Lu et al., 2016). Viral infection by Agrobacterium infiltration was performed as described (Jiang et al., 2014). The gene segments of ICS (EU257505.1), EDS5 (Niben101Scf04767g00002.1) and PAL2 (Niben101Scf05617g00005.1) were combined with the partial clones of H2B by overlap PCR to create a series of pTRV constructs for dual VIGS.
Total RNAs were isolated from TRV:00- and TRV:H2B-infected WT N. benthamiana and NahG transgenic plants with Trizol (Invitrogen, United States) according to the manufacturer’s instructions. The mRNA expression of H2B (EF189156) was analyzed by qRT-PCR. The N. benthamiana Ubiquitin C (UBC) gene (AB026056.1) was used as the internal reference gene for analysis (Rotenberg et al., 2006; Shi et al., 2016). A Roche LightCycler®480 Real-Time PCR System was used for the reaction and the results were analyzed by the ΔΔCT method (Livak and Schmittgen, 2001). The primers used for qRT-PCR of SA pathway and silencing pathway-related genes are listed in Supplementary Table S1. The stability of the UBC reference gene as a baseline for relative quantitation calculations is shown in Supplementary Table S1.
Histone proteins of plant samples were extracted with lysis buffer (10 mM Tris–HCl, pH 7.5; 2 mM EDTA; 0.25 M HCl; 5 mM DTT; 0.2 mM PMSF) as described (Qin et al., 2010). The histone protein pellet was dissolved with laemmli buffer (62.5 mM Tris–HCl, pH 6.8, 2% SDS, 25% glycerol, 0.01% bromophenol blue, and 10% β-mercaptoethanol), separated on 15% SDS–PAGE gel, detected with anti-H2B (Santa Cruz Biotechnology, Europe) and anti-actin (abbkine, Inc., WuHan, China) primary antibody and anti-rabbit (Sigma-Aldrich, St. Louis, MO, United States) secondary antibody. The antigen–antibody complexes were visualized using nitrotetrazolium blue chloride/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP) buffer (Sigma-Aldrich, St. Louis, MO, United States) under standard conditions. Total proteins of plant samples were extracted with lysis buffer (100 mM Tris–HCl, pH 8.8, 60% SDS, 2% β-mercaptoethanol). Proteins were separated in a 12% SDS-PAGE gel and detected with anti-PVX CP primary antibody (Hangzhou Huaan Biotechnology Co., Ltd., (HuaBio)) and anti-rabbit (Sigma-Aldrich, St. Louis, MO, United States) secondary antibody. After incubation with secondary antibody, proteins were visualized with the EasySee Western Blot Kit (Transgene Biotech, BeiJing, China) and imaged with the Molecular Imager ChemiDoc Touch (Bio-Rad). Quantitative analysis of digital images of Western blots was done using ImageJ software.
TRV-VIGS H2B-silenced and mock infiltrated leaves were harvested at 7 dpi. For SA quantification, approximately 50 mg leaf tissue was finely ground in liquid nitrogen and extracted with 400 μl of 10% methanol containing 1% acetic acid to which internal standards had been added (13.8 ng 2H4 SA). The quantification of SA was determined by LC-MS (Agilent 1260 Infinity-Agilent 6420A) as described previously (Forcat et al., 2008). Three independent replicates were performed with each experiment containing three biological repeats. The level of SA was measured by Zoonbio Biotechnology Co., Ltd.
Nicotiana benthamiana plants were inoculated with PVX mechanically. At 6 days post-inoculation (dpi), viral infection symptoms appeared on upper, uninoculated leaves of the PVX infected plants but not on the mock control plants (Figure 1A), and the viral infection was verified by RT-PCR with PVX CP gene-specific primers (Figure 1B). Quantitative reverse transcription (qRT)-PCR analysis at 6 dpi showed that the H2B mRNA levels in systemic PVX-infected leaves were only about 40% of those in uninfected control leaves (Figure 1C). Western blotting using an H2B-specific antibody showed that the H2B protein level was reduced in PVX-infected leaves to about 35% of that in the mock-inoculated controls (Figure 1D). However, at 13 dpi, a qRT-PCR assay showed that the expression level of H2B mRNAs in systemic leaves of PVX infected plants had recovered to about 70% of that in mock plants (Supplementary Figure S1).
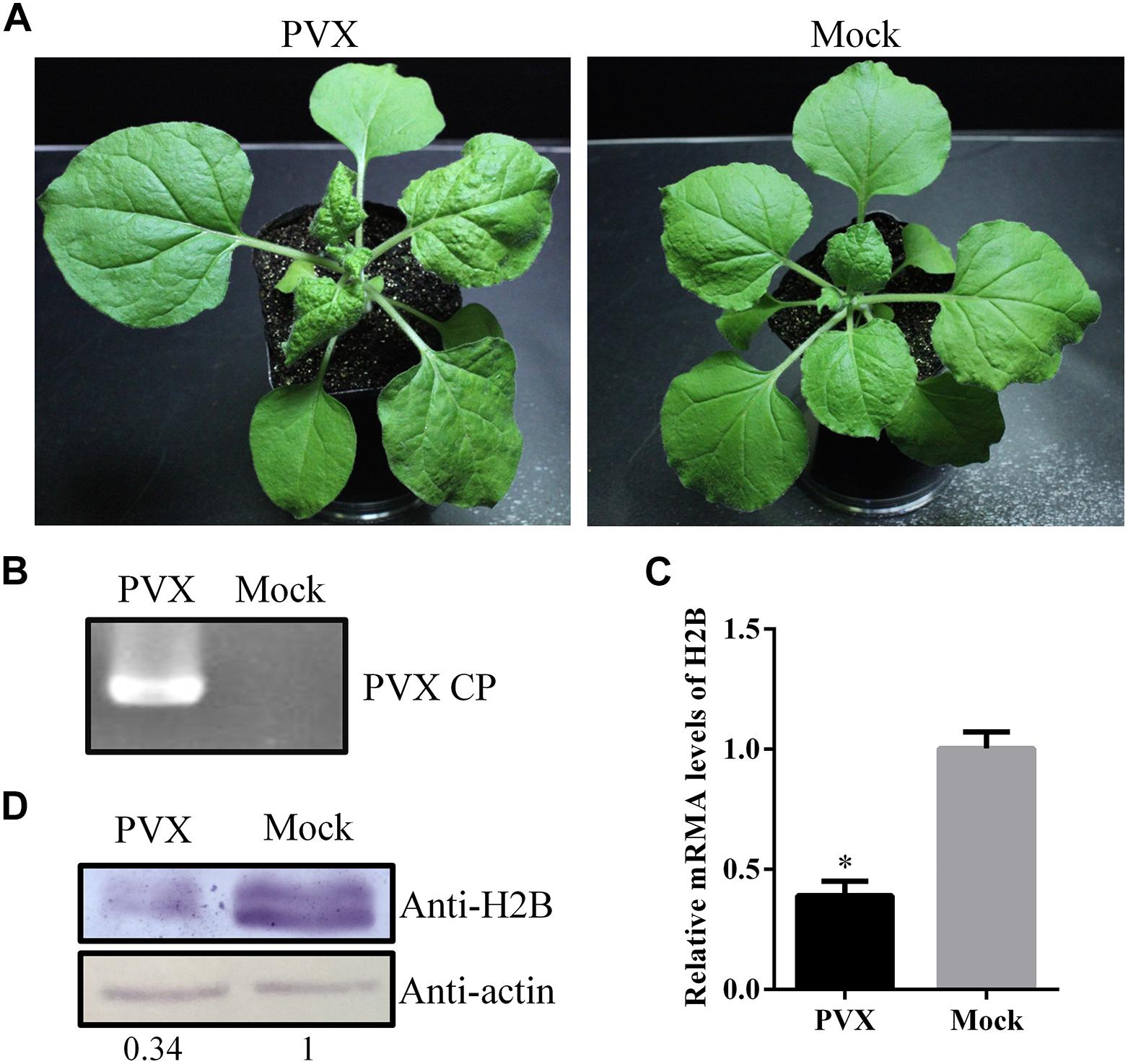
Figure 1. Expression levels of Histone H2B (H2B) transcripts and proteins in potato virus X (PVX)-infected Nicotiana benthamiana. (A) PVX symptoms at 6 dpi in N. benthamiana (left) and mock-inoculated plant (right). (B) PVX coat protein (CP) gene was detected by RT-PCR. (C) H2B proteins in PVX infected or healthy plants were detected by western blot using the H2B antibody. The relative amount of accumulated H2B protein was calculated using actin as a reference protein and is indicated below the panel. (D) After PVX infection, the expression of H2B transcripts was examined by qRT-PCR. Experiments were repeated three times. Bars represent the standard errors of the means. A two-sample unequal variance directional t-test was used to test the significance of the difference (∗P < 0.05).
