- 1National Glycoengineering Research Center and Shandong Provincial Key Laboratory of Carbohydrate Chemistry and Glycobiology, Shandong University, Qingdao, China
- 2Dongying Tiandong Pharmaceutical, Co., Ltd., Dongying, China
Glycosaminoglycan (GAG) sulfatases, which catalyze the hydrolysis of sulfate esters from GAGs, belong to a large and conserved sulfatase family. Bacterial GAG sulfatases are essential in the process of sulfur cycling and are useful for the structural analysis of GAGs. Only a few GAG-specific sulfatases have been studied in detail and reported to date. Herein, the GAG-degrading Photobacterium sp. FC615 was isolated from marine sediment, and a novel Δ4,5hexuronate-2-O-sulfatase (PB2SF) was identified from this bacterium. PB2SF specifically removed 2-O-sulfate from the unsaturated hexuronate residue located at the non-reducing end of GAG oligosaccharides produced by GAG lyases. A structural model of PB2SF was constructed through a homology-modeling method. Six conserved amino acids around the active site were chosen for further analysis using site-directed mutagenesis. N113A, K141A, K141H, H143A, H143K, H205A, and H205K mutants exhibited only feeble activity, while the H310A, H310K, and D52A mutants were totally inactive, indicating that these conserved residues, particularly Asp52 and His310, were essential in the catalytic mechanism. Furthermore, bioinformatic analysis revealed that GAG sulfatases with specific degradative properties clustered together in the neighbor-joining phylogenetic tree. Based on this finding, 60 Δ4,5hexuronate-2-O-sulfatases were predicted in the NCBI protein database, and one with relatively low identity to PB2SF was characterized to confirm our prediction. Moreover, the signature sequences of bacterial Δ4,5hexuronate-2-O-sulfatases were identified. With the reported signature motifs, the sulfatase sequence of the Δ4,5hexuronate-2-O-sulfatase family could be simply identified before cloning. Taken together, the results of this study should aid in the identification and further application of novel GAG sulfatases.
Introduction
Sulfatases, which catalyze the cleavage of sulfate esters, participate in the metabolic pathways of bacteria that utilize sulfated polysaccharides as carbon sources (Hanson et al., 2004). The desulfation of these biopolymers by sulfatases facilitates their degradation by other enzymes and is essential for the bioremediation of organosulfur compounds derived from plants and animals (Kertesz, 2000; Sogi et al., 2013; Barbeyron et al., 2016b). The marine environment is rich in heterogeneously sulfated polysaccharides, such as sulfated glycosaminoglycans (GAGs), carrageenans, and fucans/fucoidans (Berteau and Mulloy, 2003; Pavao, 2014; Vasconcelos and Pomin, 2017). GAGs are linear polyanionic polysaccharides with repeating disaccharide units and are the major compounds of the extracellular matrix of the animal cells. Chondroitin sulfate (CS)/dermatan sulfate (DS) and heparin (Hep)/heparan sulfate (HS) are highly sulfated GAG members. CS/DS polysaccharides are composed of repeating disaccharide units containing hexuronic acid and N-acetyl-D-galactosamine (GalNAc). The hexuronic acid in CS is β-D-glucuronic acid (GlcA), while GlcA is converted to α-L-iduronic acid (IdoA) by a C-5 epimerase in DS (Maccarana et al., 2006). Therefore, CS/DS polysaccharide chains usually exist in a hybrid form. In contrast, the disaccharide unit of Hep/HS is composed of hexuronic acid (GlcA/IdoA) and N-acetyl-D-glucosamine (GlcNAc) (Carlsson et al., 2008). CS/DS and Hep/HS are further modified by various sulfotransferases to form complex structures with different sulfation patterns (Sugahara and Kitagawa, 2000). Different GAG chains with unique sulfation patterns are involved in various physiological and pathological processes (Bishop et al., 2007), such as brain development, wound repair, and cancer metastasis (Poole, 1986; Afratis et al., 2012; Ghatak et al., 2015). Saccharide chains can be specifically modified via desulfation by sulfatases, making the GAG sulfatases potential tools for the preparation and structure-function analyses of oligosaccharides with unique structures.
Glycosaminoglycan sulfatases belong to a conserved sulfatase family that hydrolyzes specific sulfate esters in saccharide rings (Hanson et al., 2004). Based on the position of the sulfate group selectively removed by sulfatases (Wang et al., 2016), these enzymes can be classified into several types, such as N-acetylgalactosamine-4-O-sulfatase and N-acetylgalactosamine-6-O-sulfatase, which specifically hydrolyze sulfate groups on the C-4 and C-6 positions of GalNAc, respectively, and Δ4,5hexuronate-2-O-sulfatase, which specifically removes C-2 sulfate groups from the unsaturated hexuronic acid derived from the digestion of the β1-4 glycosidic linkage between GlcNAc/GalNAc and GlcA/IdoA units by GAG lyases (Ernst et al., 1995). Based on the sequence similarities, the GAG sulfatases were recently classified in the database SulfAtlas (Barbeyron et al., 2016a). The N-acetylglucosamine-6-O-sulfatases (EC 3.1.6.14) belong to the family S1 of sulfatases (formylglycine-dependent sulfatases), subfamily 6 (family S1_6; eukaryotic enzymes); The N-acetylgalactosamine-6-O-sulfatases (EC 3.1.6.4) belong to the family S1_5 (eukaryotic enzymes) and S1_15 (prokaryotic enzymes); The N-acetylgalactosamine-4-O-sulfatases (EC 3.1.6.12) belong to the family S1_2 (eukaryotic enzymes) and S1_27 (prokaryotic enzymes); The N-sulfoglucosamine-6-O-sulfatases (EC 3.1.6.11) belong to the family S1_11 (prokaryotic enzymes); The iduronate/glucuronate-2-O-sulfatases (EC 3.1.6.13/18) belong to the family S1_7 (eukaryotic enzymes) and the Δ4,5hexuronate-2-O-sulfatases belong to the family S1_9 (prokaryotic enzymes). Finally, the N-sulfoglucosamine-2-N-sulfatases (EC 3.10.1.1) belong to the family S1_8 (prokaryotic and eukaryotic enzymes). However, most of the identified GAG sulfatases from animals and bacteria only efficiently act on the sulfate groups at the terminal disaccharides of GAG chains and are therefore referred to exo-sulfatases (Sugahara and Kojima, 1996; Raman et al., 2003; Myette et al., 2009; Malleron et al., 2012). In recent years, several endo-sulfatases discovered in mammals (Dhoot et al., 2001; Morimoto-Tomita et al., 2002) and bacteria (Ulmer et al., 2014; Wang et al., 2015) were found to remove internal sulfate groups from polysaccharides. GAG sulfatases with distinct degradation patterns play a vital role in polysaccharide degradation, during which endo-sulfatases remove sulfate groups from GAGs to facilitate the cleavage of polysaccharide chains by GAG-degrading enzymes (Wang et al., 2015). The resulting oligosaccharides, particularly disaccharides, are further desulfated by exo-sulfatases and subsequently hydrolyzed by glucuronyl hydrolases to produce monosaccharides and sulfates, which may be used as carbon and sulfur sources for cell growth (Ernst et al., 1995; Yamada, 2015).
Arylsulfatases, including GAG-specific sulfatases, possess highly conserved N-terminal Cys/Ser-X-Pro-X-Arg sequences. The conserved Cys/Ser residue is oxidized to an aldehyde, FGly (α-formylglycine), through post-translational modification (Dierks et al., 1998; Boltes et al., 2001). The FGly residue is the critical catalytic residue in the sulfatase catalytic center (Schmidt et al., 1995; Recksiek et al., 1998). To date, only a few bacterial sulfatases acting on CS/DS have been reported, including 4-O-sulfatase (S1_27; GenBankTM Accession No. AJK90566) from Vibrio sp. FC509 (Wang et al., 2015), 4-O-sulfatase BT_3349 (S1_27; AAO78455) from Bacteroides thetaiotaomicron VPI-5482, 2-O-sulfatase BT_1596 (S1_9; NP_810509) from Bacteroides thetaiotaomicron VPI-5482 (Ulmer et al., 2014), 2-O-sulfatase Phep_2825 (S1_9; WP_015808635) from Pedobacter heparinus DSM 2366 (Myette et al., 2003), and 6-O-sulfatase BT_3333 (S1_15; NP_812245) from Bacteroides thetaiotaomicron VPI-5482. The rare protein sequences characterized in databases including GenBank and SulfAtlas do not provide enough information for the reliable assignment of GAG sulfatases via bioinformatic analysis. Therefore, although some candidate genes are predicted to be GAG sulfatases, their degradation specificity may be falsely annotated. As GAG sulfatase is an essential enzyme in the degradative metabolism and structural analysis of GAGs, it is important to identify additional types of sulfatases to enrich the sulfatase database.
In this study, a Δ4,5hexuronate-2-O-sulfatase, designated PB2SF, was identified from the Gram-negative marine bacterium Photobacterium sp. FC615, which is the first reported Photobacterium that can utilize multiple GAGs. The catalytic mechanism of this enzyme was investigated via homology modeling and site-directed mutagenesis. On this basis, we predicted another 60 Δ4,5hexuronate-2-O-sulfatases in GenBank using a bioinformatic method, and we report their signature sequences. To confirm our hypothesis, another novel 2-O-sulfatase with low identity to PB2SF was also cloned and identified.
Materials and Methods
Materials and Bacterial Strains
The strains and plasmids used in this study are listed in Table 1. PrimeSTARTM HS DNA polymerases, restriction endonuclease and other genetic engineering enzymes were purchased from Takara, Inc. (Dalian, China). A Genome Extraction Kit was purchased from Tiangen Biotech, Co., Ltd. (Beijing, China). Fast Mutagenesis System Kit was purchased from TransGen Biotech, Co., Ltd. (Beijing, China). Standard GAG unsaturated disaccharides were purchased from Iduron (Manchester, United Kingdom). CS-C and CS-D from shark cartilage, CS-E from squid cartilage and DS from porcine skin were purchased from Seikagaku, Corp. (Tokyo, Japan). Hyaluronic acid (HA) from Streptococcus equi (15–30 kDa), CS-A from bovine trachea, Hep and HS from porcine intestinal mucosa, chondroitinase ABC (CSase ABC) (EC 4.2.2.20/21), 2-aminobenzamide (2-AB) and cyanoborohydride (NaBH3CN) were purchased from Sigma-Aldrich, Inc. CS tetrasaccharides were prepared by digestion of CS-D using CSase ABC, followed by gel-filtration chromatography in a SuperdexTM Peptide 10/300 GL column, as described previously (Wang et al., 2015). Formula A reef salt was purchased from Qingdao Goe Haida Sea Salt, Co., Ltd. (Qingdao, China). All other chemicals and reagents were of the highest quality available.
Isolation of Marine CS-Degrading Bacteria
Coastal sediment samples were collected from Jiaozhou Bay, near Qingdao city in Shandong province, China. Artificial seawater was prepared by dissolving 100 g of formula A reef salt in 3000 ml of water. The seawater was subsequently filtered through a 0.45 μm membrane filter. Sole carbon source culture medium was prepared by dissolving 0.05% (w/v) CS-C, 0.15% (w/v) (NH4)2SO4, and 1.5% (w/v) agar in artificial seawater at pH 7.2. After incubation at 30°C for 72 h, colonies on the sole carbon source culture medium were randomly selected and transferred to fresh plates for further isolation. The polysaccharide-degrading abilities of the isolates were assayed via liquid culture medium without agar using CS-A, CS-C, CS-D, CS-E, DS, or HA as the sole carbon source at a final concentration of 0.5% (w/v). Bacterial growth was evaluated by measuring the absorbance at 600 nm. The concentrations of various GAGs in the culture medium were analyzed via the carbazole reaction (Bitter and Muir, 1962).
The genomic DNA of the CS-degrading bacterium was prepared using the Genome Extraction Kit (Tiangen Biotech, Co., Beijing, China). The bacterial 16S rRNA gene sequence was amplified with the bacterial universal primer pair 27F (5′-GAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-AAGGA GGTGATCCAGCC-3′). Gel-recovered polymerase chain reaction (PCR) products were cloned into the pEASY-Blunt Simple Cloning Vector (TransGen) for sequencing. The sequence was analyzed against the GenBank database using the online BLAST program to search for the most similar sequences, as described in previous papers (Han et al., 2014). A phylogenetic tree was generated using the neighbor-joining method of Kumar and coworkers with MEGA version 7.0 (Kumar et al., 2016).
