- 1Department of Microbiology, Tumor and Cell Biology, Karolinska Institutet, Bioclinicum, Stockholm, Sweden
- 2Department of Clinical Microbiology, Karolinska University Hospital, Stockholm, Sweden
- 3Singapore Centre for Environmental Life Sciences Engineering (SCELSE) and Lee Kong Chian School of Medicine (LKC), Nanyang Technological University (NTU), Singapore, Singapore
Microglia have a pivotal role in the pathophysiology of bacterial meningitis. The goal of this review is to provide an overview on how microglia respond to bacterial pathogens targeting the brain, how the interplay between microglia and bacteria can be studied experimentally, and possible ways to use gained knowledge to identify novel preventive and therapeutic strategies. We discuss the dual role of microglia in disease development, the beneficial functions crucial for bacterial clearing, and the destructive properties through triggering neuroinflammation, characterized by cytokine and chemokine release which leads to leukocyte trafficking through the brain vascular endothelium and breakdown of the blood-brain barrier integrity. Due to intrinsic complexity of microglia and up until recently lack of specific markers, the study of microglial response to bacterial pathogens is challenging. New experimental models and techniques open up possibilities to accelerate progress in the field. We review existing models and discuss possibilities and limitations. Finally, we summarize recent findings where bacterial virulence factors are identified to be important for the microglial response, and how manipulation of evoked responses could be used for therapeutic or preventive purposes. Among promising approaches are: modulations of microglia phenotype switching toward anti-inflammatory and phagocytic functions, the use of non-bacterolytic antimicrobials, preventing release of bacterial components into the neural milieu and consequential amplification of immune activation, and protection of the blood-brain barrier integrity.
Microglia in Bacterial Meningitis: Destructive or Helpful?
Bacterial meningitis is a life-threatening disease, predominantly caused by Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type b (Hib) in children and adults, and Escherichia coli K1 in neonates. Blood-borne bacteria invade and infect the brain tissue, resulting in a severe inflammatory response in the brain parenchyma and meninges (Iovino et al., 2016b). Current preventive and therapeutic strategies, including antibiotics and vaccines, have substantially improved the clinical outcome of bacterial meningitis, but the disease still represents a significant threat. This is highlighted by the emergence of resistant bacterial strains and rise of non-vaccine type strains (Doran et al., 2016). The disease burden varies between geographical regions, and prognosis is dependent on availability of health care and efficiency of vaccination programs (Grandgirard and Leib, 2010). Survivors of bacterial meningitis often suffer from neurological damage such as hearing loss, motor and cognitive impairment, largely caused by the host inflammatory response itself. While the resulting physical impairment often improves over time, the cognitive decline can persist lifelong especially in pediatric cases due to the sensitivity of the developing brain (Hoogman et al., 2007; Grandgirard and Leib, 2010).
Several mechanisms involving intrinsic virulence factors of bacteria and host defenses facilitate brain invasion and tissue injury (Gerber and Nau, 2010; Doran et al., 2016; Iovino et al., 2016a, 2017). Microglial cells are the most abundant and well-studied myeloid population of the Central Nervous System (CNS). They are ontogenetically distinct from other hematopoietic stem cell derived brain macrophages, as they arise in an earlier embryonic stage from yolk sac progenitors (Prinz and Priller, 2014). Microglias reside in the brain parenchyma and are considered the immune sentinels of the brain (Greter et al., 2015; Michels et al., 2015; Barichello et al., 2016). Once the bacteria gain access to the brain after breaching the blood-brain barrier (BBB), bacterial products such as lipopolysaccharides from Gram-negative bacteria and lipoteichoic acid from Gram-positive bacteria, are recognized by pattern recognition receptors (PRR) on microglia (Doran et al., 2016; Iovino et al., 2016a). Examples of other receptors expressed by microglia, involved in evoking inflammatory responses via damage signals derived from injured host cells, are purinergic receptors, tachykinin receptors, estrogen receptors (ER) and cannabinoid receptors (CB) (Stella, 2010; Burmeister et al., 2017; Tohidpour et al., 2017; Lee et al., 2018). Microglia can also serve as antigen presenting cells by bacterial processing and MHC class II upregulation, and together with astrocytes, microglia represent the primary source of complement in the brain (Almolda et al., 2011; Veerhuis et al., 2011).
Upon activation microglia undergo morphological changes, draw in their long ramifications associated with surveilling functions and obtain a larger amoeboid shape (Kettenmann et al., 2011; Iovino et al., 2013). In a reactive state, microglia release an array of soluble factors and exhibit migratory, proliferative and phagocytic properties adjusted to the type of activating stimuli. Reactive microglia can be further divided into M1 and M2 polarized states; the M1 “classically activated” phenotype is characterized by release of pro-inflammatory factors and the M2 “alternatively activated” is associated with enhanced phagocytosis and release of anti-inflammatory and neurotrophic factors (Cherry et al., 2014; Orihuela et al., 2016). However, such dichotomous classification is now considered oversimplified as microglia exists on a spectrum of functional phenotypes (Sousa et al., 2017). A more accurate framework to classify microglial phenotypic states under healthy, diseased and developmental stages remains to be agreed upon (Ransohoff, 2016; Holtman et al., 2017). The release of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-6, and chemokines leads to increased permeability of the brain microvascular endothelium and upregulation of adhesion molecules, facilitating leukocyte recruitment into the brain (Gerber and Nau, 2010; Mook-Kanamori et al., 2011; Coutinho et al., 2013; Tohidpour et al., 2017). Activated microglia further contributes to leakage and breakdown of the BBB by producing reactive oxygen and nitrogen species, and matrix metalloproteases (MMP) (da Fonseca et al., 2014). The increased intracranial pressure and the self-amplifying inflammatory environment ultimately results in respiratory burst and neuronal cell death (Coutinho et al., 2013; Doran et al., 2016).
Microglial activation, in particular the M1 skewed response, is a hallmark of neuroinflammation and thus microglia are fundamental players in the pathophysiology of bacterial meningitis. In the latest years, an extensive effort has been put into scrutinizing molecular mechanisms of microglial responses to brain pathogens. A handful of therapeutic strategies have been discovered and evaluated in experimental settings (Barichello et al., 2016). Attractive targets to manipulate the microglial response include modulation of phenotype switching toward M2, enhancing phagocytic functions, blocking pro-inflammatory mediators, promoting release of neurotrophic factors and protecting the BBB integrity (Hu et al., 2015). Here we discuss recent advances made in our understanding of how microglia react to bacteria and vice versa, and potential implication for improved therapeutic strategies.