Since PVX infection resulted in reduced expression of H2B, we next investigated whether down-regulation of H2B expression using tobacco rattle virus (TRV)-induced gene silencing (VIGS) would have an effect on PVX infection. qRT-PCR confirmed that the TRV:H2B VIGS construct reduced the H2B transcript level by 85% compared with TRV:00-treated control plants at 10 dpi (Figure 2A). Moreover, the H2B protein concentration in silenced plants was only 26% of that in control plants (Figure 2B).
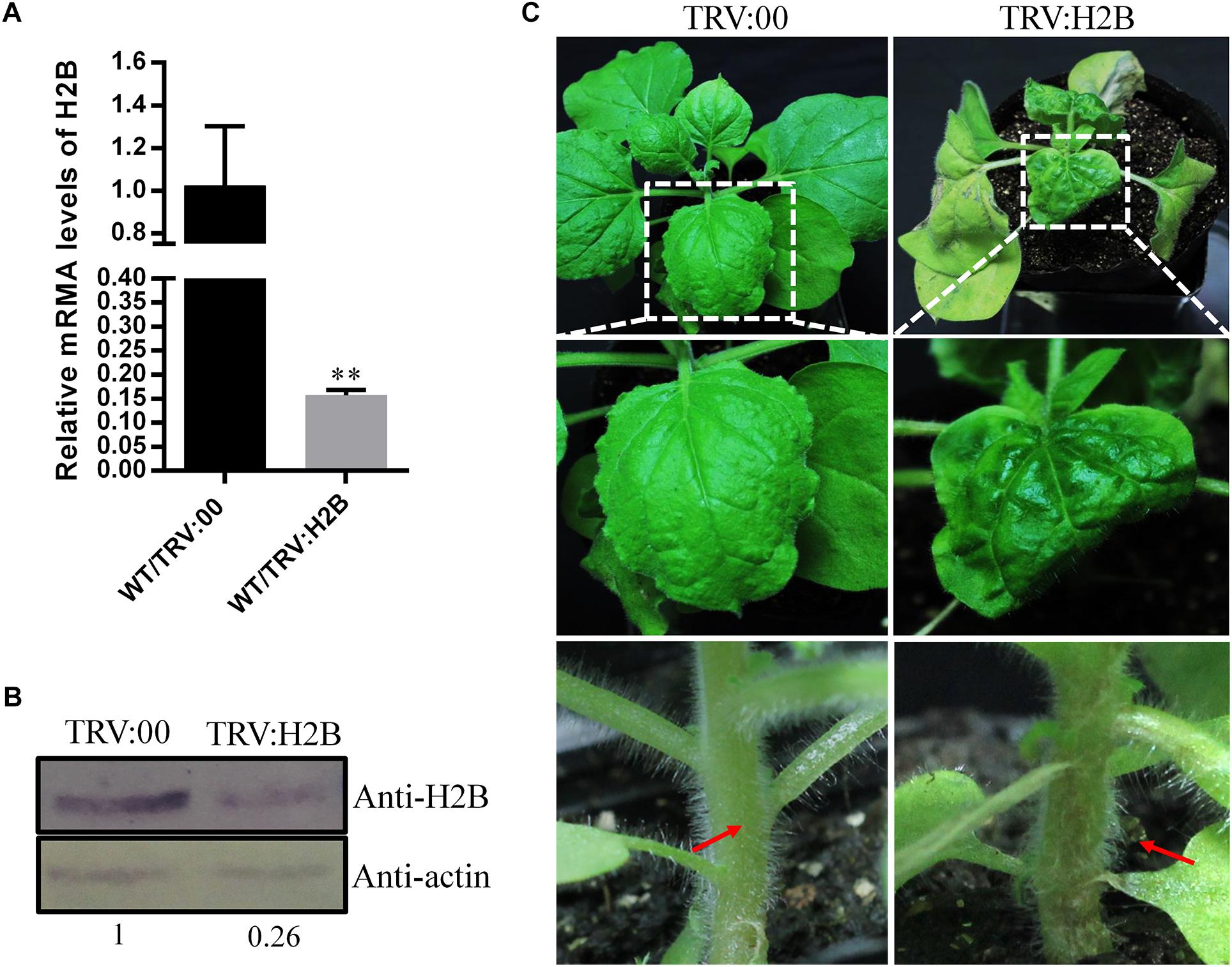
Figure 2. Effects of TRV-induced NbH2B silencing on N. benthamiana (WT) at 10 days dpi. (A) Validation of down-regulation of H2B transcripts in TRV:H2B N. benthamiana plants by qRT-PCR. Three repeat experiments were performed. Bars represent the standard errors of the means. A two-sample unequal variance directional t-test was used to test the significance of the difference (∗∗P < 0.01). (B) Western blot detection of H2B protein in H2B silenced (TRV:H2B) and non-silenced (TRV:00) plants. The relative amount of accumulated H2B protein was calculated using actin as a reference protein and is indicated below the panel. (C) H2B silencing caused the newly emerged leaves to be abnormal, yellow and curved (right upper and middle panels) compared with TRV:00 treated mock plants (left upper and middle panels) at 10 dpi. After treatment with TRV:H2B at 10 dpi, local necrosis emerged at the junction of the petioles (right bottom panel, red arrow) in H2B silenced plants, but there was no necrosis in the TRV control treated plants (left bottom panels, red arrow).
Leaf and stem malformations occurred on plants where the H2B gene was silenced by TRV VIGS, but not on TRV-treated control plants. Thus, at 10 dpi, malformed and chlorotic leaves developed, and petiole and main stem necrosis occurred on newly emerged leaves of plants agro-infiltrated with the TRV:H2B construct (Figure 2C), whereas there were no obvious symptoms on TRV:00 control plants (Figure 2C).
Semi-quantitative RT-PCR analysis showed that the levels of TRV CP were similar in both H2B-silenced and non-silenced control plants at 10 dpi (Supplementary Figure S2) suggesting that the chlorosis and necrosis of the leaves and stems was not caused by an increase in TRV titre in the silenced plants but was more likely an increased “sensitivity” of the plant to TRV infection.