Sequence Analysis of Genes and Proteins
The draft genome of Photobacterium sp. FC615 was sequenced at Meiji Biotech, Inc. (Shanghai, China) using Roche Applied Science 454 GsFLX, Illumina GAIIx technology. The sequence of Photobacterium sp. FC615 was annotated at Oak Ridge National Laboratory using their genome annotation pipeline. This included the application of a number of annotation programs, beginning with open reading frames (ORF) prediction using Prodigal, followed by manual annotation using the JGI Gene PRIMP pipeline. Automated protein function prediction was then performed using a number of databases, including the protein domains (Pfam), UniProt, TIGRFAMs, KEGG, InterPro, and COG databases; metabolic reconstruction analysis was conducted using PRIAM; tRNA prediction was carried out using tRNAscan-SE; and rRNA prediction was performed using RNAmmer as described (Han et al., 2014).
The GC content of the PB2SF ORF was calculated using BioEdit version 7.0.5.3. The molecular mass of the protein was estimated with the peptide mass tool on the ExPASy server of the Swiss Institute of Bioinformatics. Secretion signal peptides were identified using the SignalP 4.1 server. A search for similar sulfatase sequences was performed with the BLASTp algorithm online. Multiple sequence alignment and phylogenetic analysis were performed using MEGA.
Heterologous Expression of the PB2SF Gene
To express PB2SF in E. coli strains, the full-length gene without signal peptide was amplified using the primers 5′-CATATGTC CCCGGCTACTCAAACAACC-3′ (forward) and 5′-CTCGAG AGTCACTGTGTCAGCCTCTTTG-3′ (reverse). Primer pairs containing restriction enzyme sites were designed according to the insert site sequences of the expression plasmid pET-30a (+) (Invitrogen). PCR was carried out using high-fidelity Prime STARTM HS DNA polymerase (Takara, Dalian, China). Gel-recovered PCR products were cloned into the expression vector pET-30a (+) following the T7 promoter, and a His6 tag was added at the C-terminus of the protein. The recombinant expression plasmid (pET-30a-PB2SF) was transferred to E. coli BL21 (DE3) cells. The integrity of the nucleotide sequence of the constructed recombinant plasmid was confirmed via DNA sequencing.
E. coli BL21 cells harboring the recombinant plasmid (pET-30a-PB2SF) were cultured in 100 ml of LB broth at 37°C. After the cell density reached an A600 of 0.8–1.0, the inducer isopropyl 1-thio-β-D-galactopyranoside was added to the broth at a final concentration of 0.05 mM to initiate the expression of the target protein. After 24 h of additional cultivation at 16°C, the cells were harvested via centrifugation at 8000 g for 10 min, washed twice using ice-cold buffer A [50 mM Tris-HCl, 150 mM NaCl (pH 8.0)], resuspended in buffer A, and disrupted through sonication (50 repetitions, 5 s) in a low-temperature environment. The crude enzymes were collected after centrifugation at 15,000 g for 30 min.
To purify PB2SF, the soluble crude enzyme was loaded onto a column packed with nickel-SepharoseTM 6 Fast Flow (GE Healthcare). Then, the column was washed with buffer A containing 50 mM imidazole to remove impurities, and PB2SF was finally eluted from the Ni-NTA column with buffer A containing 200 mM imidazole. The eluate containing PB2SF was concentrated using an Amicon Ultra 0.5-ml 10K unit (Millipore) and then loaded onto a SuperdexTM 200 10/300 GL column and eluted with buffer A at a flow rate of 0.4 ml/min. The absorbance of the eluates was monitored at 280 nm, and the largest peak was collected. The purified protein was analyzed through SDS-PAGE in 13.2% polyacrylamide gels according to Sambrook and Russell (2006). Coomassie brilliant blue R-250 was used to stain the proteins in gels, and the protein concentration was determined via the Folin-Lowry method (Bramhall et al., 1969).
Substrate Specificity Assay for PB2SF
To determine the substrate specificity of the enzyme, different types of unsaturated disaccharides were dissolved in deionized water to prepare stock solutions (300 pmol/μl) including the ΔC unit [Δ4,5HexUAβ1-3GalNAc (6S)], ΔA unit [Δ4,5HexUAβ1-3GalNAc (4S)], ΔD unit [Δ4,5HexUA(2S)β1-3GalNAc(6S)], and ΔT unit [Δ4,5HexUA(2S)β1-3GalNAc(4S,6S)], as well as Hep/ HS unsaturated disaccharides [Δ4,5HexUAα1-4GlcNAc(6S), Δ4,5HexUAα1-4GlcNS, Δ4,5HexUAα1-4GlcNS(6S), Δ4,5Hex UA(2S)α1-4GlcNS, and Δ4,5HexUA(2S)α1-4GlcNS(6S)]. Each stock solution (10 μl) was mixed with 30 μl of 150 mM Na2HPO4-NaH2PO4 buffer (pH 8.0), 40 μl of deionized water and 10 μl of appropriately diluted enzyme (2 μg), and the solutions were incubated at 30°C for 12 h. Each enzymatic reaction included a negative control in which the active enzyme was substituted with boiled inactive enzyme. Following the enzymatic reaction, the sulfatase-treated disaccharide solutions were heated in boiling water for 10 min and then cooled in ice-cold water for 5 min. Thereafter, the reaction solutions were centrifuged at 15,000 g for 10 min, and the supernatants were collected and labeled with 2-AB in the presence of sodium cyanoborohydride, as described by Bigge et al. (1995). Next, Free 2-AB was removed through extraction with chloroform. The labeled samples were individually analyzed via anion-exchange HPLC in a YMC-Pack PA-G column (YMC-Pack PA, Kyoto, Japan) and were eluted with a linear gradient from 16 to 460 mM NaH2PO4 over 60 min at a flow rate of 1.0 ml/min at room temperature. The eluates were monitored by measuring the absorbance using a fluorescence detector at excitation and emission wavelengths of 330 and 420 nm, respectively, as described previously (Wang et al., 2015). Disaccharides were identified and quantified based on comparison with authentic unsaturated disaccharides.
To investigate whether this enzyme acts on the saturated hexuronate-2-O-sulfate, a CS tetrasaccharide, Δ4,5HexUAβ1-3GalNAc(4S)β1-4GlcUA(2S)β1-3GalNAc(6S), was prepared as described in Section “Materials and Bacterial Strains” and was used as a substrate for the activity assay described above for disaccharides.
Enzymatic Characterization of PB2SF
To determine the optimal temperature for enzyme activity, an aliquot of the ΔD unit (20 nmol) was incubated with 0.1 μg of PB2SF in 50 mM NaH2PO4-Na2HPO4 buffer (pH 8.0) in a total volume of 100 μl at temperatures ranging from 0 to 70°C for 10 min. After the optimum temperature was achieved, the optimal pH assay was carried out in buffers with different pH levels, including 50 mM HAc-NaAc buffer (pH 5.0–7.0), 50 mM NaH2PO4-Na2HPO4 buffer (pH 6.0–8.0), and 50 mM Tris-HCl buffer (pH 7.0–10.0) at 40°C for 10 min. The effects of metal ions or a chelating agent (5 mM) and the NaCl concentration (0–1 M) on the reaction rate of PB2SF were investigated under optimal reaction conditions (50 mM HAc-NaAc buffer pH 6.0 at 40°C). Finally, to investigate the thermostability of PB2SF, the enzyme in 50 mM HAc-NaAc buffer (pH 6.0) was preincubated at 0–60°C for 30–120 min, after which the residual activity was determined under optimum conditions. All reactions were performed in triplicate, and enzyme-treated samples were analyzed via anion-exchange HPLC in a YMC-Pack PA-G column (YMC-Pack PA, Kyoto, Japan) and eluted with a linear gradient from 16 to 460 mM NaH2PO4 over 60 min at a flow rate of 1.0 ml/min. The eluates were monitored by measuring the absorbance at 232 nm (Yamagata et al., 1968). The effects of pH and chemical reagents on the activity of PB2SF at 25°C were analyzed using the same method as described for 40°C.
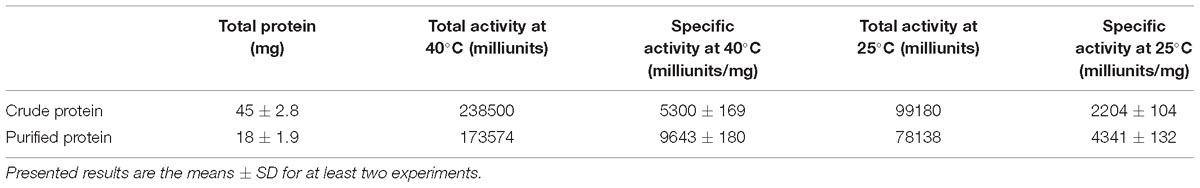
After biochemical characterization of PB2SF, its enzymatic activity against ΔD unit disaccharides was determined in the optimum buffer (50 mM HAc-NaAc buffer, pH 6.0) at 40 and 25°C. One unit of enzyme was defined as the amount of enzyme required to produce 1 μmol of free sulfate per minute.
Tertiary Structure Modeling and Key Catalytic Amino Acid Analysis of the PB2SF
The protein sequence of PB2SF was uploaded, and a three-dimensional model of PB2SF was built by the protein structure homology-modeling server SWISS-MODEL online1 (Biasini et al., 2014) using the structure of bacterial 2-O-sulfatase BT_1596 as a template (PDB code: 3b5q) (Ulmer et al., 2014). Structural figures were created and rendered using the program PyMOL (Schrödinger LLC2).
Mutants of PB2SF were produced by using the Fast Mutagenesis System Kit (TransGen) as described by Prabhakar et al. (2005). The primer sequences used for the mutations are presented in Table 2. The plasmids of the mutants were amplified by transferring them to DMT Chemically Competent Cells (TransGen), and clones were sequenced at Sangon Biotech, Co., Ltd. (Shanghai, China) to confirm the successful introduction of the mutations. Then, correct plasmids were transferred into E. coli BL21 (DE3) cells and expressed, and the enzymatic activity of the mutants was determined under optimum conditions [50 mM HAc-NaAc buffer (PH 6.0), 40°C], as described above for the wild-type enzyme PB2SF.
Prediction of Δ4,5Hexuronate- 2-O-Sulfatases
The sequences of N-acetylgalactosamine-4-O-sulfatase (endoVB4SF, GenBankTM Accession No. AJK90566) (Wang et al., 2015), N-acetylgalactosamine-4-O-sulfatase (BT_3349, AAO78 455), Δ4,5hexuronate-2-O-sulfatase (BT_1596, NP_810509) (Ulmer et al., 2014), Δ4,5hexuronate-2-O-sulfatase (Phep_2825, WP_015808635) (Myette et al., 2003), and N-acetylgalactosamine-6-O-sulfatase (BT_3333, NP_812245) (Ulmer et al., 2014) were downloaded from the NCBI protein sequence database.
Similarity searches of sulfatase sequences were performed with the BLASTp program using query sequences of reported GAG 2-O-sulfatases Phep_2825, PB2SF, and BT_1596. One hundred sequences with greater than 40% identity to each query gene were downloaded from the NCBI protein database. Among the retrieved sequences, some from different strains even species were actually the same gene. To simplify the analysis, only one of identical sequences was reserved and other “redundant identical sequences” were manually deleted. Finally, a total of 60 potential Δ4,5hexuronate-2-O-sulfatases were ultimately obtained. Then, the multiple sequence alignments were carried out using ClustalW and the phylogenetic analysis was performed using the neighbor-joining method in MEGA.
To confirm the activity of these potential 2-O-sulfatases, one (GenBankTM Accession No. WP_083232406) of these enzymes with relatively low identity to PB2SF and the two other reported Δ4,5hexuronate-2-O-sulfatases were recombinantly expressed in the expression plasmid pET-30a (+) and characterized as described for PB2SF above.
Signature Sequence Analysis of the Δ4,5Hexuronate-2-O-Sulfatase Family
To investigate the conserved signature regions of Δ4,5hexuronate-2-O-sulfatases, the sequences of bacterial CS/DS sulfatases, including three characterized 2-O-sulfatases (PB2SF from Photobacterium sp. FC615, BT_1596 from Bacteroides thetaiotaomicron VPI-5482 and Phep_2825 from Pedobacter heparinus DSM 2366), 60 potential 2-O-sulfatase genes predicted as described above and 234 sequences of family S1_9 downloaded from database SulfAtlas, were aligned using BioEdit. Then, highly conserved signature regions were selected, and logo signature sequences were built by WebLogo3 (Crooks et al., 2004).