Models and Considerations When Studying Microglial Reactivity to Pathogenic Bacteria
Patient derived post-mortem brain biopsies have provided valuable insights into the pathophysiology of bacterial meningitis, but these are only representative for fatal disease (Iovino et al., 2017). In the past few years several in vivo models have been developed, including mice, rat and rabbit models, inoculated with bacteria either intranasally, intravenously, intrathecally or by other routes (Koedel and Pfister, 1999; Hoogland et al., 2015; Iovino et al., 2016b). Disease development is usually monitored by clinical scoring of symptoms and cerebrospinal fluid (CSF) diagnosis. Pathogen inoculation into the cisterna magna and CSF sampling are more readily performed on rabbits and rats than mice, due to their larger size (Paul et al., 2003). However, mouse models represent other advantages reflected by availability of genetically modified models and biological reagents to study relevant pathways.
Several in vitro models of microglia exist to study microglia characteristics. Those include the immortalized cell lines BV2 (murine), HAPI (rat) and HMC3 (human) (Dello Russo et al., 2018; Timmerman et al., 2018). Primary cultures of freshly isolated microglia and stem cell-derived microglia represent a better biological model than cell lines in mimicking normal physiology of microglia (Timmerman et al., 2018). Ex vivo microglial response has been studied using organotypic slice cultures from mice, hamsters, rats and non-human primates (Czapiga and Colton, 1999; Burmeister et al., 2017). Microglia display a broad spectrum of different phenotypes, they are involved in different functions, and are sensitive to changes in the neural environment and adjust accordingly. Hence, the transcriptomic signature changes dramatically after a few days of culturing outside of their normal physiological niche which represents a challenge, especially when studying or comparing to homeostatic state of microglia (Galatro et al., 2017). Moreover, established protocols for microglial isolation and cell sorting have been reported to lead to artifacts in downstream analysis, by contamination of ingested material from the surroundings and induction of activation signature. RiboTag translatome profiling, a recently developed technique for gene expression analysis, has been suggested to limit artifacts and improve accuracy when studying functional states of microglia (Haimon et al., 2018).
The majority of the studies on microglial responses to peripheral inflammatory stimuli, or direct bacterial stimuli, have been conducted in mice, whereas activation has been determined by measuring ionized calcium-binding adapter molecule 1 (Iba-1), CD68, CD11b or Toll-like receptor (TLR) surface expression and TNFα and IL1β mRNA protein levels (Hoogland et al., 2015). In addition, the use of knockout mice in experimental meningitis, deficient in key cytokines, adhesion molecules and proteases, has contributed to a more complete understanding of the involvement of inflammatory pathways and which steps may be targeted for therapeutic purposes (Paul et al., 2003).
Until recently it has been difficult to distinguish microglia from other related myeloid cells due to large overlaps in genetic signature and surface markers. TMEM119 is the first marker discovered which is exclusively expressed by microglia in healthy and diseased states, in both mice and humans, opening up new venues for microglia research (Bennett et al., 2016). Other potential microglia-specific markers discovered by recent transcriptomic profiling, include P2Y12, Siglec-H, olfactomedin-like 3 and Sal1 (Colonna and Butovsky, 2017; Holtman et al., 2017). Transgenic mice with GFP labeled microglia, under the promoter of the fractalkine receptor or Iba-1, have been generated and are excellent tools for imaging the healthy brain, but less useful when studying disease states since both the fractalkine receptor and Iba-1 are expressed by peripherally derived cells present in the brain under pathological conditions (Jung et al., 2000; Hirasawa et al., 2005). Likewise, depletion of microglia has been challenging. After partial or complete eradication of microglia, other cells such as peripherally derived monocytes serve as microglia progenitors and repopulate the brain shortly after depletion (Lund et al., 2018). Sustained microglia depletion can be achieved by continuous pharmacological administration of inhibitors of colony-stimulating factor 1 receptor (CSF-1R). Microglia are the only immune cells in the CNS that express CSF-1R, and microglial development and survival is highly dependent on CSF-1R signaling (Chitu et al., 2016; Han et al., 2017). In contrast, liposomal clodronate microinjections into selected anatomical regions of the brain have been used to selectively deplete microglia within that region (Torres et al., 2016). Very recently, the specific inhibitor of colony-stimulating factor 1, PLX5622, was administered dietary to continuously deplete microglia in two studies of viral encephalitis. In both studies microglia were found to have a neuroprotective role (Waltl et al., 2018; Wheeler et al., 2018) Finally, zebrafish is emerging as a model for studying the functionality and development of microglia (Lyons and Talbot, 2014).
Appropriate modeling and availability of relevant markers is crucial to advance our understanding of the microglia-pathogen interplay. Animal models have proven useful to study the pathogenesis of bacterial meningitis, and identify and evaluate potential novel therapeutic targets. The inherent complexity of microglia, reflected by the heterogeneity of activation states, demands careful consideration of choice/combination of markers and models when monitoring physiological and pathophysiological responses of this multi-tasking cell type.
Exploring Therapeutic Targets: Inflammatory Signaling, Phagocytosis, Protecting the Blood-Brain Barrier Integrity
Various anti-inflammatory, blocking agents, and prophylactic stimulators targeting relevant signaling events, summarized in Figure 1, have shown promising results in promoting survival and preventing injury in experimental bacterial meningitis.