At 10 dpi, after TRV mediated H2B-silencing system had been fully established in WT N. benthamiana plants, the plants were further challenged with a modified clone of PVX expressing the green fluorescent protein (PVX-GFP) and monitored for symptom development. Typical PVX symptoms of vein chlorosis and leaf curling appeared at 5 dpi on the top leaves of control (non-silenced) plants inoculated with PVX-GFP (Figure 3A). Newly emerging leaves show mosaic symptoms and green fluorescence under UV illumination (Figure 3B left). PVX became systemic 2 days later in the H2B-silenced plants (Figure 3A) and the green fluorescence appearing on most of the top leaves of H2B-silenced plants was weaker and the fluorescent area was less extensive than in the control plants (Figure 3B right). Western blotting showed that the PVX coat protein (PVX CP) accumulation in the systemic leaves was nearly 50% lower in H2B-silenced plants compared to the controls (Figure 3C).
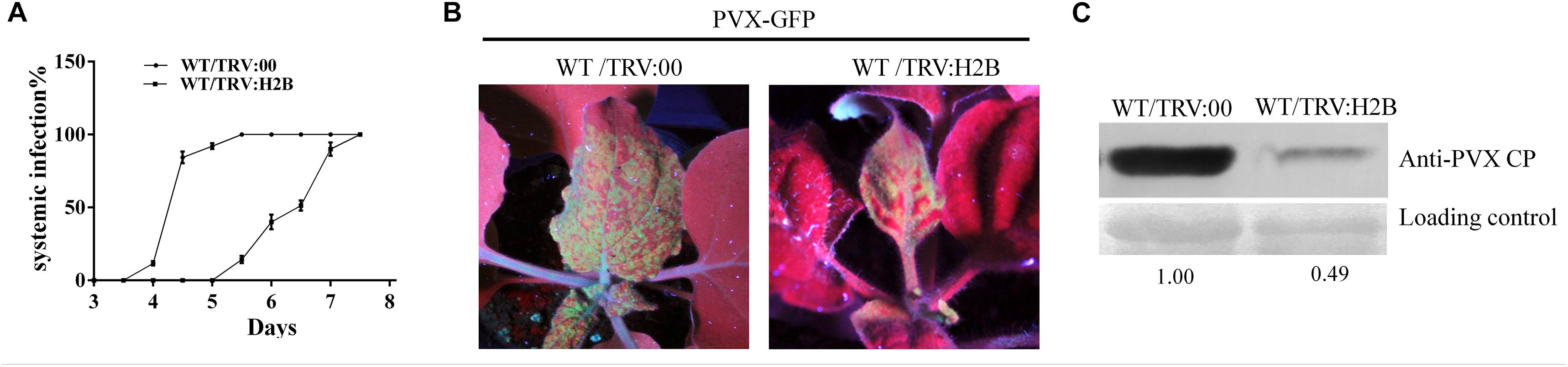
Figure 3. Decreased accumulation of PVX in H2B silenced WT plants. (A) Time-course results of PVX systemic infection index in H2B silenced and non-silenced WT plants. The proportion of systemically infected plants was determined by monitoring the green fluorescence on upper leaves under UV. The experiment was performed with 10 plants for each treatment and repeated three times. (B) Systemic PVX-GFP fluorescence at 7 dpi. The intensity of fluorescence on TRV:00 treated plants than on TRV:H2B treated plants. (C) Western blot detection of PVX CP protein at 7 dpi. The relative amount of accumulated PVX CP protein was calculated by comparing the CP band intensity with the loading control and is indicated below the panel.
One of the most well-known chemicals linked to the induction of necrosis in plants as a response to pathogen infection is SA (Alvarez, 2000; Brodersen et al., 2005; Dat et al., 2007; Straus et al., 2010). Transgenic plants expressing the bacterial enzyme salicylate hydroxylase (NahG), which degrades SA, have increased susceptibility to many plant pathogens, including viruses (Gaffney et al., 1993; Friedrich et al., 1995). We wondered whether the enhanced TRV symptoms seen in H2B-silenced plants could reflect changes to the SA synthesis and/or response pathway in these plants. We used NahG transgenic N. benthamiana plants, confirmed the expression of the transgene by RT-PCR with a specific primer pair (Supplementary Figure S3) and then also confirmed that TRV-VIGS was able to efficiently silence the H2B gene in these plants (Supplementary Figure S4). There was no obvious chlorosis on the newly emerging leaves and no necrosis of the petioles and stems of the NahG plants (Figure 4A) but these enhanced symptoms were produced in the non-transgenic control plants and also in H2B-silenced 16c plants (Voinnet et al., 2000) that were transformed with the GFP gene, which were used as a further control (Supplementary Figure S5).
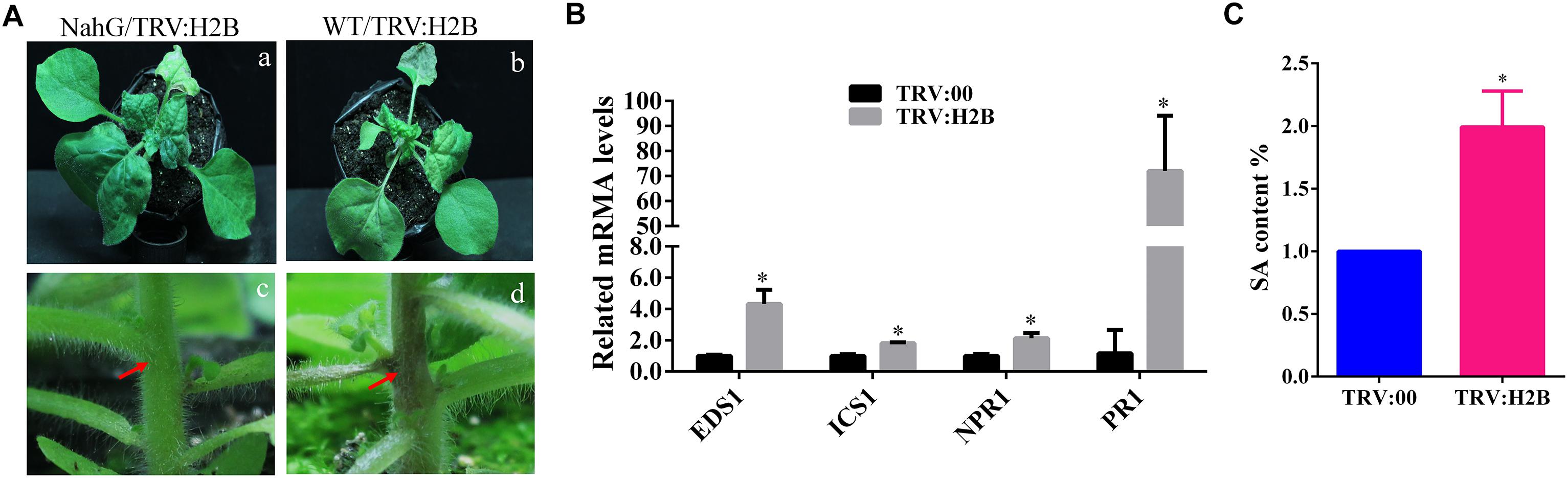
Figure 4. Salicylic acid (SA) accumulates in H2B silenced plant and is involved in petiole necrosis. (A) The symptoms caused by TRV:H2B VIGS on WT (panels a,c) or NahG (panels b,d) plants at 10 dpi. Local necrosis emerged at the junctions of stems and petioles (red arrows) in WT plants (panel d) but not NahG plants (panel c, red arrows). (B) qRT-PCR analysis of transcripts levels at 7 dpi of the EDS1, ICS1, NPR1, and PR1 genes in TRV:H2B WT plants and TRV:00 controls. A two-sample unequal variance directional t-test was used to test the significance of the difference (∗P < 0.05). (C) Relative levels of endogenous SA in TRV:00 and TRV:H2B treated WT plants as measured by LC-MS at 7 dpi. The endogenous SA in TRV:00 treated plant was set as the baseline. Three independent experiments were conducted with similar results.