Results
Isolation of a CS-Degrading Marine Bacterium
A novel bacterium strain was isolated from marine sediment using shark cartilage CS-C as the sole carbon source. BLASTn analysis of the 16S rRNA gene (GenBankTM Accession No. MK478813) showed that this bacterium belonged to the Photobacterium genus (Figure 1). The bacterium grew vigorously in simple medium with CS-A, CS-C, CS-D, CS-E, DS, and HA. Carbazole reaction analysis showed that the concentrations of various GAGs in the medium were dramatically decreased after culturing for 24 h (data not shown), suggesting that Photobacterium sp. FC615 can efficiently utilize various GAGs as a sole carbon source and contains GAG-degrading enzymes.
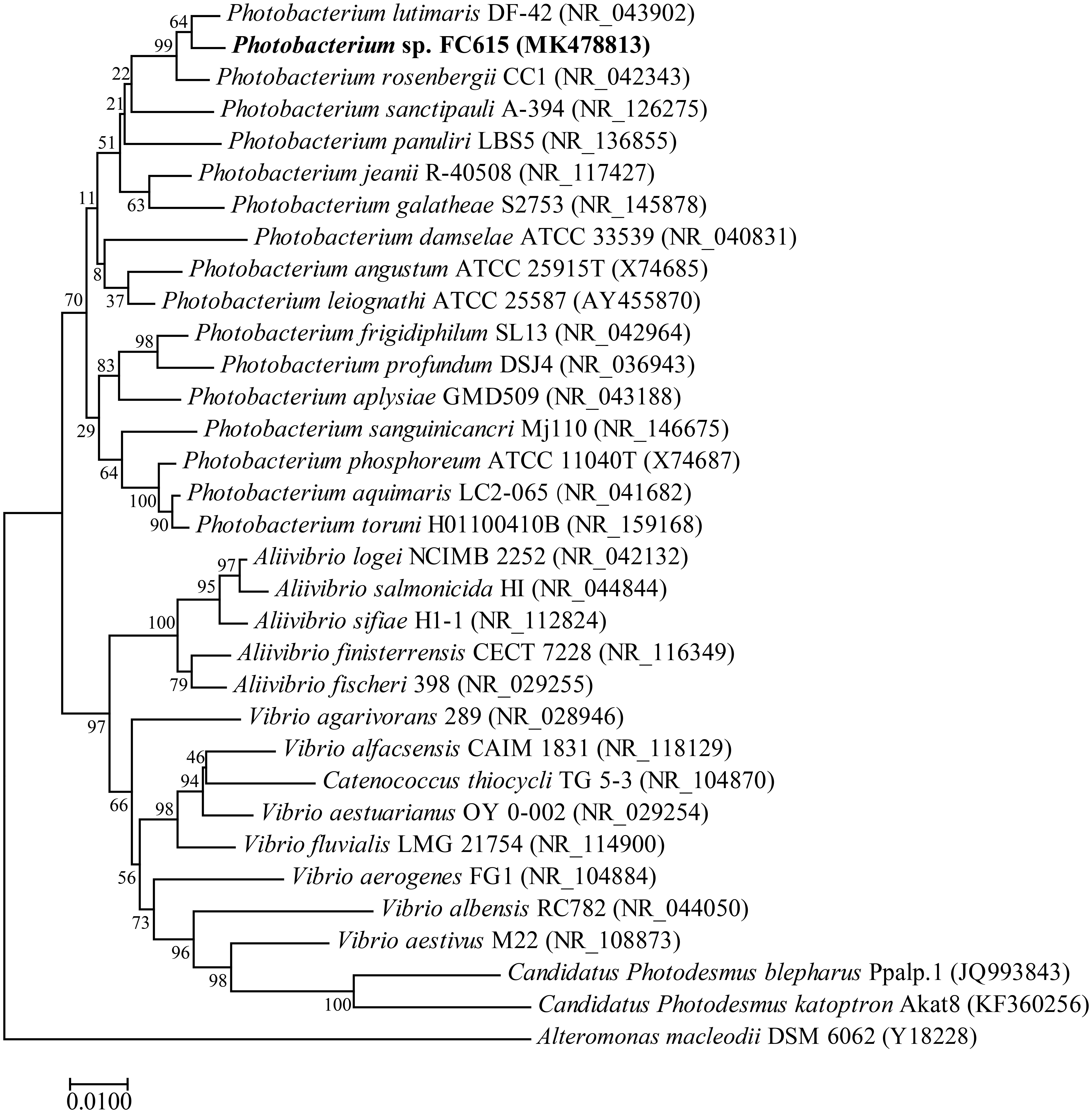
Figure 1. Phylogenetic position of Photobacterium sp. FC615 based on the 16S rRNA gene sequences. The phylogenetic analysis was performed using the neighbor-joining method in MEGA, and associated taxa clustered together in the bootstrap test of 1000 replicates.
Features of PB2SF Gene and Protein Sequence
To identify novel GAG-degrading enzymes, the genome of this isolated Photobacterium sp. FC615 was sequenced. The draft genome was ∼6.3 Mb in size and possessed at least 6400 ORFs, among which three chondroitinases and 27 sulfatase genes were predicted. One putative sulfatase gene pb2sf (GenBankTM Accession No. MH321063), which was coded by ORF 2351, was 1533 bp in length, with 52.8% GC content (G + C %), and encoded a protein (PB2SF) with a molecular weight of ∼56.8 kDa. The theoretical isoelectric point (pI) of this protein was 5.63. SignalP 4.1 server analysis revealed the presence of an N-terminal signal peptide containing 33 amino acid residues.
A BLASTp search revealed that PB2SF shared relatively low sequence identity with most identified GAG sulfatases except for the 2-O-sulfatase BT_1596 from Bacteroides thetaiotaomicron. It shared the highest sequence identity (46%, query cover 90%) with BT_1596 (GenBankTM Accession No. NP_810509) (Ulmer et al., 2014). In addition, it shared 32% sequence identity (query cover 86%) with the 2-O-sulfatase Phep_2825 from Pedobacter heparinus DSM 2366 (Myette et al., 2003) and only 25% sequence identity (query cover 72%) with human iduronate-2-O-sulfatase (S1_7; AAA92014) (Demydchuk et al., 2017).
Molecular Cloning and Recombinant Expression of PB2SF
The recombinant protein PB2SF was expressed in E. coli BL21 (DE3) cells and analyzed using SDS-PAGE (Sambrook and Russell, 2006). The results showed that BL21 (DE3) cells harboring the pET-30a-PB2SF plasmid yielded soluble protein (∼2 g/L). The protein was purified via nickel-nitrilotriacetic acid affinity chromatography and gel-filtration chromatography. SDS-PAGE showed that the purity of the PB2SF protein was greater than 98%, and the initial protein concentration was ∼5 mg/ml (Figure 2).
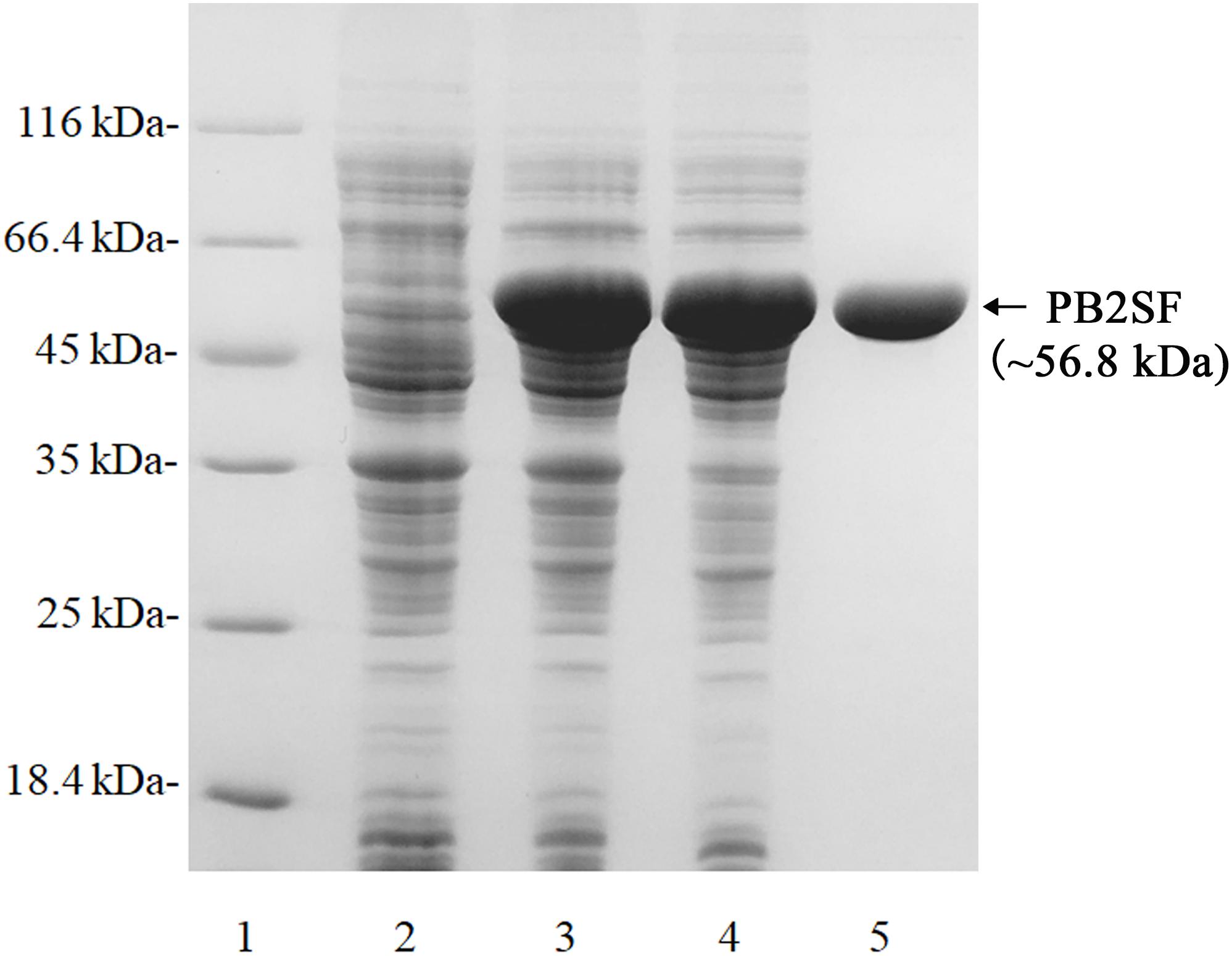
Figure 2. SDS-PAGE of the recombinant PB2SF from E. coli. PB2SF was purified through Ni2+-chelation chromatography and gel-filtration chromatography. Enzyme purity following each fractionation step was assessed by SDS-PAGE using 13.2% polyacrylamide gels, followed by staining with Coomassie brilliant blue. Lane 1, unstained protein molecular weight marker SM 0431 (Thermo); Lane 2, uninduced cell lysate; Lane 3, induced cell lysate; Lane 4, supernatant of the induced cell lysate; Lane 5, purified recombinant PB2SF.
Enzymatic Activity of PB2SF Toward Oligosaccharides
The substrate specificity of PB2SF was characterized using unsaturated CS/DS disaccharides with different sulfation patterns. The results showed that the ΔC and ΔA units were not altered (Figures 3A,B), but the ΔD unit (Figure 3C, top) and ΔT unit (Figure 3D, top) were completely transformed to a ΔC unit (Figure 3C, bottom) and ΔE unit (Figure 3D, bottom), respectively. Likewise, the 2-O-sulfate groups on the unsaturated hexuronic acid of Hep/HS disaccharides [Δ4,5HexUA(2S)α1- 4GlcNS, and Δ4,5HexUA(2S)α1-4GlcNS(6S)] were also selectively removed by this enzyme (Figure 3E, bottom). These results demonstrate that the enzyme is a hexuronate- 2-O-sulfatase.