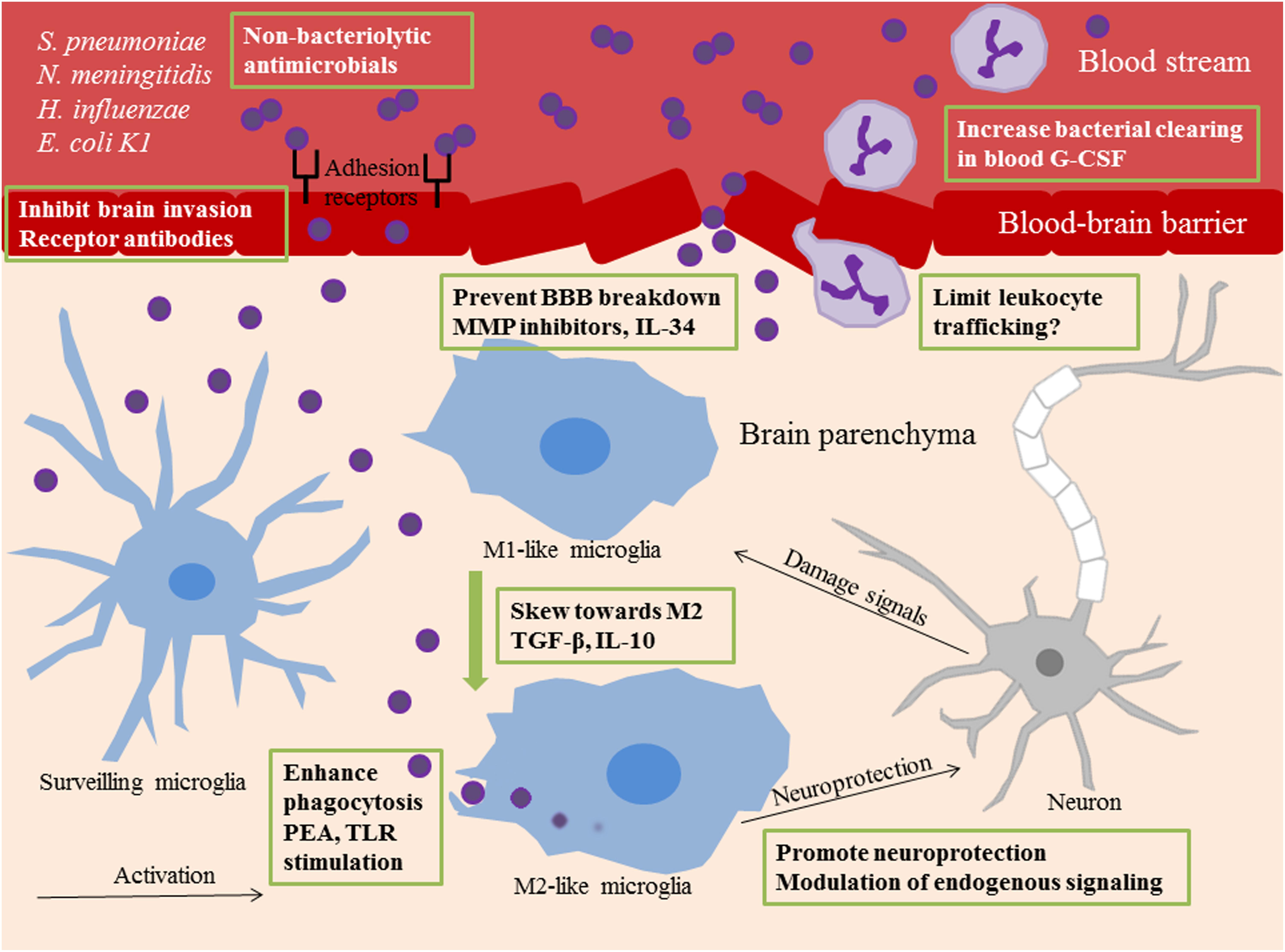
Figure 1. Summary of novel therapeutic targets in bacterial meningitis. Different therapeutic strategies are shown within green frames. Surveilling microglia recognize invading bacteria or bacterial components and undergo activation. M1 polarized microglia are potent propagators of neuroinflammation. M2 polarized microglia have phagocytic and anti-inflammatory properties. In bacterial meningitis, phenotypical shift toward M2 functions can be acquired by administration of the anti-inflammatory cytokines TGF-β and IL-10. Phagocytic uptake of bacteria by microglia can be enhanced with prophylactic TLR stimulation or PEA pretreatment. The use of non-bacteriolytic antimicrobials prevents release of bacterial components into the external milieu which can feed the inflammatory response. Blockade of receptors on the blood brain endothelium prevents bacterial invasion of the brain. BBB breakdown is a consequence of neuroinflammation and can be limited with MMP inhibitors and IL-34 administration. G-CSF increases neutrophil numbers in the circulation and enhances bacterial clearing from the blood. Pharmacological blockade of leukocyte trafficking into the brain can be deleterious for disease outcome, but different approaches in limiting infiltration may be more successful. Neurogenesis and neuroprotection can be promoted via modulation of endogenous signaling pathways. TGF-β, Transforming growth factor beta; IL-10, interleukin-10; TLR, Toll-like receptor; PEA, Palmitoylethanomide; BBB, blood-brain barrier; MMP, matrix metalloproteases; IL-34, interleukin-34; G-CSF, granulocyte colony-stimulating factor.
Inhibition of deleterious functions of microglia, associated with the classically activated phenotype M1, represents an attractive option for adjuvant therapy to antimicrobials (Barichello et al., 2015, 2016). Enhancement of the neuroprotective functions of homeostatic and the alternatively activated phenotype M2 microglia has been explored as therapeutic option in many neurological diseases, including bacterial meningitis (Cherry et al., 2014; Barichello et al., 2016; Chen and Trapp, 2016). Several strategies for skewing the inflammatory responses toward anti-inflammatory functions have been applied in experimental settings, in rodents and cell cultures in vitro, and also in the clinic in the case of corticosteroid and glycerol (Gerber and Nau, 2010). Efficacy of adjuvant Corticosteroid treatment has been debated, but has only been proven beneficial in adult patients with pneumococcal meningitis in high-income countries (Liechti et al., 2015). The anti-inflammatory cytokine IL-10 was shown to inhibit production of inflammatory cytokines in microglia and astrocytes isolated from mice previously challenged with the Gram-negative bacteria Borrelia burgdorferi and N. meningitidis, injected into the cerebroventricular space (Rasley et al., 2006). Induction of microglia of the M2 phenotype is dependent on TGF-β signaling, and it has been proposed that activated astrocytes act as source of TGF-β in the presence of IL-10, which in turn attenuates pro-inflammatory cytokine production by microglia and upregulation of anti-inflammatory mediators (Zhou et al., 2012; Norden et al., 2014). Recently, therapeutic effects of TGF-β administration were evaluated in the context of hemorrhagic stroke. TGF-β was injected directly into the brains of mice with induced intracerebral hemorrhage, and microglial responses were recorded. Microglia showed an overall dampened inflammatory profile and the treated mice underwent a quicker functional recovery. The same effects were observed in stroke patients, whereas initial increased plasma TGF-β concentrations were an indicator for improved clinical outcome (Taylor et al., 2017). TGF-β could therefore be a strong candidate as a future therapeutic agent in the treatment of acute brain injury, including bacterial meningitis. However, since activation of TGF-β has been postulated to have a role in hydrocephalus development, through induction of subarachnoid fibrosis, this approach may hold some drawbacks (Botfield et al., 2013). Microglia express PRRs able to recognize bacterial products, these include TLRs and NOD-like receptors (NLRs). NOD2, a member of the NLR family, has been shown to be crucial in propagating inflammation in response to Gram-positive and Gram-negative bacteria (Liu et al., 2010). TLR1/2, TLR4 and TLR9 have been studied most extensively in experimental bacterial meningitis. Prophylactic stimulation of these receptors resulted in increased phagocytic uptake and intracellular killing of S. pneumoniae and E. coli K1 by microglia (Ribes et al., 2009, 2010). Inhibition of TLR signaling has also been explored. The small molecule drug ibrutinib, a tyrosine kinase inhibitor, was shown to regulate TLR4 signaling in microglia by dampening LPS induced pro-inflammatory responses (Nam et al., 2018). The protein tyrosine kinase inhibitor AG126 reduced disease severity induced by pneumococcal cell walls in a similar way by blocking leukocyte influx to the brain and TNFα production (Hanisch et al., 2001). One promising novel therapeutic approach is TLR stimulation in combination with a TGF-β receptor agonist. When treated with these compounds, primary microglia cells have shown increased phagocytic capacity and clearing of E. coli K1 without increasing release of proinflammatory cytokines or affecting cell viability (Diesselberg et al., 2018).