We next performed qRT-PCR to examine the transcript levels of four SA pathway-related genes in H2B-silenced plants: Enhanced disease susceptibility 1 (EDS1), Isochorismate synthase 1 (ICS1), Non-expressor of Pathogenesis Related Genes 1 (NPR1), and Pathogenesis-Related Protein 1 (PR-1). All four genes, EDS1, ICS1, NPR1, and PR-1, were significantly up-regulated in the leaves of H2B-silenced plants compared with the non-silenced controls by 4.4, 1.7, 2.4, and 130-fold, respectively (Figure 4B). To further determine whether the biosynthesis of SA is affected by the silencing of the H2B gene, we quantified the levels of SA in H2B-silenced and non-silenced plants using high-performance liquid chromatography tandem mass spectroscopy (LC-MS). Because of the transient nature of the increase in phytohormone concentrations following stress stimuli, experiments were repeated three times, with each study containing three biological replicates. There was a 2-fold increase in SA levels in H2B silenced plants (Figure 4C). Together, these results demonstrate that knock-down of H2B leads to the generation of higher levels of SA.
Necrosis is a hallmark of the hypersensitive reaction (HR) response that functions to combat virus infection in plants and is associated with SA synthesis. The absence of necrosis in the NahG plants adds further weight to the hypothesis that silencing of H2B alters the synthesis or turnover of SA. To further investigate the functioning of the SA pathway in H2B-silenced plants, we performed silencing experiments in which both H2B and other SA pathway-related genes were targeted in the same plant simultaneously. For this purpose, TRV2 VIGS constructs were made carrying both a fragment of H2B together with a fragment (300 bp) of either ICS1, PAL2 (phenylalanine ammonia-lyase-2), or EDS5. Silencing of ICS1, PAL2, and EDS5 genes was expected to reduce SA accumulation by targeting upstream steps in the SA synthesis pathway (Nawrath et al., 2002; Brodersen et al., 2005; Catinot et al., 2008). Leaves of plants infected with these constructs (TRV-ICS/H2B, TRV-PAL2/H2B, and TRV-EDS5/H2B) displayed foliar malformations similar to those that developed in plants infected with the original TRV-H2B construct at 10 dpi (Figure 5A, upper panels). However, these plants did not have petiole necrosis and so resembled NahG plants infiltrated with TRV-H2B rather than non-transgenic plants infiltrated with TRV-H2B (Figure 5A, bottom panels, red arrows). Silencing of the ICS1, PAL2, and EDS5 genes in these plants was confirmed by qRT-PCR (Figure 5B). Expression of the NPR1 gene, which is involved in downstream signaling in the innate immunity pathway, is upregulated by increases in the SA level (An and Mou, 2011). We therefore used qRT-PCR to examine the NPR1 transcript levels in the dual-VIGS (TRV-ICS/H2B, TRV-PAL2/H2B, and TRV-EDS5/H2B) treated plants and found that NPR1 expression was reduced in these plants but increased in H2B-silenced plants (Supplementary Figure S6).

Figure 5. The symptoms of petiole and stem necrosis were compromised in TRV:ICS1-H2B, TRV:EDS5-H2B, and TRV:PAL2-H2B dual silenced plants. (A) Symptoms at 10 dpi caused by TRV mediated VIGS with TRV:ICS1-H2B, TRV:EDS5-H2B, and TRV:PAL2-H2B on WT and NahG plants. The presence of necrotic petioles (red arrow) was most apparent for the WT plant/TRV:H2B combination. TRV:ICS-H2B, TRV:EDS5-H2B, and TRV:PAL2-H2B had slight necrosis in stems on WT plants. There was almost no necrosis with the NahG plant/TRV:H2B combination. (B) Silencing of H2B, ICS, EDS5, and PAL2 genes by the dual VIGS TRV constructs. Three repeat experiments were performed. Bars represent the standard errors of the means. A two-sample unequal variance directional t-test was used to test the significance of the difference (∗P < 0.05; ∗∗P < 0.01).
Reducing either the synthesis of SA in dual VIGS-treated plants or the degradation of SA in NahG plants prevented the induction of petiole necrosis. These results corroborate the hypothesis that the petiole necrosis induced by H2B silencing is caused by activation of the SA pathway and the subsequent increase in SA content in these plants.
Previous reports showed that treatment with SA induced resistance to PVX in N. benthamiana (Lee et al., 2011). We have shown here, that silencing H2B in non-transgenic N. benthamiana plants induced the accumulation of endogenous SA and decreased the level of PVX accumulation. In further experiments we found that PVX levels were higher in NahG plants compared to WT plants, and also higher in H2B-silenced NahG plants compared to H2B-silenced WT plants (Figures 6A,B). These results show that up-regulation of SA accumulation by H2B silencing does not completely overcome the SA degradation caused by the NahG transgene.
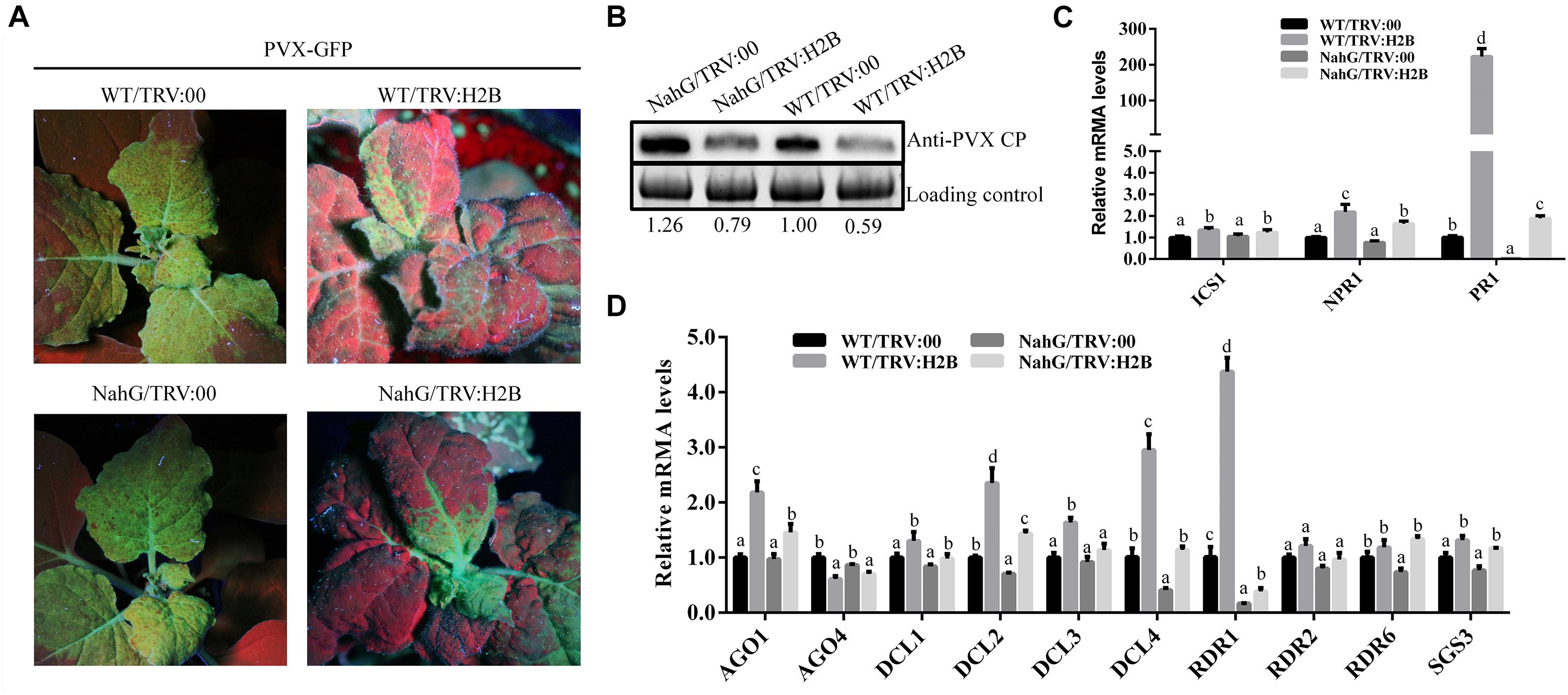
Figure 6. Expression of RNA silencing pathway genes was affected in H2B-silenced plants. (A) Systemic infection by PVX-GFP at 7 dpi on plants pre-treated with TRV:00 or TRV:H2B. (B) Western blot analysis of PVX CP protein in H2B silenced and TRV control treated WT and NahG plants. The level of PVX CP was calculated in relation to the loading control by comparing band intensities. (C) The relative expression levels of SA signaling pathways genes ICS1, NPR1 and PR1 in TRV:00 and TRV:H2B treated WT and NahG plants. (D) The relative expression levels of RNA silencing related genes AGO1, AGO4, DCL1, DCL2, DCL3, DCL4, RDR1, RDR2, RDR6, and SGS3 in TRV:00 and TRV:H2B treated WT and NahG plants at 7 dpi. Error bars show SD and the graph represents the combined data from three independent replicates. Letters on the graph denote statistically significant differences (ANOVA, P ≤ 0.05).