To determine enzyme activity toward the inner 2-O-sulfate esters of oligosaccharides, the CS-D-derived tetrasaccharide Δ4,5HexUAβ1-3GalNAc(4S)β1-4GlcUA(2S)β1-3GalNAc(6S) was used as a substrate for treatment with PB2SF. PB2SF did not affect the sulfation pattern of this tetrasaccharide (Supplementary Figure S1), indicating that this enzyme is a strict exo-sulfatase that specifically catalyzes the hydrolysis of 2-O-sulfate on the Δ4,5hexuronate residues located at the non-reducing end of GAG chains and is a Δ4,5hexuronate-2-O-sulfatase.
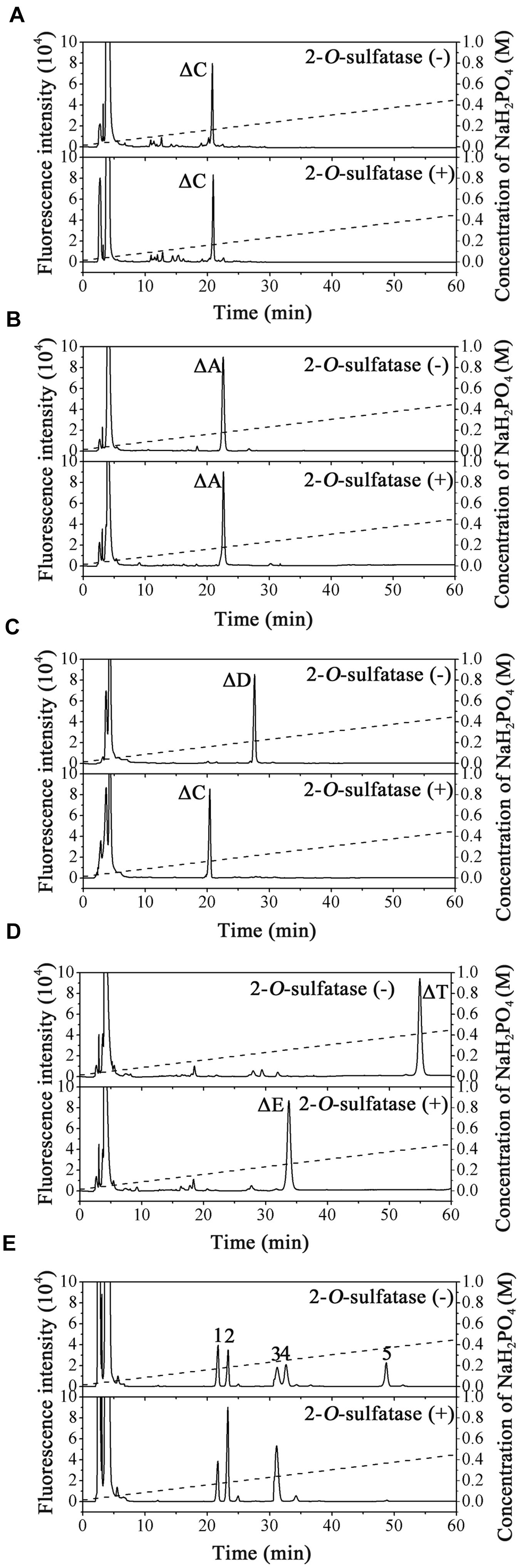
Figure 3. Analysis of the final products of unsaturated GAG disaccharides treated with PB2SF. Unsaturated CS/DS disaccharides ΔC (A), ΔA (B), ΔD (C), and ΔT (D) and unsaturated Hep/HS disaccharides (E) were incubated without (top) or with (bottom) PB2SF, labeled with 2-AB and then analyzed via anion-exchange HPLC, as described in Section “Materials and Methods.” In (A–D), the elution positions of the standard disaccharides are indicated: ΔO (Δ4,5HexUAβ1-3GalNAc), ΔC [Δ4,5HexUAβ1-3GalNAc(6S)], ΔA [Δ4,5HexUAβ1-3GalNAc(4S)], ΔD [Δ4,5HexUA(2S)β1-3GalNAc(6S)], ΔE [Δ4,5HexUAβ1-3GalNAc(4S,6S)], and ΔT [Δ4,5HexUA(2S)β1-3GalNAc(4S,6S)]. In (E), the elution positions of the following standard disaccharide components are indicated by numbers, (1) Δ4,5HexUAα1-4GlcNAc(6S); (2) Δ4,5HexUAα1-4GlcNS; (3) Δ4,5HexUAα1-4GlcNS(6S); (4) Δ4,5HexUA(2S)α1-4GlcNS; and (5) Δ4,5HexUA(2S)α1-4GlcNS(6S).
Biochemical Properties of the PB2SF
After determining substrate specificity, the optimal catalytic reaction conditions for PB2SF were further defined. Using the ΔD unit as a substrate, enzymatic reactions were carried out under different conditions, varying single factors, including temperature, pH, and the chemical components, as described in Section “Materials and Methods.” PB2SF exhibited the highest reaction rate at 40°C, and the rate did not vary greatly from 40 to 50°C (Figure 4A). The effect of the pH on enzymatic activity was investigated at the optimum temperature (40°C). The optimum pH was 6.0 in 50 mM HAc-NaAc buffer (Figure 4B). Notably, the PB2SF maintained relatively high activity in acidic buffer, especially in HAc-NaAc buffer. To determine the influence of metal ions on enzymatic activity, various metal ions and EDTA were individually added to the reaction buffer at a final concentration of 5 mM. Na+ and Li+ promoted enzymatic activity, but Ni2+, Ba2+, Hg2+, and Pb2+ strongly inhibited activity. In addition, EDTA, a divalent metal ion-chelating agent, did not inhibit sulfatase activity (Figure 4C). Considering that the enzyme was isolated from a marine bacterium and that its activity was found to be stimulated by monovalent metal ions, such as Na+ or Li+, it may be a halophilic enzyme. Thus, we further investigated the effect of the salt concentration on enzymatic activity by increasing the concentration of NaCl from 0 to 1 M. The activity was increased by 2.7-fold when the NaCl concentration was increased from 0 to 200 mM, at which point the activity gradually decreased as the salt concentration was increased. However, the relative sulfatase activity was still much higher than that in the buffer in the absence of NaCl until the NaCl concentration reached 800 mM (Figure 4D). Finally, the thermostability of this enzyme was investigated under optimum conditions. The results showed that PB2SF was very stable at 0–10°C (Figure 4E) and it maintained >90% of its activity after pre-incubated at 4°C for 12 h (data not shown). The PB2SF protein was also relatively stable at 20–30°C and the half-life at 30°C was approximately 120 min. In contrast, the stability of the enzyme quickly decreased when the temperature reaches to ≥40°C. Based on the results (Figures 4A,E), the enzyme is much more stable and has relatively high activity at 20–30°C, which should be the preferred conditions for the practical applications of this enzyme. Thus, the effects of pH and chemical reagents on the activity of PB2SF were investigated at 25°C. The results showed that the effects of these factors on the enzyme activity were similar to those obtained at 40°C (Supplementary Figure S2). The specific activity of PB2SF, which was determined in the optimum buffer (50 mM HAc-NaAc buffer, pH 6.0) was 5.3 and 2.2 units/mg of protein at 40 and 25°C, respectively (Table 3).
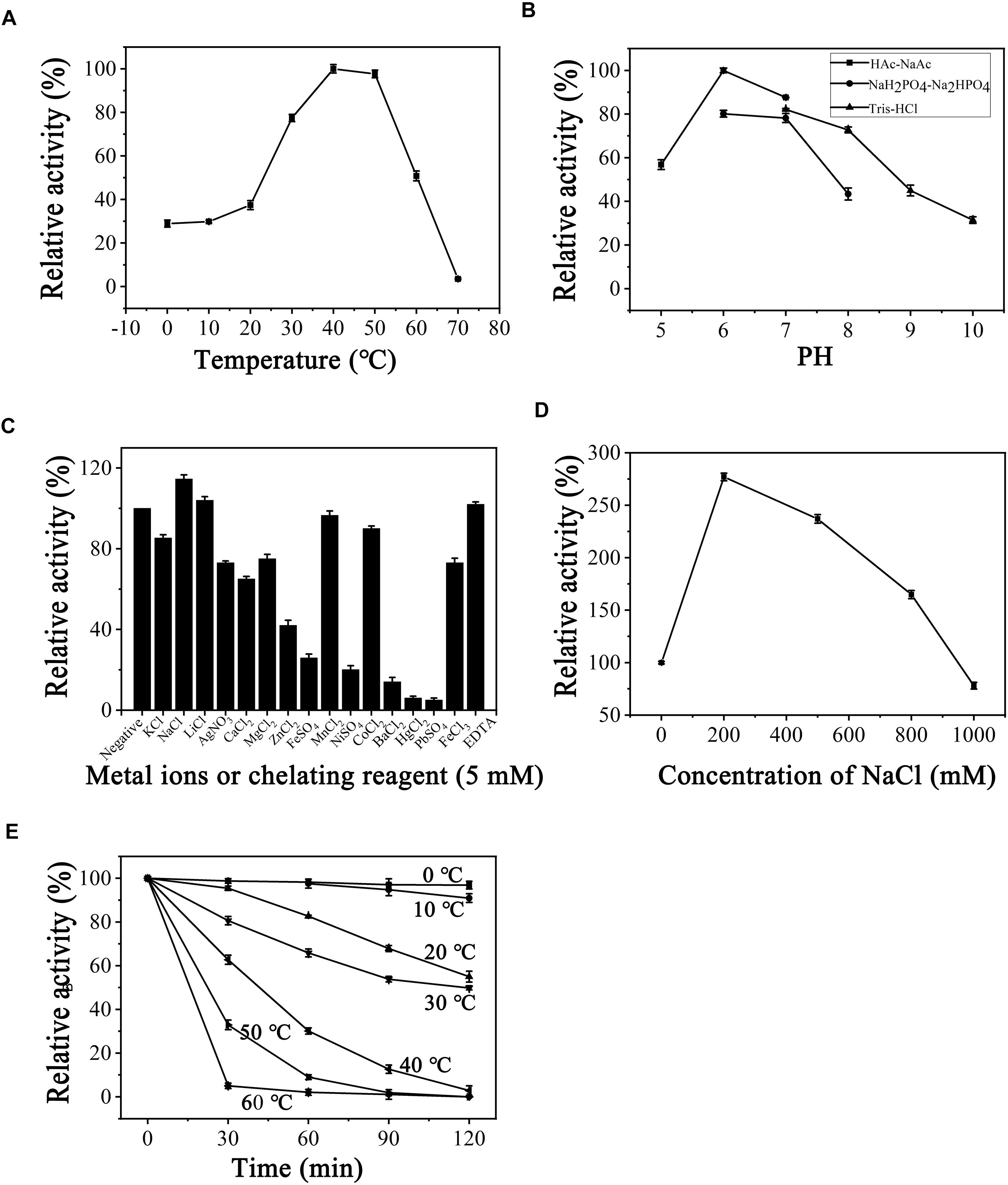
Figure 4. Biochemical conditions for recombinant PB2SF. (A) Effect of temperature. The enzyme activity of PB2SF was measured using the ΔD disaccharide as a substrate in 50 mM NaH2PO4-Na2HPO4 buffer (pH 8.0), at different temperatures for 10 min. The data are shown as percentages of the activity obtained at 40°C (100%). (B) Effect of pH. The activity of PB2SF against ΔD was measured in buffers with pH values from 5 to 10 at 40°C for 10 min. The data are shown as percentages of the activity obtained in the HAc-NaAc buffer at pH 6.0 (100%). (C) Effects of metal ions. The activities of PB2SF against ΔD were measured in the HAc-NaAc buffer (pH 6.0) containing 5 mM of various metal ions at 40°C for 10 min. Data are shown as a percentage of the activity obtained in the buffer without the tested metal ions. (D) Effects of NaCl concentrations. The activity of PB2SF against ΔD was measured in HAc-NaAc buffer (pH 6.0) containing 0–1 M NaCl at 40°C for 10 min. Data are shown as a percentage of the activity obtained in the buffer without NaCl. (E) Thermostability of PB2SF. The enzyme in 50 mM HAc-NaAc buffer (pH 6.0) was preincubated for 0–120 min at temperatures from 0 to 60°C, and the residual activity against ΔD was estimated at 40°C. Data are shown as the activity relative to that of untreated PB2SF. Error bars represent the means of triplicate analyses ± SD.