The role of the endogenous receptor for the tachykinin substance P, the neurokinin 1 (NK1) receptor, has recently been described as a potent propagator of neuroinflammation and neurotoxicity. NK1 receptor antagonists have been used as antidepressant and antiemetic drugs (Brain and Cox, 2006). Upon challenge with S. pneumoniae in mice, administration of the receptor antagonists led to reduced inflammation and increased protection of the brain (Burmeister et al., 2017). Distinctively, activation of ER-β is associated with neuroprotection through induction of brain-derived neurotrophic factor (BDNF). Some ginsenosides, bioactive molecules derived from the ginseng plant, can work as ER-β agonists. Ginsenoside Rb1 treatment of mice with experimental meningitis rescued tissue damage to some extent, with greater effect in female mice (Lee et al., 2018). The protective properties of signaling through ER might explain the gender bias in cognitive impairment persistency after bacterial meningitis, where the male sex is a risk factor (Hoogman et al., 2007).
Stimulation of phagocytic functions of microglia could help clearing the brain from bacteria quicker and in that way resolve inflammation. Palmitoylethanomide (PEA) is an endogenous lipid that has been shown to have anti-inflammatory effects on various immune cells. In experimental meningoencephalitis or sepsis induced by E. coli K1 in mice, pretreatment with PEA resulted in improved survival. Microglia and macrophages pre-stimulated with PEA in vitro showed a dose-dependent higher uptake and intracellular killing of E. coli K1 bacteria (Redlich et al., 2014). PEA was recently shown to mediate its effect on microglial activation via the endocannabinoid system, promoting CB2 expression on microglia (Guida et al., 2017).
Several experimental therapeutic interventions aim at protecting the integrity of the BBB and limit leukocyte invasion into the brain, and thus prevent excessive microglial responses (Liechti et al., 2015). These include pharmacological inhibition of MMPs, in particular MMP9, which is elevated in the CSF of meningitis patients and implicated in BBB breakdown and associated with neurological sequela in bacterial meningitis. MMP inhibition, sometimes in combination with TNFα inhibitors, have neuroprotective effects in rat models of experimental bacterial meningitis (Grandgirard and Leib, 2010). The cytokine IL-34, mainly expressed by damaged neurons in the CNS, can bind to the CSF-1R receptor of microglia and was recently found to upregulate tight junctions in brain endothelial cells as well (Jin et al., 2014). We recently proposed that blockade of the BBB endothelial receptors PECAM-1 and pIgR, which the pneumococcus uses to invade the brain, could be a novel therapeutic approach. Mice treated with blocking antibodies in combination with ceftriaxone antibiotic reached a survival rate of 100% in a 10 day survival study, which was also characterized by attenuation of microglial activation (Iovino et al., 2018). Leukocyte trafficking into the brain contributes to destruction of the BBB, and recruited neutrophils contribute to elevated MMP levels. Blocking chemotaxis of immune cells into the brain was therefore suggested to have applications in preventing brain injury. However, survival of rats with experimental pneumococcal meningitis treated with fucoidin, an inhibitor of leukocyte rolling, was compromised reflected by elevated bacterial load in the blood and inability to clear the infection (Brandt et al., 2005). Recently, adjuvant granulocyte colony-stimulating factor (G-CSF) therapy, which increases the numbers of neutrophils in the circulation, was shown to have positive effects on spatial learning and neurogenesis in mice with experimental pneumococcal meningitis. G-CSF treatment had no effect on microglia or astrocyte activation (Schmidt et al., 2015).