To further investigate this effect, the transcription levels of the SA synthesis gene ICS1 and the SA responsive genes NPR1 and PR1 were analyzed by qRT-PCR in non-silenced WT, non-silenced NahG plants, H2B-silenced WT plants and H2B-silenced NahG plants. Small but significant increases in the ICS1 transcript levels were seen when comparing H2B-silenced plants with non-silenced plants but there was no observable difference between the levels in the NahG and WT plants (Figure 6C). However, the transcription levels of the SA responsive genes NPR1 and PR1 were significantly decreased in H2B-silenced NahG plants compared with H2B-silenced WT plants (Figure 6C), reflecting the reduction of SA levels in NahG plants. In contrast, however, the transcription levels of NPR1 and PR1 were greatly up-regulated in H2B-silenced NahG plants compared with non-silenced NahG plants and in H2B-silenced WT plants compared to non-silenced WT plants (Figure 6C). These experiments show that silencing of the H2B gene increases expression of the SA responsive genes NPR1 and PR1, and that the SA degradative activity of the NahG transgene is not sufficient to overcome this effect.
In tomato, SA induces expression of the RNA silencing-related genes DCL2, RDR1, and RDR2, thereby enhancing the resistance of the plant to the tomato mosaic virus (ToMV) (Campos et al., 2014). To investigate whether the increase in endogenous SA following H2B silencing in N. benthamiana similarly altered the expression of RNA silencing-related genes, we performed a qRT-PCR analysis of the transcription of the N. benthamiana homologs of DICER 1 (NbDCL1), DICER 2 (NbDCL2), DICER 3 (NbDCL3), DICER 4 (NbDCL4), ARGONAUTE 1-1 (NbAGO1-1), ARGONAUTE 4-1 (NbAGO4-1), SGS3 (NbSGS3), RDR1 (NbRDR1), RDR2 (NbRDR2), and RDR6 (NbRDR6) (Li et al., 2014). As before, RNA samples were analyzed from H2B-silenced WT plants, non-silenced WT plants, H2B-silenced NahG plants and non-silenced NahG plants. For the non-transgenic, WT plants, silencing of the H2B gene by VIGS reproducibly and significantly increased the transcript levels of NbAGO1-1, NbDCL2, NbDCL3, NbDCL4, and NbRDR1 by 2.5, 2.7, 1.6, 3.4, and 4.1 fold, respectively, whereas there were no significant changes in expression of the other genes (AGO4, DCL1, RDR2, RDR6, and SGS3) (Figure 6D). The level of NbAGO1-1, NbDCL2, NbDCL3, NbDCL4, and NbRDR1 transcript increase was higher in WT plants as compared to NahG plants by 1.4, 2, 1.5, 2.7, and 2.2 fold, respectively, suggesting that, as before, it is the increase in endogenous SA level that leads to the increase in expression of these RNA silencing-related genes. In NahG plants the absolute level of gene expression fold-change was reduced compared to non-transgenic plants but H2B silencing still resulted in significant increase in expression of AGO1, DCL2, DCL4, and RDR1.
Although H2B histone is known to be involved in plant defense responses against fungi, no studies linking H2B and plant viruses have yet been reported (Hu et al., 2014; Li et al., 2015; Zhang et al., 2015). Here, we show that the expression level of the H2B mRNA and the accumulation of the H2B protein in N. benthamiana is decreased during infection by the RNA virus PVX. Knock down of H2B expression in N. benthamiana, also led to the abnormal development of leaves and the induction of petiole and stem necrosis. However, PVX infection, caused low expression of H2B, and resulted in milder symptoms than in H2B silenced plants (Figures 1A, 2C). We presume that the mild symptoms are due to the recovery in expression levels of H2B in PVX infected plants at 11 and 13 dpi (Supplementary Figure S1). We also found that artificially reducing H2B expression by TRV-mediated VIGS leads to a reduction in the accumulation of PVX in the plants, which was related to the induction of endogenous SA.
Deficiencies in nuclear lamina proteins CROWDED NUCLEI (CRWN) induces plant dwarfing and spontaneous cell death lesions, which are caused by over-production of SA in mutants (Choi et al., 2019). We hypothesized that the petiole necrosis in H2B silenced plants could be associated with changes in the production and/or accumulation of SA. We confirmed by LC-MS that the SA level is two-fold higher in H2B-silenced plants, and we further showed that transcription of a selection of SA pathway-related genes was up-regulated in these plants. By repeating the experiments in transgenic plants expressing the NahG gene we were able to confirm that reduction in the accumulation of SA by NahG enzyme activity inhibited the induction of petiole necrosis in H2B-silenced plants and also reduced the extent of the SA-related gene expression increase caused by the H2B silencing. A similar phenomenon was also observed for the acd11 mutant of Arabidopsis, which has constitutive activation of PCD and other genes involved in defense against pathogens such that this mutation is lethal during early plant growth (Brodersen et al., 2005). However, combining the acd11 mutation with the NahG transgene prevented the initiation of PCD and rescued the plant. It is also clear from this and other studies that the synthesis/accumulation of SA and the expression of various SA pathway genes are co-regulated, with both positive and negative feedback being identified (Shah, 2003; Loake and Grant, 2007; Chen et al., 2009; Vlot et al., 2009).
Whether the changes we observed in SA production and SA pathway gene expression are a direct or an indirect consequence of the silencing of the H2B gene, remains to be investigated. It would seem very possible that transcription of (some) SA pathway genes may be controlled directly by the binding of H2B to their promoters or by repressing the expression of other transcription factors that themselves bind to the SA pathway gene promoters.
The mechanism by which PVX infection affects the H2B level is not known. However, it was reported that histone H2B is strongly decreased in response to DNA damage (ionizing radiation) through modulation of octamer transcription factor 1 (Schild et al., 2003). Several reports indicate that virus infection, including plant viruses, can induce DNA damage (Gruhne et al., 2009; Pal et al., 2010; Cerovska et al., 2014). Particularly, there is evidence that PVX induces DNA damage in nuclei isolated from tobacco leaves (Cerovska et al., 2014). Despite being a positive-strand RNA virus that replicates in the cell cytoplasm, some viral proteins encoded by PVX can localize to the nucleus (Samuels et al., 2007). Furthermore, it has been found that SA-mediated defense gene expression is up-regulated by DNA-damaging agents and by mutation in DNA damage repair processes, which could be linked to H2B activity (Yan et al., 2013).