Site-Directed Mutagenesis of the Catalytic Site
To analyze the key amino acids involved in the catalytic mechanism of PB2SF, a structural model of the sulfatase (Figures 5A,B) was constructed via a protein homology-modeling method using the reported structure of the bacterial 2-O-sulfatase BT_1596 as a template (PDB code: 3b5q), as described in Section “Materials and Methods.” According to the structural model, six potential catalytic amino acids (Asp52, Asn113, Lys141, His143, His205, and His310) around the Cys92 residue (the precursor of FGly residue) were selected and individually mutated to Ala or similar residues to probe their roles in the catalytic mechanism. Analysis of the mutants’ enzymatic activities revealed that only the D52A, H310A, and H310K mutants were totally inactivated, indicating a key role of Asp52 and His310 in the catalytic mechanism (Figure 5C). Additionally, the mutation of His205 to Ala or Lys (H205A or H205K) caused a dramatic decrease in enzymatic activity to less than 5% of the wild-type activity. Notably, although His and Lys are both basic amino acids, the severe reduction in activity observed upon replacing His205 with Lys suggests that the imidazole group of His205, an important nucleophilic group, is essential to the catalytic mechanism. The Lys141 and His143 mutants (K141H, K141A, H143A, and H143K) also exhibited low activity, indicating that both of these sites are also involved in the catalytic mechanism. Taken together, the identification of these key residues provides a basis for future analysis of the catalytic mechanism of bacterial GAG sulfatases.
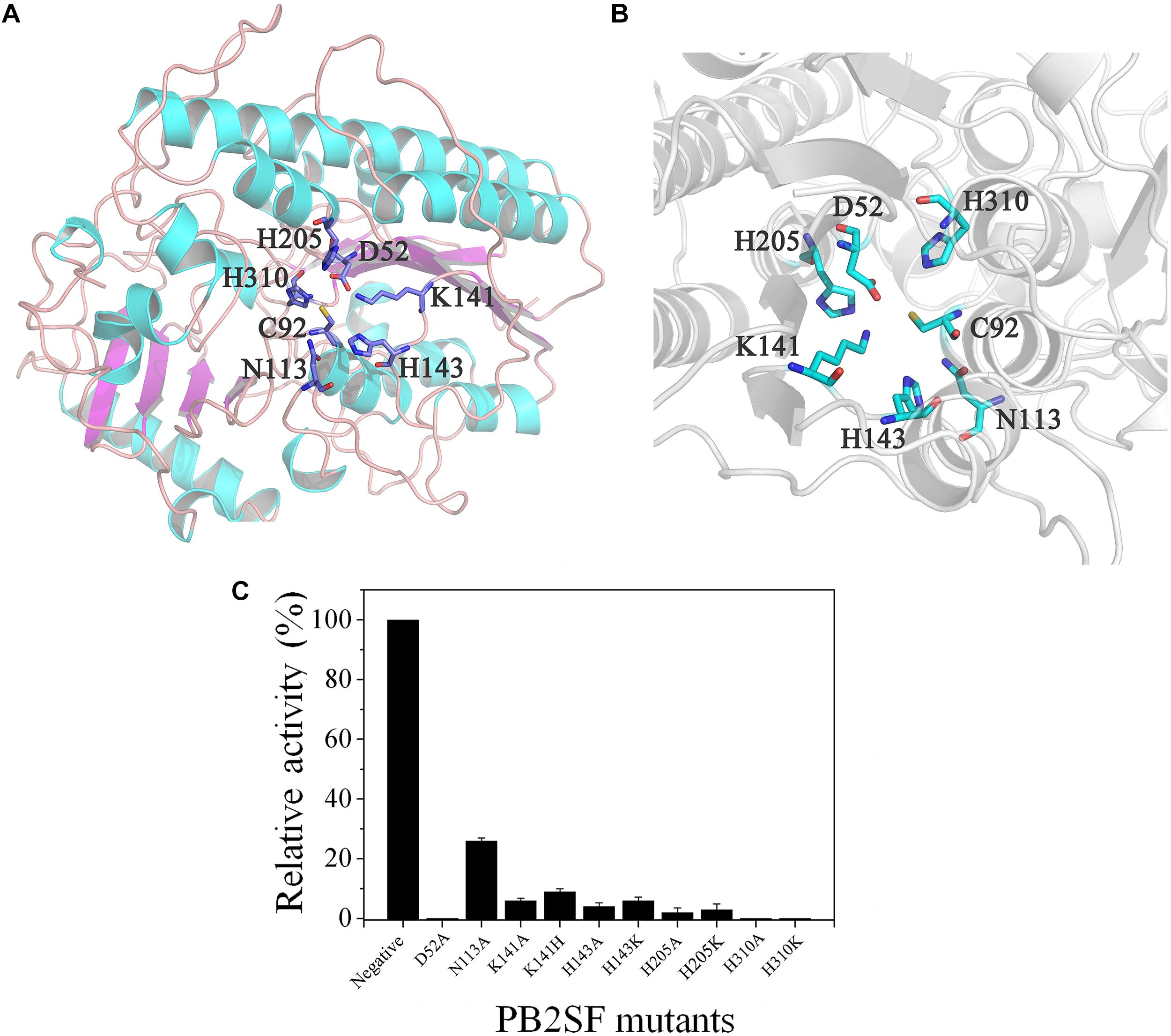
Figure 5. Structural modeling and catalytic residue analysis of PB2SF. (A) Stereo view of the entire three-dimensional model. Amino acids around the active site are shown in stick mode. Notably, the Cys92 residue was not transformed to FGly in structural modeling, as it was in the active form after post-translational modification. (B) More detailed stereo view of the active site region. Figures were created with the program PyMOL. (C) Enzymatic activity analysis of PB2SF mutants. The activities of PB2SF mutants against ΔD disaccharides were measured in HAc-NaAc buffer (pH 6.0) at 40°C for 10 min. Data are shown as a percentage of the activity of wild-type PB2SF under optimal reaction conditions.
Prediction of GAG-Specific Sulfatases via the Bioinformatic Method
To analyze the phylogenetic relationships between different bacterial CS/DS sulfatases, the sequences of the identified bacterial 4-O-sulfatases, 6-O-sulfatases and 2-O-sulfatases, including PB2SF, were aligned and analyzed. The phylogenetic tree showed that sulfatases with similar specificities clustered to form a single branch, although they descended from separate ancestral genes. This information is important for the prediction of novel GAG sulfatases with specific activities based on gene sequences. Based on this finding, additional potential Δ4,5hexuronate-2-O-sulfatases were screened from the GenBank database with the BLASTp program using three query sequences, including PB2SF and the two already reported BT_1596 and Phep_2825 sequences. For each query gene, 100 homogenous sequences were extracted from the database including some identical sequences. After eliminating the redundant identical sequences, 60 candidate genes were obtained. Among the sixty genes, 39 sequences were homologous to Phep_2825, 18 sequences were homologous to PB2SF, 14 sequences were homologous to BT_1596 and 11 sequences were homogenous to both the latter query sequences. Phylogenetic analysis showed that these 60 putative sulfatases clustered with the branches of the 2-O-sulfatase family (Figure 6), which further indicated that they were potential Δ4,5hexuronate-2-O-sulfatases.
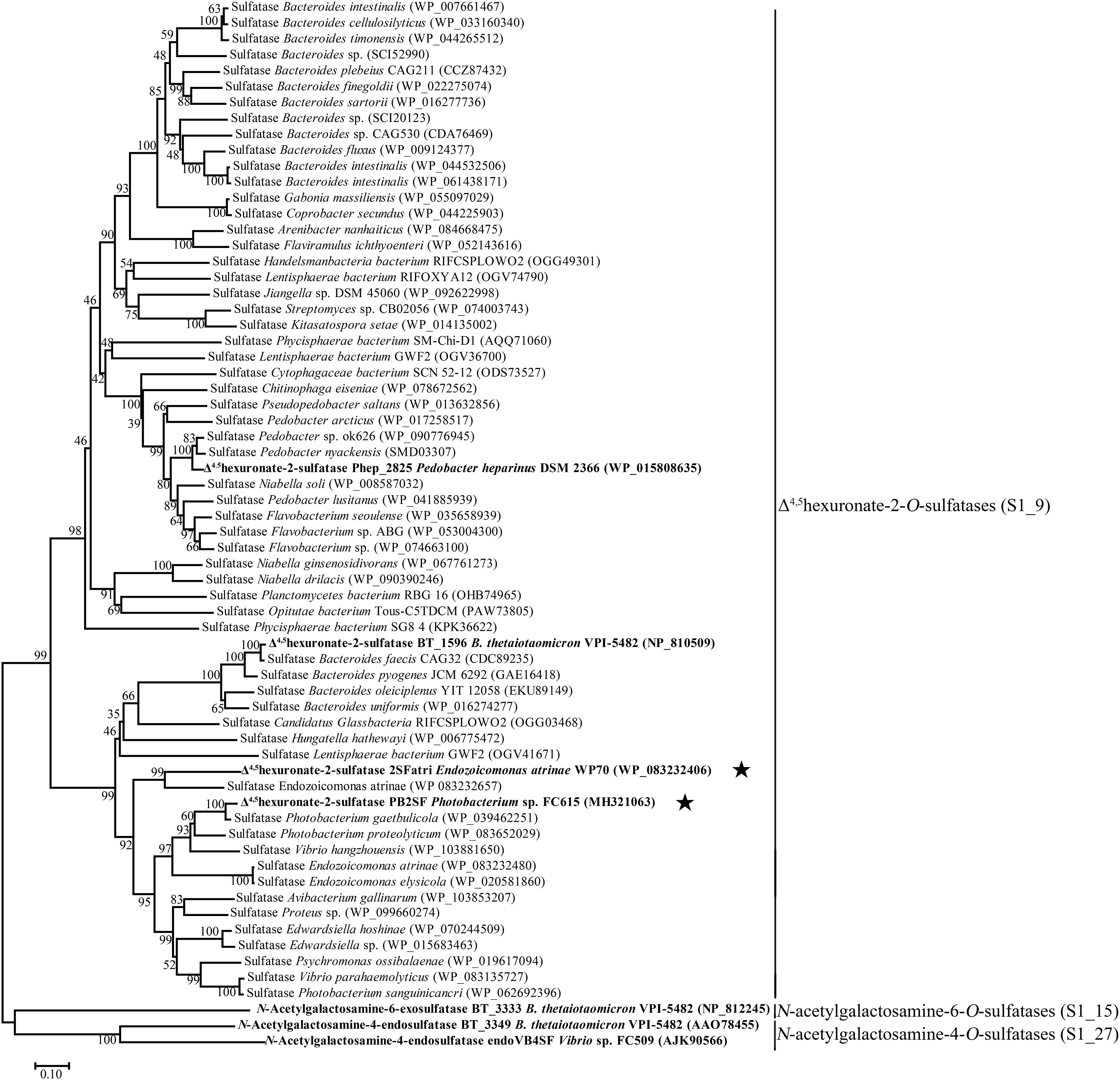
Figure 6. Phylogenetic analysis of the characterized bacterial CS/DS sulfatases and 60 potential 2-O-sulfatases. The neighbor-joining tree was constructed with MEGA. The numbers on the branches indicate the bootstrap confidence values from 1000 replicates. The bar is equal to the distance corresponding to 1 amino acid substitution per 10 amino acid residues. Sulfatases that have been previously reported are indicated in bold. PB2SF and 2SFatri elucidated in this study are labeled with five-pointed stars. Based on the classification of the database SulfAtlas, Δ4,5hexuronate-2-O-sulfatases belong to the family S1_9; N-acetylgalactosamine-4-O-sulfatases and N-acetylgalactosamine-6-O-sulfatases belong to the family S1_27 (EC 3.1.6.12) and S1_15 (EC 3.1.6.4), respectively, which are included as outgroup.
To confirm our predictions, we cloned and expressed one of these 2-O-sulfatase candidate genes. Notably, the putative protein from Endozoicomonas atrinae WP70 (GenBankTM Accession No. WP_083232406), which was annotated as a DUF4976 domain-containing protein in the NCBI protein database, shared 49 and 40% sequence identity with PB2SF and BT_1596, respectively. This putative gene, designated 2SFatri, was cloned into the pET-30a (+) vector, and the recombinant protein was expressed in E. coli BL21 (DE3) cells. SDS-PAGE analysis showed that BL21 (DE3) cells harboring the pET-30a-2SFatri plasmid yielded soluble recombinant protein (∼1.8 g/L) (Supplementary Figure S3). The substrate specificity of 2SFatri was assessed by using various unsaturated GAG disaccharides with different sulfation patterns, as described for PB2SF above. Our experiments clearly showed that, similarly to PB2SF, 2SFatri specifically removed 2-O-sulfate groups from the Δ4,5hexuronate residue (Figure 7), which strongly supports our prediction.