In the case of the Gram-positive S. pneumoniae the polysaccharide capsule is one of the most potent activator of microglial pro-inflammatory responses via PPR signaling. In fact, administration of cell walls only was enough to generate CNS inflammation in mice (Tuomanen et al., 1985). The capsule confers protection against killing by the immune system, and in microglia encapsulated bacteria are phagocytized, but resistant to intracellular killing. Intracellular pneumococci were found to upregulate capsular genes and were not fused with phagolysosomes (Peppoloni et al., 2010, 2013). The pneumococcal surface protein PspA and the novel Spr1875 were shown to be involved in protecting bacteria from intracellular killing using mutant strains (Peppoloni et al., 2013; Ricci et al., 2013). Another major pneumococcal virulence factors is pneumolysin which has been associated with neuronal and microglial cell death (Braun et al., 2002). S. pneumoniae was found to induce pyroptosis in microglial cells in a pneumolysin dependent manner, partially counteracted by increased expression of autophagy related genes (Kim et al., 2015). A current standard treatment of pneumococcal meningitis is ceftriaxone, a cephalosporin antibiotic belonging to the β-lactam family. β-lactam antibiotics induce lysis of bacteria, leading to release of bacterial components such as pneumolysin and pneumococcal cell wall, which in turn further augments microglial activation and neuroinflammation. The use of a potent bactericidal, but non-bacteriolytic antibiotic, could therefore protect the brain from further immune activation and prevent neurological damage (Koedel et al., 2010; Liechti et al., 2015). An example of such an agent is daptomycin, which has been evaluated in a rabbit and infant rat experimental model of pneumococcal meningitis and found to have favorable characteristics in bacterial killing efficiency and attenuation of neuroinflammation (Stucki et al., 2007; Grandgirard et al., 2010). However, since daptomycin cannot cross the outer membrane of Gram-negative bacteria its bactericidal activity is restricted to Gram-positive bacteria (Randall et al., 2013). Another antibiotic which may have an application as adjuvant meningitis therapy is the tetracycline antibiotic minocycline, which was shown to have anti-inflammatory and neuroprotective properties in rats challenged with LPS, proposedly via inhibition of microglial activation (Zhu et al., 2014). Two recent studies explore the therapeutic advantage of using non-bacteriolytic antibiotic in combination with a MMP inhibitor or as adjunctive ceftriaxone treatment, in combination with anti-complement C5. Both studies reported improved neurofunctional outcome in mice and rats after experimental pneumococcal meningitis (Muri et al., 2018; Klein et al., 2019). Many factors affect the selection of antimicrobial therapy in bacterial meningitis, type of pathogen and respective bacteriostatic/bactericidal efficiency, BBB penetration and antibiotic resistance perspectives. The recent notion of how the release of bacterial components into the neural environment, which activates microglia to potentiate neuroinflammation, is likely to affect future therapeutic regimens for bacterial meningitis. Hence, this important aspect of the pathophysiology of bacterial meningitis should be considered when screening for novel treatment approaches or when revisiting older ones like minocycline (Whitby and Finch, 1986).
Concluding Remarks
Taken together, a growing body of evidence implicates microglia as central players in the pathogenesis and resolution of bacterial meningitis. High mortality and morbidity rates and imminent emergence of antimicrobial resistant cases highlight the need to identify and evaluate novel therapeutic targets. Employment of improved disease models and new tools to study the dynamics of microglial responses across diverse pathological conditions, represent great advantages for future studies. Our increasing knowledge of the pathophysiology of bacterial meningitis, and bacterial and host factors involved, will certainly contribute to the goal of improved preventative and therapeutic approaches in the management of bacterial meningitis.
Author Contributions
ST and FI wrote the literature study and wrote the manuscript. BH-N contributed in writing the manuscript.
Funding
This work was supported by grants from Jeansson Foundation, Åke Wiberg Foundation, Petrus and Augusta Hedlund Foundation, HKH Kronprinsessan Lovisa Association for Child Health, KID grant from Karolinska Institutet, Knut and Alice Wallenberg Foundation, the Swedish Research Council, the Stockholm County Council, and the Swedish Foundation for Strategic Research (SSF).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Almolda, B., Gonzalez, B., and Castellano, B. (2011). Antigen presentation in EAE: role of microglia, macrophages and dendritic cells. Front. Biosci. 16, 1157–1171.
Barichello, T., Collodel, A., Generoso, J. S., Simoes, L. R., Moreira, A. P., Ceretta, R. A., et al. (2015). Targets for adjunctive therapy in pneumococcal meningitis. J. Neuroimmunol. 278, 262–270. doi: 10.1016/j.jneuroim.2014.11.015
Barichello, T., Generoso, J. S., Simoes, L. R., Goularte, J. A., Petronilho, F., Saigal, P., et al. (2016). Role of microglial activation in the pathophysiology of bacterial meningitis. Mol. Neurobiol. 53, 1770–1781. doi: 10.1007/s12035-015-9107-4
Bennett, M. L., Bennett, F. C., Liddelow, S. A., Ajami, B., Zamanian, J. L., Fernhoff, N. B., et al. (2016). New tools for studying microglia in the mouse and human CNS. Proc. Natl. Acad. Sci. U.S.A. 113, E1738–E1746. doi: 10.1073/pnas.1525528113
Botfield, H., Gonzalez, A. M., Abdullah, O., Skjolding, A. D., Berry, M., McAllister, J. P., et al. (2013). Decorin prevents the development of juvenile communicating hydrocephalus. Brain 136(Pt 9), 2842–2858. doi: 10.1093/brain/awt203
Brain, S. D., and Cox, H. M. (2006). Neuropeptides and their receptors: innovative science providing novel therapeutic targets. Br. J. Pharmacol. 147(Suppl. 1), S202–S211. doi: 10.1038/sj.bjp.0706461
Brandt, C. T., Lundgren, J. D., Frimodt-Møller, N., Christensen, T., Benfield, T., Espersen, F., et al. (2005). Blocking of leukocyte accumulation in the cerebrospinal fluid augments bacteremia and increases lethality in experimental pneumococcal meningitis. J. Neuroimmunol. 166, 126–131. doi: 10.1016/j.jneuroim.2005.05.014
Braun, J. S., Sublett, J. E., Freyer, D., Mitchell, T. J., Cleveland, J. L., Tuomanen, E. I., et al. (2002). Pneumococcal pneumolysin and H(2)O(2) mediate brain cell apoptosis during meningitis. J. Clin. Invest. 109, 19–27. doi: 10.1172/JCI12035
Burmeister, A. R., Johnson, M. B., Chauhan, V. S., Moerdyk-Schauwecker, M. J., Young, A. D., Cooley, I. D., et al. (2017). Human microglia and astrocytes constitutively express the neurokinin-1 receptor and functionally respond to substance P. J. Neuroinflammation 14:245. doi: 10.1186/s12974-017-1012-5
Chen, Z., and Trapp, B. D. (2016). Microglia and neuroprotection. J. Neurochem. 136(Suppl. 1), 10–17. doi: 10.1111/jnc.13062
Cherry, J. D., Olschowka, J. A., and O’Banion, M. K. (2014). Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J. Neuroinflammation 11:98. doi: 10.1186/1742-2094-11-98
Chitu, V., Gokhan, S., Nandi, S., Mehler, M. F., and Stanley, E. R. (2016). Emerging roles for CSF-1 receptor and its ligands in the nervous system. Trends Neurosci. 39, 378–393. doi: 10.1016/j.tins.2016.03.005
Colonna, M., and Butovsky, O. (2017). Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 35,441–468. doi: 10.1146/annurev-immunol-051116-052358
Coutinho, L. G., Grandgirard, D., Leib, S. L., and Agnez-Lima, L. F. (2013). Cerebrospinal-fluid cytokine and chemokine profile in patients with pneumococcal and meningococcal meningitis. BMC Infect. Dis. 13:326. doi: 10.1186/1471-2334-13-326
Czapiga, M., and Colton, C. A. (1999). Function of microglia in organotypic slice cultures. J. Neurosci. Res. 56, 644–651.