Finally, we showed that the expression of the RNA silencing related genes NbAGO1-1, NbDCL2, NbDCL3, NbDCL4, and NbRDR1 is up-regulated in H2B-silenced plants. We expect that these changes in gene expression are initiated by the increase in SA following H2B silencing. The tobacco RNA-dependent RNA polymerase 1 (RdRP1) gene, that functions during RNA silencing to amplify target dsRNAs, was found to be up-regulated by both TMV and SA treatment (Xie et al., 2001). More recently, in tomatoes, SA treatment was shown to up-regulate the expression of DCL1, DCL2, RDR1, RDR2, and repress the expression of DCL4 and RDR6 (Campos et al., 2014). Thus, it is becoming increasingly clear that the actions of the SA pathway and the RNA silencing pathway as a defense against viruses are coordinated in plants. In addition, other plant hormones such as jasmonic acid (JA), abscisic acid (ABA) and auxin are reported to be involved in crosstalk with SA (An and Mou, 2011; Proietti et al., 2013; Munoz-Espinoza et al., 2015), although we have not extended the work in our study to include these signaling pathways. Further work will be required to understand the precise mechanism(s) by which H2B, and perhaps other histone proteins or plant hormones, are integrated into plant defense pathways.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
XY, YL, JC, and FY designed the experiments. XY, YL, XZ, LJ, SX, JP, HZ, LL, and YW performed the experiments and interpreted the data. YL and FY drafted the manuscript. SM and JC revised the manuscript.
This work was financially supported by the National Nature Science Foundation of China (31500124), the national key research and development program of China (2018YFD0200800), the Rural and Environment Science and Analytical Services Division of the Scottish Government, the International Science and Technology Cooperation Program of China (2015DFA30700) and the K. C. Wong Magna fund from Ningbo University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.01000/full#supplementary-material
FIGURE S1 | Time course of expression levels of H2B transcripts in PVX infected systemic leaves. qRT-PCR analysis of the expression levels of H2B transcripts in PVX infected systemic leaves at 7, 9, 11, and 13 dpi.
FIGURE S2 | Levels of TRV are not altered by H2B silencing. The accumulation of the TRV CP gene in TRV:00 and TRV:H2B treated plants was analyzed by semi-quantitative RT-PCR at 10 dpi.
FIGURE S3 | NahG transgenic plant validation by RT-PCR. Expression of the NahG gene in transgenic plants was confirmed by RT-PCR in NahG/TRV:00 and NahG/TRV:H2B plants. No NahG amplification occurred in WT plants.
FIGURE S4 | Validation of H2B down regulation in TRV:H2B treated NahG plants. The expression of H2B was down-regulated by about 75% in TRV:H2B treated NahG plants. Three repeat qRT-PCR experiments were performed. Bars represent the standard errors of the means. A two-sample unequal variance directional t-test was used to test the significance of the difference (∗∗P < 0.01).
FIGURE S5 | Phenotype and petiole necrosis on H2B silenced and non-silenced WT, NahG, and 16C plants. There were no observed symptoms on non-silenced WT, NahG, and 16C plants. Abnormal foliar developments were seen on H2B silenced WT, NahG, and 16C plants. Additionally, petiole necrosis occurred on H2B silenced WT and 16C plants but not on NahG plants (red arrow).
FIGURE S6 | The NPR1 transcript level in TRV:00, TRV:H2B and three dual VIGS plants. The transcript level of NPR1 in TRV:ICS1-H2B, TRV:EDS5-H2B, and TRV:PAL2-H2B treated plants was significantly down-regulated compared with TRV:00 and TRV:H2B treated plants. Error bars show SD and the graph represents the combined data from three independent replicates. Letters on the graph denote statistically significant differences (ANOVA, P ≤ 0.05).
TABLE S1 | Primer pairs used in this study.
Alazem, M., He, M. H., Moffett, P., and Lin, N. S. (2017). Abscisic acid induces resistance against bamboo mosaic virus through argonaute2 and 3. Plant Physiol. 174, 339–355. doi: 10.1104/pp.16.00015
Alvarez, M. E. (2000). Salicylic Acid in the Machinery of Hypersensitive Cell Death and Disease Resistance. Dordrecht: Springer.
An, C., and Mou, Z. (2011). Salicylic acid and its function in plant immunity. J. Integr. Plant Biol. 53, 412–428. doi: 10.1111/j.1744-7909.2011.01043.x
Bellaïche, M., and Grégoire, J. C. (2006). Recognition and classification of histones using support vector machine. J. Comput. Biol. 13, 102–112. doi: 10.1089/cmb.2006.13.102
Brodersen, P., Malinovsky, F. G., Hématy, K., Newman, M. A., and Mundy, J. (2005). The role of salicylic acid in the induction of cell death in Arabidopsis acd11. Plant Physiol. 138, 1037–1045. doi: 10.1104/pp.105.059303
Brodersen, P., and Voinnet, O. (2006). The diversity of RNA silencing pathways in plants. Trends Genet. 22:268. doi: 10.1016/j.tig.2006.03.003
Campos, L., Granell, P., Tárraga, S., López-Gresa, P., Conejero, V., Bellés, J. M., et al. (2014). Salicylic acid and gentisic acid induce RNA silencing-related genes and plant resistance to RNA pathogens. Plant Physiol. Biochem. 77, 35–43. doi: 10.1016/j.plaphy.2014.01.016
Caplan, J. L., Mamillapalli, P., Burchsmith, T. M., Czymmek, K., and Dineshkumar, S. P. (2008). Chloroplastic protein NRIP1 mediates innate immune receptor recognition of a viral effector. Cell 132:449. doi: 10.1016/j.cell.2007.12.031
Caplan, J. L., Zhu, X., Mamillapalli, P., Marathe, R., Anandalakshmi, R., and Dinesh-Kumar, S. P. (2009). Induced ER chaperones regulate a receptor-like kinase to mediate antiviral innate immune response in plants. Cell Host Microbe 6, 457–469. doi: 10.1016/j.chom.2009.10.005
Carr, J. P., Lewsey, M. G., and Palukaitis, P. (2010). Signaling in induced resistance. Adv. Virus Res. 76, 57–121.
Catinot, J., Buchala, A., Abou-Mansour, E., and Mã©Traux, J. P. (2008). Salicylic acid production in response to biotic and abiotic stress depends on isochorismate in Nicotiana benthamiana. FEBS Lett. 582, 473–478. doi: 10.1016/j.febslet.2007.12.039
Cerovska, N., Plchova, H., Vaculik, P., Moravec, T., and Gichner, T. (2014). Potato virus X induces DNA damage in leaf nuclei of the host plant Nicotiana tabacum L. var. xanthi. Biol. Plant. 58, 783–787. doi: 10.1007/s10535-014-0448-z
Chen, Z., Zheng, Z., Huang, J., Lai, Z., and Fan, B. (2009). Biosynthesis of salicylic acid in plants. Plant Signal. Behav. 4:493.
Choi, J., Strickler, S. R., and Richards, E. J. (2019). Loss of CRWN nuclear proteins induces cell death and salicylic acid defense signaling. Plant Physiol. 179, 1315–1329. doi: doi: 10.1104/pp.18.01020
Conn, K. L., Hendzel, M. J., and Schang, L. M. (2011). Core histones H2B and H4 are mobilized during infection with herpes simplex virus 1. J. Virol. 85, 13234–13252. doi: 10.1128/JVI.06038-11
Dat, J. F., Capelli, N., Breusegem, F. V., Hayat, S., and Ahmad, A. (2007). The Interplay Between Salicylic Acid and Reactive Oxygen Species During Cell Death in Plants. Dordrecht: Springer.