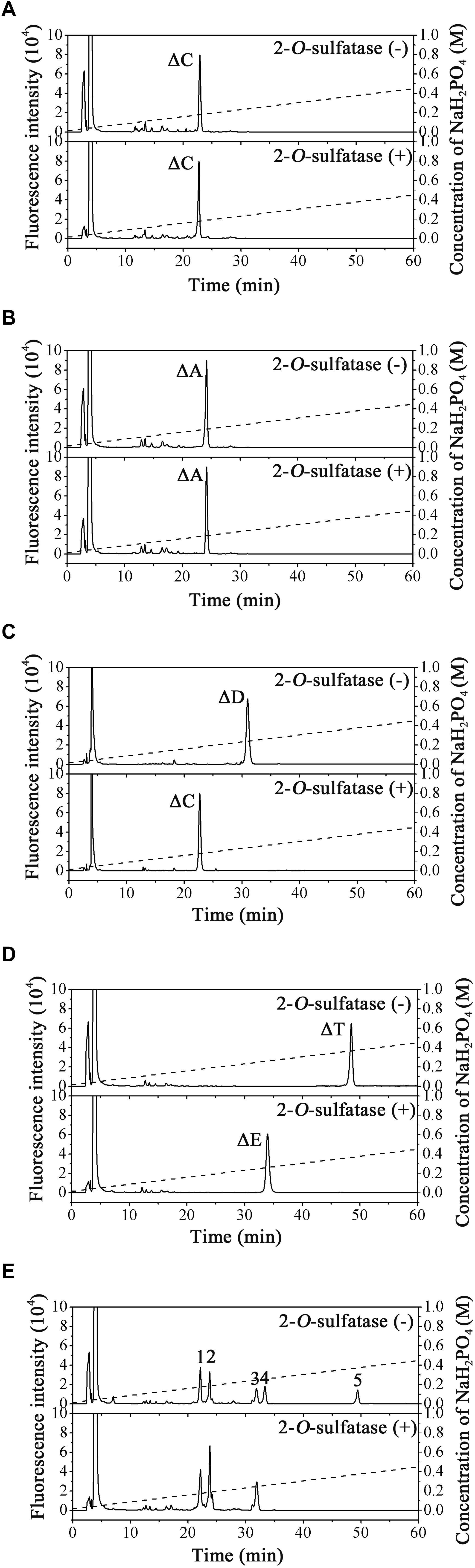
Figure 7. Final product analysis of unsaturated GAG disaccharides treated with 2SFatri. Unsaturated CS/DS disaccharides ΔC (A), ΔA (B), ΔD (C), and ΔT (D) and unsaturated Hep/HS disaccharides (E) were incubated without (top) or with (bottom) 2SFatri and analyzed via anion-exchange HPLC, as described for PB2SF.
Signature Sequences of Δ4,5Hexuronate-2-O-Sulfatases
FGly-dependent sulfatases can be preliminary identified using the signature motifs PF00884 (sulfatase) or PS00523 and PS00149, proposed at PROSITE4. Recently, Barbeyron et al. (2016a) identified three additional conserved FGly sulfatase signatures. However, there has been no specific report about the signature sequences of GAG sulfatases.
In this study, to investigate the signatures of bacterial CS/DS sulfatases, the protein sequences of CS/DS sulfatases identified from bacteria were aligned and analyzed. We found that although all the sulfatases possessed Cys/Ser-X-Pro-X-Arg as a signature sequence at the active site, the conserved sequences near the active site were distinct in each type of CS/DS sulfatase, and sulfatases with specific degradation properties possessed unique signature sequences (Figure 8). To further identify the signature sequences of Δ4,5hexuronate-2-O-sulfatases, we analyzed all identified bacterial 2-O-sulfatases including PB2SF, the 60 potential 2-O-sulfatases reported in this paper and sequences of family S1_9 predicted in SulfAtlas database. We found a series of highly conserved sequences specific to Δ4,5hexuronate-2-O-sulfatases. In the N-terminal domain, there are two conserved regions: and (the superscripts and subscripts denote the percentage of conservation in the alignment and the corresponding amino acid, respectively, at the first position of PB2SF; the catalytic amino acid is indicated in bold; Figures 9A,B). In the middle region of the 2-O-sulfatase sequences, there are also two highly homologous regions: - (Figure 9C) and (Figure 9D). Notably, although these four signature motifs are decentralized in the primary structure, they surround the catalytic key residue and are tightly concentrated at the catalytic center in the tertiary structure. In contrast, a long conserved sequence, , in the C-terminal domain (Figure 9E), located outside of the catalytic cavity, may be involved in the binding site of substrates. Overall, all of the signature sequences together contribute to the formation of the 2-O-sulfatase catalytic center, which indicates that these conserved regions may influence the substrate selectivity of GAG sulfatases. Therefore, we propose that these conserved regions play essential roles during catalysis and have therefore been highly conserved during biological evolution. These homologous sequences cluster onto a single branch in the phylogenetic tree.
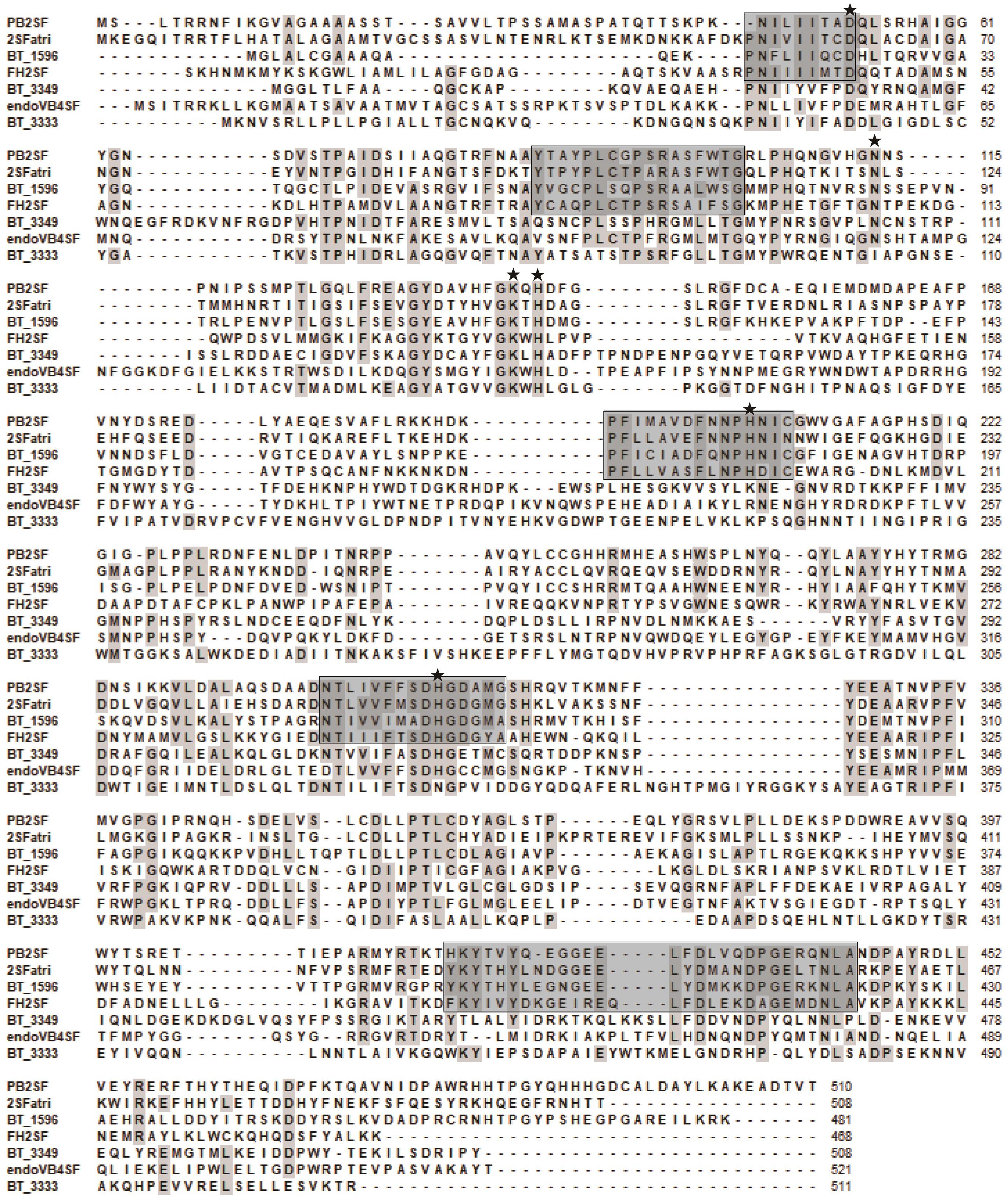
Figure 8. Protein sequence alignment of the characterized bacterial CS/DS sulfatases. Amino acids with partial identity are shaded in gray. Highly conservative amino acid residues of Δ4,5hexuronate-2-O-sulfatase family are indicated with black frames. Sulfatases are abbreviated as follows: BT_3349 from Bacteroides thetaiotaomicron VPI-5482 (GenBankTM Accession No. AAO78455), endoVB4SF from Vibrio sp. FC509 (AJK90566), BT_3333 from Bacteroides thetaiotaomicron VPI-5482 (NP_812245), BT_1596 from Bacteroides thetaiotaomicron VPI-5482 (NP_810509), Phep_2825 from Pedobacter heparinus DSM 2366 (WP_015808635). The mutated amino acids (Asp52, Asn113, Lys141, His143, His205, and His310) indicated in Figures 5A,B were marked by asterisk.
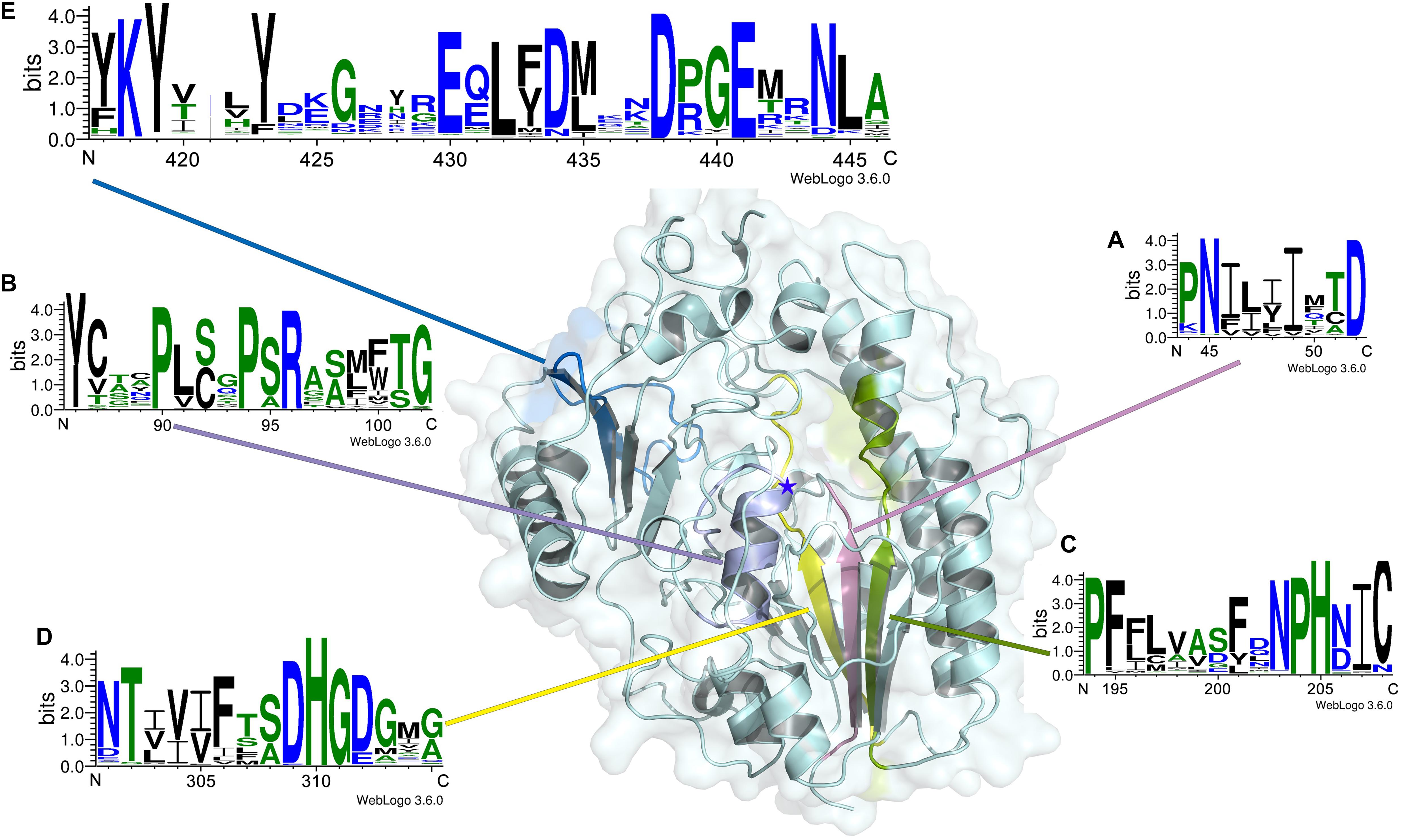
Figure 9. Signature sequence logos of Δ4,5hexuronate-2-O-sulfatases. Signature sequence logos of Δ4,5hexuronate-2-O-sulfatases were created with the WebLogo online server (http://weblogo.threeplusone.com/). The numbers below the logo sequences indicate, at the first position, the corresponding positions of the PB2SF protein sequence. The positions of signature sequences (A–E) in the structural model of PB2SF are indicated in pink, purple, green, yellow, and blue, respectively. The five-pointed star indicates the position of the key residue Cys92.