da Fonseca, A. C., Matias, D., Garcia, C., Amaral, R., Geraldo, L. H., Freitas, C., et al. (2014). The impact of microglial activation on blood-brain barrier in brain diseases. Front. Cell. Neurosci. 8:362. doi: 10.3389/fncel.2014.00362
Dello Russo, C., Cappoli, N., Coletta, I., Mezzogori, D., Paciello, F., Pozzoli, G., et al. (2018). The human microglial HMC3 cell line: where do we stand? A systematic literature review. J. Neuroinflammation 15:259. doi: 10.1186/s12974-018-1288-0
Diesselberg, C., Ribes, S., Seele, J., Kaufmann, A., Redlich, S., Bunkowski, S., et al. (2018). Activin A increases phagocytosis of Escherichia coli K1 by primary murine microglial cells activated by toll-like receptor agonists. J. Neuroinflammation 15:175. doi: 10.1186/s12974-018-1209-2
Doran, K. S., Fulde, M., Gratz, N., Kim, B. J., Nau, R., Prasadarao, N., et al. (2016). Host-pathogen interactions in bacterial meningitis. Acta Neuropathol. 131, 185–209. doi: 10.1007/s00401-015-1531-z
Galatro, T. F., Holtman, I. R., Lerario, A. M., Vainchtein, I. D., Brouwer, N., Sola, P. R., et al. (2017). Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nat. Neurosci. 20, 1162–1171. doi: 10.1038/nn.4597
Gerber, J., and Nau, R. (2010). Mechanisms of injury in bacterial meningitis. Curr. Opin. Neurol. 23, 312–318. doi: 10.1097/WCO.0b013e32833950dd
Grandgirard, D., and Leib, S. L. (2010). Meningitis in neonates: bench to bedside. Clin. Perinatol. 37, 655–676. doi: 10.1016/j.clp.2010.05.004
Grandgirard, D., Oberson, K., Bühlmann, A., Gäumann, R., and Leib, S. L. (2010). Attenuation of cerebrospinal fluid inflammation by the nonbacteriolytic antibiotic daptomycin versus that by ceftriaxone in experimental pneumococcal meningitis. Antimicrob. Agents Chemother. 54, 1323–1326. doi: 10.1128/AAC.00812-09
Greter, M., Lelios, I., and Croxford, A. L. (2015). Microglia versus myeloid cell nomenclature during brain inflammation. Front. Immunol. 6:249. doi: 10.3389/fimmu.2015.00249
Guida, F., Luongo, L., Boccella, S., Giordano, M. E., Romano, R., Bellini, G., et al. (2017). Palmitoylethanolamide induces microglia changes associated with increased migration and phagocytic activity: involvement of the CB2 receptor. Sci. Rep. 7:375. doi: 10.1038/s41598-017-00342-1
Haimon, Z., Volaski, A., Orthgiess, J., Boura-Halfon, S., Varol, D., Shemer, A., et al. (2018). Re-evaluating microglia expression profiles using RiboTag and cell isolation strategies. Nat. Immunol. 19, 636–644. doi: 10.1038/s41590-018-0110-6
Han, J., Harris, R. A., and Zhang, X. M. (2017). An updated assessment of microglia depletion: current concepts and future directions. Mol. Brain 10:25. doi: 10.1186/s13041-017-0307-x
Hanisch, U. K., Prinz, M., Angstwurm, K., Hausler, K. G., Kann, O., Kettenmann, H., et al. (2001). The protein tyrosine kinase inhibitor AG126 prevents the massive microglial cytokine induction by pneumococcal cell walls. Eur. J. Immunol. 31, 2104–2115.
Hirasawa, T., Ohsawa, K., Imai, Y., Ondo, Y., Akazawa, C., Uchino, S., et al. (2005). Visualization of microglia in living tissues using Iba1-EGFP transgenic mice. J. Neurosci. Res. 81, 357–362. doi: 10.1002/jnr.20480
Holtman, I. R., Skola, D., and Glass, C. K. (2017). Transcriptional control of microglia phenotypes in health and disease. J. Clin. Invest. 127, 3220–3229. doi: 10.1172/JCI90604
Hoogland, I. C., Houbolt, C., van Westerloo, D. J., van Gool, W. A., and van de Beek, D. (2015). Systemic inflammation and microglial activation: systematic review of animal experiments. J. Neuroinflammation 12:114. doi: 10.1186/s12974-015-0332-6
Hoogman, M., van de Beek, D., Weisfelt, M., de Gans, J., and Schmand, B. (2007). Cognitive outcome in adults after bacterial meningitis. J. Neurol. Neurosurg. Psychiatry 78, 1092–1096. doi: 10.1136/jnnp.2006.110023
Hu, X., Leak, R. K., Shi, Y., Suenaga, J., Gao, Y., Zheng, P., et al. (2015). Microglial and macrophage polarization—new prospects for brain repair. Nat. Rev. Neurol. 11, 56–64. doi: 10.1038/nrneurol.2014.207
Iovino, F., Engelen-Lee, J. Y., Brouwer, M., van de Beek, D., van der Ende, A., Valls Seron, M., et al. (2017). pIgR and PECAM-1 bind to pneumococcal adhesins RrgA and PspC mediating bacterial brain invasion. J. Exp. Med. 214, 1619–1630. doi: 10.1084/jem.20161668
Iovino, F., Orihuela, C. J., Moorlag, H. E., Molema, G., and Bijlsma, J. J. (2013). Interactions between blood-borne Streptococcus pneumoniae and the blood-brain barrier preceding meningitis. PLoS One 8:e68408. doi: 10.1371/journal.pone.0068408
Iovino, F., Hammarlof, D. L., Garriss, G., Brovall, S., Nannapaneni, P., and Henriques-Normark, B. (2016a). Pneumococcal meningitis is promoted by single cocci expressing pilus adhesin RrgA. J. Clin. Invest. 126, 2821–2826. doi: 10.1172/JCI84705
Iovino, F., Seinen, J., Henriques-Normark, B., and van Dijl, J. M. (2016b). How does Streptococcus pneumoniae invade the brain? Trends Microbiol. 24, 307–315. doi: 10.1016/j.tim.2015.12.012
Iovino, F., Thorsdottir, S., and Henriques-Normark, B. (2018). Receptor blockade: a novel approach to protect the brain from pneumococcal invasion. J. Infect. Dis. 218, 476–484. doi: 10.1093/infdis/jiy193
Jin, S., Sonobe, Y., Kawanokuchi, J., Horiuchi, H., Cheng, Y., Wang, Y., et al. (2014). Interleukin-34 restores blood-brain barrier integrity by upregulating tight junction proteins in endothelial cells. PLoS One 9:e115981. doi: 10.1371/journal.pone.0115981
Jung, S., Aliberti, J., Graemmel, P., Sunshine, M. J., Kreutzberg, G. W., Sher, A., et al. (2000). Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114.