Dhawan, R., Luo, H., Foerster, A. M., Abuqamar, S., Du, H. N., Briggs, S. D., et al. (2009). HISTONE MONOUBIQUITINATION1 interacts with a subunit of the mediator complex and regulates defense against necrotrophic fungal pathogens in Arabidopsis. Plant Cell 21, 1000–1019. doi: 10.1105/tpc.108.062364
Ding, B., and Wang, G. L. (2015). Chromatin versus pathogens: the function of epigenetics in plant immunity. Front. Plant Sci. 6:675. doi: 10.3389/fpls.2015.00675
Ding, S. W., and Voinnet, O. (2007). Antiviral immunity directed by small RNAs. Cell 130, 413–426. doi: 10.1016/j.cell.2007.07.039
Du, Y., He, W., Deng, C., Chen, X., Gou, L., Zhu, F., et al. (2016). Flowering-related RING protein 1 (FRRP1) regulates flowering time and yield potential by affecting histone H2B monoubiquitination in rice (Oryza sativa). PLoS One 11:e0150458. doi: 10.1371/journal.pone.0150458
Falcioni, T., Ferrio, J. P., del Cueto, A. I., Giné, J., Achón, M. Á, and Medina, V. (2013). Effect of salicylic acid treatment on tomato plant physiology and tolerance to potato virus X infection. Eur. J. Plant Pathol. 138, 331–345. doi: 10.1007/s10658-013-0333-1
Fleming, A. B., Kao, C. F., Hillyer, C., Pikaart, M., and Osley, M. A. (2008). H2B ubiquitylation plays a role in nucleosome dynamics during transcription elongation. Mol. Cell 31, 57–66. doi: 10.1016/j.molcel.2008.04.025
Forcat, S., Bennett, M. H., Mansfield, J. W., and Grant, M. R. (2008). A rapid and robust method for simultaneously measuring changes in the phytohormones ABA, JA and SA in plants following biotic and abiotic stress. Plant Methods 4:16. doi: 10.1186/1746-4811-4-16
Friedrich, L., Vernooij, B., Gaffney, T., Morse, A., and Ryals, J. (1995). Characterization of tobacco plants expressing a bacterial salicylate hydroxylase gene. Plant Mol. Biol. 29, 959–968. doi: 10.1007/bf00014969
Gaffney, T., Friedrich, L., Vernooij, B., Negrotto, D., Nye, G., Uknes, S., et al. (1993). Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261, 754–756. doi: 10.1126/science.261.5122.754
Gruhne, B., Sompallae, R., and Masucci, M. G. (2009). Three Epstein-Barr virus latency proteins independently promote genomic instability by inducing DNA damage, inhibiting DNA repair and inactivating cell cycle checkpoints. Oncogene 28, 3997–4008. doi: 10.1038/onc.2009.258
Hu, M., Pei, B. L., Zhang, L. F., and Li, Y. Z. (2014). Histone H2B monoubiquitination is involved in regulating the dynamics of microtubules during the defense response to Verticillium dahliae toxins in Arabidopsis. Plant Physiol. 164, 1857–1865. doi: 10.1104/pp.113.234567
Isaac, J., Hartney, S. L., Druffel, K., and Hadwiger, L. A. (2009). The non-host disease resistance in peas; alterations in phosphorylation and ubiquitination of HMGA and histone H2A/H2B. Plant Sci. 177, 439–449. doi: 10.1016/j.plantsci.2009.07.007
Jiang, S., Lu, Y., Li, K., Lin, L., Zheng, H., Yan, F., et al. (2014). Heat shock protein 70 is necessary for Rice stripe virus infection in plants. Mol. Plant Pathol. 15, 907–917.
Kyriss, M. N., Jin, Y., Gallegos, I. J., Sanford, J. A., and Wyrick, J. J. (2010). Novel functional residues in the core domain of histone H2B regulate yeast gene expression and silencing and affect the response to DNA damage. Mol. Cell Biol. 30, 3503–3518. doi: 10.1128/mcb.00290-10
Lee, W. S., Fu, S. F., Verchot-Lubicz, J., and Carr, J. P. (2011). Genetic modification of alternative respiration in Nicotiana benthamiana affects basal and salicylic acid-induced resistance to potato virus X. BMC Plant Biol. 11:41. doi: 10.1186/1471-2229-11-41
Li, F., Huang, C., Li, Z., and Zhou, X. (2014). Suppression of RNA silencing by a plant DNA virus satellite requires a host calmodulin-like protein to repress RDR6 expression. PLoS Pathog. 10:e1003921. doi: 10.1371/journal.ppat.1003921
Li, X., Jiang, Y., Ji, Z., Liu, Y., and Zhang, Q. (2015). BRHIS1 suppresses rice innate immunity through binding to monoubiquitinated H2A and H2B variants. EMBO Rep. 16, 1192–1202. doi: 10.15252/embr.201440000
Liu, Y., Schiff, M., and Dinesh-Kumar, S. P. (2002). Virus-induced gene silencing in tomato. Plant J. 31, 777–786. doi: 10.1046/j.1365-313x.2002.01394.x
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta C(T)) method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Loake, G., and Grant, M. (2007). Salicylic acid in plant defence-the players and protagonists. Curr. Opin. Plant Biol. 10, 466–472. doi: 10.1016/j.pbi.2007.08.008
Lu, Y., Yin, M., Wang, X., Chen, B., Yang, X., Peng, J., et al. (2016). The unfolded protein response and programmed cell death are induced by expression of Garlic virus X p11 in Nicotiana benthamiana. J. Gen. Virol. 97, 1462. doi: 10.1099/jgv.0.000460
Luger, K., Mäder, A. W., Richmond, R. K., Sargent, D. F., and Richmond, T. J. (1997). Crystal structure of the nucleosome core particle at 2.8 A resolution. Nat. Int. Weekly J. Sci. 389, 251–260.
Munoz-Espinoza, V. A., Lopez-Climent, M. F., Casaretto, J. A., and Gomez-Cadenas, A. (2015). Water stress responses of tomato mutants impaired in hormone biosynthesis reveal abscisic acid, jasmonic acid and salicylic acid interactions. Front. Plant Sci. 6:997. doi: 10.3389/fpls.2015.00997
Nawrath, C., Heck, S., Parinthawong, N., and Métraux, J. P. (2002). EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 14, 275–286. doi: 10.1105/tpc.010376
Pal, S., Polyak, S. J., Bano, N., Wan, C. Q., Carithers, R. L., Shuhart, M., et al. (2010). Hepatitis C virus induces oxidative stress, DNA damage and modulates the DNA repair enzyme NEIL1. J. Gastroenterol. Hepatol. 25:627. doi: 10.1111/j.1440-1746.2009.06128.x
Proietti, S., Bertini, L., Timperio, A. M., Zolla, L., Caporale, C., and Caruso, C. (2013). Crosstalk between salicylic acid and jasmonate in Arabidopsis investigated by an integrated proteomic and transcriptomic approach. Mol. Biosyst. 9, 1169–1187.