Discussion
Bacterial GAG sulfatases are essential enzymes for the biodegradation of sulfated GAGs and are potential tools for the structural and functional analysis of complex GAGs. However, only a few GAG sulfatases have been identified from soil and gut bacteria (Yamagata et al., 1968; Myette et al., 2009; Ulmer et al., 2014). Most sulfatases can be preliminary identified using common substrates such as 4-methylumbelliferyl sulfate (4MUS) or p-nitrophenyl sulfate (pNPS) (Nicholls and Roy, 1971), but further identification of GAG-specific sulfatases requires the use of GAG oligo-/polysaccharides with unique structural features as substrates. The structural complexity of substrates and the resulting products presents a large technical challenge to the study of GAG sulfatases. To identify novel enzymatic tools for studying GAG polysaccharides and enrich the database of GAG sulfatases, we searched for GAG sulfatases in marine bacteria and identified a series of novel GAG-specific sulfatases, including the first 4-O-endosulfatase from a marine bacterium, Vibrio sp. FC509 (Wang et al., 2015). The Δ4,5hexuronate-2-O-sulfatase PB2SF reported in this paper was identified from the strain Photobacterium sp. FC615, which was freshly isolated from coastal sediment using CS as the sole carbon source. It specifically hydrolyzes the 2-O-sulfate groups on the unsaturated hexuronate residues at the non-reducing end of CS/DS or Hep/HS oligosaccharides produced by GAG lyases and is similar to Phep_2825 (Myette et al., 2003). However, unlike Phep_2825 whose relative activity quickly decreased as the temperature reached 40°C, PB2SF exhibited the maximal rate at 40°C. Furthermore, the activity of Phep_2825 is strongly inhibited by NaCl concentrations exceeding 100 mM while PB2SF exhibits halophilic characteristics, which is a common feature of many marine-derived enzymes. Interestingly, divalent metal ions such as Ca2+, Mg2+, and Zn2+ did not increase enzymatic activity, and the chelator EDTA did not inhibit enzymatic activity at a concentration of 5 mM. This observation has also been reported in previous studies (Lukatela et al., 1998; Kozak et al., 2015). However, structural studies of various sulfatases have shown that divalent metal ions such as Ca2+ or Mg2+ (Waldow et al., 1999; Boltes et al., 2001) play a role in the catalytic center. Thus, the reason that the activities of PB2SF and other sulfatases were not affected by these metal ions in reaction buffer needs to be investigated further.
The core motif Cys/Ser-X-Pro-X-Arg is conserved in the sulfatase family S1 in SulfAtlas database, and the FGly residue is critical for the catalytic activity of sulfatases. The crystal structures of three human GAG sulfatases, arylsulfatase B (N-acetylgalactosamine-4-O-sulfatase; S1_2) (Bond et al., 1997), GALNS (N-acetylgalactosamine-6-O-sulfatase; S1_5) (Rivera-Colon et al., 2012) and IDS (Iduronate-2-O-sulfatase; S1_7) (Demydchuk et al., 2017), have been solved, and several key catalytic residues, including Asp, His, Lys, and Arg, have been experimentally identified in their protein structures (Waldow et al., 1999; von Bulow et al., 2001). Recently, the cocrystal of bacterial 2-O-sulfatase BT_1596 (S1_9) with unsaturated CS disaccharide was reported (PDB code: 5G2T), which revealed that a series of key residues were involved in the interaction with the disaccharide substrate. However, the role of these catalytic residues remains to be verified through biochemical methods. In this study, a three-dimensional structural model of PB2SF was constructed by homology modeling, and six conserved amino acids (Asp52, Asn113, Lys141, His143, His205, and His310) as well as the key FGly residue were found to form the groove in the catalytic center. These residues are also conserved in the catalytic center of BT_1596, as revealed by its cocrystal with disaccharide substrate. Site-directed mutagenesis analysis showed that His310 and Asp52, similar to FGly, were indispensable for enzymatic activity, and the imidazole group of His310 was also critical. Additionally, the K141A, K141H, H143A, H143K, H205A, and H205K mutants exhibited minimal activity, suggesting that these basic amino acids also participate in the catalytic mechanism and together create a basic microenvironment to facilitate the binding of the negatively charged GAG chains to the active site. In contrast, the N113A mutant maintained 30% activity, indicating that the Asn113 plays a minor role in the catalytic process though it is closely adjacent to the substrate in the structure. Thus, we experimentally demonstrated the importance of conserved residues around FGly in the active site of this bacterial GAG sulfatase.
It is noteworthy that in the draft genome of Photobacterium sp. FC615, 27 sequences were predicted to be sulfatases, but no sequence was annotated to be a Δ4,5hexuronate-2-O-sulfatase based on the sequencing results. To discover novel GAG sulfatases, several putative sulfatase genes in the genome were recombinantly expressed and characterized, among which the 2-O-sulfatase PB2SF was identified. Bacterial GAG sulfatases cannot be specifically and properly annotated in very commonly used databases such as GenBank, because few bacterial GAG sulfatases have been identified, and there is a lack of systematic studies on this class of enzyme. Notably, an interrogation in professional sulfatase database SulfAtlas showed that the PB2SF gene was homologous to the genes in family S1_9 but not others, indicating the potential of the SulfAtlas for the annotation of sulfatases. However, we found that most Δ4,5hexuronate-2-O-sulfatases predicted in this study are not included in the family S1_9 of SulfAtlas, indicating that this database still needs to be improved through more studies on sulfatases in particular the identification of novel sulfatases. In this study, we found that GAG sulfatases with identical substrate specificity show a high level of sequence identity and cluster together in the phylogenetic tree. By analyzing the sequences of Δ4,5hexuronate-2-O-sulfatases, including PB2SF and 2SFatri as well as 59 other potential genes reported in this paper, five signature sequences were obtained, which were found to construct the catalytic center of the enzymes. These findings will aid in the proper prediction and identification of GAG sulfatases, particularly Δ4,5hexuronate-2-O-sulfatases, and provide important information for research on the substrate specificity of these enzymes.
Photobacterium is a common genus of marine bacteria that is often found in association with marine organisms such as fish (Ast and Dunlap, 2005); however, we report for the first time that this bacterium can utilize animal polysaccharide GAGs, indicating that Photobacterium may be involved in some physiological and pathological processes in marine animals. Indeed, various putative genes of GAG-degrading enzymes, such as lyases and sulfatases, have been found in the genomes of the pathogenic bacteria of marine fish, including Vibrio and Photobacterium (Rivas et al., 2013; Guardiola et al., 2014). Although researchers believe that these enzymes are important virulence factors of fish as well as humans (Lafisca et al., 2008), the biochemical characteristics and pathogenic mechanisms of these enzymes remain to be investigated. Our findings will provide a basis for these types of studies.
Conclusion
Studies showing the importance of bacterial GAG sulfatases in the degradative metabolism and structural analysis of sulfated GAGs have led to an urgent demand for systematic biochemical and bioinformatic studies on various GAG sulfatases with different substrate specificities. The present study addresses this issue.
Author Contributions
SW conducted most of the experiments, analyzed the results, and wrote the manuscript under the guidance of FL. JG conducted for sulfatase site-directed mutagenesis experiments. QZ and XC conducted the experiments involving sulfatase expression. FL conceived the idea for the project and wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 31570071 and 31300664), the National Natural Science Foundation of Shandong Province (Grant No. ZR2018BC013), the Science and Technology Development Project of Shandong Province (Grant Nos. 2018GSF121002 and 2016GGH4502), the Science and Technology Special Project of Shandong Province (Grant No. 2015zdjs04002), the Independent Innovation Plan of Colleges and Universities in Jinan (Grant No. 201401242), and the Fundamental Research Funds of Shandong University (Grant No. 2015JC002).