Kettenmann, H., Hanisch, U. K., Noda, M., and Verkhratsky, A. (2011). Physiology of microglia. Physiol. Rev. 91, 461–553. doi: 10.1152/physrev.00011.2010
Kim, J. Y., Paton, J. C., Briles, D. E., Rhee, D. K., and Pyo, S. (2015). Streptococcus pneumoniae induces pyroptosis through the regulation of autophagy in murine microglia. Oncotarget 6, 44161–44178. doi: 10.18632/oncotarget.6592
Klein, M., Hohne, C., Angele, B., Hogen, T., Pfister, H. W., Tufekci, H., et al. (2019). Adjuvant non-bacteriolytic and anti-inflammatory combination therapy in pneumococcal meningitis: an investigation in a mouse model. Clin. Microbiol. Infect. 25, 108.e9–108.e15. doi: 10.1016/j.cmi.2018.03.039
Koedel, U., Klein, M., and Pfister, H. W. (2010). New understandings on the pathophysiology of bacterial meningitis. Curr. Opin. Infect. Dis. 23, 217–223. doi: 10.1097/QCO.0b013e328337f49e
Koedel, U., and Pfister, H. W. (1999). Models of experimental bacterial meningitis. Role and limitations. Infect. Dis. Clin. North Am. 13, 549–577.
Lee, S., Lee, S. O., Kim, G. L., and Rhee, D. K. (2018). Estrogen receptor-beta of microglia underlies sexual differentiation of neuronal protection via ginsenosides in mice brain. CNS Neurosci. Ther. 24, 930–939. doi: 10.1111/cns.12842
Liechti, F. D., Grandgirard, D., and Leib, S. L. (2015). Bacterial meningitis: insights into pathogenesis and evaluation of new treatment options: a perspective from experimental studies. Future Microbiol. 10, 1195–1213. doi: 10.2217/fmb.15.43
Liu, X., Chauhan, V. S., Young, A. B., and Marriott, I. (2010). NOD2 mediates inflammatory responses of primary murine glia to Streptococcus pneumoniae. Glia 58, 839–847. doi: 10.1002/glia.20968
Lund, H., Pieber, M., Parsa, R., Han, J., Grommisch, D., Ewing, E., et al. (2018). Competitive repopulation of an empty microglial niche yields functionally distinct subsets of microglia-like cells. Nat. Commun. 9:4845. doi: 10.1038/s41467-018-07295-7
Lyons, D. A., and Talbot, W. S. (2014). Glial cell development and function in zebrafish. Cold Spring Harb. Perspect. Biol. 7:a020586. doi: 10.1101/cshperspect.a020586
Michels, M., Steckert, A. V., Quevedo, J., Barichello, T., and Dal-Pizzol, F. (2015). Mechanisms of long-term cognitive dysfunction of sepsis: from blood-borne leukocytes to glial cells. Intensive Care Med. Exp. 3:30. doi: 10.1186/s40635-015-0066-x
Mook-Kanamori, B. B., Geldhoff, M., van der Poll, T., and van de Beek, D. (2011). Pathogenesis and pathophysiology of pneumococcal meningitis. Clin. Microbiol. Rev. 24, 557–591. doi: 10.1128/CMR.00008-11
Muri, L., Grandgirard, D., Buri, M., Perny, M., and Leib, S. L. (2018). Combined effect of non-bacteriolytic antibiotic and inhibition of matrix metalloproteinases prevents brain injury and preserves learning, memory and hearing function in experimental paediatric pneumococcal meningitis. J. Neuroinflammation 15:233. doi: 10.1186/s12974-018-1272-8
Nam, H. Y., Nam, J. H., Yoon, G., Lee, J. Y., Nam, Y., Kang, H. J., et al. (2018). Ibrutinib suppresses LPS-induced neuroinflammatory responses in BV2 microglial cells and wild-type mice. J. Neuroinflammation 15:271. doi: 10.1186/s12974-018-1308-0
Norden, D. M., Fenn, A. M., Dugan, A., and Godbout, J. P. (2014). TGFβ produced by IL-10 redirected astrocytes attenuates microglial activation. Glia 62, 881–895. doi: 10.1002/glia.22647
Orihuela, R., McPherson, C. A., and Harry, G. J. (2016). Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 173, 649–665. doi: 10.1111/bph.13139
Paul, R., Koedel, U., and Pfister, H. W. (2003). Using knockout mice to study experimental meningitis. Arch. Immunol. Ther. Exp. 51, 315–326.