Qin, F. J., Sun, Q. W., Huang, L. M., Chen, X. S., and Zhou, D. X. (2010). Rice SUVH histone methyltransferase genes display specific functions in chromatin modification and retrotransposon repression. Mol. Plant 3, 773–782. doi: 10.1093/mp/ssq030
Qu, F., Ye, X., Hou, G., Sato, S., Clemente, T. E., and Morris, T. J. (2005). RDR6 has a broad-spectrum but temperature-dependent antiviral defense role in Nicotiana benthamiana. J. Virol. 79, 15209. doi: 10.1128/jvi.79.24.15209-15217.2005
Qu, F., Ye, X., and Morris, T. J. (2008). Arabidopsis DRB4, AGO1, AGO7, and RDR6 participate in a DCL4-initiated antiviral RNA silencing pathway negatively regulated by DCL1. Proc. Natl. Acad. Sci. U.S.A. 105:14732. doi: 10.1073/pnas.0805760105
Ramirez-Prado, J. S., Piquerez, S. J. M., Bendahmane, A., Hirt, H., Raynaud, C., and Benhamed, M. (2018). Modify the histone to win the battle: chromatin dynamics in plant-pathogen interactions. Front. Plant Sci. 9:355. doi: 10.3389/fpls.2018.00355
Ríos-Uzeda, B., and Wallace, R. B. (2014). Histone H2B monoubiquitination is involved in the regulation of cutin and wax composition in Arabidopsis thaliana. Plant Cell Physiol. 55, 455–466. doi: 10.1093/pcp/pct182
Rønningen, T., Shah, A., Oldenburg, A. R., Vekterud, K., Delbarre, E., Moskaug, J. Ø, et al. (2015). Prepatterning of differentiation-driven nuclear lamin A/C-associated chromatin domains by GlcNAcylated histone H2B. Genome Res. 25:1825. doi: 10.1101/gr.193748.115
Rosa, S., Ntoukakis, V., Ohmido, N., Pendle, A., Abranches, R., and Shaw, P. (2014). Cell differentiation and development in Arabidopsis are associated with changes in histone dynamics at the single-cell level. Plant Cell 26, 4821–4833. doi: 10.1105/tpc.114.133793
Rotenberg, D., Thompson, T. S., German, T. L., and Willis, D. K. (2006). Methods for effective real-time RT-PCR analysis of virus-induced gene silencing. J. Virol. Methods 138, 49–59. doi: 10.1016/j.jviromet.2006.07.017
Sadakierska-Chudy, A., and Filip, M. (2015). A comprehensive view of the epigenetic landscape. Part II: histone post-translational modification, nucleosome level, and chromatin regulation by ncRNAs. Neurotox Res. 27, 172–197. doi: 10.1007/s12640-014-9508-6
Samuels, T. D., Ju, H. J., Ye, C. M., Motes, C. M., Blancaflor, E. B., and Verchotlubicz, J. (2007). Subcellular targeting and interactions among the Potato virus X TGB proteins. Virology 367:375. doi: 10.1016/j.virol.2007.05.022
Sanchez, G., Gerhardt, N., Siciliano, F., Vojnov, A., Malcuit, I., and Marano, M. R. (2010). Salicylic acid is involved in the Nb-mediated defense responses to Potato virus X in Solanum tuberosum. Mol. Plant Microbe Interact. 23, 394–405. doi: 10.1094/mpmi-23-4-0394
Schild, P. C., Shih, A., Yarymowich, N. C., and Hache, R. J. (2003). Down-regulation of histone H2B by DNA-dependent protein kinase in response to DNA damage through modulation of octamer transcription factor 1. Cancer Res. 63:7197.
Seo, J. K., Stephenson, J., and Noga, E. J. (2011). Multiple antibacterial histone H2B proteins are expressed in tissues of American oyster. Compar. Biochem. Physiol. Part B Biochem. Mol. Biol. 158:223. doi: 10.1016/j.cbpb.2010.11.011
Shah, J. (2003). The salicylic acid loop in plant defense. Curr. Opin. Plant Biol. 6, 365–371. doi: 10.1016/s1369-5266(03)00058-x
Shi, B., Lin, L., Wang, S., Guo, Q., Zhou, H., Rong, L., et al. (2016). Identification and regulation of host genes related to rice stripe virus symptom production. New Phytol. 209, 1106–1119. doi: 10.1111/nph.13699
Shilatifard, A. (2006). Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu. Rev. Biochem. 75:243. doi: 10.1146/annurev.biochem.75.103004.142422
Straus, M. R., Rietz, S., Ver, L. V. T. E., Bartsch, M., and Parker, J. E. (2010). Salicylic acid antagonism of EDS1-driven cell death is important for immune and oxidative stress responses in Arabidopsis. Plant J. 62, 628–640. doi: 10.1111/j.1365-313x.2010.04178.x
Vlot, A. C., Dempsey, D. A., and Klessig, D. F. (2009). Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 47, 177–206. doi: 10.1146/annurev.phyto.050908.135202
Voinnet, O. (2001). RNA silencing as a plant immune system against viruses. Trends Genet. Tig 17:449. doi: 10.1016/s0168-9525(01)02367-8
Voinnet, O., Lederer, C., and Baulcombe, D. C. (2000). A viral movement protein prevents spread of the gene silencing signal in Nicotiana benthamiana. Cell 103, 157–167. doi: 10.1016/s0092-8674(00)00095-7
Xie, Z., Fan, B., Chen, C., and Chen, Z. (2001). An important role of an inducible RNA-dependent RNA polymerase in plant antiviral defense. Proc. Natl. Acad. Sci. U.S.A. 98, 6516–6521. doi: 10.1073/pnas.111440998
Yan, S., Wang, W., Marques, J., Mohan, R., Saleh, A., Durrant, W. E., et al. (2013). Salicylic acid activates DNA damage responses to potentiate plant immunity. Mol. Cell 52, 602–610. doi: 10.1016/j.molcel.2013.09.019
Yang, S. J., Carter, S. A., Cole, A. B., Cheng, N. H., and Nelson, R. S. (2004). A natural variant of a host RNA-dependent RNA polymerase is associated with increased susceptibility to viruses by Nicotiana benthamiana. Proc. Natl. Acad. Sci. U.S.A. 101, 6297–6302. doi: 10.1073/pnas.0304346101
Zhang, Y. (2003). Transcriptional regulation by histone ubiquitination and deubiquitination. Genes Dev. 17, 2733–2740. doi: 10.1101/gad.1156403
Zhang, Y., Li, D., Zhang, H., Hong, Y., Huang, L., Liu, S., et al. (2015). Tomato histone H2B monoubiquitination enzymes SlHUB1 and SlHUB2 contribute to disease resistance against Botrytis cinerea through modulating the balance between SA- and JA/ET-mediated signaling pathways. BMC Plant Biol. 15:252. doi: 10.1186/s12870-015-0614-2
Keywords: H2B, potato virus X resistance, salicylic acid, VIGS, RNA silencing
Citation: Yang X, Lu Y, Zhao X, Jiang L, Xu S, Peng J, Zheng H, Lin L, Wu Y, MacFarlane S, Chen J and Yan F (2019) Downregulation of Nuclear Protein H2B Induces Salicylic Acid Mediated Defense Against PVX Infection in Nicotiana benthamiana. Front. Microbiol. 10:1000. doi: 10.3389/fmicb.2019.01000
Received: 15 February 2019; Accepted: 18 April 2019;
Published: 08 May 2019.
Edited by:
Helene Sanfacon, Agriculture and Agri-Food Canada (AAFC), CanadaReviewed by:
Shih-Feng Fu, National Changhua University of Education, TaiwanCopyright © 2019 Yang, Lu, Zhao, Jiang, Xu, Peng, Zheng, Lin, Wu, MacFarlane, Chen and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuwen Lu, bHV5dXdlbkB5ZWFoLm5ldA==; bHV5dXdlbkBuYnUuZWR1LmNu Jianping Chen, anBjaGVuMjAwMUAxMjYuY29t Fei Yan, ZmVpLnlhbkBtYWlsLnphYXMuYWMuY24=; WWFuZmVpQG5idS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.