Conflict of Interest Statement
XC was employed by company Dongying Tiandong Pharmaceutical Company, Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.00704/full#supplementary-material
Footnotes
- ^ https://swissmodel.expasy.org/
- ^ http://www.pymol.org
- ^ http://weblogo.threeplusone.com/
- ^ https://prosite.expasy.org/
References
Afratis, N., Gialeli, C., Nikitovic, D., Tsegenidis, T., Karousou, E., Theocharis, A. D., et al. (2012). Glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J. 279, 1177–1197. doi: 10.1111/j.1742-4658.2012.08529.x
Ast, J. C., and Dunlap, P. V. (2005). Phylogenetic resolution and habitat specificity of members of the Photobacterium phosphoreum species group. Environ. Microbiol. 7, 1641–1654. doi: 10.1111/j.1462-2920.2005.00859.x
Barbeyron, T., Brillet-Guéguen, L., Carré, W., Carrière, C., Caron, C., Czjzek, M., et al. (2016a). Matching the diversity of sulfated biomolecules: creation of a classification database for sulfatases reflecting their substrate specificity. PLoS One 11:e0164846. doi: 10.1371/journal.pone.0164846
Barbeyron, T., Thomas, F., Barbe, V., Teeling, H., Schenowitz, C., Dossat, C., et al. (2016b). Habitat and taxon as driving forces of carbohydrate catabolism in marine heterotrophic bacteria: example of the model algae-associated bacterium Zobellia galactanivorans DsijT. Environ. Microbiol. 18, 4610–4627. doi: 10.1111/1462-2920.13584
Berteau, O., and Mulloy, B. (2003). Sulfated fucans, fresh perspectives: structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 13, 29R–40R. doi: 10.1093/glycob/cwg058
Biasini, M., Bienert, S., Waterhouse, A., Arnold, K., Studer, G., Schmidt, T., et al. (2014). SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 42, W252–W258. doi: 10.1093/nar/gku340
Bigge, J. C., Patel, T. P., Bruce, J. A., Goulding, P. N., Charles, S. M., and Parekh, R. B. (1995). Nonselective and efficient fluorescent labeling of glycans using 2-amino benzamide and anthranilic acid. Anal. Biochem. 230, 229–238. doi: 10.1006/abio.1995.1468
Bishop, J. R., Schuksz, M., and Esko, J. D. (2007). Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446, 1030–1037. doi: 10.1038/nature05817
Bitter, T., and Muir, H. M. (1962). A modified uronic acid carbazole reaction. Anal. Biochem. 4, 330–334. doi: 10.1016/0003-2697(62)90095-7
Boltes, I., Czapinska, H., Kahnert, A., von Bulow, R., Dierks, T., Schmidt, B., et al. (2001). 1.3 A structure of arylsulfatase from Pseudomonas aeruginosa establishes the catalytic mechanism of sulfate ester cleavage in the sulfatase family. Structure 9, 483–491. doi: 10.1016/S0969-2126(01)00609-8
Bond, C. S., Clements, P. R., Ashby, S. J., Collyer, C. A., Harrop, S. J., Hopwood, J. J., et al. (1997). Structure of a human lysosomal sulfatase. Structure 5, 277–289. doi: 10.1016/S0969-2126(97)00185-8
Bramhall, S., Noack, N., Wu, M., and Loewenberg, J. R. (1969). A simple colorimetric method for determination of protein. Anal. Biochem. 31, 146–148. doi: 10.1016/0003-2697(69)90251-6
Carlsson, P., Presto, J., Spillmann, D., Lindahl, U., and Kjellen, L. (2008). Heparin/heparan sulfate biosynthesis: processive formation of N-sulfated domains. J. Biol. Chem. 283, 20008–20014. doi: 10.1074/jbc.M801652200
Crooks, G. E., Hon, G., Chandonia, J. M., and Brenner, S. E. (2004). WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190. doi: 10.1101/gr.849004
Demydchuk, M., Hill, C. H., Zhou, A., Bunkoczi, G., Stein, P. E., Marchesan, D., et al. (2017). Insights into Hunter syndrome from the structure of iduronate-2-sulfatase. Nat. Commun. 8:15786. doi: 10.1038/ncomms15786
Dhoot, G. K., Gustafsson, M. K., Ai, X., Sun, W., Standiford, D. M., and Emerson, C. J. (2001). Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science 293, 1663–1666. doi: 10.1126/science.293.5535.1663
Dierks, T., Miech, C., Hummerjohann, J., Schmidt, B., Kertesz, M. A., and von Figura, K. (1998). Posttranslational formation of formylglycine in prokaryotic sulfatases by modification of either cysteine or serine. J. Biol. Chem. 273, 25560–25564. doi: 10.1074/jbc.273.40.25560
Ernst, S., Langer, R., Cooney, C. L., and Sasisekharan, R. (1995). Enzymatic degradation of glycosaminoglycans. Crit. Rev. Biochem. Mol. Biol. 30, 387–444. doi: 10.3109/10409239509083490
Ghatak, S., Maytin, E. V., Mack, J. A., Hascall, V. C., Atanelishvili, I., Moreno, R. R., et al. (2015). Roles of proteoglycans and glycosaminoglycans in wound healing and fibrosis. Int. J. Cell Biol. 2015:834893. doi: 10.1155/2015/834893
Guardiola, F. A., Cuesta, A., Abellán, E., Meseguer, J., and Esteban, M. A. (2014). Comparative analysis of the humoral immunity of skin mucus from several marine teleost fish. Fish Shellfish Immun. 40, 24–31. doi: 10.1016/j.fsi.2014.06.018
Han, W., Wang, W., Zhao, M., Sugahara, K., and Li, F. (2014). A novel eliminase from a marine bacterium that degrades hyaluronan and chondroitin sulfate. J. Biol. Chem. 289, 27886–27898. doi: 10.1074/jbc.M114.590752
Hanson, S. R., Best, M. D., and Wong, C. (2004). Sulfatases: structure, mechanism, biological activity, inhibition, and synthetic utility. Angew. Chem. Int. Ed. 43, 5736–5763. doi: 10.1002/anie.200300632
Kertesz, M. A. (2000). Riding the sulfur cycle metabolism of sulfonates and sulfate esters in Gram-negative bacteria. FEMS Microbiol. Rev. 24, 135–175. doi: 10.1016/S0168-6445(99)00033-9
Kozak, W., Dasko, M., Maslyk, M., Kubinski, K., Rachon, J., and Demkowicz, S. (2015). Steroid sulfatase inhibitors based on phosphate and thiophosphate flavone analogs. Drug Dev. Res. 76, 450–462. doi: 10.1002/ddr.21281
Kumar, S., Stecher, G., and Tamura, K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Lafisca, A., Pereira, C. S., Giaccone, V., and Rodrigues, D. P. (2008). Enzymatic characterization of Vibrio alginolyticus strains isolated from bivalves harvested at Venice Lagoon (Italy) and Guanabara Bay (Brazil). Rev. Inst. Med. Trop. São Paulo 50, 199–202. doi: 10.1590/S0036-46652008000400002
Lukatela, G., Krauss, N., Theis, K., Selmer, T., Gieselmann, V., von Figura, K., et al. (1998). Crystal structure of human arylsulfatase A: the aldehyde function and the metal ion at the active site suggest a novel mechanism for sulfate ester hydrolysis. Biochemistry 37, 3654–3664. doi: 10.1021/bi9714924
Maccarana, M., Olander, B., Malmström, J., Tiedemann, K., Aebersold, R., Lindahl, U., et al. (2006). Biosynthesis of dermatan sulfate. J. Biol. Chem. 281, 11560–11568. doi: 10.1074/jbc.M513373200
Malleron, A., Benjdia, A., Berteau, O., and Le Narvor, C. (2012). Chondroitin-4-O-sulfatase from Bacteroides thetaiotaomicron: exploration of the substrate specificity. Carbohyd. Res. 353, 96–99. doi: 10.1016/j.carres.2012.03.033
Morimoto-Tomita, M., Uchimura, K., Werb, Z., Hemmerich, S., and Rosen, S. D. (2002). Cloning and characterization of two extracellular heparin-degrading endosulfatases in mice and humans. J. Biol. Chem. 277, 49175–49185. doi: 10.1074/jbc.M205131200
Myette, J. R., Shriver, Z., Claycamp, C., McLean, M. W., Venkataraman, G., and Sasisekharan, R. (2003). The heparin/heparan sulfate 2-O-sulfatase from Flavobacterium heparinum: molecular cloning, recombinant expression, and biochemical characterization. J. Biol. Chem. 278, 12157–12166. doi: 10.1074/jbc.M211420200
Myette, J. R., Soundararajan, V., Shriver, Z., Raman, R., and Sasisekharan, R. (2009). Heparin/heparan sulfate 6-O-sulfatase from Flavobacterium heparinum: integrated structural and biochemical investigation of enzyme active site and substrate specificity. J. Biol. Chem. 284, 35177–35188. doi: 10.1074/jbc.M109.053801
Pavao, M. S. (2014). Glycosaminoglycans analogs from marine invertebrates: structure, biological effects, and potential as new therapeutics. Front. Cell Infect. Microbiol. 4:123. doi: 10.3389/fcimb.2014.00123
Poole, A. R. (1986). Proteoglycans in health and disease: structures and functions. Biochem. J. 236, 1–14. doi: 10.1042/bj2360001
Prabhakar, V., Capila, I., Bosques, C. J., Pojasek, K., and Sasisekharan, R. (2005). Chondroitinase ABC I from Proteus vulgaris: cloning, recombinant expression and active site identification. Biochem. J. 386, 103–112. doi: 10.1042/BJ20041222
Raman, R., Myette, J. R., Shriver, Z., Pojasek, K., Venkataraman, G., and Sasisekharan, R. (2003). The heparin/heparan sulfate 2-O-sulfatase from Flavobacterium heparinum: a structural and biochemical study of the enzyme active site and saccharide substrate specificity. J. Biol. Chem. 278, 12167–12174. doi: 10.1074/jbc.M211425200
Recksiek, M., Selmer, T., Dierks, T., Schmidt, B., and von Figura, K. (1998). Sulfatases, trapping of the sulfated enzyme intermediate by substituting the active site formylglycine. J. Biol. Chem. 273, 6096–6103. doi: 10.1074/jbc.273.11.6096
Rivas, A. J., Lemos, M. L., and Osorio, C. R. (2013). Photobacterium damselae subsp. damselae, a bacterium pathogenic for marine animals and humans. Front. Microbiol. 4:283. doi: 10.3389/fmicb.2013.00283
Rivera-Colon, Y., Schutsky, E. K., Kita, A. Z., and Garman, S. C. (2012). The structure of human GALNS reveals the molecular basis for mucopolysaccharidosis IV A. J. Mol. Biol. 423, 736–751. doi: 10.1016/j.jmb.2012.08.020
Sambrook, J., and Russell, D. W. (2006). SDS-polyacrylamide gel electrophoresis of proteins. CSH Protoc. 2006:pdb.prot4540. doi: 10.1101/pdb.prot4540
Sambrook, J., and Russell, D. W. (2001). Molecular Cloning: A Laboratory Manual. New York, NY: Cold Spring Harbor Laboratory.
Schmidt, B., Selmer, T., Ingendoh, A., and von Figura, K. (1995). A novel amino acid modification in sulfatases that is defective in multiple sulfatase deficiency. Cell 82, 271–278. doi: 10.1016/0092-8674(95)90314-3
Sogi, K. M., Gartner, Z. J., Breidenbach, M. A., Appel, M. J., Schelle, M. W., and Bertozzi, C. R. (2013). Mycobacterium tuberculosis Rv3406 is a type II alkyl sulfatase capable of sulfate scavenging. PLoS One 8:e65080. doi: 10.1371/journal.pone.0065080
Sugahara, K., and Kitagawa, H. (2000). Recent advances in the study of the biosynthesis and functions of sulfated glycosaminoglycans. Curr. Opin. Struc. Biol. 10, 518–527. doi: 10.1016/S0959-440X(00)00125-1
Sugahara, K., and Kojima, T. (1996). Specificity studies of bacterial sulfatases by means of structurally defined sulfated oligosaccharides isolated from shark cartilage chondroitin sulfate D. Eur. J. Biochem. 239, 865–870. doi: 10.1111/j.1432-1033.1996.0865u.x
Ulmer, J. E., Vilen, E. M., Namburi, R. B., Benjdia, A., Beneteau, J., Malleron, A., et al. (2014). Characterization of glycosaminoglycan (GAG) sulfatases from the human gut symbiont Bacteroides thetaiotaomicron reveals the first GAG-specific bacterial endosulfatase. J. Biol. Chem. 289, 24289–24303. doi: 10.1074/jbc.M114.573303
Vasconcelos, A., and Pomin, V. (2017). The sea as a rich source of structurally unique glycosaminoglycans and mimetics. Microorganisms 5:51. doi: 10.3390/microorganisms5030051
von Bulow, R., Schmidt, B., Dierks, T., von Figura, K., and Uson, I. (2001). Crystal structure of an enzyme-substrate complex provides insight into the interaction between human arylsulfatase A and its substrates during catalysis. J. Mol. Biol. 305, 269–277. doi: 10.1006/jmbi.2000.4297
Waldow, A., Schmidt, B., Dierks, T., von Bulow, R., and von Figura, K. (1999). Amino acid residues forming the active site of arylsulfatase A. Role in catalytic activity and substrate binding. J. Biol. Chem. 274, 12284–12288. doi: 10.1074/jbc.274.18.12284
Wang, S., Sugahara, K., and Li, F. (2016). Chondroitin sulfate/dermatan sulfate sulfatases from mammals and bacteria. Glycoconj. J. 33, 841–851. doi: 10.1007/s10719-016-9720-0
Wang, W., Han, W., Cai, X., Zheng, X., Sugahara, K., and Li, F. (2015). Cloning and characterization of a novel chondroitin sulfate/dermatan sulfate 4-O-endosulfatase from a marine bacterium. J. Biol. Chem. 290, 7823–7832. doi: 10.1074/jbc.M114.629154
Yamada, S. (2015). Catabolism of chondroitin sulfate. Cell. Mol. Biol. Lett. 20, 196–212. doi: 10.1515/cmble-2015-0011
Keywords: glycosaminoglycan, chondroitin sulfate, dermatan sulfate, sulfatase, signature sequence
Citation: Wang S, Guan J, Zhang Q, Chen X and Li F (2019) Identification and Signature Sequences of Bacterial Δ4,5Hexuronate-2-O-Sulfatases. Front. Microbiol. 10:704. doi: 10.3389/fmicb.2019.00704
Received: 04 December 2018; Accepted: 20 March 2019;
Published: 05 April 2019.
Edited by:
Hari S. Misra, Bhabha Atomic Research Centre, IndiaReviewed by:
Tristan Barbeyron, Centre National de la Recherche Scientifique (CNRS), FranceRup Lal, University of Delhi, India
Copyright © 2019 Wang, Guan, Zhang, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fuchuan Li, ZnVjaHVhbmxpQHNkdS5lZHUuY24=
 Shumin Wang
Shumin Wang Jingwen Guan
Jingwen Guan Qingdong Zhang
Qingdong Zhang Xiangxue Chen2
Xiangxue Chen2 Fuchuan Li
Fuchuan Li