Peppoloni, S., Colombari, B., Beninati, C., Felici, F., Teti, G., Speziale, P., et al. (2013). The Spr1875 protein confers resistance to the microglia-mediated killing of Streptococcus pneumoniae. Microb. Pathog. 5, 42–47. doi: 10.1016/j.micpath.2013.04.002
Peppoloni, S., Ricci, S., Orsi, C. F., Colombari, B., De Santi, M. M., Messino, M., et al. (2010). The encapsulated strain TIGR4 of Streptococcus pneumoniae is phagocytosed but is resistant to intracellular killing by mouse microglia. Microbes Infect. 12, 990–1001. doi: 10.1016/j.micinf.2010.06.010
Prinz, M., and Priller, J. (2014). Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat. Rev. Neurosci. 15, 300–312. doi: 10.1038/nrn3722
Randall, C. P., Mariner, K. R., Chopra, I., and O’Neill, A. J. (2013). The target of daptomycin is absent from Escherichia coli and other gram-negative pathogens. Antimicrob. Agents Chemother. 57, 637–639. doi: 10.1128/AAC.02005-12
Ransohoff, R. M. (2016). A polarizing question: do M1 and M2 microglia exist? Nat. Neurosci. 19, 987–991. doi: 10.1038/nn.4338
Rasley, A., Tranguch, S. L., Rati, D. M., and Marriott, I. (2006). Murine glia express the immunosuppressive cytokine, interleukin-10, following exposure to Borrelia burgdorferi or Neisseria meningitidis. Glia 53, 583–592. doi: 10.1002/glia.20314
Redlich, S., Ribes, S., Schütze, S., and Nau, R. (2014). Palmitoylethanolamide stimulates phagocytosis of Escherichia coli K1 by macrophages and increases the resistance of mice against infections. J. Neuroinflammation 11:108. doi: 10.1186/1742-2094-11-108
Ribes, S., Ebert, S., Czesnik, D., Regen, T., Zeug, A., Bukowski, S., et al. (2009). Toll-like receptor prestimulation increases phagocytosis of Escherichia coli DH5alpha and Escherichia coli K1 strains by murine microglial cells. Infect. Immun. 77, 557–564. doi: 10.1128/IAI.00903-08
Ribes, S., Ebert, S., Regen, T., Agarwal, A., Tauber, S. C., Czesnik, D., et al. (2010). Toll-like receptor stimulation enhances phagocytosis and intracellular killing of nonencapsulated and encapsulated Streptococcus pneumoniae by murine microglia. Infect. Immun. 78, 865–871. doi: 10.1128/IAI.01110-09
Ricci, S., Gerlini, A., Pammolli, A., Chiavolini, D., Braione, V., Tripodi, S. A., et al. (2013). Contribution of different pneumococcal virulence factors to experimental meningitis in mice. BMC Infect. Dis. 13:444. doi: 10.1186/1471-2334-13-444
Schmidt, A. K., Reich, A., Falkenburger, B., Schulz, J. B., Brandenburg, L. O., Ribes, S., et al. (2015). Adjuvant granulocyte colony-stimulating factor therapy results in improved spatial learning and stimulates hippocampal neurogenesis in a mouse model of pneumococcal meningitis. J. Neuropathol. Exp. Neurol. 74, 85–94. doi: 10.1097/NEN.0000000000000152
Sousa, C., Biber, K., and Michelucci, A. (2017). Cellular and molecular characterization of microglia: a unique immune cell population. Front. Immunol. 8:198. doi: 10.3389/fimmu.2017.00198
Stella, N. (2010). Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 58, 1017–1030. doi: 10.1002/glia.20983
Stucki, A., Cottagnoud, M., Winkelmann, V., Schaffner, T., and Cottagnoud, P. (2007). Daptomycin produces an enhanced bactericidal activity compared to ceftriaxone, measured by [3H]choline release in the cerebrospinal fluid, in experimental meningitis due to a penicillin-resistant pneumococcal strain without lysing its cell wall. Antimicrob. Agents Chemother. 51, 2249–2252.
Taylor, R. A., Chang, C. F., Goods, B. A., Hammond, M. D., Mac Grory, B., Ai, Y., et al. (2017). TGF-β1 modulates microglial phenotype and promotes recovery after intracerebral hemorrhage. J. Clin. Invest. 127, 280–292. doi: 10.1172/JCI88647
Timmerman, R., Burm, S. M., and Bajramovic, J. J. (2018). An overview of in vitro methods to study Microglia. Front. Cell. Neurosci. 12:242. doi: 10.3389/fncel.2018.00242
Tohidpour, A., Morgun, A. V., Boitsova, E. B., Malinovskaya, N. A., Martynova, G. P., Khilazheva, E. D., et al. (2017). Neuroinflammation and infection: molecular mechanisms associated with dysfunction of neurovascular unit. Front. Cell. Infect. Microbiol. 7:276. doi: 10.3389/fcimb.2017.00276
Torres, L., Danver, J., Ji, K., Miyauchi, J. T., Chen, D., Anderson, M. E., et al. (2016). Dynamic microglial modulation of spatial learning and social behavior. Brain Behav. Immun. 55, 6–16. doi: 10.1016/j.bbi.2015.09.001
Tuomanen, E., Tomasz, A., Hengstler, B., and Zak, O. (1985). The relative role of bacterial cell wall and capsule in the induction of inflammation in pneumococcal meningitis. J. Infect. Dis. 151, 535–540.
Veerhuis, R., Nielsen, H. M., and Tenner, A. J. (2011). Complement in the brain. Mol. Immunol. 48, 1592–1603. doi: 10.1016/j.molimm.2011.04.003
Waltl, I., Kaufer, C., Gerhauser, I., Chhatbar, C., Ghita, L., Kalinke, U., et al. (2018). Microglia have a protective role in viral encephalitis-induced seizure development and hippocampal damage. Brain Behav. Immun. 74, 186–204. doi: 10.1016/j.bbi.2018.09.006
Wheeler, D. L., Sariol, A., Meyerholz, D. K., and Perlman, S. (2018). Microglia are required for protection against lethal coronavirus encephalitis in mice. J. Clin. Invest. 128, 931–943. doi: 10.1172/JCI97229
Whitby, M., and Finch, R. (1986). Bacterial meningitis. Rational selection and use of antibacterial drugs. Drugs 31, 266–278.
Zhou, X., Spittau, B., and Krieglstein, K. (2012). TGFβ signalling plays an important role in IL4-induced alternative activation of microglia. J. Neuroinflammation 9:210. doi: 10.1186/1742-2094-9-210
Keywords: microglia, bacterial meningitis, blood-brain barrier, experimental models, therapeutic strategies
Citation: Thorsdottir S, Henriques-Normark B and Iovino F (2019) The Role of Microglia in Bacterial Meningitis: Inflammatory Response, Experimental Models and New Neuroprotective Therapeutic Strategies. Front. Microbiol. 10:576. doi: 10.3389/fmicb.2019.00576
Received: 13 December 2018; Accepted: 06 March 2019;
Published: 25 March 2019.
Edited by:
Bi-Hung Peng, The University of Texas Medical Branch at Galveston, United StatesReviewed by:
Richard F. Keep, University of Michigan, United StatesTatiana Barichello, The University of Texas Health Science Center at Houston, United States
Copyright © 2019 Thorsdottir, Henriques-Normark and Iovino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Federico Iovino, ZmVkZXJpY28uaW92aW5vQGtpLnNl
 Sigrun Thorsdottir
Sigrun Thorsdottir Birgitta Henriques-Normark
Birgitta Henriques-Normark Federico Iovino
Federico Iovino